- 1Key Laboratory of Basic Pharmacology of the Ministry of Education, Zunyi Medical University, Zunyi, China
- 2Joint International Research Laboratory of Ethnomedicine of the Ministry of Education, Zunyi Medical University, Zunyi, China
- 3Key Laboratory of Basic Pharmacology of Guizhou Province, Zunyi Medical University, Zunyi, China
- 4Electron Microscopy Room of School of Basic Medicine, Zunyi Medical University, Zunyi, China
- 5Laboratory Animal Center, Zunyi Medical University, Zunyi, China
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, which is accompanied with the classical motor symptoms and a range of non-motor symptoms. Bacterial infection affects the neuroinflammation associated with the pathology of PD and various antibiotics have also been confirmed to play an important role not only in bacterial infection, but also in the PD progression. This mini-review summarized the role of common bacterial infection in PD and introduced several antibiotics that had anti-PD effects.
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases, which seriously affects patients’ health and quality of life. The clinical manifestations of PD include non-motor symptoms and motor symptoms. Motor symptoms are mainly motor retardation and static tremor, while non-motor symptoms include sleep disorder, smell loss, anxiety, depression and cognitive disorder (Homayoun, 2018). Levodopa is currently the first choice of the treatment of PD, but long-term use of levodopa will cause obvious adverse reactions, and thus could not achieve a complete cure effect (Tambasco et al., 2018). The main pathological features of PD are the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the accumulation of misfolded α-synuclein (Balestrino and Schapira, 2020). Although PD might be associated with several cellular mechanisms, including mitochondrial dysfunction, oxidative stress, neuroinflammation, and defective protein degradation, the pathogenesis of PD is still unclear (Poewe et al., 2017).
It has recently been discovered that bacteria play an important role in the pathogenesis of neurodegenerative diseases (Sampson et al., 2016; Kim et al., 2020). A large number of studies have shown a strong link between bacterial infection and neuroinflammation (Morais et al., 2021; Shen et al., 2022). Neuroinflammation is considered to be one of the causes of PD (Hirsch and Standaert, 2020), and bacterial infections have been confirmed to be closely associated with PD. For example, gastrointestinal infections increased the risk of the disease (Nerius et al., 2020). In this mini review, we summarized the association between multiple bacterial infections and PD, and then discussed the role of antibiotics in the treatment of PD.
Effects of bacterial infection on PD pathogenesis
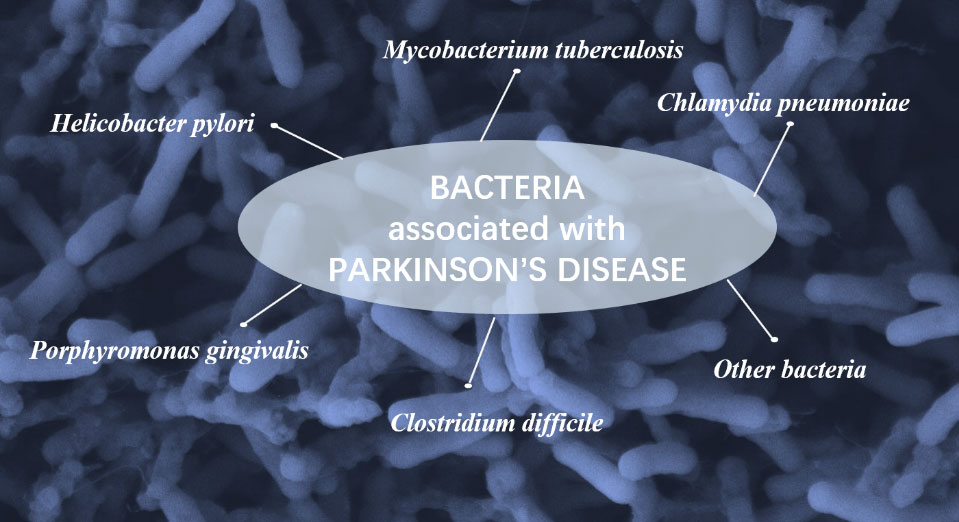
Bacteria could cause a variety of infections, most commonly in the lung, skin and gastrointestinal tract, etc. (Alby and Nachamkin, 2016; Cookson, 2017; Deusenbery et al., 2021). Several bacterial infections are closely related to the onset of PD (Figure 1).
Helicobacter pylori
Helicobacter pylori (H. pylori) is a major cause of gastritis, ulcers, gastric adenocarcinoma and MALT type lymphoma, accompanied by a variety of gastrointestinal symptoms (McGee et al., 2018). Current studies indicate that H. pylori is associated with neurodegenerative diseases (Miklossy, 2011). H. pylori infection is very common in patients with PD (Blaecher et al., 2013). It has long been found that patients with H. pylori ulcers are more likely to develop PD than healthy people of the same age (Actis, 2019). Neuroinflammation, autoimmunity and apoptosis induced by H. pylori infection might be related to the pathogenesis of PD. In addition, in the treatment of PD with levodopa, the elimination of H. pylori infection with antimicrobial could increase the absorption of levodopa in the intestinal tract (Nyholm and Hellström, 2021). However, another evidence demonstrates that eradication therapy for H. pylori does not reduce the risk of PD, even though H. pylori is a risk factor for PD (Huang et al., 2018). The mechanism of association between H. pylori infection and PD is still unclear. The main reason is that there are too many possibilities for H. pylori to cause PD, including the toxic factors, the inflammatory reaction and its influence on intestinal flora, etc. (Dobbs et al., 2016; Noto and Peek, 2017). Thus, the epidemiological investigations are needed for further study.
Mycobacterium tuberculosis
Mycobacterium tuberculosis (M. tuberculosis) is a highly infectious bacterium, and known to induce human tuberculosis (TB). A statistical meta-analysis of human gene expression in response to M. tuberculosis infection identified several enriched pathways, such as the LRRK2 pathway in PD (Wang et al., 2018), which play a critical role in regulating the central nervous system (CNS) immune milieu in PD patient (Cookson, 2017; Kim and Alcalay, 2017). Moreover, M. tuberculosis infection could induce neuroinflammation in astrocytes of PD-related brain regions in a LRRK2-dependent manner. Furtherly, the LRRK2 inhibitors are considered as a major drug development in treatment of PD patients by elevating levels of cytosolic mtDNA and chronic cGAS signaling (Arru et al., 2016; Weindel et al., 2020). Likewise, Rifampicin, an antibiotic commonly used to treat infections with M. tuberculosis, was discovered to have the ability of neuroprotective effects by reducing microglial activation and improving neuron survival against inflammation, which provides a novel therapeutic strategy of anti-Parkinson (Yulug et al., 2014; Liang et al., 2017). Recently, a therapeutic strategy with repeating bacillus Calmette-Guerin (BCG) vaccination was found to be applicable in disease with inadequate aerobic glycolysis including PD (Faustman, 2020). Therefore, basing on the therapeutic strategies with M. tuberculosis might provide a new mentality in PD treatment.
Porphyromonas gingivalis
Porphyromonas gingivalis (P. gingivalis) is a keystone pathogen for periodontitis (Hajishengallis et al., 2012). Patients with periodontal inflammatory disease (PID) are more likely to develop PD (Chen et al., 2017). It has been demonstrated that inflammation is associated with neurodegenerative diseases, and PD patients have higher levels of inflammatory cytokines in the brain compared with people don’t have PD (Adams et al., 2019). Gingipains are critical proteases encoded by P. gingivalis that could interfere or evade the host complement system. Gingipain proteases produce effects on fibrinogen that increases the risk of periodontal bleeding in patients with periodontitis (Haditsch et al., 2020; Kadowaki, 2021). Studies also found that the enzymes interfered coagulation through interacting with fibrinogen, prothrombin (Imamura et al., 2001) and the stimulation of the kallikrein/kinin pathway (Hočevar et al., 2018; Mo et al., 2020). Furthermore, amyloid fibrin (ogen) protein structure was observed in platelet poor plasma clots, and samples from PD patients contain much more amyloid-specific signal compared with the control donors (Adams et al., 2019). Thus, P. gingivalis might affect the development of PD by inducing inflammation and blood changes according to the latest research progress.
The other bacteria
There are many other bacteria verified to be associated with PD. Clostridium difficile (C. difficile) is one of the main pathogens causing diarrhea and pseudomembranous colitis which could colonize when the host has intestinal flora (Leffler and Lamont, 2015; Smits et al., 2016). The individuals with C. difficile infection (CDI) history were at higher risk of PD during the first 2 years since CDI diagnosis over a Swedish population-based cohort study (Kang et al., 2020), but there was no obviously increased PD risk in long-term follow-up. Chlamydia pneumoniae (C. pneumoniae) has been recognized as an important common respiratory pathogen causing otolaryngeal diseases, including pharyngitis, otitis media, tonsillitis and sinusitis (Roulis et al., 2013). C. pneumoniae might have close relationship with neurodegenerative, including Alzheimer’s disease (AD) due to its role in protein deposition and apoptosis in CNS (De Chiara et al., 2012). Besides, an epidemiological study demonstrated that PD risk was increased in healthy individuals who have the familiar pathogens, such as B. burgdorferi and C. pneumoniae (Patrick et al., 2019).
Antibiotics and PD
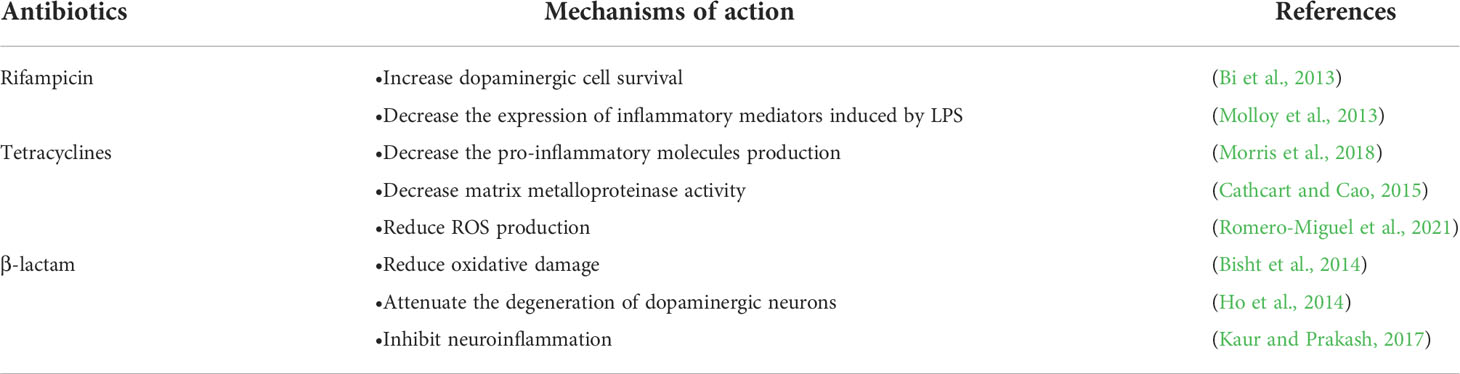
Antibiotics are various kinds of chemical compounds that kill directly or inhibit the microorganisms. Antibiotics are used widely in treating bacterial infection diseases and have decreased the mortality rates. Until now, more and more ancillary properties are found in antibiotics, such as anti-inflammatory effects (Moon et al., 2012; Rashed et al., 2022), inducing gastrointestinal motility (Lam & Ng, 2011), and neuroprotective properties against neurodegenerative and neuroinflammatory disorders (Sultan et al., 2013; Ruzza et al., 2014). Thus, antibiotics function as neuroprotective drugs may not only through treating bacterial infections, but also some other approaches. Here, several antibiotics were demonstrated to be the potential alternatives to PD drugs (Table 1).
Rifampicin
Rifampicin, a wide-spectrum antibiotic, is a semisynthetic derivative of rifamycin with the common structure of an naphthohydroquinone chromophore spanned by an aliphatic ansa chain that mainly transporting the drug to across the blood-brain barrier (BBB) into brain parenchyma. Rifampicin has been confirmed to have the apparent protection in neurodegenerative diseases by different multiple mechanisms, including with anti-apoptotic, anti-inflamatory and anti-oxidant properties (Yulug et al., 2014). In addition, rifampicin could increase the number of surviving dopaminergic neurons at different concentrations (Bi et al., 2013). Also, rifampicin pretreatment led to a dose-dependent increase in cell viability of dopaminergic neurons. Meanwhile, rifampicin decreased LP-induced expression of pro-inflammatory mediators (Molloy et al., 2013). Thus, as a macrocyclic antibiotic for the treatment of M. tuberculosis and other mycobacterial infections, rifampicin is supported to be a novel anti-inflammatory drug for PD, but the molecular and cellular mechanisms still need further investigations.
Tetracyclines
Tetracyclines and its derivatives are broad-spectrum antibiotics with inhibitory effect on most gram positive and negative bacteria and the ability of bactericidal in high concentration. In addition to the antibiotic functions, tetracyclines are reported to generate protection against neurodegenerative and neuropsychiatric diseases (Stoilova et al., 2013; Ruzza et al., 2014; Bortolanza et al., 2018) by reducing pro-inflammatory molecule production (Sultan et al., 2013; Morris et al., 2018), inhibiting matrix metalloproteinase activity and mitochondrial dysfunction (Cathcart and Cao, 2015). Furthermore, tetracycline derivatives, including doxycycline (DOX) and minocycline (MIN), are considered as an alternative therapy strategy in neurodegenerative disorders (Reglodi et al., 2015; Socias et al., 2018). Current evidence indicated that MIN mainly inhibited microglial activation, neuronal apoptosis and reactive oxygen species (ROS) production (Romero-Miguel et al., 2021). Moreover, DOX was confirmed to downregulate the expression of matrix metalloproteinases (MMPs) (Cho et al., 2011). Meanwhile, DOX could suppress the activation of microglia (Santa-Cecília et al., 2016). Therefore, there is rapidly growing evidence showing that tetracycline has the potential therapeutic benefit for PD, but clinical studies are needed to confirm its neuroprotective effect.
β-lactam
Ceftriaxone (CEF) is a β-lactam antibiotic which is most frequently used in local/systemic infection and hospital acquired infections. Recently, CEF have been highlighted the therapeutic efficacy against neurodegenerative diseases. For instance, CEF could ameliorate abnormal uncontrolled movements (Chotibut et al., 2017) in animal models of PD. Moreover, CEF attenuated oxidative damage (Bisht et al., 2014). Also, CEF was found to prevent the degeneration of dopaminergic neurons (Ho et al., 2014) and inhibit neuroinflammation (Kaur & Prakash, 2017). Thus, CEF is currently becoming a research hotspot with its multiple activities to relieve symptoms of PD. At present, more and more studies are re-interested with antibiotics due to its surprising ancillary properties in anti-inflammatory effects. With the affection of variety mental and neurological diseases in human people, drug reuse is considered as a promising new drug discovery strategy basing on the limitation of target-based drugs approaches (Lee and Kim, 2016; Corsello et al., 2017; Gooch et al., 2017).
Conclusion
Parkinson’s disease is the second most common neurodegenerative disease in the world and levodopa remains the main option for the treatment of PD. In this mini review, the relationship between common bacterial (H. pylori, M. tuberculosis, P. gingivalis, C. difficile and C. pneumoniae) infection with PD and their possible action mechanisms, such as neuroinflammation factors, LRRK2 pathway and toxic protein aggregations, were revealed. Meanwhile, the use of antibiotics in treatment of PD is worth exploration, which could provide new strategies in PD treatment. It is worth noting that levodopa is usually administered orally or enterally and the intestinal microbiota could also affect its therapeutic efficacy. Combined use of levodopa with antibiotics to regulate bacterial infection in PD patients might open a new direction to improve the therapeutic effect of levodopa. Furthermore, the underlying mechanisms of these antibiotics’ action still warrant further illumination.
Author contributions
SS conceived of the topic. SS and SZ wrote the manuscript. FZ helped editing of the manuscript. All authors contributed to this review and approved the submitted version.
Funding
The work was supported by special grant of academic new seedling cultivation and innovation exploration from Guizhou Science and Technology Department (Qian Ke He Ping Tai Ren Cai [2018]5772-036 and Qian Ke Ping Tai Ren Cai [2020]-012), Science and Technology Foundation of Guizhou Province (No. ZK[2021]-014), Science and Technology Project of Zunyi city (Zun Shi Ke He HZ Zi [2021] No. 286), Natural Science Foundation of Guizhou Province (Qian Ke He Ji Chu – ZK [2022] No. 604), and Science and Technology Project of Zunyi city (Zun Shi Ke He HZ Zi [2022] No. 372).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Actis, G. C. (2019). Helicobacter pylori infection and parkinson's disease. Minerva. Gastroenterol. Dietol. 65 (2), 164–165. doi: 10.23736/S1121-421X.19.02554-6
Adams, B., Nunes, J. M., Page, M. J., Roberts, T., Carr, J., Nell, T. A., et al. (2019). Parkinson's disease: A systemic inflammatory disease accompanied by bacterial inflammagens. Front. Aging Neurosci. 11. doi: 10.3389/fnagi.2019.00210
Alby, K., Nachamkin, I. (2016). Gastrointestinal infections. Microbiol. Spectr. 4 (3). doi: 10.1128/microbiolspec.DMIH2-0005-2015
Arru, G., Caggiu, E., Paulus, K., Sechi, G. P., Mameli, G., Sechi, L. A. (2016). Is there a role for mycobacterium avium subspecies paratuberculosis in parkinson's disease? J. Neuroimmunol. 293, 86–90. doi: 10.1016/j.jneuroim.2016.02.016
Balestrino, R., Schapira, A. H. V. (2020). Parkinson Disease. Eur. J. Neurol. 27 (1), 27–42. doi: 10.1111/ene.14108
Bisht, R., Kaur, B., Gupta, H., Prakash, A. (2014). Ceftriaxone mediated rescue of nigral oxidative damage and motor deficits in MPTP model of parkinson's disease in rats. Neurotoxicology 44, 71–79. doi: 10.1016/j.neuro.2014.05.009
Bi, W., Zhu, L., Jing, X., Liang, Y., Tao, E. (2013). Rifampicin and parkinson's disease. Neurol. Sci. 34 (2), 137–141. doi: 10.1007/s10072-012-1156-0
Blaecher, C., Smet, A., Flahou, B., Pasmans, F., Ducatelle, R., Taylor, D., et al. (2013). Significantly higher frequency of helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment. Pharmacol. Ther. 38 (11-12), 1347–1353. doi: 10.1111/apt.12520
Bortolanza, M., Nascimento, G. C., Socias, S. B., Ploper, D., Chehín, R. N., Raisman-Vozari, R., et al. (2018). Tetracycline repurposing in neurodegeneration: focus on parkinson's disease. J. Neural. Transm (Vienna). 125 (10), 1403–1415. doi: 10.1007/s00702-018-1913-1
Cathcart, J. M., Cao, J. (2015). MMP inhibitors: Past, present and future. Front. Biosci. (Landmark Ed). 20, 1164–1178. doi: 10.2741/4365
Chen, C. K., Wu, Y. T., Chang, Y. C. (2017). Periodontal inflammatory disease is associated with the risk of parkinson's disease: A population-based retrospective matched-cohort study. Peer J. 5, e3647. doi: 10.7717/peerj.3647
Cho, D. C., Cheong, J. H., Yang, M. S., Hwang, S. J., Kim, J. M., Kim, C. H. (2011). The effect of minocycline on motor neuron recovery and neuropathic pain in a rat model of spinal cord injury. J. Korean Neurosurg. Soc 49 (2), 83–91. doi: 10.3340/jkns.2011.49.2.83
Chotibut, T., Meadows, S., Kasanga, E. A., McInnis, T., Cantu, M. A., Bishop, C., et al. (2017). Ceftriaxone reduces l-dopa-induced dyskinesia severity in 6-hydroxydopamine parkinson's disease model. Mov. Disord. 32 (11), 1547–1556. doi: 10.1002/mds.27077
Cookson, M. R. (2017). Mechanisms of mutant LRRK2 neurodegeneration. Adv. Neurobiol. 14, 227–239. doi: 10.1007/978-3-319-49969-7_12
Corsello, S. M., Bittker, J. A., Liu, Z., Gould, J., McCarren, P., Hirschman, J. E., et al. (2017). The drug repurposing hub: A next-generation drug library and information resource. Nat. Med. 23 (4), 405–408. doi: 10.1038/nm.4306
De Chiara, G., Marcocci, M. E., Sgarbanti, R., Civitelli, L., Ripoli, C., Piacentini, R., et al. (2012). Infectious agents and neurodegeneration. Mol. Neurobiol. 46 (3), 614–638. doi: 10.1007/s12035-012-8320-7
Deusenbery, C., Wang, Y., Shukla, A. (2021). Recent innovations in bacterial infection detection and treatment. ACS Infect. Dis. 7 (4), 695–720. doi: 10.1021/acsinfecdis.0c00890
Dobbs, S. M., Dobbs, R. J., Weller, C., Charlett, A., Augustin, A., Taylor, D., et al. (2016). Peripheral aetiopathogenic drivers and mediators of parkinson’s disease and co-morbidities: role of gastrointestinal microbiota. J. Neurovirol. 22 (1), 22–32. doi: 10.1007/s13365-015-0357-8
Faustman, D. L. (2020). Benefits of BCG-induced metabolic switch from oxidative phosphorylation to aerobic glycolysis in autoimmune and nervous system diseases. J. Intern. Med. 288 (6), 641–650. doi: 10.1111/joim.13050
Gooch, C. L., Pracht, E., Borenstein, A. R. (2017). The burden of neurological disease in the united states: A summary report and call to action. Ann. Neurol. 81 (4), 479–484. doi: 10.1002/ana.24897
Haditsch, U., Roth, T., Rodriguez, L., Hancock, S., Cecere, T., Nguyen, M., et al. (2020). Alzheimer's disease-like neurodegeneration in porphyromonas gingivalis infected neurons with persistent expression of active gingipains. J. Alzheimers. Dis. 75 (4), 1361–1376. doi: 10.3233/JAD-200393
Hajishengallis, G., Darveau, R. P., Curtis, M. A. (2012). The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10 (10), 717–725. doi: 10.1038/nrmicro2873
Hirsch, E. C., Standaert, D. G. (2020). Ten unsolved questions about neuroinflammation in parkinson's disease. Mov. Disord. 36 (1), 16–24. doi: 10.1002/mds.28075
Hočevar, K., Potempa, J., Turk, B. (2018). Host cell-surface proteins as substrates of gingipains, the main proteases of porphyromonas gingivalis. Biol. Chem. 399 (12), 1353–1361. doi: 10.1515/hsz-2018-0215
Ho, S. C., Hsu, C. C., Pawlak, C. R., Tikhonova, M. A., Lai, T. J., Amstislavskaya, T. G., et al. (2014). Effects of ceftriaxone on the behavioral and neuronal changes in an MPTP-induced parkinson's disease rat model. Behav. Brain Res. 268, 177–184. doi: 10.1016/j.bbr.2014.04.022
Homayoun, H. (2018). Parkinson Disease. Ann. Intern. Med. 169 (5), ITC33–ITC48. doi: 10.7326/AITC201809040
Huang, H. K., Wang, J. H., Lei, W. Y., Chen, C. L., Chang, C. Y., Liou, L. S. (2018). Helicobacter pylori infection is associated with an increased risk of parkinson's disease: A population-based retrospective cohort study. Parkinsonism Relat. Disord. 47, 26–31. doi: 10.1016/j.parkreldis.2017.11.331
Imamura, T., Banbula, A., Pereira, P. J., Travis, J., Potempa, J. (2001). Activation of human prothrombin by arginine-specific cysteine proteinases (Gingipains r) from porphyromonas gingivalis. J. Biol. Chem. 276 (22), 18984–18991. doi: 10.1074/jbc.M006760200
Kadowaki, T. (2021). Enzymatic characteristics and activities of gingipains from porphyromonas gingivalis. Methods Mol. Biol. 2210, 97–112. doi: 10.1007/978-1-0716-0939-2_10
Kang, X., Ploner, A., Ludvigsson, J. F., Williams, D. M., Larsson, H., Pedersen, N. L., et al. (2020). Clostridium difficile infection and risk of parkinson's disease: a Swedish population-based cohort study. Eur. J. Neurol. 27 (11), 2134–2141. doi: 10.1111/ene.14400
Kaur, B., Prakash, A. (2017). Ceftriaxone attenuates glutamate-mediated neuro-inflammation and restores BDNF in MPTP model of parkinson's disease in rats. Pathophysiology 24 (2), 71–79. doi: 10.1016/j.pathophys.2017.02.001
Kim, C. Y., Alcalay, R. N. (2017). Genetic forms of parkinson's disease. Semin. Neurol. 37 (2), 135–146. doi: 10.1055/s-0037-1601567
Kim, M. S., Kim, Y., Choi, H., Kim, W., Park, S., Lee, D., et al. (2020). Transfer of a healthy microbiota reduces amyloid and tau pathology in an alzheimer's disease animal model. Gut 69 (2), 283–294. doi: 10.1136/gutjnl-2018-317431
Lam, H. S., Ng, P. C. (2011). Use of prokinetics in the preterm infant. Curr. Opin. Pediatr. 23 (2), 156–160. doi: 10.1097/MOP.0b013e3283431f2a
Lee, H. M., Kim, Y. (2016). ). drug repurposing is a new opportunity for developing drugs against neuropsychiatric disorders. Schizophr. Res. Treat 2016, 6378137. doi: 10.1155/2016/6378137
Leffler, D. A., Lamont, J. T. (2015). Clostridium difficile infection. N. Engl. J. Med. 373 (3), 287–288. doi: 10.1056/NEJMc1506004
Liang, Y., Zhou, T., Chen, Y., Lin, D., Jing, X., Peng, S., et al. (2017). Rifampicin inhibits rotenone-induced microglial inflammation via enhancement of autophagy. Neurotoxicology 63, 137–145. doi: 10.1016/j.neuro.2017.09.015
McGee, D. J., Lu, X. H., Disbrow, E. A. (2018). Stomaching the possibility of a pathogenic role for helicobacter pylori in parkinson's disease. J. Parkinsons. Dis. 8 (3), 367–374. doi: 10.3233/JPD-181327
Miklossy, J. (2011). Emerging roles of pathogens in Alzheimer disease. Expert. Rev. Mol. Med. 13, e30. doi: 10.1017/S1462399411002006
Molloy, D. W., Standish, T. I., Zhou, Q., Guyatt, G. (2013). A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of alzheimer's disease: the DARAD trial. Int. J. Geriatr. Psychiatry 28 (5), 463–470. doi: 10.1002/gps.3846
Mo, W., Luo, H., Wu, J., Xu, N., Zhang, F., Qiu, Q., et al. (2020). Gingipains promote RANKL-induced osteoclastogenesis through the enhancement of integrin β3 in RAW264.7 cells. J. Mol. Histol. 51 (2), 147–159. doi: 10.1007/s10735-020-09865-w
Moon, A., Gil, S., Gill, S. E., Chen, P., Matute-Bello, G. (2012). Doxycycline impairs neutrophil migration to the airspaces of the lung in mice exposed to intratracheal lipopolysaccharide. J. Inflammation (Lond). 9 (1), 31. doi: 10.1186/1476-9255-9-31
Morais, L. H., Schreiber, H. L. 4., Mazmanian, S. K. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19 (4), 241–255. doi: 10.1038/s41579-020-00460-0
Morris, G., Walker, A. J., Berk, M., Maes, M., Puri, B. K. (2018). Cell death pathways: a novel therapeutic approach for neuroscientists. Mol. Neurobiol. 55 (7), 5767–5786. doi: 10.1007/s12035-017-0793-y
Nerius, M., Doblhammer, G., Tamgüney, G. (2020). GI infections are associated with an increased risk of parkinson's disease. Gut 69 (6), 1154–1156. doi: 10.1136/gutjnl-2019-318822
Noto, J. M., Peek, R. M. (2017). The gastric microbiome, its interaction with helicobacter pylori, and its potential role in the progression to stomach cancer. PloS Pathog. 13 (10), e1006573. doi: 10.1371/journal.ppat.1006573
Nyholm, D., Hellström, P. M. (2021). Effects of helicobacter pylori on levodopa pharmacokinetics. J. Parkinsons. Dis. 11 (1), 61–69. doi: 10.3233/JPD-202298
Patrick, K. L., Bell, S. L., Weindel, C. G., Watson, R. O. (2019). Exploring the "Multiple-hit hypothesis" of neurodegenerative disease: Bacterial infection comes up to bat. Front. Cell. Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00138
Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., et al. (2017). Parkinson Disease. Nat. Rev. Dis. Primers 3, 17013. doi: 10.1038/nrdp.2017.13
Rashed, R., Valcheva, R., Dieleman, L. A. (2022). Manipulation of gut microbiota as a key target for crohn's disease. Front. Med. (Lausanne). 9. doi: 10.3389/fmed.2022.887044
Reglodi, D., Maasz, G., Pirger, Z., Rivnyak, A., Balogh, D., Jungling, A., et al. (2015). Neurochemical changes in different brain regions induced by PACAP - relations to neuroprotection. Springerplus 4 (Suppl 1), L56. doi: 10.1186/2193-1801-4-S1-L56
Romero-Miguel, D., Lamanna-Rama, N., Casquero-Veiga, M., Gómez-Rangel, V., Desco, M., Soto-Montenegro, M. L. (2021). Minocycline in neurodegenerative and psychiatric diseases: An update. Eur. J. Neurol. 28 (3), 1056–1081. doi: 10.1111/ene.14642
Roulis, E., Polkinghorne, A., Timms, P. (2013). Chlamydia pneumoniae: modern insights into an ancient pathogen. Trends Microbiol. 21 (3), 120–128. doi: 10.1016/j.tim.2012.10.009
Ruzza, C., Rizzi, A., Malfacini, D., Cerlesi, M. C., Ferrari, F., Marzola, E., et al. (2014). Pharmacological characterization of tachykinin tetrabranched derivatives. Br. J. Pharmacol. 171 (17), 4125–4137. doi: 10.1111/bph.12727
Ruzza, P., Siligardi, G., Hussain, R., Marchiani, A., Islami, M., Bubacco, L., et al. (2014). Ceftriaxone blocks the polymerization of α-synuclein and exerts neuroprotective effects in vitro. ACS Chem. Neurosci. 5 (1), 30–38. doi: 10.1021/cn400149k
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson's disease. Cell 167 (6), 1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
Santa-Cecília, F. V., Socias, B., Ouidja, M. O., Sepulveda-Diaz, J. E., Acuña, L., Silva, R. L., et al. (2016). Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox. Res. 29 (4), 447–459. doi: 10.1007/s12640-015-9592-2
Shen, S., Zhang, C., Xu, Y. M., Shi, C. H. (2022). The role of pathogens and anti-infective agents in parkinson's disease, from etiology to therapeutic implications. J. Parkinsons. Dis. 12 (1), 27–44. doi: 10.3233/JPD-212929
Smits, W. K., Lyras, D., Lacy, D. B., Wilcox, M. H., Kuijper, E. J. (2016). Clostridium difficile infection. Nat. Rev. Dis. Primers 2, 16020. doi: 10.1038/nrdp.2016.20
Socias, S. B., González-Lizárraga, F., Avila, C. L., Vera, C., Acuña, L., Sepulveda-Diaz, J. E., et al. (2018). Exploiting the therapeutic potential of ready-to-use drugs: Repurposing antibiotics against amyloid aggregation in neurodegenerative diseases. Prog. Neurobiol. 162, 17–36. doi: 10.1016/j.pneurobio.2017.12.002
Stoilova, T., Colombo, L., Forloni, G., Tagliavini, F., Salmona, M. (2013). A new face for old antibiotics: tetracyclines in treatment of amyloidoses. J. Med. Chem. 56 (15), 5987–6006. doi: 10.1021/jm400161p
Sultan, S., Gebara, E., Toni, N. (2013). Doxycycline increases neurogenesis and reduces microglia in the adult hippocampus. Front. Neurosci. 7. doi: 10.3389/fnins.2013.00131
Tambasco, N., Romoli, M., Calabresi, P. (2018). Levodopa in parkinson's disease: Current status and future developments. Curr. Neuropharmacol. 16 (8), 1239–1252. doi: 10.2174/1570159X15666170510143821
Wang, Z., Arat, S., Magid-Slav, M., Brown, J. R. (2018). Meta-analysis of human gene expression in response to mycobacterium tuberculosis infection reveals potential therapeutic targets. BMC Syst. Biol. 12 (1), 3. doi: 10.1186/s12918-017-0524-z
Weindel, C. G., Bell, S. L., Vail, K. J., West, K. O., Patrick, K. L., Watson, R. O. (2020). LRRK2 maintains mitochondrial homeostasis and regulates innate immune responses to mycobacterium tuberculosis. Elife 9, e51071. doi: 10.7554/eLife.51071
Keywords: Parkinson’s disease, bacterial infection, neuroinflammation, antibiotics, anti-PD
Citation: Sheng S, Zhao S and Zhang F (2022) Insights into the roles of bacterial infection and antibiotics in Parkinson’s disease. Front. Cell. Infect. Microbiol. 12:939085. doi: 10.3389/fcimb.2022.939085
Received: 08 May 2022; Accepted: 11 July 2022;
Published: 28 July 2022.
Edited by:
Ya-Sheng Li, Anhui Medical University, ChinaReviewed by:
Qingshan Wang, Dalian Medical University, ChinaDan Zhang, Peking Union Medical College Graduate School, China
Copyright © 2022 Sheng, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhang, emhhbmdmZW5nem1jQDE2My5jb20=
 Shuo Sheng
Shuo Sheng Shuo Zhao4
Shuo Zhao4 Feng Zhang
Feng Zhang