- 1Centre for Research in Ceramics and Composite Materials (CICECO) - Aveiro Institute of Materials & Department of Medical Sciences, University of Aveiro, Aveiro, Portugal
- 2Microbiology Department, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 3School of Allied Health Sciences and World Union for Herbal Drug Discovery [WUHeDD], Walailak University, Nakhon Si Thammarat, Thailand
- 4Institute of Biosciences, Humanities and Exact Sciences (IBILCE), São Paulo State University (UNESP), Sao Jose do Rio Preto, Brazil
Currently, less than 30% of researchers worldwide are women. Long-standing biases and gender stereotypes are discouraging girls and women from moving away from science-related fields, and STEM research in particular. Science and gender equality are, however, essential to ensure sustainable development, as highlighted by UNESCO and the United Nations. To change traditional mindsets, gender equality must be promoted, stereotypes defeated, and girls and women should be encouraged to pursue STEM careers.
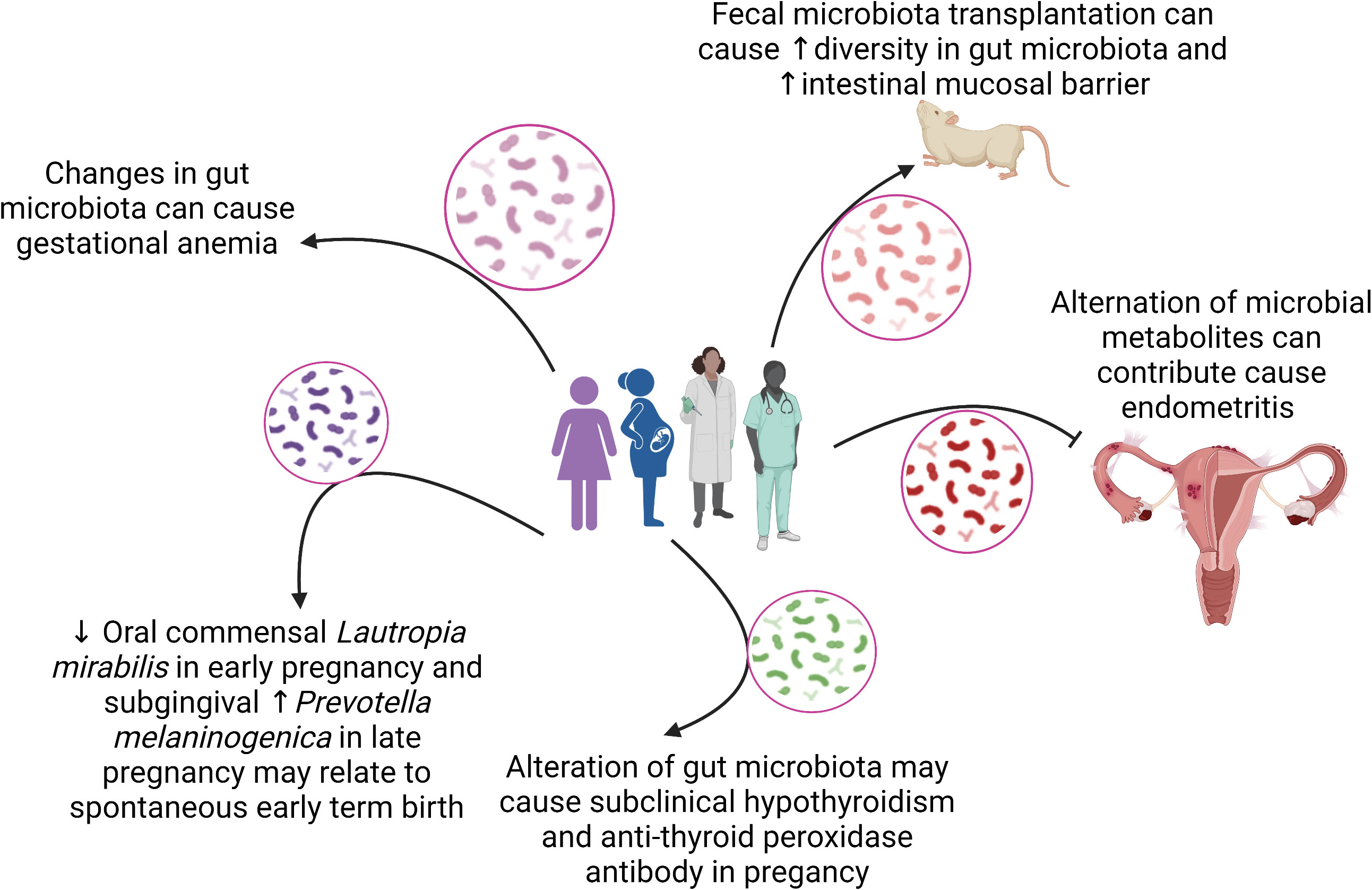
This Research Topic of Frontiers in Cellular and Infection Microbiology celebrates the achievements of women in science and highlights the diversity of research performed across the entire breadth of the microbiome in health and disease research, and presents advances in theory, experimental, and methodology with applications to compelling problems. A total of 14 articles authored by women were published and 97 researchers participated in this Research Topic. Figure 1 summarizes the main findings of the articles in relation to associations between microbiomes and women health.
Emerging findings from animal models and human pregnancy studies have shown that different factors affect maternal and fetal complications, including intrauterine or extrauterine infections, oral or intestinal dysbiosis, and dysregulated immune responses (Romero et al., 2014; MacIntyre et al., 2015; Piler et al., 2017; Fettweis et al., 2019; Serrano et al., 2019; Jehan et al., 2020; Pansieri et al., 2020; Kumar et al., 2021). Two mechanisms have been proposed to explain how gut microbes move into the uterus: 1) gram-negative microbes induce mediators of inflammation, such as prostaglandins that facilitate the ascent of microbes through the vagina; 2) intestinal hyperpermeability during pregnancy promotes bacterial translocation to the uterus or placenta (Edwards et al., 2017). Kumar et al. revised and discussed how pathogens can cross the placental barrier and promote undesirable outcomes in the pregnancy, in the childbirth and the newborn. Also, they suggest that vaginal dysbiosis can induce an abnormal immune response in pregnant women and function as predictor marker for adverse outcomes from congenital infections.
Regarding dysbiosis, some studies have suggested the connection between alterations in the diversity and function of the oral, gut and vaginal microbiota and pregnancy complications (Borgo et al., 2014; Cobb et al., 2017; Fujiwara et al., 2017; Goltsman et al., 2018). For example, oral dysbiosis with decreased Lactobacillus and increased Porphyromonas gingivalis, Neisseria, and Treponema may contribute to preeclampsia, miscarriage, preterm labor and low birth weight (Cobb et al., 2017; Fujiwara et al., 2017; Goltsman et al., 2018; Jang et al., 2021). Interestingly, the presence of Fusobacterium nucleatum in the amniotic fluid of preterm labor pregnant women indicates oral microbes translocation to the placenta (Nuriel-Ohayon et al., 2019; Amir et al., 2020). The gut microbiota of pregnant women with or without complications differs significantly, with a greater abundance of opportunistic pathogens in the first group, impacting maternal metabolism and fetal development (Koren et al., 2012; DiGiulio et al., 2015; Edwards et al., 2017; Jiang et al., 2021).
The composition of the vaginal microbiota during pregnancy changes significantly and the decrease in Lactobacillus in this environment is associated with preterm birth (Aagaard et al., 2012; Di Simone et al., 2020; Marangoni et al., 2021). Zakaria et al. revised several physiological changes in the intestinal, oral and vaginal microbiomes during pregnancy, relating these alterations to mother and fetal health. In addition, researchers finalized by discussing the effect of probiotics to manage the microbiome during pregnancy. Similarly, Dreisbach et al. provided us an updated overview of the impact of obesity on maternal gut microbiota and metabolism thought animal model studies. Researchers also discussed reports on probiotic applications in these settings.
The gestational anemia (GA) is associated with adverse maternal and fetal outcomes, including preterm birth, low birth weight, neonatal and maternal mortality (Guignard et al., 2021; Shi et al., 2022). In a prospective study, Wei et al. evaluated the gut microbiota in GA (n = 156) and in healthy pregnant women (n = 402). Alpha and beta diversities were calculated and researchers showed significant differences between microbial communities in the third trimester, compared to the second trimester, in addition to the differential relative abundance of Megamonas, Veillonella, and Haemophilus when compared to healthy controls. A microbial co-abundance group network predicted upcoming anemia in healthy pregnant women with an area under the ROC curve of 0.7738 (95%CI: 0.7171, 0.8306), suggesting the possibility of identifying women at high-risk for the GA development, and the gut microbiota as a target for therapeutic modulation, through the use of functional foods and probiotics.
In a single-center prospective observational study, Wu et al. evaluated the fecal microbiota and metabolic functions in pregnant women with subclinical hypothyroidism, with (n = 75) or without (n = 90) positivity for anti-thyroid peroxidase antibodies (TPO). The group was also subdivided according to the levothyroxine (LT4) therapy (no treatment vs. low-dose LT4 vs. high-dose LT4). Researchers did not find significant differences in microbiota richness and evenness when comparing TPO positive and TPO negative women, however, they observe significant microbial diversity between TPO+ and TPO- in the high-dose LT4 group.
Using LEfSe analysis, authors detected a microbial signature related to the LT4 replacement, such as decreased species of Ruminococcus and Bacteroides massiliensis in low-and high-dose LT4 groups, respectively, as well as nineteen differential metabolic functions involved in lipid and amino acid metabolism discriminating TPO+ and TPO- pregnant women in the second trimester. The subclinical hypothyroidism in TPO+ pregnant women was normally associated with miscarriage, preterm birth, pre-eclampsia and gestational diabetes. Since the intestinal microbiota can affect the synthesis and functions of thyroid hormones (Knezevic et al., 2020; Fenneman et al., 2020; Bargiel et al., 2021), the study suggests microbiota modulation as a therapeutic option to treat TPO+ pregnant women.
In a very interesting longitudinal descriptive study, Yang et al. evaluated the subgingival microbiota in 59 pregnant women, from 8 to 14 weeks and from 24 to 30 weeks, correlating this data with gestational age and birth outcomes. No significant differences were observed in the richness, evenness and diversity of microbiota between 8 to 14 and 24 to 30 weeks of gestation; however, in the latter group, alpha and beta diversities were different between women who had early term births and those who had delivered at term.
Interestingly, the top twenty taxa represented in the subgingival microbiota of participants throughout pregnancy include bacteria involved in the progression to periodontal disease, including Porphyromonas gingivalis, Tannerella forsythia, Prevotella, and Campylobacter species. The oral dysbiosis is associated with periodontitis in pregnant women, and this disease is an important risk factor for preterm births (Jang et al., 2021; Saadaoui et al., 2021). In the present study, researchers identified that a decrease in Lautropia mirabilis at 8 to 14 weeks and an increase in Prevotella melaninogenica at 24 to 30 weeks of pregnancy were both associated with spontaneous early term birth and represent an important target for future studies.
The dietary habits during intrauterine life and after birth have an important impact on the health of the newborn´s microbiota and on the establishment of the adulthood microbiota later in life (Cadenas-Sanchez et al., 2017; Singh and Mittal, 2020). The gut microbiota colonization begins in the uterus and at birth, acquiring microbes from the mother and the environment. The main factors for the microbiota composition in early life are delivery type (normal or C-section), breastfeeding, time of introduction of solid foods, antibiotic use, and hygiene conditions (Martin et al., 2016; Le Doare et al., 2018). In a cross-sectional study in Brazil, Freitas et al. examined early-life data, body mass index (BMI), and collected fecal samples from 114 women with a mean age of 28 years and a mean BMI of 24.5 kg/m2. Beta diversity analysis of the microbiota showed two microbiota profiles, one driven by the Blautia genus (n = 56) and another driven by Prevotella (n = 58). The breastfeeding and adequate nutritional status were positively correlated with increased abundance of Blautia, Anaerostipes, and Lachnoclostridium. The exclusive breastfeeding (≥ 6 months) is associated with Blautia-driven profile of healthy women, showing the importance of early-life events and exclusive breastfeeding for infant gut colonization and health later in life.
The human milk oligosaccharides (HMO) are indigestible glycans that acts in the microbiota establishment and immunity maturation in the infant gut, and HMO are known to have bifidogenic effects (Bode, 2012; Wiciński et al., 2020). Kijner et al. investigated the mechanisms of HMO utilization by infant gut microbes by isolating Bacteroides strains from fecal samples, and testing them with the six most common HMO, 2′-fucosyllactose (2’-FL), difucosyllactose (DFL), 3′-sialyllactose (3’-SL), 6′-sialyllactose (6’-SL), lacto-N-tetraose (LNT), and lacto-N-neotetraose (LNnT). Differential RNA sequencing analysis showed that Bacteroides dorei presents an important glycoside hydrolase (GH) activity in break carbon bonds from HMO in vitro. Seventeen GH families were upregulated when cultivated with 2’-FL, 21 in DFL, 19 in 3’-SL, 23 in 6’-SL, 15 in LNT, and 18 in LNnT (log2 fold change > 1, p adj < 0.05), expanding our knowledge on the microorganisms involved in the HMO digestion, in addition to the already known Bifidobacterium species.
Regarding women’s health, in a multicenter, randomized, blinded clinical trial, Li et al. evaluated women with mixed aerobic vaginitis with bacterial vaginosis who received an effervescent suppository (n = 39) or clindamycin (n = 41). Women aged 20 to 55 years were randomized to receive either the suppository or clindamycin, vaginal swabs were collected at three time points (V1: -2~0 days; V2: 15-17 days; V3: 40 ± 3 days), and the DNA sequenced by Accurate 16S absolute quantification. Before treatment (V1), the pathogenic species Gardnerella vaginalis, Atopobium vaginae, Sneathia amnii, and Prevotella bivia were found in both groups.
After treatment, according to Shannon and Simpson indexes, the microbiota diversity significantly decreased in V2 in both groups (p < 0.001), and slightly increased in V3 in the suppository group. The absolute abundance of Lactobacillus increased in the suppository group compared to untreated patients, and the genera Gardnerella, Prevotella, and Atopobium were enriched in V3 in the clindamycin and suppository groups. Authors concluded that the effervescent suppository is effective to treat women with mixed aerobic vaginitis with bacterial vaginosis by restoring the vaginal microbiota.
Recent studies have investigated the relationship between the vaginal microbiota and cervical cancer (CC) to better understand the involvement of dysbiosis in the establishment, progression, or remission of the disease (Bray et al., 2018; Baezconde-Garbanati et al., 2019; Martínez-Rodríguez et al., 2021). In an observational cross-sectional study in Mexico, Manzanares-Leal et al. included 120 women aged between 21 to 71 years, 60 with advanced CC and 60 without the disease. Cervicovaginal swabs were collected to obtain culturable aerobic microbes, strains were evaluated by PCR-RFLP and identified by 16S sequencing. Researchers detected a specific microbiota associated with advanced stages of CC, including Streptococcus urinalis, Escherichia coli, Bacillus safensis, Bacillus malikii, Corynebacterium jeikeium, Corynebacterium striatum, and Lactobacillus rhamnosus. They concluded that there is no causal association between the aerobic vaginitis and cervicovaginal neoplasia, but proposed that vaginitis is fundamental for the progression of preneoplastic lesions to cancer (Vieira-Baptista et al., 2016; Plisko et al., 2021). Dong et al. contributed to a research article that aimed to assess the effects of acute endometritis on intestinal microbes and their metabolites. Endometritis is generally caused by bacterial infections, and accumulating evidence has shown that the occurrence of disease may be related to the gut microbiota (Plottel and Blaser, 2011; Borella et al., 2021). Moreover, the progression of diseases has previously been shown to change the composition and diversity of the intestinal microbiota and associated metabolites. The authors used a mouse model of endometritis that involves an intrauterine administration of lipopolysaccharide (LPS). Using 16S rRNA gene sequencing and liquid chromatogram-mass spectrometry, they found that the relative abundance of some members of the microbiome was changed and resulted in the reduction of beneficial microorganisms in the intestinal tract.
At the same time, acute endometritis increased the relative abundance of pathogenic bacteria, altered the concentration of intestinal metabolites, and affected biological oxidation, energy metabolism, and biosynthesis of primary bile acids. Thus, the findings of this study have the potential to provide new strategies for the diagnosis of acute endometritis.
Amyotrophic lateral sclerosis (ALS) is a heterogeneous neuromuscular disorder with progressive degeneration of the upper and lower motor neurons (Hardiman, 2021). A combination of genetic and environmental factors, as well as age‐related dysfunctions, are hypothesized to be involved in the ALS development (Masrori and Van Damme, 2020). Women are more affected by the disease with an estimation risk of 1:400 compared to 1:350 in men (Ryan et al., 2019; Masrori and Van Damme, 2020). Martin et al. contributed a review article that provides a comprehensive overview of the role that the microbiome may play in the ALS pathogenesis of. The authors explore existing evidence of gastrointestinal symptoms and microbial alterations in ALS pathogenesis from human and animal studies and discuss the possible therapeutic approaches to target specific diets, metabolites, and intestinal microbiome in ALS patients. They highlight innovative strategies for accurate diagnosis and better treatment for this challenging disease.
The last two articles discussed important aspects of fecal microbiota transplantation (FMT) in sepsis and bioinformatics tools for use in predictive models. Gai et al. reported that the FMT in sepsis model induced by cecal ligation and puncture is able to reestablish the gut microbiota diversity and decrease mortality by modulating the inflammatory response, restoring the epithelial barrier and function by upregulating the expression of tight junction proteins. Dahan et al. presented the EasyMap, an interactive online tool allowing for (1) running multiple multivariate linear regression models, with the same features and metadata; (2) visualizing the associations between microbial features and clinical metadata found in each model; and (3) comparing between the various models to identify critical metadata variables and select the optimal model. The EasyMap provides a side-by-side visualization of association results across the various models, each with additional metadata variables, enabling us to evaluate the impact of each metadata variable on the associated feature.
Collectively, the articles in this Research Topic demonstrated important aspects of the role played by the microbiomes from different sites (oral, gut and vagina) for women´s health, for a succeed pregnancy and fetal development, and for the prevention and treatment of diseases by using strategic approaches to modulate the microbiota, including nutritional interventions, probiotics and fecal microbiota transplantation. Furthermore, studies aimed at developing software for more accurate analyzes and prediction models are also necessary for the evolution of the microbiome field.
Author contributions
GO and ML wrote the initial draft of the editorial, MP and VN revised the manuscript, and all authors approved the final version of the editorial.
Acknowledgments
We would like to thank Dr. Alok K. Paul, University of Tasmania-Australia for assistance of an infographic using Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aagaard, K., Riehle, K., Ma, J., Segata, N., Mistretta, T. A., Coarfa, C., et al. (2012). A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One 7, e36466. doi: 10.1371/journal.pone.0036466
Amir, M., Brown, J. A., Rager, S. L., Sanidad, K. Z., Ananthanarayanan, A., Zeng, M. Y. (2020). Maternal microbiome and infections in pregnancy. Microorganisms. 8, 1996. doi: 10.3390/microorganisms8121996
Baezconde-Garbanati, L., Agurto, I., Gravitt, P. E., Luciani, S., Murphy, S., Ochoa, C., et al. (2019). Barriers and innovative interventions for early detection of cervical cancer. Salud Publica Mex. 61, 456–460. doi: 10.21149/10425
Bargiel, P., Szczuko, M., Stachowska, L., Prowans, P., Czapla, N., Markowska, M., et al. (2021). Microbiome metabolites and thyroid dysfunction. J. Clin. Med. 10, 3609. doi: 10.3390/jcm10163609
Bode, L. (2012). Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22, 1147–1162. doi: 10.1093/glycob/cws074
Borella, F., Carosso, A. R., Cosma, S., Preti, M., Collemi, G., Cassoni, P., et al. (2021). Gut microbiota and gynecological cancers: A summary of pathogenetic mechanisms and future directions. ACS Infect. Dis. 7, 987–1009. doi: 10.1021/acsinfecdis.0c00839
Borgo, P. V., Rodrigues, V. A. A., Feitosa, A. C. R., Xavier, K. C. B., Avila-Campos, M. J. (2014). Association between periodontal condition and subgingival microbiota in women during pregnancy: a longitudinal study. J. Appl. Oral. Sci. 22, 528–533. doi: 10.1590/1678-775720140164
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Cadenas-Sanchez, C., Henriksson, P., Henriksson, H., Delisle Nyström, C., Pomeroy, J., Ruiz, J. R., et al. (2017). Parental body mass index and its association with body composition, physical fitness and lifestyle factors in their 4-year-old children: results from the MINISTOP trial. Eur. J. Clin. Nutr. 71, 1200–1205. doi: 10.1038/ejcn.2017.62
Cobb, C. M., Kelly, P. J., Williams, K. B., Babbar, S., Angolkar, M., Derman, R. J. (2017). The oral microbiome and adverse pregnancy outcomes. Int. J. Womens Health 9, 551–559. doi: 10.2147/IJWH.S142730
DiGiulio, D. B., Callahan, B. J., McMurdie, P. J., Costello, E. K., Lyell, D. J., Robaczewska, A., et al. (2015). Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. U S A. 112, 11060–11065. doi: 10.1073/pnas.1502875112
Di Simone, N., Santamaria Ortiz, A., Specchia, M., Tersigni, C., Villa, P., Gasbarrini, A., et al. (2020). Recent insights on the maternal microbiota: Impact on pregnancy outcomes. Front. Immunol. 11. doi: 10.3389/fimmu.2020.528202
Edwards, S. M., Cunningham, S. A., Dunlop, A. L., Corwin, E. J. (2017). The maternal gut microbiome during pregnancy. MCN Am. J. Matern Child Nurs. 42, 310–317. doi: 10.1097/NMC.0000000000000372
Fenneman, A. C., Rampanelli, E., Yin, Y. S., Ames, J., Blaser, M. J., Fliers, E., et al. (2020). Gut microbiota and metabolites in the pathogenesis of endocrine disease. Biochem. Soc. Trans. 48, 915–931. doi: 10.1042/BST20190686
Fettweis, J. M., Serrano, M. G., Brooks, J. P., Edwards, D. J., Girerd, P. H., Parikh, H. I., et al. (2019). The vaginal microbiome and preterm birth. Nat. Med. 25, 1012–1021. doi: 10.1038/s41591-019-0450-2
Fujiwara, N., Tsuruda, K., Iwamoto, Y., Kato, F., Odaki, T., Yamane, N., et al. (2017). Significant increase of oral bacteria in the early pregnancy period in Japanese women. J. Investig. Clin. Dent. 8, e12189. doi: 10.1111/jicd.12189
Goltsman, D. S. A., Sun, C. L., Proctor, D. M., DiGiulio, D. B., Robaczewska, A., Thomas, B. C., et al. (2018). Metagenomic analysis with strain-level resolution reveals fine-scale variation in the human pregnancy microbiome. Genome Res. 28, 1467–1480. doi: 10.1101/gr.236000.118
Guignard, J., Deneux-Tharaux, C., Seco, A., Beucher, G., Kayem, G., Bonnet, M. P., et al. (2021). Gestational anaemia and severe acute maternal morbidity: a population-based study. Anaesthesia. 76, 61–71. doi: 10.1111/anae.15222
Hardiman, O. (2021). Major advances in amyotrophic lateral sclerosis in 2020. Lancet Neurol. 20, 14–15. doi: 10.1016/S1474-4422(20)30447-6
Jang, H., Patoine, A., Wu, T. T., Castillo, D. A., Xiao, J. (2021). Oral microflora and pregnancy: a systematic review and meta-analysis. Sci. Rep. 11, 16870. doi: 10.1038/s41598-021-96495-1
Jehan, F., Sazawal, S., Baqui, A. H., Nisar, M. I., Dhingra, U., Khanam, R., et al. (2020). Multiomics characterization of preterm birth in low- and middle-income countries. JAMA Netw. Open 3, e2029655. doi: 10.1001/jamanetworkopen.2020.29655
Jiang, G., Zhou, Z., Li, X., Qian, Y., Wang, K. (2021). The gut microbiome during pregnancy. Matern Fetal Med. 1–9 doi: 10.1097/FM9.0000000000000091
Knezevic, J., Starchl, C., Tmava Berisha, A., Amrein, K. (2020). Thyroid-Gut-Axis: How does the microbiota influence thyroid function? Nutrients. 12, 1769. doi: 10.3390/nu12061769
Koren, O., Goodrich, J. K., Cullender, T. C., Spor, A., Laitinen, K., Bäckhed, H. K., et al. (2012). Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 150, 470–480. doi: 10.1016/j.cell.2012.07.008
Kumar, M., Murugesan, S., Singh, P., Saadaoui, M., Elhag, D. A., Terranegra, A., et al. (2021). Vaginal microbiota and cytokine levels predict preterm delivery in Asian women. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.639665
Le Doare, K., Holder, B., Bassett, A., Pannaraj, P. S. (2018). Mother’s milk: A purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 9. doi: 10.3389/fimmu.2018.00361
MacIntyre, D. A., Chandiramani, M., Lee, Y. S., Kindinger, L., Smith, A., Angelopoulos, N., et al. (2015). The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 5, 8988. doi: 10.1038/srep08988
Marangoni, A., Laghi, L., Zagonari, S., Patuelli, G., Zhu, C., Foschi, C., et al. (2021). New insights into vaginal environment during pregnancy. Front. Mol. Biosci. 8. doi: 10.3389/fmolb.2021.656844
Martínez-Rodríguez, F., Limones-González, J. E., Mendoza-Almanza, B., Esparza-Ibarra, E. L., Gallegos-Flores, P. I., Ayala-Luján, J. L., et al. (2021). Understanding cervical cancer through proteomics. Cells. 10, 1854. doi: 10.3390/cells10081854
Martin, R., Makino, H., Cetinyurek Yavuz, A., Ben-Amor, K., Roelofs, M., Ishikawa, E., et al. (2016). Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One 11, e0158498. doi: 10.1371/journal.pone.0158498
Masrori, P., Van Damme, P. (2020). Amyotrophic lateral sclerosis: a clinical review. Eur. J. Neurol. 27, 1918–1929. doi: 10.1111/ene.14393
Nuriel-Ohayon, M., Neuman, H., Ziv, O., Belogolovski, A., Barsheshet, Y., Bloch, N., et al. (2019). Progesterone increases bifidobacterium relative abundance during late pregnancy. Cell Rep. 27, 730–736.e3. doi: 10.1016/j.celrep.2019.03.075
Pansieri, C., Pandolfini, C., Clavenna, A., Choonara, I., Bonati, M. (2020). An inventory of European birth cohorts. Int. J. Environ. Res. Public Health 17, 3071. doi: 10.3390/ijerph17093071
Piler, P., Kandrnal, V., Kukla, L., Andrýsková, L., Švancara, J., Jarkovský, J., et al. (2017). Cohort profile: The European longitudinal study of pregnancy and childhood (ELSPAC) in the Czech republic. Int. J. Epidemiol. 46, 1379–1379f. doi: 10.1093/ije/dyw091
Plisko, O., Zodzika, J., Jermakova, I., Pcolkina, K., Prusakevica, A., Liepniece-Karele, I., et al. (2021). Aerobic vaginitis-underestimated risk factor for cervical intraepithelial neoplasia. Diagnostics (Basel). 11, 97. doi: 10.3390/diagnostics11010097
Plottel, C. S., Blaser, M. J. (2011). Microbiome and malignancy. Cell Host Microbe 10, 324–335. doi: 10.1016/j.chom.2011.10.003
Romero, R., Hassan, S. S., Gajer, P., Tarca, A. L., Fadrosh, D. W., Bieda, J., et al. (2014). The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2, 18. doi: 10.1186/2049-2618-2-18
Ryan, M., Heverin, M., McLaughlin, R. L., Hardiman, O. (2019). Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol. 76, 1367–1374. doi: 10.1001/jamaneurol.2019.2044
Saadaoui, M., Singh, P., Al Khodor, S. (2021). Oral microbiome and pregnancy: A bidirectional relationship. J. Reprod. Immunol. 145, 103293. doi: 10.1016/j.jri.2021.103293
Serrano, M. G., Parikh, H. I., Brooks, J. P., Edwards, D. J., Arodz, T. J., Edupuganti, L., et al. (2019). Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 25, 1001–1011. doi: 10.1038/s41591-019-0465-8
Shi, H., Chen, L., Wang, Y., Sun, M., Guo, Y., Ma, S., et al. (2022). Severity of anemia during pregnancy and adverse maternal and fetal outcomes. JAMA Netw. Open 5, e2147046. doi: 10.1001/jamanetworkopen.2021.47046
Singh, A., Mittal, M. (2020). Neonatal microbiome - a brief review. J. Matern Fetal Neonatal Med. 33, 3841–3848. doi: 10.1080/14767058.2019.1583738
Vieira-Baptista, P., Lima-Silva, J., Pinto, C., Saldanha, C., Beires, J., Martinez-de-Oliveira, J., et al. (2016). Bacterial vaginosis, aerobic vaginitis, vaginal inflammation and major pap smear abnormalities. Eur. J. Clin. Microbiol. Infect. Dis. 35, 657–664. doi: 10.1007/s10096-016-2584-1
Keywords: women in science, pregnancy, women health, microbiome, dysbiosis
Citation: Pereira MdL, Levy M, Nissapatorn V and de Oliveira GLV (2022) Editorial: Women in microbiome in health and disease 2021. Front. Cell. Infect. Microbiol. 12:1054190. doi: 10.3389/fcimb.2022.1054190
Received: 26 September 2022; Accepted: 28 September 2022;
Published: 11 October 2022.
Edited and Reviewed by:
Xin Xu, Sichuan University, ChinaCopyright © 2022 Pereira, Levy, Nissapatorn and de Oliveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gislane Lelis Vilela de Oliveira, Z2lzbGFuZS5sZWxpc0B1bmVzcC5icg==; Veeranoot Nissapatorn, bmlzc2FwYXRAZ21haWwuY29t
†These authors have contributed equally to this work
 Maria de Lourdes Pereira
Maria de Lourdes Pereira Maayan Levy
Maayan Levy Veeranoot Nissapatorn
Veeranoot Nissapatorn Gislane Lelis Vilela de Oliveira
Gislane Lelis Vilela de Oliveira