
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 21 January 2022
Sec. Microbiome in Health and Disease
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.769950
This article is part of the Research Topic The Female Reproductive Tract Microbiome - Gatekeeper for Sexual and Reproductive Health View all 20 articles
 Yonah Krakowsky1,2
Yonah Krakowsky1,2 Emery Potter2
Emery Potter2 Jason Hallarn3
Jason Hallarn3 Bern Monari4
Bern Monari4 Hannah Wilcox5
Hannah Wilcox5 Greta Bauer3
Greta Bauer3 Jacques Ravel6,7†
Jacques Ravel6,7† Jessica L. Prodger3,5*†
Jessica L. Prodger3,5*†Transgender and gender diverse individuals may seek gender-affirming medical care, such as hormone therapy or surgery, to produce primary and/or secondary sex characteristics that are more congruent with their gender. Gender-affirming medical care for transmasculine individuals can include testosterone therapy, which suppresses circulating estrogen and can lead to changes in the vaginal epithelium that are reminiscent of the post-menopausal period in cisgender females. Among transfeminine individuals, gender-affirming medical care can include vaginoplasty, which is the surgical creation of a vulva and neovaginal canal, commonly using penile and scrotal skin. The effect of gender-affirming medical care on the vagina of transmasculine individuals and on the neovagina of transfeminine individuals is poorly characterized. This review summarizes what is known of the epithelium and local microbiota of the testosterone-exposed vagina and the neovagina. We focus on potential pathogens and determinants of gynecological health and identify key knowledge gaps for future research.
Transgender and gender diverse (TGD) individuals have a gender identity that is incongruent with the sex/gender they were assigned at birth. Other key terminology used in this review is listed in Table 1. It is estimated that 0.2-0.5% of the North American adult population is TGD (Conron et al., 2012; Scheim and Bauer, 2015; Helman et al., 2016; Meerwijk and Sevelius, 2017; Zucker, 2017; Jaffray, 2020). Many TGD individuals seek gender-affirming medical care, such as hormone therapy or surgery, to produce primary and/or secondary sex characteristics that are more congruent with their gender. Gender-affirming medical care can be a critical and life-saving step for many: a meta-analysis of 28 studies reported significant improvements in gender dysphoria (80% of individuals), psychological symptoms (78%), quality of life (80%), and sexual function (72%) for those who underwent gender-affirming medical care with hormones and/or surgery (Murad et al., 2010).
Although not all TGD individuals identify as a binary gender (either a man or woman), gender-affirming medical care, including exogenous sex hormones, hormone blockers and/or surgeries, is sometimes used by TGD people to either masculinize or feminize the body. In this respect, we use the terms transfeminine (tF) for individuals assigned male at birth but who do not identify as male and may undergo feminizing gender-affirming medical care, and transmasculine (tM) for individuals who were assigned female at birth but do not identify as female and may undergo masculinizing gender-affirming care. Likewise, we use the terms cis female (cF) for individuals who were assigned female at birth and identify as female and the term cis male (cM) for individuals who were assigned male at birth and identify as male.
Hormone therapy is a common component of gender-affirming medical care for TGD individuals. The 2015 US Transgender Survey (USTS) reported that 49% of TGD individuals have received hormone therapy and a further 29% desired it (James et al., 2019). Feminizing hormone therapy usually consists of testosterone suppression, estrogen (estradiol) and occasionally progestin; these promote the development of secondary sex characteristics such as breasts, body fat redistribution, and softening of the skin, among others (T'Sjoen et al., 2019). Masculinizing hormone therapy usually consists of testosterone, which promotes secondary sex characteristics such as suppression of menstrual cycles, voice deepening, facial and body hair growth, body fat redistribution, and clitoral enlargement (T'Sjoen et al., 2019).
Genital surgery may also be a component of gender-affirming medical care. The 2015 USTS reports that 10% of tF individuals had completed vaginoplasty (the surgical creation of a neovaginal cavity) and a further 45% of respondents reported wanting to have the procedure in the future (James et al., 2019). Vaginectomy and other masculinizing gender-affirming genital surgeries are rarer, but hysterectomy is relatively common. The USTS reported 8% of tM respondents had undergone hysterectomy with a further 44% desiring to have this surgery, but only 2% had undergone metoidioplasty and/or phalloplasty (James et al., 2019). In Ontario, Canada, 2009-2010 data estimate even higher proportions, with 15% of tF Ontarians having completed vaginoplasty and 13% of tM Ontarians having undergone hysterectomy (Scheim and Bauer, 2015).
We will employ the commonly used term “neovagina” to refer to vaginas that are surgically created by vaginoplasty, and “vagina” to refer to vaginas that were present at birth. Furthermore, we will refer to the present-at-birth vaginas of those taking testosterone therapy (tM individuals) as testosterone dominant vaginas (TDV) and vaginas of reproductive-aged individuals who are not taking testosterone therapy (including both cF and TGD individuals not on testosterone therapy) as estrogen dominant vaginas (EDV). The field of transgender medicine is relatively new, and little is known of the effects of testosterone therapy on the TDV nor of estrogen therapy on the neovagina, but it is clear that both genital microenvironments are distinct from the comparatively better studied EDV. As social acceptance increases and access to gender-affirming medical care continues to improve, the number of TGD individuals who will need tailored gynecological care is increasing. The provision of inclusive healthcare is necessary to achieve optimal health and reduce inequities experienced by TGD communities (American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and American College of Obstetricians and Gynecologists’ Committee on Health Care for Underserved Women, 2021).
This review summarizes what is known of the epithelium and local microbiota of the TDV and the neovagina. We focus on potential pathogens and determinants of gynecological health and identify key knowledge gaps. Our review raises more questions than it provides answers, underscoring an urgent need for research on these distinct genital microenvironments.
The vaginal mucosa is a stratified squamous epithelium that undergoes continuous renewal through proliferation of basal cells, and thus newly formed epithelial cells are pushed outward towards the lumen by the subsequent cell generations. As basal cells lose contact with the basement membrane, they begin to differentiate, expressing cytokeratins K4/K13 and K1/K10 (which form intermediate filaments, and whose expression is organ-specific), to eventually reach full maturation in the superficial layers (Waseem et al., 1998). Maturation of vaginal epithelial cells is regulated by estrogen, which promotes epithelial cell proliferation and thus increases thickness of the epithelium (Ayehunie et al., 2015). Estrogen also promotes the production of glycogen, a glucose polysaccharide, by vaginal epithelial cells (Cruickshank, 1934; Anderson et al., 2014). Cell-cell junctions are lost during cellular maturation and the loosely connected, glycogen-rich cells of the superficial layer are readily shed into the vaginal lumen. Glycogen from shed epithelial cells is catabolized by both human and bacterial α-amylases in the vaginal lumen to smaller polymers that are a preferred carbon source of beneficial Lactobacillus spp., but also of non-desirable anaerobic bacteria (Mirmonsef et al., 2014; Spear et al., 2014; van der Veer et al., 2019; Nunn et al., 2020). Lactobacilli then metabolize glycogen-derived polymers into lactic acid, which reduces the pH of the vaginal lumen, favoring the proliferation of lactobacilli and inhibiting the growth of pathogenic organisms such as Neisseria gonorrhoeae, Chlamydia trachomatis and those associated with bacterial vaginosis (BV) (Graver and Wade, 2011; O'Hanlon et al., 2011; O'Hanlon et al., 2013; Gong et al., 2014; Mirmonsef et al., 2014; Breshears et al., 2015; Mirmonsef et al., 2016; Nardini et al., 2016; Edwards et al., 2019). In addition to reducing the pH, Lactobacillus spp. also prevent colonization by pathogens through the production of bacteriocins and biosurfactants (Valore et al., 2002). In the absence of Lactobacillus spp., the EDV is colonized by a diverse set of strict and facultative gram-positive and gram-negative anaerobes (e.g., Atopobium, Prevotella, Gardnerella) (Ravel et al., 2013; France et al., 2020; Holm et al., 2020; Turpin et al., 2021). Communities dominated by diverse anaerobes are reminiscent of BV, which is characterized by abnormal discharge, itching, malodour, and an elevated pH (Eschenbach et al., 1988; Schwebke et al., 1996; Redelinghuys et al., 2020). Even in the absence of symptoms, vaginal microbiomes deficient in lactobacilli (“molecular BV”) are associated with impaired epithelial maturation, increased mucosal inflammation, changes in epithelial barrier function, increased susceptibility to sexually transmitted infections (chlamydia, gonorrhea, HIV and HPV, among others), and reproductive risks (Leitich et al., 2003; Wiesenfeld et al., 2003; Brotman et al., 2010; Arnold et al., 2016; Zevin et al., 2016; Joag et al., 2019; Tamarelle et al., 2019; O'Hanlon et al., 2020).
Testosterone therapy is highly effective in allowing TGD individuals to develop the secondary sex characteristics associated with masculinity, but the suppression of estrogen (Table 2) can induce epithelial thinning reminiscent of the estrogen-deprived post-menopausal cF vagina (Baldassarre et al., 2013).

Table 2 Expected Serum Hormone Ranges of Populations of Interest (Leinung et al., 2018; Greene et al., 2019; Greene et al., 2020; M. C. Laboratories, 2021).
In the absence of estrogen, vaginal epithelial cell proliferation slows, and the epithelium becomes thinner and more fragile, leading to dryness, irritation, and dyspareunia (pain during intercourse) (Pessina et al., 2006; Perrone et al., 2009; Baldassarre et al., 2013). Decreased estrogen in the post-menopausal period is also associated with reduced glycogen deposition and, combined with reduced epithelial proliferation and turnover, results in a marked reduction in the availability of free glycogen in the mucosa (Mirmonsef et al., 2015). Potentially due to reduced glycogen availability, the vaginal microbiome post-menopause is significantly less likely to be dominated by lactobacilli, and instead is more likely to be colonized with a unique, diverse microbiota. This microbiota has some overlap with molecular BV in the EDV (e.g., Prevotella, Gardnerella, Dialister), but is clearly distinct, with higher abundance and prevalence of genera such as Streptococcus, Corynebacterium, Finegoldia, Peptoniphilus, Anaerococcus, and Bifidobacterium and lower abundance of BV-associated genera Atopobium, Sneathia, and Megasphaera (Brotman et al., 2014; Mirmonsef et al., 2014; Shen et al., 2016; France et al., 2020). The importance of estrogen in shaping the vaginal microbiome is underscored by the effects of local or systemic estradiol-based hormone replacement therapy post-menopause, which restores lactobacilli dominance, decreases vaginal pH, and alleviates symptoms of vaginal fragility (Brotman et al., 2014; Shen et al., 2016). In TDV, testosterone therapy has been shown to thin the epithelium, with histological evaluation revealing lowered cell proliferation, loss of the intermediate and superficial strata, and reduced glycogen deposition compared to pre-menopausal EDV (Baldassarre et al., 2013). Recently published data indicates that TDV tissue has elevated levels of inflammation, edema, collagen fibrosis, and granulation tissue (Schardein et al., 2021). Transmasculine individuals on testosterone therapy frequently experience symptoms of vaginal atrophy similar to those of the post-menopausal state, including dryness, irritation, bleeding with vaginal penetration (sex or medical examination), and dyspareunia (Peitzmeier et al., 2014; Potter et al., 2015). These symptoms can have a substantial impact on quality of life, and as such some tM individuals opt for topical estriol or estradiol administered directly to the vaginal mucosa via cream, a ring, or tablets (Santen, 2015). While local estrogen-based therapy to treat vaginal atrophy is included in multiple trans care guidelines, the efficacy of this approach has not been documented in tM (Deutsch, 2016; Obedin-Maliver and de Haan, 2017; Bourns, 2020).
To our knowledge, there has been a single study describing the TDV microbiome (Winston McPherson et al., 2019). In this study, 16S rRNA gene sequencing was used to identify the proportional abundances of bacterial species in TDV swabs collected from 28 tM individuals on testosterone therapy. Only 3/28 TDV had a Lactobacillus-dominated microbiota; instead, the majority of TDVs had microbiota composed of a diverse set of anaerobic taxa, more like microbiota observed in post-menopause cF than molecular BV (i.e., containing Anaerococcus, Corynebacterium, Finegoldia, Peptoniphilus, Streptococcus, in addition to Prevotella, Dialister, Gardnerella, but with low abundance of Atopobium, Sneathia, Megasphaera). Despite sharing some similarities with the post-menopausal vagina, the microbiota observed in the TDV was also clearly distinct, with higher abundance of Campylobacter, Fusobacterium, Parvimonas, and Porphyromonas, indicating testosterone augmentation may influence the composition of the vaginal microbiota beyond that of estrogen reduction. It is notable that, of the three individuals who had a Lactobacillus-dominated microbiota, two were prescribed topical estradiol (a total of 4 individuals in the study had been prescribed topical estradiol to treat symptoms of vaginal atrophy). Despite limited statistical power in this relatively small study, the correlation between vaginal estrogen therapy and presence of a Lactobacillus-dominated microbiota was statistically significant (p=0.045). This study provides an important first assessment of the TDV microbiota, suggesting it is distinct from the microbiota observed post-menopause, and that Lactobacillus-dominated microbiota are rare.
Despite the progress made in recent years, several key gaps remain in the literature. Additional studies are warranted to confirm and expand on the seminal publication by McPherson et al. (Winston McPherson et al., 2019). Larger longitudinal studies, including following TGD participants through the initiation of testosterone therapy and studies of individuals who have been on testosterone for decades, would provide detailed information on the specific effects of testosterone therapy. Additionally, many TGD individuals interrupt testosterone therapy to become pregnant (Obedin-Maliver and Makadon, 2016). Molecular BV in cF individuals is associated with higher risk of serious reproductive risks (Leitich et al., 2003); it is unknown how the unique microbiota of the TDV may influence reproductive outcomes. Finally, to inform appropriate clinical treatment guidelines for the medical care of TGD individuals, new studies should focus on relating microbiota composition and function to symptomology, immune status, and local energy sources available to microbes.
Another important knowledge gap is whether locally administered vaginal estrogen therapy could be used to treat vaginal atrophy and promote Lactobacillus colonization in tM individuals without interfering with the masculinizing effects of testosterone. Local estrogen therapy is commonly recommended to treat vaginal atrophy post-menopause (Kaur et al., 2020; Shim et al., 2021), and, in low doses, this therapy can alleviate symptoms without substantially increasing systemic estrogen levels (Lethaby et al., 2016). The effect of local estrogen therapy on systemic levels will likely be dependent on characteristics of the vaginal microenvironment [reviewed in (Santen, 2015)], including vaginal epithelial thickness and the local microbiome. Given the potential benefits, research is warranted to assess the acceptability and efficacy of locally administered vaginal estrogen therapy in TGD individuals on testosterone.
Third, our ability to study the effect of testosterone therapy on vaginal and cervical epithelia have been hampered by lack of appropriate model systems. Monolayers of cells in submerged culture do not replicate the stratified epithelium of the vagina and do not provide an appropriate environment for the culture of vaginal bacteria, while animal models do not replicate the relationship between the human vagina and its unique Lactobacillus-dominated microbiota (Couri et al., 2012; Barfod et al., 2013; Cassone and Sobel, 2016). Three-dimensional air-liquid interface cell culture allows for stratification of cultured vaginal epithelial cells and provides a more relevant environment for vaginal bacteria (Lee et al., 2016; Zhu et al., 2017). The utility of this model in delineating the impact of testosterone therapy on the cervicovaginal epithelium warrants further investigation.
Penile inversion vaginoplasty is the gold standard surgical technique of feminizing genital surgery (Bizic et al., 2014; Horbach et al., 2015; Buncamper et al., 2016; Dreher et al., 2018; Bustos et al., 2021; Moises da Silva et al., 2021). This surgery was first introduced in the early 1900’s and has undergone various permutations in search of the optimal outcome (Horbach et al., 2015). The ideal outcome of this surgery is a concordant vulvar anatomy, moist and hairless vagina with sufficient depth and width for types of penetration desired (if any), erogenous sensation, and requiring minimal maintenance (Garcia et al., 2020). This surgery requires many surgical steps; orchiectomy, clitoroplasty, penile de-gloving and resection of the corpora cavernosa, shortening and splaying of the urethra, surgical dissection of the space between the bladder and the rectum and the inversion of the flap of preserved penile tube skin and placement into this space. Frequently, the penile tube skin alone is insufficient to generate a vaginal canal with adequate depth and additional skin grafts, most commonly from scrotal skin, are used to augment length (Selvaggi et al., 2005; Goddard et al., 2007; Goddard et al., 2007; Dy et al., 2018). Hair from scrotal grafts is typically removed intraoperatively by thinning the scrotal graft and cauterizing visible follicles. The proportion of scrotal/penile skin used to line the canal is influenced by the amount of tissue present and the depth of the pelvic dissection and is not well identified in the literature.
Alternative procedures are used to create sufficient depth in the case that the penile and scrotal skin is insufficient. These techniques are generally recommended for revision surgeries because of their accompanying risks and complications. Bowel pedicle flaps (i.e., sigmoid colon, ileum, and transverse colon), regional and isolated skin flaps (i.e., thigh or lower abdomen), peritoneal flaps, and the incorporation of urethral mucosa into the inverted skin flap are all identified in the literature. Despite the multiple options available to line the neovagina, the penile scrotal flap is by far the most commonly used (Horbach et al., 2015; Buncamper et al., 2016), with the rectosigmoid colon bowel flap as the most common alternative procedure (Horbach et al., 2015).
Understanding the physiology and structure of neovaginal canals made with different tissues is essential, because features of the neovaginal epithelium are very likely to shape microbiome composition and function, and thus are expected to have a substantial impact on gynecological health and quality of life. It is well established that environment (i.e., local levels of oxygen, humidity, and environmental exposures) shapes the local microbiome, unambiguously demonstrated by the effect of penile circumcision on the composition of the coronal sulcus microbiota (Liu et al., 2013). Elimination of the foreskin increases water loss and oxygen tension on the coronal sulcus, which decreases the abundance of many strict anaerobes (including Prevotella, Finegoldia, Peptotreptococcus, Peptoniphilus, Porphyromonas, Dialister, Murdochiella, and Negativococcus) and increases aerobes and facultative anaerobes (e.g., Corynebacterium and Staphylococcus). However, in addition to the environment, characteristics of the epithelium itself can have a dramatic influence on the local microbiome. For example, during puberty estrogen induces changes in the vaginal epithelium, increasing thickness and glycogen content, which is associated with a dramatic shift in the local microbiome, from one dominated by a diverse set of strict and facultative anaerobes to Lactobacillus domination and an acidic pH (<4) (Schaller, 1990).
Similar to the vaginal epithelium, penile skin is a stratified squamous epithelium that is constantly renewing through proliferation of basal cells. However, the epithelial layer of penile skin is thinner than that of the pre-menopausal EDV (100 vs 300μm) (Baldassarre et al., 2013; Carias and Hope, 2019), expresses different cytokeratins (K5/K14 in intermediate layers followed by K1/10 in superficial layers), and has a soft-cornified outer layer (15-20μm thick) comprised of terminally differentiated keratinocytes that have undergone programmed cell death, lack nuclei and organelles, and are filled with keratin bundles (Stankler and Walker, 1976; Dinh et al., 2012). Fully mature skin keratinocytes limit water loss by extruding lamellar bodies to form an intercellular lipid envelope, and provide mechanical integrity through specialized cell junctions called corneodesmosomes. Desquamation of skin corneocytes is controlled by degradation of corneodesmosomes and this process frees keratin and fatty acids that are nutrient sources for bacteria and shape the microbiota (Gupta and Ramnani, 2006; Houben et al., 2008; Bragulla and Homberger, 2009; Grice and Segre, 2011). This contrasts with the outermost layer of the EDV, which has loosely connected superficial cells filled with glycogen, which when shed release glycogen and promote colonization with lactobacilli (Pask et al., 2008; Bragulla and Homberger, 2009; Menon et al., 2012; Anderson et al., 2014; Tjernlund et al., 2015). The sigmoid colon epithelium is also highly distinct from that of the EDV and the penis. It is a single-layer columnar epithelium expressing the cytokeratin pair K8/K18 and containing highly specialized epithelial cells such as goblet cells (producing mucus) and Paneth cells (producing antimicrobial peptides), among others.
Very little is known of the influence of surgical invagination and exogenous estrogen on the differentiation pattern of epithelial cells in the neovagina. It is possible that reduced water loss from surgical invagination may alter the epithelial differentiation patterns of once-penile skin, for example, through reduced lamellar body and corneodesmosome formation, resulting in an epithelial surface more similar to the EDV. However, while vaginal epithelial cells have the ability to differentiate into corneocytes in response to hormonal or mechanical signals, potentially owing to their expression of both K4/K13 (typical of non-cornified stratified epithelia) and K1/K10 (Schaller, 1990; Schaller and Genz, 1990; Schaller et al., 1993; Bragulla and Homberger, 2009), epithelial cells derived from skin and the sigmoid colon contain low levels of glycogen and do not express K4/K13 (Menon et al., 2012). One small study has examined the microstructure of the neovaginal epithelium created from penile skin (n=9) (Dekker et al., 2007). This study observed that cornification was reduced but not lost, and no glycogen production was observed, even among the three participants who had vaginoplasty more than nine years prior and had been receiving estrogen hormone therapy for >11 years. The absence of glycogen and retention of cornification suggest that it would be difficult for the neovagina to support a Lactobacillus-dominated microbiota. While a neovagina constructed from entirely penile skin and one that includes sigmoid colon may have similar environmental exposures (oxygen levels, estrogen levels, etc.), factors such as residual cornification or the presence of goblet cells producing mucus may dictate what bacteria colonize the neovagina microenvironment, and what bacteria are beneficial vs. pathogenic. Therefore, different treatment courses may be required for individuals suffering from neovaginal symptoms, depending on the tissue used to create their neovaginal canal. Altogether, our knowledge of the epithelia used for vaginoplasty does not support the notion that an optimal neovaginal microenvironment would comprise Lactobacillus spp. and an acidic pH.
Despite the enormous impact of the vaginal microbiome on cF sexual and reproductive health, there have been few reports of the microbiota colonizing the neovagina and there is no knowledge of what microbiota are optimal vs. associated with inflammation, symptoms, and STI risk. Until recently, data on the microbiota colonizing the neovagina were limited to case reports and small studies that used limited culture-based detection methods or targeted PCR to detect the presence of specific species of interest (classic STI pathogens or Lactobacillus spp.) (Bodsworth et al., 1994; Haustein, 1995; Weyers et al., 2009; Weyers et al., 2010; de Haseth et al., 2018; Radix et al., 2019). These assays fail to capture the vast majority of species present and provide no information on the composition or structure of bacterial communities in the neovagina. Recently, one study by Birse et al. (2020) used a combination of proteomics and 16S rRNA gene sequencing to examine the neovaginal microbiome in five tF individuals, four of whom had penile inversion vaginoplasty and one whom had sigmoid vaginoplasty (median 10 years post-vaginoplasty, range 4-36 years). While the small sample size limits the ability to draw conclusions, this important study is the first examination of the neovaginal microbiome and hints at interesting hypotheses (Table 3). Of the four tF individuals who underwent penile inversion vaginoplasty, Lactobacillus was detected in one individual at low abundance. Instead, highly prevalent genera in the penile-skin lined neovagina included Prevotella and Peptostreptococcus (both also prevalent in molecular BV of the EDV, the post-menopausal vagina, and the TDV); Peptoniphilus and Corynebacterium (also prevalent in the post-menopausal vagina and the TDV); and Porphyromonas and Campylobacter (also prevalent in the TDV). Interestingly, these genera are also highly abundant/prevalent within the foreskin fold of the uncircumcised penis. The skin under the foreskin fold is usually colonized with a diverse set of strict and facultative anaerobes, the most prevalent and abundant being Prevotella, Porphyromonas, and Peptoniphilus, while the circumcised penis is usually dominated by Corynebacterium (Price et al., 2010; Liu et al., 2013). The abundance of penile anaerobes is associated with inflammation and risk of STIs in uncircumcised heterosexual cM (Liu et al., 2013; Prodger et al., 2021); it remains to be investigated if the same is true in the neovagina. It is interesting to note that, based on one study of the TDV (Winston McPherson et al., 2019) and one small study of the neovagina, that the penile skin lined neovagina appears more similar in microbiota composition to the TDV and the uncircumcised penis than to the EDV or the post-menopausal vagina.
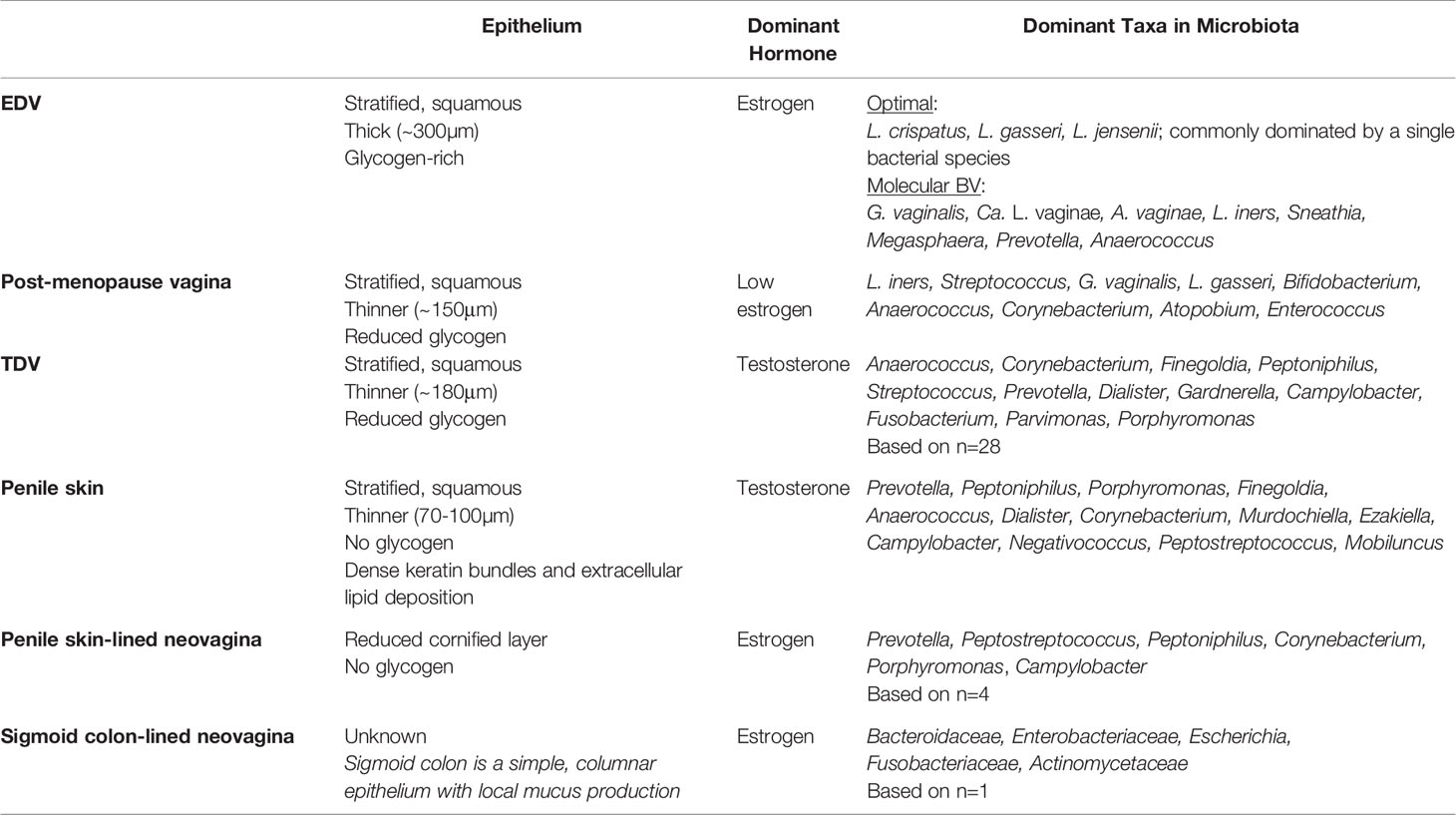
Table 3 Summary of the characteristics of the estrogen dominated vagina (EDV), post-menopause vagina, testosterone dominated vagina (TDV), sub-preputial penile skin, penile skin-lined neovagina, and sigmoid-lined neovagina.
In contrast, the neovaginal microbiome of the only participant who had sigmoid vaginoplasty was clearly distinct, completely lacking Prevotella and instead defined by taxa common in the gut microbiota, Bacteroidaceae and Enterobacteriaceae (Birse et al., 2020). These interesting data suggest that, even many years post-vaginoplasty, the origin of the tissue used defines the colonizing microbiota. It is therefore critically important that all future research of the neovaginal microenvironment consider tissue source, and ideally be powered to afford stratification by tissue source. As a field, researchers and clinicians should be aware that different standards of care and clinical recommendations may be required depending on the tissue used for neovaginal construction.
There are several important knowledge gaps in our understanding of the neovaginal microbiome that urgently need to be filled to improve neovaginal healthcare. Preliminary data from Trans PULSE Canada (n=2,873), a national survey of the TGD population in Canada, show nearly half of participants with vaginoplasty experienced gynecological symptoms in the past year, including malodor, abnormal or disturbing discharge, and itching (personal communication, Trans PULSE Canada). Such symptoms are frequently associated with BV in the EDV; however, the underlying cause of these symptoms in the neovagina remains uncharacterized. Neovaginal swabs sent for clinical diagnostics frequently return the results “altered vaginal flora inconsistent with bacterial vaginosis” and treatments established for the EDV (metronidazole) are frequently ineffective (Jain and Bradbeer, 2007; van der Sluis et al., 2020). Candidiasis is another common cause of vaginal itching in the EDV; there has been one case series published reporting neovaginal candidiasis in five individuals (de Haseth et al., 2018), warranting further characterization of neovaginal candida species and the development of clinical guidelines for prevention and treatment. The cause of neovaginal malodour also warrants further investigation. Malodour in the EDV has been associated with the production of biogenic amines by BV-associated bacteria (McMillan et al., 2015; Puebla-Barragan et al., 2021). Larger studies employing omics approaches, including metagenomics, metatranscriptomics and metabolomics to characterize the neovaginal microenvironment in symptomatic individuals are urgently needed to inform treatment options.
Equally important is defining what constitutes an optimal neovaginal microbiota post penile inversion or sigmoid vaginoplasty. This information is essential to guiding treatment options, as what the treatment leaves untouched may be just as important as what it removes. A portion of metronidazole’s efficacy in treating BV of the EDV is that it selectively spares Lactobacillus, and thus helps to promote an optimal microbiome that is resistant to re-colonization with inflammatory/pathogenic anaerobes (Petrina et al., 2017). While little is known of the microstructure of the penile-skin lined neovaginal epithelium, if it indeed lacks glycogen and retains cornification, it would be unlikely to promote Lactobacillus dominance. A minority of uncircumcised cM [~12% in Uganda (Prodger et al., 2021)] sustain a microbiota under the foreskin fold of the penis that is dominated by Corynebacterium with low abundance of Gram-negative anaerobes. This microbiota is associated with low inflammation and reduced risk of STI acquisition (Prodger et al., 2021); future larger studies will reveal if a similar microbiota is optimal in the penile skin-lined neovagina. Additional information on the microstructure of the neovaginal epithelium post penile inversion or sigmoid vaginoplasty would help to inform what type of bacterial commensals might promote an optimal, low-inflammation, protective neovaginal microenvironment.
There is paucity of data on the kind of practices that promote an optimal neovaginal microenvironment, both in the immediate post-operative period and for long-term hygiene and care. Due to a lack of evidence-based guidelines, neovaginal care recommendations vary substantially between centers (Grimstad et al., 2021). Frequent dilation is necessary post-operatively to prevent stenosis of the neovaginal canal (Goddard et al., 2007; Horbach et al., 2015; Buncamper et al., 2016; Loree et al., 2020); most centers recommend at least two dilations a day for the first six months decreasing to once weekly after a year (Buncamper et al., 2016). Ample water-based lubrication is recommended to increase the ease of the dilations and to protect the integrity of the surgical dilators. Frequently the use of a vaginal douche after dilation is recommended with varied solutions including water, soap, vinegar, or povidone iodine solutions (Goddard et al., 2007; Deutsch, 2016; Pan et al., 2019). Douching and the use of soaps or lubricants can promote molecular BV in the EDV of cF, but their effects on the neovaginal microbiota are unknown. Regular use of hygienic products and even boric acid [to lower the pH, promote colonization with Lactobacillus, and treat vaginal yeast infection (Donders et al., 2010; Iavazzo et al., 2011)] are also frequently reported, however, such efforts would be in vain, and potentially disruptive, if the optimal neovaginal microbiome is found to be dominated by Corynebacterium (penile skin-lined) or Bacteroidaceae (sigmoid-lined).
As access to gender-affirming hormone therapy and surgery increases, a growing number of TGD persons will need access to effective and evidence-informed gynecological care. There is an urgent and growing need to identify the causative agents of the unique gynecological concerns of TGD populations and to define clinical guidelines to promote gynecological health. In tM individuals on testosterone therapy, vaginal pain, bleeding, atrophy, and non-Lactobacillus-dominated vaginal microbiota are common. Further research is warranted to establish the role of testosterone augmentation beyond that of estrogen deprivation. Anecdotal evidence suggests topical estrogen therapy may promote a Lactobacillus-dominated microbiome, justifying further studies to investigate if this approach can alleviate symptoms. In tF individuals with a neovagina, gynecological symptoms such as abnormal discharge, itching and malodor are common, but the etiology of these symptoms remains unknown, and treatments designed for cF may be ineffective. The limited data we have of the neovaginal microbiome (n=5) suggests that it is very unlike that of reproductive-aged or post-menopausal cF and may have more commonalities with the microbiota of the uncircumcised penis of cM or the vagina of tM on testosterone therapy. Importantly, the limited available data suggests the tissue used to create the vaginal canal may have a substantial impact on the subsequent microbiota and should be considered and reported in future research. What defines optimal vs. non-optimal microbiota in different types of neovaginas, and what bacteria are pathogenic, is yet to be defined and this information is critically needed to improve clinical management and treatment options.
JP, JR, GB, YK, EP, HW, JH, and BM contributed to literature review, manuscript writing, and editing. All authors contributed to the article and approved the submitted version.
JP was supported by a Canada Research Chair (Canadian Institutes of Health Research: 950-233211); funds from the Schulich School of Medicine and Dentistry, Western University; and the National Institute for Allergy and Infectious Diseases of the National Institutes of Health (R01AI123002). GB is supported by a Canadian Institutes of Health Research Sex and Gender Science Chair (GSB-171372). JR and BM were supported by the National Institute for Allergy and Infectious Diseases of the National Institutes of Health (R01NR015495)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists’ Committee on Health Care for Underserved Women. (2021). Health Care for Transgender and Gender Diverse Individuals: ACOG Committee Opinion, Number 823 Obstet. Gynecol. 137 (3), e75–e88. doi: 10.1097/AOG.0000000000004294
Anderson, D. J., Marathe, J., Pudney, J. (2014). The Structure of the Human Vaginal Stratum Corneum and its Role in Immune Defense. Am. J. Reprod. Immunol. 71, 618–623. doi: 10.1111/aji.12230
Arnold, K. B., Burgener, A., Birse, K., Romas, L., Dunphy, L. J., Shahabi, K., et al. (2016). Increased Levels of Inflammatory Cytokines in the Female Reproductive Tract are Associated With Altered Expression of Proteases, Mucosal Barrier Proteins, and an Influx of HIV-Susceptible Target Cells. Mucosal Immunol. 9, 194–205. doi: 10.1038/mi.2015.51
Ayehunie, S., Islam, A., Cannon, C., Landry, T., Pudney, J., Klausner, M., et al. (2015). Characterization of a Hormone-Responsive Organotypic Human Vaginal Tissue Model: Morphologic and Immunologic Effects. Reprod. Sci. 22, 980–990. doi: 10.1177/1933719115570906
Baldassarre, M., Giannone, F. A., Foschini, M. P., Battaglia, C., Busacchi, P., Venturoli, S., et al. (2013). Effects of Long-Term High Dose Testosterone Administration on Vaginal Epithelium Structure and Estrogen Receptor-Alpha and -Beta Expression of Young Women. Int. J. Impot. Res. 25, 172–177. doi: 10.1038/ijir.2013.9
Barfod, K. K., Roggenbuck, M., Hansen, L. H., Schjorring, S., Larsen, S. T., Sorensen, S. J., et al. (2013). The Murine Lung Microbiome in Relation to the Intestinal and Vaginal Bacterial Communities. BMC Microbiol. 13, 303. doi: 10.1186/1471-2180-13-303
Birse, K. D., Kratzer, K., Zuend, C. F., Mutch, S., Noel-Romas, L., Lamont, A., et al. (2020). The Neovaginal Microbiome of Transgender Women Post-Gender Reassignment Surgery. Microbiome 8, 61. doi: 10.1186/s40168-020-00804-1
Bizic, M., Kojovic, V., Duisin, D., Stanojevic, D., Vujovic, S., Milosevic, A., et al. (2014). An Overview of Neovaginal Reconstruction Options in Male to Female Transsexuals. ScientificWorldJournal 2014, 638919. doi: 10.1155/2014/638919
Bodsworth, N. J., Price, R., Davies, S. C. (1994). Gonococcal Infection of the Neovagina in a Male-to-Female Transsexual. Sex. Transm. Dis. 21, 211–212. doi: 10.1097/00007435-199407000-00005
Bourns, A. (2020). Guidelines for Gender-Affirming Primary Care With Trans and Non-Binary Patients (Sherbourne Health, Rainbow Health Ontario).
Bragulla, H. H., Homberger, D. G. (2009). Structure and Functions of Keratin Proteins in Simple, Stratified, Keratinized and Cornified Epithelia. J. Anat. 214, 516–559. doi: 10.1111/j.1469-7580.2009.01066.x
Breshears, L. M., Edwards, V. L., Ravel, J., Peterson, M. L. (2015). Lactobacillus Crispatus Inhibits Growth of Gardnerella Vaginalis and Neisseria Gonorrhoeae on a Porcine Vaginal Mucosa Model. BMC Microbiol. 15, 276. doi: 10.1186/s12866-015-0608-0
Brotman, R. M., Klebanoff, M. A., Nansel, T. R., Yu, K. F., Andrews, W. W., Zhang, J., et al. (2010). Bacterial Vaginosis Assessed by Gram Stain and Diminished Colonization Resistance to Incident Gonococcal, Chlamydial, and Trichomonal Genital Infection. J. Infect. Dis. 202, 1907–1915. doi: 10.1086/657320
Brotman, R. M., Shardell, M. D., Gajer, P., Fadrosh, D., Chang, K., Silver, M. I., et al. (2014). Association Between the Vaginal Microbiota, Menopause Status, and Signs of Vulvovaginal Atrophy. Menopause 21, 450–458. doi: 10.1097/GME.0b013e3182a4690b
Buncamper, M. E., van der Sluis, W. B., van der Pas, R. S. D., Ozer, M., Smit, J. M., Witte, B. I., et al. (2016). Surgical Outcome After Penile Inversion Vaginoplasty: A Retrospective Study of 475 Transgender Women. Plast. Reconstr. Surg. 138, 999–1007. doi: 10.1097/PRS.0000000000002684
Bustos, V. P., Bustos, S. S., Mascaro, A., Del Corral, G., Forte, A. J., Ciudad, P., et al. (2021). Regret After Gender-Affirmation Surgery: A Systematic Review and Meta-Analysis of Prevalence. Plast. Reconstr. Surg. Glob. Open 9, e3477. doi: 10.1097/GOX.0000000000003477
Carias, A. M., Hope, T. J. (2019). Barriers of Mucosal Entry of HIV/SIV. Curr. Immunol. Rev. 15, 4–13. doi: 10.2174/1573395514666180604084404
Cassone, A., Sobel, J. D. (2016). Experimental Models of Vaginal Candidiasis and Their Relevance to Human Candidiasis. Infect. Immun. 84, 1255–1261. doi: 10.1128/IAI.01544-15
Conron, K. J., Scott, G., Stowell, G. S., Landers, S. J. (2012). Transgender Health in Massachusetts: Results From a Household Probability Sample of Adults. Am. J. Public Health 102, 118–122. doi: 10.2105/AJPH.2011.300315
Couri, B. M., Lenis, A. T., Borazjani, A., Paraiso, M. F., Damaser, M. S. (2012). Animal Models of Female Pelvic Organ Prolapse: Lessons Learned. Expert Rev. Obstet. Gynecol. 7, 249–260. doi: 10.1586/eog.12.24
Cruickshank, R. (1934). The Conversion of the Glycogen of the Vagina Into Lactic Acid. J. Pathol. Bacteriol. 39, 213–219. doi: 10.1002/path.1700390118
de Haseth, K. B., Buncamper, M. E., Ozer, M., Elfering, L., Smit, J. M., Bouman, M. B., et al. (2018). Symptomatic Neovaginal Candidiasis in Transgender Women After Penile Inversion Vaginoplasty: A Clinical Case Series of Five Consecutive Patients. Transgend. Health 3, 105–108. doi: 10.1089/trgh.2017.0045
Dekker, J. J., Hage, J. J., Karim, R. B., Bloemena, E. (2007). Do Histologic Changes in the Skin-Lined Neovagina of Male-to-Female Transsexuals Really Occur? Ann. Plast. Surg. 59, 546–549. doi: 10.1097/01.sap.0000258953.00234.50
Deutsch, M. B. (2016). Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People (San Francisco: University of California).
Dinh, M. H., Hirbod, T., Kigozi, G., Okocha, E. A., Cianci, G. C., Kong, X., et al. (2012). No Difference in Keratin Thickness Between Inner and Outer Foreskins From Elective Male Circumcisions in Rakai, Uganda. PloS One 7, e41271. doi: 10.1371/journal.pone.0041271
Donders, G. G., Bellen, G., Mendling, W. (2010). Management of Recurrent Vulvo-Vaginal Candidosis as a Chronic Illness. Gynecol. Obstet. Invest. 70, 306–321. doi: 10.1159/000314022
Dreher, P. C., Edwards, D., Hager, S., Dennis, M., Belkoff, A., Mora, J., et al. (2018). Complications of the Neovagina in Male-to-Female Transgender Surgery: A Systematic Review and Meta-Analysis With Discussion of Management. Clin. Anat. 31, 191–199. doi: 10.1002/ca.23001
Dy, G. W., Sun, J., Granieri, M. A., Zhao, L. C. (2018). Reconstructive Management Pearls for the Transgender Patient. Curr. Urol. Rep. 19, 36. doi: 10.1007/s11934-018-0795-y
Edwards, V. L., Smith, S. B., McComb, E. J., Tamarelle, J., Ma, B., Humphrys, M. S., et al. (2019). The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia Trachomatis Infection. mBio 10 (4). doi: 10.1128/mBio.01548-19
Eschenbach, D. A., Hillier, S., Critchlow, C., Stevens, C., DeRouen, T., Holmes, K. K. (1988). Diagnosis and Clinical Manifestations of Bacterial Vaginosis. Am. J. Obstet. Gynecol. 158, 819–828. doi: 10.1016/0002-9378(88)90078-6
France, M. T., Ma, B., Gajer, P., Brown, S., Humphrys, M. S., Holm, J. B., et al. (2020). VALENCIA: A Nearest Centroid Classification Method for Vaginal Microbial Communities Based on Composition. Microbiome 8, 166. doi: 10.1186/s40168-020-00934-6
Garcia, B., Scheib, S., Hallner, B., Thompson, N., Schiavo, J., Peacock, L. (2020). Cosmetic Gynecology-a Systematic Review and Call for Standardized Outcome Measures. Int. Urogynecol. J. 31, 1979–1995. doi: 10.1007/s00192-020-04294-5
Goddard, J. C., Vickery, R. M., Qureshi, A., Summerton, D. J., Khoosal, D., Terry, T. R. (2007). Feminizing Genitoplasty in Adult Transsexuals: Early and Long-Term Surgical Results. BJU Int. 100, 607–613. doi: 10.1111/j.1464-410X.2007.07017.x
Goddard, J. C., Vickery, R. M., Terry, T. R. (2007). Development of Feminizing Genitoplasty for Gender Dysphoria. J. Sex. Med. 4, 981–989. doi: 10.1111/j.1743-6109.2007.00480.x
Gong, Z., Luna, Y., Yu, P., Fan, H. (2014). Lactobacilli Inactivate Chlamydia Trachomatis Through Lactic Acid But Not H2O2. PloS One 9, e107758. doi: 10.1371/journal.pone.0107758
Graver, M. A., Wade, J. J. (2011). The Role of Acidification in the Inhibition of Neisseria Gonorrhoeae by Vaginal Lactobacilli During Anaerobic Growth. Ann. Clin. Microbiol. Antimicrob. 10, 8. doi: 10.1186/1476-0711-10-8
Greene, D. N., McPherson, G. W., Rongitsch, J., Imborek, K. L., Schmidt, R. L., Humble, R. M., et al. (2019). Hematology Reference Intervals for Transgender Adults on Stable Hormone Therapy. Clin. Chim. Acta 492, 84–90. doi: 10.1016/j.cca.2019.02.011
Greene, D. N., Schmidt, R. L., Winston-McPherson, G., Rongitsch, J., Imborek, K. L., Dickerson, J. A., et al. (2020). Reproductive Endocrinology Reference Intervals for Transgender Men on Stable Hormone Therapy. J. Appl. Lab. Med. 6, 41–50. doi: 10.1093/jalm/jfaa169
Grice, E. A., Segre, J. A. (2011). The Skin Microbiome. Nat. Rev. Microbiol. 9, 244–253. doi: 10.1038/nrmicro2537
Grimstad, F., McLaren, H., Gray, M. (2021). The Gynecologic Examination of the Transfeminine Person After Penile Inversion Vaginoplasty. Am. J. Obstet. Gynecol. 224, 266–273. doi: 10.1016/j.ajog.2020.10.002
Gupta, R., Ramnani, P. (2006). Microbial Keratinases and Their Prospective Applications: An Overview. Appl. Microbiol. Biotechnol. 70, 21–33. doi: 10.1007/s00253-005-0239-8
Haustein, U. F. (1995). [Pruritus of the Artificial Vagina of a Transsexual Patient Caused by Gonococcal Infection]. Hautarzt 46, 858–859. doi: 10.1007/s001050050354
Helman, J. L., Flores, A. R., Gates, G. J., Brown, T. N. T. (2016). How Many Adults Identify as Transgender in the United States? (Los Angeles, CA: The Williams Institute).
Holm, J. B., France, M. T., Ma, B., McComb, E., Robinson, C. K., Mehta, A., et al. (2020). Comparative Metagenome-Assembled Genome Analysis of "Candidatus Lachnocurva Vaginae", Formerly Known as Bacterial Vaginosis-Associated Bacterium-1 (Bvab1). Front. Cell Infect. Microbiol. 10, 117. doi: 10.3389/fcimb.2020.00117
Horbach, S. E., Bouman, M. B., Smit, J. M., Ozer, M., Buncamper, M. E., Mullender, M. G. (2015). Outcome of Vaginoplasty in Male-To-Female Transgenders: A Systematic Review of Surgical Techniques. J. Sex. Med. 12, 1499–1512. doi: 10.1111/jsm.12868
Houben, E., Hachem, J. P., De Paepe, K., Rogiers, V. (2008). Epidermal Ceramidase Activity Regulates Epidermal Desquamation via Stratum Corneum Acidification. Skin Pharmacol. Physiol. 21, 111–118. doi: 10.1159/000114872
Iavazzo, C., Gkegkes, I. D., Zarkada, I. M., Falagas, M. E. (2011). Boric Acid for Recurrent Vulvovaginal Candidiasis: The Clinical Evidence. J. Womens Health (Larchmt) 20, 1245–1255. doi: 10.1089/jwh.2010.2708
Jaffray, B. (2020). “Experiences of Violent Victimization and Unwanted Sexual Behaviours Among Gay, Lesbian, Bisexual and Other Sexual Minority People, and the Transgender Population, in Canada, 2018,” in Juristat / Canadian Centre for Justice Statistics No. 10 (Ottawa: Statistics Canada).
Jain, A., Bradbeer, C. (2007). A Case of Successful Management of Recurrent Bacterial Vaginosis of Neovagina After Male to Female Gender Reassignment Surgery. Int. J. STD AIDS 18, 140–141. doi: 10.1258/095646207779949790
James, S. E., Herman, J., Keisling, M., Mottet, L., Anafi, M. a. (2019). 2015 U.S. Transgender Survey (USTS) (Inter-university Consortium for Political and Social Research). [distributor].
Joag, V., Obila, O., Gajer, P., Scott, M. C., Dizzell, S., Humphrys, M., et al. (2019). Impact of Standard Bacterial Vaginosis Treatment on the Genital Microbiota, Immune Milieu, and Ex Vivo Human Immunodeficiency Virus Susceptibility. Clin. Infect. Dis. 68, 1675–1683. doi: 10.1093/cid/ciy762
Kaur, H., Merchant, M., Haque, M. M., Mande, S. S. (2020). Crosstalk Between Female Gonadal Hormones and Vaginal Microbiota Across Various Phases of Women's Gynecological Lifecycle. Front. Microbiol. 11, 551. doi: 10.3389/fmicb.2020.00551
Lee, Y., Dizzell, S. E., Leung, V., Nazli, A., Zahoor, M. A., Fichorova, R. N., et al. (2016). Effects of Female Sex Hormones on Susceptibility to HSV-2 in Vaginal Cells Grown in Air-Liquid Interface. Viruses 8 (9). doi: 10.3390/v8090241
Leinung, M. C., Feustel, P. J., Joseph, J. (2018). Hormonal Treatment of Transgender Women With Oral Estradiol. Transgend. Health 3, 74–81. doi: 10.1089/trgh.2017.0035
Leitich, H., Bodner-Adler, B., Brunbauer, M., Kaider, A., Egarter, C., Husslein, P. (2003). Bacterial Vaginosis as a Risk Factor for Preterm Delivery: A Meta-Analysis. Am. J. Obstet. Gynecol. 189, 139–147. doi: 10.1067/mob.2003.339
Lethaby, A., Ayeleke, R. O., Roberts, H. (2016). Local Oestrogen for Vaginal Atrophy in Postmenopausal Women. Cochrane Database Syst. Rev (8), CD001500. doi: 10.1002/14651858.CD001500.pub3
Liu, C. M., Hungate, B. A., Tobian, A. A., Serwadda, D., Ravel, J., Lester, R., et al. (2013). Male Circumcision Significantly Reduces Prevalence and Load of Genital Anaerobic Bacteria. MBio 4, e00076. doi: 10.1128/mBio.00076-13
Loree, J. T., Burke, M. S., Rippe, B., Clarke, S., Moore, S. H., Loree, T. R. (2020). Transfeminine Gender Confirmation Surgery With Penile Inversion Vaginoplasty: An Initial Experience. Plast. Reconstr. Surg. Glob. Open 8, e2873. doi: 10.1097/GOX.0000000000002873
McMillan, A., Rulisa, S., Sumarah, M., Macklaim, J. M., Renaud, J., Bisanz, J. E., et al. (2015). A Multi-Platform Metabolomics Approach Identifies Highly Specific Biomarkers of Bacterial Diversity in the Vagina of Pregnant and non-Pregnant Women. Sci. Rep. 5, 14174. doi: 10.1038/srep14174
Meerwijk, E. L., Sevelius, J. M. (2017). Transgender Population Size in the United States: A Meta-Regression of Population-Based Probability Samples. Am. J. Public Health 107, e1–e8. doi: 10.2105/AJPH.2016.303578
Menon, G. K., Cleary, G. W., Lane, M. E. (2012). The Structure and Function of the Stratum Corneum. Int. J. Pharm. 435, 3–9. doi: 10.1016/j.ijpharm.2012.06.005
Mirmonsef, P., Hotton, A. L., Gilbert, D., Burgad, D., Landay, A., Weber, K. M., et al. (2014). Free Glycogen in Vaginal Fluids is Associated With Lactobacillus Colonization and Low Vaginal Ph. PloS One 9, e102467. doi: 10.1371/journal.pone.0102467
Mirmonsef, P., Modur, S., Burgad, D., Gilbert, D., Golub, E. T., French, A. L., et al. (2015). Exploratory Comparison of Vaginal Glycogen and Lactobacillus Levels in Premenopausal and Postmenopausal Women. Menopause 22, 702–709. doi: 10.1097/GME.0000000000000397
Mirmonsef, P., Hotton, A. L., Gilbert, D., Gioia, C. J., Maric, D., Hope, T. J., et al. (2016). Glycogen Levels in Undiluted Genital Fluid and Their Relationship to Vaginal Ph, Estrogen, and Progesterone. PloS One 11, e0153553. doi: 10.1371/journal.pone.0153553
Moises da Silva, G. V., Lobato, M. I. R., Silva, D. C., Schwarz, K., Fontanari, A. M. V., Costa, A. B., et al. (2021). Male-To-Female Gender-Affirming Surgery: 20-Year Review of Technique and Surgical Results. Front. Surg. 8, 639430. doi: 10.3389/fsurg.2021.639430
Murad, M. H., Elamin, M. B., Garcia, M. Z., Mullan, R. J., Murad, A., Erwin, P. J., et al. (2010). Hormonal Therapy and Sex Reassignment: A Systematic Review and Meta-Analysis of Quality of Life and Psychosocial Outcomes. Clin. Endocrinol. (Oxf) 72, 214–231. doi: 10.1111/j.1365-2265.2009.03625.x
Nardini, P., Nahui Palomino, R. A., Parolin, C., Laghi, L., Foschi, C., Cevenini, R., et al. (2016). Lactobacillus Crispatus Inhibits the Infectivity of Chlamydia Trachomatis Elementary Bodies, In Vitro Study. Sci. Rep. 6, 29024. doi: 10.1038/srep29024
Nilsson, K., Risberg, B., Heimer, G. (1995). The Vaginal Epithelium in the Postmenopause–Cytology, Histology and pH as Methods of Assessment. Maturitas 21, 51–56. doi: 10.1016/0378-5122(94)00863-3
Nunn, K. L., Clair, G. C., Adkins, J. N., Engbrecht, K., Fillmore, T., Forney, L. J. (2020). Amylases in the Human Vagina. mSphere 5 (6). doi: 10.1128/mSphere.00943-20
O'Hanlon, D. E., Gajer, P., Brotman, R. M., Ravel, J. (2020). Asymptomatic Bacterial Vaginosis Is Associated With Depletion of Mature Superficial Cells Shed From the Vaginal Epithelium. Front. Cell Infect. Microbiol. 10, 106. doi: 10.3389/fcimb.2020.00106
O'Hanlon, D. E., Moench, T. R., Cone, R. A. (2011). In Vaginal Fluid, Bacteria Associated With Bacterial Vaginosis can be Suppressed With Lactic Acid But Not Hydrogen Peroxide. BMC Infect. Dis. 11, 200. doi: 10.1186/1471-2334-11-200
O'Hanlon, D. E., Moench, T. R., Cone, R. A. (2013). Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PloS One 8, e80074. doi: 10.1371/journal.pone.0080074
Obedin-Maliver, J., de Haan, G. (2017). Gynecologic Care for Transgender Adults. Curr. Obstet. Gynecol. 6, 140–148. doi: 10.1007/s13669-017-0204-4
Obedin-Maliver, J., Makadon, H. J. (2016). Transgender Men and Pregnancy. Obstet. Med. 9, 4–8. doi: 10.1177/1753495X15612658
Pan, P., Zhao, S. B., Li, B. H., Meng, Q. Q., Yao, J., Wang, D., et al. (2019). Effect of Supplemental Simethicone for Bowel Preparation on Adenoma Detection During Colonoscopy: A Meta-Analysis of Randomized Controlled Trials. J. Gastroenterol. Hepatol. 34, 314–320. doi: 10.1111/jgh.14401
Pask, A. J., McInnes, K. J., Webb, D. R., Short, R. V. (2008). Topical Oestrogen Keratinises the Human Foreskin and may Help Prevent HIV Infection. PloS One 3, e2308. doi: 10.1371/journal.pone.0002308
Peitzmeier, S. M., Reisner, S. L., Harigopal, P., Potter, J. (2014). Female-To-Male Patients Have High Prevalence of Unsatisfactory Paps Compared to non-Transgender Females: Implications for Cervical Cancer Screening. J. Gen. Intern. Med. 29, 778–784. doi: 10.1007/s11606-013-2753-1
Perrone, A. M., Cerpolini, S., Maria Salfi, N. C., Ceccarelli, C., De Giorgi, L. B., Formelli, G., et al. (2009). Effect of Long-Term Testosterone Administration on the Endometrium of Female-to-Male (FtM) Transsexuals. J. Sex. Med. 6, 3193–3200. doi: 10.1111/j.1743-6109.2009.01380.x
Pessina, M. A., Hoyt, R. F., Jr., Goldstein, I., Traish, A. M. (2006). Differential Effects of Estradiol, Progesterone, and Testosterone on Vaginal Structural Integrity. Endocrinology 147, 61–69. doi: 10.1210/en.2005-0870
Petrina, M. A. B., Cosentino, L. A., Rabe, L. K., Hillier, S. L. (2017). Susceptibility of Bacterial Vaginosis (BV)-Associated Bacteria to Secnidazole Compared to Metronidazole, Tinidazole and Clindamycin. Anaerobe 47, 115–119. doi: 10.1016/j.anaerobe.2017.05.005
Potter, J., Peitzmeier, S. M., Bernstein, I., Reisner, S. L., Alizaga, N. M., Agenor, M., et al. (2015). Cervical Cancer Screening for Patients on the Female-To-Male Spectrum: A Narrative Review and Guide for Clinicians. J. Gen. Intern. Med. 30, 1857–1864. doi: 10.1007/s11606-015-3462-8
Price, L. B., Liu, C. M., Johnson, K. E., Aziz, M., Lau, M. K., Bowers, J., et al. (2010). The Effects of Circumcision on the Penis Microbiome. PloS One 5, e8422. doi: 10.1371/journal.pone.0008422
Prodger, J. L., Abraham, A. G., Tobian, A. A., Park, D. E., Aziz, M., Roach, K., et al. (2021). Penile Bacteria Associated With HIV Seroconversion, Inflammation, and Immune Cells. JCI Insight 6 (8). doi: 10.1172/jci.insight.147363
Puebla-Barragan, S., Watson, E., van der Veer, C., Chmiel, J. A., Carr, C., Burton, J. P., et al. (2021). Interstrain Variability of Human Vaginal Lactobacillus Crispatus for Metabolism of Biogenic Amines and Antimicrobial Activity Against Urogenital Pathogens. Molecules 26 (15). doi: 10.3390/molecules26154538
Radix, A. E., Harris, A. B., Belkind, U., Ting, J., Goldstein, Z. G. (2019). Chlamydia Trachomatis Infection of the Neovagina in Transgender Women. Open Forum Infect. Dis. 6 (11). doi: 10.1093/ofid/ofz470
Ravel, J., Brotman, R. M., Gajer, P., Ma, B., Nandy, M., Fadrosh, D. W., et al. (2013). Daily Temporal Dynamics of Vaginal Microbiota Before, During and After Episodes of Bacterial Vaginosis. Microbiome 1, 29. doi: 10.1186/2049-2618-1-29
Redelinghuys, M. J., Geldenhuys, J., Jung, H., Kock, M. M. (2020). Bacterial Vaginosis: Current Diagnostic Avenues and Future Opportunities. Front. Cell Infect. Microbiol. 10, 354. doi: 10.3389/fcimb.2020.00354
Santen, R. J. (2015). Vaginal Administration of Estradiol: Effects of Dose, Preparation and Timing on Plasma Estradiol Levels. Climacteric 18, 121–134. doi: 10.3109/13697137.2014.947254
Schaller, G. (1990). Changes in Keratin Expression of Human Vaginal Epithelium During Different Female Generation Phases. Polyclonal Antibody Studies. Gynecol. Obstet. Invest. 29, 278–281. doi: 10.1159/000293334
Schaller, G., Genz, T. (1990). Immunohistochemical Detection of Keratins 1 and 13 as Differentiation Markers in the Hormone-Dependent Human Vaginal Epithelium. Gynecol. Obstet. Invest. 30, 94–96. doi: 10.1159/000293225
Schaller, G., Lengyel, E., Pantel, K., Hardt, W., Mischke, D. (1993). Keratin Expression Reveals Mosaic Differentiation in Vaginal Epithelium. Am. J. Obstet. Gynecol. 169, 1603–1607. doi: 10.1016/0002-9378(93)90444-N
Schardein, J. N., Li, G., Zaccarini, D. J., Caza, T., Nikolavsky, D. (2021). Histological Evaluation of Vaginal Cavity Remnants Excised During Neourethral Stricture Repair in Transgender Men. Urology. doi: 10.1016/j.urology.2021.06.044
Scheim, A. I., Bauer, G. R. (2015). Sex and Gender Diversity Among Transgender Persons in Ontario, Canada: Results From a Respondent-Driven Sampling Survey. J. Sex. Res. 52, 1–14. doi: 10.1080/00224499.2014.893553
Schwebke, J. R., Hillier, S. L., Sobel, J. D., McGregor, J. A., Sweet, R. L. (1996). Validity of the Vaginal Gram Stain for the Diagnosis of Bacterial Vaginosis. Obstet. Gynecol. 88, 573–576. doi: 10.1016/0029-7844(96)00233-5
Selvaggi, G., Ceulemans, P., De Cuypere, G., VanLanduyt, K., Blondeel, P., Hamdi, M., et al. (2005). Gender Identity Disorder: General Overview and Surgical Treatment for Vaginoplasty in Male-to-Female Transsexuals. Plast. Reconstr. Surg. 116, 135e–145e. doi: 10.1097/01.prs.0000185999.71439.06
Shen, J., Song, N., Williams, C. J., Brown, C. J., Yan, Z., Xu, C., et al. (2016). Effects of Low Dose Estrogen Therapy on the Vaginal Microbiomes of Women With Atrophic Vaginitis. Sci. Rep. 6, 24380. doi: 10.1038/srep24380
Shim, S., Park, K. M., Chung, Y. J., Kim, M. R. (2021). Updates on Therapeutic Alternatives for Genitourinary Syndrome of Menopause: Hormonal and Non-Hormonal Managements. J. Menopausal Med. 27, 1–7. doi: 10.6118/jmm.20034
Spear, G. T., French, A. L., Gilbert, D., Zariffard, M. R., Mirmonsef, P., Sullivan, T. H., et al. (2014). Human Alpha-Amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus. J. Infect. Dis. 210, 1019–1028. doi: 10.1093/infdis/jiu231
Stankler, L., Walker, F. (1976). Periodic Acid-Schiff (PAS) Staining for Glycogen in Clinically Normal Psoriatic and non-Psoriatic Skin. Br. J. Dermatol. 95, 599–601. doi: 10.1111/j.1365-2133.1976.tb07030.x
T'Sjoen, G., Arcelus, J., Gooren, L., Klink, D. T., Tangpricha, V. (2019). Endocrinology of Transgender Medicine. Endocr. Rev. 40, 97–117. doi: 10.1210/er.2018-00011
Tamarelle, J., Thiebaut, A. C. M., de Barbeyrac, B., Bebear, C., Ravel, J., Delarocque-Astagneau, E. (2019). The Vaginal Microbiota and its Association With Human Papillomavirus, Chlamydia Trachomatis, Neisseria Gonorrhoeae and Mycoplasma Genitalium Infections: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 25, 35–47. doi: 10.1016/j.cmi.2018.04.019
Tjernlund, A., Carias, A. M., Andersson, S., Gustafsson-Sanchez, S., Rohl, M., Petersson, P., et al. (2015). Progesterone-Based Intrauterine Device Use is Associated With a Thinner Apical Layer of the Human Ectocervical Epithelium and a Lower ZO-1 mRNA Expression. Biol. Reprod. 92, 68. doi: 10.1095/biolreprod.114.122887
Turpin, R., Slopen, N., Borgogna, J. C., Yeoman, C. J., He, X., Miller, R. S., et al. (2021). Perceived Stress and Molecular-BV in the NIH Longitudinal Study of Vaginal Flora. Am. J. Epidemiol. doi: 10.1093/aje/kwab147
Valore, E. V., Park, C. H., Igreti, S. L., Ganz, T. (2002). Antimicrobial Components of Vaginal Fluid. Am. J. Obstet. Gynecol. 187, 561–568. doi: 10.1067/mob.2002.125280
van der Sluis, W. B., Steensma, T. D., Timmermans, F. W., Smit, J. M., de Haseth, K., Ozer, M., et al. (2020). Gender-Confirming Vulvoplasty in Transgender Women in the Netherlands: Incidence, Motivation Analysis, and Surgical Outcomes. J. Sex. Med. 17, 1566–1573. doi: 10.1016/j.jsxm.2020.04.007
van der Veer, C., Hertzberger, R. Y., Bruisten, S. M., Tytgat, H. L. P., Swanenburg, J., de Kat Angelino-Bart, A., et al. (2019). Comparative Genomics of Human Lactobacillus Crispatus Isolates Reveals Genes for Glycosylation and Glycogen Degradation: Implications for In Vivo Dominance of the Vaginal Microbiota. Microbiome 7, 49. doi: 10.1186/s40168-019-0667-9
Waseem, A., Alam, Y., Dogan, B., White, K. N., Leigh, I. M., Waseem, N. H. (1998). Isolation, Sequence and Expression of the Gene Encoding Human Keratin 13. Gene 215, 269–279. doi: 10.1016/S0378-1119(98)00297-2
Weyers, S., Verstraelen, H., Gerris, J., Monstrey, S., Santiago Gdos, S., Saerens, B., et al. (2009). Microflora of the Penile Skin-Lined Neovagina of Transsexual Women. BMC Microbiol. 9, 102. doi: 10.1186/1471-2180-9-102
Weyers, S., Lambein, K., Sturtewagen, Y., Verstraelen, H., Gerris, J., Praet, M. (2010). Cytology of the 'Penile' Neovagina in Transsexual Women. Cytopathology 21, 111–115. doi: 10.1111/j.1365-2303.2009.00663.x
Wiesenfeld, H. C., Hillier, S. L., Krohn, M. A., Landers, D. V., Sweet, R. L. (2003). Bacterial Vaginosis is a Strong Predictor of Neisseria Gonorrhoeae and Chlamydia Trachomatis Infection. Clin. Infect. Dis. 36, 663–668. doi: 10.1086/367658
Winston McPherson, G., Long, T., Salipante, S. J., Rongitsch, J. A., Hoffman, N. G., Stephens, K., et al. (2019). The Vaginal Microbiome of Transgender Men. Clin. Chem. 65, 199–207. doi: 10.1373/clinchem.2018.293654
Zevin, A. S., Xie, I. Y., Birse, K., Arnold, K., Romas, L., Westmacott, G., et al. (2016). Microbiome Composition and Function Drives Wound-Healing Impairment in the Female Genital Tract. PloS Pathog. 12, e1005889. doi: 10.1371/journal.ppat.1005889
Zhu, Y., Yang, Y., Guo, J., Dai, Y., Ye, L., Qiu, J., et al. (2017). Ex Vivo 2D and 3D HSV-2 Infection Model Using Human Normal Vaginal Epithelial Cells. Oncotarget 8, 15267–15282. doi: 10.18632/oncotarget.14840
Keywords: vagina, neovagina, transgender, gender diverse, microbiome, bacterial vaginosis
Citation: Krakowsky Y, Potter E, Hallarn J, Monari B, Wilcox H, Bauer G, Ravel J and Prodger JL (2022) The Effect of Gender-Affirming Medical Care on the Vaginal and Neovaginal Microbiomes of Transgender and Gender-Diverse People. Front. Cell. Infect. Microbiol. 11:769950. doi: 10.3389/fcimb.2021.769950
Received: 02 September 2021; Accepted: 14 December 2021;
Published: 21 January 2022.
Edited by:
Lindi Masson, Burnet Institute, AustraliaReviewed by:
Lenka Vodstrcil, Monash University, AustraliaCopyright © 2022 Krakowsky, Potter, Hallarn, Monari, Wilcox, Bauer, Ravel and Prodger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica L. Prodger, anByb2RnZUB1d28uY2E=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.