
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 22 November 2021
Sec. Clinical Microbiology
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.793089
This article is part of the Research Topic Advances in Diagnosis and Therapeutic Intervention for Foodborne Parasitic Diseases, Volume II View all 20 articles
 Na Yao1,2,3†
Na Yao1,2,3† Qiong Xu1,2,3†
Qiong Xu1,2,3† Jia-Kang He4
Jia-Kang He4 Ming Pan1,2,3
Ming Pan1,2,3 Zhao-Feng Hou1,2,3
Zhao-Feng Hou1,2,3 Dan-Dan Liu1,2,3
Dan-Dan Liu1,2,3 Jian-Ping Tao1,2,3
Jian-Ping Tao1,2,3 Si-Yang Huang1,2,3,5*
Si-Yang Huang1,2,3,5*Toxoplasma gondii is a serious hazard to public health and animal husbandry. Due to the current dilemma of treatment of toxoplasmosis, it is urgent to find new anti-T. gondii drugs to treat toxoplasmosis. In this study, the anti-T. gondii activity of Origanum vulgare essential oil (Ov EO) was firstly studied, and then, carvanol (Ca), the main ingredient of Ov EO was evaluated using the MTT assay on human foreskin fibroblast (HFF) cells in vitro. The cytotoxicity was evaluated using the MTT assay on HFF cells. The CC50 of Ov EO and Ca was 134.9 and 43.93 μg/ml, respectively. Both of them exhibited anti-parasitic activity, and inhibited the growth of T. gondii in a dose-dependent manner. For the inhibition effect, Ca was better than Ov EO at the same concentration, the IC50 of Ov EO and Ca was 16.08 and 7.688 μg/ml, respectively. In addition, treatment with Ca, was found to change the morphology of T. gondii tachyzoites and made their shapes curl up. These results showed that Ca was able to inhibit the proliferation of T. gondii by reducing invasion, which may be due to its detrimental effect on the mobility of tachyzoites. Our results indicated that Ca could be a potential new and effective drug for treating toxoplasmosis.
The opportunistic pathogen Toxoplasma gondii is a serious hazard to public health and animal husbandry (Chemoh et al., 2013). One-third of the people in the world have been infected by T. gondii where tachyzoites, cysts and oocysts are three infectious stages. Human intake of raw meat or water containing T. gondii cysts or oocysts can be infectious. In a few cases, direct contact with T. gondii tachyzoites between the mucous membrane and the damaged skin can also cause an infection. Cats are intermediate hosts of T. gondii and can rule out infectious oocysts. Accidental contact between humans and cat feces is a risk of infection. For most individuals with competent immunity, infection is asymptomatic and the T. gondii eventually lies dormant as a tissue cyst. For some people primary infection can cause ocular disease, and in pregnant women, it can lead to abortion, stillbirth or brain damage in a congenitally infected fetus. Recurrence of chronic infection is a frequent cause of toxoplasmic encephalitis (TE) in an immunosuppressive patient such as advanced HIV infection, neoplastic disease, or in those receiving immunosuppressive therapies (e.g., rituximab).
As T. gondii has a wide range of hosts, apart from humans, it also infects many animals, like cattle, sheep, pig and other domestic animals. This causes the economic loss of animal husbandry and the hidden danger of public food hygiene and safety.
The drug treatment of toxoplasmosis can be traced back to the use of sulfonamides in the 1940s. In the 1950s, sulfadiazine combined with pyrimethamine successfully treated toxoplasmosis in mice. It is still the golden treatment for toxoplasmosis today (Wei et al., 2015). However, the side effects and the emergence of drug resistance have undermined the perfection of the treatment regimen (Schmidt et al., 2006). In the case of pregnant women infected with T. gondii, spiramycin is a good drug for the treatment of toxoplasmosis because of its low toxicity and it cannot penetrate the placental barrier; however it has no effect on the infected fetus (Desmonts and Couvreur, 1974). Other drugs such as Trimethoprim-sulfamethoxazole, Clindamycin, and Atovaquone also have their own disadvantages (Dunay et al., 2018). Therefore, it is urgent to find new anti-T. gondii drugs with high efficiency and low toxicity to treat toxoplasmosis.
Natural products are one of the important sources of drug development (Petrovska, 2012). In the field of cancer treatment alone, from the 1940s to now, 48.6% of the 175 small molecules are natural products or obtained directly from there (Newman and Cragg, 2012). Plants as one of the natural products usually grow outdoors, so they have to resist the infection of disease and the pressure of harsh environment in the process of growing (Weng et al., 2012). In this process of defense, the molecules they produce give plants smell, color and even toxicity (Lietava, 1992). Essential oils are a mixture of these molecules, which are a potential drug reservoir.
Origanum vulgare that is native to the Mediterranean coast, North Africa and West Asia is a perennial herb of the genus Oregon of the Lamiaceae family (Elshafie et al., 2017). The O. vulgare essential oil (Ov EO) has been proven to have certain biological activity (Argyri et al., 2021). At a concentration of 60 μg/ml, Ov EO can inhibit the invasion rate of Cryptosporidium parvum into Human colon adenocarcinoma (HCT-8) cells by 60% (Gaur et al., 2018). Ov EO can also inhibit the growth of Aeromonas hydrophila, Brevibacterium linens, Clostridium sporogenes, Leuconostoc cremoris, and Pseudomonas aeruginosa (Dorman and Deans, 2000). The crude Ov EO can decrease the activity of liver cancer cells (HepG2 cell) in a dose-dependent manner, and the IC50 value was 236 μg/μl (Elshafie et al., 2017). In addition, Ov EO also shows excellent anti-inflammatory and antioxidant activities, and also has potential functions in controlling cardiovascular diseases and metabolic syndrome (Leyva-López et al., 2017).
In this study, the anti-T. gondii activity of Ov EO was firstly studied, and then the inhibited activity of carvanol (Ca), the main ingredient of Ov EO, was selected to evaluate in vitro.
T. gondii tachyzoites of the GFP-RH strain, expressing green fluorescence protein were proliferated in human foreskin fibroblast (HFF) cells, cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37°C, in an atmosphere containing 5% CO2. To isolate the tachyzoites, heavily infected cells were scraped and the parasites were released by passing the cells through a 27-gauge needle, three to five times. Cell debris was removed by passing the mixture through a 3-µm pore membrane filter (Whatman, ThermoFisher, Waltham, MA, USA). Tachyzoites were quantified using a hemocytometer before proceeding to further experiments.
The Ov EO and Ca used in this experiment was provided by Guangxi University, EO was extracted by steam distillation and dissolved in dimethyl sulfoxide (DMSO) in a 1:1 ratio. Ca was dissolved into a suitable mother liquor with DMSO. The species number of O. vulgare used in this study is GXCM 2019023. The solutions were then diluted with DMEM, such that the final concentration of DMSO in the samples used in the experiment was lower than 1.56% v/v.
The cytotoxicity of Ov EO and Ca was evaluated in an HFF cell line with a CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corp., Madison, WI, USA), according to the manufacturer’s instructions. HFF cells (1 × 105 cells/well) were seeded in 96-well plates and cultured at 37°C, in an atmosphere containing 5% CO2, for 24 h. The cells were treated with varying concentrations of Ov EO (70, 35, 17, 9, and 4 μg/ml), Ca (70, 35, 17, 9, and 4μg/ml) or sulfamethoxazole (SMZ), and a 1.56% solution of DMSO in DMEM was used as the vehicle control. After incubating for 24 h, the HFF cells viability were measured by the MTT (3-[4,5-methylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) colorimetric method according to Costa et al. (2018). Approximately 20 μl of MTT solution (5 mg/ml) was added to each well and allowed to incubate at 37°C with 5% CO2 for 3 h and then 200 μl of DMSO was added to dissolve the formazan crystals. Absorbance was measured at 490 nm using an iMark™ Microplate Absorbance Reader (BioRad, Hercules, CA, USA). and the 50% cytotoxic concentrations (CC50) were calculated using Graph Pad Prism 8.0. The cytotoxicity experiment was performed in triplicate, using three separate plates.
One hundred freshly released GFP-RH tachyzoites were added to HFF monolayers in 6-well plates, in DMEM with 2% FBS. They were incubated at 37°C, in an atmosphere containing 5% CO2, for 4 h. Then, the extracellular parasites were removed with medium, and fresh medium containing various concentrations of Ov EOs, Ca or 1.56% DMSO (vehicle control) were added to each well. Uninfected and untreated wells were used as blank controls. After 7 days, HFF cells were washed three times with PBS, fixed with methanol for 10 min, and stained with 0.1% crystal violet for 30 min. After washing three times with phosphate buffered saline (PBS) and drying naturally (Huang et al., 2021), the plaques formed by tachyzoites were examined by microscopy.
HFF cells were incubated in 24-well plates for 48 h, then the medium was replaced by DMEM with 2% FBS, 100 freshly released GFP-RH tachyzoites were added to each well, and incubated at 37°C in an atmosphere containing 5% CO2, for 4 h. The medium containing extracellular parasites was removed and fresh medium containing either Ov EO (70, 35, 17, 9, and 4 μg/ml) or Ca (17, 9, 4, and 2 μg/ml), 1.56% DMSO (vehicle control), or 10 μg/ml SMZ (positive control) was added to each well. After 32 h, the growth of GFP-RH was observed and photographed under a fluorescence microscope. Growth of GFP-RH was calculated using Image-Pro-Express.
Invasion experiments were performed as described by Augusto et al. (2018). HFF cells was cultured in a 6-well plate, and 3 ml DMEM with 2% FBS was added to each well. Then, 104 RH and 17 μg/ml Ov EO or Ca were added simultaneously to the wells, respectively, incubating for 20, 40, or 60 min. The supernatant was gently removed, cells were fixed with 2 ml methanol for 10 min, washed three times with PBS, blocked by 5% solution of BSA in PBS (BSA/PBS) for 1 h, and washed three times with PBS. This was then incubated with mouse anti-Toxoplasma SAG1 monoclonal antibodies (mAb), diluted (1:1,000) with a 1% BSA/PBS solution, at room temperature for 2 h. Then, goat anti-mouse IgG H&L(FITC) secondary antibodies, diluted (1:1,000) in 1% BSA/PBS, were added to 6-well plates and incubated at room temperature for 2 h. After washing thrice with PBS, 300 μl of 0.2% Triton X-100 was added, and the mixture was left for 30 min. Cells were then gently washed three times with PBS, and 300 μl of a 5% BSA/PBS solution was added dropwise for a second blocking. The antibodies were added as per the procedure described earlier, this time using goat anti-mouse IgG H&L (Alexa Fluor ® 568) (ab175473) instead of the goat anti-mouse IgG H&L (FITC). Finally, 300 μl of 30% glycerol was added to each well. Five visual fields were randomly selected for observation under the 40× objective of the fluorescence microscope and the parasites in each field were counted. Three repetitions were performed to increase the accuracy of the experiment. The difference between the tachyzoites of the two colors is termed as the absolute invasion number of tachyzoites. The ratio of the invasion number to the total number of tachyzoites is termed as the invasion rate of tachyzoites.
To determine differences in the ultrastructure of tachyzoites after treatment, the purified tachyzoites were treated with 17 μg/ml Ov EO or Ca, respectively. After being cultured at 37 °C for 4 h, they were washed gently with PBS twice, and fixed overnight with 2.5% glutaraldehyde at room temperature. Gradient dehydration was carried out with 30, 50, 70, 80, 90, 95 and 100% ethanol respectively, and the critical point drying was carried out after dehydration. The tachyzoites were coated with gold (20−30 nm) and observed by scanning electron microscopy.
All data were analyzed using GraphPad Prism 8.0. The anti-parasitic activity of the Ov EO and Ca was analyzed using an unpaired t-test, while the cell invasion data were processed using multiple t-tests, to compare the results of the test groups and those of the control group (*P <0.05, **P <0.01, ***P <0.001).
The cytotoxic potential of Ov EO on the host cell was confirmed before the antiparasitic activity study. According to MTT assay result, the concentration that induced 50% HFF cells mortality (CC50) of Ov EO was 134.9 μg/ml (Figure 1). After the antiparasitic activity of Ov EO was confirmed, the cytotoxic potential of Ca was carried out, and the result indicated that the CC50 of Ca was 43.93 μg/ml (Figure 1).
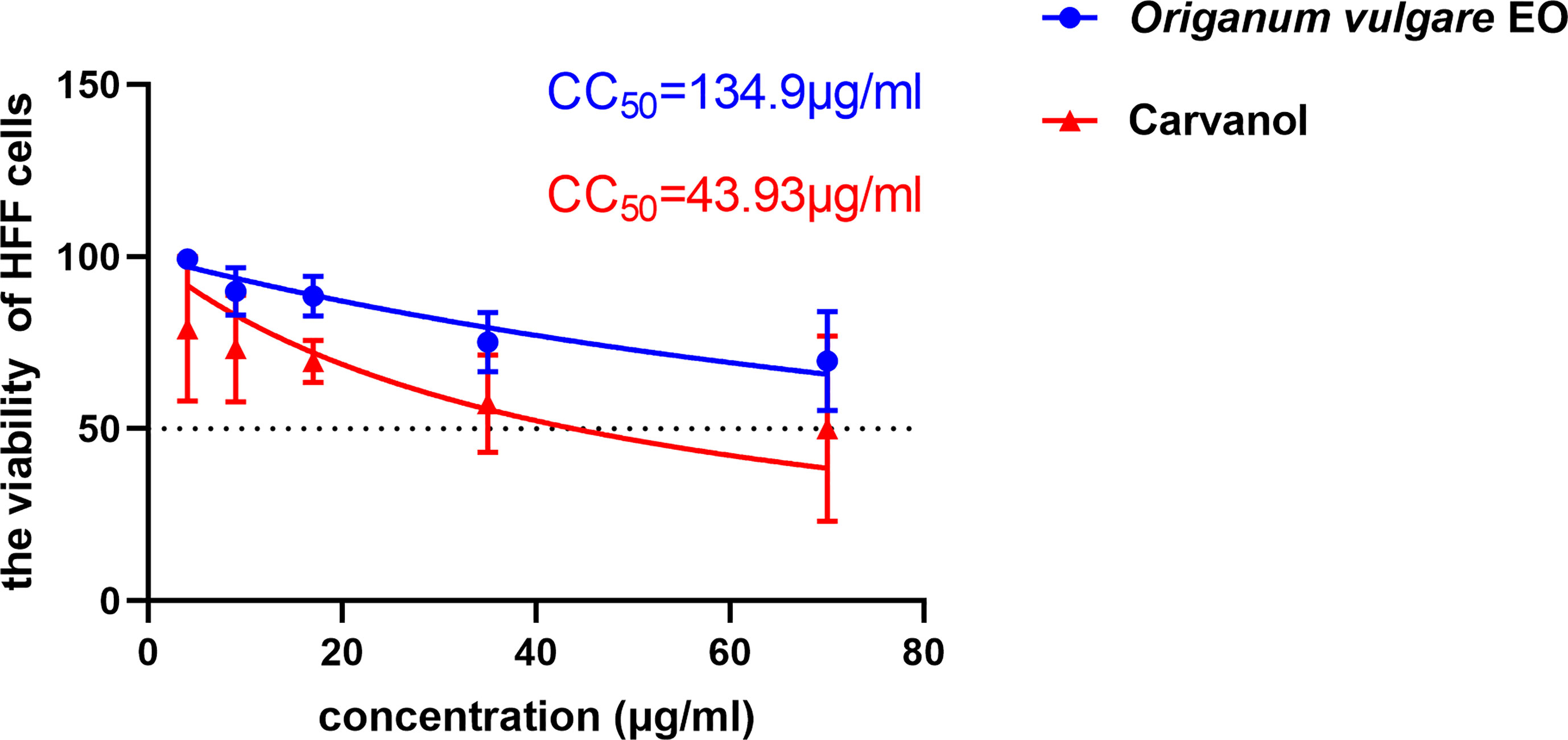
Figure 1 The 50% cytotoxic concentrations (CC50) of Ov EO and Ca. Cytotoxicity of Ov EO and Ca on HFF cells. Different concentrations of Ov EO and Ca treat HFF cells for 24 h and then cytotoxicity was evaluated using MTT Assay. All data are presented as with error bars and the experiments were performed in triplicate.
The anti-T. gondii activity of Ov EO was preliminarily evaluated by plaque test. As seen in Figure 2A, we found that the plaques visible were fewer in number and smaller in size after treatment with 9 or 17 μg/ml Ov EO (Figures 2Aa, b), as compared to those in the DMSO-treated and untreated groups. These results indicated that Ov EO was able to inhibit the growth of RH within safe concentrations. In order to find the effective ingredients in Ov EO that have the role of anti-toxoplasma, Ca was evaluated by plaque test. The results indicated that Ca was able to inhibit the growth of RH at 9 or 17 μg/ml (Figures 2Ae, f). The results indicated that the growth of T. gondii was inhibited by each of the concentrations of Ov EO and Ca tested (Figure 2B). To confirm and evaluate the effect of Ov EO concentration on anti-parasitic activity, five different concentrations were compared using an in vitro inhibition assay. The results indicated that the growth of T. gondii was inhibited by each of the concentrations of Ov EO tested (Figure 3), and the inhibition increased in a dose-dependent manner (Figure 4A), the IC50 of Ov EO was 16.08 μg/ml. We could find that the growth of T. gondii was significantly reduced after treatment with 70 and 35 μg/ml Ov EO (71 vs. 1,337; 320 vs. 1,337, P <0.001), as compared to the untreated and 1.56% DMSO-treated groups.
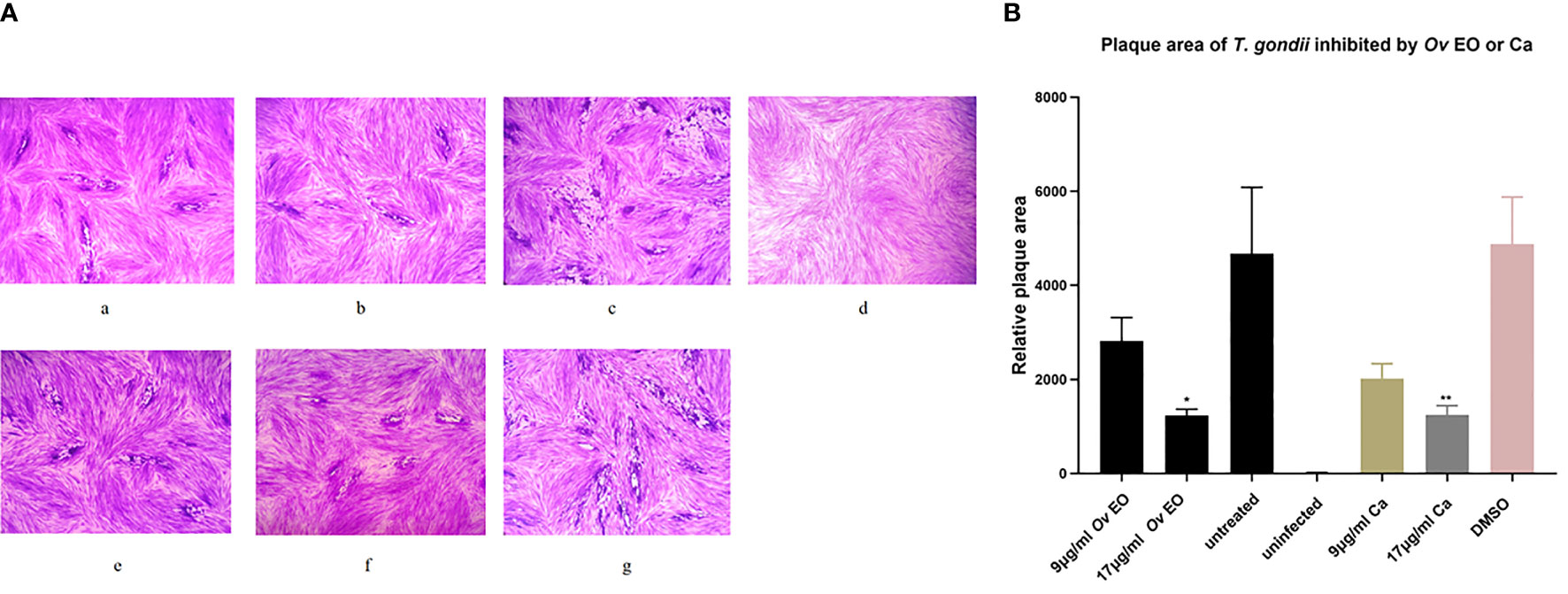
Figure 2 Plaque test for preliminary detection of anti-T. gondii activity of Ov EO and Ca. (A) Images of T. gondii plaque under different concentrations of Ov EO and Ca. (B) Statistical analysis of the images plaque. (a) HFF cells were infected by T. gondii and treated with 9 μg/ml Ov EO; (b) HFF cells were infected by T. gondii and treated with 17 μg/ml Ov EO; (c) HFF cells were infected by T. gondii and untreated; (d) HFF cells were not infected and treated; (e) HFF cells were infected by T. gondii and treated with 9 μg/ml Ca; (f) HFF cells were infected by T. gondii and treated with 17 μg/ml Ca; (g) HFF cells were infected by T. gondii and treated with 1.56% DMSO.
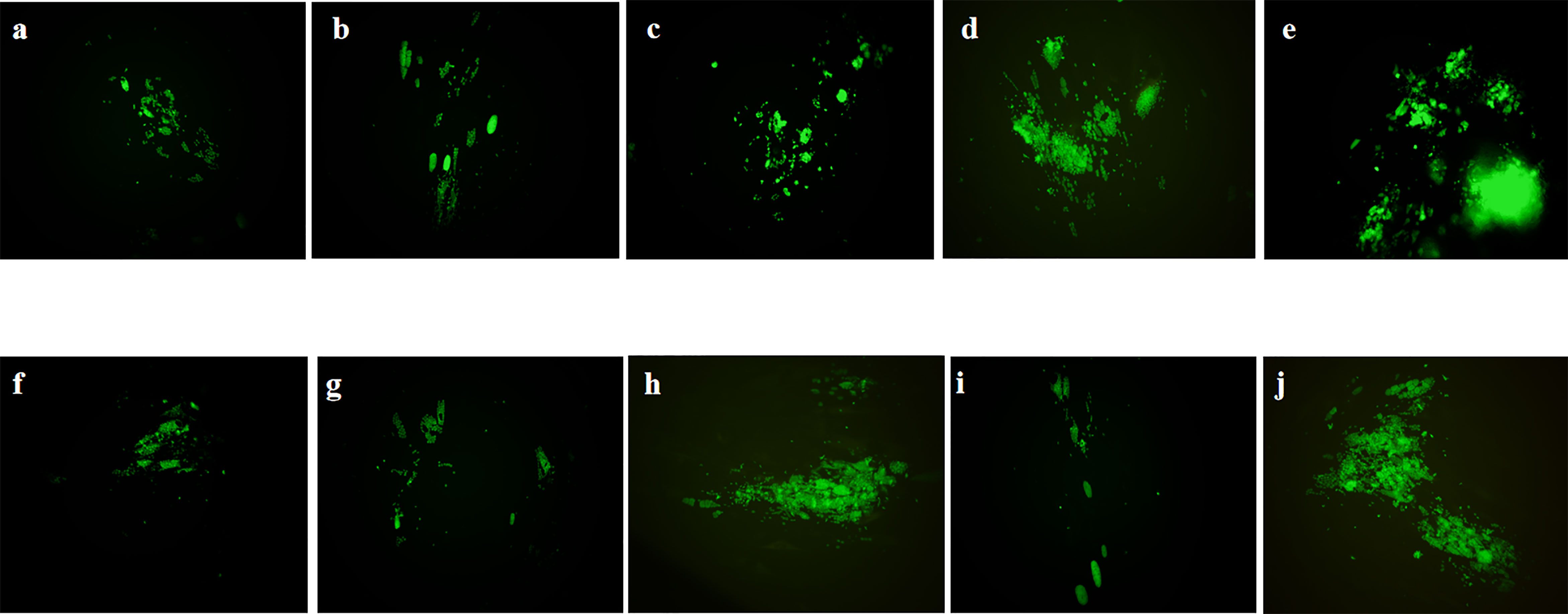
Figure 3 Anti-T. gondii activity of Ov EO and Ca evaluated by growth assay. Fluorescence area indicates the growth of T. gondii during different treatment. (A–D) Different concentrations of Ov EO, (A) 70 μg/ml, (B) 35 μg/ml, (C) 17 μg/ml, (D) 9 μg/ml, and (E) no treatment; (F–H) Different concentrations of Ca, (F) 17 μg/ml, (G) 9 μg/ml, (H) 4 μg/ml, (I) SMZ, and (J) DMSO.
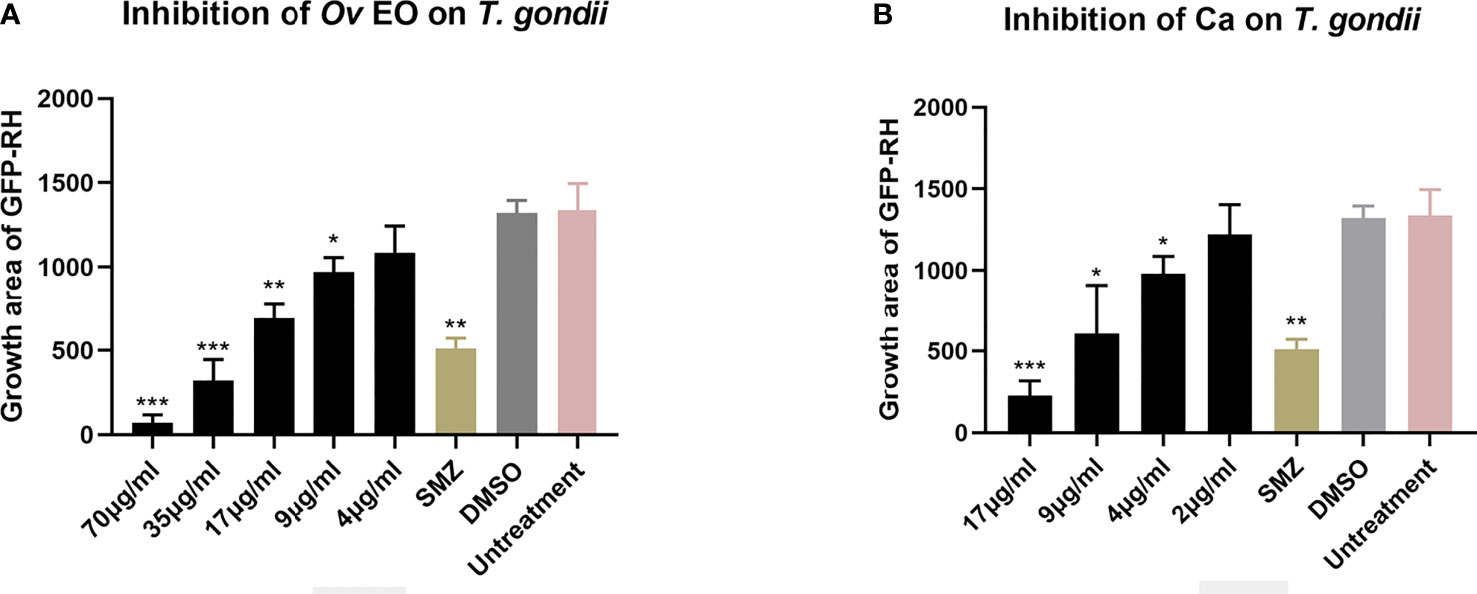
Figure 4 Statistical analysis of the inhibition effect of Ov EO and Ca in anti-T. gondii. Data analysis based on fluorescence area of GFP-RH. Each bar represents the mean ± SD of three wells per group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with untreatment group. (A) Anti-T. gondii activity of Ov EO, and (B) Anti-T. gondii activity of Ca.
To compare the effect between Ov EO and Ca on anti-parasitic activity, four different concentrations of Ca were compared using the same assay. The results indicated that the growth of T. gondii was inhibited by each of the concentrations of Ca tested (Figure 3), and the inhibition also increased in a dose-dependent manner (Figure 4B), the IC50 of Ca was 7.688 μg/ml. Comparing to the untreated group, the growth of T. gondii was also significantly reduced in low Ca concentrations treated groups (9 and 4 μg/ml) (606.8 vs 1,337; 980.7 vs. 1,337, P <0.05). Both Ov EO and Ca inhibited the growth of T. gondii in a dose-dependent manner, for the groups treated with 17 μg/ml Ov EO and 17 μg/ml Ca, the differences were also significant (692.9 vs. 1,337, P <0.01; 225.5vs. 1,337, P <0.001). For the inhibition effect, Ca was better than Ov EO at the same concentration. The inhibition of T. gondii was much more significant in the groups treated with 17 μg/ml Ca, than in those treated with SMZ (225.5 vs. 1,337, P <0.001; 490 vs. 1,337, P <0.01). There was no significant decrease in untreatment groups after treatment with 1.56% DMSO, which indicated that DMSO had no inhibitory effect on GFP-RH (1,322 vs 1,337). Due to the results of inhibition, we can calculate the IC 50 of Ov EO and Ca was 16.08 and 7.688 μg/ml, respectively (Figure 5). After statistical analysis, the selectivity index (SI) of Ov EO and Ca was 8.389 and 5.714, respectively (Table 1). From the comprehensive comparison of safety, the performance of Ov EO is better than that of Ca.
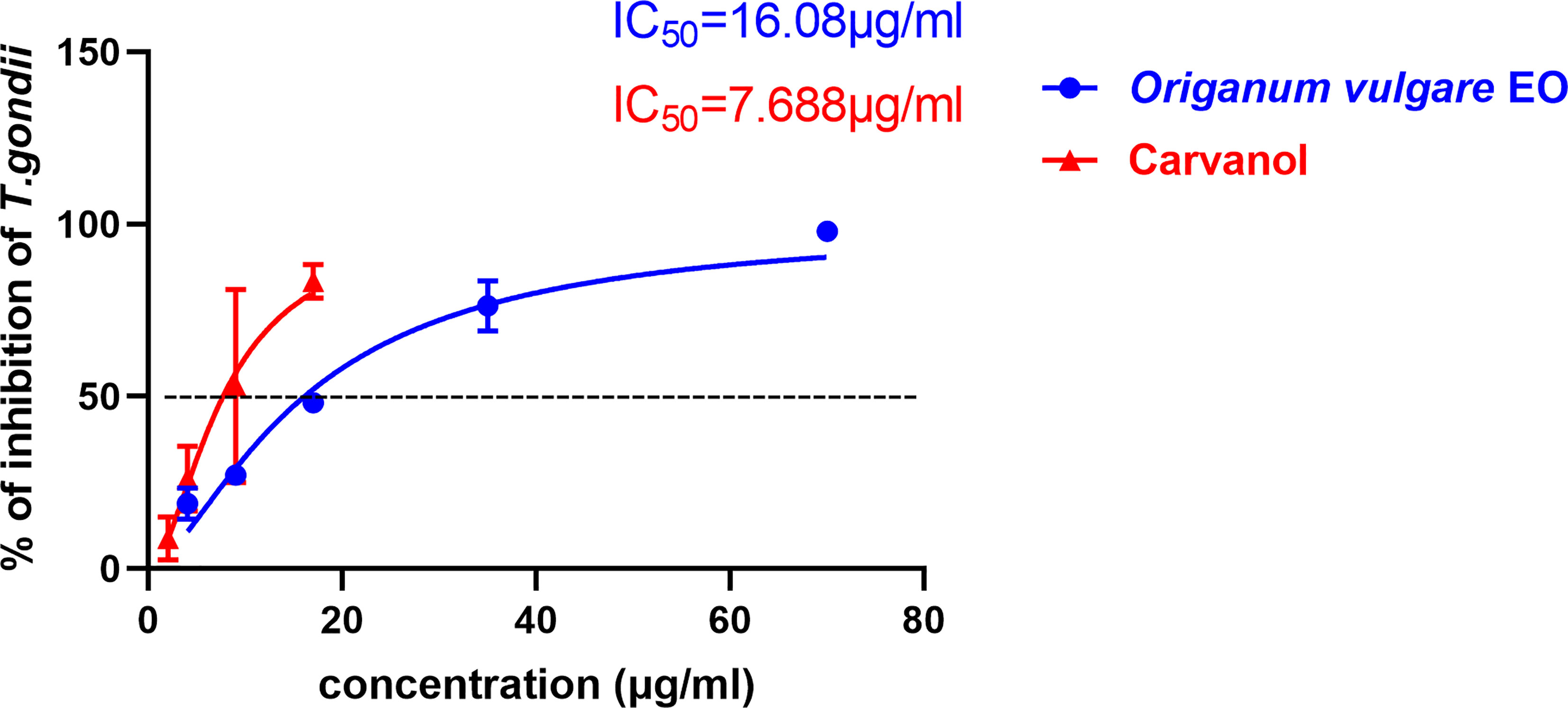
Figure 5 The 50% inhibition concentrations (CC50) of Ov EO and Ca. Inhibition of Ov EO and Ca to T. gondii. Different concentrations of Ov EO and Ca treated infected HFF cells for 32 h, the growth of GFP-RH was observed and photographed under a fluorescence microscope. Growth of GFP-RH was calculated using Image-Pro-Express. All data are presented as with error bars and the experiments were performed in triplicate.
As summarized in Figure 6, in the 17 μg/ml Ov EO treatment group, the T. gondii invasion rates at 20, 40, and 60 min post-infection were found to be 17.84, 24.10, and 28.96% respectively (Figure 6A). In the untreated group, invasion rates were found to be 38.85, 47.52, and 54.70% respectively at the three time points (Figure 5A). The invasion of T. gondii was significantly inhibited by Ov EO at 40 min (24.10% vs. 47.52%, P <0.01) and 60 min (28.96% vs. 54.70%, P <0.05). In the 17 μg/ml Ca treatment group, the T. gondii invasion rates were 21.09, 27.51, and 32.03% respectively at the three time points (Figure 6B), and the control group, invasion rates were found to be 30.52, 51.20, and 57.81% respectively (Figure 6B). Compared to the untreated group. Ca significantly reduced the invasion of T. gondii, especially after treatment for 40 and 60 min (27.51% vs. 51.20%, P <0.001; 32.03% vs. 57.81%, P <0.05, Figure 6B). The inhibitory effect was observed to increase as the treatment time increased. No change in the invasion rate of T. gondii was observed in any group treated with DMSO, across all experiments.
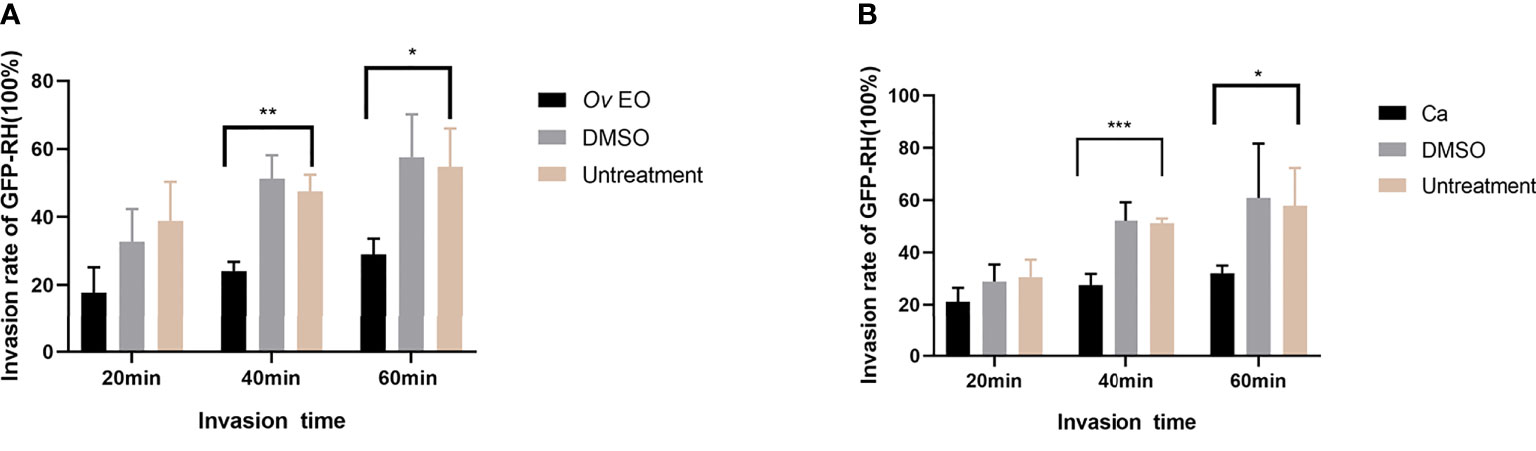
Figure 6 Effect of Ov EO on the invasion of T. gondii. Statistics of T. gondii invasion rate using two immunofluorescent dyes after treated with Ov EO (A) and Ca (B) for 20, 40, 60 min, respectively. *P < 0.05, **P < 0.01, ***P < 0.001 compared with untreated group.
The Scanning electron microscopy (SEM) results showed that the tachyzoites curled into a head-to-tail shape after the Ca treatment, but the individual was still plump and not shriveled (Figure 7B). After treatment with Ov EO, the morphology of the tachyzoites changed significantly, the worms were distorted, and showed a certain shriveled state (Figure 7A). In comparison, the tachyzoites were normal full crescent-shaped in DMSO treated group (Figure 7C) and untreated group (Figure 7D).
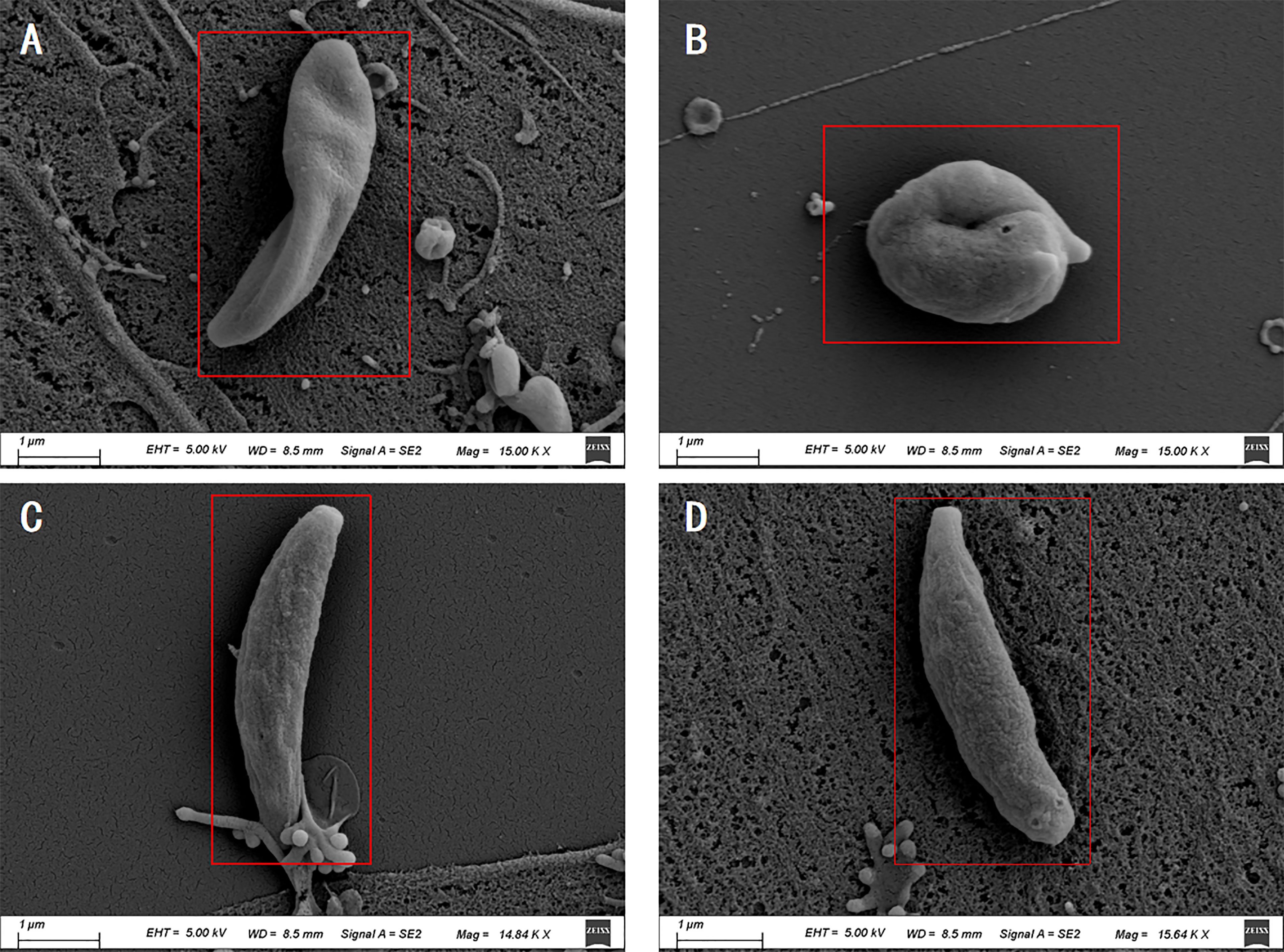
Figure 7 Scanning electron microscopy assay. The T. gondii were treated with 17 μg/ml Ov EO (A), 17 μg/ml Ca (B), DMSO (C) or untreated (D). After treated by Ov EO, the tachyzoites became sunken compared with those untreated tachyzoites. Tachyzoites treated with Ca become curled up. Scale bars: 1 μm.
T. gondii is an important zoonotic parasite, which can cause serious consequences after infected different hosts (Elsheikha, 2008; Maldonado and Read, 2017; El Bissati et al., 2018). In the face of the current dilemma of treatment and prevention, it is urgent to explore new drugs to inhibit T. gondii and control toxoplasmosis. At present, several reports indicated that plant essential oils had the inhibitory effect on T. gondii, in which, Bunium persicum (Boiss) EO (Tavakoli Kareshk et al., 2015) and Thymus broussonetii Boiss EO (Dahbi et al., 2010) played obvious roles in acute and chronic T. gondii infection in mice respectively. Ov EO is an important condiment, perfume, cosmetics, incense and preservative, with a long history of application (Diniz do Nascimento et al., 2020). The composition and content of Ov EO are affected by many factors, such as the place of production, cultivation conditions, extraction technology, etc. Research data shows that Ca is an important component in Ov EO, and its content mainly depends on the place of production, The carvacrol content in Ov EO produced in Argentina, Brazil, Greece and China is 26.7–81.92, 73.9, 63.03, and 30.73%, respectively (Leyva-López et al., 2017). This was the reason we chose the Ca study whether it plays an anti-Toxoplasma effect in Ov EO.
The cytotoxicity of Ov EO is related to the extraction method, and its toxicity to different cell lines is also different. The cytotoxicity of methanolic extracted Ov EO was more toxic, the CC50 of it was 382–374 μg/ml in MCF-7 cells. Chaouki reported that the CC50 of Ov EO was 5.5, 5.2, and 7.5 μg/ml in breast cancer cells (MCF-7), lung cancer cells (H-460) and central nervous system cancer cells (SF-268), respectively (Chaouki et al., 2010). In our experiment, the CC50 of Ov EO was 134.9 μg/mL in HFF. It has quite low cytotoxicity, so we can continue to carry out follow-up studies to evaluate its anti-Toxoplasma effect. The CC50 of Ca was 43.93 μg/ml, which is more toxic than Ov EO in HFF cells. Mostly, the active ingredients are more toxic than essential oil mixtures, and the effect will be better. From our results we found that the SI of Ov EO and Ca was not very high, which means that the cytotoxic of them are quite high, so further study should be carry on to reduce their cytotoxicity. Gaur et al. (2018) also found that Ca was more toxic than Ov EO in HCT-8 cells. In our experiment, Ca showed better anti-T. gondii activity than Ov EO at same concentration. As a kind of phenol, Ca has a significant repellent effect on many kinds of insects, such as Aedes albopictus, Culex quinquefasciatus, Ixodes scapularis, Rhipicephalus appendiculatus and so on (Lima et al., 2019). Moreover, Ca also shows anti-nematode effect on different nematodes such as Ascaris suum (Trailović et al., 2015). The main reason is that Ca can inhibit the contraction of muscle cells induced by acetylcholine, thereby inhibiting the muscle contraction of the parasite and affecting its motility (Marjanović et al., 2020). The above speculation is that the mechanism of carvacrol against T. gondii is related to the restriction of the motility of the parasite. However, its effect on the stability of calcium ions is also worthy of attention. Carvacrol has a certain regulatory effect on the stability of intracellular calcium ions. Studies have shown that carvacrol can adjust the TRP channels of transient calcium permeation receptor potentials, such as TRPV3, thereby increasing the concentration of calcium ions in the cytoplasm of primary mouse corneal epithelial cells and cultured human corneal epithelial cells (HCE-T cells) (Yamada et al., 2010). As we all know, there is a very important Ser/Thr protein kinase family in T. gondii, the CDPK family, whose activity is directly regulated by calcium ions. One family member, CDPK1, is closely related to the adhesion and invasion of T. gondii. Therefore, the reason why carvacrol can inhibit T. gondii may be achieved by inhibiting the activity of its invasion-related proteins. In our results, Ca did significantly inhibit the invasion rate of tachyzoites on HFF cells.
According to the results of SEM, the tachyzoites curled into a head-to-tail shape after the Ca treatment. Obviously, this obvious morphological change caused damage to the mobility of tachyzoites, which in turn affected its ability to invade. Studies have shown that carvacrol may induce apoptosis by reducing mitochondrial potential, releasing cytochrome C, activating caspase and carving PARP, thereby inhibiting human metastatic breast cancer cell line (MDA-MB 231) or human non-small cell lung cancer cell line Proliferation (A549) (Suntres et al., 2015). Therefore, the specific mechanism of Ca inhibiting T. gondii is not yet fully understood, and further research is needed. After Ov EO treatment, the tachyzoites showed severe dehydration and dryness. Ov EO is a mixture of different components, including terpenes, aldehydes, alcohols, esters, phenolic, ethers, and ketones and so on (Swamy et al., 2016). Among them, phenol can dehydrate the cells, resulting in the desiccation phenomenon (Samie et al., 2019). In addition, some hydrophobic compounds can pass through biological barriers and biological membranes, which may also have anti-T. gondii effects (Costa et al., 2018).
In this study, we found that Ov EO and Ca had obvious anti-Toxoplasma effect, which is likely to be achieved by changing the shape of tachyzoites, thereby limiting its movement ability, and then affecting its invasion ability. At the same time, Ca may have other biological functions, which can inhibit the proliferation of T. gondii. However, the target molecular and mechanism of action of Ov EO on T. gondii are still unclear and warrant further studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
S-YH and NY conceived and designed the study. NY, QX, J-KH, MP, and Z-FH performed the laboratory analyses. D-DL and JPT analyzed the data. All authors critically appraised and interpreted the results. NY drafted the first version of the manuscript. All authors provided feedback on the manuscript, and read and approved the final version.
The sample collection and some experiments were supported by the Outstanding Youth Foundation of Jiangsu Province of China (BK20190046), the China Postdoctoral Science Foundation (2020M671615), the Science and Technology Major Project of Zhejiang Province, China. (No. 2012C12009-2), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Veterinary Medicine).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Argyri, A. A., Doulgeraki, A. I., Varla, E. G., Bikouli, V. C., Natskoulis, P. I., Haroutounian, S. A., et al. (2021). Evaluation of Plant Origin Essential Oils as Herbal Biocides for the Protection of Caves Belonging to Natural and Cultural Heritage Sites. Microorganisms 9, 1836. doi: 10.3390/microorganisms9091836
Augusto, L., Martynowicz, J., Staschke, K. A., Wek, R. C., Sullivan, W. J., Jr. (2018). Effects of PERK Eif2α Kinase Inhibitor Against Toxoplasma Gondii. Antimicrob. Agents Chemother. 62, 1–8.doi: 10.1128/AAC.01442-18.
Chaouki, W., Leger, D. Y., Eljastimi, J., Beneytout, J. L., Hmamouchi, M. (2010). Antiproliferative Effect of Extracts From Aristolochia Baetica and Origanum Compactum on Human Breast Cancer Cell Line MCF-7. Pharm. Biol. 48, 269–274. doi: 10.3109/13880200903096588
Chemoh, W., Sawangjaroen, N., Nissapatorn, V., Suwanrath, C., Chandeying, V., Hortiwakul, T., et al. (2013). Toxoplasma Gondii Infection: What Is the Real Situation? Exp. Parasitol 135, 685–689. doi: 10.1016/j.exppara.2013.10.001
Costa, S., Cavadas, C., Cavaleiro, C., Salgueiro, L., Do Céu Sousa, M. (2018). In Vitro Susceptibility of Trypanosoma Brucei Brucei to Selected Essential Oils and Their Major Components. Exp. Parasitol 190, 34–40. doi: 10.1016/j.exppara.2018.05.002
Dahbi, A., Bellete, B., Flori, P., Hssaine, A., Elhachimi, Y., Raberin, H., et al. (2010). The Effect of Essential Oils From Thymus Broussonetii Boiss on Transmission of Toxoplasma Gondii Cysts in Mice. Parasitol Res. 107, 55–58. doi: 10.1007/s00436-010-1832-z
Desmonts, G., Couvreur, J. (1974). Toxoplasmosis in Pregnancy and its Transmission to the Fetus. Bull. N. Y. Acad. Med. 50, 146–159. doi: 10.1097/00006254-197409000-00007
Diniz do Nascimento, L., Moraes, A., Costa, K. S. D., Pereira Galúcio, J. M., Taube, P. S., Costa, C. M. L., et al. (2020). Bioactive Natural Compounds and Antioxidant Activity of Essential Oils From Spice Plants: New Findings and Potential Applications. Biomolecules 10, 988. doi: 10.3390/biom10070988
Dorman, H. J., Deans, S. G. (2000). Antimicrobial Agents From Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 88, 308–316. doi: 10.1046/j.1365-2672.2000.00969.x
Dunay, I. R., Gajurel, K., Dhakal, R., Liesenfeld, O., Montoya, J. G. (2018). Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 31, e00057–17. doi: 10.1128/CMR.00057-17
El Bissati, K., Levigne, P., Lykins, J., Adlaoui, E. B., Barkat, A., Berraho, A., et al. (2018). Global Initiative for Congenital Toxoplasmosis: An Observational and International Comparative Clinical Analysis. Emerg. Microbes Infect. 7, 165. doi: 10.1038/s41426-018-0164-4
Elshafie, H. S., Armentano, M. F., Carmosino, M., Bufo, S. A., De Feo, V., Camele, I. (2017). Cytotoxic Activity of Origanum Vulgare L. @ on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of its Biological Activity. Molecules 22, 1435. doi: 10.3390/molecules22091435.
Elsheikha, H. M. (2008). Congenital Toxoplasmosis: Priorities for Further Health Promotion Action. Public Health 122, 335–353. doi: 10.1016/j.puhe.2007.08.009
Gaur, S., Kuhlenschmidt, T. B., Kuhlenschmidt, M. S., Andrade, J. E. (2018). Effect of Oregano Essential Oil and Carvacrol on Cryptosporidium Parvum Infectivity in HCT-8 Cells. Parasitol. Int. 67, 170–175. doi: 10.1016/j.parint.2017.11.001
Huang, S. Y., Yao, N., He, J. K., Pan, M., Hou, Z. F., Fan, Y. M., et al. (2021). In Vitro Anti-Parasitic Activity of Pelargonium X. Asperum Essential Oil Against Toxoplasma Gondii. Front. Cell Dev. Biol. 9, 616340. doi: 10.3389/fcell.2021.616340
Leyva-López, N., Gutiérrez-Grijalva, E. P., Vazquez-Olivo, G., Heredia, J. B. (2017). Essential Oils of Oregano: Biological Activity Beyond Their Antimicrobial Properties. Molecules 22, 989. doi: 10.3390/molecules22060989
Lietava, J. (1992). Medicinal Plants in a Middle Paleolithic Grave Shanidar Iv? J. Ethnopharmacol. 35, 263–266. doi: 10.1016/0378-8741(92)90023-K
Lima, A. D. S., Landulfo, G. A., Costa-Junior, L. M. (2019). Repellent Effects of Encapsulated Carvacrol on the Rhipicephalus (Boophilus) Microplus (Acari: Ixodidae). J. Med. Entomol. 56, 881–885. doi: 10.1093/jme/tjy240
Maldonado, Y. A., Read, J. S. (2017). Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States. Pediatrics 139, e20163860. doi: 10.1542/peds.2016-3860
Marjanović, D. S., Zdravković, N., Milovanović, M., Trailović, J. N., Robertson, A. P., Todorović, Z., et al. (2020). Carvacrol Acts as a Potent Selective Antagonist of Different Types of Nicotinic Acetylcholine Receptors and Enhances the Effect of Monepantel in the Parasitic Nematode Ascaris Suum. Vet. Parasitol. 278, 109031. doi: 10.1016/j.vetpar.2020.109031
Newman, D. J., Cragg, G. M. (2012). Natural Products as Sources of New Drugs Over the 30 Years From 1981 to 2010. J. Nat. Prod. 75, 311–335. doi: 10.1021/np200906s
Petrovska, B. B. (2012). Historical Review of Medicinal Plants' Usage. Pharmacogn. Rev. 6, 1–5. doi: 10.4103/0973-7847.95849
Samie, S., Trollope, K. M., Joubert, L. M., Makunga, N. P., Volschenk, H. (2019). The Antifungal and Cryptococcus Neoformans Virulence Attenuating Activity of Pelargonium Sidoides Extracts. J. Ethnopharmacol. 235, 122–132. doi: 10.1016/j.jep.2019.02.008
Schmidt, D. R., Hogh, B., Andersen, O., Hansen, S. H., Dalhoff, K., Petersen, E. (2006). Treatment of Infants With Congenital Toxoplasmosis: Tolerability and Plasma Concentrations of Sulfadiazine and Pyrimethamine. Eur. J. Pediatr. 165, 19–25. doi: 10.1007/s00431-005-1665-4
Suntres, Z. E., Coccimiglio, J., Alipour, M. (2015). The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 55, 304–318. doi: 10.1080/10408398.2011.653458
Swamy, M. K., Akhtar, M. S., Sinniah, U. R. (2016). Antimicrobial Properties of Plant Essential Oils Against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat. Med. 2016, 3012462. doi: 10.1155/2016/3012462
Tavakoli Kareshk, A., Keyhani, A., Mahmoudvand, H., Tavakoli Oliaei, R., Asadi, A., Andishmand, M., et al. (2015). Efficacy of the Bunium Persicum (Boiss) Essential Oil Against Acute Toxoplasmosis in Mice Model. Iran J. Parasitol. 10, 625–631.
Trailović, S. M., Marjanović, D. S., Nedeljković Trailović, J., Robertson, A. P., Martin, R. J. (2015). Interaction of Carvacrol With the Ascaris Suum Nicotinic Acetylcholine Receptors and Gamma-Aminobutyric Acid Receptors, Potential Mechanism of Antinematodal Action. Parasitol. Res. 114, 3059–3068. doi: 10.1007/s00436-015-4508-x
Wei, H. X., Wei, S. S., Lindsay, D. S., Peng, H. J. (2015). A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma Gondii Medicines in Humans. PLoS One 10, e0138204. doi: 10.1371/journal.pone.0138204
Weng, J. K., Philippe, R. N., Noel, J. P. (2012). The Rise of Chemodiversity in Plants. Science 336, 1667–1670. doi: 10.1126/science.1217411
Keywords: Toxoplasma gondii, natural medicine, Origanum vulgare essential oil, carvacrol, in vitro
Citation: Yao N, Xu Q, He J-K, Pan M, Hou Z-F, Liu D-D, Tao J-P and Huang S-Y (2021) Evaluation of Origanum vulgare Essential Oil and Its Active Ingredients as Potential Drugs for the Treatment of Toxoplasmosis. Front. Cell. Infect. Microbiol. 11:793089. doi: 10.3389/fcimb.2021.793089
Received: 11 October 2021; Accepted: 27 October 2021;
Published: 22 November 2021.
Edited by:
Wei Cong, Shandong University, Weihai, ChinaCopyright © 2021 Yao, Xu, He, Pan, Hou, Liu, Tao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Si-Yang Huang, c2l5YW5nLmh1YW5nQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.