
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 08 July 2022
Sec. Biofilms
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.930624
This article is part of the Research Topic Novel approaches in the prevention of bacterial biofilm formation View all 8 articles
Due to the potent antibacterial properties of Cinnamomum and its derivatives, particularly cinnamaldehyde, recent studies have used these compounds to inhibit the growth of the most prevalent bacterial and fungal biofilms. By inhibiting flagella protein synthesis and swarming motility, Cinnamomum could suppress bacterial attachment, colonization, and biofilm formation in an early stage. Furthermore, by downregulation of Cyclic di‐guanosine monophosphate (c‐di‐GMP), biofilm-related genes, and quorum sensing, this compound suppresses intercellular adherence and accumulation of bacterial cells in biofilm and inhibits important bacterial virulence factors. In addition, Cinnamomum could lead to preformed biofilm elimination by enhancing membrane permeability and the disruption of membrane integrity. Moreover, this substance suppresses the Candida species adherence to the oral epithelial cells, leading to the cell wall deformities, damage, and leakages of intracellular material that may contribute to the established Candida’s biofilm elimination. Therefore, by inhibiting biofilm maturation and destroying the external structure of biofilm, Cinnamomum could boost antibiotic treatment success in combination therapy. However, Cinnamomum has several disadvantages, such as poor solubility in aqueous solution, instability, and volatility; thus, the use of different drug-delivery systems may resolve these limitations and should be further considered in future investigations. Overall, Cinnamomum could be a promising agent for inhibiting microbial biofilm-associated infection and could be used as a catheter and other medical materials surface coatings to suppress biofilm formation. Nonetheless, further in vitro toxicology analysis and animal experiments are required to confirm the reported molecular antibiofilm effect of Cinnamomum and its derivative components against microbial biofilm.
Multi-Drug Resistant (MDR) microorganisms can pose a serious threat to public health and human life if they cause bacterial infections. As a result, the microorganisms that live in biofilm become increasingly resistant to antibiotics (Jamal et al., 2018). Biofilm can protect its inside cells from the host immune system, antibiotics, and environmental factors; therefore, the biofilm community is easily identifiable in many devices and areas, such as polystyrene, glass, medical devices, bathrooms, and wastewater channels (Donlan and Costerton, 2002; Miquel et al., 2016). In general, the spread of biofilm in the environment and the human body is divided into four stages; 1- an attachment that is managed by different adhesion factors, 2- sessile growth stage that is controlled by different intracellular mediators such as Quorum Sensing (QS) signaling, 3- maturation that modulate through a synthesis of Extracellular Polymeric Substances (EPS) and, finally, 4- detachment (Kostakioti et al., 2013; Saxena et al., 2019).
According to recent studies, various microbial pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Candida species have the potential ability in biofilm formation and increased antibiotic resistance. Microorganisms with the capability of biofilm formation can escape from the immune system. Antibiotics are incapable of destroying or penetrating the inner layer of the biofilm due to the extracellular matrix’s protection, nutrient limitation, adaptive stress responses, and induction of phenotypic variability (Nuryastuti et al., 2009; Hathroubi et al., 2018; Rizzato et al., 2019). Therefore, due to the alarming occurrence of antibiotic resistance, the unavailability of appropriate antibiotics, and the chronic effects of biofilm-related diseases, new control strategies, and compounds are required that exhibit antimicrobial activity against microbial biofilms (Hong et al., 2015; Saxena et al., 2019).
To this end, alternative solutions to biofilm control, such as the use of nanoparticles (NPs), bacteriophage‐biofilm interactions, QS inhibition, enzymes, and natural products (Plant-derived essential oils), have received further attention. Natural products, including plant extracts, oils, and their derivative compounds, are known to be active against a wide variety of microorganisms and have been used to combat pathogens and infections (Kim et al., 2015; Kargaran et al., 2017; Vasconcelos et al., 2018). Cinnamomum (Cinnamon), a tropical Asian spice and a native plant of Sri Lanka, is extracted from the inner bark of a variety of trees from the Cinnamomum genus, including Cinnamomum camphora, Cinnamomum osmophloeum, Cinnamomum burmannii, Cinnamomum zeylanicum, Cinnamomum cassia, and Cinnamomum verum (Vasconcelos et al., 2018).
Cinnamomum is one of the common natural products that, in addition to being used in cooking, has received much attention due to its anti-oxidative, cardioprotective, anti-inflammatory, and antimicrobial characteristics in medical applications (Hammer et al., 1999; Yanakiev, 2020). It should be noted that the results of a study published in 2021 showed that cinnamomum at concentrations of 1000-2000 µg/ml has no toxic effects on normal human keratinocyte cell line (Wijesinghe et al., 2021).
Notably, cinnamaldehyde, one of the main Cinnamomum ingredients containing about 65% of it, due to its acrolein group (α, β-unsaturated carbonyl moiety), could be related to the antimicrobial activity of Cinnamomum. Cinnamaldehyde is not sensitive to common antibiotic resistance despite its strong effect on pathogen infections (Bae et al., 1992). In recent years, in addition to the antimicrobial effect, scientists have been interested in using Cinnamomum and its derivative components, especially cinnamaldehyde, to inhibit microbial biofilm (Kosari et al., 2020). In this regard, this review primarily focused on the role of Cinnamomum and its derivative compounds in the suppression and elimination of microbial biofilm to facilitate their possible widespread use in clinical practice.
Pseudomonas aeruginosa is a significant bacterial pathogen that causes various chronic and acute infections (Bahramian et al., 2019). Recent studies reported a high mortality rate for P. aeruginosa infection, especially in patients with underlying conditions such as severe burn injuries, cancer, cystic fibrosis, and nosocomial infections (Mah et al., 2003; Bahramian et al., 2019). Various adhesion factors such as pili, flagella, and biofilms lead to the adhesion and survival of this bacterium on medical devices, water, and diverse surfaces (Remold et al., 2011). Furthermore, P. aeruginosa biofilm results in chronic infections due to the increasing resistance to different irradiation treatments, disinfectants, immune systems, and antibiotics (Costerton et al., 1999; Stewart and Costerton, 2001; Mah et al., 2003). In this respect, recent studies reported higher antibiotic resistance in the biofilm community of P. aeruginosa compared to the planktonic cells because of antibiotic penetration reduction into the complex polysaccharide matrix (glycocalyx) (Spoering and Lewis, 2001; Ma et al., 2009). Hence, biofilms have increased the prevalence of MDR P. aeruginosa strains in recent years, and scientists are looking for new agents to manage it more effectively. After demonstrating appropriate antimicrobial function using various mechanisms, Cinnamomum and its derivative compounds have also been considered to destroy microbial biofilms (Vasconcelos et al., 2018).
To this end, Lakshmanan et al. reported that cinnamtannin B1, one of the active components of Cinnamomum tamala, inhibited biofilm formation and swarming motility of P. aeruginosa. Notably, cinnamtannin decreases the expression of fliC and rhlA associated with the synthesis of flagella protein flagellin and rhamnolipid (Lakshmanan et al., 2019). Swarming is one of the main P. aeruginosa virulence factors that aids in surface colonization and infection spread. The association between swarming motility and biofilm formation remains unknown because of conflicting results in the literature (Rampioni et al., 2009; Kerekes et al., 2013).
Moreover, the inhibition of QS was reported as the primary mechanism in inhibiting P. aeruginosa biofilm formation by Cinnamomum. Four QS systems, including PQS, IQS, Las, and Rhl, are recognized in P. aeruginosa. Rhl and Las lead to the main virulence phenotypes and physiological activities and organize nearly 10% of the P. aeruginosa genome. Las and Rhl (LasR (Transcription Activator Protein) and RhlR) receptors are stimulated in P. aeruginosa by binding to N-oxododecanoyl-L-homoserine lactone and N-butyryl-L-homoserine lactone auto-inducers. Following activation, these receptor proteins form complexes and initiate transcriptional expression further (Mukherjee et al., 2017). According to recent reviews, sub-inhibitory levels of cinnamaldehyde downregulated both the las and rhl QS systems by repressing the regulatory proteins LasR and RhlR. In addition to decreasing the production of extracellular virulence factors such as pyocyanin, elastase, and protease, this phenomenon suppressed the expression of the rhamnolipid gene and inhibited biofilm formation in P. aeruginosa strain PAO1 (PAO1). This study did not detect the exact QS inhibitory function of cinnamaldehyde, but the authors hypothesized that this substance acts as a QS antagonist (Ahmed et al., 2019).
It should be noted that lasI, in the lasI/lasR system, synthesizes 3-oxo-dodecanoyl-homoserine lactone (3-oxo-C12HSL), and this messenger subsequently binds to the cytoplasmic receptor LasR and activates the expression of genes that produce different virulence factors like elastases, proteases, and exotoxin A (Passador et al., 1993). In this regard, a recent investigation reported that whole Cinnamomum oil decreased 3-oxo-C12HSL levels in the supernatant culture of PAO1 (Kalia et al., 2015). Furthermore, this oil reduced the pyocyanin and alginate production and swarming motility of this bacterium at increasing concentrations (Kalia et al., 2015). Alginate, an essential component of extracellular polysaccharides that code by the algD gene, leads to biofilm structure integrity and confers resistance to antimicrobials by preventing entry. Therefore, inhibition of alginate production by Cinnamomum oil could repress biofilm maturation (Kalia et al., 2015).
These data support the finding by Alva et al., who reported that C. verum leaf ethanol extract significantly reduced the expression of the QS-regulatory gene RhlI, related to the signal production of N-Butanoyl-L-homoserine lactone (C4-HSL), and other QS-regulated virulence genes like PiliA, PhzH, FlagA, LasB, and algD in a clinical isolate of P. aeruginosa. In this regard, the authors detected reduced P. aeruginosa ability in producing pyocyanin, elastase, swarming motility, and biofilm formation. Lower concentrations (below 100 mg/L) of C. verum compound did not show any toxicity on zebrafish embryos (Alva et al., 2021).
Moreover, the QS-inhibitory effect of cinnamaldehyde in combination with different antibacterial agents was also performed to destroy P. aeruginosa biofilm. A recent study reported that cinnamaldehyde repressed the expression of lasB, rhlA, and pqsA; hence, demonstrating a QS-inhibitory effect. The combined use of cinnamaldehyde and tobramycin revealed strong QS inhibitory effects. Furthermore, combination therapy revealed an additive activity of cinnamaldehyde with tobramycin and colistin in the inhibition of PAO1 biofilm and preformed biofilm dispersion compared to the treatment alone (Topa et al., 2020). In another same study, Kart et al. reported that the combined use of cinnamaldehyde and ciprofloxacin showed more reduced minimum biofilm eradication concentration than ciprofloxacin alone. In this regard, the authors reported that cinnamaldehyde inhibited QS and alginate production, thereby inhibiting PAO1 biofilm formation and increasing the antibiofilm activity of ciprofloxacin (Kart et al., 2021). As a result of these findings, it is possible that cinnamaldehyde could increase the success of antibiotic treatment in combination therapy by inhibiting QS and thus increasing the susceptibility of bacterial biofilms to an antibiotic; however, this has not been tested.
Additionally, recent examination results also observed the synergism action for C. tamala essential oil (CTEO) and commercially available DNase in disrupting young and mature PAO1 biofilms and P. aeruginosa clinical isolate. The combined use of DNase and CTEO showed increased efficiency in disrupting the mature biofilms than the CTEO alone. In this respect, although CTEO inhibited QS-associated virulence factor-like alginate production, it demonstrated limited penetration into the biofilms. Hence, when the biofilm scaffold is loosened due to the degradation of extracellular DNA by the action of DNases, it could increase the CTEO penetration to the deeper layer of the bacterial biofilm (Farisa Banu et al., 2017).
In addition to the QS-inhibitory effect of Cinnamomum, a recent study reported that this compound inhibited Cyclic di‐guanosine monophosphate (c‐di‐GMP) (Figure 1) (Topa et al., 2018). C‐di‐GMP is considered a critical cytoplasmic signal and second messenger that controls virulence, cell cycle propagation, motility, and other behaviors, such as biofilm life cycle in several bacteria (Ryan et al., 2006). To this end, Topa et al. reported that cinnamaldehyde disrupted transmembrane potential, preformed biofilms, and swarming motility of PAO1. The authors suggested that the cinnamaldehyde carbon atoms may bind to nitrogen-containing components, like protein, in the cytoplasmic membrane, altering the protein structure and losing membrane integrity. Furthermore, the results demonstrated that cinnamaldehyde reduced 66.2% of c‐di‐GMP expression after 5 hours compared to the untreated control (Topa et al., 2018). However, this is the only report of cinnamaldehyde interaction with intracellular c-di-GMP levels; thus, the molecular mechanism by which cinnamaldehyde mediates changes in c-di-GMP levels remains unknown.
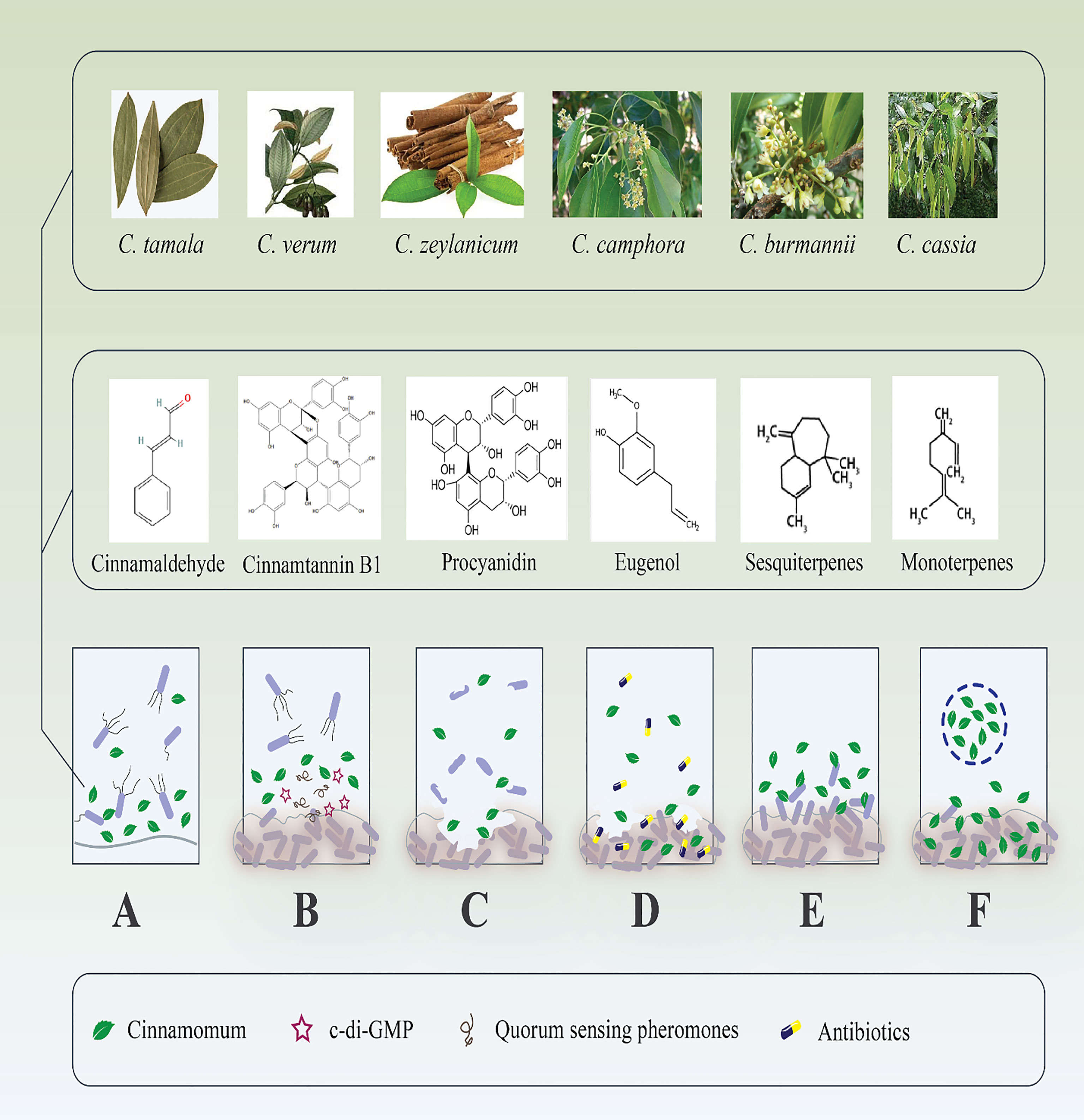
Figure 1 Antibiofilm effects of different species of Cinnamomum and their derivate components. (A) Inhibition of microbial adhesion to various surfaces. (B) Suppression of different bacterial cell signaling mediators that inhibit biofilm maturation. (C) Destruction of microbial established biofilm; consequently, (D) boost the antibiotic penetration to the dipper layer of the biofilm. (E) Handling of recalcitrant infections by repression of new biofilm formation. (F) Drug-delivery systems increase the effectiveness of Cinnamomum and their derivate components to destroy microbial biofilm.
Consequently, while the precise mechanism by which Cinnamomum acts against the QS system is unknown, it appears to act as a QS and c-di-GMP antagonist. In this regard, Cinnamomum, in addition to destroying P. aeruginosa biofilm, destroys the bacterium’s virulence factors by inhibiting QS-related factors and c-di-GMP. This phenomenon would allow the host’s innate immunity and other antibiofilm agents to function more successfully. In this respect, synergistic enhancement of antibiofilm agents via Cinnamomum administration represents an exciting future development; however, little is known about such effects at the molecular level. As a result, additional research is required to confirm mentioned findings.
In recent years, S. aureus with increased antibiotic resistance has increased morbidity, mortality, period of hospitalization, and patient cost. This bacterium results in severe nosocomial infections, and because of the extensive antibiotics usage, it has become the MDR pathogenic bacterium, most threatening to human health (Shariati et al., 2020a). In addition to the frequent occurrence of antimicrobial-resistant strains, S. aureus often resides within biofilms at the site of infection (Van den Driessche et al., 2017). Furthermore, S. aureus biofilm formation on various medical surfaces, like catheters, is a significant problem in healthcare-associated infections (Ceylan and Ugur, 2015). Accordingly, various antibiotics such as vancomycin and linezolid are used to destroy S. aureus biofilm; however, alternatives to the existing antibiotics against methicillin-resistant S. aureus (MRSA) biofilm infections are still a subject of interest (Taubes, 2008). In this regard, recent studies in this area have utilized Cinnamomum and its derivative compounds.
García-Salinas et al. discovered that cinnamaldehyde concentrations greater than 1 mg/mL eliminated the preformed biofilm of S. aureus (García-Salinas et al., 2018). In another examination, the C. zeylanicum essential oil (CZEO) and its active components, cinnamaldehyde, were used to inhibit S. aureus biofilm. Both dramatically decreased biofilm formation on stainless steel and polystyrene surfaces. Hence, the authors suggested that the anti-biofilm efficiency of CZEO is closely linked to cinnamaldehyde (its central component) (Budri et al., 2015). Furthermore, a recent study reported that cinnamaldehyde has a high antibiofilm effect because, after 48 h of treatment, the MRSA biofilms were decreased from approximately 53% to above 82% (Kot et al., 2018). As a result, recent studies have reported Cinnamomum’s antibiofilm effects against S. aureus and MRSA; however, the exact antibiofilm mechanisms of these substances were not identified in these studies.
In this regard, in other investigations, scientists evaluated molecular antibiofilm mechanisms of Cinnamomum and cinnamaldehyde against MRSA. Kot et al. reported that cinnamaldehyde efficiently reduced the biofilm formation of MRSA collected from the anus and wounds of hospitalized patients. Moreover, this compound reduced the fib, ebps, and eno genes’ expression levels that encode fibrinogen binding protein, elastin, and laminin-binding protein, respectively. Furthermore, the expression level of polysaccharide intercellular adhesin encoding genes (icaD, and icaA) decreased after cinnamaldehyde treatment. The authors proposed that by inhibiting fib, ebps, and eno, cinnamaldehyde may have an active role in MRSA adhesion inhibition to fibrinogen present in the blood, elastin, and laminin surfaces. In addition, by reducing the icaD and icaA expression, cinnamaldehyde could suppress intercellular adherence and accumulation of bacterial cells in biofilm (Kot et al., 2020).
Jia et al. also used confocal laser scanning microscopy z-section analyses and reported that cinnamaldehyde, in a dose-dependent manner, disrupted MRSA biofilm and suppressed the expression of sarA (Jia et al., 2011). Notably, biofilm-associated protein (Bap) is vital for bacterial adhesion and intercellular accumulation during biofilm formation in icaADBC-independent S. aureus. SarA regulates the expression of 120 genes in this bacterium and acts as a positive regulator of Bap-mediated biofilm formation. After bap gene activation through sarA, its expression is closely related to biofilm formation in icaADBC-independent S. aureus. Hence, inhibition of sarA through cinnamaldehyde could contribute to MRSA biofilm inhibition (Cucarella et al., 2001; Trotonda et al., 2005; Jia et al., 2011).
Finally, CTEO disrupted 60-80% of performed MRSA biofilms in another study. Microscopic examination revealed that CTEO resulted in a reduction in bio-volume and average thickness due to the EPS layer and slime synthesis disruption. Furthermore, this oil reduced MRSA hemolytic activity with a percentage inhibition of 65-80% (Rubini et al., 2018). α–hemolysin, a pore-forming toxin, lyses human red blood cells and also facilitates biofilm formation by regulating cell interactions (Caiazza and O’toole, 2003). According to studies mentioned above, Cinnamomum, through downregulation of various MRSA genes, prevents bacterial adhesion to different surfaces and prevents biofilm maturation. However, further in vitro and animal experiments are required to confirm the reported molecular interaction of Cinnamomum with MRSA biofilm.
The combination of Cinnamomum and antibiotics in inhibiting the MRSA biofilm has also demonstrated promising results. A recently published study detected synergistic effects between cinnamaldehyde, β-lactam, and non- β-lactam antibiotics. Cinnamaldehyde suppressed Penicillin-binding proteins (PBP2a) and mecA; thus, it is possible that the synergistic effect was caused by the fact that this compound inhibited the mecA transcription and translation. Additionally, cinnamaldehyde dramatically reduced the expression of the biofilm regulatory gene hld, and subsequently, the MRSA biofilm formation (Wang et al., 2021). Furthermore, Sundaramoorthy et al. discovered that their collected S. aureus was resistant to all mupirocin concentrations tested. On the other hand, Cinnamomum oil significantly eliminated S. aureus biofilm. Combining this compound and mupirocin improved the elimination of preformed biofilm compared to the Cinnamomum oil applied alone. The authors proposed that this synergistic effect could be associated with the presence of sesquiterpenes and monoterpenes with relative hydrophilicity characteristics in Cinnamomum oil that will increase biofilm penetration through the exopolysaccharide matrix. In addition, the hydrophobic nature of phenyl propenes present in this oil may interact with bacterial membrane and penetration (Sundaramoorthy et al., 2021). In this respect, through inhibition of biofilm formation in S. aureus, the resistance to antibiotics can be decreased, which may be one of the reasons that antibiotics combined with Cinnamomum have a synergistic effect. Therefore, future studies should consider using Cinnamomum in combination with antibiotics to destroy MRSA biofilms.
However, Cinnamomum essential oil and cinnamaldehyde have several limitations, such as low stability and water solubility. In this regard, in recent years, the use of these substances in various drug delivery systems has been considered (Rai et al., 2017). A recent study encapsulated Cinnamomum oil in the liposomes to increase its chemical stability. Afterward, the antibiofilm effect of this conjugation was evaluated against MRSA. The authors reported that liposome encapsulation could release Cinnamomum oil slowly, kill MRSA, and destruct its biofilms significantly on various surfaces compared to the essential oil treatment alone. These data suggested that liposome leads to the desired stability and dispersibility of Cinnamomum oil and enhances the active time of this compound in the destruction of MRSA biofilms (Cui et al., 2016).
Furthermore, Meng et al. used the combination of Gold nanocluster (Au NCs) surface ligand exchange strategy and cinnamaldehyde to inhibit MRSA biofilm. In this regard, cinnamaldehyde was performed on the surface of oxygen species (ROS) generation ability of histidine (His)-stabilized Au NCs. The results indicated that cinnamaldehyde-Au NCs removed significantly more biofilm than Au NCs. In addition, cinnamaldehyde-Au NCs exhibited better antibacterial effects in the pigskin wound infection model. Collected data from the confocal 3D fluorescence microscopy images showed that cinnamaldehyde-Au NCs enhance membrane permeability and lead to membrane integrity disruption and membrane potential dissipation. The antibacterial activity of this combination could be related to the release of the histidine-cinnamaldehyde ligand on the surface of cinnamaldehyde-Au NCs due to the occurrence of a ligand exchange reaction (Meng et al., 2021). As a result, diverse drug-delivery platforms with cinnamaldehyde or Cinnamomum could provide novel agents for the destruction of the MRSA biofilm. Finally, it should be noted that other combined uses of Cinnamomum and its derivative compounds with various drug-delivery platforms were used to inhibit S. aureus biofilm in food industries. These studies have been reported in Table 1.
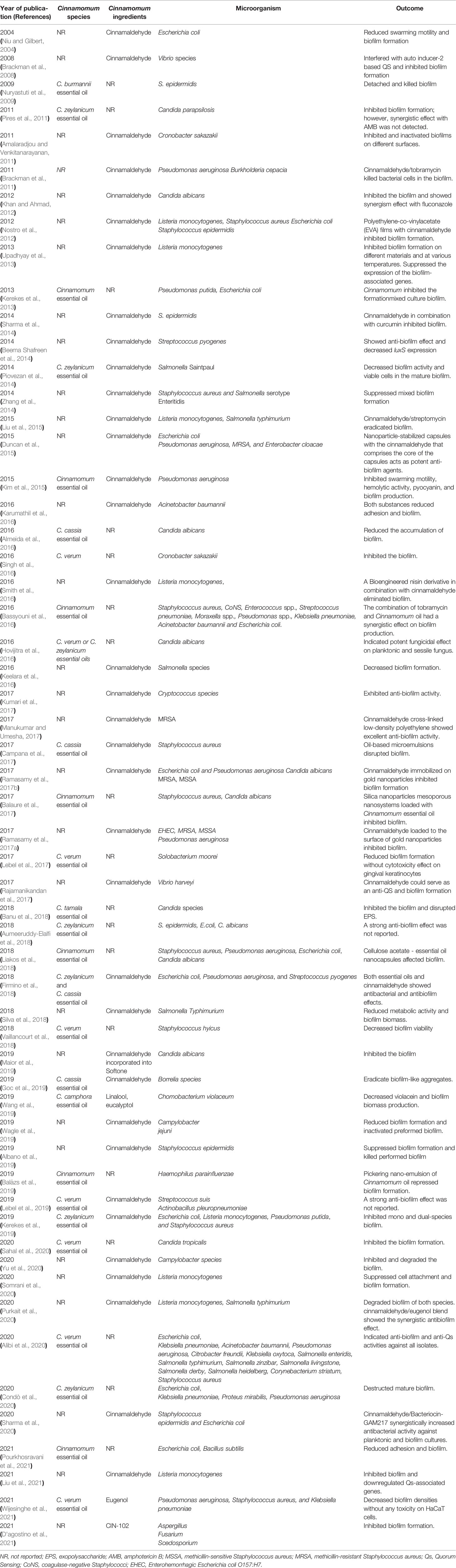
Table 1 Previous studies that evaluated the antibiofilm effect of Cinnamomum and its active components against different bacterial and fungal biofilm.
Escherichia coli is normal flora found in the human and animal digestive tracts (Sack, 2011; Sarowska et al., 2019). Diarrhea is one of the most significant diseases caused by E. coli, which leads to the deaths of thousands of people around the world, especially children (Kim et al., 2017). Once this bacterium enters the digestive system, it immediately attaches and colonizes the intestinal cells, evading the host immune system and attacking host cells by producing toxins. In this regard, E. coli frequently leads to biofilm-associated opportunistic infections like endometritis, diarrhea, and mastitis (Kim et al., 2017; Wang et al., 2020). Antibiotics can help alleviate disease symptoms and duration, but several E. coli species have developed resistance to antibiotics due to antibiotic overuse over the last 50 years (Scotti et al., 2021). Hence, Cinnamomum was used to inhibit the attachment and formation of biofilms by E. coli to manage infection caused by this bacterium.
Pourkhosravani et al. discovered that essential oil extracted from the trunk bark of Cinnamomum could inhibit E. coli from forming a biofilm. In this regard, anti-adhesion tests performed through crystal violet assay revealed that Cinnamomum completely suppressed the adhesion of this bacterium. Furthermore, biofilm metabolic activity and quantification of biofilm biomass showed that Cinnamomum suppressed the E. coli metabolic activity and biofilm formation by 99% and 100%, respectively. Notably, gas chromatography-mass spectrometry (GC-MS) analysis revealed that E-cinnamaldehyde, α-terpinyl acetate, and copaene accounted for 91.31% of the Cinnamomum essential oil (Pourkhosravani et al., 2021). Another investigation also reported that C. camphora essential oil (CCEO) killed clinical isolates of E. coli from dairy cows with clinical endometritis in both planktonic and biofilm communities. Additionally, the authors evaluated the kinetics of CCEO action against E. coli in the suspension and biofilms communities. The results indicated that the bacterial killing occurred most rapidly during the first 5 min of treatment and that the lowest level of viable bacteria was detected nearly 1 h after treatment. These data suggested that the efficiency of CCEO declined over time; thus, the pharmacodynamics time of CCEO was less than 24 h, and repressive effects on the biofilms appeared at an early stage. The microscopic analysis confirmed these results and showed that CCEO firmly suppressed the formation of E. coli biofilm, and 4 mL/mL of this essential oil could eliminate the biofilm of this bacterium (Wang et al., 2020).
Recent investigations also corroborated these findings and reported that Cinnamomum extract reduced the secretion of EPS and biofilms metabolic activity in a dose-dependent manner, consequently suppressing the E. coli strain ATCC 25922 biofilms from 24.45 to 98.09%. On the other hand, the effects on preformed biofilms ranged from 16.20 to 46.14% at various concentrations. The microscopic analysis was consistent with the above findings, indicating that the Cinnamomum extract could dramatically hinder and eliminate the E. coli biofilms (Lu et al., 2021). Furthermore, Olszewska et al. reported that cinnamaldehyde reduced almost 60% of cell metabolic activity and biofilm cell cultivability of E. coli strain CECT 434. Notably, the authors suggested that cinnamaldehyde could result in the loss of membrane integrity by biofilm cells by detecting various bacterial cell morphologies such as filamentous cells and weakened coverage of the substratum (Olszewska et al., 2020). A recently published study also reported that Cinnamomum extract and cinnamaldehyde inhibited 60% and 86.7% of the biofilm production of E. coli isolated from patients with colon cancer, respectively (Kosari et al., 2020). As a result, recent studies have reported Cinnamomum’s antibiofilm activity against a variety of E. coli isolates; however, the exact antibiofilm mechanism of these substances has not been reported.
Additionally, other researchers evaluated the inhibitory effects of Cinnamomum against E. coli strain O157:H7 (EHEC) biofilm. This bacterium belongs to the attaching and effacing (A/E) E. coli group, leading to bloody diarrhea. Antibiotics should be avoided because they induce the SOS response and activate prophages, resulting in the release of Shiga toxins (Paton and Paton, 1998; Sheng et al., 2016). The EHEC’s ability to adhere to various surfaces and form a biofilm and the absence of effective therapy against EHEC-biofilm-associated infections have led to new antibiofilm agent development. To this end, the results of recent experimentation showed that C. verum essential oil (CVEO) inhibited the biofilm formation of EHEC clinical isolates. In addition, the microscopic examination revealed the following characteristics of biofilm cells in the presence of CVEO: sparse microcolonies and individual cells with fewer and shorter interconnecting meshes between cells, but no discernible morphological changes (Scotti et al., 2021).
In another study conducted in 2019, the authors reported that sub-lethal concentrations of cinnamaldehyde increase the expression of tnaA and bssS genes that are negative regulators of biofilm formation in EHEC (Yuan and Yuk, 2019). Notably, tnaA encodes the enzyme tryptophanase that results in indole production and is a signaling molecule that suppresses E. coli biofilm formation. Moreover, BssS reduces bacterial biofilm formation by affecting cell signaling (Isaacs Jr et al., 1994; Domka et al., 2006). Nevertheless, cinnamaldehyde suppressed the expression of virulence-associated genes, including; Type III secretion systems (T3SSs) (sepD and escC), flagellar biosynthesis, and functions (fliA and motA), and chemotaxis (cheA and cheZ). Afterward, the authors evaluated the association between virulence gene expression changes and observable phenotype alterations. They detected that cinnamaldehyde remarkably decreased the biofilm-forming ability, efflux pump activity, and motility of EHEC, with no induction of antibiotic resistance in the bacterium (Yuan and Yuk, 2019).
Additionally, Kim et al. found that Cinnamomum bark oil and its constituents inhibited the formation of EHEC biofilms and virulence. Their results demonstrate that coating the biodegradable poly (lactic-co-glycolic acid) surface with cinnamaldehyde or Cinnamomum bark oil significantly inhibits EHEC biofilm formation. These compounds inhibited the expression of the csgAB and stx2 genes, which are involved in the formation of curli and the production of Shiga-like toxins, respectively. On the other hand, Cinnamomum bark oil did not demonstrate considerable effects on the expression of other biofilm-related genes such as flhD, qseB, motB, and tnaA (Kim et al., 2015). Therefore, Cinnamomum could inhibit biofilm production of one of the most important E. coli pathotypes, EHEC; therefore, it can be used as a preservative in food products. However, additional research is required to determine the precise mechanism by which these substances inhibit biofilm formation.
In addition, Cinnamomum demonstrated promising results for inhibiting Uropathogenic E. coli (UPEC)-biofilm-associated infections. By creating 80% to 85% of UTI in humans, this bacterium is known as a main etiologic factor for these infections. In addition to various virulence factors, UPEC forms a biofilm that facilitates bacterium growth, toxin secretion, and persistence in excessive pH variation (Flores-Mireles et al., 2015). UPEC biofilms also coat the catheters, in which bacteria embedded in an exopolysaccharide matrix are protected from antimicrobial agents (Manges et al., 2001; Amalaradjou et al., 2010). In this regard, in a previous study, the authors used cinnamaldehyde to treat UPEC biofilm on the polystyrene plates, latex, and urinary catheters. Cinnamaldehyde effectively prevented UPEC biofilm formation on the surfaces mentioned and, when used as an antimicrobial constituent in catheter lock solution, successfully deactivated preformed biofilm. Notably, cinnamaldehyde did not have any cytotoxic effect on bladder epithelial cells (Amalaradjou et al., 2010). This supports the findings by Kot et al. that reported cinnamaldehyde at various concentrations hindered the extension of UPEC biofilm on catheter fragments. Additionally, when this substance was used as an antimicrobial constituent in a catheter lock solution, it significantly inactivated preformed UPEC biofilms (Kot et al., 2015).
Another study used Type A procyanidin (TAP) from C. zeylanicum to inhibit biofilm formation of MDR UPEC (Vasudevan et al., 2020). Notably, procyanidin is one of Cinnamomum components with different biological activities. The results indicated that although TAP treatment did not inhibit the UPEC growth, but affected the biofilm formation. The authors hypothesized that the TAP’s anti-biofilm activity at lower concentrations could be attributed to the pentamer’s four interflavanyl linkages. In addition, TAP downregulated the expression of the focA, papG, fimH, and fimA, which mainly manage bacterial adhesion to the urinary tract. Moreover, the synergistic effect between TAP and nitrofurantoin at various pH was detected in this study. Thus, by inhibiting bacterial adhesion, TAP may act as a suppressor of biofilm formation. In addition, the use of this substance may enhance the activity of antibiotics at low concentrations (Vasudevan et al., 2020). As mentioned previously, Cinnamomum and its active components could be used to inhibit UPEC because, in addition to the appropriate antibacterial effect, it can also destroy bacterial biofilms. Furthermore, Cinnamomum could be a novel antibiofilm agent for catheter surface coatings or an ingredient in catheter lock solutions to prevent catheter-associated UTIs.
Oral candidiasis is one of the most prevalent opportunistic infections that lead to oral discomfort, dysgeusia, and pain. Due to the patients’ immunocompromised state, this infection may result in serious complications such as systemic candidiasis and esophageal candidiasis. Most oral candidiasis cases are easily treated with antifungal drugs; however, the conditions could differ in patients with underlying conditions such as HIV and dentures (Williams et al., 2012; Millsop and Fazel, 2016; Swidergall and Filler, 2017). Candida albicans, followed by Candida glabrata, are the most frequent etiology of oral candidiasis (Miranda-Cadena et al., 2018; Shariati et al., 2020b). The biofilm produced by C. albicans is resistant to treatment and outperforms it in the oral cavity. Extracellular DNA and EPS reduce the penetration of antifungals to the biofilm, which is a serious concern that is boosted by the emergence of azole-resistant isolates and the selection of Candida species with decreased antifungal susceptibility (Williams et al., 2012). As a result, recent research has focused on Cinnamomum’s ability to inhibit Candida biofilm formation, thereby limiting the extension of decreasing or resistant antifungal selective pressure.
In a recently published study, Cinnamomum oil was used to eliminate mature biofilm of C. albicans off dental devices made of heat-polymerized polymethyl methacrylate (PMMA) resin. PMMA is associated with severe candidiasis and oropharyngeal development in patients who wear it. Cinnamomum oil destroyed 99% of the Candida pre-established biofilm. Furthermore, covering the PMMA samples with this oil for 24 hours also reduced the C. albicans biofilm formation by almost 70.0% (Choonharuangdej et al., 2021). Another examination also showed that C. burmannii essential oil and its aqueous extract enriched in proanthocyanidins (Cinnulin), reduced the fungal adherence to the oral epithelial cells and had an inhibitory effect against preformed C. albicans biofilm of clinical isolates. Notably, Cinnamomum fractions boosted the oral epithelial barrier integrity and did not show cytotoxicity effects against oral epithelial cells at their effective concentrations. Further, Cinnulin decreased the secretion of interleukin (IL)-6 and IL-8 by oral epithelial cells stimulated with TNF-α (Veilleux and Grenier, 2019). Hence, different fractions of Cinnamomum could be practical agents for hindering C. albicans biofilm and subsequently for managing infections such as Candida-infected oral mucositis lesions, oral candidiasis, and denture stomatitis. Additionally, covering dental devices with these substances may be a preventive approach against Candida biofilm formation; however, more specific studies are required.
The findings of a recently published study also demonstrated severe antifungal function for cinnamaldehyde against Candida species isolated from patients with oral candidiasis. Further, cinnamaldehyde lowered the biomass and metabolic activity of mature biofilm (Miranda-Cadena et al., 2021). Collectively, the biofilm biomass reduction could play a key role in controlling MDR infections as biofilms are a source for dispersal of cells with beneficial features such as forming new biofilms and enhancing virulence plus adhesion (Uppuluri et al., 2010; Nobile and Johnson, 2015).
In addition to cinnamaldehyde, eugenol was also reported as a central component of Cinnamomum for inhibiting Candida biofilm. Eugenol is a phenylpropanoid detected in aromatic plants, especially as the main ingredient in clove oil (Marchese et al., 2017). In a 2020 study, researchers reported that CVEO indicated remarkable antifungal potency against 24-h preformed Candida species biofilms. Exposure to the CVEO could lead to cell wall deformities as well as leakages of intracellular materials in Candida biofilm. None of the CVEO-tested concentrations in this study showed any cytotoxicity on human non-cancer keratinocytes. GC-MS evaluation illustrated eugenol as the main component of CVEO (Wijesinghe et al., 2021).
Wijesinghe et al. also reported eugenol as the main compound (77.22%) of CVEO. This essential oil significantly suppressed germ tube formation, adhesion, and biofilm formation in common Candida species strains. Microscopic analysis also revealed CVEO treatment lead to leakage of intracellular materials as well as cell wall damages and deformities, plus cell density reduction for biofilm cells. The Galleria mellonella larvae experiment model did not exhibit any cytotoxicity for CVEO (Wijesinghe et al., 2020). Finally, another investigation revealed that CZEO suppressed biofilm formation and considerably decreased Candida monospecies along with multi-species preformed biofilm at 24 h and 48 h, respectively. Chemical assessment identified eugenol as the primary component (68.96%) of CZEO and confirmed previous findings. In addition, this essential oil showed low cytotoxicity effects against peripheral mononuclear and red blood cells (Rangel et al., 2018).
Eugenol could have a promising role in the degradation of Candida biofilms. Nevertheless, eugenol’s precise antifungal and antibiofilm activity has not been determined in the mentioned studies, and additional molecular and in vitro investigations are needed. Collectively, Cinnamomum species and plant material used for extraction could produce oils with different major components, suggesting that the anti-biofilm effect of each component should be evaluated separately.
On the other hand, some studies have evaluated the molecular interactions of Candida cells in biofilm community with Cinnamomum and cinnamaldehyde and discovered interesting results. A study performed by El-Baz et al. reported that CVEO has an inhibitory effect against C. albicans biofilm isolated from different clinical samples. This essential oil also suppressed the hemolysin and phospholipase activity of this fungus. Microscopic images also described the diminished biofilm formation in terms of suppressed adhesion. Note that according to molecular docking, cinnamaldehyde, as the main component of CVEO, has an impact on Als3 (El-Baz et al., 2021). The Als adhesive proteins are one of the most extensively studied virulence characteristics of C. albicans, where deletion of Als3 led to a remarkable decrease in fungal adhesion (Hoyer and Cota, 2016). Hence, the Als3 interaction and cinnamaldehyde may be a promising result for using these compounds to inhibit C. albicans adhesion and biofilm formation (El-Baz et al., 2021).
In this regard, another study discovered that cinnamaldehyde destroyed Candida cellular development and suppressed biofilm formation by detecting specific features such as the expression of rare pseudo-hyphae and absence of chlamydoconidia. Molecular docking evaluation indicated negative ligand-receptor interaction for cinnamaldehyde with the most affinity for squalene thymidylate synthase and epoxidase. Thus, the authors hypothesized that cinnamaldehyde could restrict the formation of biofilms in Candida by affecting important targets present in the fungal cell and nucleus; however, further docking studies are required for precise identification (Da Nóbrega Alves et al., 2020).
Furthermore, Gupta et al. discovered that cinnamaldehyde could destroy the biofilm community of C. glabrata clinical isolates from biomaterials’ surfaces such as contact eye lens and urinary catheter. Furthermore, cinnamaldehyde could increase ROS production, cell lysis, and plasma membrane ergosterol content. However, this compound suppressed C. glabrata enzymes’ activity such as phospholipase, catalase, and proteinase. Detailed molecular analysis showed that cinnamaldehyde downregulated the expression of FKS1, AUS1, KRE1, and CDR1 genes related to the 1,3-β-glucan synthase sterol importer, GPI-anchored protein, and multi-drug transporter, respectively. In this regard, the authors proposed that ergosterol interaction with cinnamaldehyde would change the integrity and permeability of the cell membrane, and ultimately result in intracellular content leakage and cell lysis (Gupta et al., 2018). Thus, the interaction of cinnamaldehyde with different Candida cellular pathways could suppress various virulence phenotype of this fungus like biofilm community. Accordingly, the data on this subject are scarce, necessitating additional research.
Some of the most prevalent dental disorders like periodontitis, endodontic failure, and dental caries contribute to the biofilm formation of different bacteria (Sun et al., 2013; Jhajharia et al., 2015). Dental caries is known as the most significant chronic and costly oral disorder affecting the health of children and adults worldwide (Ren et al., 2016). Streptococcus mutans are customarily found in various oral cavity sites and are the most common bacterium related to the initiation of dental caries (Lynch et al., 2013; Alshahrani and Gregory, 2020). By fermenting dietary carbohydrates, this bacterium, mainly sucrose, leads to the production of extracellular polysaccharides with high adhesion ability to the tooth surface. As a result, S. mutans may play a role in dental caries by producing a biofilm on the tooth surface f (Koo et al., 2010; Cheon et al., 2013; Klein et al., 2015). Various approaches, such as mechanical cleaning and chemical plaque control, are performed to destroy bacterial biofilm from the dental surface. However, certain limitations, such as an unpleasant taste, staining on the teeth, and the development of antimicrobial-resistant strains due to long-term use of specific antimicrobial agents, have prompted scientists to seek suitable alternative methods (Wilson and Patterson, 2008; Malhotra et al., 2011; Wiwattanarattanabut et al., 2017). Accordingly, recent studies used Cinnamomum and cinnamaldehyde to destroy S. mutans biofilm.
Alshahrani et al. reported that the water extract from C. burmannii could suppress Streptococcus mutans biofilm formation (Alshahrani and Gregory, 2020). Another investigation reported that CZEO inhibited the biofilm formation of S. mutans by up to 80% and reduced 50% of the 24-hour pre-established biofilm of this bacterium (Wiwattanarattanabut et al., 2017).
Molecular interaction of cinnamaldehyde and S. mutans biofilm have been reported in two recently published studies. The results of one of them demonstrated that cinnamaldehyde reduced S. mutans biofilm metabolism and biomass. Notably, cinnamaldehyde enhances hydrophobicity and reduces S. mutans aggregation, reducing acid production and acid tolerance. Hence, it is possible that cinnamaldehyde could suppress bacterial adherence to the tooth surfaces, and consequently, inhibit biofilm formation. Furthermore, the authors suggested that cinnamaldehyde, through inhibition of glycolytic enzymes present in the acid production pathway, may impair bacterial acidogenicity and reduce tooth demineralization. Finally, cinnamaldehyde downregulated the expression of various biofilm-associated genes such as vicR, ciaH, ciaH, and gtf cluster genes (He et al., 2019). This supports the findings of the Balasubramanian et al. study, which found that cinnamaldehyde significantly inhibited acid production and biofilm formation by S. mutans. Furthermore, the results revealed that cinnamaldehyde impaired the expression of genes related to bacteriocins production, QS, stress tolerance, metabolism, and biofilm formation in S. mutans. As a result, these data recommend that cinnamaldehyde, in addition to biofilm destruction, could suppress the various virulence factors of S. mutans. (Balasubramanian et al., 2021).
In this respect, the appropriate concentration of Cinnamomum and cinnamaldehyde in oral hygiene products such as dental floss, mouthwashes, and toothpaste could lead to the repressive of bacterial biofilm and caries incidence reduction. However, more investigations are required to understand better the molecular mechanism underlying the inhibitory effect of cinnamaldehyde on S. mutans biofilm formation. Furthermore, the inhibitory effect of Cinnamomum and cinnamaldehyde should be evaluated against multi-species dental surface biofilm. Because diverse species with varying antibiotic resistance patterns coexist in this type of biofilm, biofilm formation increases their tolerance to antibacterial agents (Worreth et al., 2021). Accordingly, a direct comparison of Cinnamomums’ inhibitory effect on mono and multi-species biofilms is not possible, and future research should place a greater emphasis on multi-species biofilms. It is worth noting that the other experiments in which Cinnamomum and its derivatives were used to inhibit dental surface biofilm formation are listed in Table 2.
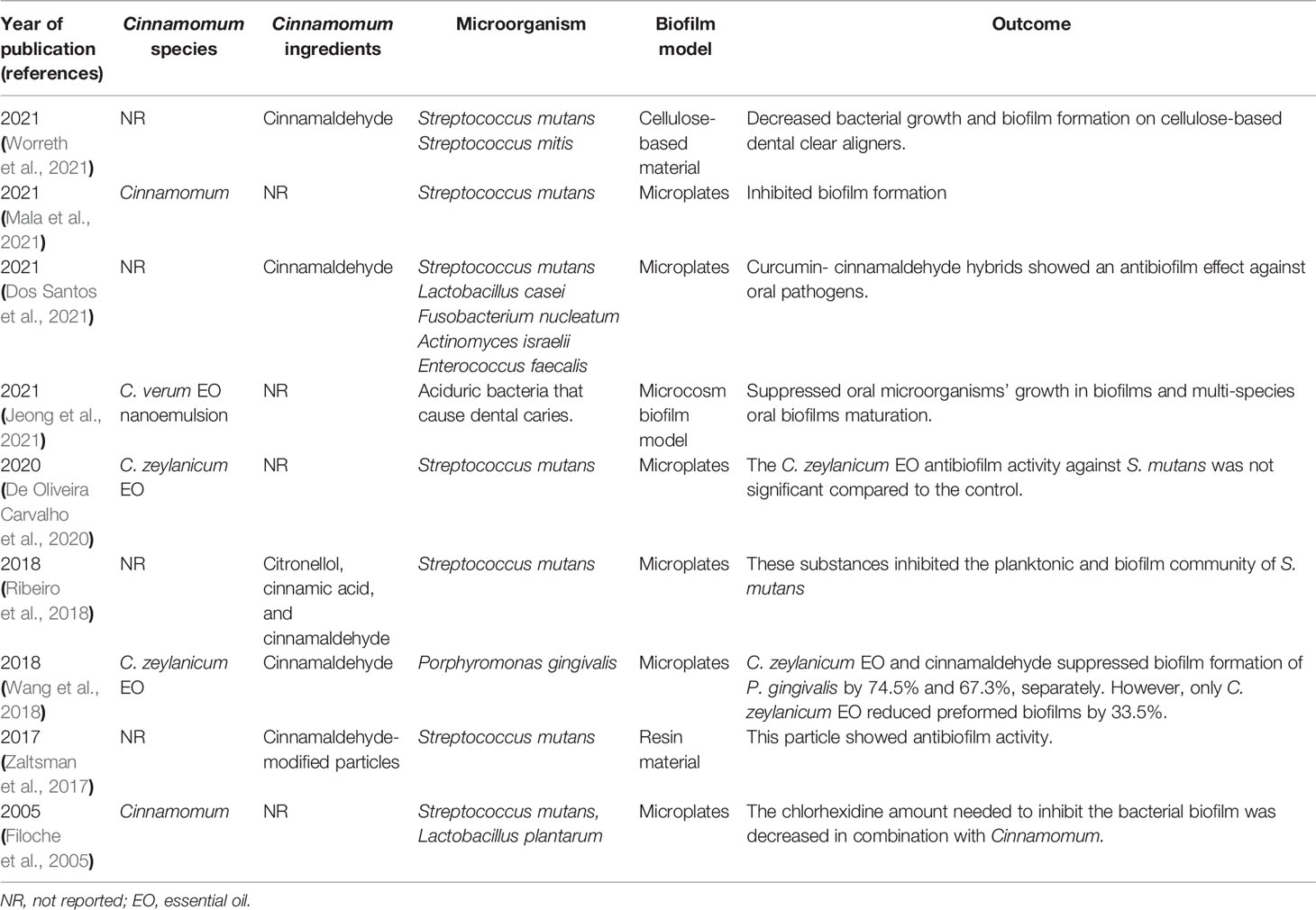
Table 2 Antibiofilm effect of Cinnamomum and its derivative compounds against biofilm of bacteria associated with dental disorders.
Bacterial removal from the root canal system is the most critical aspect of root canal treatment success (Sjögren et al., 1997). Enterococcus faecalis has a potential role in root canal treatment failure. This may be due to the significant E. faecalis potency to resist and attach to treated dentine surfaces and its ability to tolerate nutrient-deprived environments encountered inside root canals. Moreover, in addition to high antibiotic resistance characteristics, this bacterium can form biofilms on various substrates, such as hydroxyapatite, gutta-percha, dentin, and bone (Liu et al., 2010; Xu et al., 2019; Marcoux et al., 2020). In this regard, E. faecalis contributes to various peri-radicular lesions, including primary and secondary endodontic infections (Evans et al., 2002). Diverse antibacterial strategies like intracanal medicaments, diverse instrumentation techniques, and the systemic and local application of antibiotics have been used to control persistent infections. Nevertheless, these common methods are not always effective, and the systemic administration of antibiotics could exhibit several adverse effects such as allergic reactions, toxicity, and development of bacteria with higher antibiotic resistance features (Hoelscher et al., 2006; Mohammadi and Abbott, 2009; Saber and El-Hady, 2012).
Additionally, previous research has demonstrated that conventional disinfectants such as chlorhexidine and hypochlorite are incapable of completely eradicating the microbial community and bacterial biofilm from the root canal (Neelakantan et al., 2017; Ali et al., 2020). Therefore, although root canal infections are polymicrobial, E. faecalis is the most prevalent bacterium isolated in failed treatments and is thus considered the model organism to evaluate the effect of new agents [14]. In this regard, recent studies used Cinnamomum and cinnamaldehyde to eliminate E. faecalis biofilm.
Gupta et al. used an extract of C. zeylanicum to inhibit the growth of an E. faecalis biofilm. When applied to biofilms formed on cellulose nitrate membranes and tooth substrates, this substance kills all bacteria. However, the extract of C. zeylanicum used in this study was less effective against E. faecalis than sodium hypochlorite (NaOCl) (Gupta et al., 2013). Another investigation reported that intracanal application of CZEO for 14 days completely eliminated E. faecalis biofilm and was non-cytotoxic for L929 fibroblasts. Notably, GC-MS analysis showed that cinnamaldehyde was the main component of CZEO (Abbaszadegan et al., 2016).
Additionally, Abbaszadegan et al. found that CVEO killed 90.4% of E. faecalis cells embedded in biofilms, compared to 31.1% for chlorhexidine. The authors suggested that CVEO’s high efficiency could be attributed to its high terpene content, which is known for its high hydrophobicity and volatility, as well as its low molecular weight (Marcoux et al., 2020). Furthermore, the results of a 2020 study demonstrated that cinnamaldehyde significantly reduced biofilm formation and prevented biofilm recovery in a clinical strain of E. faecalis isolated from failed root canal treatment. Cinnamaldehyde treatment for 15 min had the same effect on biofilm metabolic activity as 2% chlorhexidine and 1% sodium hypochlorite. Besides, 24 h treatment with cinnamaldehyde was significantly more effective than 2% chlorhexidine at reducing biofilm viable cell counts. Notably, cinnamaldehyde inhibited the E. faecalis biofilms recovery as there was no significant enhancement in the bacterial count at day ten compared to day 0 (Ali et al., 2020). The authors suggested that the antibiofilm capacity of cinnamaldehyde could be related to its penetration and destruction of the E. faecalis hydrophobic cell membrane. Consequently, cell membrane injuries lead to intracellular contents’ leakage and suppression of the membrane-bound ATPase activity (Vasconcelos et al., 2018; Ali et al., 2020).
Finally, recently published work interestingly reported that cinnamaldehyde, at sub-inhibitory concentration, suppressed the production of exopolysaccharides and biofilm formation of E. faecalis and reduced its hemolytic and proteolytic activity. On the other hand, the authors did not observe this prohibitory effect for cinnamaldehyde against biofilm of two strains of E. faecalis with fsrB and fsrC genes insertion-deletion. Furthermore, cinnamaldehyde considerably downregulated fsrB and fsrC expression (Ali et al., 2021). It should be noted that recent studies indicated that the Fsr QS system by production of gelatinase related to the virulence and biofilm formation of E. faecalis. In this regard, the fsrB gene encodes a transmembrane protein that processes a propeptide to generate a peptide pheromone. In addition, fsrC encodes a histidine kinase sensor that responds to the peptide-signaling molecule, phosphorylates its response regulator, and subsequently induces the gelE-sprE operon’s transcription (Nakayama et al., 2006). Thus, these data suggested that cinnamaldehyde inhibits the formation of E. faecalis biofilms by targeting the Fsr QS system; however, additional complimentary research is required to confirm this hypothesis.
As a result, Cinnamomum and cinnamaldehyde may inhibit the formation of E. faecalis biofilms; thus, they may be used in endodontics to control root canal flora. However, possible interactions of these substances with the physical, chemical and pharmacological characteristics of root canal filling materials are still obscure. In addition to E. faecalis, other microorganisms such as Fusobacterium nucleatum, Porphyromonas endodontalis, Prevotella intermedia, Actinomyces israelii, and C. albicans are present in the root canal that may have a potential effect on Cinnamomums’ repressive effect. Therefore, additional research should be conducted on Cinnamomum’s anti-biofilm effect on multi-species biofilms in various environmental conditions and clinically relevant models, such as the whole tooth model for the biofilm assay.
Cinnamomum and its derivatives, particularly cinnamaldehyde, have demonstrated promising anti-biofilm properties against various microorganisms. As a result, it may be used in place of antibiotics to treat biofilm-related infections. Although some studies have demonstrated that Cinnamomum has molecular interactions with the cellular pathways of microorganisms, additional research is required to substantiate these findings. Additionally, animal models, clinical trials, and a precise assessment of cell cytotoxicity caused by long-term exposure to Cinnamomum are required.
AS and MD conceived and designed the study. AS and ZC contributed to comprehensive research. ZC, AS, and MD wrote the paper. SR and ST participated in manuscript editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
c‐di‐GMP, Cyclic di‐guanosine monophosphate; MDR, Multi-Drug Resistant; QS, Quorum Sensing; EPS, Extracellular Polymeric Substances; NPs, nanoparticles; 3-oxo-C12HSL, 3-oxo-dodecanoyl-homoserine lactone; CTEO, C. tamala essential oil; MRSA, methicillin-resistant S. aureus; CZEO, C. zeylanicum essential oil; Bap, biofilm-associated protein; PBP2a, Penicillin-binding proteins; GC-MS, gas chromatography-mass spectrometry; CCEO, C. camphora essential oil; CVEO, C. verum essential oil; TAP, Type A procyanidin; UPEC, Uropathogenic E. coli; PMMA, Polymerized Polymethyl Methacrylate.
Abbaszadegan, A., Dadolahi, S., Gholami, A., Moein, M. R., Hamedani, S., Ghasemi, Y., et al. (2016). Antimicrobial and Cytotoxic Activity of Cinnamomum Zeylanicum, Calcium Hydroxide, and Triple Antibiotic Paste as Root Canal Dressing Materials. J. Contemp. Dental Pract. 17, 105–113. doi: 10.5005/jp-journals-10024-1811
Ahmed, S., Rudden, M., Smyth, T. J., Dooley, J. S. G., Marchant, R., Banat, I. M. (2019). Natural Quorum Sensing Inhibitors Effectively Downregulate Gene Expression of Pseudomonas Aeruginosa Virulence Factors. Appl. Microbiol. Biotechnol. 103, 3521–3535. doi: 10.1007/s00253-019-09618-0
Albano, M., Crulhas, B. P., Alves, F. C. B., Pereira, A. F. M., Andrade, B., Barbosa, L. N., et al. (2019). Antibacterial and Anti-Biofilm Activities of Cinnamaldehyde Against S. Epidermidis. Microb. Pathog. 126, 231–238. doi: 10.1016/j.micpath.2018.11.009
Alibi, S., Ben Selma, W., Ramos-Vivas, J., Smach, M. A., Touati, R., Boukadida, J., et al. (2020). Anti-Oxidant, Antibacterial, Anti-Biofilm, and Anti-Quorum Sensing Activities of Four Essential Oils Against Multidrug-Resistant Bacterial Clinical Isolates. Curr. Res. Transl. Med. 68, 59–66. doi: 10.1016/j.retram.2020.01.001
Ali, I. A., Cheung, B. P., Matinlinna, J., Lévesque, C. M., Neelakantan, P. (2020). Trans-Cinnamaldehyde Potently Kills Enterococcus Faecalis Biofilm Cells and Prevents Biofilm Recovery. Microb. Pathogen. 149, 104482. doi: 10.1016/j.micpath.2020.104482
Ali, I. A., Matinlinna, J. P., Lévesque, C. M., Neelakantan, P. (2021). Trans-Cinnamaldehyde Attenuates Enterococcus Faecalis Virulence and Inhibits Biofilm Formation. Antibiotics 10, 702. doi: 10.3390/antibiotics10060702
Almeida, L.D.F.D.D., Paula, J. F. D., Almeida, R. V. D. D., Williams, D. W., Hebling, J., Cavalcanti, Y. W. (2016). Efficacy of Citronella and Cinnamon Essential Oils on Candida Albicans Biofilms. Acta Odontol. Scand. 74, 393–398. doi: 10.3109/00016357.2016.1166261
Alshahrani, A. M., Gregory, R. L. (2020). In Vitro Cariostatic Effects of Cinnamon Water Extract on Nicotine-Induced Streptococcus Mutans Biofilm. BMC Complementary Med. Therapies 20, 1–9. doi: 10.1186/s12906-020-2840-x
Alva, P. P., Suresh, S., Nanjappa, D. P., James, J. P., Kaverikana, R., Chakraborty, A., et al. (2021). Isolation and Identification of Quorum Sensing Antagonist From Cinnamomum Verum Leaves Against Pseudomonas Aeruginosa. Life Sci. 267, 118878. doi: 10.1016/j.lfs.2020.118878
Amalaradjou, M., Narayanan, A., Baskaran, S. A., Venkitanarayanan, K. (2010). Antibiofilm Effect of Trans-Cinnamaldehyde on Uropathogenic Escherichia Coli. J. Urol. 184, 358–363. doi: 10.1016/j.juro.2010.03.006
Amalaradjou, M., Venkitanarayanan, K. (2011). Effect of Trans-Cinnamaldehyde on Inhibition and Inactivation of Cronobacter Sakazakii Biofilm on Abiotic Surfaces. J. Food Prot. 74, 200–208. doi: 10.4315/0362-028X.JFP-10-296
Aumeeruddy-Elalfi, Z., Ismaël, I. S., Hosenally, M., Zengin, G., Mahomoodally, M. F. (2018). Essential Oils From Tropical Medicinal Herbs and Food Plants Inhibit Biofilm Formation In Vitro and are non-Cytotoxic to Human Cells. 3 Biotech. 8, 1–11. doi: 10.1007/s13205-018-1413-x
Bae, K.-H., Ji, J.-M., Park, K.-L. (1992). The Antibacterial Component From Cinnamomi Cortex Against a Cariogenic Bacterium Streptococcus Mutans OMZ 176. Arch. Pharmacal Res. 15, 239–241. doi: 10.1007/BF02974062
Bahramian, A., Khoshnood, S., Shariati, A., Doustdar, F., Chirani, A. S., Heidary, M. (2019). Molecular Characterization of the Pils2 Gene and its Association With the Frequency of Pseudomonas Aeruginosa Plasmid Pklc102 and PAPI-1 Pathogenicity Island. Infect. Drug Resist. 12, 221. doi: 10.2147/IDR.S188527
Balasubramanian, A., Vasudevan, S., Shanmugam, K., Lévesque, C., Solomon, A., Neelakantan, P. (2021). Combinatorial Effects of Trans-Cinnamaldehyde With Fluoride and Chlorhexidine on Streptococcus Mutans. J. Appl. Microbiol. 130, 382–393. doi: 10.1111/jam.14794
Balaure, P. C., Boarca, B., Popescu, R. C., Savu, D., Trusca, R., Vasile, B., et al. (2017). Bioactive Mesoporous Silica Nanostructures With Anti-Microbial and Anti-Biofilm Properties. Int. J. Pharm. 531, 35–46. doi: 10.1016/j.ijpharm.2017.08.062
Balázs, V. L., Horváth, B., Kerekes, E., Ács, K., Kocsis, B., Varga, A., et al. (2019). Anti-Haemophilus Activity of Selected Essential Oils Detected by TLC-Direct Bioautography and Biofilm Inhibition. Molecules 24, 3301. doi: 10.3390/molecules24183301
Banu, S. F., Rubini, D., Shanmugavelan, P., Murugan, R., Gowrishankar, S., Pandian, S. K., et al. (2018). Effects of Patchouli and Cinnamon Essential Oils on Biofilm and Hyphae Formation by Candida Species. J. Mycologie Medicale 28, 332–339. doi: 10.1016/j.mycmed.2018.02.012
Bassyouni, R. H., Kamel, Z., Abdelfattah, M. M., Mostafa, E. (2016). Cinnamon Oil: A Possible Alternative for Contact Lens Disinfection. Cont Lens Anterior Eye 39, 277–283. doi: 10.1016/j.clae.2016.01.001
Beema Shafreen, R. M., Selvaraj, C., Singh, S. K., Karutha Pandian, S. (2014). In Silico and In Vitro Studies of Cinnamaldehyde and Their Derivatives Against LuxS in Streptococcus Pyogenes: Effects on Biofilm and Virulence Genes. J. Mol. Recognit. 27, 106–116. doi: 10.1002/jmr.2339
Brackman, G., Cos, P., Maes, L., Nelis, H. J., Coenye, T. (2011). Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics In Vitro and In Vivo. Antimicrob. Agents Chemother. 55, 2655–2661. doi: 10.1128/aac.00045-11
Brackman, G., Defoirdt, T., Miyamoto, C., Bossier, P., Van Calenbergh, S., Nelis, H., et al. (2008). Cinnamaldehyde and Cinnamaldehyde Derivatives Reduce Virulence in Vibrio Spp. By Decreasing the DNA-Binding Activity of the Quorum Sensing Response Regulator LuxR. BMC Microbiol. 8, 149. doi: 10.1186/1471-2180-8-149
Budri, P. E., Silva, N. C., Bonsaglia, E. C., Júnior, A. F., Júnior, J. A., Doyama, J. T., et al. (2015). Effect of Essential Oils of Syzygium Aromaticum and Cinnamomum Zeylanicum and Their Major Components on Biofilm Production in Staphylococcus Aureus Strains Isolated From Milk of Cows With Mastitis. J. Dairy Sci. 98, 5899–5904. doi: 10.3168/jds.2015-9442
Caiazza, N. C., O'toole, G. A. (2003). Alpha-Toxin is Required for Biofilm Formation by Staphylococcus Aureus. J. Bacteriol. 185, 3214–3217. doi: 10.1128/JB.185.10.3214-3217
Campana, R., Casettari, L., Fagioli, L., Cespi, M., Bonacucina, G., Baffone, W. (2017). Activity of Essential Oil-Based Microemulsions Against Staphylococcus Aureus Biofilms Developed on Stainless Steel Surface in Different Culture Media and Growth Conditions. Int. J. Food Microbiol. 241, 132–140. doi: 10.1016/j.ijfoodmicro.2016.10.021
Ceylan, O., Ugur, A. (2015). Chemical Composition and Anti-Biofilm Activity of Thymus Sipyleus BOISS. Subsp. Sipyleus BOISS. Var. Davisianus RONNIGER Essential Oil. Arch. Pharmacal Res. 38, 957–965. doi: 10.1007/s12272-014-0516-0
Cheon, K., Moser, S. A., Wiener, H. W., Whiddon, J., Momeni, S. S., Ruby, J. D., et al. (2013). Characteristics of Streptococcus Mutans Genotypes and Dental Caries in Children. Eur. J. Oral. Sci. 121, 148–155. doi: 10.1111/eos.12044
Choonharuangdej, S., Srithavaj, T., Thummawanit, S. (2021). Fungicidal and Inhibitory Efficacy of Cinnamon and Lemongrass Essential Oils on Candida Albicans Biofilm Established on Acrylic Resin: An In Vitro Study. J. Prosthetic Dentistry 125, 707.e701–707.e706. doi: 10.1016/j.prosdent.2020.12.017
Condò, C., Anacarso, I., Sabia, C., Iseppi, R., Anfelli, I., Forti, L., et al. (2020). Antimicrobial Activity of Spices Essential Oils and its Effectiveness on Mature Biofilms of Human Pathogens. Nat. Prod. Res. 34, 567–574. doi: 10.1080/14786419.2018.1490904
Costerton, J. W., Stewart, P. S., Greenberg, E. P. (1999). Bacterial Biofilms: A Common Cause of Persistent Infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
Cucarella, C., Solano, C., Valle, J., Amorena, B., Lasa, Í., Penadés, J. R. (2001). Bap, a Staphylococcus Aureus Surface Protein Involved in Biofilm Formation. J. Bacteriol. 183, 2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001
Cui, H., Li, W., Li, C., Vittayapadung, S., Lin, L. (2016). Liposome Containing Cinnamon Oil With Antibacterial Activity Against Methicillin-Resistant Staphylococcus Aureus Biofilm. Biofouling 32, 215–225. doi: 10.1080/08927014.2015.1134516
D'agostino, M., Tesse, N., Lavergne, R. A., Le Pape, P., Frippiat, J. P., Machouart, M., et al. (2021). In Vitro Antifungal Effect of a Plant-Based Product, CIN-102, on Antifungal Resistant Filamentous Fungi and Their Biofilms. J. Med. Microbiol. 70 (9), 001399. doi: 10.1099/jmm.0.001399
Da Nóbrega Alves, D., Monteiro, A. F. M., Andrade, P. N., Lazarini, J. G., Abílio, G. M. F., Guerra, F. Q. S., et al. (2020). Docking Prediction, Antifungal Activity, Anti-Biofilm Effects on Candida Spp., and Toxicity Against Human Cells of Cinnamaldehyde. Molecules 25 (24), 5969. doi: 10.3390/molecules25245969
De Oliveira Carvalho, I., Purgato, G. A., Píccolo, M. S., Pizziolo, V. R., Coelho, R. R., Diaz-Muñoz, G., et al. (2020). In Vitro Anticariogenic and Antibiofilm Activities of Toothpastes Formulated With Essential Oils. Arch. Oral. Biol. 117, 104834. doi: 10.1016/j.archoralbio.2020.104834
Domka, J., Lee, J., Wood, T. K. (2006). YliH (BssR) and YceP (BssS) Regulate Escherichia Coli K-12 Biofilm Formation by Influencing Cell Signaling. Appl. Environ. Microbiol. 72, 2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006
Donlan, R. M., Costerton, J. W. (2002). Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 15, 167–193. doi: 10.1128/CMR.15.2.167-193
Dos Santos, V. R., Caiaffa, K. S., Oliveira, W. C. D., Pereira, J. A., Abuna, G. F., Polaquini, C. R., et al. (2021). Cytotoxicity and Effects of Curcumin and Cinnamaldehyde Hybrids on Biofilms of Oral Pathogens. Biofouling 37, 591–605. doi: 10.1080/08927014.2021.1942859
Duncan, B., Li, X., Landis, R. F., Kim, S. T., Gupta, A., Wang, L. S., et al. (2015). Nanoparticle-Stabilized Capsules for the Treatment of Bacterial Biofilms. ACS Nano 9, 7775–7782. doi: 10.1021/acsnano.5b01696
El-Baz, A. M., Mosbah, R. A., Goda, R. M., Mansour, B., Sultana, T., Dahms, T. E., et al. (2021). Back to Nature: Combating Candida Albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics 10, 81. doi: 10.3390/antibiotics10010081
Evans, M., Davies, J. K., Sundqvist, G., Figdor, D. (2002). Mechanisms Involved in the Resistance of Enterococcus Faecalis to Calcium Hydroxide. Int. Endodontic J. 35, 221–228. doi: 10.1046/j.1365-2591
Farisa Banu, S., Rubini, D., Rakshitaa, S., Chandrasekar, K., Murugan, R., Wilson, A., et al. (2017). Antivirulent Properties of Underexplored Cinnamomum Tamala Essential Oil and its Synergistic Effects With DNase Against Pseudomonas Aeruginosa Biofilms–an In Vitro Study. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01144
Filoche, S., Soma, K., Sissons, C. (2005). Antimicrobial Effects of Essential Oils in Combination With Chlorhexidine Digluconate. Oral. Microbiol. Immunol. 20, 221–225. doi: 10.1111/j.1399-302X.2005.00216.x
Firmino, D. F., Cavalcante, T. T. A., Gomes, G. A., Firmino, N. C. S., Rosa, L. D., De Carvalho, M. G., et al. (2018). Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. ScientificWorldJournal 2018, 7405736. doi: 10.1155/2018/7405736
Flores-Mireles, A. L., Walker, J. N., Caparon, M., Hultgren, S. J. (2015). Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 13, 269–284. doi: 10.1038/nrmicro3432
García-Salinas, S., Elizondo-Castillo, H., Arruebo, M., Mendoza, G., Irusta, S. (2018). Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 23, 1399. doi: 10.3390/molecules23061399
Goc, A., Niedzwiecki, A., Rath, M. (2019). Anti-Borreliae Efficacy of Selected Organic Oils and Fatty Acids. BMC Complementary Altern. Med. 19, 1–11. doi: 10.1186/s12906-019-2450-7
Gupta, A., Duhan, J., Tewari, S., Sangwan, P., Yadav, A., Singh, G., et al. (2013). Comparative Evaluation of Antimicrobial Efficacy of S Yzygium Aromaticum, O Cimum Sanctum and C Innamomum Zeylanicum Plant Extracts Against E Nterococcus Faecalis: A Preliminary Study. Int. Endodontic J. 46, 775–783. doi: 10.1111/iej.12058
Gupta, P., Gupta, S., Sharma, M., Kumar, N., Pruthi, V., Poluri, K. M. (2018). Effectiveness of Phytoactive Molecules on Transcriptional Expression, Biofilm Matrix, and Cell Wall Components of Candida Glabrata and its Clinical Isolates. ACS Omega 3, 12201–12214. doi: 10.1021/acsomega.8b01856
Hammer, K. A., Carson, C. F., Riley, T. V. (1999). Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 86, 985–990. doi: 10.1046/j.1365-2672.1999.00780.x
Hathroubi, S., Servetas, S. L., Windham, I., Merrell, D. S., Ottemann, K. M. (2018). Helicobacter Pylori Biofilm Formation and its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 82, e00001–e00018. doi: 10.1128/MMBR.00001-18
He, Z., Huang, Z., Jiang, W., Zhou, W. (2019). Antimicrobial Activity of Cinnamaldehyde on Streptococcus Mutans Biofilms. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02241
Hoelscher, A. A., Bahcall, J. K., Maki, J. S. (2006). In Vitro Evaluation of the Antimicrobial Effects of a Root Canal Sealer-Antibiotic Combination Against Enterococcus Faecalis. J. Endodontics 32, 145–147. doi: 10.1016/j.joen.2005.10.031
Hong, W., Moser, C., Hengzhuang, W., Høiby, N., Zhijun, S. (2015). Strategies for Combating Bacterial Biofilm Infections. Int. J. Oral. Sci. 7, 1–7. doi: 10.1038/ijos.2014.65
Hovijitra, R. S., Choonharuangdej, S., Srithavaj, T. (2016). Effect of Essential Oils Prepared From Thai Culinary Herbs on Sessile Candida Albicans Cultures. J. Oral. Sci. 58, 365–371. doi: 10.2334/josnusd.15-0736
Hoyer, L. L., Cota, E. (2016). Candida Albicans Agglutinin-Like Sequence (Als) Family Vignettes: A Review of Als Protein Structure and Function. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.00280
Isaacs, H., Jr., Chao, D., Yanofsky, C., Saier, J. M. H. (1994). Mechanism of Catabolite Repression of Tryptophanase Synthesis in Escherichia Coli. Microbiology 140, 2125–2134. doi: 10.1099/13500872-140-8-2125
Jamal, M., Ahmad, W., Andleeb, S., Jalil, F., Imran, M., Nawaz, M. A., et al. (2018). Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 81, 7–11. doi: 10.1016/j.jcma.2017.07.012
Jeong, Y.-J., Kim, H.-E., Han, S.-J., Choi, J.-S. (2021). Antibacterial and Antibiofilm Activities of Cinnamon Essential Oil Nanoemulsion Against Multi-Species Oral Biofilms. Sci. Rep. 11, 1–8. doi: 10.1038/s41598-021-85375-3
Jhajharia, K., Parolia, A., Shetty, K. V., Mehta, L. K. (2015). Biofilm in Endodontics: A Review. J. Int. Soc. Prev. Community Dentistry 5, 1. doi: 10.4103/2231-0762.151956
Jia, P., Xue, Y., Duan, X., Shao, S. (2011). Effect of Cinnamaldehyde on Biofilm Formation and sarA Expression by Methicillin-Resistant Staphylococcus Aureus. Lett. Appl. Microbiol. 53, 409–416. doi: 10.1111/j.1472-765X.2011.03122.x
Kalia, M., Yadav, V. K., Singh, P. K., Sharma, D., Pandey, H., Narvi, S. S., et al. (2015). Effect of Cinnamon Oil on Quorum Sensing-Controlled Virulence Factors and Biofilm Formation in Pseudomonas Aeruginosa. PLoS One 10, e0135495. doi: 10.1371/journal.pone.0135495
Kargaran, M., Moradabadi, A. R., Arjomandzadegan, M., Hosseini, H., Habibi, G., Tayebani, M., et al. (2017). Effects of the Aqueous Extract of Aloe Vera on the Morphological and Physiological Properties of E. Coli. Iranian Red Crescent Med. J. 5(3):266. doi: 10.5812/ircmj.23896
Kart, D., Reçber, T., Nemutlu, E., Sagiroglu, M. (2021). Sub-Inhibitory Concentrations of Ciprofloxacin Alone and Combinations With Plant-Derived Compounds Against P. Aeruginosa Biofilms and Their Effects on the Metabolomic Profile of P. Aeruginosa Biofilms. Antibiotics 10, 414. doi: 10.3390/antibiotics10040414
Karumathil, D. P., Surendran-Nair, M., Venkitanarayanan, K. (2016). Efficacy of Trans-Cinnamaldehyde and Eugenol in Reducing Acinetobacter Baumannii Adhesion to and Invasion of Human Keratinocytes and Controlling Wound Infection In Vitro. Phytother. Res. 30, 2053–2059. doi: 10.1002/ptr.5713
Keelara, S., Thakur, S., Patel, J. (2016). Biofilm Formation by Environmental Isolates of Salmonella and Their Sensitivity to Natural Antimicrobials. Foodborne Pathog. Dis. 13, 509–516. doi: 10.1089/fpd.2016.2145
Kerekes, E. B., Deák, É., Takó, M., Tserennadmid, R., Petkovits, T., Vágvölgyi, C., et al. (2013). Anti-Biofilm Forming and Anti-Quorum Sensing Activity of Selected Essential Oils and Their Main Components on Food-Related Micro-Organisms. J. Appl. Microbiol. 115, 933–942. doi: 10.1111/jam.12289
Kerekes, E. B., Vidács, A., Takó, M., Petkovits, T., Vágvölgyi, C., Horváth, G., et al. (2019). Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono- and Polymicrobic Bacterial Cultures. Microorganisms 7 (9), 345. doi: 10.3390/microorganisms7090345
Khan, M. S. A., Ahmad, I. (2012). Antibiofilm Activity of Certain Phytocompounds and Their Synergy With Fluconazole Against Candida Albicans Biofilms. J. Antimicrob. Chemother. 67, 618–621. doi: 10.1093/jac/dkr512
Kim, N. H., Cho, T. J., Rhee, M. S. (2017). “Current Interventions for Controlling Pathogenic Escherichia Coli,” in Advances in Applied Microbiology. (Netherlands: Elsevier), 1–47.
Kim, Y.-G., Lee, J.-H., Kim, S.-I., Baek, K.-H., Lee, J. (2015). Cinnamon Bark Oil and its Components Inhibit Biofilm Formation and Toxin Production. Int. J. Food Microbiol. 195, 30–39. doi: 10.1016/j.ijfoodmicro.2014.11.028
Klein, M. I., Hwang, G., Santos, P. H., Campanella, O. H., Koo, H. (2015). Streptococcus Mutans-Derived Extracellular Matrix in Cariogenic Oral Biofilms. Front. Cell. Infect. Microbiol. 5. doi: 10.3389/fcimb.2015.00010
Koo, H., Duarte, S., Murata, R., Scott-Anne, K., Gregoire, S., Watson, G., et al. (2010). Influence of Cranberry Proanthocyanidins on Formation of Biofilms by Streptococcus Mutans on Saliva-Coated Apatitic Surface and on Dental Caries Development In Vivo. Caries Res. 44, 116–126. doi: 10.1159/000296306
Kosari, F., Taheri, M., Moradi, A., Alni, R. H., Alikhani, M. Y. (2020). Evaluation of Cinnamon Extract Effects on clbB Gene Expression and Biofilm Formation in Escherichia Coli Strains Isolated From Colon Cancer Patients. BMC Cancer 20, 1–8. doi: 10.1186/s12885-020-06736-1
Kostakioti, M., Hadjifrangiskou, M., Hultgren, S. J. (2013). Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harbor Perspect. Med. 3, a010306. doi: 10.1101/cshperspect.a010306
Kot, B., Sytykiewicz, H., Sprawka, I., Witeska, M. (2020). Effect of Trans-Cinnamaldehyde on Methicillin-Resistant Staphylococcus Aureus Biofilm Formation: Metabolic Activity Assessment and Analysis of the Biofilm-Associated Genes Expression. Int. J. Mol. Sci. 21, 102. doi: 10.3390/ijms21010102
Kot, B., Wicha, J., Piechota, M., Wolska, K., Gruzewska, A. (2015). Antibiofilm Activity of Trans-Cinnamaldehyde, P-Coumaric, and Ferulic Acids on Uropathogenic Escherichia Coli. Turkish J. Med. Sci. 45, 919–924. doi: 10.3906/sag-1406-112
Kot, B., Wierzchowska, K., Grużewska, A., Lohinau, D. (2018). The Effects of Selected Phytochemicals on Biofilm Formed by Five Methicillin-Resistant Staphylococcus Aureus. Natural Product Res. 32, 1299–1302. doi: 10.1080/14786419.2017.1340282
Kumari, P., Mishra, R., Arora, N., Chatrath, A., Gangwar, R., Roy, P., et al. (2017). Antifungal and Anti-Biofilm Activity of Essential Oil Active Components Against Cryptococcus Neoformans and Cryptococcus Laurentii. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.02161
Lakshmanan, D., Harikrishnan, A., Vishnupriya, S., Jeevaratnam, K. (2019). Swarming Inhibitory Potential of Cinnamtannin B1 From Cinnamomum Tamala T. Nees and Eberm on Pseudomonas Aeruginosa. ACS Omega 4, 16994–16998. doi: 10.1021/acsomega.9b02471
Lebel, G., Haas, B., Adam, A. A., Veilleux, M. P., Lagha, A. B., Grenier, D. (2017). Effect of Cinnamon (Cinnamomum Verum) Bark Essential Oil on the Halitosis-Associated Bacterium Solobacterium Moorei and In Vitro Cytotoxicity. Arch. Oral. Biol. 83, 97–104. doi: 10.1016/j.archoralbio.2017.07.005
Lebel, G., Vaillancourt, K., Bercier, P., Grenier, D. (2019). Antibacterial Activity Against Porcine Respiratory Bacterial Pathogens and In Vitro Biocompatibility of Essential Oils. Arch. Microbiol. 201, 833–840. doi: 10.1007/s00203-019-01655-7
Liakos, I. L., Iordache, F., Carzino, R., Scarpellini, A., Oneto, M., Bianchini, P., et al. (2018). Cellulose Acetate - Essential Oil Nanocapsules With Antimicrobial Activity for Biomedical Applications. Colloids Surf B Biointerf. 172, 471–479. doi: 10.1016/j.colsurfb.2018.08.069
Liu, Q., Niu, H., Zhang, W., Mu, H., Sun, C., Duan, J. (2015). Synergy Among Thymol, Eugenol, Berberine, Cinnamaldehyde and Streptomycin Against Planktonic and Biofilm-Associated Food-Borne Pathogens. Lett. Appl. Microbiol. 60, 421–430. doi: 10.1111/lam.12401
Liu, H., Wei, X., Ling, J., Wang, W., Huang, X. (2010). Biofilm Formation Capability of Enterococcus Faecalis Cells in Starvation Phase and its Susceptibility to Sodium Hypochlorite. J. Endodontics 36, 630–635. doi: 10.1016/j.joen.2009.11.016
Liu, Y., Wu, L., Han, J., Dong, P., Luo, X., Zhang, Y., et al. (2021). Inhibition of Biofilm Formation and Related Gene Expression of Listeria Monocytogenes in Response to Four Natural Antimicrobial Compounds and Sodium Hypochlorite. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.617473
Lu, C., Liu, H., Shangguan, W., Chen, S., Zhong, Q. (2021). Antibiofilm Activities of the Cinnamon Extract Against Vibrio Parahaemolyticus and Escherichia Coli. Arch. Microbiol. 203, 125–135. doi: 10.1007/s00203-020-02008-5
Lynch, D. J., Michalek, S. M., Zhu, M., Drake, D., Qian, F., Banas, J. A. (2013). Cariogenicity of Streptococcus Mutans Glucan-Binding Protein Deletion Mutants. Oral. Health Dental Manage. 12, 191.
Ma, L., Conover, M., Lu, H., Parsek, M. R., Bayles, K., Wozniak, D. J. (2009). Assembly and Development of the Pseudomonas Aeruginosa Biofilm Matrix. PLoS Pathog. 5, e1000354. doi: 10.1371/journal.ppat.1000354
Mah, T.-F., Pitts, B., Pellock, B., Walker, G. C., Stewart, P. S., O'toole, G. A. (2003). A Genetic Basis for Pseudomonas Aeruginosa Biofilm Antibiotic Resistance. Nature 426, 306–310. doi: 10.1038/nature02122
Maior, L. D. F. S., Maciel, P. P., Ferreira, V. Y. N., Dantas, C. D. L. G., De Lima, J. M., Castellano, L. R. C., et al. (2019). Antifungal Activity and Shore a Hardness of a Tissue Conditioner Incorporated With Terpinen-4-Ol and Cinnamaldehyde. Clin. Oral. Invest. 23, 2837–2848. doi: 10.1007/s00784-019-02925-w
Mala, N., Sonal, S., Kumar, A., Choudhary, H. V., Songara, P., Ramesh, K. (2021). Cariostatic Efficacy of Cinnamon Water Extract on Streptococcus Mutans: An In Vitro Study. J. Pharm. Bioallied Sci. 13, S212–s216. doi: 10.4103/jpbs.JPBS_677_20
Malhotra, R., Grover, V., Kapoor, A., Saxena, D. (2011). Comparison of the Effectiveness of a Commercially Available Herbal Mouthrinse With Chlorhexidine Gluconate at the Clinical and Patient Level. J. Indian Soc. Periodontol. 15, 349. doi: 10.4103/0972-124X.92567
Manges, A. R., Johnson, J. R., Foxman, B., O'bryan, T. T., Fullerton, K. E., Riley, L. W. (2001). Widespread Distribution of Urinary Tract Infections Caused by a Multidrug-Resistant Escherichia Coli Clonal Group. New Engl. J. Med. 345, 1007–1013. doi: 10.1056/NEJMoa011265
Manukumar, H., Umesha, S. (2017). Photocrosslinker Technology: An Antimicrobial Efficacy of Cinnamaldehyde Cross-Linked Low-Density Polyethylene (Cin-C-LDPE) as a Novel Food Wrapper. Food Res. Int. 102, 144–155. doi: 10.1016/j.foodres.2017.09.095
Marchese, A., Barbieri, R., Coppo, E., Orhan, I. E., Daglia, M., Nabavi, S. F., et al. (2017). Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 43, 668–689. doi: 10.1080/1040841x.2017.1295225
Marcoux, E., Lagha, A. B., Gauthier, P., Grenier, D. (2020). Antimicrobial Activities of Natural Plant Compounds Against Endodontic Pathogens and Biocompatibility With Human Gingival Fibroblasts. Arch. Oral. Biol. 116, 104734. doi: 10.1016/j.archoralbio.2020.104734
Meng, J., Hu, Z., He, M., Wang, J., Chen, X. (2021). Gold Nanocluster Surface Ligand Exchange: An Oxidative Stress Amplifier for Combating Multidrug Resistance Bacterial Infection. J. Colloid Interface Sci. 602, 846–858. doi: 10.1016/j.jcis.2021.06.051
Millsop, J. W., Fazel, N. (2016). Oral Candidiasis. Clinics Dermatol. 34, 487–494. doi: 10.1016/j.clindermatol.2016.02.022
Miquel, S., Lagrafeuille, R., Souweine, B., Forestier, C. (2016). Anti-Biofilm Activity as a Health Issue. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.00592
Miranda-Cadena, K., Marcos-Arias, C., Mateo, E., Aguirre, J. M., Quindós, G., Eraso, E. (2018). Prevalence and Antifungal Susceptibility Profiles of Candida Glabrata, Candida Parapsilosis and Their Close-Related Species in Oral Candidiasis. Arch. Oral. Biol. 95, 100–107. doi: 10.1016/j.archoralbio
Miranda-Cadena, K., Marcos-Arias, C., Mateo, E., Aguirre-Urizar, J. M., Quindós, G., Eraso, E. (2021). In Vitro Activities of Carvacrol, Cinnamaldehyde and Thymol Against Candida Biofilms. Biomed. Pharmacother. 143, 112218. doi: 10.1016/j.biopha.2021.112218
Mohammadi, Z., Abbott, P. (2009). On the Local Applications of Antibiotics and Antibiotic-Based Agents in Endodontics and Dental Traumatology. Int. Endodontic J. 42, 555–567. doi: 10.1111/j.1365-2591.2009.01564.x
Mukherjee, S., Moustafa, D., Smith, C. D., Goldberg, J. B., Bassler, B. L. (2017). The RhlR Quorum-Sensing Receptor Controls Pseudomonas Aeruginosa Pathogenesis and Biofilm Development Independently of its Canonical Homoserine Lactone Autoinducer. PloS Pathog. 13, e1006504. doi: 10.1371/journal.ppat.1006504
Nakayama, J., Chen, S., Oyama, N., Nishiguchi, K., Azab, E. A., Tanaka, E., et al. (2006). Revised Model for Enterococcus Faecalis Fsr Quorum-Sensing System: The Small Open Reading Frame fsrD Encodes the Gelatinase Biosynthesis-Activating Pheromone Propeptide Corresponding to Staphylococcal agrD. J. Bacteriol. 188, 8321–8326. doi: 10.1128/JB.00865-06
Neelakantan, P., Romero, M., Vera, J., Daood, U., Khan, A. U., Yan, A., et al. (2017). Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 18, 1748. doi: 10.3390/ijms18081748
Niu, C., Gilbert, E. S. (2004). Colorimetric Method for Identifying Plant Essential Oil Components That Affect Biofilm Formation and Structure. Appl. Environ. Microbiol. 70, 6951–6956. doi: 10.1128/aem.70.12.6951-6956.2004
Nobile, C. J., Johnson, A. D. (2015). Candida Albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 69, 71–92. doi: 10.1146/annurev-micro-091014-104330
Nostro, A., Scaffaro, R., D'arrigo, M., Botta, L., Filocamo, A., Marino, A., et al. (2012). Study on Carvacrol and Cinnamaldehyde Polymeric Films: Mechanical Properties, Release Kinetics and Antibacterial and Antibiofilm Activities. Appl. Microbiol. Biotechnol. 96, 1029–1038. doi: 10.1007/s00253-012-4091-3
Nuryastuti, T., van der Mei, H. C., Busscher, H. J., Iravati, S., Aman, A. T., Krom, B. P. (2009). Effect of Cinnamon Oil on icaA Expression and Biofilm Formation by Staphylococcus Epidermidis. Appl. Environ. Microbiol. 75, 6850–6855. doi: 10.1128/AEM.00875-09
Olszewska, M. A., Gędas, A., Simões, M. (2020). The Effects of Eugenol, Trans-Cinnamaldehyde, Citronellol, and Terpineol on Escherichia Coli Biofilm Control as Assessed by Culture-Dependent and -Independent Methods. Molecules 25 (11), 2641. doi: 10.3390/molecules25112641
Passador, L., Cook, J. M., Gambello, M. J., Rust, L., Iglewski, B. H. (1993). Expression of Pseudomonas Aeruginosa Virulence Genes Requires Cell-to-Cell Communication. Science 260, 1127–1130. doi: 10.1126/science.8493556
Paton, J. C., Paton, A. W. (1998). Pathogenesis and Diagnosis of Shiga Toxin-Producing Escherichia Coli Infections. Clin. Microbiol. Rev. 11, 450–479. doi: 10.1128/CMR.11.3.450
Piovezan, M., Sayuri Uchida, N., Fiori Da Silva, A., Grespan, R., Regina Santos, P., Leite Silva, E., et al. (2014). Effect of Cinnamon Essential Oil and Cinnamaldehyde on Salmonella Saintpaul Biofilm on a Stainless Steel Surface. J. Gen. Appl. Microbiol. 60, 119–121. doi: 10.2323/jgam.60.119
Pires, R. H., Montanari, L. B., Martins, C. H. G., Zaia, J. E., Almeida, A. M. F., Matsumoto, M. T., et al. (2011). Anticandidal Efficacy of Cinnamon Oil Against Planktonic and Biofilm Cultures of Candida Parapsilosis and Candida Orthopsilosis. Mycopathologia 172, 453–464. doi: 10.1007/s11046-011-9448-0
Pourkhosravani, E., Dehghan Nayeri, F., Mohammadi Bazargani, M. (2021). Decoding Antibacterial and Antibiofilm Properties of Cinnamon and Cardamom Essential Oils: A Combined Molecular Docking and Experimental Study. AMB Express 11, 1–18. doi: 10.1186/s13568-021-01305-6
Purkait, S., Bhattacharya, A., Bag, A., Chattopadhyay, R. R. (2020). Evaluation of Antibiofilm Efficacy of Essential Oil Components β-Caryophyllene, Cinnamaldehyde and Eugenol Alone and in Combination Against Biofilm Formation and Preformed Biofilms of Listeria Monocytogenes and Salmonella Typhimurium. Lett. Appl. Microbiol. 71, 195–202. doi: 10.1111/lam.13308
Rai, M., Paralikar, P., Jogee, P., Agarkar, G., Ingle, A. P., Derita, M., et al. (2017). Synergistic Antimicrobial Potential of Essential Oils in Combination With Nanoparticles: Emerging Trends and Future Perspectives. Int. J. Pharmaceutics 519, 67–78. doi: 10.1016/j.ijpharm.2017.01.013
Rajamanikandan, S., Jeyakanthan, J., Srinivasan, P. (2017). Discovery of Potent Inhibitors Targeting Vibrio Harveyi LuxR Through Shape and E-Pharmacophore Based Virtual Screening and its Biological Evaluation. Microb. Pathog. 103, 40–56. doi: 10.1016/j.micpath.2016.12.003
Ramasamy, M., Lee, J. H., Lee, J. (2017a). Development of Gold Nanoparticles Coated With Silica Containing the Antibiofilm Drug Cinnamaldehyde and Their Effects on Pathogenic Bacteria. Int. J. Nanomed. 12, 2813–2828. doi: 10.2147/ijn.s132784
Ramasamy, M., Lee, J. H., Lee, J. (2017b). Direct One-Pot Synthesis of Cinnamaldehyde Immobilized on Gold Nanoparticles and Their Antibiofilm Properties. Colloids Surf B Biointerf. 160, 639–648. doi: 10.1016/j.colsurfb.2017.10.018
Rampioni, G., Schuster, M., Greenberg, E. P., Zennaro, E., Leoni, L. (2009). Contribution of the RsaL Global Regulator to Pseudomonas Aeruginosa Virulence and Biofilm Formation. FEMS Microbiol. Lett. 301, 210–217. doi: 10.1111/j.1574-6968.2009.01817.x
Rangel, M. D. L., Aquino, S. G. D., Lima, J. M. D., Castellano, L. R., Castro, R. D. D. (2018). In Vitro Effect of Cinnamomum Zeylanicum Blume Essential Oil on Candida Spp. Involved in Oral Infections. Evidence-Based Complement. Altern. Med. 2018, 4045013. doi: 10.1155/2018/4045013
Remold, S. K., Brown, C. K., Farris, J. E., Hundley, T. C., Perpich, J. A., Purdy, M. E. (2011). Differential Habitat Use and Niche Partitioning by Pseudomonas Species in Human Homes. Microb. Ecol. 62, 505. doi: 10.1007/s00248-011-9844-5
Ren, Z., Cui, T., Zeng, J., Chen, L., Zhang, W., Xu, X., et al. (2016). Molecule Targeting Glucosyltransferase Inhibits Streptococcus Mutans Biofilm Formation and Virulence. Antimicrob. Agents Chemother. 60, 126–135. doi: 10.1128/AAC.00919-15
Ribeiro, M., Malheiro, J., Grenho, L., Fernandes, M. H., Simões, M. (2018). Cytotoxicity and Antimicrobial Action of Selected Phytochemicals Against Planktonic and Sessile Streptococcus Mutans. PeerJ 6, e4872. doi: 10.7717/peerj.4872
Rizzato, C., Torres, J., Kasamatsu, E., Camorlinga-Ponce, M., Bravo, M. M., Canzian, F., et al. (2019). Potential Role of Biofilm Formation in the Development of Digestive Tract Cancer With Special Reference to Helicobacter Pylori Infection. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00846
Rubini, D., Banu, S. F., Nisha, P., Murugan, R., Thamotharan, S., Percino, M. J., et al. (2018). Essential Oils From Unexplored Aromatic Plants Quench Biofilm Formation and Virulence of Methicillin Resistant Staphylococcus Aureus. Microb. Pathogen. 122, 162–173. doi: 10.1016/j.micpath.2018.06.028
Ryan, R. P., Fouhy, Y., Lucey, J. F., Crossman, L. C., Spiro, S., He, Y.-W., et al. (2006). Cell–cell Signaling in Xanthomonas Campestris Involves an HD-GYP Domain Protein That Functions in Cyclic Di-GMP Turnover. Proc. Natl. Acad. Sci. 103, 6712–6717. doi: 10.1073/pnas.0600345103
Saber, S.E.-D.M., El-Hady, S. A. (2012). Development of an Intracanal Mature Enterococcus Faecalis Biofilm and its Susceptibility to Some Antimicrobial Intracanal Medications; an In Vitro Study. Eur. J. Dentistry 6, 43.
Sack, R. B. (2011). The Discovery of Cholera-Like Enterotoxins Produced by Escherichia Coli Causing Secretory Diarrhoea in Humans. Indian J. Med. Res. 133, 171.
Sahal, G., Woerdenbag, H. J., Hinrichs, W. L., Visser, A., Tepper, P. G., Quax, W. J., et al. (2020). Antifungal and Biofilm Inhibitory Effect of Cymbopogon Citratus (Lemongrass) Essential Oil on Biofilm Forming by Candida Tropicalis Isolates; an In Vitro Study. J. Ethnopharmacol. 246, 112188. doi: 10.1016/j.jep.2019.112188
Sarowska, J., Futoma-Koloch, B., Jama-Kmiecik, A., Frej-Madrzak, M., Ksiazczyk, M., Bugla-Ploskonska, G., et al. (2019). Virulence Factors, Prevalence and Potential Transmission of Extraintestinal Pathogenic Escherichia Coli Isolated From Different Sources: Recent Reports. Gut Pathog. 11, 10. doi: 10.1186/s13099-019-0290-0
Saxena, P., Joshi, Y., Rawat, K., Bisht, R. (2019). Biofilms: Architecture, Resistance, Quorum Sensing and Control Mechanisms. Indian J. Microbiol. 59, 3–12. doi: 10.1007/s12088-018-0757-6
Scotti, R., Stringaro, A., Nicolini, L., Zanellato, M., Boccia, P., Maggi, F., et al. (2021). Effects of Essential Oils From Cymbopogon Spp. And Cinnamomum Verum on Biofilm and Virulence Properties of Escherichia Coli O157: H7. Antibiotics 10, 113. doi: 10.3390/antibiotics10020113
Shariati, A., Moradabadi, A., Azimi, T., Ghaznavi-Rad, E. (2020a). Wound Healing Properties and Antimicrobial Activity of Platelet-Derived Biomaterials. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-57559-w
Shariati, A., Moradabadi, A., Chegini, Z., Khoshbayan, A., Didehdar, M. (2020b). An Overview of the Management of the Most Important Invasive Fungal Infections in Patients With Blood Malignancies. Infect. Drug Resist. 13, 2329. doi: 10.2147/IDR.S254478
Sharma, G., Dang, S., A, K., Kalia, M., Gabrani, R. (2020). Synergistic Antibacterial and Anti-Biofilm Activity of Nisin Like Bacteriocin With Curcumin and Cinnamaldehyde Against ESBL and MBL Producing Clinical Strains. Biofouling 36, 710–724. doi: 10.1080/08927014.2020.1804553
Sharma, G., Raturi, K., Dang, S., Gupta, S., Gabrani, R. (2014). Combinatorial Antimicrobial Effect of Curcumin With Selected Phytochemicals on Staphylococcus Epidermidis. J. Asian Natural Products Res. 16, 535–541. doi: 10.1080/10286020.2014.911289
Sheng, L., Rasco, B., Zhu, M.-J. (2016). Cinnamon Oil Inhibits Shiga Toxin Type 2 Phage Induction and Shiga Toxin Type 2 Production in Escherichia Coli O157: H7. Appl. Environ. Microbiol. 82, 6531–6540. doi: 10.1128/AEM.01702-16
Silva, A. F., Dos Santos, A. R., Coelho Trevisan, D. A., Ribeiro, A. B., Zanetti Campanerut-Sá, P. A., Kukolj, C., et al. (2018). Cinnamaldehyde Induces Changes in the Protein Profile of Salmonella Typhimurium Biofilm. Res. Microbiol. 169, 33–43. doi: 10.1016/j.resmic.2017.09.007
Singh, N., Patil, A., Prabhune, A., Goel, G. (2016). Inhibition of Quorum-Sensing-Mediated Biofilm Formation in Cronobacter Sakazakii Strains. Microbiology 162, 1708–1714. doi: 10.1099/mic.0.000342
Sjögren, U., Figdor, D., Persson, S., Sundqvist, G. (1997). Influence of Infection at the Time of Root Filling on the Outcome of Endodontic Treatment of Teeth With Apical Periodontitis. Int. Endodontic J. 30, 297–306. doi: 10.1046/j.1365-2591.1997.00092.x
Smith, M. K., Draper, L. A., Hazelhoff, P. J., Cotter, P. D., Ross, R. P., Hill, C. (2016). A Bioengineered Nisin Derivative, M21A, in Combination With Food Grade Additives Eradicates Biofilms of Listeria Monocytogenes. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01939
Somrani, M., Inglés, M.-C., Debbabi, H., Abidi, F., Palop, A. (2020). Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect Against Listeria Monocytogenes. Foods 9, 567. doi: 10.3390/foods9050567
Spoering, A. L., Lewis, K. (2001). Biofilms and Planktonic Cells of Pseudomonas Aeruginosa Have Similar Resistance to Killing by Antimicrobials. J. Bacteriol. 183, 6746–6751. doi: 10.1128/JB.183.23.6746-6751
Stewart, P. S., Costerton, J. W. (2001). Antibiotic Resistance of Bacteria in Biofilms. Lancet 358, 135–138. doi: 10.1016/s0140-6736(01)05321-1
Sundaramoorthy, M., Karuppaiah, A., Nithyanth, M., Baberoselin, R., Ramesh, S., Geetha, N., et al. (2021). Formulation Development of Cream With Mupirocin and Essential Oils for Eradication of Biofilm Mediated Antimicrobial Resistance. Arch. Microbiol. 203, 1707–1715. doi: 10.1007/s00203-020-02175-5
Sun, F., Qu, F., Ling, Y., Mao, P., Xia, P., Chen, H., et al. (2013). Biofilm-Associated Infections: Antibiotic Resistance and Novel Therapeutic Strategies. Future Microbiol. 8, 877–886. doi: 10.2217/fmb.13.58
Swidergall, M., Filler, S. G. (2017). Oropharyngeal Candidiasis: Fungal Invasion and Epithelial Cell Responses. PloS Pathog. 13, e1006056. doi: 10.1371/journal.ppat.1006056
Taubes, G. (2008). The Bacteria Fight Back. Am. Assoc. Advancement Sci. 321 (5887), 356–361. doi: 10.1126/science.321.5887.356
Topa, S. H., Palombo, E. A., Kingshott, P., Blackall, L. L. (2020). Activity of Cinnamaldehyde on Quorum Sensing and Biofilm Susceptibility to Antibiotics in Pseudomonas Aeruginosa. Microorganisms 8, 455. doi: 10.3390/microorganisms8030455
Topa, S. H., Subramoni, S., Palombo, E. A., Kingshott, P., Rice, S. A., Blackall, L. L. (2018). Cinnamaldehyde Disrupts Biofilm Formation and Swarming Motility of Pseudomonas Aeruginosa. Microbiology 164, 1087–1097. doi: 10.1099/mic.0.000692
Trotonda, M. P., Manna, A. C., Cheung, A. L., Lasa, I., Penadés, J. R. (2005). SarA Positively Controls Bap-Dependent Biofilm Formation in Staphylococcus Aureus. J. Bacteriol. 187, 5790–5798. doi: 10.1128/JB.187.16.5790-5798.2005
Upadhyay, A., Upadhyaya, I., Kollanoor-Johny, A., Venkitanarayanan, K. (2013). Antibiofilm Effect of Plant Derived Antimicrobials on Listeria Monocytogenes. Food Microbiol. 36, 79–89. doi: 10.1016/j.fm.2013.04.010
Uppuluri, P., Chaturvedi, A. K., Srinivasan, A., Banerjee, M., Ramasubramaniam, A. K., Köhler, J. R., et al. (2010). Dispersion as an Important Step in the Candida Albicans Biofilm Developmental Cycle. PloS Pathog. 6, e1000828. doi: 10.1371/journal.ppat.1000828
Vaillancourt, K., Lebel, G., Yi, L., Grenier, D. (2018). In Vitro Antibacterial Activity of Plant Essential Oils Against Staphylococcus Hyicus and Staphylococcus Aureus, the Causative Agents of Exudative Epidermitis in Pigs. Arch. Microbiol. 200, 1001–1007. doi: 10.1007/s00203-018-1512-4
Van Den Driessche, F., Brackman, G., Swimberghe, R., Rigole, P., Coenye, T. (2017). Screening a Repurposing Library for Potentiators of Antibiotics Against Staphylococcus Aureus Biofilms. Int. J. Antimicrob. Agents 49, 315–320. doi: 10.1016/j.ijantimicag.2016.11.023
Vasconcelos, N., Croda, J., Simionatto, S. (2018). Antibacterial Mechanisms of Cinnamon and its Constituents: A Review. Microb. Pathogen. 120, 198–203. doi: 10.1016/j.micpath.2018.04.036
Vasudevan, S., Thamil Selvan, G., Bhaskaran, S., Hari, N., Solomon, A. P. (2020). Reciprocal Cooperation of Type A Procyanidin and Nitrofurantoin Against Multi-Drug Resistant (MDR) UPEC: A pH-Dependent Study. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00421
Veilleux, M.-P., Grenier, D. (2019). Determination of the Effects of Cinnamon Bark Fractions on Candida Albicans and Oral Epithelial Cells. BMC Complementary Altern. Med. 19, 1–12. doi: 10.1186/s12906-019-2730-2
Wagle, B. R., Upadhyay, A., Upadhyaya, I., Shrestha, S., Arsi, K., Liyanage, R., et al. (2019). Trans-Cinnamaldehyde, Eugenol and Carvacrol Reduce Campylobacter Jejuni Biofilms and Modulate Expression of Select Genes and Proteins. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.01837
Wang, S., Kang, O.-H., Kwon, D.-Y. (2021). Trans-Cinnamaldehyde Exhibits Synergy With Conventional Antibiotic Against Methicillin-Resistant Staphylococcus Aureus. Int. J. Mol. Sci. 22, 2752. doi: 10.3390/ijms22052752
Wang, W., Li, D., Huang, X., Yang, H., Qiu, Z., Zou, L., et al. (2019). Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum Camphora Leaf Essential Oil. Molecules 24, 3792. doi: 10.3390/molecules24203792
Wang, Y., Zhang, Y., Shi, Y.-Q., Pan, X.-H., Lu, Y.-H., Cao, P. (2018). Antibacterial Effects of Cinnamon (Cinnamomum Zeylanicum) Bark Essential Oil on Porphyromonas Gingivalis. Microb. Pathogen. 116, 26–32. doi: 10.1016/j.micpath.2018.01.009
Wang, L., Zhang, K., Zhang, K., Zhang, J., Fu, J., Li, J., et al. (2020). Antibacterial Activity of Cinnamomum Camphora Essential Oil on Escherichia Coli During Planktonic Growth and Biofilm Formation. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.561002
Wijesinghe, G. K., De Oliveira, T. R., Maia, F. C., De Feiria, S. B., Barbosa, J. P., Joia, F., et al. (2021). Efficacy of True Cinnamon (Cinnamomum Verum) Leaf Essential Oil as a Therapeutic Alternative for Candida Biofilm Infections. Iran J. Basic Med. Sci. 24, 787–795. doi: 10.22038/ijbms.2021.53981.12138
Wijesinghe, G. K., Maia, F. C., De Oliveira, T. R., De Feiria, S. N. B., Joia, F., Barbosa, J. P., et al. (2020). Effect of Cinnamomum Verum Leaf Essential Oil on Virulence Factors of Candida Species and Determination of the in-Vivo Toxicity With Galleria Mellonella Model. Mem. Inst. Oswaldo Cruz 115, e200349. doi: 10.1590/0074-02760200349
Williams, D., Silva, S. C., Malic, S., Kuriyama, T., Lewis, M. A. (2012). Candida Biofilms and Oral Candidosis: Treatment and Prevention. Periodontol. 2000. 55, 250–265. doi: 10.1111/j.1600-0757.2009.00338.x
Wilson, B. C., Patterson, M. S. (2008). The Physics, Biophysics and Technology of Photodynamic Therapy. Phys. Med. Biol. 53, R61. doi: 10.1088/0031-9155/53/9/R01
Wiwattanarattanabut, K., Choonharuangdej, S., Srithavaj, T. (2017). In Vitro Anti-Cariogenic Plaque Effects of Essential Oils Extracted From Culinary Herbs. J. Clin. Diagn. Res. 11, DC30. doi: 10.7860/JCDR/2017/28327.10668
Worreth, S., Bieger, V., Rohr, N., Astasov-Frauenhoffer, M., Töpper, T., Osmani, B., et al. (2021). Cinnamaldehyde as Antimicrobial in Cellulose-Based Dental Appliances. J. Appl. Microbiol. 132(2), 1018–1024. doi: 10.1111/jam.15283
Xu, J., He, J., Shen, Y., Zhou, X., Huang, D., Gao, Y., et al. (2019). Influence of Endodontic Procedure on the Adherence of Enterococcus Faecalis. J. Endodontics 45, 943–949. doi: 10.1016/j.joen.2019.04.006
Yanakiev, S. (2020). Effects of Cinnamon (Cinnamomum Spp.) in Dentistry: A Review. Molecules 25, 4184. doi: 10.3390/molecules25184184
Yuan, W., Yuk, H.-G. (2019). Effects of Sublethal Thymol, Carvacrol, and Trans-Cinnamaldehyde Adaptation on Virulence Properties of Escherichia Coli O157: H7. Appl. Environ. Microbiol. 85, e00271–e00219. doi: 10.1128/AEM.00271-19
Yu, H. H., Song, Y. J., Yu, H. S., Lee, N. K., Paik, H. D. (2020). Investigating the Antimicrobial and Antibiofilm Effects of Cinnamaldehyde Against Campylobacter Spp. Using Cell Surface Characteristics. J. Food Sci. 85, 157–164. doi: 10.1111/1750-3841.14989
Zaltsman, N., Ionescu, A. C., Weiss, E. I., Brambilla, E., Beyth, S., Beyth, N. (2017). Surface-Modified Nanoparticles as Anti-Biofilm Filler for Dental Polymers. PloS One 12, e0189397. doi: 10.1371/journal.pone.0189397
Keywords: Cinnamomum, cinnamaldehyde, Candida species, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, biofilm
Citation: Didehdar M, Chegini Z, Tabaeian SP, Razavi S and Shariati A (2022) Cinnamomum: The New Therapeutic Agents for Inhibition of Bacterial and Fungal Biofilm-Associated Infection. Front. Cell. Infect. Microbiol. 12:930624. doi: 10.3389/fcimb.2022.930624
Received: 28 April 2022; Accepted: 17 June 2022;
Published: 08 July 2022.
Edited by:
Jayendrakumar Patel, Ganpat University, IndiaReviewed by:
Mehul Patel, Ganpat University, IndiaCopyright © 2022 Didehdar, Chegini, Tabaeian, Razavi and Shariati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aref Shariati, YXJlZnNoYXJpYXRpMDExMUBzYm11LmFjLmly
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.