
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Cell. Infect. Microbiol. , 15 December 2022
Sec. Molecular Bacterial Pathogenesis
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.1073966
This article is part of the Research Topic The pathogenic Flavobacteriaceae: recent advances in the understanding of physiology, genetics, and virulence View all 7 articles
Ayu or sweetfish, Plecoglossus altivelis altivelis, is commercially important to inland fisheries in Japan and popular as a summer delicacy owing to its unusually sweet flavor. Ayu is also a popular recreational fishing species, especially for anglers using the Japanese fishing method “tomozuri.”
Bacterial cold-water disease (BCWD) was first recorded in an ayu farm in the Tokushima Prefecture in 1987 (Wakabayashi et al., 1994; Inouye, 2000). The cause of this occurrence was believed to be the introduction of ayu from Lake Biwa because BCWD was detected only a few days after landlocked ayu stock from Lake Biwa was transported to the farm (Wakabayashi, 2009). In 1993, BCWD spread to ayu populations in Gonokawa River in Hiroshima Prefecture (Iida and Mizokami, 1996). Subsequently, BCWD epizootics in ayu populations were reported in most rivers in Japan, and a close relationship was found to exist between the occurrence of BCWD and the release and use as a decoy of landlocked ayu stocks from Lake Biwa (Inouye, 2000; Taniguchi, 2002; Imura, 2003). According to the Japanese government statistics site e-Stat (https://www.e-stat.go.jp/en), the commercial catch of ayu in 2020 has decreased by 88.3% compared with the maximum catch of 16,414 tons in 1991. In the manuscript, the current knowledge of BCWD in ayu, which inhabits Japanese rivers, was compiled.
Two forms of native ayu exist, amphidromous and landlocked ayu, with an assumed genetic distance and divergence time of approximately 100,000 years (Nishida, 1985). Amphidromous ayu is widely distributed in the Japanese Archipelago (Iguchi and Nishida, 2002) and has an annual life cycle as follows (Nishida, 1986): in autumn, mature ayu spawn and die after reproduction in the lower reaches of rivers; subsequently, newborn larvae hatch, flow to the sea, metamorphose into juveniles, and overwinter in habitats including estuarine and coastal environments (Murase et al., 2020); in spring, the juveniles migrate back into rivers and drift upstream where they grow during summer; when sexually mature, they drift downstream to the lower reaches of the river for spawning. In contrast, landlocked ayu is restricted to freshwater lakes. The largest landlocked population inhabits Lake Biwa, and the larvae and juveniles overwinter in the offshore water of the lake (Nishida, 1986).
Three types of stocking ayu exist: hatchery-born amphidromous, wild-born, and domesticated stocks. Most wild-born stocks comprise landlocked ayu caught in Lake Biwa; indeed, landlocked stock has been translocated into amphidromous populations in rivers throughout Japan for many years. Three concerns have been raised around the use of landlocked stock in relation to the conservation of natural amphidromous populations (Takamura, 2009; Kitada, 2022). First, the translocated landlocked stock poses a risk of interbreeding with wild amphidromous populations. Second, the landlocked stock cannot contribute successfully to the reproduction of the next generation. Third, the landlocked stock in Lake Biwa is infected with Flavobacterium psychrophilum. Amphidromous stock is genetically more resistant to BCWD than landlocked and domestic stocks (Nagai et al., 2004; Nagai and Sakamoto, 2006). For this reason, hatchery-born amphidromous stock is now produced and released into rivers in large quantities that surpass those of landlocked stock (Kitada, 2022).
The early release of F. psychrophilum-free amphidromous stock is recommended as a strategy for preventing the occurrence of BCWD in river-based ayu populations (Hara et al., 2007; Hara et al., 2008). The active cooperation of recreational anglers is also required to prevent BCWD, and the following recommendations have been proposed: voluntary restraints on moving ayu between rivers; cleaning fishing gears, including disinfection with alcohol and chlorine and drying in the sun or at a high temperature; and using different gears in different rivers (Katahira et al., 2019).
Most F. psychrophilum isolates from ayu in Japan are serotype O-2; therefore, the serotyping approach is useful to determine host specificity (Wakabayashi et al., 1994; Iida and Mizokami, 1996; Izumi and Wakabayashi, 1999; Izumi et al., 2003b). Various genotyping techniques have been used to characterize their variations as follows: plasmid profiling assay (Izumi, 2004; Kim et al., 2010), pulsed-field gel electrophoresis assay (Arai et al., 2007), multiplex PCR–restriction fragment length polymorphism (PCR–RFLP) assay (Izumi et al., 2003a; Izumi et al., 2007; Izumi et al., 2019), on/off switch assay (Fujiwara-Nagata et al., 2012), and multilocus sequence typing (MLST) analysis (Fujiwara-Nagata et al., 2013) (Table 1).
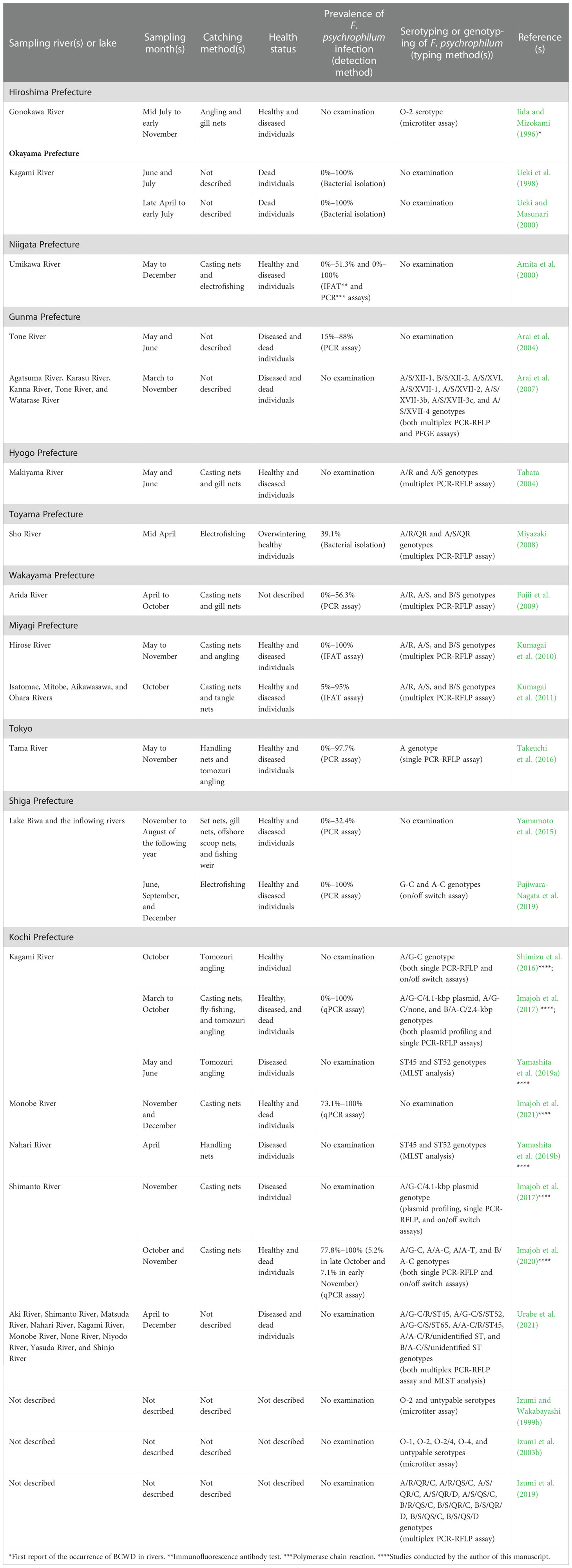
Table 1 Summary of the studies investigating the distribution of F. psychrophilum in ayu populations in Japanese rivers and Lake Biwa.
The single PCR–RFLP assay targets peptidyl-prolyl cis-trans isomerase C (PPIC) and divides the isolates into two genotypes: A and B types (Yoshiura et al., 2006). All A-type isolates are regarded as specific pathogens of ayu (Tabata, 2004), with only one B-type isolate causing BCWD in ayu in a bath infection challenge (Miwa and Nakayasu, 2005). Recently, Izumi et al. (2019) proposed the multiplex PCR–RFLP assay targeting the PPIC, DNA gyrase (gyrA and gyrB), and topoisomerase IV (parE) genes to improve genotyping performance and potentially allow the classification of 16 genotypes.
MLST provides more detailed genotyping data than the PCR–RFLP assay. MLST analysis based on seven housekeeping genes revealed that the CC-ST48, CC-ST52, and CC-ST56 lineages infect ayu in Japan and are important for the treatment and prevention of BCWD in ayu (Fujiwara-Nagata et al., 2013).
The on/off switch assay identifies two single nucleotide polymorphisms of gyrA and divides isolates into four genotypes: the G-C type isolated from ayu, the A-T type isolated from salmonid fish, and the G-T and A-C types isolated from several species including ayu (Fujiwara-Nagata et al., 2012). This assay assesses the potential pathogenicity of F. psychrophilum isolates to ayu: the G-C type shows strong pathogenicity, the A-T and G-T types show no pathogenicity, and the A-C type shows at most weak pathogenicity. Fujiwara-Nagata et al. (2019) determined the seasonal changes of the four genotypes in various samples collected from the lower basin of a river flowing into Lake Biwa, reporting that most of the isolates were the G-C type in September when ayu gathered in the lower basin for spawning and that the A-T type was only detected in December when Biwa trout (Oncorhynchus masou rhodurus) were present in the lower basin for spawning.
Ayu have been sampled to determine the distribution of F. psychrophilum in ayu populations in Japanese rivers and Lake Biwa (Table 1). Kochi Prefecture (Table 1) is located on the south coast of Shikoku Island in Japan; ayu inhabit the clear rivers of the prefecture, including the Kagami River, the Shimanto River, the Monobe River, and the Nahari River, although the commercial ayu catch has halved since 1993 due to the occurrence of BCWD (Taniguchi, 2002).
The Kagami River, located in central Kochi Prefecture, is 31 km long with a drainage basin area of 170 km2. The Kagami Dam in the middle of the river divides it into two streams, preventing ayu from drifting upstream or downstream. Thus, hatchery-born amphidromous stock is released in both the upper and lower reaches relative to the dam to enhance the natural ayu stocks. As reported by the Ministry of Agriculture, Forestry, and Fisheries, BCWD outbreaks most frequently occur from May to July at water temperatures of 14°C–21°C (http://www.maff.go.jp/j/syouan/suisan/suisan_yobo/ayu_reisui/). In late June 2014, there was a mass die-off of ayu in the upper reaches. Imajoh et al. (2017) inferred that the die-off was attributed to BCWD because they successfully isolated F. psychrophilum from all the collected dead individuals, and almost all isolates were identified as A/G-C types. Urabe et al. (2021) subsequently genotyped more isolates from more rivers in Kochi Prefecture using an on/off switch assay, a PCR–RFLP assay, and MLST analysis, finding that most isolates were the A/G-C/S/ST52 types. Therefore, this genotype is likely the main cause of BCWD in ayu in Kochi Prefecture’s rivers.
The Shimanto River, located in western Kochi Prefecture, is 196 km long with a drainage basin area of 2,186 km2. The Monobe River, located in central Kochi Prefecture, is 71 km long with a drainage basin area of 508 km2. The two rivers are considered Class A rivers, which are assigned by the government of Japan as important for the conservation of national land or for the national economy, and are famous for ayu, especially the Shimanto River, which possesses an abundance of native amphidromous ayu resources (Azuma et al., 2020). Many mature ayu drift down near the mouth of the river to lay their eggs in autumn, after which they die. Imajoh et al. (2020); Imajoh et al. (2021) collected 248 mature and 369 dead individuals at several times during the spawning season in the spawning grounds of the two rivers and used quantitative PCR to determine the prevalence of F. psychrophilum infection, which was very high at 73%–100% in the mature individuals, excluding late October and early November in the Shimanto River, and 100% in the dead individuals. Interestingly, many F. psychrophilum–infected dead individuals were prespawning fish. Sexual maturation is thought to decrease the resistance of ayu to F. psychrophilum infection as well as causing changes to nonspecific immune responses and lymphocytopenia (Minami et al., 2018; Kawashima et al., 2021). Therefore, acute F. psychrophilum infection likely occurs among mature ayu gathering on the spawning ground, resulting in septicemia due to the onset of BCWD.
Several catching methods for ayu sampling are shown in Table 1, and these methods differ according to the specific situation. There is a concern that some methods, especially tomozuri angling, may skew the catch toward healthy rather than diseased ayu because diseased fish exhibit lower physiological activity than healthy fish. Environmental DNA (eDNA) analysis enables year-round monitoring of ayu in rivers and Lake Biwa (Kono et al., 2017; Inui et al., 2018; Inui et al., 2019; Haga et al., 2020; Inui et al., 2020; Inui et al., 2021; Tsuji et al., 2022). Recently, the combined use of ayu and F. psychrophilum in eDNA analysis has attracted attention owing to its potential utility for predicting the occurrence of BCWD in rivers (Tenma et al., 2021). According to the studies of Strepparava et al. (2014) and Nguyen et al. (2018); Imajoh et al. (2020); Imajoh et al. (2021) selected the single copy gene β′ DNA-dependent RNA polymerase to detect F. psychrophilum in the water and conduct eDNA analysis in the Shimanto River and Monobe River, finding that both the eDNA concentrations of ayu and F. psychrophilum reached maximum levels in the river water of the spawning ground during the spawning season among the seasonal–annual distribution, likely reflecting the high prevalence of F. psychrophilum infection in mature and dead ayu at the spawning grounds.
The findings presented in this manuscript indicate that F. psychrophilum infection can spread widely and rapidly in spawning ayu in rivers. Thus, it is necessary to estimate the extent to which spawning ayu are lost because of F. psychrophilum infection during the spawning season. It is also possible that F. psychrophilum released from spawning ayu could survive over winter and represent the preliminary infection source in the next year. This possibility is supported by a case report in the Nahari River (Yamashita et al., 2019b), which is located in the eastern Kochi Prefecture, is 61 km long, and has a drainage basin area of 311 km2. In April 2018, BCWD caused a mass die-off of juvenile ayu beginning to drift upstream near the river mouth, which received attention for being the first such occurrence during this month in Kochi Prefecture. Importantly, F. psychrophilum infection is considered not to have been introduced into the river from an outside source because (1) ayu stocks were not released and (2) no anglers fished on the river because of a fishing ban. Yamashita et al. (2019b) isolated six F. psychrophilum isolates from the dead individuals, determined their draft genome sequences, and examined their genotypes, which were in agreement with the genotypic results of Urabe et al. (2021). The obtained draft genome data will provide insights into the survival of F. psychrophilum over winter in the Nahari River and possible reinfection of the ayu population in the next spring.
All authors contributed to the article and approved the submitted version.
I am thankful for the support received from the Kagamigawa, Monobegawa, Naharigawa Tansui, and Shimantogawa Chuo Fisheries Cooperative Associations.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amita, K., Hoshino, M., Honma, T., Wakabayashi, H. (2000). An investigation on the distribution of Flavobacterium pshchrophilum in the Umikawa River. Fish. Pathol. 35, 193–197. doi: 10.3147/jsfp.35.193
Arai, H., Morita, Y., Izumi, S., Katagiri, T., Kimura, H. (2007). Molecular typing by pulsed-field gel electrophoresis of Flavobacterium psychrophilum isolates derived from Japanese fish. J. Fish. Dis. 30, 345–355. doi: 10.1111/j.1365-2761.2007.00809.x
Arai, H., Morita, Y., Nobusawa, K., Arai, M., Boonmar, S., Kimura, H. (2004). Prevalence of Flavoacterium psychrophilum infection in ayu (Plecoglossus altivelis) in Gunma Prefecture, Japan and comparison of the gyr B sequences of isolates. Kasetsart J. 38, 523–530.
Azuma, K., Horioka, K., Ohgi, M., Iyota, T., Matsuoka, I., Iyota, K. (2020). Counting the numbers of ascending juvenile ayu based on visual observations and underwater videos in the Shimanto River, western Japan. Aquacul. Sci. 68, 375–382. doi: 10.11233/aquaculturesci.68.375
Fujii, H., Shigeo Harada, S., Kotouge, T. (2009). Flavobacterium psychrophilum carrying situation in native fish species caught from the Arida River and the pathogenicity of F. psychrophilum isolated from dark chub Zacco temminckii on ayu Plecoglossus altivelis. Aquacul. Sci. 57, 621–622. doi: 10.11233/aquaculturesci.57.621
Fujiwara-Nagata, E., Chantry-Darmon, C., Bernardet, J.-F., Eguchi, M., Duchaud, E., Nicolas, P. (2013). Population structure of the fish pathogen Flavobacterium psychrophilum at whole-country and model river levels in Japan. Vet. Res. 44, 34. doi: 10.1186/1297-9716-44-34
Fujiwara-Nagata, E., Ikeda, J., Sugahara, K., Eguchi, M. (2012). A novel genotyping technique for distinguishing between Flavobacterium psychrophilum isolates virulent and avirulent to ayu, Plecoglossus altivelis altivelis (Temminck & schlegel). J. Fish. Dis. 35, 471–480. doi: 10.1111/j.1365-2761.2012.01368.x
Fujiwara-Nagata, E., Shindoh, Y., Yamamoto, M., Okamura, T., Takegami, K., Eguchi, M. (2019). Distribution of Falvobacterium psychrophilum and its gyrA genotypes in a river. Fish. Sci. 85, 913–923. doi: 10.1007/s12562-019-01355-7
Haga, K., Takahashi, I., Yataka, H., Kitamura, Y. (2020). Evaluation of possible estimation of the sections highly used by ayu fish Plecoglossus altivelis in a large-scaled turbid river based on environmental DNA analysis-an example in the Tenryu River. Adv. Riv. Eng. 26, 307–312. doi: 10.11532/river.26.0_307
Hara, T., Kuwada, T., Kariya, T. (2008). Effectivenes of stocking with hatchery produced small-sized seedling uninfected with cold-water disease in ayu. Rep. Gifu Pref. Res. Inst. Freshw. Fish. Aquacult. Environ. 53, 1–5.
Hara, T., Kuwada, T., Saitou, K. (2007). The movement of cold-water disease in river instance of stocking of ayu not infected with cold-water disease. Rep. Gifu Pref. Res. Inst. Freshw. Fish. Aquacult. Environ. 52, 1–4.
Iguchi, K., Nishida, M. (2002). Genetic biogeography among insular populations of ayu. Fish. Sci. 1, 345–348. doi: 10.2331/fishsci.68.sup1_345
Iida, Y., Mizokami, A. (1996). Outbreaks of coldwater disease in wild ayu and pale chub. Fish. Pathol. 31, 157–164. doi: 10.3147/jsfp.31.157
Imajoh, M., Otake, K., Kato, Y., Yamamoto, K., Fukunishi, K., Matsuura, H. (2021). An epidemiological survey of Flavobacterium psychrophilum during the spawning season of ayu in the lower reaches of the Monobe River, Kochi Prefecture. Res. Rep. Kochi Univ. 70, 151–160.
Imajoh, M., Sano, A., Yamashita, H., Kato, Y., Yamamoto, K., Tsuji, Y., et al. (2020). Prediction of bacterial cold-water disease epidemics in ayu within the middle and lower reaches of the Shimanto River, Kochi Prefecture, based on quantitative detection of ayu (Plecoglossus altivelis altivelis) environmental DNA and Flavobacterium pshchrophilum in the river water, and surveillance of the infection in spawning ayu. Res. Rep. Kochi Univ. 69, 209–219.
Imajoh, M., Yamasaki, K., Yamashita, H., Monno, S., Kataoka, S., Osaki, Y., et al. (2017). A survey of Flavobacterium pshchrophilum infection in ayu Plecoglossus altivelis in the Kagami River. Fish. Pathol. 52, 141–151. doi: 10.3147/jsfp.52.141
Imura, H. (2003). Development of ayu fry supply and ayu culture industry in Shiga Prefecture. J. Reg. Fish. 53, 25–45. doi: 10.34510/jrfs.53.3_25
Inui, R., Akamatsu, Y., Kono, T., Saito, M., Miyazono, S., Nakao, R. (2021). Spatiotemporal changes of the environmental DNA concentrations of amphidromous fish Plecoglossus altivelis altivelis in the spawning grounds in the Takatsu River, western Japan. Front. Ecol. Evol. 9, 622149. doi: 10.3389/fevo.2021.622149
Inui, R., Akamatsu, Y., Okada, S., Kono, T., Nakao, R. (2020). A comparison of Plecoglossus altivelis spawning season in Shimanto River and Takatsu River using environmental DNA–the effect of water temperature. Adv. Riv. Eng. 26, 343–348. doi: 10.11532/river.26.0_343
Inui, R., Kono, T., Akamatsu, Y., Goto, M., Yamaguchi, K. (2019). Examination of an appropriate method to monitor the spawning ground of Plecoglossus altivelis using environmental DNA–focusing on timely examination. Adv. Riv. Eng. 25, 429–434. doi: 10.11532/river.25.0_429
Inui, R., Takahashi, I., Goto, M., Akamatsu, Y., Kawaguchi, Y. (2018). Monitoring the use of artificial spawning grounds for Plecoglossus altivelis altivelis at the Nahari River–focusing on the comparison between visual survey and environmental DNA analysis. Adv. Riv. Eng. 24, 333–338. doi: 10.11532/river.24.0_333
Izumi, S. (2004). Plasmid profiling of Japanese Flavobacterium psychrophilum isolates. J. Aquat. Anim. Health 16, 99–103. doi: 10.1577/H03-045.1
Izumi, S., Arai, H., Suzuki, K., Aranishi, F. (2019). A novel PCR-RFLP genotyping of Flavobacterium psychrophilum targeting the gyrB region. Fish. Pathol. 54, 37–39. doi: 10.3147/jsfp.54.37
Izumi, S., Aranishi, F., Wakabayashi, H. (2003a). Genotyping of Flavobacterium psychrophilum using PCR-RFLP analysis. Dis. Aquat. Organ. 56, 207–214. doi: 10.3354/dao056207
Izumi, S., Liu, H., Aranishi, F., Wakabayashi, H. (2003b). A novel serotype of Flavobacterium psychrophilum detected using antiserum against an isolate from amago, Oncorhynchus masou rhodurus Jordan & Gilbert, in Japan. J. Fish. Dis. 26, 677–680. doi: 10.1046/j.1365-2761.2003.00502.x
Izumi, S., Ouchi, S., Kuge, T., Arai, H., Mito, T., Fujii, H., et al. (2007). PCR-RFLP genotypes associated with quinolone resistance in isolates of Flavobacterium psychrophilum. J. Fish. Dis. 30, 141–147. doi: 10.1111/j.1365-2761.2007.00797.x
Izumi, S., Wakabayashi, H. (1999). Further study on serotyping of Flavobacterium psychrophilum. Fish. Pathol. 34, 89–90. doi: 10.3147/jsfp.34.89
Katahira, H., Yamamoto, A., Masubuchi, T., Imazu, Y., Yamaguchi, Y., Qatanabe, N., et al. (2019). Preventive measures for ayu cold-water disease, inferred from a questionnaire survey for anglers. Aquacult. Sci. 67, 191–195. doi: 10.11233/aquaculturesci.67.191
Kawashima, N., Minami, S., Suzuki, K., Watanabe, S., Nakayasu, C., Sano, M., et al. (2021). Changes in resistance against bacterial cold-water disease and in leukocyte composition along with sexual maturation in ayu Plecoglossus altivelis. Fish. Pathol. 55, 132–141. doi: 10.3147/jsfp.55.132
Kim, J. H., Gomez, D. K., Nakai, T., Park, S. C. (2010). Plasmid profiling of Flavobacterium psychrophilum isolates from ayu (Plecoglossus altivelis altivelis) and other fish species in Japan. J. Vet. Sci. 11, 85–87. doi: 10.4142/jvs.2010.11.1.85
Kitada, S. (2022). Long-term translocation explains population genetic structure of a recreationally fished iconic species in Japan: Combining current knowledge with reanalysis.. Aquacult. Fish. Fish. 2, 130–145. doi: 10.1002/aff2.34
Kono, T., Akamatsu, Y., Goto, M., Inui, R. (2017). Quantification of Plecoglossus altivelis using environmental DNA and trial of monitoring of downstream migtation. Adv. Riv. Eng. 23, 669–674. doi: 10.11532/river.23.0_669
Kumagai, A., Nawata, A., Ototake, M. (2011). The prevalence of Flavobacterium pshchrophilum among wild ayu in rivers that do not have a history of ayu stocking. Fish. Pathol. 46, 91–94. doi: 10.3147/jsfp.46.91
Kumagai, A., Nawata, A., Taniai, Y. (2010). Monitoring of outbreaks of bacterial cold water disease among ayu in a river where asymptomatic carriers of Flavobacterium pshchrophilum were released. Fish. Pathol. 45, 115–120. doi: 10.3147/jsfp.45.115
Minami, S., Suzuki, K., Watanabe, S., Sano, M., Kato, G. (2018). Maturation-associated changes in the non-specific immune response against Flavobacterium psychrophilum in ayu Plecoglossus altivelis. Fish. Shellfish Immunol. 76, 167–173. doi: 10.1016/j.fsi.2018.03.005
Ministry of Agriculture, Forestry and Fisheries (2008) Ayu reisuibyou taisakukyougikai torimatome. Available at: https://www.maff.go.jp/j/syouan/suisan/suisan_yobo/ayu_reisui/attach/pdf/index-4.pdf (Accessed September 30, 2022).
Miwa, S., Nakayasu, C. (2005). Pathogenesis of experimentally induced bacterial cold water disease in ayu Plecoglossus altivelis. Dis. Aquat. Org. 67, 93–104. doi: 10.3354/dao067093
Miyazaki, T. (2008). Flavobacterium psychrophilum isolated from overwintering ayu Plecoglossus altivelis. Fish. Pathol. 43, 167–169. doi: 10.3147/jsfp.43.167
Murase, A., Ishimaru, T., Ogata, Y., Yamasaki, Y., Kawano, H., Nakanishi, K., et al. (2020). Where is the nursery for amphidromous nekton? abundance and size comparisons of juvenile ayu among habitats and contexts. Estuar. Coast. Shelf. Sci. 241, 106831. doi: 10.1016/j.ecss.2020.106831
Nagai, T., Sakamoto, T. (2006). Susceptibility and immune response to Flavobacterium psychrophilum between different stocks of ayu Plecoglossus altivelis. Fish. Pathol. 41, 99–104.
Nagai, T., Tamura, T., Iida, Y., Yoneji, T. (2004). Differences in susceptibility to Flavobacterium psychrophilum among three stocks of ayu Plecoglossus altivelis. Fish. Pathol. 39, 159–164. doi: 10.3147/jsfp.41.99
Nguyen, P. L., Sudheesh, P. S., Thomas, A. C., Sinnesael, M., Haman, K., Cain, K. D. (2018). Rapid detection and monitoring of Flavobacterium psychrophilum in water by using a handheld, field-portable quantitative PCR system. J. Aquat. Anim. Health 30, 302–311. doi: 10.1002/aah.10046
Nishida, M. (1985). Substantial genetic differentiation in ayu Plecoglossus altivelis of the Japan and Ryukyu islands. Bull. Jpn. Soc Sci. Fish. 51, 1269–1274. doi: 10.2331/suisan.51.1269
Nishida, M. (1986). Geographic variation in the molecular, morphological and reproductive characters of the ayu Plecoglossus altivelis (Plecoglossidae) in the Japan-Ryukyu archipelago. Jpn. J. Ichthyol. 33, 232–248. doi: 10.11369/jji1950.33.232
Shimizu, M., Goda, H., Yamasaki, K., Oshima, S., Ohnishi, K., Osaki, Y., et al. (2016). Draft genome sequence of Flavobacterium psychrophilum strain KTEN-1510 with genotype A/G-C, isolated from an ayu (Plecoglossus altivelis altivelis) in the Kagami River, Kochi, Japan. Genome Announc. 4, e01762–e01715. doi: 10.1128/genomeA.01762-15
Strepparava, N., Wahli, T., Segner, H., Petrini, O. (2014). Detection and quantification of Flavobacterium psychrophilum in water and fish tissue samples by quantitative real time PCR. BMC Microbiol. 14, 105. doi: 10.1186/1471-2180-14-105
Tabata, K. (2004). Relationships of the infectivity of Flavobacterium psychrophilum between native fishes and released ayu Plecoglossus altivelis in a river. Nippon Suisan Gakkaishi 70, 318–323. doi: 10.2331/suisan.70.318
Taniguchi, N. (2002). The damage on annual production of ayu in natural waters caused by stocking seed fish with infection of the bacterial cold water disease. Fish. Pathol. 37, 220. doi: 10.3147/jsfp.37.205
Takamura, K. (2009). Invasive species from the highly endemic fish fauna of Lake Biwa threatening freshwater fish in rivers of the Kanto region. Jpn. J. Limnol. 70, 249–253. doi: 10.3739/rikusui.70.249
Takeuchi, H., Hiratsuka, M., Oinuma, H., Umino, Y., Nakano, D., Iwadare, M., et al. (2016). Infection status of ayu and other wild fish with Flavobacterium psychrophilum and Edwardsiella ictaluri in the Tama River, Japan. Fish. Pathol. 51, 184–193. doi: 10.3147/jsfp.51.184
Tenma, H., Tsunekawa, K., Fujiyoshi, R., Takai, H., Hirose, M., Masai, N., et al. (2021). Spatiotemporal distribution of Flavobacterium psychrophilum and ayu Plecoglossus altivelis in rivers revealed by environmental DNA analysis. Fish. Sci. 87, 321–330. doi: 10.2331/suisan.127
Tsuji, S., Shibata, N., Sawada, H., Watanabe, K. (2022). Differences in the genetic structure between and within two landlocked ayu groups with different migration patterns in Lake Biwa revealed by environmental DNA analysis. environ. DNA. 00, 1–12. doi: 10.1002/edn3.345
Ueki, N., Masunari, N., Fujisawa, K. (1998). On the cold water disease of the ayu Plecoglossus altivelis in the Kagami River 1996 and '97. Bull. Fish. Exp. St. Okayama Pref. 13, 33–36.
Ueki, N., Masunari, N. (2000). On the peculiarity of cold water disease in the ayu Plecoglossus altivelis in a river of Okayama Prefecture. Bull. Fish. Exp. St. Okayama Pref. 15, 47–50.
Urabe, A., Nagaiwa, R., Imajoh, M., Fujiwara-Nagata, E. (2021). Genotype identification of Flavobacterium psychrophilum isolated from ayu Plecoglossus altivelis altivelis in rivers of Kochi Prefecture. Nippon Suisan Gakkaishi 87, 31–39. doi: 10.2331/suisan.20-00022
Wakabayashi, H. (2009) Epizootic outbreaks of bacterial cold water disease among populations of river ayu, Plecoglossus altivelis, in Japan–a review In: Proceedings of the 2nd International Conference on the Members of the Genus Flavobacterium, Paris, France, September 21–23, 2009.
Wakabayashi, H., Toyama, T., Iida, T. (1994). A study on serotyping of Cytophaga psychrophilam isolated from fishes in Japan. Fish. Pathol. 29, 101–104. doi: 10.3147/jsfp.29.101
Yamamoto, M., Sugahara, K., Endo, M., Ishimaru, K., Kato, K. (2015). Epidemiological study of Flavobacterium psychrophilum in ayu Plecoglossus altivelis caught in Lake Biwa and the inflowing rivers from 1998 to 2011. Fish. Pathol. 50, 97–104. doi: 10.3147/jsfp.50.97
Yamashita, H., Wada, T., Kato, Y., Ikeda, T., Imajoh, M. (2019a). Draft genome sequences of three Flavobacterium psychrophilum strains isolated from diseased ayu (Plecoglossus altivelis altivelis) caught at three sites in the Kagami River in Kochi, Japan. Microbiol. Resour. Announc. 8, e00773–e00719. doi: 10.1128/MRA.00773-19
Yamashita, H., Wada, T., Kato, Y., Ikeda, T., Imajoh, M. (2019b). Draft genome sequences of six Flavobacterium psychrophilum strains isolated from dead juvenile ayu (Plecoglossus altivelis altivelis) near the mouth of the Nahari River, Kochi, Japan. Microbiol. Resour. Announc. 8, e00759–e00719. doi: 10.1128/MRA.00759-19
Keywords: Flavobacterium psychrophilum, bacterial cold-water disease, ayu, river, Japan
Citation: Imajoh M (2022) Bacterial cold-water disease in ayu (Plecoglossus altivelis altivelis) inhabiting rivers in Japan. Front. Cell. Infect. Microbiol. 12:1073966. doi: 10.3389/fcimb.2022.1073966
Received: 19 October 2022; Accepted: 05 December 2022;
Published: 15 December 2022.
Edited by:
David Pérez-Pascual, Institut Pasteur, FranceReviewed by:
Goshi Kato, Tokyo University of Marine Science and Technology, JapanCopyright © 2022 Imajoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masayuki Imajoh, bS1pbWFqb2hAa29jaGktdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.