
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 07 January 2022
Sec. Fungal Pathogenesis
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.805514
This article is part of the Research Topic Proceedings of the Mycology 2021 View all 6 articles
 Alessandro Busca1*†
Alessandro Busca1*† Natascia Cinatti2*†
Natascia Cinatti2*† Jessica Gill1,3*†
Jessica Gill1,3*† Roberto Passera4
Roberto Passera4 Chiara Maria Dellacasa1
Chiara Maria Dellacasa1 Luisa Giaccone1,3
Luisa Giaccone1,3 Irene Dogliotti1
Irene Dogliotti1 Sara Manetta1
Sara Manetta1 Silvia Corcione5‡
Silvia Corcione5‡ Francesco Giuseppe De Rosa5‡
Francesco Giuseppe De Rosa5‡Background: Allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients are exposed to an increased risk of invasive fungal infections (IFIs) due to neutropenia, immunosuppressive treatments, graft-versus-host disease (GvHD) and incomplete immune reconstitution. Although clinical benefit from antifungal prophylaxis has been demonstrated, IFIs remain a leading cause of morbidity and mortality in these patients. In the last decades, attention has also been focused on potential risk factors for IFI to tailor an antifungal prevention strategy based on risk stratification.
Aim of the Study: This retrospective single-center study aimed to assess the epidemiology and the prognostic factors of IFI in a large cohort of allo-HSCT patients.
Methods: Between January 2004 and December 2020, 563 patients with hematological malignancies received an allo-HSCT at the Stem Cell Transplant Unit in Turin: 191 patients (34%) received grafts from a matched sibling donor, 284 (50.5%) from a matched unrelated donor, and 87 (15.5%) from an haploidentical family member. The graft source was peripheral blood in 81.5% of the patients. Our policy for antifungal prophylaxis included fluconazole in matched related and unrelated donors, while micafungin was administered in patients receiving haploidentical transplant. According to this practice, fluconazole was administered in 441 patients (79.6%) and micafungin in 62 (11.2%), while only 9 patients received mold-active prophylaxis. Galactomannan testing was routinely performed twice a week; patients with persisting fever unresponsive to broad spectrum antibiotics were evaluated with lung high-resolution computed tomography (HRCT) scan. In case of imaging suggestive of IFI, bronchoalveolar lavage (BAL) was performed whenever feasible.
Statistical Analysis: Only probable/proven IFI (PP-IFI) occurring during the first 12 months after transplant have been evaluated. IFIs were classified as probable or proven according to the new revised European Organization for Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) consensus criteria. Multivariate competing risk regression, binary logistic, and proportional hazard models were performed to identify risk factors for PP-IFI.
Results: A total of 58 PP-IFIs (n = 47 probable; n = 11 proven) occurred in our patients resulting in a cumulative incidence of 4.1%, 8.1%, and 9.6% at 30, 180, and 365 days, respectively. Molds were the predominant agents (n = 50 Aspergillus; n = 1 Mucor), followed by invasive candidemia (n = 5 non-albicans Candida; n = 1 Candida albicans; n = 1 Trichosporon). Lung was the most frequent site involved in patients with mold infections (47/51, 92.2%). Median time from HSCT to IFI was 98.44 days (0–365 days). Only 34.5% of patients with IFI were neutropenic at the time of infection. The presence of IFI had a significant impact on overall survival at 1 year (IFI, 32.8% vs. non-IFI, 54.6%; p < 0.001). IFI-related mortality rate was 20.7% in the overall population, 17% in patients with probable IFI, and 36% in patients with proven IFI. Multivariate competing risk regression revealed that donor type was the factor significantly associated to the risk of IFI [subdistribution hazard ratio (SDHR), 1.91, IC 1.13–3.20; p = 0.015]. BAL was informative in a consistent number of cases (36/57, 63.2%) leading to the identification of fungal (21), bacterial (4), viral (3), and polymicrobial (8) infections. Overall, 79 patients (14%) received a diagnostic-driven treatment, and 63 patients (11.2%) received a fever-driven treatment. Liposomal amphoteric B was the drug used in the majority of patients receiving diagnostic-driven therapy (30/79, 38%), while caspofungin was administered more frequently in patients who received a fever-driven strategy (27/63, 42.9%).
Conclusion: According to our experience, a non-mold active prophylaxis in patients undergoing allo-HSCT is feasible when combined with an intensive diagnostic work-up including CT scan and BAL. BAL performed at the onset of the disease may provide informative results in most patients. A diagnostic-driven treatment strategy may contribute to limit the use of costly antifungal therapies.
Hematopoietic stem cell transplantation (HSCT) has proven to be a curative treatment strategy for patients with many malignant and non-malignant hematological disorders, including leukemia, lymphomas, and aplastic anemia, and indications are still expanding (Gyurkocza et al., 2010; Copelan et al., 2018).
HSCT is a procedure that restores stem cells that have been destroyed by a preparative regimen including chemotherapy with or without total body irradiation delivered before stem cell infusion to optimize tumor cell kill and, in the case of allogeneic HSCT, immunosuppress the recipient to prevent graft rejection (Gagelmann and Kröger, 2021). In addition, allogeneic HSCT recipients receive immunosuppressive agents, namely, calcineurin inhibitors, for a prolonged period of time after transplant to mitigate the graft-versus-host reaction (Zeiser and Blazar, 2017; Martinez-Cibrian et al., 2020). According to these considerations, patients undergoing HSCT are at elevated risk for severe life-threatening infections (Sahin et al., 2016; Neofytos, 2019). Indeed, invasive fungal infections (IFIs) represent one of the major limiting factors for the successful outcome of patients receiving allogeneic HSCT (Bhatti et al., 2006). As a consequence, efforts to optimize the managements of IFI in hematological patients have assumed a great relevance (Rahi et al., 2021).
The use of antifungal prophylaxis is extremely diffused among hematological patients and allogeneic HSCT recipients, in accordance with the recommendations of the most authoritative guidelines (Girmenia et al., 2014; Mellinghoff et al., 2017; Maertens et al., 2018); however, several drawbacks have emerged over the last few years, such as the occurrence of side effects, the emergence of resistance, and the overuse of antifungals (Busca and Pagano, 2016; Schmiedel and Zimmerli, 2016). The advent of new diagnostic tools including HRCT and non-culture-based microbiological (NCBM) assays have increased significantly the accuracy in the diagnosis of IFI in hematological patients and HSCT recipients (Arendrup et al., 2012; Marchetti et al., 2012; Ruhnke et al., 2018). The potential advantage of a diagnostic-driven approach incorporating imaging, biomarkers, and early antifungal therapy may be considered as a more refined strategy over an empirical treatment based on the presence of persistent fever unresponsive to broad spectrum antibiotics (De Kort et al., 2019). In this respect, several trials have evaluated the feasibility of a pre-emptive strategy; however, the introduction of mold-active prophylaxis with NCBM methods and the definition of the most appropriate antifungal treatment are still matters of debate (Cordonnier et al., 2014; Fung et al., 2015).
The aim of the present study was to investigate whether the management of HSCT recipients at risk for IFI may be optimized with azole-based antifungal prophylaxis in conjunction with an aggressive diagnostic work-up.
The study is further implemented by the analysis of risk factors that might be potentially useful to tailor an appropriate treatment for HSCT patients with IFI.
This retrospective cohort study included all consecutive adult patients who underwent an allogeneic HSCT for the treatment of a hematological malignancy at the Stem Cell Transplant Center AOU Città della Salute e della Scienza of Torino, between January 2004 and December 2020.
Indications for allogeneic HSCT were hematological malignant diseases and included acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), acute lymphoblastic leukemia (ALL), lymphomas, chronic myeloid leukemia (CML), and multiple myeloma (MM). Written consent for transplant procedures and for the use of medical records for research purposes was obtained from all patients.
Medical chart and electronic records were reviewed retrospectively, and follow-up data were obtained until April 2021.
The exclusion criteria included transplant for non-hematological malignancies or for solid tumors. Patients receiving multiple allo-HSCT during the study period were censored at the time of second or third allo-HSCT, and data were collected independently for each single transplant
We considered only proven or probably IFI (PP-IFI) occurring within the first year of transplantation. In case of multiple fungal infections, only the first episode was considered in the analysis.
All procedures performed in the present study were in accordance with the ethical standards of the Institutional Review Board of the Città della Salute e della Scienza Hospital of Torino, Torino, Italy, according to the Declaration of Helsinki.
Patients were prepared for transplant either with myeloablative or reduced intensity/non-myeloablative conditionings according to their clinical conditions and HCT-CI. Reduced intensity/non-myeloablative regimens were usually preferred in patients over 55 years of age and/or in presence of pre-transplant comorbidities. By the European Blood and Marrow Transplant Group (EBMT) criteria, myeloablative conditionings had to contain a total busulfan dose >6.4 mg/kg i.v., or a cyclophosphamide dose >120 mg/kg (or >60 mg/kg if in combination with other drugs), or a melphalan dose >140 mg/m2, or a total body irradiation dose >6 Gy. Regimens with lower doses were defined as reduced intensity/non-myeloablative conditionings.
Graft-versus-host disease (GvHD) prophylaxis regimens included cyclosporin (CSA) and short-course methotrexate or CSA combined with micophenolate mofetil (MMF). In vivo T-cell depletion with thymoglobulin (ATG) was used in transplants from unrelated donors, mainly as 5–7 mg/kg divided into two or three doses. Patients transplanted from haploidentical donors received tacrolimus, MMF, and post-transplant cyclophosphamide as described by Luznik et al. (2001).
All patients candidate to allograft were cared in rooms with high-efficiency particulate air (HEPA) filter until engraftment was achieved and received antifungal prophylaxis.
Matched related and unrelated donor transplant recipients received fluconazole from day 0 to day +75 after HSCT or posaconazole if GvHD occurred; haploidentical transplant received micafungin until discharge, then fluconazole until day +75.
All patients were screened for invasive aspergillosis using serum galactomannan before HSCT and twice weekly after HSCT until their discharge and then during the follow-up until day +120.
In case of fever, standard procedures were performed including at least two sets of blood cultures at two different sites and serum β-D-glucan antigen test.
Lung high-resolution computed tomography (HRCT) was performed within the third day of persisting fever unresponsive to broad spectrum antibiotics or in case of respiratory symptoms. Bronchoscopy with bronchoalveolar lavage (BAL) was performed whenever feasible if HRCT was positive.
Galactomannan antigen testing was performed using the PlateliaTM Aspergillus Ag kit (BIO-RAD ® Laboratories, Marnes, La Couquette, France) and was considered positive with a single value of ≥1.0 in serum or BAL fluid or with both values of ≥0.7 in serum and ≥0.8 in BAL fluid, according to new revised EORTC/MSG consensus criteria (Donnelly et al., 2020).
All fungal isolates growing in culture were identified as species according to their micromorphological characteristics and sequencing of their informative DNA target.
Broad spectrum antibiotic treatment was given on the first day of neutropenic fever.
Antifungal treatment was started on the basis of the diagnostic work-up in probable and proven fungal infections (diagnostic-driven treatment) or as empiric treatment in possible IFIs (fever-driven treatment).
We considered three different risk periods for fungal infection after allo-HSCT, defined as pre-engraftment phase (from day 0 to day +40), early post-engraftment phase (from days +41 to +100), and late post-engraftment phase (beyond day +100) (Carreras et al., 2019).
Neutropenia at the time of infection was defined as an absolute neutrophil count (ANC) <0.5 × 109/L. If a white blood cell count was lacking, the latest available blood count within the week preceding was used. Neutrophil engraftment was defined as the first of three consecutive days with neutrophils >0.5 × 109/L following allo-HSCT (Carreras et al., 2019).
IFIs were classified as probable or proven according to the new revised definition of the European Organization for Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) Consensus Group (Donnelly et al., 2020): proven disease required mycological documentation from a normally sterile site, while probable disease was supported by host factor, clinical features, and mycological evidence, like a positive galactomannan assay.
We considered the date of infection diagnosis as the day on which first positive diagnostic test was performed.
Primary antifungal therapy was defined as the first-line antifungal treatment administered after diagnosis.
Transplant risk for each patient was calculated using the hematopoietic cell transplantation-specific comorbidity index (HSCT-CI) (Sorror et al., 2005) and the revised Disease Risk Index (r-DRI) (Armand et al., 2014), respectively, for the comorbidity and the underlying disease impact.
Death was classified as IFI-related mortality if fungal infection disease was judged to be unresolved at the time of death and no alternative cause of death was identified by the treating physician nor from medical record review.
Both acute GvHD and chronic GvHD were diagnosed on the basis of clinical symptoms and/or biopsies according to standard criteria (Lee et al., 2002). Severity of chronic GvHD was assessed according to National Institutes of Health criteria and graded as mild, moderate, or severe (Filipovich et al., 2005; Jagasia et al., 2015).
The primary endpoint was the cumulative incidence (CI) for IFI (main event); competing event was the relapse/death without IFI, while alive patients were censored at the date of last contact (April 2021). The CI function was compared across groups by the Gray test, while the competing risks regression model was used to estimate the role of risk factors on IFI occurrence by the Fine–Gray test. The following covariates were tested as IFI occurrence determinants: recipient age (≥60 vs. 40–60 vs. <40 years), recipient gender (male vs. female), diagnosis (AML vs. other hematological malignancies), disease status at transplant (advanced vs. early disease), Sorror Comorbidity Index (HCT-CI, ≥3 vs. 0–2), donor type (haploidentical vs. MUD vs. MRD), Disease Risk Index (DRI, high/very high vs. intermediate vs. low), stem cell source (bone marrow vs. peripheral blood), conditioning regimen (reduced intensity vs. myeloablative), median CD34 doses infused/recipient weight (≥7.0 × 106/kg vs. <7.0 × 106/kg), median CD3 doses infused/recipient weight (≥2.5 × 108/kg vs. <2.5 × 108/kg), antifungal prophylaxis (micafungin vs. fluconazole), anti-thymocyte globulin (ATG) administration (yes vs. no), acute (grade II–IV vs. 0–I), and chronic GvHD (moderate and severe vs. no and mild) occurrence and median duration of neutropenia (≥16 vs. <16 days).
The secondary endpoint was the overall survival (OS), defined as the time from transplant to death from any cause. Survival curves were estimated by the Kaplan–Meier method and compared across groups by the log-rank test. The effect on OS of the same covariates set was analyzed by the Cox proportional hazards model, comparing the two arms by the Wald test and calculating 95% confidence intervals.
Patient characteristics were tested using the Fisher’s exact test for categorical variables and the Mann–Whitney and Kruskal–Wallis tests for continuous ones; continuous variables were described as median [interquartile range (IQR)]. All reported p-values were obtained by the two-sided exact method, at the conventional 5% significance level. Data were analyzed as of September 2021 by R 4.1.0 (R Foundation for Statistical Computing, Vienna-A, http://www.R-project.org).
The study included 523 patients receiving 563 allogeneic transplants: 36 patients had two, and 4 patients had three transplants. Three patients had a history of a previous allo-HSCT performed prior to inclusion period or in another center. Patients’ baseline characteristics are presented in Table 1.
Median age at the time of transplant was 48 years (range, 18–70) with 307 male (54.5%) and 256 female patients (45.5%).
Underlying diseases were acute leukemia (71.6%), Hodgkin and non-Hodgkin lymphomas (17.9%), chronic myeloproliferative disease (8.7%), and multiple myeloma (1.8%).
Slightly more than two-thirds of patients were transplanted in complete remission (282/563, 50.4% in first CR; 96/563, 17.1% in second CR) and 182 (32.5%) in advanced stage of disease. Patients received grafts from a matched sibling donor (191/563, 34%), from a matched unrelated donor (284/563, 50.5%), and from an haploidentical family member (87/563, 15.5%). Main graft source was peripheral blood in 81.5% of the patients.
Overall, a PP-IFI was documented in 58 of 563 transplants (10.3%) resulting in a cumulative incidence of 4.1%, 8.1%, and 9.6% at 30, 180, and 365 days, respectively (Figure 1).

Figure 1 Cumulative incidence rate of invasive fungal infections (IFIs) is 9.6% at 12 months after transplant on the whole cohort. Death or relapse without IFIs are considered the competing event.
IFIs among different types of allogeneic HSCT had a 1-year cumulative incidence of 3.2% for matched related donor, 11.4% for matched unrelated donor, and 16.8% for haploidentical donor transplants (Figure 2).
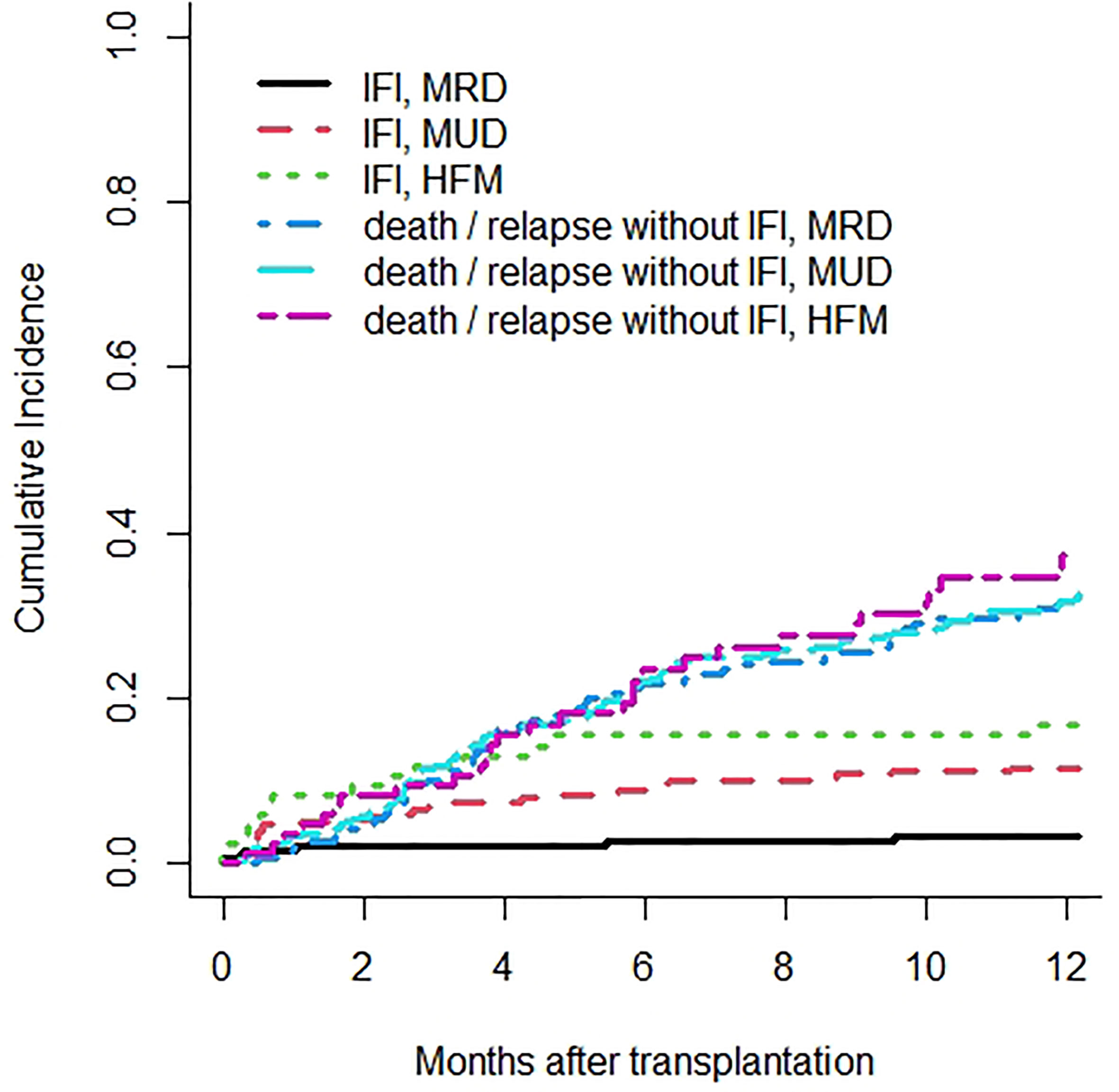
Figure 2 Cumulative incidence rates of invasive fungal infections (IFIs) are 3.2% in matched related donor (MRD), 11.4% in matched unrelated donor (MUD), and 16.8% in haploidentical family member (HFM) transplants. Death or relapse without IFIs are considered the competing event.
Median time from HSCT to IFI diagnosis was 98.44 days, 56 days for candidiasis, and 104.38 days for mold infections.
Underlying diseases in patients who developed IFI were as follows: acute leukemia, 74.1%; HL/NHL, 15.5%; MPB/LMMC, 8.6%; MM, 1.7%. More than half of infections occurred in the post-engraftment phase (10/58—17.2% plus 22/58—37.9% in early and late post-engraftment phase, respectively), while 44.8% developed in the pre-engraftment period (26/58).
Among IFIs, molds were the predominant agents (n = 50 Aspergillus; n = 1 Mucor) and primarily involved the lung (n = 47; 92.2%), central nervous system (n = 2; 3.9%), bone (n = 1; 2%), and tonsils (n = 1; 2%). Invasive candidiasis occurred in seven patients (n = 5 non-albicans Candida; n = 1 C. albicans; n = 1 Trichosporon) and were bloodstream infections in all cases.
Eleven IFIs (19%) were proven infections, and 47 (81.0%) were classified as probable. Diagnosis in these latter group was based on a combination of imaging and positive galactomannan in serum (n = 24), BAL (n = 15), or both (n = 6), and in liquor (n = 2).
In the whole cohort, a total of 57 BALs were performed and were informative in a consistent number of cases (36/57, 63.2%) leading to the identification of fungal (21), bacterial (4), viral (3), and polymicrobial (8) infections (Table 2).
Primary antifungal prophylaxis (PAP) included fluconazole in 441 patients (79.6%) and micafungin in 62 (11.2%); only 9 patients received mold-active prophylaxis (1.6%). Overall, 79 patients (14.0%) received a diagnostic-driven treatment and 63 patients (11.2%) a fever-driven treatment. Liposomal Amphotericin-B (L-AmB) and voriconazole were the drugs used in the majority of patients receiving diagnostic-driven therapy (30/79, 38% and 22/79, 27.8%, respectively), while caspofungin was administered more frequently in patients who received a fever-driven strategy (27/63, 42.9%), followed by L-AmB (17/63, 27%) and voriconazole (11/63, 17.5%).
Univariate and multivariate competing risk regression model for IFIs occurrence are summarized in Table 3.
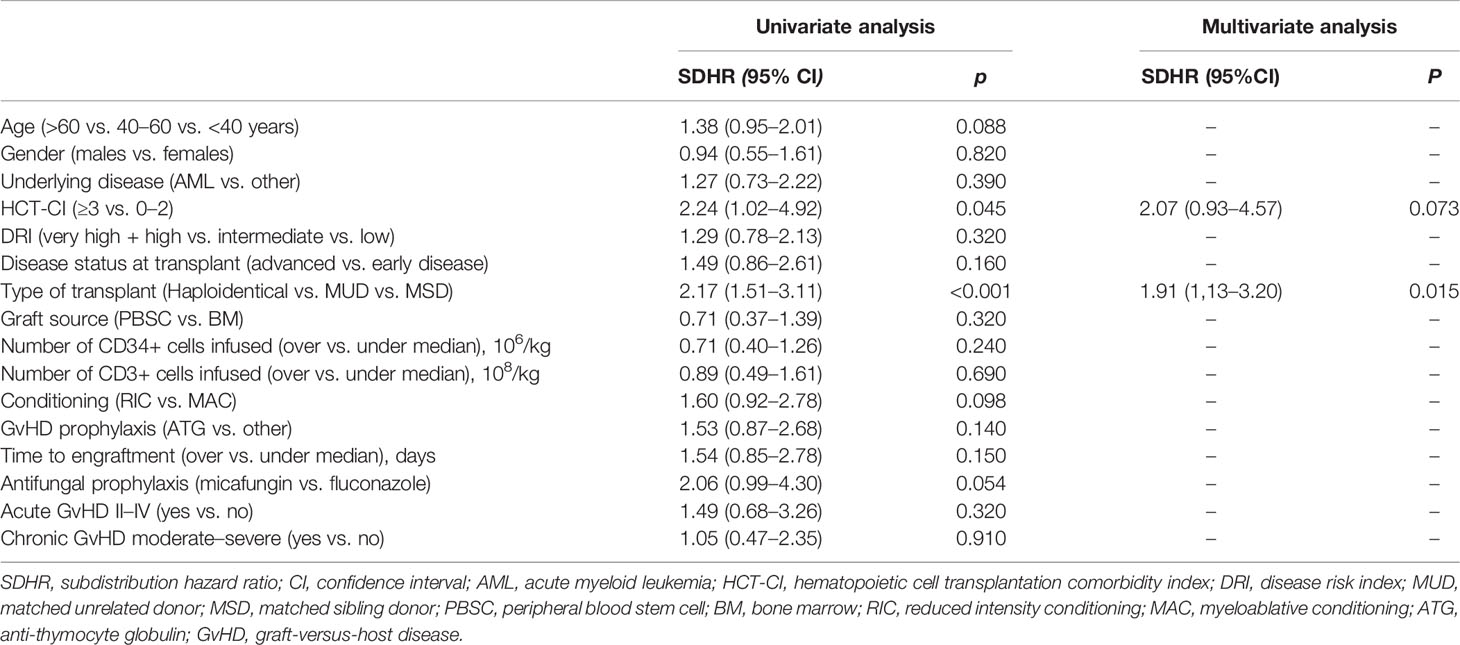
Table 3 Univariate and multivariate analysis of risk factors for invasive fungal infections after allogeneic HSCT.
A high HCT-CI (≥3), the donor type (haploidentical vs. MUD vs. MRD), and the use of micafungin as antifungal prophylaxis were associate with a higher risk for IFI [subdistribution hazard ratio (SDHR), 2.24, p = 0.045; SDHR, 2.17, p < 0.001; SDHR, 2.06, p = 0.054, respectively]. Only the donor type reached significance in the multivariate model (SDHR, 1.91; p = 0.015).
The median follow-up of surviving patients undergoing allogeneic HSCT during our study period was 6.70 years (0.14–17.02), in particular 5.68 (range, 0.28–15.48) years for patients with IFI and 6.79 (range, 0.14–17.02) years for patients without IFI.
Overall, relapse was the major cause of death (168/268, 62.7%), while 100 patients died of transplant-related complications (100/268, 37.3%). Nine patients with PP-aspergillosis (18%), 1 patient with invasive candidiasis (16.7%), and the patient with mucormycosis died of infection. IFI-related mortality rate was 20.7% in the overall population, 17% in patients with probable IFI, and 36% in patients with proven IFI.
The presence of IFI had a significant impact on overall survival at 1 year (IFI, 32.8% vs. non-IFI, 54.6%; p < 0.001) (Figure 3).
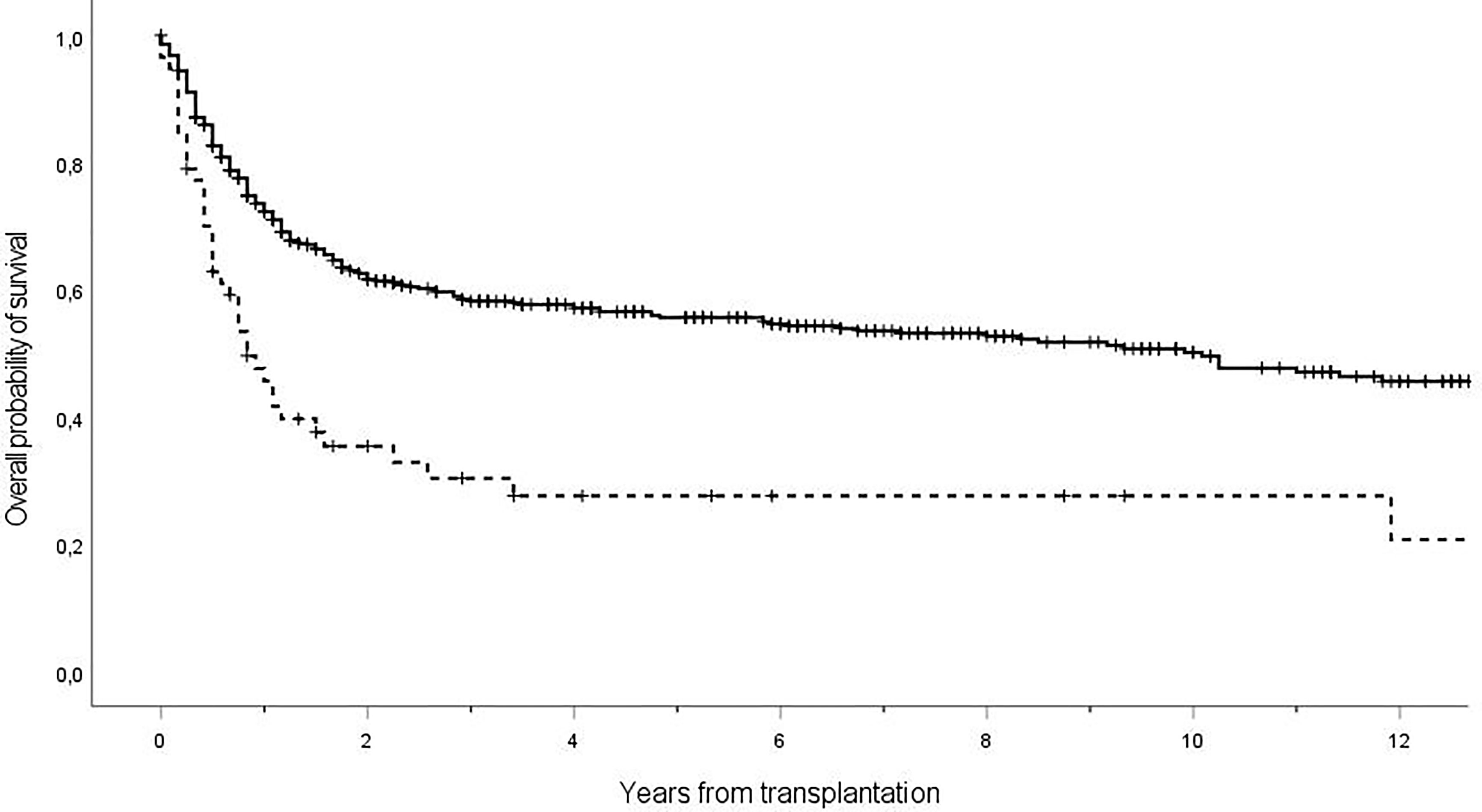
Figure 3 Overall survival of patients receiving allogeneic transplant with diagnosis of IFI (dashed line) or not (solid line). The 1-year overall survival was 32.8% in patients IFI positive vs. 54.6% in patients IFI negative, with a statistically significant difference (p < 0.001). Ticks on probability lines indicate dates of censoring at last follow-up.
In the present study, we evaluated the epidemiology and the prognostic factors of IFIs in a cohort of HSCT patients receiving PAP mainly with fluconazole and a diagnostic-driven therapy guided by screening with NCBM, BAL, and CT scan.
Overall, the cumulative incidence of PP-IFI at 1-year post-HSCT was higher as compared to the TRANSNET (Kontoyiannis et al., 2010) and SEIFEM (Pagano et al., 2007) trials (9.6% vs. 3.4% and 3.7%, respectively) but superimposable to the 7–9% rates reported in the most recent studies (Girmenia et al., 2014; Van de Peppel et al., 2018).
According to a consistent number of studies evaluating the epidemiology of IFI in hematological patients [TRANSNET (Kontoyiannis et al., 2010), SEIFEM (Pagano et al., 2007), and PATH (Neofytos et al., 2009)], invasive aspergillosis was the most predominant IFI accounting for three quarters of the infections. Non-albicans spp. were the most frequent yeast infection, underpinning that the generalized use of azoles may induce a selection pressure in favor of non-albicans spp.
According to ECIL guidelines (Maertens et al., 2018), fluconazole may be considered for PAP during the pre-engraftment phase when an intensive diagnostic work-up is part of the strategies for management of febrile patients. In our study, the median time onset of IFI was largely beyond the engraftment phase. Nevertheless, the presence of neutropenia at the time of IFI was detected in 34.5% of the patients, supporting the observation that delayed neutropenia is a frequent complication in HSCT recipients [in particular, MUD and haploidentical HSCT recipients (Di Stasi et al., 2014; Salvatore et al., 2018)] usually related to concomitant treatments for cytomegalovirus (CMV) infections and GvHD. In this respect, we have implemented our policy to include mold-active prophylaxis in patients with late cytopenias or GvHD requiring systemic immunosuppressive treatments.
In line with these observations, the high rate of IFI observed in our series of MUD and haploidentical recipients might be explained with the increased risk of viral infections and GvHD reported in many studies (Dominietto et al., 2001).
As reported in a previous study (Busca et al., 2018), a high HCT-CI (≥3) was predictive of the risk of IFIs in univariate analysis and resulted in marginal significance in the multivariate model. If prospective trials will confirm this observation, patients with high HCT-CI at the time of transplant might benefit from a more aggressive strategy including antifungal prophylaxis with mold-active agents.
The lung was the most frequent site involved in patients with mold infections. Notably, 51% of our patients with pulmonary infiltrates were explored with BAL, supporting the concept that BAL is a diagnostic procedure feasible in a consistent number of hematological patients. Even more importantly, BAL led to the identification of the causative agent in two-thirds of the cases. Our data compare favorably with the study published by the SEIFEM group, showing that 33% of patients with lung infiltrates were investigated, with BAL yielding the identification of the putative microbiological agent in 75% of the cases (Marchesi et al., 2019).
Mold-active agents include azoles (i.e., itraconazole, voriconazole, Posaconazole, and isavuconazole) targeting the fungal lanosterol 14α-demethylase, leading to an alteration in cell membrane permeability, and polyenes (i.e., amphotericin B) binding to the membrane ergosterol with consequent formation of pores and channels that irreversibly damage the fungal wall. Echinocandins represent the most effective agents for yeast infections by the inhibition of the synthesis of 1,3-β-D-glucan, which is a major component of the cell wall.
Despite the use of a non-mold-active agent as antifungal prophylaxis, our study showed that a therapeutic approach driven by a diagnostic strategy including NCBM, BAL, and CT scan is effective, resulting in a low mortality rate (21%) while avoiding unnecessary toxicity. Even in patients with proven IFI, the mortality was 36% significantly lower when compared to the 72% mortality rate reported in a recent meta-analysis of Van Peppel et al. (2018). It should be emphasized that the availability of a wide armamentarium of antifungal agents with different spectrum of activity and good safety profiles may have contributed to improve the outcome of patients with IFI. Nevertheless, it should be emphasized that the presence of IFIs had a remarkable impact on the overall survival (IFI, 32.8% vs. non-IFI, 54.6%; p < 0.001). In this respect, antifungal stewardship in hematological patients is eagerly needed: new diagnostic tools able to anticipate the identifications of patients with IFI and prognostic risk score that might predict the risk of IFI may represent resources of utmost importance.
In total, 11% of our patients received a fever-driven treatment. This negligible number of empirical treatments demonstrates that the application of an intensive work-up is able to limit the use of unnecessary treatments, even in HSCT recipients generally deemed as critical ill patients. Among these patients, 11 (17.5%) died of infection.
Several limitations of our analysis should be acknowledged. The study is retrospective and is a single-center study: accordingly, the epidemiology might not be representative of the spectrum of the fungi distributed in different transplant centers. Regrettably, the low number of proven mold infections did not allow us to investigate the possible emergence of azole resistance as described in other studies (Shariati et al., 2020).
In summary, the results of our study indicate that febrile patients after HSCT may benefit from a comprehensive diagnostic work-up including NCBM, early CT scan, and BAL. This approach unveiling a low mortality rate may be relevant to determine additional interventions aiming to improve the outcome of patients who develop IFI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All procedures performed in the present study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
AB has received honoraria from Gilead Sciences, Merck, Pfizer Pharmaceuticals, and Jazz Pharmaceuticals; he has been a speaker for Gilead Sciences, Merck, Pfizer Pharmaceuticals, and Novartis; and he is part of an Advisory Board of GILEAD and Pfizer. LG has been a speaker for Pfizer, Jazz, and Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arendrup, M. C., Bille, J., Dannaoui, E., Ruhnke, M., Heussel, C. P., Kibbler, C. (2012). ECIL-3 Classical Diagnostic Procedures for the Diagnosis of Invasive Fungal Diseases in Patients With Leukaemia. Bone Marrow Transpl. 47 (8), 1030–1045. doi: 10.1038/bmt.2011.246
Armand, P., Kim, H. T., Logan, B. R., Wang, Z., Alyea, E. P., Kalaycio, M. E., et al. (2014). Validation and Refinement of the Disease Risk Index for Allogeneic Stem Cell Transplantation. Blood 123 (23), 3664–3671. doi: 10.1182/blood-2014-01-552984
Bhatti, Z., Shaukat, A., Almyroudis, N. G., Segal, B. H. (2006). Review of Epidemiology, Diagnosis, and Treatment of Invasive Mould Infections in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Mycopathologia 162 (1), 1–15. doi: 10.1007/s11046-006-0025-x
Busca, A., Pagano, L. (2016). Antifungal Therapy in Hematopoietic Stem Cell Transplant Recipients. Mediterranean J. Hematol. Infect. Diseases 8 (1), e2016039. doi: 10.4084/MJHID.2016.039
Busca, A., Passera, R., Maffini, E., Festuccia, M., Brunello, L., Dellacasa, C. M., et al. (2018). Hematopoietic Cell Transplantation Comorbidity Index and Risk of Developing Invasive Fungal Infections After Allografting. Bone Marrow Transpl., 53, 1304–1310. doi: 10.1038/s41409-018-0161-1
Carreras, E., Dufour, C., Mohty, M., Kroger, N. (2019). The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. 7th edition (Cham (CH: Springer). Bookshelf ID: NBK553942. doi: 10.1007/978-3-030-02278-5
Copelan, E. A., Chojecki, A., Lazarus, H. M., Avalos, B. R. (2018). Allogeneic Hematopoietic Cell Transplantation; the Current Renaissance. Blood Rev. 34, 34–44. doi: 10.1016/j.blre.2018.11.001
Cordonnier, C., Robin, C., Alanio, A., Bretagne, S. (2014). Antifungal Pre-Emptive Strategy for High-Risk Neutropenic Patients: Why the Story Is Still Ongoing. Clin. Microbiol. Infect. Diseases 20 (Suppl 6), 27–35. doi: 10.1111/1469-0691.12428
De Kort, E. A., Maertens, J., Verweij, P. E., Rijnders, B. J. A., Blijlevens, N. M. A. (2019). Diagnostic-Driven Management of Invasive Fungal Disease in Hematology in the Era of Prophylaxis and Resistance Emergence: Dutch Courage? Med. Mycology 57 (Supplement_3), S267–S273. doi: 10.1093/mmy/myz026
Di Stasi, A., Milton, D. R., Poon, L. M., Hamdi, A., Rondon, G., Chen, J., et al. (2014). Similar Transplantation Outcomes for Acute Myeloid Leukemia and Myelodysplastic Syndrome Patients With Haploidentical Versus 10/10 Human Leukocyte Antigen-Matched Unrelated and Related Donors. Am. Soc. Blood Marrow Transpl. 20 (12), 1975–1981. doi: 10.1016/j.bbmt.2014.08.013
Dominietto, A., Raiola, A. M., Van Lint, M. T., Lamparelli, T., Gualandi, F., Berisso, G., et al. (2001). Factors Influencing Haematological Recovery After Allogeneic Haemopoietic Stem Cell Transplants: Graft-Versus-Host Disease, Donor Type, Cytomegalovirus Infections and Cell Dose. Br. J. Haematol. 112 (1), 219–227. doi: 10.1046/j.1365-2141.2001.02468.x
Donnelly, J. P., Chen, S. C., Kauffman, C. A., Steinbach, W. J., Baddley, J. W., Verweij, P. E., et al. (2020). Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Infect. Dis. Soc. Am. 71 (6), 1367–1376. doi: 10.1093/cid/ciz1008
Filipovich, A. H., Weisdorf, D., Pavletic, S., Socie, G., Wingard, J. R., Lee, S. J., et al. (2005). National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transpl.: J. Am. Soc. Blood Marrow Transpl. 11 (12), 945–956. doi: 10.1016/j.bbmt.2005.09.004
Fung, M., Kim, J., Marty, F. M., Schwarzinger, M., Koo, S. (2015). Meta-Analysis and Cost Comparison of Empirical Versus Pre-Emptive Antifungal Strategies in Hematologic Malignancy Patients With High-Risk Febrile Neutropenia. PloS One 10 (11), e0140930. doi: 10.1371/journal.pone.0140930
Gagelmann, N., Kröger, N. (2021). Dose Intensity for Conditioning in Allogeneic Hematopoietic Cell Transplantation: Can We Recommend "When and for Whom" in 2021? Haematologica 106 (7), 1794–1804. doi: 10.3324/haematol.2020.268839
Girmenia, C., Barosi, G., Piciocchi, A., Arcese, W., Aversa, F., Bacigalupo, A., et al. (2014). Primary Prophylaxis of Invasive Fungal Diseases in Allogeneic Stem Cell Transplantation: Revised Recommendations From a Consensus Process by Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol. Blood Marrow Transpl.: J. Am. Soc. Blood Marrow Transpl. 20 (8), 1080–1088. doi: 10.1016/j.bbmt.2014.02.018
Girmenia, C., Raiola, A. M., Piciocchi, A., Algarotti, A., Stanzani, M., Cudillo, L., et al. (2014). Incidence and Outcome of Invasive Fungal Diseases After Allogeneic Stem Cell Transplantation: A Prospective Study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol. Blood Marrow Transpl.: J. Am. Soc. Blood Marrow Transpl. 20 (6), 872–880. doi: 10.1016/j.bbmt.2014.03.004
Gyurkocza, B., Rezvani, A., Storb, R. F. (2010). Allogeneic Hematopoietic Cell Transplantation: The State of the Art. Expert Rev. Hematol. 3 (3), 285–299. doi: 10.1586/ehm.10.21
Jagasia, M. H., Greinix, H. T., Arora, M., Williams, K. M., Wolff, D., Cowen, E. W., et al. (2015). National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transpl.: J. Am. Soc. Blood Marrow Transpl. 21 (3), 389–401.e1. doi: 10.1016/j.bbmt.2014.12.001
Kontoyiannis, D. P., Marr, K. A., Park, B. J., Alexander, B. D., Anaissie, E. J., Walsh, T. J., et al. (2010). Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001-2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Infect. Dis. Soc. Am. 50 (8), 1091–1100. doi: 10.1086/651263
Lee, S. J., Klein, J. P., Barrett, A. J., Ringden, O., Antin, J. H., Cahn, J. Y., et al. (2002). Severity of Chronic Graft-Versus-Host Disease: Association With Treatment-Related Mortality and Relapse. Blood 100, 406–414. doi: 10.1182/blood.v100.2.406
Luznik, L., Jalla, S., Engstrom, L. W., Iannone, R., Fuchs, E. J. (2001). Durable Engraftment of Major Histocompatibility Complex–Incompatible Cells After Nonmyeloablative Conditioning With Fludarabine, Low- Dose Total Body Irradiation, and Posttransplantation Cyclophosphamide. Blood 98 (12), 3456–3464. doi: 10.1182/blood.v98.12.3456
Maertens, J. A., Girmenia, C., Brüggemann, R. J., Duarte, R. F., Kibbler, C. C., Ljungman, P., et al. (2018). European Guidelines for Primary Antifungal Prophylaxis in Adult Haematology Patients: Summary of the Updated Recommendations From the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 73 (12), 3221–3230. doi: 10.1093/jac/dky286
Marchesi, F., Cattaneo, C., Criscuolo, M., Delia, M., Dargenio, M., Del Principe, M. I., et al. (2019). . A Bronchoalveolar Lavage-Driven Antimicrobial Treatment Improves Survival in Hematologic Malignancy Patients With Detected Lung Infiltrates: A Prospective Multicenter Study of the SEIFEM Group. Am. J. Hematol. 94 (10), 1104–1112. doi: 10.1002/ajh.25585
Marchetti, O., Lamoth, F., Mikulska, M., Viscoli, C., Verweij, P., Bretagne, S. (2012). ECIL Recommendations for the Use of Biological Markers for the Diagnosis of Invasive Fungal Diseases in Leukemic Patients and Hematopoietic SCT Recipients. Bone Marrow Transpl. 47 (6), 846–854. doi: 10.1038/bmt.2011.178
Martinez-Cibrian, N., Zeiser, R., Perez-Simon, J. A. (2020). Graft-Versus-Host Disease Prophylaxis: Pathophysiology-Based Review on Current Approaches and Future Directions. Blood Rev. 48:100792. doi: 10.1016/j.blre.2020.100792
Mellinghoff, S. C., Panse, J., Alakel, N., Behre, G., Buchheidt, D., Christopeit, M., et al. (2017). Primary Prophylaxis of Invasive Fungal Infections in Patients With Haematological Malignancies: 2017 Update of the Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann. Hematol. 97 (2), 197–207. doi: 10.1007/s00277-017-3196-2
Neofytos, D. (2019). Antimicrobial Prophylaxis and Preemptive Approaches for the Prevention of Infections in the Stem Cell Transplant Recipient, With Analogies to the Hematologic Malignancy Patient. Infect. Dis. Clinics North Am. 33 (2), 361–380. doi: 10.1016/j.idc.2019.02.002
Neofytos, D., Horn, D., Anaissie, E., Steinbach, W., Olyaei, A., Fishman, J., et al. (2009). Epidemiology and Outcome of Invasive Fungal Infection in Adult Hematopoietic Stem Cell Transplant Recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance Registry. Infect. Dis. Soc. Am. 48 (3), 265–273. doi: 10.1086/595846
Pagano, L., Caira, M., Nosari, A., Van Lint, M. T., Candoni, A., Offidani, M. (2007). Fungal Infections in Recipients of Hematopoietic Stem Cell Transplants: Results of the SEIFEM B-2004 Study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Infect. Dis. Soc. Am. 45 (9), 1161–1170. doi: 10.1086/522189
Rahi, M. S., Jindal, V., Pednekar, P., Parekh, J., Gunasekaran, K., Sharma, S., et al. (2021). Fungal Infections in Hematopoietic Stem-Cell Transplant Patients: A Review of Epidemiology, Diagnosis, and Management. Ther. Adv. Infect. Dis. 19; 8:20499361211039050. doi: 10.1177/20499361211039050
Ruhnke, M., Behre, G., Buchheidt, D., Christopeit, M., Hamprecht, A., Heinz, W., et al. (2018). Diagnosis of Invasive Fungal Diseases in Haematology and Oncology: 2018 Update of the Recommendations of the Infectious Diseases Working Party of the German Society for Hematology and Medical Oncology (AGIHO). Mycoses 61 (11), 796–813. doi: 10.1111/myc.12838
Sahin, U., Toprak, S. K., Atilla, P. A., Atilla, E., Demirer, T. (2016). An Overview of Infectious Complications After Allogeneic Hematopoietic Stem Cell Transplantation. J. Infect. Chemother. 22 (8), 505–514. doi: 10.1016/j.jiac.2016.05.006
Salvatore, D., Labopin, M., Ruggeri, A., Battipaglia, G., Ghavamzadeh, A., Ciceri, F., et al. (2018). Outcomes of Hematopoietic Stem Cell Transplantation From Unmanipulated Haploidentical Versus Matched Sibling Donor in Patients With Acute Myeloid Leukemia in First Complete Remission With Intermediate or High-Risk Cytogenetics: A Study From the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 103 (8), 1317–1328. doi: 10.3324/haematol.2018.189258
Schmiedel, Y., Zimmerli, S. (2016). Common Invasive Fungal Diseases: An Overview of Invasive Candidiasis, Aspergillosis, Cryptococcosis, and Pneumocystis Pneumonia. Swiss Medical Weekly 146, w14281. doi: 10.4414/smw.2016.14281
Shariati, A., Moradabadi, A., Chegini, Z., Khoshbayan, A., Didehdar, M. (2020). An Overview of the Management of the Most Important Invasive Fungal Infections in Patients With Blood Malignancies. Infect. Drug Resist. 13, 2329–2354. doi: 10.2147/IDR.S25447
Sorror, M. L., Maris, M. B., Storb, R., Baron, F., Sandmaier, B. M., Maloney, D. G., et al. (2005). Hematopoietic Cell Transplantation (HCT)-Specific Comorbidity Index: A New Tool for Risk Assessment Before Allogeneic HCT. Blood 106 (8), 2912–2919. doi: 10.1182/blood-2005-05-2004
Van de Peppel, R. J., Visser, L. G., Dekkers, O. M., Boer, M. G. J. (2018). The Burden of Invasive Aspergillosis in Patients With Haematological Malignancy: A Meta-Analysis and Systematic Review. J. Infect. 76 (6), 550–562. doi: 10.1016/j.jinf.2018.02.012
Keywords: invasive fungal infections, allogeneic hematopoietic stem cell transplantation, immunocompromised host, antifungal prophylaxis, bronchoalveolar lavage
Citation: Busca A, Cinatti N, Gill J, Passera R, Dellacasa CM, Giaccone L, Dogliotti I, Manetta S, Corcione S and De Rosa FG (2022) Management of Invasive Fungal Infections in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: The Turin Experience. Front. Cell. Infect. Microbiol. 11:805514. doi: 10.3389/fcimb.2021.805514
Received: 30 October 2021; Accepted: 13 December 2021;
Published: 07 January 2022.
Edited by:
Neil Andrew Robert Gow, University of Exeter, United KingdomReviewed by:
Vishukumar Aimanianda, Institut Pasteur, FranceCopyright © 2022 Busca, Cinatti, Gill, Passera, Dellacasa, Giaccone, Dogliotti, Manetta, Corcione and De Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Busca, YWJ1c2NhQGNpdHRhZGVsbGFzYWx1dGUudG8uaXQ=; Natascia Cinatti, Y2luYXR0aW5hdGFzY2lhQGdtYWlsLmNvbQ==; Jessica Gill, amVzc2ljYS5naWxsQGVkdS51bml0by5pdA==
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.