
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 08 August 2022
Sec. Clinical Microbiology
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.955774
 Achiraya Siriphap1
Achiraya Siriphap1 Thawatchai Kitti2
Thawatchai Kitti2 Akachai Khuekankaew2
Akachai Khuekankaew2 Chalermchai Boonlao3
Chalermchai Boonlao3 Chonthida Thephinlap4
Chonthida Thephinlap4 Chutamas Thepmalee4
Chutamas Thepmalee4 Nittiya Suwannasom4
Nittiya Suwannasom4 Krissana Khoothiam1*
Krissana Khoothiam1*Background: The global emergence and spread of extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales, especially Escherichia coli and Klebsiella pneumoniae, have been recognized as a public health concern as severe infections caused by these microorganisms increase morbidity and mortality. This study aimed to assess the prevalence of ESBL-positive E. coli and K. pneumoniae strains isolated from hospitalized patients in Chiangrai Prachanukroh hospital, Chiangrai province, Thailand.
Methods: This retrospective analysis was conducted from January 2016 to December 2020. A total of 384,001 clinical specimens were collected aseptically and further cultivated on an appropriate medium. All clinical isolates (one isolate per patient) were identified based on standard laboratory methods. Antibiotic susceptibility testing was performed by the Kirby Bauer disc diffusion technique following CLSI guidelines. ESBL production was screened with ceftazidime and cefotaxime discs based on the CLSI recommendations. Phenotypic confirmation of ESBL production was carried out using a double-disc synergy technique following the CLSI standard.
Results: Of a total of 384,001 clinical samples analyzed for bacterial species identification, 11,065 (2.9%) tested positive for E. coli and 5,617 (1.5%) for K. pneumoniae. Approximately 42.5% (4,706/11,065) of E. coli and 30.2% (1,697/5,617) of K. pneumoniae isolates were classified as ESBL producers. A higher proportion of ESBL producers was found in patients older than 60 years and male groups. The highest infection rates of ESBL-positive pathogens were observed among patients in a medical unit. ESBL-producing E. coli and K. pneumoniae isolates were predominantly found in urine and sputum, respectively. ESBL producers exhibited a high resistance rate to ampicillin (99.8–100%), cefazolin (100%), cefotaxime (100%), fluoroquinolones, and trimethoprim/sulfamethoxazole.
Conclusions: This study demonstrated the high prevalence and emerging antibiotic resistance of ESBL-positive E. coli and K. pneumoniae isolates from patients admitted to a provincial hospital in northern Thailand. Most ESBL-producing strains were highly resistant to several antimicrobial agents apart from carbapenems and aminoglycosides. These findings indicated that carbapenems and aminoglycosides should be advised as the first-line drugs of choice for serious infections with ESBL-producing Enterobacterales.
Antimicrobial resistance has risen worldwide and contributes to severe morbidity and mortality in settings with limited diagnostic and healthcare (Schwaber and Carmeli, 2007; Founou et al., 2017). The prevalence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales has increased steadily worldwide, and these bacteria are recognized as a significant source of antibiotic resistance among Gram-negative bacteria (Coudron et al., 1997; Schwaber and Carmeli, 2007; Bush and Fisher, 2011; Murray and Peaper, 2015). Enterobacterales, particularly Escherichia coli and Klebsiella pneumoniae, are the primary opportunistic agents that play significant roles in hospitalized patients, causing bacteremia as well as urinary and respiratory tract infections (Kim et al., 2002; Khanfar et al., 2009). E. coli and K. pneumoniae are currently considered the two most common ESBL-producing pathogens in the hospital setting (Obeng-Nkrumah et al., 2013; Ouedraogo et al., 2016). ESBL-producing bacteria are capable of hydrolyzing third-generation cephalosporins and monobactams, but their activity is inhibited by clavulanic acid and tazobactam (Rawat and Nair, 2010). ESBLs are encoded by genes found on large plasmids that share genes for antimicrobial resistance with other pathogens. ESBLs are frequently transmitted by plasmids and can thereby be distributed among hospitalized patients, driving their spread across regions (Paterson and Bonomo, 2005; Paterson, 2006). The increasing emergence of ESBL-producing pathogens has been documented worldwide and varies among countries (Quan et al., 2017; Abayneh et al., 2018; Mineau et al., 2018; Kettani Halabi et al., 2021). In Thailand, the prevalence of ESBL-producing microorganisms in asymptomatic individuals has been recorded, with incidences ranging from 13.0% to 31.2% (Kiratisin et al., 2008; Hongsuwan et al., 2014; Sawatwong et al., 2019). However, representative studies on the emergence of ESBL producers (E. coli and K. pneumoniae) and trends in antibiotic susceptibility in Southeast Asia are currently limited. Therefore, it is essential to understand the prevalence and epidemiological features of antimicrobial resistance in this geographic region. This study aimed to assess the prevalence of ESBL-positive E. coli and K. pneumoniae isolated from microbiological samples from hospitalized patients at a provincial hospital in northern Thailand.
This retrospective descriptive study was performed over 5 years between January 2016 and December 2020 in Chiangrai Prachanukroh Hospital, a 758-bed tertiary hospital in the north of Thailand. This study was approved by the Human Ethics Committee of the University of Phayao (ethical approval number 1.1/037/63). Clinical data of individuals visiting the hospital were collected through computerized medical records and clinical chart reviews. The following data were obtained from medical records: age, gender, admission time, hospital unit of admission during infection, causative microorganisms (only the first isolates of bacterial species from each patient), specimen type, and antibiotic susceptibility profile.
Clinical specimens, including blood, urine, sputum, pus, and body fluids as per the hospital records, were cultivated on MacConkey agar plates and incubated aerobically at 37°C for 18–24 hours. Lactose-fermenting strains, which presented as pink colonies on the agar plates, were isolated. The clinical isolates were further identified as E. coli or K. pneumoniae depending on their morphology in Gram’s staining, bacterial cultures, and biochemical characteristics, as described previously (Jorgensen et al., 2015).
Antibiotic susceptibility testing was performed by the disk diffusion technique on Mueller Hinton Agar (MHA) plates following the Clinical Laboratory Standards Institute (CLSI) guidelines (Clinical and Laboratory Standards Institute, 2014). Three to four colonies were transferred into tubes containing sterile saline, and the samples were then adjusted to obtain the 0.5 McFarland turbidity standard. The bacterial suspensions were homogeneously spread on MHA agar plates with a sterile cotton swab. Antimicrobial discs were then placed on the plates. The antibiotics tested in this work include ampicillin (30 µg), amoxicillin/clavulanic acid (20/10 µg), piperacillin/tazobactam (100/10 µg), cefazolin (30 µg), cefoperazone/sulbactam (30/15 µg), cefotaxime (30 µg), ceftazidime (30 µg), ertapenem (10 µg), imipenem (10 µg), meropenem (10 µg), ciprofloxacin (5 µg), norfloxacin (10 µg), amikacin (30 µg), gentamicin (10 µg), and trimethoprim/sulfamethoxazole (1.25/23.75 µg).
Screening for ESBL production was conducted by two single disk diffusion tests with ceftazidime and cefotaxime. The positive results were defined as zones of inhibition of ≤ 22 mm or ≤ 27 mm for ceftazidime and cefotaxime, respectively. Confirmatory testing was performed using a combination disk test following the CLSI guideline (Clinical and Laboratory Standards Institute, 2014). Briefly, a disk containing cephalosporin alone (ceftazidime or cefotaxime) or in the presence of clavulanic acid was placed on MHA agar plates at a length of 20 mm (center to center). The test was considered a positive ESBL result when an increase in inhibition zone diameter (over 5 mm) of a disk containing cephalosporin plus clavulanic acid compared to a disk containing cephalosporin alone.
The data were entered into Microsoft Excel 2016. Data were analyzed using descriptive statistics and presented as frequencies and percentages. The chi-square test was used for analyzing the relationship between categorical variables. Analyses were performed using GraphPad Prism (version 5.00 for Windows). A P-value less than 0.05 was considered statistically significant.
A total of 384,001 clinical specimens were tested during the study period (blood: 212,522; urine: 70,759; sputum: 63,335; pus: 20,304; and body fluids: 17,081). A total of 11,065 (2.9%) E. coli and 5,617 (1.5%) K. pneumoniae isolates were obtained from the samples, of which 4,706/11,065 (42.5%) and 1,697/5,617 (30.2%) were ESBL-producing E. coli and K. pneumoniae strains, respectively, as seen in Table 1. Additionally, the prevalence of ESBL-producing E. coli isolates decreased significantly over the study period, from 46.2% in 2016 to 40.9% in 2020 (P < 0.05). As for K. pneumoniae, the frequency of ESBL-positive strains varied slightly over the 5-year period, ranging from 35.7% in 2017 to 27.6% in 2020 (Table 1).
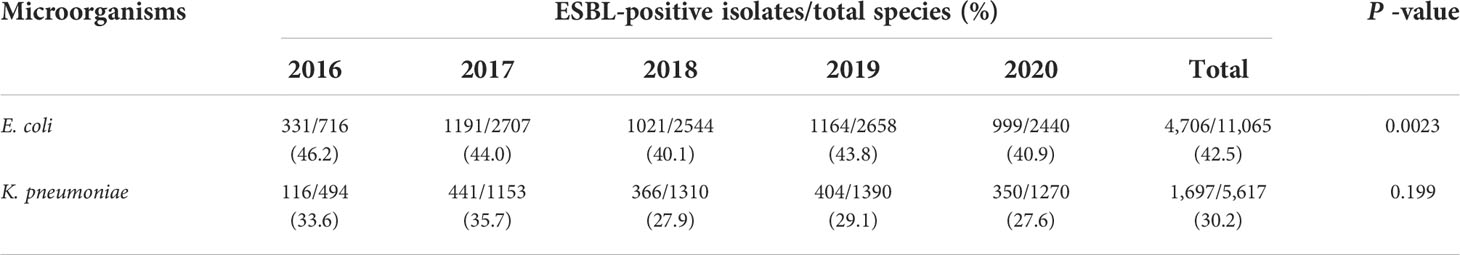
Table 1 The prevalence of ESBL-producing E. coli and K. pneumoniae isolates per year (between 2016 and 2020).
Bacterial isolates were predominantly detected in men, with a higher proportion of ESBL-producing E. coli, and K. pneumoniae isolated from male patients (E. coli: 51.1%; P<0.001, K. pneumoniae 62.1% with no significance) than in female groups (Table 2). The age distribution revealed that the maximum number of ESBL producers (E. coli and K. pneumoniae) was seen in patients over 60 years old (57.0%; P<0.001 and 56.0%with no significance, respectively). Patients in the ESBL- and non-ESBL-positive groups were further categorized by hospital unit. The prevalence of ESBL producers was significantly (P<0.001) high among isolates from individuals admitted to medical units (E. coli: 46.0%, K. pneumoniae: 40.4%) (Table 2). Among the various clinical specimens analyzed, the frequency of ESBL-positive E. coli isolates was highest in urine (53.8% with no significance) while ESBL-positive K. pneumoniae strains were predominantly detected in sputum samples (44.2%; P<0.001), as seen in (Table 2).
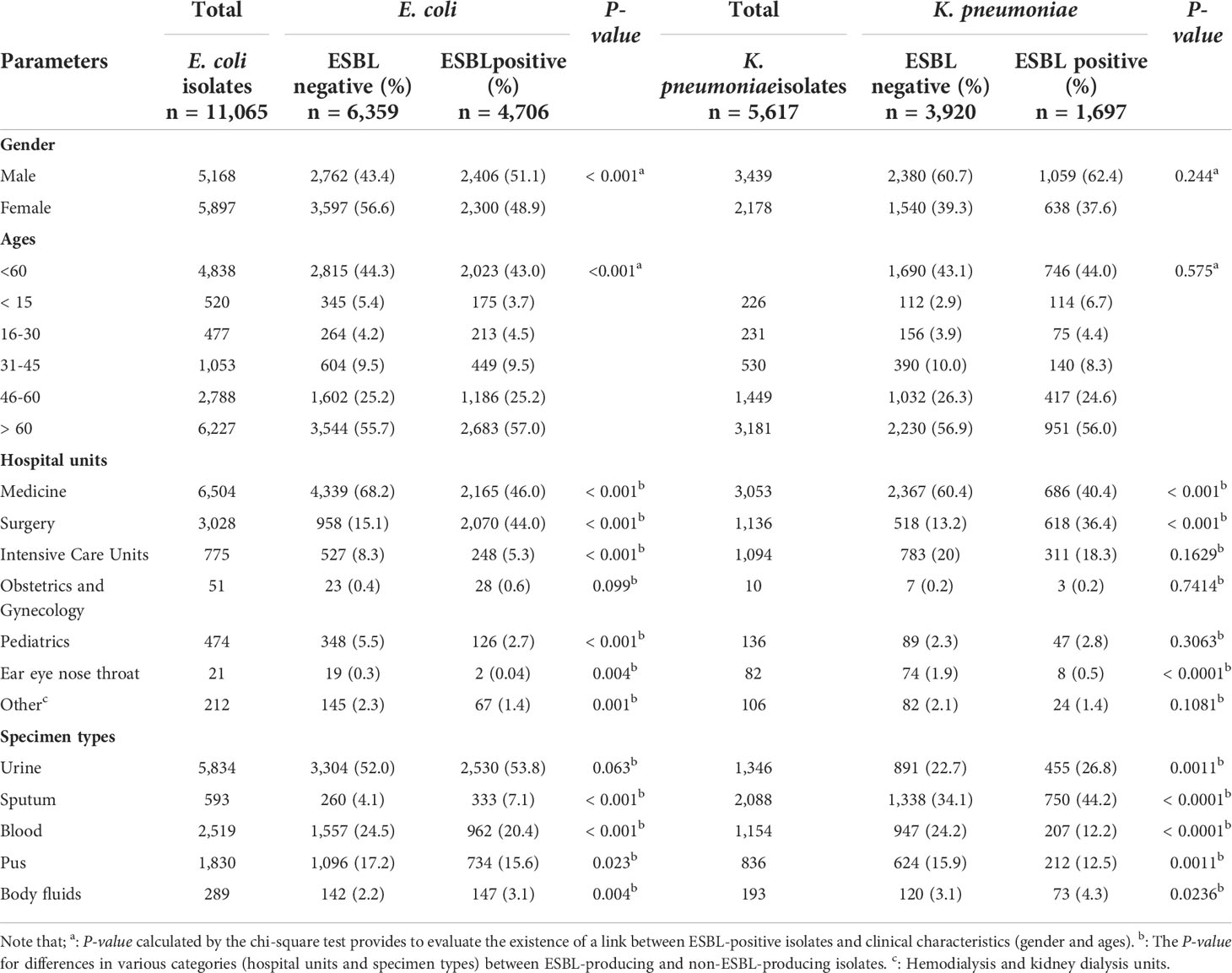
Table 2 Clinical characteristics of patients infected with ESBL-producing and non-ESBL-producing E. coli and K. pneumoniae isolates.
Antibiotic resistance patterns differed between ESBL-producing and non-ESBL-producing isolates. Overall, ESBL-positive E. coli strains had higher resistance rates than non-ESBL-producing E. coli to ceftazidime (71.3% versus 6.9%), ciprofloxacin (72.5% versus 35.1%), and trimethoprim/sulfamethoxazole (68.7% versus 46.1%). At the same time, ESBL-producing K. pneumoniae strains exhibited high resistance rates to these antibiotics as follows: ceftazidime (85.4%), ciprofloxacin (57.4%), and trimethoprim/sulfamethoxazole (79.3%). Notably, all ESBL-producing isolates were susceptible to the carbapenem group of antibiotics (imipenem, ertapenem, and meropenem; Figure 1).
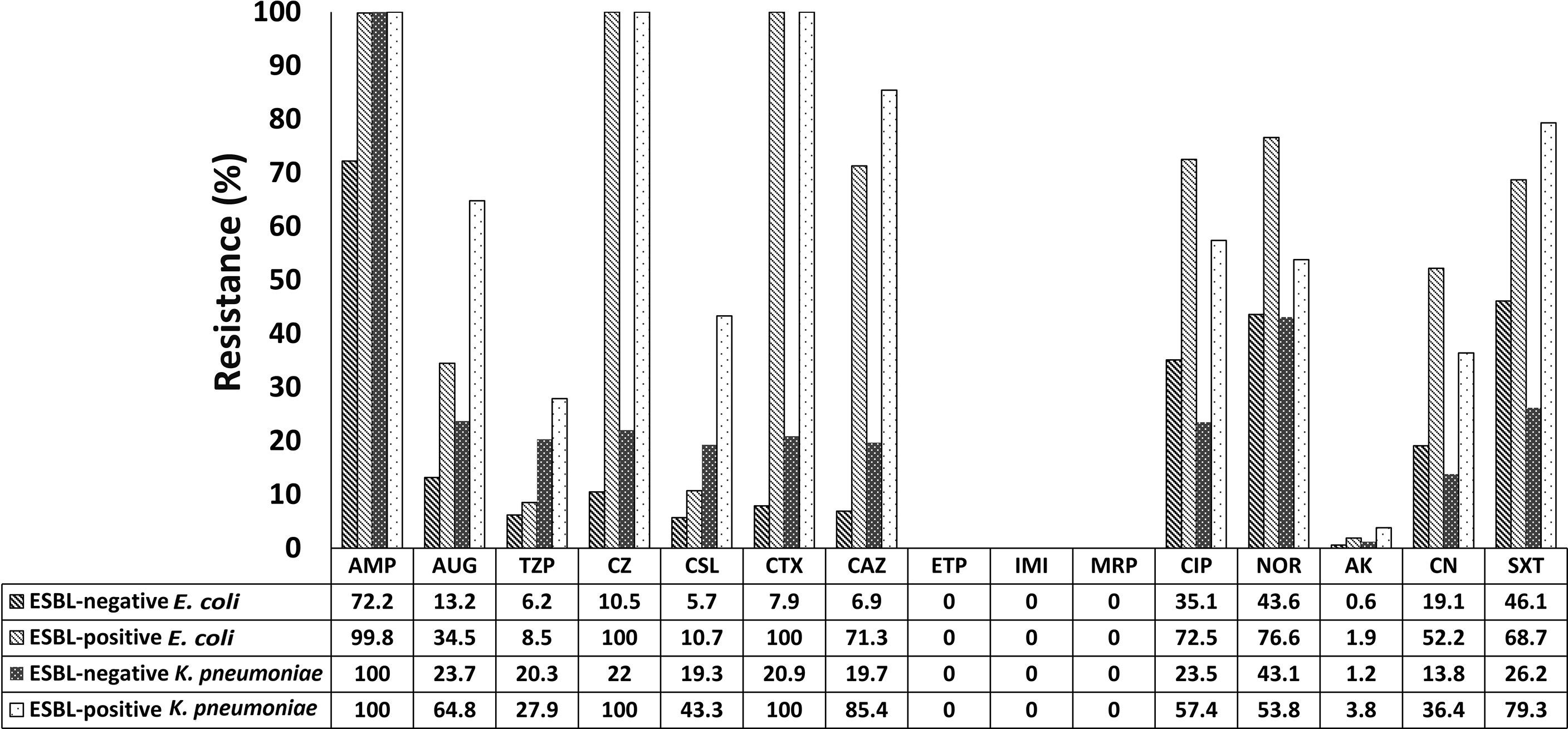
Figure 1 Antibiotic resistance profiles of ESBL-producing and non-ESBL-producing E. coli and K. pneumoniae isolated from patients admitted to Prachanukroh hospital, Chiangrai, Thailand (2016-2020). AMP, Ampicillin; AUG, Amoxicillin/Clavulanic acid; TZP, Piperacillin/Tazobactam; CZ, Cefazolin; CSL, Cefoperazone/Sulbactam; CTX, Cefotaxime; CAZ, Ceftazidime; ETP, Ertapenem; IMI, Imipenem; MRP, Meropenem; CIP, Ciprofloxacin; NOR, Norfloxacin; AK, Amikacin; CN, Gentamicin; SXT, Trimethoprim/Sulfamethoxazole.
Regarding aminoglycosides, a minority of ESBL producers showed resistance to amikacin (1.9% and 3.8% of E. coli and K. pneumoniae, respectively; Figure 1), while an increase in resistance to gentamycin was observed (E. coli: 52.2%; K. pneumoniae: 36.4%). Approximately 8.5% and 10.7% of ESBL-producing E. coli strains were resistant to piperacillin/tazobactam and cefoperazone/sulbactam, respectively; the corresponding figures for K. pneumoniae were 27.9% and 43.3%, respectively (Figure 1).
Recently, infections caused by ESBL-producing Enterobacterales, especially E. coli and K. pneumoniae, were recognized globally as a public health problem (Pitout and Laupland, 2008; Liebana et al., 2012). The emergence of ESBL-producing isolates among clinical specimens is changing over time and varies worldwide and across geographic regions. Notably, the incidence of colonization and infection rates of ESBL-producing pathogens were shown to be up to 25% in several hospitals in Thailand (Hongsuwan et al., 2014; Sawatwong et al., 2019). Therefore, it is necessary to gain knowledge of the acquisition and transmission of ESBL-producing microorganisms in different regions. Our study reported on the prevalence of ESBL-positive E. coli and K. pneumoniae isolates in clinical samples from individuals admitted to a provincial hospital in the north of Thailand between 2016 and 2020. In this study, the prevalence of ESBL-producing Enterobacterales (E. coli and K. pneumoniae) was 38.4% (6,403/16,682; Table 1), which was high compared to that documented by Hongsuwan et al. (2014; 31.2%), Kiratisin et al. (2008; 13.0%), and Sawatwong et al. (2019; 27.0%), in different regions of Thailand. However, the observed prevalence was low compared to the results of an investigation in Vietnam, which documented an ESBL producer prevalence of up to 55.1% (Suwantarat and Carroll, 2016). Additionally, the infection rates of ESBL-producing Enterobacterales in Southeast Asian countries varied widely, being 19.8% in Singapore, 36.8% in the Philippines, and 40.6% in Cambodia (Suwantarat and Carroll, 2016; Caron et al., 2018). The identified ESBL-producers were predominantly E. coli strains compared to K. pneumoniae, similar to the findings of previous studies (Kiratisin et al., 2008; Quan et al., 2017).
As noted in previous reports, patient gender is a risk factor in the distribution of ESBL producers (Najjuka et al., 2016; Xiao et al., 2019). In the present study, the prevalence of ESBL-producing isolates (E. coli and K. pneumoniae) was found more in males than females. The results are comparable to the studies done in Iran (Peerayeh et al., 2016) and elsewhere (Ouedraogo et al., 2016), but differ from other investigations that revealed that a high prevalence of ESBL producers detected in female patients (Chander and Shrestha, 2013; Abayneh et al., 2018; Kettani Halabi et al., 2021). The reason for these differences is not clear. Urinary and respiratory tract colonization by ESBL-producing bacteria in male patients could be responsible. However, there are no existing studies to explain this speculation. In this study, the highest ESBL-producing isolates (E. coli and K. pneumoniae) were detected in individuals aged over 60 years. Several investigations support that advanced age (mostly over 60 years) is a risk factor for severe infection with ESBL producers. (Hongsuwan et al., 2014; Heytens et al., 2017; Kettani Halabi et al., 2021). One potential reason for this is that old age is associated with poor health and status. Other age groups, including those under 60 years are not spared from this risk. (Halabi et al., 2021; Heytens et al., 2017). Our study observed the highest infection rates of ESBL-producers among patients in medical units. This is not surprising because the medical wards had a higher number of hospitalized patients during the study period. Patients in these wards usually have indwelling devices and extended hospital stays. These two features are well-defined risk factors for the widespread distribution of ESBL-producing pathogens among individuals admitted to hospitals (Lautenbach et al., 2001; Apisarnthanarak et al., 2007). Recently, types of clinical samples are considered as contributing factors to the spread of ESBL-positive strains (Ouedraogo et al., 2016). A high proportion of ESBL-positive E. coli strains was found in urine samples in this study, similar to a previous report by Hassuna et al. (2020), who noted that approximately 59.7% of uropathogenic E. coli were ESBL producers. On the other hand, most ESBL-producing K. pneumoniae isolates appeared in sputum specimens, which was consistent with another study in Taiwan (Cheng et al., 2016) and elsewhere (Caron et al., 2018). The predominance of these two species in urine and sputum samples may be due to their colonization as normal bacterial flora in the urinary (mainly E. coli) and respiratory tract (particularly K. pneumoniae) (Obeng-Nkrumah et al., 2013; Ouedraogo et al., 2016). The resistance profiles of ESBL-positive and ESBL-negative strains (E. coli and K. pneumoniae) differed widely based on the class of antibacterial drugs used.In our study, non-ESBL-producing isolates showed high resistant rates of 72.2% (E. coli) and 100% (K. pneumoniae) to ampicillin. Similarly, other investigations reported that a resistance rate of 100% to ampicillin was observed for non-ESBL-producing bacteria (E. coli and K. pneumoniae) (Seni et al., 2016; Abayneh et al., 2018). It is possible that these isolates may possess strategies for antibiotic resistance that include the expression of ampC lactamase and metallo-bata-lactamase (Dalela et al., 2012). These findings probably reflect the improper prevention and control of the transmission of antibiotic-resistant bacteria, and few therapeutic options will remain for individuals infected with these pathogen shortly.
All ESBL-positive E. coli strains (99.8%) were resistant to ampicillin, similar to the resistance rate of ESBL-positive K. pneumoniae strains (100%). Our results agree with another study conducted in Ethiopia (Abayneh et al., 2018). Notably, penicillin combined with a beta-lactamase inhibitor such as piperacillin/tazobactam was demonstrated to be effective against ESBL producers (resistance rates of 8.5% and 27.9% in E. coli and K. pneumoniae, respectively). Conversely, other studies documented that 40% (Kettani Halabi et al., 2021) and 74.5% (Najjuka et al., 2016) of ESBL-producing pathogens were resistant to this antimicrobial agent. In this study, some third-generation cephalosporins exhibited low effectiveness against ESBL-positive pathogens. Resistance rates of ESBL-producing E. coli isolates were 100% and 71.3% for cefotaxime and ceftazidime, respectively. In comparison, ESBL-positive K. pneumoniae strains revealed similar resistance rates to cefotaxime (100%) and ceftazidime (85.4%). These data are consistent with previous studies conducted in China (Quan et al., 2017), Nepal (Chander et al., 2013), Ethiopia (Abayneh et al., 2018) and Morocco (Kettani Halabi et al., 2021). In addition, cefoperazone/sulbactam, a third-generation cephamycin beta-lactamase inhibitor combination, had antibacterial potential against ESBL-producing isolates (resistance rates of 10.7% and 43.3% in E. coli and K. pneumoniae, respectively). Similarly, other reports noted that ESBL producers showed low resistance rates of 8.1–12.3% (E. coli) and 16.1% (K. pneumoniae) to cefoperazone/sulbactam (Yang et al., 2015; Jia et al., 2021). Carbapenems exhibited high potential against all ESBL-positive strains in this study, with a susceptibility rate of 100%, which is similar to that recorded in other studies (Abayneh et al., 2018; Chander et al., 2013). Our study supports the conclusion that carbapenems are the first-line drug of choice for severe infections with ESBL-producing bacteria.
We found that ESBL-producing isolates showed high resistance varying from 53.8% to 76.6% to quinolones, ciprofloxacin, and norfloxacin. These data parallel a previous study by Abayneh et al. (2018), who demonstrated that approximately 76.5% of ESBL producers were resistant to these drug classes. A minority of ESBL-positive isolates were resistant to amikacin (E. coli: 1.9%; K. pneumoniae: 3.8%), while moderate bacterial resistance to another aminoglycoside, gentamicin, was observed (E. coli: 52%; K. pneumoniae: 36.4%). This result is consistent with a previous study conducted in Nepal, where resistance rates of 6.6% and 12.5% to amikacin were observed for ESBL-positive E. coli and K. pneumoniae, respectively. At the same time, 23.3% and 45.3% of E. coli and K. pneumoniae strains, respectively, were resistant to gentamicin(Chander et al., 2013). Thus, aminoglycosides could be the drugs of choice for patients infected with ESBL producers. The resistance rate of ESBL-producing E. coli isolates to trimethoprim/sulfamethoxazole (68.7%) in our study was consistent with other studies done in Ethiopia (65.1%) and Tanzania (76%) (Blomberg et al., 2004; Kibret and Abera, 2011). For K. pneumoniae, the highest resistance rate was recorded against trimethoprim/sulfamethoxazole (79.3%). These results were also similar to studies performed in Ethiopia (86.4%) and Iran (91.4%) (Mansouri and Abbasi, 2010; Teklu et al., 2019). This study showed an increase in the prevalence of ESBL-producing Enterobacterales and their high resistance rate to many families of antibiotics in Chiangrai Prachanukroh Hospital, probably reflecting the overuse or misuse of antibiotics combined with unreliable diagnostic practices in resource-limited settings.
Our work also has some limitations. First, this retrospective study was conducted in a single center at a tertiary hospital in Chiang-Rai province, northern Thailand. Therefore, the high prevalence of ESBL producers found in this study does not accurately represent the prevalence of ESBL-producing isolates in the overall population of individuals admitted to hospitals in Chiang-Rai. We recommend that multicenter surveillance be conducted in the future to overcome the existing factor of these limitations in our area. Second, the lack of data on bacterial cultures due to the loss of some clinical specimens also limited our ability to assess the incidence of ESBL producers among hospitalized patients. Third, this study performed the combined disk test instead of the E-test strip (golden method) for the confirmation of ESBL producers. The reason for this is that the combination of disk tests is simple, inexpensive, and convenient for our routine microbiology laboratory. Although this method had a sensitivity of 100% for ESBL detection, but poorly specific. (Jacoby et al., 2006; Polsfuss et al., 2012). Finally, advanced molecular analysis for species classification and ESBL typing were not performed in our study. Thus, it is possible that some ESBL-producing isolates in this study were incorrectly identified and mistakenly counted. Therefore, a more sophisticated procedure for identifying ESBL producers should be available for future investigations.
Our study demonstrated a high prevalence of ESBL-producing Enterobacterales among patients in a tertiary hospital in the north of Thailand. ESBL producers were characterized primarily as being E. coli strains rather than K. pneumoniae. A high proportion of ESBL-positive isolates was detected in patients aged over 60 years and in men.ESBL-producing E. coli and K. pneumoniae isolates were predominantly found in urine and sputum specimens, respectively. This study revealed an increase in resistance to many classes of antibacterial drugs among both ESBL-positive and ESBL-negative isolates. However, carbapenems and aminoglycosides showed potential against ESBL producers. Hence, these two drugs are a good choice for treating infections of ESBL-producing microorganisms.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the University of Phayao Human Ethics Committee. Written informed consent for participation was not required for this work in accordance with national legislation and institutional requirements.
Study design: AS, TK, and KK. Data collection: AS, TK, CB, AK, CT, ChoT, NS, and KK. Data interpretation: AS, TK, CT, NS, and KK. Statistical analysis: AS, CT, and KK. All authors contributed to the initial draft of the manuscript. AS, NS, and KK approved the submitted version.
This study was partially supported by grant FF65-RIM11 from the University of Phayao, Thailand, and by the School of Medical Sciences, University of Phayao (MS 201003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abayneh, M., Tesfaw, G., Abdissa, A. (2018). Isolation of extended-spectrum β-lactamase- (ESBL-) producing Escherichia coli and Klebsiella pneumoniae from patients with community-onset urinary tract infections in jimma university specialized hospital, southwest Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2018, 4846159. doi: 10.1155/2018/4846159
Apisarnthanarak, A., Kiratisin, P., Saifon, P., Kitphati, R., Dejsirilert, S., Mundy, L. M. (2007). Risk factors for and outcomes of healthcare-associated infection due to extended-spectrum beta-Lactamase-Producing Escherichia coli or Klebsiella pneumoniae in Thailand. Infect. Control Hosp Epidemiol 28, 873–876. doi: 10.1086/518725
Blomberg, B., Mwakagile, D. S., Urassa, W. K., Maselle, S. Y., Mashurano, M., Digranes, A., et al. (2004). Surveillance of antimicrobial resistance at a tertiary hospital in Tanzania. BMC Public Health 4, 45. doi: 10.1186/1471-2458-4-45
Bush, K., Fisher, J. F. (2011). Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 65 (1), 455–478. doi: 10.1146/annurev-micro-090110-102911
Caron, Y., Chheang, R., Puthea, N., Soda, M., Boyer, S., Tarantola, A., et al. (2018). Beta-lactam resistance among enterobacteriaceae in Cambodia: The four-year itch. Int. J. Infect. Dis. 66, 74–79. doi: 10.1016/j.ijid.2017.10.025
Chander, A., Shrestha, C. D (2013). Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC. Res. Notes 6, 487. doi: 10.1186/1756-0500-6-487
Cheng, W.-L., Hsueh, P.-R., Lee, C.-C., Li, C.-W., Li, M.-J., Chang, C.-M., et al. (2016). Bacteremic pneumonia caused by extended-spectrum beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae: Appropriateness of empirical treatment matters. J. Microbiol. Immunol. Infect. 49 (2), 208–215. doi: 10.1016/j.jmii.2014.05.003
Clinical and Laboratory Standards Institute (2014). Performance standard for antimicrobial susceptibility testing: Twenty-fourth informational supplement M100-S24 (Wayne: CLSI).
Coudron, P. E., Moland, E. S., Sanders, C. C. (1997). Occurrence and detection of extended-spectrum beta-lactamases in members of the family enterobacteriaceae at a veterans medical center: Seek and you may find. J. Clin. Microbiol. 35 (10), 2593–2597. doi: 10.1128/jcm.35.10.2593-2597.199
Dalela, G., Gupta, S., Jain, D. K., Mehta, P. (2012). Antibiotic resistance patterns in uropathogens at a tertiary care hospital at jhalawar with special referenceAmpC b-lactamase and MRSA production. J. Clin. Diag.n Res. 6 (4), 645–651. doi: 10.7860/JCDR/2012/.2117
Founou, R. C., Founou, L. L., Essack, S. Y. (2017). Clinical and economic impact of antibiotic resistance in developing countries: ASystematic review and meta-analysis. PloS One 12 (12), e0189621. doi: 10.1371/journal.pone.0189621
Hassuna, N. A., Khairalla, A. S., Farahat, E. M., Hammad, A. M., Abdel-Fattah, M. (2020). Molecular characterization of extended-spectrum β lactamase- producing E. coli recovered from community-acquired urinary tract infections in upper Egypt. Sci. Rep. 10 (1), 2772. doi: 10.1038/s41598-020-59772-z
Heytens, S., Boelens, J., Claeys, G., Christiaens, T. (2017). Uropathogen distribution and antimicrobial susceptibility in uncomplicated cystitis in Belgium, a high antibiotics prescribing country: 20-year surveillance. Eur. J. Clin. Microbiol. Infect. Dis. 36 , 105–13 . doi: 10.1007/s10096-016-2776-8
Hongsuwan, M., Srisamang, P., Kanoksil, M., Luangasanatip, N., Jatapai, A., Day, N. P., et al. (2014). Increasing incidence of hospital-acquired and healthcare-associated bacteremia in northeast Thailand: AMulticenter surveillance study. PloS One 9 (10), e109324. doi: 10.1371/journal.pone.0109324
Jacoby, G. A., Walsh, K. E., Walker, V., J. (20062006). Identification of extended-spectrum, AmpC, and carbapenem- hydrolyzing beta-lactamases in Escherichia coli and Klebsiella pneumoniae by disk tests. J. Clin. Microbiol. 44 (6), 1971–1976. doi: 10.1128/JCM.00062-06
Jia, P., Zhu, Y., Li, X., Kudinha, T., Yang, Y., Zhang, G., et al. (2021). High prevalence of extended-spectrum beta-lactamases in Escherichia coli strains collected from strictly defined community-acquired urinary tract infections in adults in China: A multicenter prospective clinical microbiological and molecular study. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.663033
Jorgensen, J. H., Pfaller, M. A., Carroll, K. C., Funke, G., Landry, M. L., Richter, S. S., et al. (2015). Manual of clinical microbiology. eleventh edition (Washington, DC: ASM Press). doi: 10.1128/9781555817381
Kettani Halabi, M., Lahlou, F. A., Diawara, I., El Adouzi, Y., Marnaoui, R., Benmessaoud, R., et al. (2021). Antibiotic resistance pattern of extended spectrum beta lactamase producing Escherichia coli isolated from patients with urinary tract infection in Morocco. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.720701
Khanfar, H. S., Bindayna, K. M., Senok, A. C., Botta, G. A. (2009). Extended spectrum beta-lactamases (ESBL) in Escherichia coli and Klebsiella pneumoniae: Trends in the hospital and community settings. J. Infect. Dev. Ctries 3 (4), 295–299. doi: 10.3855/jidc.127
Kibret, M., Abera, B. (2011). Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr Health Sci. Suppl 1 (Suppl 1), S40–S45. doi: 10.4314/ahs.v11i3.70069
Kim, Y. K., Pai, H., Lee, H. J., Park, S. E., Choi, E. H., Kim, J., et al. (2002). Bloodstream infections by extended-spectrum beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae in children: Epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46 (5), 1481–1491. doi: 10.1128/aac.46.5.1481-1491.2002
Kiratisin, P., Apisarnthanarak, A., Laesripa, C., Saifon, P. (2008). Molecular characterization and epidemiology of extended-Spectrum-Beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-m family is endemic. Antimicrob. Agents Chemother. 52 (8), 2818–2824. doi: 10.1128/aac.00171-08
Lautenbach, E., Strom, B. L., Bilker, W. B., Patel, J. B., Edelstein, P. H., Fishman, N. O. (2001). Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae. Clin. Infec.t Dis. 33 (8), 1288–1294. doi: 10.1086/322667
Liebana, E., Carattoli, A., Coque, T. M., Hasman, H., Magiorakos, A.-P., Mevius, D., et al. (2012). Public health risks of enterobacterial isolates producing extended-spectrum β-lactamases or AmpC β-lactamases in food and food-producing animals: An EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin. Infect. Dis. 56 (7), 1030–1037. doi: 10.1093/cid/cis1043
Mansouri, S., Abbasi, S. (2010). Prevalence of multiple drug resistant clinical isolates of extended-spectrum beta-lactamase producing enterobacteriaceae in southeast Iran. Iran J. Med. Sci. 35 (2), 101–108. doi: 10.30476/IJMS.2010.39762
Mineau, S., Kozak, R., Kissoon, M., Paterson, A., Oppedisano, A., Douri, F., et al. (2018). Emerging antimicrobial resistance among Escherichia coli strains in bloodstream infections in toront-2016: A retrospective cohort study. CMAJ Open 6 (4), E580–e586. doi: 10.9778/cmajo.20180039
Murray, T. S., Peaper, D. R. (2015). The contribution of extended-spectrum β-lactamases to multidrug-resistant infections in children. Curr. Opin. Pediatr. 27 (1), 124–131. doi: 10.1097/mop.0000000000000182
Najjuka, C. F., Kateete, D. P., Kajumbula, H. M., Joloba, M. L., Essack, S. Y. (2016). Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res. Notes 9, 235. doi: 10.1186/s13104-016-2049-8
Obeng-Nkrumah, N., Twum-Danso, K., Krogfelt, K. A., Newman, M. J. (2013). High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistance. Am. J. Trop. Med. Hyg. 89 (5), 960–964. doi: 10.4269/ajtmh.12-0642
Ouedraogo, A. S., Sanou, M., Kissou, A., Sanou, S., Solaré, H., Kaboré, F., et al. (2016). High prevalence of extended-spectrum ß-lactamase producing enterobacteriaceae among clinical isolates in Burkina Faso. BMC Infect. Dis. 16, 326. doi: 10.1186/s12879-016-1655-3
Paterson, D. L. (2006). Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Med. 119 (6, Supplement 1), S20–S28. doi: 10.1016/j.amjmed.2006.03.013
Paterson, D. L., Bonomo, R. A. (2005). Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 18 (4), 657–686. doi: 10.1128/cmr.18.4.657-686.2005
Peerayeh, S. N., Rostami, E., Eslami, M., Rezaee, M. A (2016). High frequency of extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli isolates from male patients’ Urine. Arch. Clin. Infect. Dis 11 (2), e60127. doi: 10.5812/archcid.32696
Pitout, J. D., Laupland, K. B. (2008). Extended-spectrum beta-Lactamase-Producing enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8 (3), 159–166. doi: 10.1016/s1473-3099(08)70041-0
Polsfuss, S., Bloemberg, G. V., Giger, J., Meyer, V., Böttger, E. C., Hombach, M. (2012). Evaluation of a diagnostic flow chart for detection and confirmation of extended spectrum b-lactamases (ESBL) in Enterobacteriaceae. Clin. Microbiol. Infect. 18, 1194–1204. doi: 10.1111/j.1469-0691.2011.03737.x
Quan, J., Zhao, D., Liu, L., Chen, Y., Zhou, J., Jiang, Y., et al. (2017). High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J. Antimicrob. Chemother. 72 (1), 273–280. doi: 10.1093/jac/dkw372
Rawat, D., Nair, D. (2010). Extended-spectrum β-lactamases in gram negative bacteria. J. Glob. Infect. Dis. 2 (3), 263–274. doi: 10.4103/0974-777x.68531
Sawatwong, P., Sapchookul, P., Whistler, T., Gregory, C. J., Sangwichian, O., Makprasert, S., et al. (2019). High burden of extended-spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae bacteremia in older adults: A seven-year study in two rural Thai provinces. Am. J. Trop. Med. Hyg. 100 (4), 943–951. doi: 10.4269/ajtmh.18-0394
Schwaber, M. J., Carmeli, Y. (2007). Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 60 (5), 913–920. doi: 10.1093/jac/dkm318
Seni, J., Sweya, E., Mabewa, A., Mshana, S. E., Gilyoma, J.M (2016). Comparison of antimicrobial resistance patterns of ESBL and non ESBL bacterial isolates among patients with secondary peritonitis at Bugando medical centre, Mwanza – Tanzania. BMC. Emerg. Med 16, 41. doi: 10.1186/s12873-016-0106-1
Suwantarat, N., Carroll, K. C. (2016). Epidemiology and molecular characterization of multidrug-resistant gram-negative bacteria in southeast Asia. Antimicrob. Resist. Infect. Control 5, 15. doi: 10.1186/s13756-016-0115-6
Teklu, D. S., Negeri, A. A., Legese, M. H., Bedada, T. L., Woldemariam, H. K., Tullu, K. D. (2019). Extended-spectrum beta-lactamase production and multi-drug resistance among enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control 8, 39. doi: 10.1186/s13756-019-0488-4
Xiao, T., Wu, Z., Shi, Q., Zhang, X., Zhou, Y., Yu, X., et al. (2019). A retrospective analysis of risk factors and outcomes in patients with extended-spectrum beta-Lactamase-Producing Escherichia coli bloodstream infections. J. Glob. Antimicrob. Resist. 17, 147–156. doi: 10.1016/j.jgar.2018.12.014
Yang, Q., Zhang, H., Cheng, J., Xu, Z., Xu, Y., Cao, B., et al. (2015). In vitro activity of flomoxef and aomparators against Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis producing extended-spectrum β-lactamases in China. Int. J. Antimicrob. Agents 45 (5), 485–490. doi: 10.1016/j.ijantimicag.2014.11.012
Keywords: prevalence, ESBL, Escherichia coli, Klebsiella pneumoniae, antibiotic resistance
Citation: Siriphap A, Kitti T, Khuekankaew A, Boonlao C, Thephinlap C, Thepmalee C, Suwannasom N and Khoothiam K (2022) High prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates: A 5-year retrospective study at a Tertiary Hospital in Northern Thailand. Front. Cell. Infect. Microbiol. 12:955774. doi: 10.3389/fcimb.2022.955774
Received: 29 May 2022; Accepted: 19 July 2022;
Published: 08 August 2022.
Edited by:
Shymaa Enany, Suez Canal University, EgyptCopyright © 2022 Siriphap, Kitti, Khuekankaew, Boonlao, Thephinlap, Thepmalee, Suwannasom and Khoothiam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krissana Khoothiam, S3Jpc3NhbmEua2hAdXAuYWMudGg=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.