
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 24 November 2021
Sec. Clinical Microbiology
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.783635
This article is part of the Research Topic Insights In Clinical Microbiology: 2021 View all 8 articles
Objective: To investigate the correlation between serum immunoglobulin E (IgE) levels and the complications in children with Mycoplasma pneumoniae pneumonia (MPP).
Methods: A retrospective study of MPP patients hospitalized from May 2019 to July 2021 was performed. We analyzed the clinical manifestations, complications, laboratory findings, and treatments.
Results: A total of 275 patients who met the inclusion criteria were enrolled in the study. We divided patients into two groups based on whether there were complications. Complications occurred in 147 patients, of which pulmonary complications were more common than extrapulmonary complications. The IgE level in the complication group was higher than that in the non-complication group with p = 0.041. Patients with complications of necrotizing pneumonitis, pneumothorax, skin rash, or bronchiolitis obliterans had higher IgE levels. There was no statistically significant difference in IgE levels between pulmonary complications and extrapulmonary complications. The older the age, the greater the probability of complications (p = 0.001). The group with complications was more likely to have chest pain (p = 0.000), while the group without complications was more likely to have wheezing (p = 0.017). The use of bronchoscopy and glucocorticoids was higher in the complication group than in the non-complication group (p = 0.000).
Conclusions: MPP patients with higher IgE levels had more severe clinical symptoms and complications. We speculated that IgE might be a biomarker for complications after MP infection.
Mycoplasma pneumoniae (MP) is a common pathogen of community-acquired pneumonia in children (Shi et al., 2019). Many literature have reported that the severity of the disease caused by MP can range from mild to fatal (Waites and Talkington, 2004; Zhou et al., 2020). MP pneumonia (MPP) is mostly self-limited, but it can produce a variety of pulmonary and extrapulmonary complications and can even be life-threatening in some individuals (Poddighe, 2018). Extrapulmonary complications caused by MP can occur in any organ in the human body (Fan et al., 2015; Shah et al., 2019). It is uncertain under what circumstances MP infection is more likely to cause complications. Recently, in our clinical practice, we found that the total serum immunoglobulin E (IgE) increased in children with MP infection. Some studies also found that MPP patients had elevated total serum IgE levels (Poddighe et al., 2018; Ye et al., 2018). However, the correlations between the total serum IgE level and complications of MPP in children are still unclear. Therefore, we decided to analyze the serum IgE level in hospitalized children with MPP in order to confirm these data.
The data of patients with MPP who were admitted to Women and Children’s Hospital of Ganzhou from May 2019 to July 2021 were retrospectively collected. The inclusion criteria were as follows: 1) met the diagnostic criteria: with clinical manifestations (presence of fever, cough, tachypnea, and difficulty in breathing), abnormal lung auscultation, radiologic findings (presence of a new infiltrate on chest radiography or consolidation not attributable to some other etiologies), and MP detected to be positive by laboratory tests (Medjo et al., 2014); and 2) age less than 15 years. The exclusion criteria were as follows: 1) children with evidence of coinfection; 2) children with immune deficiency; and 3) children with a history of allergies. Severe MPP (SMPP) was defined as MPP with any one of the following: 1) a poor general condition; 2) fastidium or dehydration; 3) disturbance of consciousness; 4) an increased respiratory rate (infants >70 breaths/min and older children >50 breaths/min); 5) dyspnea; 6) cyanosis; 7) extent of infiltration on chest X-ray ≥2/3 of one lung or multilobe involvement; 8) extrapulmonary complications; 9) pleural effusion; and 10) oxygen saturation in room air ≤92% (Zheng et al., 2021).
On the day of admission, 3 ml of venous blood was collected from patients, and the passive agglutination assay was used to detect MP antibody (MP Antibody Test Kit, Fujitsu Joint Stock Company). It was defined as MP positive when the MP antibody titer was ≥1:160. Nasopharyngeal aspirates were collected on the day of admission, and MP-DNA was detected by PCR (MP Nucleic Acid Test Kit, National Sun Yat-sen University). Simultaneous detection of other pathogens was performed through the indirect immunofluorescence assay of respiratory viruses (adenovirus, respiratory syncytial virus, parainfluenza virus 1–3, and influenza virus A and B) using a D3 Ultra DFA Respiratory Virus Screening & ID Kit (Diagnostic Hybrids, Inc., OH, USA) and blood cultures for bacteria (BD FX200 blood culture system).
By consulting the electronic medical records of all patients, the demographic, clinical, and laboratory data were collected retrospectively. Clinical signs and symptoms of patients, including fever, cough, wheezing, chest pain, and complications, were obtained. All patients underwent chest X-ray or chest CT scan to confirm focal or segmental infiltration, with or without pleural effusion, atelectasis, pneumothorax, pulmonary embolism, and pulmonary necrosis.
Serum IgE levels were obtained using the automatic biochemical immunoassay analyzer produced by Roche. The test kit was also provided by Roche. The reaction was carried out according to the manufacturer’s instructions.
This study was approved by the Ethics and Research Council of Women and Children’s Hospital of Ganzhou (201905-A01) on May 15, 2019. The data from patients were collected anonymously.
The statistical analyses were carried out using SPSS 20.0 software package. Continuous variables were reported as the mean ± SD and were compared using Student’s t-test or the non-parametric Mann–Whitney U-test. The categorical variables were shown as number (%) and were compared using the chi-square test. p-Values <0.05 were considered to be significant. The figures and graphs were generated by GraphPad Prism 5.
From May 2019 to July 2021, 275 patients admitted to our hospital for MPP were enrolled in the study. The demographic and clinical characteristics are shown in Table 1. Of these, 167 (60.7%) were male and 103 (39.3%) female. The median age of the children was 4.93 years, varying from 2 months to 14 years 10 months; 9.4% of the patients were under 12 months, 24% of the patients were between 1 and 3 years, 32.4% of the patients were between 3 and 6 years, and 34.2% of the patients were older than 6 years. A total of 97 patients had SMPP. Almost all the children had cough (99.6%) and fever (95.3%), 24 (8.7%) had wheezing, and 22 (8.0%) had chest pain. Moreover, 98 (35.6%) patients received glucocorticoid therapy, 70 (25.5%) patients received electronic bronchoscopy alveolar lavage, 31 (11.3%) patients required oxygen therapy, 11 (4%) patients received immunoglobulin therapy, and eight (2.9%) patients needed intensive care unit (ICU) treatment. Further, 95 (34.5%) children experienced pulmonary complications, and 52 (18.9%) children experienced extrapulmonary complications.
The pulmonary and extrapulmonary complications are listed in Table 2. The IgE levels of patients with different complications varied from 0 to 2,500 IU/ml. Complications occurred in 147 patients, of which pulmonary complications were more common than extrapulmonary complications. Thirty (10.9%) patients had atelectasis, and 55 (20%) had pleural effusion, accounting for 89.5% of pulmonary complications. Extrapulmonary complications were more common in terms of skin rashes and hypokalemia. Further, 21 (7.64%) patients had hypokalemia, 12 (4.4%) patients had skin rashes, six (2.18%) patients had myocarditis, and six (2.18%) patients had hepatitis. Patients with complications of necrotizing pneumonitis, pneumothorax, skin rashes, eye pain, or bronchiolitis obliterans had higher IgE levels. No statistically significant difference was found in IgE levels between pulmonary complications and extrapulmonary complications with p = 0.292, as shown in Figure 1.
Patients were divided into two groups according to the presence or absence of complications. The demographics, clinical characteristics, laboratory findings, and treatments of children in the complication and non-complication groups are shown in Table 3. The children in the complication group were much older than those in the non-complication group (p = 0.001). No statistically significant difference in the appearance of fever and cough symptoms was observed between the two groups. Higher incidence of chest pain (p = 0.000) and lower incidence of wheezing (p = 0.017) were found in the complication group than in the non-complication group. Significant differences in fever duration, hospitalization duration, and incidences of SMPP between the two groups (p < 0.001) were also found. The probability of SMPP in patients with complications was higher than that in patients without complications (p = 0.000). As shown in Figure 2, the IgE level fluctuated greatly from patient to patient. The IgE level in the complication group was higher than that in the non-complication group (p = 0.041). The risk factors for complications by multifactor binary logistic regression analysis were analyzed. After age factors were adjusted, a significant difference in the IgE level was noted between the complication and non-complication groups (p = 0.047), as shown in Table 4. The threshold of IgE between these two groups was determined using the receiver operating characteristic (ROC) curve. The area under the ROC curve was 0.671. When the cutoff value for the IgE level was set at 245.6 IU/ml, the specificity and sensitivity were 75.51% and 52.27%, respectively (Figure 3). Besides the IgE level, D-dimer, lactate dehydrogenase (LDH), and ferritin levels also differed significantly between the two groups (p < 0.01). However, no significant difference was observed in white blood cell count, neutrophil percentage, hemoglobin, platelets, erythrocyte sedimentation rate, and the levels of immunoglobulin A, immunoglobulin G (IgG), immunoglobulin M, alanine transaminase (ALT), aspartate transaminase (AST), and creatine kinase isoenzymes (CK-MB). Patients in the complication group were more likely to receive oxygen therapy, glucocorticoids, and bronchoscopy as compared with those in the non-complication group (p < 0.01). Meanwhile, eight patients required ICU treatment, and one required mechanical ventilation. Except for one child who died, the rest of the children recovered and were discharged from the hospital.
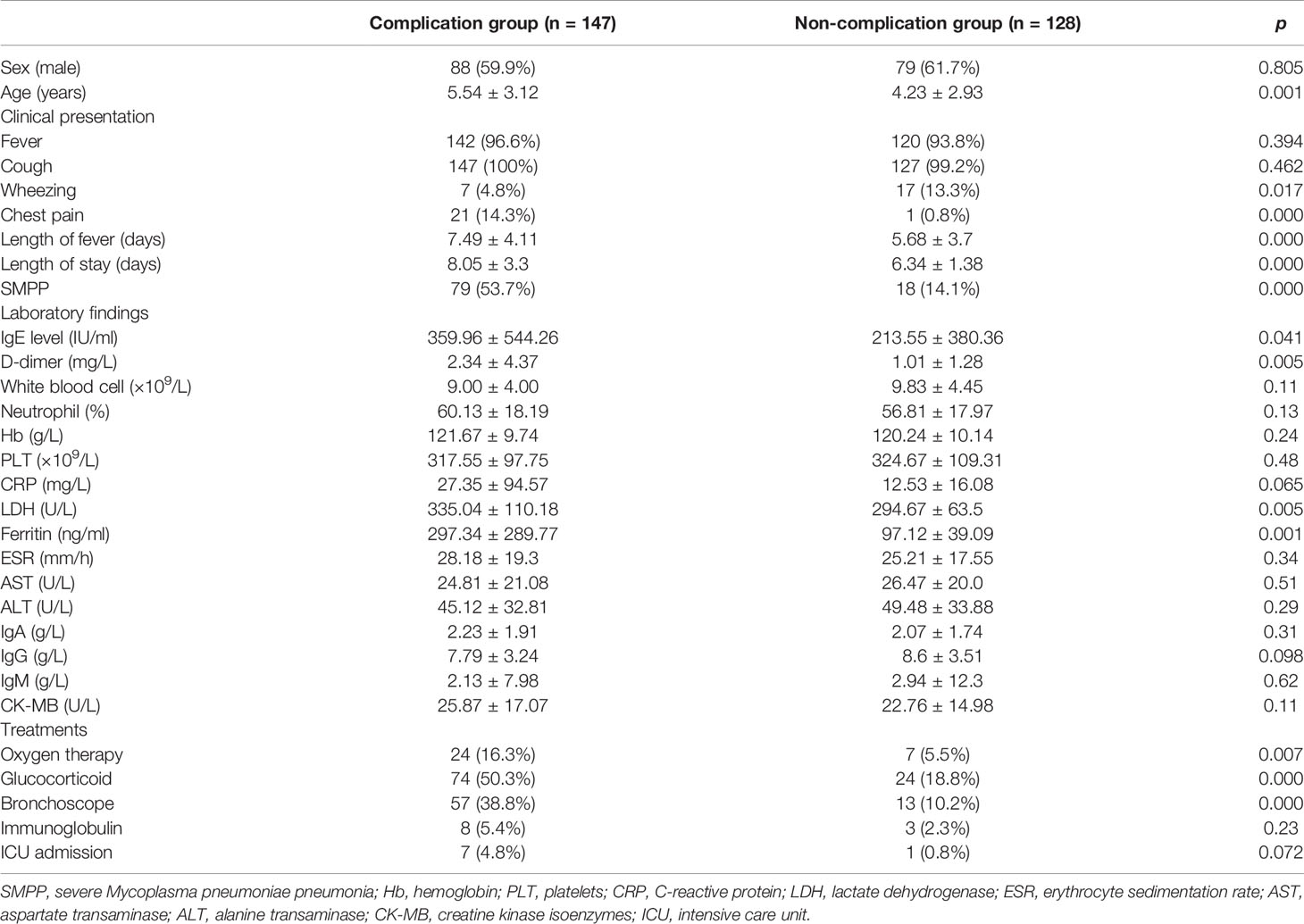
Table 3 The demographics, clinical characteristics, laboratory findings, and treatments of children in the complication group and the non-complication group.
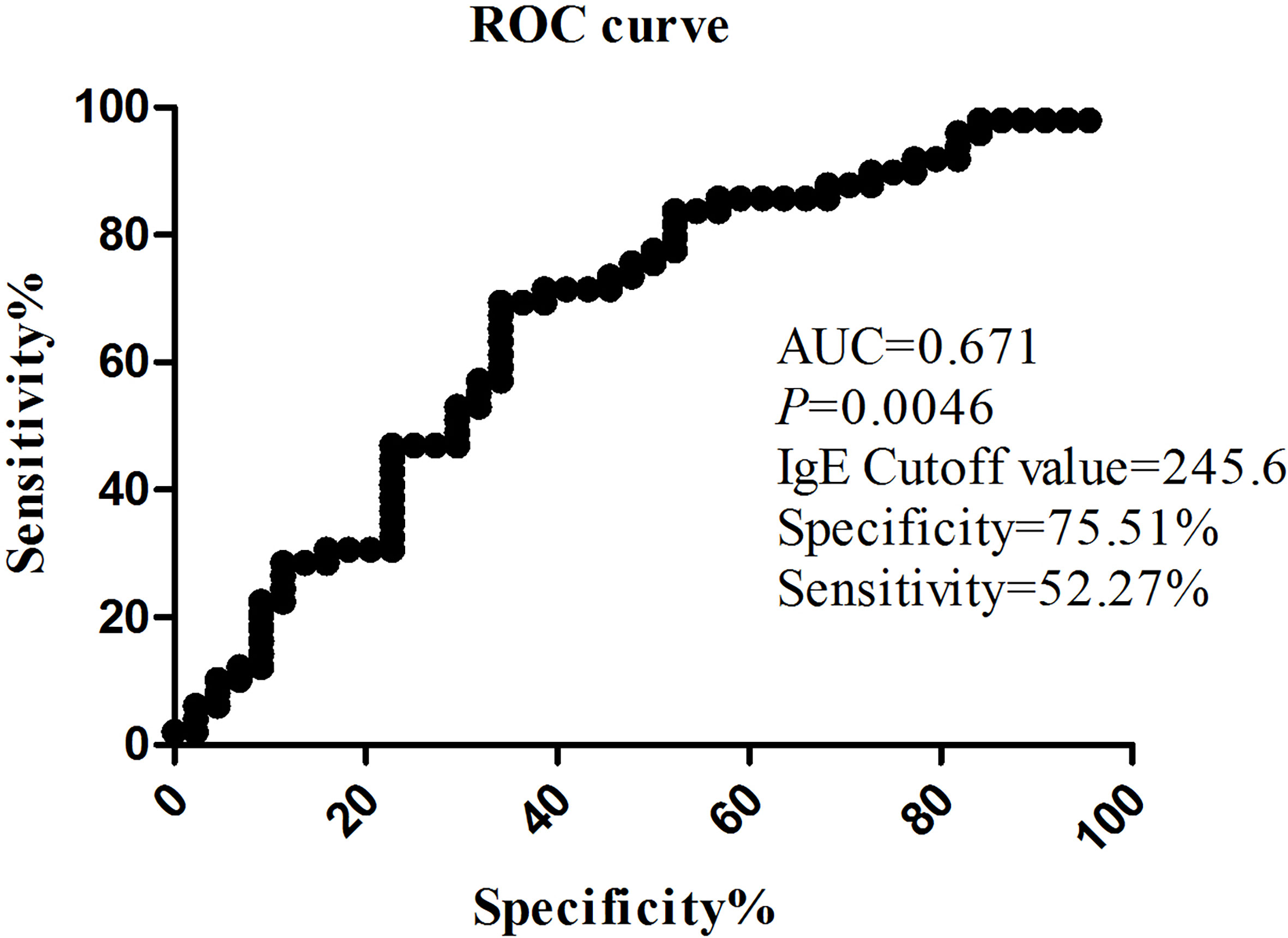
Figure 3 The value on the receiver operating characteristic (ROC) curve of the IgE level between the complication group and the non-complication group.
There were some patients with abnormally increased IgE (cases with IgE above 1,000 IU/ml) in our study. The information of these 14 patients is listed in Table 5. These patients were all acutely infected, mainly manifesting with fever and cough. There were four children younger than 3 years: two of them had wheezing, and one had complication of bronchiolitis obliterans. Two of the 14 children with elevated IgE had no complication. It was found that the older the patients, the more likely to have complications. Pulmonary complications were more common in patients with high IgE, and prognosis was good after active treatment. But unfortunately, of these 14 patients, one died due to multiple complications.
The frequency of pulmonary and extrapulmonary symptoms associated with MP infection has increased in recent decades. Few studies reported the incidence of various complications caused by MP. Most studies were in the form of case reports or reviews. The incidence of pulmonary complications in this study was 34.5%, which was slightly higher than that reported in the literature. Xu reported that the incidence of pulmonary complications in their study was 20.65% (Xu et al., 2021). The extrapulmonary complication rate in this study was 18.9%, which was a little lower than reported. Previous studies found that the incidence of extrapulmonary injury during MP infection could reach 25%–50% (Gong et al., 2021). Zheng reported that the incidence of extrapulmonary complication was 33.4% (Zheng et al., 2021). The most common extrapulmonary complication in our study was hypokalemia. However, few people pay attention to hypokalemia caused by MP. We found that 7.64% (21 in 275) patients had hypokalemia. Han reported that 15.7% (77 in 489) patients had hypokalemia, which was associated with SMPP, fever duration, and hospitalization duration (Han et al., 2021). We found that 4.4% (12 in 275) patients had skin rashes. MP infection could easily cause skin rashes, as described in previous reports (Waites and Talkington, 2004; Narita, 2010). And three patients suffered from reactive arthritis in this study, which indicated that MP could induce arthritis in some people. This was in contrast to previous reports (Shokrollahi et al., 2017; Mărginean et al., 2021). Another study showed that increased total serum IgE was associated with reactive arthritis in patients with MP infection (Poddighe et al., 2021). Encephalitis, which affected children, was the most frequent neurological complication of MP (Arighi et al., 2018). Four children suffered from encephalitis in this study. Qing reported that 18.6% (11 in 59) patients had abnormal ALT concentrations (>40 U/L) and that 55.9% (33 in 59) patients had abnormal CK-MB concentrations (>28 U/L) (Fan et al., 2015). In this study, 2.18% (six in 275) patients had myocarditis, and 2.18% (six in 275) patients had hepatitis. The incidence of myocarditis and hepatitis was much lower than previously reported, maybe because we defined abnormal elevation of ALT and CK-MB to be three times higher than normal. Also, our sample size was larger than that of previous reports.
Few studies reported both pulmonary and extrapulmonary complications, and even fewer reported on the role of IgE levels in the occurrence of complications in MPP. We found no statistically significant difference in IgE levels between pulmonary and extrapulmonary complications. However, Poddighe reported that the serum IgE level of hospitalized children with MP-related extrapulmonary diseases was significantly higher than that of children with only respiratory diseases (Poddighe et al., 2018). In this study, the serum IgE levels were relatively high in patients with MPP having complications. As far as we know, the current studies on IgE were all related to allergies, and few of them focused on the significance of elevated IgE in patients with MPP having complications. MP induced allergy by producing P1-specific IgE; therefore, MP was not only a source of infection but also an allergen for some people (Ye et al., 2018). The persistent MP antigen stimulation and/or invasion greatly increased the possibilities of severe lung lesions and pulmonary and extrapulmonary complications (Zhou et al., 2014). As an antigen, MP can produce specific antibodies in the body; it has common antigens with the body’s heart, lungs, brain, and so forth. It can produce auto-antibodies. The antigen and antibodies form complexes and then activate complement and immune cells to exert powerful immune and autoimmune responses (Waites and Talkington, 2004). It was speculated that the tissue damage and systemic inflammation in children with MPP having an increased IgE level were more severe, and therefore, the fever course was longer, and pulmonary and extrapulmonary complications were more common.
So far, the pathogenesis of MP was still not clear. It was mainly related to the theory of immunological pathogenesis, adsorption of respiratory epithelial cells, and direct invasion of MP (Meyer et al., 2014). The current theory that excessive immune response led to the progress of MPP was generally accepted (Yan et al., 2019). The humoral immunity and cellular immune dysfunction played an important role in the pathogenesis of MP, which could cause serious complications of multiple systems outside the lungs and could prolong the course of the disease (Shi et al., 2019). Investigations on IgE production are important to understand the cellular mechanisms leading to increased IgE levels in patients with MPP (Ye et al., 2014). Abnormally elevated IgE levels were considered to be one of the markers of immune imbalance (Magen et al., 2014; Poddighe and Marseglia, 2016). Thus, we speculated that IgE might be a biomarker for complications after MP infection (Gu et al., 2020).
This study had several limitations. First, this was a retrospective study with some inevitable selection biases. Second, the sample size was limited, with single-center data. Third, large-sample prospective studies needed to be designed to verify the reliability of this retrospective study in the future. In summary, patients with MPP having higher IgE levels had more severe clinical symptoms and complications. We speculated that IgE might be a biomarker for complications after MP infection. This study provided a reliable theoretical basis for early identification and intervention of MPP with elevated IgE levels. Paying attention to the increase in the total serum IgE level in children with MPP can reduce the occurrence of various pulmonary and extrapulmonary complications.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics and Research Council of Women and Children’s Hospital of Ganzhou (201905-A01). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individuals, and minors’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
LZ contributed to the concept and assisted in the critical writing. YL contributed to design of the study. ZX and XP helped in analyzing and interpreting the data. XG and LY contributed to the collection of clinical information. All authors contributed to the article and approved the submitted version.
The project was supported by grants from Bethune Foundation (SCE041BS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all colleagues in the Department of Respiratory Medicine for collecting the clinical data. We also thank all the families for their participation in this study.
MP, Mycoplasma pneumoniae; MPP, Mycoplasma pneumoniae pneumonia; SMPP, severe Mycoplasma pneumoniae pneumonia; IgE, immunoglobulin E; ICU, intensive care unit; Hb, hemoglobin; PLT, platelets; ESR, erythrocyte sedimentation rate; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; ALT, alanine transaminase; AST, aspartate transaminase; CK-MB, creatine kinase isoenzymes; ROC, receiver operating characteristic.
Arighi, P., Deregibus, M. I., Agrimbau Vázquez, J., Amoedo, D., Buchovsky, A., Costa, M., et al. (2018). Mycoplasma Pneumoniae: Neurologic Manifestations and Diagnostic Controversies. A Case Report. Archivos Argentinos Pediatria 116, e590–ee93. doi: 10.5546/aap.2018.e590
Fan, Q., Meng, J., Li, P., Liu, Z., Sun, Y., Yan, P. (2015). Pathogenesis and Association of Mycoplasma Pneumoniae Infection With Cardiac and Hepatic Damage. Microbiol. Immunol. 59, 375–380. doi: 10.1111/1348-0421.12267
Gong, H., Sun, B., Chen, Y., Chen, H. (2021). The Risk Factors of Children Acquiring Refractory Mycoplasma Pneumoniae Pneumonia: A Meta-Analysis. Medicine 100, e24894. doi: 10.1097/MD.0000000000024894
Gu, H., Zhu, Y., Zhou, Y., Huang, T., Zhang, S., Zhao, D., et al. (2020). Mycoplasma Pneumoniaelncrna MALAT1 Affects Pneumonia via NF-κb Regulation. Front. Cell Dev. Biol. 8, 563693. doi: 10.3389/fcell.2020.563693
Han, Z., Zhang, Y., Liao, S., Zhou, N. A. (2021). Mycoplasma Pneumoniaethe Clinical Characteristics of Pneumonia and its Relationship Between Hypokalemia in West China. Transl. Pediatr. 10 (2), 406–414. doi: 10.21037/tp-20-471
Magen, E., Schlesinger, M., David, M., Ben-Zion, I., Vardy, D. (2014). Selective IgE Deficiency, Immune Dysregulation, and Autoimmunity. Allergy Asthma Proc. 35, e27–e33. doi: 10.2500/aap.2014.35.3734
Mărginean, C. O., Georgescu, A. M., Meliţ, L. E. (2021). Arthritis Associated With Mycoplasma Pneumoniae in a Pediatric Patient: A Case Report. Medicine 100, e24316. doi: 10.1097/MD.0000000000024316
Medjo, B., Atanaskovic-Markovic, M., Radic, S., Nikolic, D., Lukac, M., Djukic, S. (2014). Mycoplasma Pneumoniae as a Causative Agent of Community-Acquired Pneumonia in Children: Clinical Features and Laboratory Diagnosis. Ital. J. Pediatr. 40, 104. doi: 10.1186/s13052-014-0104-4
Meyer, S. P. M., van Rossum, A. M., Vink, C. (2014). Mycoplasma Pneumoniae in Children: Carriage, Pathogenesis, and Antibiotic Resistance. Curr. Opin. Infect. Dis. 27, 220–227. doi: 10.1097/QCO.0000000000000063
Narita, M. (2010). Pathogenesis of Extrapulmonary Manifestations of Mycoplasma Pneumoniae Infection With Special Reference to Pneumonia. J. Infection Chemother. 16, 162–169. doi: 10.1007/s10156-010-0044-X
Poddighe, D. (2018). Extra-Pulmonary Diseases Related to Mycoplasma Pneumoniae in Children: Recent Insights Into the Pathogenesis. Curr. Opin. Rheumatol. 30, 380–387. doi: 10.1097/BOR.0000000000000494
Poddighe, D., Abdukhakimova, D., Dossybayeva, K., Mukusheva, Z., Assylbekova, M., Rakhimzhanova, M., et al. (2021). Mycoplasma Pneumoniae Seroprevalence and Total IgE Levels in Patients With Juvenile Idiopathic Arthritis. J. Immunol. Res. 2021, 6596596. doi: 10.1155/2021/6596596
Poddighe, D., Comi, E. V., Brambilla, I., Licari, A., Bruni, P., Marseglia, G. L. (2018). Increased Total Serum Immunoglobulin E in Children Developing Mycoplasma Pneumoniae-Related Extra-Pulmonary Diseases. Iranian J. Allergy Asthma Immunol. 17, 490–496. doi: 10.18502/ijaai.v17i5.307
Poddighe, D., Marseglia, G. L. (2016). Is There Any Relationship Between Extra-Pulmonary Manifestations of Mycoplasma Pneumoniae Infection and Atopy/Respiratory Allergy in Children? Pediatr. Rep. 8 (1), 6395.4–5. doi: 10.4081/pr.2016.6395
Shah, P. R., Williams, A. M., Pihlblad, M. S., Nischal, K. K. (2019). Ophthalmic Manifestations of Mycoplasma-Induced Rash and Mucositis. Cornea 38, 1305–1308. doi: 10.1097/ICO.0000000000001985
Shi, S., Zhang, X., Zhou, Y., Tang, H., Zhao, D., Liu, F. (2019). Immunosuppression Reduces Lung Injury Caused by Mycoplasma Pneumoniae Infection. Sci. Rep. 9, 7147. doi: 10.1038/s41598-019-43451-9
Shokrollahi, M. R., Taj Farideh, E., Noorbakhsh, S., Zarabi, V., Ashouri, S., Javadinia, S. (2017). Detection of the M. Pneumonia in Synovial Fluid of Children With Negative Culture Arthritis: A Cross Sectional Study in Tehran, Iran. Infect. Disord. Drug Targets 17 (1), 52–58. doi: 10.2174/1871526517666161121151544
Waites, K. B., Talkington, D. F. (2004). Mycoplasma Pneumoniae and its Role as a Human Pathogen. Clin. Microbiol. Rev. 17, 697–728. doi: 10.1128/CMR.17.4.697-728.2004
Xu, C., Deng, H., Zhang, J., Zhu, Y., Rong, Q., Quan, Y., et al. (2021). Mutations in Domain V of Mycoplasma Pneumoniae 23S rRNA and Clinical Characteristics of Pediatric M. Pneumoniae Pneumonia in Nanjing, China. J. Int. Med. Res. 49 (6). doi: 10.1177/03000605211016376
Yan, C., Xue, G., Zhao, H., Feng, Y., Li, S., Cui, J., et al. (2019). Molecular and Clinical Characteristics of Severe Mycoplasma Pneumoniae Pneumonia in Children. Pediatr. Pulmonol. 54, 1012–1021. doi: 10.1002/ppul.24327
Ye, Q., Mao, J. H., Shu, Q., Shang, S. Q. (2018). Mycoplasma Pneumoniae Induces Allergy by Producing P1-Specific Immunoglobulin E. Ann. Allergy Asthma Immunol. 121, 90–97. doi: 10.1016/j.anai.2018.03.014
Ye, Q., Xu, X. J., Shao, W. X., Pan, Y. X., Chen, X. J. (2014). Mycoplasma Pneumoniae Infection in Children is a Risk Factor for Developing Allergic Diseases. TheScientificWorldJournal 2014, 986527. doi: 2014.10.1155/2014/986527
Zheng, Y., Hua, L., Zhao, Q., Li, M., Huang, M., Zhou, Y., et al. (2021). Mycoplasma Pneumoniaethe Level of D-Dimer Is Positively Correlated With the Severity of Pneumonia in Children. Front. Cell. Infection Microbiol. 11, 687391. doi: 10.3389/fcimb.2021.687391
Zhou, Y., Hu, M., Ye, B., Chen, Z., Zhang, Y. (2020). Early Prediction of Necrotizing Pneumonia From Mycoplasma Pneumoniae Pneumonia With Large Pulmonary Lesions in Children. Sci. Rep. 10, 19061. doi: 10.1038/s41598-020-76083-5
Keywords: Children, Immunoglobulin E, Mycoplasma pneumoniae, Severe Mycoplasma pneumoniae pneumonia, complications
Citation: Zhou L, Li Y, Xu Z, Peng X, Gong X and Yang L (2021) Increased Total Serum Immunoglobulin E Is Likely to Cause Complications of Mycoplasma pneumoniae Pneumonia in Children. Front. Cell. Infect. Microbiol. 11:783635. doi: 10.3389/fcimb.2021.783635
Received: 26 September 2021; Accepted: 05 November 2021;
Published: 24 November 2021.
Edited by:
Max Maurin, Université Grenoble Alpes, FranceReviewed by:
Dimitri Poddighe, Nazarbayev University School of Medicine, KazakhstanCopyright © 2021 Zhou, Li, Xu, Peng, Gong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Zhou, emhvdTE5ODcxMTdAeWVhaC5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.