
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 28 April 2022
Sec. Parasite and Host
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.885348
This article is part of the Research Topic Cryptosporidium, Giardia, Cyclospora, and Toxoplasma - Insights into Their Transmission View all 7 articles
Background: Toxoplasma gondii can infect almost all warm-blooded animals, including humans and dogs. Humans can become infected with T. gondii by petting dogs that have eaten or contacted infected cat feces. The aim of this study was to evaluate T. gondii infections in dogs from central China. From 2015 to 2021, a total of 536 dog samples (195 fecal, 81 hearts, and 260 serum samples) from Henan Province were collected. Heart juice or serum samples (n = 341) were tested for T. gondii antibodies using the modified agglutination test (MAT). Fresh myocardium (n = 6) and blood (n = 2) samples were bioassayed in mice.
Results: The present study showed that 4.40% (15/341) of the dogs were seropositive for T. gondii by MAT (cut-off, 1:25) and 4.10% (8/195) of dog feces contained T. gondii DNA. No T. gondii DNA was found in any myocardium (n = 81) or blood (n = 2) samples. The viable T. gondii strain was not isolated from any myocardium or blood samples (n = 8). Compared to the prevalence of T. gondii antibodies in dogs sampled from 2015 to 2018, the prevalence significantly declined from 2020 to 2021 (P < 0.05). Gender and age were not risk factors for dogs infected with T. gondii in this study. However, compared to other sources, dogs from Zhoukou City (close to the Yellow River) or from pet shops showed significantly higher prevalence for T. gondii (P < 0.05).
Conclusion: A total of 4.29% dogs were infected by T. gondii (23/536, 8 of 195 fecal samples, 2 of 260 serum, and 13 of 81 heart juice samples). This is the first survey of T. gondii infection in dog feces from China. Dogs were exposed to T. gondii, and they could act as mechanical transmitters of T. gondii.
Toxoplasmosis is caused by an obligate, intracellular, Toxoplasma gondii. T. gondii can infect almost all warm-blooded animals, including humans and dogs. Although T. gondii infection appears asymptomatic in most species, there are severe risks for immunosuppressed individuals, pregnant women, sheep, new world monkeys, and marsupials, as it may cause severe health implications to fetuses or acute infection (Dubey, 2010; Shapiro et al., 2019; Calero-Bernal and Gennari, 2019; Dubey et al., 2020).
Recently, we reviewed T. gondii infection in dogs and found that T. gondii antibodies were found in dogs worldwide (Dubey et al., 2020). Many fatal cases of canine toxoplasmosis have been reported, where ulcerative dermatitis, rear limb paralysis, and myocarditis were the main clinical presentations (Dubey, 2010; Dubey et al., 2020; Dorsch et al., 2022). Humans can become infected with T. gondii by petting dogs that have eaten or have been in contact with infected cat feces. The oocysts of T. gondii ingested by dogs may pass through the digestive tract and remain infectious (Lindsay et al., 1997). Dogs can mechanically transmit T. gondii oocysts to humans through their body surfaces, mouth, and feet (Dubey et al., 2020). Therefore, T. gondii infection in dogs can be an indicator for the level of environmental contamination for humans (Meireles et al., 2004). Additionally, because dog meat serves as food for humans in some regions (Cui and Wang, 2001; Chevalier et al., 2021; Tasiame et al., 2021), consumption of undercooked dog meat containing T. gondii cysts also poses a health risk.
China has an estimated 27 million domestic dogs, ranking third worldwide, and has an unknown number of wild dogs. The seroepidemiology of T. gondii in dogs from China is summarized in Figure 1. For China, there are few reports on clinical toxoplasmosis in dogs, no viable T. gondii strain has been isolated, and little is known about the mechanical excretion of T. gondii oocysts from dog feces. The objective of the present study was to investigate the prevalence of T. gondii infections in dogs (bodily fluids and feces) in China, and an attempt was made to isolate viable T. gondii strains.
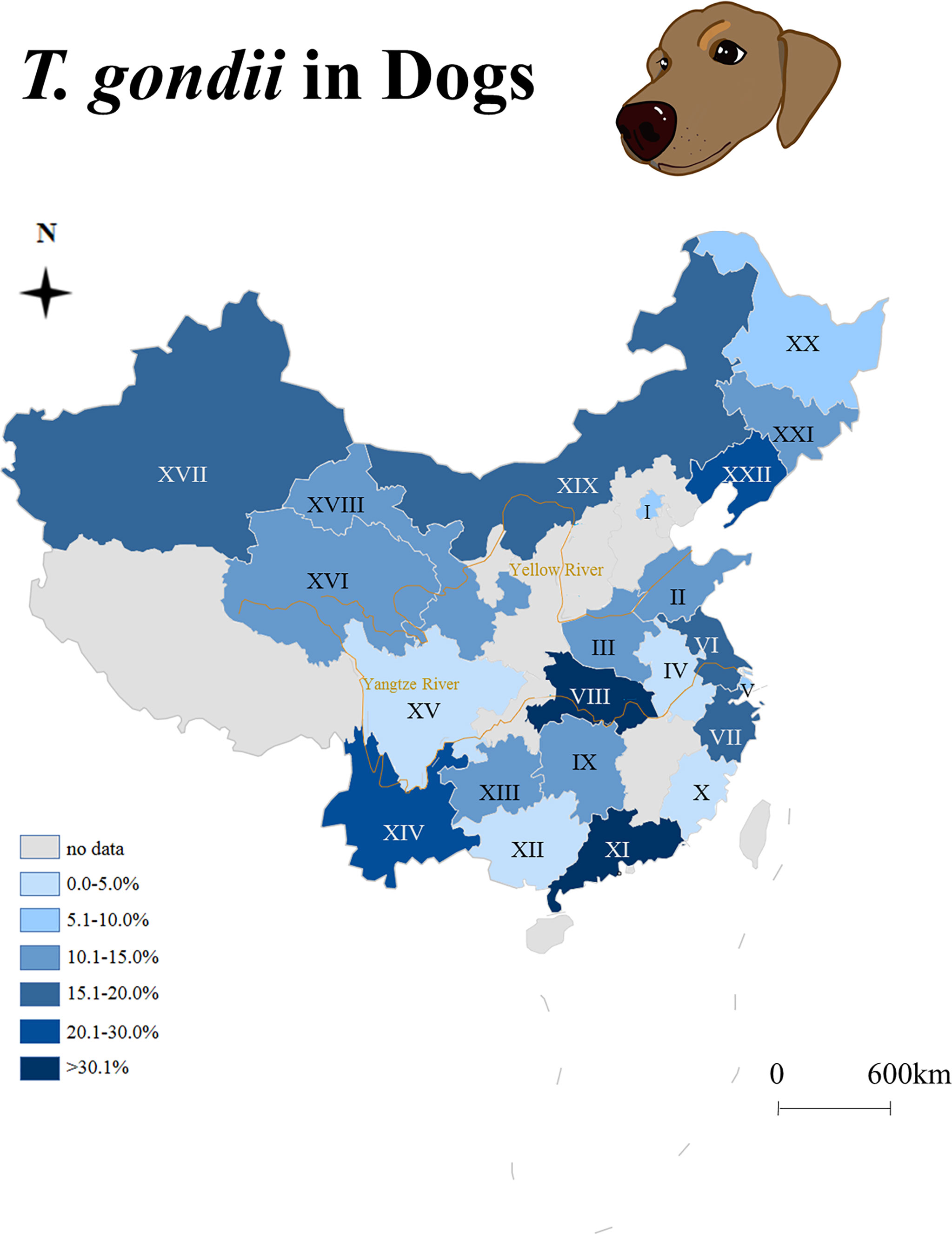
Figure 1 Seroepidemiology of Toxoplasma gondii in dogs from China (2009–2021). I: Beijing, II: Shandong, III: Henan, IV: Anhui, V: Shanghai, VI: Jiangsu, VII: Zhejiang, VIII: Hubei, IX: Hunan, X: Fujian, XI: Guangdong, XII: Guangxi, XIII: Guizhou, XIV: Yunnan, XV: Sichuan, XVI: Qinghai, XVII: Xinjiang, XVIII: Gansu, XIX: Inner Mongolia, XX: Heilongjiang, XXI: Jilin, XXII: Liaoning.
From 2015 to 2021, 81 fresh dog hearts were collected from slaughterhouses, 260 dog blood samples were collected from pet hospitals, and 195 dog fecal samples were collected from farms, shelters, pet shops, police dog-breeding bases, and hospitals (Henan Province, China) (Tables 1, 2). The fecal samples were obtained with the help of the dog owners, and the feces (5 g) were collected from rectum and placed in plastic bags. Henan Province (34.90°N, 113.50°E) is located in a warm temperate zone and in the trans-subtropical southern region. In the past 10 years, the annual temperature has ranged from 12.9°C to 16.5°C, and the annual precipitation has ranged from 464.2 to 1,193.2 mm.
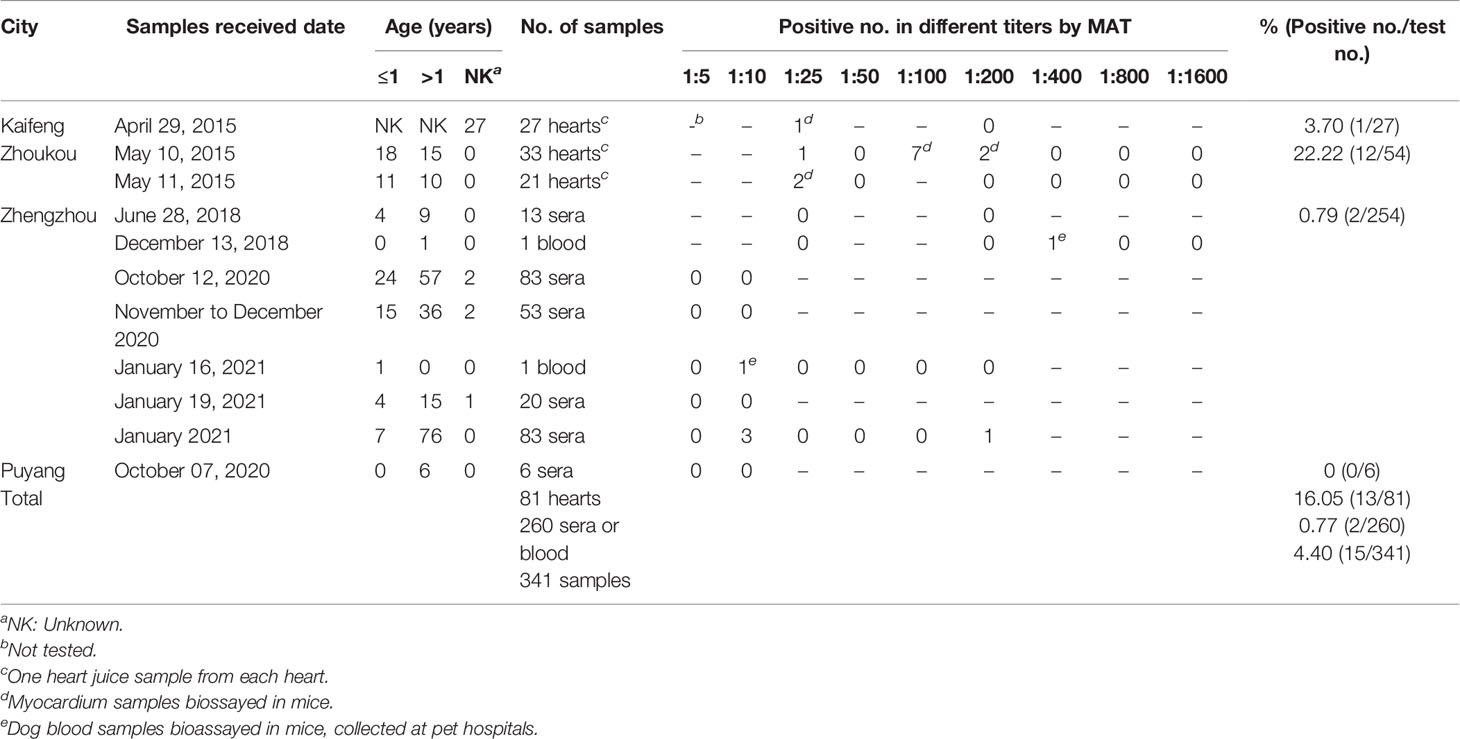
Table 1 Serological investigation of Toxoplasma gondii infection in dogs from Henan Province, China.
Heart juice samples from dog myocardium were centrifuged for 10 min at 2,000 rpm; serum samples were subsequently separated. Heart juice, serum, and myocardium samples were stored at 4°C and tested for T. gondii antibodies or bioassayed in mice within 2 days. Fecal samples were cultured at room temperature for 2 weeks so that oocysts could sporulated.
Sera (n = 260) and heart juice (n = 81) from 341 dogs were tested for T. gondii antibodies using a modified agglutination test (MAT) (Dubey and Desmonts, 1987). Whole formalin-treated T. gondii tachyzoites were obtained from the University of Tennessee Research Foundation (Dr. CL Su, Knoxville, TN, USA). All heart juice and serum samples collected from 2015 to 2018 were tested at a titer of 1:25; the dilution was subsequently doubled to a titer of 1:1,600 (Table 1). Serum samples collected from 2020 to 2021 were tested at a titer of 1:5 and 1:10. Positive samples were tested at a titer of 1:25; the dilution was subsequently doubled to a titer of 1:200 (Table 1). Negative and positive controls were included in each plate. Heart juice and serum samples with a titer of ≥1:25 were considered as a sign of exposure to this parasite.
Myocardium (n = 6) and blood (n = 2) samples from dogs were bioassayed in mice following previously described methods (Dubey, 2010). Briefly, myocardia (50 g) were digested in a pepsin solution and inoculated in Swiss mice (n = 2–3) subcutaneously. Blood samples were centrifuged for 10 min at 2,000 rpm; the sediment was suspended in saline and injected into Swiss mice (n = 2) or gamma interferon (IFN-γ) knockout mice (n = 1) subcutaneously. Swiss mice were supplied by the Zhengzhou University Laboratory Animal Center (China). IFN-γ-/- mice were purchased from the Jackson Laboratory (Stock No.: 002287; Bar Harbor, ME, USA).
Clinical symptoms in mice were recorded daily. The room temperature in the facilities was 22°C–24°C, and the humidity was 60%–70%. T. gondii tachyzoites or cysts in the lungs or brains of dead or euthanized mice were examined. If cysts or tachyzoites were not found in mouse tissues, the homogenized lung, brain, and myocardium were subcutaneously subpassaged into a new group of mice. Sera were collected from surviving mice at 30 days post-inoculation (DPI). Mouse sera at dilutions of 1:25 and 1:200 were tested for T. gondii antibodies by MAT.
Oocysts were purified from dog fecal samples by the conventional sucrose flotation method at room temperature (Dubey, 2010). Floated materials were transferred to slides and checked by light microscopy. Parasite oocysts and eggs were differentiated based on their morphological characteristics. Oocysts were stored at −20°C in a refrigerator for further analysis.
DNA was extracted from the myocardium (n = 81), pepsin-digested myocardium juice (n = 6), blood (n = 2), and fecal flotation (n = 195) samples using a DNA extraction kit (DP304; Tiangen Biotech Co., Beijing, China). T. gondii DNA was amplified by polymerase chain reaction (PCR) which targeted the 529-bp repetitive DNA fragment of T. gondii (primer pair, Tox5–Tox8) (Reischl et al., 2003; Schares et al., 2008). The length of the PCR products was estimated to be 450 bp and included negative and positive controls.
Statistical analyses were performed using GraphPad Prism version 8.4.3 software (GraphPad Software Inc., San Diego, CA, USA). The results were analyzed by the chi-square or Fisher’s exact test. The Monte Carlo test of simulated data was implemented to assess the risk factors associated with T. gondii infection. A P-value of <0.05 was considered statistically significant.
In the present study, the MAT results indicated that 4.40% (15/341, 95% CI, 2.63–7.19) of the examined dogs were seropositive with titers of 1:25 in four dogs, 1:100 in seven, 1:200 in three, and 1:400 in one (Table 1). They were 13 of 81 hearts collected at slaughterhouses, and 2 of 260 blood samples from pet hospitals (Tables 1, 3). The seroprevalence (sera, heart juice) of T. gondii infection in dogs from four cities ranged from 0% to 22%. The seroprevalence rates of T. gondii varied by region (Table 3). A significantly higher T. gondii seroprevalence was observed in Zhoukou when compared to other regions (P = 0.0323); there were no seropositive serum samples from Puyang.
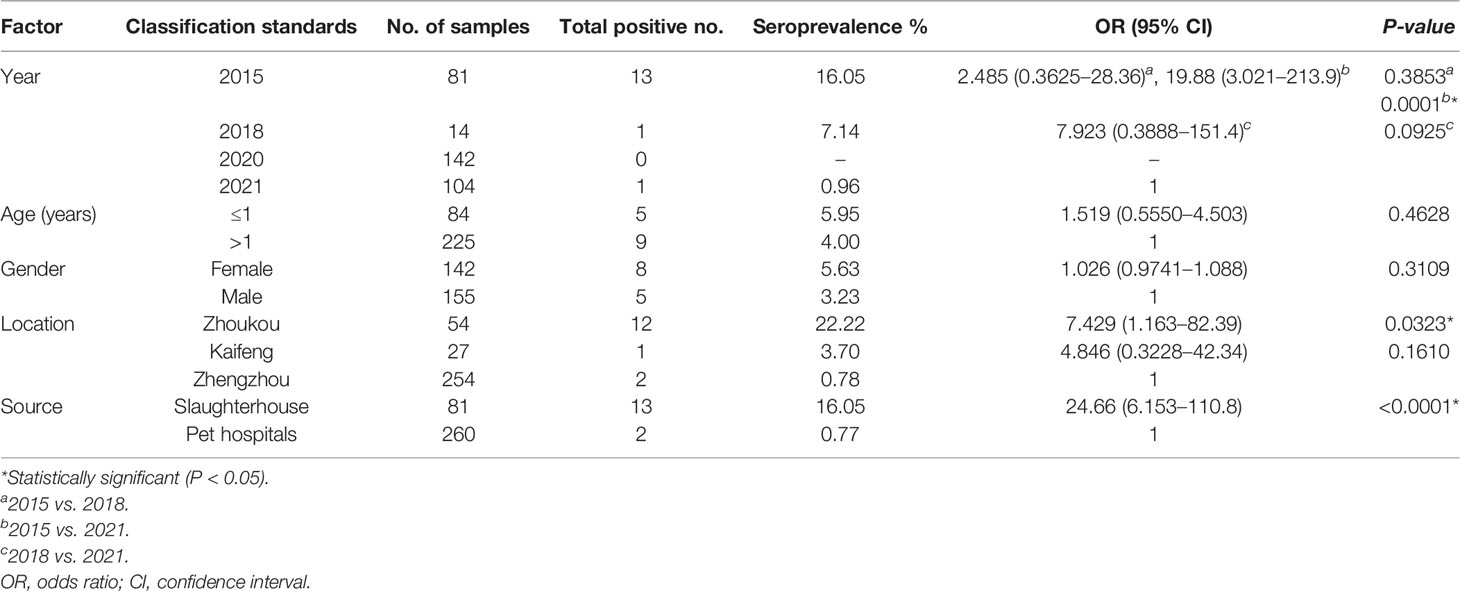
Table 3 Seroprevalence and risk factors of Toxoplasma gondii infection in dogs tested by a modified agglutination test (MAT).
The prevalence of T. gondii antibodies in dogs sampled from central China significantly declined from 2015 to 2021 (P = 0.0001) with positive serum rates of 16.05% (13/81, 95% CI, 9.49–25.69) in 2015, 7.14% (1/14, 95% CI, 0.01–33.54) in 2018, and 0.96% (1/104, 95% CI, 0.01–5.77) in 2021; there were no positive serum samples in 2020 (0/142). Regarding gender and age, females had a higher seropositive rate than males, and dogs that were ≤1-year-old had a higher seropositive rate than dogs that were >1-year-old; however, no significant differences were detected (Table 3).
In this survey, dogs were frequently mix-infected with 2–3 parasite species, including roundworm, isospora, whipworm, tapeworm, and hookworm by fecal microscopic examination. No T. gondii-like oocysts were found under light microscopy. Samples were checked for T. gondii nucleic acid by PCR. T. gondii DNA were amplified from 4.10% (8/195) dog fecal samples using the primer pair, Tox5–Tox8 (Table 2).
A significantly higher prevalence of T. gondii in fecal samples was observed in ≤1-year-old dogs when compared to dogs that were >1-year-old (P < 0.05). A significantly higher prevalence of T. gondii was observed in dogs from pet shops when compared to other sources (P = 0.0130). Fecal samples from males, urban regions, and crossbred dogs had a higher prevalence of T. gondii; however, no significant differences were observed (Table 2).
In this study, six fresh myocardium (seropositive for T. gondii) and two blood (one at 1:400, one at 1:10) samples were bioassayed in mice (Table 4). Unfortunately, the viable T. gondii strain was not successfully isolated from any tissues or blood samples. After mice were bioassayed, only one group of mouse sera (dog blood: ID# December 13, 2018; two positive mice of three inoculated mice) showed seroconversion for T. gondii at 39 DPI. However, after subpassage, none of the mice were positive for T. gondii infection by serology or etiology. None of the myocardium (n = 81), myocardium digestion fluid (n = 6), or blood (n = 2) samples contained T. gondii DNA.
Dogs are susceptible to T. gondii with a seroprevalence of 1.9% to 97.0% in the world (Dubey et al., 2020). Dogs have a keen sense of smell and the behavior of rolling in feces, thereby increasing their exposure to T. gondii (Frenkel et al., 2003; Dubey et al., 2020). Canine toxoplasmosis can also be caused by ingesting oocysts or meat containing T. gondii cysts (Yan et al., 2012; Calero-Bernal and Gennari, 2019). A previous epidemiological study detected a uniform prevalence of T. gondii between humans and dogs, which may be because they lived in the same environment, indicating that dogs may be a good sentinel species of T. gondii exposure for humans (Tenter et al., 2000; Meireles et al., 2004).
The seropositive rate was 4.40% (15/341) of the dogs by MAT. They were 13 of 81 hearts collected at slaughterhouse, and 2 of 260 blood samples collected from pet hospitals (Table 1). Hearts were collected in 2015, while blood samples were collected mainly in 2020–2021. Compared to the seroprevalence of T. gondii antibodies in dogs sampled from 2015 to 2018 in Henan Province, the prevalence significantly declined from 2020 to 2021. Previous studies have reported a T. gondii prevalence of 34.9% (83/238), 20.8% (26/125), and 3.23% (1/31) in dogs sampled from Henan Province (Wang et al., 2012; Yang et al., 2014; Qian et al., 2015). Recently, China has seen a rapid development of its economic and health industries. The increased economic and cleanliness levels may play a role in suppressing T. gondii transmission and distribution in the environment.
In the present study, geographic location was a risk factor associated with T. gondii seroprevalence in dogs. Compared to other regions, the T. gondii prevalence in dogs from Zhoukou was significantly higher. This may be due to the following: (1) Henan Province is located downstream of the Yellow River. Zhoukou is closer to the Yellow River than other cities, and T. gondii oocysts may be transported via freshwater, thereby posing a threat to animals residing close to the river. (2) Samples from Zhoukou were collected in 2015, which is relatively earlier than in cities of Zhengzhou and Puyang. Regarding T. gondii infection in this study, gender and age were not risk factors for dogs, and the lack of relationship with gender was in agreement with the findings of other studies (Lopes et al., 2011; Yang et al., 2013; Nguyen et al., 2020). However, a previous study found that seroprevalence in dogs increased with age (Dubey et al., 2020), which may be related to the low seroprevalence of T. gondii in dogs or small sample size in this study.
Environmental pollution due to dog feces is a public health concern and constitutes a health hazard to humans (Ainmode et al., 2016). When dogs ingest cat feces containing T. gondii oocysts, oocysts remain viable after passing through the digestive tract (Lindsay et al., 1997). Here, T. gondii DNA was detected in the feces of 8/195 dogs sampled from Henan Province. The seroprevalence levels of T. gondii in domestic cats in Henan Province were 50% (21/42) (Yang et al., 2015), 21% (178/843) (Wang et al., 2017), and 7% (2/28) (Yang et al., 2017). These results indicated that the environment may be polluted by T. gondii oocysts. Humid tropical climates are conducive for maintaining oocysts in the soil and water, thereby contributing to its widespread dissemination (Afonso et al., 2006). T. gondii infection in dog feces is rarely reported on a global scale. In a previous study, T. gondii DNA was detected in the feces of 4/120 dogs sampled from the United States (Munoz and Mayer, 2016). T. gondii isolates (TG-dgGER1, TG-dgGER2) were successfully isolated from 2/24,089 fecal samples (Schares et al., 2005).
Compared to older dogs, there was a significantly higher prevalence of T. gondii DNA in feces from ≤1-year-old dogs. This may be explained by the fact that puppies are more active than adults. Dogs from pet shops had a higher prevalence of T. gondii DNA in their feces than those from shelters, hospitals, or farms. Pet shops often sell many kinds of animals, including cats, dogs, birds, and fish, indicating that the pet shop environment may increase the exposure of dogs to T. gondii oocysts. Gender, breed, and source area were not risk factors associated with T. gondii-positive rates in dog fecal samples based on the T. gondii DNA results in this study. In order to investigate T. gondii infection in dogs, excluding serological tests, fecal samples may be an excellent option for etiology detection in the future. Moreover, feces are easier to obtain, especially for vigilant and fierce stray dogs or wild canines. Bioassays of dog feces via cats or mice could further verify T. gondii infectivity.
In our previous study, T. gondii was not isolated from the myocardium of 14 seropositive dogs (MAT ≥ 10) sampled from 2013 to 2014 (Yang et al., 2014). Unfortunately, in this study, T. gondii was not isolated from dog myocardium (n = 6, MAT ≥ 25) or blood (n = 2, MAT ≥ 10) samples. This may be due to the following: (1) the low virulence of T. gondii in dogs or (2) the low density of parasite loading in dog tissues. The fact that no T. gondii DNA was found in the myocardium (n = 81), myocardium digestion fluids (n = 6), or blood (n = 2) also confirms this hypothesis. Although serological results indicated contact of dogs with T. gondii, tissues were negative for the presence of the parasite. T. gondii infection might occur when consuming dog meat. The lack of detection of the parasite by molecular assays or biological assays in chronic T. gondii infection might be due to the limited sample size analyzed (0.5 g tissue or 50 g tissue), the uneven distribution of tissue cysts, and perhaps the low number of T. gondii tissue cysts in the tissues.
This is the first survey of T. gondii infection from dog fecal samples in China. The results showed that 4.40% (15/341) of the dogs were seropositive by MAT and 4.10% (8/195) dog feces contained T. gondii DNA. Dogs were exposed to T. gondii; they could act as mechanical transmitters of T. gondii.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Beijing Association for Science and Technology (SYXK [Beijing] 2007-0023).
NZ performed the laboratory tests and data analysis and wrote the manuscript. LY, SX, and WH participated in the sample collection and laboratory testing. YJ and YY designed the study protocol, analyzed the results, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was financed by the Natural Science Foundation of Henan Province, China (202300410214), and the Key Research Projects of Henan Higher Education Institutions (21A230009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Lina Qu (Henan Agricultural University, Zhengzhou, China) for performing some of the laboratory detections.
MAT, modified agglutination test; PCR, polymerase chain reaction.
Afonso, E., Thulliez, P., Gilot-Fromont, E. (2006). Transmission of Toxoplasma gondii in an Urban Population of Domestic Cats (Felis Catus). Int. J. Parasitol. 36 (13), 1373–1382. doi: 10.1016/j.ijpara.2006.07.010
Ainmode, A. B., Obebe, O. O., Olayemi, E. (2016). Prevalence of Potentially Zoonotic Gastrointestinal Parasites in Canine Faeces in Ibadan, Nigeria. Ghana Med. J. 50 (4), 201–206. doi: 10.4314/gmj.v50i4.2
Calero-Bernal, R., Gennari, S. M. (2019). Clinical Toxoplasmosis in Dogs and Cats: An Update. Front. Vet. Sci. 6, 54. doi: 10.3389/fvets.2019.00054
Chevalier, V., Davun, H., Sorn, S., Ly, P., Pov, V., Ly, S. (2021). Large Scale Dog Population Demography, Dog Management and Bite Risk Factors Analysis: A Crucial Step Towards Rabies Control in Cambodia. PloS One 16 (7), e0254192. doi: 10.1371/journal.pone.0254192
Cui, J., Wang, Z. Q. (2001). Outbreaks of Human Trichinellosis Caused by Consumption of Dog Meat in China. Parasite 8 (2 Suppl), S74–S77. doi: 10.1051/parasite/200108s2074
Dorsch, M. A., Cesar, D., Bullock, H. A., Uzal, F. A., Ritter, J. M., Giannitti, F. (2022). Fatal Toxoplasma gondii Myocarditis in an Urban Pet Dog. Veterinary Parasitol.: Regional Stud. Rep. 27, 100659. doi: 10.1016/J.VPRSR.2021.100659
Dubey, J. P., Desmonts, G. (1987). Serological Responses of Equids Fed Toxoplasma gondii Oocysts. Equine Vet. J. 19 (4), 337–339. doi: 10.1111/j.2042-3306.1987.tb01426.x
Dubey, J. P., Murata, F. H. A., Cerqueira-Cézar, C. K., Kwok, O. C. H., Yang, Y., Su, C. (2020). Toxoplasma gondii Infections in Dogs: 2009-2020. Vet. Parasitol. 287, 109223. doi: 10.1016/j.vetpar.2020.109223
Dubey, J. P., Taylor & Francis Group (2010). Toxoplasmosis of Animals and Humans (Boca Raton, Florida, USA: CRC Press).
Frenkel, J. K., Lindsay, D. S., Parker, B. B., Dobesh, M. (2003). Dogs as Possible Mechanical Carriers of Toxoplasma, and Their Fur as a Source of Infection of Young Children. Int. J. Infect. Dis. 7 (4), 292–293. doi: 10.1016/S1201-9712(03)90112-3
Lindsay, D. S., Dubey, J. P., Butler, J. M., Blagburn, B. L. (1997). Mechanical Transmission of Toxoplasma gondii Oocysts by Dogs. Vet. Parasitol. 73 (1-2), 27–33. doi: 10.1016/S0304-4017(97)00048-4
Lopes, A. P., Santos, H., Neto, F., Rodrigues, M., Kwok, O. C., Dubey, J. P., et al. (2011). Prevalence of Antibodies to Toxoplasma gondii in Dogs From Northeastern Portugal. J. Parasitol. 97 (3), 418–420. doi: 10.1645/GE-2691.1
Meireles, L. R., Galisteo, A. J., Jr, Pompeu, E., Andrade, H. F., Jr. (2004). Toxoplasma Gondii Spreading in an Urban Area Evaluated by Seroprevalence in Free-Living Cats and Dogs. Trop. Med. Int. Health 9 (8), 876–881. doi: 10.1111/j.1365-3156.2004.01280.x
Munoz, J., Mayer, D. C. (2016). Toxoplasma Gondii and Giardia Duodenalis Infections in Domestic Dogs in New York City Public Parks. Vet. J. 211, 97–99. doi: 10.1016/j.tvjl.2016.02.015
Nguyen, T. T., Kengradomkij, C., Inpankaew, T. (2020). Detection of Antibodies to Toxoplasma gondii Among Owned Dogs in Cambodia. Food Waterborne Parasitol. 22, e00103. doi: 10.1016/j.fawpar.2020.e00103
Qian, W. F., Yan, W. C., Wang, T. Q., Zhai, K., Han, L. F., Lv, C. C. (2015). Prevalence and Genetic Characterization of Toxoplasma gondii in Pet Dogs in Central China. Korean J. Parasitol. 53 (1), 125–128. doi: 10.3347/kjp.2015.53.1.125
Reischl, U., Bretagne, S., Krüger, D., Ernault, P., Costa, J. M. (2003). Comparison of Two DNA Targets for The Diagnosis of Toxoplasmosis by Real-Time PCR Using Fluorescence Resonance Energy Transfer Hybridization Probes. BMC Infect. Dis. 3, 7. doi: 10.1186/1471-2334-3-7
Schares, G., Herrmann, D. C., Beckert, A., Schares, S., Hosseininejad, M., Pantchev, N., et al. (2008). Characterization of a Repetitive DNA Fragment in Hammondia Hammondi and Its Utility for the Specific Differentiation of H. Hammondi From Toxoplasma Gondii by PCR. Mol. Cell Probes. 22 (4), 244–251. doi: 10.1016/j.mcp.2008.04.003
Schares, G., Pantchev, N., Barutzki, D., Heydorn, A. O., Bauer, C., Conraths, F. J. (2005). Oocysts of Neospora Caninum, Hammondia Heydorni, Toxoplasma gondii and Hammondia Hammondi in Faeces Collected From Dogs in Germany. Int. J. Parasitol. 35 (14), 1525–1537. doi: 10.1016/j.ijpara.2005.08.008
Shapiro, K., Bahia-Oliveira, L., Dixon, B., Dumètre, A., de Wit, L. A., VanWormer, E., et al. (2019). Environmental Transmission of Toxoplasma gondii: Oocysts in Water, Soil and Food. Food Waterborne Parasitol. 15, e00049. doi: 10.1016/j.fawpar.2019.e00049
Tasiame, W., El-Duah, P., Johnson, S. A. M., Owiredu, E. W., Bleicker, T., Veith, T., et al. (2021). Rabies Virus in Slaughtered Dogs for Meat Consumption in Ghana: A Potential Risk for Rabies Transmission. Transbound Emerg. Dis. doi: 10.1111/tbed.14266. Online ahead of print.
Tenter, A. M., Heckeroth, A. R., Weiss, L. M. (2000). Toxoplasma gondii: From Animals to Humans. Int. J. Parasitol. 30, 1217–1258. doi: 10.1016/S0020-7519(00)00124-7
Wang, H. Y., Pei, S. L., Hao, Z. F., Zhou, M. (2012). Investigation on Epidemiology of Toxoplasmosis In Dogs and Cats in Zhengzhou City. J. Henan Agr. Sci. 41, 153–154. doi: 10.3969/j.issn.1004-3268.2012.11.039
Wang, S., Zhou, Y., Niu, J., Xie, Q., Xiao, T., Chen, Y., et al. (2017). Seroprevalence of Toxoplasma gondii Infection in Domestic Cats in Central China. Parasite 24, 10. doi: 10.1051/parasite/2017010
Yan, C., Fu, L. L., Yue, C. L., Tang, R. X., Liu, Y. S., Lv, L., et al. (2012). Stray Dogs as Indicators of Toxoplasma gondii Distributed in the Environment: The First Report Across an Urban-Rural Gradient in China. Parasit Vectors 5, 5. doi: 10.1186/1756-3305-5-5
Yang, Y. R., Feng, Y. J., Lu, Y. Y., Dong, H., Li, T. Y., Jiang, Y. B., et al. (2017). Antibody Detection, Isolation, Genotyping, and Virulence of Toxoplasma gondii in Captive Felids From China. Front. Microbiol. 8, 1414. doi: 10.3389/fmicb.2017.01414
Yang, N., Mu, M., Li, H., Hu, J., Gao, W., Yang, S., et al. (2013). Seroprevalence of Toxoplasma gondii Infection in Pet Dogs in Shenyang, Northeastern China. J. Parasitol. 99 (1), 176–177. doi: 10.1645/GE-3211.1
Yang, Y., Ying, Y., Verma, S. K., Cassinelli, A. B., Kwok, O. C., Liang, H., et al. (2015). Isolation and Genetic Characterization of Viable Toxoplasma gondii From Tissues and Feces of Cats From the Central Region of China. Vet. Parasitol. 211, 283–288. doi: 10.1016/j.vetpar.2015.05.006
Keywords: Toxoplasma gondii, seroprevalence, dogs, feces, PCR, China
Citation: Zhu N, Yang L, Xin S, Huang W, Jiang Y and Yang Y (2022) Low Prevalence of Toxoplasma gondii in Dogs From Central China. Front. Cell. Infect. Microbiol. 12:885348. doi: 10.3389/fcimb.2022.885348
Received: 28 February 2022; Accepted: 29 March 2022;
Published: 28 April 2022.
Edited by:
Razakandrainibe Romy, Université de Rouen, FranceReviewed by:
Sonia Almeria, United States Food and Drug Administration, United StatesCopyright © 2022 Zhu, Yang, Xin, Huang, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yibao Jiang, eWliYW9qaWFuZ0BzaW5hLmNvbQ==; Yurong Yang, eWFuZ3l1NzcxMkBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.