- 1Department of Periodontology, Buccal Surgery and Implantology, Faculty of Medicine, Liège, Belgium
- 2Department of Periodontology , Academic Centre for Dentistry Amsterdam, Vrije Universiteit (VU) Amsterdam, Amsterdam, Netherlands
- 3Department of Oral Health Sciences, KU Leuven & Dentistry, University Hospitals Leuven, Leuven, Belgium
- 4Department of Cardiovascular and Thoracic Surgery, Faculty of Medicine, Liège, Belgium
Background: Periodontitis is a chronic inflammatory gum disease associated with systemic diseases such as cardiovascular diseases.
Aim: To investigate the association of systemic blood biomarkers, C-reactive protein (CRP), levels of lipopolysaccharide (LPS), and IgG levels against periodontal pathogens Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg) with the stability, based on the aortic diameter, the growth rate and the eligibility for surgical intervention, of patients with abdominal aortic aneurysm (AAA).
Methods: Patients with stable AAA (n = 30) and unstable AAA (n = 31) were recruited. The anti-A. actinomycetemcomitans and anti-P. gingivalis IgG levels were analyzed by ELISA, the LPS analysis was performed by using the limulus amebocyte lysate (LAL) test, and plasma levels of CRP were determined using an immune turbidimetric method. The association between these blood systemic biomarkers, AAA features, periodontal clinical parameters and oral microbial profiles were explored. Regression models were used to test the relationship between variables.
Results: The presence of antibodies against Pg and Aa, LPS and high CRP concentrations were found in all AAA patients. The IgG levels were similar in patients with stable and unstable AAA (both for Aa and Pg). Among investigated blood biomarkers, only CRP was associated with AAA stability. The amount of LPS in saliva, supra, and subgingival plaque were significantly associated with the systemic LPS (p <0.05).
Conclusions: This post-hoc study emphasizes the presence of antibodies against Pg and Aa, LPS and high CRP concentrations in all AAA patients. The presence of Pg in saliva and subgingival plaque was significantly associated with the blood LPS levels. For further studies investigating periodontitis and systemic diseases, specific predictive blood biomarkers should be considered instead of the use of antibodies alone.
Introduction
AAA is a chronic degenerative disorder of the abdominal aorta promoted by genetic and environmental risk factors such as smoking, older age, Caucasian ethnicity, and the male gender (Sakalihasan et al., 2018). The disease progresses with the increase of the abdominal aortic diameter that can lead to vessel rupture, responsible for 1 to 3% of all deaths in western countries (Sakalihasan et al., 2005). The AAA physiopathology involves an inflammatory destruction of the aortic wall structure and the presence of an intraluminal thrombus (ILT) (Hellmann et al., 2007; Morbelli et al., 2014) that can be induced by the presence of several bacteria (Halme et al., 1999; Lindholt et al., 1999; Salhi et al., 2019).
Periodontitis, a chronic inflammatory gum pathology, is the 6th most prevalent disease worldwide (Kassebaum et al., 2014) affecting more than 50% of the adult population and about 11% of the population suffers from severe form. The disease, induced by the invasion of gram negative bacteria (Socransky et al., 1998), triggering the host immune defense (Haffajee and Socransky, 1994; Amano, 2010a; Meyle and Chapple, 2015), leads to the destruction of the connective tissues supporting tooth, and ultimately to the tooth loss (Socransky et al., 1998; Meyle and Chapple, 2015). Particularly, the red complex composed of Treponema denticola (Td), Porphyromonas gingivalis (Pg), Tannarella forsythia (Tf), and Aggregatibacter actinomycetemcomitans (Aa) have been strongly associated with periodontal tissues destruction (Haffajee and Socransky, 1994; Amano, 2010a; Meyle and Chapple, 2015). Moreover, it is well documented that the release of periodontal pathogens or/and their sub-products (SPs) (Forner et al., 2006) in the bloodstream (Haffajee and Socransky, 1994) can induce a systemic inflammation and promote metastatic infection of periodontal pathogens (Loos, 2005; Salhi et al., 2019). Therefore, it has been hypothesized that periodontitis may induce bacteremia, the release of inflammatory mediators and the progression of systemic diseases (Forner et al., 2006; Amano, 2010a). Among these inflammatory mediators released by Pg, hemagglutinin can promote the platelet aggregation (Belanger et al., 2012) and gingipains can neutralized the host immune response extra-orally, in a metastatic site (Nassar et al., 2002). Other virulence factors such as the lipopolysaccharide (Bainbridge et al., 2002) (LPS) of Pg or the cytotoxin of Aa (Tan et al., 2002), can also impair systemic diseases, as CVD by disturbing the immune host response (Deshpande et al., 1998a; Deshpande et al., 1998b; Amano, 2010a). The microbial properties of specific periodontal pathogens contribute to the development of chronic non-communicable disease such as cardiovascular diseases (CVD) (Loos et al., 2000; Tonetti and Van Dyke, 2013) and diabetes (Tonetti and Van Dyke, 2013; Sanz et al., 2018). Indeed, serum antibodies against periodontal pathogens and LPS were shown to be associated with higher risk for ischemic stroke (Pussinen et al., 2004a; Tabeta et al., 2011; Hosomi et al., 2012; Palm et al., 2014), coronary heart disease (Pussinen et al., 2003; Pussinen et al., 2005; Goteiner et al., 2008) as myocardial infarction (Pussinen et al., 2004b). Therefore, in patients suffering from CVD as AAA, the concentrations of immunoglobulin G (IgG) against periodontal bacteria (Nakagawa et al., 1994; Albandar et al., 2001; Dye et al., 2009; Pussinen et al., 2011), LPS (Ebersole et al., 2010) and C-reactive protein (CRP) might be relevant periodontitis-related blood biomarkers to characterize sequelae linked to periodontitis.
The aim of this study was to investigate the relationship between blood biomarkers and serologic immunological profiles related to periodontitis (CRP, LPS, and serum anti- Aggregatibacter actinomycetemcomitans [Aa] and anti-Porphyromonas gingivalis [Pg] IgG) in abdominal aortic aneurysm patients. Secondary objectives focused on the relationship between periodontal clinical parameters, microbial profile and periodontitis-related blood biomarkers.
Material and Methods
Study Design and Ethical Committee
Unexploited data collected during a previous cross-sectional study on the periodontitis and AAA (Salhi et al., 2020) were used in the present post-hoc study to further explore the associations between AAA stability and periodontitis related blood biomarkers. The study design, sample size calculation, patient selection, and demographics of the cohort have been described in the previous report (Salhi et al., 2020). AAA imaging, periodontal, and microbiological parameters are briefly explained hereafter.
The study was approved by the human subjects’ ethics board of the University Hospital of Liege, Belgium (B707201421977), and was registered on clinicaltrial.gov (file number: NCT03767023).
Clinical Data and Blood Sample Collection
After screening the medical files of eligible AAA patients, the participants were invited to the Department of Periodontology where anamnesis and imaging were recorded. A full periodontal clinical and microbiological examination was performed by a single investigator (LS) and the blood samples were collected by the department nurse (JN M). The included AAA patients were divided into two groups according to the stability of the AAA, defined by Sweeting et al. (2012). The first group consisted of patients with stable AAA (n = 30), characterized by an antero-posterior abdominal diameter inferior to 55 mm and/or a stable growth rate inferior to 10 mm per year and not considered for surgical repair. The second group was composed of patients with an unstable AAA (n = 31) with a diameter higher than 55 mm and rapid growth rate superior to 10 mm per year), requiring short-term open surgery or endovascular aneurismal repair. The AAA growth rate was estimated from the previous AAA diameter recordings found in the patient file (at least 2 exams at a given time).
AAA Imaging Data
The AAA dimensions were assessed by measuring AAA diameters (anterior–posterior, cross-sectional, and maximal) and volumes (entire AAA, residual lumen, and thrombus), as described previously (Salhi et al., 2020). More precisely, the AAA diameters (mm) were collected in the medical file of the patient according to the last diameter based on echography (images obtained with a C5.2 convex probe, IU22 Philips Ultrasonography, Belgium) for 9 patients or computed tomography (CT) scan for 52 patients. When abdominal CT scans (slides thickness: 1.25 mm) were available, additional measurements such as the AAA diameters and volumes were collected by two independent blinded and calibrated examiners (LS and AG) using a specific imaging software (Syngovia by Siemens Healthineers, Erlangen, Germany). Aortic diameters (mm) were measured in their anterior–posterior, cross-sectional, and maximal positions based on axial acquisitions. The volumes of the entire AAA (mm³) and the thrombus were measured by using the VOI free hands tool. The lumen volume was obtained by the subtraction of the entire AAA volume and the residual lumen volume. AAA heights (mm) were recorded from the iliac bifurcation to the renal artery origin and to the neck of the AAA, respectively.
Periodontal Data
The number of teeth, the presence of healthy gingiva or gingivitis, diagnosis, and classification of periodontitis (stage, extent, and grade) were recorded for each subject according to Caton et al. (2018). Periodontal parameters were collected by a single periodontist (LS) including pocket probing depth (PD, mm; 6 sites per tooth), gingival recession (RD, mm), clinical attachment level (CAL, mm), bleeding on probing (Silness and Loe, 1964) (BOP, %), percentage (%) of PD sites ≥6 mm calculated over all PD sites of the patient, plaque score index (PI, %), furcations (Hamp et al., 1975) and tooth mobility (Miller, 1938). A graduated manual periodontal probe1 was used to measure (mm) 6 sites per tooth. The Periodontal Inflamed Surface Area (PISA), the global PISA score (Nesse et al., 2008) and the Periodontal Index for Risk of Infectiousness (PIRI) (Rompen et al., 2001), which takes into account the number and the severity of the periodontal niches in contact with blood circulation (PD and furcation impairment), were calculated. Scores were attributed to the number and depth of PD and for the number and severity of furcations (Rompen et al., 2001). By adding these 2 scores, patients were classified according their risk for metastatic injury: none or low risk (PIRI = 0), moderate risk (1 ≤PIRI≤ 5), and high risk (6 ≤PIRI≤ 10).
Microbiological Data
All microbiological samples were collected by the same investigator (LS). Saliva samples were obtained by collecting 500 µl to 1 ml of unstimulated saliva. The supragingival samples were collected from the four teeth with the deepest periodontal pockets. The sites were isolated with cotton rolls and gently dried with compressed air (Teughels et al., 2013). All supragingival plaque was taken with a periodontal curette and then placed in a sterile tube containing 0.75 ml of TE (10 mM Tris–HCl, 1 mM EDTA, pH 7.6) and an equal amount of 0.5 M NaOH. Then, the subgingival samples were harvested at the same four deepest pocket sites. The harvesting was performed by using a sterile endo paperpoint inserted into the pocket (Iso 040, Dentsply, Maillefer, Switzerland). Four paperpoints were inserted per pocket for 15 s (16 tips per patient), and were then collected as for supragingival samples. All samples were stored at −20°C. After defrosting and vigorously vortexing, 400 µl of each sample were centrifuged at 13,000g. The obtained pellets were dispersed in 200 µl Instagen. DNA was extracted with InstaGene matrix (Bio-Rad Life Science Research, Hercules, CA, USA) according to the instructions of the manufacturer. Five microliters of the purified DNA were used for the detection and quantification of Tannerella forsythia (Tf), Porphyromonas gingivalis (Pg), Aggregatibacter actinomycetemcomitans (Aa), Fusobacterium nucleatum (Fn), and Prevotella intermedia (Pi) by real time quantitative polymerase chain reaction (RT-qPCR). The RT-qPCR assay was performed with a CFX96 Real-Time System2 using a Taqman 5’ nuclease assay PCR method for detection and quantification of bacterial DNA. Assay conditions for all primer/probe sets consisted of an initial 2 min at 50°C, followed by a denaturation step for 10 min at 95°C, followed by 45 cycles of 95°C for 15 s and 60°C for 60 s. Quantification was based on a plasmid standard curve (PMID: 19930094). Results were expressed as log 10 genome equivalents (gEq)/ml. Values below the detection threshold level were recorded as 0.
Periodontitis-Related Blood Biomarkers Data
In order to induce bacteremia in blood circulation, patients were invited to have a standardized mastication on paraffin (Nicu et al., 2009) and to brush their teeth during 2 min. Afterwards, venous blood samples from antecubital fossa were taken to evaluate the blood biomarkers of interest.
IgG Against Aa and Pg
A first blood sample (10 ml red cap) was harvested to quantify the concentration of antibodies levels against Pg and Aa. The tube was kept at room temperature for 30 min and then the serum was obtained by centrifugation at 2,000g at 4°C for 10 min. Aliquots of serum were stored at −80°C. The antibodies against Pg and Aa were analyzed as previously described (Nicu et al., 2009). Briefly, a mixture was made out of five different strains of Aa (ATCC 29523, Y4, NCTC9710, 3381, and OM2 534 representing the serotypes a, b, c, d, and e) for the detection of IgG levels against Aa; and 8 different strains of Pg (W83, HG 184, A7A1-28, ATCC 49417, HG 1690, HG 1691 and 34-4 representing the capsule serotypes K1–K7, and also the uncapsulated strain 381 (Laine et al., 1997) for the detection of IgG levels against Pg. The Aa strains were grown for 18 h in brain–heart-infusion (BHI) broth (Sigma Chemical Co, St. Louis, MO, USA) aerobically at 37°C in humidified 5% CO2. The Pg strains were grown anaerobically (80% N2, 10% H2, 10% CO2) at 37°C for 18 h in BHI broth supplemented with hemin (5 mg/l) and menadione (1 mg/l) (Sigma). The bacteria were washed once with phosphate-buffered saline (PBS; 10 mM phosphate, 150 mM NaCl, pH 7.4) and then fixed overnight at 4°C in 0.5% paraformaldehyde–PBS. Further, the bacterial suspensions were washed three times in PBS, sonicated and brought to an optical density corresponding to an absorbance of 0.15 at 580 nm in ELISA-buffer (PBS, 0.5% bovine serum albumin, 0.05% Tween-20). For ELISA, 150 ml of the sonicated mixture from the five Aa or from the eight Pg strains were used to coat Microlon ELISA plates (Greiner Bio-One B.V., Alphen a/d Rijn, the Netherlands). The unspecific bindings were blocked by 5% bovine serum albumin (BSA) in PBS at room temperature for 30 min. Diluted (1:1,500) serum samples were tested in duplicate. The plates were incubated for 2 h at room temperature and washed three times in ELISA buffer. Horse-radish peroxidase-conjugated, goat anti-human IgG (Vector Laboratories Inc., Burlingame, CA, USA) diluted (1:2,000; 150 ml) were then added and the plates were incubated for 2 h at room temperature. Substrate was then added and absorbance values were measured at 450 nm with a multilabel counter (Wallac Victor 1420, Perkin-Elmer Life Sciences, Boston, MA, USA).
Endotoxin Detection Assay
A second tube of blood (10 ml EDTA plasma tube) was collected for LPS analysis and immediately placed on ice. Plasma was obtained by centrifugation at 2,000g at 4°C for 10 min. Aliquots of plasma were stored at −80°C. The LPS analysis was performed by using the EndoLISA kit according to the manufacturer’s instructions (EndoLISA®, Hyglos GmbH, Biomérieux, Bernried am Starnberger See, Germany). Briefly, all samples were defrosted and vortexed to ensure homogeneity. CSE (endotoxin standard Escherichia coli O55:B5) was diluted for the standard curve with dilution factor 10× and dissolved in endotoxin-free water. In total, 100 µl of each preparation was added in duplicate into the respective wells. A blank control was included as a negative control. Next, 20 µl of 6× Binding buffer was added to each well and was covered in aluminum foil and incubated at 37°C for 90 min at 450 rpm. Samples were washed 3× with 150 µl Wash Buffer before 100 µl of the EndoLISA assay reagent was added to each well. Fluorescent signals were measured at 37°C immediately and after 90 min.
CRP
A third blood sample (lithium-heparinate) was used for plasma cell analysis and for high sensitivity CRP (hsCRP), using a commercially available kit (Behring N latex C-reactive protein mono Analyzer, Behring Diagnostic, Marburg, Germany). This sample was analyzed within 3 h in a clinical chemistry laboratory, by using standardized and automated procedures.
Statistical Methods
The power calculation of the larger study of which this one is a post-hoc analysis showed that at least 30 patients had to be included in both stable and unstable AAA groups. Results were expressed as mean ± SD for quantitative variables and as frequency tables (numbers and percentage) for categorical variables. Non-normal distributed variables were log-transformed to normalize their distribution. Comparisons between stable and unstable AAA groups were done by Student’s t-test or Kruskal–Wallis test for continuous variables and by chi-square test or Fisher exact test for qualitative findings. Regression models were used to test the relationship between study variables and results were expressed as regression coefficients and their standard error (SE). A positive or negative regression coefficient indicated respectively an increasing and a decreasing relationship between the two variables. All regressions were adjusted for AAA stability. When the dependent variable Y was quantitative, classical linear regression was applied. When Y was binary, the logistic regression was used and when Y was ordinal then ordinal logistic regression was applied. Results were considered significant at the 5% level (p <0.05). Calculations were done in SAS (SAS Institute, Cary, NC) version 9.4.
Results
The periodontal characteristics of study patients with stable (n = 30) or unstable (n = 31) AAA were previously described (Salhi et al., 2020).
The correlation (in terms of regression coefficients) between clinical periodontal parameters, CRP and LPS blood levels, and antibodies concentrations (anti-Aa and anti-Pg) are presented in Table 1 No correlation was found neither with CRP nor with LPS whereas a trend was found between BOP and anti-Aa antibodies (p = 0.05).
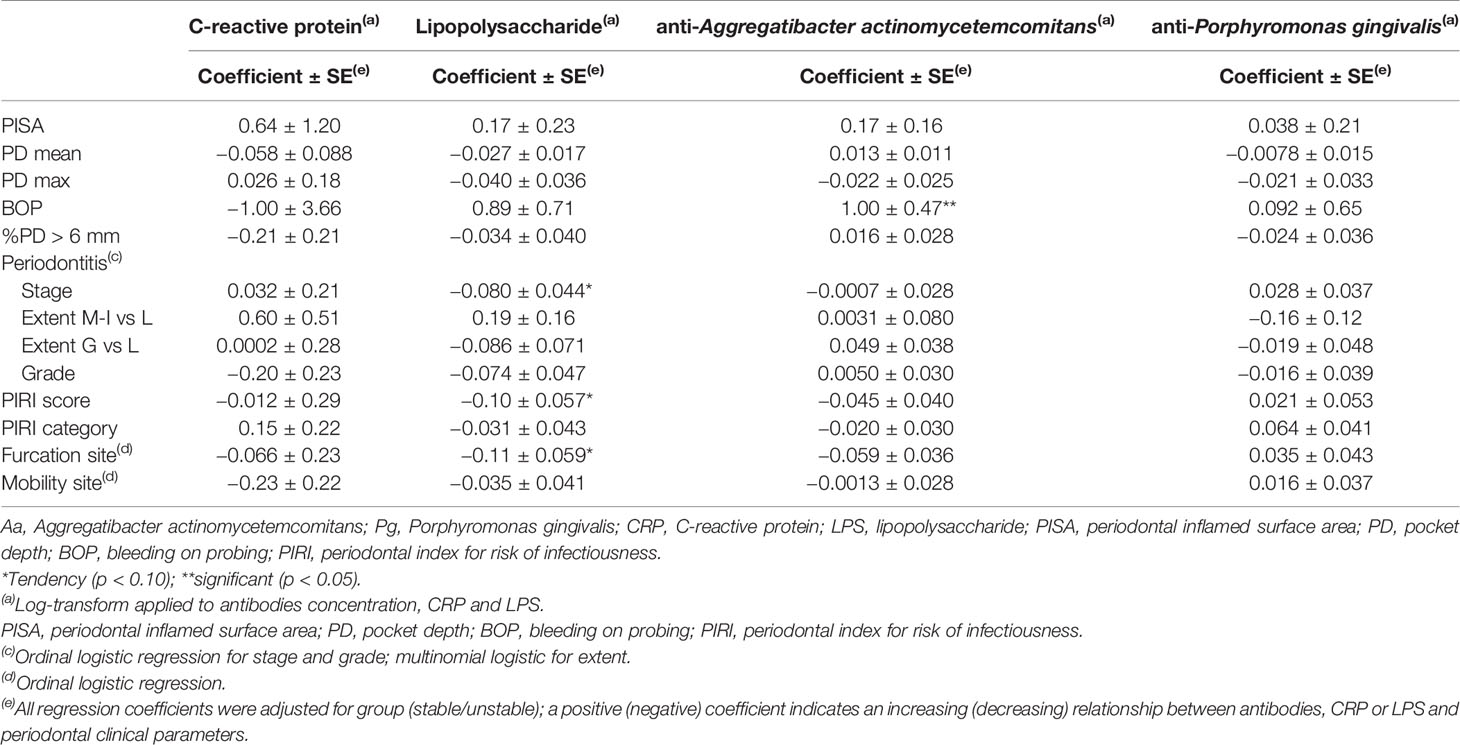
Table 1 Relationship between periodontal parameters and the blood concentrations of CRP and LPS, Aa, and Pg antibodies.
The periodontitis-related blood biomarkers analysis according to AAA stability are shown in Table 2. The CRP levels were higher in patients with unstable AAA (p = 0.017) while LPS levels did not differ between the groups. Anti-Aa and anti-Pg antibodies were found in similar concentrations in all blood samples of patients with stable and unstable AAA. The relationship between periodontal-specific blood markers, AAA diameters and volumes are shown in Table 3. No significant associations were found.
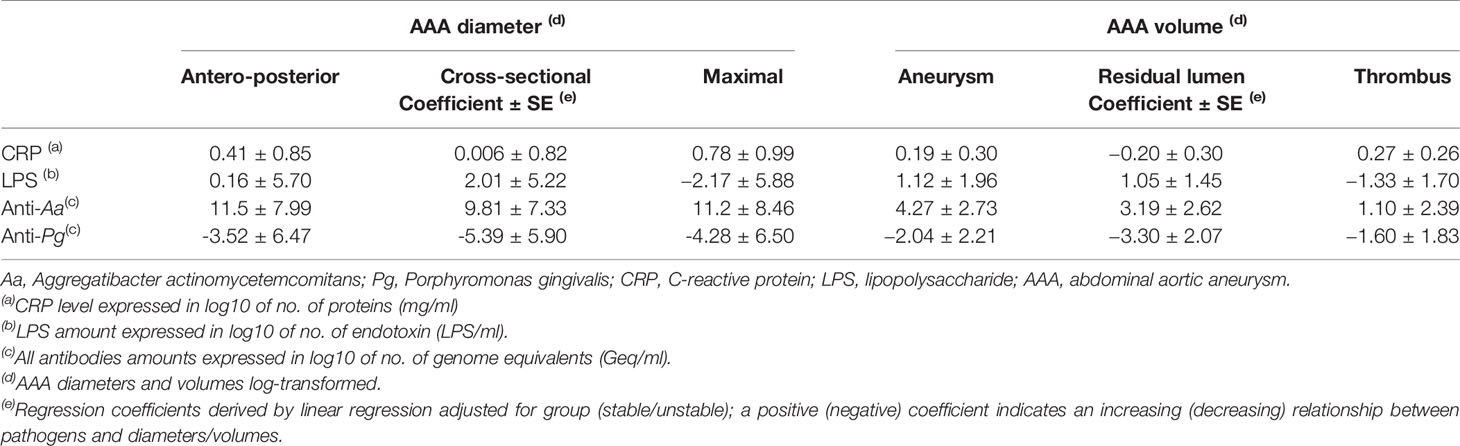
Table 3 Relationship between the blood concentrations of CRP, LPS, Aa, and Pg antibodies, and the AAA diameters and volumes (N = 57).
The relationships between Aa and Pg in saliva, supra-gingival plaque and subgingival plaque and the levels of CRP, LPS and anti-Aa/anti-Pg antibodies in blood are shown in Table 4. The presence of Pg in saliva and subgingival plaque was correlated to the LPS blood levels (p <0.05). No other significant associations were found between microbial profiles and blood biomarkers.
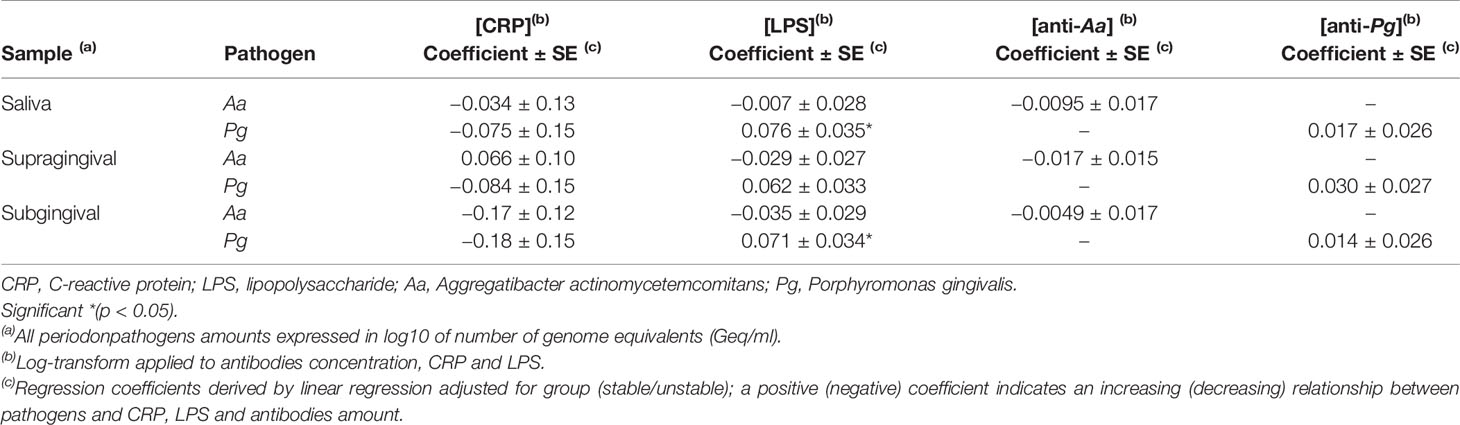
Table 4 Relationship between periodontal pathogens in saliva, supragingival plaque, and subgingival plaque with the concentrations of the Aa and Pg antibodies, CRP and LPS.
Discussion
This post-hoc study of a cross-sectional, non-interventional, study focused on the relationship between periodontitis-specific blood biomarkers and the AAA stability.
Strikingly, all the included patients presented periodontitis and periodontitis-specific blood markers (antibodies against the periodontal pathogens Pg and Aa and also LPS and CRP) although periodontitis was not an inclusion criterion of the study. Additionally, the LPS in blood was associated with the presence of Pg in saliva and subgingival plaque. Therefore, it seems that periodontitis may have induced bacteremia, endotoxemia and systemic inflammation which may eventually have a role in the physiopathology of AAA (Mealey et al., 1999).
In a previous publication, the association between the severity of the periodontal parameters, the quantity of periodontal pathogens and the severity (or the extend) of AAA was observed (Salhi et al., 2020) and the present serologic immunological profiles support these findings. Although IgG antibodies against Pg and Aa were detected in all patients, no association was found with the concentration of bacteria in subgingival plaque samples. The low levels of IgG measured in the serum may reflect an altered immune response that may contribute to the physiopathology of periodontitis and AAA (Kuivaniemi et al., 2015; Sakalihasan et al., 2018). Therefore, the quantification of seral anti-bacterial antibodies remains controversial in the diagnosis of past or current periodontitis exposure (Papapanou et al., 2000; Dye et al., 2009; Vlachojannis et al., 2010; Pussinen et al., 2011), namely due to the possible cross-reactions with other bacterial epitopes (Davison et al., 2021). Thus, the antibody titers, at least as a single marker, may not be sufficient to explore the potential association between periodontitis and CVD.
Hence, in addition to IgG detection in sera, further seral investigations should be considered, as the use of bacteria virulence (Amano, 2010b) factors. Indeed, as suggested by some authors (Loos et al., 2000; Tonetti and Van Dyke, 2013), the detection and quantification of additional markers such as fimbriae, gingipains and hemagglutinin (Amano, 2010a) and also bacteria toxins would be relevant to further understand the systemic effect of periodontitis on chronic non-communicable diseases as cardiovascular diseases (CVD) including AAA. In the present study, antibodies against Pg tended to be slightly lower in unstable AAA patients when compared to those of patients in stable AAA (p = 0.08). It could be hypothesized that virulence factors of Pg such as gingipains (Haruyama et al., 2009), fimbriae (Fan et al., 2001), capsule (Laine et al., 1997), and LPS (Bainbridge and Darveau, 2001) contribute to escape from the host immune response, lowering IgG detection in serum and, finally, contributing to periodontitis progression (Hajishengallis et al., 2012). A second interpretation could be found in the high number of tooth losses of the included patients suffering from severe form of periodontitis. Indeed, as recently suggested, the lower antibody titers against Pg were associated with an increased number of tooth loss (Aoyama et al., 2018). Therefore, tooth loss may reflect the end stage of periodontitis (Caton et al., 2018) and has been often associated with cardiovascular events (Liljestrand et al., 2015) such as myocardial infarction and stroke (Lee et al., 2019).
Additionally, the study also showed that patients with unstable AAA displayed higher CRP levels, a blood biomarker usually associated with the progression of cardiovascular disease (Loos, 2005). Although CRP is a non-specific biomarker, the present findings suggest that periodontitis may contribute to the elevated CRP concentrations and support the relationship between periodontitis, inflammation, and AAA instability. Indeed, the augmentation of systemic markers due to periodontitis (D’Aiuto et al., 2007; Paraskevas et al., 2008; Lima et al., 2011; Fedele et al., 2011; Nibali et al., 2013; Shaddox et al., 2013; Balli et al., 2014; Keles et al., 2014; Finoti et al., 2017; Chandy et al., 2017; Batschkus et al., 2017), such as specific interleukins, fibrinogen, albumin, CRP, matrix metalloproteinase-9 or tumor necrosis factor-α, participate to the vascular endothelial weakening and its dysfunction (Tonetti et al., 2007) and, therefore, can promote systemic diseases. Thus, the presence of periodontitis may enhance systemic inflammation which is involved in the AAA physiopathology (Tambyraja et al., 2007; Wallinder et al., 2009; Courtois et al., 2013; Martinez-Pinna et al., 2013; Morbelli et al., 2014).
This study suffers from some limitations, particularly because of the post-hoc design and the small sample size. Including a control group with healthy patient (without AAA), would also be of interest in future research. Findings should therefore be interpreted cautiously.
Conclusion
This post-hoc study emphasizes the presence of antibodies against Pg and Aa, LPS and high CRP concentrations in all AAA patients. However, among investigated blood biomarkers, only CRP was associated with AAA stability. The presence of Pg in saliva and subgingival plaque was significantly associated with the blood LPS levels. For further studies investigating periodontitis and systemic diseases, specific predictive blood biomarkers should be considered instead of the use of antibodies alone.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the human subjects’ ethics board of the University Hospital of Liege, Belgium (B707201421977). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Conceptualization (LS, FL); Investigation (LS); Methodology (LS, PR, WT); Supervision (ML, FL); Roles/Writing - original draft (LS); Writing – review (LS, PR, ML, DV, FL) Validation (LS, PR, DV, ML, WT, NS, FL).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are thankful to Mrs. L. Seidel (Department of Biostatistics and Medico-economic Information, University Hospital, CHU of Liège, Belgium), to Pr. A. Albert (Department of Public Health Sciences, University of Liège, Belgium), to Dr. A. Gau-Okroglic (Department of medical Imaging, University of Liège, Belgium), and to Dr. D. Deng (Department of Preventive Dentistry, ACTA, the Netherlands) for their collaboration.
Footnotes
- ^ Periodontal probe, North Carolina 2927.10, Stoma, Germany
- ^ BioRad, Hercules, California, United-States
References
Albandar, J. M., DeNardin, A. M., Adesanya, M. R., Diehl, S. R., Winn, D. M. (2001). Associations Between Serum Antibody Levels to Periodontal Pathogens and Early-Onset Periodontitis. J. Periodontol. 72, 1463–1469. doi: 10.1902/jop.2001.72.11.1463
Amano, A. (2010a). Host-Parasite Interactions in Periodontitis: Microbial Pathogenicity and Innate Immunity. Periodontol. 2000 54, 9–14. doi: 10.1111/j.1600-0757.2010.00376.x
Amano, A. (2010b). Bacterial Adhesins to Host Components in Periodontitis. Periodontol. 2000 52, 12–37. doi: 10.1111/j.1600-0757.2009.00307.x
Aoyama, N., Suzuki, J. I., Kobayashi, N., Hanatani, T., Ashigaki, N., Yoshida, A., et al. (2018). Associations Among Tooth Loss, Systemic Inflammation and Antibody Titers to Periodontal Pathogens in Japanese Patients With Cardiovascular Disease. J. Periodontal Res. 53, 117–122. doi: 10.1111/jre.12494
Bainbridge, B. W., Coats, S. R., Darveau, R. P., Dixon, D. R., Page, R. C. (2002). Porphyromonas Gingivalis Lipopolysaccharide Displays Functionally Diverse Interactions With the Innate Host Defense System Modulation of the Innate Immune Response Within the Periodontium Porphyromonas Gingivalis Lipopolysaccharide: An Unusual Pattern Recognition Receptor Ligand for the Innate Host Defense System Serum Antibodies to Porphyromonas Gingivalis Block the Prostaglandin E2 Response to Lipopolysaccharide by Mononuclear Cells. Ann. Periodontol. 7, 29–37. doi: 10.1902/annals.2002.7.1.29
Bainbridge, B. W., Darveau, R. P. (2001). Porphyromonas Gingivalis Lipopolysaccharide: An Unusual Pattern Recognition Receptor Ligand for the Innate Host Defense System. Acta Odontol. Scand. 59, 131–138. doi: 10.1080/000163501750266710
Balli, U., Keles, G. C., Cetinkaya, B. O., Mercan, U., Ayas, B., Erdogan, D. (2014). Assessment of Vascular Endothelial Growth Factor and Matrix Metalloproteinase-9 in the Periodontium of Rats Treated With Atorvastatin. J. Periodontol. 85, 178–187. doi: 10.1902/jop.2013.130018
Batschkus, S., Cingoez, G., Urlaub, H., Miosge, N., Kirschneck, C., Meyer-Marcotty, P., et al. (2018). A New Albumin-Depletion Strategy Improves Proteomic Research of Gingival Crevicular Fluid From Periodontitis Patients. Clin. Oral. Investig. 22 (3), 1375–84. doi: 10.1007/s00784-017-2213-0
Belanger, M., Kozarov, E., Song, H., Whitlock, J., Progulske-Fox, A. (2012). Both the Unique and Repeat Regions of the Porphyromonas Gingivalis Hemagglutin A Are Involved in Adhesion and Invasion of Host Cells. Anaerobe 18, 128–134. doi: 10.1016/j.anaerobe.2011.10.005
Caton, J. G., Armitage, G., Berglundh, T., Chapple, I. L. C., Jepsen, S., Kornman, K. S., et al. (2018). A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions - Introduction and Key Changes From the 1999 Classification. J. Periodontol. 89 Suppl 1, S1–S8. doi: 10.1111/jcpe.12935
Chandy, S., Joseph, K., Sankaranarayanan, A., Issac, A., Babu, G., Wilson, B., et al. (2017). Evaluation of C-Reactive Protein and Fibrinogen in Patients With Chronic and Aggressive Periodontitis: A Clinico-Biochemical Study. J. Clin. Diagn. Res. 11, Zc41–zc45. doi: 10.7860/JCDR/2017/23100.9552
Courtois, A., Nusgens, B. V., Hustinx, R., Namur, G., Gomez, P., Somja, J., et al. (2013). 18f-FDG Uptake Assessed by PET/CT in Abdominal Aortic Aneurysms Is Associated With Cellular and Molecular Alterations Prefacing Wall Deterioration and Rupture. J. Nucl. Med. 54, 1740–1747. doi: 10.2967/jnumed.112.115873
D’Aiuto, F., Parkar, M., Tonetti, M. S. (2007). Acute Effects of Periodontal Therapy on Bio-Markers of Vascular Health. J. Clin. Periodontol. 34, 124–129. doi: 10.1111/j.1600-051X.2006.01037.x
Davison, E., Johnston, W., Piela, K., Rosier, B. T., Paterson, M., Mira, A., et al. (2021). The Subgingival Plaque Microbiome, Systemic Antibodies Against Bacteria and Citrullinated Proteins Following Periodontal Therapy. Pathogens 10 (2), 193. doi: 10.3390/pathogens10020193
Deshpande, R. G., Khan, M., Genco, C. A. (1998a). Invasion Strategies of the Oral Pathogen Porphyromonas Gingivalis: Implications for Cardiovascular Disease. Invasion Metastasis 18, 57–69. doi: 10.1159/000024499
Deshpande, R. G., Khan, M. B., Genco, C. A. (1998b). Invasion of Aortic and Heart Endothelial Cells by Porphyromonas Gingivalis. Infect. Immun. 66, 5337–5343. doi: 10.1128/IAI.66.11.5337-5343.1998
Dye, B. A., Herrera-Abreu, M., Lerche-Sehm, J., Vlachojannis, C., Pikdoken, L., Pretzl, B., et al. (2009). Serum Antibodies to Periodontal Bacteria as Diagnostic Markers of Periodontitis. J. Periodontol. 80, 634–647. doi: 10.1902/jop.2009.080474
Ebersole, J. L., Stevens, J., Steffen, M. J., Dawson Iii, D., Novak, M. J. (2010). Systemic Endotoxin Levels in Chronic Indolent Periodontal Infections. J. Periodontal Res. 45, 1–7. doi: 10.1111/j.1600-0765.2008.01169.x
Fan, Q., Sims, T., Sojar, H., Genco, R., Page, R. C. (2001). Fimbriae of Porphyromonas Gingivalis Induce Opsonic Antibodies That Significantly Enhance Phagocytosis and Killing by Human Polymorphonuclear Leukocytes. Oral. Microbiol. Immunol. 16, 144–152. doi: 10.1034/j.1399-302x.2001.016003144.x
Fedele, S., Sabbah, W., Donos, N., Porter, S., D’Aiuto, F. (2011). Common Oral Mucosal Diseases, Systemic Inflammation, and Cardiovascular Diseases in a Large Cross-Sectional US Survey. Am. Heart J. 161, 344–350. doi: 10.1016/j.ahj.2010.11.009
Finoti, L. S., Nepomuceno, R., Pigossi, S. C., Corbi, S. C., Secolin, R., Scarel-Caminaga, R. M. (2017). Association Between Interleukin-8 Levels and Chronic Periodontal Disease: A PRISMA-Compliant Systematic Review and Meta-Analysis. Med. (Baltimore) 96, e6932. doi: 10.1097/MD.0000000000006932
Forner, L., Larsen, T., Kilian, M., Holmstrup, P. (2006). Incidence of Bacteremia After Chewing, Tooth Brushing and Scaling in Individuals With Periodontal Inflammation. J. Clin. Periodontol. 33, 401–407. doi: 10.1111/j.1600-051X.2006.00924.x
Goteiner, D., Craig, R. G., Ashmen, R., Janal, M. N., Eskin, B., Lehrman, N. (2008). Endotoxin Levels Are Associated With High-Density Lipoprotein, Triglycerides, and Troponin in Patients With Acute Coronary Syndrome and Angina: Possible Contributions From Periodontal Sources. J. Periodontol. 79, 2331–2339. doi: 10.1902/jop.2008.080068
Haffajee, A. D., Socransky, S. S. (1994). Microbial Etiological Agents of Destructive Periodontal Diseases. Periodontol. 2000 5, 78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x
Hajishengallis, G., Krauss, J. L., Liang, S., McIntosh, M. L., Lambris, J. D. (2012). Pathogenic Microbes and Community Service Through Manipulation of Innate Immunity. Adv. Exp. Med. Biol. 946, 69–85. doi: 10.1007/978-1-4614-0106-3_5
Halme, S., Juvonen, T., Laurila, A., Juvonen, J., Mosorin, M., Saikku, P., et al. (1999). Chlamydia Pneumoniae Reactive T Lymphocytes in the Walls of Abdominal Aortic Aneurysms. Eur. J. Clin. Invest. 29, 546–552. doi: 10.1046/j.1365-2362.1999.00463.x
Hamp, S. E., Nyman, S., Lindhe, J. (1975). Periodontal Treatment of Multirooted Teeth. Results After 5 Years. J. Clin. Periodontol. 2, 126–135. doi: 10.1111/j.1600-051X.1975.tb01734.x
Haruyama, K., Yoshimura, A., Naito, M., Kishimoto, M., Shoji, M., Abiko, Y., et al. (2009). Identification of a Gingipain-Sensitive Surface Ligand of Porphyromonas Gingivalis That Induces Toll-Like Receptor 2- and 4-Independent NF-kappaB Activation in CHO Cells. Infect. Immun. 77, 4414–4420. doi: 10.1128/IAI.00140-09
Hellmann, D. B., Grand, D. J., Freischlag, J. A. (2007). Inflammatory Abdominal Aortic Aneurysm. Jama 297, 395–400. doi: 10.1001/jama.297.4.395
Hosomi, N., Aoki, S., Matsuo, K., Deguchi, K., Masugata, H., Murao, K., et al. (2012). Association of Serum Anti-Periodontal Pathogen Antibody With Ischemic Stroke. Cerebrovasc. Dis. 34, 385–392. doi: 10.1159/000343659
Kassebaum, N. J., Bernabe, E., Dahiya, M., Bhandari, B., Murray, C. J., Marcenes, W. (2014). Global Burden of Severe Tooth Loss: A Systematic Review and Meta-Analysis. J. Dent. Res. 93, 20s–28s. doi: 10.1177/0022034514537828
Keles, Z. P., Keles, G. C., Avci, B., Cetinkaya, B. O., Emingil, G. (2014). Analysis of YKL-40 Acute-Phase Protein and Interleukin-6 Levels in Periodontal Disease. J. Periodontol. 85, 1240–1246. doi: 10.1902/jop.2014.130631
Kuivaniemi, H., Ryer, E. J., Elmore, J. R., Tromp, G. (2015). Understanding the Pathogenesis of Abdominal Aortic Aneurysms. Expert Rev. Cardiovasc. Ther. 13, 975–987. doi: 10.1586/14779072.2015.1074861
Laine, M. L., Appelmelk, B. J., van Winkelhoff, A. J. (1997). Prevalence and Distribution of Six Capsular Serotypes of Porphyromonas Gingivalis in Periodontitis Patients. J. Dent. Res. 76, 1840–1844. doi: 10.1177/00220345970760120601
Lee, H. J., Choi, E. K., Park, J. B., Han, K. D., Oh, S. (2019). Tooth Loss Predicts Myocardial Infarction, Heart Failure, Stroke, and Death. J. Dent. Res. 98, 164–170. doi: 10.1177/0022034518814829
Liljestrand, J. M., Havulinna, A. S., Paju, S., Mannisto, S., Salomaa, V., Pussinen, P. J. (2015). Missing Teeth Predict Incident Cardiovascular Events, Diabetes, and Death. J. Dent. Res. 94, 1055–1062. doi: 10.1177/0022034515586352
Lima, P. M., Souza, P. E., Costa, J. E., Gomez, R. S., Gollob, K. J., Dutra, W. O. (2011). Aggressive and Chronic Periodontitis Correlate With Distinct Cellular Sources of Key Immunoregulatory Cytokines. J. Periodontol. 82, 86–95. doi: 10.1902/jop.2010.100248
Lindholt, J. S., Juul, S., Vammen, S., Lind, I., Fasting, H., Henneberg, E. W. (1999). Immunoglobulin A Antibodies Against Chlamydia Pneumoniae Are Associated With Expansion of Abdominal Aortic Aneurysm. Br. J. Surg. 86, 634–638. doi: 10.1046/j.1365-2168.1999.01126.x
Loos, B. G. (2005). Systemic Markers of Inflammation in Periodontitis. J. Periodontol. 76, 2106–2115. doi: 10.1902/jop.2005.76.11-S.2106
Loos, B. G., Craandijk, J., Hoek, F. J., Wertheim-van Dillen, P. M., van der Velden, U. (2000). Elevation of Systemic Markers Related to Cardiovascular Diseases in the Peripheral Blood of Periodontitis Patients. J. Periodontol. 71, 1528–1534. doi: 10.1902/jop.2000.71.10.1528
Martinez-Pinna, R., Madrigal-Matute, J., Tarin, C., Burillo, E., Esteban-Salan, M., Pastor-Vargas, C., et al. (2013). Proteomic Analysis of Intraluminal Thrombus Highlights Complement Activation in Human Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 33, 2013–2020. doi: 10.1161/ATVBAHA.112.301191
Mealey, B. L., van Winkelhoff, A. J., Slots, J. (1999). Influence of Periodontal Infections on Systemic Health Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis in Nonoral Infections. Periodontol. 2000 21, 197–209. doi: 10.1111/j.1600-0757.1999.tb00176.x
Meyle, J., Chapple, I. (2015). Molecular Aspects of the Pathogenesis of Periodontitis. Periodontol. 2000 69, 7–17. doi: 10.1111/prd.12104
Morbelli, S., Ghigliotti, G., Spinella, G., Marini, C., Bossert, I., Cimmino, M., et al. (2014). Systemic Vascular Inflammation in Abdominal Aortic Aneurysm Patients: A Contrast-Enhanced PET/CT Study. Q J. Nucl. Med. Mol. Imaging 58, 299–309.
Nakagawa, S., Machida, Y., Nakagawa, T., Fujii, H., Yamada, S., Takazoe, I., et al. (1994). Infection by Porphyromonas Gingivalis and Actinobacillus Actinomycetemcomitans, and Antibody Responses at Different Ages in Humans. J. Periodontal Res. 29, 9–16. doi: 10.1111/j.1600-0765.1994.tb01085.x
Nassar, H., Chou, H. H., Khlgatian, M., Gibson, F. C., 3rd, Van Dyke, T. E., Genco, C. A. (2002). Role for Fimbriae and Lysine-Specific Cysteine Proteinase Gingipain K in Expression of Interleukin-8 and Monocyte Chemoattractant Protein in Porphyromonas Gingivalis-Infected Endothelial Cells. Infect. Immun. 70, 268–276. doi: 10.1128/IAI.70.1.268-276.2002
Nesse, W., Abbas, F., van der Ploeg, I., Spijkervet, F. K., Dijkstra, P. U., Vissink, A. (2008). Periodontal Inflamed Surface Area: Quantifying Inflammatory Burden. J. Clin. Periodontol. 35, 668–673. doi: 10.1111/j.1600-051X.2008.01249.x
Nibali, L., Pelekos, G., D’Aiuto, F., Chaudhary, N., Habeeb, R., Ready, D., et al. (2013). Influence of IL-6 Haplotypes on Clinical and Inflammatory Response in Aggressive Periodontitis. Clin. Oral. Investig. 17, 1235–1242. doi: 10.1007/s00784-012-0804-3
Nicu, E. A., Laine, M. L., Morre, S. A., van der Velden, U., Loos, B. G. (2009). Soluble CD14 in Periodontitis. Innate Immun. 15, 121–128. doi: 10.1177/1753425908101577
Palm, F., Lahdentausta, L., Sorsa, T., Tervahartiala, T., Gokel, P., Buggle, F., et al. (2014). Biomarkers of Periodontitis and Inflammation in Ischemic Stroke: A Case-Control Study. Innate Immun. 20, 511–518. doi: 10.1177/1753425913501214
Papapanou, P. N., Neiderud, A. M., Papadimitriou, A., Sandros, J., Dahlén, G. (2000). “Checkerboard” Assessments of Periodontal Microbiota and Serum Antibody Responses: A Case-Control Study. J. Periodontol. 71, 885–897. doi: 10.1902/jop.2000.71.6.885
Paraskevas, S., Huizinga, J. D., Loos, B. G. (2008). A Systematic Review and Meta-Analyses on C-Reactive Protein in Relation to Periodontitis. J. Clin. Periodontol. 35, 277–290. doi: 10.1111/j.1600-051X.2007.01173.x
Pussinen, P. J., Alfthan, G., Rissanen, H., Reunanen, A., Asikainen, S., Knekt, P. (2004a). Antibodies to Periodontal Pathogens and Stroke Risk. Stroke 35, 2020–2023. doi: 10.1161/01.STR.0000136148.29490.fe
Pussinen, P. J., Alfthan, G., Tuomilehto, J., Asikainen, S., Jousilahti, P. (2004b). High Serum Antibody Levels to Porphyromonas Gingivalis Predict Myocardial Infarction. Eur. J. Cardiovasc. Prev. Rehabil. 11, 408–411. doi: 10.1097/00149831-200410000-00008
Pussinen, P. J., Jousilahti, P., Alfthan, G., Palosuo, T., Asikainen, S., Salomaa, V. (2003). Antibodies to Periodontal Pathogens Are Associated With Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 23, 1250–1254. doi: 10.1161/01.ATV.0000072969.71452.87
Pussinen, P. J., Kononen, E., Paju, S., Hyvarinen, K, Gursoy, U. K., Huumonen, S., et al. (2011). Periodontal Pathogen Carriage, Rather Than Periodontitis, Determines the Serum Antibody Levels. J. Clin. Periodontol. 38, 405–411. doi: 10.1111/j.1600-051X.2011.01703.x
Pussinen, P. J., Nyyssonen, K., Alfthan, G., Salonen, R., Laukkanen, J. A., Salonen, J. T. (2005). Serum Antibody Levels to Actinobacillus Actinomycetemcomitans Predict the Risk for Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 25, 833–838. doi: 10.1161/01.ATV.0000157982.69663.59
Rompen, E. H., Geerts, S. O., J, C. (2001). Systemic Impactof Periodontal Infections: Definition of a New Periodon-Tal Index (in French) Belgium:Revue Médicale de Liège 29. Eds. Rompen, E. H., Geerts, S. O., Charpentier, J., 3–8.
Sakalihasan, N., Limet, R., Defawe, O. D. (2005). Abdominal Aortic Aneurysm. Lancet 365, 1577–1589. doi: 10.1016/S0140-6736(05)66459-8
Sakalihasan, N., Michel, J. B., Katsargyris, A., Kuivaniemi, H., Defraigne, J. O., Nchimi, A., et al. (2018). Abdominal Aortic Aneurysms. Nat. Rev. Dis. Primers 4, 34. doi: 10.1038/s41572-018-0030-7
Salhi, L., Rompen, E., Sakalihasan, N., Laleman, I., Teughels, W., Michel, J. B., et al. (2019). Can Periodontitis Influence the Progression of Abdominal Aortic Aneurysm? A Systematic Review. Angiology 70, 479–491. doi: 10.1177/0003319718821243
Salhi, L., Sakalihasan, N., Okroglic, A. G., Labropoulos, N., Seidel, L., Albert, A., et al. (2020). Further Evidence on the Relationship Between Abdominal Aortic Aneurysm and Periodontitis: A Cross-Sectional Study. J. Periodontol. 91, 1453–1464. doi: 10.1002/JPER.19-0671
Sanz, M., Ceriello, A., Buysschaert, M., Chapple, I., Demmer, R. T., Graziani, F., et al. (2018). Scientific Evidence on the Links Between Periodontal Diseases and Diabetes: Consensus Report and Guidelines of the Joint Workshop on Periodontal Diseases and Diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 45, 138–149. doi: 10.1111/jcpe.12808
Shaddox, L. M., Goncalves, P. F., Vovk, A., Allin, N., Huang, H., Hou, W., et al. (2013). LPS-Induced Inflammatory Response After Therapy of Aggressive Periodontitis. J. Dent. Res. 92, 702–708. doi: 10.1177/0022034513495242
Silness, J., Loe, H. (1964). Periodontal Disease in Pregnancy. II. Correlation Between Oral Hygiene and Periodontal Condtion. Acta Odontol. Scand. 22, 121–135. doi: 10.3109/00016356408993968
Socransky, S. S., Haffajee, A. D., Cugini, M. A., Smith, C., Kent, R. L., Jr. (1998). Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 25, 134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x
Sweeting, M. J., Thompson, S. G., Brown, L. C., Powell, J. T. (2012). Meta-Analysis of Individual Patient Data to Examine Factors Affecting Growth and Rupture of Small Abdominal Aortic Aneurysms. Br. J. Surg. 99, 655–665. doi: 10.1002/bjs.8707
Tabeta, K., Tanabe, N., Yonezawa, D., Miyashita, H., Maekawa, T., Takahashi, N., et al. (2011). Elevated Antibody Titers to Porphyromonas Gingivalis as a Possible Predictor of Ischemic Vascular Disease - Results From the Tokamachi-Nakasato Cohort Study. J. Atheroscler. Thromb. 18, 808–817. doi: 10.5551/jat.6957
Tambyraja, A. L., Dawson, R., Valenti, D., Murie, J. A., Chalmers, R. T. (2007). Systemic Inflammation and Repair of Abdominal Aortic Aneurysm. World J. Surg. 31, 1210–1214. doi: 10.1007/s00268-007-9014-6
Tan, K. S., Song, K. P., Ong, G. (2002). Cytolethal Distending Toxin of Actinobacillus Actinomycetemcomitans. Occurrence and Association With Periodontal Disease. J. Periodontal Res. 37, 268–272. doi: 10.1034/j.1600-0765.2002.01618.x
Teughels, W., Durukan, A., Ozcelik, O., Pauwels, M., Quirynen, M., Haytac, M. C. (2013). Clinical and Microbiological Effects of Lactobacillus Reuteri Probiotics in the Treatment of Chronic Periodontitis: A Randomized Placebo-Controlled Study. J. Clin. Periodontol. 40, 1025–1035. doi: 10.1111/jcpe.12155
Tonetti, M. S., D’Aiuto, F., Nibali, L., Donald, A., Storry, C., Parkar, M., et al. (2007). Treatment of Periodontitis and Endothelial Function. N Engl. J. Med. 356, 911–920. doi: 10.1056/NEJMoa063186
Tonetti, M. S., Van Dyke, T. E. (2013). Periodontitis and Atherosclerotic Cardiovascular Disease: Consensus Report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 84, S24–S29. doi: 10.1111/jcpe.12089
Vlachojannis, C., Dye, B. A., Herrera-Abreu, M., Pikdöken, L., Lerche-Sehm, J., Pretzl, B., et al. (2010). Determinants of Serum IgG Responses to Periodontal Bacteria in a Nationally Representative Sample of US Adults. J. Clin. Periodontol. 37, 685–696. doi: 10.1111/j.1600-051X.2010.01592.x
Keywords: periodontitis, periodontitis systemic interaction, microbiome, inflammation and innate immunity, abdominal aortic aneurysm (AAA)
Citation: Salhi L, Rijkschroeff P, Van Hede D, Laine ML, Teughels W, Sakalihasan N and Lambert F (2022) Blood Biomarkers and Serologic Immunological Profiles Related to Periodontitis in Abdominal Aortic Aneurysm Patients. Front. Cell. Infect. Microbiol. 11:766462. doi: 10.3389/fcimb.2021.766462
Received: 29 August 2021; Accepted: 20 December 2021;
Published: 14 January 2022.
Edited by:
Julien Santi-Rocca, Science and Healthcare for Oral Welfare, FranceReviewed by:
Anders Gottsäter, Lund University, SwedenAlessandro Polizzi, University of Catania, Italy
Eija Könönen, University of Turku, Finland
Copyright © 2022 Salhi, Rijkschroeff, Van Hede, Laine, Teughels, Sakalihasan and Lambert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leila Salhi, bC5zYWxoaUBjaHVsaWVnZS5iZQ==; orcid.org/0000-0003-3529-8452
 Leila Salhi
Leila Salhi Patrick Rijkschroeff
Patrick Rijkschroeff Dorien Van Hede1
Dorien Van Hede1 Marja L. Laine
Marja L. Laine Natzi Sakalihasan
Natzi Sakalihasan