
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 07 March 2022
Sec. Microbiome in Health and Disease
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.804334
This article is part of the Research TopicThe Human Microbiota in PeriodontitisView all 17 articles
 Tomomi Kawai1
Tomomi Kawai1 Tomoko Ohshima1*
Tomoko Ohshima1* Takeshi Tanaka2
Takeshi Tanaka2 Satoshi Ikawa3
Satoshi Ikawa3 Atsushi Tani4
Atsushi Tani4 Naoya Inazumi5
Naoya Inazumi5 Ryoichi Shin6
Ryoichi Shin6 Yukie Itoh6
Yukie Itoh6 Karen Meyer7
Karen Meyer7 Nobuko Maeda1†
Nobuko Maeda1†Periodontal disease develops as a result of oral microbiota in dysbiosis, followed by the growth of periodontal pathogens such as Porphyromonas gingivalis and Prevotella intermedia. In case of acute symptoms, antibacterial agents and disinfectants are administered, however the appearance of drug-resistant bacteria and allergies cause problems. In recent years, studies on the effects of probiotics have been conducted as an alternative therapy for periodontitis. However, the basic mechanism of the inhibitory effect of probiotic bacteria on periodontal disease has not been clearly elucidated. To clarify the antibacterial mechanism of probiotics against periodontal pathogens, we used Limosilactobacillus (Lactobacillus) fermentum ALAL020, which showed the strongest antibacterial activity against P. gingivalis and P. intermedia among 50 screened lactic acid bacteria strains. The antibacterial substances produced were identified and structurally analyzed. After neutralizing the MRS liquid culture supernatant of ALAL020 strain, the molecular weight (m/z) of the main antibacterial substance separated by gel filtration column chromatography and reverse phase HPLC was 226.131. This low molecular weight compound was analyzed by LC-MS and disclosed the composition formula C11H18O3N2, however the molecular structure remained unknown. Then, structural analysis by NMR revealed C11H18O3N2 as the cyclic dipeptide, “hexahydro-7-hydroxy-3- (2-methylpropyl) pyrrolo [1,2-a] pyrazine-1,4-dion cyclo (Hyp-Leu) “. Based on the results of this analysis, cyclo (Hyp-Leu) was chemically synthesized and the antibacterial activity against P. gingivalis and P. intermedia was measured. The minimum inhibitory concentration (MIC) was 2.5 g/L and the minimum bactericidal concentration (MBC) was shown to be less than 5 g/L. In addition, an in vitro epithelial tissue irritation test at 10 g/L showed no tissue toxicity. So far there are no reports of this peptide being produced by probiotic bacteria. Furthermore, antibacterial activity of this cyclic dipeptide against periodontal disease bacteria has not been confirmed. The results of this study might lead to a comprehensive understanding of the antibacterial mechanism against periodontal disease bacteria in future, and are considered applicable for the prevention of periodontal disease.
Periodontitis is an infectious disease and one of the two major dental diseases along with caries. It is caused by periodontal bacteria, mostly specific gram-negative anaerobic bacteria, which promote dysbiosis in the oral ecosystem (Houle and Grenier, 2003). Porphyromonas gingivalis and Prevotella intermedia are two of the three major periodontal pathogens and species, which were reclassified from the genus Bacteroides (Taniguchi et al., 1998). P. gingivalis is considered to be the most important periodontal pathogen. It is frequently isolated from patients with chronic periodontitis and has the strongest periodontal pathogenicity among oral bacteria (Bostanci and Belibasakis, 2012; Darveau et al., 2012). P. intermedia is not only a cause of chronic periodontitis, but also of gestational gingival inflammation, using female hormones as a growth factor (Kornman & Loesche, 1982), and it is also a causative agent of acute necrotizing ulcerative gingivitis (Bolivar et al., 2012).
Antibacterial agents and disinfectants are used for treatment, but also to prevent the onset and recurrence of periodontal disease. However, adverse events, such as the possibility of developing allergies and the emergence of drug-resistant bacteria are problematic (Watanabe, 1966; Pradier et al., 1997; van Winkelhoff et al., 2000). In addition, periodontal pathogens are present in plaque formed by indigenous bacteria in the oral cavity, and removing all indigenous bacteria leads to the onset of bacterial alternation, which in turn harms health. Therefore, the best preventive method is to eliminate only harmful pathogens, as well as dysbiosis, and to improve the microbiota.
In recent years, attention has been focused on the usefulness of probiotics represented by lactic acid bacteria in the treatment and prevention of periodontal disease (Gupta, 2011). According to Fuller probiotics are defined as “living microbes that improve the gut microbiota and have beneficial health effect to the host”. (Fuller, 1989). Attempts have been made to prevent oral diseases such as dental caries and periodontal disease by applying probiotics directly into the oral cavity (Krasse et al., 2006; Riccia et al., 2007; Vivekananda et al., 2010). For example, Ishikawa et al. (2003) reported that 4-week oral administration of the Ligilactobacillus (Lactobacillus) salivarius TI2711 strain significantly reduced the major periodontopathic bacteria, P. gingivalis and P. intermedia. Vivekananda et al. reported that the viable strains of Limosilactobacillus (Lactobacillus) reuteri DSM17938 and ATCC PTA5289 reduce the number of oral P. gingivalis in patients with periodontal disease. However, there are only few reports on the mechanism and substances of probiotics against oral bacteria.
Organic acids such as lactic acid and acetic acid, hydrogen peroxide, and bacteriocin have been reported as antibacterial substances produced by lactic acid bacteria. Takahashi et al. reported that P. gingivalis is highly acid-sensitive and growth is suppressed below pH 6.5 (Takahashi et al., 1997). Matsuoka et al. reported that the antibacterial activity of the Ligilactobacillus salivarius TI2711 strain against P. gingivalis is due to lactic acid (Matsuoka et al., 2004). However, maintaining a low pH state in the oral cavity with acid is not favored because it may induce caries and hyperesthesia. In order to solve this problem, it is preferred to apply a different antibacterial substance produced by probiotic bacteria for the treatment and prevention of periodontal disease.
In a previously reported study (Kawai et al., 2016), we selected L. fermentum ALAL020 from 50 Lactobacillus strains, which showed strong antibacterial properties against P. gingivalis in a neutral environment, and the active ingredient in the culture supernatant was extracted. When the active ingredient was fractionated and purified by high performance liquid chromatography (HPLC) using a reverse phase column, a low molecular weight substance consisting of two very adjacent peaks was obtained, which was analyzed using LC-MS. However, both peaks were found to be antibacterial substances with a molecular weight of 226.131 and a molecular formula of C11H18O3N2 (Kawai et al., 2016). Since the kind of molecular structure and properties were unclear in this study, we conducted a detailed structural analysis of the antibacterial substance C11H18O3N2, and confirmed the biological property of antibacterial activity and safety for human tissues.
We used L. fermentum ALAL020, a strain derived from fermented soymilk and provided by the Research Institute for Fermentative Microbes, A. L. A. Corporation. After suspending L. fermentum ALAL020 in Man-Rogosa-Sharpe (MRS) broth (Difco, Becton Dickinson and Company, Sparks, MD, USA), the bacteria were cultured in an anaerobic incubator (BACTRON, Sheldon Manufacturing Inc., Portland, OR, USA) in an environment of 80% N2, 10% CO2 and 10% H2 at 37°C for 24 hours.
The culture solution was centrifuged at 7000 x g for 20 minutes to prepare a culture supernatant sample. Four times volume of acetone was added to the culture supernatant, and the mixture was separated into supernatant and precipitate. The acetone extract supernatant was used as a water-soluble fraction and lyophilized. The total volume was dissolved in 150 ml of water and 1/7 volume of 21 ml was placed on a Sephadex G-25 column and eluted with ion-exchange water. The obtained fraction with antibacterial property was lyophilized and dissolved to a concentration of 100 g/L. 1/63 amount was applied to HPLC in 10 batches and analyzed by high performance liquid chromatography (HPLC), using a Wakosil II 5C18 AR Prep (20.0 mmφ x 250 mm) column, with a flow velocity of 5.0 ml/min and a concentration gradient of 10-50% acetonitrile containing 0.05% trifluoroacetic acid. The gradient was applied for 30 minutes and the elute was obtained in 11 fractions (H1-H11). Two fractions, H8-1 and H8-2 with adjacent peaks and antibacterial properties were confirmed at an absorbance of 210 nm (Kawai et al., 2016). H8 fraction was lyophilized and then dissolved in 0.6 mL of de-ionized pure water. After adjusting to 20 g/L, it was used for MS and NMR analysis.
For the structural analysis of the purified product in H8 fraction, an NMR device (AVANCE700, Bruker Biospin) was used with resonance frequency of 700.333 MHz at a temperature of 10°C. 1H NMR and 13C NMR were measured in the first dimension, and 1H-1H COSY (correlation spectroscopy), 1H-13C HSQC (hetero nuclear single-quantum correlation), and 1H-13C HMBC (hetero nuclear multiple bond correlation) were measured in the second dimension. Heavy water or deuterated acetone were used as solvent. A JNM-500A NMR spectrometer (Japan Electron Optics Laboratory) was used as the measuring instrument for the NMR analysis of chemically synthesized peptides. Heavy water or deuterated chloroform was used as the solvent, and the measurement was performed with resonance frequency of 500.00 MHz at a temperature of 20 °C.
All chemicals, reagents and solvents were used without further purification.
Condensation with COMU (El-Faham et al., 2009; El-Faham and Albericio, 2010), removal of the Boc (t-butoxycarbonyl) group (Lundt et al., 1978), intramolecular cyclization (Sollis, 2005) and removal of the Bn (benzyl) group (Hawker et al., 1992) were carried out in sequence. The details are as follows. Dimethylaminomorpholino uronium hexafluorophosphate (COMU) (1.028 g, 2.4 mmol) was added to a dimethylformamide (super dehydrated) solution (10 mL) in a round bottom flask at -10°C containing Boc-O-benzyl-L-hydroxyproline 2 (643 mg, 2.0 mmol), L-Leucine methyl ester hydrochloride 3 (727 mg, 4.0 mmol) and diisopropylethylamine (1.7 mL, 9.8 mmol) 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy). The mixture was stirred under nitrogen at the same temperature for 30 minutes. Then the mixture was continuously stirred at room temperature for 18 hours and diluted with a hexane/ethyl acetate (7/3) solution. The resulting mixture was washed with a saturated aq. NaHCO3, and the layers were separated. The organic layer was dried over Na2SO4 and concentrated via rotary evaporation. The residue was passed through a short column (silica gel, hexane/ethyl acetate = 7/3) to obtain the crude condensation product 4 (1.031 g) as a yellow oil.
To a dichloromethane solution (10 mL) in a round bottom flask containing the crude condensation product 4 (1.031 g) trifluoroacetic acid (1.5 mL, 20 mmol) was slowly added at room temperature under air and the mixture was stirred for 4 hours. Thereafter, a saturated aq. NaHCO3 was poured into the mixture. The layers were separated and extracted with chloroform. The organic extract was dried over Na2SO4 and concentrated in vacuo to obtain the deprotected product 5 (604 mg, 87% in 2 steps) as a yellow oil.
To a dimethylformamid solution (7 mL) in a round bottom flask containing the deprotected product 5 (604 mg, 1.8 mmol) piperidine (1.7 mL, 17 mmol) was added at room temperature under air and the mixture was stirred at 70°C for 12 hours. Thereafter, it was left at room temperature. Then, the mixture was concentrated in vacuo to obtain the cyclic product 6 (511 mg, 92%) as a yellow solid.
To a tetrahydrofuran (super dehydrated, stabilizer free) solution (16 mL) in a round bottom flask containing the cyclic product 6 (511 mg, 1.6 mmol) 10 wt% palladium on carbon (511 mg, 100 wt%) was added at room temperature under air and the flask was charged with hydrogen using a hydrogen filled balloon. The mixture was stirred at 55 °C for 5 hours. The balloon was removed, and the flask was left at room temperature. After dilution with methanol, a suction filtration through filter paper was carried out to eliminate the palladium carbon. The collected solution was concentrated in vacuo, and then the obtained powder was rinsed with diethyl ether to get cyclo (L-Hyp-L-Leu) 1 (283 mg, 78%).
An HPLC-purified sample was analyzed using an Ultimate 3000 UHPLC System (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and a Shim-pack VP-ODS column (150 mm × φ4.6 mm, Shimadzu, Tokyo, Japan). Gradient elution was performed using 10-50% acetonitrile with 0.05% formic acid. An MS analysis was performed with a Mass Spectrometry Q Exactive TM Quadrupole/Orbitrap hybrid mass spectrometer (Thermo Fisher Scientific). Ionization was performed using the ESI method and detection in the positive mode.
The antibacterial test with the peak fractions was performed according to the method described in a previous report (Kawai et al., 2016) using P. gingivalis ATCC 33277 as the index bacterium.
The antibacterial activity of the synthetic dipeptide cyclo (Hyp-Leu) against the P. gingivalis reference strain (ATCC 33277) and the P. intermedia reference strain (ATCC 25611) was evaluated as the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC, measuring colony forming units CFU).
-80°C cryopreserved cells of P. gingivalis and P. intermedia were thawed and suspended in brain heart infusion (BHI) medium (Difco, Becton Dickinson, Maryland, USA), supplemented with 5 μg/ml hemin, 1 μg/mL menadione, 0.5% yeast extract and 0.05% cysteine hydrochloride (Wako Pure Chemical Industries, Ltd., Osaka), and cultured in an anaerobic incubator (BACTRON, Sheldon Manufacturing Inc.) under the atmosphere of 80% N2, 10% CO2, 10% H2 at 37°C. After culturing for 2 days, the bacteria were sub-cultured in fresh medium for another 2 days to gain 107 CFU/ml each, which were used as the test bacteria.
40 μL of BHI medium supplemented with 5 μg/mL hemin and 1 μg/mL menadione were dispensed into a 96-well plate, and 5 μL of each 10 g/L, 5 g/L, and 2.5 g/L aqueous solution of the synthetic peptide was added. 20 g/L, 10 g/L, 5 g/L and 2.5 g/L sodium lactate (Lactate Na) were used as control series. 5 μL of the two test bacterium culture solutions were inoculated and cultured for 2 days under anaerobic conditions at 37 °C. The turbidity was measured at 650 nm, and a value of 0.15 or less corresponds to MIC. After MIC determination, the culture medium was inoculated on Brucella HK agar medium (KYOKUTO PHARMACEUTICAL INDUSTRIAL CO., Tokyo, Japan) supplemented with 5% sheep de-fibered blood by plating, and then anaerobically cultured at 37 °C for 5 days. MBC was determined by measuring CFU.
Epithelial stimulation tests were performed according to the standard protocol (OECD TG 439) using in vitro reconstructed human epidermal models (EPI-200SIT, KURABO, Osaka, Japan). A 10 g/L cyclo (Hyp-Leu) dipeptide aqueous solution was used as the test solution, PBS (phosphate buffered saline) as a negative control and 5% SDS (sodium dodecyl sulfate) as a positive control. The metabolic activity of the treated tissue was measured by the manufacturer specified MTT assay and compared to the described criteria.
The obtained data were statistically analyzed using IBN SPSS Statistics version 19 (IBM, Armonk, NY, USA). Comparisons between the three groups were analyzed by the Kruskal-Wallis test, and data on synthetic dipeptides compared to the positive controls were analyzed by the Mann-Whitney U test.
As in the previous report (Kawai et al., 2016), each peak was divided into 11 fractions, with H8-1 and H8-2 very adjacent (Figure 1). According to the results of the antibacterial tests against P. gingivalis, fraction H8-1 had the highest antibacterial activity, followed by H8-2. The active fractions (H8-1 and H8-2) corresponded to 9.7% of the eluted fraction and a concentration of 0.47 g/L.
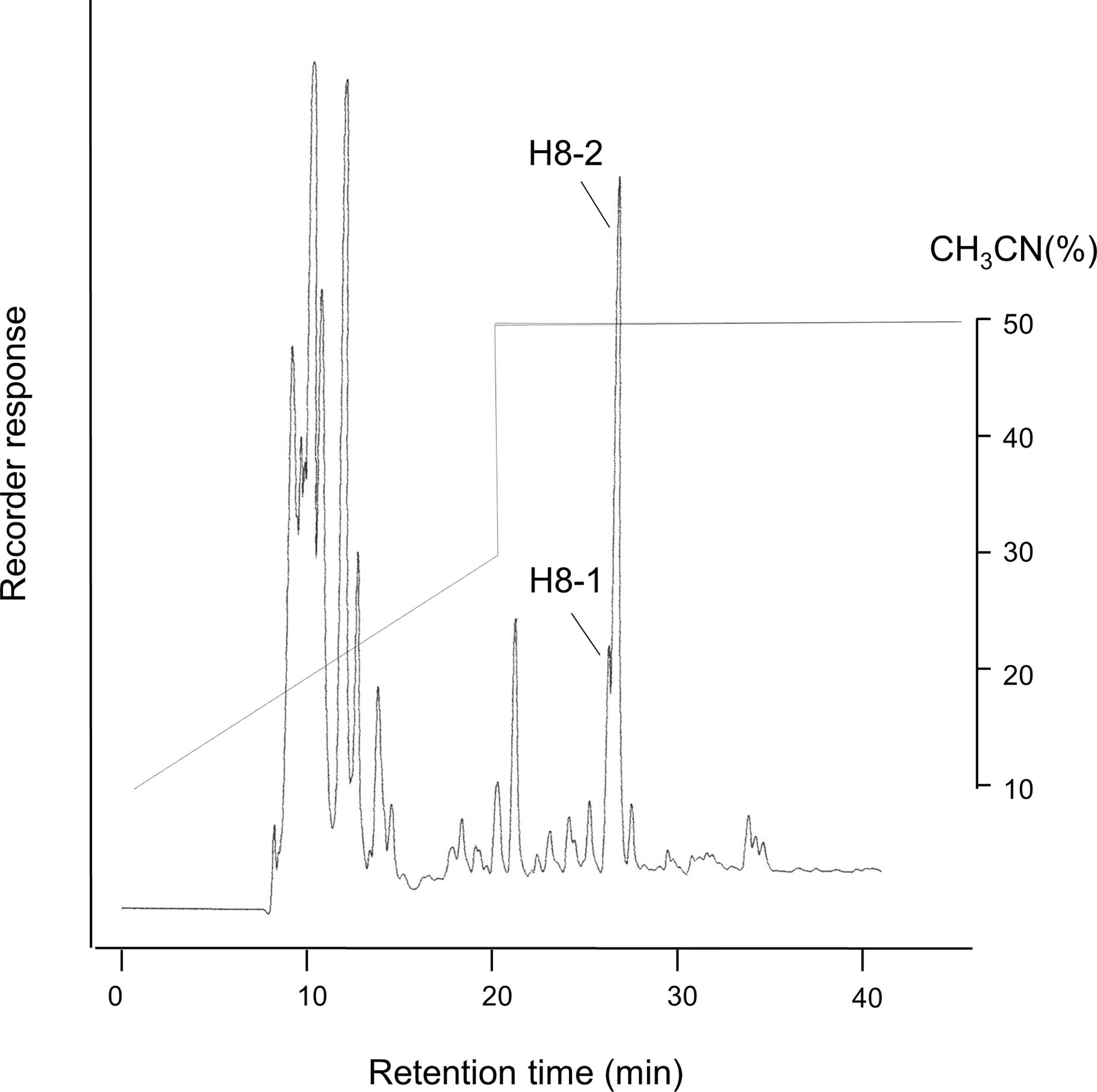
Figure 1 HPLC fractions of L. fermentum ALAL020 culture supernatant. The culture supernatant was fractionated with reverse-phase chromatography by HPLC on an ODS (C18) column.
It has also been confirmed that the molecular weights and formulas of the fractions H8-1 and H8-2 were both 226.131 Da and C11H18N2O3 respectively (Kawai et al., 2016).
The NMR spectra obtained for the antibacterial substance of H8-1 are shown in Table 1. The 1H NMR spectrum showed one NH resonance (δH: 7.49 (1H, s)), two methyl signals (δH: 0.92 (3H, d, J = 6.6Hz), 0.96 (3H, d, J = 6.7)), six methylene resonances (δH: 2.22-2.27 (1H, m), 2.38-2.42 (1H, m), 1.65-1.69 (1H, m), 1.53-1.57 (1H, m), 3.37 (1H, dd, J = 11.9, 5.4Hz), 3.65 (1H, ddd, J = 11.9, 3.7, 0.8Hz)) and four methine signals (δH: 1.75-1.82 (1H, m), 3.81-3.84 (1H, m), 4.28 (1H, dd, J = 8.6, 7.1Hz), 4.42-4.45 (1H, m)) (Figure 2A). The 13C NMR spectrum showed 11 C atoms and carbon signals (δC: 21.83, 23.17, 25.01, 37.61, 43.09, 53.90, 56.46, 56.91, 68.46, 167.28, 169.29) (Figure 2B). Comprehensive analysis results of 1H-1H COSY, 1H-13C HSQC and 1H-13C HMBC by two-dimensional NMR analysis (SF-A, B, C) revealed two amino acids, hydroxyproline and leucine (Figure 2C).
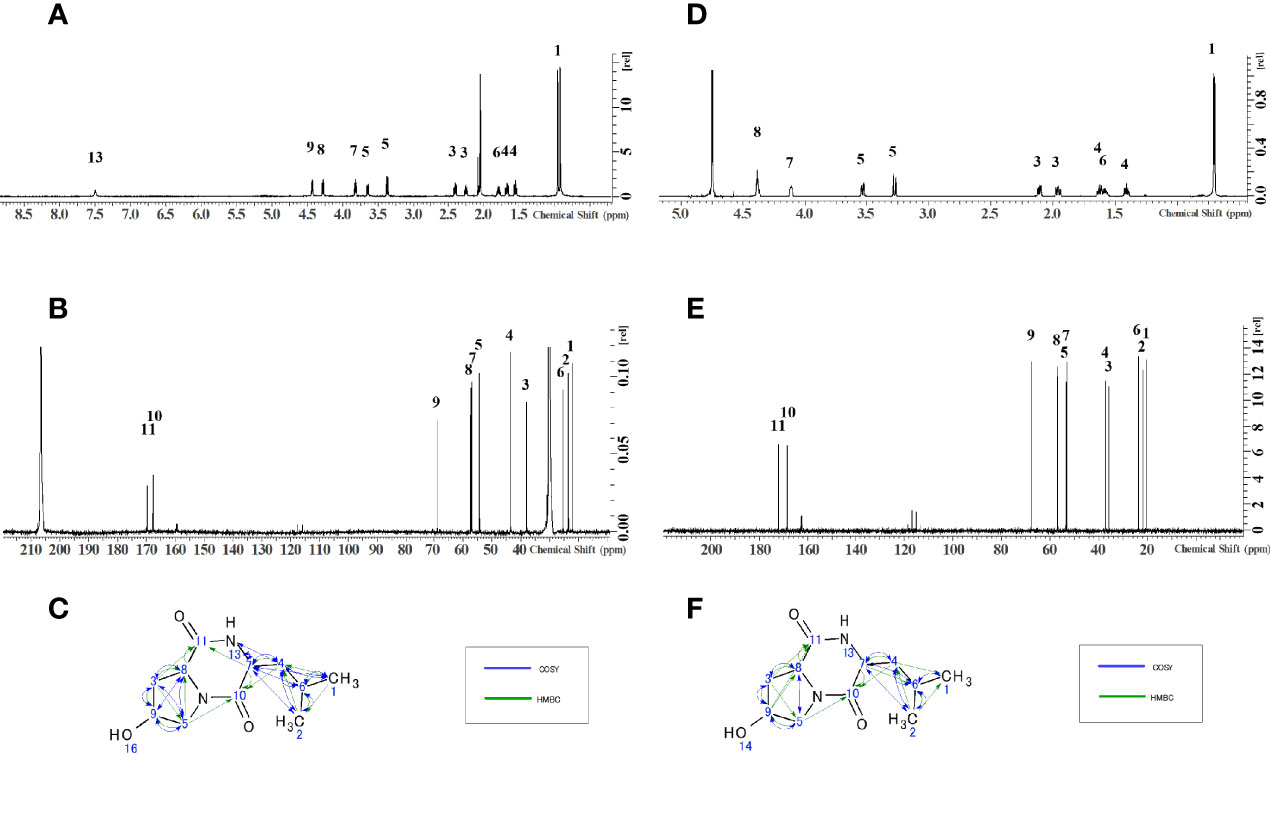
Figure 2 Structure determination by NMR. (A)1H-NMR of H8-1, (B)13C-NMR of H8-1, (C) COSY and HMBC correlations of H8-1, (D) 1H-NMR of H8-2, (E)13C-NMR of H8-2, (F) COSY and HMBC correlations of H8-2.
The NMR spectra obtained for the antibacterial substance H8-2 are shown in Table 2. The 1H NMR spectrum showed one NH resonance (δH ): two methyl signals (δH: 0.75 (6H, d, J = 6.4Hz)), six methylene resonances (δH: 2.15 (1H, dd, J = 13.5, 6.4 Hz)), 1.98-2.02 (1H, m), 1.64-1.69 (1H, m), 1.43-1.47 (1H, m), 3.57 (1H, dd, J = 13.2, 4.4Hz), 3.31 (1H, d, J = 13.2Hz)) and four methine signals (δH: 1.60-1.65 (1H, m), 4.15-4.16 (1H, m), 4.41-4.42 (1H, m), 4.43 (1H, t, J = 4.5Hz)) (Figure 2D).
The 13C NMR spectrum showed 11 C atoms and carbon signals (δC: 20.61, 21.99, 23.72, 35.92, 37.22, 53.23, 53.61, 57.04, 67.68, 168.58, 172.23) (Figure 2E). The result of comprehensive two-dimensional NMR analysis 1H-1H COSY, 1H-13C HSQC and 1H-13C HMBC showed two amino acids, hydroxyproline and leucine, similar to H8-1 (Figure 2F).
As a result of the above, the antibacterial substances corresponding to fractions H8-1 and H8-2 are both hexahydro-7-hydroxy-3- (2-metylpropyl) pyrrolo [1,2- a] pyrazine-1,4-dion as illustrated in the structural formula of Figure 3. That means, it is a cyclic dipeptide of hydroxyproline and leucine, abbreviated as “cyclo (Hyp-Leu)” (Figure 3).
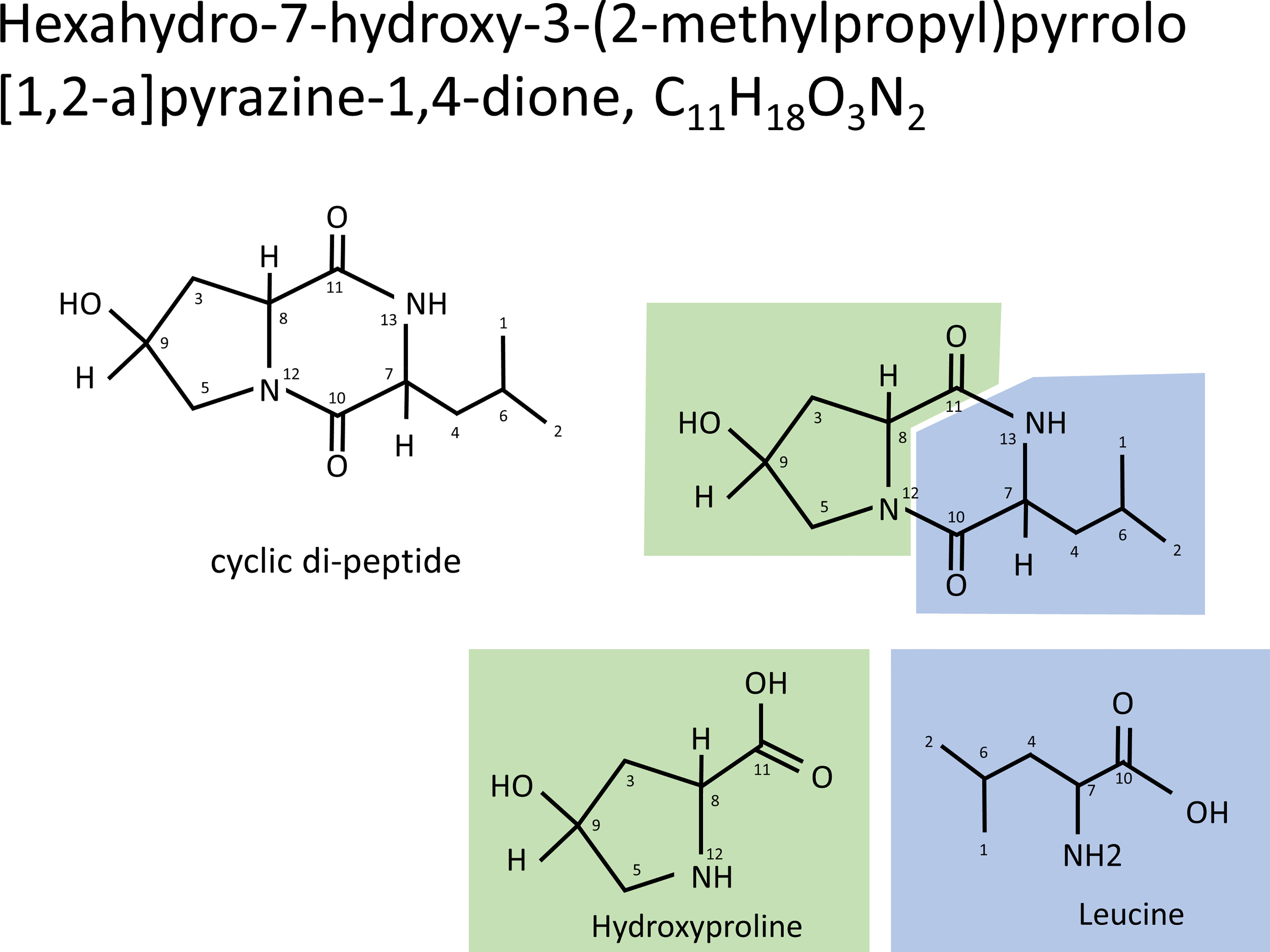
Figure 3 Structure of cyclo (Hyp-Leu). From the result of NMR analysis, the molecule of C11H18O3N2 was revealed to form a cyclic di-peptide of hydroxyproline and leucine.
Composition and structure of the cyclic dipeptide Hyp-Leu (hexahydro-7-hydroxy-3- (2-metylpropyl) pyrrolo [1,2-a] pyrazine-1,4-dion) were clarified followed by chemical synthesis. According to the scheme in Figure 4, the condensation of commercially available Boc-O-benzyl-L-hydroxyproline (2 in Figure 4) and L-leucine methyl ester hydrochloride (3 in Figure 4) was carried out with COMU as a condensing agent. The Boc group of the condensation product (4 in Figure 4) was then removed by treatment with trifluoroacetic acid, and 5 of Figure 4 was obtained. The structure was confirmed by NMR analysis (SF-D). 1H NMR (CDCl3) δ7.94 (1H, d, J = 8.5 Hz), 7.34-7.25 (5H, m), 4.57-4.52 (1H, m), 4.51-4.41 (2H, m), 4.08 (1H, s), 4.00 (1H, t, J = 7.7 Hz), 3.70 (3H, s), 3.19 (1H, d, J = 12.5 Hz), 2.74 (1H, dd, J = 12.5, 3.6 Hz), 2.48-2.43 (1H, m), 1.92-1.86 (1H, m), 1.67-1.43 (4H, m), 0.90 (6H, t, J = 5.8 Hz).
The intramolecular cyclization of the amino group and ester group in 5 was carried out under basic condition to form 2, 5-diketopiperazine. The structure was confirmed by NMR analysis (SF-E). 1H NMR (CDCl3) δ 7.45-7.26 (5H, m), 5.66 (1H, s), 4.52 (2H, s), 4.43 (1H, dd, J = 10.8, 6.3 Hz), 4.22-4.20 (1H, m), 4.03-4.01 (1H, m), 3.72 (1H, d, J = 13.1 Hz), 3.66 (1H, dd, J = 13.1, 4.6 Hz), 2.57 (1H, dd, J = 13.5, 6.3 Hz), 2.11-2.01 (2H, m), 1.75-1.46 (2H, m), 0.98 (3H, d, J = 6.4 Hz), 0.93 (3H, d, J = 6.7 Hz).
Finally, the benzyl group included in 6 was removed by hydrogenolysis to obtain the targeted compound, Cyclo (L-Hyp-L-Leu) (1 in Figure 4). The analyzed structure by NMR was as follows: 1H NMR (D2O) δ 4.42-4.39 (2H, m), 4.13 (1H, s), 3.56 (1H, dd, J = 13.1, 4.6 Hz), 3.30 (1H, d, J = 13.1 Hz), 2.14 (1H, dd, J = 13.5, 6.4 Hz), 2.01-1.95 (1H, m), 1.70-1.55 (2H, m), 1.46-1.40 (1H, m), 0.73 (6H, d, J = 6.4 Hz).
13C NMR (D2O) δ173.00, 169.33, 68.47, 57.83, 54.41, 54.05, 38.08, 36.74, 24.54, 22.78, 21.46.
When these NMR spectra were compared with the spectra of H8-1 and H8-2 derived from natural products, both 1H NMR and 13C NMR of H8-2 were in agreement (Figure 5). From this result, H8-2 is considered to be cyclo (L-Hyp-L-Leu) or cyclo (D-Hyp-D-Leu).
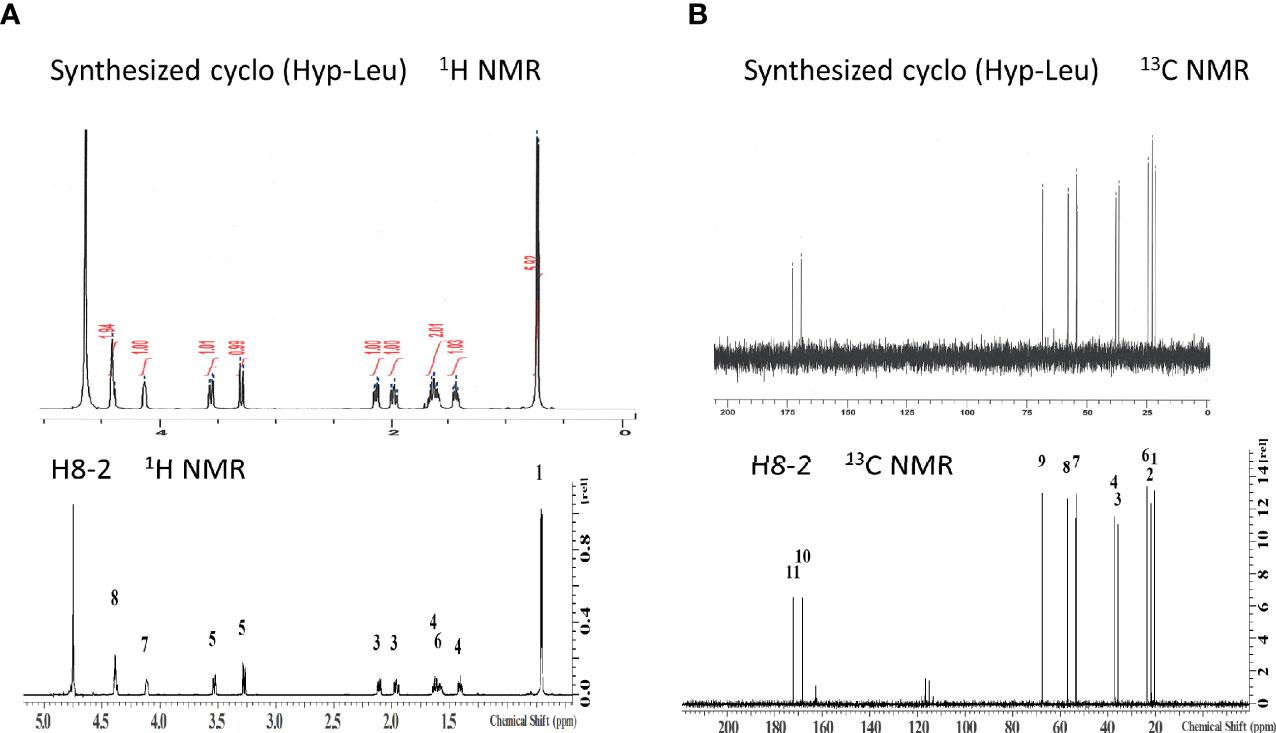
Figure 5 Structure confirmation by NMR. (A) 1H-NMR spectrum of synthesized cyclo (Hyp-Leu) was matched to that of H8-1, (B)13C-NMR spectrum of synthesized cyclo (Hyp-Leu) was matched to H8-1 13C NMR.
MS analysis for was performed and the monoisotopic mass of the protonated molecule was estimated 227.1405. Furthermore, from the results of mass spectrometry, it was found that the synthetic product was also C11H18N2O3, 226.131.
Antibacterial tests were conducted against P. gingivalis and P. intermedia. Synthetic cyclo (Hyp-Leu) showed concentration-dependent antibacterial activity against both bacteria, with a MIC of 2.5 g/L or less (Figures 5A, B). Sodium lactate used as a control, is a product of the fermentation process of lactic acid bacteria and a component widely known for its antibacterial properties, however the MIC was 20 g/L (Figures 6A, B). Furthermore, examining the MBC of synthetic cyclo (Hyp-Leu), resulted in 5 g/L or less for P. gingivalis and 2.5 g/L or less for P. intermedia (Figures 6C, D).
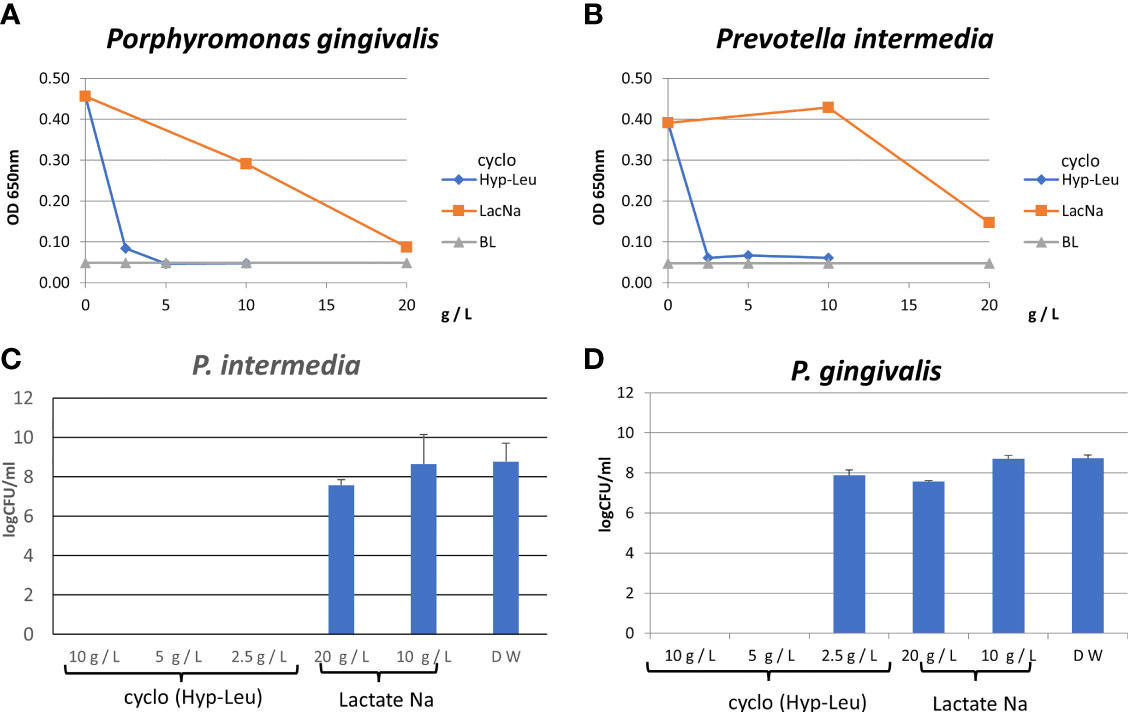
Figure 6 Antibacterial activity of synthesized cyclo (Hyp-Leu). (A, B) Show the results of the turbidity assay. BL is medium only. (C, D) Show the result of the CFU assay.
The results of the epithelial tissue irritation test are shown in Figure 7. The result of the MTT assay was 2.024 ± 0.091 for PBS used as a negative toxicity control. On the other hand, the value of 5.0% SDS used as a positive control indicated a cytotoxic level. The value of the 10 g/L synthetic cyclo (Hyp-Leu) solution was 1.964 ± 0.108, which was an equivalent level to PBS and significantly different from the 5.0% SDS of the positive control (Mann-Whitney U test p> 0.01). Therefore, no toxicity to the epithelial tissue model was observed.
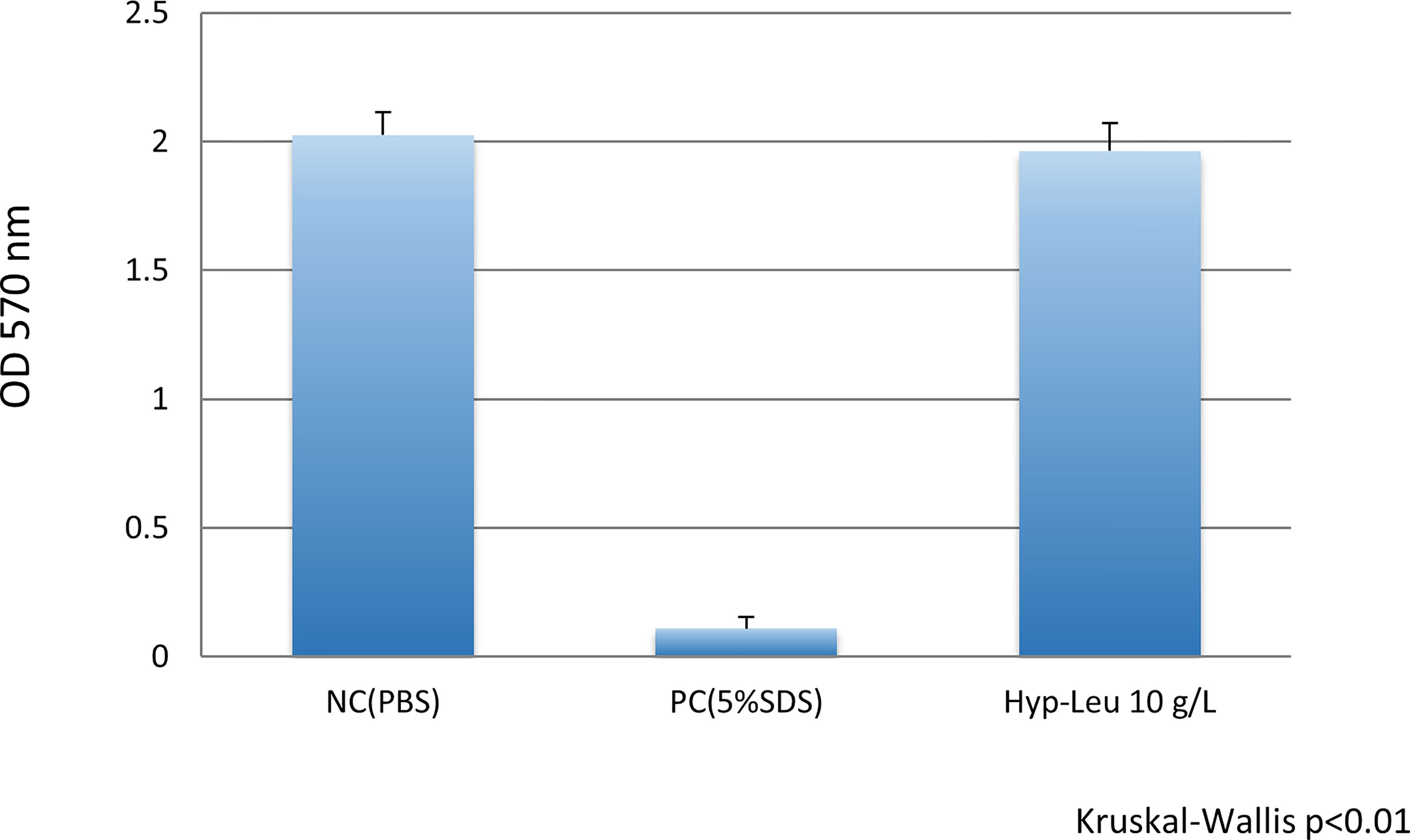
Figure 7 Epithelial tissue toxicity of cyclo (Hyp-Leu). The vertical axis shows the tissue activity level deduced form the result of the MTT assay. NC (negative control, PBS) showed no irritation, and PC (positive control, 5%SDS) showed toxicity. The level of the experimental cyclo (Hyp-Leu) group was almost the same as the NC group and significantly different from the PC group.
Generally, organic acids such as lactic acid and acetic acid, hydrogen peroxide, and bacteriocin are widely known as bactericidal or antibacterial substances produced by lactic acid bacteria. In addition, several cyclic dipeptides (also known as 2,5-diketopiperazine) have been reported (Klaenhammer, 1988; Piard and Desmazeaud, 1991; Taniguchi et al., 1998; Graz et al., 1999). Cyclic dipeptides are low molecular weight substances obtained by condensing two amino acids. They are expected to be antifungal and antibacterial (Graz et al., 1999). Cyclic dipeptides have been identified in cultures fermented with lactic acid bacteria used in food fermentation (Niku-Paavola et al., 1999; Strom et al., 2002; Magnusson et al., 2003; Ryan et al., 2011). Further, proline-based and leucine-based cyclic dipeptides have been isolated from cultures of multiple lactobacilli. For example, cyclo (L-Pro-L-Pro), cyclo (L-Leu-L-Pro), cyclo (L-Tyr-L-pro), cyclo (L-Met-L-Pro), cyclo (L-His-L-Pro) were identified from L. amylovorus, (Ryan et al., 2011), and cyclo (Gly-L-Leu), cyclo (L-Phe-L-Pro) and cyclo (L-Phe-trans-4-OH-L-Pro) have been identified from L. plantarum (Niku-Paavola et al., 1999; Strom et al., 2002). Above reports have confirmed that these cyclic dipeptides in lactobacilli cultures have antifungal activity.
The substance hexahydro-7-hydroxy-3- (2-metylpropyl) pyrrolo [1,2-a] pyrazine-1,4-dion was reported by Zhang and Lei (2015), and is a cyclic dipeptide produced by microorganisms of the genus Streptomyces classified as soil-derived actinomycetes. It was the same substance as identified in this study. However, as far as we know, the cyclic dipeptides identified in this study have not been reported as substances produced by lactic acid bacteria. This hydrophilic cyclic dipeptide was included in the acetone fraction of the culture supernatant of the L. fermentum ALAL020 strain. In a preliminary study, the acetone fraction did not show antibacterial properties against the caries-causing bacterium Streptococcus mutans or the oral fungus Candida albicans (data not shown), whereas it had excellent antibacterial properties against P. gingivalis and P. intermedia (Figure 6). Since the MIC of cyclo (Hyp-Leu) against P. gingivalis and P. intermedia was 2.5 mg/mL, the content in the supernatant was about 20% of the MIC. 2014 literature indicates the concentration of a L. brevis-produced cyclic dipeptide (Axel et al., 2014) as 10-50 ug/mL, in contrary to L. fermentum ALAL020 with 470 μg/mL, which is a production amount 5-10 times higher.
L. fermentum ALAL020 is derived from fermented food and expected to be effective when used as regular food. This strain is contained in fermented soymilk and shows an immune-stimulatory effect in an in vitro assay (unpublished data) as well as an effect of improving liver and kidney disorders in animal model experiments (Shin et al., 2009). Therefore, it can be called a probiotic candidate strain. Clinical efficacy data in humans has to be accumulated in future. In this case, if it exhibits strong bactericidal activity such as antibiotic reagents, it is possible that the bacterial microbiota might be disturbed or a bacterial replacement phenomenon might occur. Therefore, it seems appropriate to compare the antibacterial di-peptide with known antibacterial food substances. For example, the MIC of EGCG (epigallocatechin gallate), a green tea main component, against P. gingivalis has been reported to be 0.125-1.0 g/L (0.27-2.2 mM) (Sakanaka et al., 1996; Fournier-Larente et al., 2016). Since MIC of cyclo (Hyp-Leu) was 2.5 g/L (11.1 mM), it is considered the same grade as the green tea component, although the activity is slightly weaker. However, examination with multiple P. gingivalis clinical isolates, as shown in the Supplementary File (E), indicate large individual differences in susceptibility, so comparison of the MIC value of single strains is not accurate. Since we have succeeded in peptide synthesis, it seems possible to adjust the activity in future.
Probiotic L. reuteri produce reutericyclin with antibacterial property, however this substance tends to be resistant to half of the E. coli tested by Gänzle et al. (2004), confirming antibacterial activity only against Gram-positive bacteria. Therefore, reutericyclin is expected to have low activity against periodontopathic bacteria.
MIC of reuterin was reported as 7.5 to 15 mM (5.5-11.1 g/L) in Escherichia coli and that of Bacteroides 1.9 to 7.5 mM (Cleusix et al., 2007), which means that the anti-bacterial effect is almost at the same level as cyclo (Hyp-Leu) (MIC: 11.1mM) against P. gingivalis and P. prevotella. In their report, periodontal disease bacteria have not been investigated, but they might have a tendency similar to the closely related genus Bacteroides. It was reported that chemically synthesis of reuterin is very difficult because reuterin is an intermediate metabolite and degrades rapidly to various other components such as 3-Hydroxypropionaldehyde (3-HPA) hydrate, HPA dimer, and acrolein (Kang, 2014; Fujiwara et al., 2017). With this unstable property, the clinical application of reuterin seems difficult. Fujiwara et al. synthesized structural derivatives and obtained highly active and stable ones against periodontal bacteria (Fujiwara et al., 2017). The significance of our structural analysis of cyclo (Hyp-Leu) is not only to clarify the mechanism of action in the future, but also to obtain derivatives showing even better activity.
Although the effects of P. gingivalis on cell adhesion and invasion have not been investigated, it seems that bactericidal action is effective before adhesion and invasion. Further, it has been confirmed that the trypsin-like enzyme (Gingipain) activity produced by P. gingivalis, is not inhibited (data not shown), however it can be said that this is not a necessary effect if a bactericidal effect occurs.
In order to analyze the mechanism of antibacterial activity against periodontal disease bacteria, we decided to prepare a synthetic cyclo (Hyp-Leu) (Figure 4). The characteristic structure of cyclo (L-Hyp-L-Leu) 1 (Figure 4) is its 2,5-diketopiperazine skeleton within the molecule. It has been reported that the 2,5-diketopiperazine skeleton forms by condensation of 2 amino acid derivatives followed by intramolecular cyclization of the dipeptide (Wang et al., 2002). Based on this method, we attempted the synthesis of cyclo (L-Hyp-L-Leu) 1. We consider that racemization hardly occurs during condensation or intramolecular cyclization, because cyclo (L-Hyp-D-Leu) (or cyclo (D-Hyp-L-Leu)) could not be detected. A cyclic dipeptide is stereospecifically synthesized and effectively forms cyclo (L-Hyp-D-Leu) from L-Hyp and D-Leu derivatives. This time, we could confirm a diastereomer equivalent to H8-2 (Figure 5). Since antibacterial properties against periodontal disease bacteria were also confirmed (Figure 6), it is possible that this feature could be used to elucidate the mechanism of activity in future.
Furthermore, while considering future clinical applications, safety for mucosal epithelial tissues is an important aspect. In particular, for many dipeptides reports exist, which show the effects of anticancer cells, so there is concern about toxicity to eukaryotic cells. The irritation evaluation model system performed this time is widely used to evaluate short-term toxicity for drugs and cosmetics as an alternative technology to animal experiments. Since the synthetic cyclo (Hyp-Leu) seems to have low toxicity in the stimulation test using this epithelial tissue model, it might be put into practical use from the viewpoint of safety.
In conclusion, the substances isolated from fractions H8-1 and H8-2 of L. fermentum ALAL020 culture have antibacterial activity against periodontopathic bacteria P. gingivalis and P. intermedia, and were both identified as hexahydro-7-hydroxy-3-(2-metylpropyl) pyrrolo [1,2-a] pyrazine-1,4-dion. It is most likely that the slight difference in the fraction peaks of H8-1 and H8-2 was observed because they are steric isomers. Among the previously reported antibacterial substances produced by L. fermentum, no report indicates production of this substance, which is a novel finding obtained from this study. We believe that the results of this analysis will be useful for elucidating the production mechanism of this substance and the antibacterial mechanism against periodontal disease bacteria. Further studies are necessary on the mechanism of production and action, as well as the potential for clinical application.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
TK designed and coordinated the study, performed the measurement and interpretation of the data. TO contributed to the design and coordination of the study, performed the measurement and interpretation of the data. NM participated in the design and interpretation of the data; deceased Jan. 28, 2020. TT, SI, AT, NI, RS, and YI performed the measurement and interpretation of the data. TO and KM wrote and checked the manuscript. The authors read and approved the final manuscript.
The authors acknowledge financial support from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan in the form of a Grant-in-Aid for Young Scientists, No. 18K17057.
Authors RS and YI are employed by the company A. L. A. Corporation, but no-funding was offered to this study from A. L. A. Corporation. In addition, A. L. A. Corporation was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.804334/full#supplementary-material
Axel, C., Zannini, E., Arendt, E. K., Waters, D. M., Czerny, M. (2014). Quantification of Cyclic Dipeptides From Cultures of Lactobacillus Brevis R2Δ by HRGC/MS Using Stable Isotope Dilution Assay. Anal. Bioanal. Chem. 406 (9), 2433–2444. doi: 10.1007/s00216-014-7620-3
Bolivar, I., Whiteson, K., Stadelmann, B., Baratti-Mayer, D., Gizard, Y., Mombelli, A., et al. (2012). Bacterial Diversity in Oral Samples of Children in Niger With Acute Noma, Acute Necrotizing Gingivitis, and Healthy Controls. PloS Negl. Trop. Dis. 6 (3), e1556. doi: 10.1371/journal.pntd.0001556
Bostanci, N., Belibasakis, G. N. (2012). Porphyromonas Gingivalis: An Invasive and Evasive Opportunistic Oral Pathogen. FEMS Microbiol. Lett. 333 (1), 1–9. doi: 10.1111/j.1574-6968.2012.02579.x
Cleusix, V., Lacroix, C., Vollenweider, S., Duboux, M., Le Blay, G. (2007). Inhibitory Activity Spectrum of Reuterin Produced by Lactobacillus Reuteri Against Intestinal Bacteria. BMC Microbiol. 7 (1), 1–9. doi: 10.1186/1471-2180-7-101
Darveau, R. P., Hajishengallis, G., Curtis, M. A. (2012). Porphyromonas Gingivalis as a Potential Community Activist for Disease. Dent. Res. 91 (9), 816–820. doi: 10.1177/0022034512453589
El-Faham, A., Albericio, F. (2010). COMU: A Third Generation of Uronium-Type Coupling Reagents. J. Pept. Sci. 16 (1), 6–9. doi: 10.1002/psc.1204
El-Faham, A., Funosas, R. S., Prohens, R., Albericio, F. (2009). COMU: A Safer and More Effective Replacement for Benzotriazole-Based Uronium Coupling Reagents. Chemistry 15 (37), 9404–9416. doi: 10.1002/chem.200900615
Fournier-Larente, J., Morin, M. P., Grenier, D. (2016). Green Tea Catechins Potentiate the Effect of Antibiotics and Modulate Adherence and Gene Expression in Porphyromonas Gingivalis. Arch. Oral. Biol. 65, 35–43. doi: 10.1016/j.archoralbio.2016.01.014
Fujiwara, N., Murakami, K., Nakao, M., Toguchi, M., Yumoto, H., Hirota, K., et al. (2017). Antibacterial and Antibiofilm Effects of Reuterin-Related Compounds to Periodontopathic Bacteria. J. Oral. Health Biosci. 30 (1), 8–17. doi: 10.20738/johb.30.1_8
Fuller, R. (1989). Probiotics in Man and Animals. J. Appl. Bacteriol 66, 365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x
Gänzle, M. G. (2004). Reutericyclin: Biological Activity, Mode of Action, and Potential Applications. Appl. Microbiol. Biotechnol. 64 (3), 326–332. doi: 10.1007/s00253-003-1536-8
Graz, M., Hunt, A., Jamie, H., Grant, G., Milne, P. (1999). Antimicrobial Activity of Selected Cyclic Dipeptides. Pharmazie 54 (10), 772–775.
Hawker, S., Bhatti, M. A., Griffin, K. G. (1992). The Removal of Protecting Groups by Catalytic Hydrogenation. Chim. Oggi 10 (1-2), 49–51.
Houle, M. A., Grenier, D. (2003) Maladies Parodontales: Connaissances Actuelles. Curent Concepts in Periodontal Diseases. Médecine Mal Infectieuses 33, 331–340. doi: 10.1016/S0399-077X(03)00203-8
Ishikawa, H., Aiba, Y., Nakanishi, M., Oh-Hashi, Y., Koga, Y. (2003). Suppression of Periodontal Pathogenic Bacteria in the Saliva of Humans by the Administration of Lactobacillus Salivarius TI 2711. J. Jpn. Soc. Periodontol 45, 105–112. doi: 10.2329/perio.45.105
Kang, C. T. (2014). Analytical Methods for the Selected Properties Determination of 3-Hydroxypropionaldehyde (HPA). Sky J. Biochem. Res. 3, 42–46.
Kawai, T., Ohshima, T., Shin, R., Ikawa, S., Maeda, N., Gomi, K. (2016). Determination of the Antibacterial Constituents Produced by Lactobacilli Against a Periodontal Pathogen: Sodium Lactate and a Low Molecular Weight Substance. J. Prob. Health 4, 135. doi: 10.4172/2329-8901.1000135
Klaenhammer, T. R. (1988). Bacteriocins of Lactic Acid Bacteria. Biochime 70, 337–349. doi: 10.1016/0300-9084(88)90206-4
Kornman, K. S., Loesche, W. J. (1982). Effects of Estradiol and Progesterone on Bacteroides Melaninogenicus and Bacteroides Gingivalis. Infect. Immun. 35 (1), 256–263. doi: 10.1128/iai.35.1.256-263.1982
Krasse, P., Carlsson, B., Dahl, C., Paulsson, A., Nilsson, A., Sinkiewicz, G. (2006). Decreased Gum Bleeding and Reduced Gingivitis by the Probiotic Lactobacillus Reuteri. Swed. Dent. J. 30 (2), 55–60.
Lundt, B. F., Johansen, N. L., Vølund, A., Markussen, J. (1978). Removal of T-Butyl and T-Butoxycarbonyl Protecting Groups With Trifluoroacetic Acid: Mechanisms, Biproduct Formation and Evaluation of Scavengers. Int. J. Pept. Protein Res. 12 (5), 258–268. doi: 10.1111/j.1399-3011.1978.tb02896.x
Magnusson, J., Ström, K., Roos, S., Sjögren, J., Schnürer, J. (2003). Broad and Complex Antifungal Activity Among Environmental Isolates of Lactic Acid Bacteria. FEMS Microbiol. Lett. 219 (1), 129–135. doi: 10.1016/S0378-1097(02)01207-7
Matsuoka, T., Nakanishi, M., Aiba, Y., Koga, Y. (2004). Mechanism of Porphyromonas Gingivalis Killing by Lactobacillus Salivalius TI2711. J. Jpn Soc. Periodontal 46, 118–126. doi: 10.2329/perio.46.118
Niku-Paavola, M. L., Laitila, A., Mattila-Sandholm, T., Haikara, A. (1999). New Types of Antimicrobial Compounds Produced by Lactobacillus Plantarum. J. Appl. Microbiol. 86 (1), 29–35. doi: 10.1046/j.1365-2672.1999.00632.x
Piard, J. C., Desmazeaud, M. (1991). Inhibiting Factors Produced by Lactic Acid Bacteria: 1. Oxygen Metabolites and Catabolism End Products. Le Lait 71 (5), 525–541.
Pradier, C., Dunais, B., Carsenti-Etesse, H., Dellamonica, P. (1997). Pneumococcal Resistance Patterns in Europe. Pneumococcal Resistance Patterns in Europe. European J. Clin. Microbiol. Infect. Dis. 16 (9), 644–647. doi: 10.1007/BF01708553
Riccia, D. N., Bizzini, F., Perilli, M. G., Polimeni, A., Trinchieri, V., Amicosante, G., et al. (2007). Anti-Inflammatory Effects of Lactobacillus Brevis (CD2) on Periodontal Disease. Oral. Dis. 13 (4), 376–385. doi: 10.1111/j.1601-0825.2006.01291.x
Ryan, L. A., Zannini, E., Dal Bello, F., Pawlowska, A., Koehler, P., Arendt, E. K. (2011). Lactobacillus Amylovorus DSM 19280 as a Novel Food-Grade Antifungal Agent for Bakery Products. Int. J. Food Microbiol. 146 (3), 276–283. doi: 10.1016/j.ijfoodmicro.2011.02.036
Sakanaka, S., Aizawa, M., Kim, M., Yamamoto, T. (1996). Inhibitory Effects of Green Tea Polyphenols on Growth and Cellular Adherence of an Oral Bacterium, Porphyromonas Gingivalis. Biosci. Biotechnol. Biochem. 60 (5), 745–749. doi: 10.1271/bbb.60.745
Shin, R., Suzuki, M., Mizutani, T., Susa, N. (2009). Improvement of Experimentally Induced Hepatic and Renal Disorders in Rats Using Lactic Acid Bacteria-Fermented Soybean Extract (Biofermentics™). Evid.-Based Complement. Altern. Med. 6 (3), 357–363. doi: 10.1093/ecam/nem126
Sollis, S. L. (2005). Short and Novel Stereospecific Synthesis of Trisubstituted 2, 5-Diketopiperazines. J. Org Chem. 70 (12), 4735–4740. doi: 10.1021/jo0501137
Ström, K., Sjögren, J., Broberg, A., Schnürer, J. (2002). Lactobacillus Plantarum MiLAB 393 Produces the Antifungal Cyclic Dipeptides Cyclo(L-Phe-L-Pro) and Cyclo(L-Phe-Trans-4-OH-L-Pro) and 3-Phenyllactic Acid. Appl. Environ. Microbiol. 68 (9), 4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002
Takahashi, N., Saito, K., Schachtele, C. F., Yamada, T. (1997). Acid Tolerance and Acid-Neutralizing Activity of Porphyromonas Gingivalis, Prevotella Intermedia and Fusobacterium Nucleatum. Oral. Microbiol. Immunol. 12, 323–328. doi: 10.1111/j.1399-302X.1997.tb00733.x
Taniguchi, M., Nakazawa, H., Takeda, O., Kaneko, T., Hoshino, K., Tanaka, T. (1998). Production of a Mixture of Antimicrobial Organic Acids From Lactose by Co-Culture of Bifidobacterium Longum and Propionibacterium Freudenreichii. Biosci. Biotechnol. Biochem. 62, 1522–1527. doi: 10.1271/bbb.62.1522
van Winkelhoff, A. J., Herrera Gonzales, D., Winkel, E. G., Dellemijn-Kippuw, N., Vandenbroucke-Grauls, C. M., Sanz, M. (2000). Antimicrobial Resistance in the Subgingival Microflora in Patients With Adult Periodontitis. A Comparison Between The Netherlands and Spain. Clin. Periodontol 27, 79–86. doi: 10.1034/j.1600-051x.2000.027002079.x
Vivekananda, M. R., Vandana, K. L., Bhat, K. G. (2010). Effect of the Probiotic Lactobacilli Reuteri (Prodentis) in the Management of Periodontal Disease: A Preliminary Randomized Clinical Trial. J. Oral. Microbiol. Nov 2, 2. doi: 10.3402/jom.v2i0.5344
Wang, D. X., Liang, M. T., Tian, G. J., Lin, H., Liu, H. Q. (2002). A Facile Pathway to Synthesize Diketopiperazine Derivatives. Tetrahedron Lett. 43 (5), 865–867. doi: 10.1016/S0040-4039(01)02005-6
Watanabe, T. (1966). Infectious Drug Resistance in Enteric Bacteria. N. Engl. Med. 275, 888–894. doi: 10.1056/NEJM196610202751607
Keywords: antibacterial peptide, periodontal pathogen, cyclic dipeptide, probiotics, nuclear magnetic resonance analysis, chemical synthesis
Citation: Kawai T, Ohshima T, Tanaka T, Ikawa S, Tani A, Inazumi N, Shin R, Itoh Y, Meyer K and Maeda N (2022) Limosilactobacillus (Lactobacillus) fermentum ALAL020, a Probiotic Candidate Bacterium, Produces a Cyclic Dipeptide That Suppresses the Periodontal Pathogens Porphyromonas gingivalis and Prevotella intermedia. Front. Cell. Infect. Microbiol. 12:804334. doi: 10.3389/fcimb.2022.804334
Received: 29 October 2021; Accepted: 25 January 2022;
Published: 07 March 2022.
Edited by:
Julien Santi-Rocca, Science and Healthcare for Oral Welfare, FranceReviewed by:
Adolfo Contreras, University of Valle, ColombiaCopyright © 2022 Kawai, Ohshima, Tanaka, Ikawa, Tani, Inazumi, Shin, Itoh, Meyer and Maeda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomoko Ohshima, b2hzaGltYS10QGZzLnRzdXJ1bWktdS5hYy5qcA==
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.