- 1Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
- 2Department of Microbiology and Immunology, The Affiliated Hospital of GuiZhou Medical University, Guiyang, China
- 3Department of Rheumatology, Sahlgrenska University Hospital, Gothenburg, Sweden
Background: Septic arthritis is considered one of the most dangerous joints diseases and is mainly caused by the Gram-positive bacterium Staphylococcus aureus (S. aureus). Human skin commensals are known to augment S. aureus infections. The aim of this study was to investigate if human commensals could augment S. aureus-induced septic arthritis.
Method: NMRI mice were inoculated with S. aureus alone or with a mixture of S. aureus together with either of the human commensal Staphylococcus epidermidis (S. epidermidis) or Streptococcus mitis (S. mitis). The clinical, radiological and histopathological changes due to septic arthritis were observed. Furthermore, the serum levels of chemokines and cytokines were assessed.
Results: Mice inoculated with a mixture of S. aureus and S. epidermidis or S. mitis developed more severe and frequent clinical arthritis compared to mice inoculated with S. aureus alone. This finding was verified pathologically and radiologically. Furthermore, the ability of mice to clear invading bacteria in the joints but not in kidneys was hampered by the bacterial mixture compared to S. aureus alone. Serum levels of monocyte chemoattractant protein 1 were elevated at the early phase of disease in the mice infected with bacterial mixture compared with ones infected with S. aureus alone. Finally, the augmentation effect in septic arthritis development by S. epidermidis was bacterial dose-dependent.
Conclusion: The commensal bacteria dose-dependently augment S. aureus-induced septic arthritis in a mouse model of septic arthritis.
Introduction
Although major strides have been made during the last decades in treatment of bacterial infections, septic arthritis still remains a major concern for the wider health-community. Its prevalence is relatively unchanged; prognosis still remains poor with majority of patients suffering from permanent joint-dysfunction even after adequate treatment (Goldenberg, 1998; Tarkowski, 2006).
Most at risk of septic arthritis are immuno-compromised patients, the elderly as well as patients with underlying joint damages, e.g. rheumatoid arthritis (Goldenberg, 1998; Tarkowski, 2006). In fact, the prevalence among patients with underlying joint disease is up to 10 times higher than in the general population (Tarkowski, 2006). The most common causative agent isolated from septic arthritis patients is Gram-positive Staphylococcus aureus (S. aureus), accounting for almost half of all cases (Goldenberg, 1998; Tarkowski, 2006). However, other Gram-positive- as well as Gram-negative bacteria have been shown to cause septic arthritis, although to a lesser extent (Goldenberg, 1998; Tarkowski, 2006). In line with the emergence and rapid spread of methicillin-resistant S. aureus (MRSA), the prevalence of MRSA caused septic arthritis was found to be high (25-50%) in both UK and USA (Al-Nammari et al., 2007; Frazee et al., 2009). Importantly, patients with MRSA septic arthritis tended to be older and have more polyarticular involvement as well as higher mortality compared to patients with non-MRSA septic arthritis (Ross and Davidson, 2005; Al-Nammari et al., 2007).
The development of new treatments for septic arthritis has stagnated. To limit the immune response and reduce the risk of permanent joint destruction, a combination treatment of antibiotics and immunomodulatory therapy was proposed (Fei et al., 2011). However, recent data suggest that there are potential dangers associated with such combination therapies as long as the problem of antibiotic resistance persists (Ali et al., 2015a; Ali et al., 2015b). Understanding the bacterial components/host factors interaction responsible for joint inflammation and destruction is the key for the development of new therapies. It is known that antibiotic-killed S. aureus induce destructive arthritis through TNF receptor 1 and that bacterial cell walls are the culprits (Ali et al., 2015c). We have recently shown that S. aureus lipoproteins are strongly arthritogenic and cause severe bone destruction by activating monocytes/macrophages in an experimental setting (Mohammad et al., 2019). Interestingly, a recent study demonstrated that the interaction between S. aureus coagulases, especially von-Willebrand binding protein and host von-Willebrand factor is crucial for initiation and development of septic arthritis (Na et al., 2020), suggesting the new therapeutic strategies by blocking such bacteria/host interaction.
Recently, we showed that human skin commensals augment the pathogenicity of S. aureus (Boldock et al., 2018). Streptococcus mitis (S. mitis), a Gram-positive coccus, is part of the oral flora. Although S. mitis is usually not associated with septic arthritis, there have been several reported cases of septic arthritis caused by S. mitis, most likely due to transmission from health practitioners preforming joint injections (Coatsworth et al., 2013; Feder and Gruson, 2016; Cain et al., 2018). Staphylococcus epidermidis (S. epidermidis), is another Gram-positive bacterium that is part of the normal human skin flora. S. epidermidis is typically considered to be non-pathogenic, but can take advantage of the host’s compromised immune system, causing various infections (Kim and Joo, 2012). Immunosuppressed patients are well-known to be susceptible to the different infections including septic arthritis. Anti-TNF therapy used in rheumatoid arthritis is associated with increased risk of septic arthritis. Interestingly, several opportunistic species that seldom cause septic arthritis were reported in the patients treated with anti-TNF therapy (Galloway et al., 2011). Similarly, opportunistic bacteria such as atypic mycobacterium were found in the joint infections in patients with human immunodeficiency virus (Zalavras et al., 2006), suggesting that immunosuppression may increase the probability of opportunistic bacterial septic arthritis.
Since human commensals have been shown to augment the pathogenicity of S. aureus infections such as sepsis, our hypothesis for the current study was to investigate if this would be also true for specific infections, such as septic arthritis. To this end, mice were inoculated with either S. aureus, S. epidermidis, S. mitis or a combination of S. aureus and S. epidermidis or S. aureus and S. mitis. Our data demonstrate that both S. epidermidis and S. mitis augment S. aureus-induced septic arthritis.
Material and Methods
Mice
Female NMRI mice, 6– 8 weeks old, were purchased from Envigo (Venra, Netherlands). All mice were housed in the animal facility located at the Department of Rheumatology and Inflammation Research, University of Gothenburg. The mice were kept under standard conditions of temperature and light, and were fed laboratory chow and water ad libitum. The ethical committee of animal research of Gothenburg approved the experiments.
Preparation of Bacterial Solutions
The following bacterial species were used in this study: S. aureus LS-1 strain,
S. epidermidis strain CCUG 61325, and S. mitis strain CCUG 63687. The bacterial suspensions were thawed, washed in phosphate-buffered saline (PBS), and adjusted to the concentration required before conducting the experiments, as previously described (Jin et al., 2019).
Experimental Protocols for Inducing Septic Arthritis
Several separate in vivo experiments were performed to investigate if human commensals aggravate S. aureus-induced septic arthritis. In all experiments, NMRI mice were inoculated intravenously into the tail vein with 200 μl of the respective bacterial strain suspensions in PBS. To prepare bacterial mixture, each of the bacterial strains was prepared in PBS in twice as high bacterial concentration and then mixed in a 1:1 ratio volume wise. To study whether S. epidermidis aggravated S. aureus-induced septic arthritis, mice were inoculated with the following doses: S. aureus LS-1 strain, 1.2x106 colony forming units (CFU)/mouse; S. epidermidis, CCUG 61325 strain, 1.2x108 CFU/mouse; or Mixture of S. aureus LS-1 strain, 1.2x106 CFU/mouse with either S. epidermidis 1.2x106, 1.2x107 or 1.2x108 CFU/mouse.
To study whether S. mitis aggravated S. aureus-induced septic arthritis, mice were inoculated with the following doses: S. aureus 2.8x106 CFU/mouse; S. mitis, CCUG 63687, 2.4x108 CFU/mouse; or Mixture of S. aureus 2.8x106 CFU/mouse with S. mitis 2.4x108 CFU/mouse.
The mice were monitored individually and the survival, weight development and clinical arthritis were assessed regularly, as previously described (Mohammad et al., 2020). At the end of experiments, the mice were anaesthetized with ketamine hydrochloride (Pfizer AB, Sweden) and medetomidine (Orion Pharma, Finland) before blood from the axillary artery was collected. Afterwards the mice were immediately sacrificed by cervical dislocation.
Clinical Evaluation of Arthritis
Observers blinded to the treatment groups visually inspected all 4 limbs of each mouse. Arthritis was defined as erythema and/or swelling of the joints. To evaluate the severity of arthritis, a clinical scoring system ranging from 0-3 was used, as previously described (Bremell et al., 1991; Ali et al., 2015a).
Bacteriologic Examination
To study whether the bacteria invaded and persisted in the kidneys or joints, mice were inoculated with either S. aureus alone, S. epidermidis alone or a mixture of both bacterial species in 1:100 ratio, respectively. After sacrificing the mice, the kidneys of the mice were aseptically removed, homogenized, plated on blood agar plates, and quantified as CFUs on day 7 and 10 post-infection, as described elsewhere (Mohammad et al., 2016), while different joint groups (forepaws and wrists, elbows, shoulders, back paws and ankles, knees, and hips) from each animal were collected separately and homogenized for CFU counts on day 7 when the clinical arthritis became evident, as previously described (Na et al., 2020). For mixed infections, the bacteria were identified by morphological characteristics.
Microcomputed Tomography (Micro-CT)
The mice were sacrificed and joints removed, fixed in 4% paraformaldehyde for 3 days and transferred to PBS for 24 hrs. Thereafter, all limb joints were scanned with Skyscan1176 micro-CT (Bruker, Antwerp, Belgium) with a voxel size of 35 μm. The scanning was done at 55kV/455 mA, with a 0.2 mm aluminium filter. Exposure time was 47 ms. The X-ray projections were obtained at 0.7° intervals with a scanning angular rotation of 180°. The projection images were reconstructed into three-dimensional images using NRECON software (version 1.6.9.8; Bruker) and analyzed with CT-Analyzer (version 2.7.0; Bruker). After reconstruction, experienced observers (Y.F, T.J) evaluated, in a blinded manner, the extent of bone and cartilage destruction on a grading scale from 0-3, as previously described (Ali et al., 2015b; Mohammad et al., 2019).
Histopathological Examination of Joints
After the scanning, joints were decalcified, embedded in paraffin and sectioned with microtome. Tissue sections were thereafter stained with hematoxylin and eosin. All slides were coded and assessed in a blinded manner by observers (Y.F, T.J). The extent of synovitis as well as cartilage and bone destruction were judged as previously described (Ali et al., 2015c; Mohammad et al., 2019).
Measurement of Cytokine and Chemokine Levels
The levels of monocyte chemoattractant protein (MCP-1), tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in mouse blood serum were quantified, using DuoSet ELISA Kits (R&D Systems, Abingdon, UK).
Statistical analysis
Statistical significance was assessed using the Mann-Whitney U test and Fisher’s exact test as appropriate. Results are reported as the mean ± standard error of the mean (SEM) unless indicated otherwise. A p value <0.05 was considered statistically significant. Calculations were performed using GraphPad Prism version 9.3.0 software for Mac (GraphPad software, La Jolla, CA, USA).
Results
S. epidermidis and S. mitis augments S. aureus-Induced Septic Arthritis
In order to study whether the human commensal bacteria augmented S. aureus-induced septic arthritis, mice were inoculated with either S. aureus alone or with a mixture of S. aureus and S. epidermidis or S. mitis in a ratio of 1:100. Mice inoculated with a mixture of S. aureus together with S. epidermidis developed significantly more severe and more frequent clinical arthritis compared to mice inoculated with S. aureus alone. Mice infected with S. epidermidis did not develop any signs of septic arthritis during the course of infection (Figures 1A, B). Similar data were also observed when S. mitis was used. Significantly increased severity of clinical arthritis was observed in mice inoculated with combination of S. aureus together with S. mitis compared to mice inoculated with either S. aureus alone or S. mitis alone. Also, the frequency of arthritis tended to be higher in mice infected with S. aureus and S. mitis mixture than mice infected with S. aureus alone (Figures 1C, D). This strongly suggest that augmentation effect for S. aureus septic arthritis can be induced by both S. epidermidis and S. mitis.
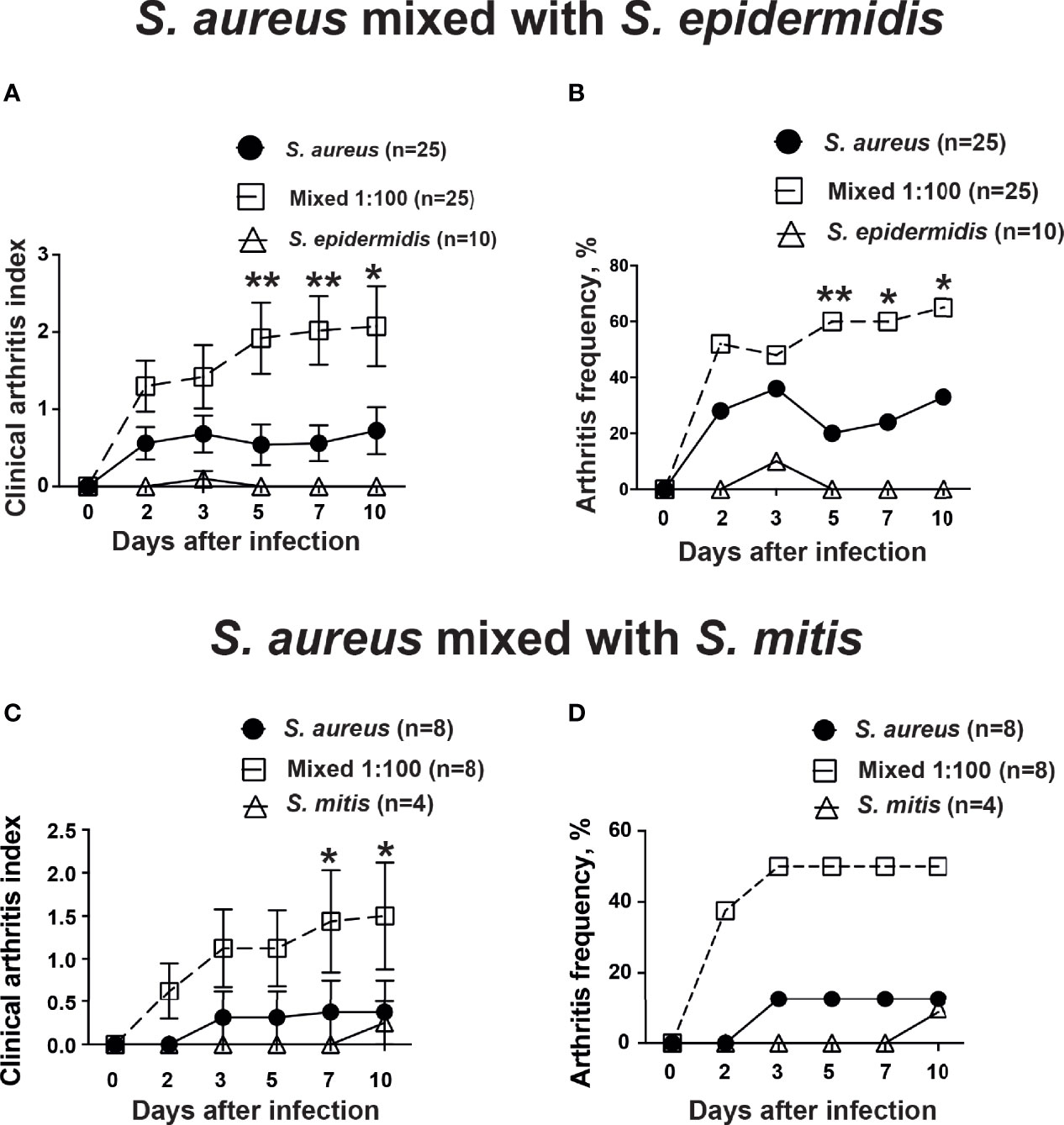
Figure 1 S. epidermidis and S. mitis augment S. aureus-induced septic arthritis. NMRI mice intravenously inoculated with either Staphylococcus aureus (S. aureus) LS-1 strain (1.2 x 106 CFU/mouse) alone, Staphylococcus epidermidis (S. epidermidis) CCUG 61325 (1.2 x 106 CFU/mouse) alone or with S. aureus LS-1 strain (1.2 x 106 CFU/mouse) mixed with S. epidermidis CCUG 61325 (1.2 x 108 CFU/mouse, denoted Mixed 1:100). The clinical severity of arthritis (A) and the frequency of arthritis (B) were followed for 10 days after infection. NMRI mice intravenously inoculated with either S. aureus LS-1 strain (2.8 x 106 CFU/mouse) alone, Streptococcus mitis (S. mitis) CCUG 63687 (2.4 x 106 CFU/mouse) alone or with S. aureus LS-1 strain (2.8 × 106 CFU/mouse) mixed with S. mitis CCUG 63687 (2.4 x 108 CFU/mouse, denoted Mixed 1:100). The clinical severity of arthritis (C) and the frequency of clinical arthritis (D) were followed for 10 days after infection. Statistical evaluations were performed using the Mann–Whitney U test, with data expressed as the mean ± standard error of the mean (A, C) or Fisher’s exact test (B, D). *P< 0.05; **P< 0.01.
The Weight Development During Infection Was Not Altered by Addition of Commensal Bacteria
Weight development is a crucial parameter in our animal model describing the general well-being of the animals. Mice inoculated with S. aureus alone or mixture of S. aureus together with S. epidermidis lost significantly more weight compared to mice inoculated with S. epidermidis alone throughout the experiment (Figure 2). However, no tangible difference was found between mice infected with S. aureus alone or mixture of S. aureus together with S. epidermidis.
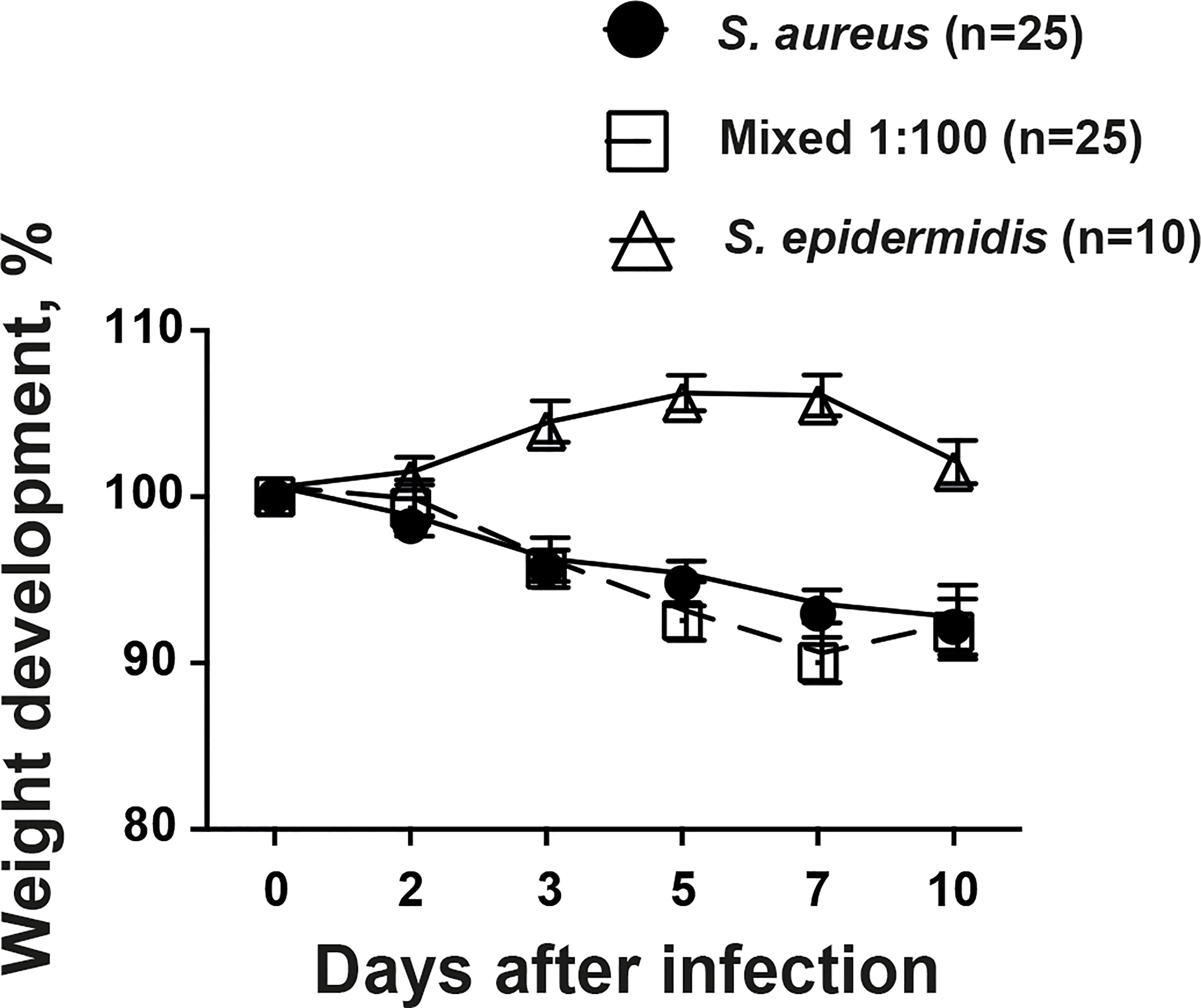
Figure 2 The weight development during infection was not altered by addition of commensal bacteria. NMRI mice intravenously inoculated with either Staphylococcus aureus (S. aureus) LS-1 strain (1.2 x 106 CFU/mouse) alone, Staphylococcus epidermidis (S. epidermidis) CCUG 61325 (1.2 x 106 CFU/mouse) alone or with S. aureus LS-1 strain (1.2 x 106 CFU/mouse) mixed with S. epidermidis CCUG 61325 (1.2 x 108 CFU/mouse, denoted Mixed 1:100). The changes in the body weight were followed for 10 days after infection. Statistical evaluations were performed using the Mann–Whitney U test, with data expressed as the mean ± standard error of the mean.
Co-Injection of S. epidermidis and S. aureus Impairs Ability of Mice to Clear Bacteria in Both Joints and Kidneys
The accumulation of bacteria in joints is diagnostic for septic arthritis. The increased bacterial counts in kidneys are associated with worse outcome in our S. aureus-induced septic arthritis animal model. Thus, we investigated the ability of mice to clear bacteria in both the joints and kidneys. Mice inoculated with the bacterial mixture had significantly higher bacteria recovered in joints compared to mice inoculated with either S. aureus or S. epidermidis alone (Figure 3A). Interestingly, no bacteria were recovered in the joints of mice inoculated with S. epidermidis alone whereas bacteria were recovered in 17% of the joints of mice inoculated with S. aureus alone (Figure 3A).
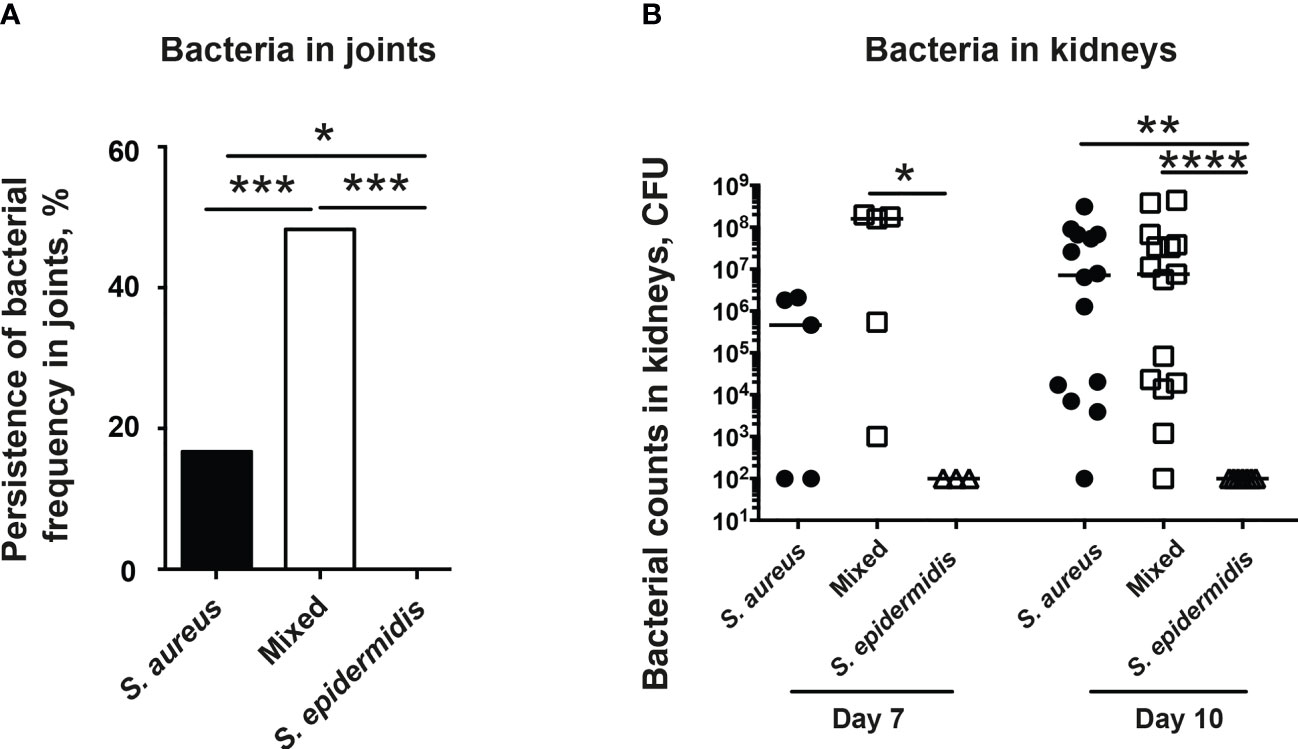
Figure 3 Co-injection of S. epidermidis and S. aureus impairs ability of mice to clear bacteria in both joints and kidneys. NMRI mice intravenously inoculated with either Staphylococcus aureus (S. aureus) LS-1 strain (1.2 x 106 CFU/mouse) alone, Staphylococcus epidermidis (S. epidermidis) CCUG 61325 (1.2 x 106 CFU/mouse) alone or with S. aureus LS-1 strain (1.2 x 106 CFU/mouse) mixed with S. epidermidis CCUG 61325 (1.2 x 108 CFU/mouse, denoted Mixed). (A) Persistence of bacterial frequency in joints including wrists, ankles, and knees of the mice (n = 3-5 mice/group) on day 7 post-infection. (B) Persistence of bacteria in kidneys of the mice on day 7 post-infection (n = 3-5 mice/group) or on day 10 post-infection (n = 7-15 mice/group). Statistical evaluations were performed using Fisher’s exact test (A) or Mann–Whitney U test, with data expressed as the median (B). *P< 0.05; **P< 0.01; ***P< 0.001; ****P< 0.0001.
In contrast, no significant differences in kidney CFU counts were observed in the mice with S. aureus alone or a mixture of S. aureus and S. epidermidis on day 7 and 10 post-infection (Figure 3B). No bacteria were found in kidneys from mice infected with high dose of S. epidermidis alone (Figure 3B).
Co-Injection of S. epidermidis and S. aureus Increases Serum Levels of Chemokines in Mice
Next step was to investigate whether co-injection of S. aureus with S. epidermidis increased the serum levels of chemokines and cytokines. Inoculation with the bacterial mixture indeed significantly increased the release of the MCP-1 compared to inoculation with either S. aureus or S. epidermidis alone on day 7 but not day 10 post-infection (Figure 4A). No difference was recorded between the groups with regard to serum levels of the pro-inflammatory cytokines, TNF-α and IL-6 on both day 7 and day 10 post-infection (Figure 4B, C).
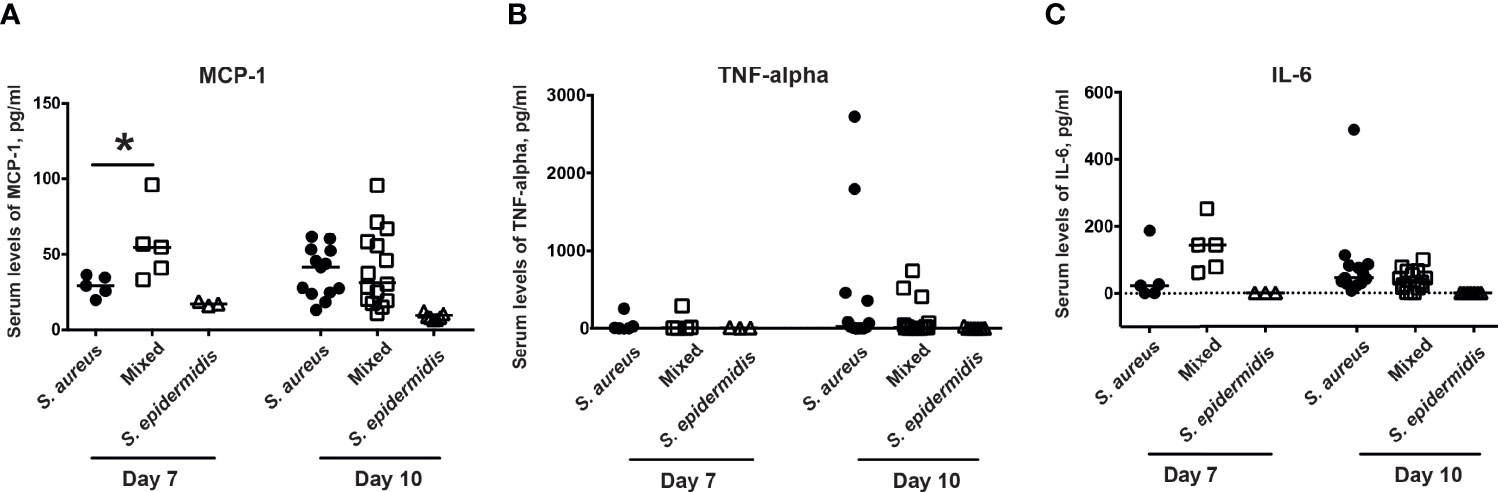
Figure 4 Co-injection of S. epidermidis and S. aureus increases serum levels of chemokines in mice. The levels of (A) monocyte chemoattractant protein 1 (MCP-1), (B) tumor necrosis factor alpha (TNF-alpha), and (C) interleukin-6 (IL-6) in the blood serum of NMRI mice intravenously inoculated with either Staphylococcus aureus (S. aureus) LS-1 strain (1.2 x 106 CFU/mouse) alone, Staphylococcus epidermidis (S. epidermidis) CCUG 61325 (1.2 x 106 CFU/mouse) alone or with S. aureus LS-1 strain (1.2 x 106 CFU/mouse) mixed with S. epidermidis CCUG 61325 (1.2 x 108 CFU/mouse, denoted Mixed) on day 7 post-infection (n = 3-5 mice/group) and on day 10 post-infection (n = 7-15 mice/group). Statistical evaluations were performed using the Mann–Whitney U test, with data expressed as the median. *P< 0.05.
S. epidermidis Augments S. aureus-Induced Septic Arthritis in a Dose-Dependent Manner
In order to study whether the human commensal S. epidermidis dose-dependently augments S. aureus-induced septic arthritis, mice were inoculated with either S. aureus alone or with a mixture of S. aureus and S. epidermidis in a ratio of 1:1, 1:10 or 1:100. Mice inoculated with a mixture of S. aureus and S. epidermidis in a ratio of 1:1 did not develop clinical arthritis (Figures 5A, B). However, mice inoculated with a mixture of S. aureus together with higher doses of S. epidermidis (1:10 and 1:100) developed much severe and more frequent clinical arthritis compared to mice inoculated with S. aureus alone (Figures 5A, B), suggesting that augmentation effect of S. epidermidis was dose-dependent. The mice infected with S. epidermidis alone did not have any sign of septic arthritis during the whole course of infection.
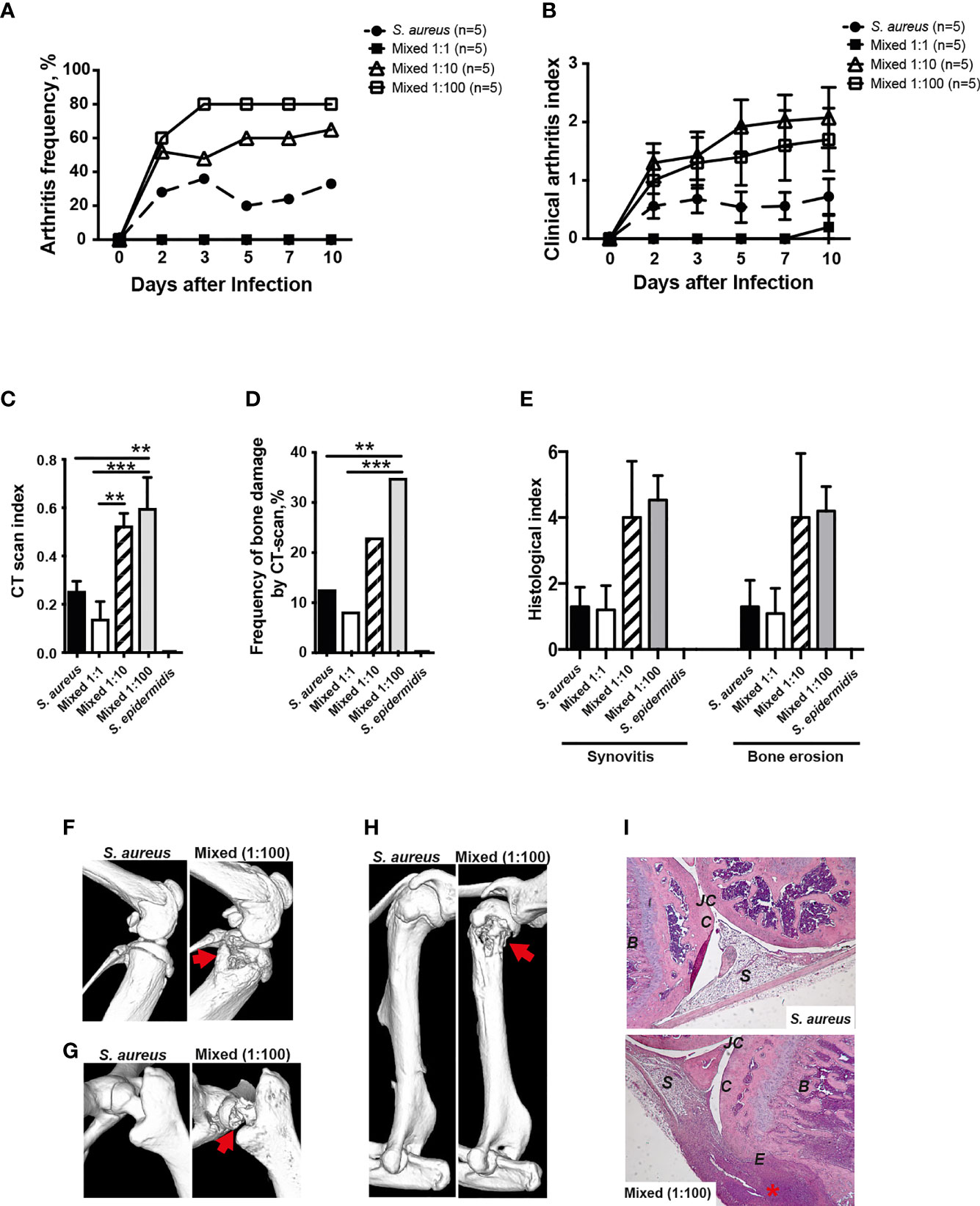
Figure 5 S. epidermidis augment S. aureus-induced septic arthritis in a dose dependent manner. NMRI mice were intravenously inoculated with either Staphylococcus aureus (S. aureus) LS-1 strain (1.2 x 106 CFU/mouse) alone, Staphylococcus epidermidis (S. epidermidis) CCUG 61325 strain (1.2 x 106 CFU/mouse) alone or with S. aureus LS-1 strain (1.2 x 106 CFU/mouse) mixed with different concentrations of S. epidermidis CCUG 61325 (1.2 x 106 CFU/mouse, denoted Mixed 1:1; 1.2 x 107 CFU/mouse, denoted Mixed 1:10; or 1.2 x 108 CFU/mouse, denoted Mixed 1:100). The frequency of clinical arthritis (A), the clinical severity of arthritis (B), and the radiological accumulative bone destruction scores (C) and the frequency of bone destructions (D) as evaluated by a microcomputed tomography (μCT)-scan on day 10 post-infection. (E) Histopathologic evaluation of synovitis and bone erosions in the joints from all 4 limbs of the 5 groups. Representative μCT-scan images of knee joints (F), hip joints (G) and shoulders (H) in NMRI mice infected with S. aureus illustrating a healthy joint (left panels) and a joint infected with a mixture of S. aureus and S. epidermidis with severe septic arthritis (right panels) on day 10 post-infection. The erosions are indicated by the arrows. (I) Illustrative photomicrographs from NMRI mice infected with S. aureus showing a healthy knee joint (upper panel) and NMRI mice infected with a mixture of S. aureus and S. epidermidis showing a heavily inflamed knee joint with severe bone and cartilage destruction (lower panel) on day 10 post-infection. Histologic staining was performed using hematoxylin and eosin. The asterisk indicates an inflamed synovium. B, bone; C, cartilage; E, erosion of bone and cartilage; JC, joint cavity; S, synovial tissue. Original magnification × 10. Statistical evaluations were performed using the Mann–Whitney U test, with data expressed as the mean ± standard error of the mean (B, C, E) or Fisher’s exact test (A, D). **P< 0.01; ***P< 0.001.
In line with the data from clinical arthritis, mice inoculated with a mixture of both S. aureus and S. epidermidis in a ratio of 1:10 and 1:100 significantly developed more severe bone destruction as well as higher frequencies of bone erosions compared to mice inoculated with S. aureus alone or a mixture of S. aureus and S. epidermidis in a ratio of 1:1 on the termination day (Figures 5C, D).
A similar trend was seen histologically whereby mice inoculated with S. aureus mixed together with S. epidermidis displayed more severe synovitis and bone erosion compared to mice inoculated with S. aureus alone (Figure 5E).
Figures 5F–H demonstrate the representative CT images of healthy joints and bone destruction in knees, hips, and shoulders. Figure I show the representative histological images of a healthy knee and a knee with severe septic arthritis.
Discussion
The presence of human commensal organism at the point of infection is known to dramatically increase the S. aureus pathogenicity in S. aureus sepsis. However, it is still largely unknown whether human commensals augment other types of S. aureus infections, such as S. aureus septic arthritis. In the current study, for the first time we show that human commensal strains such as S. epidermidis and S. mitis augment S. aureus pathogenicity in inducing septic arthritis in a dose-dependent manner.
Our data demonstrate that human commensals augment S. aureus invasion into the joints, causing more joint inflammation and bone destructions. Similarly, significantly more severe abscess formation and higher bacterial load in livers were found in previous reports in the sepsis settings (Boldock et al., 2018). In contrast, we found that bacterial load in the kidneys was not significantly increased by mixed infection. This suggest that augmentation effects by commensal bacteria are somehow tissue-specific, e.g. focal S. aureus infections in livers and joints, but not in kidneys were augmented by the commensal strains. It has been shown that augmentation effect of commensal strains on S. aureus pathogenicity is mediated through Kupffer cells (Boldock et al., 2018). Kupffer cells, resident liver macrophages, are abundant in liver tissues, serving as the first gatekeeper to eliminate the bacteria, toxins, and microbial debris transported to liver. Monocytes/macrophages are also known to be crucial for induction of septic arthritis. Depletion of monocytes in mice caused reduced severity of septic arthritis in a S. aureus septic arthritis mouse model (Verdrengh and Tarkowski, 2000). Lipoproteins, the most pro-inflammatory S. aureus component, lost their arthritogenic- as well as the bone resorptive capacities in the monocytes/macrophages depleted mice (Mohammad et al., 2019; Schultz et al., 2022). In addition, the monocytes/macrophages are the precursors of osteoclasts that is one of major bone cells in bone tissues. However, the importance and abundance of macrophages in liver and joints may not explain the organ-specificity of augmentation, as macrophages are also abundant in kidneys. The renal macrophages account for about 50% of total CD45+ leukocytes in mouse kidneys and also found in large numbers in human kidney (Liu et al., 2020). Therefore, the mechanism why augmentation effect by commensals is organ specific is warranted for the further investigations.
In our experimental settings, S. aureus were mixed with commensal strains and intravenously injected to mice. The augmentation effect was observed only when ratio of commensal strains and S. aureus mixture was higher than 1:1. Does the augmentation niche with right bacteria and right ratio of bacterial mixture exist in humans? S. aureus are commonly found on the human skin at different body sites and anterior nares are the reservoir for S. aureus skin carriage (Sollid et al., 2014). Up to 30% of the human population are colonized with nasal S. aureus (Sakr et al., 2018) and the colonized S. aureus in the nares are one of the main sources of S. aureus bacteremia (von Eiff et al., 2001). Rapid screening and decolonizing of nasal carriers of S. aureus was shown to prevent the surgical-site infections (Bode et al., 2010). The commensal bacteria found in the anterior naris are S. epidermidis, Cutibacterium acnes, Dolosigranulum pigrum, Finegoldia magna, Corynebacterium spp., Moraxella spp., Peptoniphilus spp., and Anaerococcus spp (Kumpitsch et al., 2019).. S. epidermidis that was used in the current study is one of the most predominant opportunistic bacterial species in the naris, suggesting the great possibility of coexistence of both bacteria species in the same location. Importantly, it has been shown that a broad range of microorganisms including Micrococcus luteus, Escherichia Coli, Roseomonas mucosa, and Saccharomyces cerevisiae are able to augment the S. aureus pathogenicity in sepsis (Boldock et al., 2018; Gibson et al., 2021). Indeed, a study of the overall microbial composition in anterior nare bacterial community in 40 healthy individuals revealed that in most of the cases the other commensal strains were much more prevalent in high abundance than S. aureus (Wos-Oxley et al., 2010).
How is the augmentation effect on S. aureus septic arthritis mediated by commensal strains? In our current study, both S. epidermidis and S. mitis were proven to be non-virulent, as neither kidney abscess formation nor weight loss were observed in the mice infected with those commensal strains. It is obvious that commensal strains used in our current study were much more vulnerable to the innate immune killing than S. aureus, as no commensal bacteria was recovered from kidneys and joints despite of high infection doses (100 times as S. aureus doses). In order to induce a hematogenous septic arthritis, S. aureus need to survive the bactericidal components and phagocyte attacks in the blood, disseminate to synovial tissue and finally reach the joint cavity (Jin et al., 2021). For instance, deficiency of neutrophils (Verdrengh and Tarkowski, 1997) or complement 3 (Na et al., 2016) greatly aggravated the S. aureus septic arthritis in mice. We hypothesize that as the innate immunity is non-specific, the big number of commensal strains disturbed the phagocytosis and oxygen radical burst by phagocytes against S. aureus and therefore significantly more S. aureus could survive in blood stream, reach the joint cavity and cause more severe septic arthritis. In addition, the hyperinflammatory responses including enormous leucocyte trafficking and burst of pro-inflammatory cytokines may also contribute to more severe septic arthritis due to increased multitude of infection agents, especially when much higher commensal bacteria were mixed with S. aureus during the infections.
Septic arthritis is counted as the most aggressive and devastating joint disease. For patients who have such an infection, even after they have received immediate treatment, the joint damage caused by septic arthritis is often irreversible, leading to permanent joint dysfunction for up to half of the patients (Kaandorp et al., 1995). Furthermore, the emergence of MRSA has severely complicated the available treatment options (DeLeo et al., 2010; Tong et al., 2015). The preventive strategies and novel treatments against septic arthritis are urgently needed. In this study, we have shown that commensal strains such as S. epidermidis and S. mitis dose-dependently augment S. aureus pathogenicity to induce the septic arthritis. This suggests that eradication of both S. aureus and other commensal bacteria in high-risk patients for septic arthritis might be a new preventive strategy. It is known that patient screening for S. aureus nasal carriers and decolonization of nasal/extranasal sites by topical antibiotics prevent the surgical-site infections (Bode et al., 2010). We need to keep in mind that commensal bacteria, especially microbiota in the guts modulate many vital physiological and metabolic functions as well as the development of immune system (Hooper and Gordon, 2001). The systemic eradication of commensal bacteria may break homeostasis of a balanced microbial ecosystem, which may lead to an unwanted abnormal health situation. Therefore, we propose a similar strategy for high-risk patients to prevent septic arthritis: 1) Systemic decolonization should be avoided and decolonization for nasal sites should be used; 2) Screening for the S. aureus nasal carriers should be performed for the patients with higher risk for septic arthritis, such as patients undergoing hemodialysis or peritoneal dialysis (Laupland et al., 2003); 3) Only high-risk patient with S. aureus nasal carriage will be treated with the topical antibiotics with broad spectrum covering both S. aureus and common commensal.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by The Ethics Committee of Animal Research of Gothenburg. The mouse experiments were performed in accordance with the Swedish Board of Agriculture’s regulations and recommendations on animal experiments.
Author Contributions
TJ and YF conceived and planned the experiments. YF, MM, and AA carried out the experiments. AA and TJ wrote the manuscript. All authors contributed to the interpretation of the results and provided critical feedback and helped shape the research, analysis and manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Swedish Medical Research Council [grant number 523-2013-2750 and 2019-01135 to TJ]; grants from the Swedish state under the agreement between the Swedish Government and the county councils, the ALF-agreement [grant number ALFGBG-823941 to TJ]; Rune och Ulla Amlövs Stiftelse för Neurologisk och Reumatologisk Forskning [grant number 2016-075 to TJ]; E och K.G. Lennanders stipendiestiftelse to [AA and MM]; Magnus Bergvalls Stiftelse [grant numbers 2017-01958, 2018-02797 to AA]; Sahlgrenska University Hospital Foundations to [MM] and Institute of Medicine, Gothenburg University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, A., Na, M., Svensson, M. N., Magnusson, M., Welin, A., Schwarze, J. C., et al. (2015a). IL-1 Receptor Antagonist Treatment Aggravates Staphylococcal Septic Arthritis and Sepsis in Mice. PLoS One 10 (7), e0131645. doi: 10.1371/journal.pone.0131645
Ali, A., Welin, A., Schwarze, J. C., Svensson, M. N., Na, M., Jarneborn, A., et al. (2015b). CTLA4 Immunoglobulin But Not Anti-Tumor Necrosis Factor Therapy Promotes Staphylococcal Septic Arthritis in Mice. J. Infect. Dis. 212 (8), 1308–1316. doi: 10.1093/infdis/jiv212
Ali, A., Zhu, X., Kwiecinski, J., Gjertsson, I., Lindholm, C., Iwakura, Y., et al. (2015c). Antibiotic-Killed Staphylococcus Aureus Induces Destructive Arthritis in Mice. Arthritis Rheumatol 67 (1), 107–116. doi: 10.1002/art.38902
Al-Nammari, S. S., Bobak, P., Venkatesh, R. (2007). Methicillin Resistant Staphylococcus Aureus Versus Methicillin Sensitive Staphylococcus Aureus Adult Haematogenous Septic Arthritis. Arch. Orthop Trauma Surg. 127 (7), 537–542. doi: 10.1007/s00402-007-0285-z
Bode, L. G., Kluytmans, J. A., Wertheim, H. F., Bogaers, D., Vandenbroucke-Grauls, C. M., Roosendaal, R., et al. (2010). Preventing Surgical-Site Infections in Nasal Carriers of Staphylococcus Aureus. N Engl. J. Med. 362 (1), 9–17. doi: 10.1056/NEJMoa0808939
Boldock, E., Surewaard, B. G.J., Shamarina, D., Na, M., Fei, Y., Ali, A., et al. (2018). Human Skin Commensals Augment Staphylococcus Aureus Pathogenesis. Nat. Microbiol. 3 (8), 881–890. doi: 10.1038/s41564-018-0198-3
Bremell, T., Lange, S., Yacoub, A., Ryden, C., Tarkowski, A.. (1991). Experimental Staphylococcus Aureus Arthritis in Mice. Infect. Immun. 59 (8), 2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991
Cain, S. M., Enfield, K. B., Giannetta, E. T., Sifri, C. D., Lewis, J. D.. (2018). Septic Arthritis Due to Oral Streptococci Following Intra-Articular Injection: A Case Series. Am. J. Infect. Control 46 (11), 1301–1303. doi: 10.1016/j.ajic.2018.04.227
Coatsworth, N. R., Huntington, P. G., Giuffre, B., Kotsiou, G.. (2013). The Doctor and the Mask: Iatrogenic Septic Arthritis Caused by Streptoccocus Mitis. Med. J. Aust. 198 (5), 285–286. doi: 10.5694/mja12.11695
DeLeo, F. R., Otto, M., Kreiswirth, B. N., Chambers, H. F. (2010). Community-Associated Meticillin-Resistant Staphylococcus Aureus. Lancet 375 (9725), 1557–1568. doi: 10.1016/S0140-6736(09)61999-1
Feder, O. I., Gruson, K. I. (2016). Glenohumeral Joint Sepsis Caused by Streptococcus Mitis: A Case Report. Am. J. Orthop (Belle Mead NJ) 45 (6), E343–E346.
Fei, Y., Wang, W., Kwiecinski, J., Josefsson, E., Pullerits, R., Jonsson, I. M., et al. (2011). The Combination of a Tumor Necrosis Factor Inhibitor and Antibiotic Alleviates Staphylococcal Arthritis and Sepsis in Mice. J. Infect. Dis. 204 (3), 348–357. doi: 10.1093/infdis/jir266
Frazee, B. W., Fee, C., Lambert, L. (2009). How Common is MRSA in Adult Septic Arthritis? Ann. Emerg. Med. 54 (5), 695–700. doi: 10.1016/j.annemergmed.2009.06.511
Galloway, J. B., Hyrich, K. L., Mercer, L. K., Dixon, W. G., Ustianowski, A. P., Helbert, M., et al. (2011). Risk of Septic Arthritis in Patients With Rheumatoid Arthritis and the Effect of Anti-TNF Therapy: Results From the British Society for Rheumatology Biologics Register. Ann. Rheum Dis. 70 (10), 1810–1814. doi: 10.1136/ard.2011.152769
Gibson, J. F., Pidwill, G. R., Carnell, O. T., Surewaard, B. G.J., Shamarina, D., Sutton, J. A.F., et al. (2021). Commensal Bacteria Augment Staphylococcus Aureus Infection by Inactivation of Phagocyte-Derived Reactive Oxygen Species. PLoS Pathog. 17 (9), e1009880. doi: 10.1371/journal.ppat.1009880
Goldenberg, D. L. (1998). Septic Arthritis. Lancet 351 (9097), 197–202. doi: 10.1126/science.1058709
Hooper, L. V., Gordon, J. I. (2001). Commensal Host-Bacterial Relationships in the Gut. Science 292 (5519), 1115–1118.
Jin, T., Mohammad, M., Hu, Z., Fei, Y., Moore, E. R.B., Pullerits, R., et al. (2019). A Novel Mouse Model for Septic Arthritis Induced by Pseudomonas Aeruginosa. Sci. Rep. 9 (1), 16868. doi: 10.1038/s41598-019-53434-5
Jin, T., Jin, T., Mohammad, M., Pullerits, R., Ali, A.. (2021). Bacteria and Host Interplay in Staphylococcus Aureus Septic Arthritis and Sepsis. Pathogens 10 (2). doi: 10.3390/pathogens10020158
Kaandorp, C. J., Van Schaardenburg, D., Krijnen, P., Habbema, J. D., van de Laar, M. A.. (1995). Risk Factors for Septic Arthritis in Patients With Joint Disease. A Prospective Study. Arthritis Rheum 38 (12), 1819–1825. doi: 10.1002/art.1780381215
Kim, Y. M., Joo, Y. B. (2012). Clinical Presentation of Staphylococcus Epidermidis Septic Arthritis Following Anterior Cruciate Ligament Reconstruction. Knee Surg. Relat. Res. 24 (1), 46–51. doi: 10.5792/ksrr.2012.24.1.46
Kumpitsch, C., Koskinen, K., Schopf, V., Moissl-Eichinger, C.. (2019). The Microbiome of the Upper Respiratory Tract in Health and Disease. BMC Biol. 17 (1), 87. doi: 10.1186/s12915-019-0703-z
Laupland, K. B., Church, D. L., Mucenski, M., Sutherland, L. R., Davies, H. D.. (2003). Population-Based Study of the Epidemiology of and the Risk Factors for Invasive Staphylococcus Aureus Infections. J. Infect. Dis. 187 (9), 1452–1459. doi: 10.1086/374621
Liu, F., Dai, S., Feng, D., Qin, Z., Peng, X., Sakamuri, S., et al. (2020). Distinct Fate, Dynamics and Niches of Renal Macrophages of Bone Marrow or Embryonic Origins. Nat. Commun. 11 (1), 2280. doi: 10.1038/s41467-020-16158-z
Mohammad, M., Na, M., Welin, A., Svensson, M. N., Ali, A., Jin, T., et al. (2016). RAGE Deficiency Impairs Bacterial Clearance in Murine Staphylococcal Sepsis, But Has No Significant Impact on Staphylococcal Septic Arthritis. PLoS One 11 (12), e0167287. doi: 10.1371/journal.pone.0167287
Mohammad, M., Nguyen, M. T., Engdahl, C., Na, M., Jarneborn, A., Hu, Z., et al. (2019). The YIN and YANG of Lipoproteins in Developing and Preventing Infectious Arthritis by Staphylococcus Aureus. PLoS Pathog. 15 (6), e1007877. doi: 10.1371/journal.ppat.1007877
Mohammad, M., Hu, Z., Ali, A., Kopparapu, P. K., Na, M., Jarneborn, A., et al. (2020). The Role of Staphylococcus Aureus Lipoproteins in Hematogenous Septic Arthritis. Sci. Rep. 10 (1), 7936. doi: 10.1038/s41598-020-64879-4
Na, M., Jarneborn, A., Ali, A., Welin, A., Magnusson, M., Stokowska, A., et al. (2016). Deficiency of the Complement Component 3 But Not Factor B Aggravates Staphylococcus Aureus Septic Arthritis in Mice. Infect. Immun. 84 (4), 930–939. doi: 10.1128/IAI.01520-15
Na, M., Hu, Z., Mohammad, M., Stroparo, M. D.N., Ali, A., Fei, Y., et al. (2020). The Expression of Von Willebrand Factor-Binding Protein Determines Joint-Invading Capacity of Staphylococcus Aureus, a Core Mechanism of Septic Arthritis. mBio 11 (6):e02472-20. doi: 10.1128/mBio.02472-20
Ross, J. J., Davidson, L. (2005). Methicillin-Resistant Staphylococcus Aureus Septic Arthritis: An Emerging Clinical Syndrome. Rheumatol. (Oxford) 44 (9), 1197–1198. doi: 10.1093/rheumatology/kei035
Sakr, A., Bregeon, F., Mege, J. L., Rolain, J. M., Blin, O.. (2018). Staphylococcus Aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 9, 2419. doi: 10.3389/fmicb.2018.02419
Schultz, M., Mohammad, M., Nguyen, M. T., Hu, Z., Jarneborn, A., Wienken, C.M., et al. (2022). Lipoproteins Cause Bone Resorption in a Mouse Model of Staphylococcus Aureus Septic Arthritis. Front. Microbiol. 13, 843799. doi: 10.3389/fmicb.2022.843799
Sollid, J. U., Furberg, A. S., Hanssen, A. M., Johannessen, M.. (2014). Staphylococcus Aureus: Determinants of Human Carriage. Infect. Genet. Evol. 21, 531–541. doi: 10.1016/j.meegid.2013.03.020
Tarkowski, A. (2006). Infection and Musculoskeletal Conditions: Infectious Arthritis. Best Pract. Res. Clin. Rheumatol 20 (6), 1029–1044. doi: 10.1016/j.berh.2006.08.001
Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L., Fowler, V. G., Jr. (2015). Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 28 (3), 603–661. doi: 10.1128/CMR.00134-14
Verdrengh, M., Tarkowski, A. (1997). Role of Neutrophils in Experimental Septicemia and Septic Arthritis Induced by Staphylococcus Aureus. Infect. Immun. 65 (7), 2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997
Verdrengh, M., Tarkowski, A. (2000). Role of Macrophages in Staphylococcus Aureus-Induced Arthritis and Sepsis. Arthritis Rheum 43 (10), 2276–2282. doi: 10.1002/1529-0131(200010)43:10<2276::AID-ANR15>3.0.CO;2-C
von Eiff, C., Becker, K., Machka, K., Stammer, H., Peters, G.. (2001). Nasal Carriage as a Source of Staphylococcus Aureus Bacteremia. Study Group. N Engl. J. Med. 344 (1), 11–16. doi: 10.1056/NEJM200101043440102
Wos-Oxley, M. L., Plumeier, I., von Eiff, C., Taudien, S., Platzer, M., Vilchez-Vargas, R., et al. (2010). A Poke Into the Diversity and Associations Within Human Anterior Nare Microbial Communities. ISME J. 4 (7), 839–851. doi: 10.1038/ismej.2010.15
Keywords: S. aureus, S. epidermidis, S. mitis, septic arthritis, mice
Citation: Fei Y, Ali A, Mohammad M and Jin T (2022) Commensal Bacteria Augment Staphylococcus aureus septic Arthritis in a Dose-Dependent Manner. Front. Cell. Infect. Microbiol. 12:942457. doi: 10.3389/fcimb.2022.942457
Received: 12 May 2022; Accepted: 23 June 2022;
Published: 22 July 2022.
Edited by:
Fintan Thomas Moriarty, AO Research Institute, SwitzerlandReviewed by:
Ritesh Thakare, Brown University, United StatesArif Luqman, Sepuluh Nopember Institute of Technology, Indonesia
Copyright © 2022 Fei, Ali, Mohammad and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Jin, dGFvLmppbkByaGV1bWEuZ3Uuc2U=
 Ying Fei1,2
Ying Fei1,2 Abukar Ali
Abukar Ali Majd Mohammad
Majd Mohammad Tao Jin
Tao Jin