- 1Center for Perinatal Research, Department of Pediatric Surgery, Columbus, OH, United States
- 2Nationwide Children’s Hospital, The Ohio State University, Columbus, OH, United States
Probiotics are live microorganisms that, when administered in adequate amounts, provide health benefits to the host. Some strains of the probiotic Lactobacillus reuteri (L. reuteri) have both antimicrobial and anti-inflammatory properties that may be exploited for the treatment and prevention of different gastrointestinal diseases, including necrotizing enterocolitis (NEC) and Clostridioides difficile (C. difficile) infection. Our laboratory has developed a new delivery system for L. reuteri in which the probiotic is incubated with biocompatible, semipermeable, porous dextranomer microspheres (DM) that can be loaded with beneficial and diffusible cargo. L. reuteri can be induced to form a biofilm by incubating the bacteria on the surface of these microspheres, which enhances the efficacy of the probiotic. Loading the DM with sucrose or maltose induces L. reuteri to produce more biofilm, further increasing the efficacy of the probiotic. Using a rat model of NEC, L. reuteri administered in its biofilm state significantly increases animal survival, reduces the incidence of NEC, preserves gut barrier function, and decreases intestinal inflammation. In a murine model of Clostridiodes difficile infection, L. reuteri administered in its biofilm state decreases colitis when administered either before or after C. difficile induction, demonstrating both prophylactic and therapeutic efficacy. There are currently no FDA-approved probiotic preparations for human use. An FDA-approved phase I clinical trial of L. reuteri in its biofilm state in healthy adults is currently underway. The results of this trial will be used to support a phase 1 clinical trial in neonates, with the goal of utilizing L. reuteri in its biofilm state to prevent NEC in premature neonates in the future.
Introduction
Bacteria grow and adhere to almost every surface, and form complex, multicellular communities called biofilms. Biofilms facilitate successful colonization and maintenance of a bacterial population by protecting bacteria against environmental conditions (Branda et al., 2005). Probiotic bacteria are live microorganisms that can be beneficial to the host when administered in adequate amounts. However, when consumed orally, probiotics face numerous challenges, including the acidic environment of the stomach, effectors of the host immune system, and competition with commensal and pathogenic bacteria. These factors prevent probiotics from being sufficiently sustained within the host, thereby reducing their potential beneficial effects (Navarro et al., 2017). There is extensive, ongoing research examining the use of probiotics for the treatment and prevention of intestinal diseases. In particular, our lab has been investigating the efficacy of the probiotic bacteria Lactobacillus reuteri (L. reuteri) administered in its biofilm vs. planktonic (free-living) state, to treat necrotizing enterocolitis and Clostridioides difficile (C. difficile) infections.
Lactobacillus reuteri is a Gram-positive bacterium originally isolated in 1962 by German microbiologist Gerhard Reuter. Using human fecal and intestinal samples from infants and adults, Reuter demonstrated that L. reuteri was the predominant autochthonous bacterium in both populations. He isolated multiple strains, including DSM20016 (Kandler et al., 1980; Reuter, 2001). In the years since, further genomic studies have demonstrated that human L. reuteri strains belong to two distinct multilocus sequence analysis (MLSA) clades, clades II and VI. While human strains in clade VI are more closely related to isolates from chickens, those in clade II are very specific to humans.
Certain rare strains, including DSM20016, are known to have multiple attributes beneficial to the host, including antimicrobial and anti-inflammatory properties (Spinler et al., 2014). The antimicrobial properties of L. reuteri are secondary to its ability to produce the antimicrobial compound 3-hydroxyproprionaldehyde (3-HPA), also known as reuterin (Jones and Versalovic, 2009). L. reuteri utilizes glycerol dehydratase to produce reuterin from glycerol. Reuterin inhibits the growth of multiple gastrointestinal pathogens, including C. difficile, by inducing oxidative stress (Engevik et al., 2020). L. reuteri also has extracellular glucosyltransferase (GTF) proteins that catalyze the formation of exopolysaccharides of glucose (glucans) from disaccharide sugars and possess glucan-binding domains that allow for strong binding to other glucans, important to biofilm formation. The anti-inflammatory abilities of L. reuteri are partially due to its ability to produce histamine via histidine decarboxylase. Histamine is a biologically active compound that modulates host mucosal immunity and suppresses proinflammatory tumor necrosis factor (TNF) production (Jones and Versalovic, 2009; C. M. Thomas et al., 2012). In addition, by metabolizing folate, L. reuteri can produce ethionine, which can use ethylation to modify human chromatin (Röth et al., 2019). There is also evidence that L. reuteri induces anti-inflammatory T-regulatory cells, suppresses T helper 1 (Th1) and Th2 cytokine responses, and alters dendritic cell activity; however, the mechanisms by which this occurs are poorly understood (Jones and Versalovic, 2009). Our studies use L. reuteri strain DSM20016—a strain that possesses both antimicrobial and anti-inflammatory properties.
Biofilms possess a community architecture, replete with a self-made extracellular matrix, and that is often facilitated by adherence to a surface. Biofilm formation enables bacteria to resist environmental conditions, resulting in successful colonization and maintenance of the bacterial population (Salas-Jara et al., 2016). L. reuteri grown in its biofilm state on the surface of biocompatible microspheres has an increased ability to survive in acidic environments such as that of the stomach and to adhere to intestinal epithelial cells. The biocompatible microspheres we use are composed of separation pharmacia dextran (Sephadex aka dextranomer microspheres or DM), which are beads of crosslinked dextran typically used for gel-filtration chromatography but also mimic the native glucans that L. reuteri makes. In addition, prebiotic nutrients beneficial to the probiotic bacteria can be loaded into the lumen of the Sephadex beads (Navarro et al., 2017). Harnessing the ability of L. reuteri to form a biofilm, we have developed a novel probiotic delivery system in which L. reuteri is induced to form a biofilm on the surface of DM (L. reuteri + DM), allowing for enhanced efficacy. Loading DM with maltose (DM-maltose, which is the substrate for GtfW) or sucrose (DM-sucrose, which is an inducer of gtfW) induces increased biofilm formation, enhancing the efficacy of the probiotic (Navarro et al., 2017).
Bacterial colonization of the intestine is essential for the development of a healthy gut microbiome (Harmsen et al., 2000). In contrast to that of term infants, the gut microbiome of premature infants has a notably smaller proportion of beneficial bacteria such as Lactobacillus and Bifidobacteria and higher numbers of pathogenic bacteria, likely secondary to frequent antibiotic use, exposure to the hospital environment, and artificial feeding. The intestinal dysbiosis that is present in premature infants is associated with the development of necrotizing enterocolitis (NEC) (Mshvildadze et al., 2008).
NEC is a devastating disease affecting premature infants, characterized by extensive intestinal inflammation that often progresses to tissue destruction, bacterial translocation, sepsis, and often death. Approximately 10% of infants born weighing less than 1,500 g will develop the disease, and mortality for affected infants is 20%–30%. Current treatment and preventive approaches for NEC remain suboptimal, with the mainstays of treatment being orogastric tube decompression, total parenteral nutrition (TPN), and administration of broad-spectrum antibiotics. Surgical resection is required when there is a failure of medical management and concern for bowel viability. For infants who survive, they continue to face numerous long-term complications, including gastrointestinal problems, failure to thrive, short gut syndrome, and neurodevelopmental delay (Neu and Walker, 2011).
C. difficile infection is also related to a disruption in the gut microbiome. C. difficile is one of the most common nosocomial infections. From a 2011 surveillance study in the United States, there were an estimated 453,000 C. difficile infections of which 29,300 led to death (Lessa et al., 2015). According to the American College of Gastroenterology, there was a 43% increase in C. difficile infection between 2001 and 2012, largely driven by recurrent infections which increased by 188% over the same timeframe (Ma et al., 2017; Kelly et al., 2021). This anaerobic, Gram-positive Bacillus is the primary culprit in antibiotic-driven pseudomembranous colitis, which is thought to be due to a disruption of healthy gut microbiota from a single dose or multiple doses of antibiotics. Risk factors that predispose to C. difficile infection include hospitalization, age over 65 years, and antibiotic use (Desai et al., 2016; Kelly et al., 2021). Colitis is driven by the release of toxins A and B, both of which are exotoxins that disrupt intestinal cell integrity. Treatment options for patients with active C. difficile infection range from oral or intravenous antibiotics, fecal transplantation, and, in fulminant cases of toxic megacolon, emergent total colectomy. Approximately US$5.4 billion is spent annually to treat C. difficile infections in the United States (Desai et al., 2016). A prophylactic medication would be extraordinarily cost-effective. However, to date, there is insufficient data to support the use of prophylactic treatments (Kelly et al., 2021).
Given the role of intestinal dysbiosis in the development of NEC and C. difficile infections, probiotic administration has been studied as a preventive strategy. However, the multiple studies that have been performed have been met with conflicting results. The Probiotics in Preterm Infants (PiPs) Trial was a multicenter, double-blinded, randomized, placebo-controlled trial conducted from 2010 to 2013 that examined daily dosing of Bifidobacterium breve BBG-001 in the prevention of NEC, late-onset sepsis, and death in preterm infants while monitoring for probiotic colonization of participants. They found no evidence that the use of Bifidobacterium breve BBG-001 was protective against NEC, late-onset sepsis, or death in premature infants (Costeloe et al., 2016). However, when used in combination, the probiotics Lactobacillus and Bifidobacterium spp. have been shown to prevent NEC in very low birth weight (VLBW) infants (Denkel et al., 2016). This is supported by a meta-analysis that showed a reduction in NEC incidence from a combination of Lactobacillus and Bifidobacterium species among 1,623 VLBW neonates from 8 randomized control trials (Thomas et al., 2017). Another study used the NEO-KISS database, a German surveillance system for nosocomial infections in VLBW infants, to investigate the routine use of a dual-strain probiotic formulation containing Lactobacillus acidophilus and Bifidobacterium spp. called Infloran in German NICUs from 2004 to 2014. Interestingly, while there is no FDA-approved pharmaceutical-grade probiotic in the United States, Infloran is licensed by the Swiss Agency for Therapeutic Products of the Federal Office of Public Health in Switzerland as a drug for diarrhea, and thus it is available in a pharmaceutical-grade quality. They found that the use of Infloran significantly reduced the risk of NEC, overall mortality, and nosocomial bloodstream infections (Denkel et al., 2016). This is supported by a recent meta-analysis that showed greater NEC reduction with routine multistrain probiotic use compared to routine administration of a single strain (Deshmukh & Patole, 2021). While Lactobacillus and Bifidobacterium spp. have been shown to prevent NEC in VLBW infants, the positive effects of probiotic administration appear dependent upon repetitive administration (at least daily) (Braga et al., 2011). Although rare, there have been case reports of probiotic-induced sepsis in infants (Zbinden et al., 2015), raising some concern for daily probiotic administration in VLBW infants. A Cochrane review examining 54 studies with a total of 10,604 infants who were either very preterm (born earlier than 8 weeks) or VLBW found a significant reduction in NEC (RR, 0.54; 95% CI, 0.45 to 0.65). However, evidence was assessed as low certainty due to limitations in trial design and publication bias (Sharif et al., 2020).
Lactobacillus and Bifidobacterium spp. have also been investigated for protective effects against C. difficile infection. Although some studies have found beneficial effects, results have been mixed, leading the American Gastroenterology Association to recommend against the use of probiotics for the prevention of antibiotic-induced C. difficile (Kelly et al., 2021).
Given these concerns and shortcomings of probiotics in human studies, our lab set out to develop an improved method of probiotic administration that would increase probiotic efficacy with fewer doses. Here, we review the currently published literature and progression of our laboratories’ work, which demonstrates the efficacy of a single dose of L. reuteri in its biofilm state in the treatment of NEC and C. difficile infections. This is not a general review of the field but a focused overview of our laboratories’ contributions to applied probiotic use for these diseases. We also discuss the future implications of this probiotic formulation on the treatment of intestinal diseases (Figure 1).
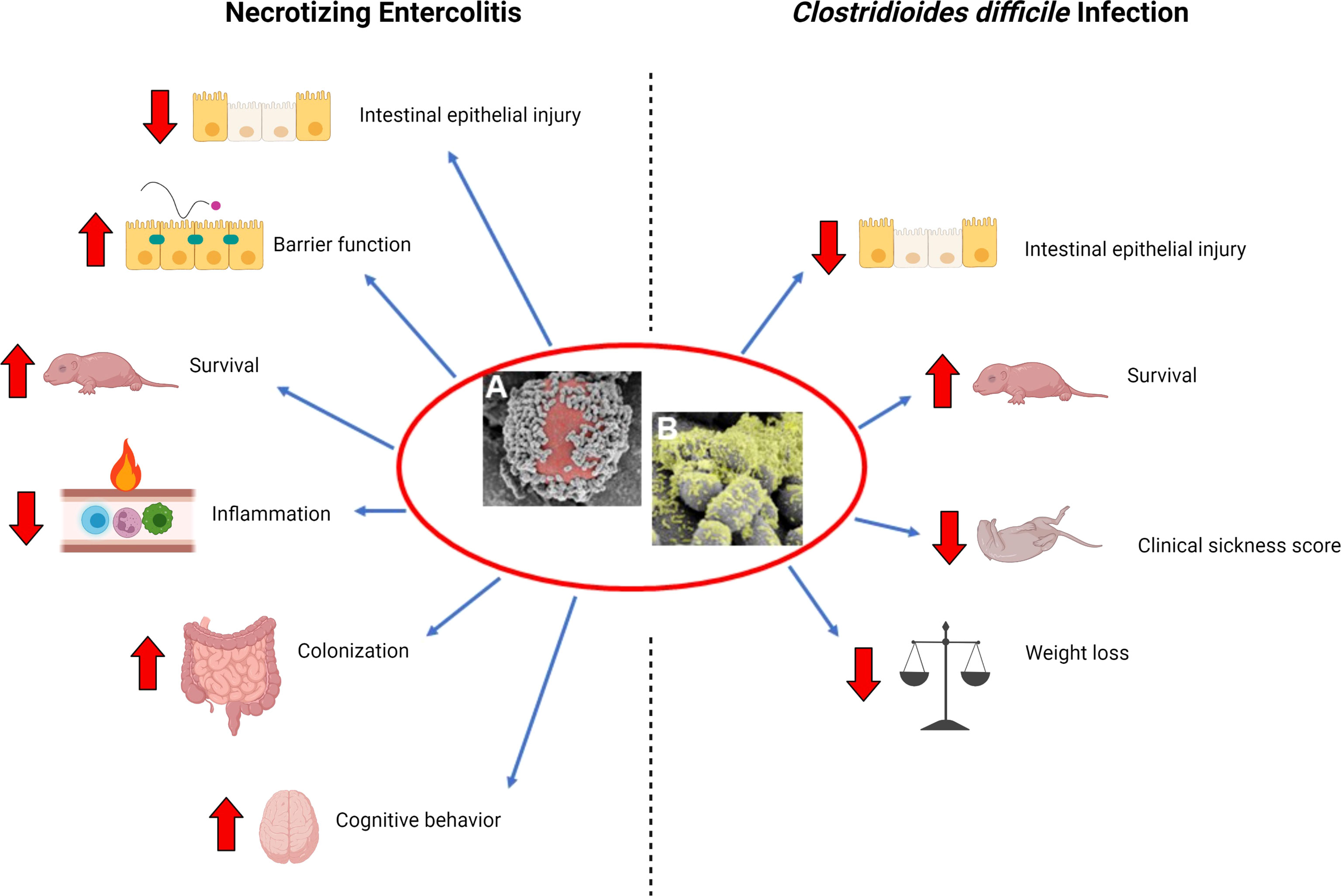
Figure 1 Beneficial effects of L. reuteri in its biofilm state. (A) Scanning electron microscopy (SEM) image demonstrating multiple L. reuteri adherent to the surface of a DM (red); (B) upon adherence to DM, L. reuteri is induced to form a biofilm (green). Necrotizing Enterocolitis: L. reuteri in its biofilm state decreases intestinal epithelial injury, increases gut barrier integrity, increases survival, decreases inflammation, increases probiotic persistence in the gastrointestinal tract, and improves cognitive behavior in a rat model of necrotizing enterocolitis (NEC). Clostridiodes difficile infection: L. reuteri in its biofilm state decreases intestinal epithelial injury, increases survival, decreases clinical sickness score, and decreases weight loss when administered either as prophylaxis or treatment in a mouse model of C. difficile infection. Created with BioRender.com.
L. reuteri in Its Biofilm State Protects the Intestines From Experimental NEC and Decreases Neurodevelopmental Impairment in Survivors of NEC
Using a rat model of NEC, we have demonstrated that a single dose of L. reuteri grown on biocompatible microspheres significantly reduces the incidence and severity of NEC and preserves gut barrier function. In our rat NEC model, premature neonatal rat pups are delivered via cesarean section and exposed to repeated episodes of hypercaloric feeding, hypoxia, and hypothermia over a 96-h time period to induce NEC. The hypercaloric diet consisted of 5 daily gavage feedings of increasing volumes of formula each day, with the first feed including a dose of lipopolysaccharide (2 mg/kg) that activates toll-like receptor 4. The first 3 feeds each day were combined with 90-s episodes of hypoxia (<1.5% oxygen) followed by hypothermia (4°C × 10 min). Experimental groups received a single enteral dose of L. reuteri grown on unloaded microspheres (biofilm state) compared to L. reuteri administered in its planktonic (free-living) state. The intestinal injury was graded using a standardized histology injury scoring system, and intestinal permeability was quantified by measuring serum levels of enterally administered fluorescein isothiocyanate-labeled dextran. Pups treated with a single dose of L. reuteri in its biofilm state demonstrated a significant reduction in histologic injury with reduced intestinal permeability, indicating improved gut barrier function, whereas a single dose of planktonic L. reuteri had no effect (Olson et al., 2016).
Preloading DM with sucrose or maltose enhances L. reuteri biofilm formation, increases L. reuteri adherence to human intestinal epithelial cells, and prolongs L. reuteri survival in acidic pH (Navarro et al., 2017). With this in mind, we next sought to test the efficacy of L. reuteri induced to produce increased biofilm formation by preloading DM with sucrose or maltose, or by mutating the L. reuteri gtfW gene to reduce the ability of L. reuteri to produce a biofilm, in a rat model of NEC (Olson et al., 2018). GtfW is a strain-specific, cell-associated extracellular enzyme involved in L. reuteri biofilm formation (Navarro et al., 2017). Pups in experimental groups received a single enteral dose of L. reuteri + DM, L. reuteri + DM-sucrose, or L. reuteri + DM-maltose. The intestinal histologic injury was graded, intestinal permeability was determined by measuring serum levels of enterally administered fluorescein isothiocyanate-labeled dextran, inflammatory gene expression was determined by quantitative real-time PCR, and preservation of the gut microbiome was determined using DNA isolation and 16S rRNA sequencing. We found that administration of a single dose of L. reuteri induced to form increased amounts of biofilm by incubation with either maltose- or sucrose-loaded DM, had significantly improved animal survival, decreased incidence of NEC, reduced intestinal mucosal barrier breakdown, and decreased intestinal inflammation, compared to L. reuteri incubated with unloaded DM or L. reuteri in its planktonic state. Importantly, L. reuteri + DM-maltose led to increased L. reuteri persistence in the intestinal tract and caused the composition of the gut microbiome to more closely resemble that of breastfed uninjured animals. Finally, the administration of L. reuteri with a mutated gtfW gene (i.e., L. reuteri is unable to readily produce a biofilm) led to a loss of protection against NEC, highlighting the importance of biofilm formation in the protective effects of L. reuteri (Olson et al., 2018).
L. reuteri has antimicrobial properties due to its ability to produce reuterin via glycerol dehydratase and anti-inflammatory properties partially attributable to its ability to produce histamine via histidine decarboxylase. We next investigated the effects of the antimicrobial and anti-inflammatory properties of L. reuteri on protecting the intestines from NEC. Prior to the induction of NEC, rat pups received either native or mutant forms of L. reuteri in either its planktonic or biofilm states. The mutant forms of L. reuteri were either reuterin- or histamine-deficient, decreasing the antimicrobial or anti-inflammatory properties of the probiotic, respectively. The intestinal injury was graded using a standardized histologic injury scoring system (Caplan et al., 1994). As in our prior studies, rat pups that received a single dose of L. reuteri in its biofilm state had a significantly decreased incidence of NEC. Administration of reuterin-deficient or histamine-deficient forms of L. reuteri, in either the planktonic or biofilm state, resulted in a significant loss of efficacy. This demonstrates the importance of both reuterin and histamine production by L. reuteri in its ability to confer intestinal protection against NEC (Shelby et al., 2021). This also highlights both an infectious and an inflammatory etiology of the disease.
There is a growing body of evidence demonstrating a connection between gut microbiota and brain function via a bidirectional gut-brain axis, which regulates communication between the enteric nervous system (ENS) and the central nervous system (CNS) (Cryan and Dinan, 2012). Given the known predisposition for infants surviving NEC to have neurodevelopmental delays and cognitive impairments (Neu and Walker, 2011), we next investigated whether our novel probiotic delivery system had neuroprotective effects on survivors of NEC. We induced NEC in rat pups using our standard rat NEC model, as described above. The incidence of death due to NEC in this model is approximately 65%. Surviving pups were placed with foster dams and subjected to daily developmental milestone testing for 23 days. These tests included daily weight, ear and eye opening, auditory startle, tests to measure labyrinthine, body righting, and coordination (air righting, cliff aversion, surface righting, and negative geotaxis), tests to measure strength (forelimb grasp), and tests to measure animal locomotion and the extinguishing of pivoting behavior (open field traversal). In addition, cognitive and memory tests were performed between 4 and 8 weeks of age. These tests included the Y-maze test to assess spatial learning and reference memory, the novel object recognition test to measure nonspatial working memory and recognition memory, the Barnes maze test to evaluate spatial learning and memory, and the elevated plus-maze test to look for anxiety-like behavior. Once collected at the end of the experiment, rat brain specimens were subjected to immunofluorescent staining, RNA isolation, and quantitative real-time PCR. We found that rat pups exposed to NEC reached developmental milestones significantly slower than breastfed pups, with mild improvement when treated with a single dose of L. reuteri in its biofilm state. While exposure to NEC was noted to have negative effects on cognitive behavior, these effects were prevented with the administration of a single dose of L. reuteri in its biofilm state. In addition, the behavioral effects of NEC were found to be associated with increased numbers of activated microglia, decreased myelin basic protein (MBP), and decreased neurotrophic gene expression. All these effects were prevented by the administration of a single dose of L. reuteri in its biofilm state (Wang et al., 2021).
L. reuteri in the Treatment and Prevention of Experimental Clostridioides difficile Infection
We have examined the use of L. reuteri as prophylaxis and treatment in a murine model of C. difficile infection, comparing L. reuteri in its biofilm state to its planktonic state (Shelby et al., 2020). To induce C. difficile infection, adult C57BL/6 mice received an oral antibiotic cocktail followed by an intraperitoneal injection of clindamycin two days later. Depending on whether the mouse was in the prophylactic or treatment arm of the study, animals received a single dose of saline, planktonic L. reuteri, or L. reuteri + DM-maltose before or after gastric gavage of 1.5 x 107 CFU of C. difficile, respectively, and clinical sickness scores (CSS) and histologic injury scores (HIS) consistent with C. difficile colitis were determined (Shelby et al., 2020). In the prophylactic experiment, mice that received a single dose of L. reuteri + DM-maltose had significantly decreased weight loss, decreased CSS, decreased HIS, and increased survival after 6 days compared to the control saline group. In addition, mice that received L. reuteri + DM-maltose had significantly increased survival after 6 days compared to those that received planktonic L. reuteri. In the treatment experiment, animals given L. reuteri + DM-maltose had significantly less weight loss, decreased HIS, and increased survival compared to the saline control group. There was a further improvement in CSS and decreased HIS in the L. reuteri + DM-maltose group compared to the L. reuteri + DM-water group. Therefore, there may be a role for both the prevention and treatment of C. difficile infection using L. reuteri in its biofilm state (Shelby et al., 2020).
L. reuteri has several characteristics that make it effective in the prevention and treatment of C. difficile infection. First, it is resistant to the antibiotics used to treat C. difficile, such as vancomycin, metronidazole, and fidaxomicin, therefore allowing it to be co-administered with these antibiotics (Spinler et al., 2017). L. reuteri is believed to induce reactive oxidative species within C. difficile through its production of reuterin, leading to altered metabolism, decreased toxin production, and increased susceptibility to vancomycin and metronidazole (Engevik et al., 2020). Although a randomized controlled trial using planktonic L. reuteri in children to prevent diarrhea and antibiotic-associated diarrhea did not show a significant effect when compared to a placebo, it is possible that there would be a clinical difference in the prevention and treatment of C. difficile in humans administered L. reuteri in its biofilm state (Kołodziej and Szajewska, 2019).
Human Clinical Trials of L. reuteri in Its Biofilm State
We have demonstrated that L. reuteri in its biofilm state can reduce the incidence of NEC and C. difficile infection in rodent models of disease. However, there are currently no FDA-approved probiotics for human administration despite the increasing use of probiotics in NICUs throughout the United States (Viswanathan et al., 2016). Published clinical trials of probiotics have all used probiotics administered in their planktonic state, with the administration of single or multiple doses daily. Two randomized controlled studies using daily doses of L. reuteri DSM17938 found a statistically significant decrease in feeding intolerance and duration of hospitalization for preterm infants weighing ≤1,500 g (Rojas et al., 2012; Oncel et al., 2014). In our animal models, we deliver our formulation of L. reuteri in its biofilm state, allowing us to deliver a single dose rather than multiple daily doses. This may help to decrease the risk of downstream bacteremia associated with probiotic administration and increase patient compliance and treatment cost-effectiveness.
In conjunction with Scioto Biosciences Inc., GMP-grade L. reuteri in its biofilm state (SB-121) has been produced, and its safety and tolerability are currently being investigated in a randomized, double-blind, crossover phase I clinical trial (NCTT04944901 (28-Day Daily-Dose Crossover Study of the Safety and Tolerability of SB-121 (Lactobacillus Reuteri With Sephadex® and Maltose) in Subjects, Ages 15 to 45 Years, Diagnosed With Autistic Disorder, 2021). Autism spectrum disorder is a neurodevelopmental disorder that is characterized by deficits in social behavior and the presence of restricted, stereotyped interests and behaviors. These behaviors involve dysregulation of the gut-brain axis as well as neuroinflammation. In mice subjected to maternal separation, L. reuteri DSM 17938 has been shown to downregulate inflammatory gene expression in the brain and enhance pro-social behavior (Park et al., 2021). In addition to neuroinflammation, oxytocin also strongly affects social behavior. Children with autism spectrum disorder have low levels of oxytocin, and in animal models, the administration of L. reuteri significantly increases oxytocin and improves social behavior (Kong et al., 2020). These findings support the current phase 1 clinical trial of SB-121 in healthy adults with an autism spectrum disorder. In this trial, volunteers with autism are blindly receiving either daily SB-121 or placebo for 28 days. Those that received SB-121 will then switch to placebo, and those that received placebo will then switch to SB-121 for an additional 28 days. Primary outcome measures will include adverse events, the presence of SB-121 in the stool, and the incidence of symptomatic L. reuteri bacteremia. Secondary outcome measures include changes in cognition, attention, and behavior; stool biomarkers; and C-reactive protein and TNF-α levels (since L. reuteri can produce anti-inflammatory compounds). The results of this trial should provide reassurance for the safe administration of L. reuteri in its biofilm state to not only treat neurodevelopmental disorders like autism spectrum disorder but also to prevent and treat NEC in premature infants and C. difficile colitis in susceptible individuals.
Discussion
L. reuteri is a beneficial Gram-positive probiotic bacterium, with strain DSM20016 possessing antimicrobial and anti-inflammatory properties. Importantly, L. reuteri can be grown in its biofilm state by using permeable biocompatible microspheres loaded with prebiotics, such as maltose and sucrose, which allows increased adherence to human intestinal epithelial cells and prolonged survival under acidic conditions in the stomach (Navarro et al., 2017). We have demonstrated the efficacy of a single dose of L. reuteri administered in its biofilm state in reducing the severity of NEC and C. difficile infections in a rat model of NEC and a murine model of C. difficile.
While using probiotics to treat intestinal disorders manifested by dysbiosis may seem logical, the reality is more complicated. There are multiple combinations of a variety of probiotics sold over the counter. However, there is currently no FDA-approved probiotic on the market. Still, there is a growing use of probiotics in NICUs in the United States. In 1997, almost no NICUs in the United States were using probiotics (Gray et al., 2020). By 2015, only approximately 14% of NICUs in the United States administered probiotics to VLBW preterm infants (Viswanathan et al., 2016). There are a number of concerns regarding probiotic administration in infants contributing to the difficulty with bringing a product of this nature to market, including probiotic-associated sepsis and contamination, as exemplified by 3 preterm infants who developed Bifidobacterium longum bacteremia after receiving the probiotic Infloran® containing viable Bifidobacterium longum (Zbinden et al., 2015). The highest profile case involved a preterm VLBW infant who succumbed to gastrointestinal mucormycosis from probiotic ABC Dophilus Powder due to mold contamination with Rhizopus oryzae (Vallabhaneni et al., 2015). Beyond concerns for probiotic-associated sepsis, there is also a concern regarding the quality of these probiotic formulations. The precise contents contained within the non-FDA-approved formulations available at present are largely unknown. As recently as 2016, a study was conducted to validate the identity of bifidobacterial species and subspecies in 16 different commercial probiotic products, with only 1 of the 16 probiotics perfectly matching its bifidobacterial label (Lewis et al., 2016). There have also been several recent recalls in the last 5 years of dietary supplement-grade probiotics due to contamination, including with Salmonella, Pseudomonas aeruginosa, Rhizopus, and Penicillium species (Liva Global, 2021). These reports highlight the importance of producing a GMP-grade probiotic preparation for human administration. To date, there is no published trial using probiotics to prevent NEC in preterm infants that have met the definition of a “probiotic drug” by the International Scientific Association for Probiotics and Prebiotics (Poindexter and Committee on Fetus and Newborn, 2021). Despite 56 randomized control trials and 30 observational trials, there remains uncertainty about the benefits of probiotics (Razak et al., 2021).
The use of probiotics in the NICU may be far more acceptable to neonatologists if an FDA-approved formulation was available. However, there are concrete reasons why an FDA-approved probiotic product is not currently available for use in the NICU. One of the main challenges is cost. The cost of producing a GMP-grade drug formulation and the effort needed to get it approved by the FDA are enormous. In addition, if the target patient population is newborns, an extra level of scrutiny is appropriately applied, requiring initial phase 1 studies in adults prior to phase 1 studies in newborns, doubling the cost. It would be very challenging to bring a new formulation to the market without the support of biopharma. This brings another level of complexity into the picture. Being an orphan disease, NEC affects a minute portion of the population compared to diseases such as cancer or cardiovascular disease. The impetus for a company to support the development of therapies for NEC is therefore diminished. It is imperative that biopharma understands the importance of cures for orphan diseases in addition to cures for more common diseases affecting humankind.
In our experimental models, a single dose of L. reuteri administered in its biofilm state has proven efficacious in reducing the severity of NEC and C. difficile infections, and the safety and tolerability of GMP-grade L. reuteri in its biofilm state (SB-121) are currently being investigated as part of a phase I clinical trial. Moving forward, further clinical trials will be needed to demonstrate the safety and efficacy of L. reuteri in its biofilm state in the prevention and treatment of NEC and C. difficile infection.
Author Contributions
MR and SW have contributed equally to this work and share first authorship. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by NIH R01 GM123482 Tunable Native Probiotic Formulations for the Treatment of Necrotizing enterocolitis (GEB, SDG, MTB).
Conflict of Interest
GB, SG, and MB have stock options in Scioto Biosciences, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Braga, T. D., da Silva, G. A. P., de Lira, P. I. C., de Carvalho Lima, M. (2011). Efficacy of Bifidobacterium breve and Lactobacillus casei Oral Supplementation on Necrotizing Enterocolitis in Very-Low-Birth-Weight Preterm Infants: A Double-Blind, Randomized, Controlled Trial. Am. J. Clin. Nutr. 93 (1), 81–86. doi: 10.3945/ajcn.2010.29799
Branda, S. S., Vik, S., Friedman, L., Kolter, R. (2005). Biofilms: The Matrix Revisited. Trends Microbiol. 13 (1), 20–26. doi: 10.1016/j.tim.2004.11.006
Caplan, M. S., Hedlund, E., Adler, L., Hsueh, W. (1994). Role of Asphyxia and Feeding in a Neonatal Rat Model of Necrotizing Enterocolitis. Pediatr. Pathol. 14 (6), 1017–1028. doi: 10.3109/15513819409037698
Costeloe, K., Bowler, U., Brocklehurst, P., Hardy, P., Heal, P., Juszczak, E., et al. (2016). A Randomised Controlled Trial of the Probiotic Bifidobacterium breve BBG-001 in Preterm Babies to Prevent Sepsis, Necrotising Enterocolitis and Death: The Probiotics in Preterm infantS (PiPS) Trial. Health Technol. Assess. 20 (66), 1–194. doi: 10.3310/hta20660
Cryan, J. F., Dinan, T. G. (2012). Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 13 (10), 701–712. doi: 10.1038/nrn3346
Denkel, L. A., Schwab, F., Garten, L., Geffers, C., Gastmeier, P., Piening, B. (2016). Protective Effect of Dual-Strain Probiotics in Preterm Infants: A Multi-Center Time Series Analysis. PLoS One 11 (6), e0158136. doi: 10.1371/journal.pone.0158136
Desai, K., Gupta, S. B., Dubberke, E. R., Prabhu, V. S., Browne, C., Mast, T. C. (2016). Epidemiological and Economic Burden of Clostridium difficile in the United States: Estimates From a Modeling Approach. BMC Infect. Dis. 16, 303. doi: 10.1186/s12879-016-1610-3
Deshmukh, M., Patole, S. (2021). Prophylactic Probiotic Supplementation for Preterm Neonates-A Systematic Review and Meta-Analysis of Nonrandomized Studies. Adv. Nutr. 12 (4), 1411–1423. doi: 10.1093/advances/nmaa164
Engevik, M. A., Danhof, H. A., Shrestha, R., Chang-Graham, A. L., Hyser, J. M., Haag, A. M., et al. (2020). Reuterin Disrupts Clostridioides difficile Metabolism and Pathogenicity Through Reactive Oxygen Species Generation. Gut. Microbes 12 (1), 1788898. doi: 10.1080/19490976.2020.1795388
Gray, K. D., Messina, J. A., Cortina, C., Owens, T., Fowler, M., Foster, M., et al. (2020). Probiotic Use and Safety in the Neonatal Intensive Care Unit: A Matched Cohort Study. J. Pediatr. 222, 59–64.e1. doi: 10.1016/j.jpeds.2020.03.051
Harmsen, H. J., Wildeboer-Veloo, A. C., Raangs, G. C., Wagendorp, A. A., Klijn, N., Bindels, J. G., et al. (2000). Analysis of Intestinal Flora Development in Breast-Fed and Formula-Fed Infants by Using Molecular Identification and Detection Methods. J. Pediatr. Gastroenterol. Nutr. 30 (1), 61–67. doi: 10.1097/00005176-200001000-00019
Jones, S. E., Versalovic, J. (2009). Probiotic Lactobacillus reuteri Biofilms Produce Antimicrobial and Anti-Inflammatory Factors. BMC Microbiol. 9, 35. doi: 10.1186/1471-2180-9-35
Kandler, O., Stetter, K.-O., Köhl, R. (1980). Lactobacillus reuteri Sp. Nov., a New Species of Heterofermentative Lactobacilli. Zentralblatt. Für. Bakteriologie.: I. Abt. Originale. C.: Allgemeine. Angewandte. Und. Ökologische. Mikrobiologie. 1 (3), 264–269. doi: 10.1016/S0172-5564(80)80007-8
Kelly, C. R., Fischer, M., Allegretti, J. R., LaPlante, K., Stewart, D. B., Limketkai, B. N., et al. (2021). ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 116 (6), 1124–1147. doi: 10.14309/ajg.0000000000001278
Kołodziej, M., Szajewska, H. (2019). Lactobacillus reuteri DSM 17938 in the Prevention of Antibiotic-Associated Diarrhoea in Children: A Randomized Clinical Trial. Clin. Microbiol. Infection. 25 (6), 699–704. doi: 10.1016/j.cmi.2018.08.017
Kong, X.-J., Liu, J., Li, J., Kwong, K., Koh, M., Sukijthamapan, P., et al. (2020). Probiotics and Oxytocin Nasal Spray as Neuro-Social-Behavioral Interventions for Patients With Autism Spectrum Disorders: A Pilot Randomized Controlled Trial Protocol. Pilot. Feasibility. Stud. 6 (1), 20. doi: 10.1186/s40814-020-0557-8
Lessa, F. C., Mu, Y., Bamberg, W. M., Beldavs, Z. G., Dumyati, G. K., Dunn, J. R., et al. (2015). Burden of Clostridium difficile Infection in the United States. New Engl. J. Med. 372 (9), 825–834. doi: 10.1056/NEJMoa1408913
Lewis, Z. T., Shani, G., Masarweh, C. F., Popovic, M., Frese, S. A., Sela, D. A., et al. (2016). Validating Bifidobacterial Species and Subspecies Identity in Commercial Probiotic Products. Pediatr. Res. 79 (3), 445–452. doi: 10.1038/pr.2015.244
Livia Global (2021). Livia Global Announces Voluntary Recall of Two Lots of Its Liviaone Liquid Probiotics Because of The Potential for Contamination With Pseudomonas Aeruginosa.
Ma, G. K., Brensinger, C. M., Wu, Q., Lewis, J. D. (2017). Increasing Incidence of Multiply Recurrent Clostridium difficile Infection in the United States: A Cohort Study. Ann. Internal Med. 167 (3), 152–158. doi: 10.7326/M16-2733
Mshvildadze, M., Neu, J., Mai, V. (2008). Intestinal Microbiota Development in the Premature Neonate: Establishment of a Lasting Commensal Relationship? Nutr. Rev. 66 (11), 658–663. doi: 10.1111/j.1753-4887.2008.00119.x
Navarro, J. B., Mashburn-Warren, L., Bakaletz, L. O., Bailey, M. T., Goodman, S. D. (2017). Enhanced Probiotic Potential of Lactobacillus reuteri When Delivered as a Biofilm on Dextranomer Microspheres That Contain Beneficial Cargo. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00489
Neu, J., Walker, W. A. (2011). Necrotizing Enterocolitis. New Engl. J. Med. 364 (3), 255–264. doi: 10.1056/NEJMra1005408
Olson, J. K., Navarro, J. B., Allen, J. M., McCulloh, C. J., Mashburn-Warren, L., Wang, Y., et al. (2018). An Enhanced Lactobacillus reuteri Biofilm Formulation That Increases Protection Against Experimental Necrotizing Enterocolitis. Am. J. Physiol. Gastrointestinal. Liver. Physiol. 315 (3), G408–G419. doi: 10.1152/ajpgi.00078.2018
Olson, J. K., Rager, T. M., Navarro, J. B., Mashburn-Warren, L., Goodman, S. D., Besner, G. E. (2016). Harvesting the Benefits of Biofilms: A Novel Probiotic Delivery System for the Prevention of Necrotizing Enterocolitis. J. Pediatr. Surg. 51 (6), 936–941. doi: 10.1016/j.jpedsurg.2016.02.062
Oncel, M. Y., Sari, F. N., Arayici, S., Guzoglu, N., Erdeve, O., Uras, N., et al. (2014). Lactobacillus reuteri for the Prevention of Necrotising Enterocolitis in Very Low Birthweight Infants: A Randomised Controlled Trial. Arch. Dis. Childhood. Fetal. Neonatal. Edition. 99 (2), F110–F115. doi: 10.1136/archdischild-2013-304745
Park, E. S., Freeborn, J., Venna, V. R., Roos, S., Rhoads, J. M., Liu, Y. (2021). Lactobacillus reuteri Effects on Maternal Separation Stress in Newborn Mice. Pediatr. Res. 90 (5), 980–988. doi: 10.1038/s41390-021-01374-0
Poindexter, B., COMMITTEE ON FETUS AND NEWBORN (2021). Use of Probiotics in Preterm Infants. Pediatrics 147 (6), 92–98. doi: 10.1542/peds.2021-051485
Razak, A., Patel, R. M., Gautham, K. S. (2021). Use of Probiotics to Prevent Necrotizing Enterocolitis: Evidence to Clinical Practice. JAMA Pediatr. 175 (8), 773–774. doi: 10.1001/jamapediatrics.2021.1077
Reuter, G. (2001). The Lactobacillus and Bifidobacterium Microflora of the Human Intestine: Composition and Succession. Curr. Issues Intestinal. Microbiol. 2 (2), 43–53.
Rojas, M. A., Lozano, J. M., Rojas, M. X., Rodriguez, V. A., Rondon, M. A., Bastidas, J. A., et al. (2012). Prophylactic Probiotics to Prevent Death and Nosocomial Infection in Preterm Infants. Pediatrics 130 (5), e1113–e1120. doi: 10.1542/peds.2011-3584
Röth, D., Chiang, A. J., Hu, W., Gugiu, G. B., Morra, C. N., Versalovic, J., et al. (2019). Two-Carbon Folate Cycle of Commensal Lactobacillus reuteri 6475 Gives Rise to Immunomodulatory Ethionine, a Source for Histone Ethylation. FASEB J. 33 (3), 3536–3548. doi: 10.1096/fj.201801848R
Salas-Jara, M. J., Ilabaca, A., Vega, M., García, A. (2016). Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 4 (3). doi: 10.3390/microorganisms4030035
Sharif, S., Meader, N., Oddie, S. J., Rojas-Reyes, M. X., McGuire, W. (2020). Probiotics to Prevent Necrotising Enterocolitis in Very Preterm or Very Low Birth Weight Infants. Cochrane Database Systematic. Rev. 10, CD005496. doi: 10.1002/14651858.CD005496.pub5
Shelby, R. D., Janzow, G. E., Mashburn-Warren, L., Galley, J., Tengberg, N., Navarro, J., et al. (2020). A Novel Probiotic Therapeutic in a Murine Model of Clostridioides Difficile Colitis. Gut. Microbes 12 (1), 1814119. doi: 10.1080/19490976.2020.1814119
Shelby, R. D., Mar, P., Janzow, G. E., Mashburn-Warren, L., Tengberg, N., Navarro, J. B., et al. (2021). Antibacterial and Anti-Inflammatory Effects of Lactobacillus reuteri in its Biofilm State Contribute to its Beneficial Effects in a Rat Model of Experimental Necrotizing Enterocolitis. J. Pediatr. Surg. 57 (7), 1382–1390. doi: 10.1016/j.jpedsurg.2021.09.001
Shelby, R. D., Tengberg, N., Conces, M., Olson, J. K., Navarro, J. B., Bailey, M. T., et al. (2020). Development of a Standardized Scoring System to Assess a Murine Model of Clostridium difficile Colitis. J. Invest. Surg. 33 (10), 887–895. doi: 10.1080/08941939.2019.1571129
Spinler, J. K., Auchtung, J., Brown, A., Boonma, P., Oezguen, N., Ross, C. L., et al. (2017). Next-Generation Probiotics Targeting Clostridium difficile Through Precursor-Directed Antimicrobial Biosynthesis. Infection. Immun. 85 (10). doi: 10.1128/IAI.00303-17
Spinler, J. K., Sontakke, A., Hollister, E. B., Venable, S. F., Oh, P. L., Balderas, M. A., et al. (2014). From Prediction to Function Using Evolutionary Genomics: Human-Specific Ecotypes of Lactobacillus reuteri Have Diverse Probiotic Functions. Genome Biol. Evol. 6 (7), 1772–1789. doi: 10.1093/gbe/evu137
Thomas, C. M., Hong, T., van Pijkeren, J. P., Hemarajata, P., Trinh, D., Hu, W., et al. (2012). Histamine Derived From Probiotic Lactobacillus reuteri Suppresses TNF via Modulation of PKA and ERK Signaling. PLoS One 7 (2), e31951. doi: 10.1371/journal.pone.0031951
Thomas, J. P., Raine, T., Reddy, S., Belteki, G. (2017). Probiotics for the Prevention of Necrotising Enterocolitis in Very Low-Birth-Weight Infants: A Meta-Analysis and Systematic Review. Acta Paediatrica. 106 (11), 1729–1741. doi: 10.1111/apa.13902
Vallabhaneni, S., Walker, T. A., Lockhart, S. R., Ng, D., Chiller, T., Melchreit, R., et al. (2015). Notes From the Field: Fatal Gastrointestinal Mucormycosis in a Premature Infant Associated With a Contaminated Dietary Supplement–Connecticut 2014. MMWR. Morbidity. Mortality. Weekly. Rep. 64 (6), 155–156.
Viswanathan, S., Lau, C., Akbari, H., Hoyen, C., Walsh, M. C. (2016). Survey and Evidence Based Review of Probiotics Used in Very Low Birth Weight Preterm Infants Within the United States. J. Perinatology. 36 (12), 1106–1111. doi: 10.1038/jp.2016.144
Wang, Y., Jaggers, R. M., Mar, P., Galley, J. D., Shaffer, T., Rajab, A., et al. (2021). Lactobacillus reuteri in Its Biofilm State Promotes Neurodevelopment After Experimental Necrotizing Enterocolitis in Rats. Brain. Behavior. Immun. - Health 14. doi: 10.1016/j.bbih.2021.100256
Keywords: Lactobacillus reuteri, biofilm, probiotics, necrotizing enterocolitis, Clostridioides difficile
Citation: Ragan MV, Wala SJ, Goodman SD, Bailey MT and Besner GE (2022) Next-Generation Probiotic Therapy to Protect the Intestines From Injury. Front. Cell. Infect. Microbiol. 12:863949. doi: 10.3389/fcimb.2022.863949
Received: 27 January 2022; Accepted: 25 May 2022;
Published: 28 June 2022.
Edited by:
Diane McDougald, University of Technology Sydney, AustraliaCopyright © 2022 Ragan, Wala, Goodman, Bailey and Besner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gail E. Besner, Z2FpbC5iZXNuZXJAbmF0aW9ud2lkZWNoaWxkcmVucy5vcmc=
†These authors have contributed equally to this work and share first authorship
 Mecklin V. Ragan
Mecklin V. Ragan