- 1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2Guangzhou Institute of Respiratory Health, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou, China
- 3The First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, China
Objective: The limited information available on mixed mycosis involving the lungs makes the understanding of mixed fungal diseases insufficient and affects prognosis. Our study aims to improve understanding by exploring experience in the successful management of mixed fungal infections.
Methods: Patients who had two types of mycosis involving the lung at the same disease course were retrospectively enrolled.
Results: Between September 2011 and December 2019, 17 patients with proven mixed mycosis were enrolled. Four patients were immunocompromised, with one case each of lung transplantation, corticosteroid treatment, STAT3 hyper-IgE syndrome, and anti-IFN-γ autoantibody-associated immunodeficiency syndrome. Among 13 patients who were not immunocompromised, 9 had type 2 diabetes mellitus. Eight cases were coinfection with Mucor and Aspergillus, 4 cases were Cryptococcus and Aspergillus, 2 cases were Talaromyces marneffei and Cryptococcus, 2 cases were Talaromyces marneffei and Aspergillus, and 1 case was Candida and Aspergillus. Seven patients were diagnosed with mixed pulmonary mycosis at almost the same time. Among the remaining 10 patients, the initial treatment was ineffective in four cases, and six patients showed a partial response to the initial antifungal treatment, but the original fungal lesions became re-enlarged. Three patients were admitted to the intensive care unit during hospitalization, and one patient died. Another Mucor coinfection patient died due to treatment refusal.
Conclusion: Mixed mycosis involving the lungs is not uncommon in patients without apparent immune deficiency diseases. During the management of mycosis, we recommend keeping mixed mycosis in mind for patients with a poor response to initial antifungal treatment, even in immunocompetent populations, and identifying the cause of illness through a rigorous procedure.
Introduction
Fungi can cause serious infections worldwide, with the number of serious cases reaching 150 million (Bongomin et al., 2017). Immunocompromised hosts, such as those with organ transplantation, hematopoietic stem cell transplantation, malignant tumors receiving radiotherapy and chemotherapy, and autoimmune disease receiving immunosuppressive therapy, are at risk of invasive pulmonary mycosis, which may lead to poor prognosis. On the basis of discharge diagnoses, the incidence of invasive fungal infections in France was 5.9/100 000 cases/year, with a mortality of 27.6%, both of which increased during the observation period (2001–2010) (von Lilienfeld-Toal et al., 2019). While, the proportion of pulmonary fungal infection increased annually from 26.5 per 1000 inpatients in 2013 to 42.6 in 2019 and from 3.07 per 1000 outpatients in 2013 to 8.48 in 2019 in Guangzhou, China (Li et al., 2021). The most common pulmonary mycoses are Aspergillus, Candida, Cryptococcus species, and Pneumocystis jirovecii (Bongomin et al., 2017; Azoulay et al., 2019; Zhan et al., 2021). Mixed fungal infections have also been reported (Wang et al., 2018; Awari et al., 2020); however, their characteristics have not yet been described in detail, and most are in immunocompromised hosts.
Existing data show that pulmonary fungal disease is not limited to immunosuppressed hosts. Reports of pulmonary fungal diseases in immunocompetent hosts are gradually increasing, such as cryptococcosis, Talaromycosis marneffei infection, and aspergillosis, although the latter is one of the common infections in primary immunodeficiency diseases such as chronic granulomatous diseases (Mortaz et al., 2019; Abd Elaziz et al., 2020). In China, most patients with cryptococcosis do not have an immunocompromised status (Ye et al., 2012; Cheng et al., 2021). Talaromycosis marneffei infection, an endemic fungal disease in Asian populations, also occurs in HIV-negative immunocompetent populations (Lee et al., 2019; Pan et al., 2020). Although some patients have been confirmed to have nonclassical immunodeficiency, such as hyper-IgE syndrome and anti-IFN-γ autoantibody-associated immunodeficiency syndrome, most patients do not have clear immunodeficiency or hypofunction (Lee et al., 2019; Pan et al., 2020). Due to the lack of specificity in the clinical manifestations of pulmonary mycosis in immunocompetent hosts, mycosis is often not the first consideration during clinical diagnosis and treatment. Incorrect diagnosis often delays treatment, which may affect the prognosis.
Diagnosis and treatment are even more difficult in mixed fungal disease because of unsatisfied positivity in culture and pathology. The need for timely inoculation, destruction of hyphae by tissue grinding, and empiric use of antifungal drugs is one of the reasons for negative culture. The morphological similarity of mold makes pathological diagnosis dependent on experienced technicians. Awari et al. and Wang et al. reported some cases of mixed mycoses of Aspergillus and Cryptococcus in both immunocompromised and immunocompetent patients (Wang et al., 2018; Awari et al., 2020). Coinfection with Talaromyces marneffei and Cryptococcus neoformans has also been reported in a non-HIV patient (He et al., 2020). However, the characteristics of fungal coinfection are still undefined. Here, we retrospectively analyzed a group of successively managed mixed mycoses to explore the clinical characteristics and main points of diagnosis and treatment of mixed mycoses.
Methods
This was a retrospective study conducted at the First Affiliated Hospital of Guangzhou Medical University. Between Jan 1, 2011 and Dec 31, 2019, patients who were hospitalized in the Department of Pulmonary and Critical Care Medicine with a diagnosis of mixed mycosis that involved pulmonary were searched from the electronic medical record database and enrolled as the study group. This study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (2018-119). Because this is a retrospective study, informed consent was not required.
Study Population
Inclusion criteria were the following: I. patients who had two types of fungal infections involving the lung at the same disease course; II. patients who had follow-up data in the outpatient medical records database; and III. patients who were HIV negative.
Mycosis was diagnosed based on the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC), which is a graded diagnostic standard, including the classifications of “proven,” “probable,” and “possible” invasive fungal disease (IFD) (Donnelly et al., 2019). The present study included only patients with proven or probable IFD. The diagnostic criteria for proven IFD included a specimen obtained by needle aspiration or biopsy showing the unique morphology of the fungus by histopathology, cytopathology or direct microscopic examination; a specimen obtained by a sterile procedure from a normally sterile and clinically or radiologically abnormal site consistent with an infectious disease process, recovering a hyaline or pigmented mold or yeast by culture; a positive blood culture for mold (Aspergillus was excluded) or yeast; or a positive test for cryptococcal antigen in cerebrospinal fluid or blood. The diagnosis of probable IFD should meet all three criteria, including host factors, clinical features and mycological evidence, with details in the referenced guideline (Donnelly et al., 2019).
Exclusion criteria included the following: I. two fungal infections did not occur at the same disease course, and the first mycosis was cured before the onset of the second mycosis; II. only one fungal infection was diagnosed; and III. there was incomplete information.
Data Collection
Clinical features of enrolled patients were collected, including medical history, underlying diseases, use of corticosteroids and/or immunosuppressants before the onset of mycosis, clinical symptoms and signs, imaging and laboratory examination data, types of culture specimens, diagnosis procedure, treatment, and outcome. If the patient went to the outpatient clinic, the follow-up and results within 1 year were also collected from the database.
Statistics
SPSS 25.0 software was used for data processing of the results of this study. Because the treatment drugs and prognosis of mucormycosis are different from those of other fungal infections, comparisons were made between Mucor mixed infection and non-Mucor mixed infection cases. All P values are two-sided tests, and P <0.05 means that the difference is statistically significant. Independent sample t-tests were used for continuous variables, and Fisher’s exact test and χ2 test were used for categorical variables.
Results
General Characteristics
Between September 2011 and December 2019, 17 cases of mixed fungal infection were confirmed in our hospital. The average age was 50.2 ± 16.9 years, ranging from 21 to 76, with 12 (70.6%) males. Four patients were immunocompromised, with one patient receiving bilateral lung transplantation for destructive pneumonophthisis, one patient receiving long-term corticosteroid therapy for nephrotic syndrome and hypoalbuminemia, one case of STAT3 hyper-IgE syndrome and one case of anti-IFN-γ autoantibody-associated immunodeficiency syndrome (Table 1). Among the 13 patients who did not have apparent immune deficiency, 10 had underlying diseases, including hypertension, diabetes mellites and hyperthyroidism (Table 1).
Among all 17 patients, 8 cases were coinfection with Mucor and Aspergillus, 4 cases with Cryptococcus and Aspergillus, 2 cases with Talaromyces marneffei and Cryptococcus, 2 cases with Talaromyces marneffei and Aspergillus, and 1 case with Candida and Aspergillus. The lesions of all patients with mucormycosis were confined to the lungs, but some patients with Talaromyces marneffei infection and cryptococcosis had involvement of extra-pulmonary organs, including bone, skin, lymph nodes, and/or central nervous system (CNS) (Table 1).
Clinical Symptoms, Signs and Laboratory Tests
Cough, expectoration and fever were the most common symptoms. More than 40% of patients had hemoptysis or shortness of breath. Only 3 patients (17.6%) had an elevated white blood cell count. There were no decreased immunoglobulin G/M/A levels or CD4+ or CD8+ T lymphocyte cell counts in patients without apparent immune deficiency diseases (Table 2).
Mycological Results and Biopsy
The culture was positive for Aspergillus fumigatus in case 6 and case 7 and for Talaromyces marneffei in case 11. A galactomannan antigen test was positive in case 11. A cryptococcal antigen test was positive in case 14 and case 16. Biopsy was positive in all patients but case 16 (Table 1).
Manifestations of CT Images
All 17 patients underwent chest CT examination. The most common features of CT images were mediastinal lymph node enlargement (12 cases, 70.6%), cavity (10 cases, 58.5%), effusion or consolidation (9 cases, 52.9%), multiple nodules (7 cases, 41.2%) and pleural effusion (7 cases, 41.2%, Table 3 and Figure 1). However, none of the 17 cases showed classical features of lung mycosis, such as halo signs and crescent signs. There was also no vascular truncation sign or anti-halo sign. Lesions were less commonly located in the lingual lobe than in other lobes (Table 3).
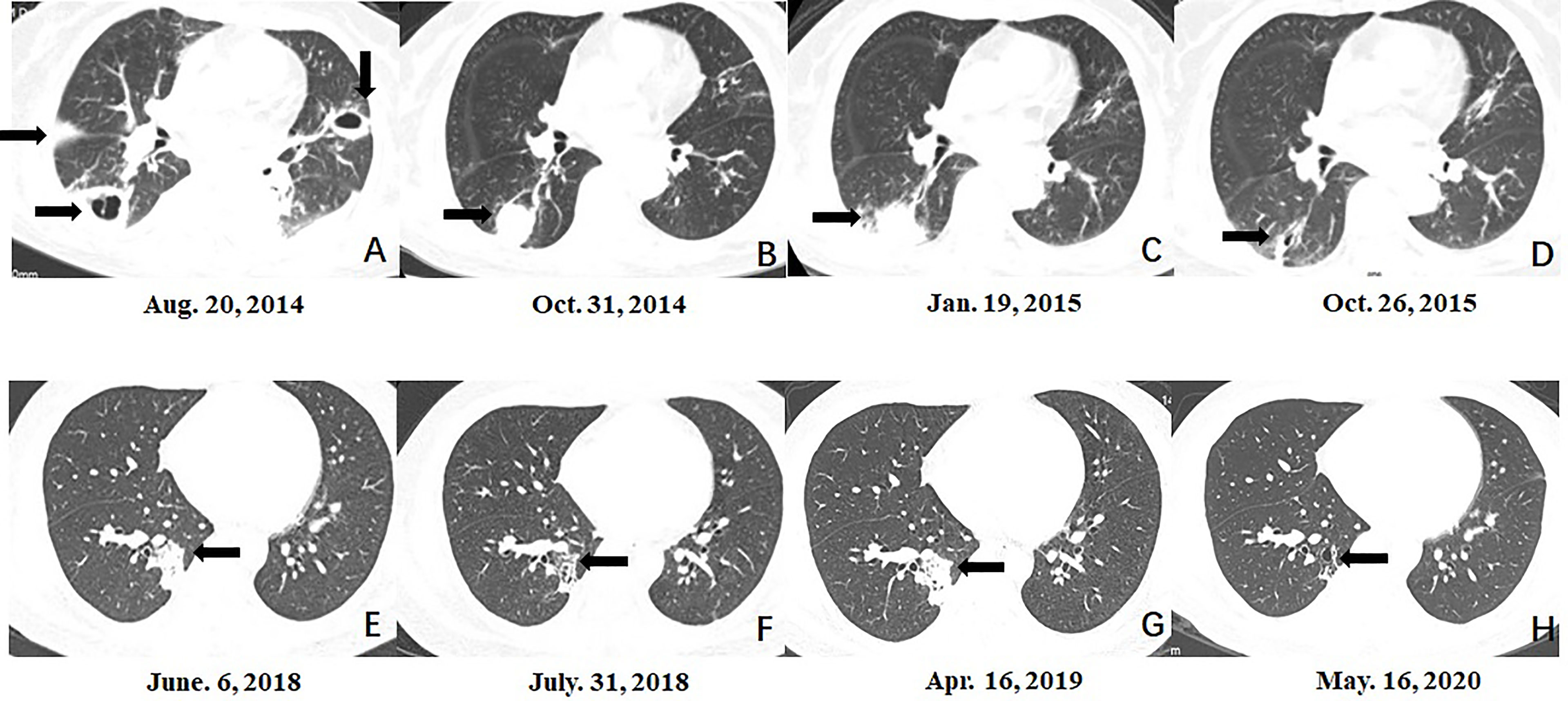
Figure 1 Serial morphologic changes on chest CT of patients with mixed mycosis. (A–D) CT images in case 3 showed that compared to the lesion at the time of pulmonary aspergillus diagnosis (A), the lesion in the right lower lobe was smaller at 3 months after voriconazole treatment (B), re-enlarged 5 months after voriconazole treatment (C), and almost disappeared at 9 months after the diagnosis of mixed mycosis and posaconazole treatment (D). (E–H) CT images in case 14 showed that compared to the lesion at the time of pulmonary cryptococcosis diagnosis (E), the lesion in the right lower lobe was smaller 8 weeks after fluconazole treatment (F), re-enlarged 10 months after fluconazole treatment (G), and almost disappeared at 1 year after the diagnosis of mixed mycosis and voriconazole treatment (H).
Clinical Course, Treatment, and Prognosis
Seven patients were diagnosed with mixed pulmonary mycosis at almost the same disease course. Among the remaining 10 patients, the initial treatment was ineffective in three cases (cases 8, 10, and 12; fluconazole in two cases and voriconazole in 1 case), and the patients were diagnosed with mixed mycoses after review of the original biopsy from the hospital to which they were first admitted (Figure 2). They showed good response to adjusted treatment. The original fluconazole, second-line amphotericin B and voriconazole were ineffective in case 2. She was diagnosed with mixed mycosis after surgical resection. In six cases (cases 3, 5, 11, 13, 14, and 15), mixed mycosis diagnosis was made after bronchoscopy biopsy of re-enlarged original fungal lesions, which showed a partial response to initial antifungal treatment (fluconazole in 1 case, voriconazole in 1 case, itraconazole in 2 cases and posaconazole in 2 cases) (Figure 1). Among the 10 cases of mixed mycoses that were not diagnosed at the same time, initial antifungal drugs in 6 cases had no activity on the second fungus.
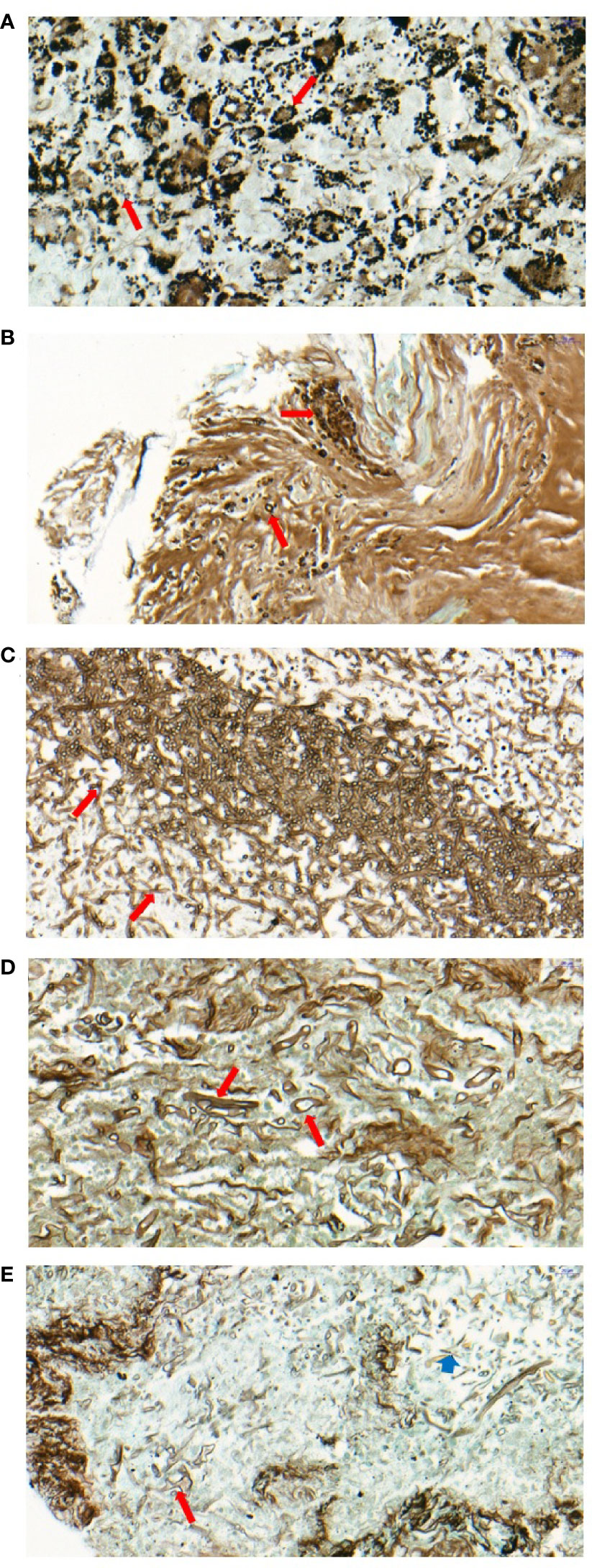
Figure 2 Pathology of patients with mixed mycosis. (A) Multiple spores can be seen in the cytoplasm of the multinucleated giant cells in the granulomatous nodules of lymphoid tissue in case 5. GMS staining (400x) showed that the fungi grew in clusters, with separation within the fungus and a small black spot in the center, which was considered Talaromyces marneffei (long red arrow). (B) Focal necrosis containing round or semilunar spores can be seen under the bronchial mucosal tissue with GMS staining (400x), which was considered to be Cryptococcus (long red arrow) in case 10. (C, D) Two forms of hyphae and spores can be seen in the necrotic foci of lung tissue by GMS staining (400x) in case 15. Fungi with the same thickness of hyphae and acute angle of branches were considered Aspergillus (long red arrow) (C), fungi with different sizes of hyphae and strange shapes of branches were considered Mucor (long red arrow) (D). (E) Two forms of hyphae and spores can be seen in the necrotic foci of lung tissue by GMS staining (400x) in case 17. Fungi with different sizes of hyphae, strange shapes of branches and thickened capsules were considered Mucor (long red arrow); fungi with the same thickness of hyphae and acute angle of branches were considered Aspergillus (short blue arrow).
Three patients were admitted to the intensive care unit during hospitalization. However, patient 7 died even after treatment in the intensive care unit, and patient 6 died due to treatment refusal. Patient 2 showed a poor response to antifungal treatment, and pneumonectomy was conducted. Patients 5 and 11 suffered from a recurrence of disseminated Talaromyces marneffei infection even under the course of antifungal treatment. The remaining patient showed improvement in the CT scan with no recurrence.
Comparisons of Patients With Mucor Coinfection and Without Mucor Coinfection
Because voriconazole is ineffective for Mucor, which has a poor prognosis, comparisons were made between patients with Mucor and without Mucor coinfection to discriminate the characteristics between these two groups. There were 8 cases with Mucor coinfection and 9 cases without Mucor coinfection. Patients with Mucor coinfection seemed to be older than patients without Mucor coinfection; however, the difference was not significant (55 ± 16 years vs. 45 ± 16 years, P=0.22). Diabetes mellitus was more common in patients with Mucor coinfection than in patients without Mucor coinfection (Figure 3). Patients with mixed Mucor infection had lower hemoglobin levels than patients without mixed Mucor infection (97 ± 15 g/L vs. 118 ± 23 g/L, P=0.037). Cavity, multiple nodule effusion/consolidation and enlarged mediastinal lymph nodes were more common in Mucor mixed infection than in non-Mucor mixed infection (Figure 3). Mortality was similar in the two groups (12.5% vs. 11.1%, P>0.05).
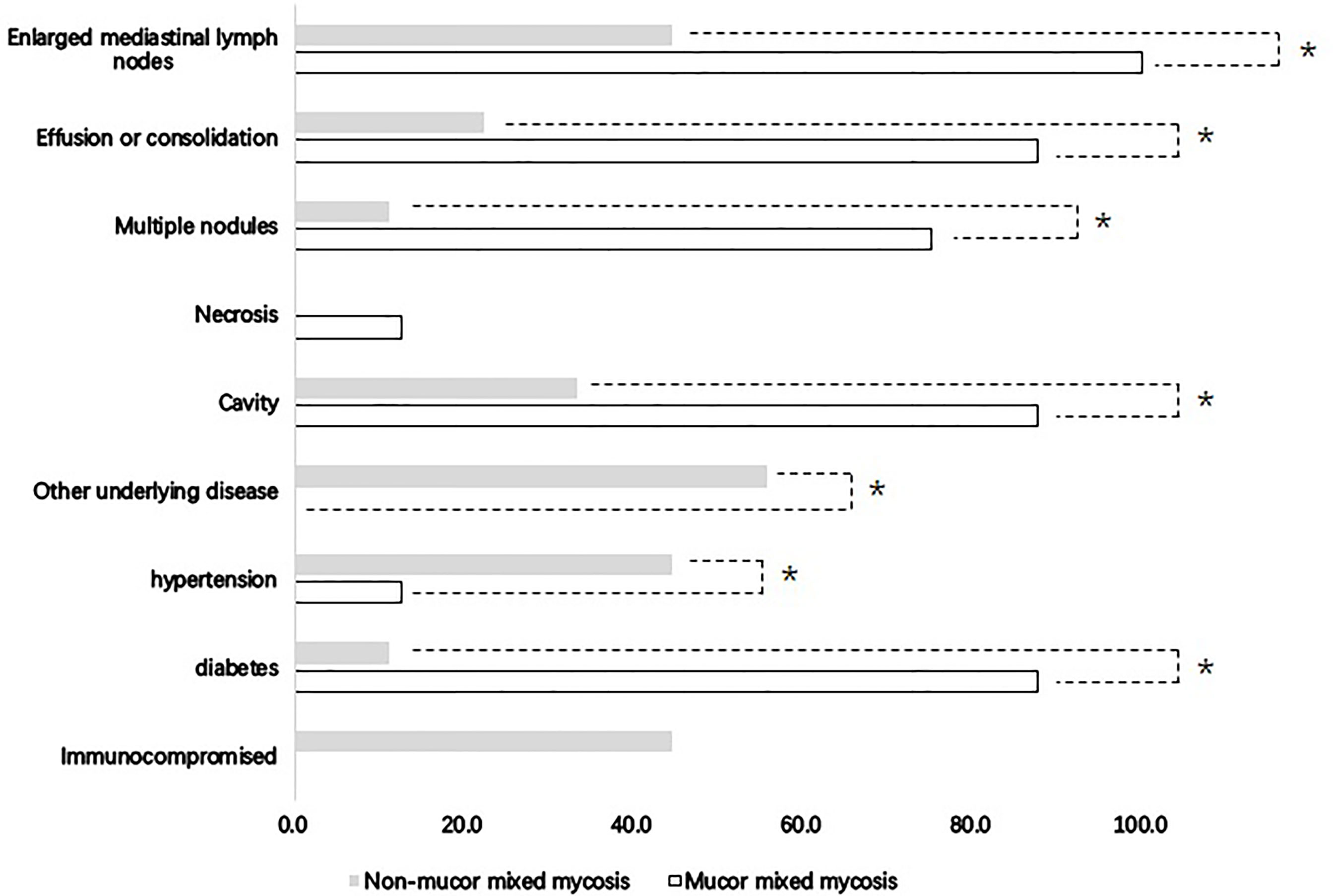
Figure 3 Clinical features of mixed mycosis patients with and without Mucor infection. *Comparisons between Mucor mixed mycosis and non-Mucor mixed mycosis showed significance (P < 0.05).
Discussion
In the past, it was believed that pulmonary mycosis occurred only in immunosuppressed hosts, with Candida, Aspergillus and Cryptococcus as the most common opportunistic fungi (Pfaller and Diekema, 2004), but increasing evidence shows that pulmonary mycosis in patients without apparent immune deficiency diseases is not uncommon, especially pulmonary cryptococcosis, which mainly occurs in immunocompetent hosts in mainland China. In our study, patients without apparent immunocompromising disease or immunosuppressive treatment accounted for an extremely large proportion.
It should be noted that only 2 cases in this study were in classical immunocompromised hosts, and one of them developed a mixed infection with Talaromyces marneffei in the present study. Another two cases of Talaromyces marneffei mixed infection were confirmed to have atypical types of immunodeficiency after the occurrence of Talaromyces marneffei infection, one with STAT3 hyper-IgE syndrome and the other with anti-IFN-γ autoantibody-associated immunodeficiency syndrome. The former was originally characterized by recurrent cold staphylococcal abscesses, pneumonia, eczema, hyperextensibility, and extreme elevation of IgE levels (Holland et al., 2007). The latter is associated with severe disseminated nontuberculous mycobacterial infections and other disseminated opportunistic infections (Salmonella, Histoplasma, and Cryptococcus) in previously healthy adults (Hong et al., 2020). However, recent studies have shown that these populations are also susceptible to infection with Talaromyces marneffei, which was previously a significant infectious complication in HIV/AIDS patients but has now increased in incidence in patients with other immune defects (Cao et al., 2019; Lee et al., 2019). Therefore, it is necessary to identify the potential cause of immunodeficiency in cases of Talaromyces marneffei infection, and people with unapparent immunodeficiency need to pay attention to the possibility of mycoses, especially those caused by Talaromyces marneffei.
In the present study, all but one patient infected by Mucor had type 2 diabetes mellites. The incidence of diabetes mellites was more common in the mucormycosis group than in the non-mucormycosis group. This result was consistent with the literature indicating that diabetes mellites, with or without ketoacidosis, is one of the risk factors for Mucor infection (Cornely et al., 2019). Although the mechanism of immunodeficiency in diabetic patients is still unclear and needs in-depth research, if diabetic patients with pulmonary mycosis do not have a good response to the existing antifungal treatment that does not cover Mucor, attention should be given to the possibility of Mucor coinfection. Additionally, there were 3 non-immunocompromised hosts with mixed fungal diseases and no underlying diseases. It is valuable information for clinicians because not only single pulmonary fungal disease but also mixed fungal diseases can occur in patients without apparent immune deficiency diseases. According to our experience, during the follow-up, if the patients with proven pulmonary fungal disease showed a poor response to antifungal treatment, the existence of another fungal disease needs to be considered even in patients without apparent immune deficiency diseases.
Regardless of the immune status, among the single IFDs, Aspergillus and Cryptococcus are the most common pathogens, while Mucor and Talaromyces marneffei are relatively rare (Letourneau et al., 2014; Yan et al., 2016; Cao et al., 2019). Pneumocystis jiroveci is also one of the most common pathogens in immunosuppressed hosts (Azoulay et al., 2019). However, the mixed mycoses reported in the literature are mainly mixed infections of Aspergillus and Cryptococcus (Lin et al., 2006; Enoki et al., 2012; Wang et al., 2018; Awari et al., 2020). There have also been reports of Aspergillus and Mucor coinfection in the lung or outside the lungs (Bergantim et al., 2013; Mahadevaiah et al., 2013; Webb et al., 2013). Most Cryptococcus and Talaromyces marneffei coinfections have been reported in HIV patients (Le et al., 2010; Groll et al., 2021). However, in the present study, mixed fungal infections with Aspergillus and Mucor were the most common types, followed by Aspergillus and Cryptococcus coinfection, and coinfection with Talaromyces marneffei and other fungi was not uncommon in HIV-negative patients. This information is valuable for clinicians. When the initial antifungal treatment is not effective and mixed fungal diseases are considered, the possibility of Mucor should be considered, especially in patients who have diabetes mellites. Therefore, fungus coinfection is worthy of consideration.
The symptoms, signs and imaging findings of mixed fungal lung infection lack specificity. There is no halo sign or crescent sign, which is not consistent with the imaging features of previous invasive pulmonary aspergillosis with a “halo sign at the early stage and crescent sign at the later stage” (Park et al., 2010). Classical chest CT images of Mucor infection, such as reversed halo signs and vascular occlusion, were also lacking in the present Mucor patients. Although Mucor patients more commonly showed multiple nodules and/or masses, effusion or consolidation and enlarged mediastinal lymph nodes in chest CT scans than non-Mucor coinfection patients, it was still difficult to diagnose Mucor coinfection, for which the prescription of targeted treatment amphotericin B liposome is recommended (Cornely et al., 2019). Hence, the diagnosis of proven invasive pulmonary mycosis mostly depends on histopathologic, cytopathologic, or direct microscopic examination of a specimen obtained by needle aspiration or biopsy.
However, the reality is that the diagnosis of pulmonary mycosis based solely on pathology may lead to misdiagnosis and missed diagnosis. Especially when distinguishing various molds, such as Aspergillus and Mucor, confusion occurs easily because of the similarity in the morphology of these two molds. Discrimination highly depends on skill and experience. In this study, the pathological judgment of some cases also exhibited this problem. It is worth noting that for 3 patients, mixed mycosis was missed in the biopsy in the hospital to which they were first admitted. The diagnosis of mucormycosis was made after reviewing the original pathological slides from the hospitals to which they were first admitted. As stated in the EORTC/MSGERC consensus definitions of IFDs, amplification of fungal DNA by PCR combined with DNA sequencing when molds are seen in formalin-fixed paraffin-embedded tissue can be used as one of the criteria for the diagnosis of infection by molds in these situations (Donnelly et al., 2019).
Additionally, lesions in 7 patients re-enlarged after the original effective treatment and were diagnosed after a second biopsy and pathological examination. Hence, based on the comprehensive patient situation, clinicians must also have rigorous procedures in the management of patients and analyze the reasons for ineffectiveness of original treatment or re-enlargement of lesions, including the sufficient triazole concentration in blood (Di Paolo et al., 2021), drug resistance (Yousefian et al., 2021), and the possibility of mixed infection with multiple pathogens such as bacteria, tuberculosis, other fungi, etc. As shown in this study, mixed mycosis is also one of the factors that needs to be considered, even in immunocompetent patients. Hence, we recommend that the following procedures can be considered in patients with pulmonary mycosis when poor response to initial antifungal treatment happens or the initial lesions grow again, including whether treatment drug monitor shows adequate concentrations of azoles (Di Paolo et al., 2021), whether there is new occurrence of symptoms or characteristic imaging of other types fungal infections other than tuberculosis or non-tuberculosis mycobacteria, whether current in use antifungal agents could cover suspected pathogens such as fluconazole is invalid for aspergillus and voriconazole is invalid for mucor. At this time, targeted fungal etiology and pathological examination are required to confirm the diagnosis.
Except for early complete surgical treatment being strongly supported for mucormycosis whenever possible, systemic antifungal treatment is recommended as the first choice for other fungi by guidelines (Cornely et al., 2019; Garcia-Vidal et al., 2019; Groll et al., 2021). In the present study, except for one patient who died because of refusing further treatment, all mucormycosis patients recovered after receiving systemic amphotericin B treatment. One mucormycosis patient also received surgery after systemic antifungal treatment. Three cases with non-Mucor mixed infection received surgery after poor response to systemic antifungal treatment or for diagnosis purposes. Hence, surgery is an alternative to systemic antifungal therapy in some mixed pulmonary mycoses with a poor response. In contrast to the high mortality in immunocompromised hosts in the literature, most mixed mycosis cases in the present study had a good response.
The major limitations of our study were that it was a retrospective, single-center study. There may have a bias in patient enrollment. Another limitation was that not all patients were screened for gene mutations, such as the STAT 3 gene and the level of anti-interferon γ antibody. In the present study, only three cases with Talaromyces marneffei infection were tested for the STAT 3 gene and anti-interferon γ antibody, with one of them positive for each.
Conclusion
Mixed pulmonary mycosis is not uncommon in immunocompetent hosts. During the management of mycosis, we recommend keeping mixed mycosis in mind for patients with a poor response to initial antifungal treatment, even in immunocompetent populations. A rigorous procedure may help to differentiate mixed mycosis from single mycosis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (2018-119). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception and design: YZ, CL, and FY. Collection and assembly of data: YZ, CL, SL, JZ, ZL, and YG. Data analysis and interpretation: YZ. Manuscript writing: YZ, CL, and FY. Final approval of manuscript: YZ, CL, SL, JZ, ZL, YG, and FY. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the ZHONGNANSHAN MEDICAL FOUNDATION OF GUANGDONG PROVINCE (ZNSA-2020003); Independent Fund of the State Key Laboratory of Respiratory Diseases (SKLRD-Z-202019); the Guangzhou Institute of Respiratory Health Open Project (2019GIRHZ06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the patients and families who contributed generously to the work that we performed.
References
Abd Elaziz, D., Abd El-Ghany, M., Meshaal, S., El Hawary, R., Lotfy, S., Galal, N., et al. (2020). Fungal Infections in Primary Immunodeficiency Diseases. Clin. Immunol. 219, 108553. doi: 10.1016/j.clim.2020.108553
Awari, D. W., Shah, A. S., Sexton, A. M., Sexton, M. A. (2020). Coinfection of Aspergillus and Cryptococcus in Immunocompromised Host: A Case Report and Review of Literature. Case Rep. Infect. Dis. 2020, 8888270. doi: 10.1155/2020/8888270
Azoulay, E., Mokart, D., Kouatchet, A., Demoule, A., Lemiale, V. (2019). Acute Respiratory Failure in Immunocompromised Adults. Lancet Respir. Med. 7 (2), 173–186. doi: 10.1016/S2213-2600(18)30345-X
Bergantim, R., Rios, E., Trigo, F., Guimarães, J. E. (2013). Invasive Coinfection With Aspergillus and Mucor in a Patient With Acute Myeloid Leukemia. Clin. Drug Investig. 33 Suppl 1, S51–S55. doi: 10.1007/s40261-012-0022-4
Bongomin, F., Gago, S., Oladele, R. O., Denning, D. W. (2017). Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi (Basel). 3 (4), 57. doi: 10.3390/jof3040057
Cao, C., Xi, L., Chaturvedi, V. (2019). Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) Marneffei: Insights Into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia 184 (6), 709–720. doi: 10.1007/s11046-019-00410-2
Cheng, K. B., Wu, Z. H., Liang, S., Li, H. P., Xu, J. F. (2021). Associations of Serum Cryptococcal Antigen With Different of Clinical Characteristics: A Comprehensive Analysis of 378 Pulmonary Cryptococcosis Patients. Ann. Palliat Med. 10 (1), 681–693. doi: 10.21037/apm-21-127
Cornely, O. A., Alastruey-Izquierdo, A., Arenz, D., Chen, S. C. A., Dannaoui, E., Hochhegger, B., et al. (2019). Global Guideline for the Diagnosis and Management of Mucormycosis: An Initiative of the European Confederation of Medical Mycology in Cooperation With the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 19 (12), e405–e421. doi: 10.1016/S1473-3099(19)30312-3
Di Paolo, M., Hewitt, L., Nwanko, E., Ni, M., Vidal-Diaz, A., Fisher, M. C., et al. (2021). A Retrospective 'Real-World' Cohort Study of Azole Therapeutic Drug Monitoring and Evolution of Antifungal Resistance in Cystic Fibrosis. JAC Antimicrob. Resist. 3 (1), dlab026. doi: 10.1093/jacamr/dlab026
Donnelly, J. P., Chen, S. C., Kauffman, C. A., et al (2019. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 71 (6), 1367–1376. doi: 10.1093/cid/ciz1008
Enoki, E., Maenishi, O., Chikugo, T., Ito, A., Kimura, M. (2012). Coinfection of Aspergillus and Cryptococcus in Post-Tuberculosis Pulmonary Cavity. Pathol. Int. 62 (8), 574–576. doi: 10.1111/j.1440-1827.2012.02839.x
Garcia-Vidal, C., Alastruey-Izquierdo, A., Aguilar-Guisado, M., Carratalà, J., Castro, C., Fernández-Ruiz, M., et al. (2019). Executive Summary of Clinical Practice Guideline for the Management of Invasive Diseases Caused by Aspergillus: 2018 Update by the GEMICOMED-SEIMC/REIPI. Enferm Infecc Microbiol. Clin. 37 (8), 535–541. doi: 10.1016/j.eimc.2018.03.018
Groll, A. H., Pana, D., Lanternier, F., Mesini, A., Ammann, R. A., Averbuch, D., et al. (2021). 8th European Conference on Infections in Leukaemia: 2020 Guidelines for the Diagnosis, Prevention, and Treatment of Invasive Fungal Diseases in Paediatric Patients With Cancer or Post-Haematopoietic Cell Transplantation. Lancet Oncol. 22, e254–e269. doi: 10.1016/S1470-2045(20)30723-3
He, S., Lv, D., Xu, Y., Wu, X., Lin, L. (2020). Concurrent Infection With Talaromyces Marneffei and Cryptococcus Neoformans in a Patient Without HIV Infection. Exp. Ther. Med. 19 (1), 160–164. doi: 10.3892/etm.2019.8172
Holland, S. M., DeLeo, F. R., Elloumi, H. Z., Hsu, A. P., Uzel, G., Brodsky, N., et al. (2007). STAT3 Mutations in the Hyper-IgE Syndrome. N Engl. J. Med. 357 (16), 1608–1619. doi: 10.1056/NEJMoa073687
Hong, G. H., Ortega-Villa, A. M., Hunsberger, S., Chetchotisakd, P., Anunnatsiri, S., Mootsikapun, P., et al. (2020). Natural History and Evolution of Anti-Interferon-γ Autoantibody-Associated Immunodeficiency Syndrome in Thailand and the United States. Clin. Infect. Dis. 71 (1), 53–62. doi: 10.1093/cid/ciz786
Lee, P. P., Lao-Araya, M., Yang, J., Chan, K. W., Ma, H., Pei, L., et al. (2019). Application of Flow Cytometry in the Diagnostics Pipeline of Primary Immunodeficiencies Underlying Disseminated Talaromyces Marneffei Infection in HIV-Negative Children. Front. Immunol. 10, 2189. doi: 10.3389/fimmu.2019.02189
Le, T., Hong Chau, T. T., Kim Cuc, N. T., Si Lam, P., Manh Sieu, T. P., Shikuma, C. M., et al. (2010). AIDS-Associated Cryptococcus Neoformans and Penicillium Marneffei Coinfection: A Therapeutic Dilemma in Resource-Limited Settings. Clin. Infect. Dis. 51 (9), e65–e68. doi: 10.1086/656685
Letourneau, A. R., Issa, N. C., Baden, L. R. (2014). Pneumonia in the Immunocompromised Host. Curr. Opin. Pulm Med. 20 (3), 272–279. doi: 10.1097/MCP.0000000000000051
Li, Z., Li, Y., Chen, Y., Li, J., Li, S., Li, C., et al. (2021). Trends of Pulmonary Fungal Infections From 2013-2019: An AI-Based Real-World Observational Study in Guangzhou, China. Emerg. Microbes Infect. 10 (1), 450–460. doi: 10.1080/22221751.2021.1894902
Lin, C. M., Tsai, Y. H., Huang, C. C., Lee, C. H., Chiang, P. C., Huang, S. F., et al. (2006). Invasive Pulmonary Aspergillosis and Pulmonary Cryptococcosis Really Coexist in Immunocompromised Host. J. Infect. 53 (2), e55–e58. doi: 10.1016/j.jinf.2005.10.017
Mahadevaiah, A. H., Rajagopalan, N., Patil, M., C, S. (2013). Coinfection of Pulmonary Mucormycosis and Aspergillosis Presenting as Bilateral Vocal Cord Palsy. BMJ Case Rep. 2013, bcr2013009615. doi: 10.1136/bcr-2013-009615
Mortaz, E., Azempour, E., Mansouri, D., Tabarsi, P., Ghazi, M., Koenderman, L., et al. (2019). Common Infections and Target Organs Associated With Chronic Granulomatous Disease in Iran. Int. Arch. Allergy Immunol. 179 (1), 62–73. doi: 10.1159/000496181
Pan, M., Qiu, Y., Zeng, W., Tang, S., Wei, X., Zhang, J. (2020). Disseminated Talaromyces Marneffei Infection Presenting as Multiple Intestinal Perforations and Diffuse Hepatic Granulomatous Inflammation in an Infant With STAT3 Mutation: A Case Report. BMC Infect. Dis. 20 (1), 394. doi: 10.1186/s12879-020-05113-4
Park, S. Y., Kim, S. H., Choi, S. H., Sung, H., Kim, M. N., Woo, J. H., et al. (2010). Clinical and Radiological Features of Invasive Pulmonary Aspergillosis in Transplant Recipients and Neutropenic Patients. Transpl Infect. Dis. 12 (4), 309–315. doi: 10.1111/j.1399-3062.2010.00499.x
Pfaller, M. A., Diekema, D. J. (2004). Rare and Emerging Opportunistic Fungal Pathogens: Concern for Resistance Beyond Candida Albicans and Aspergillus Fumigatus. J. Clin. Microbiol. 42 (10), 4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004
von Lilienfeld-Toal, M., Wagener, J., Einsele, H., Cornely, O. A., Kurzai, O. (2019). Invasive Fungal Infection. Dtsch Arztebl Int. 116 (16), 271–278. doi: 10.3238/arztebl.2019.0271
Wang, Q., Wang, Z., Hao, Y., Li, W., Xin, T., Chen, M., et al. (2018). Coinfection With Cryptococcus and Aspergillus in an Immunocompetent Adult: A Case Report. Med. (Baltimore). 97 (39), e12612. doi: 10.1097/md.0000000000012612
Webb, B. J., Blair, J. E., Kusne, S., Scott, R. L., Steidley, D. E., Arabia, F. A., et al. (2013). Concurrent Pulmonary Aspergillus Fumigatus and Mucor Infection in a Cardiac Transplant Recipient: A Case Report. Transplant. Proc. 45 (2), 792–797. doi: 10.1016/j.transproceed.2012.03.056
Yan, X., Zong, F., Kong, H., Wang, Y., Zhao, X., Liu, W., et al. (2016). Pulmonary Fungal Diseases in Immunocompetent Hosts: A Single-Center Retrospective Analysis of 35 Subjects. Mycopathologia 181 (7-8), 513–521. doi: 10.1007/s11046-016-9999-1
Ye, F., Xie, J. X., Zeng, Q. S., Chen, G. Q., Zhong, S. Q., Zhong, N. S. (2012). Retrospective Analysis of 76 Immunocompetent Patients With Primary Pulmonary Cryptococcosis. Lung 190 (3), 339–346. doi: 10.1007/s00408-011-9362-8
Yousefian, S., Dastan, F., Marjani, M., Tabarsi, P., Barati, S., Shahsavari, N., et al. (2021). Determination of Voriconazole Plasma Concentration by HPLC Technique and Evaluating Its Association With Clinical Outcome and Adverse Effects in Patients With Invasive Aspergillosis. Can. J. Infect. Dis. Med. Microbiol. 2021, 5497427. doi: 10.1155/2021/5497427
Keywords: immunocompetent, immunocompromised, Mucor, Aspergillus, Cryptococcus, Talaromyces marneffei
Citation: Zhan Y, Lu C, Li S, Zhao J, Li Z, Gu Y and Ye F (2022) Successful Management of Mixed Mycosis in HIV-Negative Patients With Different Immune Status: A Case Series Report. Front. Cell. Infect. Microbiol. 12:851891. doi: 10.3389/fcimb.2022.851891
Received: 10 January 2022; Accepted: 08 February 2022;
Published: 04 March 2022.
Edited by:
Jing Zhang, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Alexandra Freeman, National Institutes of Health (NIH), United StatesLiang He, University of California, San Francisco, United States
Ling Yin, University of Texas MD Anderson Cancer Center, United States
Copyright © 2022 Zhan, Lu, Li, Zhao, Li, Gu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Ye, eWVmZW5nQGdpcmQuY24=
†These authors have contributed equally to this work
 Yangqing Zhan
Yangqing Zhan Chun Lu
Chun Lu Shaoqiang Li
Shaoqiang Li Jin Zhao2,3
Jin Zhao2,3 Zhengtu Li
Zhengtu Li Yingying Gu
Yingying Gu Feng Ye
Feng Ye