- 1Department of Microbiology, Karpagam Academy of Higher Education, Coimbatore, India
- 2Department of Biotechnology, Karpagam Academy of Higher Education, Coimbatore, India
- 3Post Graduate Research Department of Biotechnology and Bioinformatics, Holy Cross College (Autonomous), Tiruchirappalli, India
- 4Francis Graduate School of Biomedical Sciences, Texas Tech University Health Science Center of El Paso, Texas, TX, United States
- 5National Repository for Microalgae and Cyanobacteria (NRMC)- Marine, National Facility for Marine Cyanobacteria, (Sponsored by Department of Biotechnology (DBT), Government of India), Bharathidasan University, Tiruchirappalli, India
- 6Post Graduate and Research Department of Zoology, Yadava College, Madurai, India
- 7Department of Zoology, GTN Arts College, Dindigul, India
Emerging antibiotic resistance in bacteria endorses the failure of existing drugs with chronic illness, complicated treatment, and ever-increasing expenditures. Bacteria acquire the nature to adapt to starving conditions, abiotic stress, antibiotics, and our immune defense mechanism due to its swift evolution. The intense and inappropriate use of antibiotics has led to the development of multidrug-resistant (MDR) strains of bacteria. Phytochemicals can be used as an alternative for complementing antibiotics due to their variation in metabolic, genetic, and physiological fronts as well as the rapid evolution of resistant microbes and lack of tactile management. Several phytochemicals from diverse groups, including alkaloids, phenols, coumarins, and terpenes, have effectively proved their inhibitory potential against MDR pathogens through their counter-action towards bacterial membrane proteins, efflux pumps, biofilms, and bacterial cell-to-cell communications, which are important factors in promoting the emergence of drug resistance. Plant extracts consist of a complex assortment of phytochemical elements, against which the development of bacterial resistance is quite deliberate. This review emphasizes the antibiotic resistance mechanisms of bacteria, the reversal mechanism of antibiotic resistance by phytochemicals, the bioactive potential of phytochemicals against MDR, and the scientific evidence on molecular, biochemical, and clinical aspects to treat bacterial pathogenesis in humans. Moreover, clinical efficacy, trial, safety, toxicity, and affordability investigations, current status and developments, related demands, and future prospects are also highlighted.
Introduction
Origin of Antibiotic Resistance
Bacterial penicillinase was discovered by two members of the penicillin discovery team many years before the use of penicillin as a healing agent as discovered by Alexander Fleming in 1928. Resistant strains that deactivated the drug emerged as a result of the extensive use of antibiotics. Consequently, research to chemically acclimatize penicillin to stop cleavage by penicillinases (-lactamases) began (D’Costa et al., 2006). Following penicillin, streptomycin came to practice in 1944 for the treatment of tuberculosis (TB) (TB Alliance, 2019). During the course of clinical practice with streptomycin, resistant strains of Mycobacterium tuberculosis developed. Even though innovative findings of streptomycin and isoniazid were used to fight TB, there was still rapid progress in resistance. The administration of anti-TB drugs in cocktail form has developed as an important therapeutic routine with notable recovery; however, multidrug resistance remains constant to TB treatment throughout the world for a variety of reasons. In the last two decades, M. tuberculosis strains have become extremely drug-resistant (XDR) to front-line antibiotics, including ethionamide, para-amino salicylic acid, cycloserine, ofloxacin, amikacin, and ciprofloxacin. Later, they may become totally drug-resistant strains (Velayati et al., 2009). The evolution of multidrug resistance in M. tuberculosis through horizontal gene transfer (HGT) is not evidenced by any authenticated research. Hence, it is predicted that antibiotic resistance in M. tuberculosis might be attributed to spontaneous mutation.
Similarly, the most common Gram-negative pathogens, like Escherichia coli, Salmonella enterica, and Klebsiella pneumoniae, cause many diseases in humans. Since the past half-century, antibiotic resistance development was observed towards these diseases due to antibiotic misuse and overuse. Particularly, the lactam group of antibiotics and their associated inactivating lactamase enzymes are more prevalent; nearly 1,000 resistant lactamase groups have been reported (Livermore et al., 2006). The development and transmission of resistance to lactam antibiotics among enteric groups of bacteria in the community as well as in hospital infections is majorly increased by HGT. Another major nosocomial pathogen, Pseudomonas aeruginosa, originated from a burn wound infection in which the antibiotic resistance mechanisms progressed accidentally due to treatment with new antibiotic derivatives over the existing lactam and aminoglycosides. P. aeruginosa is extremely difficult for patients infected with cystic fibrosis since the pathogen is extremely persistent and has the ability to bypass the human defense mechanism. Prolonged antibiotic regime among cystic fibrosis patients is closely linked with resistance development.
Acinetobacter baumannii, a Gram-negative nosocomial pathogen, causes severe mortality and morbidity due to its R genes and pathogenicity factors (Peleg et al., 2008). Acinetobacter obtained pathogenic determinants due to their dynamic existence and biodegradation abilities in harsh environments; moreover, several strains in nature are competent for DNA uptake and have a high chance of spontaneous transformation. Recent molecular research reported that A. baumannii has rapid evolution, with a minimum of 28 genomic islands encrypting antibiotic resistance determinants; additionally, 50% of these inserts also translate virulence in the form of type IV secretion systems (Gomez and Neyfakh, 2006).
Staphylococcus aureus, a Gram-positive superbug, is highly associated with the human population as nasal commensal in 30% of the population, and its occurrence is linked with common skin infections. Unlike M. tuberculosis, it does not have a strong historical status, but S. aureus has developed as a major multidrug-resistant nosocomial infection (Enright et al., 2002). After the discovery of penicillin, S. aureus infections became manageable, but the strain developed resistance over the course of time. The innovative discovery of methicillin in 1959 was assumed to be an effective antibiotic against penicillinases, but within 3 years, the methicillin-resistant S. aureus (MRSA) developed. At present, MRSA has started to transfer, with higher virulence and transmission features, outside the hospital and stands as a major community-acquired pathogen (DeLeo and Chambers, 2009).
Due to the frequent use of antibiotics, the majority of epidemic bacterial pathogens related to human disease developed into multidrug-resistant (MDR) strains. “Superbugs” is the term given to describe microbes with higher morbidity and mortality due to numerous mutations. These “superbugs” result in increased resistance to antibiotics exactly prescribed for their treatment. Thus, the healing choices for these microbes become less with prolonged hospitalization. Sometimes the “superbugs” attained enhanced virulence and a higher level of transmission. As a result, antibiotic resistance was considered a potential virulence factor (Davies and Davies, 2010).
Carriers of Antibiotic Resistance
Understanding of several carriers of antibiotic resistance is an important fact needed to face the global problem. The essential features which are potential carriers of antibiotic resistance include sanitation settings, infection control standards, water quality, standard of drug, diagnostics and treatments, and migration quarantine. Apart from mutations, in diverse genes of the bacterial chromosome, the direct transfer of genetic material between organisms plays an important role in the circulation of antibiotic resistance. The transfer of plasmid among bacteria is one of the vital features which may transfer genes of antibiotic resistance to the host cell (Holmes et al., 2016). Antibiotics may influence this process by inducing the transmission of resistance elements; furthermore, they employ selective pressure to the development of resistance (Munita and Arias, 2016).
Sometimes the environment can offer a path for resistant bacteria to form colonies or infect host organisms (Mazel, 2006). This is referred to as “transmission event”, while variations in their DNA sequence as well as genetic transmission among bacterial species are considered as “evolution events”. In the case of a resistant pathogen that is already common among humans, the significance of a single transmission to one more person is more restricted than for an evolutionary event, resulting in the advent of a new, potential resistance genotype in pathogens with hypothetically global significances. Even though few pathogens, like Vibrio spp., survive in the environment, it is a comparatively unfriendly environment than a human or domestic animal host. Hence, development in the environment is quite limited for those kinds of pathogens. It is possible that minor growth changes between resistant and non-resistant strains, triggered by sub-minimum inhibitory concentration (MICs) of antibiotics, are a minor factor for the opportunity that environmental exposure becomes adequately enough for the colonization or infection of a host. The rest of the living and non-living features like temperature, oxygen pressure, nutrients, predation, and competition with other species, all discrete to the antibiotic resistance habit of the bacteria, are possible to be more significant for environmental transmission chances for both resistant and non-resistant bacterial strains (Larsson and Flach, 2021).
Basically, additional genetic elements present in bacteria have the capacity of up-taking resistance genes and helping their transmission; based on the genus of the pathogen, the nature of genetic factors differs. Plasmid-mediated resistance transmission is the most common mode of HGT (Norman et al., 2009). Unexpectedly, bacteriophages taking up antibiotic resistance genes have been reported in the environment or from resistant bacteria found in hospitals; there is still no inquiry about the connection of phages with the insertional mechanisms essential for the development of mobile resistance factors or with the functions of chromosomally linked genes. Usually, they are termed as “fingerprints”, flanking genes encrypting resistance or virulence on various vectors. These actions are found to be quite common in S. aureus. Among bacterial genera like Streptococci, Meningococci, and other related genera, the exchange of both virulence and pathogenicity genes is unlimited. The main mode of DNA transfer is found to be transformation (Springman et al., 2009). Acinetobacter spp. is competent in nature to uptake DNA directly from the environment with frequent HGT (Barbe, 2004) because pathogenic bacterial strains transfer large genomic islands (Perez et al., 2007). Throughout the history of bacterial evolution, HGT has occurred; two independent sets of actions should be taken into account, which is mainly distinguished by their time span and the strength of selection pressure. Bacterial evolution over billions of years cannot be related to the mode of antibiotic resistance development and transfer over the last century. The selection pressure of intense antibiotic treatment and clearance is even higher; the selection is majorly necessary for existence in hostile environments rather than for features offering resistance in gradually developing groups of populations.
Genetic Insights of Antibiotic Resistance
Bacterial resistance towards antibiotics might be native, a unique feature of specific bacterium which is based on its biological phenomena, whereas acquired resistance is obtained through (i) the attainment of exogenous genes by plasmids through conjugation, transposons (conjugation), integrons, and bacteriophages through transduction, (ii) gene mutation, and (iii) a blend of the above-mentioned processes (Mims et al., 2004). Generally, chromosomal mutations are occasional and control resistance to structurally similar compounds (Rice et al., 2003). These kinds of spontaneous mutations take place as mistakes during replication or a damaged DNA that escaped from the repair system. The antibiotic resistance of E. coli against quinolones developed due to alterations in a minimum of seven and three amino acids in the gyrA gene or parC gene, respectively (Džidić et al., 2008). In contrast, only a single-point mutation in the rpoB gene is related to a wide-ranging resistance to rifampin (Rice et al., 2003). Through mutation, antibiotic uptake or efflux system can be altered (Hooper, 2001). Adaptive mutations take place only during the nonlethal selection of microorganisms. In this mutation, the new gene holder gets deleted at a specific recombination site (attI site) and at a promoter that starts gene transcription. The majority of class I integrons in the 3′ conserved segment has a supplementary gene suII accountable for resistance to sulphonamides (Hooper, 2001; Daikos et al., 2007).
Out of 21 reported anti-microbial resistance (AMR) genes, the vital genes accountable for MDR Salmonella and E. coli are AmpC, bla-TEM-1, bla-CTXM-15, VIM-1, NDM-1, floR, and tetG and the recently found mcr-1 gene with resistance to colistin. Diverse modes of resistance and new transmission vectors and genes are reported consistently. Bacteria carry two mechanisms for resistance, known as intrinsic resistance and acquired resistance (Lynch et al., 2013). The capacity of a bacterium to overcome the attack of a particular antibiotic by innate structural or functional phenomena is called intrinsic resistance. Pseudomonas is an outstanding example of an intrinsic resistance mechanism because of the absence of a vulnerable target site for a specific antibiotic. Triclosan is a versatile antibiotic, particularly against Gram-positive bacteria and several Gram-negative bacteria, but is unable to control the growth of Pseudomonas. Besides this, they are highly resistant to aminoglycosides, quinolones, and β-lactams.
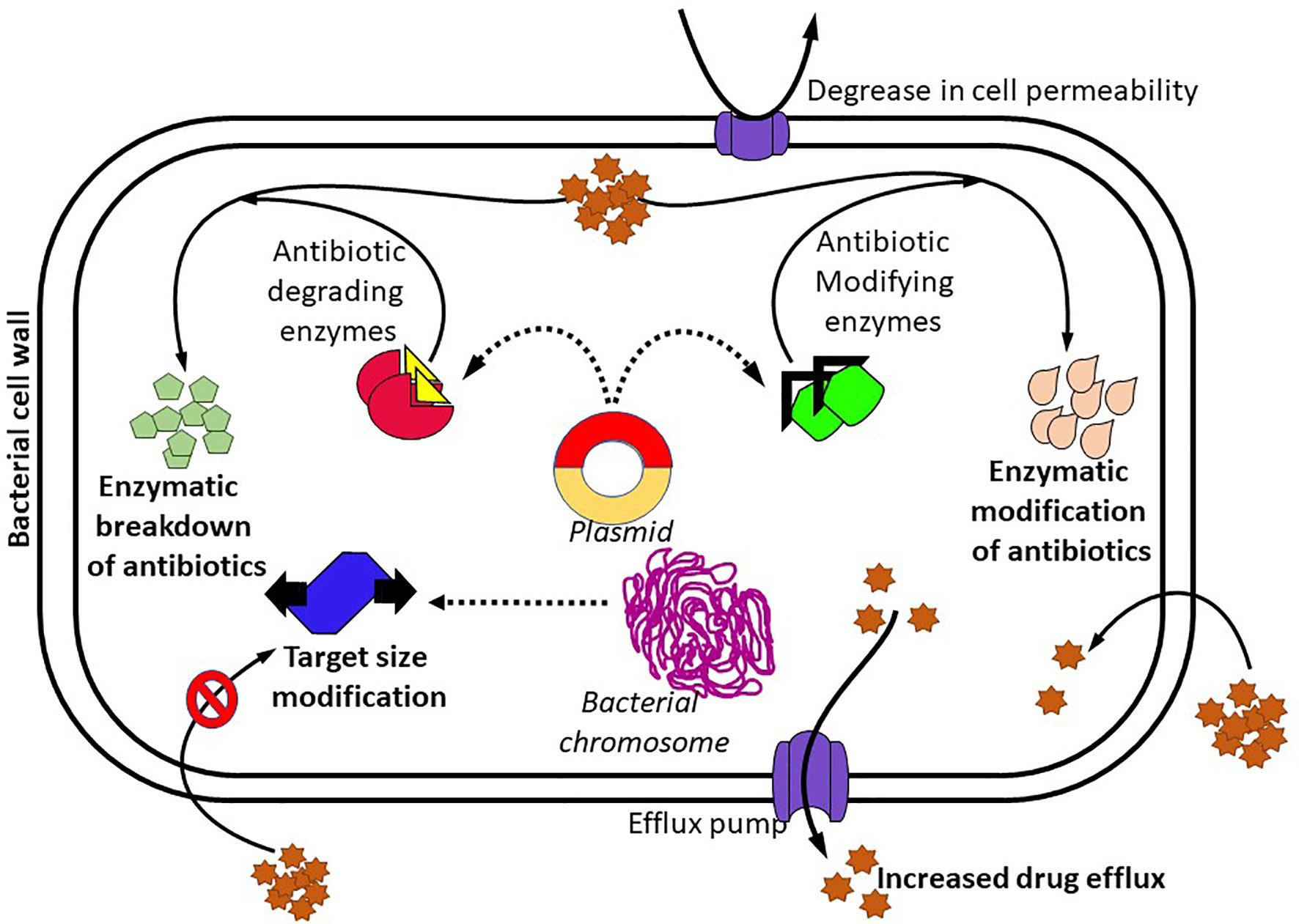
Moreover, various other processes have also been reported to be involved in microbial resistance against an antibiotic, including the upregulation of efflux pumps, structural modification of porins, enzyme synthesis, and cell-to-cell communication (Porras et al., 2020), and this is represented in Figure 1. Membrane proteins that have the ability to transfer antibiotics from the cell, thereby sustaining their low intracellular concentrations, are known as efflux pumps. When the permeability of the outer membrane (OM) gets lowered, the antibiotic uptake also gets reduced (Džidić et al., 2008). Assessment of efflux pumps is one of the most crucial factors in the analysis of antibiotic resistance. In single-component efflux systems, substrates are passed through the cytoplasmic membrane, but in Gram-negative bacteria, multicomponent pumps and a periplasmic membrane synthesis protein component transfer the substrates through the cell envelope (Alekshun and Levy, 2007; Džidić et al., 2008). Efflux pumps can be unique to each type of antibiotic. The majority of them are multidrug transporters that have the ability to pump various antibiotics like macrolides, tetracyclines, fluoroquinolones; thereby, it remarkably offers to MDR (Džidić et al., 2008). Frequently, bacteria resistant to tetracyclines secrete higher levels of membrane proteins which are used as efflux pumps for antimicrobial drugs. To remove toxic compounds from the cytoplasm and periplasm, P. aeruginosa utilizes more than four potential MDR efflux pumps (Strateva and Yordanov, 2009). MDR efflux pumps like MexV-MexW-OprM are responsible for resistance to antibiotics such as fluoroquinolones, tetracyclines, chloramphenicol, erythromycin, and acriflavine (Strateva and Yordanov, 2009). The higher-level expression of MexAB-OprM efflux pumps leads to increased inhibitory concentration against antibiotics like penicillins, cephalosporins, chloramphenicol, fluoroquinolones, macrolides, novobiocin, sulfonamides, tetracycline and trimethoprim, dyes (SYBR safe, Gelgreen), and detergents (SDS, Triton X-100) (Thomson and Bonomo, 2005). In Gram-negative bacteria, the β-lactam antibiotics can pass through a membrane protein occupied with a water molecule termed porin. When P. aeruginosa-specific OprD2 porin is absent, it results in resistance to imipenem, whereas resistance to meropenem takes place due to variations in the MexAB-OprM efflux system (Bradford, 2001; Džidić et al., 2008). Bacterial genera like Enterococcus aerogenes, Klebsiella spp., Proteus mirabilis, Serratia marcescens, Morganella morganii, H. influenzae, and Helicobacter pylori are reported to have homologs of Mex and Acr efflux systems (Piddock, 2006). The chief elimination system for macrolides, which is encrypted by the mef gene, is predominant in Gram-positive bacteria that is used for the removal of fluoroquinolones and aminoglycosides from the bacterial cell. E. coli and K. pneumoniae are comprised of an elimination system of tetracyclines and chloramphenicol, which is encoded by ramA gene. The same phenomena provide antibiotic resistance to norfloxacin (Grundmann et al, 2006).
The OM of Gram-negative bacteria encompasses an internal layer that contains phospholipids and an external layer that has the lipid A molecule. Hence, the nature of OM arrangement lessens drug uptake to a cell and passes via the OM. Antibiotics are transferred to a cell by the following mechanisms: (i) diffusion via porins, (ii) diffusion across the bilayer, and (iii) self-influenced uptake. The mode of transport is mainly based on the chemical composition of an antibiotic (Džidić et al., 2008). The reduced OM permeability of P. aeruginosa provides acquired resistance to multiple antibiotic groups. Little hydrophilic molecules, like β-lactams and quinolones, can pass through the OM only via porins. Acquired resistance is a distinctive feature of maximum resistance to almost all aminoglycosides, particularly to tobramycin, netilmicin, and gentamicin (Ferguson et al., 2007). Bacterial quorum sensing (QS), also called cell-to-cell communication, helps chemical signals, called autoinducers, activate to regulate pathogenic behaviors and assist bacteria to escape from antibiotics and host immune response. The three types of QS signals in bacteria are acyl-homoserine lactone, auto-inducing peptide, and autoinducer-2. QS signaling activation and subsequent biofilm formation lead to the antimicrobial resistance of the pathogens, thus increasing the therapy difficulty of bacterial diseases (Jiang et al., 2019).
Consequences of Antibiotic Resistance
Antibiotic-resistant bacteria are also termed as superbugs. The anxiety created by these organisms is not only relevant for the laboratory but has also emerged as a global risk responsible for the high death rate and lethal infections (Lipp et al., 2002). According to predicted statistical models, bacterial AMR caused an estimated 495 million deaths in 2019, with 127 million (95%) deaths attributed to bacterial AMR (Antimicrobial Resistance Collaborators, 2022). World Health Organization (WHO) has cautioned that a post-antibiotic period will be affected with infections often, and even minor wounds may lead to death if antibiotic resistance is not addressed properly. Multidrug-resistant bacteria cause more deaths worldwide. Several countries are fronting the problem of nosocomial infections through S. aureus as waves of clonal distribution. All over the world, MRSA strains are reported to be quickly spreading (Lowy, 2003). Assessed expenditure because of multidrug-resistant bacterial infection results in added healthcare charges with loss of outcome (Freire-Moran et al., 2011). The majority of the pharma corporations have the usual routine of antibiotic allocation, which may no longer be effective or missing regulatory sanctions (Levy and Marshall, 2004). According to the findings of the literature research, the cost of AMR is quite expensive and varies greatly by nation (Utt and Wells, 2016). According to a recent World Bank research, antibiotic resistance would increase the poverty rates and has a greater impact on low-income countries than the rest of the world (worldbank.org, 2019). According to studies, global GDP could fall by 1% year by 2050, with developing countries losing 5–7% of their GDP (Utt and Wells, 2016). This proportion equates to between 100 and 210 trillion US dollars (worldbank.org, 2019). By 2050, multidrug-resistant tuberculosis alone might cost the globe $16.7 trillion (tballiance, 2019). The World Bank research shows that global exports are increasing. The scientific report proved that more antibiotic practice may influence the increased frequency of resistant bacteria; however, the limited use of antibiotics still exhibited lower resistance rates. When antibiotics are administered too often or at random, it enhances selective pressure for bacteria to develop resistance (Laxminarayan and Brown, 2001).
Even though the excess use of antibiotics is strictly restricted all over the world, the over-prescription of antibiotics remains the same. Van Boeckel et al. (2015) reported that there will be around 67% rise in antibiotic consumption by 2030, which would nearly double in quickly developing and densely populated countries like Brazil, Russia, India, China, and South Africa (Van Boeckel et al., 2015). In modern medicine, antibiotic treatment is one of the important tactics to combat bacterial infections. The “golden era” of antibiotics extended from the 1930s to the 1960s, which gave rise to several antibiotics (Nathan and Cars, 2014). That era ended as scientists were unable to sustain the pace of antibiotic discovery in the aspect of evolving resistant bacterial pathogens. Constant failure in the discovery of new antibiotics and unlimited use of antibiotics are the influencing factors responsible for the advent of antibiotic resistance (Nathan, 2004). Hence, drug-resistant pathogens are considered the major alarm to healthcare sectors globally.
Automatous Insight on Phytochemicals to Overcome Drug Resistance
Due to the increasing efficiency of the development and spread of antibiotic-resistant strains, it is very imperative to determine a novel alternative and effective treatment measures to combat drug-resistant pathogens. Consequently, bioactive phytochemicals have been developed as an alternative to conventional antibiotics in combating such antibiotic-resistant pathogen-mediated infections. Many phytochemicals have demonstrated their potential as antimicrobial agents or antibiotic-reverting agents of prevailing antibiotics (Khare et al., 2021). These phytochemicals have proven to be suitable alternatives to address the development of antibiotic resistance associated with conventional antibiotics.
Plants are a rich source of phytochemicals with a great concern for novel drug discovery. In the present era, modern society relies on herbal medicine and ayurvedic medicine to overcome various diseases like impetigo contagiosa (Sharquie et al., 2000), chronic gastritis (Gaby, 2001), tuberculosis (Mativandlela et al., 2008), pediatric seizures (Akhondian et al., 2007), and urinary tract infections (Jepson et al., 2012). Fundamentally, phytochemicals are the chemical compounds that are synthesized in plant cells themselves to protect them from predators and pathogens. However, only a few of those plants have been explored and investigated (Gurnani et al., 2014). Crude bioactive compounds are extracted or isolated from plants or plant parts to test against various diseases and disorders due to the continuous evolution of resistant microorganisms, which is the prime risk factor to society in the present state of affairs.
Subsequently, therapeutic possibilities for the treatment of various microbial infections have become inadequate, leading to frequent infection and failure to cure or reduce the infection that increases morbidity and mortality, which was evident during the COVID-19 pandemic. Hence, it is needed to develop a novel alternative or complementary antimicrobial drug which is safer and non-toxic to health (Chitsazian-Yazdi et al., 2015). Herbal medicinal plants are a rich source of bioactive phytocompounds, which have potential against various diseases (Shakeri et al., 2018). Many of these plants or phytochemical compounds are proven to be applied in therapeutics. The satisfying medicinal properties of herbal medicinal plants are also active due to the accompanying phytocompounds, such as phenols, terpenoids, alkaloids, carotenoids, flavonoids, isothiocyanates, indoles, monoterpenes, etc. (Molyneux et al., 2007).
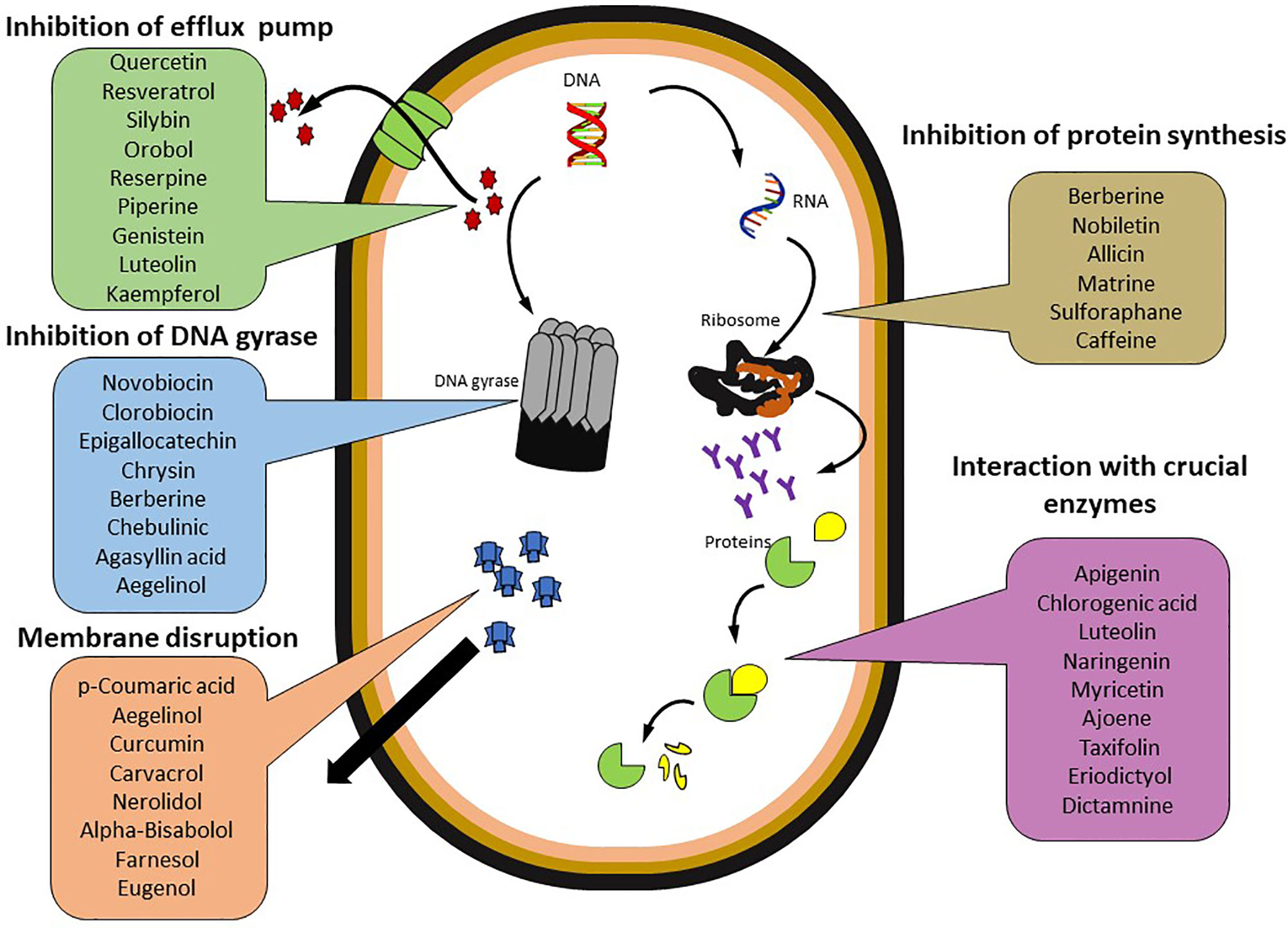
They have been shown to impede the major resistance-developing factors like efflux pumps, replication machinery, cell microstructure, membrane permeability and integrity, and other virulence mechanisms, including QS and biofilm development, which are essential for the victuals and resistance of pathogenic bacteria (Figure 2). Many of the phytochemicals have been ascertained to be effective against drug-resistant strains. Hence, by reviewing the mode of action, these phytochemical agents could pave the way towards the development of novel drugs. Besides bactericidal activity, several plant-derived compounds have also been discovered recently for their potential as adjuvants with antibiotics for re-sensitizing or reverting antibiotic resistance ability. These phytochemicals interfere with the structural membrane by increasing the cell permeability and cellular leakage, through a modification in the bacterial cell wall and cell membrane, resulting in the loss of ATP, attenuation of protein synthesis, destruction of intracytoplasmic, alteration in pH, fragmentation of DNA damage, inhibition of bacterial gene expression, ion binding, inhibition of DNA gyrase, free radical formation drug efflux pumps, mobile genetic elements, QS, and biofilm development (Bazzaz et al., 2018; Yu et al., 2020a).
Cell Membrane Inhibitors
It is a known fact that bacterial cell membranes act as a protective barrier against antimicrobial agents. Cell membrane permeability regulates the movement of antibiotics into the bacterial cell. It is believed that the mechanism of alteration in the fatty acid and membrane proteins, to monitor the cellular influx of the antibiotics, is reforming their membrane permeability (Yu et al., 2020b). Nevertheless, hydrophobic phytochemicals interact with membrane lipids in such a way to interrupt the cellular structure, eventually leading to higher membrane permeability. This makes bacterial cells unable to monitor the leakage of cellular molecules from the bacterial cells. Several research findings have confirmed the strong abilities of phytochemicals in targeting cell membrane permeability. The altered membrane permeability is possibly attributed to apparent damages to the cellular integrity and functions (Scazzocchio et al., 2017).
Cell Wall Synthesis Inhibitors
Cells are made up of peptidoglycan, which consists of repeating N‐acetylmuramic acid and N‐acetylglucosamine residues linked together by short chains of amino acids. The amino acid residues are the key components to provide strength and protection to bacteria. The synthesis of bacterial cell walls has been found to be inhibited by several phytochemicals (Upadhyay et al., 2014). The interaction of such phytochemicals with membrane proteins attached to bacterial cell walls eventually leads to an interruption in membrane penetrability. The effective antibacterial potential of phytochemicals belonging to flavonoids in counteracting infectious pathogens is attributed to their ability to complex with bacterial cell walls (AlSheikh et al., 2020).
Drug Efflux Pump Inhibitor
Bacterial efflux pumps, which diminish the concentration of the administered antibiotics by transporting the antibiotic molecules out of the cell, have evolved as important transporters in drug-resistant strains. As reviewed by Shriram et al. (2008), the bacterial efflux pumps are characterized in two super-families, namely, ATP-binding cassette multidrug transporters and secondary transporters using proton motive force based on their energy source. Further classifications are made based on the secondary transporters, which are further sub-classified into four families based on the substrate specificities; these include major facilitator superfamily, resistance nodulation cell division, multidrug and toxic compound extrusion, and small-MDR family (Putman et al., 2000; Sun et al., 2014). The presence of the efflux pumps in bacterial membranes enables the successful exclusion of the antibiotics out of the cell and thus prevents the active interaction of bacterial targets with antibiotics, leading to the development of resistance. Some phytochemicals are reported as efflux pump inhibitors (EPI) and thus revert antibiotic resistance. The antimicrobial activity of some phytochemicals against bacterial pathogens is conferred by the disruption of bacterial FtsZ Z-ring formation and the subsequent inhibition of bacterial cytokinesis (Kelley et al., 2012).
Mobile Genetic Elements
Plasmids are mobile genetic elements and are well recognized for transferring resistance genes through horizontal gene transfer among bacterial pathogens. Hence, the elimination of R-plasmid would reduce the transfer of resistance genes among bacteria. The antibacterial as well as resistance reversal potentials of phytochemicals, like essential oils, are attributed to their capability to obliterate R-plasmids. Several phytochemicals with plasmid curing ability have shown strong antibacterial activities when combined with antibiotics like amoxicillin, polymycin, and lincomycin (Si et al., 2008). Hence, the synergistic activity of phytochemicals with conventional antibiotics might possibly reduce the chance of developing drug resistance (Skalicka-Woźniak et al., 2018).
Enzyme Inhibitors
The antimicrobial potential of several phytochemicals has interconnection with nucleic acid synthesis by blocking the DNA gyrase enzyme which plays a vital role in the replication of DNA molecules (Wu et al., 2013). In some instances, phytochemicals, including flavonoids, are interrupted with helicase (DnaB and RecBCD) activity and hence prevent the DNA replication process (Xu, 2001).
Targeting Biofilm Formation and Quorum Sensing
Biofilms are the structural community of microbial populations enclosed in an exopolysaccharide matrix (Davey and O’toole, 2000), and their development is regulated by a QS mechanism, in which bacteria can communicate with each other through self-synthesized chemical signals. These signal molecules will be released into the surrounding environment. At threshold concentration, the signal molecule will bind with the appropriate receptor to form a signal receptor complex. Binding of the signal receptor complex with the promoter will, in turn, trigger the expression of virulence factors, such as secretion of virulence enzymes, antibiotic pigment production, extracellular polymeric substance production, and biofilm formation. The mechanisms of biofilm development and QS are reported to be highly effective approaches evolved by the bacteria for conferring drug resistance, its persistence, and spread. Therefore, targeting bacterial biofilms and quorum sensing are emerging as effective approaches for combatting drug resistance. Nevertheless, eliminating or impeding biofilm is challenging. However, several phytochemicals have been reported to exhibit antibiofilm and anti-QS activity. These compounds are considered as novel alternatives to antibiotics towards the prevention of biofilm formation by infectious pathogens. The attenuation of the transcription of genes critical for biofilm formation is attributed to the QS inhibitory activity of phytochemicals (Packiavathy et al., 2014).
Attenuating Bacterial Virulence
Capsular polysaccharides, produced by some bacteria, are considered as important factors and play a crucial role in the development of virulence (Taylor and Roberts, 2005) as well as to protect the bacteria from phagocytosis (Hyams et al., 2010). Capsular polysaccharides also aid in the adhesion and formation of biofilm. Additionally, capsular polysaccharides aid to enhance the survival rate of pathogens inside the host. Several bacteria displayed a reduced amount of capsular polysaccharide production upon exposure to plant-derived phytochemicals (Derakhshan et al., 2008). They are found effective in reducing the synthesis of capsule secretion by regulating the expression of bacterial regulators of capsule synthesis. Like quorum sensing, adhesion and capsular polysaccharides play a dynamic role in bacterial communication and growth inside the host; it becomes imperative to exploit them for therapeutics for overcoming the burden of increasing antibiotic resistance among microbes.
Exploring Phytochemicals for Combating Antibiotic Resistance Among Pathogenic Bacteria
Antibiotics comprise a crew of chemotherapeutic agents, either to kill (bactericidal) or to arrest (bacteriostatic) the bacteria to control microbial infections for, e.g., β-lactam antibiotics, tetracyclines, macrolide antibiotics, aminoglycosides, oxazolidinones, quinolones, lincosamides, cyclic peptides, and sulfa drugs (Gilbert and McBain, 2003). Conversely, the persistent usage of antibiotics is piloted to endure the selective pressures of their environment by the bacteria, resulting in the emergence of multi-drug resistance (Furuya and Lowy, 2006). Antibiotic-resistant infections are becoming a serious issue all over the world. A high proportion of nosocomial infections are instigated by MDR Gram-negative bacteria or by MRSA (Luyt et al., 2014). Similarly, vancomycin-resistant enterococci and an increasing number of bacterial pathogens are developing resistance to several conventional antibiotics (Golkar et al., 2014).
In 2013, the Center for Disease Control and Prevention stated the era as the “post-antibiotic era”, and the WHO warned that the emergence of antibiotic resistance is becoming a serious issue for the human race. Though the pharmaceutical industry developed diverse antibiotics to address resistance issues, the curing proportion of patients was comparatively less, making bacterial infections worse (Spellberg and Gilbert, 2014). As an alternative treatment of the bacterial resistance to antibiotics, plant-based antimicrobial agents displayed an effective role in combatting pathogenic bacteria without emerging resistance to these plant-derived phytochemicals, possibly by exploiting diverse mechanisms of action, which could prevent bacterial adaptation as reported (Essawi and Srour, 2000). The remarkable antimicrobial activity, nontoxic nature, and affordability of the discernible phytochemicals are the basis for their extensive usage as potential antimicrobial agents as well as antiseptics in clinical and industrial settings (Livermore, 2003). In the recent past, they have been employed as a source for the discovery of novel antibiotics in the pharmaceutical sector. It is noteworthy that natural products, in particular, plant extracts in the form of either pure compounds or crude extracts, offer boundless prospects towards the development of novel drug discoveries due to their unrivaled accessibility and chemical diversity.
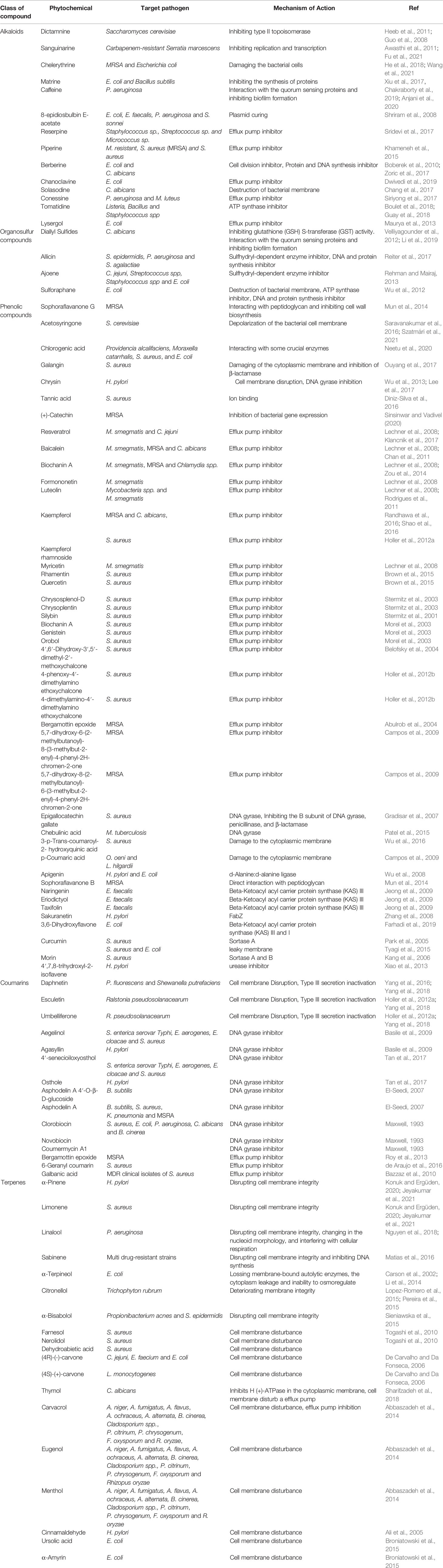
The evolution of MDR among bacterial pathogens has directed reconnoitering the perspective of phytochemicals sourced from plants as an alternative therapeutic approach to fight infectious diseases. Among the alternative and potential strategies against MDR pathogens, plant sources possibly play a vital role in offering a vast range of chemicals as secondary metabolites with potent action to combat bacterial infections (Anand et al., 2019; Anand et al., 2020). Such phytochemicals comprise various members of alkaloids, coumarins, flavonoids, quinones, etc. (Mbaveng et al., 2015; Anand et al., 2019; Anand et al., 2020; Mohammed et al., 2021). Owing to the potent applications of phytochemicals as antimicrobials, herbal medicines, food enhancements, and cosmetics, they have gained the attention of researchers; hence, several phytochemicals have been endorsed for their effective antimicrobial activities against various pathogenic bacteria, including MDR strains (Shriram et al., 2018; Anand et al., 2019; Yu et al., 2020a; Mohammed et al., 2021). Among the reported phytochemicals, the Food and Drug Administration approved a few of them based on clinical assessments. The effective role of various phytochemicals against multi-drug resistant pathogens has been reviewed (Figure 3 and Table 1).
Molecular and Biochemical Evidence of Phytochemicals to Treat Bacterial Pathogens
Attenuation of bacterial virulance is considered as a key role of phytochemicals to combat bacterial resistance potential. Interestingly, the chemical structure and the properties of natural phytocompounds reveal their antimicrobial potential by these mechanisms (Khameneh, et al., 2015). Hence, isolation and profiling of the bioactive-rich compounds, such as alkaloids, phenols, flavonoids, terpenoids, etc., owing to antimicrobial activity, is an essential part for the development of novel and natural antimicrobial drugs, and they have specific clinical importance due to their bioactivity which does not lead to resistance. Generally, these bioactive compounds are broadly classified as polyphenolics, alkaloids, tannins, glycosides, and steroids. Among these, polyphenols exhibit antimicrobial activity against a wide range of microorganisms. Particularly, polyphenolic compounds, such as flavanol and phenolic acids, were proven to have the greatest activity due to various scientific reasons, including attenuating the virulence factor of bacteria, including enzymes and toxins, dropping the extracellular polysaccharide activity, and performing as extracellular polysaccharide inhibitors. Much scientific research had evidently proved that an increase in the concentration of compounds stimulated the inhibition potential of pathogens (Bazzaz et al., 2018).
Alkaloids
Alkaloids are a cluster of heterocyclic nitrogenous compounds possessing wide-ranging antimicrobial potential. Alkaloids were proven to be an active antimicrobial agent due to the presence of heterocyclic compounds with highly flexible chemical structures. Alkaloids such as quinolone, dictamnine (Siriwong et al., 2015), and kokusagine, which are isolates of Teclea afzeli, showed antibacterial activity by enzymatic alteration, disturbing physiological processes such as restricting DNA synthesis and repair mechanisms (Yan et al., 2021). Many scientific reports suggest that the supreme groups of alkaloids, such as isoquinolines, aporphines, quinolones, and phenanthrenes, show suitable antibacterial activity against a wide range of bacterial pathogens, including B. cereus, S. aureus, and K. pneumonia (Porras et al., 2020), which can inhibit type II topoisomerase enzyme, subsequently hindering DNA replication, and reduce the consumption of O2 against bacteria. Plant-derived compounds such as curcumin, tannin, and piperine were proven to possess fantastic antimicrobial potential by directly targeting the DNA or protein. A combination of piperine, which was isolated from Piper nigrum, and ciprofloxacin attenuated the development of mutant S. aureus. Moreover, the administration of piperin and gentamicin has an inhibitory effect on multidrug-resistant organisms. Diterpenoid alkaloids, commonly isolated from plants, belong to Ranunculaceae and were reported to have antimicrobial properties. The mechanism of action of quaternary alkaloids, such as berberine and harmane, is accomplished by their ability to interpolate with DNA, thus leading to impairment in cell division and subsequent cell death (Boberek et al., 2010). Similarly, berberine has a serious antimicrobial potential against bacteria, fungi, protozoa, and even viruses by aiming at DNA intercalation, affecting RNA polymerase, gyrase, and topoisomerase, and by inhibiting cell division (Yi et al., 2007). The phytochemical compound Berberis spp. inhibited the growth of E. coli by blocking the synthesis of cell division and protein and DNA synthesis (Boberek et al., 2010). The antimicrobial compound chanoclavine, which was isolated from Ipomoea muricata, had shown synergistic activity when co-administered with tetracycline, which seems to inhibit EP, and reported as being effective and ATPase dependent (Dwivedi et al., 2019). Maurya et al. (2013) reported I. muricata-derived lysergol against E. coli by targeting the efflux pump. Another efflux pump inhibitor, reserpine, extracted from Rauwolfia serpentina, showed antimicrobial activity against Gram-positive pathogens Staphylococcus spp. and Streptococcus spp. Similarly, conessine, an alkaloid compound isolated from Holarrhena antidysenterica, displayed a potent inhibitory activity against P. aeruginosa by inhibiting the bacterial efflux pump (Siriyong et al., 2017). Sanguinarine, a benzophenanthridine alkaloid originating from the rhizomes of Sanguinaria canadensis, exhibited antimicrobial and anti-inflammatory properties. The antibacterial activity exhibited by this molecule is accomplished by the intrusion of bacterial cytokinesis (Kelley et al., 2012). The synergistic effect of this compound with vancomycin, and EDTA was found to be effective against Gram-negative bacteria (Hamoud et al., 2015). In MRSA strains, sanguinarine enables the release of membrane-bound cell wall autolytic enzymes, resulting in cell disruption (Obiang-Obounou et al., 2011).
Plasmid, a self-replicating, circular DNA coding for various gene groups, exhibits antibiotic resistance to bacteria. Some phytochemicals have been reported to target such plasmids (Buckner et al., 2018). 8-Epidiosbulbin-E-acetate, from Dioscorea bulbifera, is ascertained to cure the antibiotic-resistant R-plasmids of the clinical isolates of E. faecalis, E. coli, Shigella sonnei, and P. aeruginosa with effective curing efficacy (Shriram et al., 2008). Tomatidine, derived from Solanaceous plants, was documented to display antibacterial activity against Listeria, Bacillus, and Staphylococcus spp. The possible mechanism of action of tomatidine is postulated as an ATP synthase inhibitor (Guay et al., 2018).
Organosulfur Compounds
Allicin, an organosulfur compound from Allium sativum, has antibacterial activity against P. aeruginosa and S. epidermidis. The antibacterial action mechanism of allicin includes DNA synthesis inhibition, protein synthesis inhibition, and sulfhydryl-dependent enzyme inhibition (Reiter et al., 2017). Similarly, the investigation by Rehman and Mairaj (2013) suggested that the antimicrobial action of ajoene, from A. sativum, inhibits the sulfhydryl-dependent enzyme inhibitor of Campylobacter jejuni. The use of Diplotaxis harra-derived sulforaphane as an ATP synthase inhibitor and DNA/protein synthesis inhibitor was examined, and the results revealed that this compound effectively arrests the growth of E. coli. Furthermore, this compound has also been proven to destroy the membrane of the target pathogen (Li et al., 2017).
Phenolic Compounds
Phenolic compounds from plants are considered imperative molecules for drug discovery due to their broad spectral and important medicinal properties. The structure of phenolic compound plants includes an aromatic ring with one or more hydroxyl groups, and these are grouped into flavonoids, phenolic acids, and non-flavonoids (de Souza et al., 2019). They have been recognized as potent chemopreventive and therapeutic agents against diverse pathogenic bacteria and act as natural antimicrobial weapons by enhancing the sensitivity of MDR strains to antibiotics (Miklasińska-Majdanik et al., 2018; Makarewicz et al., 2021). Most notably, by reducing EP activity as the most significant mechanism, phenolic acids play a vital role in attenuating the resistance potential of various pathogens. Compounds such as resveratrol and flavanol are capable of inhibiting the activity of CmeABC Eps of C. jejuni or Eps of M. smegmatis (Klancnik et al., 2017). Furthermore, ferulic acid derivatives, 4-[E-2-(diethylcarbamoyl) vinyl]-2- methoxyphenyl acetate (E)-methyl 3-{4-[(p-tolylcarbamoyl) methoxy]-3-methoxyphenyl} acrylate, were found to exhibit antibacterial activity against MRSA by inhibiting the efflux pump (Sundaramoorthy et al., 2018). A similar kind of EPI activity was displayed by baicalein (Chan et al., 2011), kaempferol (Randhawa et al., 2016), and resveratrol (Klancnik et al., 2017) against MRSA and C. jejuni, respectively. The phenolic compound was also acknowledged as a beta-ketoacyl acyl carrier protein synthase inhibitor. As an example, taxifolin, from Allium cepa, showed an effective antibacterial activity against Enterococcus faecalis (Jeong et al., 2009).
Polyphenols (tannins) (Gradisar et al., 2007), chebulinic acid (Patel et al., 2015), and anthraquinones (Duan et al., 2014) are natural phenolic compounds that exhibit inhibition against DNA gyrase. Wu et al. (2016) revealed that a unique phenolic compound, 3-p-trans-coumaroyl-2-hydroxyquinic acid, extracted from Cedrus deodara showed antibacterial activity against 11 foodborne organisms. The mechanism of action of resistance against S. aureus would possibly cause damage to the cytoplasmic membrane and thereby cellular leakage of intracellular organelles due to hyperpolarization with loss of membrane integrity. It was believed that this CHA would be a better antimicrobial agent for the food and beverage industries. In general, compounds such as hydroxycinnamic acids (p-coumaric, caffeic, and ferulic acids) are other phenolic compounds that are capable of affecting membrane integrity. However, similar compounds, like p-coumaric acid, are believed to be the first prior compounds to have a potential activity due to their lipophilic nature (Campos et al., 2009; Wu et al., 2016). The results from Lanzotti et al. (2014) revealed antimicrobial activity accounting for the prevention of sulfhydryl-dependent enzymes, like alcohol dehydrogenase, thioredoxin reductase, and RNA polymerase, which was established by identifying the reduced inhibitory effect of allicin caused by the addition of cysteine and glutathione in the medium, reacting with its disulfide bond and resulting in the prevention of cellular damage. Besides this, allicin was proved to be an inhibitor of DNA and protein synthesis, which would be a possible target of allicin (Lanzotti et al., 2014). Phenolic compounds, such as pyrogallol and catechol, have been examined to show antimicrobial activity against a wide range of Gram-positive and Gram-negative bacteria. Pyrogallol and pyrocatechol were found to be effective against various oral pathogens (Shahzad et al., 2015). Additionally, halogenated catechols have also been investigated for their antimicrobial potential against various MDR strains by impeding the fatty acid synthesis of pathogenic bacteria (Liu et al., 2021). Borges et al. (2013) investigated the antibacterial activity of ferulic acid where it was found to be effective against P. aeruginosa and E. coli at MICs of 100 μg/ml. Similarly, gallic acid displayed antibacterial properties against Listeria monocytogenes, P. aeruginosa, and S. aureus. The antibacterial activity of ferulic acid and gallic acid is attributed to their ability in disrupting the cell walls of the target pathogens, leading to local damage and subsequent cellular material leakage. Similarly, gymnemic acid inhibited the biofilm development of Candida albicans and Streptococcus bordonii (Veerapandian and Vediyappan, 2019).
Flavonoids
Plant flavonoids are phenolic compounds holding a 2-phenyl-benzo-γ-pyrane nucleus and two benzene rings with potent antimicrobial activities. The various groups of flavonoids, such as flavanols, flavanones, isoflavonoids, chalcones, and dihydrochalcones, have been reported to exhibit antimicrobial properties (Górniak et al., 2019). Catechin causes membrane disruption in MRSA, which results in cell membrane damage by leakage of potassium ions. Budzyńska et al. (2011) analyzed the 3-arylideneflavanone-mediated membrane disruption, which leads to the accumulation of bacterial cells, resulting in the alteration of membrane integrity and enabling the increased permeability of pathogenic S. aureus and E. faecalis isolated from clinical samples. Interference of DNA synthesis activity was reported with flavonoid, chrysin, and kaempferol (Wu et al., 2013) and morin and myricetin (Xu, 2001). Some flavonoids have been reported as sensitizing agents. The combination of pinostrobin-a with antibiotic ciprofloxacin exhibited a synergistic effect to enhance the growth inhibitory potential of antibiotic-resistant strains P. aeruginosa and E. coli by blocking the EPI activity (Christena et al., 2015). Flavonoids are also recognized as inhibitors of quorum sensing and biofilm formation. Ouyang et al. (2016) demonstrated the QS and biofilm inhibitory activity of quercetin in P. aeruginosa PAO1. The QS activity of quercetin is attributed to the attenuated expressions of lasI, lasR, rhlI, and rhlR genes with decreased secretion of virulence factors like elastase, protease, and pyocyanin.
Terpenes
Terpenes, also called isoprenoids, are the largest single class of compounds present in essential oil and are made up of isoprene molecules (Praveen, 2018). Essential oils (EOs) consist of a combination of various phytochemicals and are highly recognized for their effective antimicrobial activity. Additionally, they have been employed as a traditional medicinal treatment to encounter antibiotic resistance since they are considered safe to consume and essential for host tissues (Yu et al., 2020b). Cox et al. (2001) reported the increased permeability of bacterial membrane upon treatment with EOs derived from Melaleuca alternifolia. Farnesol, a phytochemical isolated from essential oils, inhibited the growth of S. aureus by disrupting the cell membrane (Togashi et al., 2010). Methyl eugenol, present in the EOs of Cumium cymium, inhibited the biofilm formation and associated virulence of Gram-negative bacterial pathogens like P. aerugiosa, E. coli, Proteus mirabilis, and Serratia marcescens by attenuating the signal-based QS (Packiavathy et al., 2012). Similarly, the biofilms of uropathogenic bacteria demonstrated altered biofilm patterns in the presence of the quorum quencher molecule, Curcuma longa-derived curcumin (Packiavathy et al., 2014). The EOs of cinnamon displayed effective bactericidal activity against E. coli and Staphylococcus strains by altering the membrane permeability and structural integrity (Zhang et al., 2015). The EOs extracted from Coriandrum sativum inactivated the MDR uropathogenic E. coli strain by interrupting the cell membrane permeability (Scazzocchio et al., 2017). The striking antimicrobial activity of Plectranthus amboinicus-derived EOs against drug-resistant S. aureus is attributed to its biofilm inhibitory potential (Vasconcelos et al., 2017). The striking biofilm and QS inhibitory potential in reverting the resistance of S. aureus is attributed to the EOs of Satureja hortensis (Sharifi et al., 2018). An important compound, cis-cis-p-menthenolide, present in the EOs of Mentha suaveolens ssp. insularis was found to inhibit the signal-mediated QS system and biofilm formation of Chromobacterium violaceium. This compound exhibited a structural similarity to the natural signal molecule and hence acts as a competitive inhibitor, which could lead towards the blocking of gene expression and succeeding biofilm formation (Poli et al., 2018). EOs from Cinnamomum verum, Thymus vulgaris, and Eugenia caryophyllata were found to inhibit the growth of several MDR clinical isolates through the inhibition of biofilm and QS activities (Al1ibi et al., 2020). Very recently, a study by Önem (2022) displayed the QS-mediated biofilm inhibitory potential of Cymbopogon martini EOs and proved that the activity of these EOs is attributed to the phytochemical molecule geraniol.
As these phytochemicals have proved to inhibit the major resistance-creating factors such as efflux pumps, replication machineries, cell permeability, biofilm formation, and QS inhibition, they are considered crucial promising alternatives to overcome the decreasing activity of conventional antibiotics. The combinatorial application of these phytochemicals has proved to be highly effective against antibiotic-resistant strains. Hence, there is a pressing need for advanced research, scientific endorsement, and application of these phytochemicals to combat MDR pathogens.
Preclinical and Clinical Studies on the Antibacterial Effect of Phytochemicals
The transformation of in vitro studies to in vivo investigations and, finally, to human clinical trials is a great task in the improvement of novel phytocompounds. Various phytochemical medicinal plants exhibited antimicrobial activity, which can act as an alternative treatment to conventional medicine. However, it is expensive and time-consuming to bring a new novel drug/antibiotic to the market. Hence, the isolation of drugs from natural sources had extended its importance in the identification of chemical compounds with resistance properties (Mandal et al., 2014). The preclinical and clinical analysis guidelines for phytochemical compounds are required to safeguard the consistency in drug formulation, their efficacy, and their safety. Compounds isolated from herbal medicines, which were preclinically tested, against various infectious diseases and then licensed by completing the preclinical studies. It may be either one compound or two or more bioactive constituents being co-administered. Despite there being a vast number of bioactive compounds identified in recent centuries, only a few of them are examined via clinical studies. Moreover, most of the phytocompounds, when used as monotherapy, require a higher concentration in comparison with antibiotics. To address these problems, researchers focused on the combination of increased phytochemicals with less synthetic antibiotics to inhibit the resistance activity against various microbes (Touani et al., 2014; Santiago et al., 2015). To overcome the time consumption of these active phytocompounds as a drug on the market as a part of preclinical studies, in silico approach with natural phytocompounds were chosen on the basis of its bioactive constituents. Besides to interpret the characteristics of molecular structures such as the interactions of protein–ligand binding, an analysis of the quantitative structure activity by QSAR helps predict the compound with a specific target (Ahamad et al., 2017). Similarly, studies on pharmacophore models that simulate the 3D arrangements of particles with various physicochemical features are tangled in the interaction between ligand and target. A very common in silico approach is molecular docking, which proposes the structure–activity relationship on phytocompounds for revealing its mechanism of action and understanding the positioning of a ligand inside a protein-binding pocket (Fakhrudin et al., 2010; Zhang et al., 2011). Earlier studies revealed that more than 16,000 antimicrobial studies were registered in ClinicalTrials.gov from the year 2000. In approximately only 1 of all 10 registered scientific investigation studies were antimicrobial mediators assessed and investigated. The most common was interventional trials of drugs and biologicals, in which around 75% were randomized and about 26% were recruited for children along with adults. Diagonally between all completed interventional drug trials, only 12% had been rationalized through the investigation results (Stockmann et al., 2013). In agreement with the earlier reports, there are also some pharmacokinetic/pharmacodynamics evaluation studies which were registered in ClinicalTrials.gov since this is an essential and important component in safety and efficacy studies (Ross et al., 2012).
A standardized herbal concentration of “Tokoro Combination” and “Rehmannia and Akebia” was formulated as small granules. Both of these medicines were already approved by the ministry of health and welfare. These drugs consist of major compounds like diosgenin, yamogenin, betulin, oleanolic acid, hederagenin, akeboside, β-sitosterol, stigmasterol, inositol, catalpol, and glycyrrhizin. In the investigation of Girón et al. (1988), it was reported that the combination of Solanum nigrescens extract with nystatin showed better results in women. Both were provided as intravaginal suppositories in patients with long-established C. albicans vaginitis. The plant extract proved to be more effective when compared with nystatin. Similarly, cranberry juice was given for urinary tract infection, which was investigated in a team of elderly women who showed less bacterial infection in their urine than the untreated control groups (Avorn, 1996). A group of diverse ayurvedic formulations was examined against a placebo for their potency against acne vulgaris. Among these, Sunder Vati’s product revealed a significant reduction of lesion count in comparison with the other three formulations. Compounds such as Provir and Virend were clinically investigated against respiratory viral infection and topical antiherpes agents in 1994, and their safety and efficacy were studied in phase II studies. The extract of Opuntia streptacantha exhibited in vitro antiviral activity and was found to be safe in mice and humans (King and Tempesta, 2007), yet another compound, berberine, was proved to have a good result against various infections. A concentration above 64 µg/ml exhibited better results and was retained in the intestine, reaching an extraordinary benefit for intestinal infectious diseases and diarrhea (Lin et al., 2018). In the same way, there have been reports that Houttuynia cordata Thunb. has a medicinal property against various diseases, such as suppuration, sores, pustules, and respiratory infections, in Chinese pharmacopeia. A compound named houttuynin, which was isolated from H. cordata, exhibited antibacterial activity. The compounds isolated were used alone or in combination with conventional antibiotics to battle against infectious diseases (Hou et al., 2018; Liu et al., 2021).
Conclusion
As the emergence of antibiotic resistance among bacterial pathogens is becoming a major problem in treating infectious diseases, the progression of novel alternative treatment methods is therefore evolving rapidly against drug-resistant pathogens all over the world. As an alternative, phytochemicals have been employed to combat such infections instigated from antibiotic-resistant pathogens. So far, several plant-derived bioactive compounds (phytochemicals) have been reported for their bactericidal as well as antibiotic reversal potential. The bioactive potentials of such phytochemicals have been found to impede the important virulence factors associated with resistance development, such as cell permeability, efflux pumps, DNA replication mechanisms, and other processes linked with bacterial virulence, including biofilm formation and quorum sensing. Moreover, the synergistic effects of these phytochemicals with conventional antibiotics were found to be very effective against antibiotic-resistant pathogenic bacteria. Ultimately, several studies have proved the efficacy of phytochemicals as future drugs, the conversion success, and the scanty commercial use. Therefore, extreme progress is needed towards the commercialization of phytochemicals as proven drugs to encounter MDR-associated infections.
Author Contributions
Conceptualization: DAA. Writing—original draft: TS, IASVP, GSBA, VR, and NRD. Writing—review and editing: TS, IASVP, GSBA, SM, and DAA. Language correction and editing: AC. Supervision: DAA. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors acknowledge the University Grants Commission, India, for their support.
References
Abbaszadeh, S., Sharifzadeh, A., Shokri, H., Khosravi, A. R., Abbaszadeh, A. (2014). Antifungal Efficacy of Thymol, Carvacrol, Eugenol and Menthol as Alternative Agents to Control the Growth of Food-Relevant Fungi. J. Med. Mycol. 24 (2), e51–ee6. doi: 10.1016/j.mycmed.2014.01.063
Abulrob, A.-N., Suller, M. T. E., Gumbleton, M., Simons, C., Russell, A. D. (2004). Identification and Biological Evaluation of Grapefruit Oil Components as Potential Novel Efflux Pump Modulators in Methicillin-Resistant Staphylococcus aureus Bacterial Strains. Phytochemistry 65 (22), 3021–3027. doi: 10.1016/j.phytochem.2004.08.044
Ahamad, S., Rahman, S., Khan, F. I., Dwivedi, N., Ali, S., Kim, J., et al. (2017). QSAR Based Therapeutic Management of M. Tuberculosis. Arch. Pharmacal Res. 40, 676–694. doi: 10.1007/s12272-017-0914-1
Akhondian, J., Parsa, A., Rakhshande, H. (2007). The Effect of Nigella sativa L. (Black Cumin Seed) on Intractable Pediatric Seizures. Med. Sci. Monit. 13, CR555–CR559.
Al1ibi, S., Ben Selma, W., Ramos-Vivas, J., Smach, M. A., Touati, R., Boukadida, J., et al. (2020). Anti-Oxidant, Antibacterial, Anti-Biofilm, and Anti-Quorum Sensing Activities of Four Essential Oils Against Multidrug-Resistant Bacterial Clinical Isolates. Curr. Res. Trans. Med. 68, 59–66. doi: 10.1016/j.retram.2020.01.001
Alekshun, M. N., Levy, S. B. (2007). Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 128, 1037–1050. doi: 10.1016/j.cell.2007.03.004
Ali, S. M., Khan, A. A., Ahmed, I., Musaddiq, M., Ahmed, K. S., Polasa, H., et al. (2005). Antimicrobial Activities of Eugenol and Cinnamaldehyde Against the Human Gastric Pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 4 (20), 1–7. doi: 10.1186/1476-0711-4-20
AlSheikh, H. M. A., Sultan, I., Kumar, V., Rather, I. A., Al-Sheikh, H., Tasleem Jan, A., et al. (2020). Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 9, 480. doi: 10.3390/antibiotics9080480
Anand, U., Jacobo-Herrera, N., Altemimi, A., Lakhssassi, N. (2019). A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 9, 258. doi: 10.3390/metabo9110258
Anand, U., Nandy, S., Mundhra, A., Das, N., Pandey, D. K., Dey, A. (2020). A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents From Apocynaceae Family: Possible Therapeutic Approaches Against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist. Updates 51, 100695. doi: 10.1016/j.drup.2020.100695
Anjani, G., Widyastuti, N., Masruroh, Z., Yuliana, R. A. D., Almira, V. G., Tsani, A. F. A., et al. (2020). Bioactive Components and Antibacterial Activity in Robusta Coffee Leaves (Coffea canephora). Int. J. Pharm. Res. 12, 1374–1382. https://doc-pak.undip.ac.id/3862/3/C4-Artikel.pdf
Avorn, J. (1996). The Effect of Cranberry Juice on the Presence of Bacteria and White Blood Cells in the Urine of Elderly Women. Toward Anti Adhesion Ther. Microb. Dis. 408, 185–186. doi: 10.1007/978-1-4613-0415-9_21
Awasthi, D., Kumar, K., Ojima, I. (2011). Therapeutic Potential of FtsZ Inhibition: A Patent Perspective. Expert Opin. Ther. Patents 21, 657–679. doi: 10.1517/13543776.2011.568483
Barbe, V. (2004). Unique Features Revealed by the Genome Sequence of Acinetobacter Sp. ADP1, a Versatile and Naturally Transformation Competent Bacterium. Nucleic Acids Res. 32, 5766–5779. doi: 10.1093/nar/gkh910
Basile, A., Sorbo, S., Spadaro, V., Bruno, M., Maggio, A., Faraone, N., et al. (2009). Antimicrobial and Antioxidant Activities of Coumarins From the Roots of Ferulago campestris (Apiaceae). Molecules 14 (3), 939–952. doi: 10.3390/molecules14030939
Bazzaz, B. S. F., Khameneh, B., Ostad, M. R. Z., Hosseinzadeh, H. (2018). In Vitro Evaluation of Antibacterial Activity of Verbascoside, Lemon Verbena Extract and Caffeine in Combination With Gentamicin Against Drug-Resistant Staphylococcus aureus and Escherichia coli Clinical Isolates. Avicenna J. Phytomedicine 8, 246–253. doi: 10.22038/AJP.2018.15338.1607
Bazzaz, B. S. F., Memariani, Z., Khashiarmanesh, Z., Iranshahi, M., Naderinasab, M. (2010). Effect of Galbanic Acid, a Sesquiterpene Coumarin From Ferula szowitsiana, as an Inhibitor of Efflux Mechanism in Resistant Clinical Isolates of Staphylococcus aureus. Braz J. Microbiol. 41, 3, 574–580. doi: 10.1590/S1517-83822010000300006
Belofsky, G., Percivill, D., Lewis, K., Tegos, G. P., Ekart, J. (2004). Phenolic Metabolites of Dalea versicolor That Enhance Antibiotic Activity Against Model Pathogenic Bacteria. J. Natural Products 67 (3), 481–484. doi: 10.1021/np030409c
Boberek, J. M., Stach, J., Good, L. (2010). Genetic Evidence for Inhibition of Bacterial Division Protein FtsZ by Berberine. PloS One 5 (10), e13745. doi: 10.1371/journal.pone.0013745
Borges, A., Ferreira, C., Saavedra, M. J., Simões, M. (2013). Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 19, 256–265. doi: 10.1089/mdr.2012.0244
Boulet, M. L., Isabelle, C., Guay, I., Brouillette, E., Langlois, J. P., Jacques, P. E., et al. (2018). Tomatidine Is a Lead Antibiotic Molecule That Targets Staphylococcus aureus ATP Synthase Subunit C. Antimicrob. Agents Chemother. 62 (6), e02197–17. doi: 10.1128/AAC.02197-17
Bradford, P. A. (2001). Extended-Spectrum -Lactamases in the 21st Century: Characterization, Epidemiology, and Detection of This Important Resistance Threat. Clin. Microbiol. Rev. 14, 933–951. doi: 10.1128/cmr.14.4.933-951.2001
Broniatowski, M., Mastalerz, P., Flasiński, M. (2015). Studies of the Interactions of Ursane-Type Bioactive Terpenes With the Model of Escherichia coli Inner Membrane—Langmuir Monolayer Approach-. Biochimica Et Biophysica Acta - Biomembranes 1848, 2, 469–476. doi: 10.1016/j.bbamem.2014.10.024
Brown, A. R., Ettefagh, K. A., Todd, D., Cole, P. S., Egan, J. M., Foil, D. H., et al. (2015). A Mass Spectrometry-Based Assay for Improved Quantitative Measurements of Efflux Pump Inhibition. PloS One 10 (5), e0124814. doi: 10.1371/journal.pone.0124814
Buckner, M. M. C., Ciusa, M. L., Piddock, L. J. V. (2018). Strategies to Combat Antimicrobial Resistance: Anti-Plasmid and Plasmid Curing. FEMS Microbiol. Rev. 42, 781–804. doi: 10.1093/femsre/fuy031
Budzyńska, A., Rózalski, M., Karolczak, W., Wieckowska-Szakiel, M., Sadowska, B., Rózalska, B. (2011). Synthetic 3-Arylideneflavanones as Inhibitors of the Initial Stages of Biofilm Formation by Staphylococcus aureus and Enterococcus faecalis. Z. Naturforsch. C. J. Biosci. 66 (3-4), 104–114. doi: 10.1515/znc-2011-3-403
Campos, F. M., Couto, J. A., Figueiredo, A. R., Toth, I. V., Rangel, A. O., Hogg, T. A. (2009). Cell Membrane Damage Induced by Phenolic Acids on Wine Lactic Acid Bacteria. Int. J. Food Microbiol. 135 (2), 144–151. doi: 10.1016/j.ijfoodmicro.2009.07.031
Carson, C. F., Mee, B. J., Riley, T. V. (2002). Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 46, 1914–1920. doi: 10.1128/aac.46.6.1914-1920.2002
Chakraborty, P., Dastidar, D. G., Paul, P., Dutta, S., Basu, D., Sharma, S. R., et al. (2019). Inhibition of Biofilm Formation of Pseudomonas aeruginosa by Caffeine: A Potential Approach for Sustainable Management of Biofilm. Arch. Microbiol. 202, 623–635. doi: 10.1007/s00203-019-01775-0
Chang, W., Li, Y., Zhang, M., Zheng, S., Li, Y., Lou, H. (2017). Solasodine-3-O-Beta-D-Glucopyranoside Kills Candida albicans by Disrupting the Intracellular Vacuole. Food Chem. Toxicol. 106 (Pt A), 139–146. doi: 10.1016/j.fct.2017.05.045
Chan, B. C. L., Ip, M., Lau, C. B. S., Lui, S. L., Jolivalt, C., Ganem-Elbaz, C., et al. (2011). Synergistic Effects of Baicalein With Ciprofloxacin Against NorA Over-Expressed MRSA and Inhibition of MRSA Pyruvate Kinase. J. Ethnopharmacol. 137 (1), 767–773. doi: 10.1016/j.jep.2011.06.039
Chitsazian-Yazdi, M., Agnolet, S., Lorenz, S., Schneider, B., Es’haghi, Z., Kasaian, J., et al. (2015). Foetithiophenes C-F, Thiophene Derivatives From the Roots of Ferula foetida. Pharm. Biol. 53, 710–714. doi: 10.3109/13880209.2014.939765
Christena, L. R., Subramaniam, S., Vidhyalakshmi, M., Mahadevan, V., Sivasubramanian, A., Nagarajan, S. (2015). Dual Role of Pinostrobin-a Flavonoid Nutraceutical as an Efflux Pump Inhibitor and Antibiofilm Agent to Mitigate Food Borne Pathogens. RSC Adv. 5, 61881–61887. doi: 10.1039/c5ra07165h
Cox, S. D., Mann, C. M., Markham, J. L., Bell, H. C., Gustafson, J. E., Warmington, J. R., et al. (2001). The Mode of Antimicrobial Action of the Essential Oil of Melaleuca alternifolia (Tea Tree Oil). J. Appl. Microbiol. 88, 170–175. doi: 10.1046/j.1365-2672.2000.00943.x
Daikos, G. L., Kosmidis, C., Tassios, P. T., Petrikkos, G., Vasilakopoulou, A., Psychogiou, M., et al. (2007). Enterobacteriaceae Bloodstream Infections: Presence of Integrons, Risk Factors, and Outcome. Antimicrob. Agents Chemother. 51, 2366–2372. doi: 10.1128/aac.00044-07
Davey, M. E., O’toole, G. A. (2000). Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 64, 847–867. doi: 10.1128/mmbr.64.4.847-867.2000
Davies, J., Davies, D. (2010). Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 74, 417–433. doi: 10.1128/mmbr.00016-10
D’Costa, V. M., McGrann, K. M., Hughes, D. W., Wright, G. D. (2006). Sampling the Antibiotic Resistome. Science 311, 374–377. doi: 10.1126/science.1120800
de Araujo, R. S., Barbosa-Filho, J. M., Scotti, M. T., Scotti, L., da Cruz, R. M., Falcao-Silva Vdos, S., et al. (2016). Modulation of Drug Resistance in Staphylococcus aureus With Coumarin Derivatives. Scientifica (Cairo) 2016, 6894758. doi: 10.1155/2016/6894758
De Carvalho, C. C., Da Fonseca, M. M. R. (2006). Carvone: Why and How Should One Bother to Produce This Terpene. Food Chem. 95 (3), 413–422. doi: 10.1016/j.foodchem.2005.01.003
DeLeo, F. R., Chambers, H. F. (2009). Reemergence of Antibiotic-Resistant Staphylococcus aureus in the Genomics Era. J. Clin. Invest. 119, 2464–2474. doi: 10.1172/jci38226
Derakhshan, S., Sattari, M., Bigdeli, M. (2008). Effect of Subinhibitory Concentrations of Cumin (Cuminum cyminum L.) Seed Essential Oil and Alcoholic Extract on the Morphology, Capsule Expression and Urease Activity of Klebsiella pneumoniae. Int. J. Antimicrob. Agents 32, 432–436. doi: 10.1016/j.ijantimicag.2008.05.009
de Souza, E. L., de Albuquerque, T. M. R., dos Santos, A. S., Massa, N. M. L., de Brito Alves, J. L. (2019). Potential Interactions Among Phenolic Compounds and Probiotics for Mutual Boosting of Their Health-Promoting Properties and Food Functionalities – A Review. Crit. Rev. Food Sci. Nutr. 59, 1645–1659. doi: 10.1080/10408398.2018.1425285
Diniz-Silva, H. T., Cirino, I. C., da, S., Falcão-Silva, V.d. S., Magnani, M., de Souza, E. L., et al. (2016). Tannic Acid as a Potential Modulator of Norfloxacin Resistance in Staphylococcus aureus Overexpressing norA. Chemotherapy 61, 319–322. doi: 10.1159/000443495
Duan, F., Li, X., Cai, S., Xin, G., Wang, Y., Du, D., et al. (2014). Haloemodin as Novel Antibacterial Agent Inhibiting DNA Gyrase and Bacterial Topoisomerase I. J. Medicinal Chem. 57, 3707–3714. doi: 10.1021/jm401685f
Dwivedi, G. R., Maurya, A., Yadav, D. K., Singh, V., Khan, F., Gupta, M. K., et al. (2019). Synergy of Clavine Alkaloid 'Chanoclavine' With Tetracycline Against Multi-Drug-Resistant E. coli. J. Biomol. Struct. Dyn. 37 (5), 1307–1325. doi: 10.1080/07391102.2018.1458654
Džidić, S., Šušković, J., i Kos, B. (2008). Antibiotic Resistance Mechanisms in Bacteria: Biochemical and Genetic Aspects. Food Technol. Biotechnol. 46 (1), 11–21. doi: https://hrcak.srce.hr/file/34842
El-Seedi, H. R. (2007). Antimicrobial Arylcoumarins From Asphodelus Microcarpus. J. Natural Products 70 (1), 118–120. doi: 10.1021/np060444u
Enright, M. C., Robinson, D. A., Randle, G., Feil, E. J., Grundmann, H., Spratt, B. G. (2002). The Evolutionary History of Methicillin-Resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. 99, 7687–7692. doi: 10.1073/pnas.122108599
Essawi, T., Srour, M. (2000). Screening of Some Palestinian Medicinal Plants for Antibacterial Activity. J. Ethnopharmacol. 70, 343–349. doi: 10.1016/s0378-8741(99)00187-7
Fakhrudin, N., Ladurner, A., Atanasov, A. G., Heiss, E. H., Baumgartner, L., Markt, P., et al. (2010). Computer-Aided Discovery, Validation, and Mechanistic Characterization of Novel Neolignan Activators of Peroxisome Proliferator-Activated Receptor γ. Mol. Pharmacol. 77, 559–566. doi: 10.1124/mol.109.062141
Farhadi, F., Khameneh, B., Iranshahi, M., Iranshahy, M. (2019). Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phytother. Res. 33 (1), 13–40. doi: 10.1002/ptr.6208
Ferguson, C. J., Miguel, C. S., Kilburn, J. C., Sanchez, P. (2007). The Effectiveness of School-Based Anti-Bullying Programs. Criminal Justice Rev. 32, 401–414. doi: 10.1177/0734016807311712
Freire-Moran, L., Aronsson, B., Manz, C., Gyssens, I. C., So, A. D., Monnet, D. L., et al. (2011). Critical Shortage of New Antibiotics in Development Against Multidrug-Resistant Bacteria—Time to React is Now. Drug Resist. Updates 14, 118–124. doi: 10.1016/j.drup.2011.02.003
Fu, Y., Liu, W., Liu, M., Zhang, J., Yang, M., Wang, T., et al. (2021). In Vitro Anti-Biofilm Efficacy of Sanguinarine Against Carbapenem-Resistant Serratia marcescens. Biofouling 37, 341–351. doi: 10.1080/08927014.2021.1919649
Furuya, E. Y., Lowy, F. D. (2006). Antimicrobial-Resistant Bacteria in the Community Setting. Nat. Rev. Microbiol. 4, 36–45. doi: 10.1038/nrmicro1325
Gaby, A. R. (2001). Helicobacter pylori Eradication: Are There Alternatives to Antibiotics? Altern. Med. Rev. 6, 355–366.
Gilbert, P., McBain, A. J. (2003). Potential Impact of Increased Use of Biocides in Consumer Products on Prevalence of Antibiotic Resistance. Clin. Microbiol. Rev. 16, 189–208. doi: 10.1128/cmr.16.2.189-208.2003
Girón, L. M., Aguilar, G. A., Cáceres, A., Arroyo, G. L. (1988). Anticandidal Activity of Plants Used for the Treatment of Vaginitis in Guatemala and Clinical Trial of a Solanum nigrescens Preparation. J. Ethnopharmacol. 22, 307–313. doi: 10.1016/0378-8741(88)90241-3
Golkar, Z., Bagasra, O., Pace, D. G. (2014). Bacteriophage Therapy: A Potential Solution for the Antibiotic Resistance Crisis. J. Infect. Developing Countries 8, 129–136. doi: 10.3855/jidc.3573
Gomez, M. J., Neyfakh, A. A. (2006). Genes Involved in Intrinsic Antibiotic Resistance of Acinetobacter baylyi. Antimicrob. Agents Chemother. 50, 3562–3567. doi: 10.1128/aac.00579-06
Górniak, I., Bartoszewski, R., Króliczewski, J. (2019). Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 18, 241–272. doi: 10.1007/s11101-018-9591-z
Gradisar, H., Pristovsek, P., Plaper, A., Jerala, R. (2007). Green Tea Catechins Inhibit Bacterial DNA Gyrase by Interaction With its ATP Binding Site. J. Medicinal Chem. 50 (2), 264–271. doi: 10.1021/jm060817o
Grundmann, H., Aires-de-Sousa, M., Boyce, J., Tiemersma, E. (2006). Emergence and Resurgence of Meticillin-Resistant Staphylococcus aureus as a Public-Health Threat. Lancet 368, 874–885. doi: 10.1016/s0140-6736(06)68853-3
Guay, I., Boulanger, S., Isabelle, C., Brouillette, E., Chagnon, F., Bouarab, K., et al. (2018). Tomatidine and Analog FC04-100 Possess Bactericidal Activities Against Listeria, Bacillus and Staphylococcus Spp. BMC Pharmacol. Toxicol. 19 (1), 7. doi: 10.1186/s40360-018-0197-2
Guo, N., Yu, L., Meng, R., Fan, J., Wang, D., Sun, G., et al. (2008). Global Gene Expression Profile of Saccharomyces cerevisiae Induced by Dictamnine. Yeast 25, 631–641. doi: 10.1002/yea.1614
Gurnani, N., Mehta, D., Gupta, M., Mehta, B. K. (2014). Natural Products: Source of Potential Drugs. Afr J. of Basic and Appl. Sci. 6, 171–186.doi: 10.5829/idosi.ajbas.2014.6.6.21983
Hamoud, R., Reichling, J., Wink, M. (2015). Synergistic Antimicrobial Activity of Combinations of Sanguinarine and EDTA With Vancomycin Against Multidrug Resistant Bacteria. Drug Metab. Lett. 8, 119–128. doi: 10.2174/187231280802150212100742
Heeb, S., Fletcher, M. P., Chhabra, S. R., Diggle, S. P., Williams, P., Cámara, M. (2011). Quinolones: From Antibiotics to Autoinducers. FEMS Microbiol. Rev. 35, 247–274. doi: 10.1111/j.1574-6976.2010.00247.x
He, N., Wang, P., Wang, P., Ma, C., Kang, W. (2018). Antibacterial Mechanism of Chelerythrine Isolated From Root of Toddalia asiatica (Linn) Lam. BMC Complement. Altern. Med. 18, 1–9. doi: 10.1186/s12906-018-2317-3
Holler, J. G., Christensen, S. B., Slotved, H.-C., Rasmussen, H. B., Guzman, A., Olsen, C.-E., et al. (2012a). Novel Inhibitory Activity of the Staphylococcus aureus NorA Efflux Pump by a Kaempferol Rhamnoside Isolated From Persea lingue Nees. J. Antimicrob. Chemother. 67, 1138–1144. doi: 10.1093/jac/dks005
Holler, J. G., Slotved, H.-C., Mølgaard, P., Olsen, C. E., Christensen, S. B. (2012b). Chalcone Inhibitors of the NorA Efflux Pump in Staphylococcus aureus Whole Cells and Enriched Everted Membrane Vesicles. Bioorganic Medicinal Chem. 20 (14), 4514–4521. doi: 10.1016/j.bmc.2012.05.025
Holmes, A. H., Moore, L. S. P., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A., et al. (2016). Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 387, 176–187. doi: 10.1016/s0140-6736(15)00473-0
Hooper, D. C. (2001). Minimizing Potential Resistance: The Molecular View—A Comment on Courvalin and Trieu-Cuot. Clin. Infect. Dis. 33, S157–S160. doi: 10.1086/321842
Hou, B.-Y., Zhang, L., Du, G. H. (2018). Natural Small Molecule Drugs From Plants (Singapore: Springer), 415–420.
Hyams, C., Camberlein, E., Cohen, J. M., Bax, K., Brown, J. S. (2010). The Streptococcus pneumoniae Capsule Inhibits Complement Activity and Neutrophil Phagocytosis by Multiple Mechanisms. Infect. Immun. 78, 704–715. doi: 10.1128/iai.00881-09
Jeong, K. W., Lee, J. Y., Kang, D. I., Lee, J. U., Shin, S. Y., Kim, Y. (2009). Screening of Flavonoids as Candidate Antibiotics Against Enterococcus faecalis. J. Natural Products 72 (4), 719–724. doi: 10.1021/np800698d
Jepson, R. G., Mihaljevic, L., Craig, J. (2012). Cranberries for Preventing Urinary Tract Infections. Database Syst Rev. 4, 2012. doi: 10.1002/14651858.CD001321.pub5
Jeyakumar, G. E., Lawrence, R. (2021). Mechanisms of Bactericidal Action of Eugenol Against. Escherichia coli. J. Herbal Med. 26, 100406. doi: 10.1016/j.hermed.2020.100406
Jiang, Q., Chen, J., Yang, C., Yin, Y., Yao, K. (2019). Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed. Res. Int. 2015978, 1–15. doi: 10.1155/2019/2015978
Kang, S. S., Kim, J. G., Lee, T. H., Oh, K. B. (2006). Flavonols Inhibit Sortases and Sortase-Mediated Staphylococcus aureus Clumping to Fibrinogen. Biol. Pharm. Bull. 29 (8), 1751–1755. doi: 10.1248/bpb.29.1751
Kelley, C., Zhang, Y., Parhi, A., Kaul, M., Pilch, D. S., LaVoie, E. J. (2012). 3-Phenyl Substituted 6,7-Dimethoxyisoquinoline Derivatives as FtsZ-Targeting Antibacterial Agents. Bioorganic Medicinal Chem. 20, 7012–7029. doi: 10.1016/j.bmc.2012.10.009
Khameneh, B., Diab, R., Ghazvini, K., Fazly Bazzaz, B. S. (2016). Breakthroughs in Bacterial Resistance Mechanisms and the Potential Ways to Combat Them. Microb. Pathogen. 95, 32–42. doi: 10.1016/j.micpath.2016.02.009
Khameneh, B., Iranshahy, M., Ghandadi, M., Atashbeyk, D. G., Bazzaz, B. S. F., Iranshahi, M. (2015). Investigation of the Antibacterial Activity and Efflux Pump Inhibitory Effect of Co-Loaded Piperine and Gentamicin Nanoliposomes in Methicillin-Resistant Staphylococcus aureus. Drug Dev. Ind. Pharm. 41 (6), 989–994. doi: 10.3109/03639045.2014.920025
Khare, T., Anand, U., Dey, A., Assaraf, Y. G., Chen, Z.-S., Liu, Z., et al. (2021). Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 12. doi: 10.3389/fphar.2021.720726
King, S. R., Tempesta, M. S. (2007). From Shaman to Human Clinical Trials: The Role of Industry in Ethnobotany, Conservation and Community Reciprocity. Novartis Foundation Symp. 2007, 4–24. doi: 10.1002/9780470514634.ch14
Klancnik, A., Pogacar, M. S., Trost, K., Znidaric, M. T., Vodopivec, B. M., Smole, M. S. (2017). Anti-Campylobacter Activity of Resveratrol and an Extract From Waste Pinot Noir Grape Skins and Seeds, and Resistance of Camp. Jejuni Planktonic and Biofilm Cells, Mediated via the CmeABC Efflux Pump. J. Appl. Microbiol. 122 (1), 65–77. doi: 10.1111/jam.13315
Klančnik, A., Šikić Pogačar, M., Trošt, K., Tušek Žnidarič, M., Mozetič Vodopivec, B., Smole Možina, S. (2016). Anti-Campylobacteractivity of Resveratrol and an Extract From Waste Pinot Noir Grape Skins and Seeds, and Resistance Ofcamp. Jejuniplanktonic and Biofilm Cells, Mediated via the CmeABC Efflux Pump. J. Appl. Microbiol. 122, 65–77. doi: 10.1111/jam.13315
Konuk, H. B., Ergüden, B. (2020). Phenolic –OH Group Is Crucial for the Antifungal Activity of Terpenoids via Disruption of Cell Membrane Integrity. Folia Microbiol. 65, 775–783. doi: 10.1007/s12223-020-00787-4
Lanzotti, V., Scala, F., Bonanomi, G. (2014). Compounds From Allium Species With Cytotoxic and Antimicrobial Activity. Phytochem. Rev. 13, 769–791. doi: 10.1007/s11101-014-9366-0
Larsson, D. G. J., Flach, C.-F. (2021). Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 20, 1–13. doi: 10.1038/s41579-021-00649-x
Laxminarayan, R., Brown, G. M. (2001). Economics of Antibiotic Resistance: A Theory of Optimal Use. J. Environ. Economics Manage. 42, 183–206. doi: 10.1006/jeem.2000.1156
Lechner, D., Gibbons, S., Bucar, F. (2008). Plant Phenolic Compounds as Ethidium Bromide Efflux Inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 62 (2), 345–348. doi: 10.1093/jac/dkn178
Lee, B. W., Park, I.-H., Yim, D., Choi, S. S. (2017). Comprehensive Evaluation of the Anti-Helicobacter pylori Activity of Scutellariae radix. Natural Product Sci. 23, 46. doi: 10.20307/nps.2017.23.1.46
Levy, S.B, Marshall, B (2004). Antibacterial Resistance Worldwide: Causes, Challenges and Responses. Nat. Med. 10(12 Suppl):S122–129. doi: 10.1038/nm1145
Li, J., Koh, J.-J., Liu, S., Lakshminarayanan, R., Verma, C. S., Beuerman, R. W. (2017). Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 11. doi: 10.3389/fnins.2017.00073
Li, W.-R., Ma, Y.-K., Xie, X.-B., Shi, Q.-S., Wen, X., Sun, T.-L., et al. (2019). Diallyl Disulfide From Garlic Oil Inhibits Pseudomonas aeruginosa Quorum Sensing Systems and Corresponding Virulence Factors. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03222
Lin, Y., Si, S. Y., Jiang, J. D. (2018). Antibacterial Activity of Berberine. Yaoxue Xuebao 53, 163–168. doi: 10.16438/j.0513-4870.2017-0816
Lipp, E. K., Huq, A., Colwell, R. R. (2002). Effects of Global Climate on Infectious Disease: The Cholera Model. Clin. Microbiol. Rev. 15, 757–770. doi: 10.1128/cmr.15.4.757-770.2002
Li, L., Shi, C., Yin, Z., Jia, R., Peng, L., Kang, S., et al. (2014). Antibacterial Activity of α-Terpineol may Induce Morphostructural Alterations in Escherichia coli. Braz. J. Microbiol. 45, 1409–1413. doi: 10.1590/s1517-83822014000400035
Liu, B., Zhou, C., Zhang, Z., Roland, J. D., Lee, B. P. (2021). Antimicrobial Property of Halogenated Catechols. Chem. Eng. J. 403, 126340. doi: 10.1016/j.cej.2020.126340
Livermore, D. M. (2003). Bacterial Resistance: Origins, Epidemiology, and Impact. Clin. Infect. Dis. 36, S11–S23. doi: 10.1086/344654
Livermore, D. M., Canton, R., Gniadkowski, M., Nordmann, P., Rossolini, G. M., Arlet, G., et al. (2006). CTX-M: Changing the Face of ESBLs in Europe. J. Antimicrob. Chemother. 59, 165–174. doi: 10.1093/jac/dkl483
Lopez-Romero, J. C., González-Ríos, H., Borges, A., Simões, M. (2015). Antibacterial Effects and Mode of Action of Selected Essential Oils Components Against Escherichia coli and Staphylococcus aureus. Evidence-Based Complement. Altern. Med. 2015, 1–9. doi: 10.1155/2015/795435
Lowy, F. D. (2003). Antimicrobial Resistance: The Example of Staphylococcus aureus. J. Clin. Invest. 111, 1265–1273. doi: 10.1172/jci18535
Lu, X., Yang, X., Li, X., Lu, Y., Ren, Z., Zhao, L., et al. (2013). In Vitro Activity of Sodium New Houttuyfonate Alone and in Combination With Oxacillin or Netilmicin Against Methicillin-Resistant Staphylococcus aureus. PloS One 8, e68053. doi: 10.1371/journal.pone.0068053
Luyt, C.-E., Bréchot, N., Trouillet, J.-L., Chastre, J. (2014). Antibiotic Stewardship in the Intensive Care Unit. Crit. Care 18(480), 1–12. doi: 10.1186/s13054-014-0480-6
Lynch, J. P., Clark, N. M., Zhanel, G. G. (2013). Evolution of Antimicrobial Resistance Among Enterobacteriaceae (Focus on Extended Spectrum β-Lactamases and Carbapenemases). Expert Opin. Pharmacother. 14, 199–210. doi: 10.1517/14656566.2013.763030
Makarewicz, M., Drożdż, I., Tarko, T., Duda-Chodak, A. (2021). The Interactions Between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 10 (188), 1–70. doi: 10.3390/antiox10020188
Mandal, S. M., Roy, A., Ghosh, A. K., Hazra, T. K., Basak, A., Franco, O. L. (2014). Challenges and Future Prospects of Antibiotic Therapy: From Peptides to Phages Utilization. Front. Pharmacol. 5. doi: 10.3389/fphar.2014.00105
Matias, E. F. F., Alves, E. F., Silva, M. K. N., Carvalho, V. R. A., Figueredo, F. G., Ferreira, J. V. A., et al. (2016). Seasonal Variation, Chemical Composition and Biological Activity of the Essential Oil of Cordia verbenacea DC (Boraginaceae) and the Sabinene. Ind. Crops Products 87, 45–53. doi: 10.1016/j.indcrop.2016.04.028
Mativandlela, S. P. N., Meyer, J. J. M., Hussein, A. A., Houghton, P. J., Hamilton, C. J., Lall, N. (2008). Activity Against Mycobacterium smegmatis and M. tuberculosis by Extract of South African Medicinal Plants. Phytother. Res. 22, 841–845. doi: 10.1002/ptr.2378
Maurya, A., Dwivedi, G. R., Darokar, M. P., Srivastava, S. K. (2013). Antibacterial and Synergy of Clavine Alkaloid Lysergol and its Derivatives Against Nalidixic Acid-Resistant Escherichia coli. Chem. Biol. Drug Design 81 (4), 484–490. doi: 10.1111/cbdd.12103
Maxwell, A. (1993). The Interaction Between Coumarin Drugs and DNA Gyrase. Mol. Microbiol. 9 (4), 681–686. doi: 10.1111/j.1365-2958.1993.tb01728.x
Mazel, D. (2006). Integrons: Agents of Bacterial Evolution. Nat. Rev. Microbiol. 4, 608–620. doi: 10.1038/nrmicro1462
Mbaveng, A. T., Sandjo, L. P., Tankeo, S. B., Ndifor, A. R., Pantaleon, A., Nagdjui, B. T., et al. (2015). Antibacterial Activity of Nineteen Selected Natural Products Against Multi-Drug Resistant Gram-Negative Phenotypes. SpringerPlus 4, 823. doi: 10.1186/s40064-015-1645-8
Miklasińska-Majdanik, M., Kępa, M., Wojtyczka, R., Idzik, D., Wąsik, T. (2018). Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 15, 2321. doi: 10.3390/ijerph15102321
Mims, C., Dockrell, H. M., Goring, R. V., Roitt, I., Wakelin, D., Zuckerman, M. (2004). Medical Microbiology. 3rd Edition (Edinburgh, UK:Mosby) Pp-648. Available at: https://researchonline.lshtm.ac.uk/id/eprint/14346.
Mohammed, M. J., Anand, U., Altemimi, A. B., Tripathi, V., Guo, Y., Pratap-Singh, A. (2021). Phenolic Composition, Antioxidant Capacity and Antibacterial Activity of White Wormwood (Artemisia herba-alba). Plants 10, 164. doi: 10.3390/plants10010164
Molyneux, R. J., Lee, S. T., Gardner, D. R., Panter K.E. and James, L. F. (2007). Phytochemicals: The Good, the Bad and the Ugly? Phytochemistry 68 (22-24), 2973–2985. doi: 10.1016/j.phytochem.2007.09.004
Morel, C., Stermitz, F. R., Tegos, G., Lewis, K. (2003). Isoflavones as Potentiators of Antibacterial Activity. J. Agric. Food Chem. 51 (19), 5677–5679. doi: 10.1021/jf0302714
Munita, J. M., Arias, C. A. (2016). Mechanisms of Antibiotic Resistance. Virulence Mech. Bacterial Pathogens Fifth Edition 4, 481–511. doi: 10.1128/microbiolspec.vmbf-0016-2015
Mun, S.-H., Joung, D.-K., Kim, S.-B., Park, S.-J., Seo, Y.-S., Gong, R., et al. (2014). The Mechanism of Antimicrobial Activity of Sophoraflavanone B Against Methicillin-Resistant Staphylococcus aureus. Foodborne Pathog. Dis. 11, 234–239. doi: 10.1089/fpd.2013.1627
Nathan, C., Cars, O. (2014). Antibiotic Resistance — Problems, Progress, and Prospects. New Engl. J. Med. 371, 1761–1763. doi: 10.1056/nejmp1408040
Neetu, N., Katiki, M., Dev, A., Gaur, S., Tomar, S., Kumar, P. (2020). Structural and Biochemical Analyses Reveal That Chlorogenic Acid Inhibits the Shikimate Pathway. J. Bacteriol. 202 (18), e00248–20. doi: 10.1128/jb.00248-20
Nguyen, H. V., Meile, J.-C., Lebrun, M., Caruso, D., Chu-Ky, S., Sarter, S. (2018). Litsea cubeba Leaf Essential Oil From Vietnam: Chemical Diversity and its Impacts on Antibacterial Activity. Lett. Appl. Microbiol. 66, 207–214. doi: 10.1111/lam.12837
Norman, A., Hansen, L. H., Sørensen, S. J. (2009). Conjugative Plasmids: Vessels of the Communal Gene Pool. Philos. Trans. R. Soc. B 364, 2275–2289. doi: 10.1098/rstb.2009.0037
Obiang-Obounou, B. W., Kang, O.-H., Choi, J.-G., Keum, J.-H., Kim, S.-B., Mun, S.-H., et al. (2011). The Mechanism of Action of Sanguinarine Against Methicillin-Resistant Staphylococcus aureus. J. Toxicol. Sci. 36, 277–283. doi: 10.2131/jts.36.277
Önem, E. (2022). New Green Solutions Against Bacterial Resistance: Palmarosa (Cymbopogon martini) Essential Oil and Quorum Sensing. Sustain. Chem. Pharm. 25, 100587. doi: 10.1016/j.scp.2021.100587
Ouyang, J., Sun, F., Feng, W., Sun, Y., Qiu, X., Xiong, L., et al. (2016). Quercetin is an Effective Inhibitor of Quorum Sensing, Biofilm Formation and Virulence Factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 120, 966–974. doi: 10.1111/jam.13073
Ouyang, J., Sun, F., Feng, W., Xie, Y., Ren, L., Chen, Y. (2017). Antimicrobial Activity of Galangin and Its Effects on Murein Hydrolases of Vancomycin-Intermediate Staphylococcus aureus (VISA) Strain Mu50. Chemotherapy 63, 20–28. doi: 10.1159/000481658
Packiavathy, I.A.S.V., Agilandeswari, P, Musthafa, K.S., Pandian, S.K., Ravi, A.V. (2012). Antibiofilm and Quorum Sensing Inhibitory Potential of Cuminum cyminum and Its Secondary Metabolite Methyl Eugenol Against Gram Negative Bacterial Pathogens. Food Res. Intern. 45(1):85–92. doi: 10.1016/j.foodres.2011.10.022
Packiavathy, I. A. S. V., Priya, S., Pandian, S. K., Ravi, A. V. (2014). Inhibition of Biofilm Development of Uropathogens by Curcumin – An Anti-Quorum Sensing Agent From Curcuma longa. Food Chem. 148, 453–460. doi: 10.1016/j.foodchem.2012.08.002
Park, B. S., Kim, J. G., Kim, M. R., Lee, S. E., Takeoka, G. R., Oh, K. B., et al. (2005). Curcuma longa L. Constituents Inhibit Sortase a and Staphylococcus aureus Cell Adhesion to Fibronectin. J. Agric. Food Chem. 53 (23), 9005–9009. doi: 10.1021/jf051765z
Patel, K., Tyagi, C., Goyal, S., Jamal, S., Wahi, D., Jain, R., et al. (2015). Identification of Chebulinic Acid as Potent Natural Inhibitor of M. tuberculosis DNA Gyrase and Molecular Insights Into its Binding Mode of Action. Comput. Biol. Chem. 59, 37–47. doi: 10.1016/j.compbiolchem.2015.09.006
Peleg, A. Y., Seifert, H., Paterson, D. L. (2008). Acinetobacter Baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 21, 538–582. doi: 10.1128/cmr.00058-07
Pereira, F., de, O., Mendes, J. M., Lima, I. O., Mota, K.S. de L., Oliveira, W.A. de, et al. (2015). Antifungal Activity of Geraniol and Citronellol, Two Monoterpenes Alcohols, Againsttrichophyton Rubruminvolves Inhibition of Ergosterol Biosynthesis. Pharm. Biol. 53, 228–234. doi: 10.3109/13880209.2014.913299
Perez, F., Hujer, A. M., Hujer, K. M., Decker, B. K., Rather, P. N., Bonomo, R. A. (2007). Global Challenge of Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51, 3471–3484. doi: 10.1128/aac.01464-06
Praveen, S. (2018). Introductory Chapter: Terpenes and Terpenoids. Terpenes and Terpenoids. Intech Open. doi: 10.5772/intechopen.79683
Piddock, L. J. V. (2006). Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clin. Microbiol. Rev. 19, 382–402. doi: 10.1128/cmr.19.2.382-402.2006
Poli, J.-P., Guinoiseau, E., de Rocca Serra, D., Sutour, S., Paoli, M., Tomi, F., et al. (2018). Anti-Quorum Sensing Activity of 12 Essential Oils on Chromobacterium violaceum and Specific Action of Cis-Cis-P-Menthenolide From Corsican mentha Suaveolens Ssp. Insularis. Molecules 23, 2125. doi: 10.3390/molecules23092125
Porras, G., Chassagne, F., Lyles, J. T., Marquez, L., Dettweiler, M., Salam, A. M., et al. (2020). Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 121, 3495–3560. doi: 10.1021/acs.chemrev.0c00922
Putman, M., van Veen, H. W., Konings, W. N. (2000). Molecular Properties of Bacterial Multidrug Transporters. Microbiol. Mol. Biol. Rev. 64, 672–693. doi: 10.1128/mmbr.64.4.672-693.2000
Randhawa, H. K., Hundal, K. K., Ahirrao, P. N., Jachak, S. M., Nandanwar, H. S. (2016). Efflux Pump Inhibitory Activity of Flavonoids Isolated From Alpinia calcarata Against Methicillin-Resistant Staphylococcus aureus. Biologia 71 (5), 484–493. doi: 10.1515/biolog-2016-0073
Rehman, F., Mairaj, S. (2013). Antimicrobial Studies of Allicin and Ajoene. Int. J. Pharma Bio Sci. 4 (2), 1095–1105.
Reiter, J., Levina, N., van der Linden, M., Gruhlke, M., Martin, C., Slusarenko, A. J. (2017). Diallylthiosulfinate (Allicin), a Volatile Antimicrobial From Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 22 (10), 1–14. doi: 10.3390/molecules22101711
Rice, L. B., Carias, L., Rudin, S., Vael, C., Goossens, H., Konstabel, C., et al. (2003). A Potential Virulence Gene,hylEfm, Predominates in Enterococcus faecium of Clinical Origin. J. Infect. Dis. 187, 508–512. doi: 10.1086/367711
Rodrigues, L., Aínsa, J. A., Amaral, L., Viveiros, M. (2011). Inhibition of Drug Efflux in Mycobacteria With Phenothiazines and Other Putative Efflux Inhibitors. Recent. Pat. Antiinfect. Drug. Discov. 6 (2), 118–127. doi: 10.2174/157489111796064579
Ross, J. S., Tse, T., Zarin, D. A., Xu, H., Zhou, L., Krumholz, H. M. (2012). Publication of NIH Funded Trials Registered in ClinicalTrials.gov: Cross Sectional Analysis. BMJ 344, d7292–d7292. doi: 10.1136/bmj.d7292
Roy, S. K., Kumari, N., Pahwa, S., Agrahari, U. C., Bhutani, K. K., Jachak, S. M., et al. (2013). NorA Efflux Pump Inhibitory Activity of Coumarins From Mesua ferrea. Fitoterapia 90, 140–150. doi: 10.1016/j.fitote.2013.07.015
Santiago, C., Pang, E. L., Lim, K.-H., Loh, H.-S., Ting, K. N. (2015). Inhibition of Penicillin-Binding Protein 2a (PBP2a) in Methicillin Resistant Staphylococcus aureus (MRSA) by Combination of Ampicillin and a Bioactive Fraction From Duabanga grandiflora. BMC Complement. Altern. Med. 15, 178. doi: 10.1186/s12906-015-0699-z
Saravanakumar, T., Park, H.-S., Mo, A.-Y., Choi, M.-S., Kim, D.-H., Park, S.-M. (2016). Detoxification of Furanic and Phenolic Lignocellulose Derived Inhibitors of Yeast Using Laccase Immobilized on Bacterial Cellulosic Nanofibers. J. Mol. Catalysis B 134, 196–205. doi: 10.1016/j.molcatb.2016.11.006
Scazzocchio, F., Mondì, L., Ammendolia, M. G., Goldoni, P., Comanducci, A., Marazzato, M., et al. (2017). Coriander (Coriandrum sativum) Essential Oil: Effect on Multidrug Resistant Uropathogenic Escherichia coli. Nat. Prod. Commun 12, 623–626. doi: 10.1177/1934578x1701200438
Shahzad, M., Millhouse, E., Culshaw, S., Edwards, C. A., Ramage, G., Combet, E. (2015). Selected Dietary (Poly)Phenols Inhibit Periodontal Pathogen Growth and Biofilm Formation. Food Funct. 6, 719–729. doi: 10.1039/c4fo01087f
Shakeri, A., Sharifi, M. J., Bazzaz, B. S. F., Emami, A., Soheili, V., Sahebkar, A., et al. (2018). Bioautography Detection of Antimicrobial Compounds From the Essential Oil of Salvia Pachystachys. Curr. Bioactive Compounds 14 (1), 80–85. doi: 10.2174/1573407212666161014132503
Shao, J., Zhang, M., Wang, T., Li, Y., Wang, C. (2016). The Roles of CDR1, CDR2, and MDR1 in Kaempferol-Induced Suppression With Fluconazole-Resistant Candida albicans. Pharm. Biol. 54 (6), 984–992. doi: 10.3109/13880209.2015.1091483
Sharifi, A., Mohammadzadeh, A., Zahraei Salehi, T., Mahmoodi, P. (2018). Antibacterial, Antibiofilm and Antiquorum Sensing Effects of Thymus daenensis and Satureja hortensis Essential Oils Against Staphylococcus aureus Isolates. J. Appl. Microbiol. 124, 379–388. doi: 10.1111/jam.13639
Sharifzadeh, A., Khosravi, A. R., Shokri, H., Shirzadi, H. (2018). Potential Effect of 2- Isopropyl-5-Methylphenol (Thymol) Alone and in Combination With Fluconazole Against Clinical Isolates of Candida albicans, C. glabrata and C. krusei. J. Med. Mycol. 28 (2), 294–299. doi: 10.1016/j.mycmed.2018.04.002
Sharquie, K. E., Al-Turfi, I. A., Al-Salloum, S. M. (2000). The Antibacterial Activity of Tea In Vitro and In Vivo (in Patients With Impetigo Contagiosa). J. Dermatol., 27 (11), 706–710. doi: 10.1111/j.1346-8138.2000.tb02263.x
Shriram, V., Jahagirdar, S., Latha, C., Kumar, V., Puranik, V., Rojatkar, S., et al. (2008). A Potential Plasmid-Curing Agent, 8-Epidiosbulbin E Acetate, From Dioscorea bulbifera L. Against Multidrug-Resistant Bacteria. Int. J. Antimicrob. Agents 32, 405–410. doi: 10.1016/j.ijantimicag.2008.05.013
Shriram, V., Khare, T., Bhagwat, R., Shukla, R., Kumar, V. (2018). Inhibiting Bacterial Drug Efflux Pumps via Phyto-Therapeutics to Combat Threatening Antimicrobial Resistance. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02990
Sieniawska, E., Swatko-Ossor, M., Sawicki, R., Ginalska, G. (2015). Morphological Changes in the Overall Mycobacterium tuberculosis H37Ra Cell Shape and Cytoplasm Homogeneity Due to Mutellina purpurea L. Essential Oil and Its Main Constituents. Med. Principles Pract. 24, 527–532. doi: 10.1159/000439351
Si, H., Hu, J., Liu, Z., Zeng, Z. (2008). Antibacterial Effect of Oregano Essential Oil Alone and in Combination With Antibiotics Against Extended-Spectrum β-Lactamase-Producing. Escherichia coli 53, 190–194. doi: 10.1111/j.1574-695x.2008.00414.x
Sinsinwar, S., Vadivel, V. (2020). Catechin Isolated From Cashew Nut Shell Exhibits Antibacterial Activity Against Clinical Isolates of MRSA Through ROS-Mediated Oxidative Stress. Appl. Microbiol. Biotechnol. 104, 8279–8297. doi: 10.1007/s00253-020-10853-z
Siriwong, S., Thumanu, K., Hengpratom, T., Eumkeb, G. (2015). Synergy and Mode of Action of Ceftazidime Plus Quercetin or Luteolin on Streptococcus pyogenes. Evidence-Based Complement. Altern. Med. 2015, 1–12. doi: 10.1155/2015/759459
Siriyong, T., Srimanote, P., Chusri, S., Yingyongnarongkul, B. E., Suaisom, C., Tipmanee, V., et al. (2017). Conessine as a Novel Inhibitor of Multidrug Efflux Pump Systems in Pseudomonas aeruginosa. BMC 17 (1), 405. doi: 10.1186/s12906-017-1913-y
Skalicka-Woźniak, K., Walasek, M., Aljarba, T. M., Stapleton, P., Gibbons, S., Xiao, J., et al. (2018). The Anticonvulsant and Anti-Plasmid Conjugation Potential of Thymus vulgaris Chemistry: An In Vivo Murine and In Vitro Study. Food Chem. Toxicol. 120, 472–478. doi: 10.1016/j.fct.2018.07.045
Söderberg, T. A., Johansson, A., Gref, R. (1996). Toxic Effects of Some Conifer Resin Acids and Tea Tree Oil on Human Epithelial and Fibroblast Cells. Toxicology 107 (2), 99–109. doi: 10.1016/0300-483x(95)03242-8
Spellberg, B., Gilbert, D. N. (2014). The Future of Antibiotics and Resistance: A Tribute to a Career of Leadership by John Bartlett. Clin. Infect. Dis. 59, S71–S75. doi: 10.1093/cid/ciu392
Springman, A. C., Lacher, D. W., Wu, G., Milton, N., Whittam, T. S., Davies, H. D., et al. (2009). Selection, Recombination, and Virulence Gene Diversity Among Group B Streptococcal Genotypes. J. Bacteriol. 191, 5419–5427. doi: 10.1128/jb.00369-09
Sridevi, D., Shankar, C., Prakash, P., Park, J. H., Thamaraiselvi, K. (2017). Inhibitory Effects of Reserpine Against Efflux Pump Activity of Antibiotic Resistance Bacteria. Chem. biol. lett. 4, 2, 69–72.
Stermitz, F. R., Beeson, T. D., Mueller, P. J., Hsiang, J., Lewis, K. (2001). Staphylococcus aureus MDR Efflux Pump Inhibitors From a Berberis and a Mahonia (Sensu strictu) Species. Biochem. System. Ecol. 29 (8), 793–798. doi: 10.1016/s0305-1978(01)00025-4
Stermitz, F. R., Cashman, K. K., Halligan, K. M., Morel, C., Tegos, G. P., Lewis, K. (2003). Polyacylated Neohesperidosides From Geranium caespitosum: Bacterial Multidrug Resistance Pump Inhibitors. Bioorganic Medicinal Chem. Lett. 13 (11), 1915–1918. doi: 10.1016/s0960-894x(03)00316-0
Stockmann, C., Sherwin, C. M. T., Ampofo, K., Hersh, A. L., Pavia, A. T., Byington, C. L., et al. (2013). Characteristics of Antimicrobial Studies Registered in the USA Through ClinicalTrials.Gov. Int. J. Antimicrob. Agents 42, 161–166. doi: 10.1016/j.ijantimicag.2013.04.019
Strateva, T., Yordanov, D. (2009). Pseudomonas aeruginosa – a Phenomenon of Bacterial Resistance. J. Med. Microbiol. 58, 1133–1148. doi: 10.1099/jmm.0.009142-0
Sundaramoorthy, N. S., Mitra, K., Ganesh, J. S., Makala, H., Lotha, R., Bhanuvalli, S. R., et al. (2018). Ferulic Acid Derivative Inhibits NorA Efflux and in Combination With Ciprofloxacin Curtails Growth of MRSA In Vitro and In Vivo. Microb. Pathogen. 124, 54–62. doi: 10.1016/j.micpath.2018.08.022
Sun, J., Deng, Z., Yan, A. (2014). Bacterial Multidrug Efflux Pumps: Mechanisms, Physiology and Pharmacological Exploitations. Biochem. Biophys. Res. Commun. 453, 254–267. doi: 10.1016/j.bbrc.2014.05.090
Szatmári, Á., Móricz, Á.M., Schwarczinger, I., Kolozsváriné Nagy, J., Alberti, Á., Pogány, M., et al. (2021). A Pattern-Triggered Immunity-Related Phenolic, Acetosyringone, Boosts Rapid Inhibition of a Diverse Set of Plant Pathogenic Bacteria. BMC Plant Biol. 21, 1–20. doi: 10.1186/s12870-021-02928-4
Tan, N., Bilgin, M., Tan, E., Miski, M. (2017). Antibacterial Activities of Pyrenylated Coumarins From the Roots of Prangos hulusii. Molecules 22 (7), 1098. doi: 10.3390/molecules22071098
Taylor, C. M., Roberts, I. S. (2005). Capsular Polysaccharides and Their Role in Virulence. Contributions to Microbiol. 12, 55–66. doi: 10.1159/000081689
TB Alliance 2019. Global Pandemic. Available at www.tballiance.org/why-new-tb-drugs/global-pandemic. New York, USA: 2020.
Thomson, J. M., Bonomo, R. A. (2005). The Threat of Antibiotic Resistance in Gram-Negative Pathogenic Bacteria: β-Lactams in Peril! Curr. Opin. Microbiol. 8, 518–524. doi: 10.1016/j.mib.2005.08.014
Togashi, N., Hamashima, H., Shiraishi, A., Inoue, Y., Takano, A. (2010). Antibacterial Activities Against Staphylococcus aureus of Terpene Alcohols With Aliphatic Carbon Chains. J. Essential Oil Res. 22 (3), 263–269. doi: 10.1080/10412905.2010.9700321
Touani, F. K., Seukep, A. J., Djeussi, D. E., Fankam, A. G., Noumedem, J. A. K., Kuete, V. (2014). Antibiotic-Potentiation Activities of Four Cameroonian Dietary Plants Against Multidrug-Resistant Gram-Negative Bacteria Expressing Efflux Pumps. BMC Complement. Altern. Med. 14, 1–8. doi: 10.1186/1472-6882-14-258
Tyagi, P., Singh, M., Kumari, H., Kumari, A., Mukhopadhyay, K. (2015). Bactericidal Activity of Curcumin I is Associated With Damaging of Bacterial Membrane. PloS One 10 (3), e0121313–e. doi: 10.1371/journal.pone.0121313
Upadhyay, A., Upadhyaya, I., Kollanoor-Johny, A., Venkitanarayanan, K. (2014). Combating Pathogenic Microorganisms Using Plant-Derived Antimicrobials: A Minireview of the Mechanistic Basis. BioMed. Res. Int. 2014, 1–18. doi: 10.1155/2014/761741
Utt, E., Wells, C. (2016). The Global Response to the Threat of Antimicrobial Resistance and the Important Role of Vaccines. Pharmaceuticals Policy Law 18, 179–197. doi: 10.3233/PPL-160442
Van Boeckel, T. P., Brower, C., Gilbert, M., Grenfell, B. T., Levin, S. A., Robinson, T. P., et al. (2015). Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. 112, 5649–5654. doi: 10.1073/pnas.1503141112
Vasconcelos, S. E. C. B., Melo, H. M., Cavalcante, T. T. A., Júnior, F. E. A. C., de Carvalho, M. G., Menezes, F. G. R., et al. (2017). Plectranthus amboinicus Essential Oil and Carvacrol Bioactive Against Planktonic and Biofilm of Oxacillin- and Vancomycin-Resistant Staphylococcus aureus. Altern. Med., 17, 2–9. doi: 10.1186/s12906-017-1968-9
Veerapandian, R., Vediyappan, G. (2019). Gymnemic Acids Inhibit Adhesive Nanofibrillar Mediated Streptococcus gordonii–Candida albicans Mono-Species and Dual-Species Biofilms. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02328
Velayati, A. A., Masjedi, M. R., Farnia, P., Tabarsi, P., Ghanavi, J., ZiaZarifi, A. H., et al. (2009). Emergence of New Forms of Totally Drug-Resistant Tuberculosis bacilli. Chest 136, 420–425. doi: 10.1378/chest.08-2427
Velliyagounder, K., Ganeshnarayan, K., Velusamy, S. K., Fine, D. H. (2012). In Vitro Efficacy of Diallyl Sulfides Against the Periodontopathogen Aggregatibacter actinomycetemcomitans. . Antimicrob. Agents Chemother 56, 2397–2407. doi: 10.1128/aac.00020-12
Wang, M., Ma, B., Ni, Y., Xue, X., Li, M., Meng, J., et al. (2021). Restoration of the Antibiotic Susceptibility of Methicillin-Resistant Staphylococcus aureus and Extended-Spectrum β-Lactamases Escherichia coli Through Combination With Chelerythrine. Microb. Drug Resist 27 (3), 337–341. doi: 10.1089/mdr.2020.0044
Wu, Y., Bai, J., Zhong, K., Huang, Y., Qi, H., Jiang, Y., et al. (2016). Antibacterial Activity and Membrane-Disruptive Mechanism of 3-P-Trans-Coumaroyl-2-Hydroxyquinic Acid, a Novel Phenolic Compound From Pine Needles of Cedrus deodara, Against Staphylococcus aureus. Molecules 21 (8), 1–12. doi: 10.3390/molecules21081084
Wu, H. Z., Fei, H. J., Zhao, Y. L., Liu, X. J., Huang, Y. J., Wu, S. W. (2012). Antibacterial Mechanism of Sulforaphane on Escherichia coli. Journal of Sichuan University (Medical Science Edition) 43 (3), 386–390. doi: 10.3390/molecules21081084
Wu, D., Kong, Y., Han, C., Chen, J., Hu, L., Jiang, H., et al. (2008). D-Alanine:d-Alanine Ligase as a New Target for the Flavonoids Quercetin and Apigenin. Int. J. Antimicrob. Agents 32 (5), 421–426. doi: 10.1016/j.ijantimicag.2008.06.010
Wu, T., Zang, X., He, M., Pan, S., Xu, X. (2013). Structure–Activity Relationship of Flavonoids on Their Anti-Escherichia coli Activity and Inhibition of DNA Gyrase. J. Agric. Food Chem. 61, 8185–8190. doi: 10.1021/jf402222v
Xiao, Z. P., Peng, Z. Y., Dong, J. J., He, J., Ouyang, H., Feng, Y. T., et al. (2013). Synthesis, Structure-Activity Relationship Analysis and Kinetics Study of Reductive Derivatives of Flavonoids as Helicobacter pylori Urease Inhibitors. Eur. J. Medicinal Chem. 63, 685–695. doi: 10.1016/j.ejmech.2013.03.016
Xiu, W., Jianchun, L., Yuzhen, H., Wenyang, C., Yiguang, J. (2017). Effect of Sophora flavescens Alkaloid on Aerobic Vaginitis in Gel Form for Local Treatment. J. Tradit. Chin. Med. 37, 314–320. doi: 10.1016/s0254-6272(17)30066-3
Xu, H. (2001). Flavones Inhibit the Hexameric Replicative Helicase RepA. Nucleic Acids Res. 29, 5058–5066. doi: 10.1093/nar/29.24.5058
Yang, L., Ding, W., Xu, Y., Wu, D., Li, S., Chen, J., et al. (2016). New Insights Into the Antibacterial Activity of Hydroxycoumarins Against Ralstonia solanacearum. Molecules 21, 468. doi: 10.3390/molecules21040468
Yang, L., Wu, L., Yao, X., Zhao, S., Wang, J., Li, S., et al. (2018). Hydroxycoumarins: New, Effective Plant-Derived Compounds Reduce Ralstonia pseudosolanacearum Populations and Control Tobacco Bacterial Wilt. Microbiol. Res. 215, 15–21. doi: 10.1016/j.micres.2018.05.011
Yan, Y., Li, X., Zhang, C., Lv, L., Gao, B., Li, M. (2021). Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 10, 318. doi: 10.3390/antibiotics10030318
Yi, Z.-B., Yu, Y., Liang, Y.-Z., Bao, Z. (2007). Evaluation of the Antimicrobial Mode of Berberine by LC/ESI-MS Combined With Principal Component Analysis. J. Pharm. Biomed. Anal. 44, 301–304. doi: 10.1016/j.jpba.2007.02.018
Yu, Z., Liu, X., Chen, H., Zhu, L. (2020a). Naringenin-Loaded Dipalmitoylphosphatidylcholine Phytosome Dry Powders for Inhaled Treatment of Acute Lung Injury. J. Aerosol Med. Pulmon. Drug Delivery 33, 194–204. doi: 10.1089/jamp.2019.1569
Yu, Z., Tang, J., Khare, T., Kumar, V. (2020b). The Alarming Antimicrobial Resistance in ESKAPEE Pathogens: Can Essential Oils Come to the Rescue? Fitoterapia 140, 104433. doi: 10.1016/j.fitote.2019.104433
Zhang, L., Kong, Y., Wu, D., Zhang, H., Wu, J., Chen, J., et al. (2008). Three Flavonoids Targeting the Beta-Hydroxyacyl-Acyl Carrier Protein Dehydratase From Helicobacter pylori: Crystal Structure Characterization With Enzymatic Inhibition Assay. Protein Sci. 17 (11), 1971–1978. doi: 10.1110/ps.036186.108
Zhang, Y. B., Liu, X. Y., Jiang, P. P., Li, W. D., Wang, Y. F. (2015). Mechanism and Antibacterial Activity of Cinnamaldehyde Against Escherichia coli and Staphylococcus aureus. Modern Food Sci. Technol. 31, 31–35. doi: 10.13982/j.mfst.1673-9078.2015.5.006
Zhang, H., Xu, X., Chen, L., Chen, J., Hu, L., Jiang, H., et al. (2011). Molecular Determinants of Magnolol Targeting Both Rxrα and Pparγ. PloS One 6, e28253. doi: 10.1371/journal.pone.0028253
Zoric, N., Kosalec, I., Tomic, S., Bobnjaric, I., Jug, M., Vlainic, T., et al. (2017). Membrane of Candida albicans as a Target of Berberine. BMC 17 (1), 268. doi: 10.1186/s12906-017-1773-5
Keywords: Multi Drug Resistant (MDR), phytochemicals, bacteria, pathogenesis, antibiotics
Citation: Suganya T, Packiavathy IASV, Aseervatham GSB, Carmona A, Rashmi V, Mariappan S, Devi NR and Ananth DA (2022) Tackling Multiple-Drug-Resistant Bacteria With Conventional and Complex Phytochemicals. Front. Cell. Infect. Microbiol. 12:883839. doi: 10.3389/fcimb.2022.883839
Received: 25 February 2022; Accepted: 02 May 2022;
Published: 30 June 2022.
Edited by:
Bennett Tochukwu Amaechi, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Sivakumar Jeyarajan, University of Michigan, United StatesBalasubramanian Velramar, Amity University Chhattisgarh, India
K Sreenath, The University of Texas Health Science Center at San Antonio, United States
Copyright © 2022 Suganya, Packiavathy, Aseervatham, Carmona, Rashmi, Mariappan, Devi and Ananth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Devanesan Arul Ananth, ZC5hcnVsYW5hbnRoQGdtYWlsLmNvbQ==; YXJ1bGFuYW50aC5kZXZhbmVzYW5Aa2FoZWR1LmVkdS5pbg==; Navaneethan Renuga Devi, cmVtaTM1NzNAZ21haWwuY29t
†These authors have contributed equally to this work
 Thangaiyan Suganya1†
Thangaiyan Suganya1† Devanesan Arul Ananth
Devanesan Arul Ananth