
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 20 September 2022
Sec. Virus and Host
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.963239
This article is part of the Research Topic Interaction Between Coronavirus and Hosts View all 19 articles
Coronavirus Disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to considerable morbidity and mortality worldwide. The clinical manifestation of COVID-19 ranges from asymptomatic or mild infection to severe or critical illness, such as respiratory failure, multi-organ dysfunction or even death. Large-scale genetic association studies have indicated that genetic variations affecting SARS-CoV-2 receptors (angiotensin-converting enzymes, transmembrane serine protease-2) and immune components (Interferons, Interleukins, Toll-like receptors and Human leukocyte antigen) are critical host determinants related to the severity of COVID-19. Genetic background, such as 3p21.31 and 9q34.2 loci were also identified to influence outcomes of COVID-19. In this review, we aimed to summarize the current literature focusing on human genetic factors that may contribute to the observed diversified severity of COVID-19. Enhanced understanding of host genetic factors and viral interactions of SARS-CoV-2 could provide scientific bases for personalized preventive measures and precision medicine strategies.
Coronavirus Disease 2019 (COVID-19) was caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the first case reported in December 2019, it has been spreading worldwide and was announced a global pandemic in March 2020 (Taylor, 2022). COVID-19 presents a wide spectrum of manifestations, ranging from asymptomatic infection to critical clinical course (Figure 1). Though most cases are now known to be asymptomatic or mild, approximately 15% of infected patients developed severe disease and 5% progressed to critical status, leading to deleterious acute respiratory distress syndrome (ARDS), multi-organ dysfunction and death (Baj et al., 2020; Zhou et al., 2020).
Several risk factors that could predict the severity of disease have been identified, including age, male gender, smoking, underlying comorbidities such as hypertension, diabetes mellitus, cardiac disease, chronic lung disease and cancer, clinically apparent immunodeficiencies, local immunodeficiencies and pregnancy (Williamson et al., 2020; Grasselli et al., 2021). Nevertheless, these conditions do not fully explain the variability in COVID-19 disease severity between individuals, and severe cases were observed in young individuals without pre-existing medical conditions, sometimes clustering in families, suggesting genetic background might be a risk factor (Yousefzadegan and Rezaei, 2020).
Several gene variants of infected patients were reported to explain the different levels of severity among individuals and their outcomes, which may provide a better understanding of host protein-SARS-CoV-2 interactions. Also, it sheds light on stratifying individuals according to risk, thus allowing for the prior protection of those at greater risk, and ideally, for innovative personalized treatments. To this end, we conduct a review on current studies focusing on associations between human genetic factors and the level of severity of COVID-19.
There are two distinct biological steps relevant to the severe presentation of COVID-19: viral recognition and immune responses (Figure 2). First, the spike protein (S) on SARS-CoV-2 binds to the host ACE2 (Angiotensin-2 Conversion Enzyme) receptor (Dong et al., 2020). Following the receptor binding, Transmembrane and Serine Protease 2 (TMPRSS2) will trigger a proteolytic cleavage of the S domains to mediate membrane fusion (Hoffmann et al., 2020). Paired basic amino acid-cleaving enzyme (Furin) can also catalyze S protein proteolytic cleavage.
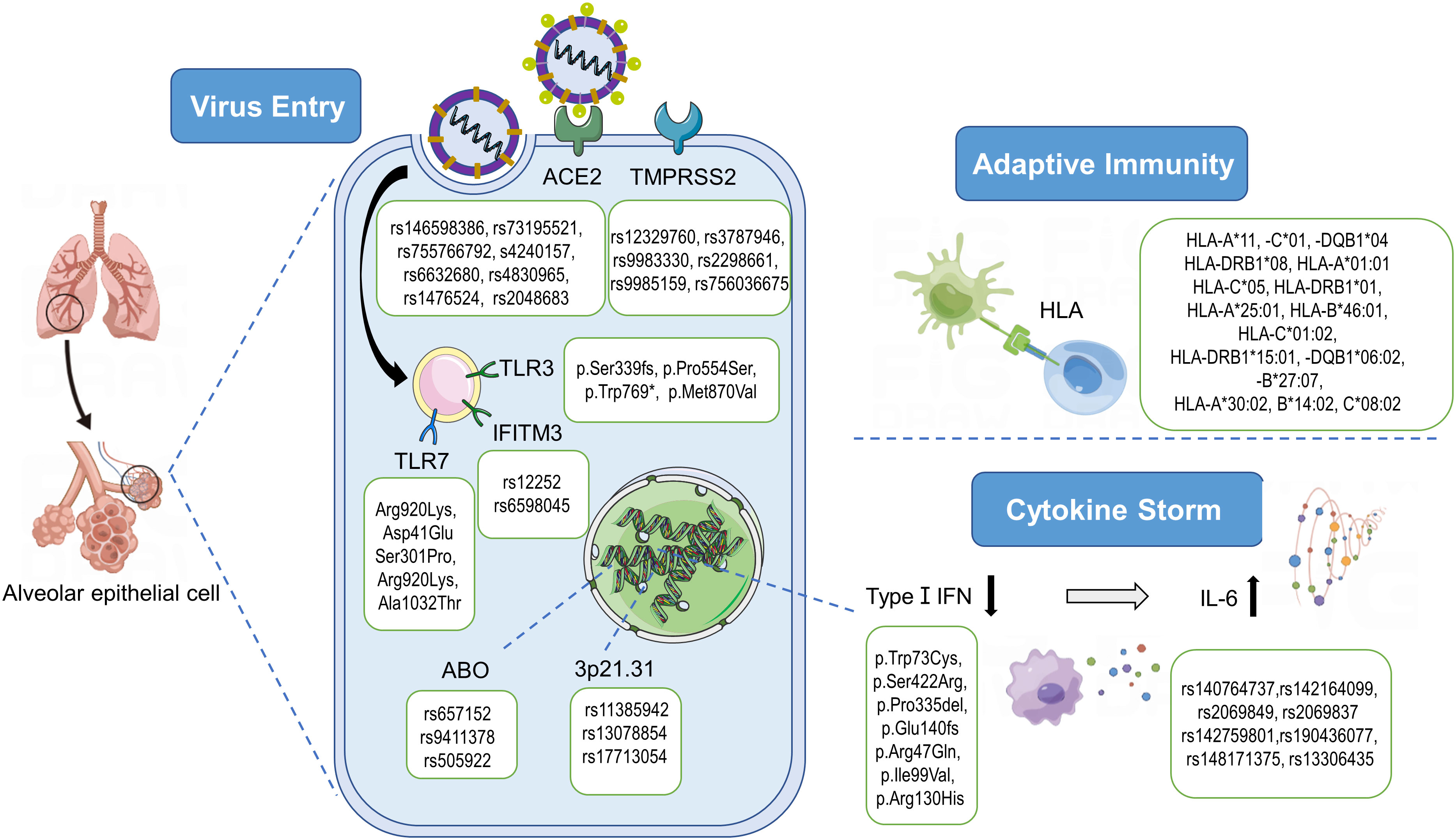
Figure 2 Pathogenesis of SARS-CoV-2 and genetic variants associated with severe COVID-19. After the recognition of ACE2 and the priming by TMPRSS2, SARS-CoV-2 enters the cell and starts the replication process. The innate immune response signaling cascade starts with the recognition of pathogen‐associated molecular patterns (PAMPs) by endosomal toll‐like receptors (TLRs). SARS-CoV-2 is able to inhibit the type I IFN responses in infected cells, leading to the cytokine storm characterized by an increase of inflammatory cytokines/chemokines such as IL-6. As an antiviral mechanism, antigen-presenting cells present antigenic peptides through the Major Histocompatibility Complex (MHC) class I and class II molecules to T cells. And 3p21.31 and ABO loci are significantly associated with Covid‐19 severity. ACE2, angiotensin‐converting enzyme‐2; TMPRSS2, transmembrane serine protease‐2; HLA, human leukocyte antigen; IFN, interferon; TLR, Toll-like receptor 7; IFITM3, interferon induced transmembrane protein 3; IL, Interleukin. By Figdraw (www.figdraw.com).
After the virus entering a target cell, the innate immune response is initiated with the recognition of SARS-CoV-2 by pattern recognition receptors such as Toll-like receptors (TLRs) 3, 7, 8 and 9. The TLR3 response triggers the activation of NOD-Like Receptor family and Pyrin domain-containing 3 protein (NLRP3) inflammasome pathway, which induces caspase-1-dependent cleavage and secretion of key proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 (Brodin, 2021), inducing inflammation and coagulopathy (Hosseini et al., 2020). In adaptive immunity, T cells recognize a bimolecular complex of an epitope bound to the Major Histocompatibility Complex (MHC) class I and II. CD4+ T cells play a critical antiviral role through promoting the secretion of pathogen-specific antibodies, whereas CD8+ T cells reduce the viral burden by killing the infected cells. It has been reported that T cells in critical patients seemed to be more active (Maamari et al., 2022). SARS-CoV-2 also triggers a robust B cell response, as IgM, IgG, IgA and neutralizing IgG antibodies can be detected in a few days after infection (Grifoni et al., 2021).
Commonly, the proinflammatory cytokines activate immune cells, notably monocytes and T lymphocytes, which clean the lung infection and help the patient recover. However, in serious cases, uncontrolled systemic hyper-inflammation, called “cytokine storm”, may occur (Kim et al., 2021). Though the pathogenesis of cytokine storm is not yet elucidated, two stages of the cytokine storm has been considered: the first stage is a temporary immune-deficient condition in which early responses of type I Interferon (IFN) are impaired; the secondary stage is an overactive immune state to compensate for the failure of target clearance (Blanco-Melo et al., 2020; McGonagle et al., 2020). A key feature of SARS-CoV-2 is its capability to shut down hosts’ IFN production, leading to a delayed or even overall suppressed type I IFN response (Zhang et al., 2021). An immune analysis performed in critical COVID-19 patients showed a low level of IFN activity and downregulation of IFN stimulated genes. On the contrary, cytokine and chemokine-related genes such as IL-6 and TNF-α were found to be increasingly expressed (Hadjadj et al., 2020). Taken together, hyper-inflammatory responses followed by impaired IFN signaling pathway are likely to determine the severity of COVID-19.
Considering the pathogenesis of COVID-19, the gene variants described for disease severity were classified whether they were related to host entry mechanism, immune system or other genes associated with severity of COVID-19. Gene variants that show protective or risk factors on the severity of COVID-19 are summarized in Table 1.
ACE2 is widely expressed in human tissues, especially in upper and lower respiratory tracts, heart, kidney, testis and gastrointestinal system (Bourgonje et al., 2020). Apart from the main receptor for SARS-CoV-2, ACE2 is also well-known for its downregulating the renin-angiotensin system (RAS), which is important for modulating the cardiovascular system (Bakhshandeh et al., 2021). However, the function of ACE2 is lost following the binding of virus, which may cause inflammation, thrombosis and death.
The expression level of ACE2 receptor, which differs among individuals across different ages, genders and ethnicities, potentially affects the severity of COVID-19. Based on the latest genome-wide association summary statistics for severe COVID-19, a recent study indicated an increased risk of severe COVID-19 for individuals who had genetically raised levels of circulating ACE2 protein (Yang et al., 2022). They also found that the variant rs4830984 was nominally significantly associated with severe COVID-19. A retrospective examination of nasal epithelium among people of different ages showed that expression level of ACE2 gene was low in younger children but increased with age (Bunyavanich et al., 2020), which may explain why children have fewer and less severe symptoms compared with adults (Patel and Verma, 2020). In addition, compared with women, men are 65% more likely to develop severe complications or even die from COVID-19. This gender difference could be explained by the well-established role of androgen receptor signaling in modulating ACE2 transcription, as well as its location on X chromosome (Samuel et al., 2020). Also, the ACE2 gene expression varies among ethnic populations. A recent study based on expression quantitative trait locus (eQTL) found that people from an Arab background had lower levels of ACE2 compared with Europeans, possibly led to lower mortality in this population (Al-Mulla et al., 2020).
Genetic variations in ACE2 may affect its binding with SARS-CoV-2 and the subsequent infection severity. Several missense changes, such as p.(Asn720Asp), p.(Lys26Arg), and p.(Gly211Arg), can affect the protein structure and stabilization, and therefore influence the internalization process of the virus (Benetti et al., 2020). Some other variants, such as rs961360700, are known to cause an increase in affinity for S protein (MacGowan et al., 2022; Ren et al., 2022). Accumulating evidence suggests that polymorphisms in ACE2 gene may modulate inflammatory responses and thus may aggravate pulmonary and systemic injuries (Li et al., 2020). In a cohort of Russian COVID-19 patients, several rare ACE2 variants (including rs146598386, rs73195521, and rs755766792) tended to cause an active inflammatory response to infection, which partially explained the variation of disease severity (Shikov et al., 2020). In another study, six variants (re4240157, rs6632680, rs1548474, rs4820965, rs1476524 and rs2048683) out of 61 evaluated ones were identified to be markedly associated with hospitalization (Wooster et al., 2020). A recent genome-wide association study (GWAS) identified a rare variant, rs190509934, that downregulated ACE2 expression and reduced disease severity among COVID-19 patients (Horowitz et al., 2022).
Nevertheless, the relationship between ACE2 polymorphism and COVID-19 severity remain controversial. A negative correlation between ACE2 expression and COVID-19 fatality at both population and molecular levels was reported (Chen et al., 2020; El Baba and Herbein, 2020). In addition, ACE2 genetic variants were analyzed by whole-exome sequencing (WES) in 137 DNA samples of COVID-19 patients, compared with the 536 age-matched controls. They found that ACE2 polymorphism was not associated with an increased risk of critical illness (Gomez et al., 2020). However, they indicated that the balance between ACE1 and ACE2 played a role in the severity of COVID-19. Another study also revealed a strong correlation between ACE1 insertion/deletion (I/D) genotype with COVID-19 mortalities (Yamamoto et al., 2020). Larger cohort of severe/critical patients and further functional studies are required to reveal the role of ACE2 genotypes in COVID-19.
The TMPRSS2 gene, located on the human chromosome 21q22.3, encodes a serine protease enzyme that primes the S protein of SARS-CoV-2, allowing fusion of viral and cellular membrane (Baughn et al., 2020). TMPRSS2 is a key gene in prostate cancer and its transcription is regulated by androgen. Thus, TMPRSS2 expression and enzymatic activity was detected significantly higher in males than in females, which may explain the male predominance of higher severity and mortality (Alshahawey et al., 2020; Okwan-Duodu et al., 2021). Also, TMPRSS2 expression increases with aging in mice and humans, and this may relatively protect children from severe illness (Rossi et al., 2021; Schuler et al., 2021). The localization of the gene on 21q22.3 place Down syndrome individuals at high risk for critical illness (De Toma and Dierssen, 2021), and its oncogenic role may be related to poor outcomes of cancer patients with COVID-19 as well (Stopsack et al., 2020).
Seven variants (rs3787946, rs9983330, rs12329760, rs2298659, rs2298661, rs9985159 and rs756036675) within TMPRSS2 were identified to be associated with severe COVID-19 (Andolfo et al., 2021; Monticelli et al., 2021; Villapalos-Garcia et al., 2022). Among them, rs12329760 (p.Val197Met) emerged as a common variant that weakened TMPRSS2 protein stability and inhibited the binding of S protein and ACE2 (Wang et al., 2020). It played a protective role and appeared less in critical patients than in mild and general cases (Wang et al., 2020; Monticelli et al., 2021; David et al., 2022). However, rs12329760 (p.Val160Met) and 2 distinct haplotypes trigger higher TMPRSS2 expression may explain the significantly higher severity and mortality rates in Italy than those in East Asia (Asselta et al., 2020). It was suggested that more genotyping studies of COVID-19 was needed to explore the contribution of TMPRSS2 variants to clinical outcomes (Stopsack et al., 2020).
Interferons (IFNs) are a family of specialized cytokines central to antiviral immunity. Viral recognition induces IFN production, which in turn triggers the transcription of IFN-stimulated genes (ISGs), mediating antiviral responses (Yang et al., 2021). Specifically, type I IFNs are the first line of defense against viral infections, and IFN-I signaling is required for the recruitment of pro-inflammatory cells in the lung (Ramasamy and Subbian, 2021). It was reported that inborn errors of type I IFNs were the genetic and immunological basis of at least 15% of cases of critical COVID-19 pneumonia (Hadjadj et al., 2020).
IFN-I signaling is initiated by the binding of IFN-I to the interferon receptor (IFNAR) complex, composed of IFNAR1 and IFNAR2 at the same proximal location (Schreiber, 2020). IFNAR1 (p.Trp73Cys, p.Ser422Arg, p.Pro335del) and IFNAR2 (p.Glu140fs) variants were identified in patients with life-threatening COVID-19, highlighting the importance of type I IFN production in severe disease (Zhang et al., 2020a). A GWAS also reported that an intron variant rs2236757 in the IFNAR2 gene increased the odds of severe COVID-19 (Pairo-Castineira et al., 2021). Loss-of-function mutations in IFNAR2 including Tyr322Ter may increase susceptibility to critical COVID-19 infection, especially Asian descent populations, where this variant is more prevalent (Smieszek et al., 2021a).
The interferon-induced transmembrane proteins (IFITM) are a group of proteins localized in the plasma and endolysosomal membranes, preventing viruses from traversing the cellular lipid bilayer (Shaath et al., 2020). Homozygosity for the C allele of rs12252 within the IFITM3 gene was associated with the severity of COVID-19 (Zhang et al., 2020c). Rs34481144, another polymorphism of IFITM3, was reported to be associated with increased severity in influenza. It has been reported that the combined haplotypes of rs12252 and rs34481144 implicated in more severe outcomes of COVID-19 (Nikoloudis et al., 2020). However, a meta-analysis indicated that rs34481144 was not correlated to COVID-19 severity (Li et al., 2022).
2′‐5′‐Oligoadenylate synthase (OAS) family genes are induced by IFNs at the early phase of viral infection. Once in the right place, OAS1 binds to dsRNA structures of the SARS-CoV-2, leading to the viral RNA degradation and inhibition of viral replication (Wickenhagen et al., 2021). A common polymorphism in OAS1 (rs10774671), where the protective allele resulted in a more active OAS1 enzyme, probably led to less severe COVID-19 (Wickenhagen et al., 2021). On the contrary, decreased expression levels of OAS1 was implicated in COVID-19 disease severity (D'Antonio et al., 2021). Three variants (p.Arg47Gln, p.Ile99Val and p.Arg130His) were detected to impair OAS1 activity and weaken its bond with RNA (Klaassen et al., 2020). Also, a recent GWAS suggested that the variant rs10735079 was associated with critical illnesses in COVID-19 (Pairo-Castineira et al., 2021). In addition, OAS1 was identified as a putative new risk gene for Alzheimer’s disease, and 4 alleles within OAS1 gene were identified to contribute to both the high incidence of Alzheimer’s disease and critical illness of COVID-19 (Magusali et al., 2021).
As mentioned above, cytokine storm plays a critical role in severe COVID-19 cases, in which increased levels of cytokines are observed in plasma blood. Interleukin 6 (IL-6) is a soluble mediator in response to infections and tissue injuries (Tanaka et al., 2014). In COVID-19, critically ill patients showed significantly higher levels of IL-6, indicating that IL-6 was a strong predictor for disease severity and survival possibility (Zhang et al., 2020b). The association of IL-6 polymorphisms with cytokine expression and disease severity have been reported. Seven variants in IL-6 (rs140764737, rs142164099, rs2069849, rs142759801, rs190436077, rs148171375, rs13306435) and five variants in IL-6R (rs2228144, rs2229237, rs2228145, rs28730735, rs143810642) appeared to alter the binding of IL-6 and IL-6R, which can be implicated in the pathogenetic mechanisms associated with COVID-19 severity and its complications (Strafella et al., 2020). A recent GWAS found that the genetic variant rs2069837 in IL-6 decreased the expression of IL-6 in the serum and was protective against critical COVID-19 (Gong et al., 2022). An Asian-common IL-6 haplotype defined by promoter SNP rs1800796 and intronic SNPs rs1524107 and rs2066992 was detected to be associated with a lower risk of severe symptoms. Mechanistically, the protective allele disrupted the CTCF-binding locus at the IL-6 intron and resulted in attenuated IL-6 induction in response to viral infection (Chen et al., 2021). On the contrary, the minor allele rs2228145 was associated with higher plasma IL-6 levels in severe COVID-19 patients (Smieszek et al., 2021b).
Beyond IL-6, IL-1 is also a highly active proinflammatory cytokine. A Chinese cohort investigated 22.2 million genetic variants among 332 COVID-19 patients, rs6020298 within TMEM189-UBE2V1, a component of IL-1 signaling pathway, was found to be the most significant SNP associated with severity (Wang et al., 2020).
TLRs are a family comprised of 11 transmembrane proteins, which are crucial components in the initiation of innate immune responses (Szeto et al., 2021). TLRs recognize pathogen-associated molecular patterns and trigger the production of pro-inflammatory cytokines as well as type I and II interferons system. TLR3 is the most widely expressed TLR that binds to double‐stranded RNA viruses, while TLR7 and TLR8 recognize single‐stranded RNA viruses (Mantovani et al., 2022). Inborn errors of TLR3-dependent type I IFN immunity have been found in life‐threatening COVID‐19 patients, and eight genetic loci have been identified (Zhang et al., 2020a). The polymorphism L412 in TLR3 inhibited autophagy and made males at risk of severe COVID-19 (Croci et al., 2021). X-linked TLR7 deficiency has been identified as a novel immunodeficiency with an increased susceptibility to severe or critical COVID-19 infection and TLR7 has been established as a critical mediator of IFN-I immunity against the virus (Solanich et al., 2021). The burden of rare variants in TLR7 was found to be significantly higher in patients with severe COVID-19 in pan-ancestry WES data from the UK biobank (Kosmicki et al., 2021). A recent study identified loss‐of‐function variants of TLR7 (c.2129_2132del; p.Gln710Argfs*18; c.2383G>T; p.Val795Phe) in four severely affected young men from two unrelated families and among them found a lower production of IFNα and IFNγ proteins following stimulation (van der Made et al., 2020). Moreover, a nested case–control study identified TLR7 loss-of-function variants in 2.1% of severely affected males but in none of the asymptomatic participants (Fallerini et al., 2021).
Since the production of IFN is mediated via the TLR7 signaling pathway, therapies that directly stimulate endogenous TLR7 could have potential therapeutic benefit for the prevention and treatment of severe COVID-19 infection (Szeto et al., 2021). In addition, genetic variations in TLR7 that located on the X chromosome, may be a possible explanation of the sex biases in COVID-19 severity. Among women, TLR7 may escape X-inactivation, leading to higher basal expression levels and elevated downstream IFN responses (van der Made et al., 2020).
The Human Leukocyte Antigen (HLA) system, containing nearly 27,000 alleles in three distinct classes of genes (Class I, II and III), is the most highly polymorphic region in the human genome. HLA Classes I and II present antigenic peptides to T lymphocytes and enable the immune system to discriminate between self and foreign proteins (Lorente et al., 2021). In patients of COVID-19, different adaptive immune responses have been observed according to disease severity, including distinct IgM levels and S protein IgG titers (Ovsyannikova et al., 2020). As HLA plays a critical role in antigen presentation, different polymorphisms may potentially alter the severity of the disease.
Specific risk and protective HLA alleles for COVID-19 severity and mortality have been detected in several studies. A study evaluated the HLA binding affinity of all possible 8-mers to 12-mers from the SARS-CoV-2 proteome and noted three peptide-presenters (HLA-A*25:01, B*46:01, and C*01:02) that were most likely associated with severe infection (Nguyen et al., 2020). Another peptide binding prediction analyses showed that HLA-DRB1*08 alleles were unable to bind any of the viral peptides with high affinity, thus individuals with those alleles were at high risk of severe COVID-19 (Amoroso et al., 2021). Several studies concluded that HLA-A*11:01, HLA-B*51:01, HLA-C*14:02, HLA-DQB1*06:02 and HLA-B*27:0 were correlated with a higher COVID-19 mortality (Novelli et al., 2020; Wang et al., 2020; Shkurnikov et al., 2021). In contrast, HLA-A*02:01, HLA-A*03:01, HLA-B*18:01, HLA-C*07:01 and HLA-DRB1*11:04 showed an inverse relationship to the number of deaths (Novelli et al., 2020; Wang et al., 2020; Shkurnikov et al., 2021). HLA-A*11 was detected to predispose worse outcome of COVID-19 patients (Lorente et al., 2021), while another study suggested that HLA-A*11:01 could generate efficient antiviral responses (Tomita et al., 2020).
Considering the high gene density of HLA locus, it was suggested that complete HLA genotypes for each individual, rather than most frequent alleles, should be analyzed (Deng et al., 2021). Based on the allele frequency data of HLA in 74 countries, HLA-C*05 was identified as the most influential allele in increasing the mortality of COVID-19. Its receptor KIR2DS4fl is expressed on natural killer (NK) cells and recognizes viral peptides bound to HLA-C*05. It was hypothesized that this HLA-KIR pair induced immune hyperactivation and caused poor outcome (Sakuraba et al., 2020). An Italian study found that haplotype HLAA*01:01, HLA-B*08:01 and HLA-DRB1*03:01 contributed to the higher COVID-19 mortality in northern Italy. In contrast, HLA-B*18:01, HLA-C*07:01 and HLA-DRB1*11:04 directly correlated with the lower mortality in southern Italy (Pisanti et al., 2020).
Nevertheless, a study based on data from 6,919 infected individuals found that HLA genotypes as well as viral T-cell epitopes were not correlated with COVID-19 severity (Schetelig et al., 2021). More uniformly designed studies with the inclusion of global data are needed to clarify the role of single HLA alleles in COVID-19 severity. Furthermore, as COVID-19 may have variable potential epitopes with HLA complex, predicting good binds across HLA alleles may contribute to the design of an efficacious vaccine against COVID-19 (Prachar et al., 2020).
Apart from genes relevant to immune and SARS-CoV-2 receptors, other genetic variations have also been identified related to the severity of COVID-19. The association of loci 3p21.31 and 9q34.2 with COVID-19 severity were identified in two independent GWAS. The first study conducted in Italy and Spain revealed that rs11385942 at locus 3p21.31 and rs657152 at locus 9q34.2 were significantly associated with severe COVID‐19 with respiratory failure (Ellinghaus et al., 2020). And the second study found that rs13078854 at locus 3p21.31 and rs9411378 at locus 9q34.2 were risk alleles for severe COVID-19 phenotypes (Shelton et al., 2021). At locus 3p21.31, the association signal compromised 6 genes (SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6 and XCR1). Among them, SLC6A20 encodes a transporter that functionally interacts with ACE2 receptor. CXCR6 and CCR9 encode chemokine receptors that are implicated in T cell differentiation and recruitment. LZTFL1 encodes a cytosolic leucine-zipper protein widely expressed in pulmonary epithelial cells and regulates epithelial-mesenchymal transition (EMT), a viral response pathway (Downes et al., 2021). Recent studies found that rs35081325 and rs1024611 in LZTFL1, appeared to strongly associated with increased infection severity (Roberts et al., 2022; Ruter et al., 2022). And a study integrating expression quantitative trait locus (eQTL) mapping identified SLC6A20 and CXCR6 as causal genes that modulate COVID-19 risk (Kasela et al., 2021). However, another study identified CCR9 and SLC6A20 as potential target genes (Yao et al., 2021). As they all have a potentially relevant role in the pathophysiology of COVID-19, further studies will be needed to delineate effector genes at the 3p21.31 locus.
The association signal at locus 9q34.2 coincided with ABO locus, suggesting the role of ABO blood type in COVID-19 severity. It has been reported that A-group was a significant risk factor for developing a severe form of COVID-19, while O-group was protective against severe COVID19 illness or death (Gomez et al., 2021; Khasayesi et al., 2021). A recent replication analysis of reported COVID-19 genetic associations with eight phenotypes found that the lead ABO SNP, rs505922, replicated in all four susceptibility phenotypes and one severity phenotype (Roberts et al., 2022). It is still unclear how ABO blood types affect outcomes of COVID-19. A proteomic profiling analysis showed that the ABO locus mediated the risk by modulating CD209/DC-SIGN, a binding site for SARS-CoV-2 (Katz et al., 2020). Another study hypothesized that ABO blood group influenced the risk of venous thromboembolism, which is frequent in severe cases, by modifying glycosyltransferase activity (Ibrahim-Kosta et al., 2020).
ApoE is one of the highly co-expressed genes in type II alveolar cells in the lungs, and the ApoE e4e4 homozygous genotype was reported to increase the risk of severe COVID-19 (Kuo et al., 2020; Kurki et al., 2021). This may be explained by a regulatory mechanism underlying SARS-CoV-2 infection through ApoE interactions with ACE2 (Zhang et al., 2022).
Pedigree analysis in a Chinese family suggested that loss-of-function variants in GOLGA3 and DPP7 implicated in critically ill and asymptomatic COVID-19 patients as a monogenic factor (Wang et al., 2020). A GWAS performed in 2,244 critically ill patients with COVID-19 found significant associations in DPP9, CCR2 and TYK2, all of which could cause inflammatory lung injury (Pairo-Castineira et al., 2021). A recent study found that patients with the TT variant in the IFIH1 had an attenuated inflammatory response to severe SARS-CoV-2 infection, leading to better outcomes (Amado-Rodriguez et al., 2022).
In this review, we provided an overview of genetic variants associated with COVID-19 severity. The variants influence at least two distinct biological progress: viral entrance to host cells and development of harmful inflammation. The world is still suffering from the COVID-19 outbreak, with high fatality rate in severe and critical patients. Therefore, identifying genetic markers associated with clinical outcomes of COVID-19 is helpful for classifying and safeguarding individuals at high risk, as well as finding potential therapeutic targets.
Future genetic studies need further sharing of individual-level data, yet ethical considerations such as perfecting genetic information-related legislation should also be considered. Furthermore, large-scale systematic investigations of the functional polymorphisms of these genes combining data among different populations would pave the way for personalized preventive measures and precision medicine strategies.
All the authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication. All authors contributed to the article and approved the submitted version.
This study was funded by the National Key Research and Development Program of China (Nos. 2021YFC2701800).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Mulla, F., Mohammad, A., Al Madhoun, A., Haddad, D., Ali, H., Eaaswarkhanth, M., et al. (2020). A comprehensive germline variant and expression analyses of ACE2, TMPRSS2 and SARSCoV-2 activator FURIN genes from the middle East: Combating SARS-CoV-2 with precision medicine. Heliyon doi: 10.1101/2020.05.16.099176
Alshahawey, M., Raslan, M., Sabri, N. (2020). Sex-mediated effects of ACE2 and TMPRSS2 on the incidence and severity of COVID-19; the need for genetic implementation. Curr. Res. Transl. Med. 68 (4), 149–150. doi: 10.1016/j.retram.2020.08.002
Amado-Rodriguez, L., Salgado Del Riego, E., Gomez de Ona, J., Lopez Alonso, I., Gil-Pena, H., Lopez-Martinez, C., et al. (2022). Effects of IFIH1 rs1990760 variants on systemic inflammation and outcome in critically ill COVID-19 patients in an observational translational study. Elife 11, e73012. doi: 10.7554/eLife.73012
Amoroso, A., Magistroni, P., Vespasiano, F., Bella, A., Bellino, S., Puoti, F., et al. (2021). HLA and AB0 polymorphisms may influence SARS-CoV-2 infection and COVID-19 severity. Transplantation 105 (1), 193–200. doi: 10.1097/Tp.0000000000003507
Andolfo, I., Russo, R., Lasorsa, V. A., Cantalupo, S., Rosato, B. E., Bonfiglio, F., et al. (2021). Common variants at 21q22.3 locus influence MX1 and TMPRSS2 gene expression and susceptibility to severe COVID-19. Iscience 24 (4), 102322. doi: 10.1016/j.isci.2021.102322
Asselta, R., Paraboschi, E. M., Mantovani, A., Duga, S. (2020). ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging-Us 12 (11), 10087–10098. doi: 10.18632/aging.103415
Baj, J., Karakula-Juchnowicz, H., Teresinski, G., Buszewicz, G., Ciesielka, M., Sitarz, R., et al. (2020). COVID-19: Specific and non-specific clinical manifestations and symptoms: The current state of knowledge. J. Clin. Med. 9 (6), 1753. doi: 10.3390/jcm9061753
Bakhshandeh, B., Sorboni, S. G., Javanmard, A. R., Mottaghi, S. S., Mehrabi, M. R., Sorouri, F., et al. (2021). Variants in ACE2; potential influences on virus infection and COVID-19 severity. Infect. Genet. Evol. 90, 104773. doi: 10.1016/j.meegid.2021.104773
Baughn, L. B., Sharma, N., Elhaik, E., Sekulic, A., Bryce, A. H., Fonseca, R. (2020). Targeting TMPRSS2 in SARS-CoV-2 infection. Mayo Clin. Proc. 95 (9), 1989–1999. doi: 10.1016/j.mayocp.2020.06.018
Benetti, E., Tita, R., Spiga, O., Ciolfi, A. A.-O., Birolo, G., Bruselles, A., et al. (2020). ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur. J. Hum. Genet. 28 (11), 1602–1614. doi: 10.1038/s41431-020-0691-z
Blanco-Melo, D., Nilsson-Payant, B. E., Liu, W. C., Uhl, S., Hoagland, D., Moller, R., et al. (2020). Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181 (5), 1036–1045.e1039. doi: 10.1016/j.cell.2020.04.026
Bourgonje, A. R., Abdulle, A. E., Timens, W., Hillebrands, J. L., Navis, G. J., Gordijn, S. J., et al. (2020). Angiotensin-converting enzyme 2 (ACE2),SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 251 (3), 228–248. doi: 10.1002/path.5471
Brodin, P. (2021). Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 27 (1), 28–33. doi: 10.1038/s41591-020-01202-8
Bunyavanich, S., Do, A., Vicencio, A. (2020). Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323 (23), 2427–2429. doi: 10.1001/jama.2020.8707
Chen, J., Jiang, Q., Xia, X., Liu, K., Yu, Z., Tao, W., et al. (2020). Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 19 (7), e13168. doi: 10.1111/acel.13168
Chen, T., Lin, Y. X., Zha, Y., Sun, Y., Tian, J., Yang, Z., et al. (2021). A low-producing haplotype of interleukin-6 disrupting CTCF binding is protective against severe COVID-19. mBio 12 (5), 2150–7511. doi: 10.1128/mBio.01372-21
Croci, S., Venneri, M. A., Mantovani, S., Fallerini, C., Benetti, E., Picchiotti, N., et al. (2021). The polymorphism L412F in TLR3 inhibits autophagy and is a marker of severe COVID-19 in males. Autophagy 18 (7), 1662–1672. doi: 10.1080/15548627.2021.1995152
D'Antonio, M., Nguyen, J. P., Arthur, T. D., Matsui, H., Initiative, C.-H. G., D'Antonio-Chronowska, A., et al. (2021). SARS-CoV-2 susceptibility and COVID-19 disease severity are associated with genetic variants affecting gene expression in a variety of tissues. Cell Rep. 37 (7), 110020. doi: 10.1016/j.celrep.2021.110020
David, A., Parkinson, N., Peacock, T. P., Pairo-Castineira, E., Khanna, T., Cobat, A., et al. (2022). A common TMPRSS2 variant has a protective effect against severe COVID-19. Curr. Res. Trans. Med. 70 (2), 103333. doi: 10.1016/j.retram.2022.103333
Deng, H., Yan, X., Yuan, L. (2021). Human genetic basis of coronavirus disease 2019. Signal Transduct Target Ther. 6 (1), 344. doi: 10.1038/s41392-021-00736-8
De Toma, I., Dierssen, M. (2021). Network analysis of down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci. Rep. 11 (1), 1930. doi: 10.1038/s41598-021-81451-w
Dong, M., Zhang, J., Ma, X., Tan, J., Chen, L., Liu, S., et al. (2020). ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. BioMed. Pharmacother. 131, 110678. doi: 10.1016/j.biopha.2020.110678
Downes, D. J., Cross, A. R., Hua, P., Roberts, N., Schwessinger, R., Cutler, A. J., et al. (2021). Identification of LZTFL1 as a candidate effector gene at a COVID-19 risk locus. Nat. Genet. 53 (11), 1606–160+. doi: 10.1038/s41588-021-00955-3
Duncan, C. J. A., Skouboe, M. K., Howarth, S., Hollensen, A. K., Chen, R., Borresen, M. L., et al. (2022). Life-threatening viral disease in a novel form of autosomal recessive IFNAR2 deficiency in the Arctic. J. Exp. Med. 219 (6), e20212427. doi: 10.1084/jem.20212427
El Baba, R., Herbein, G. (2020). Management of epigenomic networks entailed in coronavirus infections and COVID-19. Clin. Epigenet. 12 (1), 118. doi: 10.1186/s13148-020-00912-7
Ellinghaus, D., Degenhardt, F., Bujanda, L., Buti, M., Albillos, A., Invernizzi, P., et al. (2020). Genomewide association study of severe covid-19 with respiratory failure. N Engl. J. Med. 383 (16), 1522–1534. doi: 10.1056/NEJMoa2020283
Fallerini, C., Daga, S., Mantovani, S., Benetti, E., Picchiotti, N., Francisci, D., et al. (2021). Association of toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. Elife 10, e67569. doi: 10.7554/eLife.67569
Gomez, J., Albaiceta, G. M., Garcia-Clemente, M., Garcia-Gala, J. M., Coto, E. (2021). DNA Genotyping of the ABO gene showed a significant association of the a-group (A1/A2 variants) with severe COVID-19. Eur. J. Internal Med. 88, 129–132. doi: 10.1016/j.ejim.2021.02.016
Gomez, J., Albaiceta, G. M., Garcia-Clemente, M., Lopez-Larrea, C., Amado-Rodriguez, L., Lopez-Alonso, I., et al. (2020). Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene 762, 145102. doi: 10.1016/j.gene.2020.145102
Gong, B., Huang, L. L., He, Y. Q., Xie, W., Yin, Y., Shi, Y., et al. (2022). A genetic variant in IL-6 lowering its expression is protective for critical patients with COVID-19. Signal Transduction Targeted Ther. 7 (1), 112. doi: 10.1038/s41392-022-00923-1
Grasselli, G., Greco, M., Zanella, A. (2021). Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy (vol 1802020). JAMA Internal Med. 181 (7), 1021–1021, pg 1345. doi: 10.1001/jamainternmed.2021.1229
Grifoni, A., Sidney, J., Vita, R., Peters, B., Crotty, S., Weiskopf, D., et al. (2021). SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe 29 (7), 1076–1092. doi: 10.1016/j.chom.2021.05.010
Hadjadj, J., Yatim, N., Barnabei, L., Corneau, A., Boussier, J., Smith, N., et al. (2020). Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369 (6504), 718–724. doi: 10.1126/science.abc6027
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Kruger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 (2), 271–27+. doi: 10.1016/j.cell.2020.02.052
Horowitz, J. E., Kosmicki, J. A., Damask, A., Sharma, D., Roberts, G. H. L., Justice, A. E., et al. (2022). Genome-wide analysis provides genetic evidence that ACE2 influences COVID-19 risk and yields risk scores associated with severe disease. Nat. Genet. 54 (4), 382–38+. doi: 10.1038/s41588-021-01006-7
Hosseini, A., Hashemi, V., Shomali, N., Asghari, F., Gharibi, T., Akbari, M., et al. (2020). Innate and adaptive immune responses against coronavirus. BioMed. Pharmacother. 132, 110859. doi: 10.1016/j.biopha.2020.110859
Ibrahim-Kosta, M., Bailly, P., Silvy, M., Saut, N., Suchon, P., Morange, P. E., et al. (2020). ABO blood group, glycosyltransferase activity and risk of venous thromboembolism. Thromb. Res. 193, 31–35. doi: 10.1016/j.thromres.2020.05.051
Kasela, S., Daniloski, Z., Bollepalli, S., Jordan, T. X., tenOever, B. R., Sanjana, N. E., et al. (2021). Integrative approach identifies SLC6A20 and CXCR6 as putative causal genes for the COVID-19 GWAS signal in the 3p21.31 locus. Genome Biol. 22 (1), 242. doi: 10.1186/s13059-021-02454-4
Katz, Dh, Tahir, U. A., Tahir Ua Fau - Ngo, D., Ngo D Fau - Benson, M. D., Benson Md Fau - Bick, A. G., Bick Ag Fau - Pampana, A., et al. (2020). Proteomic profiling in biracial cohorts implicates DC-SIGN as a mediator of genetic risk in COVID-19. doi: 10.1101/2020.06.09.20125690
Khalilzadeh, F., Sakhaee, F., Sotoodehnejadnematalahi, F., Zamani, M. S., Ahmadi, I., Anvari, E., et al. (2022). Angiotensin-converting enzyme 2 rs2285666 polymorphism and clinical parameters as the determinants of COVID-19 severity in Iranian population. Int. J. Immunogenet. 49 (5), 325–332. doi: 10.1111/iji.12598
Khasayesi, M., Hosseini-Khah, Z., Mirzaei, N., Ghasemian, R., Eslami, M., Kashi, Z. (2021). Association of ABO and rhesus blood groups with the severity of SARS-CoV-2 infection: A cross-sectional analytical study. J. Clin. Diagn. Res. 15 (12), Oc01–Oc05. doi: 10.7860/Jcdr/2021/51103.15716
Kim, Y. C., Jeong, B. H. (2021). Strong correlation between the case fatality rate of COVID-19 and the rs6598045 single nucleotide polymorphism (SNP) of the interferon-induced transmembrane protein 3 (IFITM3) gene at the population-level. Genes 12 (1), 24. doi: 10.3390/genes12010042
Kim, J. S., Lee, J. Y., Yang, J. W., Lee, K. H., Effenberger, M., Szpirt, W., et al. (2021). Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 11 (1), 316–329. doi: 10.7150/thno.49713
Klaassen, K., Stankovic, B., Zukic, B., Kotur, N., Gasic, V., Pavlovic, S., et al. (2020). Functional prediction and comparative population analysis of variants in genes for proteases and innate immunity related to SARS-CoV-2 infection. Infect. Genet. Evol. 84, 104498. doi: 10.1016/j.meegid.2020.104498
Kosmicki, J. A., Horowitz, J. E., Banerjee, N., Lanche, R., Marcketta, A., Maxwell, E., et al. (2021). Pan-ancestry exome-wide association analyses of COVID-19 outcomes in 586,157 individuals. Am. J. Hum. Genet. 108 (7), 1350–1355. doi: 10.1016/j.ajhg.2021.05.017
Kuo, C. L., Pilling, L. C., Atkins, J. L., Masoli, J. A. H., Delgado, J., Kuchel, G. A., et al. (2020). APOE e4 genotype predicts severe COVID-19 in the UK biobank community cohort. J. Gerontol A Biol. Sci. Med. Sci. 75 (11), 2231–2232. doi: 10.1093/gerona/glaa131
Kurki, S. N., Kantonen, J., Kaivola, K., Hokkanen, L., Mayranpaa, M. I., Puttonen, H., et al. (2021). APOE epsilon4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: a Finnish biobank, autopsy and clinical study. Acta Neuropathol. Commun. 9 (1), 199. doi: 10.1186/s40478-021-01302-7
Li, G., He, X., Zhang, L., Ran, Q., Wang, J., Xiong, A., et al. (2020). Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun 112, 102463. doi: 10.1016/j.jaut.2020.102463
Littera, R., Campagna, M., Deidda, S., Angioni, G., Cipri, S., Melis, M., et al. (2020). Human leukocyte antigen complex and other immunogenetic and clinical factors influence susceptibility or protection to SARS-CoV-2 infection and severity of the disease course. the sardinian experience. Front. Immunol. 11. doi: 10.3389/fimmu.2020.605688
Li, Y., Wei, L., He, L., Sun, J., Liu, N. (2022). Interferon-induced transmembrane protein 3 gene polymorphisms are associated with COVID-19 susceptibility and severity: A meta-analysis. J. Infect. 84 (6), 825–833. doi: 10.1016/j.jinf.2022.04.029
Lorente, L., Martin, M. M., Franco, A., Barrios, Y., Caceres, J. J., Sole-Violan, J., et al. (2021). HLA genetic polymorphisms and prognosis of patients with COVID-19. Med. Intensiva (Engl Ed) 45 (2), 96–103. doi: 10.1016/j.medin.2020.08.004
Maamari, K. A., Busaidi, I. A., Kindi, M. A., Zadjali, F., BaAlawi, F., Anesta, W., et al. (2022). Short and long-term immune changes in different severity groups of COVID-19 disease. Int. J. Infect. Dis. 122, 776–784. doi: 10.1016/j.ijid.2022.07.026
MacGowan, S. A., Barton, M. I., Kutuzov, M., Dushek, O., van der Merwe, P. A., Barton, G. J. (2022). Missense variants in human ACE2 strongly affect binding to SARS-CoV-2 spike providing a mechanism for ACE2 mediated genetic risk in covid-19: A case study in affinity predictions of interface variants. PloS Comput. Biol. 18 (3), e1009922. doi: 10.1371/journal.pcbi.1009922
Magusali, N., Graham, A. C., Piers, T. M., Panichnantakul, P., Yaman, U., Shoai, M., et al. (2021). A genetic link between risk for alzheimer's disease and severe COVID-19 outcomes via the OAS1 gene. Brain 144, 3727–3741. doi: 10.1093/brain/awab337
Mantovani, S., Daga, S., Fallerini, C., Baldassarri, M., Benetti, E., Picchiotti, N., et al. (2022). Rare variants in toll-like receptor 7 results in functional impairment and downregulation of cytokine-mediated signaling in COVID-19 patients. Genes Immun. 23 (1), 51–56. doi: 10.1038/s41435-021-00157-1
McGonagle, D., Sharif, K., O'Regan, A., Bridgewood, C. (2020). The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 19 (6), 102537. doi: 10.1016/j.autrev.2020.102537
Monticelli, M., Mele, B. H., Benetti, E., Fallerini, C., Baldassarri, M., Furini, S., et al. (2021). Protective role of a TMPRSS2 variant on severe COVID-19 outcome in young males and elderly women. Genes 12 (4), 596. doi: 10.3390/genes12040596
Nguyen, A., David, J. K., Maden, S. K., Wood, M. A., Weeder, B. R., Nellore, A., et al. (2020). Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J. Virol. 94 (13), e00510-20. doi: 10.1128/JVI.00510-20
Nikoloudis, D., Kountouras, D., Hiona, A. (2020). The frequency of combined IFITM3 haplotype involving the reference alleles of both rs12252 and rs34481144 is in line with COVID-19 standardized mortality ratio of ethnic groups in England. Peerj 8, e10402. doi: 10.7717/peerj.10402
Novelli, A., Andreani, M., Biancolella, M., Liberatoscioli, L., Passarelli, C., Colona, V. L., et al. (2020). HLA allele frequencies and susceptibility to COVID-19 in a group of 99 Italian patients. Hla 96 (5), 610–614. doi: 10.1111/tan.14047
Okwan-Duodu, D., Lim, E. C., You, S., Engman, D. M. (2021). TMPRSS2 activity may mediate sex differences in COVID-19 severity. Signal Transduction Targeted Ther. 6 (1), 100. doi: 10.1038/s41392-021-00513-7
Ovsyannikova, I. G., Haralambieva, I. H., Crooke, S. N., Poland, G. A., Kennedy, R. B. (2020). The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol. Rev. 296 (1), 205–219. doi: 10.1111/imr.12897
Pairo-Castineira, E., Clohisey, S., Klaric, L., Bretherick, A. D., Rawlik, K., Pasko, D., et al. (2021). Genetic mechanisms of critical illness in COVID-19. Nature 591 (7848), 92–98. doi: 10.1038/s41586-020-03065-y
Patel, A. B., Verma, A. (2020). Nasal ACE2 levels and COVID-19 in children. JAMA 323 (23), 2386–2387. doi: 10.1001/jama.2020.8946
Pisanti, S., Deelen, J., Gallina, A. M., Caputo, M., Citro, M., Abate, M., et al. (2020). Correlation of the two most frequent HLA haplotypes in the Italian population to the differential regional incidence of covid-19. J. Trans. Med. 18 (1), 352. doi: 10.1186/s12967-020-02515-5
Prachar, M., Justesen, S., Steen-Jensen, D. B., Thorgrimsen, S., Jurgons, E., Winther, O., et al. (2020). Identification and validation of 174 COVID-19 vaccine candidate epitopes reveals low performance of common epitope prediction tools. Sci. Rep. 10 (1), 20465. doi: 10.1038/s41598-020-77466-4
Ramasamy, S., Subbian, S. (2021). Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 34 (3), e00299-20. doi: 10.1128/CMR.00299-20
Ren, W., Zhu, Y., Lan, J., Chen, H., Wang, Y., Shi, H., et al. (2022). Susceptibilities of human ACE2 genetic variants in coronavirus infection. J. Virol. 96 (1), e0149221. doi: 10.1128/JVI.01492-21
Roberts, G. H. L., Partha, R., Rhead, B., Knight, S. C., Park, D. S., Coignet, M. V., et al. (2022). Expanded COVID-19 phenotype definitions reveal distinct patterns of genetic association and protective effects. Nat. Genet. 54 (4), 374–381. doi: 10.1038/s41588-022-01042-x
Rokni, M., Heidari Nia, M., Sarhadi, M., Mirinejad, S., Sargazi, S., Moudi, M., et al. (2022). Association of TMPRSS2 gene polymorphisms with COVID-19 severity and mortality: a case-control study with computational analyses. Appl. Biochem. Biotechnol. 194 (8), 3507–3526. doi: 10.1007/s12010-022-03885-w
Rossi, A. D., de Araujo, J. L. F., de Almeida, T. B., Ribeiro-Alves, M., Velozo, C. D., de Almeida, J. M., et al. (2021). Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Sci. Rep. 11 (1), 9658. doi: 10.1038/s41598-021-88944-8
Ruter, J., Pallerla, S. R., Meyer, C. G., Casadei, N., Sonnabend, M., Peter, S., et al. (2022). Host genetic loci LZTFL1 and CCL2 associated with SARS-CoV-2 infection and severity of COVID-19. Int. J. Infect. Dis. 122, 427–436. doi: 10.1016/j.ijid.2022.06.030
Sakuraba, A., Haider, H., Sato, T. (2020). Population difference in allele frequency of HLA-C*05 and its correlation with COVID-19 mortality. Viruses 12 (11), 133. doi: 10.3390/v12111333
Samuel, R. M., Majd, H., Richter, M. N., Ghazizadeh, Z., Zekavat, S. M., Navickas, A., et al. (2020). Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell 27 (6), 876–87+. doi: 10.1016/j.stem.2020.11.009
Schetelig, J., Heidenreich, F., Baldauf, H., Trost, S., Falk, B., Hossbach, C., et al. (2021). Individual HLA-a, -b, -c, and -DRB1 genotypes are no major factors which determine COVID-19 severity. Front. Immunol. 12. doi: 10.3389/fimmu.2021.698193
Schreiber, G. (2020). The role of type I interferons in the pathogenesis and treatment of COVID-19. Front. Immunol. 11. doi: 10.3389/fimmu.2020.595739
Schuler, B. A., Habermann, A. C., Plosa, E. J., Taylor, C. J., Jetter, C., Negretti, N. M., et al. (2021). Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Invest. 131 (1), e140766. doi: 10.1172/JCI140766
Shaath, H., Vishnubalaji, R., Elkord, E., Alajez, N. M. (2020). Single-cell transcriptome analysis highlights a role for neutrophils and inflammatory macrophages in the pathogenesis of severe COVID-19. Cells 9 (11), 2374. doi: 10.3390/cells9112374
Shelton, J. F., Shastri, A. J., Ye, C., Weldon, C. H., Filshtein-Sonmez, T., Coker, D., et al. (2021). Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity. Nat. Genet. 53 (6), 801–808. doi: 10.1038/s41588-021-00854-7
Shikov, A. E., Barbitoff, Y. A., Glotov, A. S., Danilova, M. M., Tonyan, Z. N., Nasykhova, Y. A., et al. (2020). Analysis of the spectrum of ACE2 variation suggests a possible influence of rare and common variants on susceptibility to COVID-19 and severity of outcome. Front. Genet. 11. doi: 10.3389/fgene.2020.551220
Shkurnikov, M., Nersisyan, S., Jankevic, T., Galatenko, A., Gordeev, I., Vechorko, V., et al. (2021). Association of HLA class I genotypes with severity of coronavirus disease-19. Front. Immunol. 12. doi: 10.3389/fimmu.2021.641900
Smieszek, S. P., Polymeropoulos, V. M., Xiao, C., Polymeropoulos, C. M., Polymeropoulos, M. H. (2021a). Loss-of-function mutations in IFNAR2 in COVID-19 severe infection susceptibility. J. Glob Antimicrob. Resist. 26, 239–240. doi: 10.1016/j.jgar.2021.06.005
Smieszek, S. P., Przychodzen, B. P., Polymeropoulos, V. M., Polymeropoulos, C. M., Polymeropoulos, M. H. (2021b). Assessing the potential correlation of polymorphisms in the IL6R with relative IL6 elevation in severely ill COVID-19 patients'. Cytokine 148, 155662. doi: 10.1016/j.cyto.2021.155662
Solanich, X., Vargas-Parra, G., van der Made, C. I., Simons, A., Schuurs-Hoeijmakers, J., Antoli, A., et al. (2021). Genetic screening for TLR7 variants in young and previously healthy men with severe COVID-19. Front. Immunol. 12. doi: 10.3389/fimmu.2021.719115
Stopsack, K. H., Mucci, L. A., Antonarakis, E. S., Nelson, P. S., Kantoff, P. W. (2020). TMPRSS2 and COVID-19: Serendipity or opportunity for intervention? Cancer Discovery 10 (6), 779–782. doi: 10.1158/2159-8290.CD-20-0451
Strafella, C. A.-O., Caputo, V. A.-O., Termine, A. A.-O., Barati, S., Caltagirone, C., Giardina, E., et al. (2020). Investigation of genetic variations of IL6 and IL6R as potential prognostic and pharmacogenetics biomarkers: Implications for COVID-19 and neuroinflammatory disorders. Life (Basel) 10 (12), 351. doi: 10.3390/life10120351
Szeto, M. D., Maghfour, J., Sivesind, T. E., Anderson, J., Olayinka, J. T., Mamo, A., et al. (2021). Interferon and toll-like receptor 7 response in COVID-19: Implications of topical imiquimod for prophylaxis and treatment. Dermatology 237 (6), 847–856. doi: 10.1159/000518471
Tanaka, T., Narazaki, M., Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6 (10), a016295. doi: 10.1101/cshperspect.a016295
Taylor, L. (2022). Covid-19: True global death toll from pandemic is almost 15 million, says WHO. BMJ 377, o1144. doi: 10.1136/bmj.o1144
Tomita, Y., Ikeda, T., Sato, R., Sakagami, T. (2020). Association between HLA gene polymorphisms and mortality of COVID-19: An in silico analysis. Immun. Inflammation Dis. 8 (4), 684–694. doi: 10.1002/iid3.358
van der Made, C. I., Simons, A., Schuurs-Hoeijmakers, J., van den Heuvel, G., Mantere, T., Kersten, S., et al. (2020). Presence of genetic variants among young men with severe COVID-19. Jama-Journal Am. Med. Assoc. 324 (7), 663–673. doi: 10.1001/jama.2020.13719
Villapalos-Garcia, G., Zubiaur, P., Rivas-Duran, R., Campos-Norte, P., Arevalo-Roman, C., Fernandez-Rico, M., et al. (2022). Transmembrane protease serine 2 (TMPRSS2) rs75603675, comorbidity, and sex are the primary predictors of COVID-19 severity. Life Sci. Alliance 5 (10), e202201396. doi: 10.26508/lsa.202201396
Wang, F., Huang, S., Gao, R., Zhou, Y., Lai, C., Li, Z., et al. (2020). Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discovery 6 (1), 83. doi: 10.1038/s41421-020-00231-4
Wickenhagen, A., Sugrue, E., Lytras, S., Kuchi, S., Noerenberg, M., Turnbull, M. L., et al. (2021). A prenylated dsRNA sensor protects against severe COVID-19. Science 374 (6567), eabj3624. doi: 10.1126/science.abj3624
Williamson, E. J., Walker, A. J., Bhaskaran, K., Bacon, S., Bates, C., Morton, C. E., et al. (2020). Factors associated with COVID-19-related death using OpenSAFELY. Nature 584 (7821), 430–43+. doi: 10.1038/s41586-020-2521-4
Wooster, L., Nicholson, C. J., Sigurslid, H. H., Lino Cardenas, C. L., Malhotra, R. (2020). Polymorphisms in the ACE2 locus associate with severity of COVID-19 infection. medRxiv. doi: 10.1101/2020.06.18.20135152
Yamamoto, N., Ariumi, Y., Nishida, N., Yamamoto, R., Bauer, G., Gojobori, T., et al. (2020). SARS-CoV-2 infections and COVID-19 mortalities strongly correlate with ACE1 I/D genotype. Gene 758, 144944. doi: 10.1016/j.gene.2020.144944
Yang, Z., Macdonald-Dunlop, E., Chen, J., Zhai, R., Li, T., Richmond, A., et al. (2022). Genetic landscape of the ACE2 coronavirus receptor. Circulation 145 (18), 1398–1411. doi: 10.1161/CIRCULATIONAHA.121.057888
Yang, L., Wang, J., Hui, P., Yarovinsky, T. O., Badeti, S., Pham, K., et al. (2021). Potential role of IFN-alpha in COVID-19 patients and its underlying treatment options. Appl. Microbiol. Biotechnol. 105 (10), 4005–4015. doi: 10.1007/s00253-021-11319-6
Yao, Y., Ye, F., Li, K. L., Xu, P., Tan, W. J., Feng, Q. S., et al. (2021). Genome and epigenome editing identify CCR9 and SLC6A20 as target genes at the 3p21.31 locus associated with severe COVID-19. Signal Transduction Targeted Ther. 6 (1), 85. doi: 10.1038/s41392-021-00519-1
Yousefzadegan, S., Rezaei, N. (2020). Case report: Death due to COVID-19 in three brothers. Am. J. Trop. Med. Hyg 102 (6), 1203–1204. doi: 10.4269/ajtmh.20-0240
Zhang, Q., Bastard, P., Liu, Z., Le Pen, J., Moncada-Velez, M., Chen, J., et al. (2020a). Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370 (6515), eabd4570. doi: 10.1126/science.abd4570
Zhang, Y., Qin, L., Zhao, Y., Zhang, P., Xu, B., Li, K., et al. (2020c). Interferon-induced transmembrane protein 3 genetic variant rs12252-c associated with disease severity in coronavirus disease 2019. J. Infect. Dis. 222 (1), 34–37. doi: 10.1093/infdis/jiaa224
Zhang, H., Shao, L., Lin, Z., Long, Q. X., Yuan, H., Cai, L., et al. (2022). APOE interacts with ACE2 inhibiting SARS-CoV-2 cellular entry and inflammation in COVID-19 patients. Signal Transduct Target Ther. 7 (1), 261. doi: 10.1038/s41392-022-01118-4
Zhang, X., Tan, Y., Ling, Y., Lu, G., Liu, F., Yi, Z., et al. (2020b). Viral and host factors related to the clinical outcome of COVID-19. Nature 583 (7816), 437–440. doi: 10.1038/s41586-020-2355-0
Zhang, J., Zhao, C., Zhao, W. (2021). Virus caused imbalance of type I IFN responses and inflammation in COVID-19. Front. Immunol. 12. doi: 10.3389/fimmu.2021.633769
Keywords: COVID-19, disease severity, critical illness, genetic, SARS-CoV-2
Citation: Ji X-S, Chen B, Ze B and Zhou W-H (2022) Human genetic basis of severe or critical illness in COVID-19. Front. Cell. Infect. Microbiol. 12:963239. doi: 10.3389/fcimb.2022.963239
Received: 07 June 2022; Accepted: 30 August 2022;
Published: 20 September 2022.
Edited by:
Yu Chen, Wuhan University, ChinaReviewed by:
Salvatore Caradonna, Rowan University School of Osteopathic Medicine, United StatesCopyright © 2022 Ji, Chen, Ze and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Hao Zhou, emhvdXdlbmhhb0BmdWRhbi5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.