
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 21 December 2021
Sec. Clinical Microbiology
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.725035
 Sanchari Chatterjee1,2
Sanchari Chatterjee1,2 Ankita Datey1
Ankita Datey1 Soumya Sengupta1,2
Soumya Sengupta1,2 Arup Ghosh1
Arup Ghosh1 Atimukta Jha1
Atimukta Jha1 Safal Walia1
Safal Walia1 Sharad Singh1
Sharad Singh1 Sandhya Suranjika1
Sandhya Suranjika1 Gargee Bhattacharya1,2
Gargee Bhattacharya1,2 Eshna Laha1,2
Eshna Laha1,2 Supriya Suman Keshry1
Supriya Suman Keshry1 Amrita Ray1,2
Amrita Ray1,2 Sweta Smita Pani1
Sweta Smita Pani1 Amol Ratnakar Suryawanshi1
Amol Ratnakar Suryawanshi1 Rupesh Dash1
Rupesh Dash1 Shantibhusan Senapati1
Shantibhusan Senapati1 Tushar K. Beuria1
Tushar K. Beuria1 Gulam Hussain Syed1
Gulam Hussain Syed1 Punit Prasad1
Punit Prasad1 Sunil Kumar Raghav1
Sunil Kumar Raghav1 Satish Devadas1
Satish Devadas1 Rajeeb K. Swain1
Rajeeb K. Swain1 Soma Chattopadhyay1*
Soma Chattopadhyay1* Ajay Parida1*
Ajay Parida1*Purpose: The current global pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), led to the investigation with clinical, biochemical, immunological, and genomic characterization from patients to understand the pathophysiology of viral infection.
Methods: Samples were collected from six asymptomatic and six symptomatic SARS-CoV-2-confirmed hospitalized patients in Bhubaneswar, Odisha, India. Clinical details, biochemical parameters, and treatment regimen were collected from a hospital; viral load was determined by RT-PCR; and the levels of cytokines and circulating antibodies in plasma were assessed by Bio-Plex and isotyping, respectively. In addition, whole-genome sequencing of viral strains and mutational analysis were carried out.
Results: Analysis of the biochemical parameters highlighted the increased levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), serum SGPT, serum SGOT, and ferritin in symptomatic patients. Symptomatic patients were mostly with one or more comorbidities, especially type 2 diabetes (66.6%). The virological estimation revealed that there was no significant difference in viral load of oropharyngeal (OP) samples between the two groups. On the other hand, viral load was higher in plasma and serum samples of symptomatic patients, and they develop sufficient amounts of antibodies (IgG, IgM, and IgA). The levels of seven cytokines (IL-6, IL-1α, IP-10, IL-8, IL-10, IFN-α2, IL-15) were found to be highly elevated in symptomatic patients, while three cytokines (soluble CD40L, GRO, and MDC) were remarkably higher in asymptomatic patients. The whole-genome sequence analysis revealed that the current isolates were clustered with 19B, 20A, and 20B clades; however, 11 additional changes in Orf1ab, spike, Orf3a, Orf8, and nucleocapsid proteins were acquired. The D614G mutation in spike protein is linked with higher virus replication efficiency and severe SARS-CoV-2 infection as three patients had higher viral load, and among them, two patients with this mutation passed away.
Conclusions: This is the first comprehensive study of SARS-CoV-2 patients from India. This will contribute to a better understanding of the pathophysiology of SARS-CoV-2 infection and thereby advance the implementation of effective disease control strategies.
Coronavirus disease 2019 (COVID-19), which was initially reported to cause pneumonia and flu-like illness in the city of Wuhan, China, has now become considerably more perilous, with global efforts undergoing to combat the deadly disease (Yang et al., 2020). The betacoronavirus has a spherical enveloped single-stranded positive sense RNA genome with 5′ cap and 3′ poly-A-tail (Chen et al., 2020).
According to the WHO, 223 countries to date have been affected by the second wave of COVID-19, with 159,319,384 confirmed cases and an increase in the number of deaths around the world. Although the vaccination program in India is at its height, the total number of vaccinations given is 1,77,214,256. The second wave of COVID-19 cases surpassed the 23,340,938 mark and death toll continues to rise beyond 258,317 deaths. As of May 13, 2021, Odisha has 100,313 active cases with 2,304 deaths and 64.5 lakhs vaccinated (Ministry of Health and Human Welfare, GOI).
Previous investigations of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) occurrence demonstrated the correlation of disease deterioration and viral load in the nasopharynx (Cheng et al., 2004; Hung et al., 2004). Studies have now differentiated SARS-CoV-1 and SARS-CoV-2 in terms of clinical manifestations (Guan et al., 2020), virus shedding (To et al., 2020), and epidemiological aspects (Wu et al., 2020). However, investigations are required to understand the pathophysiology of SARS-CoV-2, thereby elucidating the association of viremia in different body fluids and disease severity.
Hence, the current investigation was carried out to delineate and compare the clinical manifestations, laboratory findings on viral load, immunological responses, genome sequences, treatment protocol, and its outcome of asymptomatic and symptomatic patients infected with SARS-CoV-2 to understand the pathophysiology of this virus.
In this study, all the patients were admitted to a COVID hospital in Bhubaneswar, Odisha, India, in July 2020, and biological samples including oropharyngeal (OP) swab and blood were collected. The designed study was approved by the institutional ethics committee and the signed consent forms were taken from the concerned patients. The OP swab sample from the patient was tested immediately to detect the presence of SARS-CoV-2 by RT-PCR. Next, OP swabs were collected from the patients in the first (1–3 days), second (5–7 days), and third (8–10 days) phases, while blood samples were collected in one time point (1–5 days).
The demographic and clinical details of the patients were obtained from the hospital including travel history, treatment details, comorbidities, and symptoms. The data for a few patients were missing due to the absence of tests or delayed results.
Three hundred microliters of OP swab/plasma/serum was taken for viral RNA extraction using TANBead Maelstrom 4800 as per the instructions of the manufacturer. The extracted viral RNA was stored at −80°C until further use.
The qRT-PCR was performed using 5 μl of the extracted RNA from samples using the TRUPCR SARS-CoV-2 RT qPCR Kit V-2.0. The human RNase P served as an internal control, whereas envelope (E) and nucleocapsid (N) genes were targeted for SARS-CoV-2 amplification.
The viral copy number was determined as described previously (Sanjai Kumar et al., 2021). In brief, the standard curve was generated by using the SARS-CoV-2 N gene. The percentage of copy number/ml was calculated from the corresponding Ct values of all the samples. For obtaining Ct values, cDNA was prepared from the extracted RNA using random hexamers by TaKaRa PrimeScript 1st strand cDNA synthesis kit (Kusatsu). The cDNA was subjected to qPCR (Mesa Green SYBR Green-No ROX, Eurogentec) using nucleocapsid gene-specific primers (FP: GTAACACAAGCTTTCGGCAG and RP: GTGTGACTTCCATGCCAATG).
Plasma samples were used to detect the presence of total antibodies (IgM+IgG+IgA) against SARS-CoV-2 by the COVID-19 (IgM+IgG+IgA) Microlisa kit (J. Mitra & Co. Pvt. Ltd.) as per the protocol of the manufacturer. In brief, 50-µl plasma samples were added to precoated plates and incubated for 30 min at 37°C. Following incubation, the plate was washed with 1× wash buffer five times. One hundred microliters of conjugate solution was added to each well and incubated for 30 min at 37°C. After washing five times, 100 µl of working substrate solution was added and incubated at room temperature for 30 min in the dark. The reaction was stopped using 100 µl of 1 N H2SO4 and absorbance was measured at 450 nm in a Multiskan reader (Thermo Scientific). The antibody unit was calculated as per the instruction of the manufacturer.
Twenty-five microliters of plasma sample was used to analyze the responses of 38 cytokines and chemokines using the Bio-Plex Human Cytokine/Chemokine Magnetic Bead Panel 96-Well Plate Assay (EMD Millipore) according to the instructions of the manufacturer. The samples were acquired in a Bio-Plex 200 system (Bio-Rad), and cytokine concentrations were calculated using the Bio-Plex manager software with a five-parameter curve-fitting algorithm applied for standard curve calculation.
To analyze the isotype composition of antibodies in circulation, the plasma of patients was analyzed by the ProcartaPlex Human Antibody Isotyping Panels (Invitrogen, Vienna) according to the instructions of the manufacturer. Briefly, the antibody-coated magnetic bead mixtures were incubated with 25 μl of assay buffer, kit standards, or diluted plasma samples in a ProcartaPlex 96-well plates at room temperature for 1 h. Twenty-five microliters of detection antibodies mixture was then added, and the plates were incubated on an orbital shaker at 600 rpm for 30 min. After that, each well was incubated with 50 μl of diluted streptavidin–phycoerythrin for 30 min. Plates were then washed using a handheld magnetic plate washer. All incubations were performed at room temperature in the dark. Afterwards, the samples were suspended in 120 μl reading buffer. The samples were acquired in a Bio-Plex 200 system (Bio-Rad) and cytokine concentrations were calculated using the Bio-Plex manager software with a five-parameter curve-fitting algorithm applied for standard curve calculation.
The viral amplicon libraries were prepared for whole-genome sequencing using the QIAseq FX DNA Library Kit and QIAseq SARS-CoV-2 Primer Panel as per the instructions of the manufacturer. The samples were pooled and subjected to Illumina NextSeq 550 platform in a 150 × 2 layout, as described before (Raghav et al., 2020).
The mutational and phylogenetic analysis was carried out using the Nextstrain tool as described before (Raghav et al., 2020). Sequences were aligned along with the WH01 reference strain using the Augur wrapper of MAFFT method to carry out mutational analysis, and a phylogenetic tree was constructed using the IQTREE2 tool with 1,000 bootstrap value (Raghav et al., 2020).
Statistical analysis was performed using the GraphPad Prism software, version 8.0.1. Data were presented as mean ± standard deviation (SD). The non-parametric Mann–Whitney U test was used to compare the levels of viral load, cytokines, and antibodies.
The clinical and biological features of 12 hospitalized patients (asymptomatic and symptomatic) are presented in Tables 1 and 2. The viral load was assessed after their admission to the COVID hospital (Table 3). All the asymptomatic patients (nos. 1–6) recovered and were discharged from the hospital after 10–11 days. Two symptomatic patients (nos. 10 and 11) succumbed to COVID-19 during this period, whereas patient no. 9 passed away after getting discharged from the hospital. The asymptomatic patients (nos. 1–6) were identified as COVID-19 positive during contact tracing. The analysis of the biochemical data revealed abnormalities in few clinical markers such as CRP, LDH, serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and ferritin. All the symptomatic patients had a higher level of CRP, and 50% of them displayed a high amount of serum LDH (~1.8–2.9-fold) than the normal range. Besides, they were detected with a higher percentage of serum SGPT (>2-fold) and serum SGOT (>2-fold), and almost 83% of the SARS-CoV-2 patients were detected with a higher level of ferritin than the normal range (Tables 1 and 2). These data suggest that patients with higher levels of the abovementioned biochemical parameters might experience severe pathophysiological complications after SARS-CoV-2 infection. For the treatment of this disease, all the patients were administered with pantoprazole (before food), azithromycin (once a day), ascorbic acid (2 h after food), and ivermectin (2 h after food), for a period of 10 days. Asymptomatic patients were discharged from the hospital on day 10. The symptomatic patients were found to have one or more comorbidities, especially type 2 diabetes (66.6%), indicating that other physiological conditions (Tables 1 and 2) are playing a major role in determining disease pathology. After the onset of the disease, samples were collected within 1–3, 5–7, and 8–10 days. In most of the asymptomatic (except one) and symptomatic patients (except two), the ΔΔCt values were increased in OP samples over time. However, there was no significant difference in the ΔΔCt values of asymptomatic and symptomatic patients (Figure 1A). This suggests that viral load in the OP sample does not correlate with disease severity. In contrast, the viral load estimation from plasma samples demonstrated that symptomatic patients had high viral load as compared with asymptomatic patients (Figure 1B). Three symptomatic patients (patient nos. 9, 10, and 11) who died of SARS-CoV-2 had a significantly higher amount of plasma viremia or viral load, compared with those who were discharged from the hospital. All the symptomatic patients (except one) were on ventilator support. The analysis of serum samples demonstrated that the ΔΔCt values of serum samples of asymptomatic patients were more than the symptomatic patients, and this finding correlated well with the ΔΔCt values of plasma samples for both asymptomatic and symptomatic patients (Figure 1C). Additionally, a similar pattern of ΔΔCt values for plasma and serum samples was observed for three more asymptomatic cases (data not shown). Hence, the result indicates that development of clinical complications is mostly associated to high viral load in plasma and serum samples. The viral copy number of OP samples was reduced over time in both asymptomatic and symptomatic patients, and there was no significant difference between the viral load of asymptomatic and symptomatic patients (Figure 1D). This suggests that both asymptomatic and symptomatic patients are capable of transmitting the disease equally. Plasma and serum samples of symptomatic patients had a high viral copy number than those of asymptomatic patients (Figures 1E, F). Therefore, disease severity is mostly linked with high viral copy number in plasma and serum samples.
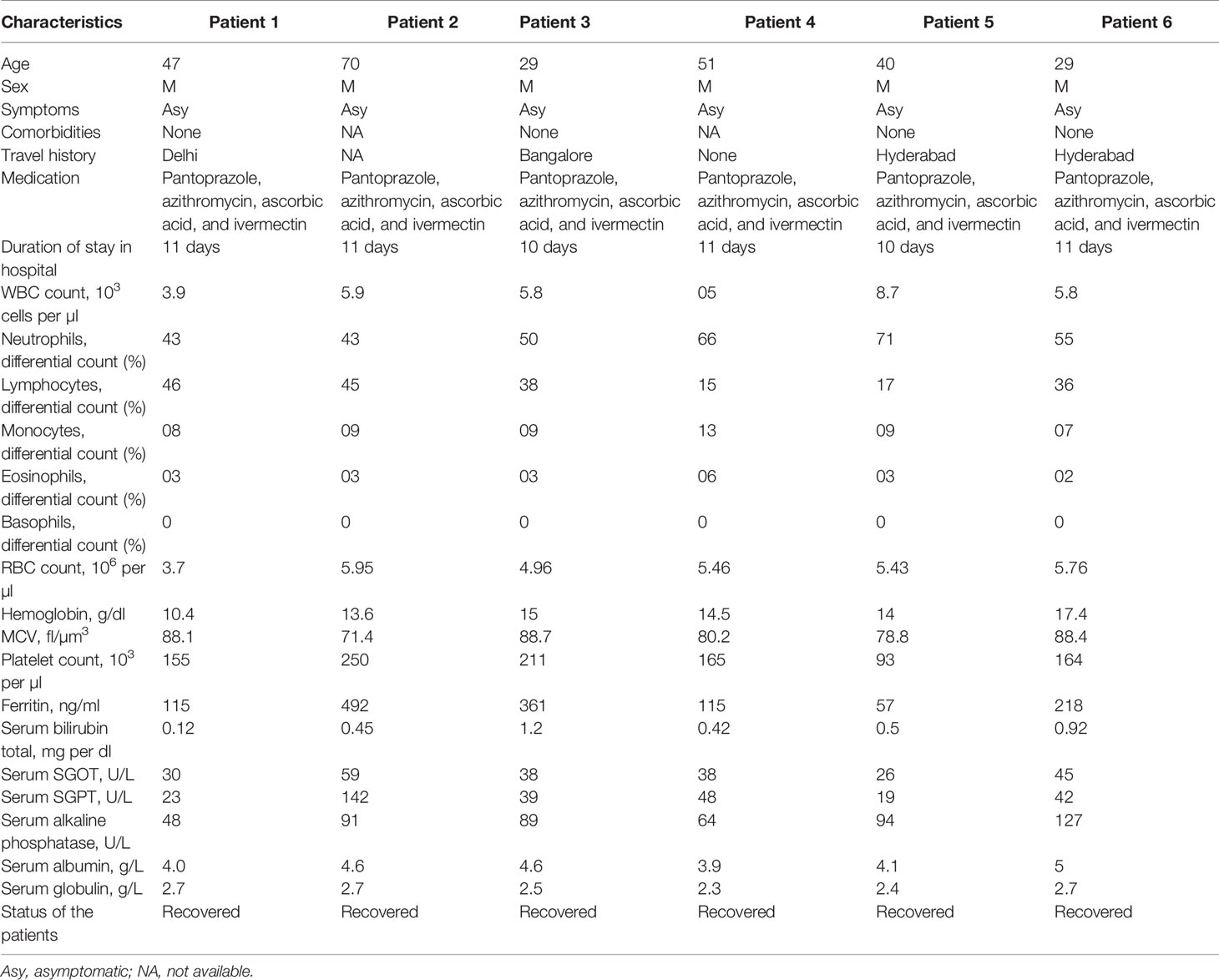
Table 1 Demographic details and laboratory findings of SARS-CoV-2-infected hospitalized asymptomatic patients.
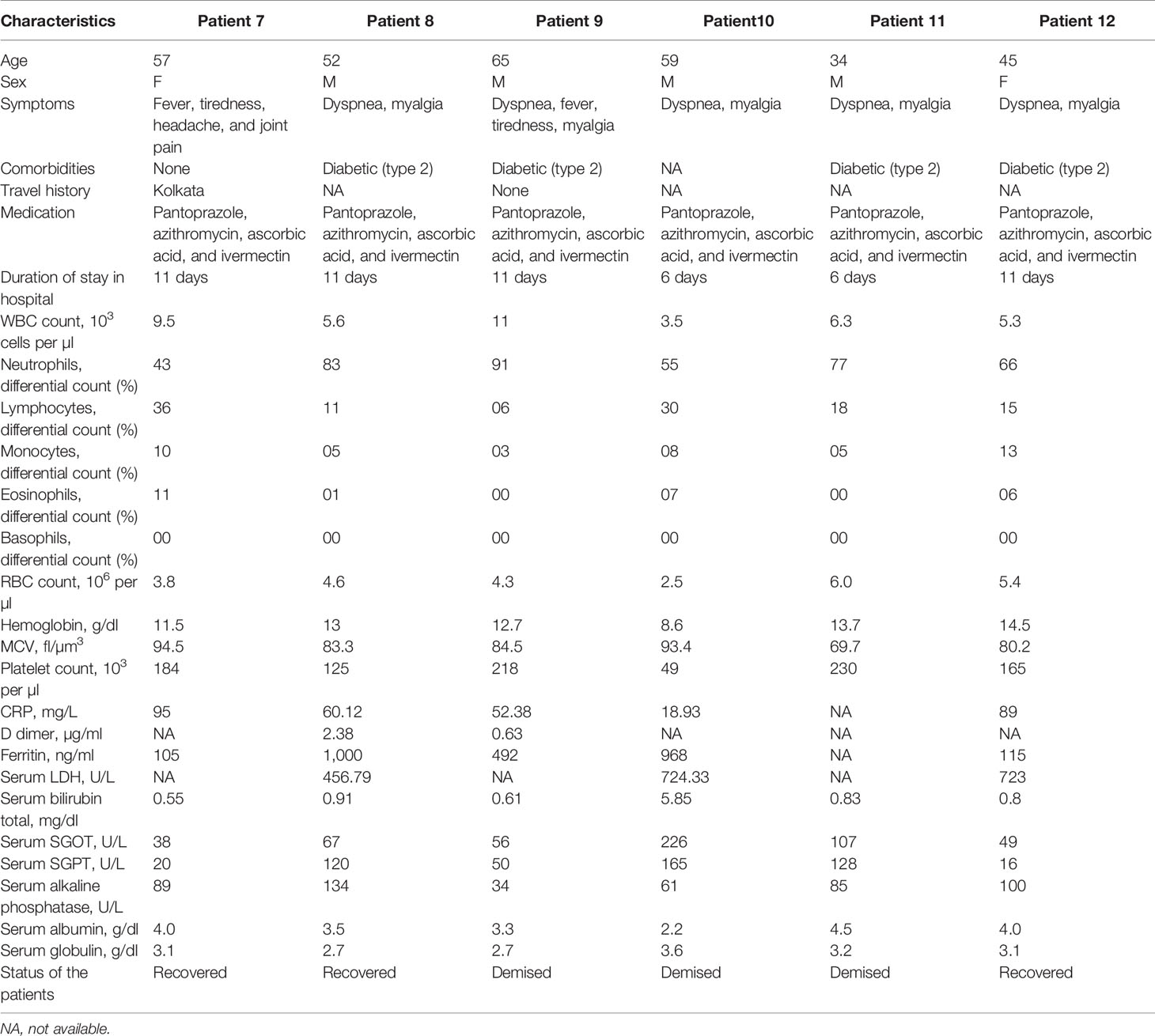
Table 2 Demographic details and laboratory findings of SARS-CoV-2-infected hospitalized symptomatic patients.

Figure 1 Viral dynamics in patients with asymptomatic and symptomatic disease. (A) Bar diagram depicting the ΔΔCT of OP samples collected from patients with asymptomatic and symptomatic COVID-19 at different days of disease onset. (B, C) Graph showing ΔΔCT of individual plasma and serum samples collected from asymptomatic and symptomatic COVID-19 patients. (D) Bar diagram representing the viral copy number of OP samples collected from patients with asymptomatic and symptomatic COVID-19 at different days of disease onset. (E, F) Graph depicting viral copy number of individual plasma and serum samples collected from asymptomatic and symptomatic COVID-19 patients. The Mann–Whitney (non-parametric, two-tailed) test was performed. All error bars were SD. p value ≤ 0.05 was considered statistically significant (*) and p value ≤ 0.01 was considered to be very significant (**).
To understand the antibody responses against SARS-CoV-2, virus-specific IgM, IgG, and IgA antibodies were estimated using plasma samples. Four out of six asymptomatic patients (patient nos. 2, 4, 5, and 6) along with all symptomatic patients were found to be ELISA positive (with low ΔΔCt values in the plasma) (Figure 2). The samples of three healthy individuals were collected as control and were negative for SARS-CoV-2-specific IgM, IgG, and IgA antibodies (data not shown). Thus, the data underline that patients with high viral titer developed a sufficient amount of antibodies. To comprehend infectivity, attempts were made to isolate the live virus from six clinical samples. However, one virus was successfully isolated from OP samples of patient 7 (viral load >107 copies/ml), which has highest viral load among all the samples. This indicates the presence of a significantly high copy number of virus in this patient.

Figure 2 Analysis of SARS-CoV-2-specific (IgG+IgM+IgA) antibodies in asymptomatic and symptomatic patients. Bar graph representing antibody units (IgG+IgM+IgA) of plasma samples from a COVID-19 patient. Line depicting the cutoff value of positive samples which was 59.3. Negative (NC) and positive (PC) controls delivered by detection kit were included to warrant test validity.
Next, the differential dynamics of circulating cytokines and chemokines in the SARS-CoV-2-infected asymptomatic and symptomatic patients were investigated. Interestingly, the levels of soluble CD40L, GRO, and MDC were higher in asymptomatic patients as compared with the symptomatic. On the other hand, the symptomatic patients showed higher levels of IL-6, IL-1α, IP-10, IL-8, IL-10, IFN-α2, and IL-15 in comparison with the asymptomatic patients (Figure 3), whereas there were no significant differences in the plasma levels of MCP-3, IL-1β, IL-17A, IL-12 p70, eotaxin, and TNF. Therefore, these data suggest that cytokines and chemokines may serve as “predictive indicators” of SARS-CoV-2 infection and contribute to understand the pathogenesis of COVID-19.
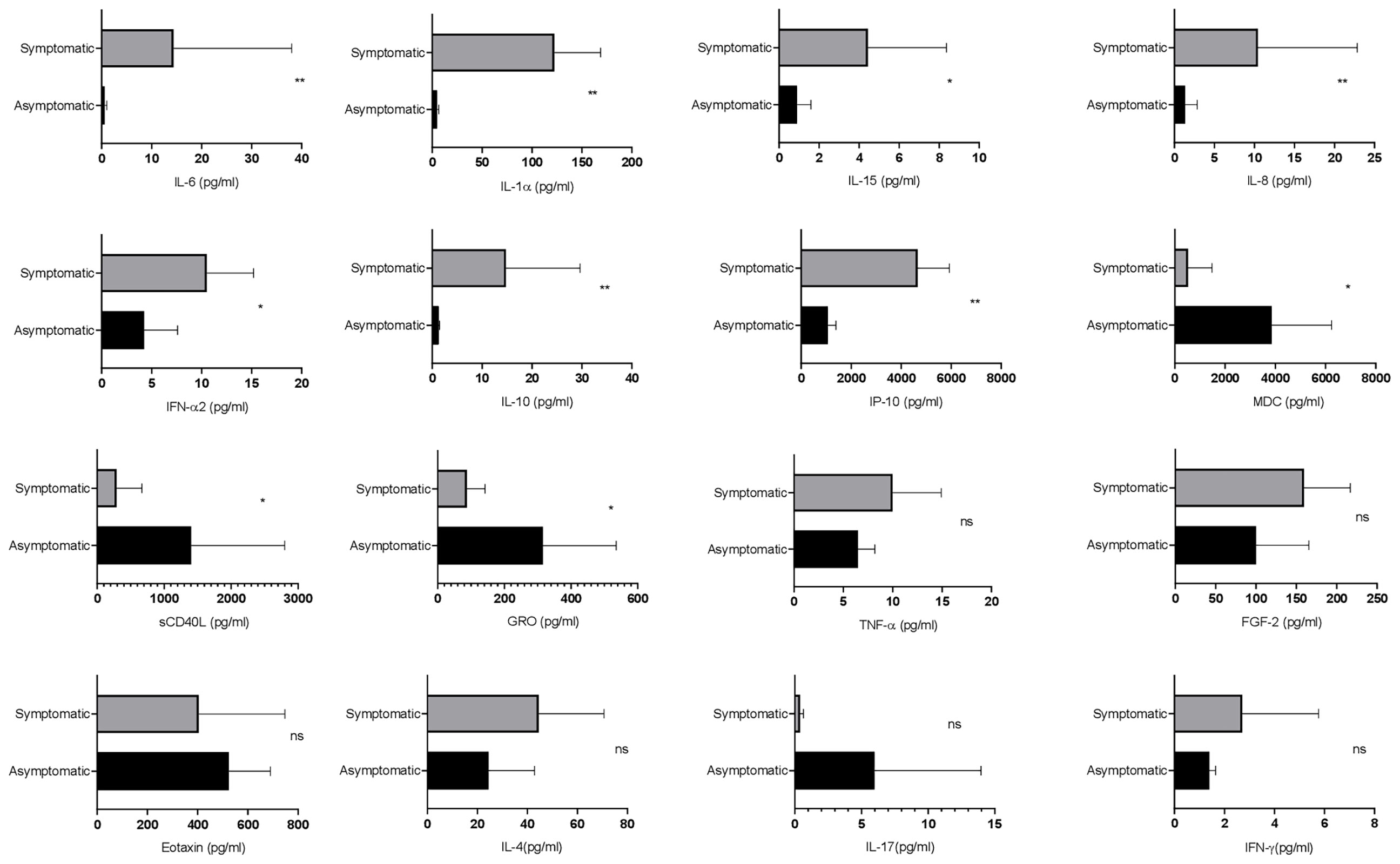
Figure 3 Evaluation of the cytokine responses between asymptomatic and symptomatic COVID-19 patients. The bar diagram depicting the expression levels of cytokines in pg/ml. Cytokines were measured after disease onset between asymptomatic and symptomatic patients. The Mann–Whitney (non-parametric, two-tailed) test was performed. p < 0.05 was considered statistically significant (*), and p < 0.005 was considered to be very significant (**). ns, not significant. All error bars were SD.
To understand the dynamics of circulating isotypes of antibody in the peripheral blood of SARS-CoV-2 patients, the Bio-Plex assay was carried out. There was no significant difference between IgA, IgM, and IgG4 in the two groups; however, IgE, IgG2, IgG3, and IgG1 were significantly higher in symptomatic than asymptomatic patients (Figure 4). This result indicates that inflammatory responses were high in these patients.
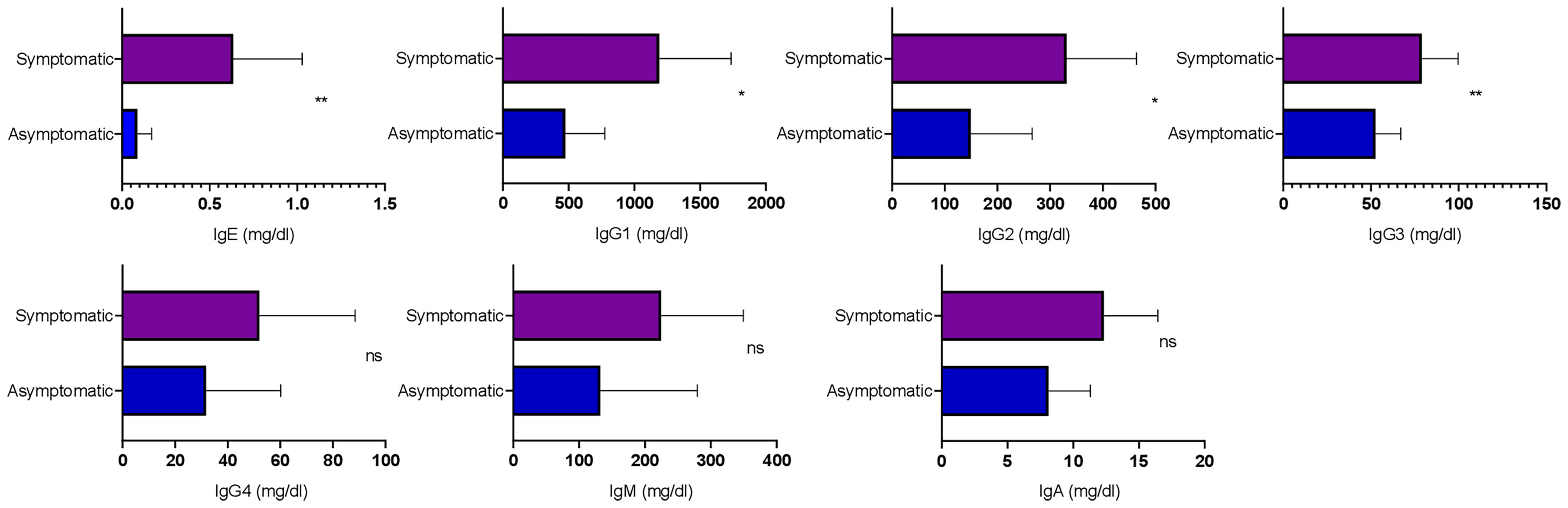
Figure 4 Analysis of the circulating antibodies in asymptomatic and symptomatic COVID-19 patients. The bar diagram representing circulating antibody isotypes (IgA, IgM, IgG1, IgG2, IgG3, IgG4, and IgE) which were evaluated from COVID-19-positive plasma samples. The Mann–Whitney (non-parametric, two-tailed) test was performed. p < 0.05 was considered statistically significant (*), and p < 0.005 was considered to be very significant (**). ns, not significant. All error bars were SD.
In the current investigation, whole-genome sequencing was also performed for four SARS-CoV-2 strains (from one asymptomatic and three symptomatic patients). All four sequences were deposited in the GISAID database and the accession numbers are listed in Table 4. The phylogenetic tree was prepared using these isolates, and the strains from Wuhan, WH-01 (NC_045512.2); USA; Brazil; Canada; Australia; South Africa; United Kingdom; and South Korea were used as references. The phylogenetic analysis revealed the presence of three main clades: two (patient nos. 7 and 9) of the strains clustered to 20A clade, whereas the rest of the two (patient nos. 1 and 10) strains belonged to 19B and 20B clades (Figure 5).
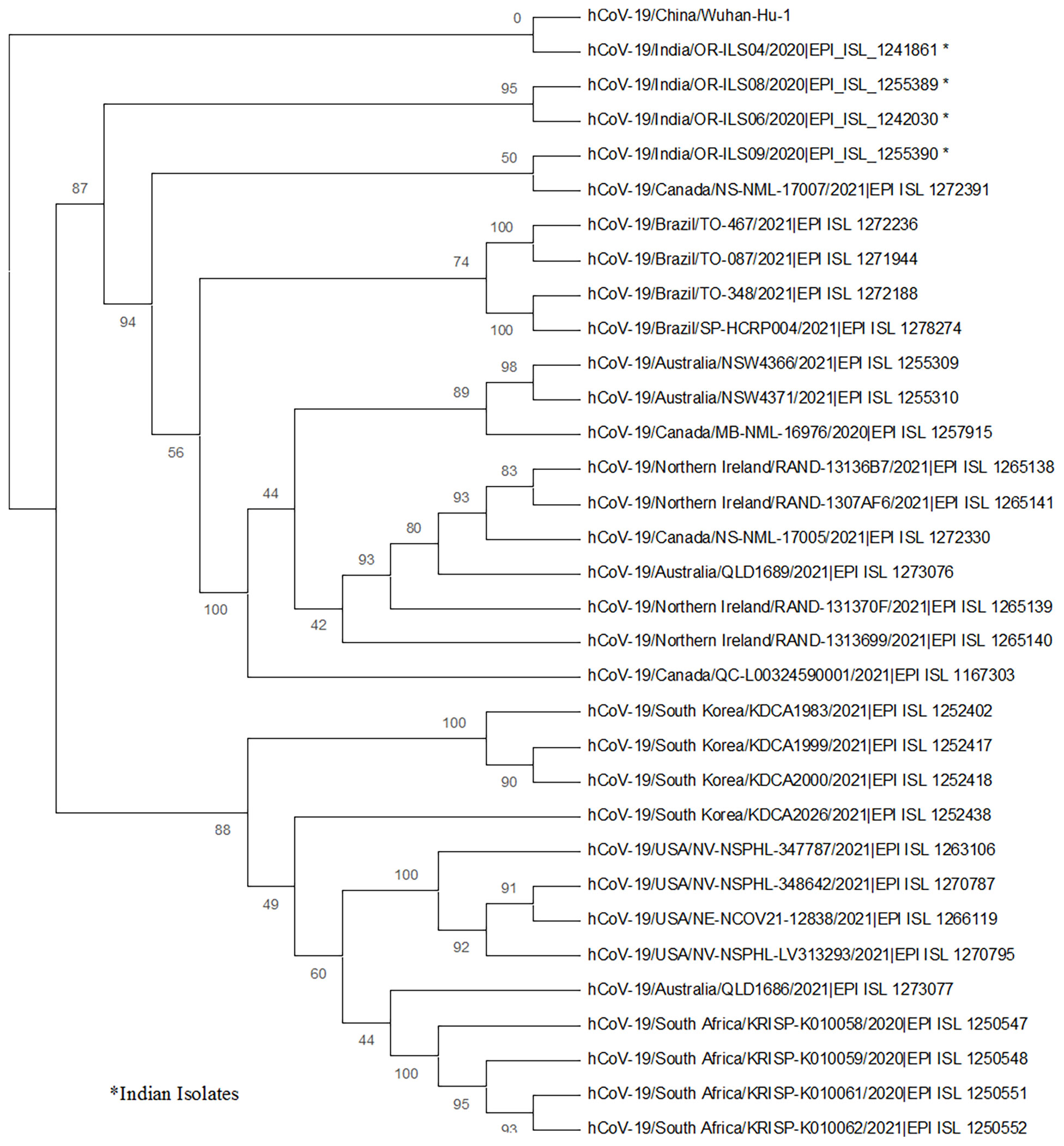
Figure 5 The phylogenetic analysis of the whole-genome sequences of four SARS-CoV-2 strains from human oropharyngeal swab samples is depicted. The phylogenetic tree was generated using the maximum likelihood method with 1,000 bootstrap value by the IQTREE2 tool. The tree was constructed using WH-01 (NC_045512.2) as a reference strain along with four strains from each of the countries, namely, USA, Brazil, Canada, Australia, South Africa, United Kingdom, and South Korea. The sequences for reference strains were retrieved from the GISAID database. The viral isolates are depicted by hCoV-19/country/strain ID/year of isolation/accession number. The bootstrap values are mentioned at major branch points of the tree.
Moreover, the sequence analysis revealed the presence of additional mutations, such as 13 in the Orf1ab region and 1 each in spike, Orf3a, Orf8, and nucleocapsid genes. All patients showed different combinations of mutations (approximately five to six) that are listed in Table 5 and Figure 6. The Ref alleles/amino acid changes represent the genotype of Wuhan-Hu-01 strain and Alt alleles/amino acid changes represent the changes that occurred in Indian strains. Mutational analysis of amino acid unveiled a total of 11 missense mutations in the Orf1ab region, whereas 4 synonymous mutations were noticed in spike, nucleocapsid, Orf3a, and Orf8 regions. Interestingly, D614G mutation of the spike protein was observed in patient numbers 7, 9, and 10, and the analysis revealed that it is mostly linked with increased replication efficiencies and severe SARS-CoV-2 infection as the patients containing this mutation had high viral load and two patients succumb to death.
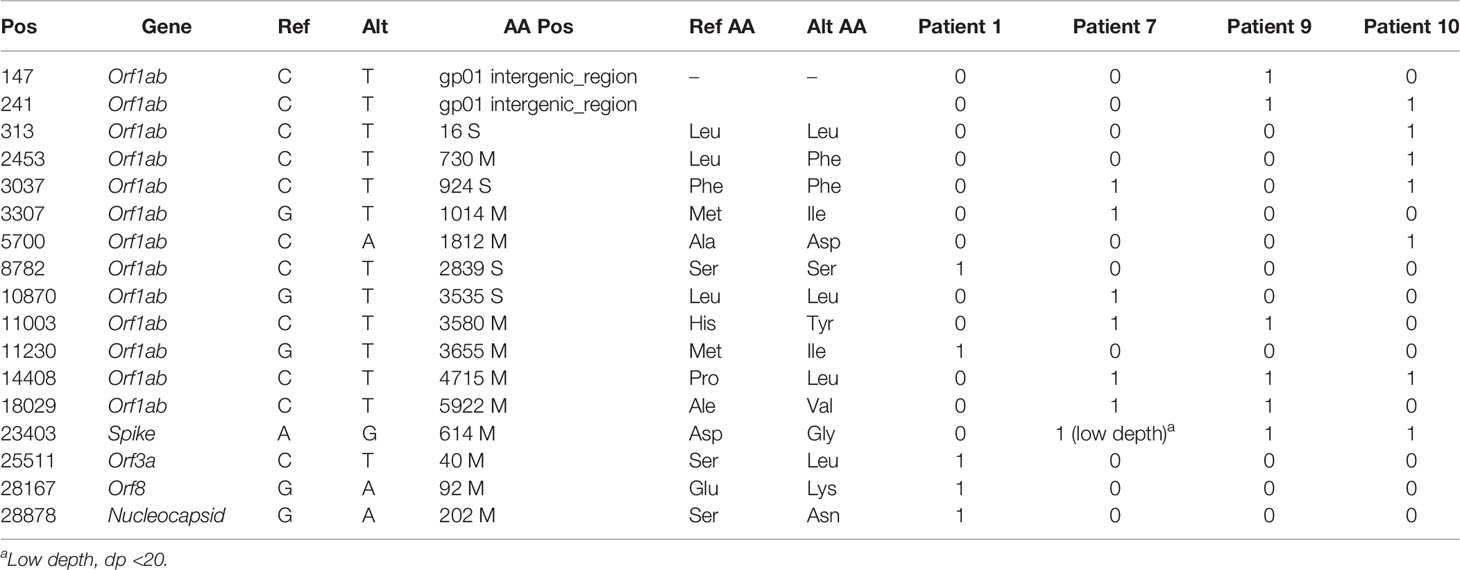
Table 5 Mutational analysis of the four SARS-CoV-2 strains compared with the Wuhan-Hu-1 (NC_045512.2) reference sequence.
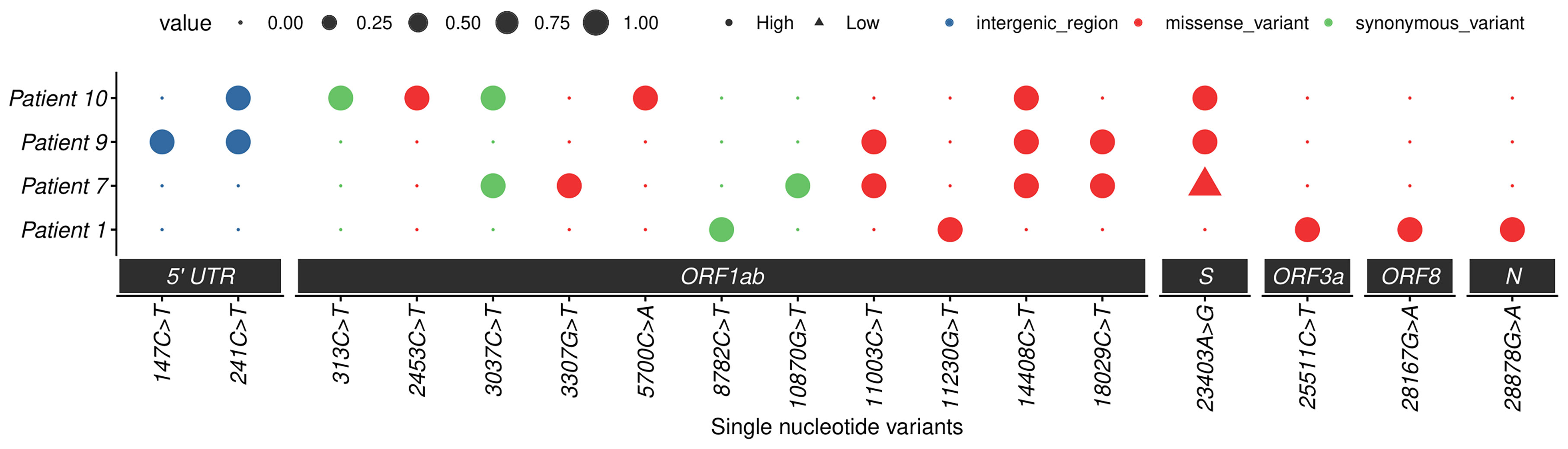
Figure 6 Mutation profiles of SARS-CoV-2 strains. Dot plot summarizing the single nucleotide variants in four studied cases. The x-axis represents the nucleotide change with position and region, and the y-axis represents cases. The dots represent the presence or absence of a mutation (small dot absent and large dot presence), and different colors represent different types of mutations. High-quality mutations (depth > 20) are represented by round dots and triangles represent low-quality mutations. PS: low-quality site only represented in spike 23403: A>G change.
A second wave of COVID-19 has grappled India, crossing 3.5 lakhs of new cases per day with an alarming death toll. An urgency persists to understand the clinical, epidemiological, immunological, and genomic characteristics of the virus for correlating with the pathophysiology of SARS-CoV-2.
The current investigation delineates and compares the clinical manifestations, laboratory findings, immunological responses, genome sequences, and treatment regimen of SARS-CoV-2 patients. Six asymptomatic and six symptomatic patients infected with this virus were studied for various parameters to understand the pathophysiology of this virus infection.
Patients with comorbidities displayed an increased severity in disease manifestation with some cases resulting in death. This observation was in accordance with a previous report where mortality as well as severity of the illness was increased for COVID-19-infected individuals with type 2diabetes as compared to non-diabetic patients (Kumar et al., 2020). This study further confirms that symptomatic patients had a higher level of CRP and ferritin than asymptomatic patients concurrent with previous reports (Velavan and Meyer, 2020; Yu et al., 2020). It was observed earlier that viral loads in nasal and throat swab samples were nearly the same in asymptomatic and symptomatic patients. The current study also confirms the above observation, suggesting that asymptomatic and symptomatic patients are equally capable of spreading the disease as there was no significant difference in viral load of both asymptomatic and symptomatic patients (Sohn et al., 2020; Zou et al., 2020). In contrast, the viral loads in plasma and serum samples were associated with clinical complications, which is consistent with other reports (Fajnzylber et al., 2020; Zhang et al., 2020). Furthermore, there might not be any threshold Ct value (viral load) responsible for clinical complications. Developing clinical complications may rely on other factors like comorbidities and cytokine profiles. A similar report by Abdulrahman et al. also suggests that just Ct values do not contribute in the disease severity or complications (Abdulrahman et al., 2021).
Additionally, the estimation of COVID-19-specific IgM, IgG, and IgA antibodies in SARS-CoV-2 patients indicated that patients with a high viral titer developed sufficient antibodies. A similar observation was reported in a study of patients from Hubei Province, China (Zhang et al., 2020).
In this study, only one strain (with >107 copies/ml) could be isolated successfully. This demonstrates that the viral load was insufficient to successfully isolate the virus from other samples. A previous study also reported difficulty in the isolation of the virus when viral load is <106 copies/ml (Wölfel et al., 2020). This explains the inability of isolating the virus from the remaining samples.
Symptomatic patients showed higher levels of IP-10, IL-8, IL-10, IFN-α2, IL-15, IL-6, and IL-1α, whereas the levels of soluble CD40L, GRO, and MDC were higher in asymptomatic patients. In addition, IL-10 and IL-6 have been associated with severe disease manifestations. On the other hand, IP-10 is considered to be a marker of ongoing inflammation, which in some cases predicts the mortality of the patients (Chi et al., 2020; Huang et al., 2020; Yang et al., 2020; Lev et al., 2021). Along with that, higher levels of IFN-α2, IL-15, IL-1α, and IL-8 in symptomatic patients indicate ongoing inflammation (Lucas et al., 2020; Qin et al., 2020). The levels of CD40L, GRO, and MDC were higher in asymptomatic patients, suggesting that they might have gone through immune suppression and repair phenomenon (Zaja-Milatovic and Richmond, 2008; Huang et al., 2012; Rapp et al., 2019). Previous reports have shown a significant difference in the expression levels of MCP-3, IL-1β, IL-17A, IL-12 p70, eotaxin, and TNF, while in the current study, there was no significant difference observed as small sample size was a limitation (Chi et al., 2020). A previous report by Ong et al. suggests that proinflammatory cytokines are increased only when the respiratory function is at the lowest level. In the current study, longitudinal profiling was not conducted; therefore, it was not very clear about the time point at which respiratory function was lowest with a significant increase of proinflammatory cytokines (Ong et al., 2020).
Symptomatic patients showed higher levels of IgE, IgG2, IgG3, and IgG1 serotypes, as compared with asymptomatic patients. IgE has already been reported to be high in COVID-19 patients, particularly those with type 2 diabetes. Out of six symptomatic patients in this study, four were suffering from type 2 diabetes and had enhanced IgE levels (Lucas et al., 2020; Zhao et al., 2020). An overall increase in IgG1, IgG2, and IgG3 responses in symptomatic patients explains the increase in inflammatory immune responses (Lucas et al., 2020). Since IgE was high in these patients, there was no significant difference in IgG4 level as they compete for fixation sites in basophils and mast cells (Kolfschoten et al., 2007). There was no difference in IgM and IgA levels of the two groups, suggesting that patients were at the early stage of infection, as the peak of virus-specific IgM is developed approximately 14–28 days after the onset of symptoms (Long et al., 2020).
The whole-genome sequencing and phylogenetic analysis revealed that the current isolates were clustered in 19B, 20A, and 20B clades; however, there were several additional unique mutations in different genes as compared with the Wuhan strain. Surprisingly, most of the changes were observed in the Orf1ab region. In India, these three clades were predominant in the months from May to July, 2020 (Maitra et al., 2020). It was observed in this study that the three symptomatic patients with high viral load, two of which had severe disease symptoms and died, were infected with the virus possessing the D614G mutation in the spike protein. This strengthens the previous hypothesis that infectivity rate and enhanced transmission of SARS-CoV-2 is associated with the D614G mutation (Raghav et al., 2020; Plante et al., 2021).
This study has indicated several interesting and significant outcomes, though with certain limitations. Primarily, all the clinical findings were correlated with viral RNA cycle threshold (ΔΔCt) value, which is capable of detecting dead virus particles also. Furthermore, the sample collection timing/interval was irregular and was solely dependent on the judgment of the physician and the condition of the patient. Finally, given the small sample size, the results must be interpreted with caution and validated in a larger cohort to fortify these conclusions.
In summary, this investigation highlights the ability of both asymptomatic and symptomatic patients to transmit the virus equally. This also demonstrated that the D614G mutation is mostly associated with higher virus replication efficiency and severe SARS-CoV-2 infection. These data also suggest that the enhanced levels of inflammatory markers such as CRP and ferritin can be predictive biomarkers for the critical condition of patients. Taken together, these results will contribute to a better understanding of the pathophysiology of SARS-CoV-2 infection and thereby advance the implementation of effective disease control strategies.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving human participants were reviewed and approved by the Institutional Human Ethics Committee, Institute of Life Sciences. The Institutional Ethics Committee (IEC)/ Institutional Review Board (IRB) reference number is 96/HEC/2020. Written consent informed to participate in this study was provided by the participants’ legal guardian/next of kin.
SoC and SaC designed the experiments. RS, SaC, SoS, ShS, SaS, GB, and AR collected the clinical specimens and demographic data. SaC, AD, and SK conducted the virological assays and RT-PCR. SoS and SaC performed the Bio-Plex and isotyping assays. SaC, AD, and SoS performed the ELISA experiment. AJ and EL prepared the samples for the whole-genome sequencing. SoC, SaC, and AD analyzed the virological data. SoS and SD analyzed the immunological data. AG, SR, and SW conducted the genomic data analysis. SoC, SaC, AD, SoS, and SP drafted the manuscript. AS, RD, SSe, TB, GS, PP, SR, SD, RS, SoC, and AP reviewed and edited the manuscript.
This study was supported by core funding of the Institute of Life Sciences, Bhubaneswar, Dept. of Biotechnology, India. SaC and GB were funded by the CSIR fellowship and SoS was funded by the DBT fellowship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully acknowledge ILS Biorepository for providing the SARS-CoV-2 samples collected from hospitals for this study. We would like to thank Dr. Subhasis Chattopadhyay and Tathagata Mukherjee for their valuable suggestions in the immunology section. We also acknowledge Mr. Paritosh Nath for collecting hospital samples, Mr. Jeky Chanwala for RNA isolation, Mr. Deepak Singh for data entry, and Ms. Swati Madhulika and Ms. Manasi Priyadarshini for their help in conducting the RT-PCR.
Abdulrahman, A., Mallah, S. I., Alqahtani, M. (2021). COVID-19 Viral Load Not Associated With Disease Severity: Findings From a Retrospective Cohort Study. BMC Infect. Dis. 21 (1), 1–4. doi: 10.1186/s12879-021-06376-1
Cheng, V. C. C., Hung, I. F. N., Tang, B. S. F., Chu, C. M., Wong, M. M. L., Chan, K. H., et al. (2004). Viral Replication in the Nasopharynx Is Associated With Diarrhea in Patients With Severe Acute Respiratory Syndrome. Clin. Infect. Dis. 38 (4), 467–475. doi: 10.1086/382681
Chen, Y., Liu, Q., Guo, D. (2020). Emerging Coronaviruses: Genome Structure, Replication, and Pathogenesis. J. Med. Virol. 92, 418–423. John Wiley and Sons Inc. doi: 10.1002/jmv.25681
Chi, Y., Ge, Y., Wu, B., Zhang, W., Wu, T., Wen, T., et al. (2020). Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 222 (5), 746–754. doi: 10.1093/infdis/jiaa363
Fajnzylber, J., Regan, J., Coxen, K., Corry, H., Wong, C., Rosenthal, A., et al. (2020). SARS-Cov-2 Viral Load Is Associated With Increased Disease Severity and Mortality. Nat. Commun. 11 (1), 1–9. doi: 10.1038/s41467-020-19057-5
Guan, W., Ni, Z., Hu, Y., Liang, W., Ou, C., He, J., et al. (2020). Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 382 (18), 1708–1720. doi: 10.1056/NEJMoa2002032
Huang, J., Jochems, C., Talaie, T., Anderson, A., Jales, A., Tsang, K. Y., et al. (2012). Elevated Serum Soluble CD40 Ligand in Cancer Patients may Play an Immunosuppressive Role. Blood 120 (15), 3030–3038. doi: 10.1182/blood-2012-05-427799
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. doi: 10.1016/S0140-6736(20)30183-5
Hung, I. F. N., Cheng, V. C. C., Wu, A. K. L., Tang, B. S. F., Chan, K. H., Chu, C. M., et al. (2004). Viral Loads in Clinical Specimens and SARS Manifestations. Emerg. Infect. Dis. 10 (9), 1550–1557. doi: 10.3201/eid1009.040058
Kolfschoten, M. V. D. N., Schuurman, J., Losen, M., Bleeker, W. K., Martínez-Martínez, P., Vermeulen, E., et al. (2007). Anti-Inflammatory Activity of Human Igg4 Antibodies by Dynamic Fab Arm Exchange. Science (80-) 317 (5844), 1554–1557. doi: 10.1126/science.1144603
Kumar, A., Arora, A., Sharma, P., Anil, S. (2020). Since January 2020 Elsevier has Created a COVID-19 Resource Centre With Free Information in English and Mandarin on the Novel Coronavirus COVID-19. The COVID-19 Resource Centre Is Hosted on Elsevier Connect, the Company’s Public News and Information. Diabetes Metab. Syndr. Clin. Res. Rev. 14 (January), 535–545.
Lev, S., Gottesman, T., Levin, G. S., Lederfein, D., Berkov, E., Diker, D., et al. (2021). Observational Cohort Study of IP-10’s Potential as a Biomarker to Aid in Inflammation Regulation Within a Clinical Decision Support Protocol for Patients With Severe COVID-19. PloS One 16, 8 (1 January). doi: 10.1371/journal.pone.0245296
Long, Q. X., Liu, B. Z., Deng, H. J., Wu, G. C., Deng, K., Chen, Y. K., et al. (2020). Antibody Responses to SARS-Cov-2 in Patients With COVID-19. Nat. Med. 26 (6), 845–848. doi: 10.1038/s41591-020-0897-1
Lucas, C., Wong, P., Klein, J., Castro, T. B. R., Silva, J., Sundaram, M., et al. (2020). Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 584 (7821), 463–469.
Maitra, A., Raghav, S., Dalal, A., Ali, F., Paynter, V. M., Paul, D., et al. (2020). PAN-INDIA 1000 SARS-Cov-2 RNA Genome Sequencing Reveals Important Insights Into the Outbreak. bioRxiv 2020.08.03.233718. doi: 10.1101/2020.08.03.233718
Ong, E. Z., Chan, Y. F. Z., Leong, W. Y., Lee, N. M. Y., Kalimuddin, S., Haja Mohideen, S. M., et al. (2020). A Dynamic Immune Response Shapes COVID-19 Progression. Cell Host Microbe 27 (6), 879–882.e2. doi: 10.1016/j.chom.2020.03.021
Plante, J. A., Liu, Y., Liu, J., Xia, H., Johnson, B. A., Lokugamage, K. G., et al. (2021). Spike Mutation D614G Alters SARS-Cov-2 Fitness. Nature 592 (7852), 116–121. doi: 10.1038/s41586-020-2895-3
Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., et al. (2020). Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 71 (15), 762–768. doi: 10.1093/cid/ciaa248
Raghav, S., Ghosh, A., Turuk, J., Kumar, S., Jha, A., Madhulika, S., et al. (2020). Analysis of Indian SARS-Cov-2 Genomes Reveals Prevalence of D614G Mutation in Spike Protein Predicting an Increase in Interaction With TMPRSS2 and Virus Infectivity. Front. Microbiol. 11, 594928. doi: 10.3389/fmicb.2020.594928
Rapp, M., Wintergerst, M. W. M., Kunz, W. G., Vetter, V. K., Knott, M. M. L., Lisowski, D., et al. (2019). CCL22 Controls Immunity by Promoting Regulatory T Cell Communication With Dendritic Cells in Lymph Nodes. J. Exp. Med. 216 (5), 1170–1181. doi: 10.1084/jem.20170277
Sanjai Kumar, P., Nayak, T. K., Mahish, C., Sahoo, S. S., Radhakrishnan, A., De, S., et al. (2021). Inhibition of Transient Receptor Potential Vanilloid 1 (TRPV1) Channel Regulates Chikungunya Virus Infection in Macrophages. Arch. Virol. 166 (1), 139–155. doi: 10.1007/s00705-020-04852-8
Sohn, Y., Jeong, S. J., Chung, W. S., Hyun, J. H., Baek, Y. J., Cho, Y., et al. (2020). Assessing Viral Shedding and Infectivity of Asymptomatic or Mildly Symptomatic Patients With COVID-19 in a Later Phase. J. Clin. Med. 9 (9), 2924. doi: 10.3390/jcm9092924
To, K. K. W., Tsang, O. T. Y., Leung, W. S., Tam, A. R., Wu, T. C., Lung, D. C., et al. (2020). Temporal Profiles of Viral Load in Posterior Oropharyngeal Saliva Samples and Serum Antibody Responses During Infection by SARS-Cov-2: An Observational Cohort Study. Lancet Infect. Dis. 20 (5), 565–574. doi: 10.1016/S1473-3099(20)30196-1
Velavan, T. P., Meyer, C. G. (2020). Mild Versus Severe COVID-19: Laboratory Markers. Int. J. Infect. Dis. 95, 304–307. doi: 10.1016/j.ijid.2020.04.061
Wölfel, R., Corman, V. M., Guggemos, W., Seilmaier, M., Zange, S., Müller, M. A., et al. (2020). Virological Assessment of Hospitalized Patients With COVID-2019. Nature 581 (7809), 465–469. doi: 10.1038/s41586-020-2196-x
Wu, J. T., Leung, K., Bushman, M., Kishore, N., Niehus, R., de Salazar, P. M., et al. (2020). Estimating Clinical Severity of COVID-19 From the Transmission Dynamics in Wuhan, China. Nat. Med. 26 (4), 506–510. doi: 10.1038/s41591-020-0822-7
Yang, Y., Shen, C., Li, J., Yuan, J., Wei, J., Huang, F., et al. (2020). Plasma IP-10 and MCP-3 Levels Are Highly Associated With Disease Severity and Predict the Progression of COVID-19. J. Allergy Clin. Immunol. 146 (1), 119–127.e4. doi: 10.1016/j.jaci.2020.04.027
Yang, X., Yu, Y., Xu, J., Shu, H., Xia, J., Liu, H., et al. (2020). Clinical Course and Outcomes of Critically Ill Patients With SARS-Cov-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 8 (5), 475–481. doi: 10.1016/S2213-2600(20)30079-5
Yu, C., Zhou, M., Liu, Y., Guo, T., Ou, C., Yang, L., et al. (2020). Characteristics of Asymptomatic COVID-19 Infection and Progression: A Multicenter, Retrospective Study. Virulence 11 (1), 1006–1014. doi: 10.1080/21505594.2020.1802194
Zaja-Milatovic, S., Richmond, A. (2008). CXC Chemokines and Their Receptors: A Case for a Significant Biological Role in Cutaneous Wound Healing. Histol. Histopathol. 23, 1399–1407.
Zhang, X., Lu, S., Li, H., Wang, Y., Lu, Z., Liu, Z., et al. (2020). Viral and Antibody Kinetics of COVID-19 Patients With Different Disease Severities in Acute and Convalescent Phases: A 6-Month Follow-Up Study. Virol. Sin. 35 (6), 820–829. doi: 10.1007/s12250-020-00329-9
Zhao, R., Sun, Y., Zhang, Y., Wang, W., Wang, S., Wang, C., et al. (2020). Distinguishable Immunologic Characteristics of COVID-19 Patients With Comorbid Type 2 Diabetes Compared With Nondiabetic Individuals. Mediators Inflamm. 2020, 9. doi: 10.1155/2020/6914878
Keywords: COVID-19, asymptomatic and symptomatic patients, cytokines, D614G mutation, disease severity, SARS-CoV-2
Citation: Chatterjee S, Datey A, Sengupta S, Ghosh A, Jha A, Walia S, Singh S, Suranjika S, Bhattacharya G, Laha E, Keshry SS, Ray A, Pani SS, Suryawanshi AR, Dash R, Senapati S, Beuria TK, Syed GH, Prasad P, Raghav SK, Devadas S, Swain RK, Chattopadhyay S and Parida A (2021) Clinical, Virological, Immunological, and Genomic Characterization of Asymptomatic and Symptomatic Cases With SARS-CoV-2 Infection in India. Front. Cell. Infect. Microbiol. 11:725035. doi: 10.3389/fcimb.2021.725035
Received: 14 June 2021; Accepted: 15 November 2021;
Published: 21 December 2021.
Edited by:
Rodolfo García-Contreras, National Autonomous University of Mexico, MexicoReviewed by:
Robert Cody Sharp, University of Florida Health, United StatesCopyright © 2021 Chatterjee, Datey, Sengupta, Ghosh, Jha, Walia, Singh, Suranjika, Bhattacharya, Laha, Keshry, Ray, Pani, Suryawanshi, Dash, Senapati, Beuria, Syed, Prasad, Raghav, Devadas, Swain, Chattopadhyay and Parida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soma Chattopadhyay, c29jaGF0Lmlsc0BnbWFpbC5jb20=; Ajay Parida, ZHJhamF5cGFyaWRhQGdtYWlsLmNvbQ==; YWpheXBhcmlkYUBpbHMucmVzLmlu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.