- 1Department of Zoology, Abdul Wali Khan University Mardan, Mardan, Pakistan
- 2King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia
- 3Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 4College of Sciences and Literature Microbiology, Nothern Border University, Rafha, Saudi Arabia
- 5College of Applied Medical Sciences, Shaqra University, Shaqra, Saudi Arabia
- 6Centro de Biotecnologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
- 7Laboratory of Infectious Diseases, Joint Faculty of Veterinary Medicine, Kagoshima University, Kagoshima, Japan
Tick sialome is comprised of a rich cocktail of bioactive molecules that function as a tool to disarm host immunity, assist blood-feeding, and play a vibrant role in pathogen transmission. The adaptation of the tick’s blood-feeding behavior has lead to the evolution of bioactive molecules in its saliva to assist them to overwhelm hosts’ defense mechanisms. During a blood meal, a tick secretes different salivary molecules including vasodilators, platelet aggregation inhibitors, anticoagulants, anti-inflammatory proteins, and inhibitors of complement activation; the salivary repertoire changes to meet various needs such as tick attachment, feeding, and modulation or impairment of the local dynamic and vigorous host responses. For instance, the tick’s salivary immunomodulatory and cement proteins facilitate the tick’s attachment to the host to enhance prolonged blood-feeding and to modulate the host’s innate and adaptive immune responses. Recent advances implemented in the field of “omics” have substantially assisted our understanding of host immune modulation and immune inhibition against the molecular dynamics of tick salivary molecules in a crosstalk between the tick–host interface. A deep understanding of the tick salivary molecules, their substantial roles in multifactorial immunological cascades, variations in secretion, and host immune responses against these molecules is necessary to control these parasites. In this article, we reviewed updated knowledge about the molecular mechanisms underlying host responses to diverse elements in tick saliva throughout tick invasion, as well as host defense strategies. In conclusion, understanding the mechanisms involved in the complex interactions between the tick salivary components and host responses is essential to decipher the host defense mechanisms against the tick evasion strategies at tick-host interface which is promising in the development of effective anti-tick vaccines and drug therapeutics.
Introduction
The natural persistence and vectorial capabilities of ticks have been assisted by the evolution of sophisticated blood-feeding mechanisms and remarkable salivary molecules that come to play in the invasion of the vertebrate host immune responses (Wikel, 1982; Ribeiro, 1995; Wikel, 1996). Ixodid ticks are particularly different from Argasidae in the duration of their attachment to the host; they feed for several days depending on the life stage, as compared to soft ticks, which repeatedly feed for short periods of time ranging from minutes to hours (Oliver, 1989; Sonenshine, 1991). Due to the prolonged feeding, hard ticks have developed myriad strategies; they create a feeding cavity by piercing the host skin through the chelicerae that induce a blood pool in the dermis, where ticks inoculate saliva to facilitate blood-feeding, and remain attached to the host (Francischetti et al., 2009; Šimo et al., 2017; Boulanger and Wikel, 2021; Pham et al., 2021; Tirloni et al., 2021; Ali et al., 2022). These pharmacologically active molecules maintain the blood-feeding cavity and suppress the host defense mechanisms at the bite site (Nuttall and Labuda, 2004; Francischetti et al., 2009; Ali et al., 2015a; Martins et al., 2020). Consequently, essential tick salivary molecules are secreted into the bite site to deploy multiple proteolytic pathways that influence the host hemostatic and immunological responses against tick bite (Xu et al., 2016; Kotál et al., 2019; Zhou et al., 2020). These molecules possess a remarkable binding affinity toward host cytokines, eicosanoids, chemokines, growth factors, and other biological molecules (Andrade et al., 2005; Francischetti et al., 2009; Kazimírová and Stibraniova, 2013); inhibit the migration of neutrophils, leukocytes, and macrophages (MPs), and inactivate dendritic cells (DCs), mast cells (MCs), lymphocytes, eosinophils, keratinocytes, and endothelial cells (Kotsyfakis et al., 2006; Sá-Nunes et al., 2007; Bullard et al., 2019; Coutinho et al., 2020). As a result, the healing of the host’s wound is delayed by degrading pain-inducing molecular signals, host hemostasis, blood clotting, and immune responses (innate and adaptive) are disrupted at the site of the tick bite (Radulović et al., 2014). Thereafter, the tick can initiate a blood meal.
During a blood meal, ticks constantly secrete salivary molecules into the blood-feeding cavity to modulate the rigorous actions of the recruited host immune cells to avoid rejection by the host (Wikel, 1982; Wikel, 1985; Ribeiro, 1987; Kaufman, 1989; Ribeiro, 1989; Ramachandra and Wikel, 1992). Tick saliva has been recognized as being comprised of a large array of molecules, exerting potent immunosuppression by targeting multiple elements in the immune system of their respective hosts (Ribeiro, 1995). Several reviews have documented the specific roles of individual tick salivary molecules in the induction of host immunomodulation and/or inhibition, vasodilation, coagulation, platelet aggregation, and inflammatory responses (Šimo et al., 2017; Parizi et al., 2018; Bullard et al., 2019; Mans, 2020). However, a major debate remained undiscussed regarding the molecular mechanisms involved in the interaction of the tick–host interface. This review focuses on updated knowledge about the molecular mechanisms of various constituents of the host immune system and their associated proteolytic activities triggered by tick sialome. The growing knowledge of the molecular mechanisms has a critical role in the understanding of tick saliva in assisting tick feeding (Hovius et al., 2008a; Kotál et al., 2015; Šimo et al., 2017; Nuttall, 2019).
Tick–Host Interface: Host Immune Components
The skin of the vertebrate host comprises a complex network of cellular interactions and exponentially relies on innate and adaptive immunity (Wikel, 2018; Boulanger and Wikel, 2021). In the early stage of tick feeding, immunosuppressive compounds are released in the tick saliva that encourages the formation of an effective feeding lesion (Ribeiro, 1987; Ribeiro, 1989; Nuttall et al., 2006; Francischetti et al., 2009). Ticks acquire blood meal by inserting their specialized mouthparts through the host skin. Fast feeding argasid deeply penetrates mouthparts to the host skin and feeds rapidly as compared to slow feeding ixodid ticks that can penetrate their mouthparts to the host skin superficially or deeply depending on tick species (Sonenshine, 1991). The inserted chelicerae extend and lacerate the epidermis, which is then followed by the insertion of the hypostome into the dermis (Richter et al., 2013). The tick-derived salivary complex cement cone proteins are secreted to facilitate tick attachment; they play a vital part in blood-sucking, sealing the feeding lesion, and immunomodulation thus enabling strong attachment mainly in hard ticks (Hollmann et al., 2018; Suppan et al., 2018). Cement cone proteins are of two types: a primary “core” (secreted early, within half an hour after attachment) and a secondary “cortex” cement (secreted for various days, having a graduated toughening procedure) (Bullard, 2016). Cement cone proteins fill any gap between the inserted mouthparts and non-intact host skin by using the polymerization of glycine-rich proteins secreted during early attachment (Bullard et al., 2019; Villar et al., 2020). Sealing improves blood-feeding and prevents fluid loss (Suppan et al., 2018). The secretion of cement cone proteins is copious in metastriate ticks (Dermacentor or Rhipicephalus genera) with short mouthparts as compared to prostriate ticks (genus Ixodes) with larger mouthparts (Ribeiro and Mans, 2020; Tahir et al., 2020). Successful attachment enables tick to conceal the scaffold of a combination of proteins, peptides, and non-peptide molecules that subsequently leads to the modulation of hemostasis, inflammation, wound healing, and the innate and adaptive immune responses of the host (Wikel et al., 1994; Reck et al., 2009; Wikel, 2018). During this course, ticks slowly start blood-feeding and ingest a small quantity during 4 to 5 days followed by a fast intake of blood meal in about 24 hours (Akov, 1982). Except for some Ixodes species, several tick species complete the final stage of their life cycle by mating on their host and subsequently take a large amount of blood in approximately 7 to 14 days before detachment and drop-off from the host (Kaufman, 2007; Anderson and Magnarelli, 2008).
A rapid cascade of events happens prior to and during tick blood-feeding (Tirloni et al., 2017). During this course, the salivary glands (SGs) yield and secrete a plethora of molecules in non-uniform time-dependent patterns (Kim et al., 2020), either to target-specific or a pleiotropic host’s molecules at the bite site, which initiates a set of mechanisms for disrupting host immune responses (Ren et al., 2019). This results in interference with several complement mediators—including complement cascade components, eicosanoids, chemokines, cytokines, growth factors, cell-signaling molecules, and antibodies (Aounallah et al., 2020) —proteolytic pathways, in particular pro-coagulants (thrombin, coagulation factors), pro-inflammatory enzymes (cathepsins S, C, B, L, and G, chymase, kallikrein, neutrophil elastase, proteinase 3, and tryptase), and complement pathway enzymes (component 2 and factors B, C, and D) (Tirloni et al., 2014; Kim et al., 2015a; Jmel et al., 2021). Meanwhile in this molecular tick–host crosstalk at the tick bite site, the dynamic responses of host hemostasis (first-line host innate immune response comprising platelet aggregation, coagulation, and vasoconstriction) are initiated against tick salivary molecules (Kim et al., 2020). This ultimately influences the composition and secretion of tick salivary components to meet the diverse requirements of tick attachment and moderate feeding, and to counter the dynamic responses generated by the host (Nuttall, 2019; Bonnet and Pollet, 2021; Boulanger and Wikel, 2021). Enhanced innate immune responses of the host against the rigorous activities of tick salivary molecules also activate the host inflammatory responses and complement cascade to counter block the tick salivary molecules (Preston et al., 2013; Kotál et al., 2015). Due to prolonged blood-intake and repetitive exposure of the host to ticks, the acquired immunity activates B lymphocytes and T lymphocytes (Olds et al., 2016; Šimo et al., 2017; Tabor et al., 2017). Ultimately, the tick–host interface leads to an arms race mechanism, where ticks try to evade the host skin and diminish the provoked immune system. In turn, the host develops early defense mechanisms against ticks (Kotál et al., 2015). The prolonged attachment and blood-feeding mechanisms of ticks are strengthened by drastic changes in the differential expression of tick salivary proteins in the repertoire of secreted saliva (Fontaine et al., 2011). During this endeavor, the host immune system takes time to recognize and counter the early secreted tick saliva–derived proteins and dynamics in its composition as a result of the hijacking of the host’s immune system (Tirloni et al., 2014; Mans, 2019). During blood-feeding, several proteins in the salivary repertoire are differentially expressed and enhances the hemoglobin digestion, heme transportation, blood coagulation, fibrinolysis, detoxification, and oxidative stress (Dickinson and Forman, 2002; Horn et al., 2009; Lara et al., 2015). Fundamentally understanding the differential expression provides significant incentives for determining the affinity and concentrations of tick salivary molecules against host components throughout the feeding period, which may be potentially useful for exploring tick-induced immunomodulation and the host’s enhanced defense mechanisms. Glycine-rich proteins present in the cement components are essential at tick feeding sites, since the RNAi-based gene knockdown induces reddening and bleeding around the mouthparts, interfering with the blood meal (Bullard et al., 2019). However, host-induced differences in the secretion of tick cement components during feeding among different tick species are mostly unexplored (Maruyama et al., 2010).
Tick-Derived Salivary Inhibitors Modulating Host Hemostasis
The fate of the host’s innate immunity has been observed to be differentially modulated or inhibited by a set of tick salivary protease inhibitors (PIs) (Martins et al., 2020). A particularly notable role of PIs has been detected in tick–host crosstalk, as the host defense mechanisms are highly regulated by specific endogenous inhibitors subsequently inhibited by tick-secreted PIs (Martins et al., 2020; Jmel et al., 2021). The salivary PIs of several ticks have been characterized and found to be differentially expressed throughout the blood-feeding process, inducing local immunosuppression by inhibiting platelet aggregation or blocking cascade elements of intrinsic, extrinsic, and common pathways of blood coagulation, tumor development, and angiogenic factors (Chmelař et al., 2017; Costa et al., 2021). The host hemostatic mechanisms are not directly hindered by the tick salivary molecules; instead, the active sites, exocites, and receptors of regulatory factors or components involved in driving mechanisms such as thrombin, plasmin, factor V (fV), factor Xa (fXa), kallikrein, and kallikrein-associated fXIIa–fXIa and fXa-TF-VIIa components are inhibited. As a result, the primary enzymatic activities and complement cascades of the host defense mechanisms are largely disrupted (Chlastáková et al., 2021).
Tick Salivary Molecules Inhibiting Host Coagulation Pathways
Tick salivary PIs are known to perform a vital role in coagulation, hemostatic inhibition, and platelet plugging (Šimo et al., 2017; Martins et al., 2020). Tick anticoagulant proteins (TAP; Kunitz-type family), including Om44 and TAI proteins derived from Ornithodoros moubata, were the first protease inhibitors discovered to specifically inhibit fXa (Waxman et al., 1990; Waxman and Connolly, 1993). TAI also blocks platelets’ adhesion to collagen (Karczewski et al., 1995), and Om44 blocks the P-selectin/PSGL-1 interaction, presumably preventing the leucocytes and platelets from adhering to vessel walls, which allows ticks to finish their blood sucking (Díaz-Martín et al., 2015). A TAP-like protein was also recognized in O. savignyi sialome that inhibited fXa and bound both exocites and active site of thrombin (Joubert et al., 1998). In vitro analysis of the recombinant tick anticoagulant peptide derived from O. moubata reduces TF/fVIIa-dependent thrombus formation (Ørvim et al., 1995). Ornithodorin of O. moubata is a highly effective and particularly selective thrombin inhibitor that specifically binds thrombin’s active site (N terminus) and exosite I (C-terminal helix) (Van de Locht et al., 1996). In addition, TAP derived from O. moubata interfere its tripeptide-containing amino terminal regions (Tyr-Asn-Arg) with both exocites and active site of thrombin. However, AsKunitz inhibition of thrombin’s activity might be due to the interaction of 2 cysteine components in the carboxy terminal region with exosite I only (Costa et al., 2021). The Amblyomma variegatum saliva protein, variegin, displays its C-terminal tail and directly binds thrombin’s active site to exosite I (Koh et al., 2007). A. variegatum-derived avathrin (homologous to variegin) is comprised of 32 residues and a competitive inhibitor that interacts with the thrombin active site and exosite I (Iyer and Goodman, 2019). Rhipicephalus (Boophilus) microplus-derived BmAP, microphilin, and boophilin (Horn et al., 2000; Ciprandi et al., 2006) can inhibit the host thrombin by binding the active site to exosite I. Additionally, boophilin also inhibits the activities of plasmin and trypsin (Macedo-Ribeiro et al., 2008). A fraction AV 16/3 of the salivary gland extracted from A. variegatum showed platelet aggregation, blood coagulation, and antithrombin activity in human platelets (Kazimirova et al., 2002). Salivary madanin 1 and 2 derived from Haemaphysalis longicornis bind anion-binding exosite 1 of the host thrombin, which inhibits the transformation of fibrinogen to fibrin by thrombin, fV and fVIII activation, and platelet aggregation without affecting its amidolytic activity (Iwanaga et al., 2003). Haemathrin 1 and 2 in the salivary gland of Haemaphysalis bispinosa specifically inhibit thrombin by cleaving its C-terminal end (Brahma et al., 2017). A. sculptum-derived amblyomin-X and Ixodes scapularis-derived Ixolaris, ixonnexin, and penthalaris salivary molecules have been proven to be inhibitors of fXa activation by inhibiting tissue factor fVIIa-Xa cascade coagulation (Francischetti et al., 2002; Francischetti et al., 2004; Chudzinski-Tavassi et al., 2010; Carneiro-Lobo et al., 2012; De Oliveira et al., 2012). The calcium-binding protein, longistatin, derived from Hae. longicornis that acts as an anticoagulant and plasminogen activator, hydrolyzes fibrinogen and delays the formation of fibrin clots (Anisuzzaman et al., 2010; Anisuzzaman et al., 2011). It also binds receptors for advanced glycation end products, mediating the stimulation of host immune cells, as a result, tempering the inflammatory and immunological responses of the host initiated due to tick bite (Anisuzzaman et al., 2014). The Hae. longicornis also secretes chimadanin in saliva that binds the active site of thrombin, inhibiting blood coagulation (Nakajima et al., 2005). Haemaphysalin, which binds to fXIIa and kallikrein BmTI-A, performs a crucial role in the inhibition of blood coagulation (Kato et al., 2005). A. sculptum secretes As8.9kDa, AsBasicTail, and AsKunitz; the former two prevent host trypsin and thrombin while inhibiting enzymatic activity derived from fXa, whereas AsKunitz inhibits only thrombin (Costa et al., 2021; Van Oosterwijk and Wikel, 2021). IxscS-1E1I of I. scapularis specifically inhibits thrombin, trypsin, cathepsin G, and fXa. It also inhibits adenosine diphosphate, thrombin’s induced platelet aggregation, and delayed the duration of plasma clotting (Ibelli et al., 2014). Other I. scapularis salivary proteins—such as penthalaris, metalloprotease, IsSMase, prostacyclin ISL1373, and ISL929—also modify blood coagulation (Ribeiro and Mather, 1998; Dai et al., 2010; Ibelli et al., 2014; Wang et al., 2016; Regmi et al., 2020). It is clearly demonstrated that the inhibitory activities and downstream dynamic responses of potent coagulant mediators against salivary molecules have an effective role in a tick’s success in blood-feeding. Approaches must be directed to design a rationale drug using an anticoagulant mediator for diverse clinical applications.
Salivary metalloproteases of I. ricinus and I. scapularis promote the delay of wound healing and angiogenesis through binding the cascade fibrinogen and fibrin and are involved in tissue disruption by digesting structural components (Francischetti et al., 2003; Decrem et al., 2008; Ali et al., 2014; Ali et al., 2015a; Ali et al., 2015b). In I. ricinus, metalloproteases interact with fXIIa, fXIa, and kallikrein mediators (Decrem et al., 2008). HLTnl derived from Hae. longicornis is a competitive inhibitor of the vascular endothelial growth factor receptor, thus delaying healing and angiogenesis (Fukumoto et al., 2006). Ixonnexin-mediated plasmin generation assists the interaction of lysine-binding sites of kringle domain(s) of plasminogen with t-PA, which promotes fibrinolysis. This protein also inhibits FeCl3-induced thrombosis in mice (Assumpção et al., 2018). Ixonnexin and salp14 derived from I. scapularis specifically bind plasmatic zymogen factor X, which impairs its binding with plasmatic or immobilized heparin, resulting in the inhibition of prothrombin-to-thrombin conversion (Narasimhan et al., 2002; Monteiro et al., 2005; Batista et al., 2010; Branco et al., 2016; Assumpção et al., 2018). Ixolaris inhibits the fVIIa/TF complex and impairs fXa binding to Sepharose-immobilized heparin by binding thrombin’s fXa heparin-binding exosite (Monteiro et al., 2005). Amblyomin-X suppress the formation of tumor growth and new blood vessels (angiogenesis) (Carneiro-Lobo et al., 2009); it also inhibits prothrombinase and tenase activities by hydrolyzing host trypsin and plasmin (substrate for trypsin and plasmin) (Branco et al., 2016). AamS6, AamAV422, and serpin19 derived from A. americanum interact with fXa and XIa trypsin, plasmin, T cells, and DCs and inhibit thrombin-initiated fibrin formation (Šimo et al., 2017) (Table 1). HT-1, HT-3, and HT-12 in the salivary secretion of I. holocyclus target presynapses by inducing muscle paralysis that inhibits the dependence of transmitter release on extracellular calcium (Chand et al., 2016). Multiple tick salivary proteins have been identified and tested in the last few decades, but the molecular mechanism behind their host immunomodulation features is largely unknown (Bensaoud et al., 2019a). The impact of these molecules on tick–host interaction requires in-depth studies at the molecular and cellular levels to validate and develop candidates for anti-tick vaccine development.
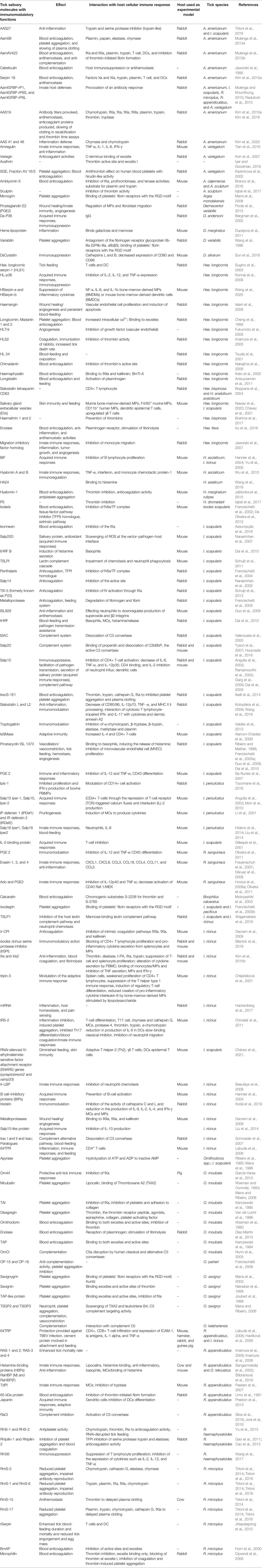
Table 1 Tick-derived immunomodulatory molecules and their effects and interaction with host immune responses.
Hyalomin 1 from Hyalomma marginatum rufipes and Hyalomin A and B from H. asciaticum deactivate host factor XI (fXI) and prevent thrombin activity by binding its active site and exosite I (Jablonka et al., 2015). Hae. longicornis longistatin binds to the V domain of receptor for advanced glycation end products, thereby inhibiting tissue inflammation (Anisuzzaman et al., 2010; Anisuzzaman et al., 2014). An anticoagulant TIX-5 (formerly known as P23) in the secretome of I. scapularis specifically prevents fV activation mediated by fXa that activates fV by fXa by involving the B-domain of fV (Schuijt et al., 2011; Aleman and Wolberg, 2013; Schuijt et al., 2013). The Ir-CPI, with one Kunitz domain interference with fXIIa, fXIa, and kallikrein, inhibits the intrinsic-specific coagulation pathway (Decrem et al., 2009). R. calcaratus, calcaratin, assists in the anticoagulation of blood by inhibiting thrombin S-2238 and fXa S-2765 chromogenic substrates (Motoyashiki et al., 2003). Lipocalin proteins are most commonly produced in tick SGs and have been implicated in host inflammation modulation by scavenging histamine and serotonin (Sangamnatdej et al., 2002; Mans et al., 2008), targeting platelet aggregation and the complement system, as well as being involved in toxicoses and as allergens (Hilger et al., 2005; Nunn et al., 2005). A. sculptum sculptin (Iqbal et al., 2017) and H. dromedarii P5 (Ibrahim and Masoud, 2018) have shown inhibition of thrombin activity.
Antiplatelet Aggregation (Tick Salivary Molecules Inhibiting Host Platelet Aggregation)
Tick-derived inhibitors target the enzymes that stimulate platelet aggregation, such as cathepsins G (Chmelař et al., 2011), C, L, S, V, and H (Chmelař et al., 2017); chymase (Chmelař et al., 2011); elastase (Cao et al., 2013; Valdés et al., 2013); and tryptase (Cao et al., 2013; Mulenga et al., 2013a). Platelets show three β1 integrins—α2β1, α5β1, and α6β1—and two β3 integrins—αIIbβ3 and αVβ3 (Joo, 2012)—which are inhibited by tick salivary molecules or bind platelet activators like ADP, serotonin, or thromboxane, thus hindering the mechanism of platelet aggregation (Chmelař et al., 2012). I. scapularis sialostatin L and L2 have been identified as specifically inhibiting cathepsins S, C, L, and papain (Kotsyfakis et al., 2006). Sialostatin L is an effective inhibitor of lysosomal cysteine cathepsins X and V (Štibrániová et al., 2019), and sialostatin L2 specifically inhibits cathepsin B and H (Kotsyfakis et al., 2010). RHcyst-2 derived from the saliva of R. haemaphysaloides inhibits cathepsin S (Wang et al., 2015). Omc2 from O. moubata inhibits cathepsins S and L (Salát et al., 2010). Iristatin of I. ricinus and BrBmcys2b and BrBmcys2c of R. microplus suppress host cathepsins C and L; however, BrBmcys2b also inhibits cathepsin B (Parizi et al., 2015; Parizi et al., 2018; Kotál et al., 2019). Iristatin diminish IL-2, IL-4, and IL-9, and IFN-γ-stimulated Th1 cells; IL-6 and IL-9; the production of nitric oxide by MPs; and anti-inflammatory cytokines IL-2 and IL-9 by Th9 cells and IL-4 by Th2 cells (Kotál et al., 2019). Salivary serpin Iris2 of I. ricinus inhibits cathepsin G and chymase to prevent inflammation (Kim et al., 2015b). Tryptogalinin secreted in the sialome of I. scapularis plays a wide variety of roles in the host immunomodulation by inhibiting α-chymotrypsin, β-tryptase, β-trypsin, elastase, matriptase, and plasmin and exerting a potentially extensive effect against several host enzymes and mast cell proteins (Valdes et al., 2013). Hae. longicornis Hlcyst-2 and Hlcyst-3 and I. persulcatus-derived JpIpcys2a, b, and c inhibit Cathepsin L and papain (Zhou et al., 2006; Rangel et al., 2017) (Table 1). Haemangin has been recognized as a Kunitz inhibitor from the saliva of Hae. longicornis that intensely prevents trypsin, chymotrypsin, and plasmin and thus supports the inhibition of plasmin-dependent fibrinolysis and angiogenic cascades (Štibrániová et al., 2019).
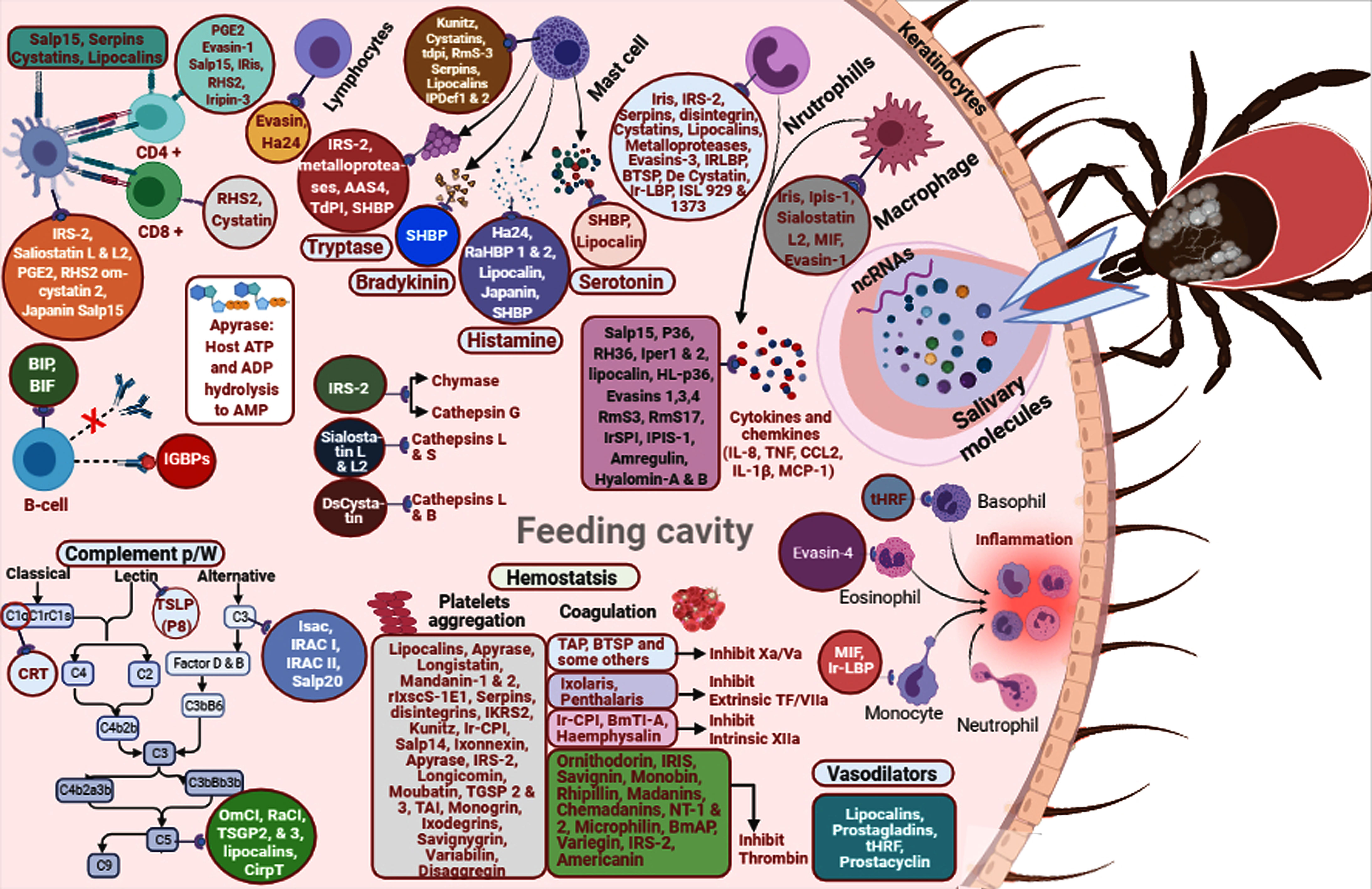
The R. appendiculatus–derived protease inhibitor TdPI effectively inhibits human β-tryptase and trypsin, as well as human plasmin (Paesen et al., 2007). Enolase from Haemaphysalis flava and O. moubata has been found to affect blood coagulation and possess anti-inflammatory and antihemostatic activities (Xu et al., 2016). R. microplus serpins have shown specific inhibition of chymotrypsin (RmS-1, RmS-3, RmS-6), and thrombin (RmS-15) (Rodriguez-Valle et al., 2015). I. scapularis and I. pacificus ixodegrin bind fibrin receptors on platelets with the RGD motif that provokes platelet aggregation (Francischetti et al., 2005b). In vivo studies have shown that YY-39 from I. pacificus and I. scapularis plays a role in platelet and thrombosis modulations by decreasing adenosine diphosphates, platelet adhesion to soluble collagen, thrombin- and TXA2-induced platelet aggregation, and binding to purified GPIIb/IIIa (Tang et al., 2015). Tick saliva–derived anti-inflammatory molecules such as AAS27 and AAS41 from A. americanum and I. scapularis and IRS-2 from I. ricinus target chymase to inhibit the inflammatory response of the host (Tirloni et al., 2019; Kim et al., 2020). In addition, AAS27 inhibits inflammation by targeting plasmin and trypsin activities (Tirloni et al., 2019), and IRS-2 targets cathepsin G activity, thus involving the inhibition of host vasodilation (Chmelař et al., 2011). DsCystatin identified in Dermacentor silvarum salivary glands weakens the activities of host cathepsins L and B of pro-inflammatory cytokine IFNγ, TNFα, and IL6 expression, and TLR2 and TLR4 signaling pathways induced NFκB activation (Sun et al., 2018). O. moubata-derived salivary protein disagregin interacts with the host’s αIIbβ3 integrin receptor that blocks its binding site to the fibrinogen, thus diminishing collagen tissue factor–mediated platelet aggregation and thrombus formation (Karczewski et al., 1994; Van den Kerkhof et al., 2021). D. variabilis variabilin blocks the binding of GPIIb-IIIa, αIIbβ3, and vitronectin receptor αvβ3 (Wang et al., 1996). O. savignyi savignygrin binds the αIIbβ3 and dissociates fibrinogen from its receptor (Mans et al., 2002); FXal binds to both exosites and active sites of thrombin (Gaspar et al., 1996). Other savignins have been observed to be involved in complement activities, vasoconstriction, and platelet and neutrophil aggregation (Mans et al., 2002; Mans and Ribeiro, 2008). BmTI-A from R. microplus hinders angiogenesis in vitro in a vessel formation assay and blocks neutrophil elastase, trypsin, plasmin, and plasma kallikrein (Soares et al., 2016). O. moubata moubatin and TSGP3 inhibit collagen-induced platelet aggregation and bind to TXA2; they also mediate vasoconstriction in the rat aorta. TXA2 is inhibited by longicornin from Hae. longicornis (Cheng et al., 1999). TSGP2 and TSGP3 from O. savignyi saliva bind host leukotrienes B4 and TXA2 with high affinity (Mans and Ribeiro, 2008). Argas monolakensis monogrin binds with fibrin receptors on platelets with the RGD motif that prevents platelet aggregation (Mans et al., 2008). Iris (elastase inhibitor) of I. ricinus hampers the intrinsic pathway and platelet aggregation (Pham et al., 2021) and inhibits the activities of elastase, thrombin, t-PA, fXa, and trypsin (Prevot et al., 2006). AAS19 is an anticoagulant protein derived from A. americanum that inhibits the activity of thrombin, plasmin, trypsin, fXa, fIXa, fXIa, fXIIa, tryptase, and chymotrypsin (Kim et al., 2015a). Dermacentor marginatus heme lipoprotein is a carbohydrate-binding protein with mannose- and galactose-binding specificity inhibiting agglutination; therefore, the presence of heme lipoproteins might lower the quantity of free heme at the feeding site by inhibiting inflammation (Dupejova et al., 2011; Tirloni et al., 2014; Tirloni et al., 2015). AM-10 and AM-38 from A. monolakensis have shown high affinity for histamine and 5-HT binding at the feeding spot, thereby preventing inflammatory actions (Mans et al., 2008). High-affinity HBPs from the saliva of R. appendiculatus have also been explored, where these proteins independently bound H1, 2, and 3 membrane receptors of histamine secreted by host basophils (Paesen et al., 1999). TIL-domain inhibitors have been reported in the salivary secretion of R. appendiculatus and I. ricinus (De Castro et al., 2017). Growth factor binding proteins—AamIGFBP-rP6L, AamIGFBP-rP-1, and AamIGFBP-rP6S—have been identified from A. americanum (Bakshi et al., 2019; Bartíková et al., 2020). R. haemaphysaloides salivary proteins RHS-1 and RHS-2 have shown antichymotrypsin activity, while RHS-1 has shown anticoagulation activity, based on activated partial thromboplastin time (APTT). Both inhibitors have been found to be involved in the inhibition of thrombin and fXa (Yu et al., 2013). Rhipilin-1 and Rhipilin-2 isolated from R. haemaphysaloides inhibit platelet aggregation by inhibiting tissue factor pathway inhibitor (TFPI) and coagulant activities involving the inhibition of serine protease elastase and trypsin (Gao et al., 2011; Cao et al., 2013). Various tick salivary-secreted molecules involved in host invasion and interfering with host defense mechanisms are displayed in Figure 1.
Tick Salivary Molecules Inhibiting the Complement Pathways of the Host
The complement cascade—including classical, lectin, and alternative pathways— is the first line of a host’s defense mechanisms that can be triggered by tick salivary molecules targeting the host’s enzymes, thereby disrupting complex assembly and downstream activation (Rosbjerg et al., 2017). The complement pathway plays a great role in controlling tissue injury and pathogen invasion when ticks have secreted several salivary effector proteins that specifically target C3 and C5 (Pham et al., 2021). The investigated anti-complementary features of tick salivary molecules include Isac, Salp9, and Salp20 from I. scapularis, and Irac I, Irac II, and IxACs from I. ricinus (Brossard and Wikel, 2004; Štibrániová et al., 2019). Among these, Isac, Irac-1 and -2, and Salp20 bind and displace properdin, thereby inhibiting C3 convertase formation (Hourcade et al., 2016; Silva et al., 2016); subsequently, dissociating C3 convertase complex (C3bBbP) from the alternative pathway prevents binding of the convertase to C3 (Francischetti et al., 2002; Monteiro et al., 2005). O. moubata OmCI and R. appendiculatus RaCI are C5 complement inhibitors that prevent its activation by C5 convertase, including MG1, MG2, and C5d domains and 5d, CUB, and C345c domains respectively (Nunn et al., 2005; Jore et al., 2016; Silva et al., 2016), probably inhibiting the rearrangement of the domains within C5, as it is necessary for activation to occur (Jore et al., 2016). Additionally, OmCI and its homologs bind complement C5 and leukotriene B4 (LTB4), inhibiting the induced inflammation (Roversi et al., 2013). C5 complement inhibitors have been reported from R. appendiculatus, R. microplus, D. andersoni, and H. marginatum (Silva et al., 2016); however, their anti-complement activity regarding binding to numerous sites at the C5 component remains to be investigated (Silva et al., 2016). O. kalahariensis TSGP2 and TSGP3 are comprised of a histidine residue His95 and a conserved βH-α2 loop in the sequences implicated in the interaction with complement C5. Both salivary components from O. savignyi have been reported to be responsible for the inactivation of the C5 mediator (Mans and Ribeiro, 2008). Saliva of A. cajennense is also involved in classical pathway inhibition; however, the inhibitor to date has not been identified (Franco et al., 2016; Silva et al., 2016). I. dammini saliva antagonizes anaphylatoxin and bradykinin, likely by the presence of a carboxypeptidase, and also blocks the deposition of C3b and the release of C3a (Andrade et al., 2005). A R. microplus salivary thiol-activated metalloendopeptidase has been shown to hydrolyse bradykinin and may have a role in relieving pain and other inflammatory signs at the tick bite site (Bastiani et al., 2002) OP-15 and OP-16 in O. parkeri from a sialome project have been identified (Francischetti et al., 2008). The molecular mechanisms of the classical pathway inhibition in R. microplus involve the binding of tick salivary calreticulin (C1-inhibitor (C1-INH)) to the C1 of the initially activated factor of C1qC1rC18 of the classical pathway, which in turn blocks the deposition of C4b (De Taeye et al., 2013); however, it does not activate the classical complement cascade in A. americanum (Kim et al., 2015b). The I. scapularis salivary lectin pathway inhibitor (TSLPI) prevents the binding of mannose-binding lectin and ficolins to their ligands, thus inactivating the cascade of the lectin pathway (Silva et al., 2016; Wagemakers et al., 2016; Vechtova et al., 2018; Coumou et al., 2019). AsKunitz and As8.9kDa are the first A. sculptum salivary inhibitory components of the classical pathway. Among these, As8.9kDa is known to inactivate the C3b factor, thus inhibiting the downstream cascade (Costa et al., 2021) (Figure 1). Salivary proteins that inhibit binding of mannan-binding lectin to the polysaccharide mannan were characterized in I. scapularis (TSLPI, Salp1, and Salp9Pac) and O. savignyi (BSAP), resulting in the inactivation of the lectin complement pathway (Denisov and Dijkgraaf, 2021). Other complement pathway inhibitors and their molecular mechanisms require further studies to be properly elucidated.
Host Cellular Response Modulating the Secretion of Tick Salivary Molecules
Cytokines and chemokines are the complement mediators that create a network and recruit leucocytes to site of the tick bite (Sangamnatdej et al., 2002). Cytokines are inhibited by salivary inhibitors (Cathepsin L and S proteins), and chemokines (CC and CXC) are bound by salivary evasins, thereby preventing the host’s inflammatory responses (Bhusal et al., 2020). Cathepsin L and S proteins diminish the phosphorylation of STAT-1 and STAT-2, thus effecting JAK/STAT signaling in DCs (Pham et al., 2021). PGE2 derived from D. variabilis, R. sanguineus, and I. scapularis inhibits DCs maturation and fibroblast migration and decreases CD40 Raf-1/MEK activation in vitro (Hovius et al., 2008a; Oliveira et al., 2011; Poole et al., 2013). Evasins from R. sanguineus—Evasin-1 (CCL18, CCL4, and CCL3), Evasin-3 (CCL1 and CCL8), and Evasin-4 (CCL11 and CCL5)—are responsible for the inhibition of chemokines (Frauenschuh et al., 2007; Guo et al., 2009). T-cell inhibitors have been found to either bind or inhibit their associated release or activation factors, including I. ricinus–derived IrSPI and Iris, I. persulcatus Ipis-1, R. microplus RmS-3 and RmS-17, and I. scapularis Salp15 (Ramamoorthi et al., 2005; Dai et al., 2009; Blisnick et al., 2019; Almazán et al., 2020). Iripin-3 from I. ricinus provides activity against the extrinsic blood coagulation pathway; moreover, this protein was shown to interfere with the adaptative immune response, since it reduces the production of interleukin-6 by MPs and the reduction of the T helper type 1 immune response (Chlastáková et al., 2021).
Similarly, tick salivary sialostatin L dampens antigen-mediating CD4+ proliferation and reduces the activation of interferon regulatory factor 4 signaling in MCs (Pham et al., 2021). Salp16 and Iper proteins are immunosuppressants that inhibit IL-8 activity, thereby impairing neutrophil chemotaxis (Sajiki et al., 2020). Salp14 inhibits T-cell proliferation and decreases the pro-inflammatory IL-6 and TNF-α production and secretion (Pham et al., 2021). Amregulin from A. variegatum inhibits the secretion of IL-1, TNF-α, IL-8, IFN-γ, and CXCL8 by LPS by stimulated rat splenocytes in vitro in a dose-dependent manner (Tian et al., 2016). Japanin immune-modulatory lipocalin derived from R. appendiculatus saliva was found to specifically reprogram DCs by blocking its differentiation from monocytes and altering the set of sequences, including pro-inflammatory, anti-inflammatory, transmembrane molecules, and cytokine secretion (Preston et al., 2013). HlSerpin-a and b derived from the saliva of Hae. longicornis suppress inflammatory cytokines by decreasing NF-a, IL6, and IL-1b bone marrow–derived MPs or mouse bone marrow–derived DCs (Wang et al., 2020).
I. scapularis and D. andersoni saliva histamine release factors were found to enhance histamine release and promote vasodilation upon binding to host basophils (Bhowmick and Han, 2020). Serotonin- and histamine-binding proteins (SHBPs) have been found in the saliva of D. reticulatus, simultaneously binding host serotonin and histamine (Sangamnatdej et al., 2002), and monotonin derived from A. monolakensis binds serotonin (Mans et al., 2008). R. appendiculatus-derived Ra-HBPs (male Ra-HBP1 and Ra-HBP3 and female Ra-HBP2) and Japanin have been identified as possessing a high affinity toward histamine binding. Functionally, Ra-HBP1 is a weak histamine binder as compared to Ra-HBP2 and 3, which display potent affinity to histamine (Štibrániová et al., 2019). H. asiaticum-derived HA24 binds specifically to histamine with a particular histamine-binding affinity demonstrated in a dose-dependent manner (Wang et al., 2016). Moubatin-like 3 stoichiometrically binds two histamine or one serotonin molecules (Mans and Ribeiro, 2008). MC migration inhibitory factor homologs have been isolated from the saliva of A. americanum, which plays a key role in the inhibition of migration of human MPs (Jaworski et al., 2001).
I. scapularis sialostatin L and L2 dampen antigen-mediated CD4+ proliferation, reduce the activation of interferon regulatory factor 4 signaling in MCs (Klein et al., 2015; Lieskovska et al., 2015), and influence the maturation of DCs by inhibiting IFN-β (Lieskovska et al., 2015). Three immunosuppressant salivary proteins—p36 from D. andersoni (Alarcon-Chaidez et al., 2003), HL-p36 from Hae. longicornis (Konnai et al., 2009), and RH36 from R. haemaphysaloides—blocked the T-lymphocyte proliferation in vitro assay (Wang et al., 2017). HL-p36 and RH36 directly inhibited the proliferation of many mitogen-stimulated cells in vivo and the expression of numerous cytokines such as IL-12, IL-2, and TNF-α (Kotsyfakis et al., 2006; Kotsyfakis et al., 2010). RHS2 can also inhibit CD4+ and CD8+ T-cell activation, leading to the inhibition of the host Th1 immune response.
Host Humoral Immune Response to Tick Salivary Molecules
Host immune responses interacting with tick salivary molecules may impede the host-associated regulatory and\or signaling pathways, evolving different mechanisms such as the suppression of the host humoral response, which in turn enhances tick blood-feeding (Andrade et al., 2005). Host humoral immunity is inhibited by tick salivary components by disrupting the B cell–derived immune responses, such as their manufacturing of specific antibody inhibitors (Andrade et al., 2005). SGs of several ixodid tick species secrete a set of immunoglobulin-G binding proteins (IGBPs) (Andrade et al., 2005). B cell–inhibitor factors derived from H. asciaticum asciaticum provoke B-cell responses (Yu et al., 2006), and B cell–inhibitory proteins derived from I. ricinus inhibit B lymphocyte proliferation, while not affecting T lymphocytes (Hannier et al., 2004). Further investigation might be necessary to discover its probable effects on B-cell receptor signaling pathways and Toll-like receptors (TLRs) such as TLR1, TLR2, and TLR4. Limited reports on B cell inhibition and the suppression of B-cell antibody production are available. The discovery of novel drugs requires components that could be a functional template for targeting B cells and their associated immune pathways. For this purpose, B-cell inhibitors in tick sialome are an essential target for determining their biological activities in host immunomodulation.
Tick Salivary-Derived Non-Proteinaceous Molecules Impairing the Host Immune System
Several tick salivary non-proteinaceous molecules have been identified, such as PGE2, prostacyclin, purine nucleoside adenosine, fatty acids, endocannabinoids, and recently discovered non-coding RNAs (ncRNAs), that induce host immunomodulation (Hermance et al., 2019). Among these, PGE2 was the first identified non-proteinaceous immunomodulatory component in tick sialome that recruits several inflammatory cells to the target site (Andrade et al., 2005). The PGE2 component was reported in saliva secreted by tick species of the following genera—Rhipicephalus, Dermacentor, Ixodes, and Amblyomma—potentially influencing the inhibition of T lymphocytes, B lymphocytes, and immune sentinel MPs (Hermance et al., 2019; Sajiki et al., 2020). Pro-inflammatory TNF-α, IL-12p40, and IL-10 by murine DCs are stimulated by saliva-derived non-proteinaceous purine nucleoside adenosine in R. sanguineus (Oliveira et al., 2011). Tick sialome-derived fatty acid organic amide, prostacyclin, and endocannabinoids have been reported to be involved in the enhancement of host vasodilation and analgesic and anti-inflammatory activities (Hackenberg et al., 2017). Tick-secreted saliva contains a set of enzymes that belong to the 5′-nucleotidase family, among whose enzymes tick salivary apyrases have been identified in the saliva of O. savignyi, O. moubata, O. kalahariensis, and I. scapularis (Ribeiro et al., 1985; Ribeiro et al., 1991; Mans et al., 1998; Valenzuela et al., 2002; Stutzer et al., 2009), which cleave to extracellular ATP and ADP to prevent platelet stimulation and aggregation. A molecular mechanism elucidated the linkage of the host’s immunomodulation with the rigorous activities of tick salivary prostaglandin E2 and purine nucleoside adenosine (Sá-Nunes et al., 2007; Oliveira et al., 2011).
Several transcribed mRNAs have been reported to show non-protein-coding parts but involving several cellular regulatory functions termed as ncRNAs (Jarroux et al., 2017). They are categorized into long non-coding RNAs and small non-coding RNAs having nucleotide ranges of <200 nucleotides and >200, respectively. They have been found to functionally participate in subverting the host’s defensive responses in the parasite–vector–host interface (Bensaoud et al., 2019b; Ahmad et al., 2021). Further investigation of long non-coding RNAs and small non-coding RNAs localization, purpose for secretion, and molecular mechanisms is necessary, as they are suggested to have a putative role in the regulation of gene expression and to disrupt the signaling between the host defense pathways in the host’s cells (Hackenberg and Kotsyfakis, 2018; Chávez et al., 2019). A wide range of extracellular vesicles (EVs) have been identified that contain molecules of modulating the host’s physiology and enabling the blood-feeding mechanism (Chávez et al., 2021). Previously, tick exosomes were suggested in the secretion of ncRNAs (Hackenberg and Kotsyfakis, 2018; Chávez et al., 2019), which was recently confirmed through the successful isolation from saliva and SGs of partially fed or unfed ixodid ticks (Zhou et al., 2020; Chávez et al., 2021). An in vivo model has shown that exosomes secreted in the saliva of A. maculatum and I. scapularis at the bite site modulate the production of cytokines or chemokines such as IL-8 and/or C-X-C motif chemokine ligand 12, which are responsible for controlling tissue injury, healing, and the wound-repair process for a successful blood-feeding mechanism (Zhou et al., 2020). This may allow and ease salivary exosomal secretions in tick feeding; understanding of its molecular mechanisms in host immunomodulation is in its infancy and will require experimental evidence to uncover its precise functional role. Future literature on ncRNAs and developing projects on the identification of the putative roles tick sialome-derived ncRNAs could be a sophisticated/possible way to describe the inhibitory activities in tick–host crosstalk.
MicroRNAs (miRNAs)
MiRNAs are a class of short non-coding RNAs with a length of approximately 22 nucleotides that are responsible for regulating gene expression at the post-transcriptional level and that can bind the 3’ UTR region of target mRNA to induce post-transcriptional inhibition (Ahmad et al., 2021). A single miRNA might bind several different mRNA targets, or multiple miRNAs can target a single mRNA transcript (Hermance et al., 2019). This phenomenon impedes the identification of miRNA-mRNA target interactions for discovering the regulatory network governed by miRNAs (Riolo et al., 2021). MiRNAs control several other cellular activities—growth and metabolisms (Malik et al., 2019) and blood-feeding (Hermance et al., 2019)—and strengthen tick–host interaction (Chavez et al., 2019; Chavez et al., 2021).
Although miRNAs have been identified and secreted via tick-derived EVs, limited studies have been conducted on the differential expression of miRNAs, with the exception of tick species that include all developmental stages of R. microplus (Barrero et al., 2011), gender-based dynamics in R. sanguineus (Shao et al., 2015), lipopolysaccharide-induced patterns in R. haemaphysaloides (Wang et al., 2015), differential expression in SG of Hae. longicornis (Zhou et al., 2013; Malik et al., 2019) and H. anatolicum (Luo et al., 2021), and the saliva of I. ricinus (Hackenberg et al., 2017). A subsequent study has shown the existence of some significant miRNAs in the EV-like structures secreted by tick sialome (36 known and 34 novel miRNAs), which may be used in tick–host interface modulation (Nawaz et al., 2020). Various tick-specific miRNAs have been identified, and their functional characteristics have been reported. For instance, the presence of miRNAs in the saliva of I. ricinus and its corresponding role in miRNA-mediated host gene expression regulation presented the first evidence in the tick–host interaction (Hackenberg et al., 2017), which was followed by the identification of several other miRNAs in several other tick species, including miR-275, miR-375, and miR-184 in Hae. longicornis, which are involved in blood digestion and oviposition (Hao et al., 2017; Malik et al., 2019); miR-133 (downregulated) and miR-79 (upregulated) in I. scapularis enhance the transmission of Anaplasma phagocytophilum from ticks to hosts (Ramasamy et al., 2020), and iri-miR-317-3p, in I. ricinus acts as a putative blood-feeding regulatory mechanism (Hackenberg et al., 2017). The same tick also secretes mir-317-3p, miR-8-3p, bantam-3p, and miR-279a-3p that could be the foci of research because of their role in KEGG pathways - “gap junction pathway” and “inflammatory mediator of TRP channels regulation of the host,” - depicting their crucial role in maintaining the host’s homeostatic activities in the tick–host interaction.
Little focus has been given to the miRNA profile in the sialome of ticks that could tackle the host’s rigorous immune responses except for recently discovered saliva-mediated EVs that strongly affect the dendritic epidermal T cells in in vitro experimentation (Chávez et al., 2021). The prospects for better understanding vector biology require the identification of miRNAs on a large scale (Malik et al., 2019). Concerning ticks, the miRNA information is limited only to characterized sequences, localized tissues, and evolutionary linkage in only five tick species. This is further scarce by the availability of annotated miRNA catalogues for only two tick species, including I. scapularis and R. microplus (Hackenberg et al., 2017). The salivary composition of other tick genera containing non-proteinaceous components—specifically miRNA expression patterns and its effects on host defense mechanisms—requires proper experiments. The identification of miRNAs in tick sialome is another advantage to demonstrating the molecular mechanisms involved in the interaction between the tick-pathogen-host interface. Their identification may also facilitate the discovery of novel targets that could potentially control ticks and their associated pathogens. Furthermore, recent updates on EVs have shown their remarkable activities in the secretion and transfer of miRNAs, lipids, and proteins intercellularly and their disposal of unimportant cellular debris (Nawaz et al., 2020). The secretion of exosomal miRNAs was further studied and found to be dependent on a ceramide-dependent pathway (O’Brien et al., 2018). Researchers have approached the objective of miRNA secretion, but the study of the uptake mechanisms of miRNAs is still in its infancy. This phenomenon has been discussed in several research articles in which multiple mechanisms were suggested/inferred; however, strong evidence is still elusive (Chen et al., 2012; Wei et al., 2017). Tick saliva-derived miRNA requires fundamentals on the molecular mechanism of its target mRNA inhibition and functional role in the immunomodulation of the tick–host interface (Malik et al., 2019). These approaches are beneficial in the development of tick miRNA-based vaccine candidates.
Differential Expression and Secretion of Tick Secretome
Tick sialome is comprised of a wide range of proteins that are secreted dynamically and that enhance host immunomodulation during blood-feeding (Narasimhan et al., 2020; Ribeiro and Mans, 2020). The dynamic expression of tick salivary secretome varies with a tick’s blood-feeding phases, tackling host immune responses, and pathogen transmission (tick–pathogen–host interaction) depending on the species and the life stage of the tick (Kim et al., 2016; Tirloni et al., 2017). This mechanism is called sialome switching (Perner et al., 2018), which has been addressed in I. scapularis (Lewis et al., 2015; Kim et al., 2016), I. ricinus (Schwarz et al., 2014), A. americanum (Radulović et al., 2014), R. microplus (Tirloni et al., 2014; Garcia et al., 2020), R. sanguineus (s.l.) (Tirloni et al., 2020), O. erraticus (Pérez-Sánchez et al., 2021; Pérez-Sánchez et al., 2022), R. pulchellus (Tan et al., 2015), R. zambeziensis (De Castro et al., 2017), and H. dromedarii (Bensaoud et al., 2019a). As a result, these experiments have proven the presence of several putative antigenic candidates in tick sialome-derived proteomes (Pérez-Sánchez et al., 2021) that have important biological functions, particularly host attachment, blood-feeding, and modulation of the host’s defense mechanisms (Kazimírová and Stibraniova, 2013; Šimo et al., 2017; Pérez-Sánchez et al., 2021). Several studies have observed the differential expression of some specific salivary molecules. For instance, AsKunitz, As8.9kDa, and AsBasicTail have been studied in A. sculptum and found to be upregulated at distinct levels (larvae and nymphs: 2.4- to 745-fold; adults: 365- to almost 20 million-fold) in early blood-feeding (Esteves et al., 2017; Costa et al., 2021). In contrast, Hae. longicornis-derived Hlcyst-2 has shown negligible expression in the sialome, which might not be involved in blood-feeding or the immunomodulation of the host (Zhou et al., 2006). I. persulcatus Ipis-1 proteins have been detected in the SGs expressed at the same level throughout all phases of feeding. However, the expression of some proteins, including HSP16, has increased from the same tick increases during engorgement (Xu et al., 2005). A homologous innexin protein was found downregulated in the salivary secretion/secretome of A. americanum (Aljamali et al., 2009), and O. moubata (Díaz-Martín et al., 2013) during blood-feeding. Female A. variegatum fed on goats expresses 336 proteins as compared to D. andersoni fed on cattle, which expressed 677 proteins (Mudenda et al., 2014) and O. moubata, which expressed 193 proteins (Díaz-Martín et al., 2013). A large set of salivary proteins have been observed to express in different feeding phases with known and unknown functions (Rodrigues et al., 2018). This phenomenon also indicates the existence of several unknown proteins in the salivary repertoire at tick–host interface. Remarkably, qualitative and quantitative variations in the saliva secretome during different feeding stages of R. microplus have been suggested to modulate the expression of proteins for successful blood-feeding, and to evade the host defense mechanism (Dai et al., 2012; Tirloni et al., 2014; Kim et al., 2016; Ribeiro and Mans, 2020). This time-dependent and dynamic expression of tick salivary proteins further indicates the participation of these molecules in different intervals of blood-feeding that are associated with feeding progression (Schwarz et al., 2013; Karim and Ribeiro, 2015; De Castro et al., 2017). Molecular mechanisms underlying the differential expression of several known and unknown salivary proteins need further investigations (Karim and Ribeiro, 2015; Sajiki et al., 2020). The rapid changes in the sialotranscriptome underlying the sialome switching of ticks may be an interesting target for the control of ticks and tick-borne pathogens.
Tick sialome also influences the acquisition, propagation, and transmission of a large array of pathogens (Pham et al., 2021). Tick-acquired pathogens exploit the activity of salivary molecules to enhance their survival and transmission (Karim and Ribeiro, 2015; Perner et al., 2016), and facilitate blood-feeding, and immune evasion at tick-host interface (Nuttall, 2019). Saliva assisted pathogens contribute to the dynamic expression of several molecules in the SGs of ticks (Kazimírová and Stibraniova, 2013). For instance, salivary Salp15 protein has been reported to selectively overexpress in the SGs of I. scapularis nymphs and bind a Borrelia burgdorferi derived OspC, a spirochete surface protein (Ramamoorthi et al., 2005). This pathogen enhances the expression of tHRF in I. scapularis (Dai et al., 2010), and transcription of TSLPI which binds the active sites of C-type mannose binding lectin of the host complement pathway (Dulipati et al., 2020). B. afzelii increases the expression of Salp15 in I. ricinus (Hovius et al., 2008b). Anaplasma phagocytophilum enhances the expression of Salp16 in the I. scapularis (Sukumaran et al., 2006). Among others, upregulation of Salp11, metis-1, prolyl 4-hydroxylase and cement proteins have been observed in ticks infected with A. phagocytophilum and B. burgdorferi (Cotté et al., 2014). Substantial up-regulate of homolog of histone deacetylase 1 protein in I. scapularis has been observed in SGs against A. phagocytophilum infection (Cabezas-Cruz et al., 2016). Rickettsia parkeri dynamically interact with A. maculatum symbionts and upregulate tick selenoproteins (Budachetri et al., 2018). Recent investigation on tick-borne Powassan virus described its ability to alter the expression of miRNAs in the SGs of I. scapularis (Hermance et al., 2019). Sialome switching could also be triggered by the epigenetic regulation in ticks; mediated by histone modification and chromatin remodeling (Adamson et al., 2013; Kotsyfakis et al., 2015; Cabezas-Cruz et al., 2016), stressor signals, internal clock of tick species, transcription factors, response to a pathogenic infection, and rigorous innate and acquired immune responses of the host, or contribution of both tick-host factors (Karim and Ribeiro, 2015; Perner et al., 2018). Moreover, the composition of saliva varies, and tick express specific proteins when they are exposed to different host species (Tirloni et al., 2017; Narasimhan et al., 2019). Several factors are involved in the induction of sialome switching at tick-host interface however, the molecular nature of sialome switching and its effects on the chain of biological processes are mostly remained unknown. Therefore, further research is essential to assess the molecular mechanisms of sialome switching during blood-feeding phases, and sialome manipulation by saliva-assisted pathogens in different tick species that might provide important information about tick salivary molecules essential for the control of ticks and tick-borne pathogens.
Role of Omics in Salivary Secretome
Knowledge of the composition and variations in the expression of tick saliva proteins is essential for understanding the tick feeding process and host immunomodulation mechanisms (Tirloni et al., 2020). Understanding the complexity of tick saliva proteins, redundant activities, putative roles, and differential gene expressions during blood-feeding has increased over the past decades as sophisticated approaches have been opted for at cellular, molecular, genomic, functional genomic, and proteomic levels (Wikel, 2018). Computational methods that enable the exploration of the immunomodulatory activities of a large set of tick salivary molecules have been identified; however, most remain to be tested in clinical trials. Currently, transcriptomics, proteomics, large-scale DNA sequencing, and bioinformatics analyses have been used to identify genes and proteins essential for resolving the complexity in the tick–host critical facets that facilitate the salivary composition and molecular dynamics throughout tick blood-feeding (Martins et al., 2020; Tirloni et al., 2020; Boulanger and Wikel, 2021). The sialoproteomes and sialotranscriptomes of several tick species have been annotated, along with their putative roles in the modulation and inhibition of host hemostatic, inflammatory, and immunity processes, in various reviews (Francischetti et al., 2008; Chmelař et al., 2012; Kotál et al., 2015; Martins et al., 2021; Pérez-Sánchez et al., 2021). The effective control of ticks and tick-borne pathogens is a long-standing and worldwide challenge (Willadsen, 2004; De la Fuente and Contreras, 2015; De la Fuente et al., 2016; De la Fuente et al., 2017; Van Oosterwijk and Wikel, 2021). Several attempts have been made to identify effective antigens in the tick sialome that could deter the blood-feeding and block the pathogen transmission (Willadsen, 2004; Ramamoorthi et al., 2005; Dai et al., 2010; Rego et al., 2019). Although commercial and experimental vaccine formulations have elicited partial protective immune responses against ticks, until now, there are no vaccine that provides adequate levels of protection against tick infestation. The use of anti-tick vaccines rises a potential alternative control method, making tick vaccine development economically essential (Parizi et al., 2012; De la Fuente and Contreras, 2015; De la Fuente et al., 2016; De la Fuente et al., 2017). Recent advances in the transcriptomic and proteomic approaches through next-generation sequencing (NGS) have increasingly resolved the complexities of tick salivary compositions during blood-feeding in different developmental stages (Bensaoud et al., 2019a). Several factors have hampered the successful development of anti-tick vaccine including the lack of understanding of the complex mechanism of tick-pathogen-host interaction, salivary molecule’s assisted microbial diversity, selection of suitable protective antigens that can induce considerable protection and potentially target a broad range of ticks and tick-borne pathogens (Rego et al., 2019; Van Oosterwijk and Wikel, 2021). Possibly, the main difficulty to obtain an adequate vaccine against ticks is the lack of knowledge about the mechanisms involved in host immune responses that induces tick rejection. Therefore, strategies should be designed to decipher the genetic basis of tick-host-pathogen interactions which are the crucial in the development of salivary derived anti-tick vaccine.
NGS further promotes the characterization of several tick sialome sequences and has opened new horizons for the discovery of mechanistic studies regarding the tick–host interface (Martins et al., 2020; Oleaga et al., 2021a). Advancements in next-generation RNA sequencing and a label-free quantitative proteomics have been used to demonstrate the quantitative gene expression in the midgut and SGs of ticks during host attachment (Schwarz et al., 2014). Massive analysis of cDNA Ends sequencing approaches in combination with RNA sequencing is useful for determining up and downstream regulation expression of tick salivary proteins during tick feeding (Rego et al., 2019; Trentelman et al., 2020). A quantitative isobaric tag for relative and absolute quantitation proteomics strategy has been developed for investigating differential protein expression in different feeding stages of tick SGs (Ren et al., 2019). Small quantities of proteins can be targeted by employing several techniques such as peptide mass fingerprinting by matrix-assisted laser desorption/ionization-mass spectrometry and shotgun proteomics by precursor ion detection and product ion detection (Rego et al., 2019). In addition, the protein half-life in the circulation can be extended by using proline/alanine-rich sequence protein conjugation with the polymeric sequence (Pro, Ala, and Ser) (Schlapschy et al., 2013; Chmelař et al., 2019).
Several complexities about the immunological interaction at tick-host interface have been increasingly resolved using genome arrays (Van der Heijden et al., 2005; Heinze et al., 2012). A complete sequence dataset of ticks is required to unravel the molecular mechanisms behind the differential expression and regulation of tick salivary proteins during feeding phases (De Castro et al., 2017). The genomic and transcriptomic data of ticks, tick-borne pathogens, and pathogen’s infected tick can provide an aid in selection and characterization of the novel therapeutic targets and vaccine candidates (Sette and Rappuoli, 2010; Antunes et al., 2012; Cramaro et al., 2015; De la Fuente and Contreras, 2015; Blecha et al., 2018; Davies et al., 2019; Ali et al., 2020a; Ali et al., 2022; Jia et al., 2020). In recent years, a number of genomic and sialotranscriptome sequences have been annotated (Hill and Wikel, 2005; Guerrero et al., 2006; Wang et al., 2007; Aljamali et al., 2009; Anatriello et al., 2010; Guerrero et al., 2010; Francischetti et al., 2011; Karim et al., 2011; Ribeiro et al., 2012; Schwarz et al., 2013; Garcia et al., 2014; Mudenda et al., 2014; Schwarz et al., 2014; Cramaro et al., 2015; Karim and Ribeiro, 2015; Xu et al., 2015; Yu et al., 2015; De Castro et al., 2016; De la Fuente et al., 2016; Gulia-Nuss et al., 2016; Barrero et al., 2017; De la Fuente et al., 2017; Maruyama et al., 2017; Ribeiro et al., 2017; Antunes et al., 2018; Miller et al., 2018; Nuss et al., 2018; Garcia et al., 2020; Jia et al., 2020; Couto et al., 2021; Oleaga et al., 2021b). Recently published annotated genome sequences of different tick species and their associated pathogens provide a valuable resource to understand blood-feeding mechanisms, tick-host-pathogen interactions, and genetic basis as tool for the control of ticks and tick-borne pathogens (Jia et al., 2020). The availability of such information affords unprecedented insight into the complex mechanisms in tick sialome and the temporal expression of several secretory protein families. Therefore, fundamental knowledge about tick genome is essential in the exploration of new horizons about tick biology, tick-host-pathogen interactions, and control strategies.
Advances in the field of computational biology have substantially improved several PIs and their targeted proteases by using 3D structural information that could be useful in permitting receptor-based design (Jmel et al., 2021). Recently discovered ncRNAs in tick saliva have been proposed to be prompted by the immune modulators of the vertebrate host (Chen et al., 2019). Many ncRNA sequences have been characterized as playing some role in the modulation of host gene expression through binding miRNAs to the host regulatory mRNAs (mRNAs) (Zhou et al., 2013; Bensaoud et al., 2019b; Malik et al., 2019; Aounallah et al., 2020; Nawaz et al., 2020). These may be concerned with the regulation of tick development or blood-feeding that has been studied in several tick species and described as gene regulators (Boulanger and Wikel, 2021). Recent updates on tick salivary miRNAs have raised growing concern regarding their redundant functions in gene expression regulation, targeting mRNAs, and tick–host interaction (Zhou et al., 2013). Studies are needed to predict miRNA molecular targets in the vertebrate host that may assist future tick-control methods (Hackenberg et al., 2017). Complexity in tick–host molecular interactions is also required for in silico screening of tick sialomes with important immunogenic properties and their directed changes in the host defense mechanism, followed by wet-lab verification (Bhowmick and Han, 2020; Ali et al., 2020a; Ali et al., 2020b). Therefore, the implementation of bioinformatics tools and bioassays to monitor the amounts of target mRNA (Ekimler and Sahin, 2014), a computational approach such as algorithms scripted from mRNA sequences and/or based on the miRNA-mRNA interactions, machine learning that describes the statistical inference (Riolo et al., 2021), and small RNA high-throughput sequencing (Hermance et al., 2019) are necessary to predict miRNA targets and rule out the molecular mechanism for maintaining the integrity of the tick–host interface. The biochemical characterization of tick salivary components in the tick–host interface is central to understanding the genes, proteins, motif, or residues and subsequently elucidate their functional mechanisms, role in the activation or inhibition of specific enzymes or receptors, blood coagulation, and platelet aggregation (Valenzuela, 2004). Biochemical analyses are useful in the identification of miRNA targets in a way more sophisticated than genetic methods (Ekimler and Sahin, 2014). Over the last two decades, tick salivary proteome has been studied, revealing that the protein profile of tick sialome is complex (Narasimhan et al., 2007; Francischetti et al., 2009; Kim et al., 2016; Perner et al., 2018; Ali et al., 2020b). Exploring the structure and functional mechanisms of tick salivary secretome and host target molecules can be used to develop synthetic peptides (Maritz-Olivier et al., 2007; Koh et al., 2018). Some examples have included the mapping of thrombin exosites by ornithodorin (Van der Locht et al., 1996), Ixolaris-derived prothrombinase complex formation (Monteiro et al., 2005), and molecular mechanisms that maintain the fV and its conversion to fVa using recombinant TIXC-5 (Aleman and Wolberg, 2013; Schuijt et al., 2013). However, novel control methods are restricted due to the lack of understanding the fundamentals of tick biology and mechanisms underlying the tick–host molecular interface (Slunge, 2015).
The omic era has advanced our understanding of the functional origin and evolution of the complex tick and host proteins that interplay during tick–host crosstalk (Vayssier-Taussat et al., 2015). The basic understanding of tick biology; the molecular interface between tick and host; the differential secretion of tick salivary secretome in the tick’s developmental stage, gender, feeding time, and behavior; and the upregulated or downregulated host genes during tick infestation have been mostly disclosed by NGS (Martins et al., 2020). Exploiting novel downregulated tick proteins and upregulated host proteins during tick feeding may be vital to hematophagy and, consequently, to suitable candidate antigens (Tabor et al., 2017). The advanced strategies increase our understanding of specific and unique tick genes and proteins, along with the annotation and classification of identified sequences (Tirloni et al., 2020). Transcriptomes and proteomes of tick sialomes have been annotated in vaccinomic pipelines for the selection and characterization of candidate protective anti-tick vaccines (Pérez-Sánchez et al., 2021), supported by techniques in the development of new proteins (Tripathi and Shrivastava, 2019), including immuno-proteome methods (Narasimhan et al., 2007), yeast surface display (Rego et al., 2019), 1D-PAGE/tryptic digestion/RPLC/MS/MS (Francischetti et al., 2008), RNA interference (RNAi), and CRISPR-Cas9 technologies (Barnard et al., 2012; Sharma et al., 2020). Broadly speaking, a number of experimental methods, including RNAi, expression library immunization and sequences tags, interactomics, proteomics, and transcriptomics, are involved in the identification of putative vaccine candidates through which a cocktail of vaccines can be selected that could increase the potential synergistic effects against tick infestation (Kocan et al., 2003; Artigas-Jerónimo et al., 2018).
Omics’ explosion of data further improves the reverse vaccinology strategies applied as an alternative for the detection of novel candidates for next-generation diagnostics and vaccines (De la Fuente et al., 2020; Couto et al., 2021). These approaches have revolutionized screening for novel natural substances. Furthermore, computational biology approaches have been introduced that assembled previously published data in a single platform such as miRNA sequences and annotations in the MicroRNA Sequence Database (miRBase) (Mans, 2019; Perez-Riverol et al., 2019), raw proteomic data in the PRoteomics IDEntifications Database (Mans, 2020), computationally assembled transcript sequences in the Transcriptome Shotgun Assembly, annotated tick sialotranscriptomes in TickSialoFam (Ribeiro and Mans, 2020), and annotated genomic assembly in VectorBase (Giraldo-Calderón et al., 2015). These databases are accessible and updated by adding new sequences and annotation details that facilitate the scientific community (Ribeiro and Mans, 2020; Marceca et al., 2021). Several research studies have provided their supplementary data comprised of putative salivary molecules derived from various tick species, which provides a valuable reference database for the current ongoing transcriptomic and proteomic studies (Oleaga et al., 2021b) and assists the process of functionally identifying unique transcripts and their associated proteins. The generation of these large data sets is the foundation for understanding the global perspective of tick–host interaction that contributes to the initiation of studies linking specifical immunomodulatory events with specific tick-derived molecules (Wikel, 2018) and high-throughput screening of compound libraries for target-oriented molecular models (Chmelař et al., 2019). In the best-case scenario, intense research efforts have been made in the last few years to achieve long-term goals for the identification of tick-derived antigens that may be useful in blocking successful feeding and pathogen transmission and that could be exploited for an anti-tick vaccine (Parizi et al., 2015; Martins et al., 2020; Pérez-Sánchez et al., 2021; Sajid et al., 2021). To date, a limited number of sialome-derived proteins have been functionally characterized in different ticks; however, the sialome repertoire and biological activities, along with post-translational gene modification by proteomic analysis, are far beyond our understanding. Subsequently, the systematic classification of the tick–host molecular interaction has remained incomplete due to less coverage of the transcriptome and proteome spaces. Comprehensive understanding may require improvement in the current technology; however, taking advantage of the recent omic era, the rich cocktail of salivary molecules in various tick species can be determined, along with the remarkable molecular mechanisms and affinity in the tick–host interface, perhaps providing a pipeline to successful functional descriptions of these molecules.
Conclusion
Updated knowledge about understanding the tick–host molecular interface is addressed, which may assist strategies to successfully deter tick blood-feeding and direct the development of effective anti-tick control. The molecular interaction between tick salivary molecules and host components throughout tick blood-feeding has been briefly discussed for understanding the roles of these molecules in the modulation of host responses. Based on growing knowledge of the complexity of tick–host molecular interaction, further experiments using functional genomics such as the CRISPR system may shape the components involved in crosstalk and control approaches. In addition, the specific roles and activities of molecules derived from ticks and their diverse means of action against host defense mechanisms in ticks remain to be investigated. Specifically, extensive studies on the molecular and cellular level of tick sialome–derived miRNAs are required to decipher their putative roles in tick–host interactions and, in turn, enhance discoveries of vaccine candidates for clinical trials. These gaps in the existing knowledge could be unraveled by introducing technological advancements that may lead to unprecedented information on the tick–host interaction and effective control strategies.
Author Contributions
AAli, IZ, and HZ searched and collected the literature and wrote the manuscript. AAli, MMA, AAlo, FA, MA, CT, IV, and TT supervised the overall investigations and helps in the manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the JST Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) Grant Number JPMJTM20SV and Takeda Science Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the financial support provided by Pakistan Science Foundation and Higher Education Commission Islamabad Pakistan, researchers supporting project number (RSP2022R494), King Saud University, Riyadh, Saudi Arabia, and the CNPq and CAPES – Brazil for providing research facilities.
References
Ørvim, U., Barstad, R. M., Vlasuk, G. P., Sakariassen, K. S. (1995). Effect of Selective Factor Xa Inhibition on Arterial Thrombus Formation Triggered by Tissue Factor/Factor VIIa or Collagen in an Ex Vivo Model of Shear-Dependent Human Thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 15 (12), 2188–2194. doi: 10.1161/01.atv.15.12.2188
Adamson, S. W., Browning, R. E., Budachetri, K., Ribeiro, J. M. C., Karim, S. (2013). Knockdown of Selenocysteine-Specific Elongation Factor in Amblyomma Maculatum Alters the Pathogen Burden of Rickettsia Parkeri With Epigenetic Control by the Sin3 Histone Deacetylase Corepressor Complex. PloS One 8 (11), e82012. doi: 10.1371/journal.pone.0082012
Ahmad, P., Bensaoud, C., Mekki, I., Rehman, M. U., Kotsyfakis, M. (2021). Long Non-Coding RNAs and Their Potential Roles in the Vector–Host–Pathogen Triad. Life 11 (1), 56. doi: 10.3390/life11010056
Akov, S. (1982). “Blood Digestion in Ticks,” in Physiology of Ticks (Pergamon: Elsevier), 197–211. doi: 10.1016/B978-0-08-024937-7.50011-1
Alarcon-Chaidez, F. J., Boppana, V. D., Hagymasi, A. T., Adler, A. J., Wikel, S. K. (2009). A Novel Sphingomyelinase-Like Enzyme in Ixodes Scapularis Tick Saliva Drives Host CD4+ T Cells to Express IL-4. Parasite Immunol. 31 (4), 210–219. doi: 10.1111/j.1365-3024.2009.01095.x
Alarcon-Chaidez, F. J., Müller-Doblies, U. U., Wikel, S. (2003). Characterization of a Recombinant Immunomodulatory Protein From the Salivary Glands of Dermacentor Andersoni. Parasite Immunol. 25 (2), 69–77. doi: 10.1046/j.1365-3024.2003.00609.x
Aleman, M. M., Wolberg, A. S. (2013). Tick Spit Shines a Light on the Initiation of Coagulation. Circulation 128 (3), 203–205. doi: 10.1161/CIRCULATIONAHA.113.003800
Ali, A., Ahmad, S., de Albuquerque, P. M. M., Kamil, A., Alshammari, F. A., Alouffi, A., et al. (2022). Prediction of Novel Drug Targets and Vaccine Candidates Against Human Lice (Insecta), Acari (Arachnida), and Their Associated Pathogens. Vaccines 10 (1), 8. doi: 10.3390/vaccines10010008
Ali, A., Ahmad, S., Wadood, A., Rehman, A. U., Zahid, H., Qayash Khan, M., et al. (2020b). Modeling Novel Putative Drugs and Vaccine Candidates Against Tick-Borne Pathogens: A Subtractive Proteomics Approach. Vet. Sci. 7, 129. doi: 10.3390/vetsci7030129
Ali, A., Fernando Parizi, L., Garcia Guizzo, M., Tirloni, L., Seixas, A., da Silva Vaz, I., Jr., et al. (2015b). Immunoprotective Potential of a Rhipicephalus (Boophilus) Microplus Metalloprotease. Vet. Parasitol. 207, 107–114. doi: 10.1016/j.vetpar.2014.11.007
Ali, A., Khan, S., Ali, I., Karim, S., da Silva Vaz, I., Jr., Termignoni, C. (2015a). Probing the Functional Role of Tick Metalloproteases. Physiol. Entomol. 40 (3), 177–188. doi: 10.1111/phen.12104
Ali, A., Mulenga, A., Vaz, I. S., Jr. (2020a). Tick and Tick-Borne Pathogens: Molecular and Immune Targets for Control Strategies. Front. Physiol. 11, 744. doi: 10.3389/fphys.2020.00744
Ali, A., Tirloni, L., Isezaki, M., Seixas, A., Konnai, S., Ohashi, K., et al. (2014). Reprolysin Metalloproteases From Ixodes Persulcatus, Rhipicephalus Sanguineus and Rhipicephalus Microplus Ticks. Exp. Appl. Acarol. 63 (4), 559–578. doi: 10.1007/s10493-014-9796-9
Aljamali, M. N., Hern, L., Kupfer, D., Downard, S., So, S., Roe, B. A., et al. (2009). Transcriptome Analysis of the Salivary Glands of the Female Tick Amblyomma Americanum (Acari: Ixodidae). Insect Mol. Biol. 18 (2), 129–154. doi: 10.1111/j.1365-2583.2009.00863.x
Almazán, C., Fourniol, L., Rakotobe, S., Šimo, L., Bornères, J., Cote, M., et al. (2020). Failed Disruption of Tick Feeding, Viability, and Molting After Immunization of Mice and Sheep With Recombinant Ixodes Ricinus Salivary Proteins IrSPI and Irlip1. Vaccines 8 (3), 475. doi: 10.3390/vaccines8030475
Anatriello, E., Ribeiro, J. M. C., de Miranda-Santos, I. K. F., Brandão, L. G., Anderson, J. M., Valenzuela, J. G., et al. (2010). An Insight Into the Sialotranscriptome of the Brown Dog Tick, Rhipicephalus Sanguineus. BMC Genom. 11, 1–17. doi: 10.1186/1471-2164-11-450
Anderson, J. F., Magnarelli, L. A. (2008). Biology of Ticks. Infect. Dis. Clin. N. Am. 22, 195–215. doi: 10.1016/j.idc.2007.12.006
Andrade, B. B., Teixeira, C. R., Barral, A., Barral-Netto, M. (2005). Haematophagous Arthropod Saliva and Host Defense System: A Tale of Tear and Blood. An. Acad. Bras. Cienc. 77, 665–693. doi: 10.1590/S0001-37652005000400008
Anguita, J., Ramamoorthi, N., Hovius, J. W., Das, S., Thomas, V., Persinski, R., et al. (2002). Salp15, an Ixodes Scapularis Salivary Protein, Inhibits CD4+ T Cell Activation. Immunity 16 (6), 849–859. doi: 10.1016/S1074-7613(02)00325-4
Anisuzzaman, M., Hatta, T., Miyoshi, T., Matsubayashi, M., Islam, M. K., Alim, M. A., et al. (2014). Longistatin in Tick Saliva Blocks Advanced Glycation End-Product Receptor Activation. J. Clin. Invest. 124 (10), 4429–4444. doi: 10.1172/JCI74917
Anisuzzaman, Islam, M. K., Alim, M. A., Miyoshi, T., Hatta, T., Yamaji, K., et al. (2011). Longistatin, a Plasminogen Activator, is Key to the Availability of Blood-Meals for Ixodid Ticks. PloS Pathog. 7 (3), e1001312. doi: 10.1371/journal.ppat.1001312
Anisuzzaman, M., Islam, K., Miyoshi, T., Alim, M. A., Hatta, T., Yamaji, K., et al. (2010). Longistatin, a Novel EF-Hand Protein From the Ixodid Tick Haemaphysalis Longicornis, is Required for Acquisition of Host Blood-Meals. Int. J. Parasitol. 40 (6), 721–729. doi: 10.1016/j.ijpara.2009.11.004
Antunes, S., Couto, J., Ferrolho, J., Rodrigues, F., Nobre, J., Santos, A. S., et al. (2018). Rhipicephalus Bursa Sialotranscriptomic Response to Blood Feeding and Babesia Ovis Infection: Identification of Candidate Protective Antigens. Front. Cell. Infect. Microbiol. 8. doi: 10.3389/fcimb.2018.00116
Antunes, S., Galindo, R. C., Almazán, C., Rudenko, N., Golovchenko, M., Grubhoffer, L., et al. (2012). Functional Genomics Studies of Rhipicephalus (Boophilus) Annulatus Ticks in Response to Infection With the Cattle Protozoan Parasite, Babesia Bigemina. Int. J. Parasitol. 42, 187–195. doi: 10.1016/j.ijpara.2011.12.003
Aounallah, H., Bensaoud, C., M’ghirbi, Y., Faria, F., Chmelar, J., Kotsyfakis, M. (2020). Tick Salivary Compounds for Targeted Immunomodulatory Therapy. Front. Immunol. 11. doi: 10.3389/fimmu.2020.583845
Artigas-Jerónimo, S., de la Fuente, J., Villar, M. (2018). Interactomics and Tick Vaccine Development: New Directions for the Control of Tick-Borne Diseases. Expert. Rev. Proteom. 15 (8), 627–635. doi: 10.1080/14789450.2018.1506701
Assumpção, T. C., Mizurini, D. M., Ma, D., Monteiro, R. Q., Ahlstedt, S., Reyes, M., et al. (2018). Ixonnexin From Tick Saliva Promotes Fibrinolysis by Interacting With Plasminogen and Tissue-Type Plasminogen Activator, and Prevents Arterial Thrombosis. Sci. Rep. 8 (1), 1–12. doi: 10.1038/s41598-018-22780-1
Bakshi, M., Kim, T. K., Porter, L., Mwangi, W., Mulenga, A. (2019). Amblyomma Americanum Ticks Utilizes Countervailing Pro and Anti-Inflammatory Proteins to Evade Host Defense. PloS Pathog. 15 (11), 1–24. doi: 10.1371/journal.ppat.1008128
Barnard, A. C., Nijhof, A. M., Fick, W., Stutzer, C., Maritz-Olivier, C. (2012). RNAi in Arthropods: Insight Into the Machinery and Applications for Understanding the Pathogen-Vector Interface. Genes 3 (4), 702–741. doi: 10.3390/genes3040702
Barrero, R. A., Guerrero, F. D., Black, M., McCooke, J., Chapman, B., Schilkey, F., et al. (2017). Gene-Enriched Draft Genome of the Cattle Tick Rhipicephalus Microplus: Assembly by the Hybrid Pacific Biosciences/Illumina Approach Enabled Analysis of the Highly Repetitive Genome. Int. J. Parasitol. 47 (9), 569–583. doi: 10.1016/j.ijpara.2017.03.007
Barrero, R. A., Keeble-Gagnère, G., Zhang, B., Moolhuijzen, P., Ikeo, K., Tateno, Y., et al. (2011). Evolutionary Conserved microRNAs are Ubiquitously Expressed Compared to Tick-Specific miRNAs in the Cattle Tick Rhipicephalus (Boophilus) Microplus. BMC Genom. 12 (1), 1–17. doi: 10.1186/1471-2164-12-328
Bartíková, P., Kazimírová, M., Štibrániová, I. (2020). Ticks and the Effects of Their Saliva on Growth Factors Involved in Skin Wound Healing. J. Venom. Res. 10, 45–52.
Bastiani, M., Hillebrand, S., Horn, F., Kist, T. B. L., Guimarães, J. A., Termignoni, C. (2002). Cattle Tick Boophilus Microplus Salivary Gland Contains a Thiol-Activated Metalloendopeptidase Displaying Kininase Activity. Insect Biochem. Mol. 32 (11), 1439–1446. doi: 10.1016/S0965-1748(02)00064-4
Batista, I. F., Ramos, O. H., Ventura, J. S., Junqueira-de-Azevedo, I. L., Ho, P. L., Chudzinski-Tavassi, A. M. (2010). A New Factor Xa Inhibitor From Amblyomma Cajennense With a Unique Domain Composition. Arch. Biochem. Biophys. 493, 151–156. doi: 10.1016/j.abb.2009.10.009
Beaufays, J., Adam, B., Menten-Dedoyart, C., Fievez, L., Grosjean, A., Decrem, Y., et al. (2008). Ir-LBP, an Ixodes Ricinus Tick Salivary LTB4-Binding Lipocalin, Interferes With Host Neutrophil Function. PloS One 3 (12), 1–13. doi: 10.1371/journal.pone.0003987
Bensaoud, C., Aounallah, H., Sciani, J. M., Faria, F., Chudzinski-Tavassi, A. M., Bouattour, A., et al. (2019a). Proteomic Informed by Transcriptomic for Salivary Glands Components of the Camel Tick Hyalomma Dromedarii. BMC Genom. 20 (1), 1–12. doi: 10.1186/s12864-019-6042-1
Bensaoud, C., Hackenberg, M., Kotsyfakis, M. (2019b). Noncoding RNAs in Parasite–Vector–Host Interactions. Trends Parasitol. 35 (9), 715–724. doi: 10.1016/j.pt.2019.06.012
Bergman, D. K., Palmer, M. J., Caimano, M. J., Radolf, J. D., Wikel, S. K. (2000). Isolation and Molecular Cloning of a Secreted Immunosuppressant Protein From Dermacentor Andersoni Salivary Gland. J. Parasitol. 86 (3), 516–525. doi: 10.1645/0022-3395(2000)086[0516:IAMCOA]2.0.CO;2
Bhowmick, B., Han, Q. (2020). Understanding Tick Biology and its Implications in Anti-Tick and Transmission Blocking Vaccines Against Tick-Borne Pathogens. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00319
Bhusal, R. P., Eaton, J. R. O., Chowdhury, S. T., Power, C. A., Proudfoot, A. E. I., Stone, M. J., et al. (2020). Evasins: Tick Salivary Proteins That Inhibit Mammalian Chemokines. Trends Biochem. Sci. 45. doi: 10.3389/fimmu.2019.03067
Blecha, I. M. Z., Csordas, B. G., Aguirre, A. D. A. R., Cunha, R. C., Garcia, M. V., Andreotti, R. (2018). Analysis of Bm86 Conserved Epitopes: Is a Global Vaccine Against Cattle Tick Rhipicephalus Microplus Possible? Rev. Bras. Parasitol. Vet. 27, 267–279. doi: 10.1590/S1984-296120180056
Blisnick, A. A., Šimo, L., Grillon, C., Fasani, F., Brûlé, S., Le Bonniec, B., et al. (2019). The Immunomodulatory Effect of IrSPI, a Tick Salivary Gland Serine Protease Inhibitor Involved in Ixodes Ricinus Tick Feeding. Vaccines 7 (4), 1–28. doi: 10.3390/vaccines7040148
Bonnet, S. I., Pollet, T. (2021). Update on the Intricate Tango Between Tick Microbiomes and Tick-Borne Pathogens. Parasite Immunol. 43 (5), 1–14. doi: 10.1111/pim.12813
Boppana, D. K., Dhinakar Raj, G., John, L., Wikel, S. K., Latha, B. R., Gomathinayagam, S. (2004). In Vivo Immunomodulatory Effects of Ixodid Ticks on Ovine Circulating T-And B-Lymphocytes. Parasite Immunol. 26 (2), 83–93. doi: 10.1111/j.0141-9838.2004.00687.x
Boulanger, N., Wikel, S. (2021). Induced Transient Immune Tolerance in Ticks and Vertebrate Host: A Keystone of Tick-Borne Diseases? Front. Immunol. 12. doi: 10.3389/fimmu.2021.625993
Brahma, R. K., Blanchet, G., Kaur, S., Kini, R. M., Doley, R. (2017). Expression and Characterization of Haemathrins, Madanin-Like Thrombin Inhibitors, Isolated From the Salivary Gland of Tick Haemaphysalis Bispinosa (Acari: Ixodidae). Thromb. Res. 152, 20–29. doi: 10.1016/j.thromres.2017.01.012
Branco, V. G., Iqbal, A., Alvarez-Flores, M. P., Sciani, J. M., de Andrade, S. A., Iwai, L. K., et al. (2016). Amblyomin-X Having a Kunitz-Type Homologous Domain, is a Noncompetitive Inhibitor of fXa and Induces Anticoagulation In Vitro and In Vivo. Biochim. Biophys. Acta 1864 (10), 1428–1435. doi: 10.1016/j.bbapap.2016.07.011
Brossard, M., Wikel, S. K. (2004). Tick Immunobiology. Parasitology 129 (S1), S161–S176. doi: 10.1017/s0031182004004834
Budachetri, K., Kumar, D., Crispell, G., Beck, C., Dasch, G., Karim, S. (2018). The Tick Endosymbiont Candidatus Midichloria Mitochondrii and Selenoproteins are Essential for the Growth of Rickettsia Parkeri in the Gulf Coast Tick Vector. Microbiome 6 (1), 1–15. doi: 10.1186/s40168-018-0524-2
Bullard, R. L. (2016). Characterization of Glycine Rich Proteins From the Salivary Glands of the Lone Star Tick Amblyomma Americanum (Hattiesburg: The University of Southern Mississippi. Dissertations), 339.
Bullard, R., Sharma, S. R., Das, P. K., Morgan, S. E., Karim, S. (2019). Repurposing of Glycine-Rich Proteins in Abiotic and Biotic Stresses in the Lone-Star Tick (Amblyomma Americanum). Front. Physiol. 10. doi: 10.3389/fphys.2019.00744
Cabezas-Cruz, A., Alberdi, P., Ayllón, N., Valdés, J. J., Pierce, R., Villar, M., et al. (2016). Anaplasma Phagocytophilum Increases the Levels of Histone Modifying Enzymes to Inhibit Cell Apoptosis and Facilitate Pathogen Infection in the Tick Vector Ixodes Scapularis. Epigenetics 11, 303–319. doi: 10.1080/15592294.2016.1163460
Cao, J., Shi, L., Zhou, Y., Gao, X., Zhang, H., Gong, H., et al. (2013). Characterization of a New Kunitz-Type Serine Protease Inhibitor From the Hard Tick Rhipicephalus Haemaphysaloides. Arch. Insect Biochem. Physiol. 84 (2), 104–113. doi: 10.1002/arch.21118
Carneiro-Lobo, T. C., Konig, S., Machado, D. E., Nasciutti, L. E., Forni, M. F., Francischetti, I. M., et al. (2009). Ixolaris, a Tissue Factor Inhibitor, Blocks Primary Tumor Growth and Angiogenesis in a Glioblastoma Model. J. Thromb. Haemost. 7 (11), 1855–1864. doi: 10.1111/j.1538-7836.2009.03553.x
Carneiro-Lobo, T. C., Schaffner, F., Disse, J., Ostergaard, H., Francischetti, I. M. B., Monteiro, R. Q., et al. (2012). The Tick-Derived Inhibitor Ixolaris Prevents Tissue Factor Signaling on Tumor Cells. J. Thromb. Haemost. 10 (9), 1849–1858. doi: 10.1111/j.1538-7836.2012.04864.x
Chand, K. K., Lee, K. M., Lavidis, N. A., Rodriguez-Valle, M., Ijaz, H., Koehbach, J., et al. (2016). Tick Holocyclotoxins Trigger Host Paralysis by Presynaptic Inhibition. Sci. Rep. 6 (1), 1–4. doi: 10.1038/srep29446
Chávez, A. S. O., O’Neal, A. J., Santambrogio, L., Kotsyfakis, M., Pedra, J. H. F. (2019). Message in a Vesicle–Trans-Kingdom Intercommunication at the Vector-Host Interface. J. Cell. Sci. 132, jcs224212. doi: 10.1242/jcs.224212
Chávez, A. S. O., Wang, X., Marnin, L., Archer, N. K., Hammond, H. L., Carroll, E. E. M., et al. (2021). Tick Extracellular Vesicles Enable Arthropod Feeding and Promote Distinct Outcomes of Bacterial Infection. Nat. Commun. 12 (1), 1–17. doi: 10.1038/s41467-021-23900-8
Chen, J., Ao, L., Yang, J. (2019). Long Non-Coding RNAs in Diseases Related to Inflammation and Immunity. Ann. Transl. Med. 7 (18), 1–11. doi: 10.21037/atm.2019.08.37
Cheng, Y., Wu, H., Li, D. (1999). An Inhibitor Selective for Collagen-Stimulated Platelet Aggregation From the Salivary Glands of Hard Tick Haemaphysalis Longicornis and its Mechanism of Action. Sci. China C Life. Sci. 42, 457–464. doi: 10.1007/BF02881768
Chen, X., Liang, H., Zhang, J., Zen, K., Zhang, C. Y. (2012). Secreted microRNAs: A New Form of Intercellular Communication. Trends Cell Biol. 22, 125–132. doi: 10.1016/j.tcb.2011.12.001
Chlastáková, A., Kotál, J., Beránková, Z., Kaščáková, B., Martins, L. A., Langhansová, H., et al. (2021). Iripin-3, a New Salivary Protein Isolated From Ixodes Ricinus Ticks, Displays Immunomodulatory and Anti-Hemostatic Properties In Vitro. Front. Immunol. 12. doi: 10.3389/fimmu.2021.626200
Chmelař, J., Calvo, E., Pedra, J. H. F., Francischetti, I. M. B., Kotsyfakis, M. (2012). Tick Salivary Secretion as a Source of Antihemostatics. J. Proteom. 75, 3842–3854. doi: 10.1016/j.jprot.2012.04.026
Chmelař, J., Kotál, J., Kovaříková, A., Kotsyfakis, M. (2019). The Use of Tick Salivary Proteins as Novel Therapeutics. Front. Physiol. 10. doi: 10.3389/fphys.2019.00812
Chmelař, J., Kotál, J., Langhansová, H., Kotsyfakis, M. (2017). Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-Host-Pathogen Interaction. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00216
Chmelař, J., Oliveira, C. J., Rezacova, P., Francischetti, I. M., Kovarova, Z., Pejler, G., et al. (2011). A Tick Salivary Protein Targets Cathepsin G and Chymase and Inhibits Host Inflammation and Platelet Aggregation. Blood 117 (2), 736–744. doi: 10.1182/blood-2010-06-293241
Chudzinski-Tavassi, A. M., De-Sá-Júnior, P. L., Simons, S. M., Maria, D. A., de Souza Ventura, J., Batista, I. D. F. C., et al. (2010). A New Tick Kunitz Type Inhibitor, Amblyomin-X, Induces Tumor Cell Death by Modulating Genes Related to the Cell Cycle and Targeting the Ubiquitin-Proteasome System. Toxicon 56 (7), 1145–1154. doi: 10.1016/j.toxicon.2010.04.019
Ciprandi, A., de Oliveira, S. K., Masuda, A., Horn, F., Termignoni, C. (2006). Boophilus Microplus: Its Saliva Contains Microphilin, a Small Thrombin Inhibitor. Exp. Parasitol. 114, 40–46. doi: 10.1016/j.exppara.2006.02.010
Costa, G. C. A., Ribeiro, I. C. T., Melo-Junior, O., Gontijo, N. F., Sant’Anna, M. R., Pereira, M. H., et al. (2021). Amblyomma Sculptum Salivary Protease Inhibitors as Potential Anti-Tick Vaccines. Front. Immunol. 11. doi: 10.3389/fimmu.2020.611104
Cotté, V., Sabatier, L., Schnell, G., Carmi-Leroy, A., Rousselle, J. C., Arsène-Ploetze, F., et al. (2014). Differential Expression of Ixodes Ricinus Salivary Gland Proteins in the Presence of the Borrelia Burgdorferi Sensu Lato Complex. J. Proteomics 96, 29–43. doi: 10.1016/j.jprot.2013.10.033
Coumou, J., Wagemakers, A., Narasimhan, S., Schuijt, T. J., Ersoz, J. I., Oei, A., et al. (2019). The Role of Mannose Binding Lectin in the Immune Response Against Borrelia Burgdorferi Sensu Lato. Sci. Rep. 9 (1), 1–11. doi: 10.1038/s41598-018-37922-8
Coutinho, M. L., Bizzarro, B., Tirloni, L., Berger, M., Oliveira, C. J. F., Sa-Nunes, A., et al. (2020). Rhipicephalus Microplus Serpins Interfere With Host Immune Responses by Specifically Modulating Mast Cells and Lymphocytes. Ticks Tick Borne Dis. 11 (4), 1–32. doi: 10.1016/j.ttbdis.2020.101425
Couto, J., Seixas, G., Stutzer, C., Olivier, N. A., Maritz-Olivier, C., Antunes, S., et al. (2021). Probing the Rhipicephalus Bursa Sialomes in Potential Anti-Tick Vaccine Candidates: A Reverse Vaccinology Approach. Biomedicines 9 (4), 363. doi: 10.3390/biomedicines9040363
Cramaro, W. J., Revets, D., Hunewald, O. E., Sinner, R., Reye, A. L., Muller, C. P., et al. (2015). Integration of Ixodes Ricinus Genome Sequencing With Transcriptome and Proteome Annotation of the Naïve Midgut. BMC Genomics 16, 871. doi: 10.1186/s12864-015-1981-7
Dai, J., Narasimhan, S., Zhang, L., Liu, L., Wang, P., Fikrig, E. (2010). Tick Histamine Release Factor is Critical for Ixodes Scapularis Engorgement and Transmission of the Lyme Disease Agent. PloS Pathog. 6 (11), e1001205. doi: 10.1371/journal.ppat.1001205
Dai, J., Wang, P., Adusumilli, S., Booth, C. J., Narasimhan, S., Anguita, J., et al. (2009). Antibodies Against a Tick Protein, Salp15, Protect Mice From the Lyme Disease Agent. Cell Host Microbe 6 (5), 82–92. doi: 10.1016/j.chom.2009.10.006
Dai, S. X., Zhang, A. D., Huang, J. F. (2012). Evolution, Expansion and Expression of the Kunitz/BPTI Gene Family Associated With Long-Term Blood Feeding in Ixodes Scapularis. BMC Evol. Biol. 12 (1), 1–16. doi: 10.1186/1471-2148-12-4
Davies, M. R., McIntyre, L., Mutreja, A., Lacey, J. A., Lees, J. A., Towers, R. J., et al. (2019). Atlas of Group A Streptococcal Vaccine Candidates Compiled Using Large-Scale Comparative Genomics. Nat. Genet. 51 (6), 1035–1043. doi: 10.1038/s41588-019-0417-8
Déruaz, M., Frauenschuh, A., Alessandri, A. L., Dias, J. M., Coelho, F. M., Russo, R. C., et al. (2008). Ticks Produce Highly Selective Chemokine Binding Proteins With Antiinflammatory Activity. J. Exp. Med. 205 (9), 2019–2031. doi: 10.1084/jem.20072689
De Castro, M. H., De Klerk, D., Pienaar, R., Latif, A. A., Rees, D. J. G., Mans, B. J. (2016). De Novo Assembly and Annotation of the Salivary Gland Transcriptome of Rhipicephalus Appendiculatus Male and Female Ticks During Blood Feeding. Ticks Tick Borne Dis. 7, 536–548. doi: 10.1016/j.ttbdis.2016.01.014
De Castro, M. H., De Klerk, D., Pienaar, R., Rees, D. J. G., Mans, B. J. (2017). Sialotranscriptomics of Rhipicephalus Zambeziensis Reveals Intricate Expression Profiles of Secretory Proteins and Suggests Tight Temporal Transcriptional Regulation During Blood-Feeding. Parasitol. Vectors 10 (1), 1–20. doi: 10.1186/s13071-017-2312-4
Decrem, Y., Beaufays, J., Blasioli, V., Lahaye, K., Brossard, M., Vanhamme, L., et al. (2008). A Family of Putative Metalloproteases in the Salivary Glands of the Tick Ixodes Ricinus. FEBS J. 275 (7), 1485–1499. doi: 10.1111/j.1742-4658.2008.06308.x
Decrem, Y., Rath, G., Blasioli, V., Cauchie, P., Robert, S., Beaufays, J., et al. (2009). Ir-CPI, a Coagulation Contact Phase Inhibitor From the Tick Ixodes Ricinus, Inhibits Thrombus Formation Without Impairing Hemostasis. J. Exp. Med. 206 (11), 2381–2395. doi: 10.1084/jem.20091007
De la Fuente, J., Antunes, S., Bonnet, S., Cabezas-Cruz, A., Domingos, A. G., Estrada-Peña, A., et al. (2017). Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00114
De la Fuente, J., Contreras, M. (2015). Tick Vaccines: Current Status and Future Directions. Expert Rev. Vaccines 14 (10), 1367–1376. doi: 10.1586/14760584.2015.1076339
De la Fuente, J., Estrada-Peña, A., Contreras, M. (2020). Modeling Tick Vaccines: A Key Tool to Improve Protection Efficacy. Expert Rev. Vaccines 19 (3), 217–225. doi: 10.1080/14760584.2020.1745635
De la Fuente, J., Waterhouse, R. M., Sonenshine, D. E., Roe, R. M., Ribeiro, J. M. C., Sattelle, D. B. (2016). Tick Genome Assembled: New Opportunities for Research on Tick-Host-Pathogen Interactions. Front. Cell. Infect. Microbiol. 6, 103. doi: 10.3389/fcimb.2016.00103
Denisov, S. S., Dijkgraaf, I. (2021). Immunomodulatory Proteins in Tick Saliva From a Structural Perspective. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.769574
De Oliveira, A. D. S., Lima, L. G., Mariano-Oliveira, A., Machado, D. E., Nasciutti, L. E., Andersen, J. F., et al. (2012). Inhibition of Tissue Factor by Ixolaris Reduces Primary Tumor Growth and Experimental Metastasis in a Murine Model of Melanoma. Thromb. Res. 130 (3), 163–170. doi: 10.1016/j.thromres.2012.05.021
De Taeye, S. W., Kreuk, L., van Dam, A. P., Hovius, J. W., Schuijt, T. J. (2013). Complement Evasion by Borrelia Burgdorferi: It Takes Three to Tango. Trends Parasitol. 29 (3), 119–128. doi: 10.1016/j.pt.2012.12.001
Díaz-Martín, V., Manzano-Román, R., Obolo-Mvoulouga, P., Oleaga, A., Pérez-Sánchez, R. (2015). Development of Vaccines Against Ornithodoros Soft Ticks: An Update. Ticks Tick Borne Dis. 6 (3), 211–220. doi: 10.1016/j.ttbdis.2015.03.006
Díaz-Martín, V., Manzano-Román, R., Valero, L., Oleaga, A., Encinas-Grandes, A., Pérez-Sánchez, R. (2013). An Insight Into the Proteome of the Saliva of the Argasid Tick Ornithodoros Moubata Reveals Important Differences in Saliva Protein Composition Between the Sexes. J. Proteom. 80. doi: 10.3389/fcimb.2017.00476
Dickinson, D. A., Forman, H. J. (2002). Cellular Glutathione and Thiols Metabolism. Biochem. Pharmacol. 64 (5-6), 1019–1026. doi: 10.1016/S0006-2952(02)01172-3
Dulipati, V., Meri, S., Panelius, J. (2020). Complement Evasion Strategies of Borrelia Burgdorferi Sensu Lato. FEBS Lett. 594, 2645–2656. doi: 10.1002/1873-3468.13894
Dupejova, J., Sterba, J., Vancova, M., Grubhoffer, L. (2011). Hemelipoglycoprotein From the Ornate Sheep Tick, Dermacentor Marginatus: Structural and Functional Characterization. Parasitol. Vectors 4 (1), 1–10. doi: 10.1186/1756-3305-4-4
Ekimler, S., Sahin, K. (2014). Computational Methods for microRNA Target Prediction. Genes 5 (3), 671–683. doi: 10.3390/genes5030671
Esteves, E., Maruyama, S. R., Kawahara, R., Fujita, A., Martins, L. A., Righi, A. A., et al. (2017). Analysis of the Salivary Gland Transcriptome of Unfed and Partially Fed Amblyomma Sculptum Ticks and Descriptive Proteome of the Saliva. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00476
Fontaine, A., Diouf, I., Bakkali, N., Missé, D., Pagès, F., Fusai, T., et al. (2011). Implication of Haematophagous Arthropod Salivary Proteins in Host-Vector Interactions. Parasitol. Vectors 4 (1), 1–57. doi: 10.1186/1756-3305-4-187
Francischetti, I., Anderson, J. M., Manoukis, N., Pham, V. M., Ribeiro, J. (2011). An Insight Into the Sialotranscriptome and Proteome of the Coarse Bontlegged Tick Hyalomma Marginatum Rufipes. J. Proteom. 74 (12), 2892–2908. doi: 10.1016/j.jprot.2011.07.015
Francischetti, I. M., Mans, B. J., Meng, Z., Gudderra, N., Veenstra, T. D., Pham, V. M., et al. (2008). An Insight Into the Sialome of the Soft Tick, Ornithodoros Parkeri. Insect. Biochem. Mol. Biol. 38, 1–21. doi: 10.1016/j.ibmb.2007.09.009
Francischetti, I. M., Mather, T. N., Ribeiro, J. M. (2003). Cloning of a Salivary Gland Metalloprotease and Characterization of Gelatinase and Fibrin(Ogen)Lytic Activities in the Saliva of the Lyme Disease Tick Vector Ixodes Scapularis. Biochem. Biophys. Res. Commun. 305, 869–875. doi: 10.1016/s0006-291x(03)00857-x
Francischetti, I. M., Mather, T. N., Ribeiro, J. M. (2004). Penthalaris, a Novel Recombinant five-Kunitz Tissue Factor Pathway Inhibitor (TFPI) From the Salivary Gland of the Tick Vector of Lyme Disease, Ixodes Scapularis. Thromb. Haemost. 91, 886–898. doi: 10.1160/TH03-11-0715
Francischetti, I. M., Mather, T. N., Ribeiro, J. M. (2005a). Tick Saliva is a Potent Inhibitor of Endothelial Cell Proliferation and Angiogenesis. Thromb. Haemost. 94, 167–174. doi: 10.1160/TH04-09-0566
Francischetti, I. M., Pham, V. M., Mans, B. J., Andersen, J. F., Mather, T. N., Lane, R. S., et al. (2005b). The Transcriptome of the Salivary Glands of the Female Western Black-Legged Tick Ixodes Pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 35, 1142–1161. doi: 10.1016/j.ibmb.2005.05.007
Francischetti, I. M., Sa-Nunes, A., Mans, B. J., Santos, I. M., Ribeiro, J. M. (2009). The Role of Saliva in Tick Feeding. Front. Biosci. 14, 2051–2088. doi: 10.2741/3363
Francischetti, I. M., Valenzuela, J. G., Andersen, J. F., Mather, T. N., Ribeiro, J. M. (2002). Ixolaris, a Novel Recombinant Tissue Factor Pathway Inhibitor (TFPI) From the Salivary Gland of the Tick, Ixodes Scapularis: Identification of Factor X and Factor Xa as Scaffolds for the Inhibition of Factor VIIa/tissue Factor Complex. Blood 99 (10), 3602–3612. doi: 10.1182/blood-2001-12-0237
Franco, P. F., Silva, N. C., do Vale, V. F., Abreu, J. F., Santos, V. C., Gontijo, N. F., et al. (2016). Inhibition of the Classical Pathway of the Complement System by Saliva of Amblyomma Cajennense (Acari: Ixodidae). Exp. Parasitol. 164, 91–96. doi: 10.1016/j.exppara.2016.03.002
Frauenschuh, A., Power, C. A., Déruaz, M., Ferreira, B. R., Silva, J. S., Teixeira, M. M., et al. (2007). Molecular Cloning and Characterization of a Highly Selective Chemokine-Binding Protein From the Tick Rhipicephalus Sanguineus. J. Biol. Chem. 282 (37), 27250–27258. doi: 10.1074/jbc.M704706200
Fukumoto, S., Sakaguchi, T., You, M., Xuan, X., Fujisaki, K. (2006). Tick Troponin I-Like Molecule is a Potent Inhibitor for Angiogenesis. Microvasc. Res. 71 (3), 218–221. doi: 10.1016/j.mvr.2006.02.003
Gao, X., Shi, L., Zhou, Y., Cao, J., Zhang, H., Zhou, J. (2011). Characterization of the Anticoagulant Protein Rhipilin-1 From the Rhipicephalus Haemaphysaloides Tick. J. Insect Physiol. 57, 339–343. doi: 10.1016/j.jinsphys.2010.12.001
Garcia, G. R., Gardinassi, L. G., Ribeiro, J. M. C., Anatriello, E., Ferreira, B. R., Moreira, H. N. S. (2014). The Sialotranscriptome of Amblyomma Triste, Amblyomma Parvum and Amblyomma Cajennense Ticks, Uncovered by 454-Based RNA-Seq. Parasitol. Vectors 7, 430. doi: 10.1186/1756-3305-7-430
Garcia, G. R., Ribeiro, J. M. C., Maruyama, S. R., Gardinassi, L. G., Nelson, K., Ferreira, B. R., et al. (2020). A Transcriptome and Proteome of the Tick Rhipicephalus Microplus Shaped by the Genetic Composition of its Hosts and Developmental Stage. Sci. Rep. 10 (1), 1–23. doi: 10.1038/s41598-020-69793-3
García-Varas, S., Manzano-Román, R., Fernández-Soto, P., Encinas-Grandes, A., Oleaga, A., Pérez-Sánchez, R. (2010). Purification and Characterisation of a P-Selectin-Binding Molecule From the Salivary Glands of Ornithodoros Moubata That Induces Protective Anti-Tick Immune Responses in Pigs. Int. J. Parasitol. 40 (3), 313–326. doi: 10.1016/j.ijpara.2009.08.011
Garg, R., Juncadella, I. J., Ramamoorthi, N., Ananthanarayanan, S. K., Thomas, V., Rincón, M., et al. (2006). Cutting Edge: CD4 is the Receptor for the Tick Saliva Immunosuppressor, Salp15. J. Immunol. 177 (10), 6579–6583. doi: 10.4049/jimmunol.177.10.6579
Gaspar, A. R., Joubert, A. M., Crause, J. C., Neitz, A. W. (1996). Isolation and Characterization of an Anticoagulant From the Salivary Glands of the Tick, Ornithodoros Savignyi (Acari Argasidae). Exp. Appl. Acarol. 20, 583–598. doi: 10.1007/BF00052809
Gillespie, R. D., Dolan, M. C., Piesman, J., Titus, R. G. (2001). Identification of an IL-2 Binding Protein in the Saliva of the Lyme Disease Vector Tick, Ixodes Scapularis. J. Immunol. 166, 4319–4326. doi: 10.4049/jimmunol.166.7.4319
Giraldo-Calderón, G. I., Emrich, S. J., MacCallum, R. M., Maslen, G., Dialynas, E., Topalis, P., et al. (2015). VectorBase: An Updated Bioinformatics Resource for Invertebrate Vectors and Other Organisms Related With Human Diseases. Nucleic Acids Res. 43 (D1), D707–D713. doi: 10.1093/nar/gku1117
Guerrero, F. D., Moolhuijzen, P., Peterson, D. G., Bidwell, S., Caler, E., Bellgard, M., et al. (2010). Reassociation Kinetics-Based Approach for Partial Genome Sequencing of the Cattle Tick, Rhipicephalus (Boophilus) Microplus. BMC Genom. 11, 374. doi: 10.1186/1471-2164-11-374
Guerrero, F. D., Nene, V. M., George, J. E., Barker, S. C., Willadsen, P. (2006). Sequencing a New Target Genome: The Boophilus Microplus (Acari: Ixodidae) Genome Project. J. Med. Entomol. 43, 9–16. doi: 10.1093/jmedent/43.1.9
Gulia-Nuss, M., Nuss, A. B., Meyer, J. M., Sonenshine, D. E., Roe, R. M., Waterhouse, R. M., et al. (2016). Genomic Insights Into the Ixodes Scapularis Tick Vector of Lyme Disease. Nat. Commun. 7 (1), 1–13. doi: 10.1038/ncomms10507
Guo, X., Booth, C. J., Paley, M. A., Wang, X., DePonte, K., Fikrig, E., et al. (2009). Inhibition of Neutrophil Function by Two Tick Salivary Proteins. Infect. Immun. 77 (6), 2320–2329. doi: 10.1128/IAI.01507-08
Hackenberg, M., Kotsyfakis, M. (2018). Exosome-Mediated Pathogen Transmission by Arthropod Vectors. Trends Parasitol. 34, 49–52. doi: 10.1016/j.pt.2018.04.001
Hackenberg, M., Langenberger, D., Schwarz, A., Erhart, J., Kotsyfakis, M. (2017). Insilico Target Network Analysis of De Novo-Discovered, Tick Saliva-Specific microRNAs Reveals Important Combinatorial Effects in Their Interference With Vertebrate Host Physiology. RNA 23, 1259–1269. doi: 10.1261/rna.061168.117
Hannier, S., Liversidge, J., Sternberg, J. M., Bowman, A. S. (2004). Characterization of the B-Cell Inhibitory Protein Factor in Ixodes Ricinus Tick Saliva: A Potential Role in Enhanced Borrelia Burgdorferi Transmission. Immunology 113 (3), 401–408. doi: 10.1111/j.1365-2567.2004.01975.x
Hao, J., Luo, J., Chen, Z., Ren, Q., Guo, J., Liu, X., et al. (2017). MicroRNA-275 and its Target Vitellogenin-2 are Crucial in Ovary Development and Blood Digestion of Haemaphysalis Longicornis. Parasitol. Vectors 10 (1), 1–9. doi: 10.1186/s13071-017-2153-1
Havlíková, S., Roller, L., Koči, J., Trimnell, A. R., Kazimírová, M., Klempa, B., et al. (2009). Functional Role of 64P, the Candidate Transmission-Blocking Vaccine Antigen From the Tick, Rhipicephalus Appendiculatus. Int. J. Parasitol. 39 (13), 1485–1494. doi: 10.1016/j.ijpara.2009.05.005
Heinze, D. M., Carmical, J. R., Aronson, J. F., Thangamani, S. (2012). Early Immunologic Events at the Tick-Host Interface. PloS One 7 (10), 1–11. doi: 10.1371/journal.pone.0047301
Hermance, M. E., Widen, S. G., Wood, T. G., Thangamani, S. (2019). Ixodes Scapularis Salivary Gland microRNAs are Differentially Expressed During Powassan Virus Transmission. Sci. Rep. 9 (1), 1–17. doi: 10.1038/s41598-019-49572-5
Hidano, A., Konnai, S., Yamada, S., Githaka, N., Isezaki, M., Higuchi, H., et al. (2014). Suppressive Effects of Neutrophil by Salp16-Like Salivary Gland Proteins From Ixodes Persulcatus Schulze Tick. Insect Mol. Biol. 23 (4), 466–474. doi: 10.1111/imb.12101
Hilger, C., Bessot, J. C., Hutt, N., Grigioni, F., De Blay, F., Pauli, G., et al. (2005). IgE-Mediated Anaphylaxis Caused by Bites of the Pigeon Tick Argas Reflexus: Cloning and Expression of the Major Allergen Arg R 1. J. Allergy Clin. Immunol. 115 (3), 617–622. doi: 10.1016/j.jaci.2004.11.052
Hill, C. A., Wikel, S. K. (2005). The Ixodes Scapularis Genome Project: An Opportunity for Advancing Tick Research. Trends Parasitol. 21, 151–153. doi: 10.1016/j.pt.2005.02.004
Hollmann, T., Kim, T. K., Tirloni, L., Radulović, Ž. M., Pinto, A. F., Diedrich, J. K., et al. (2018). Identification and Characterization of Proteins in the Amblyomma Americanum Tick Cement Cone. Int. J. Parasitol. 48 (3-4), 211–224. doi: 10.1016/j.ijpara.2017.08.018
Horn, F., dos Santos, P. C., Termignoni, C. (2000). Boophilus Microplus Anticoagulant Protein: An Antithrombin Inhibitor Isolated From the Cattle Tick Saliva. Arch. Biochem. Biophys. 384, 68–73. doi: 10.1006/abbi.2000.2076
Horn, M., Nussbaumerová, M., Šanda, M., Kovářová, Z., Srba, J., Franta, Z., et al. (2009). Hemoglobin Digestion in Blood-Feeding Ticks: Mapping a Multipeptidase Pathway by Functional Proteomics. Chem. Biol. 16 (10), 1053–1063. doi: 10.1016/j.chembiol.2009.09.009
Hourcade, D. E., Akk, A. M., Mitchell, L. M., Zhou, H. F., Hauhart, R., Pham, C. T. (2016). Anti-Complement Activity of the Ixodes Scapularis Salivary Protein Salp20. Mol. Immunol. 69, 62–69. doi: 10.1016/j.molimm.2015.11.008
Hovius, J. W. R., de Jong, M. A. P., den Dunnen, J., Litjens, M., Fikrig, E., van der Poll, T., et al. (2008a). Salp15 Binding to DC-SIGN Inhibits Cytokine Expression by Impairing Both Nucleosome Remodeling and mRNA Stabilization. PloS Pathog. 4 (2), 1–14. doi: 10.1371/journal.ppat.0040031
Hovius, J. W., Schuijt, T. J., de Groot, K. A., Roelofs, J. J., Oei, G. A., Marquart, J. A., et al. (2008b). Preferential Protection of Borrelia Burgdorferi Sensu Stricto by a Salp15 Homologue in Ixodes Ricinus Saliva. J. Infect. Dis. 198 (8), 1189–1197. doi: 10.1086/591917
Ibelli, A. M. G., Kim, T. K., Hill, C. C., Lewis, L. A., Bakshi, M., Miller, S., et al. (2014). A Blood Meal Induced Ixodes Scapularis Tick Saliva Serpin Inhibits Trypsin and Thrombin and Interferes With Platelet Aggregation and Blood Clotting. Int. J. Parasitol. 44, 369–379. doi: 10.1016/j.ijpara.2014.01.010
Ibrahim, M. A., Masoud, H. M. (2018). Thrombin Inhibitor From the Salivary Gland of the Camel Tick Hyalomma Dromedarii. Exp. Appl. Acarol. 74 (1), 85–97. doi: 10.1007/s10493-017-0196-9
Imamura, S., Da Silva Vaz Junior, I., Sugino, M., Ohashi, K., Onuma, M. (2005). A Serine Protease Inhibitor (Serpin) From Haemaphysalis Longicornis as an Anti-Tick Vaccine. Vaccine 23, 1301–1311. doi: 10.1016/j.vaccine.2004.08.041
Imamura, S., Konnai, S., da Silva Vaz, I. J., Yamada, S., Nakajima, C., Ito, Y., et al. (2008). Effects of Anti-Tick Cocktail Vaccine Against Rhipicephalus Appendiculatus. Jpn. J. Vet. Res. 56 (2), 85–98. doi: 10.14943/jjvr.56.2.85
Imamura, S., Namangala, B., Tajima, T., Tembo, M. E., Yasuda, J., Ohashi, K., et al. (2006). Two Serine Protease Inhibitors (Serpins) That Induce a Bovine Protective Immune Response Against Rhipicephalus Appendiculatus Ticks. Vaccine 24 (13), 2230–2237. doi: 10.1016/j.vaccine.2005.10.055
Iqbal, A., Goldfeder, M. B., Marques-Porto, R., Asif, H., de Souza, J. G., Faria, F., et al. (2017). Revisiting Antithrombotic Therapeutics; Sculptin, a Novel Specific, Competitive, Reversible, Scissile and Tight Binding Inhibitor of Thrombin. Sci. Rep. 7 (1), 1–14. doi: 10.1038/s41598-017-01486-w
Islam, M. K., Tsuji, N., Miyoshi, T., Alim, M. A., Huang, X., Hatta, T., et al. (2009). The Kunitz-Like Modulatory Protein Haemangin is Vital for Hard Tick Blood-Feeding Success. PloS Pathog. 5 (7), e1000497. doi: 10.1371/journal.ppat.1000497
Iwanaga, S., Okada, M., Isawa, H., Morita, A., Yuda, M., Chinzei, Y. (2003). Identification and Characterization of Novel Salivary Thrombin Inhibitors From the Ixodidae Tick, Haemaphysalis Longicornis. Eur. J. Biochem. 270, 1926–1934. doi: 10.1046/j.1432-1033.2003.03560.x
Iyer, S., Goodman, K. (2019). Congenital Babesiosis From Maternal Exposure: A Case Report. J. Emerg. Med. 56 (4), 39–41. doi: 10.1016/j.jemermed.2018.12.044
Jablonka, W., Kotsyfakis, M., Mizurini, D. M., Monteiro, R. Q., Lukszo, J., Drake, S. K., et al. (2015). Identification and Mechanistic Analysis of a Novel Tick-Derived Inhibitor of Thrombin. PloS One 10 (8), e0133991. doi: 10.1371/journal.pone.0133991
Jarroux, J., Morillon, A., Pinskaya, M. (2017). History, Discovery, and Classification of lncRNAs. Long Non Coding RNA Biol. 1008, 1–46. doi: 10.1007/978-981-10-5203-3_1
Jaworski, D. C., Jasinskas, A., Metz, C. N., Bucala, R., Barbour, A. G. (2001). Identification and Characterization of a Homologue of the Pro-Inflammatory Cytokine Macrophage Migration Inhibitory Factor in the Tick, Amblyomma Americanum. Insect Mol. Biol. 10 (4), 323–331. doi: 10.1046/j.0962-1075.2001.00271.x
Jaworski, D. C., Simmen, F. A., Lamoreaux, W., Coons, L. B., Muller, M. T., Needham, G. R. (1995). A Secreted Calreticulin Protein in Ixodid Tick (Amblyomma Americanum) Saliva. J. Insect Physiol. 41 (4), 369–375. doi: 10.1016/0022-1910(94)00107-R
Jia, N., Wang, J., Shi, W., Du, L., Sun, Y., Zhan, W., et al. (2020). Large-Scale Comparative Analyses of Tick Genomes Elucidate Their Genetic Diversity and Vector Capacities. Cell 182, 1328–1340.e13. doi: 10.1016/j.cell.2020.07.023
Jittapalapong, S., Kaewhom, P., Pumhom, P., Canales, M., de la Fuente, J., Stich, R. W. (2010). Immunization of Rabbits With Recombinant Serine Protease Inhibitor Reduces the Performance of Adult Female Rhipicephalus Microplus. Transbound Emerg. Dis. 57 (1-2), 103–106. doi: 10.1111/j.1865-1682.2010.01108.x
Jmel, M. A., Aounallah, H., Bensaoud, C., Mekki, I., Chmelař, J., Faria, F., et al. (2021). Insights Into the Role of Tick Salivary Protease Inhibitors During Ectoparasite–Host Crosstalk. Int. J. Mol. Sci. 22 (2), 892. doi: 10.3390/ijms22020892
Joo, S. J. (2012). Mechanisms of Platelet Activation and Integrin αiiβ3. Korean Circ. J. 42 (5), 295–301. doi: 10.4070/kcj.2012.42.5.295
Jore, M. M., Johnson, S., Sheppard, D., Barber, N. M., Li, Y. I., Nunn, M. A., et al. (2016). Structural Basis for Therapeutic Inhibition of Complement C5. Nat. Struct. Mol. Biol. 23 (5), 378–386. doi: 10.1038/nsmb.3196
Joubert, A. M., Louw, A. I., Joubert, F., Neitz, A. W. (1998). Cloning, Nucleotide Sequence and Expression of the Gene Encoding Factor Xa Inhibitor From the Salivary Glands of the Tick, Ornithodoros Savignyi. Exp. Appl. Acarol. 22, 603–619. doi: 10.1023/a:1006198713791
Karczewski, J., Endris, R., Connolly, T. M. (1994). Disagregin is a Fibrinogen Receptor Antagonist Lacking the Arg-Gly-Asp Sequence From the Tick, Ornithodoros Moubata. J. Biol. Chem. 269 (9), 6702–6708. doi: 10.1016/S0021-9258(17)37432-X
Karczewski, J., Waxman, L., Endris, R. G., Connolly, T. M. (1995). An Inhibitor From the Argasid Tick Ornithodoros Moubata of Cell Adhesion to Collagen. Biochem. Biophys. Res. Commun. 208, 532–541. doi: 10.1006/bbrc.1995.1371
Karim, S., Ribeiro, J. M. (2015). An Insight Into the Sialome of the Lone Star Tick, Amblyomma Americanum, With a Glimpse on its Time Dependent Gene Expression. PloS One 10 (7), e0131292. doi: 10.1371/journal.pone.0131292
Karim, S., Singh, P., Ribeiro, J. M. C. (2011). A Deep Insight Into the Sialotranscriptome of the Gulf Coast Tick, Amblyomma Maculatum. PloS One 6, e28525. doi: 10.1371/journal.pone.0028525
Kato, N., Iwanaga, S., Okayama, T., Isawa, H., Yuda, M., Chinzei, Y. (2005). Identification and Characterization of the Plasma Kallikrein-Kinin System Inhibitor, Haemaphysalin, From Hard Tick, Haemaphysalis Longicornis. J. Thromb. Haemost. 93 (02), 359–367. doi: 10.1160/TH04-05-0319
Kaufman, W. R. (1989). Tick-Host Interaction: A Synthesis of Current Concepts. Parasitol. Today 5 (2), 47–56. doi: 10.1016/0169-4758(89)90191-9
Kaufman, W. R. (2007). Gluttony and Sex in Female Ixodid Ticks: How do They Compare to Other Blood-Sucking Arthropods? J. Insect Physiol. 53 (3), 264–273. doi: 10.1016/j.jinsphys.2006.10.004
Kazimirova, M., Jancinova, V., Petrikova, M., Takac, P., Labuda, M., Nosal, R. (2002). An Inhibitor of Thrombin-Stimulated Blood Platelet Aggregation From the Salivary Glands of the Hard Tick Amblyomma Variegatum (Acari: Ixodidae). Exp. Appl. Acarol. 28, 97–105. doi: 10.1023/a:1025398100044
Kazimírová, M., Stibraniova, I. (2013). Tick Salivary Compounds: Their Role in Modulation of Host Defences and Pathogen Transmission. Front. Cell. Infect. Microbiol. 3. doi: 10.3389/fcimb.2013.00043
Kim, T. K., Ibelli, A. M. G., Mulenga, A. (2015b). Amblyomma Americanum Tick Calreticulin Binds C1q But Does Not Inhibit Activation of the Classical Complement Cascade. Ticks Tick Borne Dis. 6 (1), 91–101. doi: 10.1016/j.ttbdis.2014.10.002
Kim, T. K., Tirloni, L., Berger, M., Diedrich, J. K., Yates, J. R., III, Termignoni, C., et al. (2020). Amblyomma Americanum Serpin 41 (AAS41) Inhibits Inflammation by Targeting Chymase and Chymotrypsin. Int. J. Biol. Macromol. 156, 1007–1021. doi: 10.1016/j.ijbiomac.2020.04.088
Kim, T. K., Tirloni, L., Radulovic, Z., Lewis, L., Bakshi, M., Hill, C., et al. (2015a). Conserved Amblyomma Americanum Tick Serpin19, an Inhibitor of Blood Clotting factorsXa and XIa, Trypsin and Plasmin, has Anti-Haemostatic Functions. Int. J. Parasitol. 45 (9-10), 613–627. doi: 10.1016/j.ijpara.2015.03.009
Kim, D., Urban, J., Boyle, D. L., Park, Y. (2016). Multiple Functions of Na/K-ATPase in Dopamine-Induced Salivation of the Blacklegged Tick, Ixodes Scapularis. Sci. Rep. 6, 1–13. doi: 10.1038/srep21047
Klein, M., Brühl, T. J., Staudt, V., Reuter, S., Grebe, N., Gerlitzki, B., et al. (2015). Tick Salivary Sialostatin L Represses the Initiation of Immune Responses by Targeting IRF4-Dependent Transcription in Murine Mast Cells. J. Immunol. 195 (2), 621–631. doi: 10.4049/jimmunol.1401823
Kocan, K. M., de la Fuente, J., Guglielmone, A. A., Meléndez, R. D. (2003). Antigens and Alternatives for Control of Anaplasma Marginale Infection in Cattle. Microbiol. Rev. 16 (4), 698–712. doi: 10.1128/CMR.16.4.698-712.2003
Koh, C. Y., Kazimirova, M., Trimnell, A., Takac, P., Labuda, M., Nuttall, P. A., et al. (2007). Variegin, a Novel Fast and Tight Binding Thrombin Inhibitor From the Tropical Bont Tick. J. Biol. Chem. 282 (40), 29101–29113. doi: 10.1074/jbc.M705600200
Koh, C. Y., Modahl, C. M., Kulkarni, N., Kini, R. (2018). Toxins are an Excellent Source of Therapeutic Agents Against Cardiovascular Diseases. Semin. Thromb. Hemost. 44, 691–706. doi: 10.1055/s-0038-1661384
Konnai, S., Nakajima, C., Imamura, S., Yamada, S., Nishikado, H., Kodama, M., et al. (2009). Suppression of Cell Proliferation and Cytokine Expression by HL-P36, a Tick Salivary Gland-Derived Protein of Haemaphysalis Longicornis. Immunology 126 (2), 209–219. doi: 10.1111/j.1365-2567.2008.02890.x
Kotál, J., Langhansová, H., Lieskovská, J., Andersen, J. F., Francischetti, I. M., Chavakis, T., et al. (2015). Modulation of Host Immunity by Tick Saliva. J. Proteom. 128, 58–68. doi: 10.1016/j.jprot.2015.07.005
Kotál, J., Stergiou, N., Buša, M., Chlastáková, A., Beránková, Z., Řezáčová, P., et al. (2019). The Structure and Function of Iristatin, a Novel Immunosuppressive Tick Salivary Cystatin. Cell. Mol. Life Sci. 76 (10), 2003–2013. doi: 10.1007/s00018-019-03034-3
Kotsyfakis, M., Horka, H., Salat, J., Andersen, J. F. (2010). The Crystal Structures of Two Salivary Cystatins From the Tick Ixodes Scapularis and the Effect of These Inhibitors on the Establishment of Borrelia Burgdorferi Infection in a Murine Model. Mol. Microbiol. 77 (2), 456–470. doi: 10.1111/j.1365-2958.2010.07220.x
Kotsyfakis, M., Sá-Nunes, A., Francischetti, I. M., Mather, T. N., Andersen, J. F., Ribeiro, J. M. (2006). Antiinflammatory and Immunosuppressive Activity of Sialostatin L, a Salivary Cystatin From the Tick Ixodes Scapularis. J. Biol. Chem. 281 (36), 26298–26307. doi: 10.1074/jbc.M513010200
Kotsyfakis, M., Schwarz, A., Erhart, J., Ribeiro, J. M. (2015). Tissue-And Time-Dependent Transcription in Ixodes Ricinus Salivary Glands and Midguts When Blood Feeding on the Vertebrate Host. Sci. Rep. 5 (1), 1–10. doi: 10.1038/srep09103
Labuda, M., Trimnell, A. R., Ličková, M., Kazimírová, M., Davies, G. M., Lissina, O., et al. (2006). An Antivector Vaccine Protects Against a Lethal Vector-Borne Pathogen. PloS Pathog. 2 (4), 27. doi: 10.1371/journal.ppat.0020027
Lara, F. A., Pohl, P. C., Gandara, A. C., Ferreira, J. D. S., Nascimento-Silva, M. C., Bechara, G. H., et al. (2015). ATP Binding Cassette Transporter Mediates Both Heme and Pesticide Detoxification in Tick Midgut Cells. PloS One 10 (8), e0134779. doi: 10.1371/journal.pone.0134779
Lewis, L. A., Radulovic, Z. M., Kim, T. K., Porter, L. M., Mulenga, A. (2015). Identification of 24h Ixodes Scapularis Immunogenic Tick Saliva Proteins. Ticks Tick Borne Dis. 6 (3), 424–434. doi: 10.1016/j.ttbdis.2015.03.012
Lieskovska, J., Palenikova, J., Širmarová, J., Elsterova, J., Kotsyfakis, M., Campos Chagas, A., et al. (2015). Tick Salivary Cystatin Sialostatin L2 Suppresses IFN Responses in Mouse Dendritic Cells. Parasite Immunol. 37 (2), 70–78. doi: 10.1111/pim.12162
Limo, M. K., Voigt, W. P., Tumbo-Oeri, A. G., Njogi, R. M., Ole-Moi Yoi, O. K. (1991). Purification and Characterization of an Anticoagulant From the Salivary Glands of the Ixodid Tick Rhipicephalus Appendiculatus. Exp. Parasitol. 72, 418–429. doi: 10.1016/0014-4894(91)90088-e
Liu, J., Renneker, S., Beyer, D., Kullmann, B., Seitzer, U., Ahmed, J., et al. (2014). Identification and Partial Characterization of a Salp15 Homolog From Ixodes Ricinus. Ticks Tick Borne Dis. 5 (3), 318–322. doi: 10.1016/j.ttbdis.2013.12.004
Li, X., Yang, H., Han, Y., Yin, S., Shen, B., Wu, Y., et al. (2021). Tick Peptides Evoke Itch by Activating MrgprC11/MRGPRX1 to Sensitize TRPV1 in Pruriceptors. J. Allergy Clin. Immunol. 147 (6), 2236–2248. doi: 10.1016/j.jaci.2020.12.626
Luo, J., Ren, Q., Liu, W., Qiu, X., Zhang, G., Tan, Y., et al. (2021). MicroRNA-1 Promotes the Development of and Prolongs Engorgement Time in Hyalomma Anatolicum Anatolicum (Acari: Ixodidae) Ticks. Front. Physiol. 12. doi: 10.3389/fphys.2021.596289
Macedo-Ribeiro, S., Almeida, C., Calisto, B. M., Friedrich, T., Mentele, R., Stürzebecher, J., et al. (2008). Isolation, Cloning and Structural Characterisation of Boophilin, a Multifunctional Kunitz-Type Proteinase Inhibitor From the Cattle Tick. PloS One 3 (2), 1–16. doi: 10.1371/journal.pone.0001624
Malik, M. I., Nawaz, M., Hassan, I. A., Zhang, H., Gong, H., Cao, J., et al. (2019). A microRNA Profile of Saliva and the Role of miR-375 in Haemaphysalis Longicornis (Ixodida: Ixodidae). Parasitol. Vectors 12 (1), 1–9. doi: 10.1186/s13071-019-3318-x
Mans, B. J. (2019). Chemical Equilibrium at the Tick–Host Feeding Interface: A Critical Examination of Biological Relevance in Hematophagous Behavior. Front. Physiol. 10. doi: 10.3389/fphys.2019.00530
Mans, B. J. (2020). Quantitative Visions of Reality at the Tick-Host Interface: Biochemistry, Genomics, Proteomics, and Transcriptomics as Measures of Complete Inventories of the Tick Sialoverse. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.574405
Mans, B. J., Andersen, J. F., Schwan, T. G., Ribeiro, J. M. (2008). Characterization of Anti-Hemostatic Factors in the Argasid, Argas Monolakensis: Implications for the Evolution of Blood-Feeding in the Soft Tick Family. Insect Biochem. Mol. Biol. 38 (1), 22–41. doi: 10.1016/j.ibmb.2007.09.002
Mans, B. J., Gaspar, A. R., Louw, A. I., Neitz, A. W. (1998). Apyrase Activity and Platelet Aggregation Inhibitors in the Tick Ornithodoros Savignyi (Acari: Argasidae). Exp. Appl. Acarol. 22, 353–366. doi: 10.1023/a:1024517209621
Mans, B. J., Louw, A. I., Neitz, A. W. (2002). Amino Acid Sequence and Structure Modeling of Savignin, a Thrombin Inhibitor From the Tick, Ornithodoros Savignyi. Insect Biochem. Mol. Biol. 32 (7), 821–828. doi: 10.1016/s0965-1748(01)00169-2
Mans, B. J., Ribeiro, J. M. (2008). Function, Mechanism and Evolution of the Moubatin-Clade of Soft Tick Lipocalins. Insect Biochem. Mol. Biol. 38, 841–852. doi: 10.1016/j.ibmb.2008.06.007
Marceca, G. P., Distefano, R., Tomasello, L., Lagana, A., Russo, F., Calore, F., et al. (2021). MiREDiBase, a Manually Curated Database of Validated and Putative Editing Events in microRNAs. Data 8 (1), 1–11. doi: 10.1101/2020.09.04.283689
Maritz-Olivier, C., Stutzer, C., Jongejan, F., Neitz, A. W. H., Gaspar, A. R. M. (2007). Tick Anti-Hemostatics: Targets for Future Vaccines and Therapeutics. Trends Parasitol. 23, 397–407. doi: 10.1016/j.pt.2007.07.005
Martins, L. A., Kotál, J., Bensaoud, C., Chmelař, J., Kotsyfakis, M. (2020). Small Protease Inhibitors in Tick Saliva and Salivary Glands and Their Role in Tick-Host-Pathogen Interactions. BBA-Priteins Proteom. 1868 (2), 140336. doi: 10.1016/j.bbapap.2019.140336
Martins, T. F., Teixeira, R. H., Souza, J. C., Jr., Luz, H. R., Montenegro, M. M., Jerusalinsky, L., et al. (2021). Ticks (Parasitiformes: Ixodida) on New World Wild Primates in Brazil. Int. J. Acarology 47 (2), 95–106. doi: 10.1080/01647954.2020.1870554
Maruyama, S. R., Anatriello, E., Anderson, J. M., Ribeiro, J. M., Brandão, L. G., Valenzuela, J. G., et al. (2010). The Expression of Genes Coding for Distinct Types of Glycine-Rich Proteins Varies According to the Biology of Three Metastriate Ticks, Rhipicephalus (Boophilus) Microplus, Rhipicephalus Sanguineus and Amblyomma Cajennense. BMC Genom. 11 (1), 1–17. doi: 10.1186/1471-2164-11-363
Maruyama, S. R., Garcia, G. R., Teixeira, F. R., Brandão, L. G., Anderson, J. M., Ribeiro, J. M. C., et al. (2017). Mining a Differential Sialotranscriptome of Rhipicephalus Microplus Guides Antigen Discovery to Formulate a Vaccine That Reduces Tick Infestations. Parasitol. Vectors 10, 206. doi: 10.1186/s13071-017-2136-2
Miller, J. R., Koren, S., Dilley, K. A., Harkins, D. M., Stockwell, T. B., Shabman, R. S., et al. (2018). A Draft Genome Sequence for the Ixodes Scapularis Cell Line, ISE6. F1000Research 7, 1–12. doi: 10.12688/f1000research.13635.1
Monteiro, R. Q., Rezaie, A. R., Ribeiro, J. M. C., Francischetti, I. M. B. (2005). Ixolaris: A Factor Xa Heparin-Binding Exosite Inhibitor. Biochem. J. 387, 871–877. doi: 10.1042/BJ20041738
Mori, A., Konnai, S., Yamada, S., Hidano, A., Murase, Y., Ito, T., et al. (2010). Two Novel Salp15-Like Immunosuppressant Genes From Salivary Glands of Ixodes Persulcatus Schulze Tick. Insect Mol. Biol. 19 (3), 359–365. doi: 10.1111/j.1365-2583.2010.00994.x
Motoyashiki, T., Tu, A. T., Azimov, D. A., Ibragim, K. (2003). Isolation of Anticoagulant From the Venom of Tick, Boophilus Calcaratus, From Uzbekistan. Thromb. Res. 110, 235–241. doi: 10.1016/s0049-3848(03)00409-2
Mudenda, L., Pierlé, S. A., Turse, J. E., Scoles, G. A., Purvine, S. O., Nicora, C. D., et al. (2014). Proteomics Informed by Transcriptomics Identifies Novel Secreted Proteins in Dermacentor Andersoni Saliva. Int. J. Parasitol. 44 (13), 1029–1037. doi: 10.1016/j.ijpara.2014.07.003
Mulenga, A., Khumthong, R. (2010). Silencing of Three Amblyomma Americanum (L.) Insulin-Like Growth Factor Binding Protein-Related Proteins Prevents Ticks From Feeding to Repletion. J. Exp. Biol. 213 (7), 1153–1161. doi: 10.1242/jeb.035204
Mulenga, A., Kim, T. K., Ibelli, A. M. G. (2013a). Deorphanization and Target Validation of Cross-Tick Species Conserved Novel Amblyomma Americanum Tick Saliva Protein. Int. J. Parasitol. 43 (6), 439–451. doi: 10.1016/j.ijpara.2012.12.012
Mulenga, A., Kim, T. K., Ibelli, A. M. G. (2013b). Amblyomma Americanum Tick Saliva Serine Protease Inhibitor 6 is a Cross-Class Inhibitor of Serine Proteases and Papain-Like Cysteine Proteases That Delays Plasma Clotting and Inhibits Platelet Aggregation. Insect Mol. Biol. 22 (3), 306–319. doi: 10.1111/imb.12024
Nakajima, C., da Silva Vaz, I., Jr., Imamura, S., Konnai, S., Ohashi, K., Onuma, M. (2005). Random Sequencing of cDNA Library Derived From Partially-Fed Adult Female Haemaphysalis Longicornis Salivary Gland. J. Vet. Med. Sci. 67 (11), 1127–1131. doi: 10.1292/jvms.67.1127
Nakajima, C., Imamura, S., Konnai, S., Yamada, S., Nishikado, H., Ohashi, K., et al. (2006). A Novel Gene Encoding a Thrombin Inhibitory Protein in a cDNA Library From Haemaphysalis Longicornis Salivary Gland. J. Vet. Med. Sci. 68 (5), 447–452. doi: 10.1292/jvms.68.447
Narasimhan, S., Booth, C. J., DePonte, K., Wu, M.-J., Liang, X., Mohanty, S., et al. (2019). Host-Specific Expression of Ixodes Scapularis Salivary Genes. Ticks Tick Borne Dis. 10 (2), 386–397. doi: 10.1016/j.ttbdis.2018.12.001
Narasimhan, S., Koski, R. A., Beaulieu, B., Anderson, J. F., Ramamoorthi, N., Kantor, F., et al. (2002). A Novel Family of Anticoagulants From the Saliva of Ixodes Scapularis. Insect Mol. Biol. 11 (6), 641–650. doi: 10.1046/j.1365-2583.2002.00375.x
Narasimhan, S., Kurokawa, C., Diktas, H., Strank, N. O., Černý, J., Murfin, K., et al. (2020). Ixodes Scapularis Saliva Components That Elicit Responses Associated With Acquired Tick-Resistance. Ticks Tick-Borne Dis. 11 (3), 101369. doi: 10.1016/j.ttbdis.2019.101369
Narasimhan, S., Sukumaran, B., Bozdogan, U., Thomas, V., Liang, X., DePonte, K., et al. (2007). A Tick Antioxidant Facilitates the Lyme Disease Agent’s Successful Migration From the Mammalian Host to the Arthropod Vector. Cell. Host. Microbe 2 (1), 7–18. doi: 10.1016/j.chom.2007.06.001
Nawaz, M., Malik, M. I., Zhang, H., Hassan, I. A., Cao, J., Zhou, Y., et al. (2020). Proteomic Analysis of Exosome-Like Vesicles Isolated From Saliva of the Tick Haemaphysalis Longicornis. Front. Cell. Infect. Microbiol. 10, 1–15. doi: 10.3389/fcimb.2020.542319
Nienaber, J., Gaspar, A. R., Neitz, A. W. (1999). Savignin, a Potent Thrombin Inhibitor Isolated From the Salivary Glands of the Tick Ornithodoros Savignyi (Acari: Argasidae). Exp. Parasitol. 93, 82–91. doi: 10.1006/expr.1999.4448
Nunn, M. A., Sharma, A., Paesen, G. C., Adamson, S., Lissina, O., Willis, A. C., et al. (2005). Complement Inhibitor of C5 Activation From the Soft Tick Ornithodoros Moubata. J. Immunol. 174 (4), 2084–2091. doi: 10.4049/jimmunol.174.4.2084
Nuss, A. B., Sharma, A., Gulia-Nuss, M. (2018). Chicago and Dovetail Hi-C Proximity Ligation Yield Chromosome Length Scaffolds of Ixodes Scapularis Genome. BioRxiv, 392126. doi: 10.1101/392126
Nuttall, P. A. (2019). Tick Saliva and its Role in Pathogen Transmission. Wien. Klin. Wochenschr., 1–12. doi: 10.1007/s00508-019-1500-y
Nuttall, P. A., Labuda, M. (2004). Tick–host Interactions: Saliva-Activated Transmission. Parasitology 129 (S1), S177–S189. doi: 10.1017/S0031182004005633
Nuttall, P. A., Trimnell, A. R., Kazimirova, M., Labuda, M. (2006). Exposed and Concealed Antigens as Vaccine Targets for Controlling Ticks and Tick-Borne Diseases. Parasite Immunol. 28 (4), 155–163. doi: 10.1111/j.1365-3024.2006.00806.x
O’Brien, J., Hayder, H., Zayed, Y., Peng, C. (2018). Overview of microRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 9. doi: 10.3389/fendo.2018.00402
Olds, C. L., Mwaura, S., Odongo, D. O., Scoles, G. A., Bishop, R., Daubenberger, C. (2016). Induction of Humoral Immune Response to Multiple Recombinant Rhipicephalus Appendiculatus Antigens and Their Effect on Tick Feeding Success and Pathogen Transmission. Parasitol. Vectors 9 (1), 1–11. doi: 10.1186/s13071-016-1774-0
Oleaga, A., Carnero-Morán, A., Valero, M. L., Pérez-Sánchez, R. (2021a). Proteomics Informed by Transcriptomics for a Qualitative and Quantitative Analysis of the Sialoproteome of Adult Ornithodoros Moubata Ticks. Parasitol. Vectors 14 (1), 1–13. doi: 10.1186/s13071-021-04892-2
Oleaga, A., Soriano, B., Llorens, C., Pérez-Sánchez, R. (2021b). Sialotranscriptomics of the Argasid Tick Ornithodoros Moubata Along the Trophogonic Cycle. PLoS Negl. Trop. Dis. 15 (2), e0009105. doi: 10.1371/journal.pntd.0009105
Oliveira, C. J. F., Sá-Nunes, A., Francischetti, I. M., Carregaro, V., Anatriello, E., Silva, J. S., et al. (2011). Deconstructing Tick Saliva: non-Protein Molecules With Potent Immunomodulatory Properties. J. Biol. Chem. 286 (13), 10960–10969. doi: 10.1074/jbc.M110.205047
Oliver, J. H., Jr. (1989). Biology and Systematics of Ticks (Acari: Ixodida). Annu. Rev. Ecol. Syst. 20 (1), 397–430. doi: 10.1146/annurev.es.20.110189.002145
Paesen, G. C., Adams, P. L., Harlos, K., Nuttall, P. A., Stuart, D. I. (1999). Tick Histamine-Binding Proteins: Isolation, Cloning, and Three-Dimensional Structure. Mol. Cell. 3 (5), 661–671. doi: 10.1016/s1097-2765(00)80359-7
Paesen, G. C., Siebold, C., Harlos, K., Peacey, M. F., Nuttall, P. A., Stuart, D. I. (2007). A Tick Protein With a Modified Kunitz Fold Inhibits Human Tryptase. J. Mol. Biol. 368 (4), 1172–1186. doi: 10.1016/j.jmb.2007.03.011
Parizi, L. F., Ali, A., Tirloni, L., Oldiges, D. P., Sabadin, G. A., Coutinho, M. L., et al. (2018). Peptidase Inhibitors in Tick Physiology. Med. Vet. Entomol. 32, 129–144. doi: 10.1111/mve.12276
Parizi, L. F., Githaka, N. W., Logullo, C., Konnai, S., Masuda, A., Ohashi, K., et al. (2012). The Quest for a Universal Vaccine Against Ticks: Cross-Immunity Insights. Vet. J. 194 (2), 158–165. doi: 10.1016/j.tvjl.2012.05.023
Parizi, L. F., Sabadin, G. A., Alzugaray, M. F., Seixas, A., Logullo, C., Konnai, S., et al. (2015). Rhipicephalus Microplus and Ixodes Ovatus Cystatins in Tick Blood Digestion and Evasion of Host Immune Response. Parasitol. Vectors 8 (1), 1–11. doi: 10.1186/s13071-015-0743-3
Perez-Riverol, Y., Csordas, A., Bai, J., Bernal-Llinares, M., Hewapathirana, S., Kundu, D. J., et al. (2019). The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 47 (D1), D442–D450. doi: 10.1093/nar/gky1106
Pérez-Sánchez, R., Carnero-Morán, A., Luz Valero, M., Oleaga, A. (2022). A Proteomics Informed by Transcriptomics Insight Into the Proteome of Ornithodoros Erraticus Adult Tick Saliva. Parasitol. Vectors 15 (1), 1–22. doi: 10.1186/s13071-021-05118-1
Pérez-Sánchez, R., Carnero-Morán, Á., Soriano, B., Llorens, C., Oleaga, A. (2021). RNA-Seq Analysis and Gene Expression Dynamics in the Salivary Glands of the Argasid Tick Ornithodoros Erraticus Along the Trophogonic Cycle. Parasitol. Vectors 14 (1), 1–24. doi: 10.1186/s13071-021-04671-z
Perner, J., Kropáčková, S., Kopáček, P., Ribeiro, J. M. (2018). Sialome Diversity of Ticks Revealed by RNAseq of Single Tick Salivary Glands. PloS Negl. Trop. Dis. 12 (4), e0006410. doi: 10.1371/journal.pntd.0006410
Perner, J., Sobotka, R., Sima, R., Konvickova, J., Sojka, D., de Oliveira, P. L., et al. (2016). Acquisition of Exogenous Haem is Essential for Tick Reproduction. Elife 5, e12318. doi: 10.7554/eLife.12318.031
Pham, M., Underwood, J., Oliva Chávez, A. S. (2021). Changing the Recipe: Pathogen Directed Changes in Tick Saliva Components. Int. J. Environ. Res. Public Health 18 (4), 1806. doi: 10.3390/ijerph18041806
Poole, N. M., Mamidanna, G., Smith, R. A., Coons, L. B., Cole, J. A. (2013). Prostaglandin E2 in Tick Saliva Regulates Macrophage Cell Migration and Cytokine Profile. Parasitol. Vectors 6 (1), 1–11. doi: 10.1186/1756-3305-6-261
Preston, S. G., Majtán, J., Kouremenou, C., Rysnik, O., Burger, L. F., Cabezas Cruz, A., et al. (2013). Novel Immunomodulators From Hard Ticks Selectively Reprogramme Human Dendritic Cell Responses. PloS Pathog. 9 (6), e1003450. doi: 10.1371/journal.ppat.1003450
Prevot, P. P., Adam, B., Boudjeltia, K. Z., Brossard, M., Lins, L., Cauchie, P., et al. (2006). Anti-Hemostatic Effects of a Serpin From the Saliva of the Tick Ixodes Ricinus. J. Biol. Chem. 281, 26361–26369. doi: 10.1074/jbc.M604197200
Radulović, Ž. M., Kim, T. K., Porter, L. M., Sze, S. H., Lewis, L., Mulenga, A. (2014). A 24-48 H Fed Amblyomma Americanum Tick Saliva Immuno-Proteome. BMC Genom. 15 (1), 1–30. doi: 10.1186/1471-2164-15-518
Radulović, Ž. M., Porter, L. M., Kim, T. K., Bakshi, M., Mulenga, A. (2015). Amblyomma Americanum Tick Saliva Insulin-Like Growth Factor Binding Protein-Related Protein 1 Binds Insulin But Not Insulin-Like Growth Factors. Insect Mol. Biol. 24 (5), 539–550. doi: 10.1111/imb.12180
Ramachandrar, N., Wikel, S. K. (1992). Modulation of Host-Immune Responses by Ticks (Atari: Ixodidae): Effect of Salivary Gland Extracts on Host Marophages and Lymphocyte Cytokine Production. J. Med. Entomol. 29, 818–826. doi: 10.1093/jmedent/29.5.818
Ramamoorthi, N., Narasimhan, S., Pal, U., Bao, F., Yang, X. F., Fish, D., et al. (2005). The Lyme Disease Agent Exploits a Tick Protein to Infect the Mammalian Host. Nature 436 (7050), 573–577. doi: 10.1038/nature03812
Ramasamy, E., Taank, V., Anderson, J. F., Sultana, H., Neelakanta, G. (2020). Repression of Tick microRNA-133 Induces Organic Anion Transporting Polypeptide Expression Critical for Anaplasma Phagocytophilum Survival in the Vector and Transmission to the Vertebrate Host. PloS Genet. 16 (7), e1008856. doi: 10.1371/journal.pgen.1008856
Rangel, C. K., Parizi, L. F., Sabadin, G. A., Costa, E. P., Romeiro, N. C., Isezaki, M., et al. (2017). Molecular and Structural Characterization of Novel Cystatins From the Taiga Tick Ixodes Persulcatus. Ticks Tick-Borne Dis. 8 (3), 432–441. doi: 10.1016/j.ttbdis.2017.01.007
Reck, J., Jr., Berger, M., Terra, R. M., Marks, F. S., da Silva Vaz, I., Jr., Guimaraes, J. A., et al. (2009). Systemic Alterations of Bovine Hemostasis Due to Rhipicephalus (Boophilus) Microplus Infestation. Res. Vet. Sci. 86, 56–62. doi: 10.1016/j.rvsc.2008.05.007
Regmi, P., Khanal, S., Neelakanta, G., Sultana, H. (2020). Tick-Borne Flavivirus Inhibits Sphingomyelinase (IsSMase), a Venomous Spider Ortholog to Increase Sphingomyelin Lipid Levels for its Survival in Ixodes Scapularis Ticks. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00244
Rego, R. O., Trentelman, J. J., Anguita, J., Nijhof, A. M., Sprong, H., Klempa, B., et al. (2019). Counterattacking the Tick Bite: Towards a Rational Design of Anti-Tick Vaccines Targeting Pathogen Transmission. Parasitol. Vectors 12 (1), 1–20. doi: 10.1186/s13071-019-3468-x
Ren, S., Zhang, B., Xue, X., Wang, X., Zhao, H., Zhang, X., et al. (2019). Salivary Gland Proteome Analysis of Developing Adult Female Haemaphysalis Longicornis Ticks: Molecular Motor and TCA Cycle-Related Proteins Play an Important Role Throughout Development. Parasitol. Vectors 12 (1), 1–16. doi: 10.1186/s13071-019-3864-2
Ribeiro, J. M., Makoul, G. T., Levine, J., Robinson, D., Spielman, R. (1985). Antihemostatic, Antiinflammatory, and Immunosuppressive Properties of the Saliva of a Tick, Ixodes dammini. J. Exp. Med. 161 (2), 332–44. doi: 10.1084/jem.161.2.332
Ribeiro, J. M. C. (1987). Role of Saliva in Blood Feeding by Arthropods. Annu. Rev. Entomol. 32 (1), 463–478. doi: 10.1146/annurev.en.32.010187.002335
Ribeiro, J. M. C. (1989). Role of Saliva in Tick/Host Interactjons. Exp. Appl. Acarol. 7, 15–20. doi: 10.1007/BF01200449
Ribeiro, J. M. (1995). Blood-Feeding Arthropods: Live Syringes or Invertebrate Pharmacologists? Infect. Agents Dis. 4, 143–152.
Ribeiro, J. C., Endris, T. M., Endris, R. (1991). Saliva of the Soft Tick, Ornithodoros Moubata, Contains Anti-Platelet and Apyrase Activities. Comp. Biochem. Physiol. A Comp. Physiol. 100 (1), 109–112. doi: 10.1016/0300-9629(91)90190-n
Ribeiro, J. M. C., Labruna, M. B., Mans, B. J., Maruyama, S. R., Francischetti, I. M., Barizon, G. C., et al. (2012). The Sialotranscriptome of Antricola Delacruzi Female Ticks is Compatible With non-Hematophagous Behavior and an Alternative Source of Food. Insect Biochem. Mol. Biol. 42 (5), 332–342. doi: 10.1016/j.ibmb.2012.01.003
Ribeiro, J., Mans, B. J. (2020). TickSialoFam (TSFam): A Database That Helps to Classify Tick Salivary Proteins, a Review on Tick Salivary Protein Function and Evolution, With Considerations on the Tick Sialome Switching Phenomenon. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00374
Ribeiro, J. M., Mather, T. N. (1998). Ixodes Scapularis: Salivary Kininase Activity is a Metallo Dipeptidyl Carboxypeptidase. Exp. Parasitol. 89, 213–221. doi: 10.1006/expr.1998.4296
Ribeiro, J. M. C., Slovák, M., Francischetti, I. M. B. (2017). An Insight Into the Sialome of Hyalomma Excavatum. Ticks Tick Borne Dis. 8, 201–207. doi: 10.1016/j.ttbdis.2016.08.011
Richter, D., Matuschka, F. R., Spielman, A., Mahadevan, L. (2013). How Ticks Get Under Your Skin: Insertion Mechanics of the Feeding Apparatus of Ixodes Ricinus Ticks. Proc. R. Soc B: Biol. Sci. 280 (1773), 20131758. doi: 10.1098/rspb.2013.1758
Riolo, G., Cantara, S., Marzocchi, C., Ricci, C. (2021). miRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 4 (1), 1–20. doi: 10.3390/mps4010001
Rodriguez-Valle, M., Moolhuijzen, P., Barrero, R. A., Ong, C. T., Busch, G., Karbanowicz, T., et al (2018). Transcriptome and Toxin Family Analysis of the Paralysis Tick, Ixodes holocyclus. Int. J. Parasitol. 48 (1), 71–82. doi: 10.1016/j.ijpara.2017.07.007
Rodriguez-Valle, M., Xu, T., Kurscheid, S., Lew-Tabor, A. E. (2015). Rhipicephalus Microplus Serine Protease Inhibitor Family: Annotation, Expression and Functional Characterisation Assessment. Parasitol. Vectors 8 (1), 1–9. doi: 10.1186/s13071-014-0605-4
Rosbjerg, A., Genster, N., Pilely, K., Garred, P. (2017). Evasion Mechanisms Used by Pathogens to Escape the Lectin Complement Pathway. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00868
Roversi, P., Ryffel, B., Togbe, D., Maillet, I., Teixeira, M., Ahmat, N., et al. (2013). Bifunctional Lipocalin Ameliorates Murine Immune Complex-Induced Acute Lung Injury. J. Biol. Chem. 288 (26), 18789–18802. doi: 10.1074/jbc.M112.420331
Sajid, A., Matias, J., Arora, G., Kurokawa, C., DePonte, K., Tang, X., et al. (2021). mRNA Vaccination Induces Tick Resistance and Prevents Transmission of the Lyme Disease Agent. Sci. Transl. Med. 13 (620), eabj9827. doi: 10.1126/scitranslmed.abj9827
Sajiki, Y., Konnai, S., Ochi, A., Okagawa, T., Githaka, N., Isezaki, M., et al. (2020). Immunosuppressive Effects of Sialostatin L1 and L2 Isolated From the Taiga Tick Ixodes Persulcatus Schulze. Ticks Tick Borne Dis. 11, 101332. doi: 10.1016/j.ttbdis.2019.101332
Salát, J., Paesen, G. C., Řezáčová, P., Kotsyfakis, M., Kovářová, Z., Šanda, M., et al. (2010). Crystal Structure and Functional Characterization of an Immunomodulatory Salivary Cystatin From the Soft Tick Ornithodoros Moubata. Biochem. J. 429 (1), 103–112. doi: 10.1042/BJ20100280
Sangamnatdej, S., Paesen, G. C., Slovak, M., Nuttall, P. A. (2002). A High Affinity Serotonin-and Histamine-Binding Lipocalin From Tick Saliva. Insect Mol. Biol. 11 (1), 79–86. doi: 10.1046/j.0962-1075.2001.00311.x
Sá-Nunes, A., Bafica, A., Lucas, D. A., Conrads, T. P., Veenstra, T. D., Andersen, J. F., et al. (2007). Prostaglandin E2 is a Major Inhibitor of Dendritic Cell Maturation and Function in Ixodes Scapularis Saliva. J. Immunol. 179 (3), 1497–1505. doi: 10.4049/jimmunol.179.3.1497
Schlapschy, M., Binder, U., Börger, C., Theobald, I., Wachinger, K., Kisling, S., et al. (2013). PASylation: A Biological Alternative to PEGylation for Extending the Plasma Half-Life of Pharmaceutically Active Proteins. Protein Eng. Des. Sel. 26 (8), 489–501. doi: 10.1093/protein/gzt023
Schroeder, H., Daix, V., Gillet, L., Renauld, J. C., Vanderplasschen, A. (2007). The Paralogous Salivary Anti-Complement Proteins IRAC I and IRAC II Encoded by Ixodes Ricinus Ticks Have Broad and Complementary Inhibitory Activities Against the Complement of Different Host Species. Microbes Infect. 9 (2), 247–250. doi: 10.1016/j.micinf.2006.10.020
Schuijt, T. J., Bakhtiari, K., Daffre, S., DePonte, K., Wielders, S. J., Marquart, J. A., et al. (2013). Factor Xa Activation of Factor V is of Paramount Importance in Initiating the Coagulation System: Lessons From a Tick Salivary Protein. Circulation 128 (3), 254–266. doi: 10.1161/CIRCULATIONAHA.113.003191
Schuijt, T. J., Coumou, J., Narasimhan, S., Dai, J., DePonte, K., Wouters, D., et al. (2011). A Tick Mannose-Binding Lectin Inhibitor Interferes With the Vertebrate Complement Cascade to Enhance Transmission of the Lyme Disease Agent. Cell Host Microbe 10 (2), 136–146. doi: 10.1016/j.chom.2011.06.010
Schwarz, A., Tenzer, S., Hackenberg, M., Erhart, J., Gerhold-Ay, A., Mazur, J., et al. (2014). A Systems Level Analysis Reveals Transcriptomic and Proteomic Complexity in Ixodes Ricinus Midgut and Salivary Glands During Early Attachment and Feeding. Mol. Cell. Proteom. 13 (10), 2725–2735. doi: 10.1074/mcp.M114.039289
Schwarz, A., von Reumont, B. M., Erhart, J., Chagas, A. C., Ribeiro, J. M. C., Kotsyfakis, M. (2013). De Novo Ixodes Ricinus Salivary Gland Transcriptome Analysis Using Two Next-Generation Sequencing Methodologies. FASEB J. 27, 4745–4756. doi: 10.1096/fj.13-232140
Sette, A., Rappuoli, R. (2010). Reverse Vaccinology: Developing Vaccines in the Era of Genomics. Immunity 33 (4), 530–541. doi: 10.1016/j.immuni.2010.09.017
Shao, C. C., Xu, M. J., Chen, Y. Z., Tao, J. P., Zhu, X. Q. (2015). Comparative Profiling of microRNAs in Male and Female Rhipicephalus Sanguineus. Appl. Biochem. Biotechnol. 176 (7), 1928–1936. doi: 10.1007/s12010-015-1688-x
Sharma, A., Pham, M. N., Reyes, J. B., Chana, R., Yim, W. C., Heu, C. C., et al. (2020). Cas9-Mediated Gene-Editing in the Black-Legged Tick, Ixodes Scapularis, by Embryo Injection and Remot Control. SSRN Electron. J., 1–23. doi: 10.2139/ssrn.3691041
Silva, N. C., Vale, V. F., Franco, P. F., Gontijo, N. F., Valenzuela, J. G., Pereira, M. H., et al. (2016). Saliva of Rhipicephalus (Boophilus) Microplus (Acari: Ixodidae) Inhibits Classical and Alternative Complement Pathways. Parasitol. Vectors 9 (1), 1–14. doi: 10.1186/s13071-016-1726-8
Šimo, L., Kazimirova, M., Richardson, J., Bonnet, S. I. (2017). The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00281
Slunge, D. (2015). The Willingness to Pay for Vaccination Against Tick-Borne Encephalitis and Implications for Public Health Policy: Evidence From Sweden. PloS One 10 (12), e0143875. doi: 10.1371/journal.pone.0143875
Soares, T. S., Oliveira, F., Torquato, R. J., Sasaki, S. D., Araujo, M. S., Paschoalin, T., et al. (2016). BmTI-A, a Kunitz Type Inhibitor From Rhipicephalus Microplus Able to Interfere in Vessel Formation. Vet. Parasitol. 219, 44–52. doi: 10.1016/j.vetpar.2016.01.021
Štibrániová, I., Bartíková, P., Holíková, V., Kazimírová, M. (2019). Deciphering Biological Processes at the Tick-Host Interface Opens New Strategies for Treatment of Human Diseases. Front. Physiol. 10. doi: 10.3389/fphys.2019.00830
Stutzer, C., Mans, B. J., Gaspar, A. R., Neitz, A. W., Maritz-Olivier, C. (2009). Ornithodoros Savignyi: Soft Tick Apyrase Belongs to the 5’-Nucleotidase Family. Exp. Parasitol. 122, 318–327. doi: 10.1016/j.exppara.2009.04.007
Sugino, M., Imamura, S., Mulenga, A., Nakajima, M., Tsuda, A., Ohashi, K., et al. (2003). A Serine Proteinase Inhibitor (Serpin) From Ixodid Tick Haemaphysalis Longicornis; Cloning and Preliminary Assessment of its Suitability as a Candidate for a Tick Vaccine. Vaccine 21 (21-22), 2844–2851. doi: 10.1016/S0264-410X(03)00167-1
Sukumaran, B., Narasimhan, S., Anderson, J. F., DePonte, K., Marcantonio, N., Krishnan, M. N., et al. (2006). An Ixodes Scapularis Protein Required for Survival of Anaplasma Phagocytophilum in Tick Salivary Glands. J. Exp. Med. 203, 1507–1517. doi: 10.1084/jem.20060208
Sun, T., Wang, F., Pan, W., Wu, Q., Wang, J., Dai, J. (2018). An Immunosuppressive Tick Salivary Gland Protein DsCystatin Interferes With Toll-Like Receptor Signaling by Downregulating TRAF6. Front. Immunol. 9. doi: 10.3389/fimmu.2018.01245
Suppan, J., Engel, B., Marchetti-Deschmann, M., Nürnberger, S. (2018). Tick Attachment Cement–Reviewing the Mysteries of a Biological Skin Plug System. Biol. Rev. 93 (2), 1056–1076. doi: 10.1111/brv.12384
Tabor, A. E., Ali, A., Rehman, G., Rocha Garcia, G., Zangirolamo, A. F., Malardo, T., et al. (2017). Cattle Tick Rhipicephalus Microplus-Host Interface: A Review of Resistant and Susceptible Host Responses. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00506
Tahir, D., Meyer, L., Fourie, J., Jongejan, F., Mather, T., Choumet, V., et al. (2020). Interrupted Blood Feeding in Ticks: Causes and Consequences. Microorganisms 8 (6), 910. doi: 10.3390/microorganisms8060910
Tan, A. W., Francischetti, I. M., Slovak, M., Kini, R. M., Ribeiro, J. M. (2015). Sexual Differences in the Sialomes of the Zebra Tick, Rhipicephalus Pulchellus. J. Proteom. 117, 120–144. doi: 10.1016/j.jprot.2014.12.014
Tang, J., Fang, Y., Han, Y., Bai, X., Yan, X., Zhang, Y., et al. (2015). YY-39, a Tick Anti-Thrombosis Peptide Containing RGD Domain. Peptides 68, 99–104. doi: 10.1016/j.peptides.2014.08.008
Tian, Y., Chen, W., Mo, G., Chen, R., Fang, M., Yedid, G., et al. (2016). An Immunosuppressant Peptide From the Hard Tick Amblyomma Variegatum. Toxins 8 (5), 133. doi: 10.3390/toxins8050133
Tirloni, L., Calvo, E., Konnai, S., da Silva Vaz, I., Jr (2021). The Role of Saliva in Arthropod-Host-Pathogen Relationships. Front. Cell. Infect. Microbiol. 10, 630626. doi: 10.3389/fcimb.2020.630626
Tirloni, L., Islam, M. S., Kim, T. K., Diedrich, J. K., Yates, J. R., Pinto, A. F., et al. (2015). Saliva From Nymph and Adult Females of Haemaphysalis Longicornis: A Proteomic Study. Parasitol. Vectors 8 (1), 1–23. doi: 10.1186/s13071-015-0918-y
Tirloni, L., Kim, T. K., Berger, M., Termignoni, C., da Silva Vaz, I., Jr., Mulenga, A. (2019). Amblyomma Americanum Serpin 27 (AAS27) is a Tick Salivary Anti-Inflammatory Protein Secreted Into the Host During Feeding. PloS Negl. Trop. Dis. 13 (8), e0007660. doi: 10.1371/journal.pntd.0007660
Tirloni, L., Kim, T. K., Coutinho, M. L., Ali, A., Seixas, A., Termignoni, C., et al. (2016). The Putative Role of Rhipicephalus Microplus Salivary Serpins in the Tick-Host Relationship. Insect Biochem. Mol. Biol. 71, 12–28. doi: 10.1016/j.ibmb.2016.01.004
Tirloni, L., Kim, T. K., Pinto, A. F., Yates, J. R., III, da Silva Vaz, I., Jr., Mulenga, A. (2017). Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes Scapularis and Amblyomma Americanum Saliva Stimulated to Feed on Different Hosts. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00517
Tirloni, L., Lu, S., Calvo, E., Sabadin, G., Di Maggio, L. S., Suzuki, M., et al. (2020). Integrated Analysis of Sialotranscriptome and Sialoproteome of the Brown Dog Tick Rhipicephalus Sanguineus (Sl): Insights Into Gene Expression During Blood Feeding. J. Proteom. 229, 103899. doi: 10.1016/j.jprot.2020.103899
Tirloni, L., Reck, J., Terra, R. M. S., Martins, J. R., Mulenga, A., Sherman, N. E., et al. (2014). Proteomic Analysis of Cattle Tick Rhipicephalus (Boophilus) Microplus Saliva: A Comparison Between Partially and Fully Engorged Females. PloS One 9 (4), e94831. doi: 10.1371/journal.pone.0094831
Toyomane, K., Konnai, S., Niwa, A., Githaka, N., Isezaki, M., Yamada, S., et al. (2016). Identification and the Preliminary In Vitro Characterization of IRIS Homologue From Salivary Glands of Ixodes Persulcatus Schulze. Ticks Tick Borne Dis. 7 (1), 119–125. doi: 10.1016/j.ttbdis.2015.09.006
Trentelman, J. J., Sima, R., Krezdorn, N., Tomás-Cortázar, J., Barriales, D., Takumi, K., et al. (2020). A Combined Transcriptomic Approach to Identify Candidates for an Anti-Tick Vaccine Blocking B. Afzelii Transmission. Sci. Rep. 10 (1), 1–14. doi: 10.1038/s41598-020-76268-y
Tripathi, N. K., Shrivastava, A. (2019). Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 7. doi: 10.3389/fbioe.2019.00420
Tsuda, A., Mulenga, A., Sugimoto, C., Nakajima, M., Ohashi, K., Onuma, M. (2001). cDNA Cloning, Characterization, and Vaccine Effect Analysis of Haemaphysalis Longicornis Tick Saliva Proteins. Vaccine 19, 4287–4296. doi: 10.1016/S0264-410X(01)00148-7
Tyson, K., Elkins, C., Patterson, H., Fikrig, E., De Silva, A. (2007). Biochemical and Functional Characterization of Salp20, an Ixodes Scapularis Tick Salivary Protein That Inhibits the Complement Pathway. Insect Biochem. Mol. 16 (4), 469–479. doi: 10.1111/j.1365-2583.2007.00742.x
Valdés, J. J., Schwarz, A., Cabeza de Vaca, I., Calvo, E., Pedra, J. H., Guallar, V., et al. (2013). Tryptogalinin is a Tick Kunitz Serine Protease Inhibitor With a Unique Intrinsic Disorder. PloS One 8 (5), e62562. doi: 10.1371/journal.pone.0062562
Valenzuela, J. G. (2004). Exploring Tick Saliva: From Biochemistry to ‘Sialomes’ and Functional Genomics. Parasitology 129 (S1), S83–S94. doi: 10.1017/S0031182004005189
Valenzuela, J. G., Charlab, R., Mather, T. N., Ribeiro, J. M. (2000). Purification, Cloning, and Expression of a Novel Salivary Anticomplement Protein From the Tick, Ixodes Scapularis. J. Biol. Chem. 275 (25), 18717–18723. doi: 10.1074/jbc.M001486200
Valenzuela, J. G., Francischetti, I. M., Pham, V. M., Garfield, M. K., Mather, T. N., Ribeiro, J. M. (2002). Exploring the Sialome of the Tick Ixodes Scapularis. J. Exp. Biol. 205 (18), 2843–2864. doi: 10.1242/jeb.205.18.2843
Van de Locht, A., Stubbs, M. T., Bode, W., Friedrich, T., Bollschweiler, C., Höffken, W., et al. (1996). The Ornithodorin-Thrombin Crystal Structure, a Key to the TAP Enigma? EMBO J. 15 (22), 6011–6017. doi: 10.1002/j.1460-2075.1996.tb00989.x
Van den Kerkhof, D. L., Nagy, M., Wichapong, K., Brouns, S. L., Heemskerk, J. W., Hackeng, T. M., et al. (2021). Inhibition of Platelet Adhesion, Thrombus Formation, and Fibrin Formation by a Potent αiibβ3 Integrin Inhibitor From Ticks. Res. Pract. Thromb. Haemost. 5 (1), 231–242. doi: 10.1002/rth2.12466
Van der Heijden, K. M., Szabó, M. P. J., Egami, M. I., Pereira, M. C., Matushima, E. R. (2005). Histopathology of Tick-Bite Lesions in Naturally Infested Capybaras (Hydrochoerus Hydrochaeris) in Brazil. Exp. Appl. Acarol. 37 (3), 245–255. doi: 10.1007/s10493-005-4155-5
Van Oosterwijk, J. G., Wikel, S. K. (2021). Resistance to Ticks and the Path to Anti-Tick and Transmission Blocking Vaccines. Vaccines 9 (7), 725. doi: 10.3390/vaccines9070725
Vayssier-Taussat, M., Kazimirova, M., Hubalek, Z., Hornok, S., Farkas, R., Cosson, J. F., et al. (2015). Emerging Horizons for Tick-Borne Pathogens: From the ‘One Pathogen–One Disease’vision to the Pathobiome Paradigm. Future Microbiol. 10, 2033–2043. doi: 10.2217/fmb.15.114
Vechtova, P., Sterbova, J., Sterba, J., Vancova, M., Rego, R. O., Selinger, M., et al. (2018). A Bite So Sweet: The Glycobiology Interface of Tick-Host-Pathogen Interactions. Parasitol. Vectors 11 (1), 1–27. doi: 10.1186/s13071-018-3062-7
Villar, M., Pacheco, I., Merino, O., Contreras, M., Mateos-Hernández, L., Prado, E., et al. (2020). Tick and Host Derived Compounds Detected in the Cement Complex Substance. Biomolecules 10 (4), 555. doi: 10.3390/biom10040555
Wagemakers, A., Coumou, J., Schuijt, T. J., Oei, A., Nijhof, A. M., van ‘t Veer, C., et al. (2016). An Ixodes Ricinus Tick Salivary Lectin Pathway Inhibitor Protects Borrelia Burgdorferi Sensu Lato From Human Complement. Vector Borne Zoonotic Dis. 16 (4), 223–228. doi: 10.1089/vbz.2015.1901
Wang, X., Coons, L. B., Taylor, D. B., Stevens, S. E., Jr., Gartner, T. K. (1996). Variabilin, a Novel RGD-Containing Antagonist of Glycoprotein IIb-IIIa and Platelet Aggregation Inhibitor From the Hard Tick Dermacentor Variabilis. J. Biol. Chem. 271, 17785–17790. doi: 10.1074/jbc.271.30.17785
Wang, M., Guerrero, F. D., Pertea, G., Nene, V. M. (2007). Global Comparative Analysis of ESTs From the Southern Cattle Tick, Rhipicephalus (Boophilus) Microplus. BMC Genom. 8 (1), 1–14. doi: 10.1186/1471-2164-8-368
Wang, F., Lu, X., Guo, F., Gong, H., Zhang, H., Zhou, Y., et al. (2017). The Immunomodulatory Protein RH36 is Relating to Blood-Feeding Success and Oviposition in Hard Ticks. Vet. Parasitol. 240, 49–59. doi: 10.1016/j.vetpar.2017.03.017
Wang, X., Shaw, D. K., Sakhon, O. S., Snyder, G. A., Sundberg, E. J., Santambrogio, L., et al. (2016). The Tick Protein Sialostatin L2 Binds to Annexin A2 and Inhibits NLRC4-Mediated Inflammasome Activation. Infect. Immun. 84 (6), 1796–1805. doi: 10.1128/IAI.01526-15
Wang, F., Song, Z., Chen, J., Wu, Q., Zhou, X., Ni, X., et al. (2020). The Immunosuppressive Functions of Two Novel Tick Serpins, HlSerpin-A and HlSerpin-B, From Haemaphysalis Longicornis. Immunology 159 (1), 109–120. doi: 10.1111/imm.13130
Wang, Y., Yu, X., Cao, J., Zhou, Y., Gong, H., Zhang, H., et al. (2015). Characterization of a Secreted Cystatin From the Tick Rhipicephalus Haemaphysaloides. Exp. Appl. Acarol. 67 (2), 289–298. doi: 10.1007/s10493-015-9946-8
Waxman, L., Connolly, T. M. (1993). Isolation of an Inhibitor Selective for Collagen-Stimulated Platelet Aggregation From the Soft Tick Ornithodoros Moubata. J. Biol. Chem. 268, 5445–5449. doi: 10.1016/S0021-9258(18)53341-X
Waxman, L., Smith, D. E., Arcuri, K. E., Vlasuk, G. P. (1990). Tick Anticoagulant Peptide (TAP) is a Novel Inhibitor of Blood Coagulation Factor Xa. Science 248, 593–596. doi: 10.1126/science.2333510
Wei, F., Ma, C., Zhou, T., Dong, X., Luo, Q., Geng, L., et al. (2017). Exosomes Derived From Gemcitabine-Resistant Cells Transfer Malignant Phenotypic Traits via Delivery of miRNA-222-3. Mol. Cancer. 16, 132. doi: 10.1186/s12943-017-0694-8
Wikel, S. K. (1982). Immune Responses to Arthropods and Their Hosts. Annu. Rev. Entomol. 21, 21–48. doi: 10.1146/annurev.en.27.010182.000321
Wikel, S. K. (1985). Effects of Tick Infestation on the Plaque Forming a Cell Response to a Thymic Dependent Antigen. Ann. Trop. Med. Parasitol. 79, 195–198. doi: 10.1080/00034983.1985.11811906
Wikel, S. K. (1996). The Immunology of Host-Ectoparasitic Arthropod Relationships (Wallingford (Oxford: United Kingdom) CABI). doi: 10.1016/S0035-9203(97)90305-9
Wikel, S. K. (2018). Tick-Host-Pathogen Systems Immunobiology: An Interactive Trio. Front. Biosci. 23, 265–283. doi: 10.2741/4590
Wikel, S., Ramachandra, R. N., Bergman, D. K. (1994). Tick-Induced Modulation of the Host Immune Response. Int. J. Parasitol. 24, 59–66. doi: 10.1016/0020-7519(94)90059-0
Willadsen, P. (2004). Anti-Tick Vaccines. Parasitology 129, S367–S387. doi: 10.1017/S0031182003004657
Wu, J., Wang, Y., Liu, H., Yang, H., Ma, D., Li, J., et al. (2010). Two Immunoregulatory Peptides With Antioxidant Activity From Tick Salivary Glands. J. Biol. Chem. 285, 16606–16613. doi: 10.1074/jbc.M109.094615
Xu, Y., Bruno, J. F., Luft, B. J. (2005). Identification of Novel Tick Salivary Gland Proteins for Vaccine Development. Biochem. Biophys. Res. Commun. 326 (4), 901–904. doi: 10.1016/j.bbrc.2004.11.127
Xu, X.-L., Cheng, T. Y., Yang, H., Yan, F., Yang, Y. (2015). De Novo Sequencing, Assembly and Analysis of Salivary Gland Transcriptome of Haemaphysalis Flava and Identification of Sialoprotein Genes. Infect. Genet. Evol. 32, 135–142. doi: 10.1016/j.meegid.2015.03.010
Xu, T., Lew-Tabor, A., Rodriguez-Valle, M. (2016). Effective Inhibition of Thrombin by Rhipicephalus Microplus Serpin-15 (RmS-15) Obtained in the Yeast Pichia Pastoris. Ticks Tick Borne Dis. 7 (1), 180–187. doi: 10.1016/j.ttbdis.2015.09.007
Yu, Y., Cao, J., Zhou, Y., Zhang, H., Zhou, J. (2013). Isolation and Characterization of Two Novel Serpins From the Tick Rhipicephalus Haemaphysaloides. Ticks Tick Borne Dis. 4 (4), 297–303. doi: 10.1016/j.ttbdis.2013.02.001
Yu, X., Gong, H., Zhou, Y., Zhang, H., Cao, J., Zhou, J. (2015). Differential Sialotranscriptomes of Unfed and Fed Rhipicephalus Haemaphysaloides, With Particular Regard to Differentially Expressed Genes of Cysteine Proteases. Parasit. Vectors 8, 597. doi: 10.1186/s13071-015-1213-7
Yu, D., Liang, J., Yu, H., Wu, H., Xu, C., Liu, J., et al. (2006). A Tick B-Cell Inhibitory Protein From Salivary Glands of the Hard Tick, Hyalomma Asiaticum Asiaticum. Biochem. Biophys. Res. Commun. 343 (2), 585–590. doi: 10.1016/j.bbrc.2006.02.188
Zhou, W., Tahir, F., Wang, J. C. Y., Woodson, M., Sherman, M. B., Karim, S., et al. (2020). Discovery of Exosomes From Tick Saliva and Salivary Glands Reveals Therapeutic Roles for CXCL12 and IL-8 in Wound Healing at the Tick–Human Skin Interface. Front. Cell Dev. Biol. 8. doi: 10.3389/fcell.2020.00554
Zhou, J., Ueda, M., Umemiya, R., Battsetseg, B., Boldbaatar, D., Xuan, X., et al. (2006). A Secreted Cystatin From the Tick Haemaphysalis Longicornis and its Distinct Expression Patterns in Relation to Innate Immunity. Insect Biochem. Mol. Biol. 36 (7), 527–535. doi: 10.1016/j.ibmb.2006.03.003
Keywords: tick–host, crosstalk, salivary molecules, immune response, evasion mechanism
Citation: Ali A, Zeb I, Alouffi A, Zahid H, Almutairi MM, Ayed Alshammari F, Alrouji M, Termignoni C, Vaz IdS Jr and Tanaka T (2022) Host Immune Responses to Salivary Components - A Critical Facet of Tick-Host Interactions. Front. Cell. Infect. Microbiol. 12:809052. doi: 10.3389/fcimb.2022.809052
Received: 04 November 2021; Accepted: 04 February 2022;
Published: 16 March 2022.
Edited by:
Sukanya Narasimhan, Yale University, United StatesReviewed by:
Fabiano Oliveira, National Institute of Allergy and Infectious Diseases (NIH), United StatesShahid Karim, University of Southern Mississippi, United States
Copyright © 2022 Ali, Zeb, Alouffi, Zahid, Almutairi, Ayed Alshammari, Alrouji, Termignoni, Vaz and Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tetsuya Tanaka, azYxOTk0MzFAa2FkYWkuanA=; Abid Ali, dW9wX2FsaUB5YWhvby5jb20=
 Abid Ali
Abid Ali Ismail Zeb
Ismail Zeb Abdulaziz Alouffi
Abdulaziz Alouffi Hafsa Zahid
Hafsa Zahid Mashal M. Almutairi
Mashal M. Almutairi Fahdah Ayed Alshammari4
Fahdah Ayed Alshammari4 Itabajara da Silva Vaz Jr
Itabajara da Silva Vaz Jr Tetsuya Tanaka
Tetsuya Tanaka