- 1Department of Otolaryngology – Head & Neck Surgery, Henry Ford Health System, Detroit, MI, United States
- 2Department of Otolaryngology Head and Neck Surgery, Columbia University, New York City, NY, United States
Background: Acute exacerbations (AE) in chronic rhinosinusitis (CRS) are a common and important clinical issue. However, relatively little is known regarding the underlying microbiology that drives exacerbations or how it relates to the microbiome of CRS. The purpose of this study is to examine the literature to characterize the microbiome associated with acute exacerbations in a chronic rhinosinusitis setting. Understanding this disease process may facilitate targeted antibiotic therapy, reduced antibiotic resistance, and offer more effective disease control and treatment efficacy.
Objective: To characterize the microbiome associated with acute exacerbations of chronic rhinosinusitis (AECRS).
Methods: We conducted a systematic review of the literature on Medline, Embase, and Web of Science databases from January 1990-June 2021 to identify studies related to AE in CRS. Exclusion criteria include non-English, non-human studies, and case reports. Studies without culture or PCR data were also excluded.
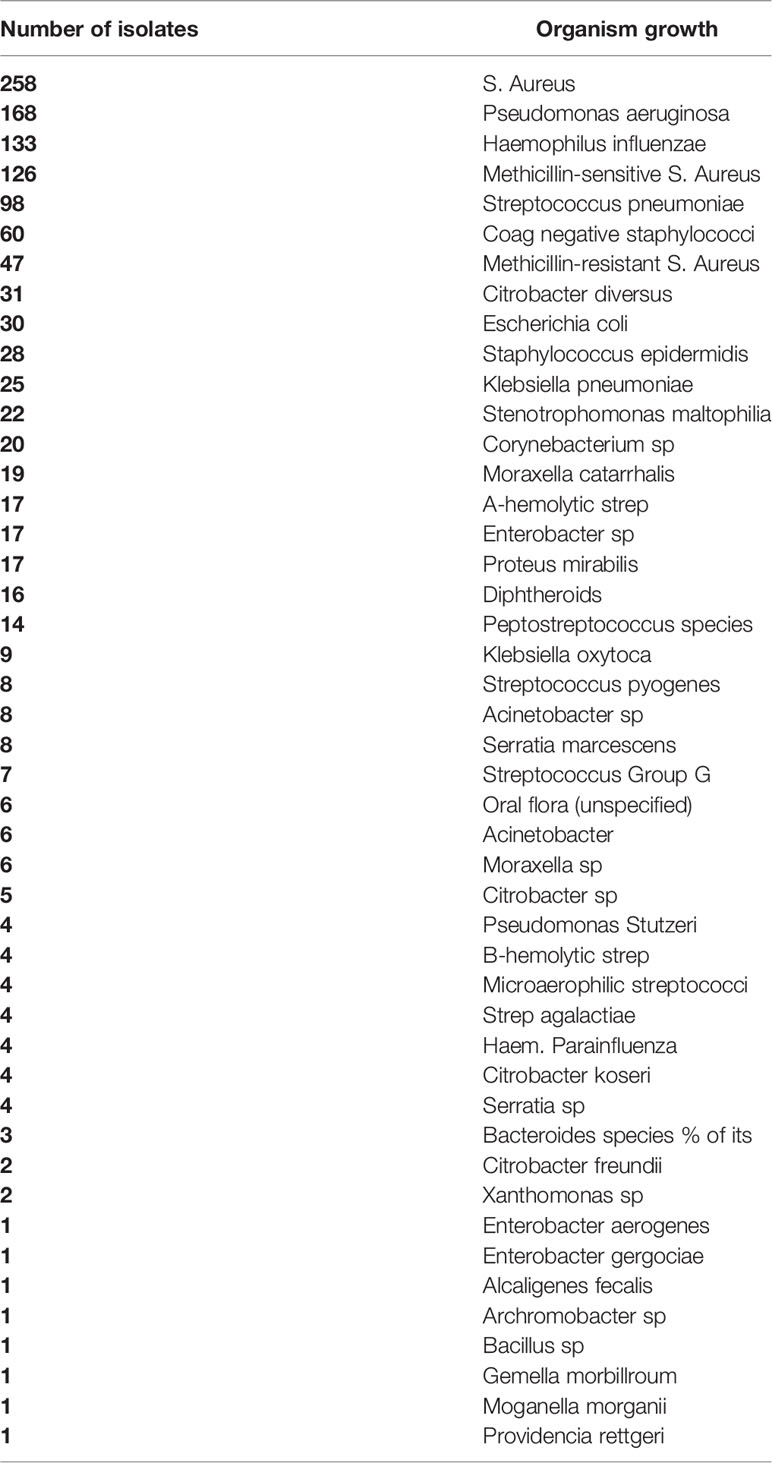
Results: Fourteen studies were identified which provided detailed data regarding sinus microbiome in AECRS patients. In these patients, a total of 1252 individual isolates were identified. While common acute pathogens were identified in high frequencies in the sinonasal cultures (Staphylococcus pneumonia, Haemophilus influenza), the predominant bacteria were Staphylococcus aureus (including methicillin-sensitive Staphylococcus aureus) and Pseudomonas aeruginosa. Patient characteristics that may represent higher risk phenotypes were not consistently collected in the studies. Discussion of antimicrobial sensitivities and/or resistance were included in 7/14 studies.
Conclusions: This systematic review identifies the predominant microbiology species that may contribute to AECRS. Further studies are needed to understand the pathogenic role of bacteria and viruses in AECRS and to identify associated comorbidities and patient phenotypes that may predispose to AE. The optimal treatment regimen for AECRS remains unclear.
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disorder of sinonasal cavity that remains one of the leading causes for patients to seek healthcare in the United States (Fokkens et al., 2020). Recent research has begun to characterize the microbiome of normal and diseased sinuses, but our understanding of the role of microbes in CRS remains limited. Mucosal dysbiosis appears to be both a central etiologic factor in the pathogenesis of CRS in addition to a consequence of CRS (Yaniv et al., 2020). Numerous other environmental and host mechanisms have been proposed to drive the pathophysiology of CRS including allergy, ciliary dysfunction, mucosal disruption, immunity derangements, and biofilm formation. Ultimately, patients with CRS tend to experience a course of illness characterized by variable degrees of chronic inflammation with periodic acute exacerbations in symptomology, known as acute exacerbations of chronic rhinosinusitis (AECRS).
Enhanced understanding of the microbiology that contributes to AECRS will facilitate the development of targeted treatment regimens to improve symptoms and disease control, while also reducing the need for inappropriate antibiotic administration and the potential for antibiotic resistance. The purpose of this study is to systematically review the published literature to characterize the underlying microbiology of AECRS.
Methods
We performed a systematic review utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A comprehensive search of Medline, Embase, Web of Science, and Google Scholar databases from January 1990-June 2021 was conducted to identify studies relating to the microbiology of acute exacerbations in CRS. A combination of terms was used to maximize the probability of finding all relevant publications, including but not limited to: “rhinitis”, “sinusitis”, “sinus”, “microbiology”, “acute exacerbation”, “chronic disease”, “bacteriology”, “cultures”, and “PCR”.
Study Selection
Titles and abstracts of all the relevant studies were reviewed by 2 independent authors (OO and AR). Included studies addressed the microbiology of AECRS with either culture or PCR data; studies without culture or PCR data were excluded. Studies were excluded if they were pediatric, non-English, non-human studies, and case reports.
Data Extraction and Analysis
Data included year of publication, study design, age range, diagnostic criteria, bacterial findings, and immune-histologic findings. After analysis of each article, summary tables were developed. In articles where various data groupings were provided, only the relevant data for the AECRS patient population were extracted and used for analysis.
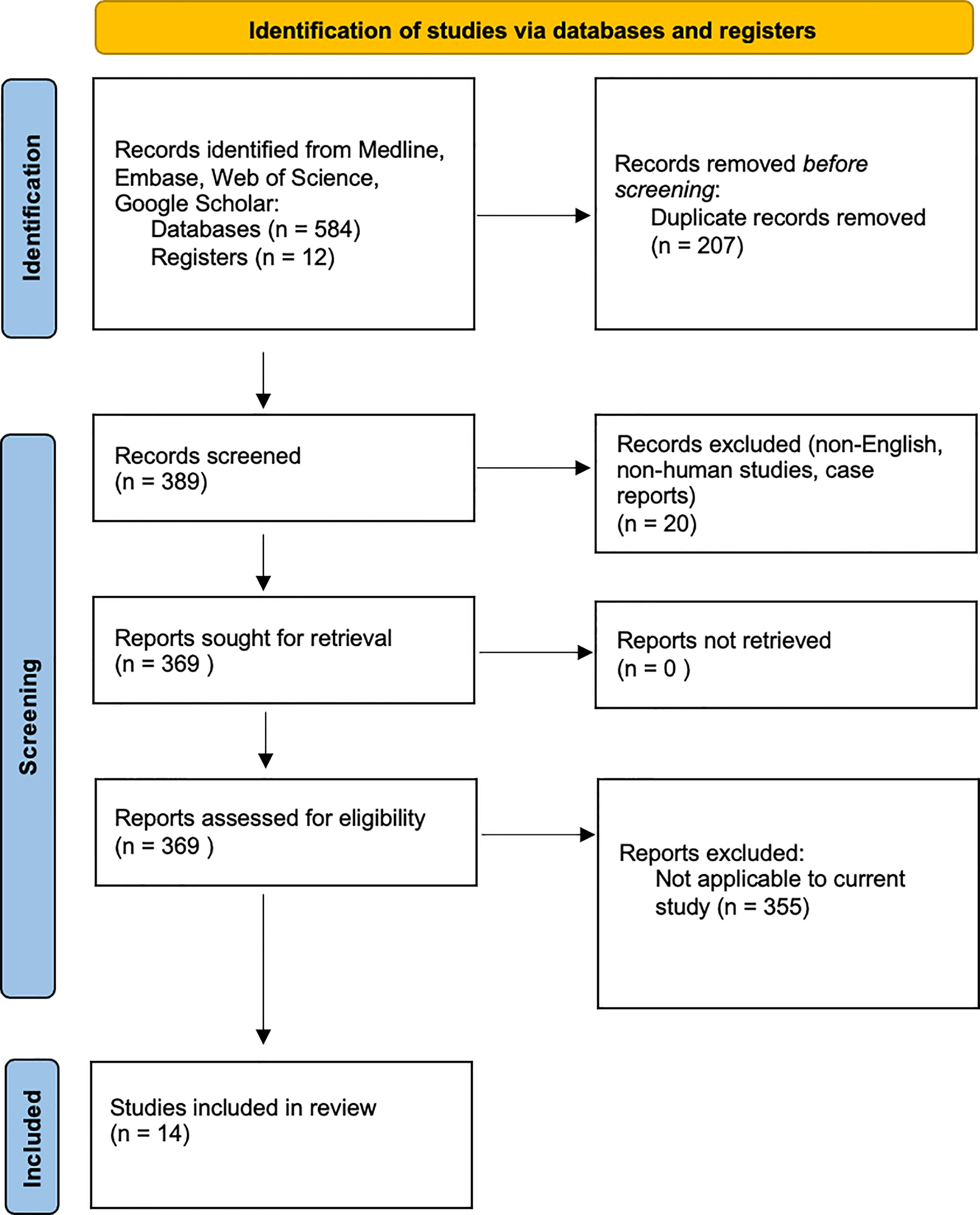
A summary of the methods is provided in Figure 1.
Results
Included Studies
Our initial database search identified 596 articles. Duplicate articles, non-English articles, those without full-text or without extractable data were excluded. A total of 14 articles met the final inclusion criteria for systematic review and underwent further full text review. These studies explored the underlying microbiology in AECRS.
Microbiology in AECRS
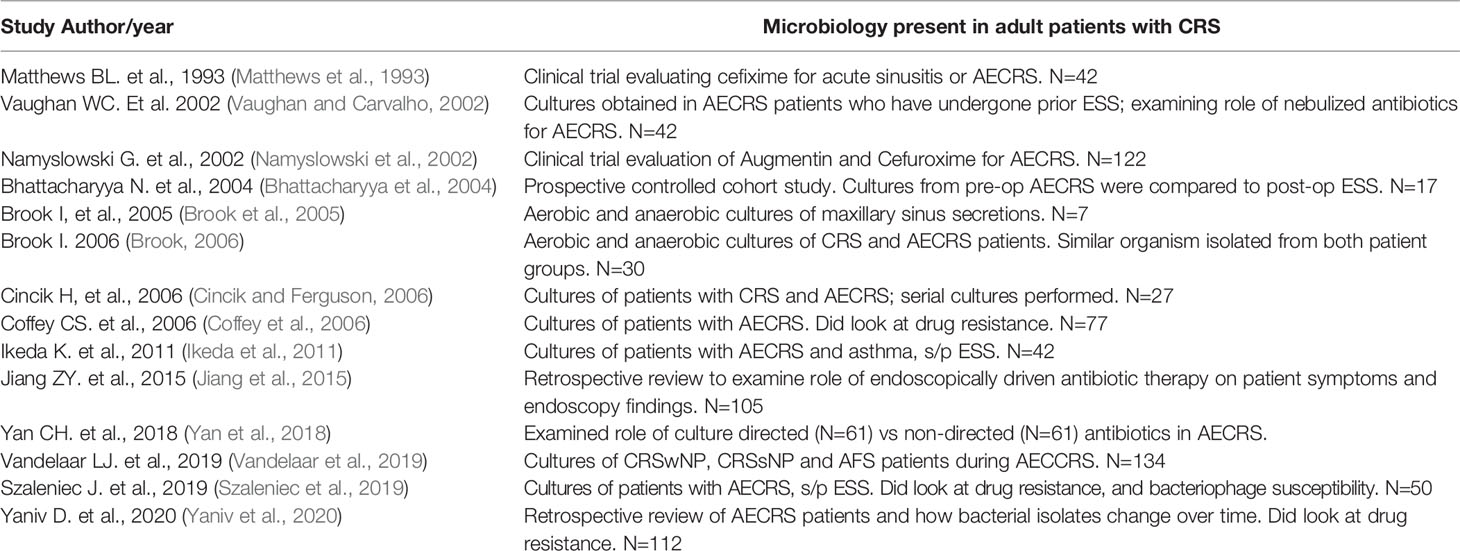
The details of the included studies exploring the microbiology of AECRS are summarized in Table 1. The bacteria that were identified with association to AECRS are listed in Table 2.
There was significant diversity in the bacteria that were associated with AECRS. The aerobic bacteria included: Staphylococci species which included both coagulase-negative Staph species and methicillin-resistant Staph species, Streptococcus species, Haemophilis influenzae, Pseudomonas aeruginosa, Enterobacteriaceae, and Moraxella catarrhalis. The predominant anaerobic bacteria that were identified included: Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, and Propionibacterium acnes, although only limited studies specifically tested or commented on anaerobic growth (Matthews et al., 1993; Vaughan and Carvalho, 2002; Brook et al., 2005; Brook, 2006). Facultative bacteria included the Escherichia species and Klebsiella pneumonia. The Staphylococci species were the most frequently identified culture-positive bacteria.
While most of the studies utilized culture data, one study did include speciation via polymerase chain reduction in addition to standard culture alone (Vandelaar et al., 2019). All included studies also commented on aerobic bacterial growth, but anaerobic growth was not routinely reported.
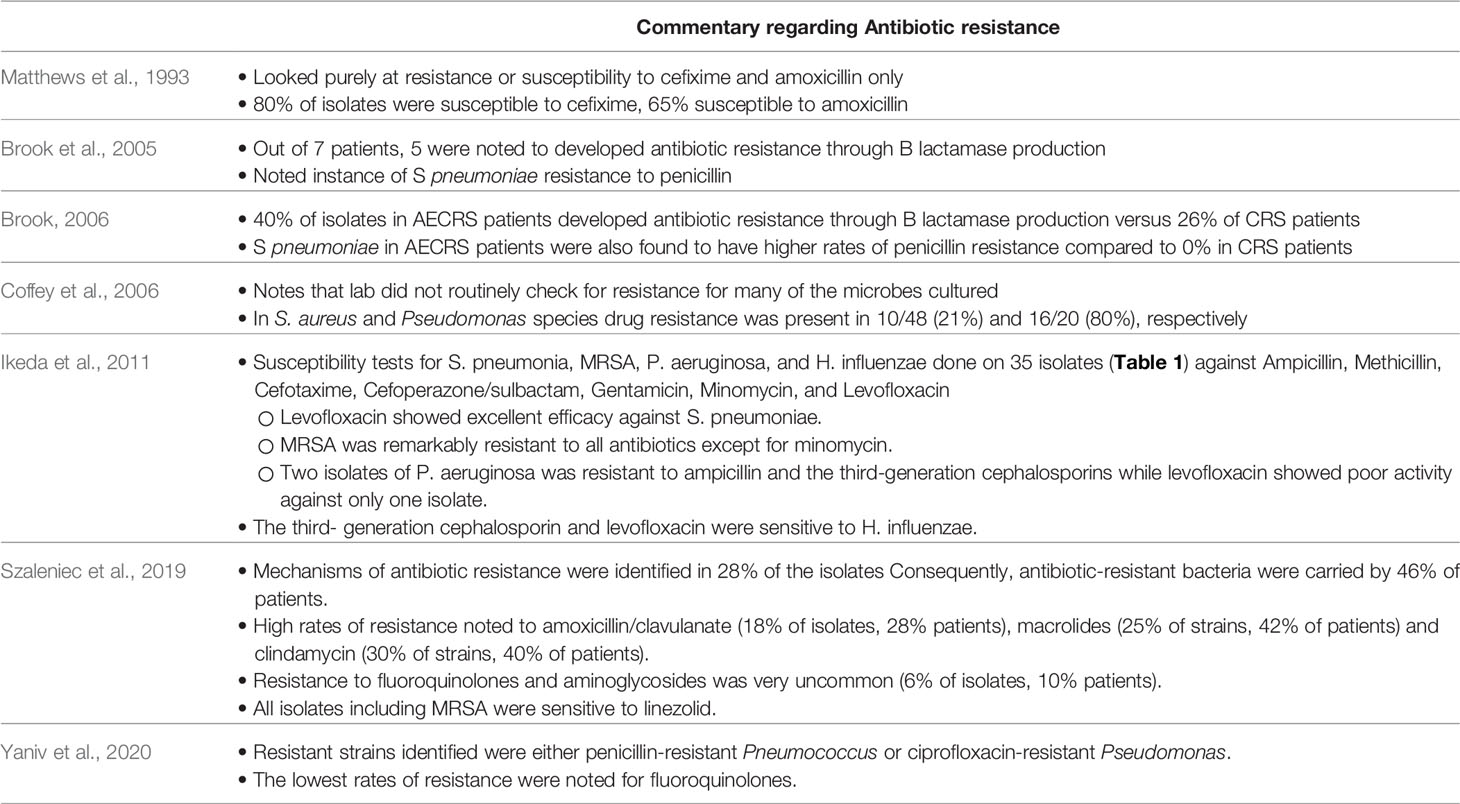
Discussion regarding antibiotic therapy, resistance, and sensitivities was noted in 7 of the 14 studies listed (Table 3), although the extent of analysis varied widely by study.
Discussion
Our review demonstrates significant diversity in the various bacteria that were associated with AECRS. Staphylococci species were the most frequently identified bacteria, followed by Pseudomonas aeruginosa, Streptococcal species, and Haemophilus influenzae. Among the Staphylococcal species, various subspecies were identified including S. aureus, MRSA, and coagulase-negative Staphylococci. Of note, Rujanavej et al. demonstrated a substantial rise in MRSA isolates from intranasal cultures since the year 2000 and beyond, underscoring the need to consider MRSA coverage in cases of AECRS (Rujanavej et al., 2013). It is also interesting to note the high prevalence of Pseudomonas species; given the Pseudomonal ability to produce biofilms and multidrug resistance, these findings underscore the value of targeted, antimicrobial therapy (Bhattacharyya and Kepnes, 1999). Of note, there were multiple studies to indicate that anerobic bacteria are present as well, suggesting that the microbial population in AECRS is a mix of aerobic and anaerobic bacteria.
AECRS likely begins with a common viral upper respiratory infection that progresses into a secondary bacterial infection, potentially in an already dysbiotic setting (Brook et al., 2005; Brook, 2006; Cho et al., 2013; Rowan et al., 2015), followed by return to baseline CRS. An exacerbation may also be characterized by worsening sinonasal symptoms, presence of purulence on nasal endoscopy, and/or endoscopically-derived bacterial cultures (Wu et al., 2019; Wu et al., 2020). However, the specific microbiology of these exacerbations remains poorly understood.
Although positive bacterial cultures are identified in up to 90.9% of patients during acute exacerbations (Ikeda et al., 2011), many of the previously utilized treatment paradigms are largely based on the microbiomes of acute or chronic rhinosinusitis states, rather than the particular dysbiome in AECRS.
Currently, there are no consistent treatment guideline for AECRS, but management usually involves short-term antibiotics and/or nasal corticosteroids. Targeted treatment for AECRS requires a better understanding of its pathophysiology. Despite being poorly understood, several factors have been noted to drive this dysbiosis including mucosal inflammation, impaired mucociliary clearance, biofilm formation, chronic mucosal disruption, atrophic rhinitis, transient viral infections and immunologic changes, and arising antibiotic resistance (Lee et al., 2018). Colonization by opportunistic pathogens such as S. aureus and Pseudomonas aeruginosa have been shown to trigger inflammation that is worsened by defects in the innate immune response.
There is significant evidence that alterations of the sinonasal microbiome are a direct driver of CRS inflammation and acute exacerbations (Rank et al., 2013; Divekar et al., 2015). While not a specific focus of this study, it should be noted that while antibiotic sensitivities were not routinely obtained in all of the included studies, significant multidrug resistance was reported. Thus, there is a growing body of literature to support culture-directed antibiotics to address microbiome shifts that are likely contributing significantly to the underlying disease process.
These intermittent and persistent disorders of the upper airway (including but not limited to asthma, allergic rhinitis, bronchitis, etc.) may represent gradients along a spectrum rather than each being a distinct pathology. In this unified airway theme, inflammatory disruptions in one subsite may affect the homeostasis in others. While this has been studied primarily in allergic disease, less is known about the impact of other adjunct upper airway disorders. For example, new evidence suggests that nasal hyperreactivity to nonspecific allergens may trigger symptoms mimicking AECRS, confounding the clinical picture (Doulaptsi et al., 2020). Additionally, in recent years, the concept of severe chronic upper airway disease (SCUAD) has been proposed to define patients with CRS (with or without polyps) and allergic, nonallergic or occupational rhinitis, whose symptoms are refractory to traditional guideline based treatments. It is worth considering whether these patients represent a group of SCUAD patients, and if so, how to best address the multifactorial underlying etiologies driving the clinical worsening of symptoms (Prokopakis et al., 2014). Thus, cultures obtained during such episodes may not necessarily reflect a true microbiome picture of pure AECRS.
This concept of microbiome shifts during acute exacerbations also mirrors findings from other unified airway subsites. For example, sputum analysis done during acute exacerbations of both chronic obstructive pulmonary disease and chronic bronchitis demonstrate dysbiosis findings similar to those observed in the paranasal sinuses (Dickson et al., 2014; Jubinville et al., 2018). Elevated IL-6 levels have also been linked to patients suffering a CRS exacerbation, suggesting either a viral infection or an altered IL-6 pathway (Yaniv et al., 2020). More robust studies on pathophysiology and treatment options, including randomized controlled trials, are needed to better understand AECRS.
In addition, the articles reviewed included patients at various stages of intervention or recent antimicrobial treatment. It is also worth noting that patients with AECRS are known to have higher prevalence of comorbid conditions including allergic rhinitis, asthma, autoimmune, or other atopic diseases (Kwah et al., 2020). Most of the included studies lacked comprehensive demographic data regarding these and other relevant comorbidities such as respiratory pathologies, diabetes, or extensive obstructive polyposis. Additionally, no study mentioned the role of an odontogenic etiology driving the patient’s CRS, which is starting to become recognized as more prevalent than previously thought (Craig et al., 2021). Thus, characterizing this subtype of CRS requires a more thoughtful and comprehensive approach to identify high risk phenotypes and incorporate preventative measures to reduce exacerbation frequency (Kuiper et al., 2018).
It is worthwhile to consider that traditional culture methods may not adequately depict the in vivo polymicrobial host community, as standard cultures offer limited, predefined conditions in which microbial growth can occur. Thus, in vitro cultures may inadvertently bias growth to select for faster growing organisms, or those without niche or symbiotic growth needs. Additionally, standard cultures may not adequately represent the microbiome due to physical limitations in obtaining the culture; for example, Miller and Davis demonstrate significant variability in pathogens in cultures from the same patient when done via standard methods compared to those obtained intraoperatively (Feazel et al., 2011; Hauser et al., 2015; Miller and Davis, 2018). Thus, it may beneficial to utilize alternative molecular methods of amplification, such as polymerase chain reaction, which can identify up to an order of magnitude more taxa that might be otherwise missed in between 25%-99% of cases. In fact, direct comparisons between sequencing and culture results find that dominant bacteria determined by sequencing is apparent in culture results less than 50% of the time (Feazel et al., 2011).
Although this discussion regarding AECRS focuses primarily on underlying bacterial pathogens, it is important to keep in mind that viruses and fungi may also be drivers of AECRS. However, the literature is limited in understanding the delicate balance of the baseline microbiome or the role of other microbes. Although rhinovirus presence has been identified as being the most prevalent virus in CRS exacerbation in some studies, its mechanism of pathogenesis and relationship to bacterial dysbiosis is unclear (Cho et al., 2013; Yaniv et al., 2020).
There is also limited and conflicting literature to describe the role endoscopic sinus surgery (ESS) may play in altering the sinus microbiome, possibly via mechanisms that alter sinonasal aeration, mucociliary clearance, inflammatory profiles, nitric oxide levels, and others. Larson and Han describe their findings in 26 patients, demonstrating that ESS does not significant alter the pre and post-surgery microbiome (Larson and Han, 2011). Hai et al. specifically examined the effect of ESS on biofilm production, finding that although ESS does not completely eradicate biofilms, it does significantly reduce their density (Hai et al., 2010). Several other studies demonstrate worse patient outcomes after ESS where biofilms are involved (Bendouah et al., 2006; Psaltis et al., 2008; Zhang et al., 2009).
The above discussion illustrates the complexity in appropriately identifying and treating AECRS. In addition to diligent and thoughtful characterization of clinical symptoms, advancements in molecular technology are already enabling research in the unique endo- and phenotypes of this disease, and allow for customized, precision treatment, termed “precision medicine” (Vlastos et al., 2019).
Conclusion
This systematic review identifies the predominant microbiology species that may contribute to AECRS. The literature supports a pathogenic role of bacteria and viruses in AECRS distinct from those cultured at baseline for patients with CRS. The optimal treatment regimen for AECRS remains unclear.
Author Contributions
OO: data collection, manuscript development. AR: data collection, manuscript development, editing and study design. DG: manuscript development, editing, and study design. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bendouah, Z., Barbeau, J., Hamad, W. A., Desrosiers, M. (2006). Biofilm Formation by Staphylococcus Aureus and Pseudomonas Aeruginosa Is Associated With an Unfavorable Evolution After Surgery for Chronic Sinusitis and Nasal Polyposis. Otolaryngol Head Neck Surg. 134 (6), 991–996. doi: 10.1016/j.otohns.2006.03.001
Bhattacharyya, N., Gopal, H. V., Lee, K. H. (2004). Bacterial Infection After Endoscopic Sinus Surgery: A Controlled Prospective Study. Laryngoscope 114 (4), 765–767. doi: 10.1097/00005537-200404000-00032
Bhattacharyya, N., Kepnes, L. J. (1999). The Microbiology of Recurrent Rhinosinusitis After Endoscopic Sinus Surgery. Arch. Otolaryngol Head Neck Surg. 125 (10), 1117–1120. doi: 10.1001/archotol.125.10.1117
Brook, I. (2006). Bacteriology of Chronic Sinusitis and Acute Exacerbation of Chronic Sinusitis. Arch. Otolaryngol Head Neck Surg. 132 (10), 1099–1101. doi: 10.1001/archotol.132.10.1099
Brook, I., Foote, P. A., Frazier, E. H. (2005). Microbiology of Acute Exacerbation of Chronic Sinusitis. Ann. Otol. Rhinol. Laryngol. 114 (7), 573–576. doi: 10.1177/000348940511400714
Cho, G. S., Moon, B. J., Lee, B. J., Gong, C. H., Kim, N. H., Kim, Y. S., et al. (2013). High Rates of Detection of Respiratory Viruses in the Nasal Washes and Mucosae of Patients With Chronic Rhinosinusitis. J. Clin. Microbiol. 51 (3), 979–984. doi: 10.1128/JCM.02806-12
Cincik, H., Ferguson, B. J. (2006). The Impact of Endoscopic Cultures on Care in Rhinosinusitis. Laryngoscope. 116 (9), 1562–1568. doi: 10.1097/01.mlg.0000230402.66579.07
Coffey, C. S., Sonnenburg, R. E., Melroy, C. T., Dubin, M. G., Senior, B. A. (2006). Endoscopically Guided Aerobic Cultures in Postsurgical Patients With Chronic Rhinosinusitis. Am. J. Rhinol. 20 (1), 72–76. doi: 10.1177/194589240602000113
Craig, J. R., Poetker, D. M., Aksoy, U., Allevi, F., Biglioli, F., Cha, B. Y., et al. (2021). Diagnosing Odontogenic Sinusitis: An International Multidisciplinary Consensus Statement. Int. Forum Allergy Rhinol. 11 (8), 1235–1248. doi: 10.1002/alr.22777
Dickson, R. P., Martinez, F. J., Huffnagle, G. B. (2014). The Role of the Microbiome in Exacerbations of Chronic Lung Diseases. Lancet. 384 (9944), 691–702. doi: 10.1016/S0140-6736(14)61136-3
Divekar, R. D., Samant, S., Rank, M. A., Hagan, J., Lal, D., O'Brien, E. K., et al. (2015). Immunological Profiling in Chronic Rhinosinusitis With Nasal Polyps Reveals Distinct VEGF and GM-CSF Signatures During Symptomatic Exacerbations. Clin. Exp. Allergy 45 (4), 767–778. doi: 10.1111/cea.12463
Doulaptsi, M., Steelant, B., Prokopakis, E., Ierodiakonou, D., Tsinaslanidou, Z., Cools, L., et al. (2020). Prevalence and Impact of Nasal Hyperreactivity in Chronic Rhinosinusitis. Allergy. 75 (7), 1768–1771. doi: 10.1111/all.14199
Feazel, L. M., Frank, D. N., Ramakrishnan, V. R. (2011). Update on Bacterial Detection Methods in Chronic Rhinosinusitis: Implications for Clinicians and Research Scientists. Int. Forum Allergy Rhinol. 1 (6), 451–459. doi: 10.1002/alr.20071
Fokkens, W. J., Lund, V. J., Hopkins, C., Hellings, P. W., Kern, R., Reitsma, S., et al. (2020). European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 58 (Suppl S29), 1–464. doi: 10.4193/Rhin20.600
Hai, P. V., Lidstone, C., Wallwork, B. (2010). The Effect of Endoscopic Sinus Surgery on Bacterial Biofilms in Chronic Rhinosinusitis. Otolaryngol Head Neck Surg. 142 (3 Suppl 1), S27–S32. doi: 10.1016/j.otohns.2009.09.022
Hauser, L. J., Feazel, L. M., Ir, D., Fang, R., Wagner, B. D., Robertson, C. E., et al. (2015). Sinus Culture Poorly Predicts Resident Microbiota. Int. Forum Allergy Rhinol. 5 (1), 3–9. doi: 10.1002/alr.21428
Ikeda, K., Yokoi, H., Kusunoki, T., Saitoh, T., Yao, T., Kase, K., et al. (2011). Bacteriology of Recurrent Exacerbation of Postoperative Course in Chronic Rhinosinusitis in Relation to Asthma. Auris Nasus Larynx. 38 (4), 469–473. doi: 10.1016/j.anl.2010.10.009
Jiang, Z. Y., Kou, Y. F., Batra, P. S. (2015). Endoscopic Culture-Directed Antibiotic Therapy: Impact on Patient Symptoms in Chronic Rhinosinusitis. Am. J. Otolaryngol. 36 (5), 642–646. doi: 10.1016/j.amjoto.2015.04.009
Jubinville, E., Veillette, M., Milot, J., Maltais, F., Comeau, A. M., Levesque, R. C., et al. (2018). Exacerbation Induces a Microbiota Shift in Sputa of COPD Patients. PloS One 13 (3), e0194355. doi: 10.1371/journal.pone.0194355
Kuiper, J. R., Hirsch, A. G., Bandeen-Roche, K., Sundaresan, A. S., Tan, B. K., Schleimer, R. P., et al. (2018). Prevalence, Severity, and Risk Factors for Acute Exacerbations of Nasal and Sinus Symptoms by Chronic Rhinosinusitis Status. Allergy. 73 (6), 1244–1253. doi: 10.1111/all.13409
Kwah, J. H., Somani, S. N., Stevens, W. W., Kern, R. C., Smith, S. S., Welch, K. C., et al. (2020). Clinical Factors Associated With Acute Exacerbations of Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 145 (6), 1598–1605. doi: 10.1016/j.jaci.2020.01.023
Larson, D. A., Han, J. K. (2011). Microbiology of Sinusitis: Does Allergy or Endoscopic Sinus Surgery Affect the Microbiologic Flora? Curr. Opin. Otolaryngol Head Neck Surg. 19 (3), 199–203. doi: 10.1097/MOO.0b013e328344f67a
Lee, D. C., Choi, H., Oh, J. M., Hong, Y., Jeong, S. H., Kim, C. S., et al. (2018). The Effect of Urban Particulate Matter on Cultured Human Nasal Fibroblasts. Int. Forum Allergy Rhinol. 8 (9), 993–1000. doi: 10.1002/alr.22167
Matthews, B. L., Kohut, R. I., Edelstein, D. R., Rybak, L. P., Rapp, M., McCaffrey, T. V., et al. (1993). Evaluation of Cefixime in the Treatment of Bacterial Maxillary Sinusitis. South Med. J. 86 (3), 329–333. doi: 10.1097/00007611-199303000-00016
Miller, C., Davis, G. E. (2018). Are Multiple Sinus Cultures Necessary During Sinus Surgery for Chronic Rhinosinusitis? Int. Forum Allergy Rhinol. 8 (4), 504–508. doi: 10.1002/alr.22068
Namyslowski, G., Misiolek, M., Czecior, E., Malafiej, E., Orecka, B., Namyslowski, P., et al. (2002). Comparison of the Efficacy and Tolerability of Amoxycillin/Clavulanic Acid 875 Mg B.I.D. With Cefuroxime 500 Mg B.I.D. In the Treatment of Chronic and Acute Exacerbation of Chronic Sinusitis in Adults. J. Chemother. 14 (5), 508–517. doi: 10.1179/joc.2002.14.5.508
Prokopakis, E. P., Vlastos, I. M., Ferguson, B. J., Scadding, G., Kawauchi, H., Georgalas, C., et al. (2014). SCUAD and Chronic Rhinosinusitis. Reinforcing Hypothesis Driven Research in Difficult Cases. Rhinology 52 (1), 3–8. doi: 10.4193/Rhino13.049
Psaltis, A. J., Weitzel, E. K., Ha, K. R., Wormald, P. J. (2008). The Effect of Bacterial Biofilms on Post-Sinus Surgical Outcomes. Am. J. Rhinol. 22 (1), 1–6. doi: 10.2500/ajr.2008.22.3119
Rank, M. A., Hagan, J. B., Samant, S. A., Kita, H. (2013). A Proposed Model to Study Immunologic Changes During Chronic Rhinosinusitis Exacerbations: Data From a Pilot Study. Am. J. Rhinol. Allergy 27 (2), 98–101. doi: 10.2500/ajra.2013.27.3850
Rowan, N. R., Lee, S., Sahu, N., Kanaan, A., Cox, S., Phillips, C. D., et al. (2015). The Role of Viruses in the Clinical Presentation of Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 29 (6), e197-e200. doi: 10.2500/ajra.2015.29.4242
Rujanavej, V., Soudry, E., Banaei, N., Baron, E. J., Hwang, P. H., Nayak, J. V. (2013). Trends in Incidence and Susceptibility Among Methicillin-Resistant Staphylococcus Aureus Isolated From Intranasal Cultures Associated With Rhinosinusitis. Am. J. Rhinol. Allergy 27 (2), 134–137. doi: 10.2500/ajra.2013.27.3858
Szaleniec, J., Gibała, A., Pobiega, M., Parasion, S., Składzień, J., Stręk, P., et al. (2019). Exacerbations of Chronic Rhinosinusitis-Microbiology and Perspectives of Phage Therapy. Antibiotics (Basel). 8 (4), 175. doi: 10.3390/antibiotics8040175
Vandelaar, L. J., Hanson, B., Marino, M., Yao, W. C., Luong, A. U., Arias, C. A., et al. (2019). Analysis of Sinonasal Microbiota in Exacerbations of Chronic Rhinosinusitis Subgroups. OTO Open 3 (3), 2473974X19875100. doi: 10.1177/2473974X19875100
Vaughan, W. C., Carvalho, G. (2002). Use of Nebulized Antibiotics for Acute Infections in Chronic Sinusitis. Otolaryngol Head Neck Surg. 127 (6), 558–568. doi: 10.1067/mhn.2002.129738
Vlastos, I., Gkouskou, K., Doulaptsi, M., Karatzanis, A., Prokopakis, E. P. (2019). Precision Medicine in Rhinosinusitis. Curr. Allergy Asthma Rep. 19 (2), 12. doi: 10.1007/s11882-019-0850-x
Wu, D., Bleier, B. S., Wei, Y. (2019). Current Understanding of the Acute Exacerbation of Chronic Rhinosinusitis. Front. Cell Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00415
Wu, D., Bleier, B., Wei, Y. (2020). Definition and Characteristics of Acute Exacerbation in Adult Patients With Chronic Rhinosinusitis: A Systematic Review. J. Otolaryngol Head Neck Surg. 49 (1), 62. doi: 10.1186/s40463-020-00459-w
Yaniv, D., Stern, D., Vainer, I., Ben Zvi, H., Yahav, D., Soudry, E. (2020). The Bacteriology of Recurrent Acute Exacerbations of Chronic Rhinosinusitis: A Longitudinal Analysis. Eur. Arch. Otorhinolaryngol. 277 (11), 3051–3057. doi: 10.1007/s00405-020-06157-7
Yan, C. H., Tangbumrungtham, N., Maul, X. A., Ma, Y., Nayak, J. V., Hwang, P. H., et al. (2018). Comparison of Outcomes Following Culture-Directed vs Non-Culture-Directed Antibiotics in Treatment of Acute Exacerbations of Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 8 (9), 1028–1033. doi: 10.1002/alr.22147
Keywords: microbiology, bacteriology, acute exacerbation, chronic rhinosinusitis, chronic sinusitis, sinus infection
Citation: Okifo O, Ray A and Gudis DA (2022) The Microbiology of Acute Exacerbations in Chronic Rhinosinusitis - A Systematic Review. Front. Cell. Infect. Microbiol. 12:858196. doi: 10.3389/fcimb.2022.858196
Received: 19 January 2022; Accepted: 24 February 2022;
Published: 24 March 2022.
Edited by:
Dawei Wu, Massachusetts Eye & Ear Infirmary and Harvard Medical School, United StatesReviewed by:
Emmanuel Prokopakis, University Hospital of Crete Heraklion, GreeceCristobal Langdon Montero, Hospital Clínic de Barcelona, Spain
Copyright © 2022 Okifo, Ray and Gudis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amrita Ray, YXJheTlAaGZocy5vcmc=
 Oghenefejiro Okifo
Oghenefejiro Okifo Amrita Ray
Amrita Ray