
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 14 November 2022
Sec. Molecular Bacterial Pathogenesis
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.1047351
This article is part of the Research Topic Aquaculture Animal Diseases: Pathogens and Control View all 17 articles
 Mingyang Xue1
Mingyang Xue1 Yeying Wu2,3
Yeying Wu2,3 Yizhan Hong4
Yizhan Hong4 Yan Meng1
Yan Meng1 Chen Xu1
Chen Xu1 Nan Jiang1
Nan Jiang1 Yiqun Li1
Yiqun Li1 Wenzhi Liu1
Wenzhi Liu1 Yuding Fan1
Yuding Fan1 Yong Zhou1*
Yong Zhou1*The influence of dietary probiotic Bacillus amyloliquefaciens on the growth performance, digestive enzyme activity, immune parameters and disease resistance of yellow catfish (Pelteobagrus fulvidraco) was evaluated. Commercial diet (C) or diet containing 106 cfu/g B. amyloliquefaciens (T) was fed for 4 weeks, and final weight (FW), specific growth rate (SGR) and feed conversion ratio (FCR) were improved (p<0.05) in the T group. Dietary B. amyloliquefaciens increased protease and amylase activities in the digestive tract after 2 and 4 weeks, respectively. Respiratory burst (RB), plasma lysozyme (LZM) activity, total antioxidant capacity (T-AOC) and superoxide dismutase (SOD) activity were also elevated (p<0.05). Immune-related genes signal transducer and activator of transcription 1 (STATA-1), immunoglobulin M (IgM) and C-type lectin (CTL) were upregulated (p<0.05), but interleukin-1 β (IL-1β) was not (p >0.05). Intestinal microbiota analysis showed that the community structure was significantly different between the two groups; the relative abundance of Cetobacterium was increased but Plesiomonas was decreased in T. Moreover, challenge tests showed that the resistance of fish fed B. amyloliquefaciens against Aeromonas veronii and Edwardsiella ictaluri was significantly enhanced (p<0.05). In conclusion, dietary supplementation of B. amyloliquefaciens can effectively improve the growth performance, digestive enzyme activity, immune responses, intestinal microbiota composition and disease resistance of yellow catfish.
Aquaculture is a rapidly growing industry, but rapid expansion of fish farming has resulted in the increased occurrence of diseases, and outbreaks have hindered aquaculture development (Bondad-Reantaso et al., 2005). There is increasing interest in the use of functional feeds that contain natural supplements such as probiotics and prebiotics to improve fish growth and health (Akhter et al., 2015; Huang et al., 2015; Dawood and Koshio, 2016). Diets supplemented with probiotics have been studied in many aquatic animals, such as tilapia (Rmm et al., 2020), shrimp (Sadat Hoseini Madani et al., 2018), grass carp (Liu et al., 2018; Qi et al., 2020) and crayfish (Xu et al., 2021), showing positive effects on improving water quality, increasing nutrient utilisation, enhancing immune status and disease resistance (Kiron, 2012; Yang et al., 2015; Hoseinifar et al., 2016). The most widely-investigated probiotics include Bacillus sp., Lactobacillus sp. and Saccharomyces cerevisiae (Newaj-Fyzul et al., 2014; Akhter et al., 2015). Bacillus is a genus of Gram-positive, aerobic or facultative anaerobic, heat-stable spore-forming bacteria (Nakagawa et al., 2003; Hong et al., 2005). Bacillus sp. exhibit strong tolerance to environmental changes and antibacterial activities. These bacteria can also facilitate digestion, promote immune responses, and help maintain a balanced intestinal microbiota in hosts (Casula et al., 2002; Reda et al., 2017; Mingmongkolchai and Panbangred, 2018).
Yellow catfish (Pelteobagrus fulvidraco), a small teleost fish with exceptional flesh quality and high commercial value, is widely cultured in China. At present, yellow catfish ranks second among freshwater fish produced in 27 provinces of China (Wang et al., 2022), with yields reaching 5.8×105 tons in 2021 (Fisheries and Fisheries Administration Bureau of Ministry of Agriculture and Industry, 2022). However, under intensive aquaculture conditions, bacterial infectious diseases occur frequently, resulting in severe economic losses (Ye et al., 2009). Aeromonas veronii and Edwardsiella ictaluri are two pathogenic bacteria in fish and could result in considerable economic losses in yellow catfish aquaculture (Zeng et al., 2021). A. veronii is a severe causative agent of ascites disease in yellow catfish (Zhou et al., 2019). E. ictaluri can cause “head perforation disease” in yellow-head catfish (Liu et al., 2010). Nevertheless, studies on using probiotics and prebiotics as dietary supplements for yellow catfish are lacking. Only Ming et al. (2016) reported studies of Taurine as a prebiotic in growth and immunity of yellow catfish (Ming et al., 2016).
The objective of the present study was to evaluate the probiotic properties of B. amyloliquefaciens on yellow catfish. Growth performance, digestive enzyme activities, immune responses, intestinal microbiota composition and disease resistance of yellow catfish were investigated. The results provide a solid base for future development of the yellow catfish farming industry.
Yellow catfish weighing 21 ± 1.2 g were obtained from a commercial yellow catfish farm located in Hubei province, China, and transported alive to Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Wuhan, China. Fish were adapted to the aquarium rearing conditions (400 L) for 14 days and fed a commercial fish feed (Tongwei, Chengdu, China). Water temperature (26 ± 0.5°C) was measured regularly during the experiment. Tanks were continuously aerated and 30% of water was renewed daily. All experimental procedures were conducted according to the guidelines of the Animal Experimental Ethical Inspection of Laboratory Animal Centre, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI2022-zhouyong-06).
The B. amyloliquefaciens strain was isolated from yellow catfish intestine and identified by cluster analysis of the 16S rDNA sequence (SRA: SRR21820145). The B. amyloliquefaciens strain was selected as a potential probiotic due to its antagonistic activity against the pathogenic strains Aeromonas veronii and Edwardsiella ictalurid (Qi et al., 2020), visualised as inhibition circles on Luria-Bertani (LB) plates (Figure 1). Prior to experiments, the bacterial strain was inoculated in LB broth medium and cultured at 30°C with shaking at 180 rpm for 24 h. Stock cultures were stored in LB broth medium containing sterile 30% (v/v) glycerol at -80°C.
Commercial feed was used as basal diet. To prepare the supplementary diet, bacterial cells were harvested after overnight culture by centrifugation at 4°C for 15 min at 6000 g and washed three times with sterile phosphate-buffered saline (PBS, pH 7.4). Subsequently, cells were re-suspended in PBS at 106/mL and mixed with basal diet. Control diet was prepared by adding the same volume of PBS to the basal diet. Diets were freeze-dried and stored at 4°C for further use (Gobi et al., 2018). Experimental diets were prepared every week to ensure the vitality of probiotics in diets.
After an acclimatisation period, fish were randomly divided into control (C, fed basal diet) and treatment (T, fed B. amyloliquefaciens supplementation diet) groups. These groups were distributed in six tanks with 60 fish per tank. Each group included three replicates. Basal diet and B. amyloliquefaciens-enriched diet were given twice a day at 2% body weight for the experimental period (4 weeks).
During the 2nd and 4th week of feeding, nine fish from each tank were randomly collected, anaesthetised with MS222 (Sigma Aldrich, St. Louis, U.S.A) at 100 mg/L, blood was collected from the caudal vein of each fish with a 1 mL syringe, and placed in plastic Eppendorf tubes containing anticoagulant solution (heparin). The tubes were kept at 4°C overnight and centrifuged at 3000 g for 10 min and the obtained serum was stored at -80°C. During the 2nd and 4th week of feeding, intestine samples from three fish per group were collected to determine digestive enzyme activity, and midgut tissue from three fish per group was obtained to determine the relative mRNA levels of immune-related genes. During the 4th week of feeding, intestine tissue was flash-frozen in liquid nitrogen and stored at -80°C for Illumina sequencing.
Weight was measured at the beginning and end of the experiment. The growth performance of fish was calculated as follows:
where Wt and W0 are the final and initial weight, respectively, t is the duration of feeding (in days), and FI is feed intake.
Intestine samples were homogenised in PBS at a ratio of 1:9 (w/v) using a glass homogeniser at 4°C. The homogenate was centrifuged at 5000 × g for 20 min at 4°C to remove tissue debris (Safari et al., 2014). The supernatant was kept on ice and used within 24 h. Aliquots of the supernatant, designated as the crude extract, were used to estimate protease and amylase activities.
Total protein content was determined using bovine serum albumin as the standard according to the Bradford method (Bradford, 1976). Protease, amylase and lipase activities were assessed with a rapid colorimetric kit described by the manufacturer’s instructions (Jiancheng, Nanjing, China).
Generation of intracellular superoxide radicals by macrophages was determined from the reduction of nitro-blue tetrazolium (Solarbio, Beijing, China) as described previously (Hong et al., 2006), with absorption measured at 620 nm using KOH/dimethylsulphoxide (DMSO) as the blank.
Plasma lysozyme (LZM) activity, total antioxidant capacity (T-AOC) and superoxide dismutase (SOD) activity were measured using appropriate kits according to the manufacturer’s instructions (Jiancheng, Nanjing, China).
Total RNA was extracted from tissues using TRIzol reagent (Invitrogen, Carlsbad, USA). The quality and purity of RNA were assessed by spectrophotometry, and 260:280 ratios were 1.8−2.0. Total RNA was reverse-transcribed into cDNA using a RevertAid First Strand cDNA Synthesis Kit (TaKaRa, Dalian, China) following the manufacturer’s instructions. Real-time quantitative PCR was performed using a SYBR Premix Ex Taq Perfect real-time Kit (TaKaRa) and a CFX96 Real-Time PCR Detection System (Bio-Rad, Berkeley, USA). The β-actin housekeeping gene served as an internal reference. Specific primers are listed in Table 1. In all cases, each PCR was performed with triplicate samples. The relative quantification of gene expression among groups was analysed by the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Bacterial genomic DNA was extracted using a Bacterial DNA Kit (Omega, Norcross, USA) following the manufacturer’s instructions. The V3−V4 region of the bacterial 16S rRNA gene was amplified by PCR using specific primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) with barcodes in 50 μL reactions. Thermal cycling consisted of initial denaturation at 95°C for 1 min, followed by 30 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, and a final extension at 72°C for 10 min. After separating by agarose gel electrophoresis, samples were assessed on an Illumina MiSeq PE250 high-throughput sequencing platform. All sequence reads were quality-filtered and assembled using the Mothur software package (Schloss et al., 2009). Reads were clustered into operational taxonomic units (OTUs) at 97% identity by RDP Classifier algorithm (http://rdp.cme.msu.edu/) (Wang et al., 2007). The abundance of corresponding OTUs in each region was calculated at the genus levels. Abundance-based coverage estimator (ACE), Chao, Shannon and Simpson alpha-diversity indices were analysed by Mothur (version v.1.30) (Elizabeth et al., 2009). Rank - abundance curve was performed with R statistical software (http://www.r-project.org/) with the aid of the packages Fields and Vegan (Bates et al., 2013).
Strains A. veronii and E. ictaluri pathogenic to yellow catfish were used in challenge tests. After the feeding trial, fish in each diet group were assigned to six tanks, fish in three tanks for each group were intraperitoneally injected with 0.2 mL A. veronii (107 cfu/mL), and fish in other tanks were intraperitoneally injected with 0.2 mL E. ictaluri (107 cfu/mL). Infected fish were observed daily and mortality was recorded for 10 days. All dead fish were examined bacteriologically to determine the presence of the pathogen.
Data were analysed by one-way analysis of variance (ANOVA) and expressed as the arithmetic mean ± standard deviation (SD). Survival curves were estimated by the Mann-Whitney U test and Kaplan-Meier method (Bland and Altman, 1998). Differences were determined by Tukey’s test in SPSS statistical software (SPSS Inc., USA) with p-values<0.05 indicating significance.
Weight gain and specific growth rate of fish fed diet supplemented with B. amyloliquefaciens were significantly higher (p<0.05) than those fed control diet (Table 2). FCR was significantly higher in fish fed control diet (p<0.05; Table 2).
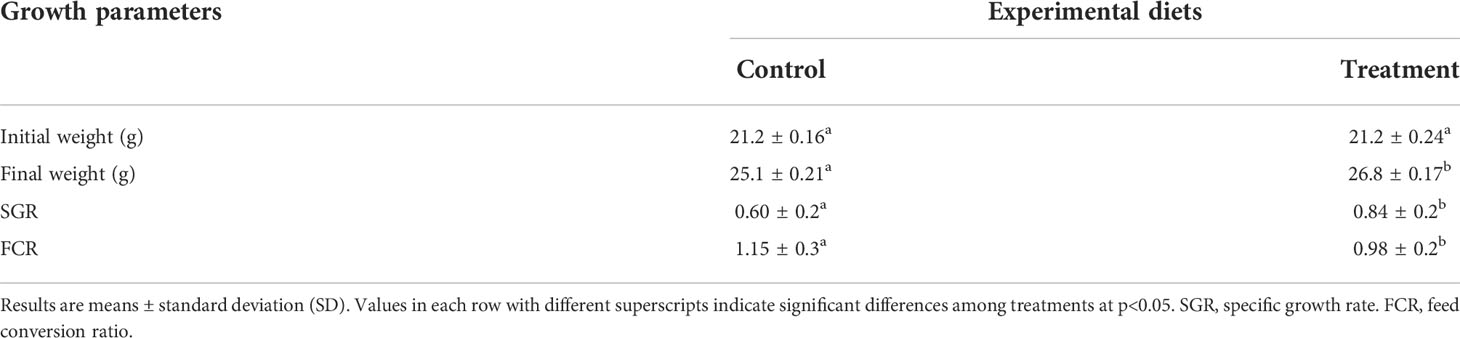
Table 2 Growth parameters of yellow catfish after 4 weeks feeding with control diet (Control, C) and diet supplemented with B. amyloliquefaciens (Treatment, T).
Amylase and protease activities in the intestine of yellow catfish fed with B. amyloliquefaciens were increased significantly (p<0.05) compared with fish fed control diet (Figure 2). Amylase and lipase activities showed no significant differences between control and treatment groups (p >0.05).
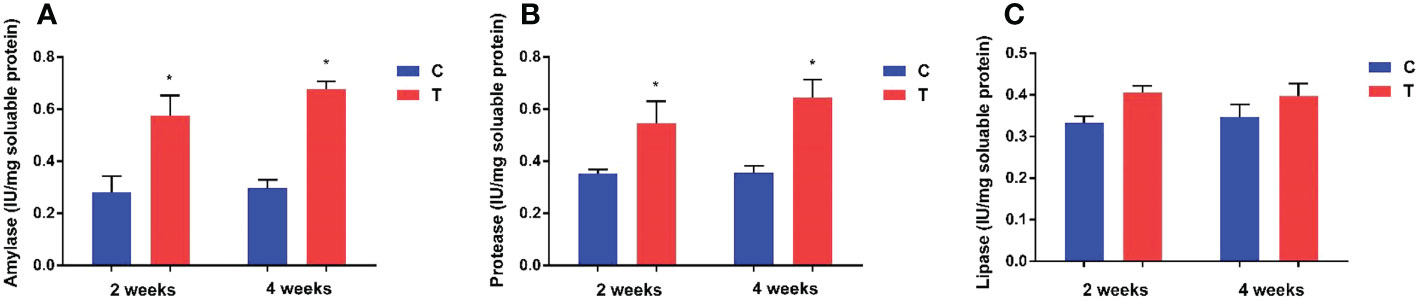
Figure 2 Effects of dietary B. amyloliquefaciens on amylase, protease and lipase activities in the gut of yellow catfish. Amylase (A), Protease (B), Lipase (C). C, control group; T, treatment group. Results are means ± SD from three individual fish (*p<0.05).
After yellow catfish were fed diet containing B. amyloliquefaciens, their respiratory burst (RB) activity increased significantly after 2 weeks (p<0.05) compared with the control diet group, but no statistically significant difference was found after 4 weeks (p >0.05; Figure 3A).
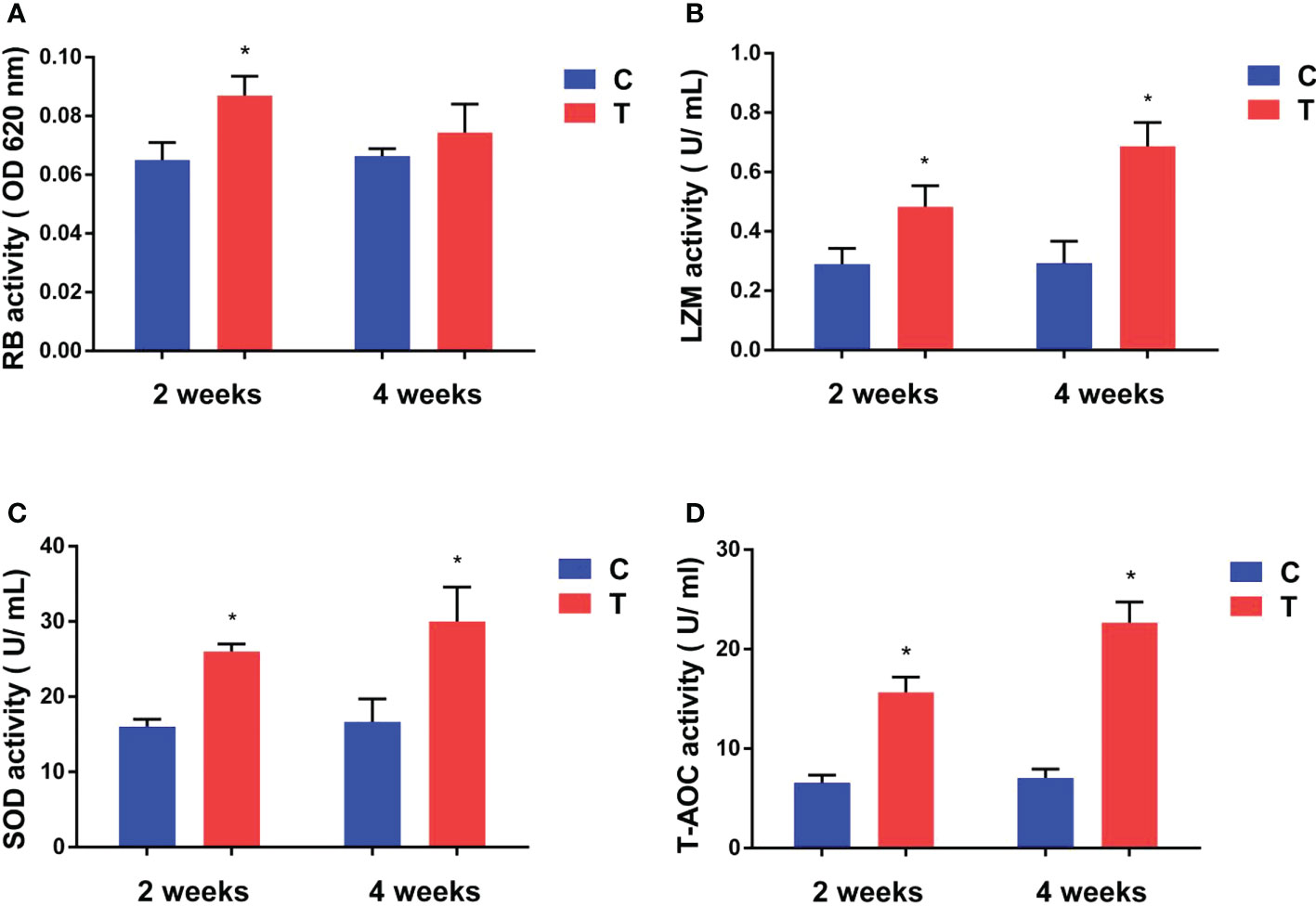
Figure 3 Effects of B. amyloliquefaciens on the respiratory burst (RB) activity (A), lysozyme (LZM) activity (B), superoxide dismutase (SOD) activity (C, D) total antioxidant capacity (T-AOC) of yellow catfish. C, control group; T, treatment group. Results are means ± SD from three individual fish (*p<0.05).
LZM, and SOD activities and T-AOC of yellow catfish were increased significantly in the treatment diet group compared with the control group after 2 and 4 weeks of feeding (p<0.05; Figures 3B–D).
Expression levels of immune-related genes at the 2nd and 4th week in the intestine of yellow catfish supplemented with different levels of B. amyloliquefaciens are presented in Figure 4. Signal transducer and activator of transcription 1 (STAT-1), immunoglobulin M (IgM) and C-type lectin (CTL) were significantly upregulated in fish fed with B. amyloliquefaciens compared with control diet (p<0.05). By contrast, interleukin-1β (IL-1β) mRNA levels were not significantly different among control and treatment groups (p >0.05).
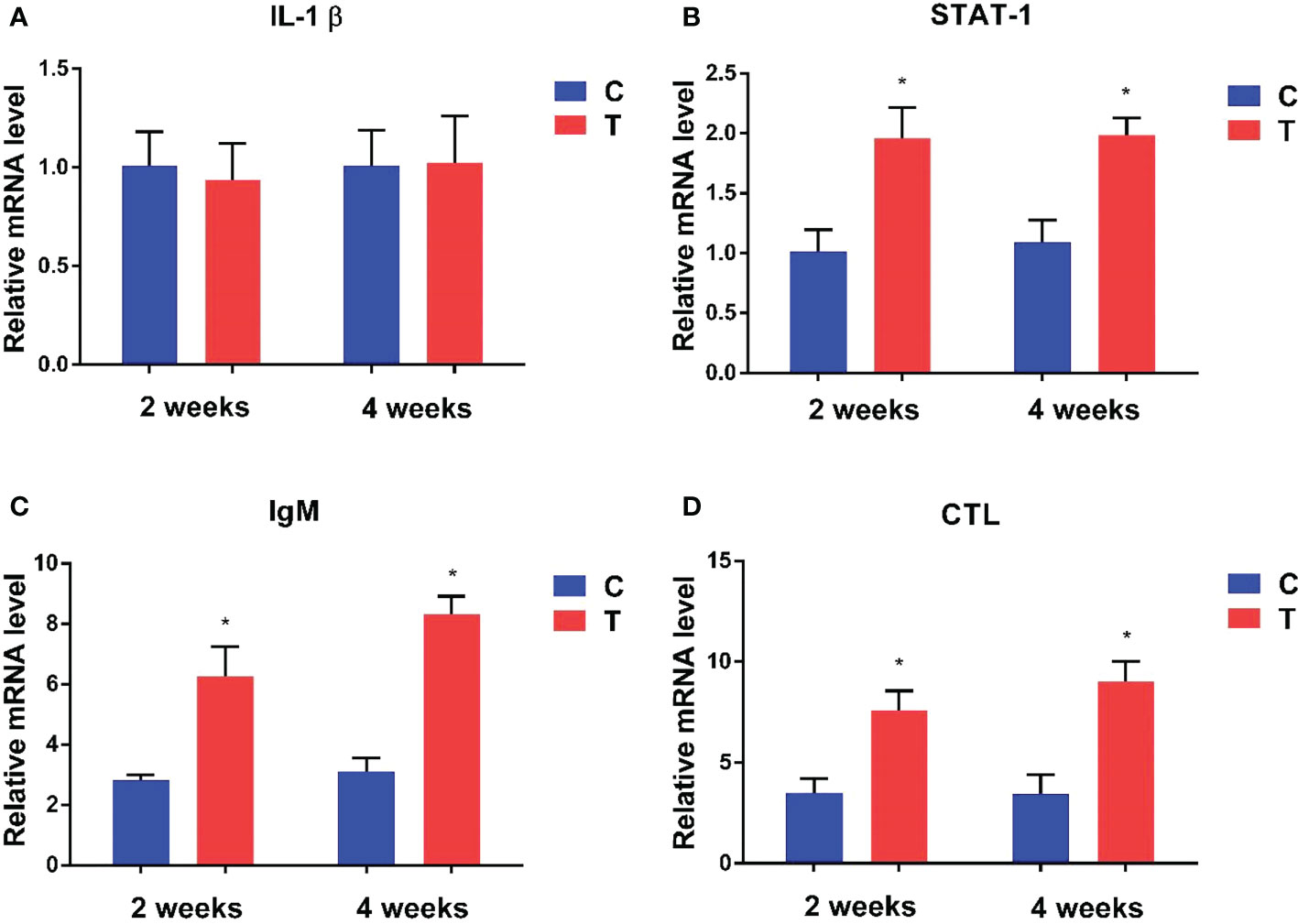
Figure 4 Relative expression levels of immune-related genes in the intestine of fish treated with B. amyloliquefaciens and P. fulvidraco. IL-1β (A), STAT-1 (B), IgM (C), CTL (D). C, control group; T, treatment group (*p<0.05).
To explore changes in the gut microbiota community of yellow catfish fed a diet supplemented with B. amyloliquefaciens, OTUs of the intestinal microbiota were determined for each group according to the abundances of taxa at the genus level. At the genus level, the primary intestinal microbiota in all groups were Plesiomonas, Cetobacterium and Romboutsia (Figure 5). The relative abundance of Cetobacterium was increased significantly in the gut of yellow catfish fed B. amyloliquefaciens (p<0.05). Conversely, Plesiomonas was decreased significantly in the gut of yellow catfish fed B. amyloliquefaciens (p<0.05; Figure 5).
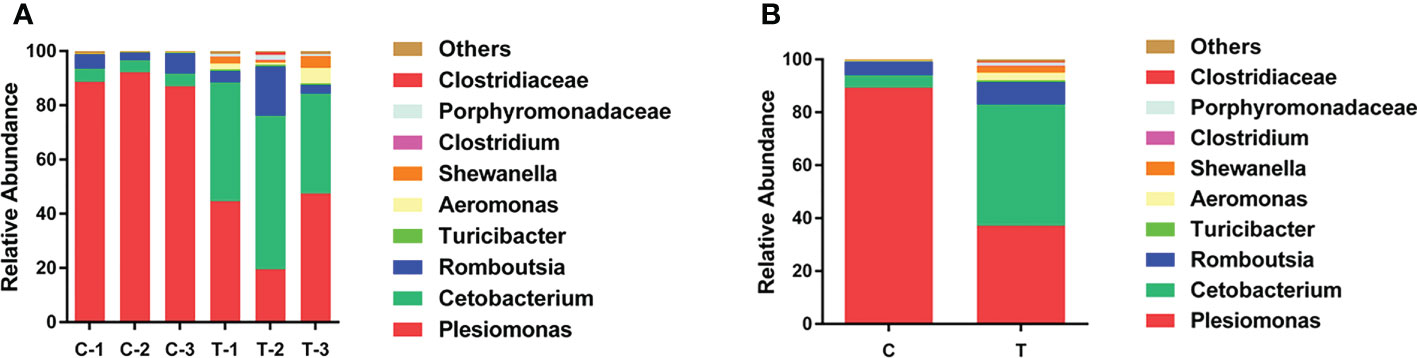
Figure 5 Structure and composition of the intestinal bacterial communities in yellow catfish at the genus level. (A) appearing in each sample, (B) means representing as two groups. C and C1−C3, control groups; T and T1−T3, treatment groups.
Good’s coverage ranged from 0.996 to 0.999, which indicated that the gut microbiota of the samples was reliably identified. There were no significant differences between ACE and Chao1 indices among control and treatment groups (p >0.05; Figures 6A, B). However, Shannon and Simpson indices were significantly higher and lower (p<0.01), respectively, in the treatment group than the control group (Figures 6C, D). The range of the curve on the horizontal axis in the treatment group was significantly higher than that in the control group (p<0.05; Figure 6E).
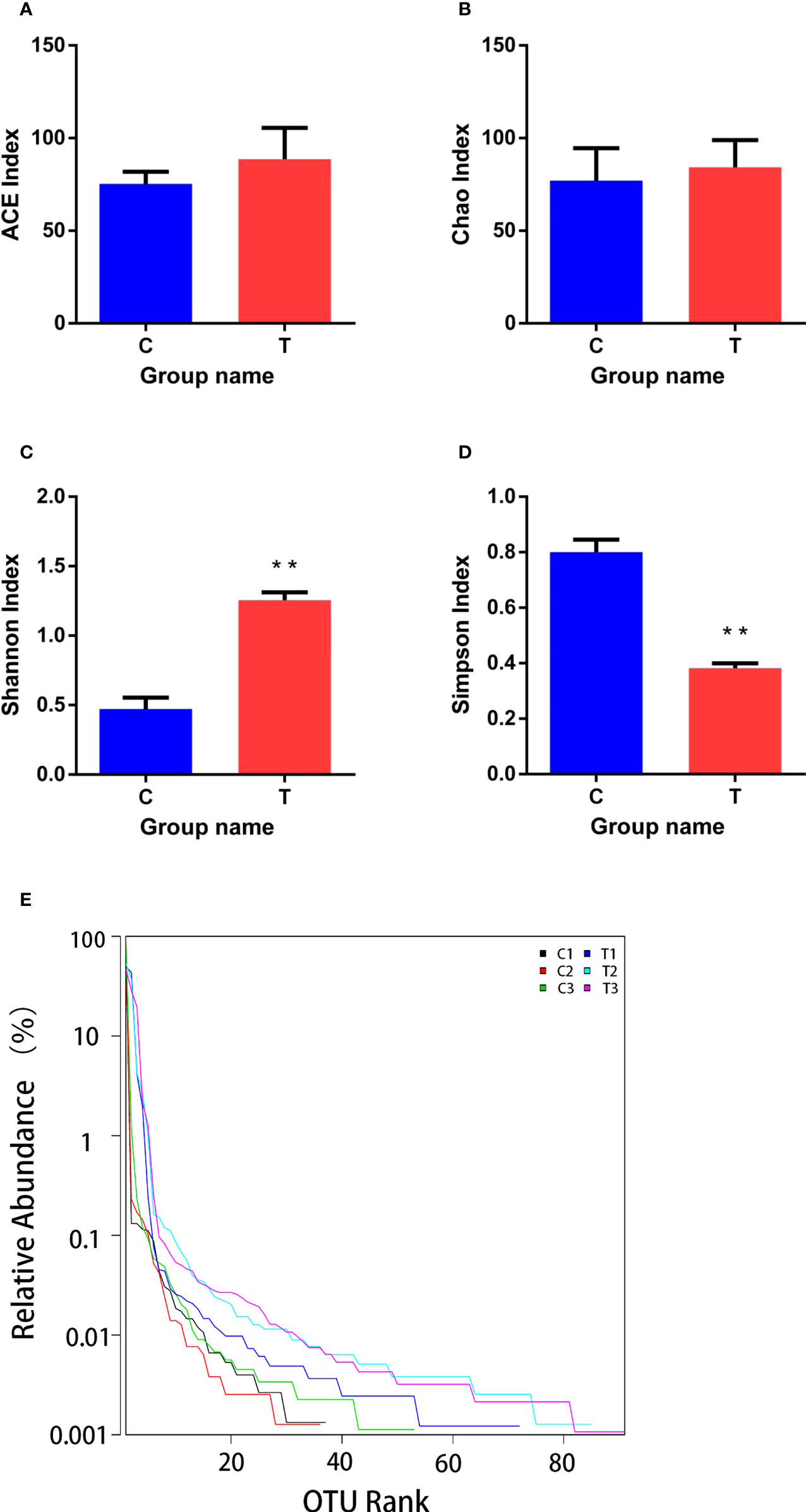
Figure 6 Richness and diversity of bacterial species in treatment and control groups. (A–D) are ACE, Chao, Shannon and Simpson indices of OTUs, respectively. (E) was Rank - abundance curve. C, control group; T, treatment group. Results are means ± SD from three individual fish (**p<0.01).
The cumulative survival rates of yellow catfish challenged with A. veronii and E. ictaluri for 10 days are shown in Figure 7. At the end of the 10-day challenge test, the cumulative survival rate of fish fed with B. amyloliquefaciens diet was significantly higher than that of fish fed with control diet (p<0.05). Furthermore, A. veronii and E. ictaluri were respectively re-isolated from the artificially infected fish.
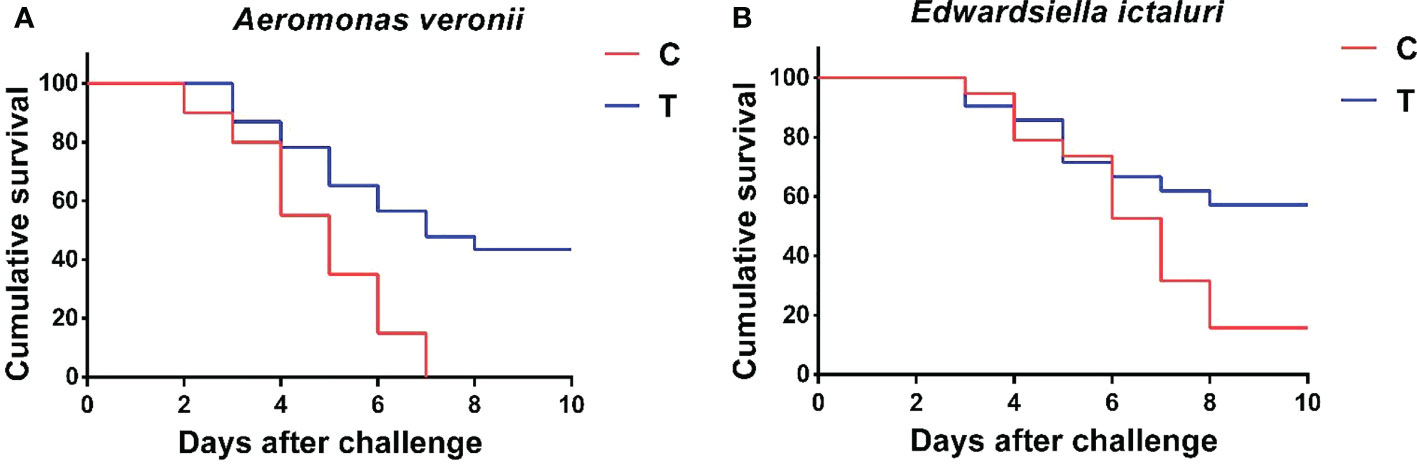
Figure 7 Cumulative survival rate of P. fulvidraco after challenge with A. veronii (A) and E. ictaluri (B) for 10 days. C, control group; T, treatment group.
Research on the dietary supplementation of probiotics in aquaculture is receiving increasing attention due to the demand for eco-friendly prophylactic measures for fish growth performance and health improvement (Geng et al., 2011; Lee et al., 2016; Asaduzzaman et al., 2018). Probiotics, such as Bacillus spp., are being used increasingly in aquaculture (Gobi et al., 2018). The effects of dietary supplementation of Bacillus subtilis HAINUP40 on growth, immunity and disease resistance of tilapia (Oreochromis niloticus) were investigated (Liu et al., 2017). In another study, Muthukrishnan et al. (2016)found that Bacillus cereus BP-MBRG/1b significantly improved growth performance, intestinal propionic acid production and haemolymph SOD activity in prawns (Muthukrishnan et al., 2021). In the present study, dietary supplementation of B. amyloliquefaciens improved growth performance, immune responses, the structure of the intestinal microbiota and resistance to pathogens in yellow catfish.
Previous studies show that many Bacillus spp. can effectively improve the host’s growth performance, such as percentage weight gain and feed efficiency. For instance, Madani et al. reported that oral administration of commercial probiotic Bacillus (B. subtilis and B. licheniformis) had beneficial effects on growth performance parameters and feed utilisation in Litopenaeus vannamei post-larvae (Sadat Hoseini Madani et al., 2018). Similarly, B. amyloliquefaciens significantly improved the specific growth rate and percentage weight gain of yellow catfish in the present study. It is known that increasing body weight gain in fish fed a probiotic supplemented diet can be attributed to increased digestive enzyme activity (Irianto and Austin, 2010). In the present study, B. amyloliquefaciens dietary supplementation significantly increased amylase and protease activities of yellow catfish. However, the proportion of enzymes produced by probiotics cannot be assessed, since probiotics can produce enzymes and may also stimulate the production of endogenous enzymes in fish (Wu et al., 2012; Dawood et al., 2016; Liu et al., 2017). Our results revealed that B. amyloliquefaciens could enhance the digestive enzyme activity of yellow catfish, and thus improve growth.
RBs produced by phagocytes are considered an important indicator of cellular immunity mechanisms in fish when evaluating their defence abilities against pathogens (Wu et al., 2015). Our study revealed that RBs of phagocytes in the experimental group were increased significantly after 2 weeks of feeding on a B. amyloliquefaciens-supplemented diet. LZM is an important component of the innate immune defence system against invasive pathogens. The rise of serum LZM levels suggests the elevation of various humoral factors that can protect the host during pathogen invasion. Liu et al. (2017) demonstrated that LZM activity of tilapia was increased significantly after fish were fed with B. subtilis HAINUP40 for 8 weeks (Liu et al., 2017). A similar result was observed in our study; feeding on B. amyloliquefaciens significantly increased LZM activity in yellow catfish. Therefore, B. amyloliquefaciens may induce disease resistance in yellow catfish against A. veronii and E. ictaluri by promoting RB and LZM activities.
Under normal physiological conditions, animal cells maintain a balance between generation and removal of reactive oxygen species. T-AOC includes enzymatic and non-enzymatic antioxidant activities. SOD is the first line of the antioxidant enzymatic defence system, and T-AOC is useful to evaluate the capacity of all antioxidants. In the present study, activities of SOD and TAOC were increased in the serum of yellow catfish whose diets contained B. amyloliquefaciens. SOD and T-AOC are indicators of the antioxidant status of fish, and are utilised as oxidative stress biomarkers. Therefore, oral administration of B. amyloliquefaciens promoted the antioxidant activity of yellow catfish. Similarly, Esteban et al. (2014) demonstrated that oral administration of Shewanella putrefaciens and Bacillus on gilthead significantly enhanced its SOD activity (Esteban et al., 2014). Significantly higher SOD levels were reported after 8 weeks of feeding on Lactobacillus rhamnosus in red sea bream (Dawood et al., 2016) and B. subtilis HAINUP40 in tilapia (Liu et al., 2017).
Cytokines are cell signalling molecules involved in many physiological processes, including the regulation of immune and inflammatory responses, which are important to maintain the health of hosts (Xiao et al., 2018). In the present study, mRNA expression levels of immune-related genes in intestine were measured after dietary administration of probiotic B. amyloliquefaciens. Pro-inflammatory cytokines such as IL-1β mediate powerful inflammatory responses in fish after infection (Julio et al., 2014). Our results showed that Bacillus did not cause inflammation in yellow catfish. Additionally, oral administration of B. amyloliquefaciens upregulated the expression of IgM, CTL and STAT-1 in intestine of yellow catfish. IgM and CTL play key roles in controlling pathogens and maintaining homeostasis in fish. A number of probiotics can effectively modulate the expression of inflammatory cytokines in many animals (Rebeca et al., 2013; Guo et al., 2016). These results suggest that immune cytokines are influenced by B. amyloliquefaciens, and further promote disease resistance in yellow catfish.
The intestinal microbiota plays important roles in host health due to its critical influence on metabolism and immune function (Xu et al., 2022). The richness and diversity of gut bacteria are closely linked to the stability of intestinal microbial communities in animals (Piazzon et al., 2019; Xue et al., 2022). Significantly increased Shannon index and significantly decreased Simpson index values were observed for the treatment group in the present study, while ACE and Chao1 index values among control and treatment groups were not significantly different. These results indicate that the diversity of the intestinal microbiota in treatment group was significantly increased, but there was no significant change in bacterial richness. B. amyloliquefaciens may improve intestinal stability and health by increasing the diversity of intestinal microbiota. As the largest immune organ in the body, the intestinal tract plays an important role in reducing the invasion of pathogenic bacteria. An increase in the diversity and stability of gut microbes also contributes to host immunity, this also contributes to the host’s resistance to disease. The relative abundance of Cetobacterium was increased significantly and Plesiomonas was decreased significantly in the gut of treated yellow catfish vs. controls. Cetobacterium is an important beneficial bacteria in the gut of aquatic animals, and it can produce large quantities of vitamin B-12 (Tsuchiya et al., 2008). Members of the genus Plesiomonas are ubiquitous opportunistic pathogens in aquaculture systems, and can cause infections in humans (Ekundayo and Okoh, 2019; Xi et al., 2019). In the present study, diets containing B. amyloliquefaciens increased the proportion of beneficial bacteria and decreased the proportion of harmful bacteria in yellow catfish.
The current study revealed a higher survival rate in P. fulvidraco challenged with A. veronii or E. ictaluri when fed a B. amyloliquefaciens-supplemented diet compared with a basal diet. The ability of probiotics to inhibit the growth of pathogenic bacteria and elevate the immune response of hosts might be important for reducing the percentage cumulative mortality and protecting yellow catfish against these pathogens.
In summary, B. amyloliquefaciens exhibits many properties of a good probiotic, including the ability to secrete extracellular enzymes and inhibit the growth of pathogenic bacteria. Oral administration of B. amyloliquefaciens can improve growth performance, digestive enzyme activities, immune responses, the structure of the intestinal microbiota and disease resistance against A. veronii and E. ictaluri in yellow catfish. These results indicate that B. amyloliquefaciens can be used as a potential probiotic in yellow catfish farming.
The data presented in the study are deposited in the NCBI (https://www.ncbi.nlm.nih.gov/) repository, accession number SRR21820145.
The animal study was reviewed and approved by Animal Experimental Ethical Inspection of Laboratory Animal Centre, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI2022-zhouyong-06).
MX conceived and designed the study, performed the data collection, analysis, statistical analysis, and wrote the manuscript. YW and CX conducted the software analysis and literature review. YH and NJ conducted the animal management and sample collections. YM, WL and YL performed the microbial analysis, immunity analysis, and literature review. YF and YZ contributed to acquisition of funding, conceptualization, writing - review & editing, and supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research Development Program of China (2019YFD0900105), and the Central Public-interest Scientific Institution Basal Research Fund (2020TD44).
We thank Wuhan Dynamic Life Science and the Hainan Yonghe Biotechnology Co., Ltd. for support in carrying out this study.
Author YW is employed by Wuhan Dynamic Life Science Co, Ltd. Author YH is employed by Hainan Yonghe Biotechnology Co, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akhter, N., Wu, B., Memon, A. M., Mohsin, M. (2015). Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 45, 733–741. doi: 10.1016/j.fsi.2015.05.038
Asaduzzaman, M., Iehata, S., Akter, S., Kader, M. A., Ghosh, S. K., Khan, M. N. A., et al. (2018). Effects of host gut-derived probiotic bacteria on gut morphology, microbiota composition and volatile short chain fatty acids production of Malaysian mahseer tor tambroides. Aquaculture Rep. 9, 53–61. doi: 10.1016/j.aqrep.2017.12.003
Bates, S. T., Clemente, J. C., Flores, G. E., Walters, W. A., Parfrey, L. W., Knight, R., et al. (2013). Global biogeography of highly diverse protistan communities in soil. ISME J. 7, 652–659. doi: 10.1038/ismej.2012.147
Bland, J. M., Altman, D. G. (1998). Statistics notes - survival probabilities (the Kaplan-Meier method). BMJ Clin. Res. 317, 1572–1572. doi: 10.1136/bmj.317.7172.1572
Bondad-Reantaso, M. G., Subasinghe, R. P., Arthur, J. R., Ogawa, K., Chinabut, S., Adlard, R., et al. (2005). Disease and health management in Asian aquaculture ☆. Veterinary Parasitol. 132, 249–272. doi: 10.1016/j.vetpar.2005.07.005
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Casula, Gabriella, Cutting, Simon, M. (2002). Bacillus probiotics: Spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 68, 2344–2352. doi: 10.1128/AEM.68.5.2344-2352.2002
Dawood, M., Koshio, S. (2016). Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture 454, 243–251. doi: 10.1016/j.aquaculture.2015.12.033
Dawood, M., Koshio, S., Ishikawa, M., El-Sabagh, M., Esteban, M. A., Zaineldin, A. I. (2016). Probiotics as an environment-friendly approach to enhance red sea bream, pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol. 57, 170–178. doi: 10.1016/j.fsi.2016.08.038
Ekundayo, T. C., Okoh, A. I. (2019). Antimicrobial resistance in freshwater plesiomonas shigelloides isolates: Implications for environmental pollution and risk assessment. Environ. pollut. 257, 113493. doi: 10.1016/j.envpol.2019.113493
Elizabeth, Grice, Heidi, Kong, Sean, Conlan, et al. (2009). Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–11192. doi: 10.1126/science.1171700
Esteban, M. A., Cordero, H., Martínez-Tomé, M., Jiménez-Monreal, A. M., Bakhrouf, A., Mahdhi, A. (2014). Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata l.). Fish Shellfish Immunol. 39, 532–540. doi: 10.1016/j.fsi.2014.06.012
Fisheries and Fisheries Administration Bureau of Ministry of Agriculture and Industry. (2022). In China Fisheries Yearbook (Vol. 27). Beijing, Chinese: China Agriculture Press.
Geng, X., Dong, X. H., Tan, B. P., Yang, Q. H., Chi, S. Y., Liu, H. Y., et al. (2011). Effects of dietary chitosan and bacillus subtilis on the growth performance, non-specific immunity and disease resistance of cobia, rachycentron canadum. Fish Shellfish Immunol. 31, 400–406. doi: 10.1016/j.fsi.2011.06.006
Gobi, N., Vaseeharan, B., Chen, J. C., Rekha, R., Vijayakumar, S., Anjugam, M., et al. (2018). Dietary supplementation of probiotic bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against aeromonas hydrophila in tilapia oreochromis mossambicus. Fish Shellfish Immunol. 74, S105046481730815X. doi: 10.1016/j.fsi.2017.12.066
Guo, X., Chen, D. D., Peng, K. S., Cui, Z. W., Zhang, X. J., Li, S., et al. (2016). Identification and characterization of bacillus subtilis from grass carp ( ctenopharynodon idellus ) for use as probiotic additives in aquatic feed. Fish Shellfish Immunol. 52, 74–84. doi: 10.1016/j.fsi.2016.03.017
Hong, H. A., Le, H. D., Cutting, S. M. (2005). The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 29, 813–835. doi: 10.1016/j.femsre.2004.12.001
Hong, X. T., Xiang, L. X., Shao, J. Z. (2006). The immunostimulating effect of bacterial genomic DNA on the innate immune responses of bivalve mussel, hyriopsis cumingii lea. Fish Shellfish Immunol. 21, 357–364. doi: 10.1016/j.fsi.2005.12.013
Hoseinifar, S. H., Ringø, E., Shenavar Masouleh, A., Esteban, M.Á. (2016). Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: a review. Rev. Aquaculture 8, 89–102. doi: 10.1111/raq.12082
Huang, L., Ran, C., He, S., Ren, P., Hu, J., Zhao, X., et al. (2015). Effects of dietary saccharomyces cerevisiae culture or live cells with bacillus amyloliquefaciens spores on growth performance, gut mucosal morphology, hsp70 gene expression, and disease resistance of juvenile common carp ( cyprinus carpio ). Aquaculture 438, 33–38. doi: 10.1016/j.aquaculture.2014.12.029
Irianto, A., Austin, B. (2010). Use of probiotics to control furunculosis in rainbow trout, oncorhynchus mykiss (Walbaum). J. Fish Dis. 25, 333–342. doi: 10.1046/j.1365-2761.2002.00375.x
Julio, P. D., Carolina, G. L., Luis, F., Angel, G. (2014). Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 20, 15632–15649. doi: 10.3748/wjg.v20.i42.15632
Kiron, V. (2012). Fish immune system and its nutritional modulation for preventive health care ☆. Anim. Feed Sci. Technol. 173, 111–133. doi: 10.1016/j.anifeedsci.2011.12.015
Lee, S., Katya, K., Park, Y., Won, S., Seong, M., Hamidoghli, A., et al. (2016). Comparative evaluation of dietary probiotics bacillus subtilis WB60 and lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 61, 201–210. doi: 10.1016/j.fsi.2016.12.035
Liu, H., Li, J., Guo, X., Liang, Y., Wang, W. (2018). Yeast culture dietary supplementation modulates gut microbiota, growth and biochemical parameters of grass carp. Microb. Biotechnol. 11, 551–565. doi: 10.1111/1751-7915.13261
Liu, H., Wang, S., Yan, C., Guo, X., Cao, Z., Zhang, Y., et al. (2017). Dietary administration of bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, oreochromis niloticus. Fish Shellfish Immunol. 60, 326–333. doi: 10.1016/j.fsi.2016.12.003
Liu, J., Li, A., Zhou, D., Wen, Z., and Ye, X. (2010). Isolation and characterization of Edwardsiella ictaluri strains as pathogens from diseased yellow catfish Pelteobagrus fulvidraco (Richardson) cultured in China. Aquac. Res. 41, 1835–1844. doi: 10.1111/j.1365-2109.2010.02571.x
Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ming, L., Hang, L., Li, Q., Gong, S., Wang, R. (2016). Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish pelteobagrus fulvidraco fed all-plant protein diets - ScienceDirect. Aquaculture 450, 349–355. doi: 10.1016/j.aquaculture.2015.08.013
Mingmongkolchai, S., Panbangred, W. (2018). Bacillus probiotics: an alternative to antibiotics for livestock production. J. Appl. Microbiol. 124, 1334–1346. doi: 10.1111/jam.13690
Muthukrishnan, S., Hoong, M. C., Chen, W. W., Natrah, I. (2021). Efficacy of bacillus cereus strain BP-MBRG/1b and prebiotic fructooligosaccharides dietary supplementation on growth performance and disease resistance of macrobrachium rosenbergii (De Mann) towards aeromonas hydrophila AH-1N. Aquaculture Res. 52, 1657–1665. doi: 10.1111/are.15018
Nakagawa, M., Kawano, Y., Akasaka, Y., Takabayashi, T., Miyazawa, N. (2003). Resistance of bacillus endospores to extreme terrestrial and extraterrestrial environments. Digestive Endoscopy 16, 84–87. doi: 10.1111/j.1443-1661.2004.00314.x
Newaj-Fyzul, A., Al-Harbi, A. H., Austin, B. (2014). Review: Developments in the use of probiotics for disease control in aquaculture. Aquaculture 431, 1–11. doi: 10.1016/j.aquaculture.2013.08.026
Piazzon, M. C., Naya-Català, F., Simó-Mirabet, P., Picard-Sánchez, A., Pérez-Sánchez, J. (2019). Sex, age, and bacteria: How the intestinal microbiota is modulated in a protandrous hermaphrodite fish. Front. Microbiol. 10, 2512. doi: 10.3389/fmicb.2019.02512
Qi, X., Xue, M., Cui, H., Yang, K., Song, K., Zha, J., et al. (2020). Antimicrobial activity of pseudomonas monteilii JK-1 isolated from fish gut and its major metabolite, 1-hydroxyphenazine, against aeromonas hydrophila. Aquaculture 526, 735366. doi: 10.1016/j.aquaculture.2020.735366
Rebeca, C., José, M., M Ángeles, E. (2013). Effects of dietary inulin, bacillus subtilis and microalgae on intestinal gene expression in gilthead seabream (Sparus aurata l.). Fish Shellfish Immunol. 34, 843–848. doi: 10.1016/j.fsi.2012.12.026
Reda, R. M., Seli, K. M., El-Sayed, H. M., El-Hady, M. A. (2017). In vitro selection and identification of potential probiotics isolated from the gastrointestinal tract of Nile tilapia, oreochromis niloticus. Probiotics Antimicrobial Proteins 10, 692–703. doi: 10.1007/s12602-017-9314-6
Rmm, A., Mcjv, A., Sd, A., Lm, B., Jws, A. (2020). Effect of enzymes (phytase and xylanase), probiotics (B. amyloliquefaciens) and their combination on growth performance and nutrient utilisation in Nile tilapia - ScienceDirect. Aquaculture 10, 736226–736226. doi: 10.1016/j.aquaculture.2020.736226
Sadat Hoseini Madani, N., Adorian, T. J., Ghafari Farsani, H., Hoseinifar, S. H. (2018). The effects of dietary probiotic bacilli (Bacillus subtilis and bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) postlarvae. Aquaculture Res. 49, 1926–1933. doi: 10.1111/are.13648
Safari, O., Shahsavani, D., Paolucci, M., Atash, M. M. S. (2014). Single or combined effects of fructo- and mannan oligosaccharide supplements on the growth performance, nutrient digestibility, immune responses and stress resistance of juvenile narrow clawed crayfish, astacus leptodactylus leptodactylus eschscholtz, 182. Aquaculture 432, 192–203. doi: 10.1016/j.aquaculture.2014.05.012
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537. doi: 10.1128/AEM.01541-09
Tsuchiya, C., Sakata, T., Sugita, H. (2008). Novel ecological niche of cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 46, 43–48. doi: 10.1111/j.1472-765X.2007.02258.x
Wang, M., Feng, W., Wang, Y., Li, B., Wang, J., Zhu, X., et al. (2022). Water quality, plankton composition, and growth performance of juvenile yellow catfish (Pelteobagrus fulvidraco) in mono- and polyculture systems. Aquaculture 552, 738017–738017. doi: 10.1016/j.aquaculture.2022.738017
Wang, Q., Garrity, G. M., Tiedje, J. M., Cole, J. R. (2007). Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Wu, Z. X., Feng, X., Xie, L. L., Peng, X. Y., Yuan, J., Chen, X. X. (2012). Effect of probiotic bacillus subtilis Ch9 for grass carp, ctenopharyngodon idella (Valenciennes 1844), on growth performance, digestive enzyme activities and intestinal microflora. J. Appl. Ichthyology 28, 721–727. doi: 10.1111/j.1439-0426.2012.01968.x
Wu, Z. Q., Jiang, C., Ling, F., Wang, G. X. (2015). Effects of dietary supplementation of intestinal autochthonous bacteria on the innate immunity and disease resistance of grass carp ( ctenopharyngodon idellus ). Aquaculture 438, 105–114. doi: 10.1016/j.aquaculture.2014.12.041
Xiao, Xiaozhou, Aiguo, Huang, Fei, Ling, et al. (2018). Cytokine gene expression profiles in goldfish (Carassius auratus) during gyrodactylus kobayashii infection. Fish shellfish Immunol. 86, 116–124. doi: 10.1016/j.fsi.2018.11.035
Xi, D., Jing, F., Liu, Q., Cao, B. (2019). Plesiomonas shigelloides sipD mutant, generated by an efficient gene transfer system, is less invasive. J. Microbiological Methods 159, 75–80. doi: 10.1016/j.mimet.2019.02.017
Xue, M., Jiang, N., Fan, Y., Yang, T., Li, M., Liu, W., et al. (2022). White spot syndrome virus (WSSV) infection alters gut histopathology and microbiota composition in crayfish (Procambarus clarkii). Aquaculture Rep. 22, 101006. doi: 10.1016/j.aqrep.2022.101006
Xu, Y., Li, Y., Xue, M., Xiao, Z., Fan, Y., Zeng, L., et al. (2022). Effects of dietary enterococcus faecalis YFI-G720 on the growth, immunity, serum biochemical, intestinal morphology, intestinal microbiota, and disease resistance of crucian carp (Carassius auratus). Fishes 7, 18. doi: 10.3390/fishes7010018
Xu, Y., Li, Y., Xue, M., Yang, T., Luo, X., Fan, Y., et al. (2021). Effects of dietary saccharomyces cerevisiae YFI-SC2 on the growth performance, intestinal morphology, immune parameters, intestinal microbiota, and disease resistance of crayfish (Procambarus clarkia). Anim. (Basel) 11, 1963. doi: 10.3390/ani11071963
Yang, G., Tian, X., Dong, S., Peng, M., Wang, D. (2015). Effects of dietary bacillus cereus G19, b. cereus BC-01, and paracoccus marcusii DB11 supplementation on the growth, immune response, and expression of immune-related genes in coelomocytes and intestine of the sea cucumber (Apostichopus japonicus selenka). Fish Shellfish Immunol. 45, 800–807. doi: 10.1016/j.fsi.2015.05.032
Ye, S., Li, H., Qiao, G., Li, Z. (2009). First case of edwardsiella ictaluri infection in China farmed yellow catfish pelteobagrus fulvidraco. Aquaculture 292, 6–10. doi: 10.1016/j.aquaculture.2009.03.036
Zeng, J., Ouyang, A., Wang, H., Liu, W., Xue, M., Zhou, Y., et al. (2021). A bivalent vaccine comprised of inactivated aeromonas veronii and edwardsiella ictaluri stimulates protective immune responses in yellow-head catfish, pelteobagrus fulvidraco. Aquaculture Res. 52, 5673–5681. doi: 10.1111/are.15441
Zhou, Y., Jiang, N., Zeng, J., Fan, Y., Liu, W., Kaige, S. I., et al. (2019). Isolation and identification of pathogenic bacterium from ascites disease of yellow catfish,Pelteobagrus fulvidraco. Chin. Fishery Qual. Standards. 9, 18–26. doi: 10.3969/j.issn.2095–1833
Keywords: Bacillus amyloliquefaciens, intestinal microbiota, disease resistance, Pelteobagrus fulvidraco, yellow catfish, digestive enzyme activity, dietary supplementation
Citation: Xue M, Wu Y, Hong Y, Meng Y, Xu C, Jiang N, Li Y, Liu W, Fan Y and Zhou Y (2022) Effects of dietary Bacillus amyloliquefaciens on the growth, immune responses, intestinal microbiota composition and disease resistance of yellow catfish, Pelteobagrus fulvidraco. Front. Cell. Infect. Microbiol. 12:1047351. doi: 10.3389/fcimb.2022.1047351
Received: 18 September 2022; Accepted: 24 October 2022;
Published: 14 November 2022.
Edited by:
Pengfei Li, Guangxi Academy of Sciences, ChinaReviewed by:
Fei Ling, Northwest A&F University, ChinaCopyright © 2022 Xue, Wu, Hong, Meng, Xu, Jiang, Li, Liu, Fan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhou, emhvdXlAeWZpLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.