
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell. Infect. Microbiol. , 22 July 2022
Sec. Clinical Microbiology
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.926154
Pannonibacter phragmitetus (P. phragmitetus) is rarely related with human disease. We reported a case of catheter-related infection caused by P. phragmitetus in a 68-year-old woman on hemodialysis. The patient developed recurrent fever during hemodialysis and blood cultures were positive for P. phragmitetus. The patient’s body temperature returned to normal after intravenous cefoperazone/sulbactam treatment, and the hemodialysis catheter was locked with gentamicin and urokinase. The potential anti-infective treatment against P. phragmitetus was discussed.
Pannonibacter phragmitetus (P. phragmitetus) is a gram-negative rod, which is recognized from a Hungarian soda lake in 2003 (Borsodi et al., 2003). P. phragmitetus is commonly applied to environmental pollution control due to its ability to remove nitrogen and metals in water and soil (Bai et al., 2019; Chai et al., 2019; Wang et al., 2019; Liao et al., 2020; Saravanan et al., 2021). Although it was first identified as Achromobacter groups B and E from human blood (Holmes et al., 1990; Holmes et al., 1993; Holmes et al., 2006), little is known about its pathogenicity. After searching in PubMed and Web of Science, we found only 4 cases of infection caused by P. phragmitetus: one case of late replacement valve endocarditis (McKinley et al., 1990), one case of recurrent septicemia (Jenks and Shaw, 1997), one case of liver abscess (Wang et al., 2017), and one case of bacteriemia (Gallardo et al., 2020). Here, we presented probably the first case of hemodialysis catheter infection caused by P. phragmitetus in China.
A 68-year-old woman with end-stage renal disease was admitted to our hospital for hemodialysis on September 16, 2021. The patient had a history of left kidney cyst, atrophy of both kidneys, chronic kidney disease, obstructive pulmonary disease, and hypertension. A rectal resection was performed in 2002. Her normal medication consisted of nifedipine (20 mg, bid), benazepril (10 mg, bid) and terazosin (2 mg, tid). Since January 19, 2021, the patient started hemodialysis three times a week via a catheter in the right internal jugular vein at a local hospital. On August 17, the patient had chills during hemodialysis. After returning home, she developed fever and sweating with a body temperature of 39.1°C. Her body temperature returned to normal after she received diclofenac and anti-infective treatment. On September 1, she became febrile again after hemodialysis. She was hospitalized at the local hospital for 10 days and she was recovered after receiving anti-infective therapy. On September 13, the patient developed chills, fever and sweating again with a body temperature of 38.6°C during hemodialysis. Her body temperature returned to normal after physical cooling.
Upon admission, the patients’ body temperature was 36.9°C, pulse rate was 82 beats/min, respiratory rate was 18 breaths/min, and blood pressure was 200/110 mmHg. Laboratory tests (see Table 1) showed impaired kidney function (CO2 21.4 mmol/L, creatinine 958.3 μmol/L, uric acid 460 μmol/L, BUN 23.2mmol/L), anemia (RBC 3.29×1012/L, Hb 102 g/L), vitamin D deficiency (28.11 nmol/L), and hyperparathyroidism (PTH 226.1 pg/mL).
On the next day of admission, the patient developed fever during hemodialysis, and she was treated with intravenous demethylvancomycin (400 mg, qd). A pair of venous blood samples were collected prior to antimicrobial therapy, as well as a pair of blood sample drawn from the catheter. Aerobic blood cultures were positive after 2 days of incubation (48 h 25 min). A gram staining revealed gram-negative rods. Positive cultures were then transferred to Columbia blood agar plates, MacConkey agar plates, chocolate agar plates and Sabouraud’s agar plates at 35°C, and Sabouraud’s agar plates at 28°C. After incubation overnight, P. phragmitetus (Figure 1) was identified by matrix-assisted laser desorption ionization/time of flight mass spectrometry (LVD MALDI Biotyper System, Bruker Daltonik GmbH, Germany). P. phragmitetus was also verified by DNA sequencing (Supplementary Figure 1 and Data Sheet 1), using bacterial 16S rDNA primers (forward primer: 5’-AGTTTGATCMTGGCTCAG-3’, reverse primer: 5’-GGTTACCTTGTTACGACTT-3’). Antimicrobial susceptibility test (AST) was performed by the Kirby-Bauer disc diffusion method (Thermo Fisher Scientific, USA). The minimum inhibitory concentration (MIC) of P. phragmitetus for antibiotics were determined by BD Phoenix 100 system using NMIC/ID-4 susceptibility panels (Table 2). P. phragmitetus was sensitive to cefoxitin, imipenem, meropenem, amikacin, gentamicin, tetracycline, minocycline, ciprofloxacin, levofloxacin, chloramphenicol, but resistant against penicillin, ampicillin, piperacillin, piperacillin/tazobactam, aztreonam, trimethoprim/sulfamethoxazole, vancomycin, teicoplanin, clindamycin and linezolid amine. The patient was then changed to intravenous cefoperazone/sulbactam (500mg/500mg, bid) treatment. The hemodialysis catheter was locked with gentamicin (1.8×104 IU) and urokinase (1×105 IU).
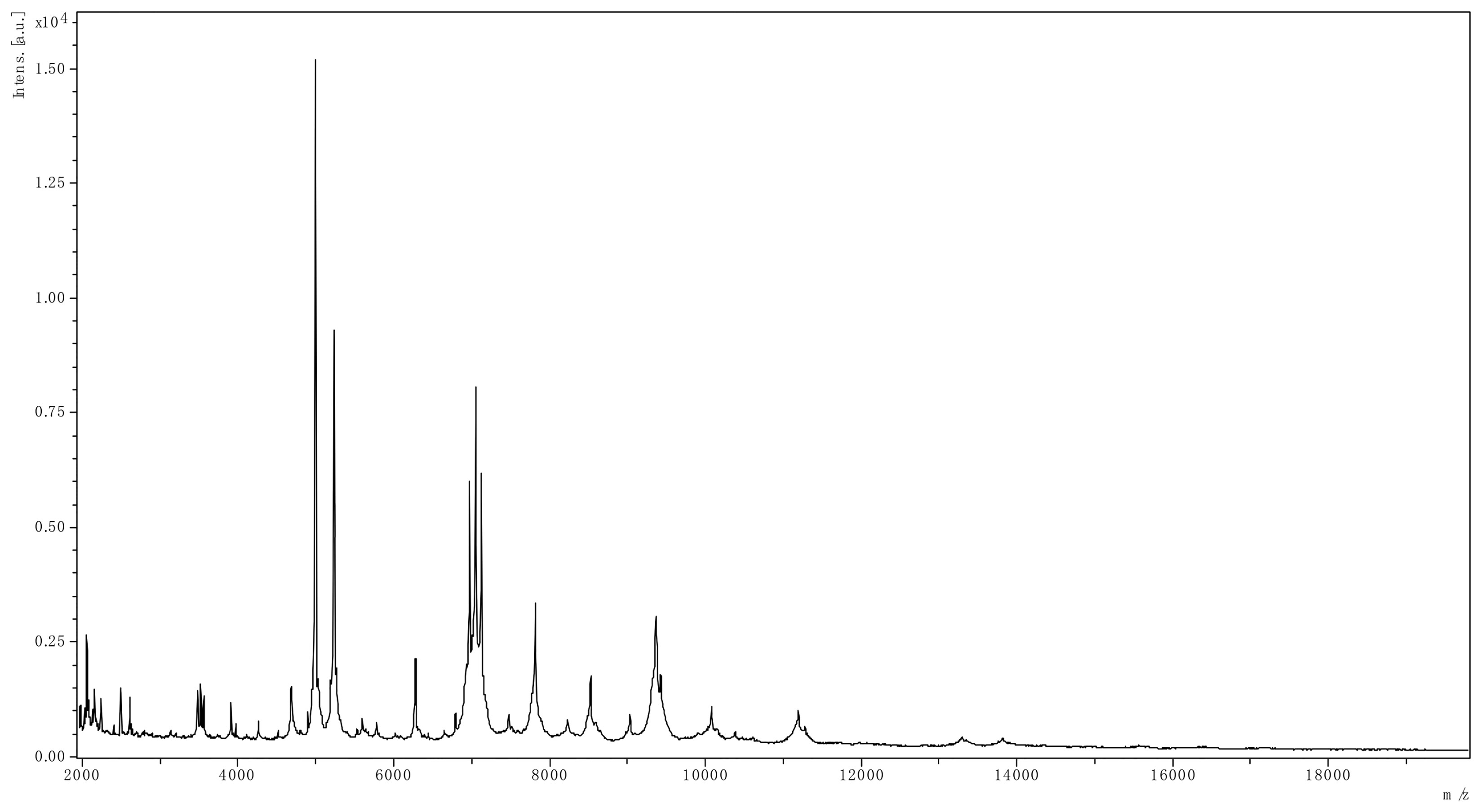
Figure 1 The spectrum of P. phragmitetus analyzed by MALDI Biotyper 3.1 (Bruker Daltonik GmbH, Germany).
Although clinical results were improved, but blood tests still showed a sign of infection (PCT 1.04 ng/ml, see Table 1). However, the patient refused to replace the hemodialysis catheter. On September 23, the patient was discharged from our hospital with treatment on nifedipine (20 mg, bid), benazepril (10 mg, bid), terazosin (2 mg, tid) and cefdinir (100 mg, tid). The patient underwent regular hemodialysis at the local hospital and developed no fever in the following 3 months (the patient’s medical timeline was shown in Figure 2).
The first case of infection caused by P. phragmitetus was reported in a male patient who developed a low-grade fever in 1988 (McKinley et al., 1990). The patient underwent pulmonary autograft replacement in 1987 and P. phragmitetus was probably introduced during the previous surgery. In 1997, a male patient appeared fever after urinary catheterization, and then P. phragmitetus was identified in blood cultures (Jenks and Shaw, 1997). The patient had clinically important improvement after antibiotic therapy and removal of the catheter, but the patient developed fever again after recatheterization. The infection of P. phragmitetus was thought to be caused by insertion of the urinary catheter. In 2017, a male patient with liver abscess was diagnosed with P. phragmitetus. However, P. phragmitetus was only identified from blood cultures, but not from abscess fluid (Wang et al., 2017).
The first case of bacteriemia caused by P. phragmitetus in a hemodialysis patient was reported in 2020 (Gallardo et al., 2020). A male patient started hemodialysis due to impaired kidney function in Spain. After one month and a half, the patient made a trip to Cuba and did his regular hemodialysis at a local hospital. When he returned to Spain and continued hemodialysis, he developed fever during the session. The infection of P. phragmitetus was probably introduced during his stay in Cuba.
Highly like the case described in 2020, we reported the first hemodialysis related infection caused by P. phragmitetus in China. Fever occurred 4 times during or after hemodialysis in our case. However, the patient is also not engaged in environmental pollution treatment, and there is no lake near her house. Since 2022, the patient has been undergoing hemodialysis at our hospital, and there is currently no sign of fever or infection. Therefore, we speculate that P. phragmitetus was probably induced via the catheter during her regular hemodialysis at her local hospital.
Among total 5 cases of P. phragmitetus infection, fever appeared in 4 cases, and catheter‐related infection (CRI) was suspected to be involved in 3 cases (Table 3). CRI is a common but terrified complication in hemodialysis patients (Labriola, 2019). Sepsis accounts for 6.5% of deaths for patients receiving hemodialysis in USA (System USRD., 2021). Genomic sequencing showed P. phragmitetus is multidrug-resistant (Zhou et al., 2017), but there is no standard treatment against P. phragmitetus. Combining AST results from literatures and the present case, imipenem, amikacin, ciprofloxacin, and gentamicin would be ideal options for anti-infective monotherapy or combination therapy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Central Hospital of Wuhan. The patients/participants provided their written informed consent to participate in this study.
RT, JW, and YZ collected the samples, performed the laboratory analyses, and wrote the manuscript, KW performed CT image analysis, HW revised the manuscript, ZL designed and revised the manuscript. All authors read and approved the final manuscript.
This work was supported by the discipline construction program (2021XK071) of the Central Hospital of Wuhan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.926154/full#supplementary-material
Bai, H., Liao, S., Wang, A., Huang, J., Shu, W., Ye, J. (2019). High-efficiency inorganic nitrogen removal by newly isolated pannonibacter phragmitetus B1. Bioresour. Technol. 271, 91–99. doi: 10.1016/j.biortech.2018.09.090
Borsodi, A. K., Micsinai, A., Kovács, G., Tóth, E., Schumann, P., Kovács, A. L., et al. (2003). Pannonibacter phragmitetus gen. nov., sp. nov., a novel alkalitolerant bacterium isolated from decomposing reed rhizomes in a Hungarian soda lake. Int. J. Syst. Evol. Microbiol. 53, 555–561. doi: 10.1099/ijs.0.02356-0
Chai, L., Ding, C., Li, J., Yang, Z., Shi, Y. (2019). Multi-omics response of pannonibacter phragmitetus BB to hexavalent chromium. Environ. pollut. 249, 63–73. doi: 10.1016/j.envpol.2019.03.005
Gallardo, A., Merino Bueno, M. D. C., Sango Merino, C., Suárez Laurés, A. M., de la Torre-Fernández, M., Sánchez Álvarez, E. (2020). First case of bacteriemia caused by pannonibacter phragmitetus in a haemodialysis patient. Nefrologia (Engl Ed) S0211-6995 (20), 30183–1. doi: 10.1016/j.nefro.2020.08.014
Holmes, B., Costas, M., Wood, A. C., Kersters, K. (1990). Numerical analysis of electrophoretic protein patterns of “Achromobacter” group b, e and f strains from human blood. J. Appl. Bacteriol 68, 495–504. doi: 10.1111/j.1365-2672.1990.tb02902.x
Holmes, B., Moss, C. W., Daneshvar, M. I. (1993). Cellular fatty acid compositions of “achromobacter groups b and e”. J. Clin. Microbiol. 31, 1007–1008. doi: 10.1128/jcm.31.4.1007-1008.1993
Holmes, B., Segers, P., Coenye, T., Vancanneyt, M., Vandamme, P. (2006). Pannonibacter phragmitetus, described from a Hungarian soda lake in 2003, had been recognized several decades earlier from human blood cultures as achromobacter groups b and e. Int. J. Syst. Evol. Microbiol. 56, 2945–2948. doi: 10.1099/ijs.0.64563-0
Jenks, P. J., Shaw, E. J. (1997). Recurrent septicaemia due to “achromobacter group b”. J. Infection 34, 143–145. doi: 10.1016/S0163-4453(97)92490-7
Labriola, L. (2019). Antibiotic locks for the treatment of catheter-related blood stream infection: Still more hope than data. Semin. Dial 32, 402–405. doi: 10.1111/sdi.12807
Liao, Q., Tang, J., Wang, H., Yang, W., He, L., Wang, Y., et al. (2020). Dynamic proteome responses to sequential reduction of Cr(VI) and adsorption of Pb(II) by pannonibacter phragmitetus BB. J. Hazard Mater 386, 121988. doi: 10.1016/j.jhazmat.2019.121988
McKinley, K. P., Laundy, T. J., Masterton, R. G. (1990). Achromobacter group b replacement valve endocarditis. J. Infection 20, 262–263. doi: 10.1016/0163-4453(90)91294-N
Saravanan, A., Kumar, P. S., Yaashikaa, P. R., Karishma, S., Jeevanantham, S., Swetha, S. (2021). Mixed biosorbent of agro waste and bacterial biomass for the separation of Pb(II) ions from water system. Chemosphere 277, 130236. doi: 10.1016/j.chemosphere.2021.130236
System USRD (2021). “USRDS annual data report,” in Epidemiology of kidney disease in the united states (Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases). Available at: https://adr.usrds.org/2021.
Wang, M., Zhang, X., Jiang, T., Hu, S., Yi, Z., Zhou, Y., et al. (2017). Liver abscess caused by pannonibacter phragmitetus: case report and literature review. Front. Med. (Lausanne) 4. doi: 10.3389/fmed.2017.00048
Wang, X., Zhu, H., Shutes, B., Fu, B., Yan, B., Yu, X., et al. (2019). Identification and denitrification characteristics of a salt-tolerant denitrifying bacterium pannonibacter phragmitetus F1. AMB Express 9, 193. doi: 10.1186/s13568-019-0918-y
Keywords: Pannonibacter phragmitetus, hemodialysis, catheter infection, recurrent fever, anti-infective treatment
Citation: Tang R, Wang J, Zhan Y, Wu K, Wang H and Lu Z (2022) Hemodialysis catheter-related infection caused by Pannonibacter phragmitetus: a rare case report in China. Front. Cell. Infect. Microbiol. 12:926154. doi: 10.3389/fcimb.2022.926154
Received: 22 April 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Bin Cao, Nanyang Technological University, SingaporeReviewed by:
Yichao Wu, Huazhong Agricultural University, ChinaCopyright © 2022 Tang, Wang, Zhan, Wu, Wang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongxin Lu, bHV6aG9uZ3hpbkB6eGhvc3BpdGFsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.