
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 29 September 2021
Sec. Bacteria and Host
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.714229
Sex steroid hormones (SSH) are cholesterol-derived molecules. They are secreted into saliva and enter the oral cavity, triggering physiological responses from oral tissues, with possible clinical implications, such as gingival inflammation and bleeding. SSH and hormonal changes affect not only oral host cells but also oral microorganisms.
Historically, most research has focused on the effect of hormonal changes on specific bacteria and yeasts. Recently a broader effect of SSH on oral microorganisms was suggested. In order to assess the role of SSH in host-microbe interactions in the oral cavity, this review focuses on how and up to what extent SSH can influence the composition and behavior of the oral microbiome. The available literature was reviewed and a comprehensive hypothesis about the role of SSH in host-microbiome interactions is presented. The limited research available indicates that SSH may influence the balance between the host and its microbes in the oral cavity.
Sex steroid hormones (SSH) are regulatory molecules synthesized or converted by several different living organisms ranging from mammals to plants, and from parasites to bacteria (Winter and Bokkenheuser, 1987; Kristan and Rizner, 2012; Romano et al., 2015; Chiang et al., 2019; Tarkowská, 2019). In mammals, these molecules derive from cholesterol and are secreted by the testes, ovaries, adrenal cortex and – during pregnancy – by the placenta (Figure 1). After being secreted, they are released into the bloodstream to reach different target tissues, where they induce various physiological functions (Chan and O'Malley, 1976; Hu et al., 2010). Interestingly, even though SSH are structurally similar, they induce very different responses in target tissues (Figure 1). The oral cavity is not exempt from the effect of these molecules. In fact, SSH can diffuse into saliva through capillaries and salivary ducts (Vining et al., 1983). Consequently, they are capable of reaching intra-oral target tissues such as the gingiva and the periodontium, where they induce a response (Markou et al., 2009; Mariotti and Mawhinney, 2013). Likewise, microorganisms present in the oral cavity are exposed to SSH and specific oral bacteria and yeast have been shown to be affected by them (Kornman and Loesche, 1982; Powell et al., 1984).
During pregnancy, the surge in hormonal levels triggers responses by oral tissues, with 30-100% of women undergoing gingival changes such as inflammation and increased bleeding (Mealey and Moritz, 2003). Similar changes have also been observed during physiological and artificially induced hormonal shift periods, as well as an effect on oral bacteria (Ozcelik et al., 2006; Kumar, 2013; Brusca et al., 2014).
Researchers have tried to explain why oral clinical changes take place, which mechanisms are involved, and the possible implications of both for the host. It has been proposed that SSH are capable of affecting periodontal tissues and their surrounding environment through four different mechanisms: (1) by locally influencing proliferation of fibroblasts and epithelial cells; (2) by increasing vascular permeability; (3) by elevating levels of immune cells in the periodontium; and (4) by inducing changes in certain oral microorganisms (Mariotti and Mawhinney, 2013). However, despite the available evidence, it is not yet known how these mechanisms are connected and what the exact influence of SSH is on periodontal tissues and the oral microbiota.
Studies in females and males on the effects of SSH on oral bacteria and fungi have been inconclusive. Moreover, the study of the oral microbiome, understood as the microorganisms present in the oral cavity, their genetic information and the environment in which they interact (Cho and Blaser, 2012) has gained interest (Cornejo Ulloa et al., 2019) and represents a gap in our knowledge that needs to be addressed. Developments in the field of microbial endocrinology will especially help to better understand periodontal health and disease. In addition, current omics-based techniques open up vast possibilities to study the effects of SSH on not only single-species but also the oral microbial composition in depth.
The purpose of this review was to assess the role of SSH in host-microbe interactions in the oral cavity, and the extent to which SSH can influence the composition and behavior of the oral microbiome. Our review of in vitro and clinical studies provided the basis for a hypothesis about host-microbiome interactions mediated by SSH. Possible implications for the host’s oral health and future perspectives are discussed.
Just like other microorganisms, some oral bacteria are capable of transforming or degrading SSH (Kornman and Loesche, 1982; Clark and Soory, 2007; Donova and Egorova, 2012; Holert et al., 2018; Horinouchi et al., 2019). This has been tested in vitro using estradiol, progesterone and testosterone on different oral microorganisms, especially periodontal bacteria. The specific focus on periodontal bacteria derives from earlier studies showing that SSH can compensate for the absence of vitamin K (Kornman and Loesche, 1982). Vitamin K is produced in vivo by certain microorganisms and further used as an electron carrier during the anaerobic reduction of fumarate to succinate by periodontal bacteria (Glick et al., 1959; Thauer et al., 1977; Marcotte and Lavoie, 1998; Hojo et al., 2007).
Several studies have investigated the response of different oral bacteria and the fungus Candida to the presence of SSH and other similar compounds (Ojanotko-Harri et al., 1991; Soory, 1995; Clark and Soory, 2005; Clark and Soory, 2007; Fteita et al., 2014) and have also suggested that the exposure of certain microorganisms to SSH can modify bacterial metabolism and the growth and expression of virulence factors (Garcia-Gomez et al., 2013), and have effects on the host (Vom Steeg and Klein, 2017).
Unfortunately, most in vitro studies have used single-species models (Kornman and Loesche, 1982; Clark and Soory, 2005; Yokoyama et al., 2005), while fewer studies have worked with polymicrobial models (Soory, 1995; Soory and Ahmad, 1997) to mimic a more realistic oral environment. While this has simplified the understanding of single-species behavior, it has also created reductionistic inferences – which, as a matter of fact, do not necessarily apply when looking at the bigger picture: the oral microbiome and its interplay with the host.
To date, in vitro research has sought to establish the direct and indirect effects of certain oral species, i.e. (1) the interaction between oral microorganisms and SSH; and (2) the response of host oral cells to the presence of both hormone metabolizing microorganisms and SSH. These direct and indirect influences of SSH on oral microbiota are discussed below.
Several oral bacteria and Candida species have been reported to respond to the presence of SSH. For the purpose of this review, SSH-responsive microorganisms have been classified into four groups based on their putative role in oral health and disease and in line with the classification used in the majority of the reviewed literature. These are: (1) periodontal-disease-associated bacteria; (2) caries-associated bacteria; (3) oral-health-associated bacteria; and (4) oral fungi. Their characteristics and known response to SSH are summarized in Table 1.
In the early 1980s, Kornman and Loesche conducted the first study which showed that, in the absence of vitamin K, two periodontal bacteria – Bacteroides melaninogenicus subsp. intermedius (now Prevotella intermedia), and Bacteroides melaninogenicus subsp. melaninogenicus (now Prevotella melaninogenica) – used estradiol and progesterone as growth factors in the absence of vitamin K (Kornman and Loesche, 1982). They also showed that Bacteroides gingivalis (now Porphyromonas gingivalis) and P. intermedia were able to nearly quintuplicate their SSH uptake when fumarate was added to the medium, significantly enhancing their metabolism.
Later studies have confirmed these observations and identified other effects of estradiol on bacteria from the Prevotella intermedia group. Estradiol positively increases protein levels and formation of polysaccharides in biofilms as well as enhancing its coaggregation with Fusobacterium nucleatum. This could have an influence in biofilm formation (Fteita et al., 2014). Moreover, Prevotella intermedia group bacteria express enzymes that act as virulence factors. One of these enzymes is the protease dipeptidyl peptidase IV (DPPIV) which is involved in the destruction of periodontal tissue. The activity of this protease is increased by the presence of estradiol, which thus enhances its virulence (Fteita et al., 2015).
The ability of certain periodontal pathogens to metabolize testosterone has also been tested. Subgingival plaque samples as well as single-species cultures of Aggregatibacter actinomycetemcomitans, Prevotella intermedia and Porphyromonas gingivalis were grown in the presence of testosterone. Both subgingival plaque samples and single-species cultures were able to convert testosterone into dihydrotestosterone (DHT) or 4-androstenedione (Soory, 1995) (Figure 1). Two years later, a similar experiment was conducted to assess the effect of these bacterial species alone, in pairs or together in the conversion of 4-androstenedione to testosterone or DHT. It was observed that A. actinomycetemcomitans, P. intermedia and P. gingivalis were able to convert 4-androstenedione into testosterone and DHT. Additionally, when grown in pairs or combined, they were able to suppress this conversion (Soory and Ahmad, 1997).
One study assessed the response of Bacillus cereus strain Socransky 67 to the presence of progesterone and testosterone (Ojanotko-Harri et al., 1990). This bacterium expressed 17β-hydroxysteroid dehydrogenase activity, and thus appeared capable of metabolizing progesterone and testosterone.
Yokoyama et al. (2005) investigated the response of various periodontal pathogens when treated with estradiol or progesterone. In this study, the growth of Campylobacter rectus – a periodontal pathogen that has been associated with preterm low birth weight (Varadan and Ramamurthy, 2015) – was shown to be influenced by estrogen and progesterone. The presence of these SSH increased its growth similarly to P. intermedia as described by Kornman and Loesche (1982).
Treponema denticola has also received attention due to its presence in subgingival biofilms. This microorganism has the ability to 5α-reduce SSH in vitro (Clark and Soory, 2005). This finding is relevant specially for testosterone, whose 5α-reduced form – DHT – is a powerful androgen in the human body (Swerdloff et al., 2017). The growth of this oral spirochete can be inhibited by progesterone at concentrations found in plasma (Clark and Soory, 2006; Clark and Soory, 2007), showing that Treponema denticola is sensitive to changes in concentrations of SSH present in the medium.
Ojanotko-Harri et al. investigated the metabolism of progesterone and testosterone by Streptococcus mutans strain Ingbritt cultures (Ojanotko-Harri et al., 1990). They showed that S. mutans is capable of metabolizing testosterone and progesterone by measuring the presence of metabolites in the culture medium. Based on the previously obtained results, the same group published their work on metabolism of 17β-estradiol by oral S. mutans, amongst other microorganisms (Ojanotko-Harri et al., 1991), showing 17β-hydroxysteroid dehydrogenase activity of this bacterium. Together, these publications are the only available evidence that S. mutans is capable of metabolizing SSH in vitro.
The same study that tested the ability of S. mutans to metabolize 17β-estradiol also analyzed the response of S. sanguis (Ojanotko-Harri et al., 1991), which also showed 17β-hydroxysteroid dehydrogenase activity. This is the only available evidence supporting the hypothesis that oral-health-related bacteria are also capable of metabolizing SSH in vitro.
Earlier studies have reported that Candida species possess estrogen (C. albicans and C. glabrata) and progesterone (C. albicans) binding sites (Powell et al., 1984; Skowronski and Feldman, 1989; Gujjar et al., 1997). Estrogen has been described to stimulate growth in C. albicans, as well as the enhancement of several virulence factors (Zhang et al., 2000). This includes the formation of hyphae (Cheng et al., 2006; Prasad et al., 2012) and expression of CDR1 and CDR2 (Krishnamurthy et al., 1998; Cheng et al., 2006; Larsen et al., 2006), important genes in drug resistance. Progesterone is known to alter gene expression, both favoring resistance to drugs (Larsen et al., 2006) as well as impairing the yeast’s ability to form biofilms, colonize and invade the host (Alves et al., 2014).
Unfortunately, most of the available evidence on Candida has focused on the development of vaginitis (Kinsman et al., 1988) and few reports have explored the role of SSH in the oral cavity and C. albicans behavior (Theaker et al., 1993; Kravtsov et al., 2014). This comes to no surprise, since fungi have been systematically excluded from oral environmental studies and little is known about their commensal behavior in contrast to their role in oral pathology (Krom et al., 2014). Until now, evidence indicates that Candida can benefit from the presence of SSH though in a different way than some oral bacteria do.
Up until now, only three studies have investigated the effect of SSH on the response of human gingival fibroblasts to oral bacteria in vitro (Soory, 1995; Soory and Ahmad, 1997; Yokoyama et al., 2005). The dynamics between SSH, oral bacteria and other cell types is not known.
In 1995, Soory cultured gingival fibroblasts from healthy gingival tissue in the presence of testosterone and supernatants obtained from bacterial cultures of A. actinomycetemcomitans, P. intermedia and P. gingivalis (Soory, 1995). The presence of these bacterial supernatants increased the cell’s ability to convert testosterone into dihydrotestosterone (DHT).
In a later study, gingival fibroblasts from chronically inflamed gingival tissue were cultured in the presence of 4-androstenedione and supernatants from the same bacterial cultures, separately (Soory and Ahmad, 1997). This interaction resulted in an increased conversion rate of 4-androstenedione to testosterone by gingival fibroblasts when grown in the presence of each bacterial culture’s supernatant.
Yokoyama et al. assessed the reaction of gingival fibroblasts in the presence of C. rectus in combination with estradiol (Yokoyama et al., 2005). An additive effect was observed in the production of vascular endothelial growth factor (VEGF) by these gingival fibroblasts. VEGF is a potent blood vessel function regulator that affects the endothelium, including the promotion of hyperpermeability, angiogenesis and endothelial cell growth (Connolly, 1991).
The existence of SSH metabolic pathways can have an evolutionary explanation. Host and microbiome have co-evolved and remain to do so, adapting their behavior and response to the existing circumstances. Available evidence has mostly reported effects on the growth of oral microorganisms, catabolic effects on SSH and effects on the virulence factors of the tested bacteria. This steroid use can be convenient for the microorganisms in different ways, which have been earlier described for gut bacteria, including: (1) synthesis of surface capsular proteins; (2) persistence and dissemination in their human host; (3) co- and inter-aggregation and; (4) production of energy via electron transfer chain (Bokkenheuser et al., 1983). It has also been proposed that steroid metabolites that result from the reduction of SSH by some bacteria could serve as nutritional requirements for themselves and other microorganisms as well as enabling synthesis of matrices associated with host evasion mechanisms (Soory, 2000).
Since the beginning of the exploration of the effect of SSH on oral bacteria and fungi, it has repeatedly been observed that a number of sub- and supragingival bacteria are capable of changing their behavior in the presence of these molecules. This has also been observed for the fungus Candida. The different responses triggered by SSH not only in disease-associated bacteria but also health-associated and commensal microorganisms hint a versatile influence of SSH in an oral microcosm.
Changes in the metabolism of fibroblasts have also been documented. This has shown that SSH are not only capable of affecting the microorganisms in the environment, but may also as a result, change the response of the host.
In vitro studies have set the fundamentals to question the role of the oral microbiome during periods of changing hormone levels, giving enough reason to presume that not only inflammation and changes in the immune response of the host are responsible for the observable clinical changes. As explained above, there are unfortunately no in vitro studies that have tested the response of an oral microcosm to the presence of SSH. The hypothesis of changes in a polymicrobial setting have only been investigated in vivo and these results will be discussed below.
Since gingival alterations during pregnancy were linked to hormonal changes (Ziskin, 1938; Ziskin and Nesse, 1946) different factors have been pinpointed as contributors to this phenomenon. Immunological, vascular, cellular and microbiological changes have been and continue to be studied and until today, results involving the oral microbiome remain contradictory.
The contribution of female SSH to the alteration of periodontal bacteria has been thoroughly reviewed (Kumar, 2013) and it was concluded that evidence remains insufficient. Even though studies suggest a preferential colonization by specific periodontal bacteria during periods of fluctuating hormones, several confounding factors make it difficult to positively link these changes to a selective enhancement of the subgingival microbiome.
This, paired with differences in study design and microbiological analysis methods have made interpretation of the results a challenging task. However, since the publication of the aforementioned review, several new studies have been published. Recent studies have focused on endogenous fluctuations (physiological and pathological) as well as exogenous administration of hormones. Species affected during these changes are listed on Tables 2–4, and the corresponding studies are discussed below.
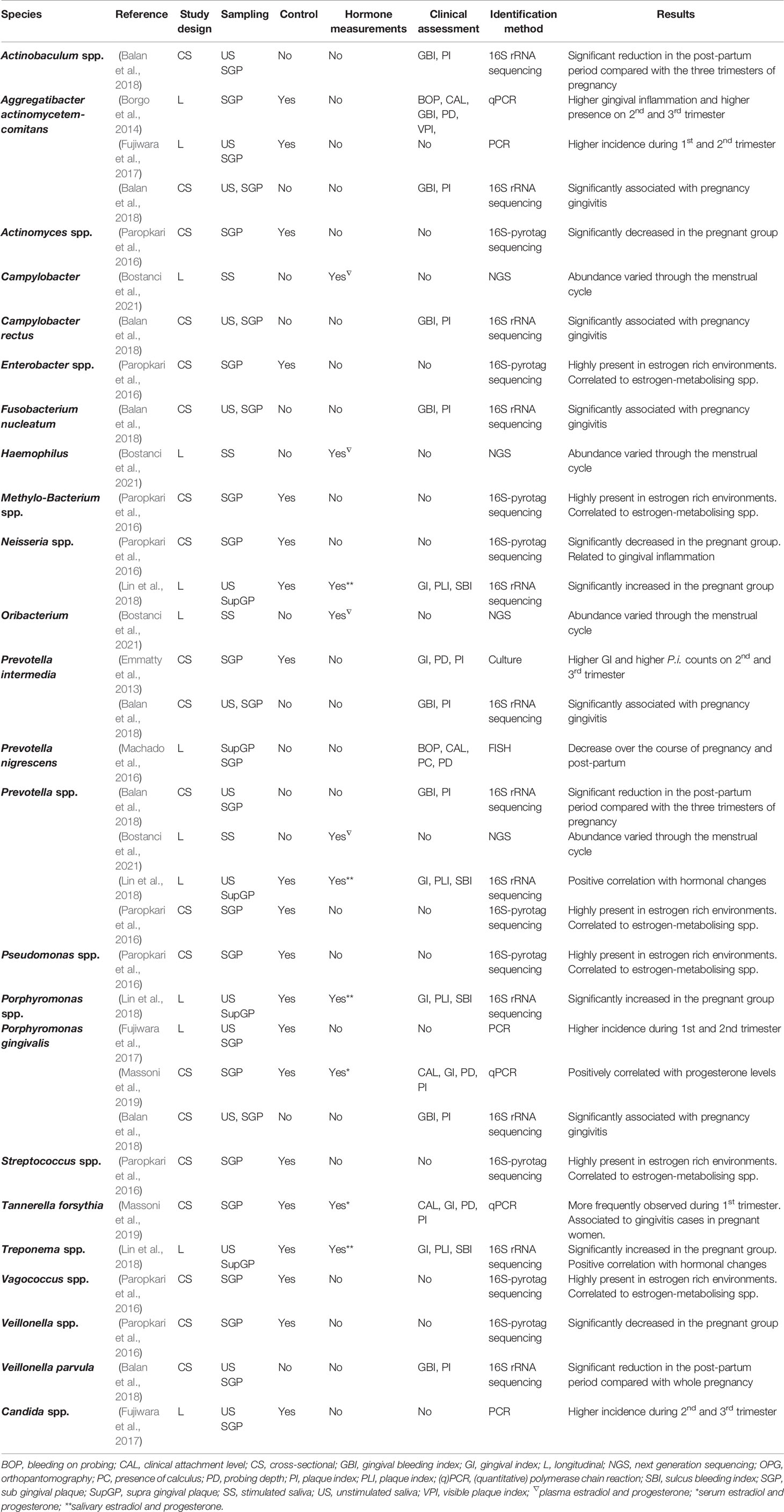
Table 2 Bacteria and fungi affected by the menstrual cycle, pregnancy and postpartum [Not included in (Kumar, 2013)].
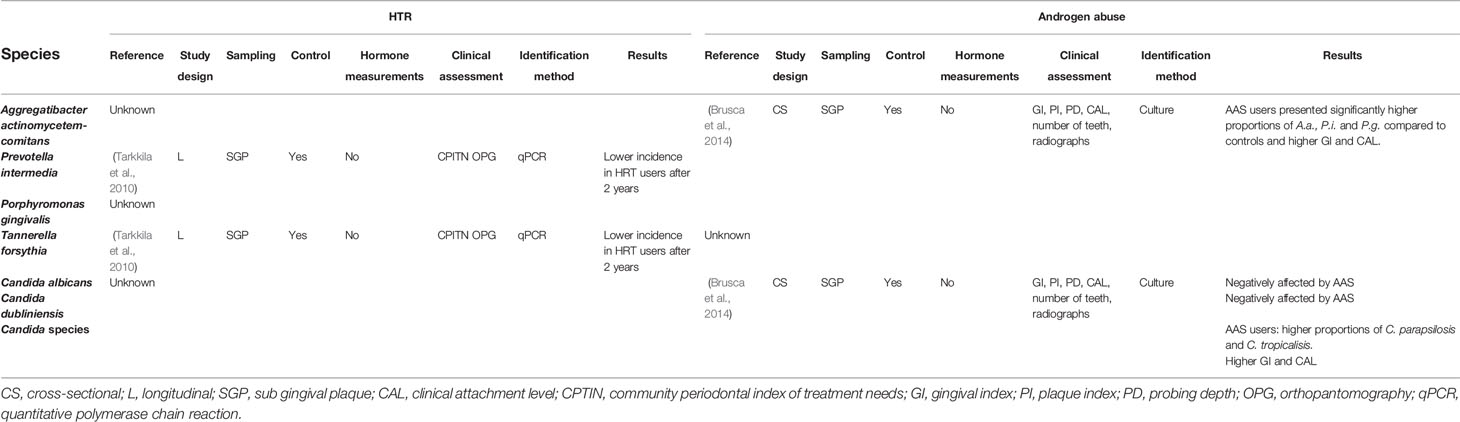
Table 4 Bacteria and fungi affected by exogenous administration of SSH [Not included in (Kumar, 2013)].
Physiological and physical changes taking place during lifetime are regulated by various biological processes, including important fluctuations in SSH. These fluctuations occur differently depending on the sex of the individual. While females experience marked increases or decreases of SSH -especially during pregnancy- males face progressive and steady changes (Jones and Boelaert, 2015). This could explain why studies assessing the effects of SSH on the oral cavity and oral microorganisms have mainly focused on females of reproductive age. A limited number of studies have included males as study subjects (Kumar, 2013) representing an important shortcoming. Recent studies have addressed the effect of SSH fluctuations on the oral microbiome during the menstrual cycle, pregnancy and postpartum, and these are presented below. The existing studies focusing on changes during puberty have already been reviewed by Kumar and thus have not been included in this review.
The menstrual cycle is a periodic shift in hormone production paired with physical changes of the uterus and ovaries in women of reproductive age (Reed and Carr, 2000; Bates and Bowling, 2013). These cyclic changes take place in 25–30-day periods and can be divided into two phases: (1) a follicular or proliferative phase, and (2) a luteal or secretory phase (Reed and Carr, 2000). The follicular phase starts with the first day of the menstrual period and ends with the ovulation, understood as the release of a mature egg from the ovary. The luteal phase, starts with the ovulation and ends just before the menstrual period (Bates and Bowling, 2013). During the follicular phase, estrogen levels in blood steadily increase until just before ovulation, reaching between 130 and 200 pg/mL from an initial concentration of approximately 50 pg/mL (Saucier, 2009). After ovulation, the luteal phase begins and progesterone levels in blood start to increase (Bates and Bowling, 2013), raising from less than 1 ng/mL to values between 10-35 ng/mL (Taraborrelli, 2015). If the egg is not fertilized, both estrogen and progesterone will sharply decrease to baseline levels, leading to the menstrual period (Bates and Bowling, 2013).
A limited number of studies have investigated the effects of the menstrual cycle on both gingival and oral microbiological changes, without reaching definite consensus (Kumar, 2013). This could be explained by small sample-size studies and methodologies that either targeted specific oral species or provided non-specific information about microbiological changes during the menstrual cycle (Prout and Hopps, 1970; Jensen et al., 1981; Calil et al., 2008; Fischer et al., 2008). To overcome this the use of new techniques can provide us with a better understanding of the influence of the menstrual cycle on the oral microbiome. A recently published study, investigated this using next generation sequencing (Bostanci et al., 2021). This study reported stability of the oral microbiome through the menstrual cycle of 103 women of reproductive age. However, they did observe variations in abundance of certain genera during the menstrual cycle (e.g., Campylobacter, Haemophilus, Prevotella and Oribacterium) and a higher diversity of species during the luteal phase. These results support the hypothesis that periodic hormonal shifts can influence the oral microbiome, but more studies are needed to confirm this and assess the possible clinical implications.
During the course of pregnancy, the female body experiences several physiological and physical changes. Estradiol and progesterone play a major role during pregnancy. Both are produced by the corpus luteum until the 6th – 7th week of pregnancy after which the production is taken over by the placenta. Estrogen increases steadily until delivery, rising from 0.1 ng/mL during the follicular phase of a normal menstrual cycle to 6-30 ng/mL at the end of the third trimester of pregnancy. Progesterone rises from less than 1 ng/mL during the follicular phase to 100-300 ng/mL at term (Tal et al., 2000). These values decrease back to normal levels at post-partum (Raber-Durlacher et al., 1994).
These considerable fluctuations have been linked to clinical changes in the periodontium: estradiol has been reported to increase gingival inflammation and progesterone to increase vascular permeability (Güncü et al., 2005). However, these direct effects on the oral tissues may not be the only ones. Based on the available in vitro evidence, it becomes apparent that hormones can directly and indirectly affect the oral microbiome.
Recent studies have mostly targeted specific species known for their affinity for SSH by means of selective media, PCR, qPCR and Fluorescence in situ hybridization (FISH). Two studies have analyzed the microbiome in depth by means of sequencing techniques.
In vitro studies have not reported any effect of either estrogen or progesterone on Aggregatibacter actinomycetemcomitans. However, this bacterium appears to be more prevalent during the second and third trimester of pregnancy according to Borgo et al. (2014) and during the first and second trimester according to Fujiwara et al. (2017). Even though both studies had a longitudinal design and used PCR techniques, the sample sizes varied greatly (9 and 125 subjects, respectively).
A cross-sectional study reported increased counts of Prevotella intermedia paired with increased gingival inflammation during the 2nd and 3rd trimester of pregnancy (Emmatty et al., 2013), confirming similar results earlier documented (Kornman and Loesche, 1980; Raber-Durlacher et al., 1994). In vitro studies also support these observations, given that estrogen and progesterone promote P. intermedia’s growth (Kornman and Loesche, 1982; Fteita et al., 2014). Nevertheless, this was not observed on a recent longitudinal study, where control subjects showed higher P. intermedia counts instead (Borgo et al., 2014).
During a longitudinal study, Prevotella nigrescens was noted to decrease significantly from pregnancy to post-partum (Machado et al., 2016). A high variability between pregnancy and post-partum was reported for other bacteria (Fusobacterium nucleatum and Prevotella intermedia) but not to a significant level. This could be related to the small sample size and a high drop-out rate. In vitro, P. nigrescens shows enhanced biofilm formation at estradiol concentrations present during the second trimester of pregnancy and enhanced planktonic growth at estradiol concentrations corresponding to the third trimester of pregnancy (Fteita et al., 2014). Based on this, it seems logical that after estradiol levels have dropped, P. nigrescens’ counts decline.
Increased microbial counts of Porphyromonas gingivalis have been reported during the first and second trimester of pregnancy compared to non-pregnant controls (Fujiwara et al., 2017). Also, a correlation between bacterial counts and progesterone levels in the first trimester of pregnancy has been observed (Massoni et al., 2019). In vitro studies have reported effects of estrogen and progesterone on P. gingivalis, which could partially explain this observation (Kornman and Loesche, 1982).
A higher prevalence of Tannerella forsythia has been noted to take place during the first trimester of pregnancy. This bacterium, during the same study, also correlated with the diagnosis of gingivitis among all pregnant participants independent of the pregnancy stage (Massoni et al., 2019). Unfortunately, no in vitro studies have reported effects of SSH on T. forsythia.
Fujiwara et al., also reported a significant increase of Candida species during the second and third trimester (Fujiwara et al., 2017). These results suggest an increased susceptibility during pregnancy for the colonization of certain periodontal bacteria and Candida species, which has also been observed in vitro (Powell et al., 1984; Skowronski and Feldman, 1989; Gujjar et al., 1997).
Studies using a more comprehensive approach to assess the effects on the oral microbiome have been recently carried out.
So far, three studies using sequencing techniques have been published and results show that microbial shifts occur during periods of SSH fluctuations and not only periodontal species are involved, but also novel phylotypes.
One cross-sectional study assessed the subgingival flora of 44 pregnant and non-pregnant women (smokers and non-smokers) using 16S-pyrotag sequencing, to investigate the synergistic effects of smoking during pregnancy (Paropkari et al., 2016). A significant clustering of pregnancy and smoking status was observed, indicating a different effect on the subgingival microbiome for each condition alone and combined. Results also indicated that pregnancy promoted the growth of Gram-negative facultative anaerobes to the detriment of anaerobes. This does not agree with previous studies (Gürsoy et al., 2009; Emmatty et al., 2013) which could be partially explained by the inclusion of only periodontally healthy participants. Additionally, a co-occurrence network analysis –aimed to visualize possible relations between bacteria based on environmental factors – showed that among others, Pseudomonas, Prevotella, Methylobacterium, Vagococcus and Enterobacter are highly present in estrogen rich environments and strongly correlated to species known to metabolize estrogen. Therefore, despite the small sample size, this study provides insight into unexplored species that might also be affected by the presence of SSH.
A second cross-sectional study aimed to present a snapshot of the oral microbiome during all trimesters of pregnancy and post-partum using 16S rRNA gene sequencing (Balan et al., 2018). Diversity remained stable across pregnancy and post-partum whereas an increase in pathogenic species during pregnancy was documented as well as a marked decrease after delivery with a reestablishment of oral health. An interesting observation was the co-occurrence of gingival bleeding and novel phylotypes rather than the usually-associated species.
Only one longitudinal study has used sequencing methods to assess the oral microbiome of pregnant women (Lin et al., 2018). Unlike most studies, supragingival samples were used for the microbiological analysis and hormonal levels were also assessed.
It was reported that several genera are responsible for the clustering according to the pregnancy trimester and that different genera were positively correlated with the surge in SSH, including Prevotella, Treponema, Cardiobacterium, Aggregatibacter, Leptotrichia among others. Even though the study sample was small, a shift could be observed, suggesting that hormonal changes during pregnancy could push the biofilm towards an unbalanced or dysbiotic state.
Two cross-sectional studies have explored the microbial composition of post-menopausal women (Brennan et al., 2007; Hernández-Vigueras et al., 2016). Even though both studies reported the co-occurrence of a similar group of periodontal pathogens, lack of follow-up and different inclusion criteria together with the absence of hormonal measurements makes drawing conclusions difficult.
Polycystic ovary syndrome (PCOS) is an endocrine, reproductive and metabolic condition affecting women of reproductive age, with a prevalence between 15-20% (Sirmans and Pate, 2013). Recently, it was reported that individuals suffering from PCOS may also experience a higher prevalence and risk of developing periodontal disease (Machado et al., 2020). Subsequently, microbiological changes have been pointed as one of the causes (Akcalı et al., 2014; Lindheim et al., 2016; Wendland et al., 2020). To date, three studies have investigated changes in oral bacterial species in individuals suffering from PCOS. In the first study, salivary samples from individuals with PCOS (with and without gingivitis) were compared to a matched control group, using PCR for bacterial identification (Akcalı et al., 2014). It was observed that F. nucleatum, P. gingivalis, P. intermedia, S. oralis and T. forsythia were significantly higher in individuals presenting PCOS and gingivitis, compared to controls. Also, serum antibody levels were measured using ELISA. Individuals with PCOS and gingivitis showed an increased antibody-mediated immunological response to P. gingivalis, and periodontally healthy individuals with PCOS, showed an increased response to P. intermedia and S. oralis.
One more study used PCR to evaluate the presence of nine different periodontal bacteria in young individuals with and without PCOS (Wendland et al., 2020). In this study, no particular microbiome alterations could be linked to PCOS. Interestingly, the control group showed a significantly higher prevalence of pathogenic and potentially pathogenic bacteria. These results could have been affected by a small sample size and less strict diagnostic criteria for the selected population. Nevertheless, this is the only study done in young individuals (2 years after the onset of menstruation) and more studies are needed to confirm these observations.
To date, only one study has used next generation sequencing to investigate possible changes in the oral microbiome of individuals with PCOS (Lindheim et al., 2016). This study reported a decreased relative abundance of the phylum Actinobacteria without further significant variation in community composition and diversity between the studied groups. This reduction could favor an environment suitable for disease-associated bacteria, increasing the risk of dysbiosis. A small sample size and the absence of more severe forms of PCOS could have influenced the obtained results.
Existing research remains contradictory regarding the role of PCOS in the composition of the oral microbiome and its contribution to inflammation and periodontal health/disease.
Hormonal contraceptive methods consist of synthetic forms of progestagens and/or estrogens at levels aimed to suppress ovulation, similar to the ones achieved during pregnancy. These come in different dosages and formulations and can be administered orally (oral contraceptive pills), in the form of injections, implants, intravaginal, topical patches and intra uterine devices (Colquitt and Martin, 2015).
Previous studies have investigated the possible influence of oral contraceptives (OC) on the composition of the subgingival microbiome (Jensen et al., 1981; Klinger et al., 1998). These studies have been reviewed by Kumar (2013) and even though results indicate that OC promote a selection of certain periodontal microorganisms, the applicability of these conclusions today is not feasible. Differences in the formulation, dosage and routes of administration of contraceptives as well as changes these have undergone through the years, make standardization nor generalization possible.
Higher counts of P. gingivalis, P. intermedia and A. actinomycetemcomitans have been observed in women using oral contraceptives together with an increased risk of developing periodontitis (Brusca et al., 2010). Candida species have also been reported to increase in counts for individuals using OC for at least three years (Brusca et al., 2010). In this recent publication smokers were included as participants but not separately analyzed. It is well known that tobacco negatively affects oral health and the oral microbiome resulting in an increased risk of developing periodontal disease (Gloria et al., 2002; Mason et al., 2015) and this must be considered when weighing these results.
The existing evidence points towards a role of oral contraceptives on the composition of the subgingival microbiome. Nevertheless, more studies assessing this interaction and taking into account the different formulations, dosages and administration routes are needed.
HRT is the treatment of choice for acute climacteric syndrome and to prevent long-term estrogen deficiency. It consists of single (estrogen) or combined (estrogen-progesterone) preparations. Administration can be oral, transdermal, percutaneous, intramuscular, intranasal or local with dosing and timing depending on the case (Fait, 2019).
Only one study has investigated the effect of HRT on oral health. Samples were taken at baseline and after two years of therapy (Tarkkila et al., 2010). Both clinical and microbiological parameters were evaluated and it was concluded that the use of HRT does not have a significant effect on the overall composition of the oral microbiome. Additionally, there was a decrease of certain species usually found in periodontal disease – namely P. gingivalis, P. intermedia and T. forsythia – after 2 years of HRT use. Even though these results seem optimistic and could indicate a positive influence of HRT on periodontal health, the role of HRT on clinical attachment loss (CAL) remains debatable and inconclusive (Chaves et al., 2020).
More studies are needed to assess the risks and benefits of undergoing HRT from an oral health perspective.
Androgens – also known as male steroid hormones – include testosterone and its metabolites as well as synthetic preparations. Synthetic preparations are largely used to treat androgen deficiency but these are also (mis)used by individuals looking forward to gaining muscular mass, leading to different complications (Sadaie et al., 2018).
Studies evaluating oral health and the use of androgens are scarce. The reason behind this could be that androgens do not experiment extreme fluctuations like female SSH do. This results in a more stable concentration throughout the life of an individual (Harman et al., 2001).
It can be expected that androgens have an effect in the oral cavity as androgen receptors have been described in diverse oral tissues such as gingiva (Southren et al., 1978), oral mucosa (Ojanotko-Harri et al., 1992) and salivary glands (Laine et al., 1993) indicating that they can also act as modulating agents in the oral cavity. Earlier reports have noted that DHT can be metabolized by oral fibroblasts resulting in a collagen matrix stimulatory effect (Soory and Ahmad, 1997). This is consistent with later studies reporting that individuals consuming anabolic steroids experience gingival inflammation compared to controls (Ozcelik et al., 2006). One study investigated the effect of anabolic steroids on the oral microbiota of a group of body builders (Brusca et al., 2014). Results showed that users had a significantly higher prevalence of severe periodontal disease, higher gingival inflammation and attachment loss as well as a higher prevalence of A. actinomycetemcomitans, P. intermedia, P. gingivalis and Candida species associated to a diseased oral state, compared to a control group of anabolic steroids non-users.
Taken together, the above-described studies indicate that it is likely that SSH affect the oral cavity, however more studies are needed to understand how endogenous and exogenous androgens can affect the balance between the host and the oral microbiome.
We set out to review the data available on the effect of SSH in the oral cavity. Various in vitro and in vivo studies have aimed to clarify the direct and indirect effects of SSH on the oral microbiota and host tissues. It is important to note that both these approaches have advantages and limitations. In vitro studies provide a controlled environment and can explain the mechanisms involved in microbial responses to SSH. Nonetheless, they provide only a simplified interpretation of reality and lack important factors that play a role in the complex host-microbiome interaction. And while in vivo studies offer real-life conditions whose results can be directly applicable to the clinical field, there is a high degree of subject variability and, more importantly, a real risk of bias. Both in vitro and in vivo studies have mostly targeted specific periodontal pathogens and failed to look at the oral microbiome in its totality. The incorporation of both approaches is therefore most suitable to grasp the whole picture.
The great variability between the methodologies applied to date in in vivo and in vitro studies makes them difficult to compare. The technologies used to classify microorganisms in early studies were less sensitive and did not document all species correctly.
The available evidence makes it evident that hormones can affect the oral microbiome both directly and indirectly. The remaining question is if and to what extent these effects play a role in oral health and disease and what the underlying mechanisms are.
Recent studies have approached this question using clinical evaluation paired with current technologies such as sequencing, that focus not only on classic periodontal pathogens but also on the oral microbiome (Balan et al., 2018; Lin et al., 2018; Bostanci et al., 2021). These analyses have yielded interesting results regarding the diversity of the oral microbiome and how SSH can promote a shift in its composition that is not exclusive to known periodontal pathogens (Short et al., 2014).
Building on the available evidence, we propose a hypothesis that describes the mechanism of this interplay between SSH, host and microbiome.
By metabolizing SSH, certain bacteria can directly and indirectly influence SSH concentration in the environment (Kornman and Loesche, 1982; Soory and Ahmad, 1997). Their conversion into more active molecules (i.e. by forming DHT from testosterone by means of 5α-reduction) can directly affect oral tissues. Bacterial enzymes can also stimulate the natural conversion of testosterone into DHT by oral fibroblasts (Soory, 1995). While this bidirectional interaction has been proposed only for certain bacterial species, we believe that it is not an exclusive response of a limited number of microorganisms, and more generally occurs in the oral microbiome. Specifically, this interaction evidences a bidirectional response, supporting the hypothesis that biofilm-mediated hormonal metabolism can result in a cellular response.
We therefore propose a hypothesis to explain how SSH concentrations in saliva can lead to shifts in the oral microbiome and the oral milieu that directly induce a response from the host. These shifts include the proliferation of certain species and the metabolism of SSH into other molecules. Meanwhile, the host responds to these changes and – in turn – metabolizes SSH in the medium, adapting its physiological response to the environmental conditions (Figure 2). This interaction generates a local feedback loop whose consequences are yet to be investigated.
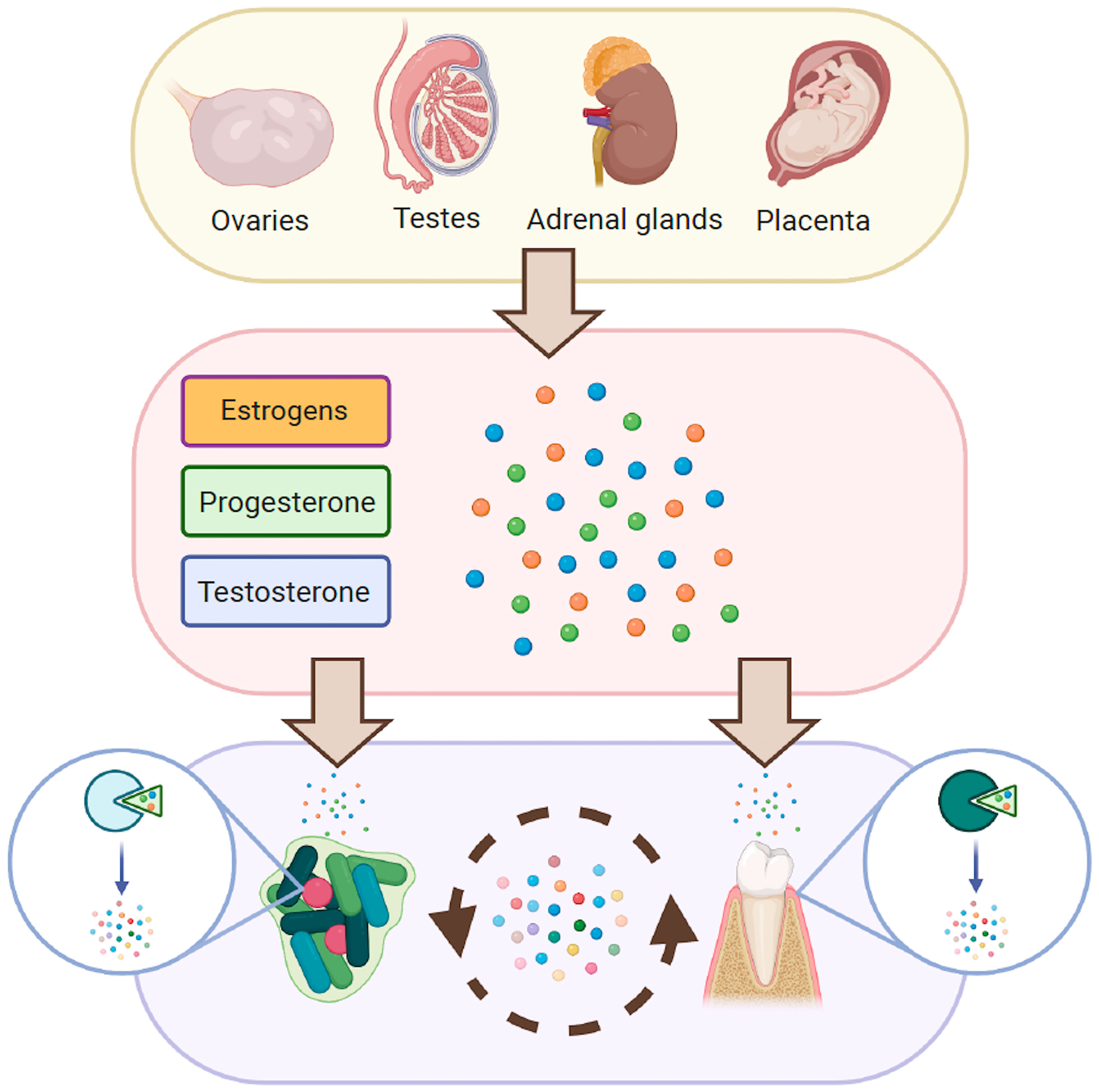
Figure 2 Proposed interaction between SSH, the host and its microbiome. Solid arrows represent known interactions between SSH, oral microorganisms and oral tissues. Dashed arrows represent hypothesized interactions.
There is evidence of a bidirectional interaction between hormone-metabolizing as well as producing bacteria and the host in gut bacteria (Neuman et al., 2015; Tetel et al., 2018). In rats it has been documented that Toxoplasma gondii – a protozoan parasite – can induce the steroidogenesis of testosterone, supporting the hypothesis that microorganisms can also modulate the synthesis of SSH to their own benefit (Lim et al., 2013).
To conclude, in vitro and in vivo studies support a direct and indirect effect of SSH on oral microbes during hormonal shifts. The classical perspective has tried to link presence of specific species to a surge or decline in SSH and subsequently to clinical changes in the periodontium. Even though this has contributed to our understanding of this complex interaction, newer studies considering the dynamics between the oral microbiome and the host are needed to grasp the underlying mechanisms. Emerging research in endocrinology suggests that there is an active and bidirectional interaction between the host and its microbiome, mediated by SSH. A fuller understanding of these events will offer immense possibilities in the treatment and prevention of disease.
Unlimited perspectives are yielded by the emerging understanding of the oral microbiome as an active player in some of the host’s vital physiological processes. These microbial communities have co-evolved – and continue to co-evolve – with the human host, developing mechanisms that are mutually beneficial. This interaction, when balanced, prevents a drift towards a dysbiotic or pathological state.
To date, there is sufficient ground for stating that the presence of SSH influences the oral milieu and its inhabitants. Nevertheless, it is yet to be discovered if and how this is involved in the maintenance of an oral ecological balance.
Hormone concentrations in saliva can be altered during periods of intrinsic SSH hormone fluctuations such as puberty, menstrual cycle, pregnancy, or by extrinsic fluctuations such as the use of oral contraceptives, hormone replacement therapy, gender affirming therapy or androgen use. By contributing to our understanding of oral health and how to maintain it, a better understanding of the role of SSH in the oral cavity will allow clinicians to deliver more personalized care for their patients.
PC proposed the topic to be reviewed, performed the search, extracted and analyzed the data and wrote and edited the original draft. BK and MV reviewed and edited the original draft and supervised the work. All authors contributed to the article and approved the submitted version.
The research was funded by the Academic Centre for Dentistry Amsterdam (ACTA), University of Amsterdam and VU University Amsterdam.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
All figures were created with BioRender.com.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.714229/full#supplementary-material
Akcalı, A., Bostanci, N., Özçaka, Ö., Öztürk-Ceyhan, B., Gümüş, P., Buduneli, N., et al. (2014). Association Between Polycystic Ovary Syndrome, Oral Microbiota and Systemic Antibody Responses. PloS One 9 (9), e108074. doi: 10.1371/journal.pone.0108074
Alves, C. T., Silva, S., Pereira, L., Williams, D. W., Azeredo, J., Henriques, M. (2014). Effect of Progesterone on Candida Albicans Vaginal Pathogenicity. Int. J. Med. Microbiol. 304 (8), 1011–1017. doi: 10.1016/j.ijmm.2014.07.004
Balan, P., Chong, Y. S., Umashankar, S., Swarup, S., Loke, W. M., Lopez, V., et al. (2018). Keystone Species in Pregnancy Gingivitis: A Snapshot of Oral Microbiome During Pregnancy and Postpartum Period. Front. Microbiol. 9, 2360. doi: 10.3389/fmicb.2018.02360
Bates, G. W., Bowling, M. (2013). Physiology of the Female Reproductive Axis. Periodontol. 2000 61 (1), 89–102. doi: 10.1111/j.1600-0757.2011.00409.x
Bokkenheuser, V. D., Winter, J., Cohen, B. I., O'Rourke, S., Mosbach, E. H. (1983). Inactivation of Contraceptive Steroid Hormones by Human Intestinal Clostridia. J. Clin. Microbiol. 18 (3), 500–504. doi: 10.1128/jcm.18.3.500-504.1983
Borgo, P. V., Rodrigues, V. A., Feitosa, A. C., Xavier, K. C., Avila-Campos, M. J. (2014). Association Between Periodontal Condition and Subgingival Microbiota in Women During Pregnancy: A Longitudinal Study. J. Appl. Oral. Sci. 22 (6), 528–533. doi: 10.1590/1678-775720140164
Bostanci, N., Krog, M. C., Hugerth, L. W., Bashir, Z., Fransson, E., Boulund, F., et al. (2021). Dysbiosis of the Human Oral Microbiome During the Menstrual Cycle and Vulnerability to the External Exposures of Smoking and Dietary Sugar. Front. Cell Infect. Microbiol. 11, 625229. doi: 10.3389/fcimb.2021.625229
Brennan, R. M., Genco, R. J., Wilding, G. E., Hovey, K. M., Trevisan, M., Wactawski-Wende, J. (2007). Bacterial Species in Subgingival Plaque and Oral Bone Loss in Postmenopausal Women. J. Periodontol. 78 (6), 1051–1061. doi: 10.1902/jop.2007.060436
Brusca, M. I., Rosa, A., Albaina, O., Moragues, M. D., Verdugo, F., Pontón, J. (2010). The Impact of Oral Contraceptives on Women's Periodontal Health and the Subgingival Occurrence of Aggressive Periodontopathogens and Candida Species. J. Periodontol. 81 (7), 1010–1018. doi: 10.1902/jop.2010.090575
Brusca, Verdugo, F., Amighini, C., Albaina, O., Moragues, M. D. (2014). Anabolic Steroids Affect Human Periodontal Health and Microbiota. Clin. Oral. Investig. 18 (6), 1579–1586. doi: 10.1007/s00784-013-1126-9
Calil, C. M., Lima, P. O., Bernardes, C. F., Groppo, F. C., Bado, F., Marcondes, F. K. (2008). Influence of Gender and Menstrual Cycle on Volatile Sulphur Compounds Production. Arch. Oral. Biol. 53 (12), 1107–1112. doi: 10.1016/j.archoralbio.2008.06.008
Chan, L., O'Malley, B. W. (1976). Mechanism of Action of the Sex Steroid Hormones (First of Three Parts). N. Engl. J. Med. 294 (24), 1322–1328. doi: 10.1056/nejm197606102942405
Chaves, J. D. P., Figueredo, T. F. M., Warnavin, S., Pannuti, C. M., Steffens, J. P. (2020). Sex Hormone Replacement Therapy in Periodontology-A Systematic Review. Oral. Dis. 26 (2), 270–284. doi: 10.1111/odi.13059
Cheng, G., Yeater, K. M., Hoyer, L. L. (2006). Cellular and Molecular Biology of Candida Albicans Estrogen Response. Eukaryotic. Cell 5 (1), 180–191. doi: 10.1128/EC.5.1.180-191.2006
Chiang, Y. R., Wei, S. T., Wang, P. H., Wu, P. H., Yu, C. P. (2019). Microbial Degradation of Steroid Sex Hormones: Implications for Environmental and Ecological Studies. Microb. Biotechnol. 13 (4), 926–949. doi: 10.1111/1751-7915.13504
Cho, I., Blaser, M. J. (2012). The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 13 (4), 260–270. doi: 10.1038/nrg3182
Clark, D. T., Soory, M. (2005). The Metabolism of Cholesterol and Certain Hormonal Steroids by Treponema Denticola. Steroids 71 (5), 352–363. doi: 10.1016/j.steroids.2005.11.006
Clark, D. T., Soory, M. (2006). The Influence of Cholesterol, Progesterone, 4-Androstenedione and Testosterone on the Growth of Treponema Denticola ATCC 33520 in Batch Cultures. Anaerobe 12 (5-6), 267–273. doi: 10.1016/j.anaerobe.2006.08.002
Clark, D. T., Soory, M. (2007). Steroid 5alpha-Reductase Activity of Treponema Denticola. Oral. Microbiol. Immunol. 22 (5), 326–332. doi: 10.1111/j.1399-302X.2007.00364.x
Colquitt, C. W., Martin, T. S. (2015). Contraceptive Methods: A Review of Nonbarrier and Barrier Products. J. Pharm. Pract. 30 (1), 130–135. doi: 10.1177/0897190015585751
Connolly, D. T. (1991). Vascular Permeability Factor: A Unique Regulator of Blood Vessel Function. J. Cell Biochem. 47 (3), 219–223. doi: 10.1002/jcb.240470306
Cornejo Ulloa, P., van der Veen, M. H., Krom, B. P. (2019). Review: Modulation of the Oral Microbiome by the Host to Promote Ecological Balance. Odontology 107 (4), 437–448. doi: 10.1007/s10266-019-00413-x
Donova, M. V., Egorova, O. V. (2012). Microbial Steroid Transformations: Current State and Prospects. Appl. Microbiol. Biotechnol. 94 (6), 1423–1447. doi: 10.1007/s00253-012-4078-0
Emmatty, R., Mathew, J. J., Kuruvilla, J. (2013). Comparative Evaluation of Subgingival Plaque Microflora in Pregnant and non-Pregnant Women: A Clinical and Microbiologic Study. J. Indian Soc. Periodontol. 17 (1), 47–51. doi: 10.4103/0972-124X.107474
Fait, T. (2019). Menopause Hormone Therapy: Latest Developments and Clinical Practice. Drugs Context 8, 212551–212551. doi: 10.7573/dic.212551
Fischer, C. C., Persson, R. E., Persson, G. R. (2008). Influence of the Menstrual Cycle on the Oral Microbial Flora in Women: A Case-Control Study Including Men as Control Subjects. J. Periodontol. 79 (10), 1966–1973. doi: 10.1902/jop.2008.080057
Fteita, D., Kononen, E., Gursoy, M., Soderling, E., Gursoy, U. K. (2015). Does Estradiol Have an Impact on the Dipeptidyl Peptidase IV Enzyme Activity of the Prevotella Intermedia Group Bacteria? Anaerobe 36, 14–18. doi: 10.1016/j.anaerobe.2015.09.002
Fteita, D., Kononen, E., Soderling, E., Gursoy, U. K. (2014). Effect of Estradiol on Planktonic Growth, Coaggregation, and Biofilm Formation of the Prevotella Intermedia Group Bacteria. Anaerobe 27, 7–13. doi: 10.1016/j.anaerobe.2014.02.003
Fujiwara, N., Tsuruda, K., Iwamoto, Y., Kato, F., Odaki, T., Yamane, N., et al. (2017). Significant Increase of Oral Bacteria in the Early Pregnancy Period in Japanese Women. J. Investig. Clin. Dent. 8 (1), e12189. doi: 10.1111/jicd.12189
Garcia-Gomez, E., Gonzalez-Pedrajo, B., Camacho-Arroyo, I. (2013). Role of Sex Steroid Hormones in Bacterial-Host Interactions. BioMed. Res. Int. 2013, 928290. doi: 10.1155/2013/928290
Glick, M. C., Zilliken, F., Gyorgy, P. (1959). Supplementary Growth Promoting Effect of 2-Methyl-1,4-Naphthoquinone of Lactobacillus Bifidus Var. Pennsylvanicus. J. Bacteriol. 77 (2), 230–236. doi: 10.1128/jb.77.2.230-236.1959
Gloria, C., Ramón, J.-M., Echeverría, J.-J. (2002). Effects of Smoking on Periodontal Tissues. J. Clin. Periodontol. 29 (8), 771–776. doi: 10.1034/j.1600-051X.2002.290815.x
Gujjar, P. R., Finucane, M., Larsen, B. (1997). The Effect of Estradiol on Candida Albicans Growth. Ann. Clin. Lab. Sci. 27 (2), 151–156.
Güncü, G. N., Tözüm, T. F., Cağlayan, F. (2005). Effects of Endogenous Sex Hormones on the Periodontium–Review of Literature. Aust. Dent. J. 50 (3), 138–145. doi: 10.1111/j.1834-7819.2005.tb00352.x
Gürsoy, M., Haraldsson, G., Hyvönen, M., Sorsa, T., Pajukanta, R., Könönen, E. (2009). Does the Frequency of Prevotella Intermedia Increase During Pregnancy? Oral. Microbiol. Immunol. 24 (4), 299–303. doi: 10.1111/j.1399-302X.2009.00509.x
Harman, S. M., Metter, E. J., Tobin, J. D., Pearson, J., Blackman, M. R. (2001). Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 86 (2), 724–731. doi: 10.1210/jcem.86.2.7219
Hernández-Vigueras, S., Martínez-Garriga, B., Sánchez, M. C., Sanz, M., Estrugo-Devesa, A., Vinuesa, T., et al. (2016). Oral Microbiota, Periodontal Status, and Osteoporosis in Postmenopausal Females. J. Periodontol. 87 (2), 124–133. doi: 10.1902/jop.2015.150365
Hojo, K., Nagaoka, S., Murata, S., Taketomo, N., Ohshima, T., Maeda, N. (2007). Reduction of Vitamin K Concentration by Salivary Bifidobacterium Strains and Their Possible Nutritional Competition With Porphyromonas Gingivalis. J. Appl. Microbiol. 103 (5), 1969–1974. doi: 10.1111/j.1365-2672.2007.03436.x
Holert, J., Cardenas, E., Bergstrand, L. H., Zaikova, E., Hahn, A. S., Hallam, S. J., et al. (2018). Metagenomes Reveal Global Distribution of Bacterial Steroid Catabolism in Natural, Engineered, and Host Environments. mBio 9 (1), e02345–17. doi: 10.1128/mBio.02345-17
Horinouchi, M., Koshino, H., Malon, M., Hirota, H., Hayashi, T. (2019). Steroid Degradation in Comamonas Testosteroni TA441: Identification of the Entire β-Oxidation Cycle of the Cleaved B Ring. Appl. Environ. Microbiol. 85 (20), e01204–e01219. doi: 10.1128/AEM.01204-19
Hu, J., Zhang, Z., Shen, W.-J., Azhar, S. (2010). Cellular Cholesterol Delivery, Intracellular Processing and Utilization for Biosynthesis of Steroid Hormones. Nutr. Metab. 7 (1), 47. doi: 10.1186/1743-7075-7-47
Jensen, J., Liljemark, W., Bloomquist, C. (1981). The Effect of Female Sex Hormones on Subgingival Plaque. J. Periodontol. 52 (10), 599–602. doi: 10.1902/jop.1981.52.10.599
Jones, C. M., Boelaert, K. (2015). The Endocrinology of Ageing: A Mini-Review. Gerontology 61 (4), 291–300. doi: 10.1159/000367692
Kinsman, O. S., Pitblado, K., Coulson, C. J. (1988). Effect of Mammalian Steroid Hormones and Luteinizing Hormone on the Germination of Candida Albicans and Implications for Vaginal Candidosis. Mycoses 31 (12), 617–626. doi: 10.1111/j.1439-0507.1988.tb04416.x
Klinger, G., Eick, S., Klinger, G., Pfister, W., Gräser, T., Moore, C., et al. (1998). Influence of Hormonal Contraceptives on Microbial Flora of Gingival Sulcus. Contraception 57 (6), 381–384. doi: 10.1016/s0010-7824(98)00044-4
Kornman, K. S., Loesche, W. J. (1980). The Subgingival Microbial Flora During Pregnancy. J. Periodontol. Res. 15 (2), 111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x
Kornman, K. S., Loesche, W. J. (1982). Effects of Estradiol and Progesterone on Bacteroides Melaninogenicus and Bacteroides Gingivalis. Infect. Immun. 35 (1), 256–263. doi: 10.1128/iai.35.1.256-263.1982
Kravtsov, E. G., Anokhina, I. V., Rybas, Y. A., Sachivkina, N. P., Ermolaev, A. V., Brodskaya, S. B. (2014). Effects of Female Sex Hormones on Adhesion of Candida Albicans Yeast-Like Fungi to the Buccal Epithelium. Bull. Exp. Biol. Med. 157 (2), 246–248. doi: 10.1007/s10517-014-2536-7
Krishnamurthy, S., Gupta, V., Prasad, R., Panwar, S. L., Prasad, R. (1998). Expression of CDR1, a Multidrug Resistance Gene of Candida Albicans: Transcriptional Activation by Heat Shock, Drugs and Human Steroid Hormones. FEMS Microbiol. Lett. 160 (2), 191–197. doi: 10.1111/j.1574-6968.1998.tb12910.x
Kristan, K., Rizner, T. L. (2012). Steroid-Transforming Enzymes in Fungi. J. Steroid Biochem. Mol. Biol. 129 (1-2), 79–91. doi: 10.1016/j.jsbmb.2011.08.012
Krom, B. P., Kidwai, S., Ten Cate, J. M. (2014). Candida and Other Fungal Species: Forgotten Players of Healthy Oral Microbiota. J. Dent. Res. 93 (5), 445–451. doi: 10.1177/0022034514521814
Kumar, P. S. (2013). Sex and the Subgingival Microbiome: Do Female Sex Steroids Affect Periodontal Bacteria? Periodontol 200061 (1), 103–124. doi: 10.1111/j.1600-0757.2011.00398.x
Laine, M., Bläuer, M., Ylikomi, T., Tuohimaa, P., Aitasalo, K., Happonen, R. P., et al. (1993). Immunohistochemical Demonstration of Androgen Receptors in Human Salivary Glands. Arch. Oral. Biol. 38 (4), 299–302. doi: 10.1016/0003-9969(93)90136-A
Larsen, B., Anderson, S., Brockman, A., Essmann, M., Schmidt, M. (2006). Key Physiological Differences in Candida Albicans CDR1 Induction by Steroid Hormones and Antifungal Drugs. Yeast 23 (11), 795–802. doi: 10.1002/yea.1394
Lim, A., Kumar, V., Hari Dass, S. A., Vyas, A. (2013). Toxoplasma Gondii Infection Enhances Testicular Steroidogenesis in Rats. Mol. Ecol. 22 (1), 102–110. doi: 10.1111/mec.12042
Lindheim, L., Bashir, M., Münzker, J., Trummer, C., Zachhuber, V., Pieber, T. R., et al. (2016). The Salivary Microbiome in Polycystic Ovary Syndrome (PCOS) and Its Association With Disease-Related Parameters: A Pilot Study. Front. Microbiol. 7, 1270 (1270). doi: 10.3389/fmicb.2016.01270
Lin, W., Jiang, W., Hu, X., Gao, L., Ai, D., Pan, H., et al. (2018). Ecological Shifts of Supragingival Microbiota in Association With Pregnancy. Front. Cell Infect. Microbiol. 8, 24. doi: 10.3389/fcimb.2018.00024
Machado, F. C., Cesar, D. E., Apolonio, A. C., Ribeiro, L. C., Ribeiro, R. A. (2016). Longitudinal Study on Clinical and Microbial Analysis of Periodontal Status in Pregnancy. Braz. Oral. Res. 30 (1), e87. doi: 10.1590/1807-3107BOR-2016.vol30.0087
Machado, V., Escalda, C., Proença, L., Mendes, J. J., Botelho, J. (2020). Is There a Bidirectional Association Between Polycystic Ovarian Syndrome and Periodontitis? A Systematic Review and Meta-Analysis. J. Clin. Med. 9 (6), 1961. doi: 10.3390/jcm9061961
Marcotte, H., Lavoie, M. C. (1998). Oral Microbial Ecology and the Role of Salivary Immunoglobulin A. Microbiol. Mol. Biol. Rev. 62 (1), 71. doi: 10.1128/MMBR.62.1.71-109.1998
Mariotti, A., Mawhinney, M. (2013). Endocrinology of Sex Steroid Hormones and Cell Dynamics in the Periodontium. Periodontol. 2000 61 (1), 69–88. doi: 10.1111/j.1600-0757.2011.00424.x
Markou, E., Eleana, B., Lazaros, T., Antonios, K. (2009). The Influence of Sex Steroid Hormones on Gingiva of Women. Open Dent. J. 3, 114–119. doi: 10.2174/1874210600903010114
Mason, M. R., Preshaw, P. M., Nagaraja, H. N., Dabdoub, S. M., Rahman, A., Kumar, P. S. (2015). The Subgingival Microbiome of Clinically Healthy Current and Never Smokers. ISME J. 9 (1), 268–272. doi: 10.1038/ismej.2014.114
Massoni, R., Aranha, A. M. F., Matos, F. Z., Guedes, O. A., Borges, Á. H., Miotto, M., et al. (2019). Correlation of Periodontal and Microbiological Evaluations, With Serum Levels of Estradiol and Progesterone, During Different Trimesters of Gestation. Sci. Rep. 9 (1), 11762–11762. doi: 10.1038/s41598-019-48288-w
Mealey, B. L., Moritz, A. J. (2003). Hormonal Influences: Effects of Diabetes Mellitus and Endogenous Female Sex Steroid Hormones on the Periodontium. Periodontol. 2000 32, 59–81. doi: 10.1046/j.0906-6713.2002.03206.x
Neuman, H., Debelius, J. W., Knight, R., Koren, O. (2015). Microbial Endocrinology: The Interplay Between the Microbiota and the Endocrine System. FEMS Microbiol. Rev. 39 (4), 509–521. doi: 10.1093/femsre/fuu010
Ojanotko-Harri, A., Forssell, H., Laine, M., Hurttia, H., Bläuer, M., Tuohimaa, P. (1992). Immunohistochemical Detection of Androgen Receptors in Human Oral Mucosa. Arch. Oral. Biol. 37 (6), 511–514. doi: 10.1016/0003-9969(92)90108-K
Ojanotko-Harri, A., Laine, M., Tenovuo, J. (1991). Metabolism of 17B-Estradiol by Oral Streptococcus Mutans, Streptococcus Sanguis, Bacillus Cereus and Candida Albicans. Oral. Microbiol. Immunol. 6 (2), 126–128. doi: 10.1111/j.1399-302X.1991.tb00465.x
Ojanotko-Harri, A., Nikkari, T., Harri, M. P., Paunio, K. U. (1990). Metabolism of Progesterone and Testosterone by Bacillus Cereus Strain Socransky 67 and Streptococcus Mutans Strain Ingbritt. Oral. Microbiol. Immunol. 5 (4), 237–239. doi: 10.1111/j.1399-302x.1990.tb00653.x
Ozcelik, O., Haytac, M. C., Seydaoglu, G. (2006). The Effects of Anabolic Androgenic Steroid Abuse on Gingival Tissues. J. Periodontol. 77 (7), 1104–1109. doi: 10.1902/jop.2006.050389
Paropkari, A. D., Leblebicioglu, B., Christian, L. M., Kumar, P. S. (2016). Smoking, Pregnancy and the Subgingival Microbiome. Sci. Rep. 6 (1), 30388. doi: 10.1038/srep30388
Powell, B. L., Frey, C. L., Drutz, D. J. (1984). Identification of A17β-Estradiol Binding Protein in Candida Albicans and Candida (Torulopsis) Glabrata. Exp. Mycol. 8 (4), 304–313. doi: 10.1016/0147-5975(84)90054-9
Prasad, R., Devaux, F., Dhamgaye, S., Banerjee, D. (2012). Response of Pathogenic and non-Pathogenic Yeasts to Steroids. J. Steroid Biochem. Mol. Biol. 129 (1-2), 61–69. doi: 10.1016/j.jsbmb.2010.11.011
Prout, R. E., Hopps, R. M. (1970). A Relationship Between Human Oral Bacteria and the Menstrual Cycle. J. Periodontol. 41 (2), 98–101. doi: 10.1902/jop.1970.41.2.98
Raber-Durlacher, J. E., van Steenbergen, T. J., van der Velden, U., de Graaff, J., Abraham-Inpijn, L. (1994). Experimental Gingivitis During Pregnancy and Post-Partum: Clinical, Endocrinological, and Microbiological Aspects. J. Clin. Periodontol. 21 (8), 549–558. doi: 10.1111/j.1600-051x.1994.tb01172.x
Reed, B. G., Carr, B. R. (2000). “The Normal Menstrual Cycle and the Control of Ovulation,” in Endotext. Eds. Feingold, K. R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W. W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J. M., Hofland, J., Kalra, S., Kaltsas, G., Koch, C., Kopp, P., Korbonits, M., Kovacs, C. S., Kuohung, W., Laferrère, B., McGee, E. A., McLachlan, R., Morley, J. E., New, M., Purnell, J., Sahay, R., Singer, F., Stratakis, C. A., Trence, D. L., Wilson, D. P. (South Dartmouth (MA: MDText.com, Inc.Copyright © 2000-2021, MDText.com, Inc.)
Romano, M. C., Jiménez, P., Miranda-Brito, C., Valdez, R. A. (2015). Parasites and Steroid Hormones: Corticosteroid and Sex Steroid Synthesis, Their Role in the Parasite Physiology and Development. Front. Neurosci. 9, 224. doi: 10.3389/fnins.2015.00224
Sadaie, M. R., Farhoudi, M., Zamanlu, M., Aghamohammadzadeh, N., Amouzegar, A., Rosenbaum, R. E., et al. (2018). What Does the Research Say About Androgen Use and Cerebrovascular Events? Ther. Adv. Drug Saf. 9 (8), 439–455. doi: 10.1177/2042098618773318
Saucier, D. (2009). Sex Differences in the Brain: From Genes to Behaviour. Laterality 14 (1), 102–104. doi: 10.1080/13576500802366829
Short, F. L., Murdoch, S. L., Ryan, R. P. (2014). Polybacterial Human Disease: The Ills of Social Networking. Trends Microbiol. 22 (9), 508–516. doi: 10.1016/j.tim.2014.05.007
Sirmans, S. M., Pate, K. A. (2013). Epidemiology, Diagnosis, and Management of Polycystic Ovary Syndrome. Clin. Epidemiol. 6, 1–13. doi: 10.2147/CLEP.S37559
Skowronski, R., Feldman, D. (1989). Characterization of an Estrogen-Binding Protein in the Yeast Candida Albicans. Endocrinology 124 (4), 1965–1972. doi: 10.1210/endo-124-4-1965
Soory, M. (1995). Bacterial Steroidogenesis by Periodontal Pathogens and the Effect of Bacterial Enzymes on Steroid Conversions by Human Gingival Fibroblasts in Culture. J. Periodontol. Res. 30 (2), 124–131. doi: 10.1111/j.1600-0765.1995.tb01261.x
Soory, M. (2000). Targets for Steroid Hormone Mediated Actions of Periodontal Pathogens, Cytokines and Therapeutic Agents Some Implications on Tissue Turnover in the Periodontium. Curr. Drug Targets 1 (4), 309–325. doi: 10.2174/1389450003349119
Soory, M., Ahmad, S. (1997). 5α-Reductase Activity in Human Gingiva and Gingival Fibroblasts in Response to Bacterial Culture Supernatants, Using [14C]4-Androstenedione as Substrate. Arch. Oral. Biol. 42 (4), 255–262. doi: 10.1016/S0003-9969(97)00028-9
Southren, A. L., Rappaport, S. C., Gordon, G. G., Vittek, J. (1978). Specific 5α-Dihydrotestosterone Receptors in Human Gingiva*. J. Clin. Endocrinol. Metab. 47 (6), 1378–1382. doi: 10.1210/jcem-47-6-1378
Swerdloff, R. S., Dudley, R. E., Page, S. T., Wang, C., Salameh, W. A. (2017). Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 38 (3), 220–254. doi: 10.1210/er.2016-1067
Tal, R., Taylor, H. S., Burney, R. O., Mooney, S. B., Giudice, L. C. (2000). “Endocrinology of Pregnancy” in Endotext. Eds. Feingold, K. R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W. W., Dungan, K., Grossman, A., Hershman, J. M., Hofland, H. J., Kaltsas, G., Koch, C., Kopp, P., Korbonits, M., McLachlan, R., Morley, J. E., New, M., Purnell, J., Singer, F., Stratakis, C. A., Trence, D. L., Wilson, D. P. (South Dartmouth MA: MDText.com, Inc.). Available from: https://www.ncbi.nlm.nih.gov/books/NBK278962/.
Taraborrelli, S. (2015). Physiology, Production and Action of Progesterone. Acta Obstet. Gynecol. Scand. 94 Suppl 161, 8–16. doi: 10.1111/aogs.12771
Tarkkila, L., Kari, K., Furuholm, J., Tiitinen, A., Meurman, J. H. (2010). Periodontal Disease-Associated Micro-Organisms in Peri-Menopausal and Post-Menopausal Women Using or Not Using Hormone Replacement Therapy. A Two-Year Follow-Up Study. BMC Oral. Health 10, 10. doi: 10.1186/1472-6831-10-10
Tarkowská, D. (2019). Plants are Capable of Synthesizing Animal Steroid Hormones. Molecules (Basel Switzerland) 24 (14):2585. doi: 10.3390/molecules24142585
Tetel, M. J., de Vries, G. J., Melcangi, R. C., Panzica, G., O'Mahony, S. M. (2018). Steroids, Stress and the Gut Microbiome-Brain Axis. J. Neuroendocrinol. 30 (2), 10.1111/jne.12548. doi: 10.1111/jne.12548
Thauer, R. K., Jungermann, K., Decker, K. (1977). Energy Conservation in Chemotrophic Anaerobic Bacteria. Bacteriol. Rev. 41 (1), 100–180. doi: 10.1128/br.41.1.100-180.1977
Theaker, E. D., Drucker, D. B., Gibbs, A. C. C. (1993). The Possible Influence of the Menstrual Cycle on the Adherence of Candida Albicans to Human Buccal Epithelial Cells In Vitro. Arch. Oral. Biol. 38 (4), 353–355. doi: 10.1016/0003-9969(93)90143-A
Varadan, M., Ramamurthy, J. (2015). Association of Periodontal Disease and Pre-Term Low Birth Weight Infants. J. Obstet. Gynaecol. India 65 (3), 167–171. doi: 10.1007/s13224-014-0581-9
Vining, R. F., McGinley, R. A., Symons, R. G. (1983). Hormones in Saliva: Mode of Entry and Consequent Implications for Clinical Interpretation. Clin. Chem. 29 (10), 1752–1756. doi: 10.1093/clinchem/29.10.1752
Vom Steeg, L. G., Klein, S. L. (2017). Sex Steroids Mediate Bidirectional Interactions Between Hosts and Microbes. Horm. Behav. 88, 45–51. doi: 10.1016/j.yhbeh.2016.10.016
Wendland, N., Opydo-Szymaczek, J., Mizgier, M., Jarząbek-Bielecka, G (2020). Subgingival Microflora in Adolescent Females With Polycystic Ovary Syndrome and its Association With Oral Hygiene, Gingivitis, and Selected Metabolic and Hormonal Parameters. Clin. Oral. Investig. 25 (3), 1485–1496. doi: 10.1007/s00784-020-03456-5
White, S., Larsen, B. (1997). Candida Albicans Morphogenesis is Influenced by Estrogen. Cell. Mol. Life Sci. CMLS 53 (9), 744–749. doi: 10.1007/s000180050094
Winter, J., Bokkenheuser, V. D. (1987). Bacterial Metabolism of Natural and Synthetic Sex Hormones Undergoing Enterohepatic Circulation. J. Steroid Biochem. 27 (4-6), 1145–1149. doi: 10.1016/0022-4731(87)90201-9
Yokoyama, M., Hinode, D., Masuda, K., Yoshioka, M., Grenier, D. (2005). Effect of Female Sex Hormones on Campylobacter Rectus and Human Gingival Fibroblasts. Oral. Microbiol. Immunol. 20 (4), 239–243. doi: 10.1111/j.1399-302X.2005.00222.x
Zhang, X., Essmann, M., Burt, E. T., Larsen, B. (2000). Estrogen Effects on Candida Albicans: A Potential Virulence-Regulating Mechanism. J. Infect. Dis. 181 (4), 1441–1446. doi: 10.1086/315406
Ziskin, D. E. (1938). Effects of Certain Hormones on Gingival and Oral Mucous Membranes. J. Am. Dental Assoc. Dental Cosmos. 25 (3), 422–426. doi: 10.14219/jada.archive.1938.0375
Keywords: sex steroid hormones, host-microbiome interactions, microbial endocrinology, oral microbiome, oral bacteria and fungi
Citation: Cornejo Ulloa P, Krom BP and van der Veen MH (2021) Sex Steroid Hormones as a Balancing Factor in Oral Host Microbiome Interactions. Front. Cell. Infect. Microbiol. 11:714229. doi: 10.3389/fcimb.2021.714229
Received: 24 May 2021; Accepted: 13 September 2021;
Published: 29 September 2021.
Edited by:
Natarajaseenivasan Kalimuthusamy, Bharathidasan University, IndiaReviewed by:
Woojung Shin, Wyss Institute for Biologically Inspired Engineering and Harvard Medical School, United StatesCopyright © 2021 Cornejo Ulloa, Krom and van der Veen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monique H. van der Veen, bS52ZC52ZWVuQGFjdGEubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.