- 1Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, United States
- 2Section of Nephrology, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, United States
The molecular and cellular pathogenesis of leptospirosis remains poorly understood. Based on comparative bacterial genomics data, we recently identified the hypothetical PF07598 gene family as encoding secreted exotoxins (VM proteins) that mediate cytotoxicity in vitro. To address whether VM proteins mediate in vivo leptospirosis pathogenesis, we tested the hypothesis that VM protein immunization of mice would protect against lethal challenge infection and reduce bacterial load in key target organs. C3H/HeJ mice were immunized with recombinant E. coli-produced, endotoxin-free, leptospiral VM proteins (derived from L. interrogans serovar Lai) in combination with the human-compatible adjuvant, glucopyranoside lipid A/squalene oil-in-water. Mice receiving full length recombinant VM proteins were protected from lethal challenge infection by L. interrogans serovar Canicola and had a 3-4 log10 reduction in bacterial load in the liver and kidney. These experiments show that immunization with recombinant VM proteins prevents leptospirosis clinical pathogenesis and leads to markedly reduced key target organ infection in this animal model. These data support the role of leptospiral VM proteins as virulence factors and suggest the possibility that a VM protein-based, serovar-independent, pan-leptospirosis vaccine may be feasible.
Introduction
The discoveries of the etiological agent of leptospirosis and the rodent reservoir of transmission were first reported in 1915 and 1917, respectively, and even then, was recognized as a disease of global importance (Inada et al., 1916; Noguchi, 1917). The most recent estimate of the global burden of leptospirosis conservatively estimates that this disease annually affects more than one million people, causing at least 60,000 deaths annually. Despite effective antibiotic treatment, leptospirosis still has a 5-20% case fatality rate (Costa et al., 2015; Torgerson et al., 2015; Rudd et al., 2020). There is no safe and effective vaccine generally approved for humans (Bharti et al., 2003; Brown et al., 2003; Balakrishnan and Roy, 2014; Bouvet et al., 2020; Martin et al., 2014). The lack of a human vaccine is due to several factors including reactogenicity of current bacterin-based vaccines, current concepts regarding limitations of serovar coverage, and most importantly, a lack of knowledge regarding specific vaccine antigens targeting conserved pathogenetic mechanisms (Palaniappan et al., 2007; Dellagostin et al., 2017; Bashiru and Bahaman, 2018; Felix et al., 2020).
Previous work with live (Fish and Kingscote, 1973), bacterin (Stringfellow et al., 1983; Bolin et al., 1991; Adler, 2015), lipopolysaccharide (Jost et al., 1986; Jost et al., 1989; Midwinter et al., 1990; Wang et al., 2007), recombinant proteins vaccines (Haake et al., 1999; Branger et al., 2001; Faisal et al., 2009; Grassmann et al., 2012; Llanos Salinas et al., 2020), recombinant subunit vaccine [LigA7-13, LigB0-7 (Evangelista et al., 2017), LigB131-645 (Conrad et al., 2017), and LigA DNA (Faisal et al., 2008)] in animals has not led to definitive vaccine candidates. Most recently an attenuated live Leptospira vaccine and fcpA- mutant confer cross-protective immunity against heterologous leptospiral serovars affecting humans (Murray et al., 2018; Wunder et al., 2021).
We have recently identified a family of conserved paralogous proteins encoded by the PF07598 gene family that encode virulence factors potentially involved in the pathogenesis of leptospirosis. PF07598-encoded proteins, denominated virulence modifying (VM) proteins are found only in Group 1 pathogenic Leptospira (Lehmann et al., 2013; Fouts et al., 2016), are secreted exotoxins with bona fide N-terminal ricin B domains and a C-terminal toxin domain; and their expression is variably upregulated in vivo in a hamster model of acute infection (Lehmann et al., 2013; Lehmann et al., 2014). After cell-surface binding, mCherry-labeled VM protein LA3490 localizes to the nucleus and mediates cytotoxicity because of potent DNase activity (Chaurasia et al., 2022).
Given these observations, we tested the hypothesis that the Leptospira interrogans VM proteins (encoded by the PF07598 gene family) mediate severe disease in an animal model. Targeted gene knockout is not feasible to interrupt the 12+ L. interrogans PF07598 paralogs to evaluate the role of individual VM proteins in mediating leptospirosis pathogenesis. Previous transposon mutagenesis experiments in which individual VM family members were disrupted did not yield informative phenotypes of disease-induction or tissue colonization (Murray et al., 2009; Marcsisin et al., 2013).
Therefore, we took an immunological approach to determine whether leptospiral VM proteins might be protective vaccine antigens in preventing severe leptospirosis in a susceptible murine model (Viriyakosol et al., 2006). We immunized mice with VM proteins identified in the L. interrogans serovar Lai genome: a ricin B domain (RBL-1, called t3490 here) from one VM protein (LA3490), or combinations of full length recombinant VM proteins [LA3490 (UniProt ID: Q8F0K3), LA0620 (Q8F8D7), LA1402 (Q8F6A7), LA1400 (Q8F6A9), and LA0591 (a VM protein with a similar C-terminal domain but lacking ricin B domains at the amino terminus, (Q8F8G6)], chosen according to their variable upregulation in the blood, liver, and kidney in vivo (Lehmann et al., 2013). After lethal challenge infection with a different serovar of L. interrogans, Canicola, we determined the effect of heterologous VM protein vaccination on the protection of mice from severe clinical disease.
Results
Conservation of PF07598 Protein Family and Their Orthologs in Pathogenic Leptospira
The PF07598-encoded VM paralogous protein family has an expanded repertoire within L. interrogans, with at least 12 distinct paralogs in serovars Lai, Copenhageni, and Canicola. Orthologs have >90% amino acid amino acid identity (Table 1). Most VM proteins are comprised of ~640 amino acids with an AB domain architecture comprised of two tandemly arrayed β-trefoil, N-terminal ricin B-like lectin domains, and a C-terminal toxin domain that has DNase activity (Supplementary Figure 1). L. interrogans serovars also encode a single unique ortholog that lacks a N-terminal ricin B-like domain (typified by LA0591, of ~313 aa) but which contains a signal sequence (Table 1 and Supplementary Figure 1).
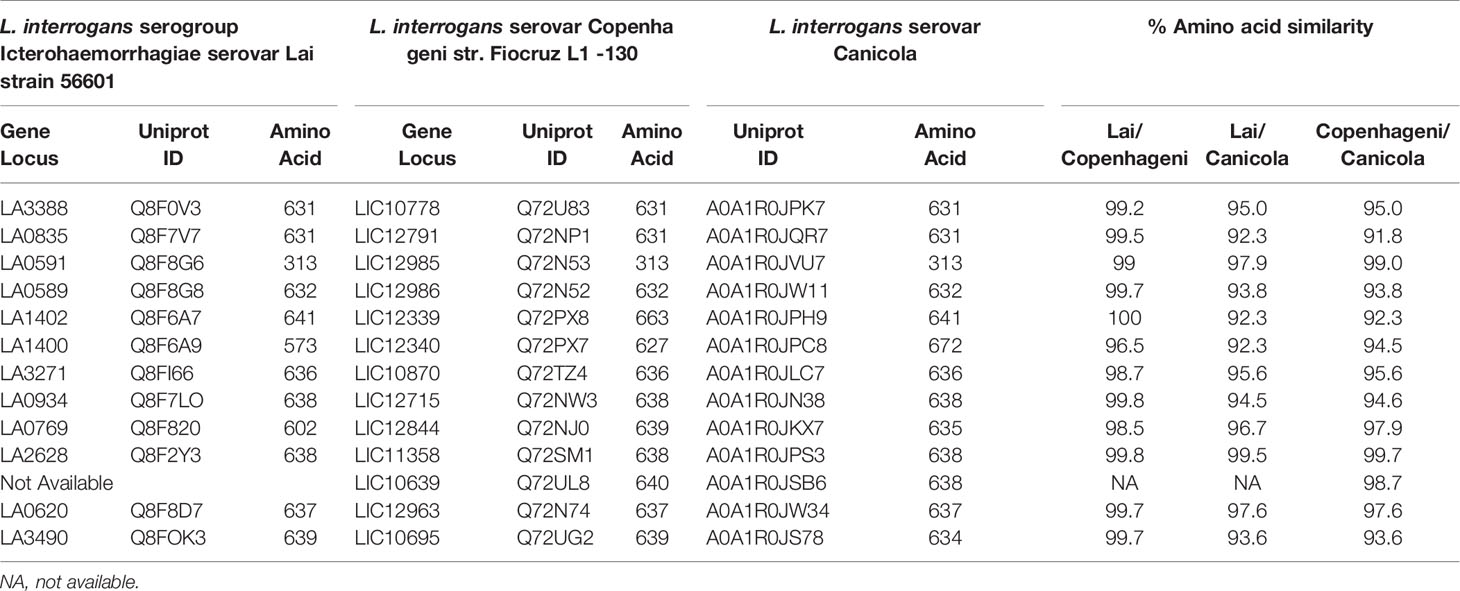
Table 1 Orthologs and percentage amino acid similarity of PF07598 gene family members in Group I pathogenic Leptospira.
Immunization With Full Length VM Proteins Prevented Severe Leptospirosis in Mice
Full length recombinant VM proteins LA3490, LA0620, LA1402, LA1400, and LA0591 (following L. interrogans serovar Lai nomenclature) were expressed in E. coli as N-terminal fusions with thioredoxin (TRX)-His6 affinity tags to facilitate solubility and affinity purification, and C-terminal fusions with mCherry-His6 to facilitate affinity purification and fluorescence microscopy visualization of the protein, respectively (Figure 1A, B). The homogeneity of recombinant VM proteins was verified by SDS-PAGE and Western immunoblot (Figure 1C).
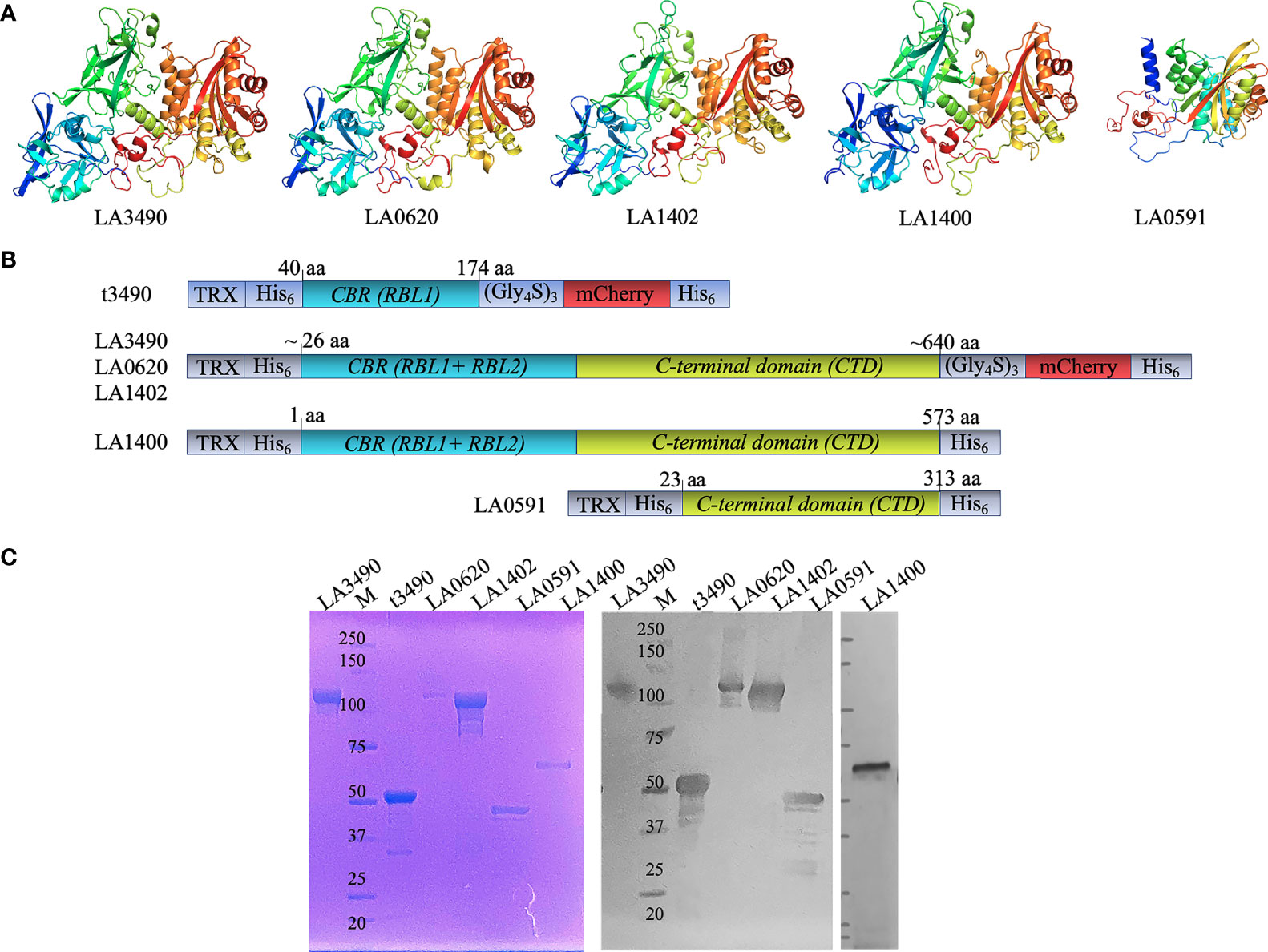
Figure 1 DeepMind AlphaFold algorithm derived structure, strategy for cloning, purification, and antigenicity of recombinant His-tagged VM proteins. (A) Artificial intelligence-based high-resolution structural modeling of (LA3490, LA0620, LA1402, LA1400 and LA0591) using AlphaFold algorithm [Callaway, E. (2020)]. (B) Schematic diagram depicting the organization of the recombinant mCherry (mC) fusion VM proteins used in the current study; t3490, amino acid positions 40 aa -147 aa (minus signal sequence); LA3490 (19 aa – 639 aa), LA0620 (32 aa – 637 aa), LA1402 (28 aa - 641 aa), LA1400 (1 aa - 573 aa), and LA0591 (23 aa – 313 aa). The clones were designed without signal sequences. LA1400 naturally lack signal sequence. Recombinant fusions include a glycine-serine (Gly4S)3 linker (for flexibility), N-and C-terminal His6 tag (purification), and N-terminal thioredoxin. (C) AKTA purified soluble His-tagged VM proteins (LA3490, t3490, LA0620, LA1402, LA0591, and LA1400) were analyzed by 4 ± 12% SDS-PAGE followed by Coomassie staining. A replicate gel was run for immunoblot analysis. The proteins were transferred to a nitrocellulose membrane and the blot was probed with mouse anti-His monoclonal-ALP conjugate (1:2,000 dilution; Santa Cruz Biotechnology, USA). M represents molecular weight marker.
Mice were injected intramuscularly with recombinant proteins or PBS control mixed with glucopyranosyl lipid A/squalene oil-in-water (GLA-SE) adjuvant (schematically depicted in Figure 2). This adjuvant was chosen for the present experiments because it is compatible for human use, hence useful to test in animal models towards eventual vaccine development for humans. The GLA component (a synthetic, non-toxic moiety with six acyl chains on a disaccharide backbone and a single phosphate group (Pantel et al., 2012) would not be expected to have a TLR4 agonist immunostimulatory effect in C3H/HeJ mice, which are genetically hyporesponsive to lipid A due to a mutation in the gene encoding a functional Toll-like receptor (TLR4) (Beutler and Poltorak, 2000).
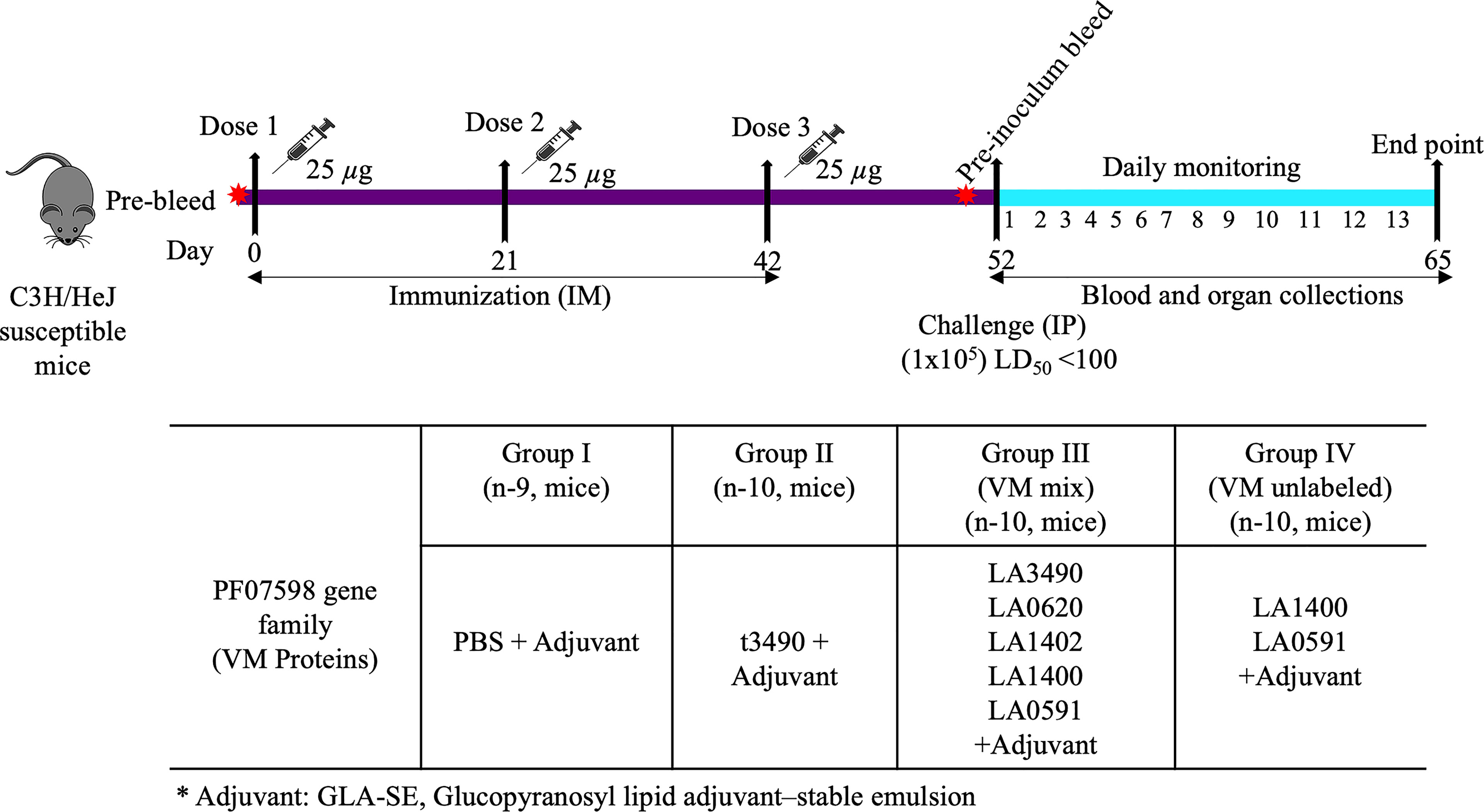
Figure 2 Mouse immunization schedule and sample collection. C3H/HeJ mice were immunized with 25 μg of total antigen along with adjuvant (5 µg GLA–squalene–oil-in-water emulsion) on days 0, 21 and 42 respectively by intramuscular route. They were pre-bled prior to each immunization and prior to challenge infection and blood was obtained on day of necropsy. Control mice were immunized with PBS buffer plus adjuvant. Following immunization on day 52, mice were infected with live L. interrogans serovar Canicola (~1x105 leptospires, LD50 <100) by the intraperitoneal route. Blood and organs were collected after subsequent infection.* represent Adjuvant: GLA-SE, Glucopyranosyl lipid adjuvant–stable emulsion.
The primary outcome of this immunization study was whether mice developed severe manifestations of leptospirosis after lethal challenge infection (105 organisms of a low passage (P3) with L. interrogans serovar Canicola strain LOCaS46 strain, which has a median lethal dose LD50 <100 (Llanos Salinas et al., 2020). Mice were euthanized and considered having arrived at a severe disease endpoint if they developed severe manifestations after a challenge infection as defined by weight loss of >15% from the beginning of the experiment or if they were unable to groom, eat, drink, or developed severe lassitude/hunching. The secondary outcomes were 1) quantitative bacterial load in the liver and kidney as measured by quantitative real time PCR, and 2) antibody responses measured by ELISA and Western immunoblots.
No mouse developed severe disease after the immunization protocol. Mouse groups receiving PBS (G-I) plus adjuvant or the ricin B-domain RBL1 [t3490, (G-II)] plus adjuvant showed a modest decrease body weight after challenge infection but had to be euthanized on days 6 and 5, respectively, because of severe illness manifested by lethargy and inability to feed/drink. Vaccination with Full length VM proteins, either a mix of 5 (G-III) or a mix of 2 (G-IV), prevented all observable clinical illness (Figure 3A). This observation indicates that protection from severe leptospirosis required full length VM proteins.
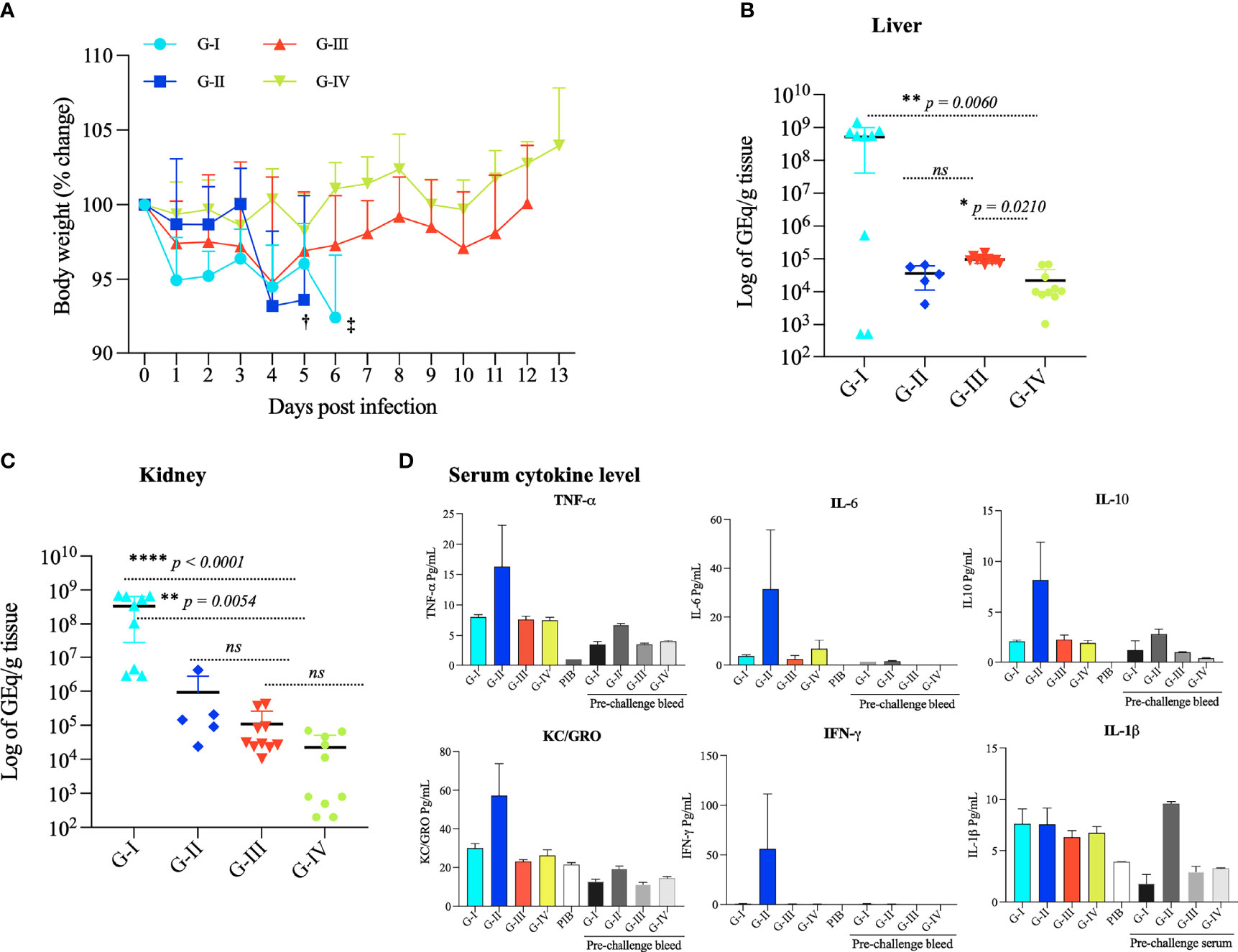
Figure 3 Body weight change, bacterial load, and pro-inflammatory cytokine response of mice challenged with L. interrogans serovar Canicola. (A) Mouse body weight (% change) was recorded from 0 day to 13 days upon infection; concurrent assessment of clinical status (grooming, eating, drinking, energy level) was also observed. G-I and G-II mice were sacrificed at 6th and 5th day (‡ and †,). Statistical analysis was performed to determine statistical significance in body weight between the PBS control and vaccinated groups using two-tailed unpaired, Mann-Whitney T-Test. p values: VM mix vs PBS, p - 0.0152 *: VM unlabeled vs PBS, p – 0.0005*: VM unlabeled vs VM mix, p < 0.0001 ****: t3490 vs PBS, p - 0.3869, ns. Error bars indicate the standard error. Total genomic DNA was extracted from the kidney (B) and liver (C) and analyzed by qPCR performed in duplicates with lipL32 primers and SYBER Green probes to quantify leptospiral tissue load. Statistical analysis was performed using the Kruskal–Wallis test and Dunn’s multiple comparisons test. p < 0.0001 was considered significant. (D) Pro-inflammatory cytokine response in pooled serum samples from each group: G-I (PBS control), G-II (t3490), G-III (mix of 5 VM proteins), and G-IV mice (mix of 2 VM proteins) pre-challenge and post-challenge were used to measure the levels of TNF-α, IL-6, IL10, KC/GRO, IFN-γ and IL-1β by V-PLEX Proinflammatory Panel 1 Mouse Kit (Meso Scale Discovery, MD, USA), an immunoassay based on electrochemiluminescence. PIB denotes pre-immunized bleed. *p < 0.05, **p < 0.001, ****p < 0.0001.
Immunization With rVM Proteins Significantly Reduced Bacterial Load in the Liver and Kidney
The leptospiral load of Leptospira in the liver and kidney in the four experimental groups was quantified by qPCR. After challenge infection, the three groups immunized with recombinant proteins plus adjuvant (G-II, G-III and G-III) had ~103-104 -fold fewer genome equivalents (Geq) per gram of tissue in the liver and kidney (Kruskal-Wallis test, ANOVA result: liver p < 0.0001, kidney p = 0.0003) compared to the PBS control group (G-I) (Figures 3B, C). Dunn’s multiple comparisons statistical test with control group PBS (G-I), VM mix (G-III) p = 0.0054, and VM unlabeled protein (G-IV) p < 0.0001 confirmed this statistically significant difference.
Immunization With t3490 Led to Severe Disease Caused by Pro-Inflammatory Cytokines Despite Significantly Reducing Bacterial Load in the Liver and Kidney
To determine whether immunization with the first highly conserved ricin B-like domain (RBL1) would confer protection from lethal challenge and as a control for the Full length VM protein LA3490, E. coli-produced recombinant RBL1 domain (truncated 3490, t3490) was produced and purified using identical procedures as for full length LA3490, and used for the immunization study. Surprisingly, mice (G-2) immunized with t3490 developed accelerated clinical disease after challenge infection, yet had decreased bacterial load in the liver and kidney (Figures 3B, C). Disease enhancement in G-2 was associated with high levels of TNF-α, IFN-γ, IL-6, IL-10, and the chemokine KC/GRO compared to the PBS and Full length protein recipient groups (Figure 3D).
Antibody Profile and Cross-Reactivity of Mice Response to PF07598 (VM) Proteins Pre – and Post– Challenge
To determine whether mice immunized with VM proteins developed an IgG antibody response, sera from pre-and post-immunized mice were collected and antibody profiles were examined by ELISA using all 6 antigens used in the study (Figure 4A). Control group (G-I) and pre-immunized sera did not show detectable IgG antibody against any of the VM antigens. The antibody response against t3490 antigens was observed in sera from t3490 immunized mice (G-II) and cross-reactivity was seen with LA3490 (p = 0.0010) and LA1402 (p = 0.0010) antigens.
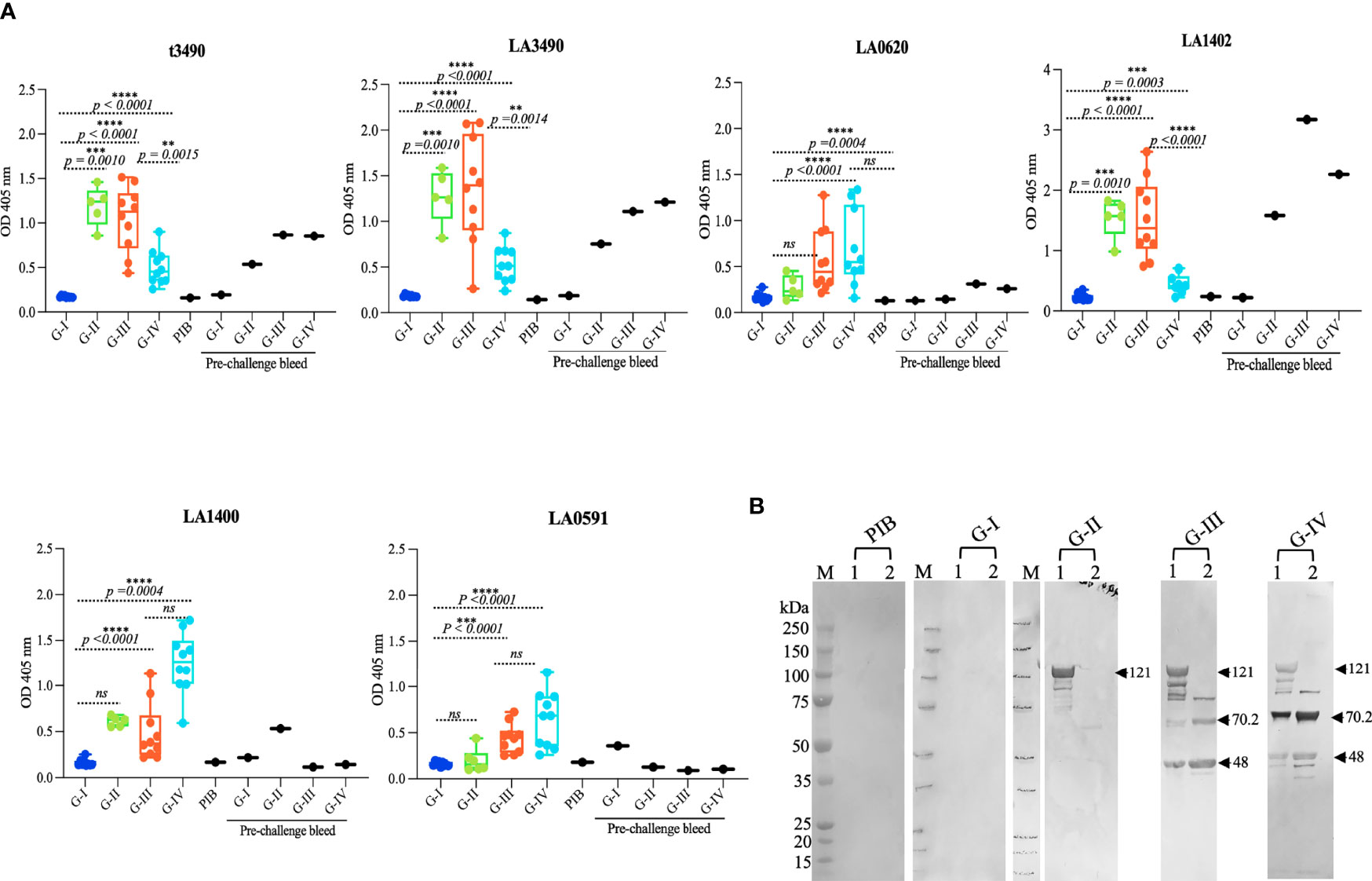
Figure 4 IgG responses to recombinant VM protein immunization. (A) Antibody titers were measured in each study group pre-and post-challenge against individual VM proteins in triplicate using ELISA. Each data line represents the average IgG response of each animal (n-10). Box and whiskers plots represent the antibody titers against t3490, LA3490, LA0620, LA1402, LA1400, and LA0591, respectively. The four-study groups include G-I: PBS, G-II: t3490, G-III: VM mix and G-IV: VM unlabeled. The box boundaries indicate the median and interquartile ranges and the whiskers denote the maximum and minimum values. Statistical analysis was performed by t-test and the non-parametric, unpaired, two tailed Mann Whitney test p < 0.0001 values were considered significant. (B) Aliquot from immunized recombinant purified VM proteins were run in 4 ± 12% SDS-PAGE then transferred to nitrocellulose membrane for Western blot analysis. The membrane was probed probed with pooled sera (1:100 dilution) collected post-challenge. PIB denotes pre-immunized bleed, served as control. VM proteins were recognized by sera from G-II, G-III, and G-IV. Lane 1 shows VM mix protein (LA3490, LA0620, LA1402, LA1400 and LA0591) and Lane 2 shows VM unlabeled proteins (LA1400 and LA0591). Arrows shows expected size of VM proteins. M represents molecular weight markers. **p < 0.05, ***p < 0.001, ****p < 0.0001.
Sera from the VM mix-immunized mice (G-III) reacted with all VM antigens tested [t3490, LA3490, LA0620, LA1402, LA1400, and LA0591, (p < 0.0001)]; highest titers were seen against LA3490 and LA1402 antigens. The antibody responses against each antigen in the VM mix antigen group [t3490 (p = 0.0015), LA3490 (p < 0.0001), LA0620 (p = 0.0004), LA1402 (p < 0.0001), LA1400 (p = 0.0003), LA0591 (p < 0.0001)] were observed with sera from VM unlabeled group (G-IV) and highest titer was detected with LA1400, LA0591, and LA0620 antigens. Antibody responses against t3490, LA3490, and LA1402 antigens were also observed in post-immunized, pre-challenge mice. Pre-challenge antibody titers to LA1400, LA0591, and LA0620 were lower than post-infection titers after challenge with live L. interrogans serovar Canicola. Further experimental investigation of the direct effect of VM proteins and their immune profiling in vivo is warranted. Despite having >90% amino acid similarity, each VM proteins showed unique reactivity with pre-and post-challenge sera and may well have different in vivo function. The differences in the reactivity of VM proteins indicates differences in immunogenicity and because of high level of amino acid similarity, they cross-react with pre-and post-challenge sera. Generation of VM protein-specific monoclonal antibodies and identification of protective epitopes would help to distinguish the roles of, and mechanisms by, which different VM proteins contribute to leptospirosis pathogenesis.
Cross-reactivity was confirmed by Western immunoblot analysis, probing recombinant VM proteins immobilized on nitrocellulose membrane with pooled sera from immunized animals (Figure 4B). The pre-bleed sera and PBS control (G-1) group did not show reactivity with a cocktail of VM mix and VM unlabeled recombinant antigens (5 and 2 proteins, respectively). Sera from t3490 immunized mice showed significant antibody titers against t3490 antigens (not shown) and cross-reacted with Full length VM proteins but faintly with LA1400, and no reactivity with LA0591, which lacks N-terminal, ricin B-domain, and suggests that t3490 only cross reacts with the epitope shared at N-terminal region of VM proteins. Sera from the VM mix group (G-III) cross-reacted with all five antigens and the reactivity pattern was consistent with each. The reactivity of LA1400 with sera from the VM unlabeled group (G-IV) was highest among all the VM proteins and in the same cocktail lot of VM antigens immunized to G-III and G-IV mice. The finding that high titer antibodies against the LA1400 antigen (as determined by both ELISA and Western blot) were induced in the VM unlabeled group sera (G-IV) suggests that LA1400 elicits the strongest humoral immune response in mice compared to other VM proteins and may be responsible mediating protective immunity. These data do provide strong confidence, however, that one or more of these VM proteins mediate the pathogenesis in this animal model. Future optimization of which VM proteins should be used for vaccination based on these observations is supported by these data.
VM Protein Expression in In Vitro and In Vivo and Cross-Reaction Among Pathogenic Serovars
Protein extracts from L. interrogans serovar Lai, Canicola, Copenhageni, and non-pathogenic strain L. biflexa serovar Patoc induced with and without 120 mM NaCl were probed on Western blots using polyclonal anti-LA3490 antibodies. Native VM protein expression was seen at the expected size of ~70 kDa molecular weight by pathogenic serovars Lai, Canicola, and Copenhageni but not with serovar Patoc (a negative control given the absence of PF07598 gene family members in this saprophytic species) (Figure 5A).
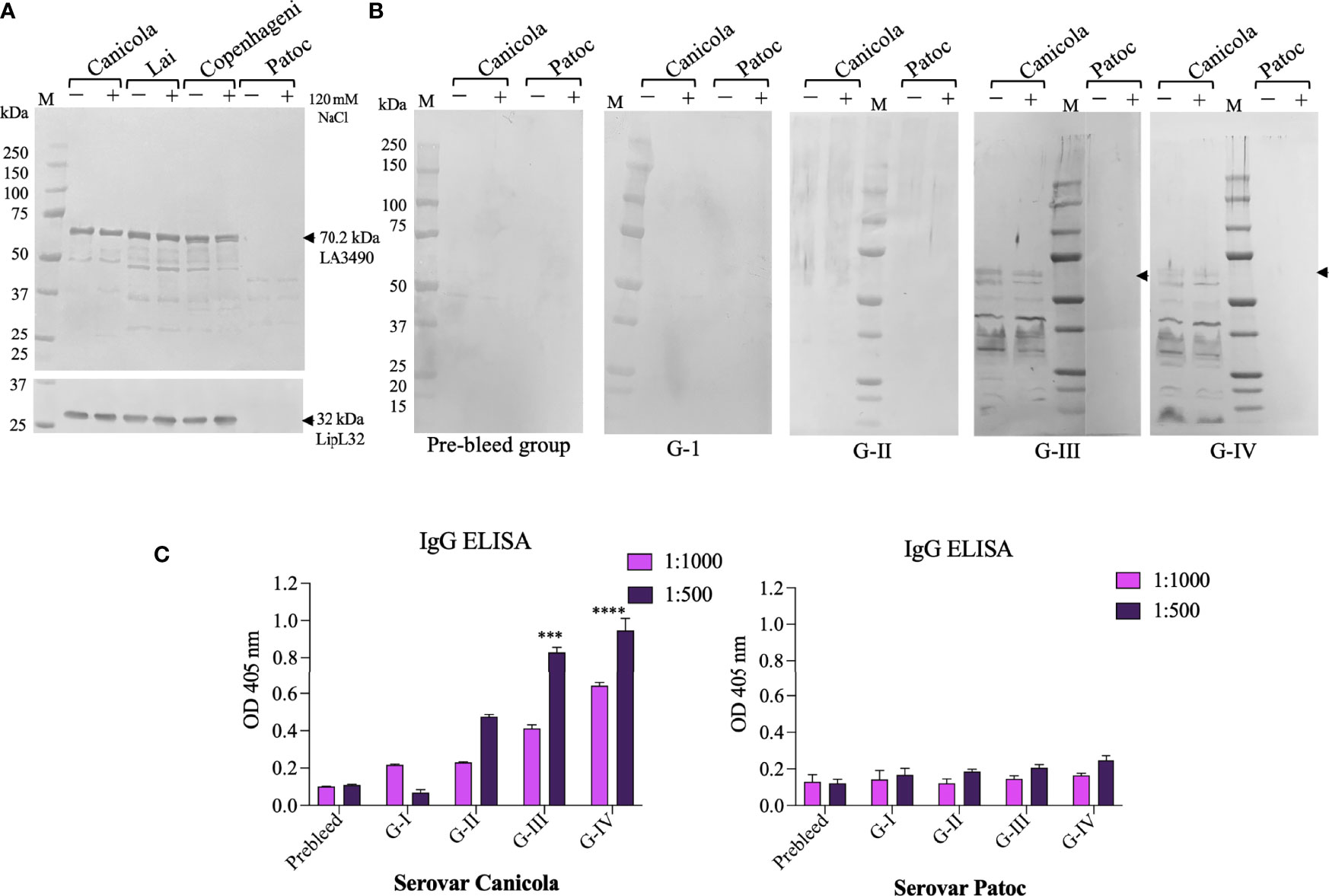
Figure 5 In vitro and in vivo recognition of VM proteins in Leptospira cell free lysate by sera from immunized mouse groups. Pathogenic L. interrogans serovars Canicola, Lai, Copenhagni, and non-pathogenic L. biflexa serovar Patoc were grown in conditional EMJH medium which was induced with 120 mM NaCl for 4 h in log phase and unconditional EMJH medium and cells were harvested. Cell-free lysates were analyzed by 4-12% SDS-PAGE, then transferred to a nitrocellulose membrane for Western blot analysis. (A) The membrane was probed with polyclonal LA3490 antibodies (1:2,000 dilution) and LipL32 monoclonal antibody (1:10,000) which was served as loading control. (B) The other set of membrane were probed with pooled sera (1:100 dilution) collected before immunization (pre-bleed) and after challenge Group I (PBS+ adjuvant), Group II (t3490), Group III (VM Mix) and Group IV (VM Unlabeled. (A, B) Leptospira grown in EMJH medium without addition of NaCl, represented by minus (–), and Leptospira grown in EMJH medium to log phase, at which time 120 nM NaCl was added, represented by plus (+). Arrows indicate the expression of 70.29 kDa native VM proteins. (C) Anti-Leptospira immunoglobulins generated against with serovars Canicola after experimental infection of C3H/HeJ suspectable mice. Whole cell IgG ELISA were performed with pre-bleed and sera from immunized mice post-challenge. Serovar Patoc served as negative control. ***p < 0.001, ****p < 0.0001.
To determine whether immunization with a limited set of VM proteins leads to broadly cross-reactive anti-VM protein antibodies, in vivo VM protein expression and cross-reactive serovar immune profiles was inferred by Western immunoblot analysis using cell-free protein extracts from Leptospira interrogans serovar Canicola and the non-infectious saprophyte L. biflexa serovar Patoc. Antibodies from the immunized groups showed antibodies against serovar Canicola recognized the predicted size of VM proteins (~70 kDa), suggesting in vivo VM protein expression during challenge infection. This antibody reactivity also reacted with post-challenge G-III and G-IV sera. However, reactivity was not seen with the negative control, serovar Patoc cell-free lysate (Figure 5B). We observed lower molecular weight reactive proteins detected with sera from G-III and G-IV, suggesting the possibility that VM proteins undergo proteolytic processing (Figure 5B). Further study is warranted to determine whether these low molecular weight proteins play a role in leptospiral pathogenesis.
We quantified IgG antibody profiles against homologous and heterologous VM proteins. Cell-free protein extracts from L. interrogans serovar Canicola and the non-pathogenic strain L. biflexa serovar Patoc were used as solid phase antigen adsorbed to ELISA plates. ELISA confirmed reactivity of serovar Canicola with sera from the VM mix (G-III) and VM unlabeled (G-IV) post-challenge mouse groups. L. interrogans serovar Lai-encoded VM proteins that were used to immunize the G-III and G-IV groups cross-reacted with lysates of serovar Canicola. Notably, orthologs of VM protein are highly conserved in L. interrogans serovars and consistent with this observed cross reactivity. Non-pathogenic serovar Patoc did not cross-react with sera from control group and immunized mouse group mice either pre- or post-challenge (Figure 5C).
Discussion
Mechanisms by which pathogenic Leptospira cause severe disease has remained elusive ever since the initial description of the etiology of leptospirosis (Inada et al., 1915; Noguchi, 1917). Although historically, the nomenclature of Leptospira has been confusing, recent genomic and molecular approaches have clarified the relationships among species and serovars. Of high importance is the discovery that the PF07598 gene family is present only in pathogenic, Group 1, Leptospira and expanded in the most pathogenic species, L. interrogans, as well as in L. kirschneri and L. noguchii. Severe human disease is primarily attributed to infection by serovars belonging to L. interrogans; such data are limited because of insufficient isolates obtained from cases of severe leptospirosis that enable definitive identification of infecting Leptospira. Because current gene knockout approaches to Leptospira remain limited, especially as applied to multi-gene families, we used an immunological approach to demonstrate whether the leptospiral PF07598 gene family-encoded VM proteins might be virulence factors contributing to severe leptospirosis disease manifestations in a mouse model. The data presented here support the hypothesis that VM proteins have central importance as virulence factors in the pathogenesis of severe leptospirosis.
Vaccination of C3H/HeJ mice with as few as two L. interrogans serovar Lai VM proteins (G-IV, LA1400 and LA0591) but as many as five (G-III, LA1400, LA0591, LA3490, LA0620, and LA1402) protected mice from any clinical manifestations of disease and led to ~3-4 log10 reduction in bacterial load in the liver and kidney, two key organs in pathogenesis of leptospirosis and transmission of Leptospira, respectively. Previous data indicate that all PF07598 gene family members are variably upregulated in the hamster model of acute, severe leptospirosis (Lehmann et al., 2013). We chose the specific VM protein antigens for groups G-III and G-IV based on previously data describing those with the highest and lowest expression in vivo (Lehmann et al., 2013). The present findings suggest that VM protein vaccination with a minimum complement of cross-reactive VM proteins might confer protective immunity but, as of yet, we do not know whether LA1400 and LA0591 are both needed as immunogens. Curiously, we found that post-immunization/pre-challenge sera from G-IV heterologously cross-reacted with highest titers against with LA1402 and LA3490 despite low titers against their homologous proteins. LA1400, an ancestral VM protein in Group 1 pathogenic Leptospira belonging to cluster A (Fouts et al., 2016), has 2 N-terminal, tandemly repeated ricin B-like lectin domains (RBLs), and a C-terminal toxin domain (CTD). LA0591 has a CTD but lacks RBLs. These domains might a priori be expected to cross react most strongly with homologous VM proteins but the experimental data indicate that heterologous cross-reactivity was instead found to be strongest. Further experiments are in progress to determine further whether immunization with either of these proteins alone, concatenated, or isolated subdomains of various VM proteins, or even one more full length VM proteins such as LA1402 and LA3490, might confer pan-leptospiral immunity. A likely scenario is that the general cross-reactivity to VM proteins induced by vaccination with LA1400 and LA0591 mediates protection against lethal challenge infection and tissue colonization. This possibility is suggested by bioinformatic analysis indicating that VM proteins are highly conserved at the amino acid level within L. interrogans and is supported experimentally (Supplementary Figure 1) (Chaurasia et al., 2022).
While immunization of leptospirosis disease-susceptible C3H/HeJ mice (Viriyakosol et al., 2006) with Full length leptospiral VM proteins protected against severe disease, vaccination with an isolated RBL, t3490, and a recombinant protein containing only the N-terminal ricin B domain (G-II), led to disease enhancement while simultaneously reducing the bacterial load in the liver and kidney. Multiplex cytokine analysis on serum showed that Group II mice had unique elevations in pro-inflammatory cytokine markers (IL-ß, IL-6, IL-10, IFN-γ, TNF-α and KC/GRO (Wolpe et al., 1989) (neutrophil chemoattractant related to IL-8 in rodents) which suggest that this cytokine storm might lead to mice death (Figure 3D). The mechanism by which RBD-domain-induced immune enhancement leads to severe disease is unclear. We speculate that one potential mechanism by which t3490 immunization led to disease enhancement could be the induction of antibodies to the N-terminus RBLs of Leptospira-secreted VM proteins that, in vivo, carry the Full length protein to the pro-inflammatory pathway in Fc receptor-containing cells but this hypothesis requires experimental testing. Nonetheless, cross-reactive antibody generated against RBD in G-II immunized mice did not protect against severe disease. These observations suggest that the N-terminal RBD alone should not be used for a VM protein-based leptospirosis vaccine studies. Further work to study RBD-mediated immune enhancement is needed.
In the present study, ELISA and Western blot analysis of post-vaccination sera on using recombinant VM proteins and osmolarity-induced in vitro cultivated L. interrogans serovar Lai indicates that vaccination results in both homologous and heterologous VM protein recognition associated with protective immunity. These experimental results confirm bioinformatic predictions of cross-reactivity of polyclonal antisera for VM proteins within the genus L. interrogans. Further work to confirm protection against challenge infection in rodent models by other L. interrogans serovars is essential. Cross-species protection experiments after VM protein vaccination against challenge with virulent isolates of closely related L. kirschneri and L. noguchii, the other group 1 highly pathogenic Leptospira species (Fouts et al., 2012; Lehmann et al., 2013) are planned. Cross-species protection experiments after homologous, or heterologous VM protein vaccination followed by challenge infection with virulent isolates of other group 1 pathogens such as L. borgpetersenii, which have few PF07598 paralogs in their genomes, will contribute to determining which VM proteins might be appropriate for further development into a pan-leptospirosis vaccine.
A serovar-independent pan-leptospirosis vaccine that confers protection against leptospirosis is a major priority in the leptospirosis field (Ko et al., 2009; Wunder et al., 2021). Various inactivated whole bacterial cell-based vaccines (bacterins) are serovar-specific and limited to animal use, where this legacy technology remains incompletely effective. Subunit vaccines, and more recently a spontaneously-arising attenuation mutant of L. interrogans serovar Copenhageni (Wunder et al., 2021) have been proposed amidst the search for pan-leptospirosis vaccine candidates (Govindan et al 2021.; Haake et al., 1999; Coutinho et al., 2011; Conrad et al., 2017; Techawiwattanaboon et al., 2019; Haake and Matsunaga, 2020; Teixeira et al., 2020; Phoka et al., 2021; Rodrigues de Oliveira et al., 2021). Bacterins are limited from wider use because of adverse effects and suboptimal efficacy, including the lack of durable protective and sterilizing immunity (Levett, 2001; Techawiwattanaboon et al., 2019; Felix et al., 2020; Zaugg and Ottiger, 2021).
The present study has several limitations. First, the clinical endpoints were visual and for ethical reasons did not use death as an endpoint. Second, the current experiments did not include histopathological analysis, nor did we attempt to isolate viable Leptospira from the different vaccine groups. Third, while challenge infection used a leptospiral serovar (L. interrogans serovar Canicola serogroup Canicola) from a different serogroup that the one from which the VM protein sequences were derived (L. interrogans serovar Lai, serogroup Icterohaemorrhagiae (Masuzawa et al., 1988), challenge infections using other serovars/species were not performed. This gap limits generalizability at this time but will be addressed in pending experiments. Fourth, vaccine dosing regimens—indeed the minimal set of protective VM protein antigens—have yet to be optimized. Finally, challenge infections were performed using the intraperitoneal route which is not the natural route of infection (Coutinho et al., 2014). While protective immunity in the present experiments is presumed to be mediated by antibody against Leptospira-secreted VM proteins, further experiments, including passive immunization and mapping of protective epitopes requiring monoclonal antibodies or putative epitope-specific polyclonal sera are needed. Nonetheless, the present report provides a strong basis for such experiments given the protective immunity induced by vaccination with a subset of L. interrogans VM proteins against lethal challenge infection. The immunization strategy to induce anti-VM protein antibodies validates the VM protein role in mediating leptospirosis pathogenesis.
Methods
Bacterial Cultures
Leptospira interrogans serovar Canicola strain LOCaS46 were grown at 30°C in liquid Ellinghausen-McCullough-Johnson-Harris (EMJH, BD Biosciences, USA) (Ellinghausen and McCullough, 1965). Leptospira were grown under conditions mimicking the in vivo host environment known to induce virulence gene expression in vitro (Matsunaga et al., 2005). Briefly, mid-logarithmic cultures in unmodified EMJH medium were harvested by centrifugation at 18,514 g. Pelleted cells were washed twice with 1X phosphate buffered saline, resuspended in liquid EMJH medium supplemented with 120 mM NaCl, and then incubated at 37°C for 4 h (Sigma Aldrich, USA). The LD50 of LOCaS46 strain has a median lethal dose LD50 <100 (Llanos Salinas et al., 2020).
Chemically competent E. coli strain DH5α (New England Biolabs, USA) was used for gene cloning, and strain SHuffle®T7 competent E. coli cells (New England Biolabs, USA) was used for protein expression and purification. E. coli were grown in Luria-Bertani (LB) medium (BD Biosciences, USA) supplemented with 100 μg/mL ampicillin (Sigma-Aldrich, USA).
Preparation of Leptospiral Whole Cell Lysate
The L. interrogans serovars Lai, Canicola, Copenhageni, and the non-pathogenic serovar L. biflexa serovar Patoc were grown in liquid EMJH medium and harvested by centrifugation at 18,514 g for 10 mins. Cells were washed twice with 1X PBS pH 7.4 and pellets were resuspended in 5 mL/gram of BugBuster® Protein Extraction Reagent (Sigma-Aldrich, USA) containing “Protease Inhibitor Cocktail with EDTA” (Roche, USA). Cell lysates were incubated on a rotating mixer for 15 minutes at room temperature. Insoluble cell debris was removed by centrifugation at 18,514 g for 20 minutes at 4°C. Supernatant were stored at -20°C until analysis.
Computational Biology
N-and C-terminal amino acid sequences (LA3490, LA0620, LA1402, LA1400, and LA0591) of the PF07598 family were aligned using MAFFT (Multiple Alignment using Fast Fourier Transform) with using L-INS-i (accuracy-oriented) and visualized in Jalview v2.11.5 (https://www.jalview.org). The originally deposited LA1400 sequence was found to be incomplete in that it lacked sequence encoding the first 54 amino acids of the complete encoded protein. This conclusion was based on the use of clustal analysis to compare the amino acid sequences of L. interrogans serovar Lai LA1400 to LIC12340, the LA1400 ortholog in L. interrogans serovar Copenhageni strain FioCruz L1-130 (Supplementary Figure 2). The recombinant protein referred to in the present work as LA1400 is comprised of amino acids 31 to 54 derived from LIC12340, then LA1400-derived amino acids from position 55 to the end.
Animals and Ethics Statement
All the animal experiments performed in this study were approved by the institutional Animal Care and Use Committee at Yale University (Protocol 2022-20243). All animal experiments were performed under Animal Biosafety Level (ABSL-2) conditions. All animals were under the supervision of an attending veterinarian and procedures were used to reduce pain and distress.
Three-week-old, specific pathogen-free, female C3H/HeJ mice were purchased from the Jackson Laboratories (ME, USA) and were maintained in a specific-pathogen-free environment at Yale Animal Resources Center. The mice were housed in individually ventilated microisolator cages with sterile, absorbent beddings changed twice weekly. The animals were fed and watered throughout the course of the experiment. Following L. interrogans serovar Canicola challenge, mice were weighed and monitored twice daily until the final endpoint. They were observed for loss of appetite, severe lassitude, difficulty in breathing, prostration, ruffled fur, and weight loss of 10%. Mice with these manifestations were euthanized by CO2 according to AAALAC/AVMA-approved procedures and considered to have met the endpoint of severe/lethal leptospirosis.
Plasmid Constructs and Cloning
Synthetic E. coli codon-optimized genes were constructed by Gene Universal (https://www.geneuniversal.com) consisting of either the complete PF07598 genes encoding NCBI locus tag LA3490 (Uniprot: Q8F0K3), LA0620 (Q8F8D7), and LA1402 (Q8F6A7) from serovar Lai, and locus tag LIC12340 (Q72PX7) (Lai ortholog: LA1400), and LIC12985 (Q72N53) (Lai ortholog: LA0591) from serovar Copenhageni. Coding sequence, minus the predicted signal peptide or truncated 3490, an N-terminal domain, was synthesized and cloned into pET32b (+) (Gene Universal Inc., USA). LA3490, LA0620, LA1402, and t3490 were linked to mCherry (AST15061.1) via a glycine-serine hinge (Gly4Ser)3 and cloned into pET32b (+) (Gene Universal Inc., USA) between enterokinase cleavage sites for convenient removal of the mCherry fluorescent tag. Full-length LA1400 and LA0591 constructs were made without the mCherry fusion (Figure 1B, C). Prior to use, the sequence and the orientation of the genes in the constructs were verified by restriction digestion and sequencing.
Expression and Purification of Recombinant Soluble PF07598 Antigens
Recombinant PF07598 protein constructs were expressed in SHuffle®T7 competent E. coli cells (New England Biolabs, USA). Transformants were sub-cultured into Luria-Bertani (LB) medium containing 100 µg/mL ampicillin. Expression of PF07598 proteins were induced at OD of 0.6 via addition of 1 mM isopropyl-β-D-thiogalactoside (IPTG; Sigma-Aldrich, USA) and allowed to incubate at 16°C and 250 rpm for 24 h. Upon induction, cells were harvested and pellets were lysed in CelLytic™ B (Cell Lysis Reagent; Sigma-Aldrich, USA) containing 50 units benzonase nuclease (Sigma-Aldrich, USA), 0.2 µg/mL lysozyme, non-EDTA protease inhibitor cocktail (Roche, USA) plus100 mM PMSF (Sigma-Aldrich, USA) for 30 minutes at 37°C. Supernatants and pellets were separated and then analyzed by 4-12% bis-tris sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentrations were determined by BCA assay (Bio-Rad, USA).
Recombinant PF07598 fusion and without fusion proteins were purified using a 5 mL pre-packed Ni-Sepharose AKTA Hi-TRAP column (GE Healthcare, USA) pre-equilibrated with a buffer containing 100 mM NaH2PO4, 10 mM Tris-HCl, 25 mM imidazole, and pH 8.0. PF07598 proteins bound to Hi-TRAP column were then eluted in the presence of 500 mM imidazole, and pH 8.0. Eluates were pooled, concentrated via a 10 kDa Amicon® Ultra centrifugal filter, and further dialyzed overnight against 1X PBS (pH 7.4) with gentle stirring (350 rpm) at 4°C (10 kDa cutoff, Slide-A-Lyzer, Thermo Scientific™, USA). Purified recombinant PF07598 proteins were resolved in SDS-PAGE, verified by immunoblotting with mouse anti-His monoclonal-ALP conjugate (1:2,000 dilution; Santa Cruz Biotechnology, USA). Aliquot for boosters and SDS-PAGE were prepared from the single preparation and stored at −80°C to prevent repeated freeze-thawing.
Animal Immunization, Leptospira Challenge and Sample Collection
C3H/HeJ mice were immunized via intramuscular (IM) route with recombinant PF07598 proteins (Viriyakosol et al., 2006). GLA–squalene–oil-in-water emulsion adjuvants (0.25 mg/mL) were procured from Infectious Disease Research Institute (IDRI), Seattle, WA, USA (http://www.idri.org). Immediately before injections, adjuvant was added to the recombinant protein or PBS to a final volume of 100 μL and mixed by brief vortexing (Patra et al., 2015).
Mice were divided into four groups; G-I served as negative control and was injected with 1X phosphate buffer saline (PBS) mixed with adjuvant (EM082; 5 µg GLA–squalene– oil-in-water emulsion). Similarly, G-II (t3490), G-III [VM mix, (LA3490, LA0620, LA1400, LA1402 and LA0591] and G-IV [VM unlabeled, (LA1400 and LA0591] were immunized with 25 μg total antigen in equimolar ratio along with adjuvant (5 µg GLA–squalene–oil-in-water emulsion) followed by two injections of 25 μg of total antigen at 3-week intervals (Figure 2). Immunized mice were bled two weeks after the final immunization and to smooth out individual differences with groups, serum samples were pooled and measured for anti-VM antibodies in a serum known as pre-challenged bleed. All the groups were experimentally infected by intraperitoneal (IP) injection with 1x105 organisms of a virulent, low passage isolate of L. interrogans serovar Canicola, strain LOCaS46, kindly provided by Dr. Alejandro de la Peña Moctezuma. Mice that survived infection were euthanized 13 days after infectious challenge. Blood was collected by terminal cardiac puncture and serum was isolated from whole blood. Serum was allowed to clot at room temperature and stored overnight at 4°C. Samples were then centrifuged at 11, 292 g for 15 minutes at 4°C. Serum was collected and stored at -80°C. Organs were collected and stored in RNALater at 4°C. Kidney and liver tissues were used for quantification of L. interrogans by quantitative PCR (qPCR).
Evaluation of PF07598 Proteins-Induced Immunity by ELISA
Serum antibody responses to recombinant PF07598 proteins in immunized groups were quantified by ELISA (Chaurasia et al., 2018). Briefly, PF07598 antigens (LA3490, LA0620, LA1402, LA1400, and LA0598, respectively) in 100 μL of bicarbonate/carbonate coating buffer were coated (250 ng) in 96-well microtiter ELISA plate (Corning, USA) and incubated at 4°C for overnight. Each set of antigens were incubated with pre–and post–immunized serum group (Group I–IV, 1:1000) for 1 h followed by goat anti-mouse IgG (Fc specific)–alkaline phosphatase conjugate (1:5000; KPL, USA) for 1 h, washed thrice with TBST and developed with p-Nitrophenyl phosphate (1-Step™ PNPP Substrate Solution; KPL, USA). The reaction was stopped with 2 M NaOH, and absorbance was read at 405 nm using a SpectraMax® M2e Microplate Reader (Molecular Devices, USA). For whole cell ELISA, plate was coated with 500 ng/well cell free lysates. The controls included pre-bleed, pre-immunized serum samples, and antigen and antibody blanks.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Immunoblot Analysis
SDS-PAGE was done according to the method of Laemmli (1970). Immunoblot analysis was performed to determine whether sera from immunized animals recognize recombinant or native leptospiral PF07598 proteins. Purified recombinant PF07598 proteins or leptospiral whole cell lysate (120 mM NaCl induced and without induced) were transferred to nitrocellulose membranes and blocked for 2 h with 5% nonfat dry milk dissolved in 1X TBST buffer (AmericanBio, USA). The membrane was incubated with pooled sera from immunized groups (Group I–IV, 1:100) and controlled as pre-bleed and pre-immunize bleed for overnight at 4°C on rocker. They were probed with goat anti-mouse IgG (Fc specific)–alkaline phosphatase conjugate (1:5,000; KPL, USA) for 2.5 h, washed thrice with TBST, and developed with p-Nitrophenyl phosphate (1-Step™ PNPP Substrate Solution; KPL, USA). Monoclonal LipL32 antibody served as loading control (1:10,000 dilution).
Quantitative PCR
DNA was extracted by dicing 40 ± 50 mg of kidney and liver tissues and suspending in 500 μL of 1X PBS and all work was done under positive pressure in a location separate from the handling of Leptospira and PCR products in order to reduce risk of cross-contamination. Following tissue homogenization, total genomic DNA was extracted from the equivalent of 25 mg tissue using the DNeasy Blood and Tissue Kit (Qiagen, USA) per manufacturer’s instructions and eluted in 50 μL of elution buffer. L. interrogans serovar Canicola at a density of 2x107 leptospires/mL grown in 5 mL of EMJH culture medium. Cells were harvested and DNA was extracted for standard curves using the same DNeasy Blood and Tissue Kit (Qiagen, USA).
The concentration of eluted DNA was determined using a NanoDrop Spectrophotometer ND-1000 (NanoDrop Technologies, USA). All DNA samples were kept at -80°C until use. Serial dilution (1x10° to 1x107 genomic equivalents (GEq)/5 μL) of DNA was prepared and L. interrogans Serovar Canicola genome was quantified by qPCR using 2X iQ5 SYBR Green supermix (Bio-Rad, CA, USA) with 5 pmol forward (5’-TCT GTG ATC AAC TAT. TAC GGA TAC-3’) and reverse (5’-ATC CAA GTA TCA AAC CAA TGT GG -3’) LipL32 primer. Four microliters of standard or sample DNA was added to 10 μL PCR mix and the reaction was subjected to amplification in the CFX96 Real-time PCR Detection System (Bio-Rad, USA) using the following program: 3 min at 95°C, 0.10 min at 95°C, 0.30 min at 62°C, followed by 44 cycles at 1.00 min at 72°C then final extension 7 min at 72°C. A standard curve was generated using Bio-Rad iQCycler5 software and the number of GEq was extrapolated from the threshold cycle (CT) values. A negative result was assigned where no amplification occurred or if the CT value was greater than 3 SD+Ct. Data are presented as the number of L. interrogans GEq per gram of tissue.
Statistical Analysis
All experiments were performed in triplicate and repeated twice. The Kruskal-Wallis test was used to determine significant differences in the number of bacteria in the kidney or liver among the survivors from different immunization groups. The results were analyzed by the non-parametric Mann–Whitney test to determine significant differences between individual groups and were considered as statistically significant when p < 0.05, p < 0.001 p < 0.0001. All analyses and graphs were generated using Graph Prism version 8 (GraphPad Software, Inc., USA).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Yale Institutional Animal Care and Use Committee.
Author Contributions
Conceptualization: RC and JV. Data curation: RC, XG, and JV. Formal analysis: RC, XG, GD, and JV. Funding acquisition: JV. Investigation: RC, AS, XG, and JV. Methodology: RC, AS, XG, and JV, Resources: GD and JV. Supervision: GD and JV. Visualization: RC, AS, XG, and JV. Writing - original draft: RC. Writing - review & editing: RC, AS, XG, GD, and JV. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the United States Public Health Service through National Institutes of Health, NIAID grants R01AI108276 and U19AI115658.
Conflict of Interest
The work reported here has been filed in patent applications from Yale University. JV and spouse have an equity interest in LeptoX BioPharma, Inc. which may have a future interest in licensing this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Dr. Tony Tong for help with statistical analysis, and Dr. Michael M. Matthias for discussions and technical assistance in animal work. We thank Dr. Alejandro de la Pena Moctezuma at School of Veterinary Medicine and Zootechnics, UNAM, Mexico for providing low-passage Leptospira interrogans serovar Canicola strain LOCaS46.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.926994/full#supplementary-material
References
Adler, B. (2015). Vaccines Against Leptospirosis. Curr. Topics Microbiol. Immunol. 387, 251–272. doi: 10.1007/978-3-662-45059-8_10
Balakrishnan, G., Roy, P. (2014). Comparision of Efficacy of Two Experimental Bovine Leptospira Vaccines Under Laboratory and Field. Vet. Immunol. Immunopathol. 159 (1-2), 11–15. doi: 10.1016/j.vetimm.2014.03.002
Bashiru, G., Bahaman, A. R. (2018). Advances & Challenges in Leptospiral Vaccine Development. Indian J. Med. Res. 147 (1), 15–22. doi: 10.4103/ijmr.IJMR_1022_16
Beutler, B., Poltorak, A. (2000). Positional Cloning of Lps, and the General Role of Toll-Like Receptors in the Innate Immune Response. Eur. Cytokine Netw. 11 (2), 143–152.
Bharti, A. R., Nally, J. E., Ricaldi, J. N., Matthias, M. A., Diaz, M. M., Lovett, M. A., et al. (2003). Leptospirosis: A Zoonotic Disease of Global Importance. Lancet Infect. Dis. 3 (12), 757–771. doi: 10.1016/S1473-3099(03)00830-2
Bolin, C. A., Cassells, J. A., Zuerner, R. L., Trueba, G. (1991). Effect of Vaccination With a Monovalent Leptospira Interrogans Serovar Hardjo Type Hardjo-Bovis Vaccine on Type Hardjo-Bovis Infection of Cattle. Am. J. Vet. Res. 52 (10), 1639–1643.
Bouvet, J., Lemaitre, L., Cariou, C., Scotto, M., Blain, C., Oberli, F., et al. (2020). A Canine Vaccine Against Leptospira Serovars Icterohaemorrhagiae, Canicola and Grippotyphosa Provides Cross Protection Against Leptospira Serovar Copenhageni. Vet. Immunol. Immunopathol. 219, 109985. doi: 10.1016/j.vetimm.2019.109985
Branger, C., Sonrier, C., Chatrenet, B., Klonjkowski, B., Ruvoen-Clouet, N., Aubert, A., et al. (2001). Identification of the Hemolysis-Associated Protein 1 as a Cross-Protective Immunogen of Leptospira Interrogans by Adenovirus-Mediated Vaccination. Infect. Immun. 69 (11), 6831–6838. doi: 10.1128/IAI.69.11.6831-6838.2001
Brown, R. A., Blumerman, S., Gay, C., Bolin, C., Duby, R., Baldwin, C. L. (2003). Comparison of Three Different Leptospiral Vaccines for Induction of a Type 1 Immune Response to Leptospira Borgpetersenii Serovar Hardjo. Vaccine 21 (27-30), 4448–4458. doi: 10.1016/S0264-410X(03)00439-0
Callaway, E. (2020). ‘It Will Change Everything’: Deepmind’s AI Makes Gigantic Leap in Solving Protein Structures. Nature 588, 203—204. doi: 10.1038/d41586-020-03348-4
Chaurasia, R., Marroquin, A., Vinetz, J., Matthias, M. (2022). Pathogenic Leptospira Evolved a Unique Gene Family Comprised of Ricin B-Like Lectin Domain-Containing Cytotoxins. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.859680
Chaurasia, R., Thresiamma, K. C., Eapen, C. K., Zachariah, B. J., Paul, R., Sritharan, M. (2018). Pathogen-Specific Leptospiral Proteins in Urine of Patients With Febrile Illness Aids in Differential Diagnosis of Leptospirosis From Dengue. Eur. J. Clin. Microbiol. Infect. Dis. 37 (3), 423–433. doi: 10.1007/s10096-018-3187-9
Conrad, N. L., Cruz McBride, F. W., Souza, J. D., Silveira, M. M., Felix, S., Mendonca, K. S., et al. (2017). LigB Subunit Vaccine Confers Sterile Immunity Against Challenge in the Hamster Model of Leptospirosis. PLos Negl. Trop. Dis. 11 (3), e0005441. doi: 10.1371/journal.pntd.0005441
Costa, F., Hagan, J. E., Calcagno, J., Kane, M., Torgerson, P., Martinez-Silveira, M. S., et al. (2015). Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLos Negl. Trop. Dis. 9 (9), e0003898. doi: 10.1371/journal.pntd.0003898
Coutinho, M. L., Choy, H. A., Kelley, M. M., Matsunaga, J., Babbitt, J. T., Lewis, M. S., et al. (2011). A LigA Three-Domain Region Protects Hamsters From Lethal Infection by Leptospira Interrogans. PLos Negl. Trop. Dis. 5 (12), e1422. doi: 10.1371/journal.pntd.0001422
Coutinho, M. L., Matsunaga, J., Wang, L. C., de la Pena Moctezuma, A., Lewis, M. S., Babbitt, J. T., et al. (2014). Kinetics of Leptospira Interrogans Infection in Hamsters After Intradermal and Subcutaneous Challenge. PLos Negl. Trop. Dis. 8 (11), e3307. doi: 10.1371/journal.pntd.0003307
Dellagostin, O. A., Grassmann, A. A., Rizzi, C., Schuch, R. A., Jorge, S., Oliveira, T. L., et al. (2017). Reverse Vaccinology: An Approach for Identifying Leptospiral Vaccine Candidates. Int. J. Mol. Sci. 18 (1), 158. doi: 10.3390/ijms18010158
Ellinghausen, H. C., Jr., McCullough, W. G. (1965). Nutrition of Leptospira Pomona and Growth of 13 Other Serotypes: A Serum-Free Medium Employing Oleic Albumin Complex. Am. J. Vet. Res. 26, 39–44.
Evangelista, K. V., Lourdault, K., Matsunaga, J., Haake, D. A. (2017). Immunoprotective Properties of Recombinant LigA and LigB in a Hamster Model of Acute Leptospirosis. PLos One 12 (7), e0180004. doi: 10.1371/journal.pone.0180004
Faisal, S. M., Yan, W., Chen, C. S., Palaniappan, R. U., McDonough, S. P., Chang, Y. F. (2008). Evaluation of Protective Immunity of Leptospira Immunoglobulin Like Protein A (LigA) DNA Vaccine Against Challenge in Hamsters. Vaccine 26 (2), 277–287. doi: 10.1016/j.vaccine.2007.10.029
Faisal, S. M., Yan, W., McDonough, S. P., Chang, Y. F. (2009). Leptospira Immunoglobulin-Like Protein A Variable Region (LigAvar) Incorporated in Liposomes and PLGA Microspheres Produces a Robust Immune Response Correlating to Protective Immunity. Vaccine 27 (3), 378–387. doi: 10.1016/j.vaccine.2008.10.089
Felix, C. R., Siedler, B. S., Barbosa, L. N., Timm, G. R., McFadden, J., McBride, A. J. A. (2020). An Overview of Human Leptospirosis Vaccine Design and Future Perspectives. Expert Opin. Drug Discovery 15 (2), 179–188. doi: 10.1080/17460441.2020.1694508
Fish, N. A., Kingscote, B. (1973). Protection of Gilts Against Leptospirosis by Use of a Live Vaccine. Can. Vet. J. 14 (1), 12–15.
Fouts, D. E., Matthias, M. A., Adhikarla, H., Adler, B., Amorim-Santos, L., Berg, D. E., et al. (2016). What Makes a Bacterial Species Pathogenic?:Comparative Genomic Analysis of the Genus Leptospira. PLos Negl. Trop. Dis. 10 (2), e0004403. doi: 10.1371/journal.pntd.0004403
Fouts, D. E., Torralba, M., Nelson, K. E., Brenner, D. A., Schnabl, B. (2012). Bacterial Translocation and Changes in the Intestinal Microbiome in Mouse Models of Liver Disease. J. Hepatol. 56 (6), 1283–1292. doi: 10.1016/j.jhep.2012.01.019
Govindan, P., Manjusha, P., Saravanan, K. M., Natesan, V., Salmen, S. H., Alfarraj, S., et al. (2021) Expression and Preliminary Characterization of the Potential Vaccine Candidate LipL32 of Leptospirosis. Appl. Nanosci. 2021, 1–15. doi: 10.1007/s13204-021-02097-8
Grassmann, A. A., Felix, S. R., dos Santos, C. X., Amaral, M. G., Seixas Neto, A. C., Fagundes, M. Q., et al. (2012). Protection Against Lethal Leptospirosis After Vaccination With LipL32 Coupled or Coadministered With the B Subunit of Escherichia Coli Heat-Labile Enterotoxin. Clin. Vaccine Immunol. 19 (5), 740–745. doi: 10.1128/CVI.05720-11
Haake, D. A., Matsunaga, J. (2020). Leptospiral Immunoglobulin-Like Domain Proteins: Roles in Virulence and Immunity. Front. Immunol. 11, 579907. doi: 10.3389/fimmu.2020.579907
Haake, D. A., Mazel, M. K., McCoy, A. M., Milward, F., Chao, G., Matsunaga, J., et al. (1999). Leptospiral Outer Membrane Proteins OmpL1 and LipL41 Exhibit Synergistic Immunoprotection. Infect. Immun. 67 (12), 6572–6582. doi: 10.1128/IAI.67.12.6572-6582.1999
Inada, R., Ido, Y., Hoki, R., Al, E. (1915). The Etiology, Mode of Infection, and Specific Therapy of Weil's Disease (Spirochaetosis Icterohaemorrhagica). J. Exp. Med. XXIII, 377–402.
Inada, R., Ido, Y., Hoki, R., Kaneko, R., Ito, H. (1916). The Etiology, Mode of Infection, and Specific Therapy of Weil's Disease (Spirochaetosis Icterohaemorrhagica). J. Exp. Med. 23 (3), 377–402. doi: 10.1084/jem.23.3.377
Jost, B. H., Adler, B., Faine, S. (1989). Experimental Immunisation of Hamsters With Lipopolysaccharide Antigens of Leptospira Interrogans. J. Med. Microbiol. 29 (2), 115–120. doi: 10.1099/00222615-29-2-115
Jost, B. H., Adler, B., Vinh, T., Faine, S. (1986). A Monoclonal Antibody Reacting With a Determinant on Leptospiral Lipopolysaccharide Protects Guinea Pigs Against Leptospirosis. J. Med. Microbiol. 22 (3), 269–275. doi: 10.1099/00222615-22-3-269
Ko, A. I., Goarant, C., Picardeau, M. (2009). Leptospira: The Dawn of the Molecular Genetics Era for an Emerging Zoonotic Pathogen. Nat. Rev. Microbiol. 7 (10), 736–747. doi: 10.1038/nrmicro2208
Laemmli, U. K. (1970). Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 227 (5259), 680–685. doi: 10.1038/227680a0
Lehmann, J. S., Fouts, D. E., Haft, D. H., Cannella, A. P., Ricaldi, J. N., Brinkac, L., et al. (2013). Pathogenomic Inference of Virulence-Associated Genes in Leptospira Interrogans. PLos Negl. Trop. Dis. 7 (10), e2468. doi: 10.1371/journal.pntd.0002468
Lehmann, J. S., Matthias, M. A., Vinetz, J. M., Fouts, D. E. (2014). Leptospiral Pathogenomics. Pathogens 3 (2), 280–308. doi: 10.3390/pathogens3020280
Levett, P. N. (2001). Leptospirosis. Clin. Microbiol. Rev. 14 (2), 296–326. doi: 10.1128/CMR.14.2.296-326.2001
Llanos Salinas, S. P., Castillo Sanchez, L. O., Castaneda Miranda, G., Rodriguez Reyes, E. A., Ordonez Lopez, L., Mena Banuelos, R., et al. (2020). GspD, The Type II Secretion System Secretin of Leptospira, Protects Hamsters Against Lethal Infection With a Virulent L. Interrogans Isolate. Vaccines (Basel) 8 (4), 759. doi: 10.3390/vaccines8040759
Marcsisin, R. A., Bartpho, T., Bulach, D. M., Srikram, A., Sermswan, R. W., Adler, B., et al. (2013). Use of a High-Throughput Screen to Identify Leptospira Mutants Unable to Colonize the Carrier Host or Cause Disease in the Acute Model of Infection. J. Med. Microbiol. 62 (Pt 10), 1601–1608. doi: 10.1099/jmm.0.058586-0
Martin, L. E., Wiggans, K. T., Wennogle, S. A., Curtis, K., Chandrashekar, R., Lappin, M. R. (2014). Vaccine-Associated Leptospira Antibodies in Client-Owned Dogs. J. Vet. Intern. Med. 28 (3), 789–792. doi: 10.1111/jvim.12337
Masuzawa, T., Kumagai, M., Shimizu, T., Yanagihara, Y. (1988). Classification of Leptospira Interrogans Serovar Lai Strain 017 by Using Monoclonal Antibodies. J. Clin. Microbiol. 26 (11), 2332–2337. doi: 10.1128/jcm.26.11.2332-2337.1988
Matsunaga, J., Sanchez, Y., Xu, X., Haake, D. A. (2005). Osmolarity, a Key Environmental Signal Controlling Expression of Leptospiral Proteins LigA and LigB and the Extracellular Release of LigA. Infect. Immun. 73 (1), 70–78. doi: 10.1128/IAI.73.1.70-78.2005
Midwinter, A., Faine, S., Adler, B. (1990). Vaccination of Mice With Lipopolysaccharide (LPS) and LPS-Derived Immuno-Conjugates From Leptospira Interrogans. J. Med. Microbiol. 33 (3), 199–204. doi: 10.1099/00222615-33-3-199
Murray, G. L., Morel, V., Cerqueira, G. M., Croda, J., Srikram, A., Henry, R., et al. (2009). Genome-Wide Transposon Mutagenesis in Pathogenic Leptospira Species. Infect. Immun. 77 (2), 810–816. doi: 10.1128/IAI.01293-08
Murray, G. L., Simawaranon, T., Kaewraemruaen, C., Adler, B., Sermswan, R. W. (2018). Heterologous Protection Elicited by a Live, Attenuated, Leptospira Vaccine. Vet. Microbiol. 223, 47–50. doi: 10.1016/j.vetmic.2018.07.018
Noguchi, H. (1917). Spirochaeta Icterohaemorrhagiae in American Wild Rats and Its Relation to the Japanese and European Strains : First Paper. J. Exp. Med. 25 (5), 755–763. doi: 10.1084/jem.25.5.755
Palaniappan, R. U., Ramanujam, S., Chang, Y. F. (2007). Leptospirosis: Pathogenesis, Immunity, and Diagnosis. Curr. Opin. Infect. Dis. 20 (3), 284–292. doi: 10.1097/QCO.0b013e32814a5729
Pantel, A., Cheong, C., Dandamudi, D., Shrestha, E., Mehandru, S., Brane, L., et al. (2012). A New Synthetic TLR4 Agonist, GLA, Allows Dendritic Cells Targeted With Antigen to Elicit Th1 T-Cell Immunity In Vivo. Eur. J. Immunol. 42 (1), 101–109. doi: 10.1002/eji.201141855
Patra, K. P., Li, F., Carter, D., Gregory, J. A., Baga, S., Reed, S. G., et al. (2015). Alga-Produced Malaria Transmission-Blocking Vaccine Candidate Pfs25 Formulated With a Human Use-Compatible Potent Adjuvant Induces High-Affinity Antibodies That Block Plasmodium Falciparum Infection of Mosquitoes. Infect. Immun. 83 (5), 1799–1808. doi: 10.1128/IAI.02980-14
Phoka, T., Techawiwattanaboon, T., Sangjun, N., Komanee, P., Murray, G. L., Wongratanacheewin Sermswan, R., et al. (2021). Identification of In Vivo Expressed Proteins in Live Attenuated Lipopolysaccharide Mutant That Mediates Heterologous Protection Against Leptospira Spp. Vet. Microbiol. 262, 109220. doi: 10.1016/j.vetmic.2021.109220
Rodrigues de Oliveira, N., Jorge, S., Andrade Colares Maia, M., Thurow Bunde, T., Kurz Pedra, A. C., Pinto Seixas Neto, A. C., et al. (2021). Protective Efficacy of Whole-Cell Inactivated Leptospira Vaccines Made Using Virulent or Avirulent Strains in a Hamster Model. Vaccine 39 (39), 5626–5634. doi: 10.1016/j.vaccine.2021.08.014
Rudd, K. E., Johnson, S. C., Agesa, K. M., Shackelford, K. A., Tsoi, D., Kievlan, D. R., et al. (2020). Global, Regional, and National Sepsis Incidence and Mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 395 (10219), 200–211. doi: 10.1016/S0140-6736(19)32989-7
Stringfellow, D. A., Brown, R. R., Hanson, L. E., Schnurrenberger, P. R., Johnson, J. (1983). Can Antibody Responses in Cattle Vaccinated With a Multivalent Leptospiral Bacterin Interfere With Serologic Diagnosis of Disease? J. Am. Vet. Med. Assoc. 182 (2), 165–167.
Techawiwattanaboon, T., Barnier-Quer, C., Palaga, T., Jacquet, A., Collin, N., Sangjun, N., et al. (2019). Reduced Renal Colonization and Enhanced Protection by Leptospiral Factor H Binding Proteins as a Multisubunit Vaccine Against Leptospirosis in Hamsters. Vaccines (Basel) 7 (3), 95. doi: 10.3390/vaccines7030095
Teixeira, A. F., Cavenague, M. F., Kochi, L. T., Fernandes, L. G., Souza, G. O., de Souza Filho, A. F., et al. (2020). Immunoprotective Activity Induced by Leptospiral Outer Membrane Proteins in Hamster Model of Acute Leptospirosis. Front. Immunol. 11, 568694. doi: 10.3389/fimmu.2020.568694
Torgerson, P. R., Hagan, J. E., Costa, F., Calcagno, J., Kane, M., Martinez-Silveira, M. S., et al. (2015). Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLos Negl. Trop. Dis. 9 (10), e0004122. doi: 10.1371/journal.pntd.0004122
Viriyakosol, S., Matthias, M. A., Swancutt, M. A., Kirkland, T. N., Vinetz, J. M. (2006). Toll-Like Receptor 4 Protects Against Lethal Leptospira Interrogans Serovar Icterohaemorrhagiae Infection and Contributes to In Vivo Control of Leptospiral Burden. Infect. Immun. 74 (2), 887–895. doi: 10.1128/IAI.74.2.887-895.2006
Wang, Z., Jin, L., Wegrzyn, A. (2007). Leptospirosis Vaccines. Microb. Cell Fact 6, 39. doi: 10.1186/1475-2859-6-39
Wolpe, S. D., Sherry, B., Juers, D., Davatelis, G., Yurt, R. W., Cerami, A. (1989). Identification and Characterization of Macrophage Inflammatory Protein 2. Proc. Natl. Acad. Sci. U. S. A. 86 (2), 612–616. doi: 10.1073/pnas.86.2.612
Wunder, E. A., Adhikarla, H., Hamond, C., Owers Bonner, K. A., Liang, L., Rodrigues, C. B., et al. (2021). A Live Attenuated-Vaccine Model Confers Cross-Protective Immunity Against Different Species of the Leptospira Genus. Elife 10, e64166. doi: 10.7554/eLife.64166
Keywords: leptospirosis, AB toxins, immunity, vaccine, host – bacteria interaction
Citation: Chaurasia R, Salovey A, Guo X, Desir G and Vinetz JM (2022) Vaccination With Leptospira interrogans PF07598 Gene Family-Encoded Virulence Modifying Proteins Protects Mice From Severe Leptospirosis and Reduces Bacterial Load in the Liver and Kidney. Front. Cell. Infect. Microbiol. 12:926994. doi: 10.3389/fcimb.2022.926994
Received: 23 April 2022; Accepted: 24 May 2022;
Published: 28 June 2022.
Edited by:
Angela Silva Barbosa, Butantan Institute, BrazilReviewed by:
Kanitha Patarakul, Chulalongkorn University, ThailandPaulo Lee Ho, Butantan Institute, Brazil
Copyright © 2022 Chaurasia, Salovey, Guo, Desir and Vinetz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph M. Vinetz, am9zZXBoLnZpbmV0ekB5YWxlLmVkdQ==
 Reetika Chaurasia
Reetika Chaurasia Aryeh Salovey1
Aryeh Salovey1 Gary Desir
Gary Desir Joseph M. Vinetz
Joseph M. Vinetz