- 1College of Natural and Health Sciences, Zayed University, Dubai, United Arab Emirates
- 2Department of Pharmaceutical Sciences, School of Pharmacy, Lebanese International University, Bekaa, Lebanon
The serious challenge of antimicrobial resistance continues to threaten public health and lingers in the era of the coronavirus disease 2019 (COVID-19), declared pandemic by the World Health Organization. While the pandemic has triggered the importance of infection control practices and preventive measures such as physical distancing, hand hygiene, travel reduction and quarantine, the ongoing alarm of antimicrobial resistance seems to accompany the pandemic too. Antimicrobial resistance has been fostered during COVID-19, possibly due to high rate of empirical antibiotic utilization in COVID-19 patients, increased use of biocides, and the disruption of proper healthcare for other conditions. Specifically, carbapenemase-producing Gram-negative bacteria have shown to cause secondary bacterial infections in patients hospitalized for COVID-19. Clinical and microbiological evidence of such infections is accumulating in different parts of the world. With the resilient nature of carbapenemases, their association with mortality, and the limited treatment options available, concerns regarding this group of antibiotic-hydrolyzing enzymes during the pandemic are expected to upsurge. While the additional burden carbapenemases exert on healthcare is worrisome, it remains hidden or abandoned among the various health consequences of the pandemic. The purpose of this minireview is to shed a light on carbapenemase-associated infections during such unprecedented time of COVID-19. A focused insight shall be made into carbapenemases, their implications for COVID-19 patients, and the features and consequences of co-infection, with a review of available evidence from pertinent literature. The importance of increased surveillance for carbapenemase-producers and optimizing their management in relation to the pandemic, shall be addressed as well.
Introduction to Carbapenem Resistance and Implications in COVID-19
In its ranking of bacterial pathogens into critical, high, and medium priority, the World Health Organization (WHO) classifies carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa, and carbapenem-resistant and third-generation cephalosporin-resistant Enterobacteriaceae, as critical priority bacteria (Tacconelli et al., 2018). Such definition reflects urgent need to develop new antibiotics against these pathogens, and indicates their multidrug resistant nature, making them a particular menace in hospitals, nursing homes, and among critically-ill patients (WHO, 2017). As such, high-resistance rates to carbapenems in Gram-negative pathogens inescapably complicate infections, and are propagated across different countries by mobile genetic elements (Brink, 2019). Hence, carbapenems, last resort antibiotics for complicated bacterial infections, are now threatened by widespread resistance (Potter et al., 2016). A primary mechanism of carbapenem resistance in gram-negative bacteria is expression of acquired carbapenemases, enzymes that hydrolyze these antibiotics. Carbapenemase production is especially challenging when encountered in members of Enterobacteriaceae family, which can rapidly spread and colonize patients in healthcare environments, and are often resistant to multiple antibiotic classes, resulting in restricted treatment options (Bonomo et al., 2018).
Parallel to the ongoing health overload of antimicrobial resistance in general and carbapenemases in particular, the world witnessed, in 2020, the unprecedented emergency caused by a novel coronavirus, that first appeared in Wuhan, China (Wang et al., 2020). This virus was identified as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of the coronavirus disease 2019 (COVID-19) (Wu et al., 2020), which became domestically and internationally spread, and shortly declared pandemic by the WHO with additional impact on society, environment, economy, politics and global biosafety (Barbuddhe et al., 2020; Benach, 2021). Essentially an infectious disease, many other ailments remained hidden in shadows of COVID-19, namely pediatric health (Zemrani et al., 2021), geriatric health (Dhama et al., 2020), and mental well-being (Gambin et al., 2021; Magson et al., 2021). The pandemic has caused a remarkable disruption in healthcare and emergency services, drug shortages, and a surge in misinformation (Rusic et al., 2021), described as infodemic (Orso et al., 2020).
Antimicrobial resistance continues to flourish as another adversity during the pandemic, even though certain measures that prevent and control infections are accompanying COVID-19, and may cause decreased antimicrobial resistance (Rusic et al., 2021). These measures include enhanced hand hygiene, reduced travel, decreased incidence of infections due to social distancing, disinfection and protective measures, introduction of novel biomarkers (i.e., procalcitonin), decreased antimicrobial consumption due to fewer patient consultations, and reduction of critically ill patient transfer among countries (Donà et al., 2020; Monnet and Harbarth, 2020; Rawson et al., 2020; Martin et al., 2021). However, the pandemic is exacerbating antimicrobial resistance through increased use of environmental biocidal agents, potential risk of co-infection, unlicensed use of antibiotics, increased rate of empirical antimicrobial treatment for respiratory illness, decreased resistance surveillance due to diagnosis focus on COVID-19, and overloading of healthcare systems. Unfortunately, the weight of these factors on antimicrobial resistance appears to be major, and their negative impact should be monitored (Cantón et al., 2020).
The exponential growth in antibiotic use during COVID-19 is possibly inducing further pressure, contributing to the selection of antibiotic-resistant bacteria, including carbapenemase producers (Sellera et al., 2021). For example, in 2020, 68.9% of COVID-19 patients reported use of antibiotics prior to hospital admission, with a self-medication rate of 33.0% (Zavala-Flores and Salcedo-Matienzo, 2020). Evidence exists that COVID-19 patients experience co-infection with carbapenemase producers (Arcari et al., 2021), and that the pandemic impacted control and surveillance programs previously established against these organisms (Belvisi et al., 2021). As such, meticulous investigation of carbapenemase producers in COVID-19 patients and analysis of pandemic implications on these resilient pathogens are needed.
Concise Review of Carbapenemases
The global dissemination of carbapenemases continues at alarming rates, threatening the once effective carbapenems. The most prevalent types KPC, VIM, IMP, NDM, and OXA-48 are routinely reported in many infections worldwide (Hansen, 2021). Historically, the first isolated carbapenemases early in the 1990s were the chromosomal imipenem-hydrolyzing enzyme (Yang et al., 1990) and NmcA (Nordmann et al., 1993) in Serratia marcescens and Enterobacter cloacae respectively. Later, plasmid-encoded carbapenemases emerged, and an epidemic involving KPC, MBLs, and OXA-48 carbapenemases occurred at the beginning of the 21st century (Bush, 2018). Carbapenemases are classified within classes A, B and D of the Ambler structural classification (Suay-García and Pérez-Gracia, 2019), although some plasmid-encoded class C enzymes (AmpC variants) are linked to reduced imipenem sensitivity (Sawa et al., 2020). A concise presentation of the three major Ambler types of carbapenemases is shown below; further reading (Hammoudi Halat and Ayoub Moubareck, 2020) can be sought for a comprehensive review.
Ambler Class A Carbapenemases
Presented initially by the chromosomal enzymes NmcA (Nordmann et al., 1993) and SME-1 (Naas et al., 1994), these are non-metallo β-lactamases with serine active moiety, and wide hydrolytic profile for all β-lactamases except cephamycins. They are inhibited by clavulanate, tazobactam and boronic acid derivatives (Hammoudi et al., 2014). The more important class A carbapenemases are the plasmid-encoded KPC and GES. KPC enzymes were initially described in Klebsiella pneumoniae but are now detected in various members of the Enterobacteriaceae family. The blaKPC genes can be carried on mobile genetic elements such as transposons (e.g., Tn4401b) and plasmids (IncFII, IncL/M, and IncN). Often, organisms expressing KPC genes are simultaneously resistant to other antibiotics, such as quinolones and aminoglycosides, creating further challenges (Logan and Weinstein, 2017). Although over 20 different KPC variants are known, KPC-2 and KPC-3 persist as most common, and are linked to the major K. pneumoniae clone of sequence type (ST)-258 (Naas et al., 2016). The GES family of enzymes (Naas et al., 2016) includes many extended-spectrum β-lactamases (ESBLs), with few displaying carbapenemase activity and being prevalent, like GES-2 (Stewart et al., 2015), GES-4 (Yamasaki et al., 2017), GES-5 (Ayoub Moubareck et al., 2019), GES-6 (Botelho et al., 2015) and GES-20 (Garza-Ramos et al., 2015). The numerous point-mutant derivatives of GES-enzymes and their geographic spread indicate ongoing evolution and sheds darkness on antibiotic resistance, as these enzymes can become true carbapenemases by single point mutations.
Ambler Class B Carbapenemases
These are metallo-β-lactamases (MBLs) that diverge from serine enzymes by their active site and catalytic features. MBLs require a bivalent metal ion like zinc for activity, and hydrolyze all β-lactams, including carbapenems, with the exception of aztreonam (Bahr et al., 2021). MBLs are not inhibited by clavulanic acid, tazobactam, or vaborbactam. The most well-known MBLs are the Verona integron–encoded MBL (VIM), Pseudomonas (IMP)-type and the New Delhi MBL (NDM). The spread of MBLs is attributed to their expression on mobile genetic elements such as integrons, plasmids and transposons (Hansen, 2021). The remarkable global spread of NDM remains a worrisome manifestation; initially isolated from a Swedish patient traveling to New Delhi (Yong et al., 2009), the prototype enzyme, NDM-1, became prominent in K. pneumoniae and Escherichia coli, then in A. baumannii and P. aeruginosa (Dortet et al., 2014). Currently, about 28 NDM variants have been described and spread despite efforts of containment (Farhat and Khan, 2020). Serious outbreaks from China, India, Pakistan and Bangladesh caused by NDM-producing Enterobacteriaceae are reported, and will cripple carbapenem utility (Usman Qamar et al., 2020).
Ambler Class D Carbapenemases
Initially known as oxacillinases because of hydrolytic activity on oxacillin, methicillin and cloxacillin, the class D β-lactamases became a clinical concern. They possess variable but significant carbapenemase activity and are generally not inhibited by clavulanic acid, tazobactam, and sulbactam (Hansen, 2021). Currently, important OXA enzymes with carbapenemase activity belong to groups OXA-23-like, OXA-24/40-like, OXA-48-like, OXA-58-like, OXA-143-like and OXA-235 (Hammoudi Halat and Ayoub Moubareck, 2020). The first carbapenem-hydrolyzing oxacillinase, OXA-23, was plasmid-mediated and isolated from A. baumannii early upon clinical use of imipenem (Scaife et al., 1995). It is capable of hydrolysis of oxyiminocephalosporins, aminopenicillins, piperacillin, oxacillin, aztreonam and carbapenems (Kaitany et al., 2013). The locations of OXA-23 are both plasmid and chromosomal-based, usually flanked by the insertion sequence ISAba1, which likely acts as strong promotor of gene expression (Turton et al., 2006). Aside to the A. baumannii-derived group of OXA carbapenemases, the OXA-48-type carbapenemases, first isolated in Turkey from K. pneumoniae (Poirel et al., 2004), are increasingly reported in enterobacterial species. These enzymes hydrolyze penicillins at a high level and carbapenems at a low level, sparing broad-spectrum cephalosporins, and are not susceptible to β-lactamase inhibitors (Poirel et al., 2012). OXA-48, OXA-181, OXA-232, OXA-204, OXA-162, and OXA-244, in this order, are the most common enzymes among the group. Genetically, OXA-48 is associated with different Tn1999 variants on IncL plasmids; it is endemic in North Africa and the Middle East, while OXA-162 and OXA-244 predominate in Europe. OXA-181 and OXA-232 are associated with ISEcp1, Tn2013 on ColE2, and IncX3 plasmids, and are endemic in the Indian subcontinent and certain sub-Saharan African regions (Pitout et al., 2019).
Carbapenemase-Producers Causing Co-Infections in COVID-19 Patients: A Review of Available Evidence
Previous experience shows that carbapenemase-producing bacteria can thrive in times of chaos (Livermore, 2021), as seen in countries with war, socioeconomic and immigrant challenges (Lafeuille et al., 2013; Rafei et al., 2014; Dandachi et al., 2019). In COVID-19 era, with all associated inconveniences, stressed healthcare systems, and collateral damage, an analogous portrait is expected. For example, a statistically significant difference in the resistance to carbapenems was noted in one study from Serbia (Despotovic et al., 2021) when COVID-19 patients were compared to non-COVID-19 patients in the prepandemic year. A significant association between the diagnosis of COVID-19 and a more exaggerated resistance to different carbapenems (imipenem, 56.8% vs. 24.5%, p < 0.001; meropenem, 61.1% vs. 24.3%, p < 0.001; and ertapenem, 26.1% vs. 21.7%, p = 0.03) was observed upon comparison. Such observation indicates the significant shift of resistance profiles with COVID-19, and may be attributed to the inability to completely conform with standard practices of infection control during such unprecedented time (Pascale et al., 2021). Also, the in-hospital use of antibiotics has known to be a driver of resistance in a time-dependent manner whether or not a pandemic takes place, highlighting the need for rational antimicrobial use across all levels of healthcare (Kousovista et al., 2021), and this indeed, applies to carbapenems. In one retrospective single-center, case-control study, authors from Spain reported that COVID-19 patients had increased risk of nosocomial infections with carbapenemase-producing Enterobacteriaceae, with infections being severe, appearing in critically-ill patients, and associated with a high mortality. The aforementioned infection was the cause of death in about two thirds of the studied patients (Pintado et al., 2022). In Brazil, the COVID-19 pandemic was correlated to an increase in incidence of carbapenem-resistant A. baumannii both in ICU and non-ICU settings (Polly et al., 2021). Likewise, the number of carbapenem-resistant Enterobacteriaceae isolated from COVID-19 patients in a Turkish hospital was higher than that isolated during an equal time period prior to the pandemic (Karataş et al., 2021). Taken together, such annotations indicate that institutions and regions with a high prevalence of carbapenemase-producers should be primed for a significant increase in the prevalence of these strains with the progressive expansion of the pandemic. Although similar carbapenemase groups are being isolated as in the pre-pandemic era, the implementation of increased surveillance and antimicrobial stewardship programs should continue throughout the pandemic. Additionally, with COVID-19, carbapenemase-producers are expected to renew their challenge (Livermore, 2021). Accordingly, and with subsequent pandemic waves and emergence of new SARS-CoV-2 variants, focused efforts to control carbapenemase-producers should be an essential component of management strategies for COVID-19 patients.
In an attempt to review the available literature on infections caused by carbapenemase-producers in COVID-19 patients, a PubMed search was conducted using the search terms carbapenemase, COVID-19, Gram-negative bacteria, Enterobacteriaceae, Pseudomonas, and Acinetobacter. All retrieved studies in 2020 and 2021 discussing infection of COVID-19 patients with carbapenemase-producers among these three bacterial categories were included, whether as co-infecting or secondary infecting bacteria. Positive results from samples collected within 2 days of admission were categorized as co-infections, and those collected more than 2 days after admission as secondary infections (Russell et al., 2021). Studies not mentioning carbapenemase production among co-infecting or secondary infecting bacteria, and studies in which COVID-19 patients were not distinguished for infection or colonization by carbapenemase-producers were excluded. According to this search, observation of bacterial infection with carbapenemase-producers in patients with COVID-19 started with the pandemic (Hughes et al., 2020; Ling et al., 2020; Nori et al., 2021), with detection of multiresistant Gram-negative pathogens (Chen et al., 2020) as well as carbapenemase producers (Cultrera et al., 2021; Sang et al., 2021; Senok et al., 2021). Early during COVID-19, a report from China (Zhang et al., 2020) found that more than half of COVID-19 patients in intensive care unit (ICU) of one hospital were coinfected with carbapenem-resistant A. baumanni, more common in patients who died compared to survivors. In a single-center experience from Italy, 50% critically ill COVID-19 patients developed multi-drug resistant infections, of which 32% were carbapenem-resistant K. pneumoniae (KPC-producing), 19% were carbapenem-resistant A. baumannii, and 14% were carbapenem-resistant P. aeruginosa (Karruli et al., 2021). With carbapenem-resistant bacteria considered critical priority pathogens, there is an urgency to revise available literature about carbapenemases in bacterial co-infections with COVID-19 and to refine findings for better surveillance and control. We present below a summary of studies on carbapenemase-producing Enterobacteriaceae and A. baumannii in COVID-19 patients. Regarding P. aeruginosa, and while this multiresistant pathogen is known to produce carbapenemases (VIM-2, GES-7, VEB-1, IMP-1, KPC-2, PER-1) (Cantón et al., 2020), and despite being among top pathogens co-infecting COVID-19 patients (Ramadan et al., 2020; Senok et al., 2021; Westblade et al., 2021), sometimes associated with severe pyogenic infection (Meguins et al., 2021), literature has not yet extensively reported carbapenemases from this organism in COVID-19 patients. This calls for vigilant investigations to fill such gap of knowledge regarding this significant nosocomial pathogen. In a small cohort of Italian COVID-19 patients on assisted ventilation, carbapenem-resistant P. aeruginosa in bronchial aspirates was the most common agent causing superinfection, and resistance was attributed to OprD porin mutation/deletion rather than production of carbapenemases (Mazzariol et al., 2021).
Studies Involving Enterobacteriaceae
A summary of studies reporting co-infection of COVID-19 patients with carbapenemase-producing Gram-negative pathogens is shown in Table 1. Evidence obtained from studies on carbapenemase-producing Enterobacteriaceae is quite heterogeneous. Although some findings published early in the pandemic on COVID-19 patients didn’t detect significant rise in carbapenemase-producing Enterobacteriaceae compared to previous years (Cuntrò et al., 2021; Pascale et al., 2021), this depiction changed later with rise in these pathogens observed in many regions of the world. For example, colonization/infection with Enterobacteriaceae producing NDM in a cohort of COVID-19 patients in an Italian teaching hospital increased versus other patients, and co-infection significantly increased duration of hospital stay by about 17 days (Porretta et al., 2020). Also, incidence of acquisition of KPC-producing K. pneumoniae in COVID-19 patients from the ICU of another hospital increased from 6.7% in 2019 to 50% in 2020 (Tiri et al., 2020). It is possible that overloading of facilities, presence of many healthcare workers in a high risk area, extended and prolonged contact with patients, presence of untrained personnel, and increased use of empirical antimicrobial therapy contribute to such increase in colonization (Donà et al., 2020; Rawson et al., 2020; Tiri et al., 2020). In some instances, and in the setting of a recent decrease of carbapenemase-producing Enterobacteriaceae in the institution and the region, their rise in COVID-19 patients was notable. This supports concerns regarding emergence of carbapenemases in the wake of the global pandemic (Gomez-Simmonds et al., 2021). In other settings, a depicted change occurred in K. pneumoniae ST and its virulence traits over 5 years since 2015, where 2020 witnessed a dominant shift from ST11 to ST15, and this may be ascribed to COVID-19 (Chen et al., 2021). A review of studies on carbapenem-resistant K. pneumoniae in COVID-19 patients from 6 countries found that majority of infected patients (84%) were males with mean age of 61 years, and predominant carbapenemases were KPC and NDM. Cases described were from Italy (4 studies), China (2 studies), Egypt, United States, Spain, and Peru (one study from each). Different factors contributed to variable prevalence of carbapenemase-producers, ranging between 0.35-53%, with lowest prevalence reported in US and highest in China. It was hypothesized that contaminated protective equipment may be the main cause of cross-transmission, namely use of same protective facemasks to care for different patients, and use of double gloves where outer gloves were changed while inner gloves were disinfected with alcohol. Other factors included employment of additional medical staff unexperienced in infection control, use of broad-spectrum antibiotics in COVID-19 patients, and medical personnel burnout, decreasing commitment to infection prevention and relaxing rules of infection prophylaxis and surveillance (Mędrzycka-Dąbrowska et al., 2021).
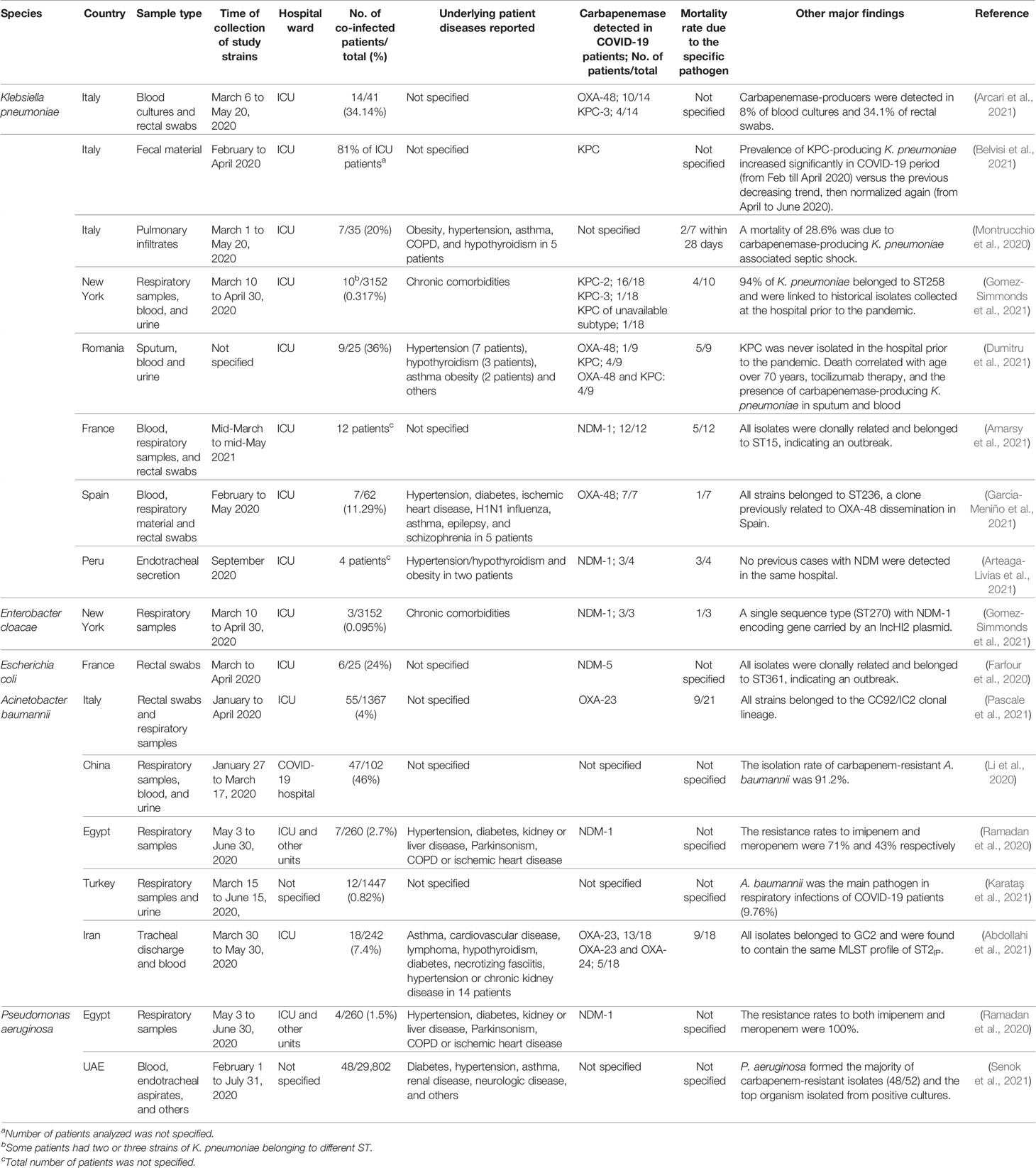
Table 1 Summary of studies describing carbapenemase-producing Gram-negative pathogens reported in COVID-19 patients as of August 2021.
As such, international literature indicates that carbapenemase-producing Enterobacteriaceae may have lingered in the context of COVID-19, and focused studies may be needed to assess changes in their prevalence, molecular genetics, and dissemination. Also, rational antibiotic therapy should be used, as well as continuous assessment of co-infections in patients with COVID-19, to limit outbreaks with carbapenemase-producers that may ascend while managing the pandemic.
Studies Involving A. baumannii
An agent of pneumonia, septicemia, meningitis, urinary tract and wound infections, carbapenemase-producing A. baumannii readily contaminates hospital facilities and healthcare personnel’s hands, survives on dry surfaces, and spreads from asymptomatic colonization. Collectively, these factors make its outbreaks challenging to control (Ayoub Moubareck and Hammoudi Halat, 2020). During surge in COVID-19 admissions in a hospital in New Jersey, 26 patients were co-infected with OXA-23-producing A. baumannii, 4 of which co-harbored NDM, rarely identified in A. baumannii from the US. As COVID-19 hospitalizations decreased, and standard infection control practices resumed, prevalence of carbapenem-resistant A. baumannii returned to pre-COVID-19 baseline of zero to two per month in New Jersey (Perez et al., 2020). Such shift indicates potential for carbapenemase producers to spread during events when typical hospital practices are disorganized, but limiting and decreasing spread are possible too. Similarly, an Italian multicenter before-after cross-sectional study that compared colonization and infection with carbapenem-resistant A. baumannii, detected increase of 7.5 and 5.5 folds respectively, with predominance of OXA-23 (Pascale et al., 2021). In Wuhan, China, among 159 strains of bacteria isolated form 102 COVID-19 patients with secondary bacterial infections, A. baumannii was the most common pathogen (35.8%) with carbapenem resistance rates above 90% (Li et al., 2020). In another COVID-19 report from Egypt, 100% of A. baumannii isolates harbored NDM-1, with 71% and 43% resistance rates to imipenem and meropenem respectively (Ramadan et al., 2020).
Recommendations for Stewardship and Surveillance of Carbapenemase-Producers
Combating surge of carbapenemases during COVID-19, while conforming with general measures of antimicrobial stewardship, warrants additional alertness and investigation. Detection of carbapenemase producers in COVID-19 patients calls for infection control activities both intra- and inter-hospitals, and if necessary re-modulating them according to new organizational structures imposed by the pandemic (Pascale et al., 2021). In areas where carbapenemase producers have declined substantially in recent years, increased detection in patients with COVID-19 may signal a re-emergence, prompting need for increased surveillance and identification of optimal treatments (Gomez-Simmonds et al., 2021). The experience gained during the pandemic should be built upon to improve strict surveillance for carbapenemase producers colonization and co-infection in COVID-19 patients (Arcari et al., 2021). Consensus statements published recently by professional societies (Centers for Disease Control and Prevention (CDC), 2009; Tacconelli et al., 2019) and independent research groups (Chea et al., 2015; Puleston et al., 2020; Yarbrough et al., 2020) focused on detection of carbapenemase producers, which should be reinforced during COVID-19.
The optimal treatment of infections due to carbapenemase-producing organisms is uncertain, and options are quite limited (Rodríguez-Baño et al., 2018). In general, for infections caused by KPC- or OXA-48-producers, ceftazidime-avibactam (van Duin et al., 2018), or a novel beta-lactam-beta-lactamase inhibitor combination, such as meropenem-vaborbactam, imipenem-cilastain-relebactam (Zhanel et al., 2018), or cefiderocol (Zhanel et al., 2019) are suggested. Overall, clinical experience in treating carbapenemase-producing organisms with these agents is limited; most has been with ceftazidime-avibactam (Rodríguez-Baño et al., 2018). For MBL producers, and since these confer resistance to all beta-lactam-type antibiotics except cefiderocol and aztreonam, combining ceftazidime-avibactam and aztreonam can have a synergistic effect, as avibactam can inactivate other beta-lactamases to preserve aztreonam activity. This combination was used to successfully treat patients with extremely resistant MBL-producing pathogens (Marshall et al., 2017; Jayol et al., 2018). Alternative regimens include polymyxin with a second agent like meropenem (Paul et al., 2018), tigecycline or eravacylcine (Karakonstantis et al., 2020). Evidence indicates that these regimens were used in treatment of infections caused by carbapenemase-producers with variable outcomes (Montrucchio et al., 2020; Gomez-Simmonds et al., 2021). In one report, despite ceftazidime/avibactam therapy, five of nine patients infected with K. pneumoniae producing KPC and/or OXA-48 died, contributing to poor prognosis for patients with COVID-19, especially for high-risk populations (Dumitru et al., 2021). Further analysis is warranted to understand outcomes of treatment with these antibiotics in COVID-19 patients upon co-infection with carbapenemase-producers. It is crucial to establish a rigorous program of antibiotic administration and to maintain rational antibiotic use and continuous surveillance.
Concluding Remarks
Carbapenemases contributing to antimicrobial resistance form a collateral and unavoidable consequence of COVID-19 and combating them remains demanding. In the aftermath of the pandemic, calls for global health security measures to limit spread of these enzymes will take on a new urgency, and the more powerful these measures are, the more delineated their outcomes will be on dissemination and thriving of carbapenemases. In pandemic emergencies, like the current one, antimicrobial stewardship shall contribute to better use of resources, limit unnecessary use of antimicrobials, and restrain antibiotic resistance trends including formidable challenges instigated by carbapenemases.
Author Contributions
DHH and CAM designed this minireview. DHH wrote the first draft of the manuscript. CAM was responsible for editing, reviewing, and acquiring the funds. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahi, A., Aliramezani, A., Salehi, M., Norouzi Shadehi, M., Ghourchian, S., Douraghi, M. (2021). Co-Infection of ST2IP Carbapenem-Resistant Acinetobacter Baumannii With SARS-CoV-2 in the Patients Admitted to a Tehran Tertiary Referral Hospital. BMC Infect. Dis. 21, 927. doi: 10.1186/s12879-021-06642-2
Amarsy, R., Jacquier, H., Munier, A.-L., Merimèche, M., Berçot, B., Mégarbane, B. (2021). Outbreak of NDM-1-Producing Klebsiella Pneumoniae in the Intensive Care Unit During the COVID-19 Pandemic: Another Nightmare. Am. J. Infect. Control 49 (10), 1324–1326. doi: 10.1016/j.ajic.2021.07.004
Arcari, G., Raponi, G., Sacco, F., Bibbolino, G., Di Lella, F. M., Alessandri, F., et al. (2021). Klebsiella Pneumoniae Infections in COVID-19 Patients: A 2-Month Retrospective Analysis in an Italian Hospital. Int. J. Antimicrob. Agents 57, 106245. doi: 10.1016/j.ijantimicag.2020.106245
Arteaga-Livias, K., Pinzas-Acosta, K., Perez-Abad, L., Panduro-Correa, V., Rabaan, A. A., Pecho-Silva, S., et al. (2021). A Multidrug-Resistant Klebsiella Pneumoniae Outbreak in a Peruvian Hospital: Another Threat From the COVID-19 Pandemic. Infect. Control Hosp. Epidemiol. 1–2. doi: 10.1017/ice.2020.1401
Ayoub Moubareck, C., Hammoudi Halat, D. (2020). Insights Into Acinetobacter Baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiot (Basel) 9, E119. doi: 10.3390/antibiotics9030119
Ayoub Moubareck, C., Hammoudi Halat, D., Akkawi, C., Nabi, A., AlSharhan, M. A., AlDeesi, Z. O., et al. (2019). Role of Outer Membrane Permeability, Efflux Mechanism, and Carbapenemases in Carbapenem-Nonsusceptible Pseudomonas Aeruginosa From Dubai Hospitals: Results of the First Cross-Sectional Survey. Int. J. Infect. Dis. 84, 143–150. doi: 10.1016/j.ijid.2019.04.027
Bahr, G., González, L. J., Vila, A. J. (2021). Metallo-β-Lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design. Chem. Rev. 121, 7957–8094. doi: 10.1021/acs.chemrev.1c00138
Barbuddhe, S. B., Rawool, D. B., Gaonkar, P. P., Vergis, J., Dhama, K., Malik, S. S. (2020). Global Scenario, Public Health Concerns and Mitigation Strategies to Counter Current Ongoing SARS-CoV-2 / COVID-19 Pandemic. Hum. Vaccin Immunother. 16, 3023–3033. doi: 10.1080/21645515.2020.1810496
Belvisi, V., Del Borgo, C., Vita, S., Redaelli, P., Dolce, P., Pacella, D., et al. (2021). Impact of SARS CoV-2 Pandemic on Carbapenemase-Producing Klebsiella Pneumoniae Prevention and Control Programme: Convergent or Divergent Action? J. Hosp Infect. 109, 29–31. doi: 10.1016/j.jhin.2020.11.030
Benach, J. (2021). We Must Take Advantage of This Pandemic to Make a Radical Social Change: The Coronavirus as a Global Health, Inequality, and Eco-Social Problem. Int. J. Health Serv. 51, 50–54. doi: 10.1177/0020731420946594
Bonomo, R. A., Burd, E. M., Conly, J., Limbago, B. M., Poirel, L., Segre, J. A., et al. (2018). Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 66, 1290–1297. doi: 10.1093/cid/cix893
Botelho, J., Grosso, F., Sousa, C., Peixe, L. (2015). Characterization of a New Genetic Environment Associated With GES-6 Carbapenemase From a Pseudomonas Aeruginosa Isolate Belonging to the High-Risk Clone ST235. J. Antimicrob. Chemother. 70, 615–617. doi: 10.1093/jac/dku391
Brink, A. J. (2019). Epidemiology of Carbapenem-Resistant Gram-Negative Infections Globally. Curr. Opin. Infect. Dis. 32, 609–616. doi: 10.1097/QCO.0000000000000608
Bush, K. (2018). Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 62, e01076-18. doi: 10.1128/AAC.01076-18
Cantón, R., Gijón, D., Ruiz-Garbajosa, P. (2020). Antimicrobial Resistance in ICUs: An Update in the Light of the COVID-19 Pandemic. Curr. Opin. Crit. Care 26, 433–441. doi: 10.1097/MCC.0000000000000755
Centers for Disease Control and Prevention (CDC) (2009). Guidance for Control of Infections With Carbapenem-Resistant or Carbapenemase-Producing Enterobacteriaceae in Acute Care Facilities. MMWR Morb Mortal Wkly Rep. 58, 256–260.
Chea, N., Bulens, S. N., Kongphet-Tran, T., Lynfield, R., Shaw, K. M., Vagnone, P. S., et al. (2015). Improved Phenotype-Based Definition for Identifying Carbapenemase Producers Among Carbapenem-Resistant Enterobacteriaceae. Emerg. Infect. Dis. 21, 1611–1616. doi: 10.3201/eid2109.150198
Chen, J., Hu, C., Wang, R., Li, F., Sun, G., Yang, M., et al. (2021). Shift in the Dominant Sequence Type of Carbapenem-Resistant Klebsiella Pneumoniae Bloodstream Infection From ST11 to ST15 at a Medical Center in Northeast Chin-2020. Infect. Drug Resist. 14, 1855–1863. doi: 10.2147/IDR.S311968
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 395, 507–513. doi: 10.1016/S0140-6736(20)30211-7
Cultrera, R., Barozzi, A., Libanore, M., Marangoni, E., Pora, R., Quarta, B., et al. (2021). Co-Infections in Critically Ill Patients With or Without COVID-19: A Comparison of Clinical Microbial Culture Findings. Int. J. Environ. Res. Public Health 18, 4358. doi: 10.3390/ijerph18084358
Cuntrò, M., Manisco, A., Guarneri, D., Zuglian, G., Vailati, F., Passera, M., et al. (2021). Blood Stream Infections During the First Wave of COVID-19. A Short Microbiological Retrospective Picture at Papa Giovanni XXIII Hospital, Bergamo, Italy. New Microbiol. 44, 51–58.
Dandachi, I., Azar, E., Hamouch, R., Maliha, P., Abdallah, S., Kanaan, E., et al. (2019). Acinetobacter Spp in a Third World Country With Socio-Economic and Immigrants Challenges. J. Infect. Dev. Ctries 13, 948–955. doi: 10.3855/jidc.11341
Despotovic, A., Milosevic, B., Cirkovic, A., Vujovic, A., Cucanic, K., Cucanic, T., et al. (2021). The Impact of COVID-19 on the Profile of Hospital-Acquired Infections in Adult Intensive Care Units. Antibiotics 10, 1146. doi: 10.3390/antibiotics10101146
Dhama, K., Patel, S. K., Kumar, R., Rana, J., Yatoo, M. I., Kumar, A., et al. (2020). Geriatric Population During the COVID-19 Pandemic: Problems, Considerations, Exigencies, and Beyond. Front. Public Health 8, 574198. doi: 10.3389/fpubh.2020.574198
Donà, D., Di Chiara, C., Sharland, M. (2020). Multi-Drug-Resistant Infections in the COVID-19 Era: A Framework for Considering the Potential Impact. J. Hosp Infect. 106, 198–199. doi: 10.1016/j.jhin.2020.05.020
Dortet, L., Poirel, L., Nordmann, P. (2014). Worldwide Dissemination of the NDM-Type Carbapenemases in Gram-Negative Bacteria. BioMed. Res. Int. 2014, 249856. doi: 10.1155/2014/249856
Dumitru, I. M., Dumitrascu, M., Vlad, N. D., Cernat, R. C., Ilie-Serban, C., Hangan, A., et al. (2021). Carbapenem-Resistant Klebsiella Pneumoniae Associated With COVID-19. Antibiot. (Basel) 10, 561. doi: 10.3390/antibiotics10050561
Farfour, E., Lecuru, M., Dortet, L., Le Guen, M., Cerf, C., Karnycheff, F., et al. (2020). Carbapenemase-Producing Enterobacterales Outbreak: Another Dark Side of COVID-19. Am. J. Infect. Control 48, 1533–1536. doi: 10.1016/j.ajic.2020.09.015
Farhat, N., Khan, A. U. (2020). Evolving Trends of New Delhi Metallo-Betalactamse (NDM) Variants: A Threat to Antimicrobial Resistance. Infect. Genet. Evol. 86, 104588. doi: 10.1016/j.meegid.2020.104588
Gambin, M., Sękowski, M., Woźniak-Prus, M., Wnuk, A., Oleksy, T., Cudo, A., et al. (2021). Generalized Anxiety and Depressive Symptoms in Various Age Groups During the COVID-19 Lockdown in Poland. Specific Predictors and Differences in Symptoms Severity. Compr. Psychiatry 105, 152222. doi: 10.1016/j.comppsych.2020.152222
García-Meniño, I., Forcelledo, L., Rosete, Y., García-Prieto, E., Escudero, D., Fernández, J. (2021). Spread of OXA-48-Producing Klebsiella Pneumoniae Among COVID-19-Infected Patients: The Storm After the Storm. J. Infect Public Health 14, 50–52. doi: 10.1016/j.jiph.2020.11.001
Garza-Ramos, U., Barrios, H., Reyna-Flores, F., Tamayo-Legorreta, E., Catalan-Najera, J. C., Morfin-Otero, R., et al. (2015). Widespread of ESBL- and Carbapenemase GES-Type Genes on Carbapenem-Resistant Pseudomonas Aeruginosa Clinical Isolates: A Multicenter Study in Mexican Hospitals. Diagn. Microbiol. Infect. Dis. 81, 135–137. doi: 10.1016/j.diagmicrobio.2014.09.029
Gomez-Simmonds, A., Annavajhala, M. K., McConville, T. H., Dietz, D. E., Shoucri, S. M., Laracy, J. C., et al. (2021). Carbapenemase-Producing Enterobacterales Causing Secondary Infections During the COVID-19 Crisis at a New York City Hospital. J. Antimicrob. Chemother. 76, 380–384. doi: 10.1093/jac/dkaa466
Hammoudi Halat, D., Ayoub Moubareck, C. (2020). The Current Burden of Carbapenemases: Review of Significant Properties and Dissemination Among Gram-Negative Bacteria. Antibiot (Basel) 9. doi: 10.3390/antibiotics9040186
Hammoudi, D., Moubareck, C. A., Sarkis, D. K. (2014). How to Detect Carbapenemase Producers? A Literature Review of Phenotypic and Molecular Methods. J. Microbiol. Methods 107, 106–118. doi: 10.1016/j.mimet.2014.09.009
Hansen, G. T. (2021). Continuous Evolution: Perspective on the Epidemiology of Carbapenemase Resistance Among Enterobacterales and Other Gram-Negative Bacteria. Infect. Dis. Ther. 10, 75–92. doi: 10.1007/s40121-020-00395-2
Hughes, S., Troise, O., Donaldson, H., Mughal, N., Moore, L. S. P. (2020). Bacterial and Fungal Coinfection Among Hospitalized Patients With COVID-19: A Retrospective Cohort Study in a UK Secondary-Care Setting. Clin. Microbiol. Infect. 26, 1395–1399. doi: 10.1016/j.cmi.2020.06.025
Jayol, A., Nordmann, P., Poirel, L., Dubois, V. (2018). Ceftazidime/avibactam Alone or in Combination With Aztreonam Against Colistin-Resistant and Carbapenemase-Producing Klebsiella Pneumoniae. J. Antimicrob. Chemother. 73, 542–544. doi: 10.1093/jac/dkx393
Kaitany, K.-C. J., Klinger, N. V., June, C. M., Ramey, M. E., Bonomo, R. A., Powers, R. A., et al. (2013). Structures of the Class D Carbapenemases OXA-23 and OXA-146: Mechanistic Basis of Activity Against Carbapenems, Extended-Spectrum Cephalosporins, and Aztreonam. Antimicrob. Agents Chemother. 57, 4848–4855. doi: 10.1128/AAC.00762-13
Karakonstantis, S., Kritsotakis, E. I., Gikas, A. (2020). Treatment Options for K. Pneumoniae, P. Aeruginosa and A. Baumannii Co-Resistant to Carbapenems, Aminoglycosides, Polymyxins and Tigecycline: An Approach Based on the Mechanisms of Resistance to Carbapenems. Infection 48, 835–851. doi: 10.1007/s15010-020-01520-6
Karataş, M., Yaşar-Duman, M., Tünger, A., Çilli, F, Aydemir, Ş, Özenci, V (2021). Secondary Bacterial Infections and Antimicrobial Resistance in COVID-19: Comparative Evaluation of Pre-Pandemic and Pandemic-Era, a Retrospective Single Center Study. Ann. Clin. Microbiol. Antimicrob. 20, 51. doi: 10.1186/s12941-021-00454-7
Karruli, A., Boccia, F., Gagliardi, M., Patauner, F., Ursi, M. P., Sommese, P., et al. (2021). Multidrug-Resistant Infections and Outcome of Critically Ill Patients With Coronavirus Disease 2019: A Single Center Experience. Microb. Drug Resist. 27 (9), 1167–1175. doi: 10.1089/mdr.2020.0489
Kousovista, R., Athanasiou, C., Liaskonis, K., Ivopoulou, O., Ismailos, G., Karalis, V. (2021). Correlation Between Acinetobacter Baumannii Resistance and Hospital Use of Meropenem, Cefepime, and Ciprofloxacin: Time Series Analysis and Dynamic Regression Models. Pathogens 10, 480. doi: 10.3390/pathogens10040480
Lafeuille, E., Decré, D., Mahjoub-Messai, F., Bidet, P., Arlet, G., Bingen, E. (2013). OXA-48 Carbapenemase-Producing Klebsiella Pneumoniae Isolated From Libyan Patients. Microb. Drug Resist. 19, 491–497. doi: 10.1089/mdr.2012.0219
Ling, L., So, C., Shum, H. P., Chan, P. K. S., Lai, C. K. C., Kandamby, D. H., et al. (2020). Critically Ill Patients With COVID-19 in Hong Kong: A Multicentre Retrospective Observational Cohort Study. Crit. Care Resusc 22, 119–125. doi: 10.51893/2020.2.oa1
Livermore, D. M. (2021). Antibiotic Resistance During and Beyond COVID-19. JAC Antimicrob. Resist. 3, i5–i16. doi: 10.1093/jacamr/dlab052
Li, J., Wang, J., Yang, Y., Cai, P., Cao, J., Cai, X., et al. (2020). Etiology and Antimicrobial Resistance of Secondary Bacterial Infections in Patients Hospitalized With COVID-19 in Wuhan, China: A Retrospective Analysis. Antimicrob. Resist. Infect. Control 9, 153. doi: 10.1186/s13756-020-00819-1
Logan, L. K., Weinstein, R. A. (2017). The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 215, S28–S36. doi: 10.1093/infdis/jiw282
Mędrzycka-Dąbrowska, W., Lange, S., Zorena, K., Dąbrowski, S., Ozga, D., Tomaszek, L. (2021). Carbapenem-Resistant Klebsiella Pneumoniae Infections in ICU COVID-19 Patients-A Scoping Review. J. Clin. Med. 10, 2067. doi: 10.3390/jcm10102067
Magson, N. R., Freeman, J. Y. A., Rapee, R. M., Richardson, C. E., Oar, E. L., Fardouly, J. (2021). Risk and Protective Factors for Prospective Changes in Adolescent Mental Health During the COVID-19 Pandemic. J. Youth Adolesc. 50, 44–57. doi: 10.1007/s10964-020-01332-9
Marshall, S., Hujer, A. M., Rojas, L. J., Papp-Wallace, K. M., Humphries, R. M., Spellberg, B., et al. (2017). Can Ceftazidime-Avibactam and Aztreonam Overcome β-Lactam Resistance Conferred by Metallo-β-Lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 61, e02243–e02216. doi: 10.1128/AAC.02243-16
Martin, E., Philbin, M., Hughes, G., Bergin, C., Talento, A. F. (2021). Antimicrobial Stewardship Challenges and Innovative Initiatives in the Acute Hospital Setting During the COVID-19 Pandemic. J. Antimicrob. Chemother. 76, 272–275. doi: 10.1093/jac/dkaa400
Mazzariol, A., Benini, A., Unali, I., Nocini, R., Smania, M., Bertoncelli, A., et al. (2021). Dynamics of SARS-CoV2 Infection and Multi-Drug Resistant Bacteria Superinfection in Patients With Assisted Mechanical Ventilation. Front. Cell. Infect. Microbiol. 11, 683409. doi: 10.3389/fcimb.2021.683409
Meguins, L. C., Rocha, A. S., Laurenti, M. R., de Morais, D. F. (2021). Ventricular Empyema Associated With Severe Pyogenic Meningitis in COVID-19 Adult Patient: Case Report. Surg. Neurol. Int. 12, 346. doi: 10.25259/SNI_514_2021
Monnet, D. L., Harbarth, S. (2020). Will Coronavirus Disease (COVID-19) Have an Impact on Antimicrobial Resistance? Euro Surveill 25. doi: 10.2807/1560-7917.ES.2020.25.45.2001886
Montrucchio, G., Corcione, S., Sales, G., Curtoni, A., De Rosa, F. G., Brazzi, L. (2020). Carbapenem-Resistant Klebsiella Pneumoniae in ICU-Admitted COVID-19 Patients: Keep an Eye on the Ball. J. Glob Antimicrob. Resist. 23, 398–400. doi: 10.1016/j.jgar.2020.11.004
Naas, T., Dortet, L., Iorga, B. I. (2016). Structural and Functional Aspects of Class A Carbapenemases. Curr. Drug Targets 17, 1006–1028. doi: 10.2174/1389450117666160310144501
Naas, T., Vandel, L., Sougakoff, W., Livermore, D. M., Nordmann, P. (1994). Cloning and Sequence Analysis of the Gene for a Carbapenem-Hydrolyzing Class A Beta-Lactamase, Sme-1, From Serratia Marcescens S6. Antimicrob. Agents Chemother. 38, 1262–1270. doi: 10.1128/AAC.38.6.1262
Nordmann, P., Mariotte, S., Naas, T., Labia, R., Nicolas, M. H. (1993). Biochemical Properties of a Carbapenem-Hydrolyzing Beta-Lactamase From Enterobacter Cloacae and Cloning of the Gene Into Escherichia Coli. Antimicrob. Agents Chemother. 37, 939–946. doi: 10.1128/AAC.37.5.939
Nori, P., Cowman, K., Chen, V., Bartash, R., Szymczak, W., Madaline, T., et al. (2021). Bacterial and Fungal Coinfections in COVID-19 Patients Hospitalized During the New York City Pandemic Surge. Infect. Control Hosp. Epidemiol. 42, 84–88. doi: 10.1017/ice.2020.368
Orso, D., Federici, N., Copetti, R., Vetrugno, L., Bove, T. (2020). Infodemic and the Spread of Fake News in the COVID-19-Era. Eur. J. Emerg. Med. 27 (5), 327–328. doi: 10.1097/MEJ.0000000000000713
Pascale, R., Bussini, L., Gaibani, P., Bovo, F., Fornaro, G., Lombardo, D., et al. (2021). Carbapenem Resistant Bacteria in Intensive Care Unit During COVID-19 Pandemic: Multicenter Before-After Cross Sectional Study. Infect. Control Hosp Epidemiol., 1–25. doi: 10.1017/ice.2021.144
Paul, M., Daikos, G. L., Durante-Mangoni, E., Yahav, D., Carmeli, Y., Benattar, Y. D., et al. (2018). Colistin Alone Versus Colistin Plus Meropenem for Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria: An Open-Label, Randomised Controlled Trial. Lancet Infect. Dis. 18, 391–400. doi: 10.1016/S1473-3099(18)30099-9
Perez, S., Innes, G. K., Walters, M. S., Mehr, J., Arias, J., Greeley, R., et al. (2020). Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter Baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions - New Jersey, February-July 2020. MMWR Morb. Mortal Wkly. Rep. 69, 1827–1831. doi: 10.15585/mmwr.mm6948e1
Pintado, V., Ruiz-Garbajosa, P., Escudero-Sanchez, R., Gioia, F., Herrera, S., Vizcarra, P., et al. (2022). Carbapenemase-Producing Enterobacterales Infections in COVID-19 Patients. Infect. Dis. (Lond) 54 (1), 1–10. doi: 10.1080/23744235.2021.1963471
Pitout, J. D. D., Peirano, G., Kock, M. M., Strydom, K.-A., Matsumura, Y. (2019). The Global Ascendency of OXA-48-Type Carbapenemases. Clin. Microbiol. Rev. 33, e00102-19. doi: 10.1128/CMR.00102-19
Poirel, L., Héritier, C., Tolün, V., Nordmann, P. (2004). Emergence of Oxacillinase-Mediated Resistance to Imipenem in Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 48, 15–22. doi: 10.1128/AAC.48.1.15-22.2004
Poirel, L., Potron, A., Nordmann, P. (2012). OXA-48-Like Carbapenemases: The Phantom Menace. J. Antimicrob. Chemother. 67, 1597–1606. doi: 10.1093/jac/dks121
Polly, M., Almeida, B. L., de Lennon, R. P., Cortês, M. F., Costa, S. F., Guimarães, T. (2021). Impact of the COVID-19 Pandemic on the Incidence of Multidrug-Resistant Bacterial Infections in an Acute Care Hospital in Brazil. Am. J. Infect Control 50 (1), 32–38. doi: 10.1016/j.ajic.2021.09.018
Porretta, A. D., Baggiani, A., Arzilli, G., Casigliani, V., Mariotti, T., Mariottini, F., et al. (2020). Increased Risk of Acquisition of New Delhi Metallo-Beta-Lactamase-Producing Carbapenem-Resistant Enterobacterales (NDM-CRE) Among a Cohort of COVID-19 Patients in a Teaching Hospital in Tuscany, Italy. Pathogens 9, E635. doi: 10.3390/pathogens9080635
Potter, R. F., D’Souza, A. W., Dantas, G. (2016). The Rapid Spread of Carbapenem-Resistant Enterobacteriaceae. Drug Resist. Update 29, 30–46. doi: 10.1016/j.drup.2016.09.002
Puleston, R., Brown, C. S., Patel, B., Fry, C., Singleton, S., Robotham, J. V., et al. (2020). Recommendations for Detection and Rapid Management of Carbapenemase-Producing Enterobacterales Outbreaks. Infect. Prev. Pract. 2, 100086. doi: 10.1016/j.infpip.2020.100086
Rafei, R., Dabboussi, F., Hamze, M., Eveillard, M., Lemarié, C., Mallat, H., et al. (2014). First Report of blaNDM-1-Producing Acinetobacter Baumannii Isolated in Lebanon From Civilians Wounded During the Syrian War. Int. J. Infect. Dis. 21, 21–23. doi: 10.1016/j.ijid.2014.01.004
Ramadan, H. K.-A., Mahmoud, M. A., Aburahma, M. Z., Elkhawaga, A. A., El-Mokhtar, M. A., Sayed, I. M., et al. (2020). Predictors of Severity and Co-Infection Resistance Profile in COVID-19 Patients: First Report From Upper Egypt. Infect. Drug Resist. 13, 3409–3422. doi: 10.2147/IDR.S272605
Rawson, T. M., Moore, L. S. P., Castro-Sanchez, E., Charani, E., Davies, F., Satta, G., et al. (2020). COVID-19 and the Potential Long-Term Impact on Antimicrobial Resistance. J. Antimicrob. Chemother. 75, 1681–1684. doi: 10.1093/jac/dkaa194
Rodríguez-Baño, J., Gutiérrez-Gutiérrez, B., Machuca, I., Pascual, A. (2018). Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 31, e00079–e00017. doi: 10.1128/CMR.00079-17
Rusic, D., Vilovic, M., Bukic, J., Leskur, D., Seselja Perisin, A., Kumric, M., et al. (2021). Implications of COVID-19 Pandemic on the Emergence of Antimicrobial Resistance: Adjusting the Response to Future Outbreaks. Life (Basel) 11, 220. doi: 10.3390/life11030220
Russell, C. D., Fairfield, C. J., Drake, T. M., Turtle, L., Seaton, R. A., Wootton, D. G., et al. (2021). Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalised With COVID-19 During the First Pandemic Wave From the ISARIC WHO CCP-UK Study: A Multicentre, Prospective Cohort Study. Lancet Microbe 2, e354–e365. doi: 10.1016/S2666-5247(21)00090-2
Sang, L., Xi, Y., Lin, Z., Pan, Y., Song, B., Li, C.-A., et al. (2021). Secondary Infection in Severe and Critical COVID-19 Patients in China: A Multicenter Retrospective Study. Ann. Palliat Med., apm–21-833. doi: 10.21037/apm-21-833
Sawa, T., Kooguchi, K., Moriyama, K. (2020). Molecular Diversity of Extended-Spectrum β-Lactamases and Carbapenemases, and Antimicrobial Resistance. J. Intensive Care 8, 13. doi: 10.1186/s40560-020-0429-6
Scaife, W., Young, H. K., Paton, R. H., Amyes, S. G. (1995). Transferable Imipenem-Resistance in Acinetobacter Species From a Clinical Source. J. Antimicrob. Chemother. 36, 585–586. doi: 10.1093/jac/36.3.585
Sellera, F. P., Da Silva, L. C. B. A., Lincopan, N. (2021). Rapid Spread of Critical Priority Carbapenemase-Producing Pathogens in Companion Animals: A One Health Challenge for a Post-Pandemic World. J. Antimicrob. Chemother. dkab169. doi: 10.1093/jac/dkab169
Senok, A., Alfaresi, M., Khansaheb, H., Nassar, R., Hachim, M., Al Suwaidi, H., et al. (2021). Coinfections in Patients Hospitalized With COVID-19: A Descriptive Study From the United Arab Emirates. Infect. Drug Resist. 14, 2289–2296. doi: 10.2147/IDR.S314029
Stewart, N. K., Smith, C. A., Frase, H., Black, D. J., Vakulenko, S. B. (2015). Kinetic and Structural Requirements for Carbapenemase Activity in GES-Type β-Lactamases. Biochemistry 54, 588–597. doi: 10.1021/bi501052t
Suay-García, B., Pérez-Gracia, M. T. (2019). Present and Future of Carbapenem-Resistant Enterobacteriaceae (CRE) Infections. Antibiot. (Basel) 8, E122. doi: 10.3390/antibiotics8030122
Tacconelli, E., Carrara, E., Savoldi, A., Harbarth, S., Mendelson, M., Monnet, D. L., et al. (2018). Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 18, 318–327. doi: 10.1016/S1473-3099(17)30753-3
Tacconelli, E., Mazzaferri, F., de Smet, A. M., Bragantini, D., Eggimann, P., Huttner, B. D., et al. (2019). ESCMID-EUCIC Clinical Guidelines on Decolonization of Multidrug-Resistant Gram-Negative Bacteria Carriers. Clin. Microbiol. Infect. 25, 807–817. doi: 10.1016/j.cmi.2019.01.005
Tiri, B., Sensi, E., Marsiliani, V., Cantarini, M., Priante, G., Vernelli, C., et al. (2020). Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 9, E2744. doi: 10.3390/jcm9092744
Turton, J. F., Ward, M. E., Woodford, N., Kaufmann, M. E., Pike, R., Livermore, D. M., et al. (2006). The Role of ISAba1 in Expression of OXA Carbapenemase Genes in Acinetobacter Baumannii. FEMS Microbiol. Lett. 258, 72–77. doi: 10.1111/j.1574-6968.2006.00195.x
Usman Qamar, M., Lopes, S., Hassan, B., Khurshid, M., Shafique, M., Atif Nisar, M., et al. (2020). The Present Danger of New Delhi Metallo-β-Lactamase: A Threat to Public Health. Future Microbiol. 15, 1759–1778. doi: 10.2217/fmb-2020-0069
van Duin, D., Lok, J. J., Earley, M., Cober, E., Richter, S. S., Perez, F., et al. (2018). Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 66, 163–171. doi: 10.1093/cid/cix783
Wang, C., Horby, P. W., Hayden, F. G., Gao, G. F. (2020). A Novel Coronavirus Outbreak of Global Health Concern. Lancet 395, 470–473. doi: 10.1016/S0140-6736(20)30185-9
Westblade, L. F., Simon, M. S., Satlin, M. J. (2021). Bacterial Coinfections in Coronavirus Disease 2019. Trends Microbiol., S0966-842X(21)00094–9. doi: 10.1016/j.tim.2021.03.018
World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available at: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (Accessed July 29, 2021).
Wu, Y., Ho, W., Huang, Y., Jin, D.-Y., Li, S., Liu, S.-L., et al. (2020). SARS-CoV-2 Is an Appropriate Name for the New Coronavirus. Lancet 395, 949–950. doi: 10.1016/S0140-6736(20)30557-2
Yamasaki, K., Komatsu, M., Ono, T., Nishio, H., Sueyoshi, N., Kida, K., et al. (2017). Nosocomial Spread of Klebsiella Pneumoniae Isolates Producing blaGES-4 Carbapenemase at a Japanese Hospital. J. Infect. Chemother. 23, 40–44. doi: 10.1016/j.jiac.2016.09.006
Yang, Y. J., Wu, P. J., Livermore, D. M. (1990). Biochemical Characterization of a Beta-Lactamase That Hydrolyzes Penems and Carbapenems From Two Serratia Marcescens Isolates. Antimicrob. Agents Chemother. 34, 755–758. doi: 10.1128/AAC.34.5.755
Yarbrough, M. L., Wallace, M. A., Potter, R. F., D’Souza, A. W., Dantas, G., Burnham, C.-A. D. (2020). Breakpoint Beware: Reliance on Historical Breakpoints for Enterobacteriaceae Leads to Discrepancies in Interpretation of Susceptibility Testing for Carbapenems and Cephalosporins and Gaps in Detection of Carbapenem-Resistant Organisms. Eur. J. Clin. Microbiol. Infect. Dis. 39, 187–195. doi: 10.1007/s10096-019-03711-y
Yong, D., Toleman, M. A., Giske, C. G., Cho, H. S., Sundman, K., Lee, K., et al. (2009). Characterization of a New Metallo-Beta-Lactamase Gene, Bla(NDM-1), and a Novel Erythromycin Esterase Gene Carried on a Unique Genetic Structure in Klebsiella Pneumoniae Sequence Type 14 From India. Antimicrob. Agents Chemother. 53, 5046–5054. doi: 10.1128/AAC.00774-09
Zavala-Flores, E., Salcedo-Matienzo, J. (2020). Medicación Prehospitalaria En Pacientes Hospitalizados Por COVID-19 En Un Hospital Público De Lima-Perú. Acta Med. PERUANA 37. doi: 10.35663/amp.2020.373.1277
Zemrani, B., Gehri, M., Masserey, E., Knob, C., Pellaton, R. (2021). A Hidden Side of the COVID-19 Pandemic in Children: The Double Burden of Undernutrition and Overnutrition. Int. J. Equity Health 20, 44. doi: 10.1186/s12939-021-01390-w
Zhanel, G. G., Golden, A. R., Zelenitsky, S., Wiebe, K., Lawrence, C. K., Adam, H. J., et al. (2019). Cefiderocol: A Siderophore Cephalosporin With Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs 79, 271–289. doi: 10.1007/s40265-019-1055-2
Zhanel, G. G., Lawrence, C. K., Adam, H., Schweizer, F., Zelenitsky, S., Zhanel, M., et al. (2018). Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations. Drugs 78, 65–98. doi: 10.1007/s40265-017-0851-9
Keywords: carbapenemases, antimicrobial resistance, COVID-19, NDM, KPC
Citation: Ayoub Moubareck C and Hammoudi Halat D (2022) The Collateral Effects of COVID-19 Pandemic on the Status of Carbapenemase-Producing Pathogens. Front. Cell. Infect. Microbiol. 12:823626. doi: 10.3389/fcimb.2022.823626
Received: 27 November 2021; Accepted: 27 January 2022;
Published: 17 March 2022.
Edited by:
Sophia Vourli, University General Hospital Attikon, GreeceReviewed by:
Saoussen Oueslati, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2022 Ayoub Moubareck and Hammoudi Halat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carole Ayoub Moubareck, Q2Fyb2xlLkF5b3ViTW91YmFyZWNrQHp1LmFjLmFl
 Carole Ayoub Moubareck
Carole Ayoub Moubareck Dalal Hammoudi Halat
Dalal Hammoudi Halat