- Grupo de Genômica Funcional de Parasitos – Instituto René Rachou, Fundação Oswaldo Cruz, Belo Horizonte, Brazil
Leishmaniasis is one of the major public health concerns in Latin America, Africa, Asia, and Europe. The absence of vaccines for human use and the lack of effective vector control programs make chemotherapy the main strategy to control all forms of the disease. However, the high toxicity of available drugs, limited choice of therapeutic agents, and occurrence of drug-resistant parasite strains are the main challenges related to chemotherapy. Currently, only a small number of drugs are available for leishmaniasis treatment, including pentavalent antimonials (SbV), amphotericin B and its formulations, miltefosine, paromomycin sulphate, and pentamidine isethionate. In addition to drug toxicity, therapeutic failure of leishmaniasis is a serious concern. The occurrence of drug-resistant parasites is one of the causes of therapeutic failure and is closely related to the diversity of parasites in this genus. Owing to the enormous plasticity of the genome, resistance can occur by altering different metabolic pathways, demonstrating that resistance mechanisms are multifactorial and extremely complex. Genetic variability and genome plasticity cause not only the available drugs to have limitations, but also make the search for new drugs challenging. Here, we examined the biological characteristics of parasites that hinder drug discovery.
Introduction
Leishmaniases are a complex of diseases caused by different species of protozoan parasites of the genus Leishmania, which are transmitted to humans by the bite of infected female sand fly insects. Leishmaniases are found in 90 countries and territories (WHO, 2021) and are a major public health concern because they have high death rates among all neglected diseases, and they mostly affect the poorest populations (Kaufer et al., 2017; Parthasarathy and Kalesh, 2020).
Currently, no vaccine for human use is available to prevent leishmaniasis. Controlling reservoirs and vectors is advised; however, this is extremely complicated to accomplish. Thus, rapid diagnosis and treatment of patients is the main form of disease control (WHO, 2021). Only a small number of compounds are used to treat leishmaniasis, including pentavalent antimonials (SbV), amphotericin B and its formulations, miltefosine, paromomycin sulphate, and pentamidine isethionate (Muraca et al., 2020). Therapeutic failure in leishmaniasis is a growing problem and may be related to the occurrence of treatment-resistant parasites, but also to other factors, such as patient immunity (Lopez-Velez et al., 1998; Alvar et al., 2008; Van Griensven et al., 2014); nutritional status, age, and gender of the patient (Dorlo et al., 2012; Ostyn et al., 2014); and whether the parasites are infected with any RNA viruses, such as LRV1 (Bourreau et al., 2015; Adaui et al., 2016). Here, we provide an overview of how genetic variation in the genus Leishmania influences the emergence of drug-resistant parasites, as well as the main tools for studying drug resistance mechanisms and searching for new drugs. The topics covered in this study are summarised in Figure 1.
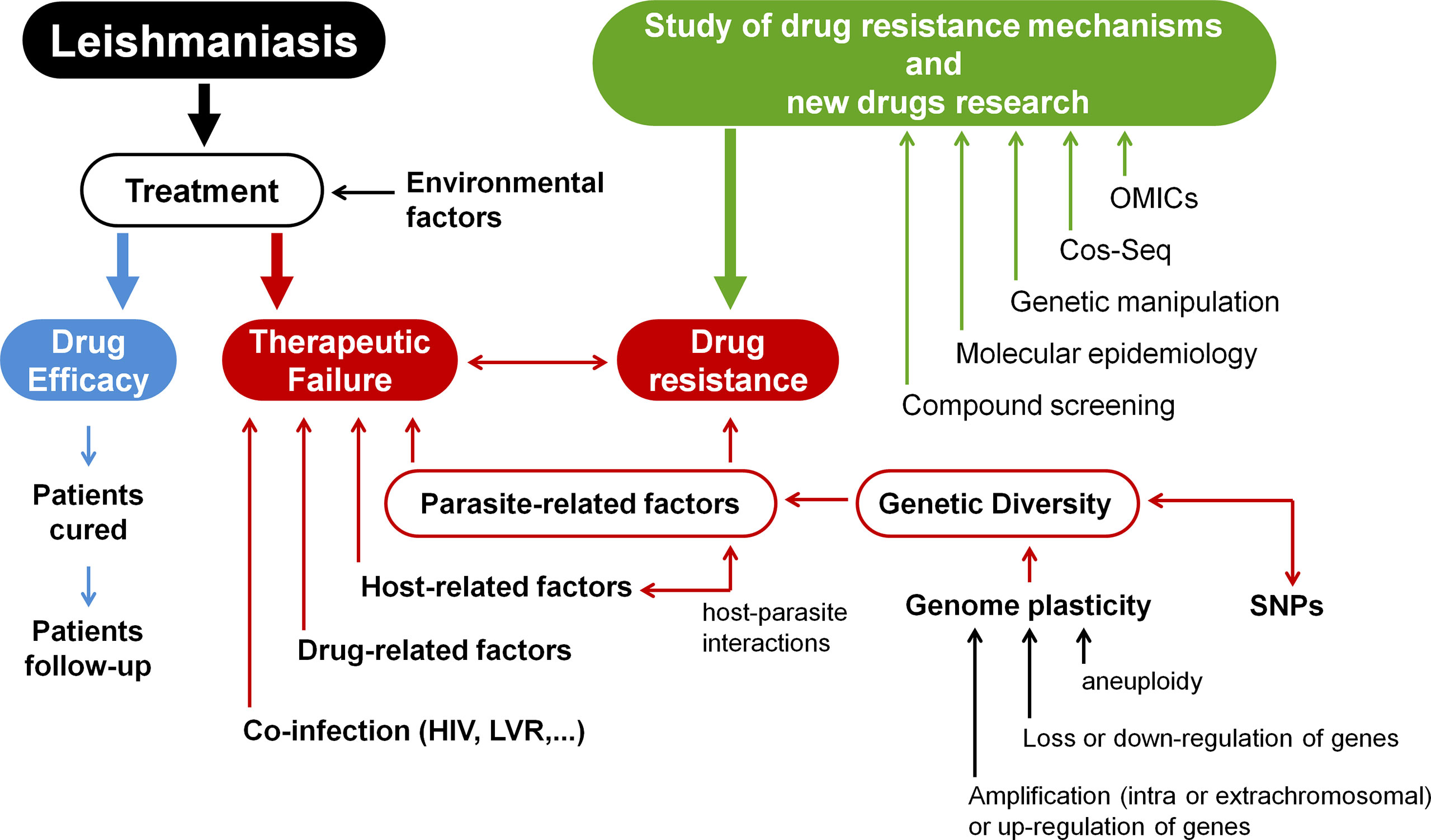
Figure 1 Research into resistance mechanisms and the development of new drugs for leishmaniases treatment. Environmental variables, parasite-related factors, host-related factors, drug-related factors, and co-infections (HIV, LVR) can all affect how well a patient responds to therapy. This demonstrates the significance of studying host-Leishmania interactions in the context of developing novel chemotherapeutic agents for leishmaniasis. The parasite’s diversity is another key element in treatment outcomes since diversity is closely related to drug resistance. Because Leishmania’s genome is highly plastic, there is a great deal of variety across samples. Different Leishmania isolates display single-nucleotide polymorphisms (SNPs), as well as structural variations (such as changes in the numbers of genes, clusters of genes, or even whole chromosomes). Thus, genome plasticity involves different molecular modifications, such as amplification or up-regulation of genes, loss or down-regulation of genes, or aneuploidy. These modifications contribute to drug-resistant phenotypes in Leishmania samples. Consequently, drug resistance mechanisms are multifactorial and exceedingly complicated. Different approaches can be used to study resistance mechanisms in Leishmania and also to search for new molecular targets and novel drugs. OMICs integration (such as genomics, transcriptomics, proteomics, and metabolomics) can be used to better understand pathways associated with drug metabolism. However, due to post-transcriptional control, genomic and transcriptomic data should be interpreted with caution. In addition, data from promastigotes should be carefully analysed since such amastigote forms have distinct transcriptomic and proteomic profiles. The high-throughput Cos-Seq method can be used to identify gain-of-function resistance mechanisms and drug targets. Genetic manipulation of parasites can be performed using different molecular tools (such as deletion by allelic substitution, overexpression and heterologous expression, plasmid shuffling, RNAi, DiCRE, DD domain, Cos-Seq method, and CRISPR/Cas9) which allow the identification of novel chemotherapeutic targets. Molecular epidemiology is also important to better understand the diversity of the genus Leishmania and the variables that drive diversification. Finally, compound screening is a critical component in the search for novel drugs (Modified from Ponte-Sucre et al., 2017).
The Genome of Leishmania Is Atypical
Different species of Leishmania have different numbers of chromosomes as well as diverse sets of genes (Ivens et al., 2005; Peacock et al., 2007). A recent assembly of the genome of L. major revealed a 32.8 Mb genome involving 11,238 genes distributed across 36 chromosomes (Camacho et al., 2021). Previously, Leishmania populations were thought to have an average diploid genome, but mosaic aneuploidy is now thought to be the norm in the genomes of these parasites, with the degree of aneuploidy varying depending on species or strain (Sterkers et al., 2012; Lachaud et al., 2014).
Almost all protein-coding genes in trypanosomatids do not contain introns and are organised in unidirectional polycistronic transcription units with no functionally linked genes. Pre-mRNAs generated by polycistronic transcription are processed to produce mature mRNAs (Bartholomeu et al., 2021). All genes are constitutively expressed, mainly by RNA polymerase II; however, the start of transcription is not well understood because canonical promoter sequences have not yet been found in these parasites (Martínez-Calvillo et al., 2003; Martínez-Calvillo et al., 2004; Thomas et al., 2009; Iantorno et al., 2017). Epigenetic mechanisms appear to play a role in Leishmania transcription initiation by influencing DNA accessibility (Thomas et al., 2009; Chandra et al., 2017; Saha, 2020; Grünebast et al., 2021). Transcription termination occurs at the end of each polycistronic transcription unit and is determined by the presence of the base J (van Luenen et al., 2012; Reynolds et al., 2016; Kieft et al., 2020). However, it is commonly stated in the literature that in the absence of transcriptional regulation, the regulation of protein expression in these parasites is mediated by post-transcriptional events, such as RNA degradation, translation control, and protein degradation (Clayton and Shapira, 2007; Grünebast and Clos, 2020; Karamysheva et al., 2020). Several studies have shown that chromosomal copy number can be associated with transcription levels, supporting the notion that expression control occurs after transcription (Dumetz et al., 2017; Iantorno et al., 2017; Patino et al., 2019). In contrast, transcript and protein levels are not necessarily correlated (Alcolea et al., 2019).
Importance of Genetic Diversity and Genome Plasticity of Leishmania
Variations in the parasite genome can be associated with its geographical distribution and clinical manifestations, which can influence leishmaniasis management. Interestingly, large-scale research involving a large number of samples and wide geographic range has revealed that genetic diversity is significantly higher than that previously reported (Bussotti et al., 2018; Zackay et al., 2018; Franssen et al., 2020; Imamura et al., 2020; Patino et al., 2020; Salloum et al., 2020; Zheng et al., 2020; Patino et al., 2021; Schwabl et al., 2021). Recently, single-cell sequencing has demonstrated the presence of several distinct karyotypes within the same Leishmania clone (Imamura et al., 2020; Negreira et al., 2020), and multiple-genotype infections have been demonstrated to occur even within the same host and tissue (Cupolillo et al., 2020).
In addition to the role of mutations in parasite diversity (Downing et al., 2011; Domagalska et al., 2019; Franssen et al., 2020), the Leishmania genome is highly plastic and constantly rearranges, resulting in variations in gene copy number, clusters of genes, or even whole chromosomes (Ubeda et al., 2008; Leprohon et al., 2009; Mannaert et al., 2012; Laffitte et al., 2016b; Iantorno et al., 2017). As a result, mosaic aneuploidy is not only widespread in Leishmania but is also an essential adaptive mechanism that allows a certain genome structure to be quickly selected in the face of adverse conditions (Sterkers et al., 2012; Dujardin et al., 2014; Lachaud et al., 2014; Sterkers et al., 2014; Reis-Cunha et al., 2018). Alterations in ploidy are not random, but seem to follow the same pattern in samples subjected to different stressors, and each strain tends to follow the same pattern, which indicates the occurrence of selective processes (Dumetz et al., 2017; Bussotti et al., 2018; Restrepo et al., 2019).
The copy number of a gene can be changed by adding or deleting genes in tandem, or by creating extrachromosomal copies of genes, which can be linear or circular. These extrachromosomal gene copies are commonly found in Leishmania under stress, but are also found in wild-type populations (Ouellette and Borst, 1991; Grondin et al., 1993; Leprohon et al., 2009). It has been reported that there are pairs of repeated sequences surrounding sets of genes in Leishmania (Leprohon et al., 2009; Ubeda et al., 2014; Bussotti et al., 2018; Carnielli et al., 2018) and DNA double-strand breaks near or within these repeated sequences may induce homologous recombination, which is associated with an increase in gene rearrangements (Genois et al., 2014; da Silva, 2021).
Subtelomeric DNA is more sensitive to replicative stress (Damasceno et al., 2013; Damasceno et al., 2016; Damasceno et al., 2018), and telomeric amplification has also been identified as a genetic adaptability mechanism in Leishmania (Bussotti et al., 2018). Interestingly, aneuploidy may arise from the duplication of subtelomeres outside the S phase (Damasceno et al., 2020a).
Leishmania replicates by clonal expansion; however, several studies have reported genetic exchanges between parasites, suggesting that Leishmania may reproduce sexually during the life cycle (Gutiérrez-Corbo et al., 2021). It has been proposed that this exchange may be important for the long-term survival of these parasites (Van den Broeck et al., 2020; Kato et al., 2021).
Genomic Diversity Among Drug-Resistant Parasite Strains
Drug resistance is not an unexpected finding in these parasites, and it has been proposed that genetic variation is the primary driving force in the emergence of diverse drug-resistant phenotypes (Decuypere et al., 2012; Reis-Cunha et al., 2018). Because a variety of alterations might result in resistance to currently available treatments, there is no unique marker to evaluate resistance in clinical isolates. Here, we focus on certain examples where genetic diversity led to resistance in both clinical isolates and in in vitro studies.
Antimony
Antimonials include meglumine antimoniate (Glucantime®) and sodium stibogluconate (Pentostam®). Several studies have shown that clinical isolates have increased resistance to pentavalent antimony (Peters, 1981; Sundar, 1994; Romero et al., 2001; Sundar, 2001; Croft et al., 2006; Azeredo-Coutinho et al., 2007; Perry et al., 2015; Mohebali et al., 2019). These clinical isolates have high variability in terms of antimonial resistance mechanisms, and the same sample can present several different alterations. For example, it was demonstrated that L. braziliensis and L. panamensis resistant to trivalent antimony (SbIII) exhibit differences in chromosomal somy and gene copy number when compared to their respective susceptible lines; however, such changes are more prominent in L. braziliensis (Patino et al., 2019). Different antimony-resistance mechanisms have been reported, including decreased cellular antimony entry, decreased drug reduction/activation, increased antimony efflux, and sequestration of the metal-thiol conjugate into intracellular vesicles of Leishmania (Croft et al., 2006).
An intriguing example of how genetic diversity might aid in the development of resistant parasite strains is how high levels of As (III) in Indian waters may have assisted in the selection of antimony-resistant parasites (Perry et al., 2015). Several different mechanisms can be involved in the SbIII resistant phenotype in India, as it was shown, for example, that L. donovani from India has pre-adaptative aneuploidies involving various chromosomes (Dumetz et al., 2018). As MRPA (previously known as PGPA) is an ATP-binding cassette gene implicated in SbIII sequestration into intracellular vesicles (Leprohon et al., 2009), it has been suggested that amplification of the MRPA gene copy number could generate the SbIII or AsIII resistance phenotype in samples, because both have the same sequestration mechanism (Maciaszczyk-Dziubinska et al., 2012; Dumetz et al., 2018). Similarly, other studies have shown that aneuploidy is related to the acquisition of antimony resistance in Leishmania by altering the MRPA copy number (Haimeur et al., 2000; Légaré et al., 2001; Anacleto et al., 2003; Mukherjee et al., 2007; Leprohon et al., 2009; Moreira et al., 2013).
However, other mechanisms can also participate in parasite resistance to SbIII, such as changes in the aquaglyceroporin (AQP1) coding sequence or down-regulation of expression of this gene in clinical samples (Mandal et al., 2010; Dumetz et al., 2018; Potvin et al., 2021) because it is known that differences in AQP1 transporter levels can influence both SbIII and AsIII uptake (Gourbal et al., 2004; Marquis et al., 2005; Mandal et al., 2010). On the other hand, amplification of trypanothione synthetase (TryS) may also play a role in resistance because an increase in trypanothione levels favours the formation of conjugates with SbIII or AsIII, which increases its sequestration into intracellular vesicles (Mukhopadhyay et al., 1996; Frézard et al., 2014; Dumetz et al., 2018).
Mutations can also generate resistance against SbIII; for example, mutations in the multidrug resistance 1 gene (MDR1) have been associated with drug resistance in samples from individuals who do not respond to treatment (Abadi et al., 2021), while calcium-dependent protein kinase (CDPK1) mutations are linked to resistance to both paromomycin and antimony (Bhattacharya et al., 2019).
Another useful technique for assessing potential resistance pathways is to obtain resistant parasites in vitro. It is worth noting that the detected genes are frequently similar to those found in clinical samples. In vitro SbIII resistance selection results in parasites with amplification of many genes, including MRPA, ascorbate-dependent peroxidase (APX), and a putative glucose-6-phosphate dehydrogenase (G6PDH) (Leprohon et al., 2009; Mukherjee et al., 2013; Monte-Neto et al., 2015). Obtaining SbIII-resistant parasites in vitro can also result in deletions or point mutations in a region containing AQP1 (Mukherjee et al., 2013; Monte-Neto et al., 2015).
Furthermore, overexpression of antioxidant defence enzymes, such as iron superoxide dismutase-A (Tessarollo et al., 2015), tryparedoxin peroxidase (Andrade and Murta, 2014), or APX (Moreira et al., 2018) are involved in the SbIII-resistant phenotype in L. braziliensis.
Miltefosine
Miltefosine is the only oral medicine available for the treatment of leishmaniasis and was first used in India to replace antimonials (Sundar et al., 2000; Sundar et al., 2002). Despite the usefulness of this drug in treating diseases caused by some species of Leishmania, the limited efficacy of miltefosine in treating visceral leishmaniasis in Brazil has been related to the loss of the Miltefosine Sensitivity Locus (MSL) (De Morais-Teixeira and Damasceno, 2011; Carnielli et al., 2018; Carnielli et al., 2019). Mutations in the miltefosine transporter confer resistance to both miltefosine and amphotericin B (Coelho et al., 2012; Fernandez-Prada et al., 2016; Laffitte et al., 2016a). In contrast, it was recently demonstrated that increasing the number of copies of the Ros3 (Lem3p/CDC50) gene in clinical isolates of L. braziliensis increases miltefosine uptake, rendering these parasites more sensitive to treatment (Espada et al., 2021).
Amphotericin B
Analysis of clinical isolates of L. donovani resistant to amphotericin B revealed a greater rate of amphotericin B efflux due to increased expression of the multidrug resistance gene MDR1 (Purkait et al., 2012). Studies have also shown that amphotericin B-resistant parasites can be easily selected for in vitro (Mbongo et al., 1998; Al-Mohammed et al., 2005). Loss of the gene encoding 24-sterol methyltransferase (SMT) has also been associated with L. donovani in vitro resistance to amphotericin B (Rastrojo et al., 2018). Similarly, another study selected four L. mexicana lines with amphotericin B resistance induced in vitro, one of which showed a resistance-associated mutation in the sterol biosynthesis gene sterol C5-desaturase (SC5D), and three lines revealed loss of expression of SMT due to genomic copy number variants (Pountain et al., 2019). Amphotericin B resistance has also been reported in L. mexicana owing to mutations in the sequence of sterol 14-demethylase (CYP51) (Mwenechanya et al., 2017).
Drug Combinations
Combinations of drugs to treat leishmaniasis, in which different drugs inhibit different metabolic pathways, seem to represent a promising option for overcoming resistance in parasites that are as adaptable as Leishmania. Drug combinations can reduce the overall dose of drugs required and duration of treatment, and can lead to lower toxicity and improved patient compliance (Uliana et al., 2018). However, drug combinations for leishmaniasis treatment must be used with caution as resistance to multiple drugs and cross-resistance can occur (García-Hernández et al., 2012; Berg et al., 2015; Fernandez-Prada et al., 2016).
Impact of Genetic Diversity on New Drug Research
The search for new molecular targets for leishmaniasis treatment is an urgent task. Compound screening is an important approach for identifying new drugs. However, because of the tremendous diversity of these parasites, it is exceedingly difficult to identify drugs that are effective in treating infections caused by different species or strains. In this sense, many “-omics” (such as genomics, transcriptomics, proteomics, and metabolomics) have demonstrated diversity among the Leishmania genus and how various pathways play important roles in the resistant phenotype. In addition, the use of “-omics” to study host-Leishmania interactions should not be underestimated because such interactions are also important in terms of treatment outcomes. However, as Leishmania exhibits post-transcriptional control, there is a risk of extrapolating genomic or transcriptomic data in the context of drug resistance. Sample preparation, in contrast, is the key concealing element in proteome and metabolomic analysis. Furthermore, data from promastigotes should be interpreted with caution, because amastigote forms have distinct transcriptomic and proteomic profiles (De Pablos et al., 2016). Other “-omics” obstacles, such as operating costs, complexity, and the broad range of samples, will most likely be addressed in the next years as “-omics” and bioinformatic technologies progress (Dos Santos et al., 2016).
In addition to the evaluation of “-omics”, gene manipulation in parasites is an interesting option to better understand the pathways associated with drug metabolism. Currently, several tools can be used for genetic manipulation of Leishmania, such as the classic method of deletion by allelic substitution (Cruz et al., 1991), overexpression and heterologous expression (Kapler et al., 1990), plasmid shuffling (Murta et al., 2009), RNAi (Lye et al., 2010), DiCRE (Duncan et al., 2016), DD domain (Madeira et al., 2009), and CRISPR/Cas9 (Sollelis et al., 2015; Zhang and Matlashewski, 2015). In particular, the LeishGEdit toolkit has allowed researchers to explore the function of hundreds of genes swiftly and effectively, propelling the research of gene function and regulation of Leishmania metabolic pathways to unprecedented levels (Beneke et al., 2017; Beneke and Gluenz, 2019; Beneke and Gluenz, 2020). Another novel approach is the high-throughput Cos-Seq method, which can be used to identify gain-of-function resistance mechanisms and drug targets (Gazanion et al., 2016; Fernandez-Prada et al., 2018). In addition to these tools, sequencing of the parasite genome is also fundamental (Ivens et al., 2005), and the availability of parasite genomes in the TritrypDB database provides extremely easy access to all relevant data (Aslett et al., 2010).
However, despite all of the tools available, conducting studies involving gene manipulation in these parasites is not always straightforward, mainly because of the remarkable plasticity of their genomes and its high sensitivity to environmental changes. The parasites can undergo several adaptations for survival in vitro, and it has been recommended that genetic manipulations should be conducted directly in clinical samples to avoid experimental artefacts (Dumetz et al., 2017). Similarly, gene deletion can lead to the selection of parasites that display aneuploidies and altered phenotypes that may not match those observed in nature (Santi et al., 2021). Unfortunately, inducible systems to turn gene expression on and off in Leishmania have not been explored, and there is limited literature regarding the use of these systems (Yan et al., 2001; Kushnir et al., 2005; Yao et al., 2007; Kraeva et al., 2014). Although the implementation of the DiCRE system offers significant advancement in this regard, there are still certain limitations because it is neither reversible nor tunable (Duncan et al., 2016; Santos et al., 2017; Damasceno et al., 2020b; Damianou et al., 2020).
Conclusions and Future Directions
Here, we emphasised the relevance of research into the diversity of the genus Leishmania and the variables that drive diversification. We reaffirm the relevance of using different approaches, such as “-omics” technologies, genetic manipulation, and compound screening, to elucidate drug resistance mechanisms and identify novel chemotherapeutic targets for leishmaniasis. Single-cell sequencing will reveal many more varieties previously submerged under the parasite pool. We further emphasise the need for molecular techniques to explore pathway regulation and establish novel inducible systems for Leishmania. An integrated study using data provided by these different approaches and aspects of host-Leishmania interactions will contribute to a better understanding of the complexity of drug resistance mechanisms, therapeutic failure, and great adaptability of these insidious parasites.
Author Contributions
SM and AS designed the work, collected data, wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work received financial support from the following agencies: Programa INOVA FIOCRUZ - Fundação Oswaldo Cruz (VPPCB-007-FIO-18-2-94); Convênio Fiocruz-Institut Pasteur-USP (no grant number); Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG – APQ-02816-21), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 304158/2019-4), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. SM is supported by CNPq. A.M.M. Santi is supported by CAPES.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the Oswaldo Cruz Foundation (FIOCRUZ) the Graduate Program in Health Science (IRR/FIOCRUZ) for providing an environment for scientific excellence and commitment to public health.
References
Abadi, M. F. S., Moradabadi, A., Vahidi, R., Shojaeepour, S., Rostami, S., Rad, I., et al. (2021). High Resolution Melting Analysis and Detection of Leishmania Resistance: The Role of Multi Drug Resistance 1 Gene. Genes Environ. 43, 4–11. doi: 10.1186/s41021-021-00210-5
Adaui, V., Lye, L., Akopyants, N. S., Zimic, M., Llanos-cuentas, A., Garcia, L., et al. (2016). Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania Braziliensis in Peru and Bolivia. J. Infect. Dis. 213, 112–121. doi: 10.1093/infdis/jiv354
Alcolea, P. J., Alonso, A., Molina, R., Jiménez, M., Myler, P. J., Larraga, V. (2019). Functional Genomics in Sand Fly–Derived Leishmania Promastigotes. PloS Negl. Trop. Dis. 13, 1–30. doi: 10.1371/journal.pntd.0007288
Al-Mohammed, H. I., Chance, M. L., Bates, P. A. (2005). Production and Characterization of Stable Amphotericin-Resistant Amastigotes and Promastigotes of Leishmania Mexicana. Antimicrob. Agents Chemother. 49, 3274–3280. doi: 10.1128/AAC.49.8.3274
Alvar, J., Aparicio, P., Aseffa, A., Boer, M. D., Can, C., Dedet, J., et al. (2008). The Relationship Between Leishmaniasis and AIDS: The Second 10 Years. Clin. Microbiol. Rev. 21, 334–359. doi: 10.1128/CMR.00061-07
Anacleto, C., Abdo, M. C. B., Ferreira, A. V. B., Murta, S. M. F., Romanha, A. J., Fernandes, A. P., et al. (2003). Structural and Functional Analysis of an Amplification Containing a PGPA Gene in a Glucantime-Resistant Leishmania (Viannia) Guyanensis Cell Line. Parasitol. Res. 90, 110–118. doi: 10.1007/s00436-002-0798-x
Andrade, J. M., Murta, S. M. F. (2014). Functional Analysis of Cytosolic Tryparedoxin Peroxidase in Antimony-Resistant and -Susceptible Leishmania Braziliensis and Leishmania Infantum Lines. Parasitol. Vectors 7, 406. doi: 10.1186/1756-3305-7-406
Aslett, M., Aurrecoechea, C., Berriman, M., Brestelli, J., Brunk, B. P., Carrington, M., et al. (2010). TriTrypDB: A Functional Genomic Resource for the Trypanosomatidae. Nucleic Acids Res. 38, 457–462. doi: 10.1093/nar/gkp851
Azeredo-Coutinho, R. B. G., Mendonça, S. C. F., Callahan, H., Portal, A. C., Max, G. (2007). Sensitivity of Leishmania Braziliensis Promastigotes to Meglumine Antimoniate (Glucantime) Is Higher Than That of Other Leishmania Species and Correlates With Response to Therapy in American Tegumentary Leishmaniasis. J. Parasitol. 93, 688–693. doi: 10.1645/GE-1031R.1
Bartholomeu, D. C., Teixeira, S. M. R., Cruz, A. K. (2021). Genomics and Functional Genomics in Leishmania and Trypanosoma Cruzi: Statuses, Challenges and Perspectives. Mem. Inst. Oswaldo. Cruz. 116, e200634–e200634. doi: 10.1590/0074-02760200634
Beneke, T., Gluenz, E. (2019). LeishGEdit: A Method for Rapid Gene Knockout and Tagging Using CRISPR-Cas9. Methods Mol. Biol. 1971, 189–210. doi: 10.1007/978-1-4939-9210-2_9
Beneke, T., Gluenz, E. (2020). Bar-Seq Strategies for the LeishGEdit Toolbox. Mol. Biochem. Parasitol. 239, 111295. doi: 10.1016/j.molbiopara.2020.111295
Beneke, T., Madden, R., Makin, L., Valli, J., Sunter, J., Gluenz, E. (2017). A CRISPR Cas9 High-Throughput Genome Editing Toolkit for Kinetoplastids. R. Soc Open Sci. 4, 170095. doi: 10.1098/rsos.170095
Berg, M., García-hernández, R., Cuypers, B., Vanaerschot, M., Manzano, J. I., Poveda, J. A., et al. (2015). Experimental Resistance to Drug Combinations in Leishmania Donovani: Metabolic and Phenotypic Adaptations. Antimicrob. Agents Chemother. 59, 2242–2255. doi: 10.1128/AAC.04231-14
Bhattacharya, A., Leprohon, P., Bigot, S., Padmanabhan, P. K., Mukherjee, A., Roy, G., et al. (2019). Coupling Chemical Mutagenesis to Next Generation Sequencing for the Identification of Drug Resistance Mutations in Leishmania. Nat. Commun. 10, 5627. doi: 10.1038/s41467-019-13344-6
Bourreau, E., Ginouves, M., Hartley, M., Gangneux, P., Robert-gangneux, F., Dufour, J., et al. (2015). Presence of Leishmania RNA Virus 1 in Leishmania Guyanensis Increases the Risk of First-Line Treatment Failure and Symptomatic Relapse. J. Infect. Dis. 13, 105–111.
Bussotti, G., Gouzelou, E., Boité, M. C., Kherachi, I., Harrat, Z., Eddaikra, N., et al. (2018). Leishmania Genome Dynamics During Environmental Adaptation Reveal Strain-Specific Differences in Gene Copy. MBio 9, 1–18. doi: 10.1128/mBio.01399-18
Camacho, E., González-de la Fuente, S., Solana, J. C., Rastrojo, A., Carrasco-Ramiro, F., Requena, J. M., et al. (2021). Gene Annotation and Transcriptome Delineation on a De Novo Genome Assembly for the Reference Leishmania Major Friedlin Strain. Genes (Basel) 12, 1359. doi: 10.3390/genes12091359
Carnielli, J. B. T., Crouch, K., Forrester, S., Costa, V., Carvalho, S. F. G., Damasceno, J. D., et al. (2018). A Leishmania Infantum Genetic Marker Associated With Miltefosine Treatment Failure for Visceral Leishmaniasis. EBioMedicine 36, 83–91. doi: 10.1016/j.ebiom.2018.09.029
Carnielli, J. B. T., Monti-rocha, R., Costa, D. L., Sesana, A. M., Pansini, L. N. N., Segatto, M., et al. (2019). Natural Resistance of Leishmania Infantum to Miltefosine Contributes to the Low Efficacy in the Treatment of Visceral Leishmaniasis in Brazil. Am. J. Trop. Med. Hyg. 101, 789–794. doi: 10.4269/ajtmh.18-0949
Chandra, U., Yadav, A., Kumar, D., Saha, S. (2017). Cell Cycle Stage-Specific Transcriptional Activation of Cyclins Mediated by HAT2-Dependent H4K10 Acetylation of Promoters in Leishmania Donovani. PloS Pathog. 13, e1006615. doi: 10.1371/journal.ppat.1006615
Clayton, C., Shapira, M. (2007). Post-Transcriptional Regulation of Gene Expression in Trypanosomes and Leishmanias. Mol. Biochem. Parasitol. 156, 93–101. doi: 10.1016/j.molbiopara.2007.07.007
Coelho, A. C., Boisvert, S., Mukherjee, A., Leprohon, P., Corbeil, J., Ouellette, M. (2012). Multiple Mutations in Heterogeneous Miltefosine- Resistant Leishmania Major Population as Determined by Whole Genome Sequencing. PloS Negl. Trop. Dis. 6, e1512. doi: 10.1371/journal.pntd.0001512
Croft, S. L., Sundar, S., Fairlamb, A. H. (2006). Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 19, 111–126. doi: 10.1128/CMR.19.1.111
Cruz, A., Coburn, C. M., Beverley, S. M. (1991). Double Targeted Gene Replacement for Creating Null Mutants. Proc. Natl. Acad. Sci. 88, 7170–7174. doi: 10.1073/pnas.88.16.7170
Cupolillo, E., Cavalcanti, A. S., Ferreira, G. E. M., Boité, M. C., Morgado, F. N., Porrozzi, R. (2020). Occurrence of Multiple Genotype Infection Caused by Leishmania Infantum in Naturally Infected Dogs. PloS Negl. Trop. Dis. 14, 1–15. doi: 10.1371/journal.pntd.0007986
Damasceno, J. D., Marques, C. A., Beraldi, D., Crouch, K., Lapsley, C., Obonaga, R., et al. (2020a). Genome Duplication in Leishmania Major Relies on Persistent Subtelomeric DNA Replication. Elife 9, e58030. doi: 10.7554/eLife.58030
Damasceno, J. D., Nunes, V. S., Tosi, L. R. O. (2013). LmHus1 Is Required for the DNA Damage Response in Leishmania Major and Forms a Complex With an Unusual Rad9 Homologue. Mol. Microbiol. 90, 1074–1087. doi: 10.1111/mmi.12418
Damasceno, J. D., Obonaga, R., Santos, E. V., Scott, A., McCulloch, R., Tosi, L. R. O. (2016). Functional Compartmentalization of Rad9 and Hus1 Reveals Diverse Assembly of the 9-1-1 Complex Components During the DNA Damage Response in Leishmania. Mol. Microbiol. 101, 1054–1068. doi: 10.1111/mmi.13441
Damasceno, J. D., Obonaga, R., Silva, G. L. A., Reis-Cunha, J. L., Duncan, S. M., Bartholomeu, D. C., et al. (2018). Conditional Genome Engineering Reveals Canonical and Divergent Roles for the Hus1 Component of the 9-1-1 Complex in the Maintenance of the Plastic Genome of Leishmania. Nucleic Acids Res. 46, 11835–11846. doi: 10.1093/nar/gky1017
Damasceno, J. D., Reis-Cunha, J., Crouch, K., Beraldi, D., Lapsley, C., Tosi, L. R. O., et al. (2020b). Conditional Knockout of RAD51-Related Genes in Leishmania Major Reveals a Critical Role for Homologous Recombination During Genome Replication. PloS Genet. 16, e1008828. doi: 10.1371/journal.pgen.1008828
Damianou, A., Burge, R. J., Catta-Preta, C. M. C., Geoghegan, V., Nievas, Y. R., Newling, K., et al. (2020). Essential Roles for Deubiquitination in Leishmania Life Cycle Progression. PloS Pathog. 16, e1008455. doi: 10.1371/journal.ppat.1008455
da Silva, M. S. (2021). DNA Double-Strand Breaks: A Double-Edged Sword for Trypanosomatids. Front. Cell Dev. Biol. 9:669041. doi: 10.3389/fcell.2021.669041
Decuypere, S., Vanaerschot, M., Brunker, K., Imamura, H., Müller, S., Khanal, B., et al. (2012). Molecular Mechanisms of Drug Resistance in Natural Leishmania Populations Vary With Genetic Background. PloS Negl. Trop. Dis. 6, e1514. doi: 10.1371/journal.pntd.0001514
De Morais-Teixeira, E., Damasceno, Q. S. (2011). The In Vitro Leishmanicidal Activity of Hexadecylphosphocholine (Miltefosine) Against Four Medically Relevant Leishmania Species of Brazil. Mem. Inst. Oswaldo. Cruz. 106, 475–478. doi: 10.1590/S0074-02762011000400015
De Pablos, L. M., Ferreira, T. R., Walrad, P. B. (2016). Developmental Differentiation in Leishmania Lifecycle Progression: Post-Transcriptional Control Conducts the Orchestra. Curr. Opin. Microbiol. 34, 82–89. doi: 10.1016/j.mib.2016.08.004
Domagalska, M. A., Imamura, H., Sanders, M., Van den Broeck, F., Bhattarai, N. R., Vanaerschot, M., et al. (2019). Genomes of Leishmania Parasites Directly Sequenced From Patients With Visceral Leishmaniasis in the Indian Subcontinent. PloS Negl. Trop. Dis. 13, 1–22. doi: 10.1371/JOURNAL.PNTD.0007900
Dorlo, T. P. C., Huitema, A. D. R., Beijnen, J. H., de Vries, P. J. (2012). Optimal Dosing of Miltefosine in Children and Adults With Visceral. Antimicrob. Agents Chemother. 56, 3864–3872. doi: 10.1128/AAC.00292-12
Dos Santos, B. S., da Silva, L. C. N., da Silva, T. D., Rodrigues, J. F. S., Grisotto, M. A. G., Correia, M. T. D. S., et al. (2016). Application of Omics Technologies for Evaluation of Antibacterial Mechanisms of Action of Plant-Derived Products. Front. Microbiol. 7, 1466. doi: 10.3389/fmicb.2016.01466
Downing, T., Imamura, H., Decuypere, S., Clark, T. G., Coombs, G. H., Cotton, J. A., et al. (2011). Whole Genome Sequencing of Multiple Leishmania Donovani Clinical Isolates Provides Insights Into Population Structure and Mechanisms of Drug Resistance. Genome Res. 21, 2143–2156. doi: 10.1101/gr.123430.111.Freely
Dujardin, J. C., Mannaert, A., Durrant, C., Cotton, J. A. (2014). Mosaic Aneuploidy in Leishmania: The Perspective of Whole Genome Sequencing. Trends Parasitol. 30, 554–555. doi: 10.1016/j.pt.2014.09.004
Dumetz, F., Cuypers, B., Imamura, H., Zander, D., D’Haenens, E., Maes, I., et al. (2018). Molecular Preadaptation to Antimony Resistance in Leishmania Donovani on the Indian Subcontinent. mSphere 3, e00548–17. doi: 10.1128/mSphere.00548-17
Dumetz, F., Imamura, H., Sanders, M., Seblova, V., Myskova, J., Pescher, P., et al. (2017). Modulation of Aneuploidy in Leishmania Donovani During Adaptation to Different In Vitro and In Vivo Environments and Its Impact on Gene Expression. MBio 8, e00599-17. doi: 10.1128/mBio.00599-17
Duncan, S. M., Myburgh, E., Philipon, C., Brown, E., Meissner, M., Brewer, J., et al. (2016). Conditional Gene Deletion With DiCre Demonstrates an Essential Role for CRK3 in Leishmania Mexicana Cell Cycle Regulation. Mol. Microbiol. 100, 931–944. doi: 10.1111/mmi.13375
Espada, C. R., Albuquerque-Wendt, A., Hornillos, V., Gluenz, E., Coelho, A. C., Uliana, S. R. B. (2021). Ros3 (Lem3p/CDC50) Gene Dosage Is Implicated in Miltefosine Susceptibility in Leishmania (Viannia) Braziliensis Clinical Isolates and in Leishmania (Leishmania) Major. ACS Infect. Dis. 7, 849–858. doi: 10.1021/acsinfecdis.0c00857
Fernandez-Prada, C., Sharma, M., Plourde, M., Bresson, E., Roy, G., Leprohon, P., et al. (2018). High-Throughput Cos-Seq Screen With Intracellular Leishmania Infantum for the Discovery of Novel Drug-Resistance Mechanisms. IJP Drugs Drug Resist. 8, 165–173. doi: 10.1016/j.ijpddr.2018.03.004
Fernandez-Prada, C., Vincent, I. M., Brotherton, M., Rivas, L., Leprohon, P., Smith, T. K., et al. (2016). Different Mutations in a P-Type ATPase Transporter in Leishmania Parasites Are Associated With Cross-Resistance to Two Leading Drugs by Distinct Mechanisms. PloS Negl. Trop. Dis. 10, e0005171. doi: 10.1371/journal.pntd.0005171
Franssen, S. U., Durrant, C., Stark, O., Moser, B., Downing, T., Imamura, H., et al. (2020). Global Genome Diversity of the Leishmania Donovani Complex. Elife 9, 1–44. doi: 10.7554/eLife.51243
Frézard, F., Monte-Neto, R., Reis, P. G. (2014). Antimony Transport Mechanisms in Resistant Leishmania Parasites. Biophys. Rev. 6, 119–132. doi: 10.1007/s12551-013-0134-y
García-Hernández, R., Manzano, J. I., Castanys, S., Gamarro, F. (2012). Leishmania Donovani Develops Resistance to Drug Combinations. PloS Negl. Trop. Dis. 6, e1974. doi: 10.1371/journal.pntd.0001974
Gazanion, É., Fernández-Prada, C., Papadopoulou, B., Leprohon, P., Ouellette, M. (2016). Cos-Seq for High-Throughput Identification of Drug Target and Resistance Mechanisms in the Protozoan Parasite Leishmania. Proc. Natl. Acad. Sci. U. S. A. 113, E3012–E3021. doi: 10.1073/pnas.1520693113
Genois, M.-M., Paquet, E. R., Laffitte, M.-C. N., Maity, R., Rodrigue, A., Ouellette, M., et al. (2014). DNA Repair Pathways in Trypanosomatids: From DNA Repair to Drug Resistance. Microbiol. Mol. Biol. Rev. 78, 40–73. doi: 10.1128/MMBR.00045-13
Gourbal, B., Sonuc, N., Bhattacharjee, H., Legare, D., Sundar, S., Ouellette, M., et al. (2004). Drug Uptake and Modulation of Drug Resistance in Leishmania by an Aquaglyceroporin. J. Biol. Chem. 279, 31010–31017. doi: 10.1074/jbc.M403959200
Grondin, K., Papadopoulou, B., Ouellette, M. (1993). Homologous Recombination Between Direct Repeat Sequences Yields P-Glycoprotein Containing Amplicons in Arsenite Resistant Leishmania. Nucleic Acids Res. 21, 1895–1901. doi: 10.1093/nar/21.8.1895
Grünebast, J., Clos, J. (2020). Leishmania: Responding to Environmental Signals and Challenges Without Regulated Transcription. Comput. Struct. Biotechnol. J. 18, 4016–4023. doi: 10.1016/j.csbj.2020.11.058
Grünebast, J., Lorenzen, S., Zummack, J., Clos, J. (2021). Life Cycle Stage-Specific Accessibility of Leishmania Donovani Chromatin at Transcription Start Regions. mSystems 6, e0062821–e0062821. doi: 10.1128/mSystems.00628-21
Gutiérrez-Corbo, C., Domínguez-Asenjo, B., Martínez-Valladares, M., Pérez-Pertejo, Y., García-Estrada, C., Balaña-Fouce, R., et al. (2021). Reproduction in Trypanosomatids: Past and Present. Biol. (Basel) 10, 471. doi: 10.3390/biology10060471
Haimeur, A., Brochu, C., Papadopoulou, B., Ouellette, M. (2000). Amplification of the ABC Transporter Gene PGPA and Increased Trypanothione Levels in Potassium Antimonyl Tartrate (SbIII) Resistant Leishmania Tarentolae. Mol. Biochem. Parasitol. 108, 131–135. doi: 10.1016/S0166-6851(00)00187-0
Iantorno, S. A., Durrant, C., Khan, A., Sanders, M. J., Beverley, S. M., Warren, W. C., et al. (2017). Gene Expression in Leishmania Is Regulated Predominantly by Gene Dosage. MBio 8, 1–20. doi: 10.1128/mBio.01393-17
Imamura, H., Monsieurs, P., Jara, M., Sanders, M., Maes, I., Vanaerschot, M., et al. (2020). Evaluation of Whole Genome Amplification and Bioinformatic Methods for the Characterization of Leishmania Genomes at a Single Cell Level. Sci. Rep. 10, 15043. doi: 10.1038/s41598-020-71882-2
Ivens, A. C., Peacock, C. S., Worthey, E. A., Murphy, L., Aggarwal, G., Berriman, M., et al. (2005). The Genome of the Kinetoplastid Parasite, Leishmania Major. Science (80-.) 309, 436–442. doi: 10.1126/science.1112680
Kapler, G. M., Coburn, C. M., Beverley, S. M. (1990). Stable Transfection of the Human Parasite Leishmania Major Delineates a 30-Kilobase Region Sufficient for Extrachromosomal Replication and Expression. Mol. Cell. Biol. 10, 1084–1094. doi: 10.1128/mcb.10.3.1084
Karamysheva, Z. N., Guarnizo, S. A. G., Karamyshev, A. L. (2020). Regulation of Translation in the Protozoan Parasite Leishmania. Int. J. Mol. Sci. 21, 1–15. doi: 10.3390/ijms21082981
Kato, H., Cáceres, A. G., Gomez, E. A., Tabbabi, A., Mizushima, D., Yamamoto, D. S., et al. (2021). Prevalence of Genetically Complex Leishmania Strains With Hybrid and Mito-Nuclear Discordance. Front. Cell. Infect. Microbiol. 11, 625001. doi: 10.3389/fcimb.2021.625001
Kaufer, A., Ellis, J., Stark, D., Barratt, J. (2017). The Evolution of Trypanosomatid Taxonomy. Parasitol. Vectors 10, 1–17. doi: 10.1186/s13071-017-2204-7
Kieft, R., Zhang, Y., Marand, A. P., Moran, J. D., Bridger, R., Wells, L., et al. (2020). Identification of a Novel Base J Binding Protein Complex Involved in RNA Polymerase II Transcription Termination in Trypanosomes. PloS Genet. 16, e1008390. doi: 10.1371/journal.pgen.1008390
Kraeva, N., Ishemgulova, A., Lukeš, J., Yurchenko, V. (2014). Tetracycline-Inducible Gene Expression System in Leishmania Mexicana. Mol. Biochem. Parasitol. 198, 11–13. doi: 10.1016/j.molbiopara.2014.11.002
Kushnir, S., Gase, K., Breitling, R., Alexandrov, K. (2005). Development of an Inducible Protein Expression System Based on the Protozoan Host Leishmania Tarentolae. Protein Expr. Purif. 42, 37–46. doi: 10.1016/j.pep.2005.03.004
Lachaud, L., Bourgeois, N., Kuk, N., Morelle, C., Crobu, L., Merlin, G., et al. (2014). Constitutive Mosaic Aneuploidy Is a Unique Genetic Feature Widespread in the Leishmania Genus. Microbes Infect. 16, 61–66. doi: 10.1016/j.micinf.2013.09.005
Laffitte, M. N., Leprohon, P., Papadopoulou, B., Ouellette, M. (2016b). Plasticity of the Leishmania Genome Leading to Gene Copy Number Variations and Drug Resistance [Version 1; Referee: 5 Approved] Referee Status. F1000Res 5, 2350. doi: 10.12688/f1000research.9218.1
Laffitte, M. C. N., Leprohon, P., Légaré, D., Ouellette, M. (2016a). Deep-Sequencing Revealing Mutation Dynamics in the Miltefosine Transporter Gene in Leishmania Infantum Selected for Miltefosine Resistance. Parasitol. Res. 115, 3699–3703. doi: 10.1007/s00436-016-5195-y
Légaré, D., Richard, D., Mukhopadhyay, R., Stierhof, Y. D., Rosen, B. P., Haimeur, A., et al. (2001). The Leishmania ATP-Binding Cassette Protein PGPA Is an Intracellular Metal-Thiol Transporter ATPase. J. Biol. Chem. 276, 26301–26307. doi: 10.1074/jbc.M102351200
Leprohon, P., Le, D., Hardiman, G., Corbeil, J., Ouellette, M. (2009). Gene Expression Modulation Is Associated With Gene Amplification, Supernumerary Chromosomes and Chromosome Loss in Antimony-Resistant Leishmania Infantum. Nucleic Acids Res. 37, 1387–1399. doi: 10.1093/nar/gkn1069
Lopez-Velez, R., Perez-Molina, J. A., Guerrero, A., Baquero, F., Escribano, L., Bellas, C., et al. (1998). Clinicoepidemiologic Characteristics, Prognostic Factors, and Survival Analysis of Patients Coinfected With Human Immunodeficiency Virus and Leishmania in an Area of Madrid, Spain. Am. J. Trop. Med. Hyg. 58, 436–443. doi: 10.4269/ajtmh.1998.58.436
Lye, L., Owens, K., Shi, H., Murta, S. M. F., Vieira, A. C., Turco, S. J., et al. (2010). Retention and Loss of RNA Interference Pathways in Trypanosomatid Protozoans. PloS Pathog. 6, e1001161. doi: 10.1371/journal.ppat.1001161
Maciaszczyk-Dziubinska, E., Wawrzycka, D., Wysocki, R. (2012). Arsenic and Antimony Transporters in Eukaryotes. Int. J. Mol. Sci. 13, 3527–3548. doi: 10.3390/ijms13033527
Madeira, L., Owens, K. L., Murta, S. M. F., Beverley, S. M. (2009). Regulated Expression of the Leishmania Major Surface Virulence Factor Lipophosphoglycan Using Conditionally Destabilized Fusion Proteins. Proc. Natl. Acad. Sci. U. S. A. 106, 7583–7588. doi: 10.1073/pnas.0901698106
Mandal, S., Maharjan, M., Singh, S., Chatterjee, M., Madhubala, R. (2010). Assessing Aquaglyceroporin Gene Status and Expression Profile in Antimony-Susceptible and -Resistant Clinical Isolates of Leishmania Donovani From India. J. Antimicrob. Chemoter. 65, 496–507. doi: 10.1093/jac/dkp468
Mannaert, A., Downing, T., Imamura, H., Dujardin, J. C. (2012). Adaptive Mechanisms in Pathogens: Universal Aneuploidy in Leishmania. Trends Parasitol. 28, 370–376. doi: 10.1016/j.pt.2012.06.003
Marquis, N., Gourbal, B., Rosen, B. P., Mukhopadhyay, R., Ouellette, M. (2005). Modulation in Aquaglyceroporin AQP1 Gene Transcript Levels in Drug-Resistant Leishmania. Mol. Immunol. 57, 1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x
Martínez-Calvillo, S., Nguyen, D., Stuart, K., Myler, P. J. (2004). Transcription Initiation and Termination on Leishmania Major Chromosome 3. Eukaryot. Cell 3, 506–517. doi: 10.1128/EC.3.2.506
Martínez-Calvillo, S., Yan, S., Nguyen, D., Fox, M., Stuart, K., Myler, P. J., et al. (2003). Transcription of Leishmania Major Friedlin Chromosome 1 Initiates in Both Directions Within a Single Region. Mol. Cell 5, 1291–1299. doi: 10.1016/S1097-2765(03)00143-6
Mbongo, N., Loiseau, P. M., Billion, M. A. (1998). Mechanism of Amphotericin B Resistance in Leishmania Donovani Promastigotes. Antimicrob. Agents Chemother. 42, 352–357. doi: 10.1128/AAC.42.2.352
Mohebali, M., Kazemirad, E., Hajjaran, H., Kazemirad, E., Oshaghi, M. A., Raoofian, R., et al. (2019). Gene Expression Analysis of Antimony Resistance in Leishmania Tropica Using Quantitative Real-Time PCR Focused on Genes Involved in Trypanothione Metabolism and Drug Transport. Arch. Dermatol. Res. 311, 9–17. doi: 10.1007/s00403-018-1872-2
Monte-Neto, R., Laffitte, M. N., Leprohon, P., Reis, P. (2015). Intrachromosomal Amplification, Locus Deletion and Point Mutation in the Aquaglyceroporin AQP1 Gene in Antimony Resistant Leishmania (Viannia) Guyanensis. PloS Negl. Trop. Dis. 9, e0003476. doi: 10.1371/journal.pntd.0003476
Moreira, D., de, S., Xavier, M. V., Murta, S. M. F. (2018). Ascorbate Peroxidase Overexpression Protects Leishmania Braziliensis Against Trivalent Antimony Effects. Mem. Inst. Oswaldo. Cruz. 113, e180377. doi: 10.1590/0074-02760180377
Moreira, D. S., Monte Neto, R. L., Andrade, J. M., Santi, A. M. M., Reis, P. G., Frézard, F., et al. (2013). Molecular Characterization of the MRPA Transporter and Antimony Uptake in Four New World Leishmania Spp. Susceptible and Resistant to Antimony. Int. J. Parasitol. Drugs Drug Resist. 3, 143–153. doi: 10.1016/j.ijpddr.2013.08.001
Mukherjee, A., Boisvert, S., do Monte-Neto, R. L., Coelho, A. C., Raymond, F., Mukhopadhyay, R., et al. (2013). Telomeric Gene Deletion and Intrachromosomal Amplification in Antimony-Resistant Leishmania. Mol. Microbiol. 88, 189–202. doi: 10.1111/mmi.12178
Mukherjee, A., Padmanabhan, P. K., Singh, S., Girard, I., Chatterjee, M., Ouellette, M., et al. (2007). Role of ABC Transporter MRPA, G -Glutamylcysteine Synthetase and Ornithine Decarboxylase in Natural Antimony-Resistant Isolates of Leishmania Donovani. J. Antimicrob. Chemoter. 59, 204–211. doi: 10.1093/jac/dkl494
Mukhopadhyay, R., Dey, S., Xu, N., Gage, D., Lightbody, J., Ouellette, M., et al. (1996). Trypanothione Overproduction and Resistance to Antimonials and Arsenicals in Leishmania. Proc. Natl. Acad. Sci. U. S. A. 93, 10383–10387. doi: 10.1073/pnas.93.19.10383
Muraca, G., Berti, I. R., Sbaraglini, M. L., Fávaro, W. J., Durán, N., Castro, G. R., et al. (2020). Trypanosomatid-Caused Conditions: State of the Art of Therapeutics and Potential Applications of Lipid-Based Nanocarriers. Front. Chem. 8:601151. doi: 10.3389/fchem.2020.601151
Murta, S. M. F., Vickers, T. J., Scott, D. A., Beverley, S. M. (2009). Methylene Tetrahydrofolate Dehydrogenase/Cyclohydrolase and the Synthesis of 10-CHO-THF Are Essential in Leishmania Major. Mol. Microbiol. 71, 1386–1401. doi: 10.1111/j.1365-2958.2009.06610.x.Methylene
Mwenechanya, R., Kovářová, J., Dickens, N. J., Mudaliar, M., Herzyk, P., Vincent, I. M., et al. (2017). Sterol 14α-Demethylase Mutation Leads to Amphotericin B Resistance in Leishmania Mexicana. PloS Negl. Trop. Dis. 11, e0005649. doi: 10.1371/journal.pntd.0005649
Negreira, G. H., Monsieurs, P., Imamura, H., Maes, I., Kuk, N., Yagoubat, A., et al. (2020). Exploring the Evolution and Adaptive Role of Mosaic Aneuploidy in a Clonal Leishmania Donovani Population Using High Throughput Single Cell Genome Sequencing. bioRxiv. 2020.03.05.976233. doi: 10.1101/2020.03.05.976233
Ostyn, B., Hasker, E., Dorlo, T. P. C., Rijal, S., Sundar, S., Dujardin, J., et al. (2014). Failure of Miltefosine Treatment for Visceral Leishmaniasis in Children and Men in South-East Asia. PloS One 9, e100220. doi: 10.1371/journal.pone.0100220
Ouellette, M., Borst, P. (1991). Drug Resistance and P-Glycoprotein Gene Amplification in the Protozoan Parasite Leishmania. Res. Microbiol. 142, 737–746. doi: 10.1016/0923-2508(91)90089-S
Parthasarathy, A., Kalesh, K. (2020). Defeating the Trypanosomatid Trio: Proteomics of the Protozoan Parasites Causing Neglected Tropical Diseases. RSC. Med. Chem. 11, 625–645. doi: 10.1039/d0md00122h
Patino, L. H., Castillo-Castañeda, A., Muñoz, M., Muskus, C., Rivero-Rodríguez, M., Pérezdoria, A., et al. (2021). Revisiting the Heterogeneous Global Genomic Population Structure of Leishmania Infantum. Microb. Genomics 7, 000640. doi: 10.1099/MGEN.0.000640
Patino, L. H., Imamura, H., Cruz-saavedra, L., Pavia, P., Muskus, C., Méndez, C., et al. (2019). Major Changes in Chromosomal Somy, Gene Expression and Gene Dosage Driven by Sb III in Leishmania Braziliensis and Leishmania Panamensis. Sci. Rep. 9, 1–13. doi: 10.1038/s41598-019-45538-9
Patino, L. H., Muskus, C., Muñoz, M., Ramírez, J. D. (2020). Genomic Analyses Reveal Moderate Levels of Ploidy, High Heterozygosity and Structural Variations in a Colombian Isolate of Leishmania (Leishmania) Amazonensis. Acta Trop. 203, 105296. doi: 10.1016/j.actatropica.2019.105296
Peacock, C. S., Seeger, K., Harris, D., Murphy, L., Ruiz, J. C., Quail, M. A., et al. (2007). Comparative Genomic Analysis of Three Leishmania Species That Cause Diverse Human Disease. Nat. Genet. 39, 839–847. doi: 10.1038/ng2053
Perry, M. R., Prajapati, V. K., Menten, J., Raab, A., Feldmann, J., Chakraborti, D., et al. (2015). Arsenic Exposure and Outcomes of Antimonial Treatment in Visceral Leishmaniasis Patients in Bihar, India : A Retrospective Cohort Study. PloS Negl. Trop. Dis. 9, 1–17. doi: 10.1371/journal.pntd.0003518
Peters, W. (1981). The Treatment of Kala-Azar–New Approaches to an Old Problem. Indian J. Med. Res. 73 Suppl, 1–18.
Ponte-Sucre, A., Gamarro, F., Dujardin, J., Barrett, M. P., Garcı, R., Pountain, A. W., et al. (2017). Drug Resistance and Treatment Failure in Leishmaniasis : A 21st Century Challenge. PloS Negl. Trop. Dis. 11, e0006052. doi: 10.1371/journal.pntd.0006052
Potvin, J.-E., Leprohon, P., Queffeulou, M., Sundar, S., Ouellette, M. (2021). Mutations in an Aquaglyceroporin as a Proven Marker of Antimony Clinical Resistance in the Parasite Leishmania Donovani. Clin. Infect. Dis. an. Off. Publ. Infect. Dis. Soc Am. 72, e526–e532. doi: 10.1093/cid/ciaa1236
Pountain, A. W., Weidt, S. K., Regnault, C., Bates, P. A., Donachie, A. M., Dickens, N. J., et al. (2019). Genomic Instability at the Locus of Sterol C24-Methyltransferase Promotes Amphotericin B Resistance in Leishmania Parasites. PloS Negl. Trop. Dis. 13, e0007052. doi: 10.1371/journal.pntd.0007052
Purkait, B., Kumar, A., Nandi, N., Sardar, H., Das, S., Kumar, S., et al. (2012). Mechanism of Amphotericin B Resistance in Clinical Isolates of Leishmania Donovani. Antimicrob. Agents Chemother. 56, 1031–1041. doi: 10.1128/AAC.00030-11
Rastrojo, A., García-Hernández, R., Vargas, P., Camacho, E., Corvo, L., Imamura, H., et al. (2018). Genomic and Transcriptomic Alterations in Leishmania Donovani Lines Experimentally Resistant to Antileishmanial Drugs. Int. J. Parasitol. Drugs Drug Resist. 8, 246–264. doi: 10.1016/j.ijpddr.2018.04.002
Reis-Cunha, J. L., Valdivia, H. O., Bartholomeu, D. C. (2018). Gene and Chromosomal Copy Number Variations as an Adaptive Mechanism Towards a Parasitic Lifestyle in Trypanosomatids. Curr. Genomics 19, 87–97. doi: 10.2174/1389202918666170911161311
Restrepo, C. M., Llanes, A., Cedeño, E. M., Chang, J. H., Álvarez, J., Ríos, M., et al. (2019). Environmental Conditions May Shape the Patterns of Genomic Variations in Leishmania Panamensis. Genes (Basel) 10, 838. doi: 10.3390/genes10110838
Reynolds, D. L., Hofmeister, B. T., Cliffe, L., Siegel, T. N., Anderson, B. A., Beverley, S. M., et al. (2016). Base J Represses Genes at the End of Polycistronic Gene Clusters in Leishmania Major by Promoting RNAP II Termination. Mol. Microbiol. 101, 559–574. doi: 10.1111/mmi.13408
Romero, G. A., Guerra, M. V., Paes, M. G., Macêdo, V. O. (2001). Comparison of Cutaneous Leishmaniasis Due to Leishmania (Viannia) Braziliensis and L. (V.) Guyanensis in Brazil: Therapeutic Response to Meglumine Antimoniate. Am. J. Trop. Med. Hyg. 65, 456–465. doi: 10.4269/ajtmh.2001.65.456
Saha, S. (2020). Histone Modifications and Other Facets of Epigenetic Regulation in Trypanosomatids: Leaving Their Mark. MBio 11, e01079-20. doi: 10.1128/mBio.01079-20
Salloum, T., Moussa, R., Rahy, R., Al Deek, J., Khalifeh, I., El Hajj, R., et al. (2020). Expanded Genome-Wide Comparisons Give Novel Insights Into Population Structure and Genetic Heterogeneity of Leishmania Tropica Complex. PloS Negl. Trop. Dis. 14, e0008684. doi: 10.1371/journal.pntd.0008684
Santi, A. M. M., Silva, P. A., Santos, I. F. M., Murta, S. M. F. (2021). Downregulation of FeSOD-A Expression in Leishmania Infantum Alters Trivalent Antimony and Miltefosine Susceptibility. Parasitol. Vectors 14, 366. doi: 10.1186/s13071-021-04838-8
Santos, R. E. R. S., Silva, G. L. A., Santos, E. V., Duncan, S. M., Mottram, J. C., Damasceno, J. D., et al. (2017). A DiCre Recombinase-Based System for Inducible Expression in Leishmania Major. Mol. Biochem. Parasitol. 216, 45–48. doi: 10.1016/j.molbiopara.2017.06.006
Schwabl, P., Boité, M. C., Bussotti, G., Jacobs, A., Andersson, B., Moreira, O., et al. (2021). Colonization and Genetic Diversification Processes of Leishmania Infantum in the Americas. Commun. Biol. 4, 1–13. doi: 10.1038/s42003-021-01658-5
Sollelis, L., Ghorbal, M., Macpherson, C. R., Martins, R. M., Kuk, N., Crobu, L., et al. (2015). Breaking R Eport First Efficient CRISPR-Cas9-Mediated Genome Editing in Leishmania Parasites. Cell. Microbiol. 17, 1405–1412. doi: 10.1111/cmi.12456
Sterkers, Y., Crobu, L., Lachaud, L., Pagès, M., Bastien, P. (2014). Parasexuality and Mosaic Aneuploidy in Leishmania: Alternative Genetics. Trends Parasitol. 30, 429–435. doi: 10.1016/j.pt.2014.07.002
Sterkers, Y., Lachaud, L., Bourgeois, N., Crobu, L., Bastien, P., Pagès, M. (2012). Novel Insights Into Genome Plasticity in Eukaryotes : Mosaic Aneuploidy in Leishmania Micro Review Novel Insights Into Genome Plasticity in Eukaryotes: Mosaic Aneuploidy in Leishmania. Mol. Microbiol. 86, 15–23. doi: 10.1111/j.1365-2958.2012.08185.x
Sundar, S. (1994). Clinicoepidermiological Study of Drug Resistance in Indian Kala- Azar. BMJ 308, 307. doi: 10.1136/bmj.308.6924.307
Sundar, S. (2001). Drug Resistance in Indian Visceral Leishmaniasis. Trop. Med. Int. Health 6, 849–854. doi: 10.1046/j.1365-3156.2001.00778.x
Sundar, S., Jha, T., Thakur, C., Engel, J., Sindermann, H., Fischer, C., et al. (2002). Oral Miltefosine For Indian Visceral Leishmaniasis. N. Engl. J. Med. 347, 1739–1746. doi: 10.1056/NEJMoa021556
Sundar, S., Makharia, A., More, D., Agrawal, G., Voss, A., Fischer, C., et al. (2000). Short-Course of Oral Miltefosine for Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 31, 1110–1113. doi: 10.1086/318122
Tessarollo, N. G., Andrade, J. M., Moreira, D. S., Murta, S. M. F. (2015). Functional Analysis of Iron Superoxide Dismutase-A in Wild-Type and Antimony-Resistant Leishmania Braziliensis and Leishmania Infantum Lines. Parasitol. Int. 64, 125–129. doi: 10.1016/j.parint.2014.11.001
Thomas, S., Green, A., Sturm, N. R., Campbell, D. A., Myler, P. J. (2009). Histone Acetylations Mark Origins of Polycistronic Transcription in Leishmania Major. BMC Genomics 10, 1–15. doi: 10.1186/1471-2164-10-152
Ubeda, J. M., Légaré, D., Raymond, F., Ouameur, A. A., Boisvert, S., Rigault, P., et al. (2008). Modulation of Gene Expression in Drug Resistant Leishmania Is Associated With Gene Amplification, Gene Deletion and Chromosome Aneuploidy. Genome Biol. 9, R115. doi: 10.1186/gb-2008-9-7-r115
Ubeda, J.-M., Raymond, F., Mukherjee, A., Plourde, M., Gingras, H., Roy, G., et al. (2014). Genome-Wide Stochastic Adaptive DNA Amplification at Direct and Inverted DNA Repeats in the Parasite Leishmania. PloS Biol. 12, e1001868. doi: 10.1371/journal.pbio.1001868
Uliana, S. R. B., Trinconi, C. T., Coelho, A. C. (2018). Chemotherapy of Leishmaniasis: Present Challenges. Parasitology 145, 464–480. doi: 10.1017/S0031182016002523
Van den Broeck, F., Savill, N. J., Imamura, H., Sanders, M., Maes, I., Cooper, S., et al. (2020). Ecological Divergence and Hybridization of Neotropical Leishmania Parasites. Proc. Natl. Acad. Sci. U. S. A. 117, 25159–25168. doi: 10.1073/pnas.1920136117
Van Griensven, J., Carrillo, E., Lopez-Velez, R., Lynen, L., Moreno, J. (2014). Leishmaniasis in Immunosuppressed Individuals. Clin. Microbiol. Infect. 20, 286–299. doi: 10.1111/1469-0691.12556
van Luenen, H. G. A. M., Farris, C., Jan, S., Genest, P.-A., Tripathi, P., Velds, A., et al. (2012). Glucosylated Hydroxymethyluracil, DNA Base J, Prevents Transcriptional Readthrough in Leishmania. Cell 150, 909–921. doi: 10.1016/j.cell.2012.07.030
WHO. (2021). Leishmaniasis. 20 May 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (Accessed August 6, 2021).
Yan, S., Myler, P. J., Stuart, K. (2001). Tetracycline Regulated Gene Expression in Leishmania Donovani. Mol. Biochem. Parasitol. 112, 61–69. doi: 10.1016/S0166-6851(00)00345-5
Yao, C., Luo, J., Hsiao, C. C., Donelson, J. E., Wilson, M. E. (2007). Leishmania Chagasi: A Tetracycline-Inducible Cell Line Driven by T7 RNA Polymerase. Exp. Parasitol. 116, 205–213. doi: 10.1016/j.exppara.2007.01.001
Zackay, A., Cotton, J. A., Sanders, M., Hailu, A., Nasereddin, A., Warburg, A., et al. (2018). Genome Wide Comparison of Ethiopian Leishmania Donovani Strains Reveals Differences Potentially Related to Parasite Survival. PloS Genet. 14, e1007133. doi: 10.1371/journal.pgen.1007133
Zhang, W., Matlashewski, G. (2015). CRISPR-Cas9-Mediated Genome Editing in Leishmania Donovani. MBio 6, e00861. doi: 10.1128/mBio.00861-15.Editor
Keywords: Leishmania, chemotherapy, drug resistance, genetic diversity, genome plasticity
Citation: Santi AMM and Murta SMF (2022) Impact of Genetic Diversity and Genome Plasticity of Leishmania spp. in Treatment and the Search for Novel Chemotherapeutic Targets. Front. Cell. Infect. Microbiol. 12:826287. doi: 10.3389/fcimb.2022.826287
Received: 30 November 2021; Accepted: 04 January 2022;
Published: 24 January 2022.
Edited by:
Luiz Tosi, University of São Paulo, BrazilReviewed by:
Karunakaran Kalesh, Durham University, United KingdomCopyright © 2022 Santi and Murta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvane Maria Fonseca Murta, c2lsdmFuZS5tdXJ0YUBmaW9jcnV6LmJy
 Ana Maria Murta Santi
Ana Maria Murta Santi Silvane Maria Fonseca Murta
Silvane Maria Fonseca Murta