
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 22 April 2022
Sec. Clinical Microbiology
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.788692
Background: In this study, we evaluated and compared the accuracy of blood and cerebrospinal fluid (CSF) interferon release tests [interferon-gamma release assays (IGRAs)] in the diagnosis of tuberculous meningitis (TBM) by a meta-analysis of the relevant literature.
Methods: We searched for studies published before 2021 in Medline, Embase, the Cochrane database, and Chinese databases. All studies used the QuantiFERON-TB Gold In-Tube and/or T-SPOT.TB method. Blood and/or CSF tests that met the guidelines for the quality assessment of studies with diagnostic accuracy were included. We used the revised diagnostic accuracy study quality assessment to assess the quality of the included studies. Begg’s funnel plots were used to assess publication bias in the meta-analysis of the diagnostic studies, and statistical analyses were performed by using Stata (Version 12) software.
Results: A total of 12 blood and/or CSF IGRA studies were included in this meta-analysis, with 376 patients and 493 controls. The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and area under the summary receiver operating characteristic curve (SROC) of the blood IGRAs in the pooled data from 12 studies were 74% (95% CI: 0.65-0.82), 78% (95% CI: 0.68-0.86), 3.38 (95% CI 2.26-5.06), 0.33 (95% CI: 0.23-0.46), 10.25 (95% CI: 5.46-19.25), and 0.83 (95% CI: 0.79-0.86), respectively. For CSF IGRAs, these values for the pooled data from the 10 studies included were 79% (95% CI: 0.71-0.85), 95% (95% CI: 0.88-0.98), 16.30 (95% CI 6.5-40.83), 0.22 (95% CI: 0.16-0.31), 57.93 (95% CI: 22.56-148.78), and 0.91 (95% CI: 0.88-0.93), respectively.
Conclusion: CSF IGRAs exhibited a better diagnostic accuracy than blood IGRAs in diagnosing TBM.
The World Health Organization (WHO) Global Tuberculosis Report 2020 estimates the number of people living with tuberculosis (TB) at around 10 million in 2019, which makes it the most common cause of death due to a single infectious agent (Chakaya et al., 2021). Tuberculous meningitis (TBM), the most serious form of extrapulmonary TB (Brancusi et al., 2012), is caused by Mycobacterium tuberculosis (M. tuberculosis, MTB) and is associated with significant morbidity and mortality, especially among children and people living with HIV (Ho et al., 2013). Early diagnosis and treatment of TBM is crucial for its prognosis (Garg, 2010). Unfortunately, early diagnosis of TBM is often difficult. Cerebrospinal fluid (CSF) smear, M. tuberculosis culture, and polymerase chain reaction are the gold standards for detecting M. tuberculosis in the CSF (Marais et al., 2010). However, the possibility of identifying acid-fast bacilli in CSF smears is very low, and the culture of M. tuberculosis in CSF is time consuming (Thwaites et al., 2004; Garg, 2019). Although polymerase chain reaction has a higher sensitivity in detecting M. tuberculosis DNA in CSF samples, it also has a higher false-positive rate (Donovan et al., 2020). In response to these challenges, a new technique for the rapid detection of M. tuberculosis has been developed.
In the past decade, the interferon-gamma release assay (IGRA) has become widely used as an immunodiagnostic method for M. tuberculosis infection (Bergot et al., 2018). It detects interferon (IFN)-gamma produced by T cells as a reaction to M. tuberculosis-specific antigens, such as early secretory antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10, which are thought to be present only in M. tuberculosis but not in M. bovis bacille Calmette–Guérin (BCG) vaccine and other mycobacteria (Pai et al., 2006). QuantiFERON-TB Gold In-Tube (QFT-G-IT) (Cellestis, Carnegie, VIC, Australia) and T-SPOT.TB (T-SPOT) (Oxford Immunotec, Abingdon, United Kingdom) are the two most widely used IGRA systems to date, using enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot assay (ELISPOT) to detect IFN-gamma (Pai et al., 2006).
Blood IGRAs are most commonly used. However, an alternative approach of IGRAs using effector T cells from infected TB site specimens may be more likely to detect IFN against TB infection than using peripheral blood mononuclear cells (PBMCs). Recent studies have evaluated the use of CSF IGRAs for the diagnosis of TBM; however, the sample sizes of these included studies were insufficient, and their accuracy was disputable. Therefore, this study aimed to perform a meta-analysis to systematically evaluate and compare the accuracy of blood and CSF IGRAs in the diagnosis of TBM and to review relevant literature.
Relevant works of literature in English were retrieved using Web of Science, PubMed, EBSCO, Medline, Elsevier, and Cochrane Library, while those in Chinese were retrieved using Wanfang Data, China Biology Medicine discs, and China Knowledge Resource Integrated Database. The following keywords were used as search terms: “Tuberculosis meningitis”, “Mycobacterium tuberculosis”, “Tuberculosis”, “Interferon-gamma release assay”, “T cell-based assay”, “T-SPOT.TB”, “ELISPOT”,”IGRA”, “Quantiferen”, “ESAT-6”, “CFP-10”, “Cerebrospinal fluid”, “Sensitivity”, “Specificity”, and “Accuracy”. The study included all diagnostic studies published before 2021.
The inclusion criteria were as follows: (1) IGRAs including T-SPOT (ELISPOT) and/or QFT-GIT were used for the diagnosis of TBM; (2) blood and/or CSF IGRAs were performed; and (3) research articles with original data were included. The exclusion criteria include the following: (1) duplicated studies, case reports, reviews, animal studies, and abstracts; (2) studies without any control group; (3) IGRA systems performed other than QFT-GIT and T-SPOT (ELISPOT); and (4) the fourfold table was not presented or could not be provided.
Two reviewers independently extracted the data using standard data extraction forms (Table 1). The data extracted from these selected articles were as follows: study sites, the date of publication, author’s name, population studied, assay type, diagnostic method of TB, and cut-off of IGRAs. Inconclusive results have been eliminated. Discrepancies were resolved by a third examiner.
The quality of the studies that aimed to calculate the accuracy of the analyses was assessed independently by two reviewers using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) checklist, in which each assessment item was assigned the labels “yes”, “no”, or “unclear” (Whiting et al., 2011).
The degree of heterogeneity of the selected studies was evaluated using the Q-test, and the size of the heterogeneity was quantified by computing for I2. For each study, 2 × 2 tables representing true-positive, true-negative, false-positive, and false-negative values were identified. The sensitivity, specificity, diagnostic odds ratio (DOR), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and area under the summary receiver operating characteristic (SROC) curve from the pooled data were calculated by meta-analysis and expressed in 95% confidence intervals (CIs). Begg’s funnel plot was used to assess publication bias in nine of the included studies. Fagan’s nomogram was used to calculate the post-test probability for each group. All data were analyzed using STATA version 12.0 software. Statistical significance was set at P <0.05.
A total of 832 published studies were screened using the process shown in Figure 1. Among these, 12 studies with 854 human subjects (386 TB patients and 468 controls) from five countries satisfied the inclusion criteria (Kim et al., 2008; Thomas et al., 2008; Kim et al., 2010; Patel et al., 2010; Cho et al., 2011; Vidhate et al., 2011; Park et al., 2012; Zhang et al., 2013; Caliman-Sturdza et al., 2015; Qin et al., 2015; Lu et al., 2016; Pan et al., 2017) including six prospective case-controlled studies (Kim et al., 2008; Thomas et al., 2008; Kim et al., 2010; Patel et al., 2010; Cho et al., 2011; Park et al., 2012; Pan et al., 2017). Most of the studies included were from Asia, of which four were from South Korea (Kim et al., 2008; Kim et al., 2010; Cho et al., 2011; Park et al., 2012), four from China (Zhang et al., 2013; Qin et al., 2015; Lu et al., 2016; Pan et al., 2017), and two from India (Kim et al., 2008; Vidhate et al., 2011). The remaining studies were conducted either in South Africa (Patel et al., 2010) or Romania (Caliman-Sturdza et al., 2015). Blood IGRAs were performed in all, CSF IGRAs (Kim et al., 2008; Thomas et al., 2008; Kim et al., 2010; Patel et al., 2010; Park et al., 2012; Zhang et al., 2013; Caliman-Sturdza et al., 2015; Qin et al., 2015; Lu et al., 2016; Pan et al., 2017) in ten, IGRAs using the T-Spot or ELISPOT system (Kim et al., 2008; Thomas et al., 2008; Kim et al., 2010; Patel et al., 2010; Cho et al., 2011; Park et al., 2012; Zhang et al., 2013; Qin et al., 2015; Pan et al., 2017) in nine, and the QFT-G-IT system (Vidhate et al., 2011; Caliman-Sturdza et al., 2015; Lu et al., 2016) in three of the 12 included studies (Table 1). Eleven studies (Kim et al., 2008; Thomas et al., 2008; Kim et al., 2010; Patel et al., 2010; Cho et al., 2011; Vidhate et al., 2011; Zhang et al., 2013; Caliman-Sturdza et al., 2015; Qin et al., 2015; Lu et al., 2016; Pan et al., 2017) were performed on patients with culture-confirmed TB. In this study, testing of 60 case individuals yielded a positive result for TBM via CSF culture and/or AFB, with a sensitivity of only 16% (Table 2).
This meta-analysis assessed the quality of the included studies using the QUADAS-2 tool, which contains 14 items in the checklist. Among the 12 included studies, one (Thomas et al., 2008) met 14 items, four (Kim et al., 2008; Vidhate et al., 2011; Caliman-Sturdza et al., 2015; Qin et al., 2015) met 13 items, two (Kim et al., 2010; Pan et al., 2017) met 12 items, another two (Patel et al., 2010; Lu et al., 2016) met 11 items, two more (Cho et al., 2011; Zhang et al., 2013) met 10 items, and the last one (Park et al., 2012) met 6 items in the checklist.
The Q-test and I2 statistic results showed high heterogeneity among the included studies (P=0.000, I2>50%). As a result, a random-effects model was used for the meta-analysis. The sensitivity and specificity of blood IGRAs obtained from pooled data were 74% (95% CI: 0.65-0.82) and 78% (95% CI: 0.68-0.86), respectively. In addition, the PLR, NLR, and DOR were 3.38 (95% CI: 2.26-5.06), 0.33 (95% CI: 0.23-0.46), and 10.25 (95% CI: 5.46-19.25), respectively. The false-positive rate of blood detected by T-SPOT was 0.307 and the false-negative rate was 0.298, while QFT-GIT is 0.159 and 0.317, respectively. The area under the SROC curve of blood IGRA was 0.83 (95% CI: 0.79-0.86) (Figures 2A and 3A).
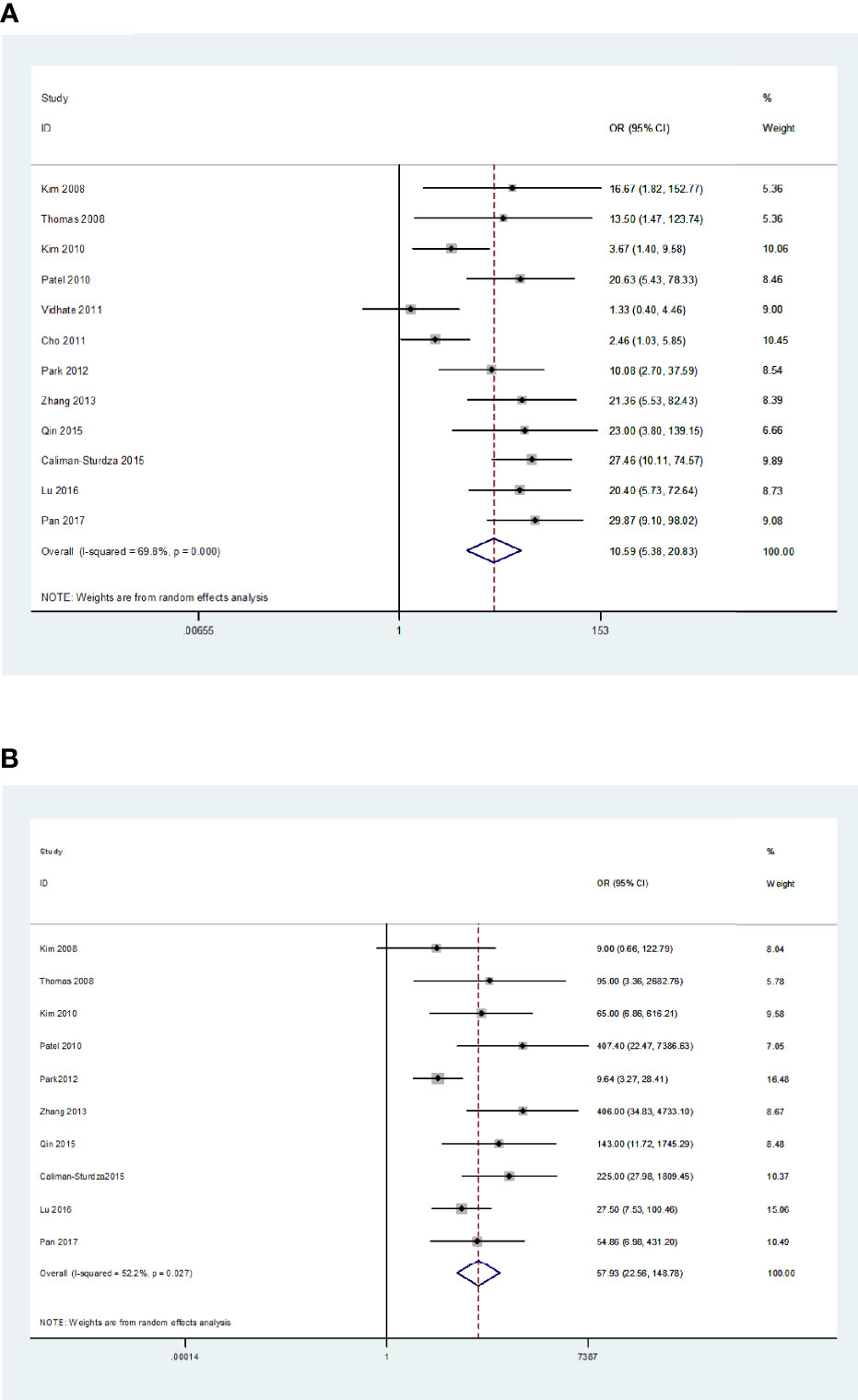
Figure 2 Diagnostic accuracy of the IGRA. For blood IGRAs (A), pooled sensitivity and specificity were 74% (95% CI: 0.65-0.82) and 78% (95% CI: 0.68-0.86), respectively. Moreover, PLR, NLR, and DOR were 3.38 (95% CI: 2.26-5.06), 0.33 (95% CI: 0.23-0.46), and 10.25 (95% CI: 5.46-19.25). For CSF IGRAs (B), pooled sensitivity and specificity were 79% (95% CI: 0.71-0.85) and 95% (95% CI: 0.88-0.98), respectively. In addition, PLR, NLR, and DOR were 16.30 (95% CI: 6.50-40.83), 0.22 (95% CI: 0.16-0.31), and 57.93 (95% CI: 22.56-148.78), respectively.
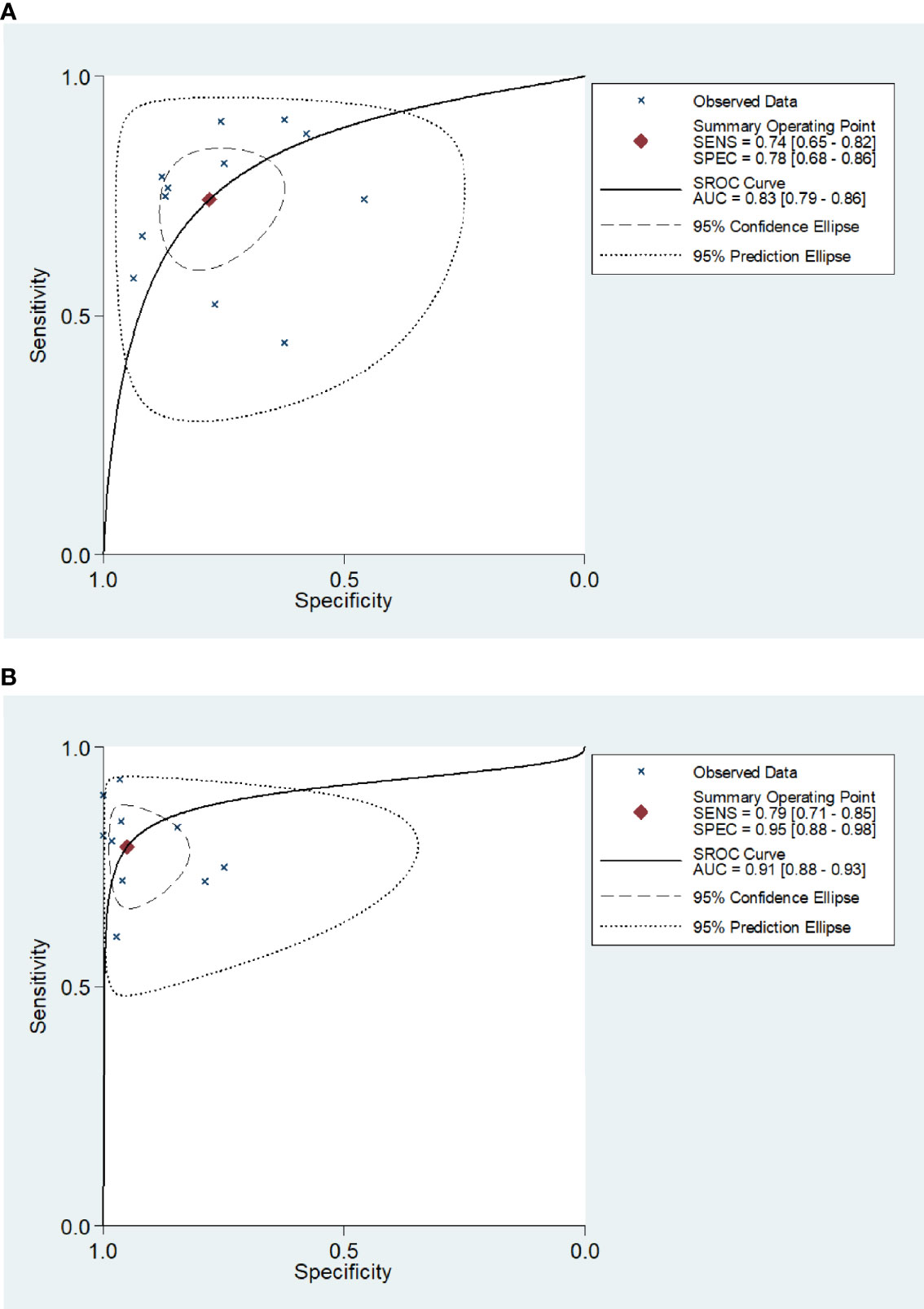
Figure 3 Receiver operating characteristic curve of the IGRA for diagnosis of tuberculosis meningitis. The SROC curves for blood (A) and CSF IGRAs (B). The AUCs were 0.83 (95% CI: 0.79-0.86) for blood IGRAs (A) and 0.91 (95% CI: 0.88-0.93) for CSF IGRAs (B).
The Q-test and I2 statistic results also showed high heterogeneity among the included studies that utilized CSF as the medium analyzed (P=0.03, I2>50%). Therefore, a random-effects model was used for the meta-analysis. The sensitivity and specificity of CSF IGRAs obtained from pooled data were 79% (95% CI: 0.71-0.85) and 95% (95% CI: 0.88-0.98), respectively. In addition, the PLR, NLR, and DOR were 16.30 (95% CI: 6.50-40.83), 0.22 (95% CI: 0.16-0.31), and 57.93 (95% CI: 22.56-148.78), respectively. The false-positive rate of CSF detected by T-SPOT was 0.078 and the false-negative rate was 0.24, while QFT-GIT is 0.074 and 0.21, respectively. The AUC of the SROC curve was 0.91 (95% CI: 0.88-0.93) for CSF IGRAs (Figures 2B and 3B). Therefore, CSF IGRAs showed higher diagnostic sensitivity and specificity than blood IGRAs.
The publication bias of the included studies was determined using Begg’s funnel plot. The Pr>|z| values of blood and CSF were 0.815 (Figure 4A) and 0.458 (Figure 4B), respectively, indicating that no publication bias was observed in blood or CSF IGRAs.
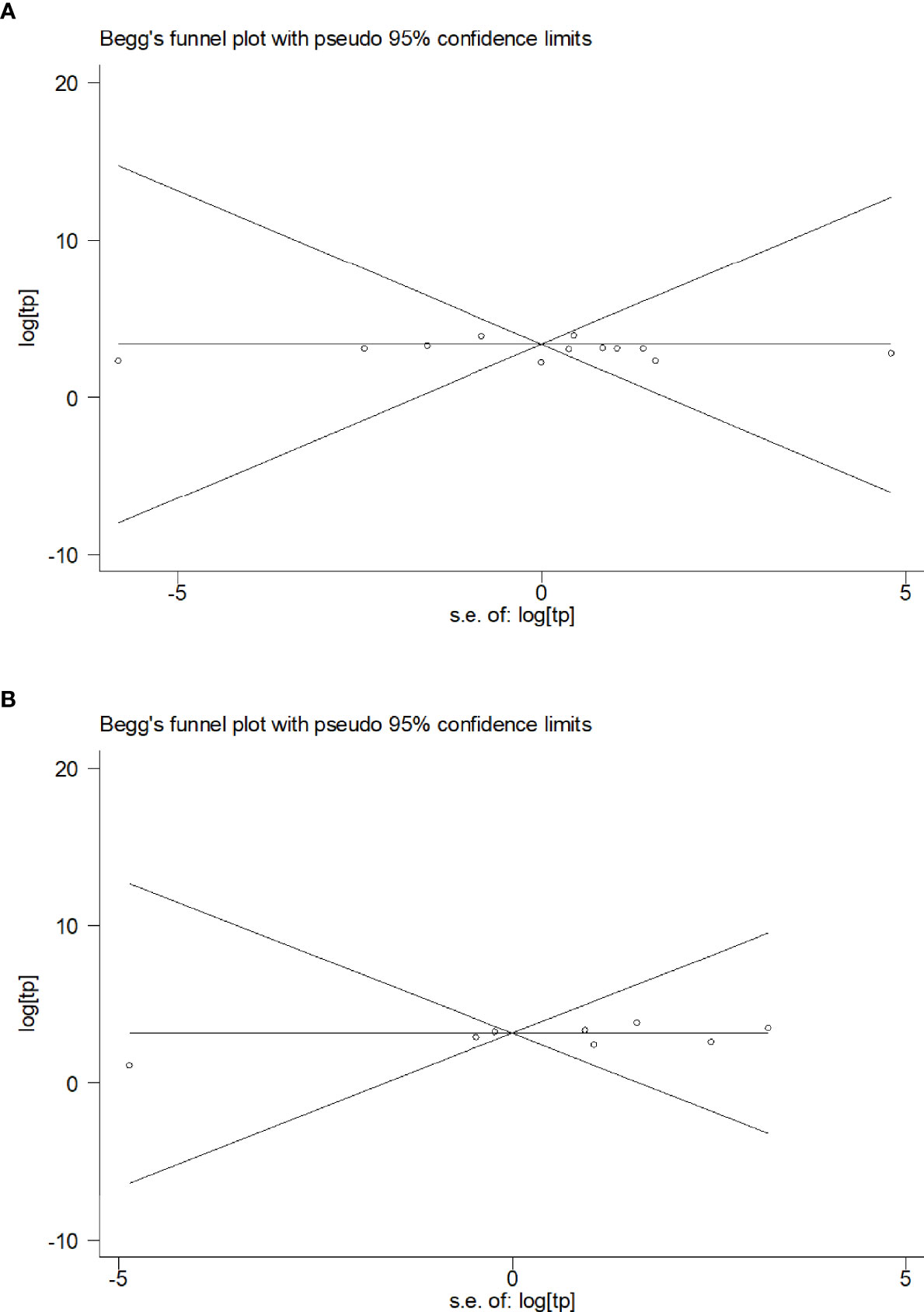
Figure 4 Funnel plot of the included studies. (A) Funnel plots of the included studies on blood (A) and CSF samples (B).
Fagan’s nomogram statistics showed that the positive post-test probabilities of TBM after either blood or CSF IGRAs were 46% and 80%, respectively (Figures 5A, B). A higher but limited probability of body fluids indicates that a positive IGRA result should not be used solely for the diagnosis of TBM, either from blood or from CSF. On the contrary, the negative post-test probabilities were 8% for blood and 5% for CSF IGRAs (Figures 5A, B), which indicates that negative body fluid IGRA results would be more reliable in excluding suspected TBM.
Since early diagnosis and treatment of TBM save lives, tests must be performed to diagnose TBM quickly and accurately, especially in its early stages (Ho et al., 2013). Unfortunately, the diagnosis of TBM is often ambiguous because of nonspecific clinical manifestations and the low sensitivity of available diagnostic methods (Thwaites et al., 2013). The absolute and most widely used diagnostic tools for TBM are Ziehl-Neelsen staining and culture (Marais et al., 2010), but they are negative in the majority of TBM cases. Our results showed that the sensitivity for CSF culture and/or AFB was 16% in the TBM group. Increasing the volume of CSF (>6 ml) obtained and meticulous microscopy (for at least half an hour) further increases the chance of positive diagnosis (Heemskerk et al., 2018; Bahr et al., 2019). For this reason, we did not restrict diagnostic criteria to microbiological confirmation, which is usually not possible in a routine clinical setting. This may produce bias. The detection of M. tuberculosis DNA in CSF samples using nucleic acid amplification tests (NAATs) are a widely used diagnostic method (Marais et al., 2010). In recent years, some studies on NAATs (Modi et al., 2016 Jyothy et al., 2019; Kwizera et al., 2019; Pormohammad et al., 2019; Siddiqi et al., 2019; Poplin et al., 2020; Slane and Unakal, 2021) reported a potential to rule in or confirm diagnosis (specificity, 80%-100%), but low sensitivity (~40%-96%) precludes the use of these tests to rule out TBM. Up to now, adenosine deaminase (ADA) is still a hot spot in TB diagnosis. Numerous studies have been published regarding the usefulness in TBM. Performance varies according to the assay and cut-off used, the mean sensitivity and specificity of ADA assays were 60%-90% and 80%-90%, respectively (Pormohammad et al., 2017). ADA assays may be useful in identifying TBM, but raised levels may also be seen in other central nervous system diseases such as purulent meningitis (Ekermans et al., 2017) (Table 3).
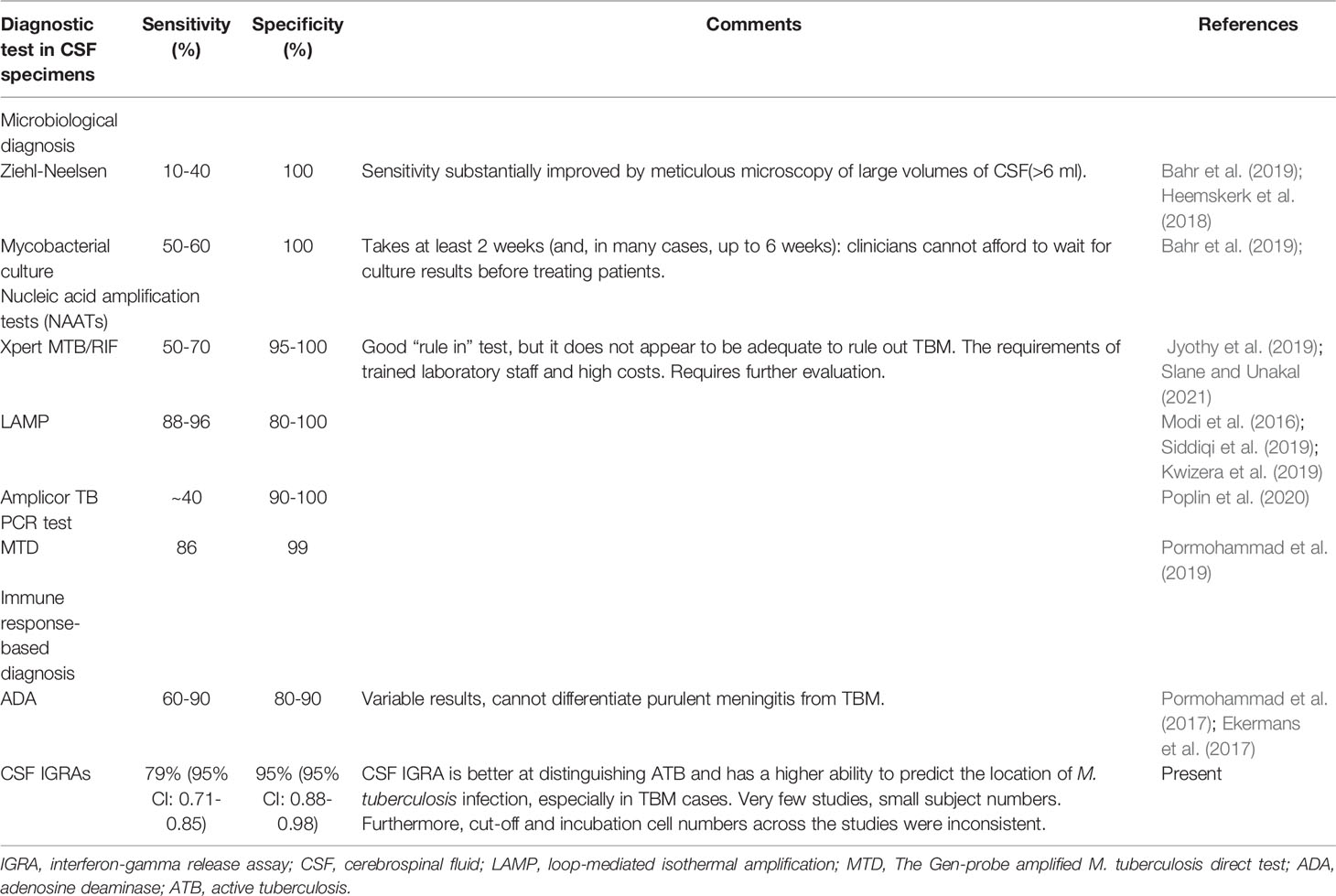
Table 3 Comparison of conventional and novel diagnostic tests for tuberculous meningitis performed on CSF specimens.
To address this, newer tests have emerged for the early diagnosis of TBM, such as IGRAs. The design and development of IGRAs were based on the fact that T lymphocytes release IFN-gamma in response to the stimulation of M. tuberculosis-specific antigens such as ESAT-6 and CFP-10 (Andersen et al., 2000; Pai et al., 2007; Stevens et al., 2019). Moreover, blood-based tests have been widely evaluated and are considered promising tools for the rapid detection of M. tuberculosis (Westermann and Pabst, 1992; Meier et al., 2005; Kawamura et al., 2012; Rangaka et al., 2012). In addition, the accuracy of CSF IGRAs has also been evaluated for the diagnosis of TBM (Kim et al., 2008; Thomas et al., 2008; Kim et al., 2010; Patel et al., 2010; Park et al., 2012; Zhang et al., 2013; Caliman-Sturdza et al., 2015; Qin et al., 2015; Lu et al., 2016; Pan et al., 2017). However, no studies comparing blood and CSF IGRAs have been performed yet. Therefore, we performed this meta-analysis to systematically evaluate and compare the diagnostic accuracy of blood and CSF IGRAs for diagnosing TBM. The obtained overall sensitivity, specificity, PLR, NLR, DOR, and the SROC AUC in blood samples were 74% (95% CI: 0.65-0.82), 78% (95% CI: 0.68-0.86), 3.38 (95% CI: 2.26-5.06), 0.33 (95% CI: 0.23-0.46), 10.25 (95% CI: 5.46-19.25), and 0.83 (95% CI: 0.79-0.86), respectively, and for CSF, these values were 79% (95% CI: 0.71-0.85), 95% (95% CI: 0.88-0.98), 16.30 (95% CI: 6.50-40.83), 0.22 (95% CI: 0.16-0.31), 57.93 (95% CI: 22.56-148.78), and 0.91 (95% CI: 0.88-0.93), respectively. The post-test probability of blood samples was 46%/8% and 80%/5% in CSF samples. These results suggest that the IGRAs carried out in CSF have better diagnostic sensitivity and specificity than IGRAs in blood for the diagnosis of TBM.
However, even if the sensitivity, specificity, and post-test probability were higher for CSF IGRAs than for blood IGRAs in this analysis, the diagnostic sensitivity of CSF IGRAs alone was not high enough to support the diagnosis of TBM. Nevertheless, CSF IGRAs have some advantages over blood IGRAs. When peripheral blood IGRAs are used alone, it becomes difficult to distinguish active tuberculosis (ATB) from latent tuberculosis infection in a clinical setting (Pai et al., 2004; Gao et al., 2017). In an ATB stimulation, antigen-specific T lymphocytes are recruited to the sites of infection and proliferate rapidly (Schwander et al., 1998; Hirsch et al., 2001). Because of this, CSF IGRA is better at distinguishing ATB and has a higher ability to predict the location of the M. tuberculosis infection, especially in TBM cases.
The current analysis had some limitations. First, among the ten included studies that utilized CSF IGRA, the sample size was fairly small. Second, the cut-off and incubation cell numbers across the studies were inconsistent. This is an important consideration because the cut-off values greatly influence the sensitivity and specificity. Finally, in some of the included studies, the diagnosis of TBM patients was not confirmed by microbiological methods (smear or culture).
The results of this meta-analysis showed that CSF IGRAs had higher sensitivity and specificity in the diagnosis of TBM than peripheral blood IGRAs. However, carefully designed higher-quality independent studies are required to reliably compare the diagnostic accuracies of blood and CSF IGRAs.
AW, E-LL, and FH designed the study. The manuscript was written by AW, E-LL, and FH with the final approval. S-ML and Y-LZ did the data searches and study selection. W-FC and D-YY did the data synthesis and created the tables and figures. All authors contributed to the article and approved the submitted version.
This study was supported by Jiangxi Provincial Health Commission Science and Technology Foundation grants 20203025.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Thanks for the effort made by all authors of studies included in this meta-analysis. At the same time, We would like to thank Editage (www.editage.cn) for English language editing.
Andersen, P., Munk, M. E., Pollock, J. M., Doherty, T. M. (2000). Specific Immune-Based Diagnosis of Tuberculosis. Lancet 356, 1099–1104. doi: 10.1016/S0140-6736(00)02742-2
Bahr, N. C., Meintjes, G., Boulware, D. R. (2019). Inadequate Diagnostics: The Case to Move Beyond the Bacilli for Detection of Meningitis Due to Mycobacterium Tuberculosis. J. Med. Microbiol. 68, 755–760. doi: 10.1099/jmm.0.000975
Bergot, E., Abiteboul, D., Andréjak, C., Antoun, F., Barras, E., Blanc, F. X., et al. (2018). [Practice Recommendations for the Use and Interpretation of Interferon Gamma Release Assays in the Diagnosis of Latent and Active Tuberculosis]. Rev. Mal. Respir. 35, 852–858. doi: 10.1016/j.rmr.2018.08.007
Brancusi, F., Farrar, J., Heemskerk, D. (2012). Tuberculous Meningitis in Adults: A Review of a Decade of Developments Focusing on Prognostic Factors for Outcome. Future Microbiol. 7, 1101–1116. doi: 10.2217/fmb.12.86
Caliman-Sturdza, O.-A. -., Mihalache, D., Luca, C.-M. (2015). Performance of an Interferon-Gamma Release Assay in the Diagnosis of Tuberculous Meningitis in Children. ROM J. LEG Med. 23, 199–212. doi: 10.1515/rrlm–2015–0016
Chakaya, J., Khan, M., Ntoumi, F., Aklillu, E., Fatima, R., Mwaba, P., et al. (2021). Global Tuberculosis Report 2020 - Reflections on the Global TB Burden, Treatment and Prevention Efforts. Int. J. Infect. Dis. 113 (suppl 1), S7–S12. doi: 10.1016/j.ijid.2021.02.107
Cho, O. H., Park, K. H., Kim, S. M., Park, S. J., Moon, S. M., Chong, Y. P., et al. (2011). Diagnostic Performance of T-SPOT.TB for Extrapulmonary Tuberculosis According to the Site of Infection. J. Infect. 63, 362–369. doi: 10.1016/j.jinf.2011.06.010
Donovan, J., Thwaites, G. E., Huynh, J. (2020). Tuberculous Meningitis: Where to From Here? Curr. Opin. Infect. Dis. 33, 259–266. doi: 10.1097/QCO.0000000000000648
Ekermans, P., Dusé, A., George, J. (2017). The Dubious Value of Cerebrospinal Fluid Adenosine Deaminase Measurement for the Diagnosis of Tuberculous Meningitis. BMC Infect. Dis. 17, 104. doi: 10.1186/s12879-017-2221-3
Gao, L., Li, X., Liu, J., Wang, X., Lu, W., Bai, L., et al. (2017). Incidence of Active Tuberculosis in Individuals With Latent Tuberculosis Infection in Rural China: Follow-Up Results of a Population-Based, Multicentre, Prospective Cohort Study. Lancet Infect. Dis. 17, 1053–1061. doi: 10.1016/S1473-3099(17)30402-4
Garg, R. K. (2010). Tuberculous Meningitis. Acta Neurol. Scand. 122, 75–90. doi: 10.1111/j.1600–0404.2009.01316.X
Garg, R. K. (2019). Microbiological Diagnosis of Tuberculous Meningitis: Phenotype to Genotype. Indian J. Med. Res. 150, 448–457. doi: 10.4103/ijmr.IJMR_1145_19
Heemskerk, A. D., Donovan, J., Thu, D. D. A., Marais, S., Chaidir, L., Dung, V. T. M., et al. (2018). Improving the Microbiological Diagnosis of Tuberculous Meningitis: A Prospective, International, Multicentre Comparison of Conventional and Modified Ziehl-Neelsen Stain, GeneXpert, and Culture of Cerebrospinal Fluid. J. Infect. 77, 509–515. doi: 10.1016/j.jinf.2018.09.003
Hirsch, C. S., Toossi, Z., Johnson, J. L., Luzze, H., Ntambi, L., Peters, P., et al. (2001). Augmentation of Apoptosis and Interferon-Gamma Production at Sites of Active Mycobacterium Tuberculosis Infection in Human Tuberculosis. J. Infect. Dis. 183, 779–788. doi: 10.1086/318817
Ho, J., Marais, B. J., Gilbert, G. L., Ralph, A. P. (2013). Diagnosing Tuberculous Meningitis - Have We Made Any Progress? Trop. Med. Int. Health 18, 783–793. doi: 10.1111/tmi.12099
Jyothy, A., Ratageri, V. H., Illalu, S., Fattepur, S. R., Wari, P. K. (2019). The Utility of CSF Xpert MTB/RIF in Diagnosis of Tubercular Meningitis in Children. Indian J. Pediatr. 86, 1089–1093. doi: 10.1007/s12098-019-03032-0
Kawamura, L. M., Grinsdale, J. A., Ho, C. S., Jereb, J. A. (2012). Interferon-γ Release Assays for Prediction of Tuberculosis. Lancet Infect. Dis. 12, 584. doi: 10.1016/S1473-3099(12)70169-X
Kim, S. H., Cho, O. H., Park, S. J., Lee, E. M., Kim, M. N., Lee, S. O., et al. (2010). Rapid Diagnosis of Tuberculous Meningitis by T Cell-Based Assays on Peripheral Blood and Cerebrospinal Fluid Mononuclear Cells. Clin. Infect. Dis. 50, 1349–1358. doi: 10.1086/652142
Kim, S. H., Chu, K., Choi, S. J., Song, K. H., Kim, H. B., Kim, N. J., et al. (2008). Diagnosis of Central Nervous System Tuberculosis by T-Cell-Based Assays on Peripheral Blood and Cerebrospinal Fluid Mononuclear Cells. Clin. Vaccine Immunol. 15, 1356–1362. doi: 10.1128/CVI.00040-08
Kwizera, R., Cresswell, F. V., Mugumya, G., Okirwoth, M., Kagimu, E., Bangdiwala, A. S., et al. (2019). Performance of Lipoarabinomannan Assay Using Cerebrospinal Fluid for the Diagnosis of Tuberculous Meningitis Among HIV Patients. Wellcome Open Res. 4, 123. doi: 10.12688/wellcomeopenres.15389.2
Lu, D., Chen, C., Yu, S., Chen, S. (2016). Diagnosis of Tuberculous Meningitis Using a Combination of Peripheral Blood T-SPOT.TB and Cerebrospinal Fluid Interferon-γ Detection Methods. Lab. Med. 47, 6–12. doi: 10.1093/labmed/lmv010
Marais, S., Thwaites, G., Schoeman, J. F., Török, M. E., Misra, U. K., Prasad, K., et al. (2010). Tuberculous Meningitis: A Uniform Case Definition for Use in Clinical Research. Lancet Infect. Dis. 10, 803–812. doi: 10.1016/S1473-3099(10)70138-9
Meier, T., Eulenbruch, H. P., Wrighton-Smith, P., Enders, G., Regnath, T. (2005). Sensitivity of a New Commercial Enzyme-Linked Immunospot Assay (T SPOT-TB) for Diagnosis of Tuberculosis in Clinical Practice. Eur. J. Clin. Microbiol. Infect. Dis. 24, 529–536. doi: 10.1007/s10096-005-1377-8
Modi, M., Sharma, K., Sharma, M., Sharma, A., Sharma, N., Sharma, S., et al. (2016). Multitargeted Loop-Mediated Isothermal Amplification for Rapid Diagnosis of Tuberculous Meningitis. Int. J. Tuberc. Lung Dis. 20, 625–630. doi: 10.5588/ijtld.15.0741
Pai, M., Dheda, K., Cunningham, J., Scano, F., O'brien, R. (2007). T-Cell Assays for the Diagnosis of Latent Tuberculosis Infection: Moving the Research Agenda Forward. Lancet Infect. Dis. 7, 428–438. doi: 10.1016/S1473-3099(07)70086-5
Pai, M., Kalantri, S., Dheda, K. (2006). New Tools and Emerging Technologies for the Diagnosis of Tuberculosis: Part I. Latent Tuberculosis. Expert Rev. Mol. Diagn. 6, 413–422. doi: 10.1586/14737159.6.3.413
Pai, M., Riley, L. W., Colford, J. M., Jr. (2004). Interferon-Gamma Assays in the Immunodiagnosis of Tuberculosis: A Systematic Review. Lancet Infect. Dis. 4, 761–776. doi: 10.1016/S1473-3099(04)01206-X
Pan, L., Liu, F., Zhang, J., Yang, X., Zheng, S., Li, J., et al. (2017). Interferon-Gamma Release Assay Performance of Cerebrospinal Fluid and Peripheral Blood in Tuberculous Meningitis in China. BioMed. Res. Int. 2017, 8198505. doi: 10.1155/2017/8198505
Park, K. H., Cho, O. H., Lee, E. M., Lee, S. O., Choi, S. H., Kim, Y. S., et al. (2012). T-Cell-Based Assays on Cerebrospinal Fluid and PBMCs for Rapid Diagnosis of TB Meningitis in Non-HIV Patients. Eur. Respir. J. 39, 768–770. doi: 10.1183/09031936.00098111
Patel, V. B., Singh, R., Connolly, C., Coovadia, Y., Peer, A. K., Parag, P., et al. (2010). Cerebrospinal T-Cell Responses Aid in the Diagnosis of Tuberculous Meningitis in a Human Immunodeficiency Virus- and Tuberculosis-Endemic Population. Am. J. Respir. Crit. Care Med. 182, 569–577. doi: 10.1164/rccm.200912-1931OC
Poplin, V., Boulware, D. R., Bahr, N. C. (2020). Methods for Rapid Diagnosis of Meningitis Etiology in Adults. Biomark. Med. 14, 459–479. doi: 10.2217/bmm-2019-0333
Pormohammad, A., Nasiri, M. J., Mchugh, T. D., Riahi, S. M., Bahr, N. C. (2019). A Systematic Review and Meta-Analysis of the Diagnostic Accuracy of Nucleic Acid Amplification Tests for Tuberculous Meningitis. J. Clin. Microbiol. 57, e01113–18. doi: 10.1128/JCM.01113-18
Pormohammad, A., Riahi, S. M., Nasiri, M. J., Fallah, F., Aghazadeh, M., Doustdar, F., et al. (2017). Diagnostic Test Accuracy of Adenosine Deaminase for Tuberculous Meningitis: A Systematic Review and Meta-Analysis. J. Infect. 74, 545–554. doi: 10.1016/j.jinf.2017.02.012
Qin, L., Zhang, L., Zhang, Y., Shi, X., Zhang, Y., Liu, X. (2015). Diagnostic Value of T-Cell Interferon-γ Release Assays on Cerebrospinal Fluid for Tuberculous Meningitis. PloS One 10, e0141814. doi: 10.1371/journal.pone.0141814
Rangaka, M. X., Wilkinson, K. A., Glynn, J. R., Ling, D., Menzies, D., Mwansa-Kambafwile, J., et al. (2012). Predictive Value of Interferon-γ Release Assays for Incident Active Tuberculosis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 12, 45–55. doi: 10.1016/S1473-3099(11)70210-9
Schwander, S. K., Torres, M., Sada, E., Carranza, C., Ramos, E., Tary-Lehmann, M., et al. (1998). Enhanced Responses to Mycobacterium Tuberculosis Antigens by Human Alveolar Lymphocytes During Active Pulmonary Tuberculosis. J. Infect. Dis. 178, 1434–1445. doi: 10.1086/314454
Siddiqi, O. K., Birbeck, G. L., Ghebremichael, M., Mubanga, E., Love, S., Buback, C., et al. (2019). Prospective Cohort Study on Performance of Cerebrospinal Fluid (CSF) Xpert MTB/RIF, CSF Lipoarabinomannan (LAM) Lateral Flow Assay (LFA), and Urine LAM LFA for Diagnosis of Tuberculous Meningitis in Zambia. J. Clin. Microbiol. 57, e00652–19. doi: 10.1128/JCM.00652-19
Slane, V. H., Unakal, C. G. (2021). “Tuberculous Meningitis,” in StatPearls (Treasure Island FL: StatPearls Publishing LLC).
Stevens, J. P., Ballengee, C. R., Chandradevan, R., Thompson, A. B., Schoen, B. T., Kugathasan, S., et al. (2019). Performance of Interferon-Gamma Release Assays for Tuberculosis Screening in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 69, e162. doi: 10.1097/MPG.0000000000002501
Thomas, M. M., Hinks, T. S., Raghuraman, S., Ramalingam, N., Ernst, M., Nau, R., et al. (2008). Rapid Diagnosis of Mycobacterium Tuberculosis Meningitis by Enumeration of Cerebrospinal Fluid Antigen-Specific T-Cells. Int. J. Tuberc. Lung Dis. 12, 651–657. doi: 10.1080/01902140802022526
Thwaites, G. E., Chau, T. T., Farrar, J. J. (2004). Improving the Bacteriological Diagnosis of Tuberculous Meningitis. J. Clin. Microbiol. 42, 378–379. doi: 10.1128/JCM.42.1.378-379.2004
Thwaites, G. E., Van Toorn, R., Schoeman, J. (2013). Tuberculous Meningitis: More Questions, Still Too Few Answers. Lancet Neurol. 12, 999–1010. doi: 10.1016/S1474-4422(13)70168-6
Vidhate, M. R., Singh, M. K., Garg, R. K., Verma, R., Shukla, R., Goel, M. M., et al. (2011). Diagnostic and Prognostic Value of Mycobacterium Tuberculosis Complex Specific Interferon Gamma Release Assay in Patients With Tuberculous Meningitis. J. Infect. 62, 400–403. doi: 10.1016/j.jinf.2011.03.009
Westermann, J., Pabst, R. (1992). Distribution of Lymphocyte Subsets and Natural Killer Cells in the Human Body. Clin. Investig. 70, 539–544. doi: 10.1007/BF00184787
Whiting, P. F., Rutjes, A. W., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., et al. (2011). QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 155, 529–536. doi: 10.7326/0003-4819-155-8-201110180-00009
Keywords: tuberculous meningitis, cerebrospinal fluid, meta-analysis, interferon-release assays, tuberculosis
Citation: Wen A, Leng E-L, Liu S-M, Zhou Y-L, Cao W-F, Yao D-Y and Hu F (2022) Diagnostic Accuracy of Interferon-Gamma Release Assays for Tuberculous Meningitis: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 12:788692. doi: 10.3389/fcimb.2022.788692
Received: 03 October 2021; Accepted: 24 March 2022;
Published: 22 April 2022.
Edited by:
Yang Zhang, University of Pennsylvania, United StatesReviewed by:
Jiabo Ding, China Institute of Veterinary Drug Control, ChinaCopyright © 2022 Wen, Leng, Liu, Zhou, Cao, Yao and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Hu, aHVkYW1pbmcyMDA1QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.