- 1Department of Clinical Laboratory, Zibo Central Hospital, Zibo, China
- 2Department of Respiratory Medicine, Zibo Central Hospital, Zibo, China
- 3Department of Gastroenterology, Zibo Central Hospital, Zibo, China
- 4Key Laboratory of Biomedical Engineering & Technology of Shandong High School, Qilu Medical University, Zibo, China
- 5College of Sport and Health, Shandong Sport University, Jinan, China
Hematopoietic stem cell transplant (HSCT) recipients are vulnerable to Clostridium difficile infection (CDI) due to risk factors such as immunosuppression, antimicrobial use, and frequent hospitalization. We systematically searched PubMed and Embase to screen relevant studies from April 2014 to November 2021. A meta-analysis was performed to identify the association between CDI and hematopoietic transplantation based on the standard mean difference and 95% confidence intervals (CIs). Among the 431 retrieved citations, we obtained 43 eligible articles, which included 15,911 HSCT patients at risk. The overall estimated prevalence of CDI was 13.2%. The prevalence of CDI among the 10,685 allogeneic transplantation patients (15.3%) was significantly higher than that among the 3,840 autologous HSCT recipients (9.2%). Different incidence rates of CDI diagnosis over the last 7 years were found worldwide, of which North America (14.1%) was significantly higher than Europe (10.7%) but not significantly different from the prevalence among Asia (11.6%). Notably, we found that the estimated prevalence of CDI diagnosed by polymerase chain reaction (PCR) (17.7%) was significantly higher than that diagnosed by enzyme immunoassay (11.5%), indicating a significant discrepancy in the incidence rate of CDI owing to differences in the sensibility and specificity of the detection methods. Recurrence of CDI was found in approximately 15% of the initial patients with CDI. Furthermore, 20.3% of CDI cases were severe. CDI was found to be a common complication among HSCT recipients, displaying an evident increase in the morbidity of infection.
Introduction
Clostridium difficile infections (CDI) remain the leading cause of infectious diarrhea among hospitalized patients across the world. The rates of CDI in industrialized countries have increased with the emergence of the NAP1/RT027 strain in 2002, which is responsible for the outbreaks of severe diseases in North America and Europe ( (Loo et al., 2005; Kuijper et al., 2006). Patients with hematologic malignancies—particularly those who undergo hematopoietic stem cell transplants (HSCT)—are at risk of developing CDI because of prolonged hospital stay, exposure to broad-spectrum antibiotics, and compromise of the gastrointestinal mucosal barrier ( (Alonso et al., 2013; Shah et al., 2017).
Given a set of important factors, such as the transplant population, follow-up period, and testing method, the incidence of confirmed CDI among autologous HSCT (auto-HSCT) recipients varies from 5% to 24% (Bruminhent et al., 2014; Pilcante et al., 2015), whereas the incidence among allogeneic HSCT (allo-HSCT) recipients varies from 9% to 34% (Lavallee et al., 2017; Dubberke et al., 2017). An earlier systematic review of published literature until 2014 showed that the pooled prevalence of CDI among 12,025 HSCT patients was 7.9%, and an increasing trend of CDI diagnosis was also found worldwide and across studies conducted in North America over the last 34 years (Zacharioudakis et al., 2014).
Recently, with the widely implemented antibiotic prophylaxis and progress in the diagnostic strategy of CDI, it is unknown how CDI trends change in HSCT recipients during the peri-transplantation and late post-transplantation periods. Therefore, this study evaluated and updated the epidemiology of CDI in the hematopoietic transplantation setting from April 2014 to November 2021.
Methods
All procedures used in this meta-analysis were consistent with the guidelines of the Meta-analysis of Observational Studies in Epidemiology ( (Stroup et al., 2000) and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.
Literature Search
We searched PubMed and Embase (April 1, 2014, to November 30, 2021) medical databases to identify publications reporting the prevalence of CDI among patients who received hematopoietic stem cell transplantation. The concise search term was transplant * AND [clostrid * OR difficile OR infect * OR diarrhea OR (clostridium difficile) OR (pseudomembranous colitis)] AND ([stem cell] OR marrow OR chord OR autologous OR allogeneic) referring to previous systematic reviews (Zacharioudakis et al., 2014). We also manually searched the bibliographies of relevant papers to retrieve additional studies. Articles that were considered eligible following title and abstract reading were assessed in full text.
Selection Criteria
Studies were considered eligible if they reported the prevalence of CDI among HSCT patients during their hospitalization after stem cell transplantation. A restriction for English literature was imposed.
Outcomes of Interest
The prevalence of CDI among HSCT patients was the primary outcome of interest in this meta-analysis. CDI was defined as the presence of symptoms (usually diarrhea), and either a stool test positive for C. difficile toxins or the presence of toxigenic C. difficile, or colonoscopic, or histopathologic findings demonstrating pseudomembranous colitis (McDonald et al., 2018). The prevalence was calculated as the proportion of patients diagnosed with CDI among HSCT recipients. The subgroup analyses included the geographical region, study population, year of study implementation, transplantation type (i.e., autologous or allogeneic), study design, duration of follow-up, and detection methods used in the lab. The recurrence rate of CDI in infected patients was the secondary outcome of interest. Recurrent CDI was defined as a complete elimination of CDI and other symptoms with appropriate therapy, followed by the reappearance of diarrhea and positive result of toxigenic C. difficile after the cessation of treatment.
The peri-transplantation period for HSCT patients was divided into four periods: pre-transplantation (pre-T, hospitalization before transplantation), pre-engraftment (pre-E, approximately 0 to 30 days after transplantation), post-engraftment (post-E, approximately 30 to 100 days after transplantation), and late post-transplantation (Lpost-T, generally the day after +100 day of transplantation). Furthermore, to understand the effect of follow-up duration on the estimated prevalence of CDI, we distinguished the duration of follow-up as early- (pre-T + pre-E), middle- (pre-T + pre-E + post-E), and long-term (pre-T + pre-E + post-E + Lpost-T).
Data Extraction
Two reviewers (YL and QW) independently assessed the studies that were considered for inclusion in the meta-analysis. A spreadsheet was used to summarize the relevant information from the figures, tables, and text of the eligible articles. The trial data published in duplicate were included only once, and the maximum relevant information was extracted. Any disagreements or uncertainties regarding data extraction were resolved in consensus with a third reviewer (BZ). The extracted data included the region of source; study period; patient population; HSCT types (autologous or allogeneic); study design (prospective versus retrospective); laboratory detection methods; source of stem cells; duration of follow-up; the total number of patients who underwent HSCT during the study period; the total number of CDI cases among such patients; the number of NAP1/027 strains; the severity of CDI; and the number of recurrent episodes. If CDI recurred more than once, only the data of the first one were used in the analysis and assessed for the incidence. The severity of CDI in each patient was assessed as severe by the following clinical features: evidence of sepsis, gastrointestinal perforation, pseudomembranous colitis, toxic megacolon, ileus, intensive care unit admission, surgery for colitis, or death because of colitis (Kaltsas et al., 2012). Only studies that mentioned the outcome and severity of CDI were coverage initiated in the analysis.
Quality Assessment
Two reviewers (YL and QW) independently evaluated the methodological quality of the eligible studies using the Newcastle–Ottawa Quality Assessment Scale, which was a “star-based” rating system. The parameters used to assess the quality of each eligible study were as follows: representative of the exposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study, assessment of outcome, duration of follow-up for outcomes to occur, and adequacy of follow-up cohorts (Zacharioudakis et al., 2014). Two parameters, selection of the non-exposed cohort and comparability between cohorts, were not applicable to our analysis. Therefore, each study could obtain up to six stars. As representative of the study population in the exposed cohort, we considered the occurrence of CDI among all available transplantation patients rather than a specific subpopulation. We assessed the outcome by presenting the symptoms and laboratory diagnosis of CDI. The follow-up time was viewed as adequate for outcomes to occur, if it was at least 100 days or it included the entire period of hospitalization. Studies that received at least 4 stars were considered adequate quality to extract relevant information.
Data Analysis
A random-effects model, estimating the pooled prevalence and 95% confidence intervals (CIs), was performed in the meta-analysis (DerSimonian and Laird, 1986). The Freeman–Tukey arcsine methodology was used to remove an excessively large weight for studies with extremely low (close to 0) or extremely high (close to 100%) prevalence (Ziakas et al., 2015). Egger’s test was used to assess the publication bias (Egger et al., 1997). Between-study variance τ2 estimation was used to assess statistical heterogeneity (Rucker et al., 2008). Subgroup analyses were used to account for possible sources of heterogeneity. Statistical analysis was implemented by R language software and SPSS software (version 18.0, IBM, New York, USA). The statistical significance threshold was set at 0.05.
Results
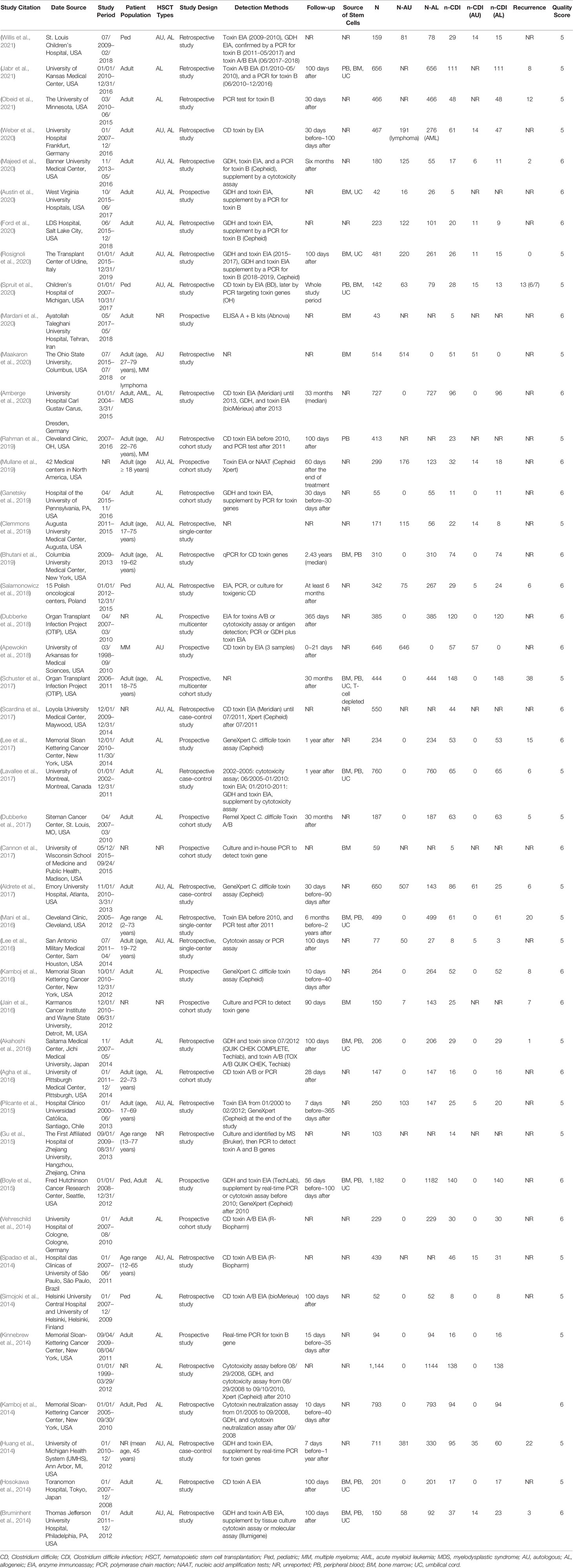
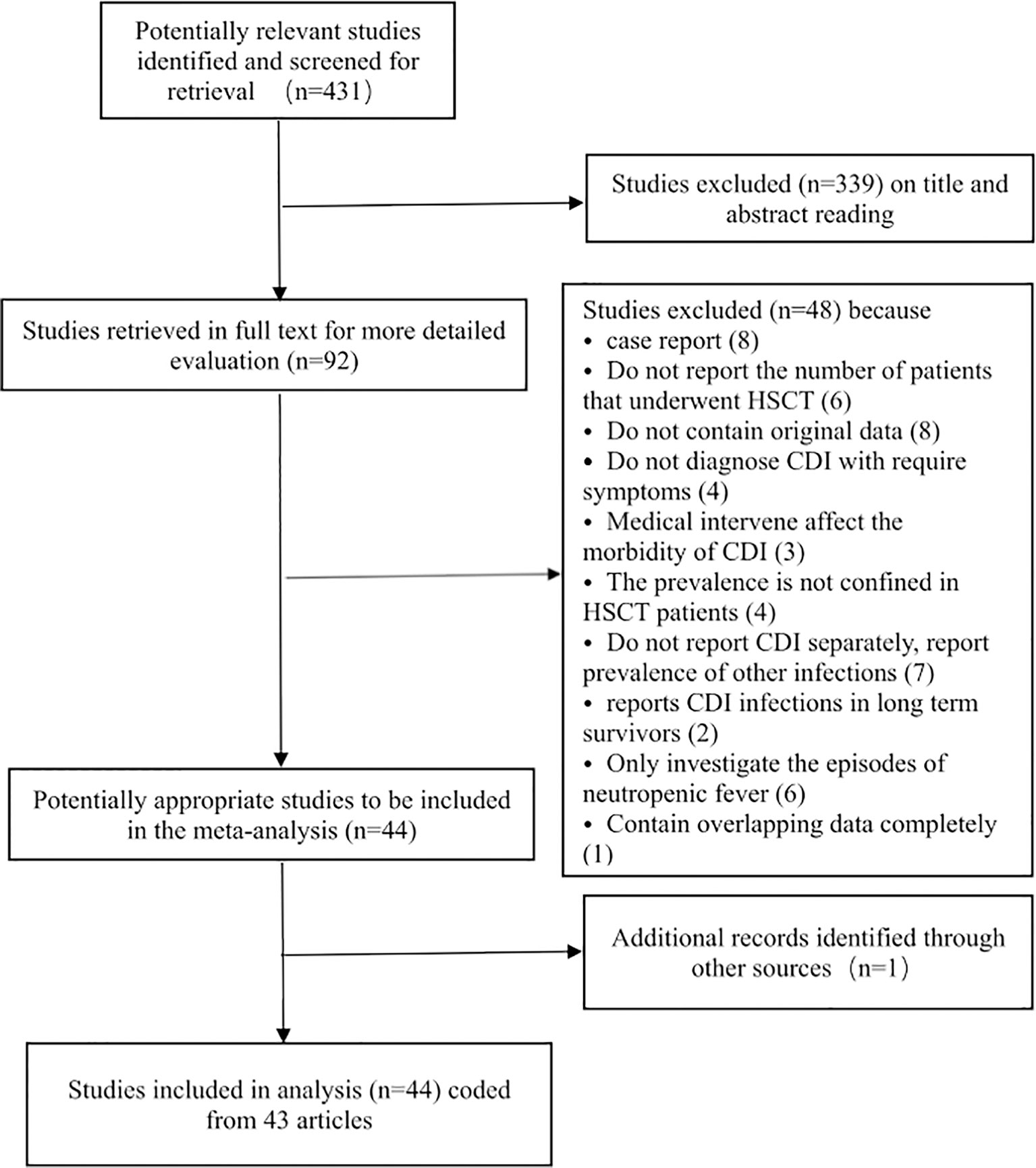
Our search generated 431 publications by accessing the databases between April 1, 2014, and November 30, 2021. After scrutinizing the titles and abstracts of the retrieved articles, 431 studies were excluded from our analysis, and 92 studies were retrieved in full text for more detailed evaluation. Among these, 49 articles were excluded because of the absence of extractable data on the prevalence of CDI among HSCT patients. Of the remaining 43 articles considered suitable for our meta-analysis, two contained partially overlapping data (Schuster et al., 2017; Dubberke et al., 2018), and the maximum available data were extracted from each article. Finally, 44 analyses were included in the final analysis coded from 43 articles (Table 1). We presented the details for selecting eligible articles in a flowchart presented in Figure 1.
The 44 analyses (coded from 43 articles) included in our analysis were published from April 2014 to November 2021, and data on 15,911 HSCT patients were reported from 1998 to 2019. The characteristics of each study are presented in Table 1. In the study containing intervention or prophylaxis that could affect the incidence of CDI among HSCT patients, only the data from the un-intervened cohort were used in the analysis. All studies were considered to possess the adequate quality to be included in the analysis based on the Newcastle–Ottawa Scale (Supplementary Table 1).
Among the 44 included analyses, 13 were prospective and 31 were retrospective, and one contained both prospectively and retrospectively collected data. The included studies varied by location, of which 32 were conducted in North America, 6 in Europe, 4 in Asia, and 2 in South America.
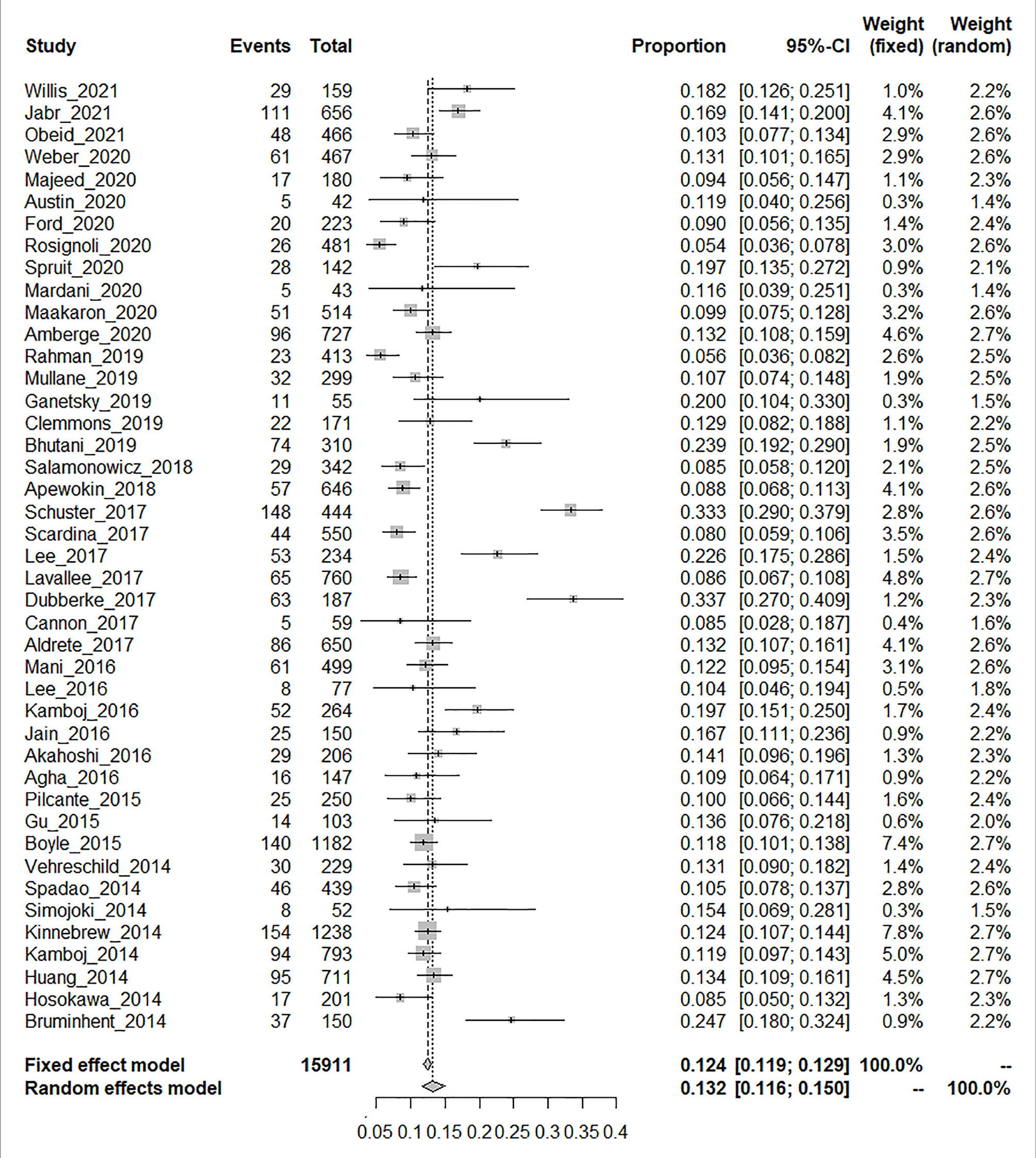
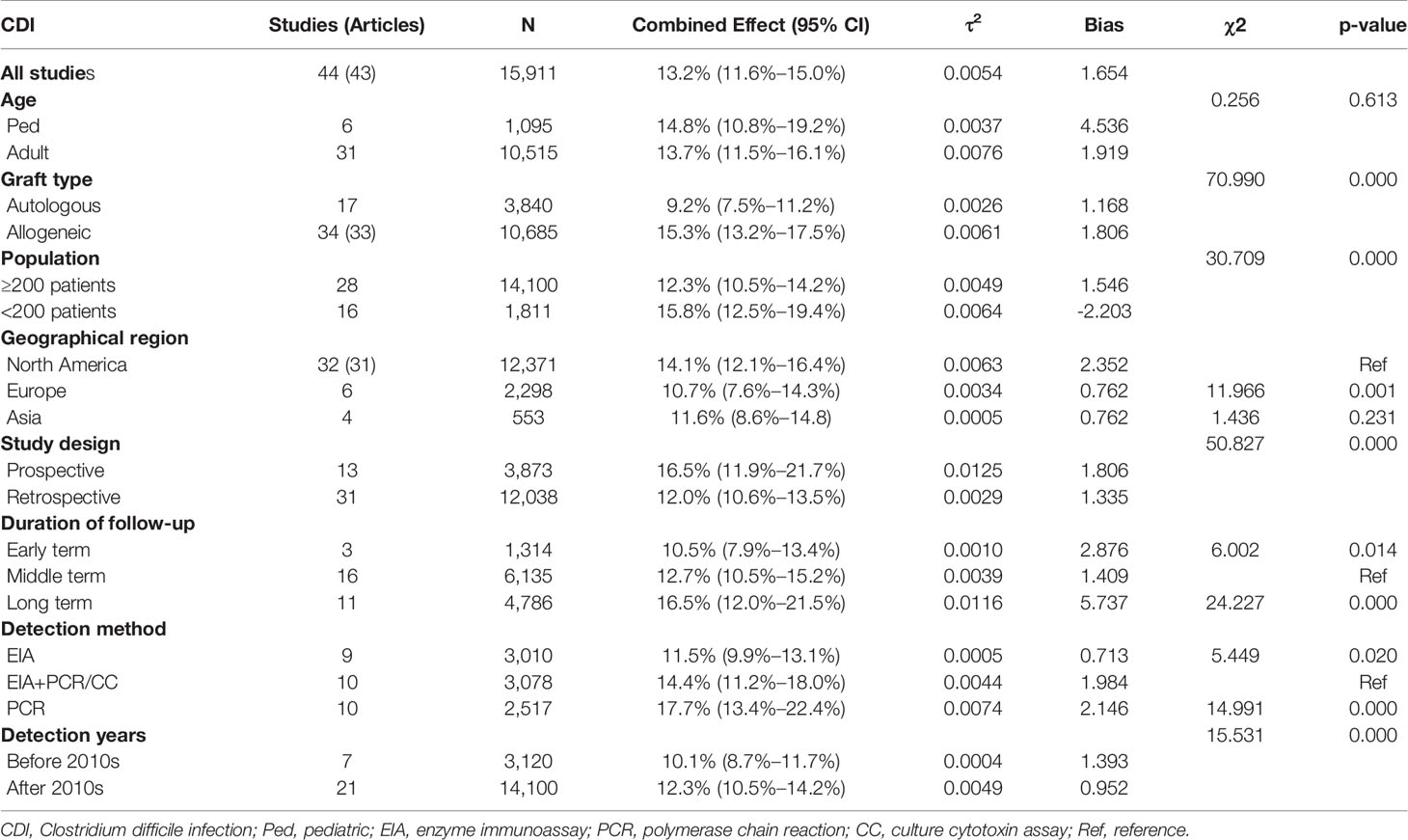
The laboratory detection methods of CDI used in each included study are displayed in Table 1. The pooled prevalence of CDI among the 15,911 HSCT recipients was 13.2% [95% CI, (11.6% to 15.0%), τ2 = 0.0054] according to the random-effects model (Figure 2). No evidence of publication bias was found for the overall estimated prevalence according to Egger’s test (bias: 1.654, p value = 0.176).
The HSCT patients included in the study were stratified based on age (pediatric or adult) and the type of HSCT (autologous or allogeneic). The included 15,911 HSCT patients included 1,095 pediatric patients extracted from six studies, 10,515 adult patients from 31 studies, and 4,301 patients unidentified by age. No significant diffidence was found in the pooled prevalence of CDI between the pediatric patients [14.8% (95% CI, 10.8% to 19.2%), τ2 = 0.0037] and adult patients [13.7% (95% CI, 11.5% to 16.1%), τ2 = 0.613] (Supplementary Figure 1). Seventeen studies reported relevant data on 3,840 auto-HSCT patients, whereas 34 studies provided extractable data on 10,685 allo-HSCT patients. The prevalence of patients with CDI who underwent allogeneic transplantation was 15.3% [95% CI (13.2% to 17.5%), τ2 = 0.0061], which was significantly higher than the corresponding prevalence among auto-HSCT recipients [9.2%, (95% CI, 7.5% to 11.2%), τ2 = 0.0026, p < 0.01] (Supplementary Figure 2).
Among the 43 studies, the estimated prevalence of CDI in North America [14.1% (95% CI, 12.1% to 16.4%), τ2 = 0.0063] was higher than the estimated prevalence among European studies [10.7% (95% CI, 7.6% to 14.3%), τ2 = 0.0034, p = 0.001] but not significantly different from the prevalence among Asian studies [11.6% (95% CI, 8.6% to 14.8%), τ2 = 0.0005, p = 0.231] (Supplementary Figure 3). We also conducted a subgroup analysis on the basis of the population and found that the estimated prevalence of 16 studies with <200 patients [15.8% (95% CI, 12.5% to 19.4%), τ2 = 0.0064] was statistically significantly higher than that of 28 studies with ≥200 patients [12.3% (95% CI, 10.5% to 14.2%), τ2 = 0.0049, p < 0.01] (Supplementary Figure 4).
We stratified our data based on the study design (prospective or retrospective) and found that the estimated prevalence of CDI in 13 prospective studies was 16.5% (95% CI, 11.9% to 21.7%), which was significantly higher than that of the 31 retrospective studies [12.0% (95% CI, 10.6% to 13.5%), p < 0.01] (Supplementary Figure 5). Based on the duration of follow-up, the estimated prevalence of CDI surveyed in the middle term [12.7% (95% CI, 10.5% to 15.2%)] was significantly higher than that in the early term [10.5% (95% CI, 7.9% to 13.4%), p = 0.014] and lower than that in the long term [16.5% (95% CI, 12.0% to 21.5%), p < 0.01] (Supplementary Figure 6).
We also stratified the studies on the detection methods. Forty-one studies expounded on the laboratory detection methods of CDI. The laboratory detection methods of CDI used in each included study are displayed in Table 1. Approximately half of the included studies used two or more detection methods to test the C. difficile toxin; thereinto, twelve studies altered the detection methods of CDI with time. One or more of the following methods were used in the laboratory detection of CDI: enzyme immunoassay (EIA), tissue culture cytotoxin assay (CC), and polymerase chain reaction (PCR). Ten studies used GDH and/or toxin EIA, supplemented by a PCR for toxin B or culture cytotoxin assay, abbreviated as EIA + PCR/CC. The estimated prevalence of CDI in studies that used EIA + PCR/CC was 14.4% (95% CI, 11.2% to 18%), which was significantly higher than studies that used EIA only [11.5% (95% CI, 9.9% to 13.1%), p = 0.02] as well as significantly lower than that in studies that used PCR only [17.7% (95% CI, 13.4% to 22.4%), p < 0.01] (Supplementary Figure 7 and Table 2).
Eighteen studies reported data on recurrence of CDI among 990 infected patients, among which 11 studies included the definition of recurrence. The reported recurrence rate was estimated to be 14.9% [95% CI (9.8% to 20.7%), τ2 = 0.0193] (Supplementary Figure 8). The individual study data of the first recurrent case are presented in Table 1. Further analyses were performed for the estimated prevalence of CDI patients from 1998 to 2010 and from 2011 to 2021; the results showed that the estimated prevalence of CDI in 1998–2010 patients was 10.1% (95% CI, 8.7% to 11.7%), which was significantly lower than that of the 2011–2021 patients [13.0% (95% CI, 10.9% to 15.3%), p < 0.01] (Supplementary Figure 9).
Finally, seven studies which included the definition of CDI severity, and 11 studies which reported data on the severity of CDI among infected patients, were included in the analysis. Among 524 CDI patients, 107 (20.3%, 107/524) severe cases, 26 (5.0%, 26/524) ICU admissions, 9 (1.7%, 9/524) CDI-related colectomies, 7 (1.3%, 7/524) gastrointestinal perforations, 13 (2.5%, 13/524) pseudomembranous colitis cases, and 13 (2.5%, 13/524) deaths were reported in the remaining 11 studies. Two studies reported high-virulent NAP1/027 strains, in one of which NAP1/027 strains account for 24.5% (23/94), in the other one only 2.7% (1/37) (Supplementary Table 2).
Discussion
CDI has been increasingly discerned among HSCT recipients because of the fragility of the immune system, graft-versus-host disease (GVHD), and antibiotic usage or prophylaxis (Ilett et al., 2019; Rosignoli et al., 2020; Jabr et al., 2021). Along with the growing cognition on CDI for clinical physicians and improving diagnostic capacity of laboratories on CDI, the relevant data on the prevalence of reported CDI have gradually increased in recent years. This study aimed to update the previous analysis on the prevalence of CDI among HSCT patients by Zacharioudakis et al. (Zacharioudakis et al., 2014) and investigate the variation in the estimated prevalence and subgroup analysis of CDI among HSCT recipients reported from April 1, 2014, to November 30, 2021.
In our study, the estimated prevalence of CDI in HSCT patients was 13.2%, which was approximately two times higher than the corresponding morbidity reported in the previous analysis (7.9%) (Zacharioudakis et al., 2014) and approximately 15 times higher than the general hospital population (0.9% reported in 2009) (Lucado et al., 2006). In the analysis by Zacharioudakis, the actual change in C. difficile epidemiology was attributed to the emergency of more virulent strains (Zacharioudakis et al., 2014). However, we had different findings in the variability of C. difficile epidemiology because of several factors, including spectrum antibiotics, immunosuppression, strain, and the diagnostic sensitivity of CDI (Alonso et al., 2013; Shah et al., 2017). Analysis of our data between 1998–2010 and 2011–2021 also showed a gradual increase in prevalence of CDI among HSCT recipients.
The prevalence of allogeneic transplantation patients was 15.3%, which was significantly higher than autologous graft (9.2%), indicating that the graft type was one of the primary elements to influence the prevalence of CDI among HSCT recipients. The risk factors for CDI in allo-HSCT patients included receipt of chemotherapy before conditioning for HSCT, broad-spectrum antimicrobial use, acute GVHD, and greater immunosuppression caused by allo-HSCT conditioning regimens (Alonso et al., 2012). A greater deviation in the prevalence of CDI compared to the overall estimated prevalence (13.2%) was found for smaller studies (<200 patients, 15.8% vs. ≥200 patients, 12.3%), highlighting that a reasonable and large sample size was necessary for reducing the random error and being representative.
In our analysis, we observed that most of the studies (72.1%, 31/43) were obtained from North America, and the estimated prevalence of CDI among HSCT patients in North America was 14.1%, which was significantly higher than that in Europe (10.7%) but did not reach statistical significance than that in Asia (11.6%). It revealed the regional epidemic characteristics of CDI over the last 7 years. Another national discharge data also indicated that the USA had a 10-fold higher CDI rate than England among overall inpatients (King et al., 2017). The regional difference might be associated with the national infection control policy or epidemic of a hypervirulent strain. Therefore, continuous regional surveillance was necessary to investigate the presumed association between vulnerability and CDI in the different ethnic groups and regions.
In our study, we only included data on the first post-transplant hospitalization, which may have resulted in the higher overall estimated prevalence. Most studies were followed up from pre-transplantation to 100 days post-transplantation, and the estimated prevalence of CDI with the middle term of follow-up was 12.7%, which was significantly higher than the early term (p = 0.014) and significantly lower than the long term (p < 0.01). However, most cases of CDI among HSCT recipients were diagnosed in the early term of transplantation because of more intense antimicrobial exposure, high immunosuppression, accelerated antimicrobial exposure, and increased transmission in the hospital environment (Schuster et al., 2017). Our study indicated that the risk of CDI among the middle and late periods cannot be ignored.
The diagnosis of CDI is a complicated process, incorporating clinical diagnosis, defined by the presence of symptoms (usually diarrhea), with laboratory diagnosis, assured by either a stool test positive for C. difficile toxin or detection of toxigenic C. difficile or colonoscopic or histopathologic findings revealing pseudomembranous colitis (McDonald et al., 2018). In our studies, the estimated prevalence of CDI diagnosed by EIA (11.5%) was significantly lower than that diagnosed by EIA+PCR/CC (14.4%, p = 0.02), and the CDI diagnosed by EIA+PCR/CC was significantly lower than that diagnosed by PCR (17.7%, p < 0.01), indicating that a significant discrepancy in the incidence rate of CDI was observed because of the different sensibility and specificity of the detection methods of CDI. The related laboratory indices of CDI diagnosis detected by EIA were glutamate dehydrogenase (GDH) and C. difficile toxin A and/or B (CDAB). One of our previous studies revealed that the sensitivity of the detection method combining GDH and CDAB for the diagnosis of CDI was only 54.2% (39/72), and with further addition of PCR to the scheme, the sensitivity for the diagnosis of CDI could be increased to 100% (Luo et al., 2018). This mate analysis showed that a PCR for CD toxin was the most sensitive detection method for CDI. A conventional PCR for CD toxin needs to be combined with time-consuming and demanding anaerobic culture, increasing the difficulty of its universal use. In recent years, some commercially nucleic acid amplification test (NAAT) products were approved by the FDA, such as the Gene Xpert CD assay (Cepheid, Sunnyvale, USA) directly detecting the tcdB gene in feces by RT-PCR, and widely used in the national world. The Gene Xpert was notable because of its high sensitivity (97%) and specificity (95%) in diagnosing toxigenic CDI both rapidly and simply ( (Bai et al., 2017).
The recurrence of CD infection occurred in approximately 15% of the initial patients with CDI, with a large variation from 3% to 46% in our analysis. Antecedent antibiotic usage and neutropenia were considered independent predictors of recurrent CDI (Huang et al., 2014; Mani et al., 2016). Notably, 20.3% of CDI cases were severe. However, because of failing raw data on each risk factor, further statistical statements could not be implemented in our analysis. Infection control measures and regional epidemiology possess a significant role in the prevalence of CDI among individual medical centers, and our pooled estimation does not reduce the need for local centers to understand local prevalence. The meta-analysis showed that fecal microbiota transplantation, as an innovative strategy to reduce CDI occurrence, was recommended in patients with recurrent CDI in whom appropriate antibiotic treatments failed (Pession et al., 2021).
Our study estimated the pooled prevalence of CDI among HSCT recipients to be almost 2-fold higher than that in the previous analysis (Zacharioudakis et al., 2014). The increased prevalence of CDI with the high rate of severe cases highlighted the necessity for prophylactic policies, such as antimicrobial stewardship programs, strict hand hygiene procedures, and environmental decontamination that is specifically aimed at this patient population. Furthermore, future studies were required to recognize immunosuppressive and preventive antimicrobial regimens that were presumedly associated with a lower risk of CDI.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization: QW. Data curation: YL, BZ, SZ, and HS. Software: YL and WC. Writing—original draft: YL, BZ, and QW. Writing—review and editing: YL, SZ, and HS. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Natural Scientific Foundation of Shandong Province, China (ZR2018MH038, ZR2019PC053) and the Zibo City Innovation Development Key Project (2018CX04A007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.801475/full#supplementary-material
Supplementary Table 1 | Newcastle Ottawa Quality assessment of individual studies.
Supplementary Table 2 | Recurrence and outcomes of CDI in HSCT recipients.
Supplementary Figure 1 | Prevalence of CDI among adult (A) and pediatric (B) HSCT patients.
Supplementary Figure 2 | Prevalence of CDI among allogeneic (A) and autologous (B) HSCT recipients.
Supplementary Figure 3 | Prevalence of CDI among studies in North America (A), Asia (B), and Europe (C).
Supplementary Figure 4 | Prevalence of CDI among studies with < 200 patients (A) and studies with ≥ 200 patients (B).
Supplementary Figure 5 | Prevalence of CDI in prospective studies (A) and retrospective studies (B).
Supplementary Figure 6 | Prevalence of CDI surveyed in the Early term (A), Middle term (B), and Long term (C).
Supplementary Figure 7 | Prevalence of CDI in studies with EIA used only (A), EIA + PCR/CC (B), and PCR used only (C).
Supplementary Figure 8 | Prevalence of recurrent CDI in studies.
Supplementary Figure 9 | Prevalence analysis of CDI in 1998-2010 (A) and 2010-2020 (B) year.
References
Agha, A., Sehgal, A., Lim, M. J., Weber, D., Hou, J. Z., Farah, R., et al. (2016). Peri-Transplant Clostridium Difficile Infections in Patients Undergoing Allogeneic Hematopoietic Progenitor Cell Transplant. Am. J. Hematol. 91, 291–294. doi: 10.1002/ajh.24263
Akahoshi, Y., Kimura, S., Nakano, H., Harada, N., Kameda, K., Ugai, T., et al. (2016). Significance of a Positive Clostridium Difficile Toxin Test After Hematopoietic Stem Cell Transplantation. Clin. Transplant. 30, 703–708. doi: 10.1111/ctr.12737
Aldrete, S. D., Kraft, C. S., Magee, M. J., Chan, A., Hutcherson, D., Langston, A. A., et al. (2017). Risk Factors and Epidemiology of Clostridium Difficile Infection in Hematopoietic Stem Cell Transplant Recipients During the Peritransplant Period. Transpl Infect. Dis. 19, e12649. doi: 10.1111/tid.12649
Alonso, C. D., Dufresne, S. F., Hanna, D. B., Labbe, A. C., Treadway, S. B., Neofytos, D., et al. (2013). Clostridium Difficile Infection After Adult Autologous Stem Cell Transplantation: A Multicenter Study of Epidemiology and Risk Factors. Biol. Blood Marrow Transplant. 19, 1502–1508. doi: 10.1016/j.bbmt.2013.07.022
Alonso, C. D., Treadway, S. B., Hanna, D. B., Huff, C. A., Neofytos, D., Carroll, K. C., et al. (2012). Epidemiology and Outcomes of Clostridium Difficile Infections in Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 54, 1053–1063. doi: 10.1093/cid/cir1035
Amberge, S., Kramer, M., Schrottner, P., Heidrich, K., Schmelz, R., Middeke, J. M., et al. (2020). Clostridium Difficile Infections in Patients With AML or MDS Undergoing Allogeneic Hematopoietic Stem Cell Transplantation Identify High Risk for Adverse Outcome. Bone Marrow Transplant. 55, 367–375. doi: 10.1038/s41409-019-0678-y
Apewokin, S., Lee, J. Y., Goodwin, J. A., McKelvey, K. D., Stephens, O. W., Zhou, D., et al. (2018). Host Genetic Susceptibility to Clostridium Difficile Infections in Patients Undergoing Autologous Stem Cell Transplantation: A Genome-Wide Association Study. Support Care Cancer 26, 3127–3134. doi: 10.1007/s00520-018-4173-6
Austin, K., Sweet, M., Likar, E., LaSala, P. R., Murray, A., Wen, S., et al. (2020). Prospective Assessment of Clostridioides (Formerly Clostridium) Difficile Colonization and Acquisition in Hematopoietic Stem Cell Transplant Patients. Transpl Infect. Dis. 22, e13438. doi: 10.1111/tid.13438
Bai, Y., Sun, X., Jin, Y., Wang, Y., Li, J. (2017). Accuracy of Xpert Clostridium Difficile Assay for the Diagnosis of Clostridium Difficile Infection: A Meta Analysis. PLoS One 12, e0185891. doi: 10.1371/journal.pone.0185891
Bhutani, D., Jaiyeoba, C., Kim, S., Naylor, P., Uberti, J. P., Ratanatharathorn, V., et al. (2019). Relationship Between Clostridium Difficile Infection and Gastrointestinal Graft Versus Host Disease in Recipients of Allogeneic Stem Cell Transplantation. Bone Marrow Transplant. 54, 164–167. doi: 10.1038/s41409-018-0270-x
Boyle, N. M., Magaret, A., Stednick, Z., Morrison, A., Butler-Wu, S., Zerr, D., et al. (2015). Evaluating Risk Factors for Clostridium Difficile Infection in Adult and Pediatric Hematopoietic Cell Transplant Recipients. Antimicrob. Resist. Infect. Control 4, 41. doi: 10.1186/s13756-015-0081-4
Bruminhent, J., Wang, Z. X., Hu, C., Wagner, J., Sunday, R., Bobik, B., et al. (2014). Clostridium Difficile Colonization and Disease in Patients Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 20, 1329–1334. doi: 10.1016/j.bbmt.2014.04.026
Cannon, C. M., Musuuza, J. S., Barker, A. K., Duster, M., Juckett, M. B., Pop-Vicas, A. E., et al. (2017). Risk of Clostridium Difficile Infection in Hematology-Oncology Patients Colonized With Toxigenic C. Difficile. Infect. Control Hosp Epidemiol. 38, 718–720. doi: 10.1017/ice.2017.48
Clemmons, A. B., Gandhi, A. S., Albrecht, B., Jacobson, S., Pantin, J. (2019). Impact of Fluoroquinolone Prophylaxis on Infectious-Related Outcomes After Hematopoietic Cell Transplantation. J. Oncol. Pharm. Pract. 25, 326–332. doi: 10.1177/1078155217735153
DerSimonian, R., Laird, N. (1986). Meta-Analysis in Clinical Trials. Control Clin. Trials 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Dubberke, E. R., Reske, K. A., Olsen, M. A., Bommarito, K., Cleveland, A. A., Silveira, F. P., et al. (2018). Epidemiology and Outcomes of Clostridium Difficile Infection in Allogeneic Hematopoietic Cell and Lung Transplant Recipients. Transpl Infect. Dis. 20, e12855. doi: 10.1111/tid.12855
Dubberke, E. R., Reske, K. A., Olsen, M. A., Bommarito, K. M., Seiler, S., Silveira, F. P., et al. (2017). Risk for Clostridium Difficile Infection After Allogeneic Hematopoietic Cell Transplant Remains Elevated in the Postengraftment Period. Transplant. Direct 3, e145. doi: 10.1097/TXD.0000000000000662
Egger, M., Davey Smith, G., Schneider, M., Minder, C. (1997). Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Ford, C. D., Lopansri, B. K., Coombs, J., Webb, B. J., Asch, J., Hoda, D. (2020). Are Clostridioides Difficile Infections Being Overdiagnosed in Hematopoietic Stem Cell Transplant Recipients? Transpl Infect. Dis. 22, e13279. doi: 10.1111/tid.13279
Ganetsky, A., Han, J. H., Hughes, M. E., Babushok, D. V., Frey, N. V., Gill, S. I., et al. (2019). Oral Vancomycin Prophylaxis Is Highly Effective in Preventing Clostridium Difficile Infection in Allogeneic Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 68, 2003–2009. doi: 10.1093/cid/ciy822
Gu, S. L., Chen, Y. B., Lv, T., Zhang, X. W., Wei, Z. Q., Shen, P., et al. (2015). Risk Factors, Outcomes and Epidemiology Associated With Clostridium Difficile Infection in Patients With Haematological Malignancies in a Tertiary Care Hospital in China. J. Med. Microbiol. 64, 209–216. doi: 10.1099/jmm.0.000028
Hosokawa, K., Takami, A., Tsuji, M., Araoka, H., Ishiwata, K., Takagi, S., et al. (2014). Relative Incidences and Outcomes of Clostridium Difficile Infection Following Transplantation of Unrelated Cord Blood, Unrelated Bone Marrow, and Related Peripheral Blood in Adult Patients: A Single Institute Study. Transpl Infect. Dis. 16, 412–420. doi: 10.1111/tid.12224
Huang, A. M., Marini, B. L., Frame, D., Aronoff, D. M., Nagel, J. L. (2014). Risk Factors for Recurrent Clostridium Difficile Infection in Hematopoietic Stem Cell Transplant Recipients. Transpl Infect. Dis. 16, 744–750. doi: 10.1111/tid.12267
Ilett, E. E., Helleberg, M., Reekie, J., Murray, D. D., Wulff, S. M., Khurana, M. P., et al. (2019). Incidence Rates and Risk Factors of Clostridioides Difficile Infection in Solid Organ and Hematopoietic Stem Cell Transplant Recipients. Open Forum Infect. Dis. 6, ofz086. doi: 10.1093/ofid/ofz086
Jabr, R., El Atrouni, W., Shune, L., Telfah, M., Gao, G., He, J., et al. (2021). Clostridioides Difficile Infection and Risk of Acute Graft-Versus-Host Disease Among Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Transplant. Cell Ther. 27, 176.e1–176.e8. doi: 10.1016/j.jtct.2020.10.009
Jain, T., Croswell, C., Urday-Cornejo, V., Awali, R., Cutright, J., Salimnia, H., et al. (2016). Clostridium Difficile Colonization in Hematopoietic Stem Cell Transplant Recipients: A Prospective Study of the Epidemiology and Outcomes Involving Toxigenic and Nontoxigenic Strains. Biol. Blood Marrow Transplant. 22, 157–163. doi: 10.1016/j.bbmt.2015.07.020
Kaltsas, A., Simon, M., Unruh, L. H., Son, C., Wroblewski, D., Musser, K. A., et al. (2012). Clinical and Laboratory Characteristics of Clostridium Difficile Infection in Patients With Discordant Diagnostic Test Results. J. Clin. Microbiol. 50, 1303–1307. doi: 10.1128/JCM.05711-11
Kamboj, M., Sheahan, A., Sun, J., Taur, Y., Robilotti, E., Babady, E., et al. (2016). Transmission of Clostridium Difficile During Hospitalization for Allogeneic Stem Cell Transplant. Infect. Control Hosp Epidemiol. 37, 8–15. doi: 10.1017/ice.2015.237
Kamboj, M., Xiao, K., Kaltsas, A., Huang, Y. T., Sun, J., Chung, D., et al. (2014). Clostridium Difficile Infection After Allogeneic Hematopoietic Stem Cell Transplant: Strain Diversity and Outcomes Associated With NAP1/027. Biol. Blood Marrow Transplant. 20, 1626–1633. doi: 10.1016/j.bbmt.2014.06.025
King, A., Mullish, B. H., Williams, H. R. T., Aylin, P. (2017). Comparative Epidemiology of Clostridium Difficile Infection: England and the USA. Int. J. Qual Health Care 29, 785–791. doi: 10.1093/intqhc/mzx120
Kinnebrew, M. A., Lee, Y. J., Jenq, R. R., Lipuma, L., Littmann, E. R., Gobourne, A., et al. (2014). Early Clostridium Difficile Infection During Allogeneic Hematopoietic Stem Cell Transplantation. PLoS One 9, e90158. doi: 10.1371/journal.pone.0090158
Kuijper, E. J., van den Berg, R. J., Debast, S., Visser, C. E., Veenendaal, D., Troelstra, A., et al. (2006). Clostridium Difficile Ribotype 027, Toxinotype III, the Netherlands. Emerg. Infect. Dis. 12, 827–830. doi: 10.3201/eid1205.051350
Lavallee, C., Labbe, A. C., Talbot, J. D., Alonso, C. D., Marr, K. A., Cohen, S., et al. (2017). Risk Factors for the Development of Clostridium Difficile Infection in Adult Allogeneic Hematopoietic Stem Cell Transplant Recipients: A Single-Center Study in Quebec, Canada. Transpl Infect. Dis. 19, 10.1111/tid.12648. doi: 10.1111/tid.12648
Lee, Y. J., Arguello, E. S., Jenq, R. R., Littmann, E., Kim, G. J., Miller, L. C., et al. (2017). Protective Factors in the Intestinal Microbiome Against Clostridium Difficile Infection in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. J. Infect. Dis. 215, 1117–1123. doi: 10.1093/infdis/jix011
Lee, L. E., Barsoumian, A. E., Brown, A. W., Wiggins, M. A., Renshaw, J. S., Osswald, M. B., et al. (2016). Rates of Microbiologically Diagnosed Infection and Pathogen Detection in Hematopoietic Stem Cell Transplant Patients. Mil Med. 181, e1685–e1691. doi: 10.7205/MILMED-D-15-00553
Loo, V. G., Poirier, L., Miller, M. A., Oughton, M., Libman, M. D., Michaud, S., et al. (2005). A Predominantly Clonal Multi-Institutional Outbreak of Clostridium Difficile-Associated Diarrhea With High Morbidity and Mortality. N Engl. J. Med. 353, 2442–2449. doi: 10.1056/NEJMoa051639
Lucado, J., Gould, C., Elixhauser, A. (2006). “Clostridium Difficile Infections (CDI) in Hospital Stay: Statistical Brief #124,” in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs (US: Rockville (MD): Agency for Healthcare Research and Quality).
Luo, Y., Zhang, W., Cheng, J. W., Xiao, M., Sun, G. R., Guo, C. J., et al. (2018). Molecular Epidemiology of Clostridium Difficile in Two Tertiary Care Hospitals in Shandong Province, China. Infect. Drug Resist. 11, 489–500. doi: 10.2147/IDR.S152724
Maakaron, J. E., C.Maakaron Liscynesky, J. E., Boghdadly, Z. E., Huang, Y., Agyeman, A., Brammer, J., et al. (2020). Fluoroquinolone Prophylaxis in Autologous Stem Cell Transplantation: Worthy of a Second Look. Biol. Blood Marrow Transplant. 26, e198–e201. doi: 10.1016/j.bbmt.2020.03.027
Majeed, A., Larriva, M. M., Iftikhar, A., Mushtaq, A., Campbell, P., Nadeem Malik, M., et al. (2020). A Single-Center Experience and Literature Review of Management Strategies for Clostridium Difficile Infection in Hematopoietic Stem Cell Transplant Patients. Infect. Dis. Clin. Pract. (Baltim Md) 28, 10–15. doi: 10.1097/ipc.0000000000000798
Mani, S., Rybicki, L., Jagadeesh, D., Mossad, S. B. (2016). Risk Factors for Recurrent Clostridium Difficile Infection in Allogeneic Hematopoietic Cell Transplant Recipients. Bone Marrow Transplant. 51, 713–717. doi: 10.1038/bmt.2015.311
Mardani, M., Abolghasemi, S., Shabani, S. (2020). Impact of an Antimicrobial Stewardship Program in the Antimicrobial-Resistant and Prevalence of Clostridioides Difficile Infection and Amount of Antimicrobial Consumed in Cancer Patients. BMC Res. Notes 13, 246. doi: 10.1186/s13104-020-05085-3
McDonald, L. C., Gerding, D. N., Johnson, S., Bakken, J. S., Carroll, K. C., Coffin, S. E., et al. (2018). Clinical Practice Guidelines for Clostridium Difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 66, e1–e48. doi: 10.1093/cid/cix1085
Mullane, K. M., Winston, D. J., Nooka, A., Morris, M. I., Stiff, P., Dugan, M. J., et al. (2019). A Randomized, Placebo-Controlled Trial of Fidaxomicin for Prophylaxis of Clostridium Difficile-Associated Diarrhea in Adults Undergoing Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 68, 196–203. doi: 10.1093/cid/ciy484
Obeid, K. M., Sapkota, S., Cao, Q., Richmond, S., Watson, A. P., Karadag, F. K., et al. (2021). Early Clostridioides Difficile Infection Characterizations, Risks, and Outcomes in Allogeneic Hematopoietic Stem Cell and Solid Organ Transplant Recipients. Transpl Infect. Dis., e13720. doi: 10.1111/tid.13720
Pession, A., Zama, D., Muratore, E., Leardini, D., Gori, D., Guaraldi, F., et al. (2021). Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Systematic Review. J. Pers. Med. 11, 100. doi: 10.3390/jpm11020100
Pilcante, J., Rojas, P., Ernst, D., Sarmiento, M., Ocqueteau, M., Bertin, P., et al. (2015). Clostridium Difficile Infection in Chilean Patients Submitted to Hematopoietic Stem Cell Transplantation. Rev. Bras. Hematol. Hemoter 37, 388–394. doi: 10.1016/j.bjhh.2015.07.010
Rahman, S., Rybicki, L., Ky Hamilton, B., Pohlman, B., Jagadeesh, D., Cober, E., et al. (2019). Early Infectious Complications After Autologous Hematopoietic Cell Transplantation for Multiple Myeloma. Transpl Infect. Dis. 21, e13114. doi: 10.1111/tid.13114
Rosignoli, C., Petruzzellis, G., Radici, V., Facchin, G., Girgenti, M., Stella, R., et al. (2020). Risk Factors and Outcome of C. Difficile Infection After Hematopoietic Stem Cell Transplantation. J. Clin. Med. 9, 3673. doi: 10.3390/jcm9113673
Rucker, G., Schwarzer, G., Carpenter, J. R., Schumacher, M. (2008). Undue Reliance on I(2) in Assessing Heterogeneity may Mislead. BMC Med. Res. Methodol 8, 79. doi: 10.1186/1471-2288-8-79
Salamonowicz, M., Ociepa, T., Fraczkiewicz, J., Szmydki-Baran, A., Matysiak, M., Czyzewski, K., et al. (2018). Incidence, Course, and Outcome of Clostridium Difficile Infection in Children With Hematological Malignancies or Undergoing Hematopoietic Stem Cell Transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 37, 1805–1812. doi: 10.1007/s10096-018-3316-5
Scardina, T. L., Kang Martinez, E., Balasubramanian, N., Fox-Geiman, M., Smith, S. E., Parada, J. P. (2017). Evaluation of Risk Factors for Clostridium Difficile Infection in Hematopoietic Stem Cell Transplant Recipients. Pharmacotherapy 37, 420–428. doi: 10.1002/phar.1914
Schuster, M. G., Cleveland, A. A., Dubberke, E. R., Kauffman, C. A., Avery, R. K., Husain, S., et al. (2017). Infections in Hematopoietic Cell Transplant Recipients: Results From the Organ Transplant Infection Project, a Multicenter, Prospective, Cohort Study. Open Forum Infect. Dis. 4, ofx050. doi: 10.1093/ofid/ofx050
Shah, N. N., McClellan, W., Flowers, C. R., Lonial, S., Khoury, H., Waller, E. K., et al. (2017). Evaluating Risk Factors for Clostridium Difficile Infection In Stem Cell Transplant Recipients: A National Study. Infect. Control Hosp Epidemiol. 38, 651–657. doi: 10.1017/ice.2017.12
Simojoki, S. T., Kirjavainen, V., Rahiala, J., Kanerva, J. (2014). Surveillance Cultures in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation. Pediatr. Transplant. 18, 87–93. doi: 10.1111/petr.12177
Spadao, F., Gerhardt, J., Guimaraes, T., Dulley, F., Almeida Junior, J. N., Batista, M. V., et al. (2014). Incidence of Diarrhea by Clostridium Difficile in Hematologic Patients and Hematopoietic Stem Cell Transplantation Patients: Risk Factors for Severe Forms and Death. Rev. Inst Med. Trop. Sao Paulo 56, 325–331. doi: 10.1590/s0036-46652014000400010
Spruit, J. L., Knight, T., Sweeney, C., Salimnia, H., Savasan, S. (2020). Clostridium Difficile Infection in a Children's Hospital With Specific Patterns Among Pediatric Oncology and Hematopoietic Stem Cell Transplantation Populations. Pediatr. Hematol. Oncol. 37, 211–222. doi: 10.1080/08880018.2019.1711473
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 283, 2008–2012. doi: 10.1001/jama.283.15.2008
Vehreschild, M. J., Weitershagen, D., Biehl, L. M., Tacke, D., Waldschmidt, D., Tox, U., et al. (2014). Clostridium Difficile Infection in Patients With Acute Myelogenous Leukemia and in Patients Undergoing Allogeneic Stem Cell Transplantation: Epidemiology and Risk Factor Analysis. Biol. Blood Marrow Transplant. 20, 823–828. doi: 10.1016/j.bbmt.2014.02.022
Weber, S., Scheich, S., Magh, A., Wolf, S., Enssle, J. C., Brunnberg, U., et al. (2020). Impact of Clostridioides Difficile Infection on the Outcome of Patients Receiving a Hematopoietic Stem Cell Transplantation. Int. J. Infect. Dis. 99, 428–436. doi: 10.1016/j.ijid.2020.08.030
Willis, D. N., Huang, F. S., Elward, A. M., Wu, N., Magnusen, B., Dubberke, E. R., et al. (2021). Clostridioides Difficile Infections in Inpatient Pediatric Oncology Patients: A Cohort Study Evaluating Risk Factors and Associated Outcomes. J. Pediatr. Infect. Dis. Soc. 10, 302–308. doi: 10.1093/jpids/piaa090
Zacharioudakis, I. M., Ziakas, P. D., Mylonakis, E. (2014). Clostridium Difficile Infection in the Hematopoietic Unit: A Meta-Analysis of Published Studies. Biol. Blood Marrow Transplant. 20, 1650–1654. doi: 10.1016/j.bbmt.2014.06.001
Keywords: Clostridium difficile infection, hematopoietic stem cell transplantation, meta-analysis, Asia, detection methods, allogeneic transplantation patients
Citation: Luo Y, Zhang S, Shang H, Cui W, Wang Q and Zhu B (2022) Prevalence of Clostridium difficile Infection in the Hematopoietic Transplantation Setting: Update of Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 12:801475. doi: 10.3389/fcimb.2022.801475
Received: 25 October 2021; Accepted: 17 January 2022;
Published: 21 February 2022.
Edited by:
Dazhi Jin, Hangzhou Medical College, ChinaReviewed by:
Jakob Passweg, University Hospital of Basel, SwitzerlandRiccardo Masetti, University of Bologna, Italy
Copyright © 2022 Luo, Zhang, Shang, Cui, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinglu Wang, d3FsX3pjcUAxMjYuY29t
 Ying Luo
Ying Luo Sumei Zhang2
Sumei Zhang2 Qinglu Wang
Qinglu Wang