
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 17 November 2022
Sec. Bacteria and Host
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.1026457
The association between periodontal disease and systemic disease has become a research hotspot. Porphyromonas gingivalis (P. gingivalis), a crucial periodontal pathogen, affects the development of systemic diseases. The pathogenicity of P. gingivalis is largely linked to interference with the host’s immunity. This review aims to discover the role of P. gingivalis in the modulation of the host’s adaptive immune system through a large number of virulence factors and the manipulation of cellular immunological responses (mainly mediated by T cells). These factors may affect the cause of large numbers of systemic diseases, such as atherosclerosis, hypertension, adverse pregnancy outcomes, inflammatory bowel disease, diabetes mellitus, non-alcoholic fatty liver disease, rheumatoid arthritis, and Alzheimer’s disease. The point of view of adaptive immunity may provide a new idea for treating periodontitis and related systemic diseases.
Periodontal diseases include chronic inflammatory diseases such as gingivitis and periodontitis (PD). With 11% of the world’s population suffering from severe periodontitis, periodontitis is one of the most common periodontal disorders (Hajishengallis et al., 2012). The clinical symptoms and effects of periodontitis include red and swollen gums, bleeding gums, alveolar bone resorption, and even teeth loss, which affect the patient’s ability to chew. Furthermore, there is growing evidence of a strong link between systemic diseases and periodontitis, including atherosclerosis, adverse pregnancy outcomes, inflammatory bowel disease, diabetes mellitus, rheumatoid arthritis, and others.
Periodontal disease is characterized by the immune-mediated destruction of the osseous support surrounding the dentition in the oral mucosa (Darveau, 2010). Periodontitis is an infectious disease resulting from the invasion of periodontal pathogens that activate innate and adaptive immune responses. As periodontitis persists, many immune cells participate in the inflammatory process. While these immune cells deter bacterial invasion, they also secrete various cytokines that cause the periodontal tissues to deteriorate. In this case, a necessary trigger for the initiation and persistence of the inflammatory response is the colonization of the dentition by a microbial biofilm rich in gram-negative bacteria. One of them is Porphyromonas gingivalis, an obligate anaerobic bacterium, which is also known as P. gingivalis (pg). P. gingivalis is believed to be the primary pathogen of periodontal disease because of its capacity to modify the normal oral microbiome to enhance its virulence, which significantly accelerates the process of bone loss (Honda, 2011; Kassebaum et al., 2014). P. gingivalis has been closely linked to the emergence of remote inflammatory responses associated with chronic diseases and autoimmune disorders (Pussinen et al., 2007; Martinez-Martinez et al., 2009) in addition to periodontal disease and its consequences (Hajishengallis, 2009). Additionally, P. gingivalis is considered a master at subverting the immune system, exploiting several types of sabotage techniques to escape and weaken the immune system (Krauss et al., 2010b). Although having a variety of virulence factors, such as gingipains, lipopolysaccharide, and fimbriae, may seem significant, P. gingivalis’ pathogenicity is mainly determined by its capacity to undermine the host’s immune system’s defense (Hajishengallis, 2011). Through mechanisms that allow pathogen persistence inside the local inflammatory environment of periodontitis, P. gingivalis interferes with the host’s immune system response, leading to pathology or complications at systemic sites. P. gingivalis and its virulence factors have been discovered in all parts of the body, including atherosclerotic plaque, placenta, intestine, and joints. Further research has revealed that P. gingivalis is a unique bacterium that is capable of infecting myeloid dendritic cells and reprogramming them to cause an immunosuppressive response (Zeituni et al., 2009; Zeituni et al., 2010). There is multiple evidence proving that distant systemic effects are possibly influenced by local microbial dysbiosis and the ensuing immune response within periodontitis (Chapple et al., 2013; Tonetti et al., 2013). Reports have indicated that patients suffering from periodontal diseases may have systemic exposure to P. gingivalis, as evidenced by the discovery of P. gingivalis-specific T-cells within the peripheral blood (Gemmell et al., 1999; Nakajima et al., 1999). The report also described that in patients suffering from periodontitis and experimental models, the T and B cells were activated in the gingival tissues. These outcomes of the adaptive immune response are antigen-specific to several P. gingivalis strains are communicated locally and systemically (Ebersole et al., 2016). A growing number of studies have also suggested that P. gingivalis can influence the development of periodontitis-related systemic disorders by affecting the adaptive immune response and inducing inflammation and innate immune responses (Table 1).

Table 1 P. gingivalis-induced adaptive immune responses (mainly mediated by T cells) in various systemic diseases.
Previous review articles (Hajishengallis, 2015; Olsen et al., 2016; Bui et al., 2019) have discussed the association of inflammation and the innate immune response with periodontal pathogens and systemic disease. However, there has been no comprehensive analysis of the impact of adaptive immunity on the relationship between P. gingivalis and periodontitis-related conditions. This review elaborates on how P. gingivalis modifies the host’s adaptive immune system by controlling cellular immunological responses, particularly T cells, in relevant systemic diseases. It also offers new ideas for future research and developing treatments for these systemic diseases using adoptive immunotherapy as a treatment strategy.
As previously mentioned, P. gingivalis is considered a major pathogen in periodontitis, which means that after initially colonizing the host, it can induce dysbiosis in the oral microbiota (Hajishengallis et al., 2012). P. gingivalis expresses various virulence factors, such as lipopolysaccharide (LPS), fimbriae, ceramide, nucleoside diphosphate kinase (NDK), capsule, gingipains, and outer membrane vesicles (OMVs) (How et al., 2016), which can trigger host immune responses (summarized in Table 2). This periodontopathogen is regarded as an expert at tricking the immune system, and it uses a variety of sabotage strategies to avoid detection by the host immune system or to weaken or trick it (Krauss et al., 2010b). Here, we briefly reviewed how P. gingivalis subvert adaptive immune response.
Ivanyi and Lehner were the first to discover that oral bacterium encouraged lymphocytes mobilization in patients with mild periodontal disease, whereas this activation was reduced in individuals with severe periodontitis (Ivanyi and Lehner, 1970). Recent studies have demonstrated that the presence of many T cell subsets contributes to the complex roles of T cell immunity in periodontitis. During the development of periodontitis, various subsets of CD4+ T cells support or inhibit the host’s immunological responses (Jin et al., 2014; Wang et al., 2015). In a study by Baker et al., it was shown that rats lacking MHC-II-restricted CD4+ T cells, but not MHC-I-restricted CD8+ T cells, were sensitive to the resorption of oral alveolar bone brought on by P. gingivalis infection (Baker et al., 1999). This finding suggests that CD4+ T cells are involved in bone demineralization. CD4+ T cells are divided into distinct functional lineages such as Th1, Th2, Th17, Treg, and some other subsets (Vernal et al., 2014).
Th1 and Th2 cells have diverse responses to various stimuli and lead to various results in inflammatory and infectious disorders. It is still controversial that which specific Th subsets are selectively activated during periodontitis. On the one hand, it has been shown that Porphyromonas gingivalis and its virulence factors promote Th1 differentiation (Gemmell et al., 1999; Jotwani and Cutler, 2004). On the other hand, Porphyromonas gingivalis and its virulence factors have also been reported to induce a Th2 response (Jotwani et al., 2003; Gaddis et al., 2013). It’s interesting to notice that in older animals, a reducing in Th1/Th2 cytokines such IFN-γ, IL-4, IL-12p40, or IL-10 might lead to a more significant loss of alveolar bone (Alayan et al., 2007). Given that IFN-γ and IL-12 are crucial for bacterial cleaning and that IL-4 and IL-10 are well known for their anti-inflammatory properties, the incongruent reports may be explicable by the fact that a delicate balance between pathogens and various host immune elements is necessary to preserve periodontal health.
Th17 cells have a critical role in both increasing inflammation and defending against extracellular infections and fungi (O'Connor et al., 2010). Early studies have found that patients with periodontitis had the Th17-specific cytokine IL-17 and other relevant cytokines identified in their gingival tissues (Lester et al., 2007). Increased Th17 cell infiltration in periodontal lesions further demonstrated the link between Th17 and periodontitis (Adibrad et al., 2012). Periodontal lesions were encouraged to produce Th17 by P. gingivalis, particularly the strain W83 (Moutsopoulos et al., 2012). Different K-serotypes of P. gingivalis strains primed dendritic cells, with strains W83 of serotype K1 and HG184 of K2 activating a Th1/Th17 pattern of immune response while strains K3, K4, and K5 stimulated a Th2 response (Vernal et al., 2014). In addition to releasing the pro-inflammatory cytokine IL-17, Th17 cells also affect bone loss by increasing the production of RANKL, which is necessary for osteoclastogenesis (Stadhouders et al., 2018).
Regulatory T cells (Treg) are a distinct population of suppressive lymphocytes that prevent other immune cells from becoming activated, proliferating, and performing their effector roles. By directing and intensifying both innate and adaptive immunity as well as moderating a variety of host immunological responses, Treg cells are essential for the preservation of host immune homeostasis (Chaudhry and Rudensky, 2013). Treg cells were shown to be enriched in periodontitis lesions in numerous publications on human patients (Nakajima et al., 2005; Cardoso et al., 2008). Interesting results also showed that periodontitis lesions had fewer Foxp3+CD25+ cells (Ernst et al., 2007). It is still unknown what led to the contradiction. The well-known anti-inflammatory cytokines TGF-β and IL-10 are also important cytokines for Tregs, while their levels of expression were inversely linked with the severity of the periodontitis (Dutzan et al., 2012). In IL-10-deficient animals, P. gingivalis infection resulted in more severe alveolar bone loss (Sasaki et al., 2004), and the treatment of IL-10 inhibited alveolar bone loss (Zhang et al., 2014), demonstrating the protective role of IL-10. Research conducted both in vivo and in vitro demonstrated that P. gingivalis can promote the formation of Treg cells (Kobayashi et al., 2011). It’s interesting to note that P. gingivalis’s impact on Treg growth depends on the environment. For instance, compared to non-pregnant mice, pregnant mice had less Treg cells stimulated by P. gingivalis. Also, the reduction of Tregs in atherosclerotic patients following infection with P. gingivalis, particularly in those with type II FimA, suggests that type II FimA may be linked to Treg dysregulation (Yang et al., 2022). As a whole, additional study is needed to comprehend the functions of T-cell subsets in periodontitis and the biological significance of P. gingivalis’s modulation of those functions in the context of its function as a keystone pathogen.
Atherosclerosis is a disorder characterized by inflammation and multifaceted conditions, which is caused by the accumulation of lipid droplets and different types of immune cells in the arterial wall. These cells include macrophages and T and B lymphocytes (Pirro and Mannarino, 2019). Despite being the minority, T and B cells are crucial for the modulation of immune responses during the progression of atherosclerosis (Gounopoulos et al., 2007; Libby, 2012). The subsets of T cells have all been identified in the atherosclerotic plaque of the human body (Andersson et al., 2010; Hansson and Hermansson, 2011; Libby et al., 2011). Additionally, there are growing efforts to find immunomodulatory treatments that target T or B cells with potential antiatherosclerotic effects. Different subtypes of T and B lymphocytes that control various branches of the adaptive immune system have been discovered throughout the progression of atherosclerosis (Pirro and Mannarino, 2019). Although these ideas of immunomodulatory techniques are appealing, clinical translation is greatly hampered by the lack of understanding of the functions of autoantibodies, B and T cells, and the low predictive value of animal models (Libby, 2012).
A 1993 study discovered an association between periodontitis and cardiovascular disease (Mattila et al., 1993; Wolf and Ley, 2019). In 2012, the American Heart Association endorsed these findings and asserted the relationship is unrelated to the earlier established criteria (Mattila et al., 1989). Although the pathogenesis of atherosclerosis is uncertain, P. gingivalis is currently gaining attention due to its potential role in accelerating the progression of the disease (Tonetti, 2009; Lockhart et al., 2012). By altering the host’s lipid profile, P. gingivalis infections in the mouth have been shown by Maekawa et al. to facilitate the growth of atheroma. Patients with cardiovascular illness were also shown to have high titers of P. gingivalis antibodies. (Maekawa et al., 2011). Previous studies have suggested that the invasion of cardiovascular cells and tissues by P. gingivalis may contribute to the onset of atherosclerosis (Pussinen et al., 2007; Olsen and Progulske-Fox, 2015).
HSP60 is a chaperonin type, also known as the 60 kDa heat shock protein 60. A chaperonin is a group of proteins that aids in the assembly of intracellular molecules and protein folding. On the cell surface and in the extracellular milieu, HSP60 chaperones also function as danger signals for stressed and injured cells (Pockley and Multhoff, 2008), serving as potent stimulators of the immune responses (Pockley et al., 2008; Cai et al., 2014). Previous research has demonstrated that HSP60 is present in atherosclerotic lesion regions (Kol et al., 1998). High titers of HSP60 antibodies were discovered in individuals with coronary heart disease, carotid atherosclerosis, and stroke. Additionally, a strong association between anti-HSP antibody levels and the prevalence of atherosclerosis was identified. (Mandal et al., 2004). Studies have also revealed that P. gingivalis can express GroEL-like proteins from the HSP60 family (Hotokezaka et al., 1994). Furthermore, the immune system may be unable to distinguish between the host and bacterial HSP. Yamazaki et al. discovered that cross-reactive T cells against human HSP60 and P. gingivalis HSP60 (GroEL) exist in both periodontitis and atherosclerotic aneurysm tissue (Yamazaki et al., 2004). In addition, Choi et al. discovered that atherosclerosis patients had elevated titers of anti-P. gingivalis HSP60 IgG antibody (Choi et al., 2002) and P. gingivalis Hsp-specific T-cell lines, which were formed from peripheral blood and atherosclerotic lesions. These data imply that the P. gingivalis HSP60-specific T-cell immunological response may be a component of the immunopathologic process underlying atherosclerotic disease.
Atherosclerosis was thought to occur due to a Th1/Th2 imbalance (Cheng et al., 2008). However, recent views have changed due to new evidence that patients with acute coronary syndrome have an imbalance of Th17/Treg (Yang et al., 2017). According to Cai et al., during the development of periodontitis, an infection caused by P. gingivalis increases the responses of Th17 cells (Cai et al., 2014). Since a Th17/Treg imbalance causes inflammation, it is possible to propose that plaque destabilization may also be influenced by this imbalance. Moreover, Tregs are known to produce plenty of TGF-β and IL-10, and by repressing immune cell functions, these proteins play a vital role in the pathogenesis of atherosclerosis. The study also revealed that patients with P. gingivalis who develop atherosclerosis have lower Treg cell levels than a control group who do not (Yang et al., 2014). A collection of TGF-β1 was reduced in those infected with P. gingivalis. According to Cai et al. (2014), P. gingivalis-induced atherosclerosis in ApoE-/- mice increased the amount of Th17 cells and Th17-related chemicals in the heart relative to Th1 and Treg cells. The finding may indicate that Th17 cells have potent pro-inflammatory functions to exacerbate the progression of atherosclerosis. Additionally, in P. gingivalis-infected ApoE-/- mice, Yang et al. (2017) found that P. gingivalis enhances the formation of atherosclerotic lesions and plaque instability. Such a finding was also associated with decreased Treg cell frequency and increased Th17 cell frequency. The evidence suggests that P. gingivalis oral infection may contribute to a Th17/Treg imbalance by impacting T-cell differentiation during atherosclerosis, acting as a vital factor in expanding lesion areas and reducing plaque instability.
Interleukin-2(IL-2) regulates regulatory T cells and type-2 innate lymphoid cells, the two cells that are crucial for atherosclerosis and myocardial healing (Zhao et al., 2020). Additionally, Khalaf et al. reported that the virulent P. gingivalis inhibits the expression and accumulation of IL-2 (Khalaf and Bengtssown, 2012), which results in the production of active oxygen species and the elevation of Ca2+ in blood, as well as the depreciation of activity of transcription factors, namely AP-1 and NF-κB. AP-1 has been proven to be an essential regulator of IL-2. The partial suppression of arginine-specific (Rgp) gingipains, a protease secreted by P. gingivalis, could also result in the inhibition of IL-2 accumulation. In summary, P. gingivalis infection and the inhibition of AP-1 protein both impact the accumulation of IL-2 at the protein level (Khalaf and Bengtssown, 2012). Therefore, it has been proposed that the alteration of adaptive immune response and the fluctuation of IL-2 levels are responsible for the internal inflammatory state of atherosclerosis.
Avoiding periodontal inflammation may be an effective way to reduce the formation and progression of atherosclerosis since the periodontal infections that cause it may involve various potential mechanisms. Nasal immunization with a 40 kDa outer membrane protein of P. gingivalis effectively restrained atherosclerosis and inflammation in C57BL/6 mice fed with ApoEshl and an HFD (Koizumi et al., 2008; Fukasawa et al., 2012). Furthermore, mucosal administration of related autoantigens is an effective way to lessen autoimmune diseases by inducing an unresponsive state of tolerance (Calder et al., 2006) because Hsp60 (GroEL) from P. gingivalis can trigger a connection between atherosclerosis and periodontitis (Choi et al., 2004; Choi et al., 2011). Because the acceleration of P. gingivalis infection is controlled by P. gingivalis GroEL sublingual immunization (Hagiwara et al., 2014), mucosal vaccination with P. gingivalis Hsp60 may also be able to regulate the inflammation and the development of atherosclerosis due to the periodontal pathogen infection.
The possible mechanisms through which P. gingivalis affects the state of arteriosclerosis (mainly adaptive immune response) are summarised in Figure 1.
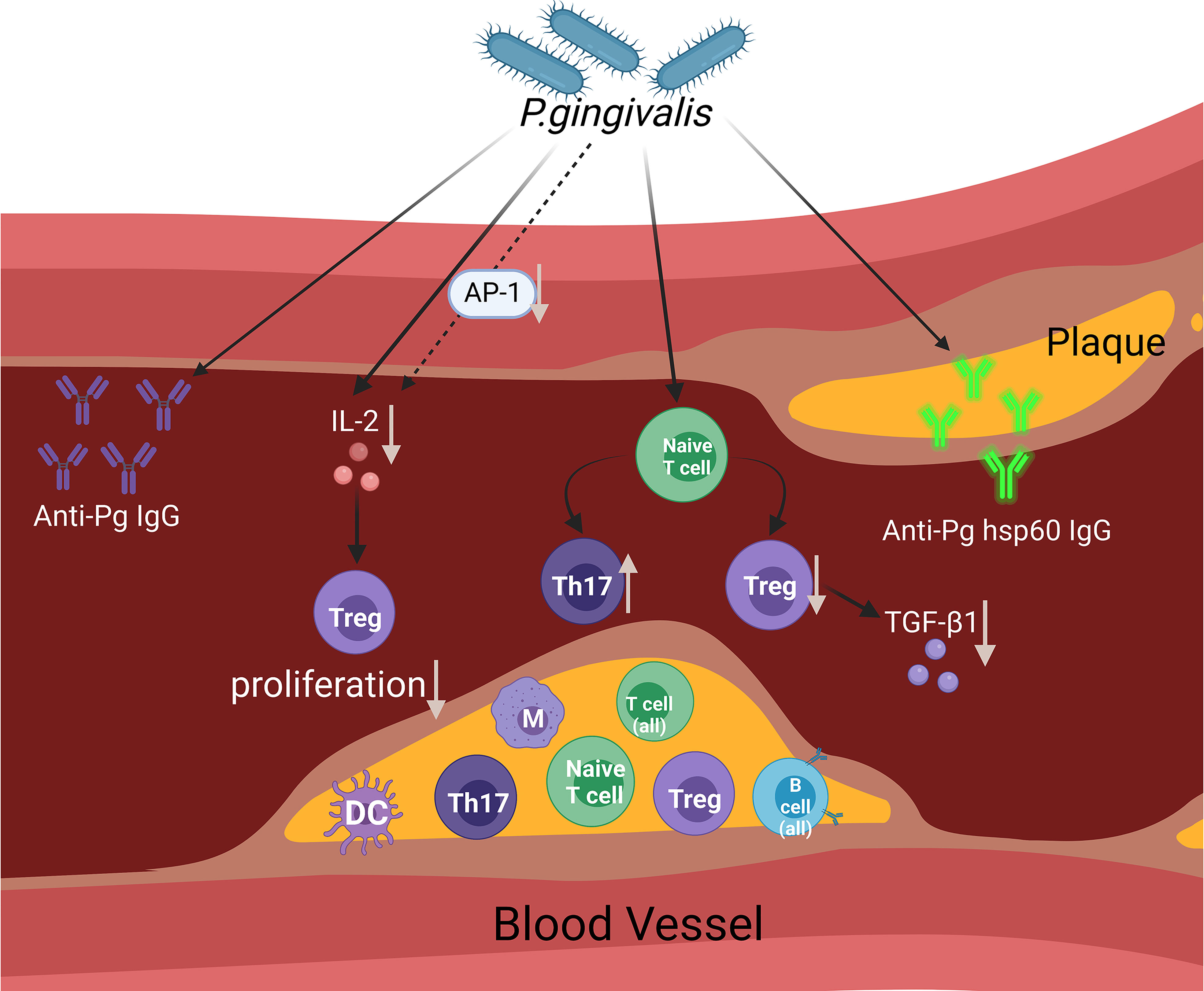
Figure 1 This figure aims to display the presumed contribution of P. gingivalis to atherosclerosis via the regulation of the adaptive immune system. The figure shows a diagram of an open blood vessel affected by atherosclerotic plaque. Within the diagram, we can see that there are different types of immune cells affected in the blood vessels and atherosclerotic plaques. As infection rises within the blood vessels caused by P. gingivalis, the Th17/Treg cell population becomes imbalanced (an increase in the Th17 cell population and a decrease in the Treg cell population) and reduces TGF-β1 levels. In reaction to P. gingivalis, AP-1 transcription factor activity was reduced. The accumulation of IL-2 is influenced by P. gingivalis and partially affects the suppression of AP-1, which affects Treg proliferation. The involvement of adaptive immune responses of the host is proven by the evidence of increased anti-Pg lgG and anti-Pg hsp60 lgG. (Created with BioRender.com).
Hypertension is primarily caused by functional changes in blood vessels, such as increased vasoconstrictor responses and endothelial dysfunction. Recent studies have examined the link between periodontitis and hypertension and successfully linked the two conditions epidemiologically. While there are no simple mechanisms that can fully explain the aetiology behind hypertension, the involvement of both inflammation and immune response has been reported in many studies. P. gingivalis infection influences the host’s inflammatory state and immune responses, promoting hypertension, as evidenced by the findings demonstrating the higher risk of hypertension development in patients suffering from periodontitis (Aguilera et al., 2020). Additionally, the outcomes of clinical trials and periodontal intervention have demonstrated a considerable reduction in systolic and diastolic blood pressure after periodontal therapy is provided (Vidal et al., 2013). This outcome offers preliminary evidence that periodontitis affects hypertension.
The findings demonstrate that the immune response is a crucial factor in developing hypertension and related organ injury, with both innate and adaptive immunity associated with hypertension (Harrison et al., 2011). The presence of T cells in the kidneys and vessels of patients suffering from hypertension has also been reported, in addition to the accumulation of circulating T cells that trigger the production of cytokines in Th1 cells, type 1 T helper cells, and Th17 and IL-17 producer cells (Youn et al., 2013; Itani et al., 2016). Although there are ongoing studies on the T-cell activation process and its influence on hypertension and vascular dysfunction, the results are insufficient to fully understand the mechanisms (Meissner et al., 2017). Chronic infections such as periodontitis have also been suggested to alter the population of T-cells. Such a change in the environment primes the host to exhibit heightened immune responses and promotes disorders like hypertension, such as the elevated production of Th1 that leads to hypertension (Shao et al., 2003). In patients suffering from periodontitis, gingival bacterial infection alters the innate and adaptive immune responses, heightening the level of pro-inflammatory cytokines in the systemic circulation. In the event of chronic infection, T cell memories are formed, which can be activated to divide and generate in a heterologous way through the actions of nonspecific antigens and cytokines (Kim et al., 2002; Freeman et al., 2012). The cells can then migrate towards the vasculature and kidneys, altering the function of the vasculature, leading to renal damage and potentially causing hypertension. This discovery provides preliminary evidence for the relationship between periodontitis and hypertension.
A recent animal study supported the hypothesis that high blood pressure is caused by the Th1 immune response triggered by P. gingivalis antigens (Czesnikiewicz-Guzik et al., 2019). Studies have also discovered that the activation of systemic T-cells seems characteristic of hypertension and is aggravated by antigen stimulation by P. gingivalis. This characteristic later shifted to increased leukocyte infiltration, particularly T-cell and macrophage infiltration, and aortic vascular inflammation. Studies have revealed the elevated expression of the Th1 cytokines IFN-γ, TNF‐α, and TBX21 in the aortas of P. gingivalis/IL-12/aluminum oxide-immunized mouse. While the cytokines continued to show elevated expression, IL-4 and TGF‐β expression was invariant. Furthermore, the induced Th1 mice exhibited a more significant rise in blood pressure and endothelial dysfunction in response to a 2-week infusion of a suppressant or a dose of angiotensin II than the control mice. According to the research, Th1 immune responses to bacterial antigens like P. gingivalis appear more sensitive to low-dose angiotensin II and other suppressive or pro-hypertensive insults.
Maternal, fetal, and neonatal complications that occur during pregnancy, labor, and postpartum are all classified as adverse pregnancy outcomes (APOs). Modern epidemiologic studies have consistently implied positive connections between periodontitis and APOs (Ide and Papapanou, 2013). Evidence has depicted that P. gingivalis DNA and antigens are present within the placenta, umbilical cord (Vanterpool et al., 2016), and amniotic fluid (Leon et al., 2007), which are associated with multiple types of pregnancy complications (Chaparro et al., 2013). These researches suggest that P. gingivalis directly invades and damages uterine placental tissues, contributing to the development of APOs. A study confirmed that P. gingivalis infection could increase the risk of uterine-placental pathologies, including endometrial arteritis, mild chorioamnionitis, and uterine-placental hemorrhage accompanied by placental structural disorders. Thus, we further sought to prove that P. gingivalis infection contributes to the development of APOs.
It is known that periodontitis causes immune responses to switch from Th2 to Th1 and releases cytokines that stimulate the Th1 immune response (Raghupathy, 2001). Fetal growth restriction, spontaneous abortion, and maternal rejection of the implanted fetus have been linked with an increase in the expression of Th1-mediated cytokines such as TNF-α, IL-1, IL-8, IL-2, IL-12, and IFN-γ, as well as a decrease in the expression of Th2-mediated cytokines such as IL-10, TGF-β2, and IL-6 (Liang et al., 2006).
Multiple studies have proposed that an imbalanced Th17 profile (surplus) and Treg cells (reduced) may lead to the development of APOs (Figueiredo and Schumacher, 2016). Pregnancy-induced hypertension, fetal growth restriction, and recurrent miscarriage are the pregnancy-related complications associated with such imbalance. These pregnancy-related complications and maternal inflammation have been associated with the increasing of Th17-mediated cytokines (Dechend et al., 2000; Pongcharoen et al., 2006; Keeler et al., 2009; Dhillion et al., 2012; Ozkan et al., 2015). Additionally, the production of Th17-related cytokines, such as IL-17, was reportedly increased following the activation of Th17 cells by P. gingivalis. According to the main genotype subunit of fimbriae FimA, the strains of P. gingivalis can be classified into six types: I, Ib, II, III, IV, and V. The W83 strain, a type IV strain, is classified as a highly virulent strain of P. gingivalis, which is also a potent inducer of Th17-mediated IL-17 production. A study demonstrated that the activation of NF-κb and RORγ by P. gingivalis caused the elevated production of IL-17 (Manel et al., 2008). Furthermore, P. gingivalis can selectively suppress IL-12 an and boost the Th17 lineage by varying the Th17 to Treg cell ratio (Moutsopoulos et al., 2012), a mechanism associated with the pathogenesis of preeclampsia and spontaneous abortion (Keeler et al., 2009). Additionally, Pngcharoen et al. discovered a significant increase in progesterone secretion during labor due to Th17-induced IL-17 (Pongcharoen et al., 2006).
IL-17 regulates the remodeling of the uterine spiral artery and the modification of the superficial invasion of EVTS into uterine tissues. During the pregnancy period, the progression of atherosclerosis, FGR, and preeclampsia are believed to be associated with the remodeling of the altered spiral artery (Keeler et al., 2009; Ozkan et al., 2015). Ozkan et al. discovered that compared with the control groups, patients suffering recurrent/idiopathic miscarriages and patients with preeclampsia exhibited relatively high levels of IL-17, IL-23, IL-6, soluble IL-6 receptor, and RORγ (Park et al., 2012b; Ozkan et al., 2015). In addition, the production of IL-17 in fetal and placental tissues was increased due to the regulation of angiotensin II type I receptor (AT1-AA) and increased placental oxidative stress caused by P. gingivalis (Dechend et al., 2000). Furthermore, IL-17 production enhanced by AT1-AA has also been linked with the progression of atypical cytotrophoblast invasion and the remodeling of inferior spiral arteries. Recently, a correlation between the increased expression of IL-17 and IL-17R and abnormal cortical progression and autism-like behavioral abnormalities in the developing embryo has been revealed (Dechend et al., 2000; Dhillion et al., 2012).
Inflammatory bowel disease (IBD) is a class of chronic idiopathic diseases, primarily composed of: Crohn’s disease (CD) and ulcerative colitis (UC). Today, multiple epidemiological studies have suggested a link between IBD and periodontitis. Compared with non-IBD patients, patients suffering from IBD have been found to have a relatively high risk of periodontitis and weak oral health (Papageorgiou et al., 2017; Lauritano et al., 2019). A study on SAMP1/YitFc mice revealed a spontaneous model of Crohn’s disease generated by natural periodontitis (Pietropaoli et al., 2014). Additonally, IBD patients’ oral symptoms also have an impact on the composition of the oral microbiota (Xun et al., 2018), indicating a connection between IBD and PD.
Despite the findings, the pathogenesis of inflammatory bowel disease is still poorly understood, which may be related to environmental factors and genetic elements, intestinal disorders, and immune regulation disorders (de Souza and Fiocchi, 2016). Furthermore, the combination of a genetic predisposition, an excessive host response, and the presence of environmental stimuli, which are the primary factors in the pathogenesis of periodontitis, are comparable to those that cause IBD (mainly pathogenic microflora). Multiple factors affecting IBD have also been identified as risk factors for periodontitis (D'Aiuto et al., 2004; Kinane and Bartold, 2007; Indriolo et al., 2011). Additionally, studies have proven that both IBD and periodontitis diseases exhibit the characteristics of immunoinflammatory and tissue destruction (Brandtzaeg, 2001; Kinane and Bartold, 2007). The underlying mechanisms that connect these two diseases may include common bacterial aetiology and shared immune pathways (Baima et al., 2022). First, intestinal ecology becomes dysbiotic due to the migration of periodontal pathobionts from the mouth cavity to the gut. Second, a disturbed gut microbiota results in an intestinal immune response that manifests as intestinal and systemic inflammation, which can cause periodontitis to develop or worsen. And last, the original causes of PD or IBD can be dysbiosis of the intestinal or oral microbiota. Subsequent modifications, such as pathogens, virulence factors, toxic metabolites, and other proinflammatory elements, might travel via the circulatory system between the intestines and the mouth cavity. Also, Stein et al. conducted a study to examine periodontal pathogens in the subgingival plaque of 147 Crohn’s disease patients (Stein et al., 2010), which revealed that among all 147 patients, 76.9% were found infected by Aggregatibacter actinomycetemcomitans and 62.6% were found infected by P. gingivalis. Combined with the recent animal studies showing that periodontal pathogens can cause intestinal inflammation, it is safe to conclude that there is a bidirectional relationship between the two diseases.
A survey by Arimatsu et al. depicted that the oral administration of P. gingivalis changed the C57BL/6N mouse gut microbiota (Arimatsu et al., 2014), in addition to the change in the function of the gut epithelial barrier, increasing gut permeability. P. gingivalis infection also disrupted the colonic epithelial barrier due to decreased tight junction protein expression (Tsuzuno et al., 2021). In addition to the impairment in intestinal barrier function, the administration of P. gingivalis also induced the overexpression of pro-inflammatory cytokine mRNA in the intestine. The mRNA expression of IL-6 and TNF-α was also observed in the small and large intestines. An insignificant increase in the expression of Foxp3, a characteristic regulatory T cell marker, was also reported, in addition to the reduced expression of Rorγt, a distinct Th17 cell marker (Nakajima et al., 2015). Additionally, in the DSS-induced colitis model, it was discovered that the intravenous injection of P. gingivalis extract-stimulated CD4+ T cells aggravated the inflammatory response and increased the Th17/Treg ratio in the colon and lamina propria lymphocytes. And CD4+ T cells stimulated with P. gingivalis and Lactobacillus rhamnosus GG were found to alleviate colitis by reducing the Th17/Treg ration via JAK-STAT signaling pathway (Jia et al., 2020). Zhao et al. found that P. gingivalis exacerbated the severity of UC, in part through Porphyromonas gingivalis peptidylarginine deiminase (PPAD). The main mechanisms are stimulation of Th17 numbers and IL-17 production, and reduction of Treg numbers and IL-10 production (Zhao et al., 2021). According to a recent study, P. gingivalis modifies the gut microbiota, which eventually stimulates intestinal IL-9+CD4+T cells and inflammation (Sohn et al., 2022). The finding implies that the rise in regulatory T cells may counteract the rise in intestinal tissue inflammation brought on by P. gingivalis, but more research in this area is still needed.
Diabetes mellitus (DM) is a category of metabolic illnesses distinguished by persistent hyperglycemia that develops as a result of irregular insulin production and/or insulin resistance over an extended period of time. Type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are the two most common kinds of DM. T1DM results from autoimmune destruction of β-cells, which typically causes an utter lack of insulin and latent autoimmune diabetes of maturity. T2DM results from insulin resistance and a progressive loss of sufficient β-cell insulin production (American Diabetes Association, 2021). Diabetes mellitus raises the risk of periodontitis, despite the absence of any phenotypic characteristics specific to periodontitis in patients with the condition. Periodontitis also has an impact on the complications of glycemic control. Due to the “two-way” nature of the association between periodontitis and diabetes mellitus, numerous clinical and experimental research have been conducted to better understand the molecular mechanisms underlying these two diseases and how they might interact. Here, we mainly reviewed the important role played by P. gingivalis in the bidirectional relationship between periodontitis and diabetes.
The advancement of next-generation sequencing technologies has aided in the research of the human microbiome, especially the oral microbiome and associated systemic disorders, in recent years. Recent studies have shown that people with diabetes mellitus are at risk for developing periodontitis because of a decline in the relative abundance and prevalence of species compatible with health and an increase in the pathogenic content of the periodontitis-associated microorganisms (including Porphyromonas, Prevotella, Campylobacter, and Fusobacterium) (Ganesan et al., 2017; Long et al., 2017). Some authors, however, present findings that type 2 diabetes mellitus decreases reduces the variety and density of the subgingival microbiome, that this decline is even connected to insufficient glycemic management (Longo et al., 2018; Saeb et al., 2019). Another glycemic control study of the periodontal microbiota showed that in patients with moderate or severe periodontitis, the orange-red cluster (Prevotella melaningenica, Prevotella intermedia, Prevotella nigrescen, and P. gingivalis mixture) was inversely associated with fasting glucose levels (Merchant et al., 2014). This study has demonstrated a relationship between periodontal antibody titers and hyperglycemia. Despite being highly relevant, there are still a lot of uncertainties about the makeup of the subgingival microbial community in diabetes circumstances.
Increased inflammation is typically associated with diabetic problems, and there is strong evidence that DM enhances periodontal tissue inflammation. In human periodontal tissues, T1DM and T2DM both cause an increase in the release of inflammatory cytokines and chemokines (Bastos et al., 2012; Polak and Shapira, 2018). These cytokines include IL-1β, IL-17, IL-23, IL-6, and tumour necrosis factor (Salvi et al., 1998; Graves et al., 2020). Contrarily, diabetics have lower levels of anti-inflammatory factors including IL-4, IL-10, and TGF-β, along with M2 macrophages and anti-inflammatory regulatory T cells (Acharya et al., 2017; Van Dyke, 2017).
The effects of periodontitis on diabetes mellitus may generally be correlated with bacteria and the incidence of inflammatory cytokines in systemic circulation. Therefore, a heightened systemic inflammatory response to subgingival bacteria results in systemically elevated levels of pro-inflammatory cytokines that promote insulin resistance. An interesting experimental study of periodontitis in C57BL/6 female mice induced by various oral pathogens (P. gingivalis, F. nucleatum, P. intermedia) depicted that pathogen-induced periodontitis elevated insulin resistance in high-fat diet-fed mice. Such an event is believed to be caused by the adaptive immune system’s response that explicitly targets periodontal disorders-causing pathogens. Empirical evidence also demonstrates that periodontitis simultaneously affects local and systemic immune responses, weakening glucose metabolism. Moreover, it was found that the periodontitis-aggravated metabolic disease is protected through the transfer of cervical lymph node cells from the infected mice to the noninfected recipients. Treatment with inactivated P. gingivalis before the periodontal infection resulted in the generation of specific antibodies against P. gingivalis, which protects the mouse from periodontitis-induced dysmetabolism. The findings prove the relationship between P. gingivalis antibodies caused by periodontitis, the decrease in such specific antibodies, and defective glucose metabolism in HFD-fed mice. This leads to the conclusion that regional (cervical) adaptive immune system modulation is a partial cause of insulin resistance induced by periodontitis (Blasco-Baque et al., 2017). The findings suggest that vaccination against P. gingivalis may lessen the effect of periodontitis on glucose metabolism. However, the role of P. gingivalis monomers in the two-way relationship between periodontitis and diabetes requires a lot of in-depth research.
Today, it is acknowledged that non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent chronic liver illnesses in people who consume little or no alcohol, also having a fatty liver (Chalasani et al., 2018). NAFLD is an overall term for non-alcoholic steatohepatitis (NASH), a risk factor for fibrosis and liver cancer, and simple fatty liver with little or no inflammation. Numerous studies on NAFLD have been conducted, including both cross-sectional and prospective epidemiological studies, which have proven that periodontal disease is a risk factor for NAFLD (Alazawi et al., 2017; Iwasaki et al., 2018; Shin, 2020). Evidence from the in vivo study of animal models demonstrates that the development of NAFLD is accelerated, and steatosis is strengthened by the periodontopathic bacterial infection (Yoneda et al., 2012; Furusho et al., 2013; Komazaki et al., 2017). In addition, the presence of periodontopathic bacteria was identified in the liver, further suggesting the direct impact of P. gingivalis on NAFLD development. Another study showed significantly higher levels of P. gingivalis in patients with NAFLD than in those without NAFLD (Yoneda et al., 2012).
Fim A type 2 P. gingivalis, which exhibits significant adherence and invasion to host cells and is linked to severe periodontitis (Amano et al., 1999; Nakagawa et al., 2002), was found half of the individuals with NAFLD (Yoneda et al., 2012). Previously, 200 individuals with NAFLD had their livers biopsied and their serum anti-P. gingivalis antibody titers were measured. The degree of fibrosis and antibody titers against P. gingivalis fim A types 1 and 4 were found to be positively correlated. Additionally, progressive fibrosis was linked to antibody titers against P. gingivalis fim A type 4. (Nakahara et al., 2018). Immunohistochemistry was used to identify P. gingivalis in the hepatocytes of liver biopsy samples taken from 40 NAFLD patients. According to the findings, those who tested positive for P. gingivalis progressed more quickly toward hepatic fibrosis than those who tested negative (Furusho et al., 2013). In addition, many studies have shown that P. gingivalis lipopolysaccharide can induce hepatocyte inflammation and intracellular lipid formation (Furusho et al., 2013; Zaitsu et al., 2016; Ding et al., 2019).
However, findings of P. gingivalis affecting the mechanism of adaptive immunity in NAFLD remain limited. Recent studies have revealed that Th17 cells are abundant in animal models’ liver and peripheral blood with NAFLD (Fabbrini et al., 2013; Guo et al., 2016; Rolla et al., 2016). The Th17 cell count in periodontal disease tissues was reportedly high due to P. gingivalis infection. Therefore, when examining the connection from an immunological perspective, P. gingivalis infection may activate the Th17 axis in vivo, and Th17 cells produced by periodontitis may move to the liver and worsen NAFLD (Hatasa et al., 2021). A recent study also found that oral P. gingivalis treatment can directly induce NAFLD in mice, which may be reliant on ferroptosis of liver cells that took place through the imbalance of Th17/Treg brought on by disrupted microbial metabolism (Yao et al., 2022). Meanwhile, insulin resistance is a significant pathogenic factor for NAFLD. Mice suffering from endotoxaemia caused by the injection of P. gingivalis exhibited enhanced NAFLD and damaged glucose tolerance and insulin resistance (Sasaki et al., 2018). The findings demonstrate that the P. gingivalis mechanism related to insulin resistance discussed above may also impact NAFLD, although more research is needed to identify associated processes.
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that damages the tissues in the joints, sometimes resulting in functional impairment (Scott et al., 2010). Recently, several epidemiological studies have found an association between periodontal disease and RA, suggesting that individuals with periodontal disease have a higher prevalence of RA than healthy controls (Ranade and Doiphode, 2012; Fuggle et al., 2016). These findings encourage more research into the similarities between pathogenic and clinical traits of these disorders (Potempa et al., 2017). Patients were found to have autoantibodies, including anti-citrullinated peptide antibodies (ACPAs), years before the disease even manifests itself, suggesting that the immune reactions toward the condition are triggered at sites other than the joints (Rantapaa-Dahlqvist et al., 2003; van de Sande et al., 2011). The development of the immune response in RA is believed to originate from the infection by lung microbiota, periodontal disease, and oral microbiota.
Due to their numerous similarities in pathological and immunological characteristics, the link between periodontitis and RA has been noted. First, Porphyromonas gingivalis peptidylarginine deiminase (PPAD) was directly related to the formation of ACPA. Moreover, the production of pro-inflammatory mediators and the increased infiltration of immune cells induced Th17 cell response, which affect the development of RA. In addition, immune cells were induced to release receptor activator for nuclear factor-κB ligand (RANK-L) that resulted in activating osteoclasts (de Pablo et al., 2009). The possible pathogenic mechanisms linking P. gingivalis to RA are briefly summarised in Figure 2.
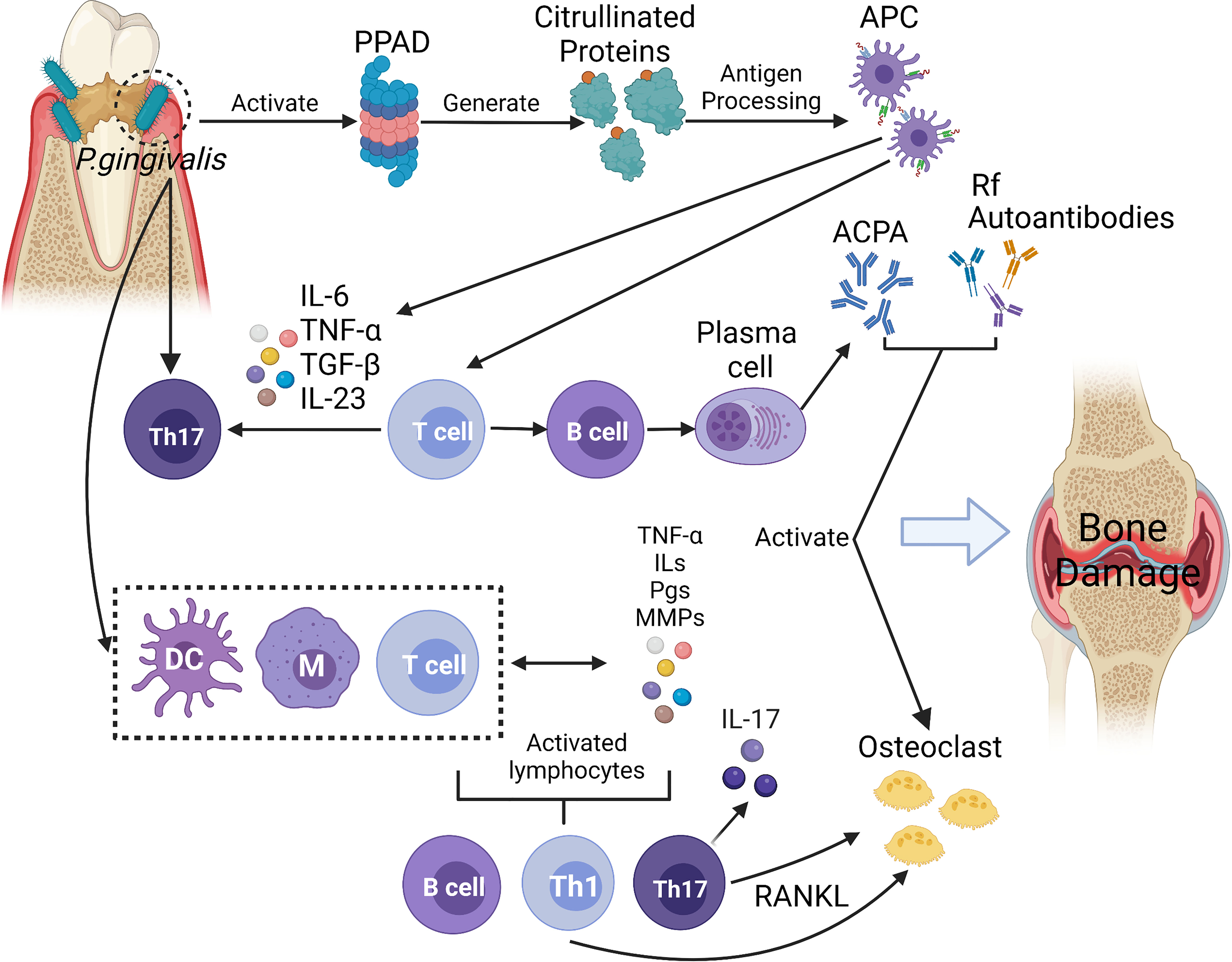
Figure 2 In this diagram, P. gingivalis infection displays a series of activities and effects that lead to bone and cartilage damage. The infection activates proteases and peptidylarginine deiminase (PPADs), which generates citrullinated proteins and triggers the synthesis of anti-citrullinated protein antibodies (ACPAs). In the above reaction, antigen presenting cells (APC) can not only activate T cells and promote B cells’ autoantibodies production, but also produce inflammatory cytokines (such as IL-6, IL-23, IL-1β) to induce Th17 cells, which also affect the development of RA. With the combination of the inflammatory process stimulated by macrophages, dendritic cells (DCs), and T cells, the host response towards citrullinated proteins occurs. With this response, immune cells begin to produce proinflammatory mediators such as interleukins (ILs), prostaglandins (PGs), tumour necrosis factor (TNF), and metalloproteinases (MMPs). The mediators mentioned above also contribute to the irritation of immune responses. IL-17, an important cytokine of Th17 cells, induces osteoblast expression of receptor activator of the factor nuclear kappa B ligand (RANKL), which stimulates osteoclast activation. Enhanced expression of rheumatoid factor (RF) and ACPAs is caused by a resultant signal against citrullinated epitopes in the joints, and such enhanced expression then aids in the formation of immune complexes. These immune complexes form to empower the host’s inflammatory development, which may further complicate RA. Furthermore, ACPAs, RF and other autoantibodies (such as antibodies against carbamylated proteins (anti-CarP), anti-acetylated protein antibodies (AAPA)) may also contribute to the inflammatory process by activating osteoclasts directly and resulting in bone and cartilage damage. (Created with BioRender.com).
P. gingivalis is thought to be the causative agent of the reactions described above because of its unique ability to citrullinate proteins via endogenous Porphyromonas gingivalis peptidylarginine deiminase (PPAD) (McGraw et al., 1999a; Rodríguez et al., 2009). The highly conserved PPAD gene is widespread within P. gingivalis but is absent from P. gingivalis-related species. Lappin et al. discovered that the levels of ACPA serum in patients suffering from periodontitis induced by P. gingivalis were higher than in patients without P. gingivalis (Lappin et al., 2013). It was also found that despite the distribution of P. gingivalis, antibody responses against P. gingivalis were more potent in patients diagnosed with RA and severe periodontitis than in patients in the control group who suffered only from the periodontal condition (de Smit et al., 2012). In contrast to human PAD, PPAD can be activated at higher pH levels and largely functions by citrullinating C-terminal arginines. Citrullinated peptides are produced when arginine gingipains (Rgp) collaborate with another enzyme to degrade polypetides into short peptides with C-terminal arginines, which is subsequently followed by a speedy citrullination process assisted by PPAD (McGraw et al., 1999a; Rodríguez et al., 2009). The continuous exposure to citrullinated peptides that are localized at the level of the periodontium may be caused by P. gingivalis’ posttranslational changes, which may cause immunological tolerance in people with certain genetic dispositions to break down. Lastly, this might influence the creation of ACPA (Valesini et al., 2015). After the infection of P. gingivalis, neutrophils have the ability to extract neutrophil extracellular traps, which are structures with active proteases and PADs that produce citrullinated epitopes and cause ACPA to be produced. Additionally, neutrophils may be drawn to a gingival fissure that is undergoing necrosis, releasing molecules that are associated with damage and causing both local and systemic inflammation (Hahn et al., 2013; Khandpur et al., 2013; Pratesi et al., 2014). Collagen-induced arthritis (CIA) is the most universally studied model of rheumatoid arthritis. A model was created using an inflammation-prone mouse strain by inoculating the mouse with Aggregatibacter, Actinomycetemcomitans, and P. gingivalis orally to induce arthritis and experimental periodontitis. In the study, neither periodontitis nor arthritis progression was altered in the control mice carrying pristine-induced arthritis. The findings reveal that the exacerbation of CIA in DBA/1 mice seems to be dependent on the expression of PPAD (Maresz et al., 2013), demonstrating the crucial role of PPAD in the relationship between periodontitis and RA. Gully et al. found that in the BALB/c mice orally inoculated with a PPAD knockout P. gingivalis W50 strain; the CIA intensity was lower than in the P. gingivalis W50 wild-type strain (Gully et al., 2014). In addition, mice infected with PPAD-KO P. gingivalis exhibited a lower level of ACPA serum than the wild-type strain. Multiple studies have suggested PPAD as a critical factor linking the pathogenic hypothesis between RA and periodontal disease.
P. gingivalis can also stimulate immune cells to produce pro-inflammatory mediators, such as IL-6, IL-1β, TNF-α, IL-17, and matrix-degrading enzymes (MMPs) (Gonzales et al., 2014). These overproduced pro-inflammatory mediators were also the critical pathogenesis of RA, which led to the cartilage and bone destruction (McInnes and Schett, 2011). In addition, through a model of T-cell-dependent experimental arthritis, evidence of the relationship between periodontitis and RA via cellular immunity was discovered. In a study on DBA/1 mice, the severity of CIA was significantly enhanced by periodontitis induced by P. gingivalis and Prevotella nigrescens, as characterized by the increase in arthritic bone erosion via an antigen-induced Th17 response (de Aquino et al., 2014). Th17-mediated immunity was observed to intensify the inflammatory responses to both periodontitis and RA in mice carrying both conditions, and it was reliant on a common hyperinflammatory genotype (Trombone et al., 2010; de Aquino et al., 2014). Thus, the bond between RA and periodontitis may be linked to the mutual aggravation of inflammatory and immunity responses (de Smit et al., 2012). Th17-mediated immunity has gained more attention due to research on the relationships between human periodontitis and RA (Leipe et al., 2010; Lin et al., 2017).
Other virulence components, such as lipopolysaccharide, gingipains, lipoproteins, and fimbriae, are also produced by P. gingivalis. Receptors such as protease-activated receptors, Toll-like receptors, and NOD2 receptors recognize these virulence components in gingival epithelial cells and phagocytes, leading to the activation of RANK-L signaling pathways, complement system, and helper T cell differentiation, which eventually contribute to the development of osteoclastogenesis (Lu et al., 2018; Narayan et al., 2018). Persistent P. gingivalis infection induced the increase of pro-inflammatory mediators and chemokines such as TNF-α, IL-17, IL-6, IL-1β, RANK-L and so on (de Pablo et al., 2009). RANK-L was the main regulator of osteoclastogenesis (McInnes and Schett, 2007; McInnes and Schett, 2011). In addition, activated lymphocytes (especially Th1 and Th17 cells) further stimulated the production of RANK-L (de Pablo et al., 2009), ultimately exacerbating inflammation and osteoarticular destruction in RA.
Alzheimer’s disease (AD), a progressive neurological disease, is a common type of dementia, accounting for between 60 and 80 percent of all cases (Alzheimer’s Association, 2016). AD is characterized by disrupted cognition, classically amyloid-beta (Aβ) deposits, and hyperphosphorylated neurofibrillary tangles (Goedert et al., 1991). Numerous clinical and experimental research have suggested that systemic peripheral inflammation or infection may be the crucial factor in the pathophysiology of AD, which supports bidirectional communication between the brain and the systemic immune response (Perry et al., 2007; Poole et al., 2013; Cunningham and Hennessy, 2015).
Early research on Alzheimer’s disease has typically assumed that innate immunity is the primary factor contributing to neuropatholog. Recent studies have revealed a different perspective, suggesting that the adaptive immune system influences the mechanisms related to suppressing neuropathology in AD (Marsh et al., 2016; Olsen et al., 2016). Although the innate immune system is thought to be linked to inflammatory components in AD, adaptive immunity was not previously thought of. Additionally, the role of adaptive immunity has been proposed based on genome-wide studies, which reveal the association between the inability of microglia to clear Aβ from the brain and several genes involved in the immune system (Guerreiro et al., 2013). Pathogens can typically increase Aβ deposition, including P. gingivalis lipopolysaccharide (Wu et al., 2017). Aβ is a protein with various antimicrobial effects against bacteria, fungi, and viruses, and its deposition has been linked to the immune response in the host towards the cerebral invasion of pathogens, which are associated with AD (Soscia et al., 2010; Bourgade et al., 2015; Kumar et al., 2016). The peripheral pools of Aβ in the body can be increased by systemic infection with P. gingivalis (Nie et al., 2019), and chronic P. gingivalis infection may interact synergistically with cerebral RAGE expression and peripheral Aβ production (Zeng et al., 2021). In mice study, an oral P. gingivalis infection led to brain colonization and increased production of Aβ1-42, a substance found in amyloid plaques (Dominy et al., 2019a).
More clinical research has now examined the link between periodontitis and AD, yielding better findings (Stein et al., 2012; Kamer et al., 2015; Chen et al., 2017). A recent research used an animal model of periodontitis and human brain tissues suffering from AD after death. According to the study, P. gingivalis and gingipain were translocated into the brain (Ilievski et al., 2018; Dominy et al., 2019a). After receiving oral P. gingivalis inoculations every two days for five months, Ilievski et al. found that C57BL/6 mice developed neuropathology similar to Alzheimer’s disease (Ilievski et al., 2018). Dominy et al. also reported evidence showing the influence of P. gingivalis and gingipains on the pathogenesis of AD. The study also provided strong evidence proving the presence of P. gingivalis DNA and gingipain antigens in the host brain of AD patients (Dominy et al., 2019a). Furthermore, the same study also discovered that by providing small-molecule gingipain inhibitors or the wide-range antibiotic moxifloxacin, the neurodegeneration caused by gingipain could be blocked. Doing so significantly decreased the P. gingivalis load in the aged mouse brain and reduced the host Aβ1–42 response towards P. gingivalis brain infection. This discovery has created a new framework for treating AD (Dominy et al., 2019a).
As more findings from the studies are revealed, it is possible to suggest that periodontitis-related low-grade systemic inflammation could increase the blood-brain barrier’s permeability (BBB). The increase in BBB permeability causes an surge of peripheral immune cells and pathogens toward the host, which can lead to activate the neuroinflammatory reaction result in the neural network breakdown (Sadrameli et al., 2020). The ability of pathogens to evade the peripheral compartment has further been proven by the discovery of LPS in the neocortex of AD patients (Zhao et al., 2017). Additionally, Poole et al. discovered the presence of P. gingivalis lipopolysaccharide in the brains of patients suffering from AD (Poole et al., 2013). Moreover, TLR-2 was found to mediate IL-10 production, which inhibited the IFN-γ and T cell response after the initial systemic exposure to P. gingivalis, allowing the pathogen to evade the host’s immune response in the brain (Poole et al., 2013). Through studies conducted in mice, IFN-γ has been found to regulate the protection and repair of neurons. Increased neurogenesis could be an indication of how the immune system normally regulates inflammation and healing in the brain (Baron et al., 2008; Hohlfeld, 2008). Given the evidence, the systemic and oral connections between periodontitis and AD are impossible to ignore. More in-depth studies are required to understand the interactions between the host and pathogen throughout AD development.
In summary, many epidemiological studies, in vivo experiments, and in vitro experiments have demonstrated multiple correlations between periodontitis and many systematic chronic diseases. P. gingivalis can affect the adaptive immune system in various ways in periodontitis and many other related systemic disorders. Additionally, strategies that promote immune-subverting and pro-inflammatory mechanisms in periodontal bacteria will harm the periodontium and affect the association of periodontitis with systemic diseases. However, current studies on P. gingivalis in affecting systemic diseases through regulating adaptive immunity are insufficient, particularly on neurological disorders, respiratory diseases, and kidney diseases, which are closely related to periodontitis.
Key findings from these studies must be applied in clinical applications, e.g., through the host modulation therapies that combat the disruptive immune mechanisms of periodontal bacteria, thereby assisting in the management of periodontitis and related systemic inflammatory diseases. The host modification strategies may be more effective than direct antibacterial treatments. It is especially crucial when targeting keystone pathogens as they are highly virulent even in low abundance and have a lower chance of being entirely eradicated, partly due to their ability to conceal themselves within permissive host cells. Immunotherapy may be a viable new treatment for chronic inflammatory diseases, which demands further research into the field of oral microbiome modulation and its implications on systemic disease.
CL and YD participated in the review selection and design. CL and RY wrote the first draft of the review. YD edited and finalized the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China(No.81800984), Fundamental Research Funds for the Central Universities(No.2020kfyXGYJ082), National Science Foundation of Hubei Province(No.2020CFB787). Free Innovation pre-Research Foundation of Wuhan Union Hospital(2021xhyn090).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acharya, A. B., Thakur, S., Muddapur, M. V., Kulkarni, R. D. (2017). Cytokine ratios in chronic periodontitis and type 2 diabetes mellitus. Diabetes Metab. Syndr. 11 (4), 277–278. doi: 10.1016/j.dsx.2016.12.007
Adibrad, M., Deyhimi, P., Ganjalikhani Hakemi, M., Behfarnia, P., Shahabuei, M., Rafiee, L. (2012). Signs of the presence of Th17 cells in chronic periodontal disease. J. Periodontal. Res. 47 (4), 525–531. doi: 10.1111/j.1600-0765.2011.01464.x
Aguilera, E. M., Suvan, J., Buti, J., Czesnikiewicz-Guzik, M., Ribeiro, A. B., Orlandi, M., et al. (2020). Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 116 (1), 28–39. doi: 10.1093/cvr/cvz201
Alayan, J., Ivanovski, S., Farah, C. S. (2007). Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient mice. J. Periodontal. Res. 42 (2), 97–103. doi: 10.1111/j.1600-0765.2006.00920.x
Alazawi, W., Bernabe, E., Tai, D., Janicki, T., Kemos, P., Samsuddin, S., et al. (2017). Periodontitis is associated with significant hepatic fibrosis in patients with non-alcoholic fatty liver disease. PLos One 12 (12), e185902. doi: 10.1371/journal.pone.0185902
Aliko, A., Kamińska, M., Bergum, B., Gawron, K., Benedyk, M., Lamont, R. J., et al. (2018). Impact of porphyromonas gingivalis peptidylarginine deiminase on bacterial biofilm formation, epithelial cell invasion, and epithelial cell transcriptional landscape. Sci. Rep. 8 (1), 14144. doi: 10.1038/s41598-018-32603-y
Amano, A., Nakagawa, I., Kataoka, K., Morisaki, I., Hamada, S. (1999). Distribution of porphyromonas gingivalis strains with fimA genotypes in periodontitis patients. J. Clin. Microbiol. 37 (5), 1426–1430. doi: 10.1128/JCM.37.5.1426-1430.1999
Amano, A., Nakagawa, I., Okahashi, N., Hamada, N. (2004). Variations of porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal. Res. 39 (2), 136–142. doi: 10.1111/j.1600-0765.2004.00719.x
Andersson, J., Libby, P., Hansson, G. K. (2010). Adaptive immunity and atherosclerosis. Clin. Immunol. 134 (1), 33–46. doi: 10.1016/j.clim.2009.07.002
Arimatsu, K., Yamada, H., Miyazawa, H., Minagawa, T., Nakajima, M., Ryder, M. I., et al. (2014). Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 4, 4828. doi: 10.1038/srep04828
Alzheimer’s Association (2016). 2016 alzheimer's disease facts and figures. Alzheimers Dement. 12 (4), 459–509. doi: 10.1016/j.jalz.2016.03.001
American Diabetes Association. (2021). 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 44 (Suppl 1), S15–s33. doi: 10.2337/dc21-S002
Baba, A., Abe, N., Kadowaki, T., Nakanishi, H., Ohishi, M., Asao, T., et al. (2001). Arg-gingipain is responsible for the degradation of cell adhesion molecules of human gingival fibroblasts and their death induced by porphyromonas gingivalis. Biol. Chem. 382 (5), 817–824. doi: 10.1515/bc.2001.099
Baima, G., Massano, A., Squillace, E., Caviglia, G. P., Buduneli, N., Ribaldone, D. G., et al. (2022). Shared microbiological and immunological patterns in periodontitis and IBD: A scoping review. Oral. Dis. 28(4), 1029–1041. doi: 10.1111/odi.13843
Bainbridge, B., Verma, R. K., Eastman, C., Yehia, B., Rivera, M., Moffatt, C., et al. (2010). Role of porphyromonas gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect. Immun. 78 (11), 4560–4569. doi: 10.1128/iai.00703-10
Baker, P. J., Dixon, M., Evans, R. T., Dufour, L., Johnson, E., Roopenian, D. C. (1999). CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67 (6), 2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999
Baron, R., Nemirovsky, A., Harpaz, I., Cohen, H., Owens, T., Monsonego, A. (2008). IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of alzheimer's disease. FASEB J. 22 (8), 2843–2852. doi: 10.1096/fj.08-105866
Bastos, A. S., Graves, D. T., Loureiro, A. P., Rossa Júnior, C., Abdalla, D. S., Faulin Tdo, E., et al. (2012). Lipid peroxidation is associated with the severity of periodontal disease and local inflammatory markers in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 97 (8), E1353–E1362. doi: 10.1210/jc.2011-3397
Blasco-Baque, V., Garidou, L., Pomie, C., Escoula, Q., Loubieres, P., Le Gall-David, S., et al. (2017). Periodontitis induced by porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 66 (5), 872–885. doi: 10.1136/gutjnl-2015-309897
Bourgade, K., Garneau, H., Giroux, G., Le Page, A. Y., Bocti, C., Dupuis, G., et al. (2015). β-amyloid peptides display protective activity against the human alzheimer's disease-associated herpes simplex virus-1. Biogerontology 16 (1), 85–98. doi: 10.1007/s10522-014-9538-8
Brandtzaeg, P. (2001). Inflammatory bowel disease: clinics and pathology. do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odontol. Scand. 59 (4), 235–243. doi: 10.1080/00016350152509265
Bui, F. Q., Almeida-da-Silva, C. L.C., Huynh, B., et al. (2019). Association between periodontal pathogens and systemic disease. Biomed J. 42 (1), 27–35. doi: 10.1016/j.bj.2018.12.001
Cai, Y., Kobayashi, R., Hashizume-Takizawa, T., Kurita-Ochiai, T. (2014). Porphyromonas gingivalis infection enhances Th17 responses for development of atherosclerosis. Arch. Oral. Biol. 59 (11), 1183–1191. doi: 10.1016/j.archoralbio.2014.07.012
Calder, C. J., Nicholson, L. B., Dick, A. D. (2006). Mechanisms for inducing nasal mucosal tolerance in experimental autoimmune uveoretinitis. Methods 38 (2), 69–76. doi: 10.1016/j.ymeth.2005.09.008
Cardoso, C. R., Garlet, G. P., Moreira, A. P., Júnior, W. M., Rossi, M. A., Silva, J. S. (2008). Characterization of CD4+CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J. Leukoc. Biol. 84 (1), 311–318. doi: 10.1189/jlb.0108014
Chalasani, N., Younossi, Z., Lavine, J. E., Charlton, M., Cusi, K., Rinella, M., et al. (2018). The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology 67 (1), 328–357. doi: 10.1002/hep.29367
Chaparro, A., Blanlot, C., Ramirez, V., Sanz, A., Quintero, A., Inostroza, C., et al. (2013). Porphyromonas gingivalis, treponema denticola and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: a case-control study. J. Periodontal. Res. 48 (6), 802–809. doi: 10.1111/jre.12074
Chapple, I. L. C., Genco, R., Worksh, W.G.J.E.A. (2013). Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 40, S106–S112. doi: 10.1111/jcpe.12077
Chaudhry, A., Rudensky, A. Y. (2013). Control of inflammation by integration of environmental cues by regulatory T cells. J. Clin. Invest. 123 (3), 939–944. doi: 10.1172/jci57175
Cheng, W. C., Hughes, F. J., Taams, L. S. (2014). The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J. Clin. Periodontol. 41 (6), 541–549. doi: 10.1111/jcpe.12238
Cheng, X., Yu, X., Ding, Y. J., Fu, Q. Q., Xie, J. J., Tang, T. T., et al. (2008). The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 127 (1), 89–97. doi: 10.1016/j.clim.2008.01.009
Chen, C. K., Wu, Y. T., Chang, Y. C. (2017). Association between chronic periodontitis and the risk of alzheimer's disease: a retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 9 (1), 56. doi: 10.1186/s13195-017-0282-6
Choi, J. I., Chung, S. W., Kang, H. S., Rhim, B. Y., Kim, S. J., Kim, S. J. (2002). Establishment of porphyromonas gingivalis heat-shock-protein-specific T-cell lines from atherosclerosis patients. J. Dent. Res. 81 (5), 344–348. doi: 10.1177/154405910208100511
Choi, J. I., Chung, S. W., Kang, H. S., Rhim, B. Y., Park, Y. M., Kim, U. S., et al. (2004). Epitope mapping of porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J. Dental Res. 83 (12), 936–940. doi: 10.1177/154405910408301209
Choi, J., Lee, S. Y., Kim, K., Choi, B. K. (2011). Identification of immunoreactive epitopes of the porphyromonas gingivalis heat shock protein in periodontitis and atherosclerosis. J. Periodontal. Res. 46 (2), 240–245. doi: 10.1111/j.1600-0765.2010.01339.x
Cunningham, C., Hennessy, E. (2015). Co-Morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimers Res. Ther. 7, 33. doi: 10.1186/s13195-015-0117-2
Curtis, M. A., Aduse-Opoku, J., Rangarajan, M. (2001). Cysteine proteases of porphyromonas gingivalis. Crit. Rev. Oral. Biol. Med. 12 (3), 192–216. doi: 10.1177/10454411010120030101
Czesnikiewicz-Guzik, M., Nosalski, R., Mikolajczyk, T. P., Vidler, F., Dohnal, T., Dembowska, E., et al. (2019). Th1-type immune responses to porphyromonas gingivalis antigens exacerbate angiotensin II-dependent hypertension and vascular dysfunction. Br. J. Pharmacol. 176 (12), 1922–1931. doi: 10.1111/bph.14536
D'Aiuto, F., Parkar, M., Brett, P. M., Ready, D., Tonetti, M. S. (2004). Gene polymorphisms in pro-inflammatory cytokines are associated with systemic inflammation in patients with severe periodontal infections. Cytokine 28 (1), 29–34. doi: 10.1016/j.cyto.2004.06.005
d'Empaire, G., Baer, M. T., Gibson, F. C. (2006). The K1 serotype capsular polysaccharide of porphyromonas gingivalis elicits chemokine production from murine macrophages that facilitates cell migration. Infect. Immun. 74 (11), 6236–6243. doi: 10.1128/iai.00519-06
Darveau, R. P. (2010). Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8 (7), 481–490. doi: 10.1038/nrmicro2337
Davey, M., Liu, X., Ukai, T., Jain, V., Gudino, C., Gibson, F. C., et al. (2008). Bacterial fimbriae stimulate proinflammatory activation in the endothelium through distinct TLRs. J. Immunol. 180 (4), 2187–2195. doi: 10.4049/jimmunol.180.4.2187
de Aquino, S. G., Abdollahi-Roodsaz, S., Koenders, M. I., van de Loo, F. A., Pruijn, G. J., Marijnissen, R. J., et al. (2014). Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J. Immunol. 192 (9), 4103–4111. doi: 10.4049/jimmunol.1301970
Dechend, R., Homuth, V., Wallukat, G., Kreuzer, J., Park, J. K., Theuer, J., et al. (2000). AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation 101 (20), 2382–2387. doi: 10.1161/01.CIR.101.20.2382
de Pablo, P., Chapple, I. L., Buckley, C. D., Dietrich, T. (2009). Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 5 (4), 218–224. doi: 10.1038/nrrheum.2009.28
de Smit, M., Westra, J., Vissink, A., Doornbos-van der Meer, B., Brouwer, E., van Winkelhoff, A. J. (2012). Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res. Ther. 14 (5), R222. doi: 10.1186/ar4061
de Souza, H. S. P., Fiocchi, C. (2016). Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13 (1), 13–27. doi: 10.1038/nrgastro.2015.186
Dhillion, P., Wallace, K., Herse, F., Scott, J., Wallukat, G., Heath, J., et al. (2012). IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 303 (4), R353–R358. doi: 10.1152/ajpregu.00051.2012
Ding, L. Y., Liang, L. Z., Zhao, Y. X., Yang, Y. N., Liu, F., Ding, Q. R., et al. (2019). Porphyromonas gingivalis-derived lipopolysaccharide causes excessive hepatic lipid accumulation via activating NF-κB and JNK signaling pathways. Oral. Dis. 25 (7), 1789–1797. doi: 10.1111/odi.13153
Diya, Z., Lili, C., Shenglai, L., Zhiyuan, G., Jie, Y. (2008). Lipopolysaccharide (LPS) of porphyromonas gingivalis induces IL-1beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of escherichia coli LPS. Innate Immun. 14 (2), 99–107. doi: 10.1177/1753425907088244
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019a). Porphyromonas gingivalis in alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5 (1), eaau3333. doi: 10.1126/sciadv.aau3333
Dutzan, N., Vernal, R., Vaque, J. P., García-Sesnich, J., Hernandez, M., Abusleme, L., et al. (2012). Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J. Periodontol. 83 (7), 948–954. doi: 10.1902/jop.2011.110482
Ebersole, J. L., Kirakodu, S. S., Novak, M. J., Orraca, L., Martinez, J. G., Cunningham, L. L., et al. (2016). Transcriptome analysis of b cell immune functions in periodontitis: Mucosal tissue responses to the oral microbiome in aging. Front. Immunol. 7. doi: 10.3389/fimmu.2016.00272
Ernst, C. W., Lee, J. E., Nakanishi, T., Karimbux, N. Y., Rezende, T. M., Stashenko, P., et al. (2007). Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease. Clin. Exp. Immunol. 148 (2), 271–280. doi: 10.1111/j.1365-2249.2006.03318.x
Fabbrini, E., Cella, M., Mccartney, S. A., Fuchs, A., Abumrad, N. A., Pietka, T. A., et al. (2013). Association between specific adipose tissue CD4(+) T-cell populations and insulin resistance in obese individuals. Gastroenterology 145 (2), 366–36+. doi: 10.1053/j.gastro.2013.04.010
Figueiredo, A. S., Schumacher, A. (2016). The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 148 (1), 13–21. doi: 10.1111/imm.12595
Fleetwood, A. J., Lee, M. K. S., Singleton, W., Achuthan, A., Lee, M. C., O'Brien-Simpson, N. M., et al. (2017). Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by porphyromonas gingivalis and its outer membrane vesicles. Front. Cell Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00351
Freeman, B. E., Hammarlund, E., Raué, H. P., Slifka, M. K. (2012). Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl. Acad. Sci. U.S.A. 109 (25), 9971–9976. doi: 10.1073/pnas.1203543109
Fuggle, N. R., Smith, T. O., Kaul, A., Sofat, N. (2016). Hand to mouth: A systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front. Immunol. 7. doi: 10.3389/fimmu.2016.00080
Fukasawa, A., Kurita-Ochiai, T., Hashizume, T., Kobayashi, R., Akimoto, Y., Yamamoto, M. (2012). Porphyromonas gingivalis accelerates atherosclerosis in C57BL/6 mice fed a high-fat diet. Immunopharmacol. Immunotoxicol. 34 (3), 470–476. doi: 10.3109/08923973.2011.627866
Furusho, H., Miyauchi, M., Hyogo, H., Inubushi, T., Ao, M., Ouhara, K., et al. (2013). Dental infection of porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J. Gastroenterol. 48 (11), 1259–1270. doi: 10.1007/s00535-012-0738-1
Gabarrini, G., Palma Medina, L. M., Stobernack, T., Prins, R. C., du Teil Espina, M., Kuipers, J., et al. (2018). There's no place like OM: Vesicular sorting and secretion of the peptidylarginine deiminase of porphyromonas gingivalis. Virulence 9 (1), 456–464. doi: 10.1080/21505594.2017.1421827
Gaddis, D. E., Maynard, C. L., Weaver, C. T., Michalek, S. M., Katz, J. (2013). Role of TLR2-dependent IL-10 production in the inhibition of the initial IFN-γ T cell response to porphyromonas gingivalis. J. Leukoc. Biol. 93 (1), 21–31. doi: 10.1189/jlb.0512220
Ganesan, S. M., Joshi, V., Fellows, M., Dabdoub, S. M., Nagaraja, H. N., O'Donnell, B., et al. (2017). A tale of two risks: smoking, diabetes and the subgingival microbiome. Isme J. 11 (9), 2075–2089. doi: 10.1038/ismej.2017.73
Gemmell, E., Grieco, D. A., Cullinan, M. P., Westerman, B., Seymour, G. J. (1999). The proportion of interleukin-4, interferon-gamma and interleukin-10-positive cells in Porphyromonas gingivalis--specific T-cell lines established from P. gingivalis-positive subjects. Oral. Microbiol. Immunol. 14 (5), 267–274. doi: 10.1034/j.1399-302x.1999.140501.x
Goedert, M., Sisodia, S. S., Price, D. L. (1991). Neurofibrillary tangles and beta-amyloid deposits in alzheimer's disease. Curr. Opin. Neurobiol. 1 (3), 441–447. doi: 10.1016/0959-4388(91)90067-h
Gonzales, J. R., Groeger, S., Johansson, A., Meyle, J. (2014). T Helper cells from aggressive periodontitis patients produce higher levels of interleukin-1 beta and interleukin-6 in interaction with porphyromonas gingivalis. Clin. Oral. Investig. 18 (7), 1835–1843. doi: 10.1007/s00784-013-1162-5
Gounopoulos, P., Merki, E., Hansen, L. F., Choi, S. H., Tsimikas, S. (2007). Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva cardioangiologica 55 (6), 821–837.
Graves, D. T., Ding, Z., Yang, Y. (2020). The impact of diabetes on periodontal diseases. Periodontol 2000 82 (1), 214–224. doi: 10.1111/prd.12318
Guerreiro, R., Wojtas, A., Bras, J., Carrasquillo, M., Rogaeva, E., Majounie, E., et al. (2013). TREM2 variants in alzheimer's disease. N Engl. J. Med. 368 (2), 117–127. doi: 10.1056/NEJMoa1211851
Gully, N., Bright, R., Marino, V., Marchant, C., Cantley, M., Haynes, D., et al. (2014). Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLos One 9 (6), e100838. doi: 10.1371/journal.pone.0100838
Guo, Y., Nguyen, K. A., Potempa, J. (2010). Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 54 (1), 15–44. doi: 10.1111/j.1600-0757.2010.00377.x
Guo, H., Xu, B. C., Yang, X. G., Peng, D., Wang, Y., Liu, X. B., et al. (2016). A high frequency of peripheral blood IL-22(+)CD4(+) T cells in patients with new onset type 2 diabetes mellitus. J. Clin. Lab. Anal. 30 (2), 95–102. doi: 10.1002/jcla.21821
Hagiwara, M., Kurita-Ochiai, T., Kobayashi, R., Hashizume-Takizawa, T., Yamazaki, K., Yamamoto, M. (2014). Sublingual vaccine with GroEL attenuates atherosclerosis. J. Dent. Res. 93 (4), 382–387. doi: 10.1177/0022034514523784
Hahn, S., Giaglis, S., Chowdhury, C. S., Hösli, I., Hasler, P. (2013). Modulation of neutrophil NETosis: interplay between infectious agents and underlying host physiology. Semin. Immunopathol. 35 (4), 439–453. doi: 10.1007/s00281-013-0380-x
Hajishengallis, G. (2009). Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 11 (6-7), 637–645. doi: 10.1016/j.micinf.2009.03.009
Hajishengallis, G. (2011). Immune evasion strategies of porphyromonas gingivalis. J. Oral. Biosci. 53 (3), 233–240. doi: 10.2330/joralbiosci.53.233
Hajishengallis, G., Darveau, R. P., Curtis, M. A. (2012). The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10 (10), 717–725. doi: 10.1038/nrmicro2873
Hajishengallis, G. (2015). Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 15 (1), 30–44. doi: 10.1038/nri3785doi
Hajishengallis, G., Wang, M., Liang, S., Triantafilou, M., Triantafilou, K. (2008). Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl. Acad. Sci. U.S.A. 105 (36), 13532–13537. doi: 10.1073/pnas.0803852105
Hamedi, M., Belibasakis, G. N., Cruchley, A. T., Rangarajan, M., Curtis, M. A., Bostanci, N. (2009). Porphyromonas gingivalis culture supernatants differentially regulate interleukin-1beta and interleukin-18 in human monocytic cells. Cytokine 45 (2), 99–104. doi: 10.1016/j.cyto.2008.11.005
Hansson, G. K., Hermansson, A. (2011). The immune system in atherosclerosis. Nat. Immunol. 12 (3), 204–212. doi: 10.1038/ni.2001
Harrison, D. G., Guzik, T. J., Lob, H. E., Madhur, M. S., Marvar, P. J., Thabet, S. R., et al. (2011). Inflammation, immunity, and hypertension. Hypertension 57 (2), 132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576
Hatasa, M., Yoshida, S., Takahashi, H., Tanaka, K., Kubotsu, Y., Ohsugi, Y., et al. (2021). Relationship between NAFLD and periodontal disease from the view of clinical and basic research, and immunological response. Int. J. Mol. Sci. 22 (7), 3728. doi: 10.3390/ijms22073728
Hohlfeld, R. (2008). Neurotrophic cross-talk between the nervous and immune systems: relevance for repair strategies in multiple sclerosis? J. Neurol. Sci. 265 (1-2), 93–96. doi: 10.1016/j.jns.2007.03.012
Honda, K. (2011). Porphyromonas gingivalis sinks teeth into the oral microbiota and periodontal disease. Cell Host Microbe 10 (5), 423–425. doi: 10.1016/j.chom.2011.10.008
Hotokezaka, H., Hayashida, H., Ohara, N., Nomaguchi, H., Kobayashi, K., Yamada, T. (1994). Cloning and sequencing of the groESL homologue from porphyromonas gingivalis. Biochim. Biophys. Acta 1219 (1), 175–178. doi: 10.1016/0167-4781(94)90265-8
How, K. Y., Song, K. P., Chan, K. G. (2016). Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.00053
Ide, M., Papapanou, P. N. (2013). Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes–systematic review. J. Clin. Periodontol. 40 (Suppl 14), S181–S194. doi: 10.1111/jcpe.12063
Ilievski, V., Zuchowska, P. K., Green, S. J., Toth, P. T., Ragozzino, M. E., Le, K., et al. (2018). Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLos One 13 (10), e204941. doi: 10.1371/journal.pone.0204941
Indriolo, A., Greco, S., Ravelli, P., Fagiuoli, S. (2011). What can we learn about biofilm/host interactions from the study of inflammatory bowel disease. J. Clin. Periodontol. 38, 36–43. doi: 10.1111/j.1600-051X.2010.01680.x
Itani, H. A., McMaster, W. G., Saleh, M. A., Nazarewicz, R. R., Mikolajczyk, T. P., Kaszuba, A. M., et al. (2016). Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68 (1), 123. doi: 10.1161/HYPERTENSIONAHA.116.07237
Ivanyi, L., Lehner, T. (1970). Stimulation of lymphocyte transformation by bacterial antigens in patients with periodontal disease. Arch. Oral. Biol. 15 (11), 1089–1096. doi: 10.1016/0003-9969(70)90121-4
Iwasaki, T., Hirose, A., Azuma, T., Ohashi, T., Watanabe, K., Obora, A., et al. (2018). Correlation between ultrasound-diagnosed non-alcoholic fatty liver and periodontal condition in a cross-sectional study in Japan. Sci. Rep. 8(1), 7496. doi: 10.1038/s41598-018-25857-z
Jia, L., Wu, R., Han, N., Fu, J., Luo, Z., Guo, L., et al. (2020). Porphyromonas gingivalis and lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin. Transl. Immunol. 9 (11), e1213. doi: 10.1002/cti2.1213
Jin, Y., Wang, L., Liu, D., Lin, X. (2014). Tamibarotene modulates the local immune response in experimental periodontitis. Int. Immunopharmacol. 23 (2), 537–545. doi: 10.1016/j.intimp.2014.10.003
Jotwani, R., Cutler, C. W. (2004). Fimbriated porphyromonas gingivalis is more efficient than fimbria-deficient p. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infect. Immun. 72 (3), 1725–1732. doi: 10.1128/iai.72.3.1725-1732.2004
Jotwani, R., Pulendran, B., Agrawal, S., Cutler, C. W. (2003). Human dendritic cells respond to porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur. J. Immunol. 33 (11), 2980–2986. doi: 10.1002/eji.200324392
Kamer, A. R., Pirraglia, E., Tsui, W., Rusinek, H., Vallabhajosula, S., Mosconi, L., et al. (2015). Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging 36 (2), 627–633. doi: 10.1016/j.neurobiolaging.2014.10.038
Kanzaki, H., Movila, A., Kayal, R., Napimoga, M. H., Egashira, K., Dewhirst, F., et al. (2017). Phosphoglycerol dihydroceramide, a distinctive ceramide produced by porphyromonas gingivalis, promotes RANKL-induced osteoclastogenesis by acting on non-muscle myosin II-a (Myh9), an osteoclast cell fusion regulatory factor. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862 (5), 452–462. doi: 10.1016/j.bbalip.2017.01.008
Kassebaum, N. J., Bernabe, E., Dahiya, M., Bhandari, B., Murray, C. J., Marcenes, W. (2014). Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J. Dent. Res. 93 (11), 1045–1053. doi: 10.1177/0022034514552491
Keeler, S. M., Kiefer, D. G., Rust, O. A., Vintzileos, A., Atlas, R. O., Bornstein, E., et al. (2009). Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am. J. Obstet. Gynecol. 201 (3), 276.e271–276. doi: 10.1016/j.ajog.2009.05.045
Khalaf, H., Bengtssown, T. (2012). Altered T-cell responses by the periodontal pathogen porphyromonas gingivalis. PLos One 7 (9), e45192. doi: 10.1371/journal.pone.0045192
Khandpur, R., Carmona-Rivera, C., Vivekanandan-Giri, A., Gizinski, A., Yalavarthi, S., Knight, J. S., et al. (2013). NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5 (178), 178ra140. doi: 10.1126/scitranslmed.3005580
Kim, S. K., Brehm, M. A., Welsh, R. M., Selin, L. K. (2002). Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J. Immunol. 169 (1), 90–98. doi: 10.4049/jimmunol.169.1.90
Kinane, D. F., Bartold, P. M. (2007). Clinical relevance of the host responses of periodontitis. Periodontology 2000 43, 278–293. doi: 10.1111/j.1600-0757.2006.00169.x
Kitamura, Y., Matono, S., Aida, Y., Hirofuji, T., Maeda, K. (2002). Gingipains in the culture supernatant of porphyromonas gingivalis cleave CD4 and CD8 on human T cells. J. Periodontal. Res. 37 (6), 464–468. doi: 10.1034/j.1600-0765.2002.01364.x
Kobayashi, R., Kono, T., Bolerjack, B. A., Fukuyama, Y., Gilbert, R. S., Fujihashi, K., et al. (2011). Induction of IL-10-producing CD4+ T-cells in chronic periodontitis. J. Dent. Res. 90 (5), 653–658. doi: 10.1177/0022034510397838
Koizumi, Y., Kurita-Ochiai, T., Oguchi, S., Yamamoto, M. (2008). Nasal immunization with porphyromonas gingivalis outer membrane protein decreases p-gingivalis-induced atherosclerosis and inflammation in spontaneously hyperlipidemic mice. Infection Immun. 76 (7), 2958–2965. doi: 10.1128/IAI.01572-07
Kol, A., Sukhova, G. K., Lichtman, A. H., Libby, P. (1998). Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98 (4), 300–307. doi: 10.1161/01.cir.98.4.300
Komazaki, R., Katagiri, S., Takahashi, H., Maekawa, S., Shiba, T., Takeuchi, Y., et al. (2017). Periodontal pathogenic bacteria, aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci. Rep. 7 (1), 13950. doi: 10.1038/s41598-017-14260-9
Krauss, J. L., Potempa, J., Lambris, J. D., Hajishengallis, G. (2010b). Complementary tolls in the periodontium: how periodontal bacteria modify complement and toll-like receptor responses to prevail in the host. Periodontol 2000 52 (1), 141–162. doi: 10.1111/j.1600-0757.2009.00324.x
Kumar, D. K., Choi, S. H., Washicosky, K. J., Eimer, W. A., Tucker, S., Ghofrani, J., et al. (2016). Amyloid-β peptide protects against microbial infection in mouse and worm models of alzheimer's disease. Sci. Transl. Med. 8 (340), 340ra372. doi: 10.1126/scitranslmed.aaf1059
Lappin, D. F., Apatzidou, D., Quirke, A. M., Oliver-Bell, J., Butcher, J. P., Kinane, D. F., et al. (2013). Influence of periodontal disease, porphyromonas gingivalis and cigarette smoking on systemic anti-citrullinated peptide antibody titres. J. Clin. Periodontol. 40 (10), 907–915. doi: 10.1111/jcpe.12138
Lauritano, D., Boccalari, E., Di Stasio, D., Della Vella, F., Carinci, F., Lucchese, A., et al. (2019). Prevalence of oral lesions and correlation with intestinal symptoms of inflammatory bowel disease: A systematic review. Diagnostics (Basel) 9 (3), 77. doi: 10.3390/diagnostics9030077
Leipe, J., Grunke, M., Dechant, C., Reindl, C., Kerzendorf, U., Schulze-Koops, H., et al. (2010). Role of Th17 cells in human autoimmune arthritis. Arthritis Rheumatism 62 (10), 2876–2885. doi: 10.1002/art.27622
Leon, R., Silva, N., Ovalle, A., Chaparro, A., Ahurnada, A., Gajardo, M., et al. (2007). Detection of porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J. Periodontol. 78 (7), 1249–1255. doi: 10.1902/jop.2007.060368
Lester, S. R., Bain, J. L., Johnson, R. B., Serio, F. G. (2007). Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J. Periodontol. 78 (8), 1545–1550. doi: 10.1902/jop.2007.060458
Liang, S. C., Tan, X. Y., Luxenberg, D. P., Karim, R., Dunussi-Joannopoulos, K., Collins, M., et al. (2006). Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203 (10), 2271–2279. doi: 10.1084/jem.20061308
Libby, P. (2012). Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32 (9), 2045–2051. doi: 10.1161/ATVBAHA.108.179705
Libby, P., Ridker, P. M., Hansson, G. K. (2011). Progress and challenges in translating the biology of atherosclerosis. Nature 473 (7347), 317–325. doi: 10.1038/nature10146
Lin, C. H., Chen, D. Y., Chao, W. C., Liao, T. L., Chen, Y. M., Chen, H. H. (2017). Association between periodontitis and the risk of palindromic rheumatism: A nationwide, population-based, case-control study. PLos One 12 (8), e0182284. doi: 10.1371/journal.pone.0182284
Lockhart, P. B., Bolger, A. F., Papapanou, P. N., Osinbowale, O., Trevisan, M., Levison, M. E., et al. (2012). Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American heart association. Circulation 125 (20), 2520–2544. doi: 10.1161/CIR.0b013e31825719f3
Long, J., Cai, Q., Steinwandel, M., Hargreaves, M. K., Bordenstein, S. R., Blot, W. J., et al. (2017). Association of oral microbiome with type 2 diabetes risk. J. Periodontal. Res. 52 (3), 636–643. doi: 10.1111/jre.12432
Longo, P. L., Dabdoub, S., Kumar, P., Artese, H. P. C., Dib, S. A., Romito, G. A., et al. (2018). Glycaemic status affects the subgingival microbiome of diabetic patients. J. Clin. Periodontol. 45 (8), 932–940. doi: 10.1111/jcpe.12908
Lu, W., Gu, J. Y., Zhang, Y. Y., Gong, D. J., Zhu, Y. M., Sun, Y. (2018). Tolerance induced by porphyromonas gingivalis may occur independently of TLR2 and TLR4. PLos One 13 (7), e200946. doi: 10.1371/journal.pone.0200946
Maekawa, T., Takahashi, N., Tabeta, K., Aoki, Y., Miyashita, H., Miyauchi, S., et al. (2011). Chronic oral infection with porphyromonas gingivalis accelerates atheroma formation by shifting the lipid profile. PLos One 6 (5), e20240. doi: 10.1371/journal.pone.0020240
Mandal, K., Jahangiri, M., Xu, Q. (2004). Autoimmunity to heat shock proteins in atherosclerosis. Autoimmun Rev. 3 (2), 31–37. doi: 10.1016/S1568-9972(03)00088-0
Manel, N., Unutmaz, D., Littman, D. R. (2008). The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 9 (6), 641–649. doi: 10.1038/ni.1610
Maresz, K. J., Hellvard, A., Sroka, A., Adamowicz, K., Bielecka, E., Koziel, J., et al. (2013). Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLos Pathog. 9 (9), e1003627. doi: 10.1371/journal.ppat.1003627
Marsh, S. E., Abud, E. M., Lakatos, A., Karimzadeh, A., Yeung, S. T., Davtyan, H., et al. (2016). The adaptive immune system restrains alzheimer's disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. U.S.A. 113 (9), E1316–E1325. doi: 10.1073/pnas.1525466113
Mattila, K. J., Nieminen, M. S., Valtonen, V. V., Rasi, V. P., Kesäniemi, Y. A., Syrjälä, S. L., et al. (1989). Association between dental health and acute myocardial infarction. Br. Med. J. 298 (6676), 779–781. doi: 10.1136/bmj.298.6676.779
Mattila, K. J., Valle, M. S., Nieminen, M. S., Valtonen, V. V., Hietaniemi, K. L. (1993). Dental infections and coronary atherosclerosis. Atherosclerosis 103 (2), 205–211. doi: 10.1016/0021-9150(93)90263-t
Martinez-Martinez, R. E., Abud-Mendoza, C., Patiño-Marin, N., Rizo-Rodríguez, J. C., Little, J. W., Loyola-Rodríguez, J. P. (2009). Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. 36 (12), 1004–1010. doi: 10.1111/j.1600-051X.2009.01496.x
McGraw, W. T., Potempa, J., Farley, D., Travis, J. (1999a). Purification, characterization, and sequence analysis of a potential virulence factor from porphyromonas gingivalis, peptidylarginine deiminase. Infect. Immun. 67 (7), 3248–3256. doi: 10.1128/iai.67.7.3248-3256.1999
McInnes, I. B., Schett, G. (2007). Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7 (6), 429–442. doi: 10.1038/nri2094
McInnes, I. B., Schett, G. (2011). The pathogenesis of rheumatoid arthritis. N Engl. J. Med. 365 (23), 2205–2219. doi: 10.1056/NEJMra1004965
Meissner, A., Miro, F., Jiménez-Altayó, F., Jurado, A., Vila, E., Planas, A. M. (2017). Sphingosine-1-phosphate signalling-a key player in the pathogenesis of angiotensin II-induced hypertension. Cardiovasc. Res. 113 (2), 123–133. doi: 10.1093/cvr/cvw256
Merchant, A. T., Shrestha, D., Chaisson, C., Choi, Y. H., Hazlett, L. J., Zhang, J. (2014). Association between serum antibodies to oral microorganisms and hyperglycemia in adults. J. Dent. Res. 93 (8), 752–759. doi: 10.1177/0022034514538451
Miyata, Y., Takeda, H., Kitano, S., Hanazawa, S. (1997). Porphyromonas gingivalis lipopolysaccharide-stimulated bone resorption via CD14 is inhibited by broad-spectrum antibiotics. Infect. Immun. 65 (9), 3513–3519. doi: 10.1128/iai.65.9.3513-3519.1997
Monteiro, A. C., Scovino, A., Raposo, S., Gaze, V. M., Cruz, C., Svensjö, E., et al. (2009). Kinin danger signals proteolytically released by gingipain induce fimbriae-specific IFN-gamma- and IL-17-producing T cells in mice infected intramucosally with porphyromonas gingivalis. J. Immunol. 183 (6), 3700–3711. doi: 10.4049/jimmunol.0900895
Moutsopoulos, N. M., Kling, H. M., Angelov, N., Jin, W., Palmer, R. J., Nares, S., et al. (2012). Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J. Autoimmun 39 (4), 294–303. doi: 10.1016/j.jaut.2012.03.003
Nakagawa, I., Amano, A., Kuboniwa, M., Nakamura, T., Kawabata, S., Hamada, S. (2002). Functional differences among FimA variants of porphyromonas gingivalis and their effects on adhesion to and invasion of human epithelial cells. Infection Immun. 70 (1), 277–285. doi: 10.1128/IAI.70.1.277-285.2002
Nakahara, T., Hyogo, H., Ono, A., Nagaoki, Y., Kawaoka, T., Miki, D., et al. (2018). Involvement of porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 53 (2), 269–280. doi: 10.1007/s00535-017-1368-4
Nakajima, M., Arimatsu, K., Kato, T., Matsuda, Y., Minagawa, T., Takahashi, N., et al. (2015). Oral administration of p. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLos One 10 (7), e0134234. doi: 10.1371/journal.pone.0134234
Nakajima, T., Ueki-Maruyama, K., Oda, T., Ohsawa, Y., Ito, H., Seymour, G. J., et al. (2005). Regulatory T-cells infiltrate periodontal disease tissues. J. Dent. Res. 84 (7), 639–643. doi: 10.1177/154405910508400711
Nakajima, T., Yamazaki, K., Cullinan, M. P., Gemmell, E., Seymour, G. J. (1999). T-Cell antigen specificity in humans following stimulation with porphyromonas gingivalis. Arch. Oral. Biol. 44 (12), 1045–1053. doi: 10.1016/S0003-9969(99)00094-1
Narayan, I., Gowda, T. M., Mehta, D. S., Kumar, B. T. (2018). Estimation of toll-like receptor 9 in gingival tissues of patients with chronic periodontitis with or without hyperlipidemia and its association with the presence of porphyromonas gingivalis. J. Indian Soc. Periodontol. 22 (4), 298–303. doi: 10.4103/jisp.jisp_124_18
Niederman, R., Brunkhorst, B., Smith, S., Weinreb, R. N., Ryder, M. I. (1990). Ammonia as a potential mediator of adult human periodontal infection: inhibition of neutrophil function. Arch. Oral. Biol. 35Suppl), 205s–209s. doi: 10.1016/0003-9969(90)90159-8
Nie, R., Wu, Z., Ni, J., Zeng, F., Yu, W., Zhang, Y., et al. (2019). Porphyromonas gingivalis infection induces amyloid-β accumulation in Monocytes/Macrophages. J. Alzheimers Dis. 72 (2), 479–494. doi: 10.3233/jad-190298
O'Connor, W., Jr., Zenewicz, L. A., Flavell, R. A. (2010). The dual nature of T(H)17 cells: shifting the focus to function. Nat. Immunol. 11 (6), 471–476. doi: 10.1038/ni.1882
Olsen, I., Progulske-Fox, A. (2015). Invasion of porphyromonas gingivalis strains into vascular cells and tissue. J. Oral. Microbiol. 7,28788. doi: 10.3402/jom.v7.28788
Olsen, I., Taubman, M. A., Singhrao, S. K. (2016). Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and alzheimer's disease. J. Oral. Microbiol. 8, 33029. doi: 10.3402/jom.v8.33029
Ozkan, Z. S., Deveci, D., Simsek, M., Ilhan, F., Risvanli, A., Sapmaz, E. (2015). What is the impact of SOCS3, IL-35 and IL17 in immune pathogenesis of recurrent pregnancy loss? J. Matern. Fetal. Neonatal. Med. 28 (3), 324–328. doi: 10.3109/14767058.2014.916676
Papageorgiou, S. N., Hagner, M., Nogueira, A. V. B., Franke, A., Jager, A., Deschner, J. (2017). Inflammatory bowel disease and oral health: systematic review and a meta-analysis. J. Clin. Periodontol. 44 (4), 382–393. doi: 10.1111/jcpe.12698
Park, Y. D., Kim, Y. S., Jung, Y. M., Lee, S. I., Lee, Y. M., Bang, J. B., et al. (2012b). Porphyromonas gingivalis lipopolysaccharide regulates interleukin (IL)-17 and IL-23 expression via SIRT1 modulation in human periodontal ligament cells. Cytokine 60 (1), 284–293. doi: 10.1016/j.cyto.2012.05.021
Perry, V. H., Cunningham, C., Holmes, C. (2007). Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 7 (2), 161–167. doi: 10.1038/nri2015
Pietropaoli, D., Del Pinto, R., Corridoni, D., Rodriguez-Palacios, A., Di Stefano, G., Monaco, A., et al. (2014). Occurrence of spontaneous periodontal disease in the SAMP1/YitFc murine model of crohn disease. J. Periodontol. 85 (12), 1799–1805. doi: 10.1902/jop.2014.140316
Pirro, M., Mannarino, M. R. (2019). Editorial commentary: Atherosclerosis and immunity: A perspective. Trends Cardiovasc. Med. 29 (6), 372–373. doi: 10.1016/j.tcm.2018.11.005
Pockley, A. G., Multhoff, G. (2008). Cell stress proteins in extracellular fluids: friend or foe? Novartis Found. Symp. 291, 86–95. doi: 10.1002/9780470754030.ch7
Pockley, A. G., Muthana, M., Calderwood, S. K. (2008). The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci. 33 (2), 71–79. doi: 10.1016/j.tibs.2007.10.005
Polak, D., Shapira, L. (2018). An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 45 (2), 150–166. doi: 10.1111/jcpe.12803
Pongcharoen, S., Niumsup, P., Sanguansermsri, D., Supalap, K., Butkhamchot, P. (2006). The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am. J. Reprod. Immunol. 55 (4), 291–300. doi: 10.1111/j.1600-0897.2006.00366.x
Poole, S., Singhrao, S. K., Kesavalu, L., Curtis, M. A., Crean, S. (2013). Determining the presence of periodontopathic virulence factors in short-term postmortem alzheimer's disease brain tissue. J. Alzheimers Dis. 36 (4), 665–677. doi: 10.3233/jad-121918
Potempa, J., Mydel, P., Koziel, J. (2017). The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 13 (10), 606–620. doi: 10.1038/nrrheum.2017.132
Pratesi, F., Dioni, I., Tommasi, C., Alcaro, M. C., Paolini, I., Barbetti, F., et al. (2014). Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 73 (7), 1414–1422. doi: 10.1136/annrheumdis-2012-202765
Pussinen, P. J., Alfthan, G., Jousilahti, P., Paju, S., Tuomilehto, J. (2007). Systemic exposure to porphyromonas gingivalis predicts incident stroke. Atherosclerosis 193 (1), 222–228. doi: 10.1016/j.atherosclerosis.2006.06.027
Raghupathy, R. (2001). Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Semin. Immunol. 13 (4), 219–227. doi: 10.1006/smim.2001.0316
Ranade, S. B., Doiphode, S. (2012). Is there a relationship between periodontitis and rheumatoid arthritis? J. Indian Soc. Periodontol. 16 (1), 22–27. doi: 10.4103/0972-124x.94599
Rangarajan, M., Aduse-Opoku, J., Paramonov, N., Hashim, A., Bostanci, N., Fraser, O. P., et al. (2008). Identification of a second lipopolysaccharide in porphyromonas gingivalis W50. J. Bacteriol. 190 (8), 2920–2932. doi: 10.1128/jb.01868-07
Rantapaa-Dahlqvist, S., de Jong, B. A. W., Berglin, E., Hallmans, G., Wadell, G., Stenlund, H., et al. (2003). Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheumatism 48 (10), 2741–2749. doi: 10.1002/art.11223
Reife, R. A., Coats, S. R., Al-Qutub, M., Dixon, D. M., Braham, P. A., Billharz, R. J., et al. (2006). Porphyromonas gingivalis lipopolysaccharide lipid a heterogeneity: differential activities of tetra- and penta-acylated lipid a structures on e-selectin expression and TLR4 recognition. Cell Microbiol. 8 (5), 857–868. doi: 10.1111/j.1462-5822.2005.00672.x
Rodríguez, S. B., Stitt, B. L., Ash, D. E. (2009). Expression of peptidylarginine deiminase from porphyromonas gingivalis in escherichia coli: enzyme purification and characterization. Arch. Biochem. Biophys. 488 (1), 14–22. doi: 10.1016/j.abb.2009.06.010
Rolla, S., Alchera, E., Imarisio, C., Bardina, V., Valente, G., Cappello, P., et al. (2016). The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin. Sci. 130 (3), 193–203. doi: 10.1042/CS20150405
Rosenstein, E. D., Greenwald, R. A., Kushner, L. J., Weissmann, G. (2004). Hypothesis: The humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation 28 (6), 311–318. doi: 10.1007/s10753-004-6641-z
Sadrameli, M., Bathini, P., Alberi, L. (2020). Linking mechanisms of periodontitis to alzheimer's disease. Curr. Opin. Neurol. 33 (2), 230–238. doi: 10.1097/wco.0000000000000797
Saeb, A. T. M., Al-Rubeaan, K. A., Aldosary, K., Udaya Raja, G. K., Mani, B., Abouelhoda, M., et al. (2019). Relative reduction of biological and phylogenetic diversity of the oral microbiota of diabetes and pre-diabetes patients. Microb. Pathog. 128, 215–229. doi: 10.1016/j.micpath.2019.01.009
Salvi, G. E., Beck, J. D., Offenbacher, S. (1998). PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Ann. Periodontol. 3 (1), 40–50. doi: 10.1902/annals.1998.3.1.40
Sasaki, N., Katagiri, S., Komazaki, R., Watanabe, K., Maekawa, S., Shiba, T., et al. (2018). Endotoxemia by porphyromonas gingivalis injection aggravates non-alcoholic fatty liver disease, disrupts Glucose/Lipid metabolism, and alters gut microbiota in mice. Front. Microbiol. 9, 13950. doi: 10.3389/fmicb.2018.02470
Sasaki, H., Okamatsu, Y., Kawai, T., Kent, R., Taubman, M., Stashenko, P. (2004). The interleukin-10 knockout mouse is highly susceptible to porphyromonas gingivalis-induced alveolar bone loss. J. Periodontal. Res. 39 (6), 432–441. doi: 10.1111/j.1600-0765.2004.00760.x
Scott, D. L., Wolfe, F., Huizinga, T. W. (2010). Rheumatoid arthritis. Lancet. 376 (9746), 1094–1108. doi: 10.1016/s0140-6736(10)60826-4
Shao, J., Nangaku, M., Miyata, T., Inagi, R., Yamada, K., Kurokawa, K., et al. (2003). Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42 (1), 31–38. doi: 10.1161/01.hyp.0000075082.06183.4e
Shin, H. S. (2020). Association between periodontal status and non-alcoholic fatty liver disease in a Korean adult population: A nationwide cross-sectional study. J. Periodontol. 91 (4), 524–532. doi: 10.1002/JPER.19-0291
Sohn, J., Li, L., Zhang, L., Settem, R. P., Honma, K., Sharma, A., et al. (2022). Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4+ T cells. Mol. Oral. Microbiol. 37 (2), 42–52. doi: 10.1111/omi.12359
Soscia, S. J., Kirby, J. E., Washicosky, K. J., Tucker, S. M., Ingelsson, M., Hyman, B., et al. (2010). The alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLos One 5 (3), e9505. doi: 10.1371/journal.pone.0009505
Stadhouders, R., Lubberts, E., Hendriks, R. W. (2018). A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun 87, 1–15. doi: 10.1016/j.jaut.2017.12.007
Stein, J. M., Lammert, F., Zimmer, V., Granzow, M., Reichert, S., Schulz, S., et al. (2010). Clinical periodontal and microbiologic parameters in patients with crohn's disease with consideration of the CARD15 genotype. J. Periodontol. 81 (4), 535–545. doi: 10.1902/jop.2009.090563
Stein, P. S., Steffen, M. J., Smith, C., Jicha, G., Ebersole, J. L., Abner, E., et al. (2012). Serum antibodies to periodontal pathogens are a risk factor for alzheimer's disease. Alzheimers Dementia 8 (3), 196–203. doi: 10.1016/j.jalz.2011.04.006
Sugawara, S., Nemoto, E., Tada, H., Miyake, K., Imamura, T., Takada, H. (2000). Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J. Immunol. 165 (1), 411–418. doi: 10.4049/jimmunol.165.1.411
Sundqvist, G., Figdor, D., Hänström, L., Sörlin, S., Sandström, G. (1991). Phagocytosis and virulence of different strains of porphyromonas gingivalis. Scand. J. Dent. Res. 99 (2), 117–129. doi: 10.1111/j.1600-0722.1991.tb01874.x
Takeuchi, H., Hirano, T., Whitmore, S. E., Morisaki, I., Amano, A., Lamont, R. J. (2013). The serine phosphatase SerB of porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-κB RelA/p65. PLos Pathog. 9 (4), e1003326. doi: 10.1371/journal.ppat.1003326
Tam, V., O'Brien-Simpson, N. M., Chen, Y. Y., Sanderson, C. J., Kinnear, B., Reynolds, E. C. (2009). The RgpA-kgp proteinase-adhesin complexes of porphyromonas gingivalis inactivate the Th2 cytokines interleukin-4 and interleukin-5. Infect. Immun. 77 (4), 1451–1458. doi: 10.1128/iai.01377-08
Tonetti, M. S. (2009). Periodontitis and risk for atherosclerosis: an update on intervention trials. J. Clin. Periodontol. 36 (Suppl10), 15–19. doi: 10.1111/j.1600-051X.2009.01417.x
Tonetti, M. S., Van Dyke, T. E., Workshop, J. E. A. (2013). Periodontitis and atherosclerotic cardiovascular disease: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 40, S24–S29. doi: 10.1111/jcpe.12089
Trombone, A. P., Claudino, M., Colavite, P., de Assis, G. F., Avila-Campos, M. J., Silva, J. S., et al. (2010). Periodontitis and arthritis interaction in mice involves a shared hyper-inflammatory genotype and functional immunological interferences. Genes Immun. 11 (6), 479–489. doi: 10.1038/gene.2010.13
Tsuzuno, T., Takahashi, N., Yamada-Hara, M., Yokoji-Takeuchi, M., Sulijaya, B., Aoki-Nonaka, Y., et al. (2021). Ingestion of porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J. Periodontal. Res. 56 (2), 275–288. doi: 10.1111/jre.12816
Valesini, G., Gerardi, M. C., Iannuccelli, C., Pacucci, V. A., Pendolino, M., Shoenfeld, Y. (2015). Citrullination and autoimmunity. Autoimmun Rev. 14 (6), 490–497. doi: 10.1016/j.autrev.2015.01.013
van de Sande, M. G. H., de Hair, M. J. H., van der Leij, C., Klarenbeek, P. L., Bos, W. H., Smith, M. D., et al. (2011). Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann. Rheumatic Dis. 70 (5), 772–777. doi: 10.1136/ard.2010.139527
Van Dyke, T. E. (2017). Pro-resolving mediators in the regulation of periodontal disease. Mol. Aspects Med. 58, 21–36. doi: 10.1016/j.mam.2017.04.006
van Hamburg, J. P., Asmawidjaja, P. S., Davelaar, N., Mus, A. M., Colin, E. M., Hazes, J. M., et al. (2011). Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 63 (1), 73–83. doi: 10.1002/art.30093
Vanterpool, S. F., Been, J. V., Houben, M. L., Nikkels, P. G. J., De Krijger, R. R., Zimmermann, L. J. I., et al. (2016). Porphyromonas gingivalis within placental villous mesenchyme and umbilical cord stroma is associated with adverse pregnancy outcome. PLos One 11 (1), e146157. doi: 10.1371/journal.pone.0146157
Vernal, R., Diaz-Guerra, E., Silva, A., Sanz, M., Garcia-Sanz, J. A. (2014). Distinct human T-lymphocyte responses triggered by porphyromonas gingivalis capsular serotypes. J. Clin. Periodontol. 41 (1), 19–30. doi: 10.1111/jcpe.12176
Vidal, F., Cordovil, I., Figueredo, C. M. S., Fischer, R. G. (2013). Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J. Clin. Periodontol. 40 (7), 681–687. doi: 10.1111/jcpe.12110
Wang, L., Guan, N., Jin, Y., Lin, X., Gao, H. (2015). Subcutaneous vaccination with porphyromonas gingivalis ameliorates periodontitis by modulating Th17/Treg imbalance in a murine model. Int. Immunopharmacol. 25 (1), 65–73. doi: 10.1016/j.intimp.2015.01.007
Wang, P. L., Ohura, K. (2002). Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and toll-like receptors. Crit. Rev. Oral. Biol. Med. 13 (2), 132–142. doi: 10.1177/154411130201300204
Wegner, N., Wait, R., Sroka, A., Eick, S., Nguyen, K. A., Lundberg, K., et al. (2010). Peptidylarginine deiminase from porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62 (9), 2662–2672. doi: 10.1002/art.27552
Wolf, D., Ley, K. (2019). Immunity and inflammation in atherosclerosis. Circ. Res. 124 (2), 315–327. doi: 10.1161/CIRCRESAHA.118.313591
Wu, Z., Ni, J. J., Liu, Y. C., Teeling, J. L., Takayama, F., Collcutt, A., et al. (2017). Cathepsin b plays a critical role in inducing alzheimer's disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from porphyromonas gingivalis in mice. Brain Behav. Immun. 65, 350–361. doi: 10.1016/j.bbi.2017.06.002
Xun, Z., Zhang, Q., Xu, T., Chen, N., Chen, F. (2018). Dysbiosis and ecotypes of the salivary microbiome associated with inflammatory bowel diseases and the assistance in diagnosis of diseases using oral bacterial profiles. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01136
Yamazaki, K., Ohsawa, Y., Itoh, H., Ueki, K., Tabeta, K., Oda, T., et al. (2004). T-Cell clonality to porphyromonas gingivalis and human heat shock protein 60s in patients with atherosclerosis and periodontitis. Oral. Microbiol. Immunol. 19 (3), 160–167. doi: 10.1111/j.0902-0055.2004.00134.x
Yang, J., Hao, T., Liu, Y., Huang, J., Wu, W., Wu, J., et al. (2022). Th17/Treg balance and indoleamine 2,3 dioxygenase activity in periodontitis-associated atherosclerotic patients. J. Int. Med. Res. 50 (2), 3000605221080877. doi: 10.1177/03000605221080877
Yang, J., Wu, J., Liu, Y., Huang, J., Lu, Z., Xie, L., et al. (2014). Porphyromonas gingivalis infection reduces regulatory T cells in infected atherosclerosis patients. PLos One 9 (1), e86599. doi: 10.1371/journal.pone.0086599
Yang, J., Wu, J., Zhang, R., Yao, M., Liu, Y., Miao, L., et al. (2017). Porphyromonas gingivalis oral infection promote T helper 17/Treg imbalance in the development of atherosclerosis. J. Dent. Sci. 12 (1), 60–69. doi: 10.1016/j.jds.2016.10.003
Yao, C., Lan, D., Li, X., Wang, Y., Qi, S., Liu, Y. (2022). Porphyromonas gingivalis is a risk factor for the development of nonalcoholic fatty liver disease via ferroptosis. Microbes Infection, in press. doi: 10.1016/j.micinf.2022.105040
Yoneda, M., Naka, S., Nakano, K., Wada, K., Endo, H., Mawatari, H., et al. (2012). Involvement of a periodontal pathogen, porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 12, 16. doi: 10.1186/1471-230X-12-16
Youn, J. C., Yu, H. T., Lim, B. J., Koh, M. J., Lee, J., Chang, D. Y., et al. (2013). Immunosenescent CD8+ T cells and c-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62 (1), 126–133. doi: 10.1161/hypertensionaha.113.00689
Yun, P. L., Decarlo, A. A., Chapple, C. C., Collyer, C. A., Hunter, N. (2005). Binding of porphyromonas gingivalis gingipains to human CD4(+) T cells preferentially down-regulates surface CD2 and CD4 with little affect on co-stimulatory molecule expression. Microb. Pathog. 38 (2-3), 85–96. doi: 10.1016/j.micpath.2005.01.001
Zaitsu, Y., Iwatake, M., Sato, K., Tsukuba, T. (2016). Lipid droplets affect elimination of porphyromonas gingivalis in HepG2 cells by altering the autophagy-lysosome system. Microbes Infection 18 (9), 565–571. doi: 10.1016/j.micinf.2016.05.004
Zeituni, A. E., Carrion, J., Cutler, C. W. (2010). Porphyromonas gingivalis-dendritic cell interactions: consequences for coronary artery disease. J. Oral. Microbiol. 2, 5782. doi: 10.3402/jom.v2i0.5782
Zeituni, A. E., Jotwani, R., Carrion, J., Cutler, C. W. (2009). Targeting of DC-SIGN on human dendritic cells by minor fimbriated porphyromonas gingivalis strains elicits a distinct effector T cell response. J. Immunol. 183 (9), 5694–5704. doi: 10.4049/jimmunol.0901030
Zeng, F., Liu, Y., Huang, W., Qing, H., Kadowaki, T., Kashiwazaki, H., et al. (2021). Receptor for advanced glycation end products up-regulation in cerebral endothelial cells mediates cerebrovascular-related amyloid β accumulation after porphyromonas gingivalis infection. J. Neurochem. 158 (3), 724–736. doi: 10.1111/jnc.15096
Zhang, Q., Chen, B., Yan, F., Guo, J., Zhu, X., Ma, S., et al. (2014). Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. BioMed. Res. Int. 2014, 284836. doi: 10.1155/2014/284836
Zhao, Y. H., Cong, L., Lukiw, W. J. (2017). Lipopolysaccharide (LPS) accumulates in neocortical neurons of alzheimer's disease (AD) brain and impairs transcription in human neuronal-glial primary Co-cultures. Front. Aging Neurosci. 9. doi: 10.3389/fnagi.2017.00407
Zhao, X., Liu, J., Zhang, C., Yu, N., Lu, Z., Zhang, S., et al. (2021). Porphyromonas gingivalis exacerbates ulcerative colitis via porphyromonas gingivalis peptidylarginine deiminase. Int. J. Oral. Sci. 13 (1), 31. doi: 10.1038/s41368-021-00136-2
Keywords: Porphyromonas gingivalis, periodontal diseases, adaptive immune response, systemic diseases, atherosclerosis, adverse pregnancy outcomes, inflammatory bowel disease, rheumatoid arthritis
Citation: Li C, Yu R and Ding Y (2022) Association between Porphyromonas Gingivalis and systemic diseases: Focus on T cells-mediated adaptive immunity. Front. Cell. Infect. Microbiol. 12:1026457. doi: 10.3389/fcimb.2022.1026457
Received: 23 August 2022; Accepted: 25 October 2022;
Published: 17 November 2022.
Edited by:
Daniel Miller, Virginia Commonwealth University, United StatesCopyright © 2022 Li, Yu and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumei Ding, eXVtZWlkaW5nQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.