- 1Department of Basic Medical Laboratory, The 980th Hospital of the PLA Joint Logistical Support Force (Bethune International Peace Hospital), Shijiazhuang, China
- 2Department of Respiratory Medicine, The 980th Hospital of the PLA Joint Logistical Support Force (Bethune International Peace Hospital), Shijiazhuang, China
- 3Department of Radiology, The 980th Hospital of the PLA Joint Logistical Support Force (Bethune International Peace Hospital), Shijiazhuang, China
- 4Department of Clinical Laboratory, The 980th Hospital of the PLA Joint Logistical Support Force (Bethune International Peace Hospital), Shijiazhuang, China
- 5Department of Nutrition, Beidaihe Rehabilitation and Recuperation Center, Qinhuangdao, China
- 6Department of Microbiology and Biochemical Pharmacy, College of Pharmacy, Army Medical University, Chongqing, China
Objective: The multicenter literature review and case studies of 3 patients were undertaken to provide an updated understanding of nocardiosis, an opportunistic bacterial infection affecting immunosuppressed nephrotic syndrome (NS) patients receiving long-term glucocorticoid and immunosuppressant treatment. The results provided clinical and microbiological data to assist physicians in managing nocardiosis patients.
Methods: Three cases between 2017 and 2018 from a single center were reported. Additionally, a systematic review of multicenter cases described in the NCBI PubMed, Web of Science, and Embase in English between January 1, 2001 and May 10, 2021 was conducted.
Results: This study described three cases of Nocardia infection in NS patients. The systematic literature review identified 24 cases with sufficient individual patient data. A total of 27 cases extracted from the literature review showed that most patients were > 50 years of age and 70.4% were male. Furthermore, the glucocorticoid or corticosteroid mean dose was 30.9 ± 13.7 mg per day. The average time between hormone therapy and Nocardia infection was 8.5 ± 9.7 months. Pulmonary (85.2%) and skin (44.4%) infections were the most common manifestations in NS patients, with disseminated infections in 77.8% of patients. Nodule/masses and consolidations were the major radiological manifestations. Most patients showed elevated inflammatory biomarkers levels, including white blood cell counts, neutrophils percentage, and C-reactive protein. Twenty-five patients received trimethoprim-sulfamethoxazole monotherapy (18.5%) or trimethoprim-sulfamethoxazole-based multidrug therapy (74.1%), and the remaining two patients (7.4%) received biapenem monotherapy. All patients, except the two who were lost to follow-up, survived without relapse after antibiotic therapy.
Conclusions: Nephrotic syndrome patients are at high risk of Nocardia infection even if receiving low-dose glucocorticoid during the maintenance therapy. The most common manifestations of nocardiosis in NS patients include abnormal lungs revealing nodules and consolidations, skin and subcutaneous abscesses. The NS patients have a high rate of disseminated and cutaneous infections but a low mortality rate. Accurate and prompt microbiological diagnosis is critical for early treatment, besides the combination of appropriate antibiotic therapy and surgical drainage when needed for an improved prognosis.
Introduction
Nocardia species are aerobic, Gram-positive, filamentous, beaded, weakly acid-fast branching bacilli found worldwide in soil and water (Brown-Elliott et al., 2006; Wilson, 2012). More than 100 different Nocardia species have been identified by phenotypic identifications, molecular methods and 16S rRNA gene sequencing (https://www.bacterio.net) up to now, and over 50 of them have been reported pathogenic to humans (Conville et al., 2018).
Nocardia, an opportunistic pathogen, infects humans via respiratory inhalation and injured skin. The organism causes pulmonary, superficial cutaneous, and subcutaneous infections, and can spread through the blood causing disseminated infection (Beaman and Beaman, 1994; Wang et al., 2015). Chronic lung disease and immunosuppression caused by glucocorticoids or other immunosuppressive therapies, human immunodeficiency virus (HIV) infection, solid organ transplantation, and chemotherapy for neoplasm are the common risk factors for Nocardia infections (Conville et al., 2003; Liu et al., 2011; Molina et al., 2018; Zia et al., 2019). Recent years have seen an increase in nocardiosis incidences with extensive immunosuppressive therapies.
Nephrotic syndrome (NS), caused by glomerular permeability abnormality, is a clinical syndrome with massive proteinuria responsible for hypoalbuminemia (<30 g/L), hyperlipidemia, edema, and various complications. Impaired renal function, administration of glucocorticoids, and immunosuppressant use in NS patients may lead to immune disorders, making them more susceptible to various infections (Li et al., 2017; Wang et al., 2019). NS patients with long-term glucocorticoids and (or) immunosuppressants treatments have high morbidity rates of Nocardia infection. Nocardia infection in NS patients was first described in 1962 by Kerbel NC (Kerbel, 1962). Only a few case reports of Nocardia infection in NS patients are available up to now, and the two latest single-center literature reviews were published in 2020 (Guo et al., 2020; Han et al., 2020). However, there are hardly any multicenter retrospective reviews. The present multicenter study reports Nocardia infection in 3 NS patients between 2017 and 2018 in our hospital, summarizes the available literature to understand the infectious disease, and provides clinical and microbiological data to assist physicians in managing nocardiosis patients.
Materials and Methods
Single-Center Case Report
Three patients with a medical history of NS and diagnosed with nocardiosis at the 980th Hospital of the PLA Joint Logistical Support Force from 2017 to 2018 were retrospectively reviewed for clinical history and characteristics, laboratory data, imaging features, microbiological data, and treatment data. The Nocardia infection was defined by clinical features, radiographic manifestations, pathogen identification, and post-treatment radiological or clinical condition improvements. Disseminated nocardiosis was defined as the involvement of at least two noncontiguous organs or demonstration of bloodstream infection (Margalit et al., 2021).
Different clinical specimens, including sputum, pus, pleural, and bronchoalveolar lavage, were inoculated on the blood-containing medium and Lowenstein-Jensen medium at 35°C for 3-21 days under aerobic conditions. Presumptive Nocardia species were identified by Gram staining, modified acid-fast staining, and acid-fast staining, and then confirmed by 16S rRNA gene sequencing. Based on Clinical and Laboratory Standards Institute (CLSI, 2018) (Woods et al., 2011) guidelines, antimicrobial susceptibility testing (AST) was performed by broth microdilution (BMD), and the minimum inhibitory concentrations (MICs) were interpreted according to the CLSI susceptibility breakpoints.
Inflammatory biomarkers, including white blood cell (WBC) counts, neutrophils percentage, C-reactive protein (CRP), procalcitonin (PCT), and erythrocyte sedimentation rate (ESR), were analyzed to evaluate the infection. Serum albumin and creatinine were measured as the renal function indicators.
Literature Review
We searched Pubmed, Web of Science, and Embase to identify original research, case reports, case series, and review articles with detail medical history and laboratory data published between January 1, 2001 and May 10, 2021. The search keywords included “Nephrotic syndrome”, “Nocardia”, “Nocardiosis”, “steroid therapy”, “glucocorticoid therapy”, “Kidney disease”, and “infection”. All relevant, available full texts published in English were extracted.
Statistical Analysis
Data analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as the means with standard deviations (SDs). Significant differences between any two groups were tested using the χ2 test and the Fisher’s exact test when the value in any group was below 5. The P-value of <0.05 was considered to be statistically significant.
Results
Single-Center Three Case Reports
Three cases of nocardiosis with NS identified between January 1, 2017 and December 31, 2018 were studied. Table S1 presents the antimicrobial susceptibility patterns for Nocardia; N.cyriacigeorgica, N.brasiliensis, and N.farcinica. The three cases are discussed as follows.
Case 1
In May 2017, a 71-year-old women was admitted to our hospital with an aggravated cough and intermittent fever for 14 days. Three days before the admission, the patient was initially treated with cefoperazone/sulbactam in a local hospital because the radiographic manifestations suspected bacterial pneumonia. However, her symptoms did not improve, and the fever was persistent; she was then transferred to our hospital for further diagnosis and treatment. She had a 3-month history of NS, and the renal biopsy confirmed focal segmental glomerulersclerosis. Subsequently, she was on steroid therapy (methylprednisolone, 32 mg/d) for 3 months. On admission, the patient’s body temperature was 36.2°C. The WBC was 15.8×109/L with 89% neutrophils; ESR and CRP were elevated to 96 mm/h and 87.3 mg/L, respectively. The 24-h urine protein stood at 6687.5 mg/d, the serum albumin was 25.7 g/L, and the serum β-D-glucan was normal. Chest computed tomography (CT) scan revealed cord-like high-density shadows in both lungs and consolidation in the left lower lobe with left-sided pleural effusion (Figure 1). Bronchoscopy showed white purulent mucus found on the left lower bronchi (Supplementary Figure S1). Bronchoalveolar lavage fluid (BALF) showed branched neutrophilia, and the Gram staining and modified acid-fast staining revealed filamentous, beaded, branching bacilli (Figure 1). The BALF culture on the blood agar revealed Nocardia spp., identified as N.cyriacigeorgica by 16S rRNA sequencing (Supplementary Figure S2). Further investigations of the disseminated infection, including brain CT (Supplementary Figure S1) and blood culture, were negative. Pulmonary nocardiosis was diagnosed based on the clinical data, imageological characteristics, and pathogen identification. The patient was administered intravenous cefoperazone/sulbactam (3 g twice daily) and oral trimethoprim-sulfamethoxazole (TMP-SMX, 0.96 g thrice daily) for 14 days based on the bacterial species and AST results. A follow-up CT after 2 weeks showed the disappearance of left pleural effusion and flake consolidation in the left lower lobe. She was discharged on a 6-month course of oral TMP-SMX therapy after 20 days of hospitalization. Figure 2 shows the treatment flow diagrams of the case.
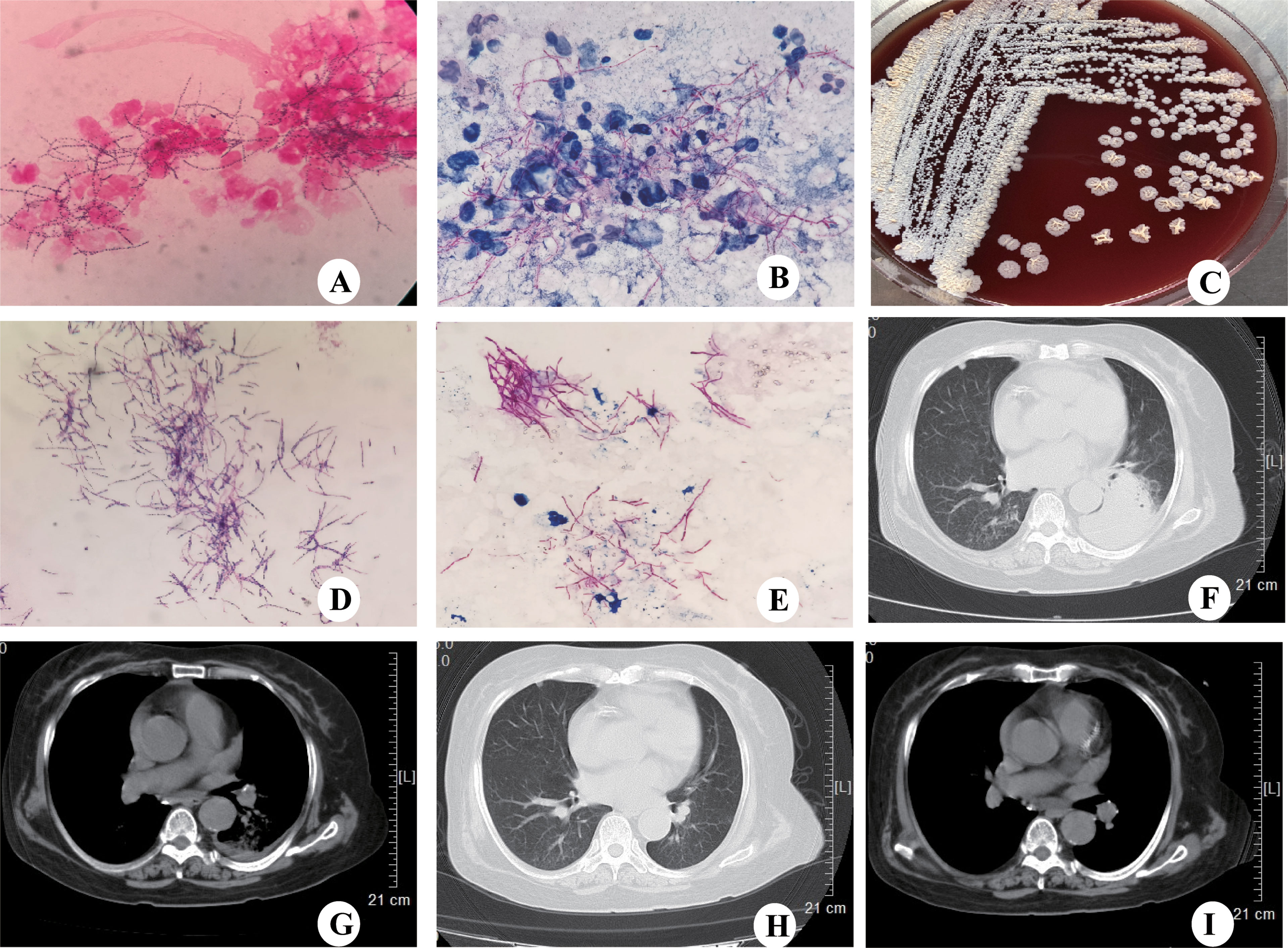
Figure 1 CT scanning and microbiological identification of case 1. (A) Gram staining of BALF showing Gram-positive, filamentous branching bacilli (magnification, ×100). (B) Modified acid-fast staining of BALF showing filamentous, weakly acid-fast branching bacilli (magnification,×100). (C) Colonies of N.cyriacigeorgica cultured on blood agar for 72h. (D) Gram staining showing the Gram-positive, filamentous branching and beaded structure of N.cyriacigeorgica (magnification,×100). (E) Modified acid-fast staining showing filamentous, weakly acid-fast branching bacilli (magnification,×100). (F) Chest CT showing consolidation in the left lower lobe with left-sided pleural effusion. (G) Chest CT showing left-sided pleural effusion. (H) Chest CT showing consolidation in the left lower lobe disappeared following treatment. (I) Chest CT showing left-sided pleural effusion disappeared following treatment.
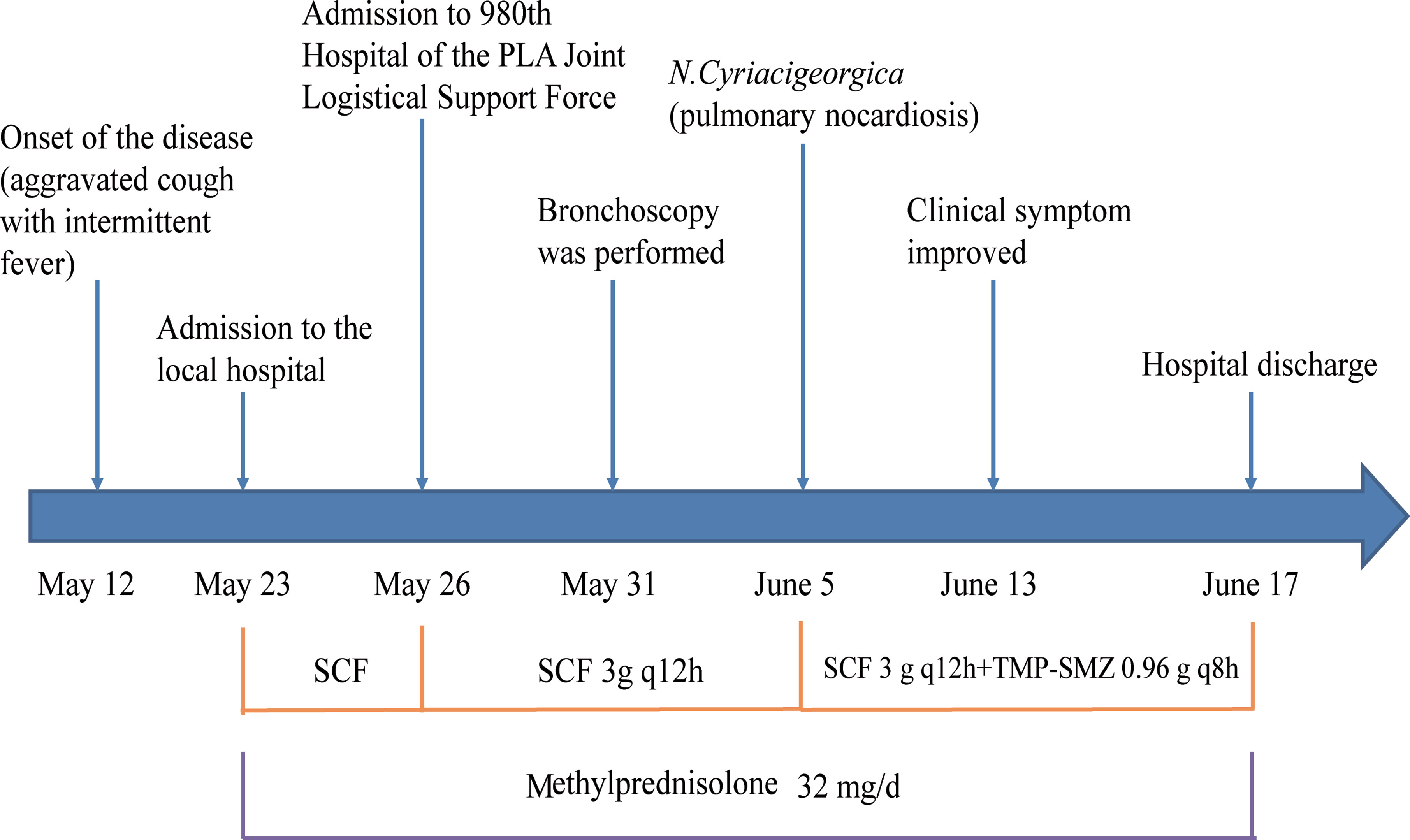
Figure 2 Treatment flow diagram of case 1. TMP-SMX, trimethoprim-sulfamethoxazole; SCF, cefoperazone/sulbactam.
Case 2
A 68-year-old man presented with fever and coughing up purulent sputum. He had been diagnosed with NS one year prior. Renal biopsy showed membranous nephropathy, and he was administered a daily dose of 30 mg prednisone and 100 mg cyclosporine each. One month prior to the admission, he was initially diagnosed with Aspergillus fumigatus pneumonia and received the oral voriconazole treatment. The 2-week antifungal therapy improved the patient’s symptoms both clinically and radiologically. He was discharged on a regimen of prednisone (30 mg/d), cyclosporine (100 mg/d), and voriconazole (400 mg/d). On admission, a physical examination revealed low fever (37.6°C). After one-year-steroid therapy, the 24-h urinary protein loss was reduced to 656 mg/d, and the serum albumin levels rose to 24.6 g/L. The WBC count was 10.7×109/L with 91% neutrophils, and the CRP and PCT were elevated to 188 mg/L and 1.6 ng/mL, respectively. His fasting blood sugar was 9.2 mmol/L and the glycosylated hemoglobin was 10.6%. Laboratory investigations regarding lymphocyte count were abnormal: CD3+ 53.6%, CD3+CD4+ 12.8%, CD3+CD8+ 40.6%, CD4/CD8 0.32, indicating suppressed immunity. Serum β-D-glucan and galactomannan were normal. Chest CT revealed multiple flake and cord-like high-density shadows in both lungs near the pleura, multiple cavities in the right upper lobe, bilateral pleural effusion, and emphysema in both lungs (Supplementary Figure S3). Pathogens isolated from sputum were ultimately identified as N.brasiliensis by 16S rRNA gene sequencing (Supplementary Figure S4), but the blood cultures were negative. The patient was administered intravenous moxifloxacin (0.4 g/d) and oral TMP-SMX (0.96 g thrice daily) for 14 days. A follow-up chest CT revealed the reduced size of the previously detected cavities (Supplementary Figure S3) and improved clinical symptoms after 21-day hospitalization. Unfortunately, the patient refused further medication and was discharged on a 6-month course of oral TMP-SMX therapy but was lost to follow-up. Supplementary Figure S5 presents the treatment flow diagrams of the case.
Case 3
During the autumn of 2017, a 69-year-old man suffering from edema in both lower legs was admitted to our hospital. He was diagnosed with NS (membranous nephropathy), and an intravenous methylprednisolone therapy (32 mg/d) was initiated. There was no improvement 1 month post-treatment, so the patient was started on a combination therapy of oral prednisone (25 mg/d) and hemodialysis. His renal disease stabilized, so the prednisone dosage was reduced to 15 mg/d as the maintenance therapy. After 2 months of combination therapy, the 24-h urinary protein loss was reduced to 378 mg/d, while the serum albumin level rose to 22.9 g/L. However, he developed low fever, cough, and chest pain. The laboratory examination showed elevated WBC count (11.8×109/L) with 91% neutrophils, CRP (26 mg/L), PCT (2.9 ng/ml), and ESR (90 mm/h). Serum β-D-glucan and galactomannan were normal. Chest CT revealed bilateral inflammation, cavities in the right upper lobe, and multiple nodules in both lungs. Mycobacterium tuberculosis-specific T lymphocyte (T spot-TB) was positive, but M tuberculosis was not founded in the sputum. Based on the clinical features, imageological characteristics, and lab tests, the patient was administrated a combination of isoniazid and rifampin because M tuberculosis was suspected of etiological agent. Nevertheless, his symptoms did not improve, and the chest CT revealed enlarged nodules in the right middle lobe and aggravated inflammation in the left lower lobe. A firm abscess (2cm×1cm in diameter) which manifested as tender was founded in the left upper groin. Ultrasonography (USG) of the abscess showed thickened subcutaneous soft tissue and non-uniform internal echo (Supplementary Figure S6). He had no history of trauma. The abscess was drained, and the yellowish purulent discharge was sent to the laboratory for culture. The Gram-and modified acid-fast staining of the sputum showed filamentous, beaded, branching bacilli which was suspected of Nocardia spp. (Supplementary Figure S6). The sputum was resubmitted, cultured aerobically on the blood agar after digestion with 2% potassium hydroxide (KOH), and incubated at 37°C to confirm the presence of Nocardia spp. The pus and sputum culture presented yellowish Nocardia spp., identified as N.farcinica by gene sequencing (Supplementary Figure S7). Pus and sputum cultures, and morphological evidence of sputum were positive for Nocardia spp. and disseminated nocardiosis was diagnosed. Antibiotic therapy was replaced with intravenous cefatriaxone (2 g/d) and oral TMP-SMX (0.96 g thrice daily) for 2 weeks. The patient was discharged as his clinical condition improved after 43 days of hospitalization. He continued oral TMP-SMX monotherapy for 6 months and remained in good condition. The treatment flow diagrams of the case are presented in Supplementary Figure S8.
Literature Review
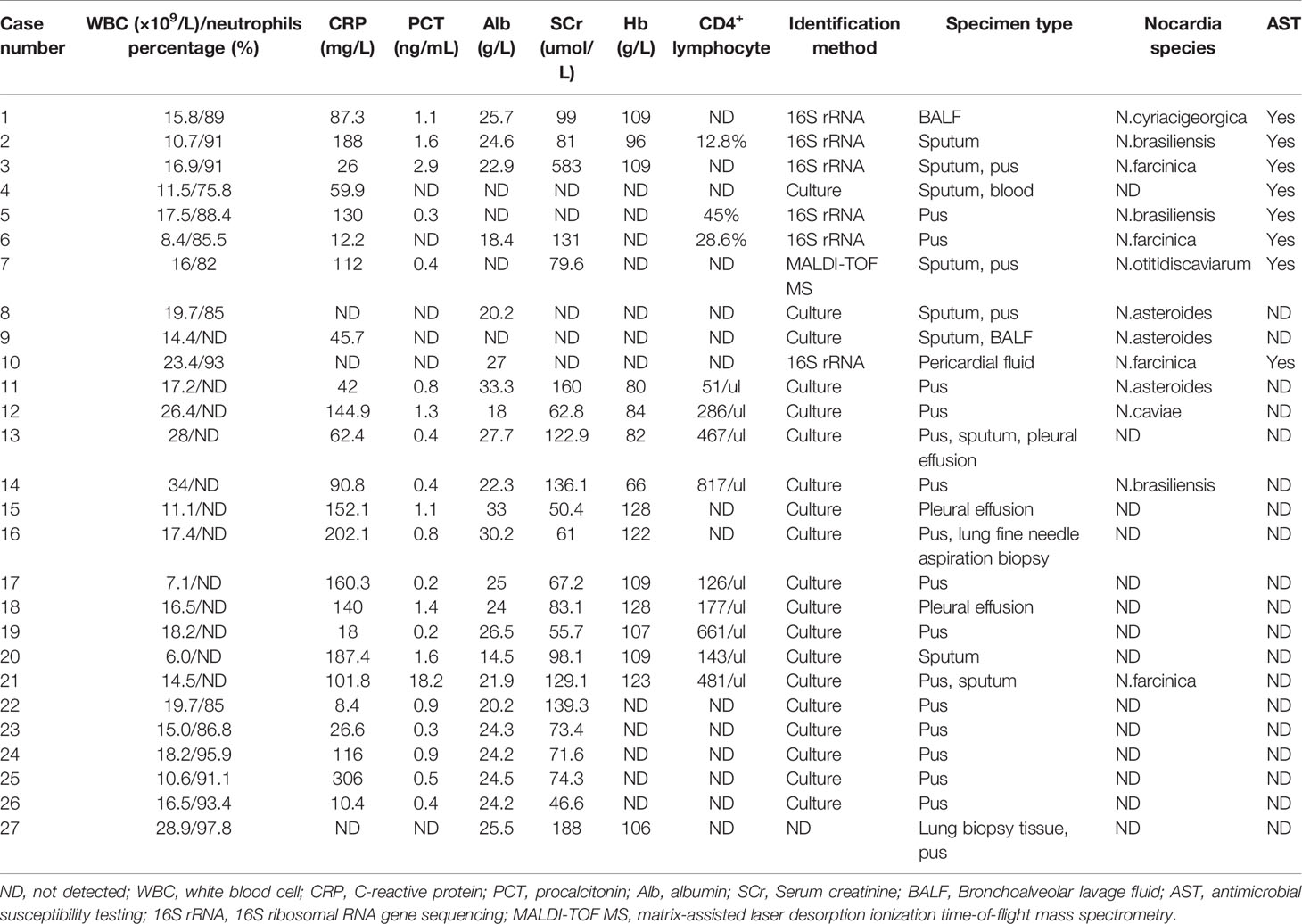
We performed a systematic literature search of previous articles and identified 13 articles on nocardiosis with NS; 3 of them were excluded (1 article published in Japanese, 1 article published in Danish, 1 article published in Turkish). Ten articles described 24 cases that had sufficient individual patient data. Table S2 summarizes these publications (Hwang et al., 2004; Sirijatuphat et al., 2013; Zhou et al., 2014; Chen et al., 2016; Zhu et al., 2017; Xu et al., 2018; Guo et al., 2020; Han et al., 2020; Sah et al., 2020; Yang et al., 2020). A total of 27 cases of nocardiosis with NS were assessed, including the three cases of nocardiosis in our hospital. Demographic characteristics, clinical features, laboratory data, radiological features, microbiological results, treatment data, and patient outcomes were collected and summarized (Tables 1, 2).
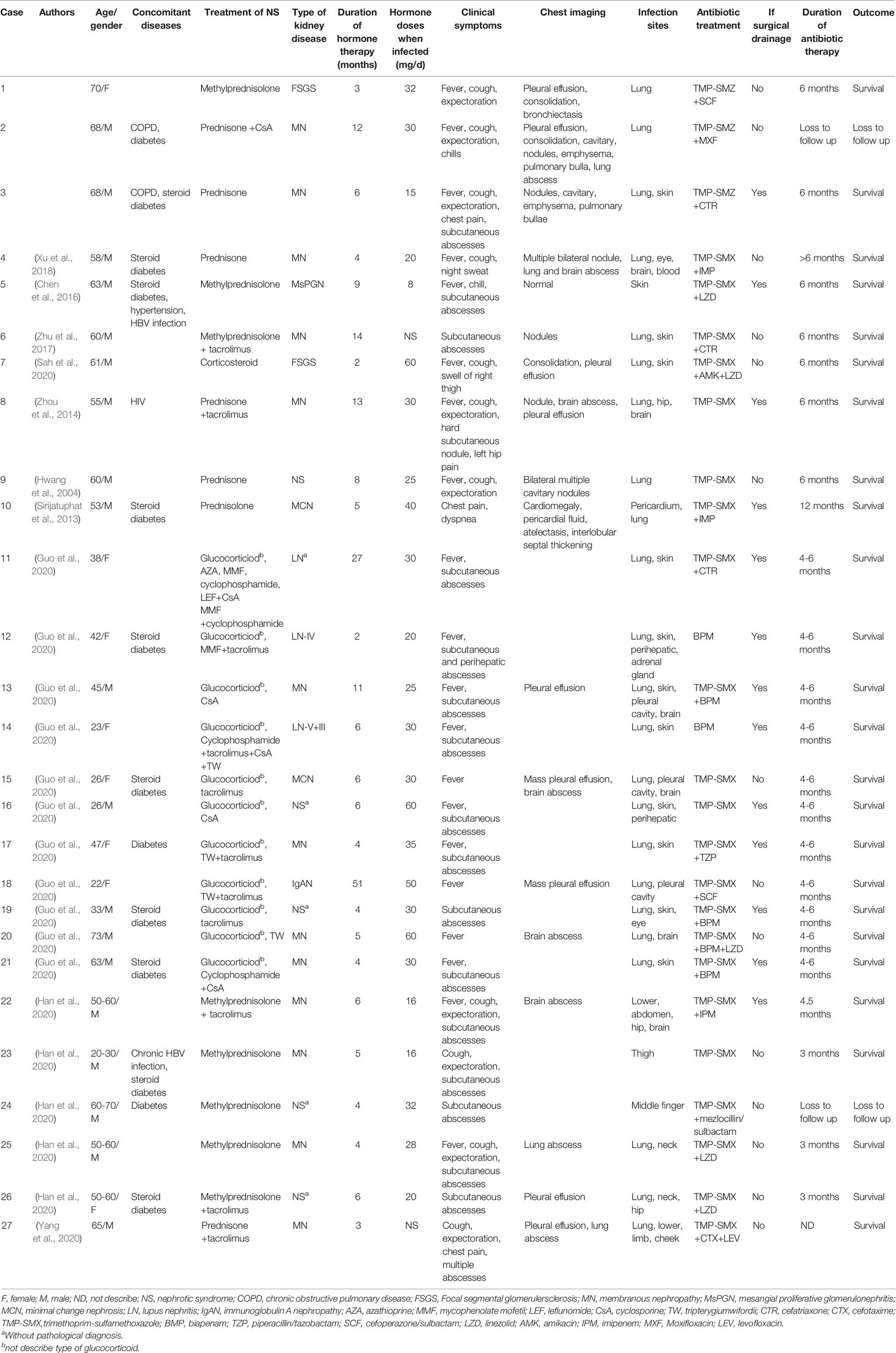
Table 1 Epidemiographical and characteristic data of 27 nocardiosis patients with nephrotic syndrome.
Epidemiographical Data of NS Patients Infected With Nocardia
The demographic characteristics of the patients in the articles reviewed included 19 males (70.4%) and 8 females (29.6%) (Figure 3A). A slight increase in the numbers of patients was observed in the age group 51-60 and > 60 years of age (Figure 3B). Concomitant diseases observed were chronic obstructive pulmonary disease (COPD) in 2 patients (case 2 and 3), HIV infection in 1 patient (case 8), chronic hepatitis B virus (HBV) infection in 2 patients (case 5 and 23), and underlying diabetes mellitus in 3 patients (case 2, 17 and 24) (Table 1). Furthermore, 10 cases were diagnosed as steroid diabetes after glucocorticoid treatment.
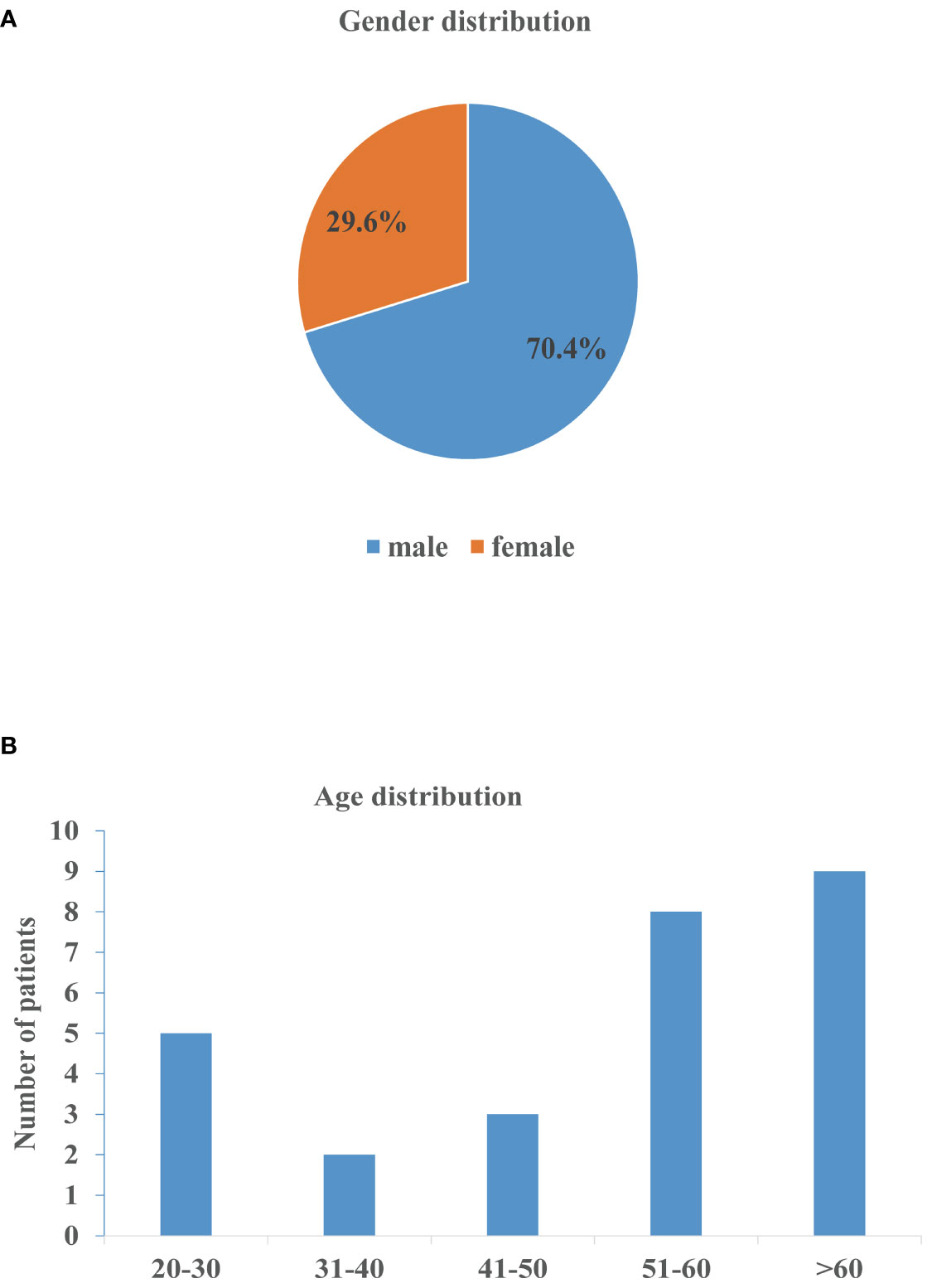
Figure 3 Gender and age distribution of nocardiosis with nephrotic syndrome. (A) Gender distribution of patients. (B) Age distribution of patients.
Characteristics and Treatment of NS
The NS type was confirmed by renal needle biopsy. The pathological examination confirmed 13 cases of membranous nephropathy, 3 cases of lupus nephritis, 2 cases of focal segmental glomerulosclerosis, 2 cases of minimal change nephrosis, 1 case of mesangial proliferative glomerulonephritis, and 1 case of immunoglobulin A nephropathy (Table 1). All patients had received glucocorticoid or corticosteroid therapy (case 7) for NS with mean doses of 30.9 ± 13.7 mg/d when infected (case 6 and 27 without doses of glucocorticoid). Combination treatments of glucocorticoid and immunosuppressor were administrated in 17 patients. The average time between hormone therapy and Nocardia infection was 8.5 ± 9.7 months. Nineteen patients developed nocardiosis within 6 months after glucocorticoid or corticosteroid therapy. Case 7 was diagnosed as disseminated nocardiosis within 2 months of corticosteroid therapy.
Clinical and Radiographic Manifestations of Nocardiosis
Serum albumin levels were reported in 23/27 cases, with all 23 patients having hypoalbuminemia; 68% (15/22) of the patients had increased serum creatinine levels (Table 2); and 13 cases had anemia, with the average hemoglobin level at 103.9 ± 18.1 g/L. The elevated WBC counts were noted in 24 out of the 27 cases (88.9%), and the average level was 17.0 ± 6.5×109/L. The neutrophils percentage was reported in 15/26 cases, and all had elevated neutrophil percentages. Case 6 had a normal WBC count but with an elevated neutrophil percentage. The CRP levels were reported in 24/27 cases; all but 1 (95.8%) had elevated CRP levels. Serum PCT levels were reported in 21/27 cases, and the PCT levels were ≥0.5 ng/mL in 13 patients; however, the PCT level was significantly elevated in only one patient (case 21). The average CRP and PCT were 101.3 ± 73.8 mg/L and 1.7 ± 3.7 ng/mL, respectively. The CD4+ T-lymphocyte counts were tested in 12 patients, and 9 of them had decreased CD4+ counts.
In our retrospective study, pulmonary infection was the most common manifestation in NS patients, with 85.2% (23/27) of patients affected (Table 1). The disseminated infection occurred in 77.8% (21/27) patients, 29.6% (8/27) patients suffered from multiorgan dissemination (>2 organ), and 5 cases had evidence of central nervous system (CNS) infection, and only 1 patient developed Nocardia bacteremia, a rare occurrence (case 4). Other infection sites of nocardiosis were skin (12/27), pleural cavity (3/27), hip (3/27), eye (2/27), and neck (2/27).
The main respiratory symptoms included fever (74.1%, 20/27), cough (48.1%, 13/27), expectoration (44.4%, 12/27), chest pain (29.6%, 8/27), dyspnea (11.1%, 3/27), chest tightness (7.4%, 2/27), and chill (7.4%, 2/27) (Table 1). Multiple pulmonary symptoms manifested in one patient. Subcutaneous or skin abscesses were observed in 18 patients. Subcutaneous abscesses were observed in 7 patients as the first symptoms.
Chest CT images revealed that pulmonary nodules/mass (48.1%, 13/27), consolidation (37%, 10/27), and pleural effusion (37%, 10/27) were the most common manifestations. Other pulmonary radiological findings included pleural thickening (18.5%, 5/27), cavities (18.5%, 5/27), bronchiectasis (18.5%, 5/27), pericardial effusion (18.5%, 5/27), pulmonary abscesses (14.8%, 4/27), emphysema (7.4%, 2/27), pulmonary bullae (7.4%, 2/27), and ground-glass opacity (3.7%, 1/27). Magnetic resonance imaging (MRI) and CT showed 5 patients with cerebral abscesses; but 2 had no CNS symptoms.
The etiological diagnosis of nocardiosis was established by Gram staining, modified acid-fast staining, microbial culture, gene sequencing, and matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). The most common specimen types for microbiological identification were pus (66.7%, 18/27) and sputum (33.3%, 9/27). Six different Nocardia species, including N.cyriacigeorgica, N.brasiliensis, N.farcinica, N.otitidiscaviarum, N.asteroides and N.caviae, were identified in 13 cases, 6 cases by 16S rRNA gene sequencing, 6 cases by culture and biochemical tests, and 1 case by MALDI-TOF MS. AST was performed in 8 cases.
Nocardiosis Treatment and Patient Prognosis
All 27 patients received antibiotic treatment once they were diagnosed, of which 25 patients were treated with TMP-SMX monotherapy (18.5%, 5/27) or TMP-SMX-based multidrug therapy (74.1%, 20/27). Only 2 patients (case 12 and 14) received biapenem monotherapy. Thirteen patients having subcutaneous abscesses or pericardial fluid received surgical drainage in addition to antibiotic therapy. During follow-up, except for case 27 (which lacked the treatment duration), the antibiotic therapy duration in other cases lasted from 3 months to 1 year. In terms of prognosis, all patients improved after antibiotic therapy and abscess drainage, but 2 patients (case 2 and 24) were lost to follow-up. In case 24, the abscess in the right middle finger diminished after 3-day antibiotic treatment, but he was lost to follow-up. Case 2 refused further treatment despite clinical improvements after 21 days of hospitalization.
Discussion
Nocardiosis is a rare infectious disease caused by the filamentous Gram-positive bacteria Nocardia spp. present in the environment. Nocardiosis most frequently presents with pulmonary, cutaneous, and subcutaneous infections, leading to invasive and potentially disseminated infections in severe cases.
Previous research (Martinez-Barricarte, 2020) showed that Nocardia infection had gender biases, possibly due to differences in distinct lifestyles and professions of man and women; nocardiosis mainly occurred in the age groups 31-40 and 51-60 years. The present study observed males as a more high-risk infection category than females with an incidence of 2:1, similar to the observations in immunocompromised individuals (Beaman et al., 1976). We also found that there was no age predilection for infection in NS patients in the age group 31-40 years, however, those over 50 years were impacted.
Long-term glucocorticoids and immunosuppressive therapy, which is proposed to be the leading risk factor for nocardiosis, can inhibit host cell-mediated immunity against infection (Ercibengoa et al., 2020). Patients with deficient cell-mediated immunity predispose to nocardiosis (Oh et al., 1988; Penkert et al., 2016; Sheikh-Taha and Corman, 2017). Previous studies confirmed that impaired renal function might compromise normal immune function, and the elevated serum creatinine levels in NS patients was an independent risk factor for pulmonary infection (Xu et al., 2017; Wang et al., 2019). Other predisposing factors, including organ transplantation, diabetes mellitus, HIV infection, and COPD (Saubolle & Sussland, 2003; Filice, 2005; Rosen et al., 2015; Coussement et al., 2017; Haussaire et al., 2017), could further destroy the immune system of patients and increase the risk of Nocardia infection. In our retrospective study, the treatment of glucocorticoids and (or) immunosuppressant and elevated serum creatinine indicated impaired immune function, and the patients were predisposed to infection. Additionally, 14 patients (51.9%) had two or three other risk factors, suggesting that these patients were more likely to be at a higher risk of Nocardia infection. Case 3 developed nocardiosis 1 month after the low-dose (15 mg/d) prednisone therapy, suggesting that opportunistic infection by rare pathogens may also occur during low-dose glucocorticoid maintenance therapy.
The lung is the most common site of infection because inhalation is the primary entry route for Nocardia spp., accounting for 62-86% of infection. The frequently involved organs include the CNS and skin, and concerns 2%-42.9% and 8%-31% of patients with Nocardia infection, respectively (Minero et al., 2009; Coussement et al., 2016; Haussaire et al., 2017; Yagishita et al., 2021). In our study, the NS patients did not have any trauma history, so it was assumed that the pathogen might infect patients through the respiratory tracts and then disseminate into the skin. Our data indicated that cutaneous nocardiosis was more common than CNS nocardiosis in NS patients. However, Yagishita et al. (Yagishita et al., 2021) reported that brain infection (35.7%, 5/14) was more than skin/cutaneous infection (28.6%, 4/14) in patients with connective tissue diseases. The possible explanation for this difference in the infected organs needs further studies. Interestingly, compared with other immunosuppressed patients, such as Cushing’s Syndrome patients [77.8% (21/27) vs 38.9% (7/18), P=0.013] (Zhang et al., 2021) and solid organ transplant recipients [77.8% (21/27) vs 42.7% (50/117), P=0.001] (Coussement et al., 2016), NS patients had a higher rate of disseminated infection. A possible explanation could be the presence of disseminated infections already even before the appearance of apparent symptoms due to its low virulence, slow growth, and long infection duration. Therefore, if cutaneous lesions were identified, disseminated Nocardia infection should be considered. Virulent Nocardia gets rapidly cleared from the blood (Beaman and Beaman, 1994), making its spread detection through the bloodstream difficult. Our study showed that only 3.7% (1/27) of the patients experiencing Nocardia bacteremia by the automated blood culture system were detected. Nocardia bacteremia might be underestimated due to its slow growth, low grade or intermittent bacteremia, bacteremia only at very initial moments of the infections. A study of solid organ transplant recipients with CNS nocardiosis showed that 43.3% of cases had no CNS manifestations, and unrecognized CNS involvement might potentially lead to the treatment failure (Coussement et al., 2016). We found that 3 CNS nocardiosis patients with cerebral abscesses had no neurological symptoms, highlighting the importance of performing routine contrast-enhanced brain imaging for all patients with demonstrated or suspected nocardiosis.
The main clinical characteristics and symptoms of pulmonary Nocardia infection in NS patients include fever, cough, expectoration, chest pain, dyspnea, chest tightness, and chill, indicating that it is non-specific and difficult to distinguish from other bacterial infections. Cutaneous infection in NS patients manifest as ulcers, abscesses and nodules, and can spread to lymph nodes. If the infection spreads to the CNS, the symptoms include weakness, ataxia, and sudden obnubilation. Previous research demonstrated that primary skin and subcutaneous lesions were rare manifestations of Nocardia infection (Shimizu et al., 1998). In NS patients, both respiratory symptoms and multiple subcutaneous indurations or abscesses could be the first symptoms. Some patients had no pulmonary symptoms, while some experienced respiratory distress. Case 7 was shifted to ICU and intubated because of respiratory distress. Case 6 had inflammatory nodules in the lung without fever or any pulmonary symptoms; his infection manifested as the primary subcutaneous abscess. Therefore, a physician should consider the Nocardia infection when the skin and subcutaneous lesions are identified in NS patients receiving immunosuppressive therapy.
In our study, nodule/mass and consolidations were the major radiologic manifestations in NS patients with Nocardia infections, consistent with the previous single-center review (Guo et al., 2020; Han et al., 2020). A retrospective study of Cushing’s syndrome with nocardiosis reported that cavitary lesions and nodules were the most common radiological findings (Zhang et al., 2021), indicating that the main radiologic manifestations may have slight differences in different diseases. Han et al. (Han et al., 2020) proposed that pulmonary abscesses were important signs of nocardiosis in glomerular disease patients, whereas only 4 patients (case 2, 4, 25, 27) presented with pulmonary abscesses in our review. Therefore, further studies with more cases are needed to assess if pulmonary abscesses were important characteristics in NS patients and the radiological differences between NS patients and other nocardiosis.
Procalcitonin is a well-known inflammatory biomarker for early detection of bacterial infections, with its levels increasing from systemic inflammatory response syndrome (0.6-2 ng/ml) to severe sepsis (2-10 ng/ml) and septic shock (>10 ng/ml) (Markanday, 2015). The antibiotics administration is usually encouraged at the PCT levels ≥0.5 ng/mL (Bouadma et al., 2010). Nevertheless, previous research demonstrated inadequate sensitivity of PCT for the early diagnosis of Gram-positive bacterial infections (Koizumi et al., 2020). In our study, normal or slightly elevated PCT levels in most nocardiosis patients could be because Nocardia is Gram-positive bacteria and most Nocardia species have low virulence. Further research on the role of PCT levels in the early diagnosis of Nocardia infections requires more clinical data.
Non-specific and diverse clinical characteristics and radiological manifestations of nocardiosis make its diagnosis difficult as these findings are similar to fungal, mycobacterial, and other bacterial infections. Therefore, microbiological identification plays a vital role in the diagnosis of nocardiosis. Microscopic examination, performed mainly by Gram-staining and modified acid-fast staining, can provide an early suspicion of nocardiosis. If Nocardia infection is suspected, prolonged incubation and the use of specific media, such as mycobacterial medium, buffered charcoal-yeast extract (BCYE), and fungal medium, are recommended (Saubolle and Sussland, 2003; Brown-Elliott et al., 2006). Most cases in our retrospective study were diagnosed through the microbial culture of clinical specimens. Case 3 proved that mycobacterial medium and digestion with 2% KOH before inoculation were useful for inhibiting the oropharyngeal flora growth to improve the separation rate. In addition to cultures, Nocardia may be identified by polymerase chain reaction (PCR)-based rapid molecular techniques on clinical samples with high sensitivity but relatively lower specificity (Couble et al., 2005; Rouzaud et al., 2018). Therefore, positive PCR results of respiratory samples might be an indication of colonization and not of infection (Brown-Elliott et al., 2006; Coussement et al., 2019). Reliable species identification can predict antimicrobial susceptibility and guide the initial antimicrobial therapy before obtaining antibiotic susceptibility patterns (Wallace et al., 1991; McTaggart et al., 2015; Body et al., 2018; Durand et al., 2020). The gold standard for identifying Nocardia species is molecular biology, such as PCR-based assay and 16S rRNA gene sequencing (Conville et al., 2012; Valdezate et al., 2017; Martinez-Barricarte, 2020). In addition, MALDI-TOF MS is being used increasingly to identify Nocardia species (Girard et al., 2017; Body et al., 2018). In case 7, initial multidrug therapy (amikacin, meropenem, and cotrimoxazole) was effective; however, meropenem was then modified to linezolid as the species identified, N.otitidiscaviarum, was less susceptible to beta-lactam antibiotics. Thus, accurate species identification will facilitate the selection of initial antimicrobial therapy.
To date, there is no consensus for optimal management of nocardiosis. Carbapenem monotherapy has been found effective for nocardiosis (Ameen et al., 2010); 2 cases (case 12 and 14) in our study confirmed the effectiveness of carbapenem monotherapy of disseminated nocardia infection in NS patients. Sulphonamides, most commonly TMP-SMX, have been the mainstay treatment for Nocardia infections since the 1950s (Wilson, 2012). Additionally, combination drug therapy (two or three drugs) had been suggested, especially for patients with serious or disseminated infections (Liu et al., 2017). The companion regimens in TMP-SMX-based multidrug therapy include third-generation cephalosporins (ceftriaxone, cefotaxime), carbapenems (imipenem, meropenem, ertapenem), amikacin, and linezolid. Linezolid may be used as monotherapy for nocardiosis treatment because of its excellent coverage against all pathogenic Nocardia species (Shen et al., 2011; Davidson et al., 2020). If antimicrobial treatment fails to control the infection, we should take therapeutic modifications into account. In case 4, the patient was administered with oral TMP-SMX in combination with intravenous imipenem/cilastatin because the initial antibiotic treatment was ineffective. In case 15, antibiotic treatment was switched to intravenous biapenem and linezolid for 2 weeks because of aggravated pulmonary inflammation and multiple cerebral abscesses after initial TMP-SMX monotherapy. An antibiotic duration of 3-12 months is recommended depending on the therapeutic response, infection site, and immune status of patients (Wallace et al., 1982; Hui et al., 2003; Wilson, 2012). It is seemed that the mortality rate of nocardiosis in NS patients is far lower than that in other immunocompromised patients of 40-66.7% (Xu et al., 2016; Williams et al., 2020; Zhang et al., 2021). One reason could be no co-infections with other pathogens, associated with increased mortality in nocardiosis (Huang et al., 2019). Another reason could be the effective treatment of CNS infection. The mortality rate of CNS infection in SLE (Systemic Lupus Erythematosus) and Cushing’s Syndrome was more than 50% (Mc-Nab et al., 2000; Zhang et al., 2021). Multidrug therapy is preferable, and neurosurgical drainage should be especially considered for patients with large brain abscesses or not responding to antimicrobial therapy, or if a co-infection is suspected (Anagnostou et al., 2014; Rafiei et al., 2016; Restrepo et al., 2019). In our retrospective study, CNS infection occurred in 5 of 27 patients; 4 of them received TMP-SMX-based multidrug therapy (two or three drugs). All patients with CNS nocardia infection recovered without surgical drainage and did not relapse during the follow-up period, indicating that multidrug therapy was effective. Martinez-Barricarte (Martinez-Barricarte, 2020) demonstrated that antibiotic treatment was used in combination with debridement or drainage of affected area in the lymph nodes, CNS, lungs, or extremities in 18.3% of cases. In our study, antibiotic therapy was combined with surgical drainage in nearly 50% of cases with subcutaneous abscesses or pericardial fluid, and these results demonstrated the combination strategy might play an important role in patients’ therapeutic outcomes.
Our retrospective study has a few limitations. First, despite a multicenter retrospective study, the number and area of reported nocardiosis cases with NS are limited. Second, some laboratory and clinical testing, such as brain imaging, AST, species identification, were not performed in some cases, which may generate some bias when analyzing clinical data.
Conclusions
NS patients who received low-to-high doses of immuno-suppressive therapies within 6 months are at high risk of Nocardia infection. Nocardiosis in NS patients had a high rate of disseminated and cutaneous infection but a low mortality rate. Physicians should be alert about Nocardia infection in NS patients presenting subcutaneous abscesses or nodules and inform the microbiology laboratory of the suspected nocardiosis. As demonstrated in our case reports, the improvement of laboratory diagnostic methods, including careful microscopic examination, prolonged incubation, use of appropriate media, accurate species identification, and antimicrobial susceptibility testing, should be highlighted for timely diagnosis and correct treatment of nocardiosis. A combination of accurate antibiotic therapy and surgical drainage might be essential for the successful treatment and prognosis of patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the first author.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of 980th Hospital of the PLA Joint Logistical Support Force (No.2021-KY-121). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors contributed to this work. All authors have read and agreed to the published version of the manuscript. YC and JG designed the study. T-yW, H-lY, WL, and Z-xH collected the data. J-pS, JC, J-yG, and F-kW interpreted the data. YC wrote the first draft of the paper. YC and JG reviewed and approved the final report.
Funding
This research was funded by the National Natural Science Foundation of China (No.31200142).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.789754/full#supplementary-material
Supplementary Figure S1 | Bronchoscopic images and magnetic resonance imaging of brain in case 1. (A) Bronchoscopic image at the left lower lobe. (B) Bronchoscopic image at the right middle and lower lobe. (C) Magnetic resonance imaging of brain.
Supplementary Figure S2 | 16S rRNA gene sequencing of Nocardia spp. isolated from BALF in case 1. (A) Gene sequencing of N.cyriacigeorgica identified from BALF. (B) Sequence blast description.
Supplementary Figure S3 | CT scanning and microbiological identification of case 2. (A) Gram staining of sputum showing Gram-positive, filamentous branching bacilli (magnification, ×100). (B) Modified acid-fast staining of sputum showing filamentous, weakly acid-fast branching bacilli (magnification,×100). (C) Colonies of N.brasiliensis cultured on blood agar for 72h. (D) Gram staining showing the Gram-positive, filamentous branching and beaded structure of N.brasiliensis (magnification,×100). (E) Modified acid-fast staining showing filamentous, weakly acid-fast branching bacilli (magnification,×100). (F) Chest CT showing multiple cavities in the right upper lobe and bilateral pleural effusion. (G) Chest CT showing the size of abscesses decreased gradually following treatment.
Supplementary Figure S4 | 16S rRNA gene sequencing of Nocardia spp. isolated from sputum in case 2. (A) Gene sequencing of N.brasiliensis identified from sputum. (B) Sequence blast description.
Supplementary Figure S5 | Treatment flow diagram of case 2. TMP-SMX: trimethoprim-sulfamethoxazole.
Supplementary Figure S6 | CT scanning, ultrasonography and microbiological identification of case 3. (A) Gram staining of sputum showing Gram-positive, filamentous branching bacilli (magnification, ×100). (B) Modified acid-fast staining of sputum showing filamentous, weakly acid-fast branching bacilli (magnification,×100). (C) Colonies of N.farcinica cultured on blood agar for 72h. (D) Colonies of N.farcinica cultured on mycobacterial medium (Lowenstein-Jensen medium) for 72h. (E) Gram staining showing the Gram-positive, filamentous branching and beaded structure of N.farcinica (magnification,×100). (F) Modified acid-fast staining showing filamentous, weakly acid-fast branching bacilli (magnification,×100). (G) Chest CT showing cavities in the right upper lobe. (H) Chest CT showing nodules in the left lung. (I) Chest CT showing nodules in the right lung. (J) USG of abscess showed thickened subcutaneous soft tissue and the internal echo was not uniform.
Supplementary Figure S7 | 16S rRNA gene sequencing of Nocardia spp. isolated from sputum and pus in case 3. (A) Gene sequencing of N.farcinica identified from sputum. (B) Sequence blast description.
Supplementary Figure S8 | Treatment flow diagram of case 3. TMP-SMX: trimethoprim-sulfamethoxazole. T spot-TB: Mycobacterium tuberculosis specific T lymphocyte.
References
Ameen, M., Arenas, R., Vasquez del Mercado, E., Fernandez, R., Torres, E., Zacarias, R. (2010). Efficacy of Imipenem Therapy for Nocardia Actinomycetomas Refractory to Sulfonamides. J. Am. Acad. Dermatol. 62, 239–246. doi: 10.1016/j.jaad.2009.06.043
Anagnostou, T., Arvanitis, M., Kourkoumpetis, T. K., Desalermos, A., Carneiro, H. A., Mylonakis, E. (2014). Nocardiosis of the Central Nervous System: Experience From a General Hospital and Review of 84 Cases From the Literature. Med. (Baltimore) 93, 19–32. doi: 10.1097/MD.0000000000000012
Beaman, B. L., Beaman, L. (1994). Nocardia Species: Host-Parasite Relationships. Clin. Microbiol. Rev. 7, 213–264. doi: 10.1128/CMR.7.2.213
Beaman, B. L., Burnside, J., Edwards, B., Causey, W. (1976). Nocardial Infections in the United States 1972-1974. J. Infect. Dis. 134, 286–289. doi: 10.1093/infdis/134.3.286
Body, B. A., Beard, M. A., Slechta, E. S., Hanso, K. E., Barker, A. P., Babady, N. E., et al. (2018). Evaluation of the Vitek MS V3.0 Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry System for Identification of Mycobacterium and Nocardia Species. J. Clin. Microbiol. 56(6), e00237–18. doi: 10.1128/JCM.00237-18
Bouadma, L., Luyt, C. E., Tubach, F., Cracco, C., Alvarez, A., Schwebel, C., et al. (2010). Use of Procalcitonin to Reduce Patients' Exposure to Antibiotics in Intensive Care Units (PRORATA Trial): A Multicentre Randomised Controlled Trial. Lancet 375, 463–474. doi: 10.1016/S0140-6736(09)61879-1
Brown-Elliott, B. A., Brown, J. M., Conville, P. S., Wallace, R. J., Jr. (2006). Clinical and Laboratory Features of the Nocardia Spp. Based on Current Molecular Taxonomy. Clin. Microbiol. Rev. 19, 259–282. doi: 10.1128/CMR.19.2.259-282.2006
Chen, B., Tang, J., Lu, Z., Wang, N., Gao, X., Wang, F. (2016). Primary Cutaneous Nocardiosis in a Patient With Nephrotic Syndrome: A Case Report and Review of the Literature. Med. (Baltimore) 95, e2490. doi: 10.1097/MD.0000000000002490
Conville, P. S., Brown-Elliott, B. A., Smith, T., Zelazny, A. M. (2017). The Complexities of Nocardia Taxonomy and Identification. J. Clin. Microbiol. 56 (1), e01419–17. doi: 10.1128/JCM.01419-17
Conville, P. S., Brown-Elliott, B. A., Wallace, R. J., Jr., Witebsky, F. G., Koziol, D., Hall, G. S., et al. (2012). Multisite Reproducibility of the Broth Microdilution Method for Susceptibility Testing of Nocardia Species. J. Clin. Microbiol. 50, 1270–1280. doi: 10.1128/JCM.00994-11
Conville, P. S., Brown, J. M., Steigerwalt, A. G., Lee, J. W., Byrer, D. E., Anderson, V. L., et al. (2003). Nocardia Veterana as a Pathogen in North American Patients. J. Clin. Microbiol. 41, 2560–2568. doi: 10.1128/JCM.41.6.2560-2568.2003
Couble, A., Rodriguez-Nava, V., de Montclos, M. P., Boiron, P., Laurent, F. (2005). Direct Detection of Nocardia Spp. In Clinical Samples by a Rapid Molecular Method. J. Clin. Microbiol. 43, 1921–1924. doi: 10.1128/JCM.43.4.1921-1924.2005
Coussement, J., Lebeaux, D., El Bizri, N., Claes, V., Kohnen, M., Steensels, D., et al. (2019). Nocardia Polymerase Chain Reaction (PCR)-Based Assay Performed on Bronchoalveolar Lavage Fluid After Lung Transplantation: A Prospective Pilot Study. PLoS One 14, e0211989. doi: 10.1371/journal.pone.0211989
Coussement, J., Lebeaux, D., Rouzaud, C., Lortholary, O. (2017). Nocardia Infections in Solid Organ and Hematopoietic Stem Cell Transplant Recipients. Curr. Opin. Infect. Dis. 30, 545–551. doi: 10.1097/QCO.0000000000000404
Coussement, J., Lebeaux, D., van Delden, C., Guillot, H., Freund, R., Marbus, S., et al. (2016). Nocardia Infection in Solid Organ Transplant Recipients: A Multicenter European Case-Control Study. Clin. Infect. Dis. 63, 338–345. doi: 10.1093/cid/ciw241
Davidson, N., Grigg, M. J., McGuinness, S. L., Baird, R. J., Anstey, N. M. (2020). Safety and Outcomes of Linezolid Use for Nocardiosis. Open Forum Infect. Dis. 7, ofaa090. doi: 10.1093/ofid/ofaa090
Durand, T., Vautrin, F., Bergeron, E., Girard, V., Polsinelli, S., Monnin, V., et al. (2020). Assessment of VITEK(R) MS IVD Database V3.0 for Identification of Nocardia Spp. Using Two Culture Media and Comparing Direct Smear and Protein Extraction Procedures. Eur. J. Clin. Microbiol. Infect. Dis. 39, 559–567. doi: 10.1007/s10096-019-03758-x
Ercibengoa, M., Camara, J., Tubau, F., Garcia-Somoza, D., Galar, A., Martin-Rabadan, P., et al. (2020). A Multicentre Analysis of Nocardia Pneumonia in Spain: 2010-2016. Int. J. Infect. Dis. 90, 161–166. doi: 10.1016/j.ijid.2019.10.032
Filice, G. A. (2005). Nocardiosis in Persons With Human Immunodeficiency Virus Infection, Transplant Recipients, and Large, Geographically Defined Populations. J. Lab. Clin. Med. 145, 156–162. doi: 10.1016/j.lab.2005.01.002
Girard, V., Mailler, S., Polsinelli, S., Jacob, D., Saccomani, M. C., Celliere, B., et al. (2017). Routine Identification of Nocardia Species by MALDI-TOF Mass Spectrometry. Diagn. Microbiol. Infect. Dis. 87, 7–10. doi: 10.1016/j.diagmicrobio.2016.09.024
Guo, J., Li, S., Xu, S., Jiang, L., Gao, E., Liu, Z. (2020). Nocardiosis in Patients With Nephrotic Syndrome: A Retrospective Analysis of 11 Cases and a Literature Review. Int. Urol. Nephrol. 52, 731–738. doi: 10.1007/s11255-020-02415-z
Han, Y., Huang, Z., Zhang, H., He, L., Sun, L., Liu, Y., et al. (2020). Nocardiosis in Glomerular Disease Patients With Immunosuppressive Therapy. BMC Nephrol. 21, 516. doi: 10.1186/s12882-020-02179-9
Haussaire, D., Fournier, P. E., Djiguiba, K., Moal, V., Legris, T., Purgus, R., et al. (2017). Nocardiosis in the South of France Over a 10-Years Perio-2014. Int. J. Infect. Dis. 57, 13–20. doi: 10.1016/j.ijid.2017.01.005
Huang, L., Sun, L., Yan, Y. (2019). Characteristics of Nocardiosis Patients With Different Immune Status From a Chinese Tertiary General Hospital During 8-Year Period: A STROBE-Compliment Observational Study. Med. (Baltimore) 98, e17913. doi: 10.1097/MD.0000000000017913
Hui, C. H., Au, V. W., Rowland, K., Slavotinek, J. P., Gordon, D. L. (2003). Pulmonary Nocardiosis Re-Visited: Experience of 35 Patients at Diagnosis. Respir. Med. 97, 709–717. doi: 10.1053/rmed.2003.1505
Hwang, J. H., Koh, W. J., Suh, G. Y., Chung, M. P., Kim, H., Kwon, O. J., et al. (2004). Pulmonary Nocardiosis With Multiple Cavitary Nodules in a HIV-Negative Immunocompromised Patient. Intern. Med. 43, 852–854. doi: 10.2169/internalmedicine.43.852
Kerbel, N. C. (1962). Long-Term Steroid Therapy of the Nephrotic Syndrome in an Adult Complicated by Fatal Nocardiosis. Can. Med. Assoc. J. 87, 129–132.
Koizumi, Y., Sakanashi, D., Ohno, T., Nakamura, A., Yamada, A., Shibata, Y., et al. (2021). Plasma Procalcitonin Levels Remain Low at the Onset of Gram-Positive Bacteremia Regardless of Severity or the Presence of Shock: A Retrospective Analysis of Patients With Detailed Clinical Characteristics. J. Microbiol. Immunol. Infect. 54 (6), 1028–1037. doi: 10.1016/j.jmii.2020.08.015
Liu, C., Feng, M., Zhu, J., Tao, Y., Kang, M., Chen, L. (2017). Severe Pneumonia Due to Nocardia Otitidiscaviarum Identified by Mass Spectroscopy in a Cotton Farmer: A Case Report and Literature Review. Med. (Baltimore) 96, e6526. doi: 10.1097/MD.0000000000006526
Liu, W. L., Lai, C. C., Hsiao, C. H., Hung, C. C., Huang, Y. T., Liao, C. H., et al. (2011). Bacteremic Pneumonia Caused by Nocardia Veterana in an HIV-Infected Patient. Int. J. Infect. Dis. 15, e430–e432. doi: 10.1016/j.ijid.2011.03.001
Li, J., Zhang, Q., Su, B. (2017). Clinical Characteristics and Risk Factors of Severe Infections in Hospitalized Adult Patients With Primary Nephrotic Syndrome. J. Int. Med. Res. 45, 2139–2145. doi: 10.1177/0300060517715339
Margalit, I., Lebeaux, D., Tishler, O., Goldberg, E., Bishara, J., Yahav, D., et al. (2021). How do I Manage Nocardiosis? Clin. Microbiol. Infect. 27, 550–558. doi: 10.1016/j.cmi.2020.12.019
Markanday, A. (2015). Acute Phase Reactants in Infections: Evidence-Based Review and a Guide for Clinicians. Open Forum Infect. Dis. 2, ofv098. doi: 10.1093/ofid/ofv098
Martinez-Barricarte, R. (2020). Isolated Nocardiosis, an Unrecognized Primary Immunodeficiency? Front. Immunol. 11, 590239. doi: 10.3389/fimmu.2020.590239
Mc-Nab, P., Fuentealba, C., Ballesteros, F., Pacheco, D., Alvarez, M., Dabanch, J., et al. (2000). Nocardia Asteroides Infection in a Patient With Systemic Lupus Erythematosus. Rev. Med. Chil. 128, 526–528.
McTaggart, L. R., Doucet, J., Witkowska, M., Richardson, S. E. (2015). Antimicrobial Susceptibility Among Clinical Nocardia Species Identified by Multilocus Sequence Analysis. Antimicrob. Agents Chemother. 59, 269–275. doi: 10.1128/AAC.02770-14
Minero, M. V., Marin, M., Cercenado, E., Rabadan, P. M., Bouza, E., Munoz, P. (2009). Nocardiosis at the Turn of the Century. Med. (Baltimore) 88, 250–261. doi: 10.1097/MD.0b013e3181afa1c8
Molina, A., Winston, D. J., Pan, D., Schiller, G. J. (2018). Increased Incidence of Nocardial Infections in an Era of Atovaquone Prophylaxis in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol. Blood Marrow Transplant. 24, 1715–1720. doi: 10.1016/j.bbmt.2018.03.010
Oh, C. S., Stratta, R. J., Fox, B. C., Sollinger, H. W., Belzer, F. O., Maki, D. G. (1988). Increased Infections Associated With the Use of OKT3 for Treatment of Steroid-Resistant Rejection in Renal Transplantation. Transplantation 45, 68–73. doi: 10.1097/00007890-198801000-00016
Penkert, H., Delbridge, C., Wantia, N., Wiestler, B., Korn, T. (2016). Fulminant Central Nervous System Nocardiosis in a Patient Treated With Alemtuzumab for Relapsing-Remitting Multiple Sclerosis. JAMA Neurol. 73, 757–759. doi: 10.1001/jamaneurol.2016.0146
Rafiei, N., Peri, A. M., Righi, E., Harris, P., Paterson, D. L. (2016). Central Nervous System Nocardiosis in Queensland: A Report of 20 Cases and Review of the Literature. Med. (Baltimore) 95, e5255. doi: 10.1097/MD.0000000000005255
Restrepo, A., Clark, N. M., Infectious Diseases Community of Practice of the American Society of T (2019). Nocardia Infections in Solid Organ Transplantation: Guidelines From the Infectious Diseases Community of Practice of the American Society of Transplantation. Clin. Transplant. 33, e13509. doi: 10.1111/ctr.13509
Rosen, L. B., Rocha Pereira, N., Figueiredo, C., Fiske, L. C., Ressner, R. A., Hong, J. C., et al. (2015). Nocardia-Induced Granulocyte Macrophage Colony-Stimulating Factor is Neutralized by Autoantibodies in Disseminated/Extrapulmonary Nocardiosis. Clin. Infect. Dis. 60, 1017–1025. doi: 10.1093/cid/ciu968
Rouzaud, C., Rodriguez-Nava, V., Catherinot, E., Mechai, F., Bergeron, E., Farfour, E., et al. (2018). Clinical Assessment of a Nocardia PCR-Based Assay for Diagnosis of Nocardiosis. J. Clin. Microbiol. 56 (6), e00002–18. doi: 10.1128/JCM.00002-18
Sah, R., Khadka, S., Neupane, S., Nepal, G., Singla, S., Kumari, P., et al. (2020). Disseminated Infection With Nocardia Otitidiscaviarum in a Patient Under Steroid Therapy. Clin. Case Rep. 8, 369–373. doi: 10.1002/ccr3.2640
Saubolle, M. A., Sussland, D. (2003). Nocardiosis: Review of Clinical and Laboratory Experience. J. Clin. Microbiol. 41, 4497–4501. doi: 10.1128/JCM.41.10.4497-4501.2003
Sheikh-Taha, M., Corman, L. C. (2017). Pulmonary Nocardia Beijingensis Infection Associated With the Use of Alemtuzumab in a Patient With Multiple Sclerosis. Mult. Scler. 23, 872–874. doi: 10.1177/1352458517694431
Shen, T., Wu, L., Geng, L., Wei, Z., Zheng, S. (2011). Successful Treatment of Pulmonary Nocardia Farcinica Infection With Linezolid: Case Report and Literature Review. Braz. J. Infect. Dis. 15, 486–489. doi: 10.1016/s1413-8670(11)70234-3
Shimizu, T., Furumoto, H., Asagami, C., Kanaya, K., Mikami, Y., Muto, M. (1998). Disseminated Subcutaneous Nocardia Farcinica Abscesses in a Nephrotic Syndrome Patient. J. Am. Acad. Dermatol. 38, 874–876. doi: 10.1016/S0190-9622(98)70479-7
Sirijatuphat, R., Niltwat, S., Tiangtam, O., Tungsubutra, W. (2013). Purulent Pericarditis and Cardiac Tamponade Caused by Nocardia Farcinica in a Nephrotic Syndrome Patient. Intern. Med. 52, 2231–2235. doi: 10.2169/internalmedicine.52.0453
Valdezate, S., Garrido, N., Carrasco, G., Medina-Pascual, M. J., Villalon, P., Navarro, A. M., et al. (2017). Epidemiology and Susceptibility to Antimicrobial Agents of the Main Nocardia Species in Spain. J. Antimicrob. Chemother. 72, 754–761. doi: 10.1093/jac/dkw489
Wallace, R. J., Jr., Brown, B. A., Tsukamura, M., Brown, J. M., Onyi, G. O. (1991). Clinical and Laboratory Features of Nocardia Nova. J. Clin. Microbiol. 29, 2407–2411. doi: 10.1128/jcm.29.11.2407-2411.1991
Wallace, R. J., Jr., Septimus, E. J., Williams, T. W., Jr., Conklin, R. H., Satterwhite, T. K., Bushby, M. B., et al. (1982). Use of Trimethoprim-Sulfamethoxazole for Treatment of Infections Due to Nocardia. Rev. Infect. Dis. 4, 315–325. doi: 10.1093/clinids/4.2.315
Wang, H. K., Sheng, W. H., Hung, C. C., Chen, Y. C., Lee, M. H., Lin, W. S., et al. (2015). Clinical Characteristics, Microbiology, and Outcomes for Patients With Lung and Disseminated Nocardiosis in a Tertiary Hospital. J. Formos Med. Assoc. 114, 742–749. doi: 10.1016/j.jfma.2013.07.017
Wang, T., Zhang, Y., Ping, F., Zhao, H., Yan, L., Lin, Q., et al. (2019). Predicting Risk of Pulmonary Infection in Patients With Primary Membranous Nephropathy on Immunosuppressive Therapy: The AIM-7C Score. Nephrol. (Carlton) 24, 1009–1016. doi: 10.1111/nep.13544
Williams, E., Jenney, A. W., Spelman, D. W. (2020). Nocardia Bacteremia: A Single-Center Retrospective Review and a Systematic Review of the Literature. Int. J. Infect. Dis. 92, 197–207. doi: 10.1016/j.ijid.2020.01.011
Wilson, J. W. (2012). Nocardiosis: Updates and Clinical Overview. Mayo Clin. Proc. 87, 403–407. doi: 10.1016/j.mayocp.2011.11.016
Woods, G. L., Brown-Elliott, B. A., Conville, P. S., Desmond, E. P., Hall, G. S., Lin, G., et al. (2011). Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. Pennsylvania, USA: Wayne (PA).
Xu, H., Fu, B., Xu, L., Sun, J. (2018). Disseminated Nocardiosis With Subretinal Abscess in a Patient With Nephrotic Syndrome-a Case Report. BMC Ophthalmol. 18, 234. doi: 10.1186/s12886-018-0883-2
Xu, H., Gasparini, A., Ishigami, J., Mzayen, K., Su, G., Barany, P., et al. (2017). eGFR and the Risk of Community-Acquired Infections. Clin. J. Am. Soc. Nephrol. 12, 1399–1408. doi: 10.2215/CJN.00250117
Xu, L., Xu, Q., Yang, M., Gao, H., Xu, M., Ma, W. (2016). Nocardiosis in Ectopic ACTH Syndrome: A Case Report and Review of 11 Cases From the Literature. Exp. Ther. Med. 12, 3626–3632. doi: 10.3892/etm.2016.3846
Yagishita, M., Tsuboi, H., Tabuchi, D., Sugita, T., Nishiyama, T., Okamoto, S., et al. (2021). Clinical Features and Prognosis of Nocardiosis in Patients With Connective Tissue Diseases. Mod. Rheumatol. 31, 636–642. doi: 10.1080/14397595.2020.1823070
Yang, W., Zhao, L., Tang, M., Jiang, Y., Zhu, Z., Brady, T. J., et al. (2020). Disseminated Nocardia Infection in an Old Male Patient With Nephrotic Syndrome. Clin. Lab. 66 (1), 193–196. doi: 10.7754/Clin.Lab.2019.190638
Zhang, D., Jiang, Y., Lu, L., Lu, Z., Xia, W., Xing, X., et al. (2021). Cushing's Syndrome With Nocardiosis: A Case Report and a Systematic Review of the Literature. Front. Endocrinol. (Lausanne) 12, 640998. doi: 10.3389/fendo.2021.640998
Zhou, L., Liu, H., Yuan, F., Yuan, S. (2014). Disseminated Nocardiosis in a Patient With Nephrotic Syndrome Following HIV Infection. Exp. Ther. Med. 8, 1142–1144. doi: 10.3892/etm.2014.1883
Zhu, N., Zhu, Y., Wang, Y., Dong, S. (2017). Pulmonary and Cutaneous Infection Caused by Nocardia Farcinica in a Patient With Nephrotic Syndrome: A Case Report. Med. (Baltimore) 96, e7211. doi: 10.1097/MD.0000000000007211
Keywords: Nocardia, nocardiosis, nephrotic syndrome, immunosuppression, infection
Citation: Cheng Y, Wang T-y, Yuan H-l, Li W, Shen J-p, He Z-x, Chen J, Gao J-y, Wang F-k and Gu J (2022) Nocardia Infection in Nephrotic Syndrome Patients: Three Case Studies and A Systematic Literature Review. Front. Cell. Infect. Microbiol. 11:789754. doi: 10.3389/fcimb.2021.789754
Received: 15 October 2021; Accepted: 29 December 2021;
Published: 24 January 2022.
Edited by:
Max Maurin, Université Grenoble Alpes, FranceReviewed by:
Beiwen Zheng, Zhejiang University, ChinaJose Maria Marimon, Osakidetza Hospital Universitario Donostia, Spain
Copyright © 2022 Cheng, Wang, Yuan, Li, Shen, He, Chen, Gao, Wang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Gu, amlhbmdndTIwMTJAMTYzLmNvbQ==
 Yan Cheng
Yan Cheng Tian-yi Wang2
Tian-yi Wang2 Zheng-xin He
Zheng-xin He Jiang Gu
Jiang Gu