
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 05 November 2021
Sec. Microbes and Innate Immunity
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.733564
This article is part of the Research Topic Innate Immune Cells in the Control of Intracellular Bacteria View all 6 articles
 Noha Mousaad Elemam1,2*†
Noha Mousaad Elemam1,2*† Rakhee K. Ramakrishnan1,2†
Rakhee K. Ramakrishnan1,2† Jennifer E. Hundt3
Jennifer E. Hundt3 Rabih Halwani1,2,4
Rabih Halwani1,2,4 Azzam A. Maghazachi1,2
Azzam A. Maghazachi1,2 Qutayba Hamid1,2,5*
Qutayba Hamid1,2,5*Infectious diseases represent one of the largest medical challenges worldwide. Bacterial infections, in particular, remain a pertinent health challenge and burden. Moreover, such infections increase over time due to the continuous use of various antibiotics without medical need, thus leading to several side effects and bacterial resistance. Our innate immune system represents our first line of defense against any foreign pathogens. This system comprises the innate lymphoid cells (ILCs), including natural killer (NK) cells that are critical players in establishing homeostasis and immunity against infections. ILCs are a group of functionally heterogenous but potent innate immune effector cells that constitute tissue-resident sentinels against intracellular and extracellular bacterial infections. Being a nascent subset of innate lymphocytes, their role in bacterial infections is not clearly understood. Furthermore, these pathogens have developed methods to evade the host immune system, and hence permit infection spread and tissue damage. In this review, we highlight the role of the different ILC populations in various bacterial infections and the possible ways of immune evasion. Additionally, potential immunotherapies to manipulate ILC responses will be briefly discussed.
Bacterial infections were, are and will continue to remain a pertinent health challenge and burden. The human body is under constant exposure to a plethora of bacteria, including commensal and pathogenic bacterial species. The immune system is equipped to ward off pathogenic bacteria while maintaining symbiosis with the commensal flora. However, some bacterial species have evolved to evade the host protective responses and establish infections.
Innate lymphoid cells (ILCs), are innate lymphocytes that lack adaptive antigen receptors (Artis and Spits, 2015). Nevertheless, they are equipped with a wide array of activating and inhibitory receptors. During fetal development, a subset of ILCs functions as lymphoid tissue-inducer cells (LTi cells), that induce lymphoid organogenesis and are involved in the formation of secondary lymphoid organs (Mebius et al., 1997). Although ILCs do not undergo antigen priming, they immediately respond in an antigen-independent manner either upon engaging their germline-encoded receptors or through cytokine stimulation resulting in effector cytokine secretion (Glatzer et al., 2013).
ILCs are basically classified into three subgroups, namely group 1 innate lymphoid cells (ILC1s) including natural killer (NK) cells and interferon (IFN)-γ secreting ILC1s, group 2 innate lymphoid cells (ILC2s), and group 3 innate lymphoid cells (ILC3s), based on their lineage-defining transcription factors and cytokine secretion profiles (Spits et al., 2013; Elemam et al., 2017). These subgroups are largely considered as the innate counterparts of CD4+ T helper (Th)1, Th2, and Th17 cells respectively, while the NK cells are analogous to CD8+ cytotoxic T cells (Vivier et al., 2018). As such, the differentiation and function of ILC1s in response to IL-12 and IL-18 depend on the expression of transcription factor T-box expressed in T cells (T-bet, also called as TBX21), while NK cells depend on the transcription factor eomesodermin (Eomes) resulting in the production of IFN-γ and tumor necrosis factor (TNF)-α (Spits et al., 2013). Like Th1 cells, ILC1s respond to intracellular pathogens such as bacteria and viruses. On the other hand, ILC2s express GATA binding protein 3 (GATA3) and produce cytokines IL-4, IL-13, IL-5 and IL-9, in response to IL-25 and IL-33. Together with Th2 cells, ILC2s are involved in responses to extracellular parasites/helminths, allergens and tissue repair. ILC3s express retinoic acid-related orphan receptor gamma t (ROR-γt) in response to IL-23 and IL-1β, and produce IL-22, IL-17, granulocyte-macrophage colony-stimulating factor (GM-CSF), and lymphotoxin (LT)-α1β2. ILC3s and Th17 cells respond to extracellular pathogenic bacteria and fungi. Therefore, the various ILC subsets, respond to a wide array of pathogens ranging from bacteria, parasites, viruses and fungi, albeit in a subset-specific manner. It is also worth mentioning that plasticity exists among these cells in order for them to adapt their transcriptional profile to the local microenvironment cues and specific cytokine exposure (Vivier et al., 2018).
In contrast to T cells, ILCs respond quickly to stress signals from tissue-resident cells. Their production of effector cytokines helps activate and regulate the activity of both innate and adaptive immune cells such as T cells, B cells, dendritic cells (DCs), eosinophils, neutrophils, macrophages, and epithelial cells (Artis and Spits, 2015). ILCs work in synergy with T cells where they interact and cross-regulate each other, thus amplifying their response. Nevertheless, they also compete with each other for the same growth factors and inducer cytokines.
Developmentally, ILCs are programed to migrate, differentiate and populate mucosal tissues and lymphoid tissues. They are primarily tissue-resident cells that are constitutively present in mucosal tissues, such as the respiratory and gastrointestinal tracts. The presence of ILCs in close proximity to mucosal barriers leads to their exposure to a wide variety of both commensal and pathogenic bacteria. Their interaction with the microbiome is important for the maintenance of tissue homeostasis. The orchestration of the relationship between host and commensal bacteria in turn influences the homeostasis of ILCs (Sonnenberg and Artis, 2012). Further, these cells are characterized by their rapid response in mucosal defense against pathogenic bacteria and in orchestrating other immune cells.
ILCs are crucial for mucosal tissue homeostasis and any dysregulation of these cells could lead to a broad spectrum of diseases, including bacterial infections. Multiple interactions with various cell types regulate the function of ILCs. For instance, neuroimmune circuits have been shown to integrate extrinsic environmental signals such as light-dark cycles and nutrient intake, to orchestrate ILC responses at barrier surfaces to harmonize immunity. There is a reported interaction between ILCs and the enteric nervous system (ENS), leading to ILC activation and cytokine secretion (Han et al., 2019). On another note, neurons and enteric glial cells (EGCs) were found to interact with ILC3s through neurotrophic factor signals, thus protecting the intestinal lining against inflammation and microbial infection (Bessac et al., 2018). Also, enteric neurons express and sense cytokines such as TSLP, IL-4, and IL-31, where they crosstalk with ILCs, thus promoting a type 2 response (Wilson et al., 2013; Oetjen et al., 2017). In addition, the circadian rhythm controls ILC2 and ILC3 activation in the intestine to regulate intestinal homeostasis and gut defense (Godinho-Silva et al., 2019; Talbot et al., 2020). There is a proposed connection between ENS, gut microbiota and ILC (Rolig et al., 2017). Furthermore, the neuropeptide vasoactive intestinal peptide (VIP) expressed by enteric neurons exerts both stimulatory and inhibitory effects on CCR6+ ILC3s and its functional role appears to be context-dependent and impacted by the commensal microbiota. On the other hand, a direct stimulation of ILC2s by neurons through the released neuronal messenger neuromedin U was reported, causing an induced immune response (Han et al., 2019). In this review, we highlight the role of ILCs in various bacterial infections and their possible evasion by bacteria. Additionally, potential immunotherapies to manipulate ILC responses will be briefly discussed.
ILCs are a crucial component of the innate and adaptive immune responses to bacterial infections. The type of pathogen largely determines the selective ILC response during infections. As pointed out earlier, intracellular bacteria elicit mainly an ILC1 and some ILC3 response, while extracellular bacterial and fungal infections stimulate primarily the ILC3 subset. ILC2s are involved in response to parasitic infections and tissue repair in response to viral-induced tissue injury. ILC secretion of a variety of chemokines and cytokines results in the recruitment of other immune players and amplification of the inflammatory response against these pathogens. There has been extensive research on the role of ILCs in viral and parasitic infections as reviewed in (Diefenbach, 2013; Vivier et al., 2018; Hildreth and O'Sullivan, 2019; Hirose et al., 2019; Loser et al., 2019; Panda and Colonna, 2019; Seo et al., 2020). Here, we discuss the involvement of the ILC subsets across various bacterial infections (Figure 1).
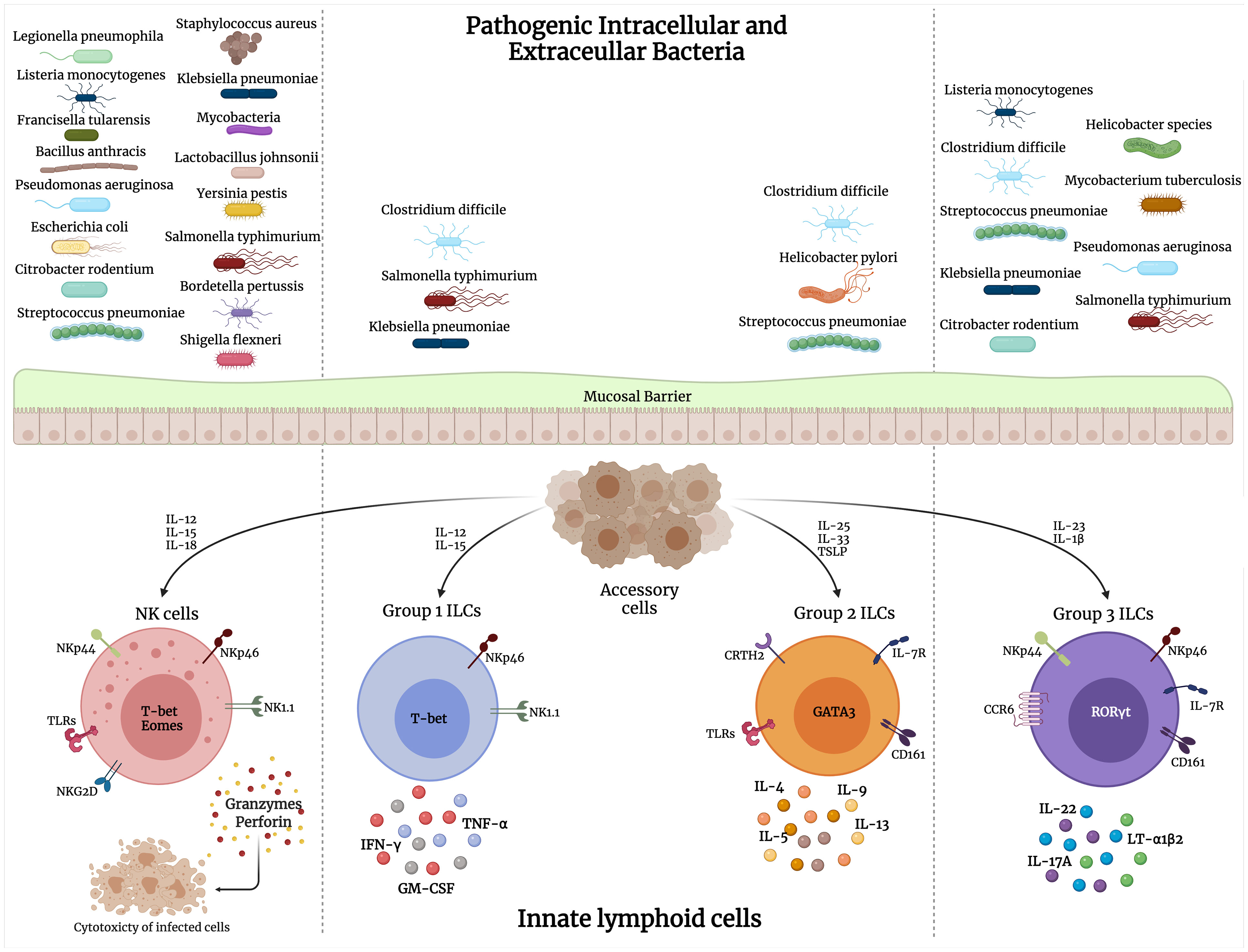
Figure 1 Interaction of pathogenic intracellular and extracellular bacteria with different groups of innate lymphoid cells (ILCs). Upon bacterial stimulation, various accessory cells secrete cytokines that activate ILCs to perform their direct cytolytic activity on bacteria/infected cells, or release cytokines that mediate bacterial clearance.
Numerous studies have reported that intracellular and extracellular bacteria activate NK cells (Harrington et al., 2007; Small et al., 2008; Souza-Fonseca-Guimaraes et al., 2012; Schmidt et al., 2016). These include Listeria monocytogenes, Francisella tularensis, Chlamydia pneumoniae, and Yersinia enterocolitica as well as sepsis (Bohn and Autenrieth, 1996; López et al., 2004; Berg et al., 2005; Thäle and Kiderlen, 2005; Etogo et al., 2008). The anti-bacterial potential of NK cells was reported in various bacterial infections, where NK cells were able to lyse Shigella flexneri, Legionella pneumophila, Mycobacterium lepraemurium or Mycobacterium avium infected monocytes (Klimpel et al., 1986; Blanchard et al., 1987; Katz et al., 1990; Denis, 1991). Also, NK cells have displayed bactericidal effects against macrophages infected with intracellular bacilli (Bermudez et al., 1990). Activated NK cells were found in the airways of mice infected with Staphylococcus aureus or Bordetella pertussis where they were found to play crucial roles in bacterial clearance (Byrne et al., 2004; Small et al., 2008). The immunoregulatory role of NK cells include cytokine production, including IFN-γ, GM-CSF, and TNF-α, that contribute to the inflammatory environment during an infection (Huntington et al., 2007; Lünemann et al., 2009). Further, upon activation with cytokines such as IL-12, IL-15 or IL-18, NK cells can also release IL-6, IL-10, transforming growth factor (TGF)-β, IL-17 and IL-22 (Jewett et al., 1996; Cella et al., 2009; Perona-Wright et al., 2009; Hall et al., 2010; Passos et al., 2010), as well as various chemokines (Maghazachi and Al-Aoukaty, 1998; Fehniger et al., 1999; Maghazachi, 2010). Additionally, the cytotoxic molecules released by NK cells (perforin and granzymes) possess an anti-bacterial effect on intracellular and extracellular bacteria such as L. monocytogenes, Salmonella typhimurium, Bacillus anthracis, Escherichia coli, S. aureus and Mycobacterium tuberculosis (Stenger et al., 1998; Ernst et al., 2000; Endsley et al., 2009; Gonzales et al., 2012; Lu et al., 2014). Another cytotoxic molecule secreted by NK cells is granulysin that disrupts the membrane of bacteria and has potent anti-microbial activity against various gram-positive and gram-negative bacterial species (Krensky and Clayberger, 2009; McSharry and Gardiner, 2010). A possible mechanism of action of the bactericidal granulysin could be via inducing lesions and distortions in the bacterial membrane (Stenger et al., 1998). Further, other studies reported that granulysin interferes with oxidative metabolism and energy generation by the bacteria (Krensky and Clayberger, 2009). For instance, granulysin was reported to directly kill the extracellular M. tuberculosis by altering the membrane integrity of the bacillus, while it further decreased the viability of intracellular M. tuberculosis when combined with perforin (Stenger et al., 1998).
During an infection, the crosstalk between NK cells and other accessory cells, such as DCs or macrophages, enables them to perform their anti-microbial activity. There was a reported indirect activation of NK cells by various types of bacteria including L. monocytogenes, S. aureus, Lactobacillus johnsonii, and Mycobacterium infections. This could be due to the recognition by mature DCs and secretion of cytokines such as IL-12, IL-18, and type-1 interferons (Nomura et al., 2002; Newman et al., 2006). IL-18 is a pro-inflammatory cytokine that is crucial in restriction of bacterial growth as reported by studies with hindrance of neutrophil-mediated lung damage in M. tuberculosis infected mice as well as Legionella pneumophila infection (Spörri et al., 2008; Schneider et al., 2010). Moreover, IL-18 triggered NK cell activity and IFN-γ production upon administration of lipopolysaccharide (LPS) and in Propioni-bacterium acnes infection (Takeda et al., 1998). Also, IL-18 was found to trigger γδT cells to produce IL-17A, which promotes IFN-γ production by NK cells upon injection of LPS (Andrews et al., 2011).
The secreted mediators by NK cells possess potent anti-bacterial activity against a variety of gram-negative and gram-positive bacteria (Garcia-Peñarrubia et al., 1989). At the same time, NK cells are able to halt their own activation in fighting bacterial infections including L. monocytogenes and Yersinia pestis. For instance, NK cells can secrete the regulatory cytokine IL-10 which inhibits IL-12 secretion by DCs (Perona-Wright et al., 2009). Such a reaction could be required to prevent immune pathology during systemic infections. In enteric bacterial infections, it was found that NK cells play a vital role in bacterial clearance such as that observed in Citrobacter rodentium infection (Hall et al., 2013). This could be done through cytokine release, direct cytotoxic effects to C. rodentium and activation of other innate and adaptive immune cells, leading to prevention of bacterial dissemination into the systemic circulation (Hall et al., 2013). Similarly, this NK cell behavior was observed in the infection of the lungs with M. tuberculosis (Feng et al., 2006). Further, NK cells are able to control murine M. tuberculosis infections upon their activation with various cytokines, such as IL-12, IL-18, and IL-2 from CD4+ T cells (Evans et al., 2011). Another possible way of NK cell elimination of M. tuberculosis is through the secretion of IL-22 upon stimulation with IL-15 and IL-23 (Dhiman et al., 2009). Upon activation with IL-12, NK cells secrete IFN-γ, leading to the elimination of bacterial infections including those caused by Y. enterocolitica, S. typhimurium, and L. monocytogenes, as well as stimulation with LPS (Hunter et al., 1995; Bohn and Autenrieth, 1996; Mastroeni et al., 1996; van de Wetering et al., 2009). Furthermore, macrophages promote NK cell activation by upregulation of surface CD69 upon exposure to LPS as well as NK cell production of IFN-γ in Legionella pneumophila and L. monocytogenes infections (Blanchard et al., 1988; Wherry et al., 1991; Scott et al., 2004). In turn, IFN-γ can favor the production of IL-12 from macrophages as reported in Mycobacterium bovis infection (Matsumoto et al., 1997). Being the main cytokine released by NK cells, IFN-γ, was reported to play a crucial role in fighting bacterial infections by inducing macrophage-mediated phagocytosis of bacteria or infected cells (McSharry and Gardiner, 2010; Horowitz et al., 2012). This was highlighted by a study where IFN-γ deficient mice exhibited an increase in the bacterial load and impairment in the anti-bacterial immune response upon infection with Legionella pnemophilia, L. monocytogenes, or mycobacteria (Cooper et al., 1993; Huang et al., 1993; Spörri et al., 2006). Moreover, IFN-γ could affect chemokine production and recruitment of immune cells to S. aureus infected tissues (McLoughlin et al., 2008). Besides, IFN-γ affected the availability of iron that is an essential nutrient needed for bacterial replication, as reported in S. typhimurium infected macrophages (Nairz et al., 2008). In addition, NK cells and specifically IFN-γ can modulate the maturation and activation of other adaptive immune cells populations by regulating the antigen presentation function (Degli-Esposti and Smyth, 2005; Nedvetzki et al., 2007; Hall et al., 2010; Horowitz et al., 2012). Another possible mechanism of host protection by IFN-γ, is the formation of granuloma post-infection with intracellular bacteria, such as Mycobacterium avium and Francisella tularensis, thus isolating infectious lesions (Smith et al., 1997; Bokhari et al., 2008).
Another way of activation of NK cells is through their expression of pathogen recognition receptors (PRRs) which bind to pathogen-associated molecular patterns (PAMPs) on the surface of certain bacteria (Chalifour et al., 2004; Souza-Fonseca-Guimaraes et al., 2012; Adib-Conquy et al., 2014). In response, several cytokines are released by NK cells that contribute to the cytokine storm present in infections (Cavaillon et al., 2003; Hargreaves and Medzhitov, 2005). Also, this could lead to production of α-defensins which are anti-microbial peptides that cause bacterial death by disruption of the bacterial membrane (Agerberth et al., 2000; Doss et al., 2010). This may also represent a direct cytotoxic pathway involved in NK cell-mediated protection against bacteria such as that observed in C. rodentium. Furthermore, NK cells can secrete cathelicidin (LL37) as well as indoleamine 2,3-dioxygenase (IDO) and nitric oxide (NO), that possess anti-bacterial effects and can limit infections (Agerberth et al., 2000; Bogdan, 2001; Zelante et al., 2009).
Among these PRRs are toll-like receptors (TLRs), a family composed of ten receptors that recognize various microbial components (Akira and Sato, 2003). TLR expression was controversial in literature depending on the investigated NK cell population (Adib-Conquy et al., 2014). However, the majority of the TLR family (TLR1-9) are expressed on NK cells (Hornung et al., 2002; Lauzon et al., 2006; Muhammad et al., 2019). It is noteworthy that post-translational modifications in NK cells could be the reason for the variability in TLR expression in literature. TLR2 on NK cells was found to be stimulated by peptidoglycan from M. tuberculosis and protein A from Klebsiella pneumoniae (KpOmpA) (Chalifour et al., 2004; Esin et al., 2013). On the other hand, the anti-bacterial role of NK cells could be triggered upon binding of TLR4 with the FimH protein of E. coli, TLR5 binding to flagellin of E. coli, and TLR9 binding to bacterial unmethylated CpG motifs (Sivori et al., 2004; Tsujimoto et al., 2005; Mian et al., 2010; Esin et al., 2013). LPS, a major component of the outer membranes of gram-negative bacteria, was found to stimulate TLRs on NK cells, resulting in their activation (Schmidt et al., 2016). Further, TLR4 agonists stimulate the production of IFN-α/β, which contribute to the NK cell activation in bacterial infections (Nguyen et al., 2002). Furthermore, such type 1 interferon secretion is associated with the production of the chemokine CXCL10 from infected cells, which promotes the chemotaxis of NK cells (Lande et al., 2003). Previous work by Muhammad J.S. et al. reported that NK cells are stimulated by LPS leading to the release of the proinflammatory cytokine IL-1β through pyroptosis signaling pathway (Muhammad et al., 2019). As mentioned earlier, another possible way of NK cell mediated bacterial elimination is through their indirect interaction with DCs. For instance, a study by Oth T. et al. demonstrated that beside the direct sensing of bacterial pathogens by NK cells and the induction of their cytotoxic capacity, there is also an enhancement of NK cell-mediated help for DC maturation (Oth et al., 2018). This was mainly attributed to the soluble factors released by PAMP-triggered NK cells, especially IFN-γ, that was able to intensify the pro-inflammatory cytokine response of DCs.
Other PRRs include the nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) and the retinoic acid inducible gene I (RIG-I)-like receptors, that are expressed on NK cells. For instance, NOD1 and NOD2 receptors bind to motifs derived from peptidoglycan of gram-negative bacteria and gram-positive bacteria, thus promoting NK cell activation as indicated by CD69 expression and IFN-γ production. Also, NLRP3, the key element of an inflammasome, was reported to be expressed in NK cells (Qiu et al., 2011). Further, NLRP3 activation in macrophages during Bordetella pertussis infection, resulted in the production of IL-18 and IL-1β, thus promoting NK cell activation and proinflammatory response against the bacteria (Kroes et al., 2019).
NK cell receptors were reported to recognize and directly interact with host cell proteins as well as viral and bacterial proteins. For instance, studies have shown that the natural cytotoxicity receptor, NKp44, could directly bind to ligands on the surface of M. tuberculosis and Pseudomonas aeruginosa (Esin et al., 2008). Similarly, NK cells were able to recognize and directly interact with bacteria such as Mycobacterium bovis BCG, leading to their activation and release of cytokines such as IFN-γ and TNF-α, as well as cytolytic activity of target cells (Marcenaro et al., 2008). This was attributed to the expression and function of TLR2 on NK cells (Marcenaro et al., 2008). Besides, NKp46 was found to be a potential receptor for vimentin protein that is expressed on the surface of infected monocytes with M. tuberculosis (Vankayalapati et al., 2002; Garg et al., 2006). Additionally, UL16-binding proteins (ULBPs) and MHC class I polypeptide–related sequence A/B(MICA/MICB), ligands of NKG2D receptor were found to be expressed on infected monocytes, leading to NK cell activation to perform their cytolytic activity. Also, LPS-stimulated macrophages induce NK cell proliferation, IFN-γ production, and cytotoxicity as well as increase the surface expression of ULBPs 1, 2, 3 and MICA/B (Nedvetzki et al., 2007). Further, ULBP1 was upregulated in M. tuberculosis-infected monocytes and alveolar macrophages (Vankayalapati et al., 2005), while MICA was elevated on the surface of epithelial cells in E. coli infection (Tieng et al., 2002).
Bacteria release toxins that are so called superantigens, that activate NK cells. For example, the streptococcal pyrogenic exotoxin A (SPEA) was found to induce IFN-γ production as well as NK cell cytotoxic activity (Cavaillon et al., 1982; Sacks et al., 1991; Dobashi et al., 1999). Similarly, the staphylococcal enterotoxin B and the exotoxin A produced by Pseudomonas aeruginosa were reported to activate NK cells and their function including cytotoxicity and IFN-γ release (D'Orazio et al., 1995; Mühlen et al., 2004).
NK cells could be a friend or foe for bacterial infections, depending on the environment. In fact, the detrimental effects of NK cells in fighting bacterial infection were previously reported. Some studies claim that depleting NK cells may be beneficial and result in bacterial clearance including E.coli, Streptococcus pneumoniae and Pseudomonas aeruginosa (McSharry and Gardiner, 2010). Also, excessive LPS stimulation of IFN-γ production by NK cells could lead to an uncontrolled secretion of pro-inflammatory cytokines that could lead to lethal septic shock (Doherty et al., 1992; Emoto et al., 2002; Sherwood et al., 2004; Etogo et al., 2008; Souza-Fonseca-Guimaraes et al., 2012; Adib-Conquy et al., 2014). Therefore, a balance should be maintained in the immune response in order to have a beneficial anti-bacterial effect. On the contrary, NK cell cytotoxic activity was found to be impaired in patients with sepsis (Maturana et al., 1991). Additionally, some pathological effects of IFN-γ were reported in bacterial infections. For example, it was found that IFN-γ could cause death in polymicrobial peritonitis, P. aeruginosa infection and upon administration of LPS in mice (Miles et al., 1994; Heremans et al., 2000; Murphey et al., 2004).
Besides NK cells, group 1 ILCs have shown to be central players in the protection against bacterial infections (Beck et al., 2020). ILC1s are potent producers of TNF-α and IFN-γ upon stimulation with IL-12, IL-15 and IL-18, that allow them to play key roles in immune protection and chronic inflammation (Fuchs, 2016). Additionally, such cytokine stimulation, and especially IL-15 aids in their development and contribution to enhanced immunity against infectious diseases including several viruses and bacteria (Diefenbach et al., 2014; Klose et al., 2014; Kwon et al., 2019; Poniewierska-Baran et al., 2019). For example, Rag1−/− mice lacking T and B cells when infected with Clostridium difficile bacteria, displayed ILC1-associated proteins such as IFN-γ, TNF-α and nitric oxide synthase (NOS)2. On the contrary, mice lacking all innate lymphoid cells, especially ILC1s, witnessed an increased susceptibility to C. difficile infection (Abt et al., 2015). In vitro studies of the gram-negative and pathogenic S. typhimurium infection revealed that infection of human colonic lamina propria cells led to IFN-γ production by ILC1s and NK cells (Klose et al., 2013). Similarly, the ILC response and their respective cytokines such as TNF, IL-23, IL-17, and IFN-γ were critical players in the clearance of Klebsiella pneumoniae infection (Moore et al., 2002; Xiong et al., 2016). Also, there was a significant decrease in ILC1 and ILC3 populations in the peripheral blood of sepsis patients (Cruz-Zárate et al., 2018). On the contrary, another study reported an increase in ILC1 but a decrease in ILC3 in the peripheral blood of patients with septic shock (Carvelli et al., 2019). Such a discrepancy in the studies could be attributed to the plasticity of ILC populations.
Analogous to Th2 cells, ILC2s mediate a type 2 immune response. They produce characteristic Th2 cytokines, including IL-4, IL-5, IL-9 and IL-13 (Moro et al., 2010; Neill et al., 2010; Price et al., 2010). Human ILC2s are characterized by the expression of chemoattractant receptor, CRTH2 and NK cell marker, CD161 (Mjösberg et al., 2011) and respond to cytokine cues including IL-25, thymic stromal lymphopoietin (TSLP), and IL-33. ILC2s are known to play a vital role in extracellular parasitic infections (Bouchery et al., 2015), allergic diseases such as asthma, rhinitis, atopic dermatitis (Tojima et al., 2019; Akdis et al., 2020), and tissue repair (Monticelli et al., 2015; Rak et al., 2016).
Recently, their role in bacterial infection is gaining interest. ILC2 activity has been reported to be regulated by various triggers, including signaling via cytokine receptors, lipid-, metabolite-driven, neuro-immune and microRNA modulation (Burrows et al., 2019). IL-33 production by the gut and lung epithelium is important for maintaining gut and lung homeostasis through the recruitment of ILC2s. Tuft cells are important responders to bacterial presence in the intestinal mucosa. During type 2 immune responses, tuft cells are capable of influencing the gut microbiome by regulating the intestinal ILC2-epithelial response circuit. Upon chemosensory-like sensing of pathogens, tuft cell-derived IL-25 triggers IL-13 secretion by resident ILC2s which in turn activated goblet cells to release mucus that aided in the clearance of bacterial pathogens (von Moltke et al., 2016). Metabolite-triggered small intestinal tuft cell-ILC2 circuit also orchestrated epithelial remodeling in the small intestine thereby shaping epithelial responses to intestinal pathogen that impaired their infestation (Schneider et al., 2018). The surface expression of TLR1, 4 and 6, on ILC2s enables them to respond to TLR ligands by secreting cytokines, such as IL-5 and IL-13, and inducing the production of immunoglobulins IgM, IgG, IgA, and IgE by B cells, which are important in shaping the microbial flora (Maggi et al., 2017).
Gastric Helicobacter pylori infection of the gut is a highly prevalent condition. H. pylori infection induces a skewed type 2 immunity and immunosuppressive microenvironment that is mediated by ILC2s (Li et al., 2017a). Commensal stomach bacteria favor an ILC2 environment by inducing the production of IL-7 and IL-33 cytokines, thereby making it the predominant ILC subset in the stomach (Satoh-Takayama et al., 2020). ILC2-dependent IgA response protected the stomach by eliminating IgA-coated bacteria including pathogenic H. pylori (Satoh-Takayama et al., 2020). The increasing prevalence of antibiotic heteroresistance among H. pylori strains is a matter of grave concern (Rizvanov et al., 2019). Better understanding of the underlying involvement of ILC2s may therefore, lead to better therapeutic approaches.
Similarly, Clostridium difficile colonizes the epithelial cells of the gut, releasing toxins that triggers cell death pathways and colonic inflammation. C. difficile infection upregulated IL-33 production that in turn activated ILC2s leading to prevention of epithelial death and disruption (Frisbee et al., 2019). Therefore, IL-33-mediated ILC2 activation is a key defense mechanism against C. difficile colitis.
Lungs constitute a unique organ as they are under constant exposure to the external environment since birth. The immunological milieu in the lungs is specialized to protect them from damage and infection. In a study by Saluzzo S. et al., the epithelium-derived IL-33 was found to be increased after the first day of life in newborn mice and this was closely followed by IL-13 secretion from ILC2s (Saluzzo et al., 2017). The homeostatic role of ILC2s in the lungs entailed the recruitment of alveolar macrophages and their IL-13 driven-polarization to an anti-inflammatory M2 phenotype (Saluzzo et al., 2017). However, this led to a delayed immune response to S. pneumoniae infection in mice during adult life.
Abundantly found in the intestines, ILC3s are indispensable in the maintenance of intestinal immunity and microbiota-host homeostasis. Microbial stimulation leads to the development of these cells after birth and various environmental signals, such as bacterial and dietary metabolites, regulate ILC3 differentiation and function (Qiu et al., 2012; Mielke et al., 2013). ILC3s are characterized by their production of IL-22 and/or IL-17, and are thus, the innate equivalent of Th17 cells. IL-22, however, is the predominant cytokine produced by these cells which contributes to intestinal homeostasis.
ILC3s are crucial in wading off bacterial infections as well as in the interactions with commensal bacteria. Occasionally, commensal bacteria can penetrate the mucosal barrier and thus, the human body has evolved mechanisms to re-establish homeostasis by minimizing inflammation. The intestinal microbiome interacts indirectly with ILC3s by promoting crosstalk between innate myeloid and lymphoid cells (Mortha et al., 2014; Gury-BenAri et al., 2016; Castleman et al., 2019). Both commensal as well as pathogenic bacteria induce accessory cells in the milieu, such as CD11c+ myeloid DCs to generate IL-23 and IL-1β which then contribute to IL-22 secretion from ILC3s (Castleman et al., 2019). In turn, IL-22 helps restrict the dissemination of commensal bacteria by inducing the expression of anti-microbial peptides (AMPs), including peptides of the S100 family, RegIIIβ and RegIIIγ (Sonnenberg et al., 2011a). AMPs possess potent anti-microbial as well as anti-biofilm activity. Also termed host defense peptides, these positively charged amphipathic molecules selectively target a broad spectrum of bacteria and kill them via several mechanisms. Their main mechanism of action is attributed to disrupting the bacterial cell membrane causing cell lysis and death. Additionally, they also form transmembrane channels in the membrane initiating cytoplasm leakage and cell death. Furthermore, they have demonstrated intracellular inhibitory activities by inhibiting essential intracellular functions by binding to intracellular proteins or nucleic acids (Le et al., 2017). In addition to their involvement in the innate immune response to extracellular bacteria, these IL-22 producing cells promote selective anatomical containment of lymphoid-resident commensal bacteria thereby preventing systemic inflammation (Cella et al., 2009; Sonnenberg et al., 2012; Hepworth et al., 2013). Along with IL-22 production, they also secrete IL-17 and regulate adaptive Th17 responses (Hepworth et al., 2013). GM-CSF is another cytokine that is produced by both mice and human ILC3s (Mortha et al., 2014). While the cell surface expression of NKp46 characterizes ILC3s in mice, NKp44 expression is observed in humans (Cupedo et al., 2009; Sanos et al., 2009). ILC3s also express CD127 (IL-7 receptor α-chain) and CD161. Although ILC3s are predominantly dependent on the transcription factor RORγt, T-bet expression is observed in a subset of NKp46+ cells and is essential for IL-22 and IFN-γ production in these cells (Sciumé et al., 2012; Klose et al., 2013; Rankin et al., 2013). Further, expression of major histocompatibility complex class II (MHC class II) by CCR6-expressing lymphoid tissue inducer (LTi)-like ILC3 is another mechanism by which they downregulate pathological CD4+ T cell responses against commensal bacteria thereby limiting spontaneous intestinal inflammation (Hepworth et al., 2013).
Infection by S. typhimurium causes diarrhea and gastroenteritis. IFN-γ response of innate origin is crucial in restricting the growth of S. typhimurium (Muotiala and Mäkelä, 1990; Songhet et al., 2011). In addition to expanding the armory in the fight against intracellular pathogens (Yrlid et al., 2001), IFN-γ also modulates goblet cell function during S. typhimurium infection (Songhet et al., 2011). Mucosal RORγt+ ILCs are emerging as important players in the immunity against intestinal infections. Studies indicate that ILC3s and not NK cells are the major source of IFN-γ during S. typhimurium infection (Vonarbourg et al., 2010; Klose et al., 2013). Graded expression of T-bet was found to determine the fate of a distinct lineage of CCR6- RORγt+ ILCs by influencing their expression of IFN-γ and the natural cytotoxicity receptor NKp46 (Klose et al., 2013). During Salmonella enterica infection, IFN-γ from these CCR6- RORγt+ ILCs was essential for the secretion of mucus-forming glycoproteins that ensures epithelial barrier integrity (Klose et al., 2013). IL-23 orchestrated inflammatory mucosal response during S. typhimurium infection. This involved the early production of IL-22 and IL-17A by T cells and ILC3s (Godinez et al., 2009; Siegemund et al., 2009).
Another possible anti-bacterial mechanism of ILC3s is through the induction of the expression and secretion of lipocalin-2 via IFN-γ and IL-22 at barrier surfaces (Sonnenberg et al., 2010; Zhao et al., 2014). This limits bacterial growth by sequestrating the iron scavenged by bacteria during infection (Flo et al., 2004). Commensal bacteria are known to induce fucosylation of intestinal epithelial cells by adding L-fucose to glycolipids and glycoproteins on epithelial cells. The fucose moiety serves as a dietary carbohydrate for these bacteria, where they are metabolized into beneficial metabolites such as short-chain fatty acids. In addition, ILC3s induce the IL-22-mediated intestinal expression of fucosyltransferase 2 and subsequent epithelial fucosylation that promote barrier integrity in the intestinal tract (Goto et al., 2014). ILC3-mediated intestinal epithelial cell glycosylation reduces the susceptibility and improves host tolerance to S. typhimurium infection.
NK-derived IFN-γ is largely implicated in controlling the dissemination of intracellular bacteria such as L. monocytogenes. Oral infection by L. monocytogenes was observed to induce IFN-γ production by NKp46+ RORγt- ILCs or NK cells and IL-22 production by NKp46+ RORγt+ ILC3s as well (Reynders et al., 2011).
Citrobacter rodentium is known to cause acute infection of the colonic epithelium leading to mild colitis. Infection with C. rodentium is associated with IL-23 dependent CD4+ LTi cell responses (Sonnenberg et al., 2011b). Further, these cells are early responders to infection through the production of IL-22. Depletion of CD4+ LTi cells led to a decline in the expression of infection-induced IL-22 and anti-microbial peptides that impaired innate immunity in the intestine. While ILC3s are important responders in the initial phase of C. rodentium infection, B lymphocytes and CD4+ T cells are crucial for resolution of C. rodentium infection (Simmons et al., 2003). In addition to activation of the surface receptors, various other mechanisms such as surrounding phagocytes, diet- and bacteria-derived metabolites can contribute to ILC3 activation during C. rodentium infection (Beck et al., 2020). LTi-ILC3s are equipped with a wide variety of receptors, including MHCII, NK cell receptor P1 (NKR-P1R), G-protein-coupled receptors (GPCRs) (GPR183, free fatty acid receptor 2 (Ffar2)), aryl hydrocarbon receptor (AHR) that sense environmental cues to mount an appropriate response against C. rodentium when triggered by the pathogen (Lee et al., 2011; Chu et al., 2018; Li et al., 2018; Chun et al., 2019; Melo-Gonzalez et al., 2019). Myeloid-ILC3 crosstalk also shapes the ILC3 response against C. rodentium infection. For example, the release of CXCL16 from DCs activates CXCR6 signaling in ILC3s stimulating the release of IL-22 and secretion of antimicrobial peptides (Longman et al., 2014; Satoh-Takayama et al., 2014). Further, depletion of the chemokine receptor CX3CR1 led to reduced expression of IL-22, antimicrobial peptides RegIIIβ and RegIIIγ, and subsequently delayed clearance of C. rodentium (Manta et al., 2013). Similarly, depletion of these CX3CR1+ mononuclear phagocytes led to increased severity of colitis and mortality upon C. rodentium infection (Longman et al., 2014). In addition to nutrient sensing AHR, dietary vitamin A promotes intestinal homeostasis by recruiting immune cells and improving the integrity of the mucosal barrier (Li et al., 2017b; Xiao et al., 2019). Furthermore, the active vitamin A metabolite retinoic acid regulates the transcription factor RORγt thereby controlling the ILC3 response against C. rodentium infection (Goverse et al., 2016).
C. difficile is an opportunistic enteric pathogen that causes infection upon antibiotic-induced gut microbiota alterations. While ILC1s are critical for protection against acute C. difficile infection, ILC3s also play a supporting role where their depletion of IL-22 production was associated with a minor contribution to resistance (Abt et al., 2015). Also, a recent study demonstrated the role of short-chain fatty acids (SCFAs), in particular acetate, in ameliorating the infection by activating the free fatty acid receptor 2 (FFAR2) on ILC3s and neutrophils (Fachi et al., 2020). This ligand-receptor signaling led to increased neutrophil-mediated inflammasome activation and release of IL-1β, which boosted IL-1R expression on ILC3s and IL-22 production.
Non-gastric Helicobacter species such as Helicobacter apodemus and Helicobacter typhlonius, while activating ILCs and inducing gut inflammation, were found to negatively regulate RORγt+ ILC3s and weaken their proliferative capacity in immunocompromised mice (Bostick et al., 2019). Further, antigen-presenting ILC3s through their interaction with T follicular helper cells (Tfh) and B cells limited mucosal IgA responses to H. typhlonius in order to preserve mucosal-dwelling commensal microbiota (Melo-Gonzalez et al., 2019).
In response to S. pneumoniae infection, the release of IL-23 from DCs led to rapid accumulation of ILC3s in the lungs and their activation in an MyD88-dependent manner leading to IL-22 secretion (Kinnebrew et al., 2012; Van Maele et al., 2014). Further treatment with TLR5 agonist flagellin exacerbated ILC3-mediated IL-22 production, that helped provide defense against lethal infection (Van Maele et al., 2014). Furthermore, intestinal commensal bacteria protect neonatal mice against bacterial pneumonia immediately after birth by directing ILC3s influx into the lungs and IL-22-dependent host resistance to pneumonia (Gray et al., 2017). ILC3-derived IL-22 and IL-17 have also been implicated in host defense against Klebsiella pneumoniae, a bacteria that displays high-level of acquired antibiotic resistance (Chen et al., 2016; Murakami et al., 2016). During K. pneumoniae infection in mice, inflammatory monocytes rapidly migrate to the lung and secrete TNF, leading to the increased activation of IL-17-producing ILCs (Xiong et al., 2016). IL-17 and IL-22-producing ILC3s are essential for host response and defense against chronic pulmonary infection caused by Pseudomonas aeruginosa, possibly by moderating neutrophil-mediated lung damage (Bayes et al., 2016; Broquet et al., 2017). The crosstalk between myeloid cells and ILCs promotes clearance of pneumonia.
ILC3s are known to mediate an early protective response to tuberculosis, and in particular to M. tuberculosis (Ardain et al., 2019). Acute infection with pulmonary tuberculosis is associated with a depletion of circulating ILC subsets which are later restored upon treatment. In response to infection, ILC subsets, in particular ILC3s, accumulate in the lungs resulting in a robust innate immune response in containing the infection (Ardain et al., 2019).
Bacterial pathogens have evolved strategies to evade, inhibit or manipulate the innate immune response to their advantage. Some of these strategies include subversion of antimicrobial peptides, modulation of innate immune signal transduction cascades, immune receptor localization and cytokine secretion (Figure 2). These mechanisms have been comprehensively reviewed in (Reddick and Alto, 2014).
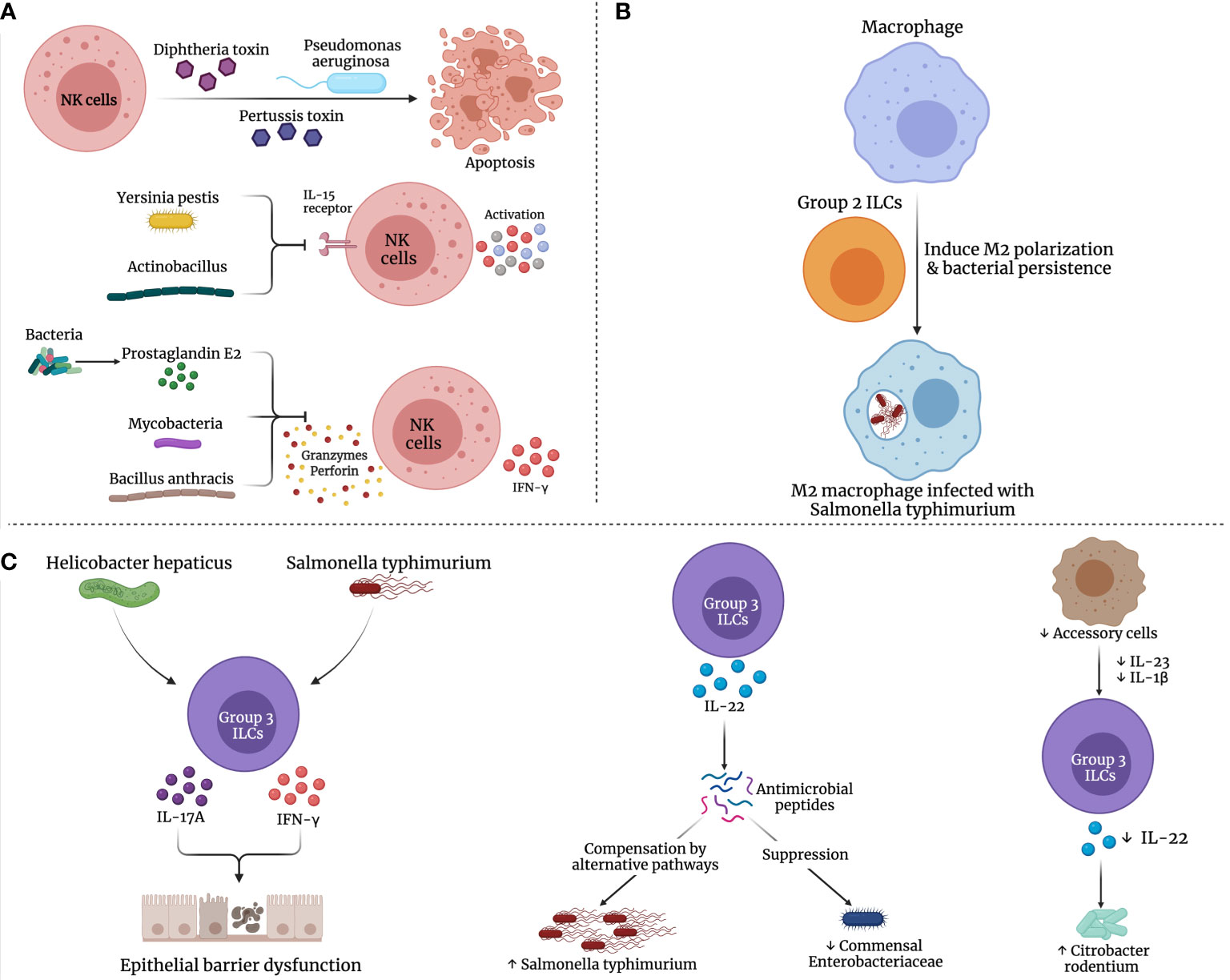
Figure 2 Possible mechanisms for dysregulation of ILCs in bacterial infections. Several bacterial species may manipulate the immune (A) NK cells, (B) Group 2, and (C) Group 3 ILCs, promoting bacterial persistence and inhibiting their elimination.
Over the past decades, numerous studies have reported the strategies developed by bacteria to evade the NK cell response leading to bacterial persistence. For example, Pseudomonas aeruginosa could eliminate NK cells via phagocytosis-induced apoptosis (Chung et al., 2009). Further, toxins from various bacteria including diphtheria toxin, pertussis toxin and P. aeruginosa exotoxin A halt NK cell activity and promote NK cell apoptosis (Waters et al., 1990; Whalen et al., 1992; Michałkiewicz et al., 1999). Bacillus anthracis toxin was reported to inhibit NK cell cytotoxicity and IFN-γ secretion (Klezovich-Bénard et al., 2012). Similarly, cell wall components of mycobacteria affect DC maturation, hinder NK cell activity and IFN-γ production as well as promote immunosuppressive IL-10 secretion (Geijtenbeek et al., 2003). Also, leukotoxin produced by Actinobacillus actino-mycetemcomitans as well as the membrane virulence protein of Yersinia pestis, inhibit the expression of NK cell activation markers and IL-15 receptor, respectively (Shenker et al., 1994; Kerschen et al., 2004). Another approach by various bacterial species is promoting the production of prostaglandin E2 (PGE2), which suppresses NK cell response to cytokines, migration, IFN-γ production and cytolytic function, as illustrated in Figure 2A (Walker and Rotondo, 2004; Szymanski et al., 2012).
Bacterial infections have evolved to cause substantial damage to epithelial barrier surfaces and its subsequent loss of protective function. Interestingly, this correlated with infection-induced perturbations in ILC frequency and function. The effect of ILCs on intestinal homeostasis is largely dependent on the bacteria-specific protective or deleterious cytokine response. In addition to fostering intestinal homeostasis in response to bacteria, ILCs may also promote an exaggerated inflammatory response. Inflamed mucosal tissues and inflammatory diseases of the gut [inflammatory bowel diseases (IBDs) and Crohn’s disease (CD)] were associated with increased frequency of IFN-γ/IL-17A-producing ILCs (Geremia et al., 2011; Bernink et al., 2013) and reduced frequency of IL-22-producing ILCs (Takayama et al., 2010; Bernink et al., 2015). The increased presence of IFN-γ at mucosal surfaces compromises the epithelial tight junctions and upregulates TNF-α receptor expression on epithelial cells, which induces intestinal epithelial barrier dysfunction (Wang et al., 2005; Beaurepaire et al., 2009).
S. typhimurium is known to manipulate macrophage polarization to a M2 state which enables their persistence within the macrophage leading to the establishment of persistent infection (Pham et al., 2020). ILC2s preferentially mediated the alternate activation of macrophages (Kim et al., 2019) that reportedly enhanced bacterial dissemination and long-term persistence in S. typhimurium infection (Figure 2B) (Eisele et al., 2013).
Depending on the cytokines in the milieu, ILC3s demonstrate plasticity in their effector cytokine production (Bernink et al., 2015). IFN-γ-producing ILC3s may contribute to the breakdown of the gut epithelial barrier. A compromised epithelial barrier promotes intrusion of gut bacteria into the lamina propria resulting in immune cell exposure to a wide variety of bacterial species at varying magnitudes and induction of potentially pathogenic immune responses (Pastorelli et al., 2013). The production of IFN-γ/IL-17 in response to S. typhimurium or Helicobacter hepaticus reportedly promotes bacteria-driven innate colitis (Buonocore et al., 2010; Klose et al., 2013). Therefore, IL-23-responsive ILC3s could also mediate intestinal immune-mediated pathology.
As discussed above, ILC3-derived IL-22 is induced in response to bacterial infections and plays an important role in host defense at mucosal surfaces. At the same time, IL-22 has been reported to suppress the commensal bacteria while promoting the colonization of the pathogenic bacteria (Behnsen et al., 2014). In the study by Behnsen, J. et al., it was demonstrated that IL-22 induced AMPs, including lipocalin-2 and calprotectin, that are responsible for sequestering essential metal ions from microbes, were compensated in S. typhimurium by alternative pathways. Moreover, IL-22 preferentially boosted the colonization of S. typhimurium, while suppressing commensal Enterobacteriaceae species that are susceptible to AMPs (Figure 2C). Thus, the production of IL-22 by ILC3s is exploited by pathogenic bacteria to enhance their colonization on mucosal surfaces at the cost of their competing commensals.
Furthermore, the production of IL-23 and IL-1β by accessory cells such as mDCs, monocytes and macrophages, helps induce and regulate IL-22 secretion from ILC3s (Manta et al., 2013; Castleman et al., 2019). The depletion of CX3CR1+ phagocytes in mice reduced IL-22 expression in ILC3s, leading to increased microbial translocation and delayed clearance of C. rodentium (Manta et al., 2013). Thus, pathogenic bacteria may potentially exploit this mechanism by either reducing the frequency of accessory cells or depleting the cytokines responsible for IL-22 production by ILC3s in order to promote bacterial dissemination.
Considering their relatively recent discovery, ILCs are now actively studied to decipher their contribution to immune response in health and disease, and to manipulate them for clinical benefit. However, therapeutic strategies targeting ILCs should follow after firmly establishing a unique and non-redundant protective or deteriorating role for ILCs in diseases. Variety of strategies are now available to therapeutically target ILCs, including cytokine administration, adoptive transfer, anti-cytokine antibodies, antibody depletion of ILCs, modulating ILC plasticity and/or function, inhibiting ILC migration and function, and immune checkpoint modulation, as elaborately described in (Cobb and Verneris, 2021). With increasing preclinical and clinical studies, it is evident that ILCs play a role in the initiation, regulation and resolution of bacterial infections suggesting a potential beneficial role in therapeutically targeting ILCs in bacterial infections.
NK cells were heavily investigated and utilized as a therapeutic modality in various diseases including infections and cancer. Several studies have suggested using certain bacterial strains to activate NK cells, thus boosting their cytotoxicity effects against cancer cells. For example, the live vaccine strain BCG was proposed as a successful immunotherapy for bladder cancer due to the induced inflammation and recruitment of NK cells (Koga et al., 1988). Another example is L. monocytogenes infection which was found to initiate the anti-tumor NK cell response specifically against hepatic metastasis, due to their tropism in the liver (Yoshimura et al., 2007).
On the other hand, the role of ILC1s in Crohn’s disease highlighted its potential in being a therapeutic target. In CD patients, the frequency of CD127+ ILC1s was found to increase at the cost of ILC3s in inflamed intestinal tissues (Bernink et al., 2015). Here, the plasticity of ILCs can be exploited to differentiate IFN-γ-producing CD127+ T-bet+ c-Kit− NKp44− ILC1s into IL-22-producing NKp44+ ILC3s in the presence of IL-1β and IL-23, thereby re-establishing homeostasis which may demonstrate a therapeutic effect in Crohn’s disease.
While ILC3s are essential for the maintenance of gut homeostasis (Vivier et al., 2018), their dysregulation may also contribute to intestinal inflammation. For instance, increased colonic secretion of IL-17 and IFN-γ by ILC3s was associated with bacteria-driven innate colitis (Buonocore et al., 2010). In cases of redundancy between ILCs and T cells, neutralizing the common effector cytokines from ILCs and T cells may prove beneficial. Although targeting both IL-17 and IFN-γ appeared promising in pre-clinical studies (Buonocore et al., 2010), neutralizing these cytokines or blockade of IL-17R failed to demonstrate clinical efficacy against Crohn’s disease (Hueber et al., 2012; Kaser, 2014). In this case, targeting the upstream cytokines, such as IL-12 and IL-23 simultaneously or IL-23 alone, that stimulate the ILCs to produce IL-17 and IFN-γ may serve as a more effective strategy in treating patients with Crohn’s disease (Sands et al., 2017; Rutgeerts et al., 2018).
In addition, ILC3-derived GM-CSF is an important element in ILC-driven colitis, where they are responsible for the recruitment and maintenance of intestinal inflammatory monocytes (Pearson et al., 2016). The IL-23/GM-CSF–mediated autocrine feedback loop may sustain the crosstalk between myeloid cells and ILC3s. Further, GM-CSF may mobilize the ILC3s from within lymphoid aggregate cryptopatches into adjacent intestinal mucosa, as seen following the induction of colitis. Neutralization of GM-CSF prevented the egress of ILC3 from cryptopatches and bore promising results in mouse models (Pearson et al., 2016). However, GM-CSF may not be a straightforward target in IBD as GM-CSF governs clear host protective functions in the intestine (Bernasconi et al., 2010; Hirata et al., 2010).
In fact, several biological therapies that target ILCs are currently approved for the treatment of CD. Patients with CD are generally characterized by high intestinal levels of ILC1s and low ILC3s. Ustekinumab, monoclonal antibody against IL-12/23 p40, normalized the ILC frequencies, thereby contributing to intestinal mucosal healing in these patients (Li et al., 2016). In addition to ustekinumab, the inhibition of TNF-α and α4β7 integrin inhibitor (vedolizumab) are approved for Crohn’s disease (Crohn’s and Colitis Foundation, 2021). NCR+ ILC3s constitute an important source of intestinal IL-22 and were found to be reduced in the intestinal mucosa of CD patients in favor of pro-inflammatory ILC1s. In a study of 54 CD patients, increased ILC1 levels and significantly lower NCR+ ILC3 levels were detected at baseline (Creyns et al., 2019). However, biological therapy with anti-TNF, ustekinumab or vedolizumab was found to restore the NCR+ ILC3 levels to homeostatic proportions in the intestine. Interestingly, the circulating NCR+ ILC3s increased only in the anti-TNF and ustekinumab treatment groups but not with vedolizumab therapy. Taking into consideration the critical role of α4β7 integrin in the development and migration of ILCs (Sonnenberg and Artis, 2015; Tufa et al., 2020), the effect of vedolizumab on the frequency of peripheral ILC3s may suggest the lack of ILC homing from the blood to the gut (Forkel et al., 2019) but rather a selective inhibition of ILC migration from cryptopatches into intestinal mucosa. The biological efficacy of these therapeutics could thus, be attributed at least partially to its impact on ILC differentiation, migration and/or function, and hence re-establishing homeostatic intestinal conditions.
Various other therapies that are currently in the pipeline may also target ILCs. Multiple agents targeting IL-23 are in late-phase clinical trials for patients with CD and/or ulcerative colitis (Moschen et al., 2019) (NCT03650413). JAK inhibitors, through their ability to modulate IL-12 and IL-23 cytokine signaling may affect ILC plasticity and are promising candidates in trials. A monoclonal antibody targeting NKG2D is also being tested in a phase II clinical trial (NCT02877134). Since the gut microbiome is known to reciprocally regulate ILCs (Gury-BenAri et al., 2016), a small-molecule FimH antagonist, Sibofimloc, that was created to impede bacterial adherence to the gut, thus decreasing intestinal permeability and reducing innate immune activation, is being investigated in a phase II study (https://www.enterome.com/). Sphingosine-1-phosphate receptor 1 (S1PR1) regulates ILC egress from secondary lymphoid organs (Eken et al., 2019) and S1PR1 agonists, such as fingolimod, are expected to modulate their migration and function (Bell et al., 2018). With parallel action to regulatory T cells, regulatory ILCs (ILCregs) are proposed to promote the resolution of intestinal inflammation by suppressing the activation of ILC1 and ILC3 through IL-10 secretion. These therapeutic approaches have been reviewed in (Moschen et al., 2019).
Bacterial molecules can activate ILCs via a direct and indirect mechanisms, hence highlighting the crucial roles for these innate cells in bacterial infections (Table 1). The past 10 years or so have been instrumental in shaping our understanding of the functional diversity of ILCs. Nevertheless, there are still a lot of open-ended questions that need to be answered to fully comprehend the complex roles of these cells in health and disease. ILCs are known to regulate key signaling circuits to establish tissue homeostasis; however, upon further dissection, new circuits may be revealed. Furthermore, unraveling the crosstalk between ILCs and the various other innate, adaptive immune players and non-hematopoietic cells may open up further avenues of research. Similarities in the molecular profiles between ILCs and T cells limits the specific targeting of ILCs without simultaneously affecting lymphocytes. ILCs are important mediators in the innate immune response to bacterial infections, particularly by regulating tissue-specific immunity. The traditional treatment regimens of bacterial infections are usually antibiotics. Over the past decades, bacteria have managed to develop evasion strategies including antibiotic resistance. Hence, alternative therapeutic modalities are needed. Being the first innate defense members in the anti-bacterial response, ILCs represent a new promising target for anti-bacterial therapy. Further research needs to focus on the immunoregulatory pathways controlled by ILCs and ways of therapeutically harnessing them.
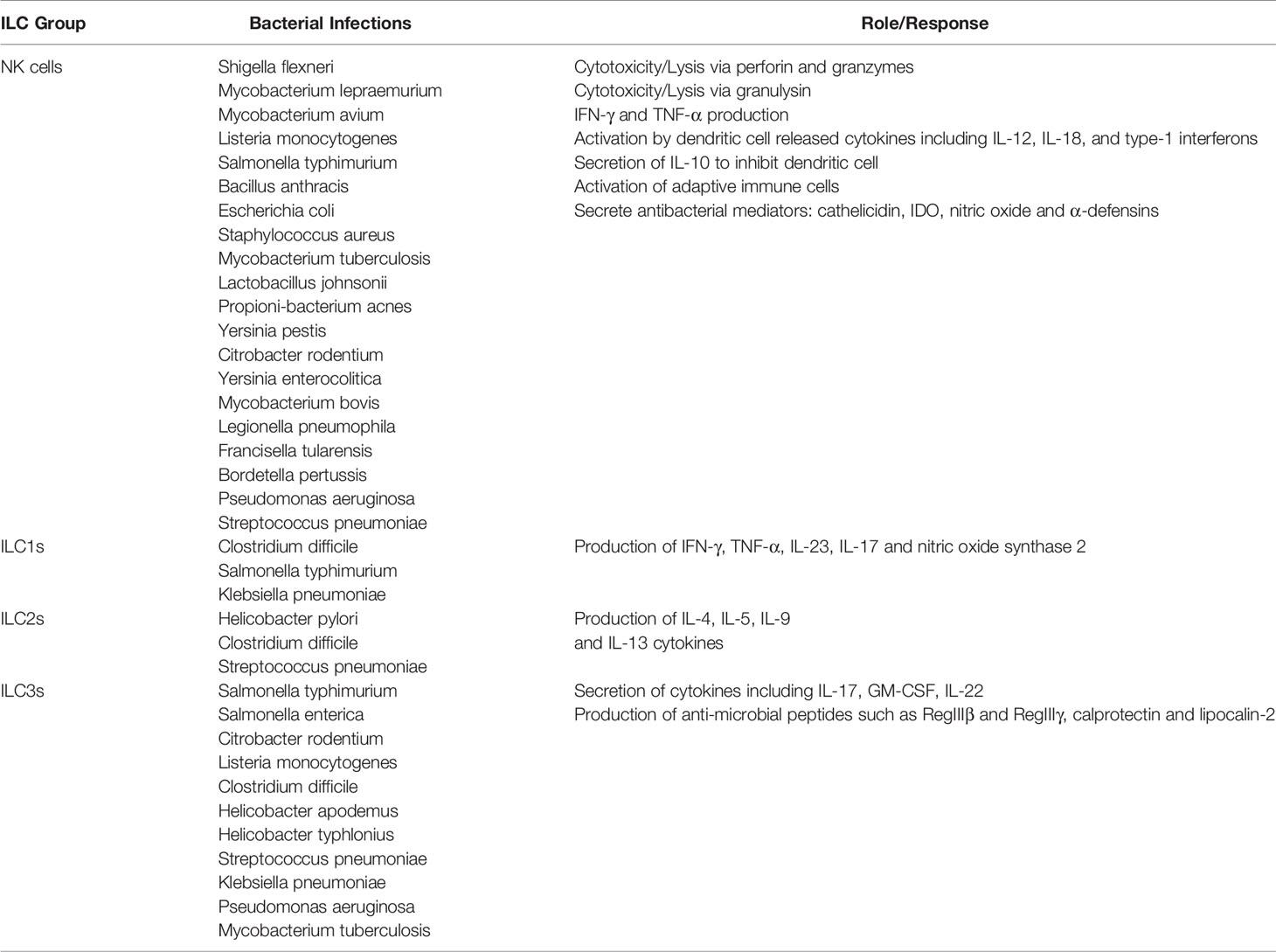
Table 1 Response of different ILC groups in various intracellular and extracellular bacterial infections.
NE and RR wrote the original manuscript. JH, AM, RH, and QH revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the University of Sharjah for the continuous support to the researchers in this article.
Abt, M. C., Lewis, B. B., Caballero, S., Xiong, H., Carter, R. A., Sušac, B., et al. (2015). Innate Immune Defenses Mediated by Two ILC Subsets Are Critical for Protection Against Acute Clostridium Difficile Infection. Cell Host Microbe 18 (1), 27–37. doi: 10.1016/j.chom.2015.06.011
Adib-Conquy, M., Scott-Algara, D., Cavaillon, J.-M., Souza-Fonseca-Guimaraes, F. (2014). TLR-Mediated Activation of NK Cells and Their Role in Bacterial/Viral Immune Responses in Mammals. Immunol. Cell Biol. 92 (3), 256–262. doi: 10.1038/icb.2013.99
Agerberth, B., Charo, J., Werr, J., Olsson, B., Idali, F., Lindbom, L., et al. (2000). The Human Antimicrobial and Chemotactic Peptides LL-37 and Alpha-Defensins are Expressed by Specific Lymphocyte and Monocyte Populations. Blood 96 (9), 3086–3093. doi: 10.1182/blood.V96.9.3086
Akdis, C. A., Arkwright, P. D., Brüggen, M. C., Busse, W., Gadina, M., Guttman-Yassky, E., et al. (2020). Type 2 Immunity in the Skin and Lungs. Allergy 75 (7), 1582–1605. doi: 10.1111/all.14318
Akira, S., Sato, S. (2003). Toll-Like Receptors and Their Signaling Mechanisms. Scand. J. Infect. Dis. 35 (9), 555–562. doi: 10.1080/00365540310015683
Andrews, D. M., Chow, M. T., Ma, Y., Cotterell, C. L., Watt, S. V., Anthony, D. A., et al. (2011). Homeostatic Defects in Interleukin 18-Deficient Mice Contribute to Protection Against the Lethal Effects of Endotoxin. Immunol. Cell Biol. 89 (6), 739–746. doi: 10.1038/icb.2010.168
Ardain, A., Domingo-Gonzalez, R., Das, S., Kazer, S. W., Howard, N. C., Singh, A., et al. (2019). Group 3 Innate Lymphoid Cells Mediate Early Protective Immunity Against Tuberculosis. Nature 570 (7762), 528–532. doi: 10.1038/s41586-019-1276-2
Artis, D., Spits, H. (2015). The Biology of Innate Lymphoid Cells. Nature 517(7534), 293–301. doi: 10.1038/nature14189
Bayes, H. K., Ritchie, N. D., Evans, T. J. (2016). Interleukin-17 Is Required for Control of Chronic Lung Infection Caused by Pseudomonas Aeruginosa. Infect. Immun. 84 (12), 3507–3516. doi: 10.1128/iai.00717-16
Beaurepaire, C., Smyth, D., McKay, D. M. (2009). Interferon-Gamma Regulation of Intestinal Epithelial Permeability. J. Interferon Cytokine Res. 29 (3), 133–144. doi: 10.1089/jir.2008.0057
Beck, K., Ohno, H., Satoh-Takayama, N. (2020). Innate Lymphoid Cells: Important Regulators of Host-Bacteria Interaction for Border Defense. Microorganisms 8 (9), 1342. doi: 10.3390/microorganisms8091342
Behnsen, J., Jellbauer, S., Wong, C. P., Edwards, R. A., George, M. D., Ouyang, W., et al. (2014). The Cytokine IL-22 Promotes Pathogen Colonization by Suppressing Related Commensal Bacteria. Immunity 40 (2), 262–273. doi: 10.1016/j.immuni.2014.01.003
Bell, M., Foley, D., Naylor, C., Robinson, C., Riley, J., Epemolu, O., et al. (2018). Discovery of Super Soft-Drug Modulators of Sphingosine-1-Phosphate Receptor 1. Bioorg Med. Chem. Lett. 28 (19), 3255–3259. doi: 10.1016/j.bmcl.2018.07.044
Berg, R. E., Crossley, E., Murray, S., Forman, J. (2005). Relative Contributions of NK and CD8 T Cells to IFN-Gamma Mediated Innate Immune Protection Against Listeria Monocytogenes. J. Immunol. (Baltimore Md. 1950) 175 (3), 1751–1757. doi: 10.4049/jimmunol.175.3.1751
Bermudez, L. E., Kolonoski, P., Young, L. S. (1990). Natural Killer Cell Activity and Macrophage-Dependent Inhibition of Growth or Killing of Mycobacterium Avium Complex in a Mouse Model. J. Leukoc. Biol. 47 (2), 135–141. doi: 10.1002/jlb.47.2.135
Bernasconi, E., Favre, L., Maillard, M. H., Bachmann, D., Pythoud, C., Bouzourene, H., et al. (2010). Granulocyte-Macrophage Colony-Stimulating Factor Elicits Bone Marrow-Derived Cells That Promote Efficient Colonic Mucosal Healing. Inflamm. Bowel Dis. 16 (3), 428–441. doi: 10.1002/ibd.21072
Bernink, J. H., Krabbendam, L., Germar, K., de Jong, E., Gronke, K., Kofoed-Nielsen, M., et al. (2015). Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 43 (1), 146–160. doi: 10.1016/j.immuni.2015.06.019
Bernink, J. H., Peters, C. P., Munneke, M., te Velde, A. A., Meijer, S. L., Weijer, K., et al. (2013). Human Type 1 Innate Lymphoid Cells Accumulate in Inflamed Mucosal Tissues. Nat. Immunol. 14 (3), 221–229. doi: 10.1038/ni.2534
Bessac, A., Cani, P. D., Meunier, E., Dietrich, G., Knauf, C. (2018). Inflammation and Gut-Brain Axis During Type 2 Diabetes: Focus on the Crosstalk Between Intestinal Immune Cells and Enteric Nervous System. Front. Neurosci. 12, 725. doi: 10.3389/fnins.2018.00725
Blanchard, D. K., Friedman, H., Stewart, W. E., 2nd, Klein, T. W., Djeu, J. Y. (1988). Role of Gamma Interferon in Induction of Natural Killer Activity by Legionella Pneumophila In Vitro and in an Experimental Murine Infection Model. Infect. Immun. 56 (5), 1187–1193. doi: 10.1128/iai.56.5.1187-1193.1988
Blanchard, D. K., Stewart, W. E., 2nd, Klein, T. W., Friedman, H., Djeu, ,. J. Y. (1987). Cytolytic Activity of Human Peripheral Blood Leukocytes Against Legionella Pneumophila-Infected Monocytes: Characterization of the Effector Cell and Augmentation by Interleukin 2. J. Immunol. 139 (2), 551–556.
Bogdan, C. (2001). Nitric Oxide and the Immune Response. Nat. Immunol. 2 (10), 907–916. doi: 10.1038/ni1001-907
Bohn, E., Autenrieth, I. B. (1996). IL-12 is Essential for Resistance Against Yersinia Enterocolitica by Triggering IFN-Gamma Production in NK Cells and CD4+ T Cells. J. Immunol. 156 (4), 1458–1468.
Bokhari, S. M., Kim, K.-J., Pinson, D. M., Slusser, J., Yeh, H.-W., Parmely, M. J. (2008). NK Cells and Gamma Interferon Coordinate the Formation and Function of Hepatic Granulomas in Mice Infected With the Francisella Tularensis Live Vaccine Strain. Infect Immun. 76 (4), 1379–1389. doi: 10.1128/IAI.00745-07
Bostick, J. W., Wang, Y., Shen, Z., Ge, Y., Brown, J., Chen, Z. E., et al. (2019). Dichotomous Regulation of Group 3 Innate Lymphoid Cells by Nongastric Helicobacter Species. Proc. Natl. Acad. Sci. U. S. A. 116 (49), 24760–24769. doi: 10.1073/pnas.1908128116
Bouchery, T., Kyle, R., Camberis, M., Shepherd, A., Filbey, K., Smith, A., et al. (2015). ILC2s and T Cells Cooperate to Ensure Maintenance of M2 Macrophages for Lung Immunity Against Hookworms. Nat. Commun. 6, 6970. doi: 10.1038/ncomms7970
Broquet, A., Jacqueline, C., Davieau, M., Besbes, A., Roquilly, A., Martin, J., et al. (2017). Interleukin-22 Level is Negatively Correlated With Neutrophil Recruitment in the Lungs in a Pseudomonas Aeruginosa Pneumonia Model. Sci. Rep. 7 (1), 11010. doi: 10.1038/s41598-017-11518-0
Buonocore, S., Ahern, P. P., Uhlig, H. H., Ivanov, I. I., Littman, D. R., Maloy, K. J., et al. (2010). Innate Lymphoid Cells Drive Interleukin-23-Dependent Innate Intestinal Pathology. Nature 464 (7293), 1371–1375. doi: 10.1038/nature08949
Burrows, K., Ngai, L., Wong, F., Won, D., Mortha, A. (2019). ILC2 Activation by Protozoan Commensal Microbes. Int. J. Mol. Sci. 20 (19), 4865. doi: 10.3390/ijms20194865
Byrne, P., McGuirk, P., Todryk, S., Mills, K. H. (2004). Depletion of NK Cells Results in Disseminating Lethal Infection With Bordetella Pertussis Associated With a Reduction of Antigen-Specific Th1 and Enhancement of Th2, But Not Tr1 Cells. Eur. J. Immunol. 34 (9), 2579–2588. doi: 10.1002/eji.200425092
Carvelli, J., Piperoglou, C., Bourenne, J., Farnarier, C., Banzet, N., Demerlé, C., et al. (2019). Imbalance of Circulating Innate Lymphoid Cell Subpopulations in Patients With Septic Shock. Front. Immunol. 10, 2179. doi: 10.3389/fimmu.2019.02179
Castleman, M. J., Dillon, S. M., Purba, C. M., Cogswell, A. C., Kibbie, J. J., McCarter, M. D., et al. (2019). Commensal and Pathogenic Bacteria Indirectly Induce IL-22 But Not Ifnγ Production From Human Colonic ILC3s via Multiple Mechanisms. Front. Immunol. 10:649. doi: 10.3389/fimmu.2019.00649
Cavaillon, J. M., Adib-Conquy, M., Fitting, C., Adrie, C., Payen, D. (2003). Cytokine Cascade in Sepsis. Scand. J. Infect. Dis. 35 (9), 535–544. doi: 10.1080/00365540310015935
Cavaillon, J. M., Riviere, Y., Svab, J., Montagnier, L., Alouf, J. E. (1982). Induction of Interferon by Streptococcus Pyogenes Extracellular Products. Immunol. Lett. 5 (6), 323–326. doi: 10.1016/0165-2478(82)90121-3
Cella, M., Fuchs, A., Vermi, W., Facchetti, F., Otero, K., Lennerz, J. K., et al. (2009). A Human Natural Killer Cell Subset Provides an Innate Source of IL-22 for Mucosal Immunity. Nature 457 (7230), 722–725. doi: 10.1038/nature07537
Chalifour, A., Jeannin, P., Gauchat, J. F., Blaecke, A., Malissard, M., N'Guyen, T., et al. (2004). Direct Bacterial Protein PAMP Recognition by Human NK Cells Involves TLRs and Triggers Alpha-Defensin Production. Blood 104 (6), 1778–1783. doi: 10.1182/blood-2003-08-2820
Chen, K., Eddens, T., Trevejo-Nunez, G., Way, E. E., Elsegeiny, W., Ricks, D. M., et al. (2016). IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense Against K. Pneumoniae. Cell Host Microbe 20 (5), 596–605. doi: 10.1016/j.chom.2016.10.003
Chu, C., Moriyama, S., Li, Z., Zhou, L., Flamar, A. L., Klose, C. S. N., et al. (2018). Anti-Microbial Functions of Group 3 Innate Lymphoid Cells in Gut-Associated Lymphoid Tissues Are Regulated by G-Protein-Coupled Receptor 183. Cell Rep. 23 (13), 3750–3758. doi: 10.1016/j.celrep.2018.05.099
Chung, J. W., Piao, Z.-H., Yoon, S. R., Kim, M. S., Jeong, M., Lee, S. H., et al. (2009). Pseudomonas Aeruginosa Eliminates Natural Killer Cells via Phagocytosis-Induced Apoptosis. PLoS Pathog. 5 (8), e1000561. doi: 10.1371/journal.ppat.1000561
Chun, E., Lavoie, S., Fonseca-Pereira, D., Bae, S., Michaud, M., Hoveyda, H. R., et al. (2019). Metabolite-Sensing Receptor Ffar2 Regulates Colonic Group 3 Innate Lymphoid Cells and Gut Immunity. Immunity 51 (5), 871–884.e876. doi: 10.1016/j.immuni.2019.09.014
Cobb, L. M., Verneris, M. R. (2021). Therapeutic Manipulation of Innate Lymphoid Cells. JCI Insight 6 (6), e146006. doi: 10.1172/jci.insight.146006
Cooper, A. M., Dalton, D. K., Stewart, T. A., Griffin, J. P., Russell, D. G., Orme, I. M. (1993). Disseminated Tuberculosis in Interferon Gamma Gene-Disrupted Mice. J. Exp. Med. 178 (6), 2243–2247. doi: 10.1084/jem.178.6.2243
Creyns, B., Verstockt, B., Cremer, J., Ballet, V., Ferrante, M., Vermeire, S., et al. (2019). DOP26 Biological Therapy Increases NCR+ ILC3 Levels in IBD Patients. J. Crohn's Colitis 13 (Supplement_1), S040–S040. doi: 10.1093/ecco-jcc/jjy222.061
Crohn’s and Colitis Foundation (2021) Fact Sheet.News From the IBD Help Center. Biologics [Online]. Available at: https://www.crohnscolitisfoundation.org/sites/default/files/2020-02/Biologics%201.2020.pdf (Accessed 01/06/2021).
Cruz-Zárate, D., Cabrera-Rivera, G. L., Ruiz-Sánchez, B. P., Serafín-López, J., Chacón-Salinas, R., López-Macías, C., et al. (2018). Innate Lymphoid Cells Have Decreased HLA-DR Expression But Retain Their Responsiveness to TLR Ligands During Sepsis. J. Immunol. 201 (11), 3401–3410. doi: 10.4049/jimmunol.1800735
Cupedo, T., Crellin, N. K., Papazian, N., Rombouts, E. J., Weijer, K., Grogan, J. L., et al. (2009). Human Fetal Lymphoid Tissue-Inducer Cells are Interleukin 17-Producing Precursors to RORC+ CD127+ Natural Killer-Like Cells. Nat. Immunol. 10 (1), 66–74. doi: 10.1038/ni.1668
D'Orazio, J. A., Burke, G. W., Stein-Streilein, J. (1995). Staphylococcal Enterotoxin B Activates Purified NK Cells to Secrete IFN-Gamma But Requires T Lymphocytes to Augment NK Cytotoxicity. J. Immunol. 154 (3), 1014–1023.
Degli-Esposti, M. A., Smyth, M. J. (2005). Close Encounters of Different Kinds: Dendritic Cells and NK Cells Take Centre Stage. Nat. Rev. Immunol. 5 (2), 112–124. doi: 10.1038/nri1549
Denis, M. (1991). Activated Murine Natural Killer Cells Control Growth of Mycobacterium Lepraemurium in Mouse Macrophages; In Vitro and In Vivo Evidence. Int. J. Immunopharmacol 13 (7), 881–887. doi: 10.1016/0192-0561(91)90040-e
Dhiman, R., Indramohan, M., Barnes, P. F., Nayak, R. C., Paidipally, P., Rao, L. V., et al. (2009). IL-22 Produced by Human NK Cells Inhibits Growth of Mycobacterium Tuberculosis by Enhancing Phagolysosomal Fusion. J. Immunol. 183 (10), 6639–6645. doi: 10.4049/jimmunol.0902587
Diefenbach, A. (2013). Innate Lymphoid Cells in the Defense Against Infections. Eur. J. Microbiol. Immunol. (Bp) 3 (3), 143–151. doi: 10.1556/EuJMI.3.2013.3.1
Diefenbach, A., Colonna, M., Koyasu, S. (2014). Development, Differentiation, and Diversity of Innate Lymphoid Cells. Immunity 41 (3), 354–365. doi: 10.1016/j.immuni.2014.09.005
Dobashi, H., Seki, S., Habu, Y., Ohkawa, T., Takeshita, S., Hiraide, H., et al. (1999). Activation of Mouse Liver Natural Killer Cells and NK1.1(+) T Cells by Bacterial Superantigen-Primed Kupffer Cells. Hepatology 30 (2), 430–436. doi: 10.1002/hep.510300209
Doherty, G. M., Lange, J. R., Langstein, H. N., Alexander, H. R., Buresh, C. M., Norton, J. A. (1992). Evidence for IFN-Gamma as a Mediator of the Lethality of Endotoxin and Tumor Necrosis Factor-Alpha. J. Immunol. 149 (5), 1666–1670.
Doss, M., White, M. R., Tecle, T., Hartshorn, K. L. (2010). Human Defensins and LL-37 in Mucosal Immunity. J. Leukoc. Biol. 87 (1), 79–92. doi: 10.1189/jlb.0609382
Eisele, N. A., Ruby, T., Jacobson, A., Manzanillo, P. S., Cox, J. S., Lam, L., et al. (2013). Salmonella Require the Fatty Acid Regulator Pparδ for the Establishment of a Metabolic Environment Essential for Long-Term Persistence. Cell Host Microbe 14 (2), 171–182. doi: 10.1016/j.chom.2013.07.010
Eken, A., Yetkin, M. F., Vural, A., Okus, F. Z., Erdem, S., Azizoglu, Z. B., et al. (2019). Fingolimod Alters Tissue Distribution and Cytokine Production of Human and Murine Innate Lymphoid Cells. Front. Immunol. 10, 217. doi: 10.3389/fimmu.2019.00217
Elemam, N. M., Hannawi, S., Maghazachi, A. A. (2017). Innate Lymphoid Cells (ILCs) as Mediators of Inflammation, Release of Cytokines and Lytic Molecules. Toxins (Basel) 9 (12), 398. doi: 10.3390/toxins9120398
Emoto, M., Miyamoto, M., Yoshizawa, I., Emoto, Y., Schaible, U. E., Kita, E., et al. (2002). Critical Role of NK Cells Rather Than V Alpha 14(+)NKT Cells in Lipopolysaccharide-Induced Lethal Shock in Mice. J. Immunol. 169 (3), 1426–1432. doi: 10.4049/jimmunol.169.3.1426
Endsley, J. J., Torres, A. G., Gonzales, C. M., Kosykh, V. G., Motin, V. L., Peterson, J. W., et al. (2009). Comparative Antimicrobial Activity of Granulysin Against Bacterial Biothreat Agents. Open Microbiol. J. 3, 92–96. doi: 10.2174/1874285800903010092
Ernst, W. A., Thoma-Uszynski, S., Teitelbaum, R., Ko, C., Hanson, D. A., Clayberger, C., et al. (2000). Granulysin, a T Cell Product, Kills Bacteria by Altering Membrane Permeability. J. Immunol. 165 (12), 7102–7108. doi: 10.4049/jimmunol.165.12.7102
Esin, S., Batoni, G., Counoupas, C., Stringaro, A., Brancatisano, F. L., Colone, M., et al. (2008). Direct Binding of Human NK Cell Natural Cytotoxicity Receptor NKp44 to the Surfaces of Mycobacteria and Other Bacteria. Infect. Immun. 76 (4), 1719–1727. doi: 10.1128/iai.00870-07
Esin, S., Counoupas, C., Aulicino, A., Brancatisano, F. L., Maisetta, G., Bottai, D., et al. (2013). Interaction of Mycobacterium Tuberculosis Cell Wall Components With the Human Natural Killer Cell Receptors NKp44 and Toll-Like Receptor 2. Scand. J. Immunol. 77 (6), 460–469. doi: 10.1111/sji.12052
Etogo, A. O., Nunez, J., Lin, C. Y., Toliver-Kinsky, T. E., Sherwood, E. R. (2008). NK But Not CD1-Restricted NKT Cells Facilitate Systemic Inflammation During Polymicrobial Intra-Abdominal Sepsis. J. Immunol. 180 (9), 6334–6345. doi: 10.4049/jimmunol.180.9.6334
Evans, J. H., Horowitz, A., Mehrabi, M., Wise, E. L., Pease, J. E., Riley, E. M., et al. (2011). A Distinct Subset of Human NK Cells Expressing HLA-DR Expand in Response to IL-2 and can Aid Immune Responses to BCG. Eur. J. Immunol. 41 (7), 1924–1933. doi: 10.1002/eji.201041180
Fachi, J. L., Sécca, C., Rodrigues, P. B., Mato, F. C. P., Di Luccia, B., Felipe, J. S., et al. (2020). Acetate Coordinates Neutrophil and ILC3 Responses Against C. Difficile Through FFAR2. J. Exp. Med. 217 (3), jem.20190489. doi: 10.1084/jem.20190489
Fehniger, T. A., Shah, M. H., Turner, M. J., VanDeusen, J. B., Whitman, S. P., Cooper, M. A., et al. (1999). Differential Cytokine and Chemokine Gene Expression by Human NK Cells Following Activation With IL-18 or IL-15 in Combination With IL-12: Implications for the Innate Immune Response. J. Immunol. 162 (8), 4511–4520.
Feng, C. G., Kaviratne, M., Rothfuchs, A. G., Cheever, A., Hieny, S., Young, H. A., et al. (2006). NK Cell-Derived IFN-Gamma Differentially Regulates Innate Resistance and Neutrophil Response in T Cell-Deficient Hosts Infected With Mycobacterium Tuberculosis. J. Immunol. 177 (10), 7086–7093. doi: 10.4049/jimmunol.177.10.7086
Flo, T. H., Smith, K. D., Sato, S., Rodriguez, D. J., Holmes, M. A., Strong, R. K., et al. (2004). Lipocalin 2 Mediates an Innate Immune Response to Bacterial Infection by Sequestrating Iron. Nature 432 (7019), 917–921. doi: 10.1038/nature03104
Forkel, M., van Tol, S., Höög, C., Michaëlsson, J., Almer, S., Mjösberg, J. (2019). Distinct Alterations in the Composition of Mucosal Innate Lymphoid Cells in Newly Diagnosed and Established Crohn's Disease and Ulcerative Colitis. J. Crohns Colitis 13 (1), 67–78. doi: 10.1093/ecco-jcc/jjy119
Frisbee, A. L., Saleh, M. M., Young, M. K., Leslie, J. L., Simpson, M. E., Abhyankar, M. M., et al. (2019). IL-33 Drives Group 2 Innate Lymphoid Cell-Mediated Protection During Clostridium Difficile Infection. Nat. Commun. 10 (1), 2712. doi: 10.1038/s41467-019-10733-9
Fuchs, A. (2016). ILC1s in Tissue Inflammation and Infection. Front. Immunol. 7, 104. doi: 10.3389/fimmu.2016.00104
Garcia-Peñarrubia, P., Koster, F. T., Kelley, R. O., McDowell, T. D., Bankhurst, A. D. (1989). Antibacterial Activity of Human Natural Killer Cells. J. Exp. Med. 169 (1), 99–113. doi: 10.1084/jem.169.1.99
Garg, A., Barnes, P. F., Porgador, A., Roy, S., Wu, S., Nanda, J. S., et al. (2006). Vimentin Expressed on ≪Em<Mycobacterium Tuberculosis≪/Em<-Infected Human Monocytes Is Involved in Binding to the NKp46 Receptor. J. Immunol. 177 (9), 6192. doi: 10.4049/jimmunol.177.9.6192
Geijtenbeek, T. B., Van Vliet, S. J., Koppel, E. A., Sanchez-Hernandez, M., Vandenbroucke-Grauls, C. M., Appelmelk, B., et al. (2003). Mycobacteria Target DC-SIGN to Suppress Dendritic Cell Function. J. Exp. Med. 197 (1), 7–17. doi: 10.1084/jem.20021229
Geremia, A., Arancibia-Cárcamo, C. V., Fleming, M. P., Rust, N., Singh, B., Mortensen, N. J., et al. (2011). IL-23-Responsive Innate Lymphoid Cells are Increased in Inflammatory Bowel Disease. J. Exp. Med. 208 (6), 1127–1133. doi: 10.1084/jem.20101712
Glatzer, T., Killig, M., Meisig, J., Ommert, I., Luetke-Eversloh, M., Babic, M., et al. (2013). Rorγt+ Innate Lymphoid Cells Acquire a Proinflammatory Program Upon Engagement of the Activating Receptor Nkp44. Immunity 38 (6), 1223–1235. doi: 10.1016/j.immuni.2013.05.013
Godinez, I., Raffatellu, M., Chu, H., Paixão, T. A., Haneda, T., Santos, R. L., et al. (2009). Interleukin-23 Orchestrates Mucosal Responses to Salmonella Enterica Serotype Typhimurium in the Intestine. Infect. Immun. 77 (1), 387–398. doi: 10.1128/iai.00933-08
Godinho-Silva, C., Domingues, R. G., Rendas, M., Raposo, B., Ribeiro, H., da Silva, J. A., et al. (2019). Light-Entrained and Brain-Tuned Circadian Circuits Regulate ILC3s and Gut Homeostasis. Nature 574 (7777), 254–258. doi: 10.1038/s41586-019-1579-3
Gonzales, C. M., Williams, C. B., Calderon, V. E., Huante, M. B., Moen, S. T., Popov, V. L., et al. (2012). Antibacterial Role for Natural Killer Cells in Host Defense to Bacillus Anthracis. Infect. Immun. 80 (1), 234–242. doi: 10.1128/IAI.05439-11
Goto, Y., Obata, T., Kunisawa, J., Sato, S., Ivanov, I. I., Lamichhane, A., et al. (2014). Innate Lymphoid Cells Regulate Intestinal Epithelial Cell Glycosylation. Science 345 (6202), 1254009. doi: 10.1126/science.1254009
Goverse, G., Labao-Almeida, C., Ferreira, M., Molenaar, R., Wahlen, S., Konijn, T., et al. (2016). Vitamin A Controls the Presence of Rorγ+ Innate Lymphoid Cells and Lymphoid Tissue in the Small Intestine. J. Immunol. 196 (12), 5148–5155. doi: 10.4049/jimmunol.1501106
Gray, J., Oehrle, K., Worthen, G., Alenghat, T., Whitsett, J., Deshmukh, H. (2017). Intestinal Commensal Bacteria Mediate Lung Mucosal Immunity and Promote Resistance of Newborn Mice to Infection. Sci. Transl. Med. 9 (376), eaaf9412. doi: 10.1126/scitranslmed.aaf9412
Gury-BenAri, M., Thaiss, C. A., Serafini, N., Winter, D. R., Giladi, A., Lara-Astiaso, D., et al. (2016). The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell 166 (5), 1231–1246.e1213. doi: 10.1016/j.cell.2016.07.043
Hall, L. J., Clare, S., Dougan, G. (2010). NK Cells Influence Both Innate and Adaptive Immune Responses After Mucosal Immunization With Antigen and Mucosal Adjuvant. J. Immunol. 184 (8), 4327–4337. doi: 10.4049/jimmunol.0903357
Hall, L. J., Murphy, C. T., Hurley, G., Quinlan, A., Shanahan, F., Nally, K., et al. (2013). Natural Killer Cells Protect Against Mucosal and Systemic Infection With the Enteric Pathogen Citrobacter Rodentium. Infect Immun. 81 (2), 460–469. doi: 10.1128/IAI.00953-12
Han, L., Wang, X.-M., Di, S., Gao, Z.-Z., Li, Q.-W., Wu, H.-R., et al. (2019). Innate Lymphoid Cells: A Link Between the Nervous System and Microbiota in Intestinal Networks. Mediators Inflamm. 2019, 1978094–1978094. doi: 10.1155/2019/1978094
Hargreaves, D. C., Medzhitov, R. (2005). Innate Sensors of Microbial Infection. J. Clin. Immunol. 25 (6), 503–510. doi: 10.1007/s10875-005-8065-4
Harrington, L., Srikanth, C. V., Antony, R., Shi, H. N., Cherayil, B. J. (2007). A Role for Natural Killer Cells in Intestinal Inflammation Caused by Infection With Salmonella Enterica Serovar Typhimurium. FEMS Immunol. Med. Microbiol. 51 (2), 372–380. doi: 10.1111/j.1574-695X.2007.00313.x
Hepworth, M. R., Monticelli, L. A., Fung, T. C., Ziegler, C. G., Grunberg, S., Sinha, R., et al. (2013). Innate Lymphoid Cells Regulate CD4+ T-Cell Responses to Intestinal Commensal Bacteria. Nature 498 (7452), 113–117. doi: 10.1038/nature12240
Heremans, H., Dillen, C., Groenen, M., Matthys, P., Billiau, A. (2000). Role of Interferon-Gamma and Nitric Oxide in Pulmonary Edema and Death Induced by Lipopolysaccharide. Am. J. Respir. Crit. Care Med. 161 (1), 110–117. doi: 10.1164/ajrccm.161.1.9902089
Hildreth, A. D., O'Sullivan, T. E. (2019). Tissue-Resident Innate and Innate-Like Lymphocyte Responses to Viral Infection. Viruses 11 (3), 272. doi: 10.3390/v11030272
Hirata, Y., Egea, L., Dann, S. M., Eckmann, L., Kagnoff, M. F. (2010). GM-CSF-Facilitated Dendritic Cell Recruitment and Survival Govern the Intestinal Mucosal Response to a Mouse Enteric Bacterial Pathogen. Cell Host Microbe 7 (2), 151–163. doi: 10.1016/j.chom.2010.01.006
Hirose, S., Wang, S., Tormanen, K., Wang, Y., Tang, J., Akbari, O., et al. (2019). Roles of Type 1, 2, and 3 Innate Lymphoid Cells in Herpes Simplex Virus 1 Infection In Vitro and In Vivo. J. Virol. 93 (13), e00523–e00519. doi: 10.1128/JVI.00523-19
Hornung, V., Rothenfusser, S., Britsch, S., Krug, A., Jahrsdörfer, B., Giese, T., et al. (2002). Quantitative Expression of Toll-Like Receptor 1-10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 168 (9), 4531–4537. doi: 10.4049/jimmunol.168.9.4531
Horowitz, A., Stegmann, K. A., Riley, E. M. (2012). Activation of Natural Killer Cells During Microbial Infections. Front. Immunol. 2, 88. doi: 10.3389/fimmu.2011.00088
Huang, S., Hendriks, W., Althage, A., Hemmi, S., Bluethmann, H., Kamijo, R., et al. (1993). Immune Response in Mice That Lack the Interferon-Gamma Receptor. Science 259 (5102), 1742–1745. doi: 10.1126/science.8456301
Hueber, W., Sands, B. E., Lewitzky, S., Vandemeulebroecke, M., Reinisch, W., Higgins, P. D., et al. (2012). Secukinumab, a Human Anti-IL-17A Monoclonal Antibody, for Moderate to Severe Crohn's Disease: Unexpected Results of a Randomised, Double-Blind Placebo-Controlled Trial. Gut 61 (12), 1693–1700. doi: 10.1136/gutjnl-2011-301668
Hunter, C. A., Chizzonite, R., Remington, J. S. (1995). IL-1 Beta is Required for IL-12 to Induce Production of IFN-Gamma by NK Cells. A Role for IL-1 Beta in the T Cell-Independent Mechanism of Resistance Against Intracellular Pathogens. J. Immunol. 155 (9), 4347–4354.
Huntington, N. D., Vosshenrich, C. A., Di Santo, J. P. (2007). Developmental Pathways That Generate Natural-Killer-Cell Diversity in Mice and Humans. Nat. Rev. Immunol. 7 (9), 703–714. doi: 10.1038/nri2154
Jewett, A., Gan, X. H., Lebow, L. T., Bonavida, B. (1996). Differential Secretion of TNF-Alpha and IFN-Gamma by Human Peripheral Blood-Derived NK Subsets and Association With Functional Maturation. J. Clin. Immunol. 16 (1), 46–54. doi: 10.1007/bf01540972
Kaser, A. (2014). Not All Monoclonals are Created Equal - Lessons From Failed Drug Trials in Crohn's Disease. Best Pract. Res. Clin. Gastroenterol. 28 (3), 437–449. doi: 10.1016/j.bpg.2014.04.005
Katz, P., Yeager, H., Jr., Whalen, G., Evans, M., Swartz, R. P., Roecklein, J. (1990). Natural Killer Cell-Mediated Lysis of Mycobacterium-Avium Complex-Infected Monocytes. J. Clin. Immunol. 10 (1), 71–77. doi: 10.1007/BF00917500
Kerschen, E. J., Cohen, D. A., Kaplan, A. M., Straley, S. C. (2004). The Plague Virulence Protein YopM Targets the Innate Immune Response by Causing a Global Depletion of NK Cells. Infect. Immun. 72 (8), 4589–4602. doi: 10.1128/iai.72.8.4589-4602.2004
Kim, J., Chang, Y., Bae, B., Sohn, K. H., Cho, S. H., Chung, D. H., et al. (2019). Innate Immune Crosstalk in Asthmatic Airways: Innate Lymphoid Cells Coordinate Polarization of Lung Macrophages. J. Allergy Clin. Immunol. 143 (5), 1769–1782.e1711. doi: 10.1016/j.jaci.2018.10.040
Kinnebrew, M. A., Buffie, C. G., Diehl, G. E., Zenewicz, L. A., Leiner, I., Hohl, T. M., et al. (2012). Interleukin 23 Production by Intestinal CD103(+)CD11b(+) Dendritic Cells in Response to Bacterial Flagellin Enhances Mucosal Innate Immune Defense. Immunity 36 (2), 276–287. doi: 10.1016/j.immuni.2011.12.011
Klezovich-Bénard, M., Corre, J. P., Jusforgues-Saklani, H., Fiole, D., Burjek, N., Tournier, J. N., et al. (2012). Mechanisms of NK Cell-Macrophage Bacillus Anthracis Crosstalk: A Balance Between Stimulation by Spores and Differential Disruption by Toxins. PLoS Pathog. 8 (1), e1002481. doi: 10.1371/journal.ppat.1002481
Klimpel, G. R., Niesel, D. W., Klimpel, K. D. (1986). Natural Cytotoxic Effector Cell Activity Against Shigella Flexneri-Infected HeLa Cells. J. Immunol. 136 (3), 1081–1086.
Klose, C. S. N., Flach, M., Möhle, L., Rogell, L., Hoyler, T., Ebert, K., et al. (2014). Differentiation of Type 1 ILCs From a Common Progenitor to All Helper-Like Innate Lymphoid Cell Lineages. Cell 157 (2), 340–356. doi: 10.1016/j.cell.2014.03.030
Klose, C. S., Kiss, E. A., Schwierzeck, V., Ebert, K., Hoyler, T., d'Hargues, Y., et al. (2013). A T-Bet Gradient Controls the Fate and Function of CCR6-Rorγt+ Innate Lymphoid Cells. Nature 494 (7436), 261–265. doi: 10.1038/nature11813
Koga, S., Kiyohara, T., Taniguchi, K., Nishikido, M., Kubota, S., Sakuragi, T., et al. (1988). BCG Induced Killer Cell Activity. Urol Res. 16 (5), 351–355. doi: 10.1007/BF00256041
Krensky, A. M., Clayberger, C. (2009). Biology and Clinical Relevance of Granulysin. Tissue Antigens 73 (3), 193–198. doi: 10.1111/j.1399-0039.2008.01218.x
Kroes, M. M., Mariman, R., Hijdra, D., Hamstra, H. J., van Boxtel, K., van Putten, J. P. M., et al. (2019). Activation of Human NK Cells by Bordetella Pertussis Requires Inflammasome Activation in Macrophages. Front. Immunol. 10, 2030. doi: 10.3389/fimmu.2019.02030
Kwon, K. W., Kim, S. J., Kim, H., Kim, W. S., Kang, S. M., Choi, E., et al. (2019). IL-15 Generates IFN-γ-Producing Cells Reciprocally Expressing Lymphoid-Myeloid Markers During Dendritic Cell Differentiation. Int. J. Biol. Sci. 15 (2), 464–480. doi: 10.7150/ijbs.25743
Lande, R., Giacomini, E., Grassi, T., Remoli, M. E., Iona, E., Miettinen, M., et al. (2003). IFN-Alpha Beta Released by Mycobacterium Tuberculosis-Infected Human Dendritic Cells Induces the Expression of CXCL10: Selective Recruitment of NK and Activated T Cells. J. Immunol. 170 (3), 1174–1182. doi: 10.4049/jimmunol.170.3.1174
Lauzon, N. M., Mian, F., MacKenzie, R., Ashkar, A. A. (2006). The Direct Effects of Toll-Like Receptor Ligands on Human NK Cell Cytokine Production and Cytotoxicity. Cell Immunol. 241 (2), 102–112. doi: 10.1016/j.cellimm.2006.08.004
Lee, J. S., Cella, M., McDonald, K. G., Garlanda, C., Kennedy, G. D., Nukaya, M., et al. (2011). AHR Drives the Development of Gut ILC22 Cells and Postnatal Lymphoid Tissues via Pathways Dependent on and Independent of Notch. Nat. Immunol. 13 (2), 144–151. doi: 10.1038/ni.2187
Le, C. F., Fang, C. M., Sekaran, S. D. (2017). Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 61 (4), e02340–16. doi: 10.1128/AAC.02340-16
Li, S., Bostick, J. W., Ye, J., Qiu, J., Zhang, B., Urban, J. F., Jr., et al. (2018). Aryl Hydrocarbon Receptor Signaling Cell Intrinsically Inhibits Intestinal Group 2 Innate Lymphoid Cell Function. Immunity 49 (5), 915–928.e915. doi: 10.1016/j.immuni.2018.09.015
Li, J., Doty, A. L., Iqbal, A., Glover, ,. S. C. (2016). The Differential Frequency of Lineage(-)CRTH2(-)CD45(+)NKp44(-)CD117(-)CD127(+)ILC Subset in the Inflamed Terminal Ileum of Patients With Crohn's Disease. Cell Immunol. 304–305, 63–68. doi: 10.1016/j.cellimm.2016.05.001
Li, Y., Gao, Y., Cui, T., Yang, T., Liu, L., Li, T., et al. (2017b). Retinoic Acid Facilitates Toll-Like Receptor 4 Expression to Improve Intestinal Barrier Function Through Retinoic Acid Receptor Beta. Cell Physiol. Biochem. 42 (4), 1390–1406. doi: 10.1159/000479203
Li, R., Jiang, X. X., Zhang, L. F., Liu, X. M., Hu, T. Z., Xia, X. J., et al. (2017a). Group 2 Innate Lymphoid Cells Are Involved in Skewed Type 2 Immunity of Gastric Diseases Induced by Helicobacter Pylori Infection. Mediators Inflamm. 2017, 4927964. doi: 10.1155/2017/4927964
Longman, R. S., Diehl, G. E., Victorio, D. A., Huh, J. R., Galan, C., Miraldi, E. R., et al. (2014). CX3CR1+ Mononuclear Phagocytes Support Colitis-Associated Innate Lymphoid Cell Production of IL-22. J. Exp. Med. 211 (8), 1571–1583. doi: 10.1084/jem.20140678
López, M. C., Duckett, N. S., Baron, S. D., Metzger, D. W. (2004). Early Activation of NK Cells After Lung Infection With the Intracellular Bacterium, Francisella Tularensis LVS. Cell Immunol. 232 (1-2), 75–85. doi: 10.1016/j.cellimm.2005.02.001
Loser, S., Smith, K. A., Maizels, R. M. (2019). Innate Lymphoid Cells in Helminth Infections-Obligatory or Accessory? Front. Immunol. 10, 620. doi: 10.3389/fimmu.2019.00620
Lünemann, A., Lünemann, J. D., Münz, C. (2009). Regulatory NK-Cell Functions in Inflammation and Autoimmunity. Mol. Med. 15 (9-10), 352–358. doi: 10.2119/molmed.2009.00035
Lu, C. C., Wu, T. S., Hsu, Y. J., Chang, C. J., Lin, C. S., Chia, J. H., et al. (2014). NK Cells Kill Mycobacteria Directly by Releasing Perforin and Granulysin. J. Leukoc. Biol. 96 (6), 1119–1129. doi: 10.1189/jlb.4A0713-363RR
Maggi, L., Montaini, G., Mazzoni, A., Rossettini, B., Capone, M., Rossi, M. C., et al. (2017). Human Circulating Group 2 Innate Lymphoid Cells can Express CD154 and Promote IgE Production. J. Allergy Clin. Immunol. 139 (3), 964–976.e964. doi: 10.1016/j.jaci.2016.06.032
Maghazachi, A. A. (2010). Role of Chemokines in the Biology of Natural Killer Cells. Curr. Top. Microbiol. Immunol. 341, 37–58. doi: 10.1007/82_2010_20
Maghazachi, A. A., Al-Aoukaty, A. (1998). Chemokines Activate Natural Killer Cells Through Heterotrimeric G-Proteins: Implications for the Treatment of AIDS and Cancer. FASEB J. 12 (11), 913–924. doi: 10.1096/fasebj.12.11.913
Manta, C., Heupel, E., Radulovic, K., Rossini, V., Garbi, N., Riedel, C. U., et al. (2013). CX(3)CR1(+) Macrophages Support IL-22 Production by Innate Lymphoid Cells During Infection With Citrobacter Rodentium. Mucosal Immunol. 6 (1), 177–188. doi: 10.1038/mi.2012.61
Marcenaro, E., Ferranti, B., Falco, M., Moretta, L., Moretta, A. (2008). Human NK Cells Directly Recognize Mycobacterium Bovis via TLR2 and Acquire the Ability to Kill Monocyte-Derived DC. Int. Immunol. 20 (9), 1155–1167. doi: 10.1093/intimm/dxn073
Mastroeni, P., Harrison, J. A., Chabalgoity, J. A., Hormaeche, C. E. (1996). Effect of Interleukin 12 Neutralization on Host Resistance and Gamma Interferon Production in Mouse Typhoid. Infect. Immun. 64 (1), 189–196. doi: 10.1128/iai.64.1.189-196.1996
Matsumoto, H., Suzuki, K., Tsuyuguchi, K., Tanaka, E., Amitani, R., Maeda, A., et al. (1997). Interleukin-12 Gene Expression in Human Monocyte-Derived Macrophages Stimulated With Mycobacterium Bovis BCG: Cytokine Regulation and Effect of NK Cells. Infect. Immun. 65 (11), 4405–4410. doi: 10.1128/iai.65.11.4405-4410.1997
Maturana, P., Puente, J., Miranda, D., Sepulveda, C., Wolf, M. E., Mosnaim, A. D. (1991). Natural Killer Cell Activity in Patients With Septic Shock. J. Crit. Care 6 (1), 42–45. doi: 10.1016/0883-9441(91)90032-O
McLoughlin, R. M., Lee, J. C., Kasper, D. L., Tzianabos, A. O. (2008). IFN-Gamma Regulated Chemokine Production Determines the Outcome of Staphylococcus Aureus Infection. J. Immunol. 181 (2), 1323–1332. doi: 10.4049/jimmunol.181.2.1323
McSharry, B. P., Gardiner, C. M. (2010). “The Role of NK Cells in Bacterial Infections,” in Natural Killer Cells: At the Forefront of Modern Immunology. Ed. Zimmer, J. (Berlin, Heidelberg: Springer Berlin Heidelberg), 153–175.
Mebius, R. E., Rennert, P., Weissman, I. L. (1997). Developing Lymph Nodes Collect CD4+CD3- LTbeta+ Cells That can Differentiate to APC, NK Cells, and Follicular Cells But Not T or B Cells. Immunity 7 (4), 493–504. doi: 10.1016/s1074-7613(00)80371-4
Melo-Gonzalez, F., Kammoun, H., Evren, E., Dutton, E. E., Papadopoulou, M., Bradford, B. M., et al. (2019). Antigen-Presenting ILC3 Regulate T Cell-Dependent IgA Responses to Colonic Mucosal Bacteria. J. Exp. Med. 216 (4), 728–742. doi: 10.1084/jem.20180871
Mian, M. F., Lauzon, N. M., Andrews, D. W., Lichty, B. D., Ashkar, A. A. (2010). FimH can Directly Activate Human and Murine Natural Killer Cells via TLR4. Mol. Ther. J. Am. Soc. Gene Ther. 18 (7), 1379–1388. doi: 10.1038/mt.2010.75
Michałkiewicz, J., Stachowski, J., Barth, C., Patzer, J., Dzierzanowska, D., Madaliński, K. (1999). Effect of Pseudomonas Aeruginosa Exotoxin A on IFN-Gamma Synthesis: Expression of Costimulatory Molecules on Monocytes and Activity of NK Cells. Immunol. Lett. 69 (3), 359–366. doi: 10.1016/s0165-2478(99)00121-2
Mielke, L. A., Jones, S. A., Raverdeau, M., Higgs, R., Stefanska, A., Groom, J. R., et al. (2013). Retinoic Acid Expression Associates With Enhanced IL-22 Production by γδ T Cells and Innate Lymphoid Cells and Attenuation of Intestinal Inflammation. J. Exp. Med. 210 (6), 1117–1124. doi: 10.1084/jem.20121588
Miles, R. H., Paxton, T. P., Dries, D. J., Gamelli, R. L. (1994). Interferon-Gamma Increases Mortality Following Cecal Ligation and Puncture. J. Trauma 36 (5), 607–611. doi: 10.1097/00005373-199405000-00001
Mjösberg, J. M., Trifari, S., Crellin, N. K., Peters, C. P., van Drunen, C. M., Piet, B., et al. (2011). Human IL-25- and IL-33-Responsive Type 2 Innate Lymphoid Cells are Defined by Expression of CRTH2 and CD161. Nat. Immunol. 12 (11), 1055–1062. doi: 10.1038/ni.2104
Monticelli, L. A., Osborne, L. C., Noti, M., Tran, S. V., Zaiss, D. M., Artis, D. (2015). IL-33 Promotes an Innate Immune Pathway of Intestinal Tissue Protection Dependent on Amphiregulin-EGFR Interactions. Proc. Natl. Acad. Sci. U. S. A. 112 (34), 10762–10767. doi: 10.1073/pnas.1509070112
Moore, T. A., Perry, M. L., Getsoian, A. G., Newstead, M. W., Standiford, T. J. (2002). Divergent Role of Gamma Interferon in a Murine Model of Pulmonary Versus Systemic Klebsiella Pneumoniae Infection. Infect. Immun. 70 (11), 6310–6318. doi: 10.1128/iai.70.11.6310-6318.2002
Moro, K., Yamada, T., Tanabe, M., Takeuchi, T., Ikawa, T., Kawamoto, H., et al. (2010). Innate Production of T(H)2 Cytokines by Adipose Tissue-Associated C-Kit(+)Sca-1(+) Lymphoid Cells. Nature 463 (7280), 540–544. doi: 10.1038/nature08636
Mortha, A., Chudnovskiy, A., Hashimoto, D., Bogunovic, M., Spencer, S. P., Belkaid, Y., et al. (2014). Microbiota-Dependent Crosstalk Between Macrophages and ILC3 Promotes Intestinal Homeostasis. Science 343 (6178):1249288. doi: 10.1126/science.1249288
Moschen, A. R., Tilg, H., Raine, T. (2019). IL-12, IL-23 and IL-17 in IBD: Immunobiology and Therapeutic Targeting. Nat. Rev. Gastroenterol. Hepatol. 16 (3), 185–196. doi: 10.1038/s41575-018-0084-8
Muhammad, J. S., Jayakumar, M. N., Elemam, N. M., Venkatachalam, T., Raju, T. K., Hamoudi, R. A., et al. (2019). Gasdermin D Hypermethylation Inhibits Pyroptosis And LPS-Induced IL-1β Release From NK92 Cells. Immunotargets Ther. 8, 29–41. doi: 10.2147/itt.S219867
Mühlen, K. A., Schümann, J., Wittke, F., Stenger, S., Van Rooijen, N., Van Kaer, L., et al. (2004). NK Cells, But Not NKT Cells, are Involved in Pseudomonas Aeruginosa Exotoxin A-Induced Hepatotoxicity in Mice. J. Immunol. 172 (5), 3034–3041. doi: 10.4049/jimmunol.172.5.3034
Muotiala, A., Mäkelä, P. H. (1990). The Role of IFN-Gamma in Murine Salmonella Typhimurium Infection. Microb. Pathog. 8 (2), 135–141. doi: 10.1016/0882-4010(90)90077-4
Murakami, T., Hatano, S., Yamada, H., Iwakura, Y., Yoshikai, Y. (2016). Two Types of Interleukin 17a-Producing γδ T Cells in Protection Against Pulmonary Infection With Klebsiella Pneumoniae. J. Infect. Dis. 214 (11), 1752–1761. doi: 10.1093/infdis/jiw443
Murphey, E. D., Herndon, D. N., Sherwood, E. R. (2004). Gamma Interferon Does Not Enhance Clearance of Pseudomonas Aeruginosa But Does Amplify a Proinflammatory Response in a Murine Model of Postseptic Immunosuppression. Infect. Immun. 72 (12), 6892–6901. doi: 10.1128/iai.72.12.6892-6901.2004
Nairz, M., Fritsche, G., Brunner, P., Talasz, H., Hantke, K., Weiss, G. (2008). Interferon-Gamma Limits the Availability of Iron for Intramacrophage Salmonella Typhimurium. Eur. J. Immunol. 38 (7), 1923–1936. doi: 10.1002/eji.200738056
Nedvetzki, S., Sowinski, S., Eagle, R. A., Harris, J., Vely, F., Pende, D., et al. (2007). Reciprocal Regulation of Human Natural Killer Cells and Macrophages Associated With Distinct Immune Synapses. Blood 109 (9), 3776–3785. doi: 10.1182/blood-2006-10-052977
Neill, D. R., Wong, S. H., Bellosi, A., Flynn, R. J., Daly, M., Langford, T. K., et al. (2010). Nuocytes Represent a New Innate Effector Leukocyte That Mediates Type-2 Immunity. Nature 464 (7293), 1367–1370. doi: 10.1038/nature08900
Newman, K. C., Korbel, D. S., Hafalla, J. C., Riley, E. M. (2006). Cross-Talk With Myeloid Accessory Cells Regulates Human Natural Killer Cell Interferon-Gamma Responses to Malaria. PLoS Pathog. 2 (12), e118. doi: 10.1371/journal.ppat.0020118
Nguyen, K. B., Salazar-Mather, T. P., Dalod, M. Y., Van Deusen, J. B., Wei, X. Q., Liew, F. Y., et al. (2002). Coordinated and Distinct Roles for IFN-Alpha Beta, IL-12, and IL-15 Regulation of NK Cell Responses to Viral Infection. J. Immunol. 169 (8), 4279–4287. doi: 10.4049/jimmunol.169.8.4279
Nomura, T., Kawamura, I., Tsuchiya, K., Kohda, C., Baba, H., Ito, Y., et al. (2002). Essential Role of Interleukin-12 (IL-12) and IL-18 for Gamma Interferon Production Induced by Listeriolysin O in Mouse Spleen Cells. Infect. Immun. 70 (3), 1049–1055. doi: 10.1128/iai.70.3.1049-1055.2002
Oetjen, L. K., Mack, M. R., Feng, J., Whelan, T. M., Niu, H., Guo, C. J., et al. (2017). Sensory Neurons Co-Opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171 (1), 217–228 e213. doi: 10.1016/j.cell.2017.08.006
Oth, T., Habets, T., Germeraad, W. T. V., Zonneveld, M. I., Bos, G. M. J., Vanderlocht, J. (2018). Pathogen Recognition by NK Cells Amplifies the Pro-Inflammatory Cytokine Production of Monocyte-Derived DC. via IFN-γ. BMC Immunol. 19 (1), 8. doi: 10.1186/s12865-018-0247-y
Panda, S. K., Colonna, M. (2019). Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 10, 861. doi: 10.3389/fimmu.2019.00861
Passos, S. T., Silver, J. S., O'Hara, A. C., Sehy, D., Stumhofer, J. S., Hunter, C. A. (2010). IL-6 Promotes NK Cell Production of IL-17 During Toxoplasmosis. J. Immunol. 184 (4), 1776–1783. doi: 10.4049/jimmunol.0901843
Pastorelli, L., De Salvo, C., Mercado, J. R., Vecchi, M., Pizarro, T. T. (2013). Central Role of the Gut Epithelial Barrier in the Pathogenesis of Chronic Intestinal Inflammation: Lessons Learned From Animal Models and Human Genetics. Front. Immunol. 4, 280. doi: 10.3389/fimmu.2013.00280
Pearson, C., Thornton, E. E., McKenzie, B., Schaupp, A. L., Huskens, N., Griseri, T., et al. (2016). ILC3 GM-CSF Production and Mobilisation Orchestrate Acute Intestinal Inflammation. Elife 5, e10066. doi: 10.7554/eLife.10066
Perona-Wright, G., Mohrs, K., Szaba, F. M., Kummer, L. W., Madan, R., Karp, C. L., et al. (2009). Systemic But Not Local Infections Elicit Immunosuppressive IL-10 Production by Natural Killer Cells. Cell Host Microbe 6 (6), 503–512. doi: 10.1016/j.chom.2009.11.003
Pham, T. H. M., Brewer, S. M., Thurston, T., Massis, L. M., Honeycutt, J., Lugo, K., et al. (2020). Salmonella-Driven Polarization of Granuloma Macrophages Antagonizes TNF-Mediated Pathogen Restriction During Persistent Infection. Cell Host Microbe 27 (1), 54–67.e55. doi: 10.1016/j.chom.2019.11.011
Poniewierska-Baran, A., Tokarz-Deptuła, B., Deptuła, W. (2019). The Role of Innate Lymphoid Cells in Selected Disease States - Cancer Formation, Metabolic Disorder and Inflammation. Arch. Med. Sci. 17 (1), 196–206. doi: 10.5114/aoms.2019.89835
Price, A. E., Liang, H. E., Sullivan, B. M., Reinhardt, R. L., Eisley, C. J., Erle, D. J., et al. (2010). Systemically Dispersed Innate IL-13-Expressing Cells in Type 2 Immunity. Proc. Natl. Acad. Sci. U. S. A. 107 (25), 11489–11494. doi: 10.1073/pnas.1003988107
Qiu, J., Heller, J. J., Guo, X., Chen, Z. M., Fish, K., Fu, Y. X., et al. (2012). The Aryl Hydrocarbon Receptor Regulates Gut Immunity Through Modulation of Innate Lymphoid Cells. Immunity 36 (1), 92–104. doi: 10.1016/j.immuni.2011.11.011
Qiu, F., Maniar, A., Diaz, M. Q., Chapoval, A. I., Medvedev, A. E. (2011). Activation of Cytokine-Producing and Antitumor Activities of Natural Killer Cells and Macrophages by Engagement of Toll-Like and NOD-Like Receptors. Innate Immun. 17 (4), 375–387. doi: 10.1177/1753425910372000
Rak, G. D., Osborne, L. C., Siracusa, M. C., Kim, B. S., Wang, K., Bayat, A., et al. (2016). IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J. Invest. Dermatol. 136 (2), 487–496. doi: 10.1038/jid.2015.406
Rankin, L. C., Groom, J. R., Chopin, M., Herold, M. J., Walker, J. A., Mielke, L. A., et al. (2013). The Transcription Factor T-Bet is Essential for the Development of NKp46+ Innate Lymphocytes via the Notch Pathway. Nat. Immunol. 14 (4), 389–395. doi: 10.1038/ni.2545
Reddick, L. E., Alto, N. M. (2014). Bacteria Fighting Back: How Pathogens Target and Subvert the Host Innate Immune System. Mol. Cell 54 (2), 321–328. doi: 10.1016/j.molcel.2014.03.010
Reynders, A., Yessaad, N., Vu Manh, T. P., Dalod, M., Fenis, A., Aubry, C., et al. (2011). Identity, Regulation and In Vivo Function of Gut Nkp46+Rorγt+ and Nkp46+Rorγt- Lymphoid Cells. EMBO J. 30 (14), 2934–2947. doi: 10.1038/emboj.2011.201
Rizvanov, A. A., Haertlé, T., Bogomolnaya, L., Talebi Bezmin Abadi, A. (2019). Helicobacter Pylori and Its Antibiotic Heteroresistance: A Neglected Issue in Published Guidelines. Front. Microbiol. 10, 1796. doi: 10.3389/fmicb.2019.01796
Rolig, A. S., Mittge, E. K., Ganz, J., Troll, J. V., Melancon, E., Wiles, T. J., et al. (2017). The Enteric Nervous System Promotes Intestinal Health by Constraining Microbiota Composition. PLoS Biol. 15 (2), e2000689. doi: 10.1371/journal.pbio.2000689
Rutgeerts, P., Gasink, C., Chan, D., Lang, Y., Pollack, P., Colombel, J. F., et al. (2018). Efficacy of Ustekinumab for Inducing Endoscopic Healing in Patients With Crohn's Disease. Gastroenterology 155 (4), 1045–1058. doi: 10.1053/j.gastro.2018.06.035
Sacks, L. V., Petermann, F. G., Lapham, C. K., John, P. A., Yuille, M. A., Tomar, R. H. (1991). A Streptococcal Erythrogenic Toxin Preparation Augments Natural Killer Activity of Peripheral Blood Mononuclear Cells. J. Infect. Dis. 164 (3), 522–526. doi: 10.1093/infdis/164.3.522
Saluzzo, S., Gorki, A. D., Rana, B. M. J., Martins, R., Scanlon, S., Starkl, P., et al. (2017). First-Breath-Induced Type 2 Pathways Shape the Lung Immune Environment. Cell Rep. 18 (8), 1893–1905. doi: 10.1016/j.celrep.2017.01.071
Sands, B. E., Chen, J., Feagan, B. G., Penney, M., Rees, W. A., Danese, S., et al. (2017). Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn's Disease: A Phase 2a Study. Gastroenterology 153 (1), 77–86.e76. doi: 10.1053/j.gastro.2017.03.049
Sanos, S. L., Bui, V. L., Mortha, A., Oberle, K., Heners, C., Johner, C., et al. (2009). RORgammat and Commensal Microflora are Required for the Differentiation of Mucosal Interleukin 22-Producing NKp46+ Cells. Nat. Immunol. 10 (1), 83–91. doi: 10.1038/ni.1684
Satoh-Takayama, N., Kato, T., Motomura, Y., Kageyama, T., Taguchi-Atarashi, N., Kinoshita-Daitoku, R., et al. (2020). Bacteria-Induced Group 2 Innate Lymphoid Cells in the Stomach Provide Immune Protection Through Induction of IgA. Immunity 52 (4), 635–649.e634. doi: 10.1016/j.immuni.2020.03.002
Satoh-Takayama, N., Serafini, N., Verrier, T., Rekiki, A., Renauld, J. C., Frankel, G., et al. (2014). The Chemokine Receptor CXCR6 Controls the Functional Topography of Interleukin-22 Producing Intestinal Innate Lymphoid Cells. Immunity 41 (5), 776–788. doi: 10.1016/j.immuni.2014.10.007
Schmidt, S., Ullrich, E., Bochennek, K., Zimmermann, S. Y., Lehrnbecher, T. (2016). Role of Natural Killer Cells in Antibacterial Immunity. Expert Rev. Hematol. 9 (12), 1119–1127. doi: 10.1080/17474086.2016.1254546
Schneider, B. E., Korbel, D., Hagens, K., Koch, M., Raupach, B., Enders, J., et al. (2010). A Role for IL-18 in Protective Immunity Against Mycobacterium Tuberculosis. Eur. J. Immunol. 40 (2), 396–405. doi: 10.1002/eji.200939583
Schneider, C., O'Leary, C. E., von Moltke, J., Liang, H. E., Ang, Q. Y., Turnbaugh, P. J., et al. (2018). A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 174 (2), 271–284.e214. doi: 10.1016/j.cell.2018.05.014
Sciumé, G., Hirahara, K., Takahashi, H., Laurence, A., Villarino, A. V., Singleton, K. L., et al. (2012). Distinct Requirements for T-Bet in Gut Innate Lymphoid Cells. J. Exp. Med. 209 (13), 2331–2338. doi: 10.1084/jem.20122097
Scott, M. J., Hoth, J. J., Stagner, M. K., Gardner, S. A., Peyton, J. C., Cheadle, W. G. (2004). CD40-CD154 Interactions Between Macrophages and Natural Killer Cells During Sepsis are Critical for Macrophage Activation and are Not Interferon Gamma Dependent. Clin. Exp. Immunol. 137 (3), 469–477. doi: 10.1111/j.1365-2249.2004.02547.x
Seo, G.-Y., Giles, D. A., Kronenberg, M. (2020). The Role of Innate Lymphoid Cells in Response to Microbes at Mucosal Surfaces. Mucosal Immunol. 13 (3), 399–412. doi: 10.1038/s41385-020-0265-y
Shenker, B. J., Vitale, L. A., Keiba, I., Harrison, G., Berthold, P., Golub, E., et al. (1994). Flow Cytometric Analysis of the Cytotoxic Effects of Actinobacillus Actinomycetemcomitans Leukotoxin on Human Natural Killer Cells. J. Leukoc. Biol. 55 (2), 153–160. doi: 10.1002/jlb.55.2.153
Sherwood, E. R., Enoh, V. T., Murphey, E. D., Lin, C. Y. (2004). Mice Depleted of CD8+ T and NK Cells are Resistant to Injury Caused by Cecal Ligation and Puncture. Lab. Invest. 84 (12), 1655–1665. doi: 10.1038/labinvest.3700184
Siegemund, S., Schütze, N., Schulz, S., Wolk, K., Nasilowska, K., Straubinger, R. K., et al. (2009). Differential IL-23 Requirement for IL-22 and IL-17A Production During Innate Immunity Against Salmonella Enterica Serovar Enteritidis. Int. Immunol. 21 (5), 555–565. doi: 10.1093/intimm/dxp025
Simmons, C. P., Clare, S., Ghaem-Maghami, M., Uren, T. K., Rankin, J., Huett, A., et al. (2003). Central Role for B Lymphocytes and CD4+ T Cells in Immunity to Infection by the Attaching and Effacing Pathogen Citrobacter Rodentium. Infect. Immun. 71 (9), 5077–5086. doi: 10.1128/iai.71.9.5077-5086.2003
Sivori, S., Falco, M., Della Chiesa, M., Carlomagno, S., Vitale, M., Moretta, L., et al. (2004). CpG and Double-Stranded RNA Trigger Human NK Cells by Toll-Like Receptors: Induction of Cytokine Release and Cytotoxicity Against Tumors and Dendritic Cells. Proc. Natl. Acad. Sci. U. S. A. 101 (27), 10116–10121. doi: 10.1073/pnas.0403744101
Small, C.-L., McCormick, S., Gill, N., Kugathasan, K., Santosuosso, M., Donaldson, N., et al. (2008). NK Cells Play a Critical Protective Role in Host Defense Against Acute Extracellular Staphylococcus Aureus Bacterial Infection in the Lung. J. Immunol. 180 (8), 5558. doi: 10.4049/jimmunol.180.8.5558
Smith, D., Hänsch, H., Bancroft, G., Ehlers, S. (1997). T-Cell-Independent Granuloma Formation in Response to Mycobacterium Avium: Role of Tumour Necrosis Factor-Alpha and Interferon-Gamma. Immunology 92 (4), 413–421. doi: 10.1046/j.1365-2567.1997.00384.x
Songhet, P., Barthel, M., Stecher, B., Müller, A. J., Kremer, M., Hansson, G. C., et al. (2011). Stromal IFN-γr-Signaling Modulates Goblet Cell Function During Salmonella Typhimurium Infection. PLoS One 6 (7), e22459. doi: 10.1371/journal.pone.0022459
Sonnenberg, G. F., Artis, D. (2012). Innate Lymphoid Cell Interactions With Microbiota: Implications for Intestinal Health and Disease. Immunity 37 (4), 601–610. doi: 10.1016/j.immuni.2012.10.003
Sonnenberg, G. F., Artis, D. (2015). Innate Lymphoid Cells in the Initiation, Regulation and Resolution of Inflammation. Nat. Med. 21 (7), 698–708. doi: 10.1038/nm.3892
Sonnenberg, G. F., Fouser, L. A., Artis, D. (2010). Functional Biology of the IL-22-IL-22R Pathway in Regulating Immunity and Inflammation at Barrier Surfaces. Adv. Immunol. 107, 1–29. doi: 10.1016/b978-0-12-381300-8.00001-0
Sonnenberg, G. F., Fouser, L. A., Artis, D. (2011a). Border Patrol: Regulation of Immunity, Inflammation and Tissue Homeostasis at Barrier Surfaces by IL-22. Nat. Immunol. 12 (5), 383–390. doi: 10.1038/ni.2025
Sonnenberg, G. F., Monticelli, L. A., Alenghat, T., Fung, T. C., Hutnick, N. A., Kunisawa, J., et al. (2012). Innate Lymphoid Cells Promote Anatomical Containment of Lymphoid-Resident Commensal Bacteria. Science 336 (6086), 1321–1325. doi: 10.1126/science.1222551
Sonnenberg, G. F., Monticelli, L. A., Elloso, M. M., Fouser, L. A., Artis, D. (2011b). CD4(+) Lymphoid Tissue-Inducer Cells Promote Innate Immunity in the Gut. Immunity 34 (1), 122–134. doi: 10.1016/j.immuni.2010.12.009
Souza-Fonseca-Guimaraes, F., Adib-Conquy, M., Cavaillon, J.-M. (2012). Natural Killer (NK) Cells in Antibacterial Innate Immunity: Angels or Devils? Mol. Med. (Cambridge Mass.) 18 (1), 270–285. doi: 10.2119/molmed.2011.00201
Spits, H., Artis, D., Colonna, M., Diefenbach, A., Di Santo, J. P., Eberl, G., et al. (2013). Innate Lymphoid Cells–a Proposal for Uniform Nomenclature. Nat. Rev. Immunol. 13 (2), 145–149. doi: 10.1038/nri3365
Spörri, R., Joller, N., Albers, U., Hilbi, H., Oxenius, A. (2006). MyD88-Dependent IFN-γ Production by NK Cells Is Key for Control of ≪Em<Legionella Pneumophila≪/Em< Infection. J. Immunol. 176 (10):6162. doi: 10.4049/jimmunol.176.10.6162
Spörri, R., Joller, N., Hilbi, H., Oxenius, A. (2008). A Novel Role for Neutrophils as Critical Activators of NK Cells. J. Immunol. 181 (10), 7121–7130. doi: 10.4049/jimmunol.181.10.7121
Stenger, S., Hanson, D. A., Teitelbaum, R., Dewan, P., Niazi, K. R., Froelich, C. J., et al. (1998). An Antimicrobial Activity of Cytolytic T Cells Mediated by Granulysin. Science 282 (5386), 121–125. doi: 10.1126/science.282.5386.121
Szymanski, K. V., Toennies, M., Becher, A., Fatykhova, D., Guessan, P. D., Gutbier, B., et al. (2012). ≪Em<Streptococcus Pneumoniae≪/Em<-Induced Regulation of Cyclooxygenase-2 in Human Lung Tissue. Eur. Respir. J. 40 (6), 1458. doi: 10.1183/09031936.00186911
Takayama, T., Kamada, N., Chinen, H., Okamoto, S., Kitazume, M. T., Chang, J., et al. (2010). Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) Natural Killer Cells in the Intestinal Mucosa of Patients With Crohn's Disease. Gastroenterology 139882-892 (3), 892.e881–883. doi: 10.1053/j.gastro.2010.05.040
Takeda, K., Tsutsui, H., Yoshimoto, T., Adachi, O., Yoshida, N., Kishimoto, T., et al. (1998). Defective NK Cell Activity and Th1 Response in IL-18-Deficient Mice. Immunity 8 (3), 383–390. doi: 10.1016/s1074-7613(00)80543-9
Talbot, J., Hahn, P., Kroehling, L., Nguyen, H., Li, D., Littman, D. R. (2020). Feeding-Dependent VIP Neuron-ILC3 Circuit Regulates the Intestinal Barrier. Nature 579 (7800), 575–580. doi: 10.1038/s41586-020-2039-9
Thäle, C., Kiderlen, A. F. (2005). Sources of Interferon-Gamma (IFN-Gamma) in Early Immune Response to Listeria Monocytogenes. Immunobiology 210 (9), 673–683. doi: 10.1016/j.imbio.2005.07.003
Tieng, V., Le Bouguénec, C., du Merle, L., Bertheau, P., Desreumaux, P., Janin, A., et al. (2002). Binding of Escherichia Coli Adhesin AfaE to CD55 Triggers Cell-Surface Expression of the MHC Class I-Related Molecule MICA. Proc. Natl. Acad. Sci. U. S. A. 99 (5), 2977–2982. doi: 10.1073/pnas.032668099
Tojima, I., Matsumoto, K., Kikuoka, H., Hara, S., Yamamoto, S., Shimizu, S., et al. (2019). Evidence for the Induction of Th2 Inflammation by Group 2 Innate Lymphoid Cells in Response to Prostaglandin D(2) and Cysteinyl Leukotrienes in Allergic Rhinitis. Allergy 74 (12), 2417–2426. doi: 10.1111/all.13974
Tsujimoto, H., Uchida, T., Efron, P. A., Scumpia, P. O., Verma, A., Matsumoto, T., et al. (2005). Flagellin Enhances NK Cell Proliferation and Activation Directly and Through Dendritic Cell-NK Cell Interactions. J. Leukoc. Biol. 78 (4), 888–897. doi: 10.1189/jlb.0105051
Tufa, D. M., Yingst, A. M., Trahan, G. D., Shank, T., Jones, D., Shim, S., et al. (2020). Human Innate Lymphoid Cell Precursors Express CD48 That Modulates ILC Differentiation Through 2B4 Signaling. Sci. Immunol. 5 (53), eaay4218. doi: 10.1126/sciimmunol.aay4218
van de Wetering, D., de Paus, R. A., van Dissel, J. T., van de Vosse, E. (2009). Salmonella Induced IL-23 and IL-1beta Allow for IL-12 Production by Monocytes and Mphi1 Through Induction of IFN-Gamma in CD56 NK/NK-Like T Cells. PLoS One 4 (12), e8396. doi: 10.1371/journal.pone.0008396
Vankayalapati, R., Garg, A., Porgador, A., Griffith, D. E., Klucar, P., Safi, H., et al. (2005). Role of NK Cell-Activating Receptors and Their Ligands in the Lysis of Mononuclear Phagocytes Infected With an Intracellular Bacterium. J. Immunol. 175 (7), 4611–4617. doi: 10.4049/jimmunol.175.7.4611
Vankayalapati, R., Wizel, B., Weis, S. E., Safi, H., Lakey, D. L., Mandelboim, O., et al. (2002). The NKp46 Receptor Contributes to NK Cell Lysis of Mononuclear Phagocytes Infected With an Intracellular Bacterium. J. Immunol. 168 (7), 3451. doi: 10.4049/jimmunol.168.7.3451
Van Maele, L., Carnoy, C., Cayet, D., Ivanov, S., Porte, R., Deruy, E., et al. (2014). Activation of Type 3 Innate Lymphoid Cells and Interleukin 22 Secretion in the Lungs During Streptococcus Pneumoniae Infection. J. Infect. Dis. 210 (3), 493–503. doi: 10.1093/infdis/jiu106
Vivier, E., Artis, D., Colonna, M., Diefenbach, A., Di Santo, J. P., Eberl, G., et al. (2018). Innate Lymphoid Cells: 10 Years on. Cell 174 (5), 1054–1066. doi: 10.1016/j.cell.2018.07.017
Vonarbourg, C., Mortha, A., Bui, V. L., Hernandez, P. P., Kiss, E. A., Hoyler, T., et al. (2010). Regulated Expression of Nuclear Receptor Rorγt Confers Distinct Functional Fates to NK Cell Receptor-Expressing Rorγt(+) Innate Lymphocytes. Immunity 33 (5), 736–751. doi: 10.1016/j.immuni.2010.10.017
von Moltke, J., Ji, M., Liang, H. E., Locksley, R. M. (2016). Tuft-Cell-Derived IL-25 Regulates an Intestinal ILC2-Epithelial Response Circuit. Nature 529(7585), 221–225. doi: 10.1038/nature16161
Walker, W., Rotondo, D. (2004). Prostaglandin E2 is a Potent Regulator of Interleukin-12- and Interleukin-18-Induced Natural Killer Cell Interferon-Gamma Synthesis. Immunology 111 (3), 298–305. doi: 10.1111/j.1365-2567.2004.01810.x
Wang, F., Graham, W. V., Wang, Y., Witkowski, E. D., Schwarz, B. T., Turner, J. R. (2005). Interferon-Gamma and Tumor Necrosis Factor-Alpha Synergize to Induce Intestinal Epithelial Barrier Dysfunction by Up-Regulating Myosin Light Chain Kinase Expression. Am. J. Pathol. 166 (2), 409–419. doi: 10.1016/s0002-9440(10)62264-x
Waters, C. A., Schimke, P. A., Snider, C. E., Itoh, K., Smith, K. A., Nichols, J. C., et al. (1990). Interleukin 2 Receptor-Targeted Cytotoxicity. Receptor Binding Requirements for Entry of a Diphtheria Toxin-Related Interleukin 2 Fusion Protein Into Cells. Eur. J. Immunol. 20 (4), 785–791. doi: 10.1002/eji.1830200412
Whalen, M. M., Doshi, R. N., Bankhurst, A. D. (1992). Effects of Pertussis Toxin Treatment on Human Natural Killer Cell Function. Immunology 76 (3), 402–407.
Wherry, J. C., Schreiber, R. D., Unanue, E. R. (1991). Regulation of Gamma Interferon Production by Natural Killer Cells in Scid Mice: Roles of Tumor Necrosis Factor and Bacterial Stimuli. Infect. Immun. 59 (5), 1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991
Wilson, S. R., The, L., Batia, L. M., Beattie, K., Katibah, G. E., McClain, S. P., et al. (2013). The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 155 (2), 285–295. doi: 10.1016/j.cell.2013.08.057
Xiao, L., Cui, T., Liu, S., Chen, B., Wang, Y., Yang, T., et al. (2019). Vitamin A Supplementation Improves the Intestinal Mucosal Barrier and Facilitates the Expression of Tight Junction Proteins in Rats With Diarrhea. Nutrition 57, 97–108. doi: 10.1016/j.nut.2018.06.007
Xiong, H., Keith, J. W., Samilo, D. W., Carter, R. A., Leiner, I. M., Pamer, E. G. (2016). Innate Lymphocyte/Ly6C(hi) Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance. Cell 165 (3), 679–689. doi: 10.1016/j.cell.2016.03.017
Yoshimura, K., Laird, L. S., Chia, C. Y., Meckel, K. F., Slansky, J. E., Thompson, J. M., et al. (2007). Live Attenuated Listeria Monocytogenes Effectively Treats Hepatic Colorectal Cancer Metastases and is Strongly Enhanced by Depletion of Regulatory T Cells. Cancer Res. 67 (20), 10058–10066. doi: 10.1158/0008-5472.CAN-07-0573
Yrlid, U., Svensson, M., Håkansson, A., Chambers, B. J., Ljunggren, H. G., Wick, M. J. (2001). In Vivo Activation of Dendritic Cells and T Cells During Salmonella Enterica Serovar Typhimurium Infection. Infect. Immun. 69 (9), 5726–5735. doi: 10.1128/iai.69.9.5726-5735.2001
Zelante, T., Fallarino, F., Bistoni, F., Puccetti, P., Romani, L. (2009). Indoleamine 2,3-Dioxygenase in Infection: The Paradox of an Evasive Strategy That Benefits the Host. Microbes Infect. 11 (1), 133–141. doi: 10.1016/j.micinf.2008.10.007
Keywords: innate lymphoid cells (ILCs), natural killer cells (NKs), bacterial infection, mucosal immunity, intracellular bacteria, extracellular bacteria
Citation: Elemam NM, Ramakrishnan RK, Hundt JE, Halwani R, Maghazachi AA and Hamid Q (2021) Innate Lymphoid Cells and Natural Killer Cells in Bacterial Infections: Function, Dysregulation, and Therapeutic Targets. Front. Cell. Infect. Microbiol. 11:733564. doi: 10.3389/fcimb.2021.733564
Received: 30 June 2021; Accepted: 19 October 2021;
Published: 05 November 2021.
Edited by:
Luciana Balboa, Academia Nacional de Medicina, ArgentinaReviewed by:
Surya Prakash Pandey, University of Pittsburgh, United StatesCopyright © 2021 Elemam, Ramakrishnan, Hundt, Halwani, Maghazachi and Hamid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noha Mousaad Elemam, bm9oYS5lbGVtYW0yMTFAZ21haWwuY29t; bmVsZW1hbUBzaGFyamFoLmFjLmFl; Qutayba Hamid, cWFsaGVpYWx5QHNoYXJqYWguYWMuYWU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.