
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 19 August 2021
Sec. Clinical Microbiology
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.704169
Background: Diagnosis of Mycobacterium tuberculosis (MTB) infection can be confirmed by Xpert assays within hours. However, when sample size does not allow performing both culture and Xpert, or if Xpert is negative, then formal diagnosis of MTB relies on culture and time to detection of growth (TDG) becomes critical for clinical management.
Objectives: To determine TDG in Xpert negative samples, or in samples in which Xpert could not be performed, in a low-incidence area for MTB.
Methods: Retrospective analysis (2015-2020) of a database including all cultures for mycobacteria in a University Hospital covering approximately 500’000 inhabitants. Analysis was restricted to culture positive (C+) samples for MTB for which 1/Xpert was negative or could not be performed because of limited sample volume, and 2/collected from subjects treated less than 24 hours. TDG was analyzed according to microscopy, origin of sample (pulmonary or not) and presence of cavitation.
Results: Among 837 C+ samples for MTB, 236 samples (80% of respiratory origin) from 147 patients fulfilled study criteria; 78 samples (49 patients, 33%) were acid-fast bacilli (AFB) positive. Median (IQR) TDG was 25 (17; 40) days for all samples. TDG exceeded 28 days in 43% of samples and was significantly shorter in AFB+ vs AFB- samples, and samples from cavitary vs non cavitary or extra-thoracic disease.
Conclusions: In Xpert negative samples, or samples for which Xpert could not be performed, TDG exceeded 4 weeks in 43% of samples. AFB+ and samples from cavitary lung disease had a significantly shorter TDG.
Since the publication by Boehme et al. of the contribution of rapid molecular diagnostic techniques for detecting TB (referred to as NAAT: Nuclear acid amplification techniques), and their rapid implementation worldwide, the diagnosis of tuberculosis has changed dramatically (Boehme et al., 2010). NAAT have not only increased the analytical sensitivity as compared to microscopy (auramine stain and Ziehl-Neelsen for acid-fast bacilli: AFB): they allow a positive diagnosis of tuberculosis within hours, while requiring only basic levels of laboratory skills. Multicentric studies in high prevalence areas show that NAAT allow early identification in approximately 80% of all culture positive TB cases irrespective of microscopy (Theron et al., 2014; Calligaro et al., 2017). Boehme et al. showed in a large multicentric field study that Xpert/MTB-RIF could detect 90.3% of culture positive samples (76.9% in smear negative samples) (Boehme et al., 2011). Because of the low skills and the minimal hands-on time involved, there is a trend towards favoring the use of NAAT instead of microscopy: indeed, in some centers, microscopy for detecting AFB may not always be available, or not on the spot. The present contribution of microscopy is mainly to assess risk of transmission, a role that may in a near future, be replaced by sensitive quantitative PCR assays.
In patients with clinical suspicion of MTB, a certain number of cases remain NAAT negative (or samples are insufficient to perform NAAT and culture): in these cases, diagnosis relies on a positive identification by culture, since, in low incidence settings, microscopy alone cannot formally discriminate between MTB and nontuberculous mycobacteria (NTM). Time to detection of growth (TDG) in liquid and/or solid media is then critical to determine when a case of TB can either be confirmed or reasonably excluded.
While time to culture conversion (i.e.: time to negative culture after initiating tuberculostatic treatment) is frequently reported and used as a surrogate marker for response to treatment (Olaru et al., 2014), recent data concerning TDG in samples taken before initiation of treatment are scarce. TDG is inversely correlated with number of acid-fast bacilli (AFB) on sputum smears (Perrin et al., 2007; Bark et al., 2011; Olaru et al., 2014), number of colony forming units in sputum specimens (Diacon et al., 2014) and risk of relapse after therapy in clinical trials (Bark et al., 2012). Baseline TDG is also correlated to time to conversion (Visser et al., 2012) and inversely related to transmissibility (Khan et al., 2018). TDG in treatment-naïve subjects are, as expected, lower in smear positive and NAAT positive subjects (Khan et al., 2018) with median values ranging between 5 (Bisognin et al., 2020; Ma et al., 2020) and 23 days (Boehme et al., 2011; Bark et al., 2012; Pfyffer and Wittwer, 2012; Tyrrell et al., 2012; Visser et al., 2012; Olaru et al., 2014; Ogwang et al., 2015; Diriba et al., 2017; Khan et al., 2018; Zhao et al., 2019). A few recent studies suggest that, in the majority of clinical samples, growth of MTB could be detected within 28 days of incubation (Pfyffer and Wittwer, 2012; Ogwang et al., 2015). A US multicentric study reported a median TDG of 14 days (using the Mycobacterium Growth Indicator Tube (MGIT) system, Becton Dickinson, Allschwil, Switzerland) with a 100% detection of all positive samples within 28 days, thus suggesting that there was no additional benefit of growing MTB on solid or liquid media for more than 5 weeks (Tyrrell et al., 2012).
The aims of this study were: 1/to quantify, in a real-life low TB incidence clinical setting, time to detection of growth for MTB and 2/to determine if 4 weeks were sufficient to detect all or most MTB growth in patients for whom a diagnosis could not be established by Xpert/MTB-RIF assays. Because of the systematic implementation of Xpert techniques in our area, we restricted our analysis to culture positive clinical samples identified as MTB for which 1/Xpert/MTB-RIF was negative or could not be performed because of limited sample volume, and 2/collected from subjects either naïve to tuberculostatic drugs or treated for less than 24 hours. Duration of TDG was analyzed according to results of microscopy when performed, site of infection and presence of cavitation in pulmonary TB.
This study was performed at Geneva University Hospitals (HUG), a 2008-bed complex providing care for a population of approximately 500’000 inhabitants. Switzerland has a low incidence for TB (7/105 inhabitants). All clinical samples sent for microbiological analysis within HUG – whether provided by inpatients or outpatients - are treated only by the Bacteriology Laboratory of HUG. An online database is kept up-to-date and includes all samples processed by the laboratory.
We analyzed all clinical samples with positive cultures for mycobacteria included in this database over a 5-year period (2015-2020). This report focuses only on mycobacteria belonging to the M. tuberculosis complex (MTB).
The following items were recorded from the electronic patient files: age, gender, site of infection (when pulmonary, we recorded presence of cavitation), presence and type of immunosuppression, relevant comorbidities, results of microscopy, and MTB sub-species identified. Details of treatment and treatment outcome are not reported.
The study protocol was approved by our local ethics committee (CCER 2020-00872) and registered at Clinicaltrials.gov (NCT04718571).
Samples sent for detection of mycobacteria are processed as follows, according to WHO/CDC guidelines:
Culture is favored over direct testing (PCR, microscopy) whenever the sample volume is limited. When the sample volume is sufficient, direct testing is performed using the Xpert MTB/RIF ULTRA on the GeneXpert System (Cepheid) for the detection of Mycobacterium tuberculosis and of potential mutations in the rpoB gene conferring rifampin-resistance. Likewise, a direct microscopic examination is performed using first an auramine based stain followed by a Ziehl-Neelsen stain. Cultivation of samples is performed on a combination of liquid and solid media: samples are inoculated into Mycobacterium Growth Indicator Tube (MGIT) bottles (MGIT 960, Becton Dickinson, Allschwil, Switzerland) and on the following two solid media: Coletsos (Bio-Rad, Marnes-la-Coquette, France) and Loewenstein-Jensen (Becton Dickinson), all of which are incubated for 12 weeks. Whenever positive, the supernatant of the MGIT bottle is subjected to an immunochromatographic assay detecting protein MPT64 (Becton Dickinson), as a surrogate marker for the identification of Mycobacterium tuberculosis. Supernatant is also inoculated on two separate solid media: Coletsos (bioRad) and Loewenstein-Jensen (Becton Dickinson), as described above, for further characterization.
Solid cultures permit further identification by using the Xpert MTB/RIF ULTRA for detecting M. tuberculosis or by rpoB gene sequencing for the identification of NTM.
Samples sent for culture of mycobacteria came from inpatient and outpatient hospital sites, all within HUG. Bronchial aspirates and bronchoalveolar lavages are also routinely cultivated for mycobacteria in our institution, irrespective of whether there is or not a clinical suspicion of tuberculosis or NTM infection.
Were excluded from the analysis: 1/external quality control strains; 2/all positive cultures for NTM; 3/all samples for which Xpert MTB/RIF ULTRA was positive for M. tuberculosis complex; 4/all samples collected more than 24 hours after introduction of anti-TB treatment.
Thus, we included only: samples with 1/a positive culture for M. tuberculosis complex, and 2/either a negative or no Xpert/MTB-RIF, irrespectively of direct microscopy.
Descriptive data are reported for patient characteristics, type and origin of samples, and results of microscopy according to clinical presentation. Quantitative variables were described as median and interval interquartile (IQR) and qualitative variables were described as counts, percentages.
Time to detection of growth (TDG) is reported as the time between inoculation to liquid and solid media, and first signal of growth. TDG was described overall, according to results of microscopy (presence or absence of AFB) and according to case presentation (cavitary vs. non cavitary pulmonary tuberculosis, vs. extra-pulmonary tuberculosis). Descriptive statistics of TDG included mean (SD), median (IIQ), 90th centile, and counts and percentages of samples for which TDG was > 28 days. Distribution of TDG was represented using Empirical cumulative distribution function (ECDF) plots and compared between groups (positive vs. negative microscopy findings; cavitary vs. non cavitary pulmonary tuberculosis vs. extra-thoracic tuberculosis) using linear regression models (samples were considered as independent observations).
Statistical significance was assessed at the two-sided 0.05 level for all analyses.
All analyses were performed using R software version R-4.0.2 (R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
A total of 236 samples were analyzed, originating from 147 patients (Figure 1). Number of samples per patient ranged from 1 to 5 (Mean: 1.6; SD: 0.9). All strains were identified as M. tuberculosis except for one M. africanum, and four M. bovis.
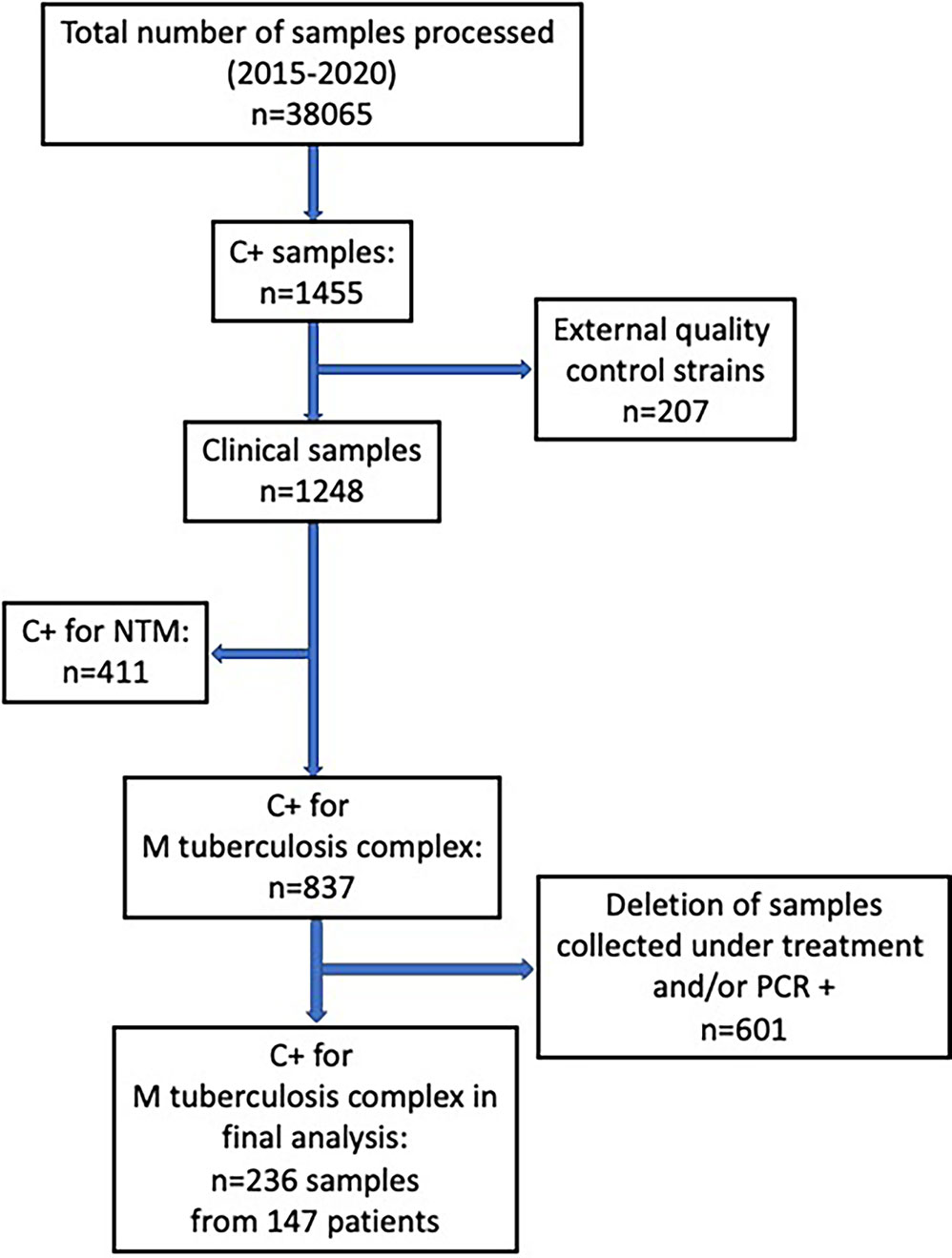
Figure 1 Flow-chart of samples analyzed from the database of all positive culture results for mycobacteria at our institution over a 5-year period (May 2015- May 2020).
Xpert was performed on 96 samples (41%). Of all samples in which Xpert was performed (and negative), 6/96 showed AFB on microscopic examination (all confirmed as M. tuberculosis by culture, all of pulmonary origin) yielding 6.3% of false negative Xpert results.
Table 1 shows baseline characteristics of the study population, main site of infection, comorbidities, factors of immunosuppression, origin, and resistance profile to first line tuberculostatic drugs. Samples came from a relatively young population, mostly of foreign origin (only 24% were from Western Europe including Switzerland) with few comorbidities, or factors of immunosuppression. Isolated resistance to tuberculostatic drugs or MDR strains were rare (n=14, 9%).
Table 2 shows the source of the 236 samples: 80% of all samples (n=189) were of respiratory origin, and 20% (n=47) were extra-pulmonary. Microscopy was performed on 230 samples (97.5%) and was positive for AFB on 78 samples (34%), and negative on 152 samples (66%); in 6 cases microscopy was not performed due to insufficient sample volume (Table 3).
For all samples, median (IQR) time to detection of growth (TDG) of M tuberculosis complex was 25 days (IQR: 17; 40); range: 6-129 days; 90th centile: 55 days. For 101 samples (43%), time to detection of growth exceeded 28 days. Table 4 provides TDG according to result of clinical presentation, and microscopy. Figure 2 shows TDG distribution according to A) case presentation (cavitary vs. non cavitary pulmonary tuberculosis, vs. extra-thoracic tuberculosis, p<0.001) and B) microscopy (positive i.e. presence of acid-fast bacilli (AFB), vs negative: absence of AFB, p>0.001). Figure 3 shows TDG distribution according to both items combined.
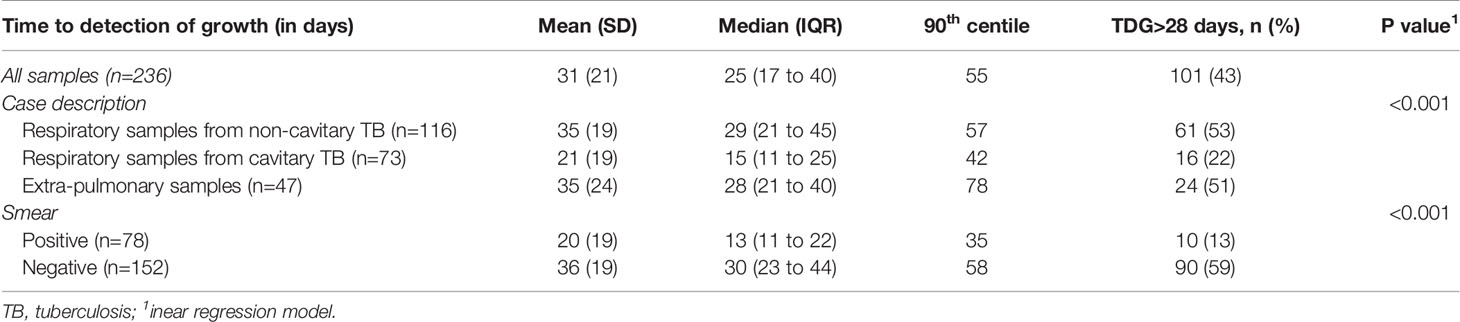
Table 4 Time to detection of growth (TDG) according to results of microscopy and clinical presentation.
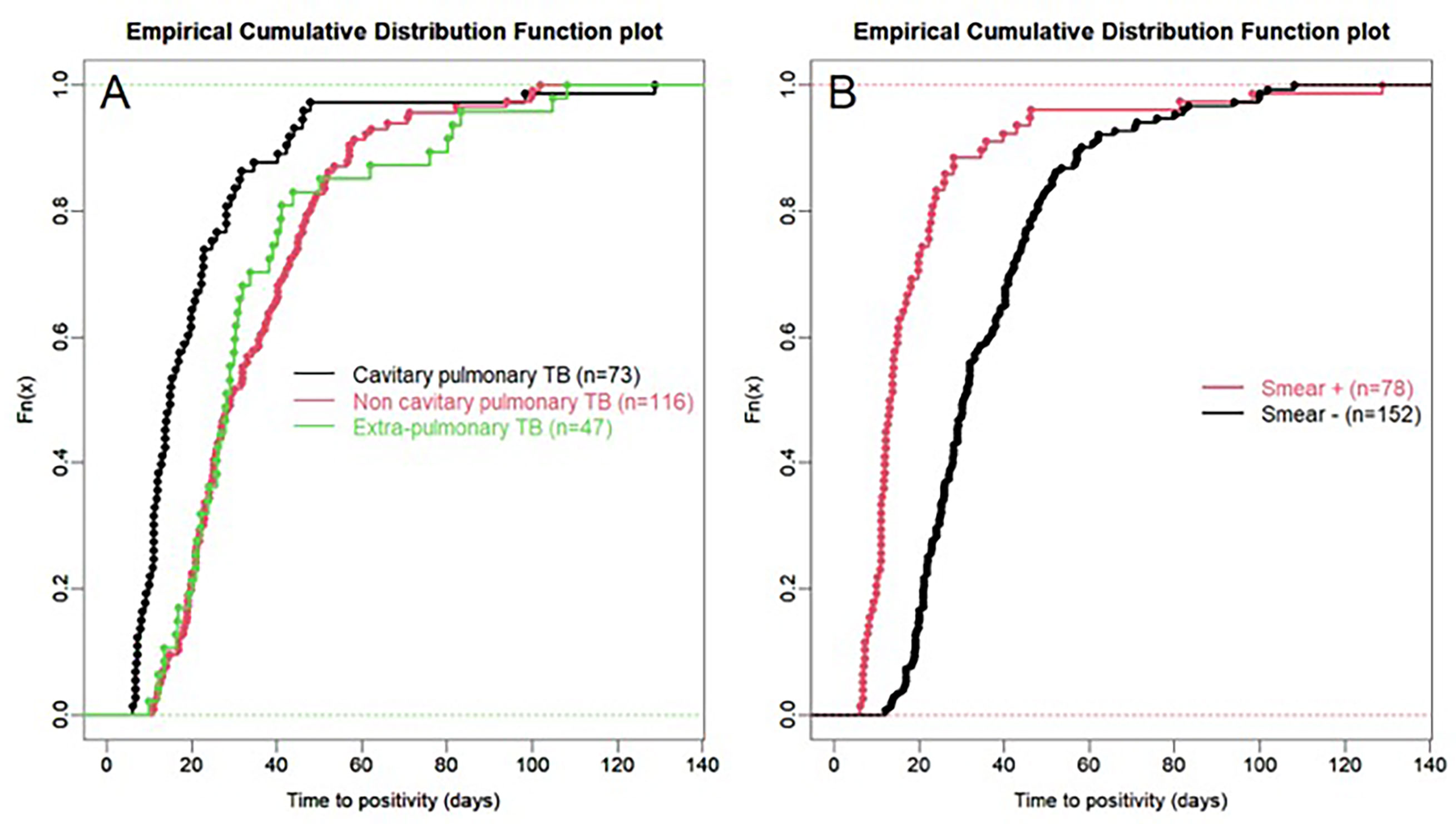
Figure 2 (A) Empirical cumulative distribution function plot of time to detection of growth in samples according to case presentation: cavitary vs. non-cavitary pulmonary tuberculosis vs. extra-pulmonary tuberculosis. (B) Empirical cumulative distribution function plot of time to detection of growth in samples according to microscopy (positive i.e. presence of acid-fast bacilli (AFB), vs negative: absence of AFB) irrespective of case presentation. All samples are PCR negative.
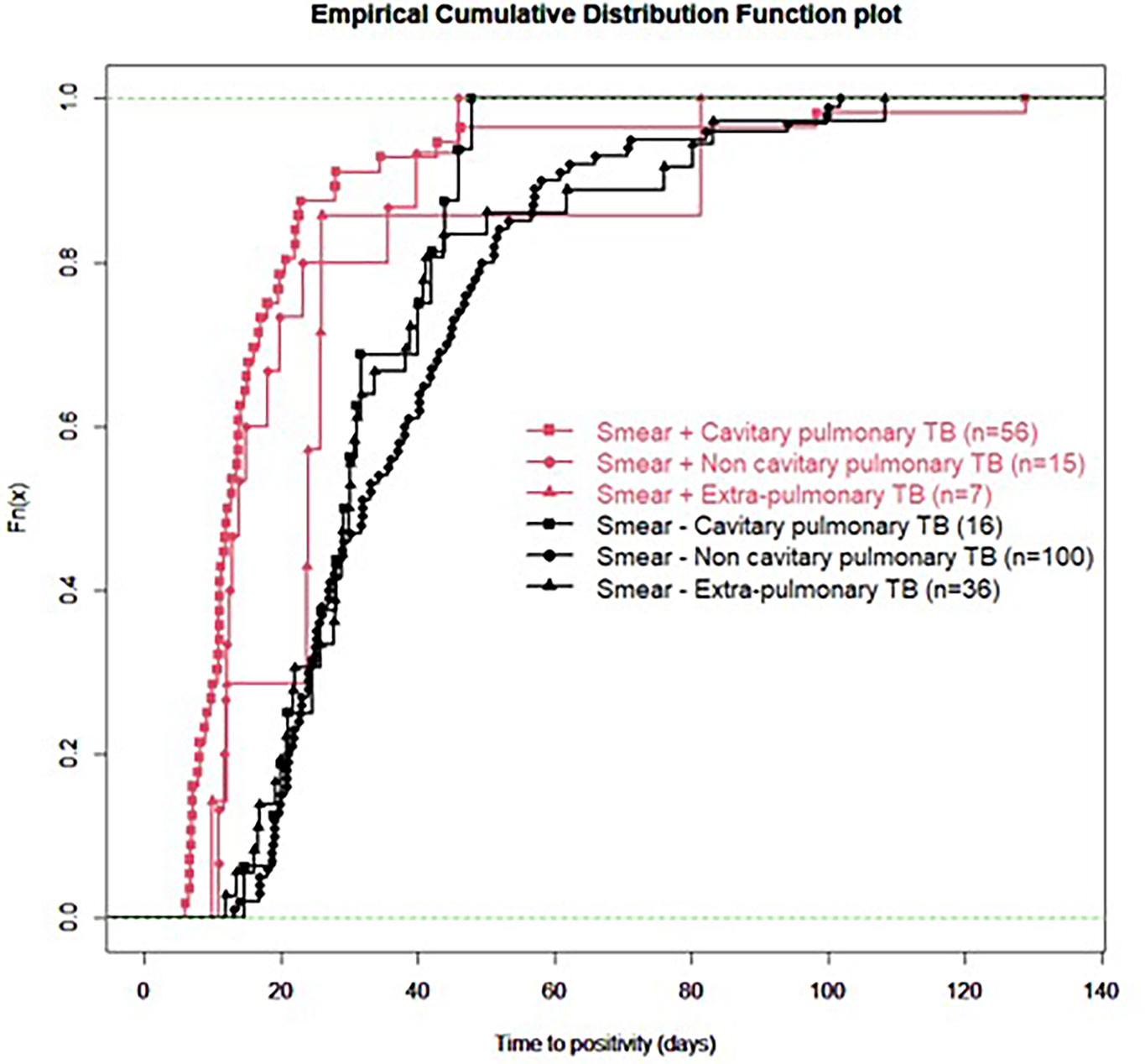
Figure 3 Empirical cumulative distribution function plot of time to detection of growth in samples according to microscopy (positive i.e. presence of acid-fast bacilli (AFB), vs negative: absence of AFB) and clinical presentation (cavitary vs. non-cavitary pulmonary tuberculosis vs. extra-pulmonary tuberculosis). All samples are PCR negative.
In this retrospective study, we aimed to establish, in a real-life setting, what was the time to detection of growth (TDG) in liquid and/or solid growth media for M. tuberculosis complex for clinical samples which were either Xpert negative or for which Xpert could not be performed because of sample size and/or quality. We excluded from our analyses samples from patients who had received tuberculostatic treatment for more than 24 hours to minimize impact of drugs on TDG. Our results show that in this specific group of clinical samples, median TDG was 25 (IQR: 17; 40) days. In 43% of samples studied, TDG exceeded 28 days. Cavitation, pulmonary vs extra-pulmonary samples and presence of acid-fast bacilli (AFB) on microscopy had a significant impact on TDG (Figures 2, 3, Table 4).
The rationale of this selection of samples was as follows: 1/large scale implementation of Xpert/MTB-RIF has increased same-day diagnosis, with a diagnostic sensitivity exceeding 80% in culture positive samples, irrespective of microscopy (Boehme et al., 2011; Khan et al., 2018); 2/in patients who do not have a same-day diagnosis by Xpert/MTB-RIF (negative test or not performed because of sample size), and who will eventually become culture positive, TDG determines when a diagnosis MTB can either be confirmed (as well as drug sensitivity profile), or reasonably excluded (treatment will most often have been started based on clinical suspicion). Our focus was therefore on TDG for all culture positive clinical samples fulfilling these criteria.
In this study, TDG exceeded the 4-5 week duration reported by several authors as sufficient for identifying all culture positive samples (Pfyffer and Wittwer, 2012; Tyrrell et al., 2012; Ogwang et al., 2015; Khan et al., 2018). Indeed, 90th centile for TDG of all samples was 55 days, and even 58 days in smear negative cases. There are several possible explanations for this. Firstly, both presence of AFB on microscopy and positive Xpert assay results are suggestive of a higher organism burden, which is associated with a shorter TDG (Tyrrell et al., 2012; Visser et al., 2012; Diriba et al., 2017). In this study, all samples were all either Xpert/MTB-RIF negative, or could not be processed for Xpert/MTB-RIF; furthermore, 64% of all samples did not show AFB on microscopic examination. This suggests that the samples selected probably had a low organism burden, after excluding all cases for which a rapid diagnosis had been provided by Xpert/MTB-RIF. Secondly, 20% of all samples were extra-pulmonary: these samples have a lower number of bacilli, which can impact on TDG (Mazza-Stalder et al., 2012). This selection was deliberate, to conform with the diagnostic process since Xpert/MTB-RIF systems are systematically used. Our data do not support therefore the assumption that 4-5 weeks suffice to exclude the possibility of a positive culture: when empirical treatment is initiated, and if being culture negative is decisive to interrupt the treatment, then treatment should be pursued for at least 2 months before considering interrupting tuberculostatic drugs.
The fact that 33% of all samples (and patients) were smear positive for AFB could be considered as a confirmation of MTB. However, we did not delete these samples from our analyses for the following reasons. On-the spot immediate microscopy (auramine and Ziehl-Neelsen stains for AFB) is not available in all centers, and requires expertise, as opposed to Xpert assays. Also, in a low incidence country such as Switzerland (7/105 inhabitants), with NTM cases being more and more frequently identified in clinical practice as in neighbor countries (Hoefsloot et al., 2013; Ringshausen et al., 2016; Vongthilath-Moeung et al., 2021), presence of AFB cannot be considered as diagnostic of MTB although it does increase the probability of MTB. Our analyses reflect a pragmatic approach in which microscopy may in a near future play a decreasing role in diagnosing MTB.
The present data show very clearly that TDG is significantly longer in AFB negative vs positive samples (Median, IQR: 30 (23; 44) vs 13 (11; 22) days), and 59% of AFB negative samples had a TDG > 28 days. Similar differences were found between samples from cavitary pulmonary TB vs samples from non-cavitary or extra-thoracic TB (Table 4 and Figures 2, 3). TDG for samples from non-cavitary or extra-thoracic TB were similar. This information is therefore crucial for clinicians to anticipate TDG.
This study has a few limitations. Firstly, it is based on a retrospective analysis of laboratory data, and although the data are comprehensive and include all samples processed during the 5-year period defined, recording errors cannot be excluded. Secondly, we do not know how many samples were sent per patient all together, and how many negative results occurred for each patient, and thus we can only comment on TDG in culture positive samples with little or no impact of tuberculostatic treatment. This is a sample-based and not a patient-based analysis. Data provided suggest that, if Xpert/MTB-RIF is negative or unavailable, 43% of samples collected within the first 24 hours of an empirical treatment will become positive after more than 4 weeks. It will be important through future studies to determine at a national and local level what is the present percentage of cases which are diagnosed only by culture or treated without bacteriological confirmation in the NAAT/Xpert era as compared to data provided for 2005-2011 (Publique OFdlS, 2013). Thirdly, because of the restrictive design of our study, sample size is limited. Finally, these results are representative of TDG in a specific setting: the organism burden is dependent on local epidemiology, socio-economic status of the population, health care system, access to care and average time to diagnosis (Auer et al., 2018).
This study does not corroborate previous observations suggesting that the vast majority of clinical samples cultures for mycobacteria become positive after 4 weeks. In fact, in a high proportion of samples (43%), TDG exceeded 4 weeks. It must however be taken into account that we restricted our analysis clinical samples for which Xpert was either negative or unavailable, for clinical relevance. Case selection thus differs from previous studies. Based on this observation, when a presumptive diagnosis of TB cannot confirmed within hours by NAAT, it is mandatory to wait for at least 8 weeks before reasonably considering cultures as negative. Identification of AFB, cavitation, having an extra-thoracic vs pulmonary form of TB, and the combination of these elements all affect significantly TDG.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study protocol was approved by the ethics committee of the Geneva University Hospitals (CCER 2020-00872) and registered at Clinicaltrials.gov (NCT04718571). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
RV-M was implicated in the conception and design of the study, data processing, writing and revision of the manuscript; she approved the final version and agrees to be accountable for all aspects of the work including its accuracy and integrity. AP was implicated in the conception and design of the study, data processing, methodological support and statistics, writing and revision of the manuscript; he approved the final version and agrees to be accountable for all aspects of the work including its accuracy and integrity. GR was implicated in the conception and design of the study, data processing, writing and revision of the manuscript; he approved the final version and agrees to be accountable for all aspects of the work including its accuracy and integrity. JS was implicated in the conception and design of the study, data processing, writing and revision of the manuscript; he approved the final version and agrees to be accountable for all aspects of the work including its accuracy and integrity. J-PJ was implicated in the conception and design of the study, data processing, writing and revision of the manuscript; he approved the final version and agrees to be accountable for all aspects of the work including its accuracy and integrity. All authors contributed to the article and approved the submitted version.
This work was supported by the Pulmonary League of Geneva, a non-profit health-care provider involved in patients with chronic pulmonary disorders and mycobacterial infection.
None of the authors received any financial support. The Pulmonary League of Geneva was not implicated in the conception, content or writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Auer, C., Kiefer, S., Zuske, M., Schindler, C., Wyss, K., Blum, J., et al. (2018). Health-Seeking Behaviour and Treatment Delay in Patients With Pulmonary Tuberculosis in Switzerland: Some Slip Through the Net. Swiss. Med. Wkly. 148, w14659. doi: 10.4414/smw.2018.14659
Bark, C. M., Okwera, A., Joloba, M. L., Thiel, B. A., Nakibali, J. G., Debanne, S. M., et al. (2011). Time to Detection of Mycobacterium tuberculosis as an Alternative to Quantitative Cultures. Tuberc. (Edinb.) 91 (3), 257–259. doi: 10.1016/j.tube.2011.01.004
Bark, C. M., Thiel, B. A., Johnson, J. L. (2012). Pretreatment Time to Detection of Mycobacterium tuberculosis in Liquid Culture is Associated With Relapse After Therapy. J. Clin. Microbiol. 50 (2), 538. doi: 10.1128/JCM.06193-11
Bisognin, F., Lombardi, G., Lombardo, D., Re, M. C., Dal Monte, P. (2020). Comparison of MycoPrep and the New MYCO-TB Kit: Rapid and Efficient Digestion and Decontamination of Respiratory Specimens for the Detection of Mycobacteria. New Microbiol. 43 (1), 13–16.
Boehme, C. C., Nabeta, P., Hillemann, D., Nicol, M. P., Shenai, S., Krapp, F., et al. (2010). Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N. Engl. J. Med. 363 (11), 1005–1015. doi: 10.1056/NEJMoa0907847
Boehme, C. C., Nicol, M. P., Nabeta, P., Michael, J. S., Gotuzzo, E., Tahirli, R., et al. (2011). Feasibility, Diagnostic Accuracy, and Effectiveness of Decentralised Use of the Xpert MTB/RIF Test for Diagnosis of Tuberculosis and Multidrug Resistance: A Multicentre Implementation Study. Lancet 377 (9776), 1495–1505. doi: 10.1016/S0140-6736(11)60438-8
Calligaro, G. L., Zijenah, L. S., Peter, J. G., Theron, G., Buser, V., McNerney, R., et al. (2017). Effect of New Tuberculosis Diagnostic Technologies on Community-Based Intensified Case Finding: A Multicentre Randomised Controlled Trial. Lancet Infect. Dis. 17 (4), 441–450. doi: 10.1016/S1473-3099(16)30384-X
Diacon, A. H., van der Merwe, L., Demers, A. M., von Groote-Bidlingmaier, F., Venter, A., Donald, P. R. (2014). Time to Positivity in Liquid Culture Predicts Colony Forming Unit Counts of Mycobacterium tuberculosis in Sputum Specimens. Tuberc. (Edinb.) 94 (2), 148–151. doi: 10.1016/j.tube.2013.12.002
Diriba, G., Kebede, A., Yaregal, Z., Getahun, M., Tadesse, M., Meaza, A., et al. (2017). Performance of Mycobacterium Growth Indicator Tube BACTEC 960 With Lowenstein-Jensen Method for Diagnosis of Mycobacterium tuberculosis at Ethiopian National Tuberculosis Reference Laboratory, Addis Ababa, Ethiopia. BMC Res. Notes 10 (1), 181. doi: 10.1186/s13104-017-2497-9
Hoefsloot, W., van Ingen, J., Andrejak, C., Angeby, K., Bauriaud, R., Bemer, P., et al. (2013). The Geographic Diversity of Nontuberculous Mycobacteria Isolated From Pulmonary Samples: An NTM-NET Collaborative Study. Eur. Respir. J. 42 (6), 1604–1613. doi: 10.1183/09031936.00149212
Khan, S., Nakasone, A., Ghajar, M., Zhowandai, M., Prabhu, S., Alexander, R., et al. (2018). Time to Detection in Culture Supports Prediction of Low Transmissibility of Tuberculosis and Discontinuation of Isolation for Low-Risk Patients With A Single AFB-Negative and NAAT-Negative Respiratory Specimen. Infect. Control Hosp. Epidemiol. 39 (5), 619–621. doi: 10.1017/ice.2018.37
Ma, Y., Fan, J., Li, S., Dong, L., Li, Y., Wang, F., et al. (2020). Comparison of Lowenstein-Jensen Medium and MGIT Culture System for Recovery of Mycobacterium tuberculosis From Abscess Samples. Diagn. Microbiol. Infect. Dis. 96 (4), 114969. doi: 10.1016/j.diagmicrobio.2019.114969
Mazza-Stalder, J., Nicod, L., Janssens, J. P. (2012). Extrapulmonary Tuberculosis. Rev. Mal. Respir. 29 (4), 566–578. doi: 10.1016/j.rmr.2011.05.021
Office fédéral de la Santé Publique (Federal Office of Public Health) (2013). La Tuberculose En Suisse, 2005-2011 (Etat Des Données: 17 Octobre 2012); Tuberculosis in Switzerland, 2005-2011; Situation Up to October 17, 2012. Bulletin de l’OFSP, Vol. 21). 343–353.
Ogwang, S., Mubiri, P., Bark, C. M., Joloba, M. L., Boom, W. H., Johnson, J. L. (2015). Incubation Time of Mycobacterium tuberculosis Complex Sputum Cultures in BACTEC MGIT 960: 4weeks of Negative Culture Is Enough for Physicians to Consider Alternative Diagnoses. Diagn. Microbiol. Infect. Dis. 83 (2), 162–164. doi: 10.1016/j.diagmicrobio.2015.07.002
Olaru, I. D., Heyckendorf, J., Grossmann, S., Lange, C. (2014). Time to Culture Positivity and Sputum Smear Microscopy During Tuberculosis Therapy. PloS One 9 (8), e106075. doi: 10.1371/journal.pone.0106075
Perrin, F. M., Lipman, M. C., McHugh, T. D., Gillespie, S. H. (2007). Biomarkers of Treatment Response in Clinical Trials of Novel Antituberculosis Agents. Lancet Infect. Dis. 7 (7), 481–490. doi: 10.1016/S1473-3099(07)70112-3
Pfyffer, G. E., Wittwer, F. (2012). Incubation Time of Mycobacterial Cultures: How Long is Long Enough to Issue a Final Negative Report to the Clinician? J. Clin. Microbiol. 50 (12), 4188–4189. doi: 10.1128/JCM.02283-12
Ringshausen, F. C., Wagner, D., de Roux, A., Diel, R., Hohmann, D., Hickstein, L., et al. (2016). Prevalence of Nontuberculous Mycobacterial Pulmonary Disease, Germany, 2009-2014. Emerg. Infect. Dis. 22 (6), 1102–1105. doi: 10.3201/eid2206.151642
Theron, G., Zijenah, L., Chanda, D., Clowes, P., Rachow, A., Lesosky, M., et al. (2014). Feasibility, Accuracy, and Clinical Effect of Point-of-Care Xpert MTB/RIF Testing for Tuberculosis in Primary-Care Settings in Africa: A Multicentre, Randomised, Controlled Trial. Lancet. 383 (9915), 424–435. doi: 10.1016/S0140-6736(13)62073-5
Tyrrell, F. C., Budnick, G. E., Elliott, T., Gillim-Ross, L., Hildred, M. V., Mahlmeister, P., et al. (2012). Probability of Negative Mycobacterium tuberculosis Complex Cultures Based on Time to Detection of Positive Cultures: A Multicenter Evaluation of Commercial-Broth-Based Culture Systems. J. Clin. Microbiol. 50 (10), 3275–3282. doi: 10.1128/JCM.01225-12
Visser, M. E., Stead, M. C., Walzl, G., Warren, R., Schomaker, M., Grewal, H. M., et al. (2012). Baseline Predictors of Sputum Culture Conversion in Pulmonary Tuberculosis: Importance of Cavities, Smoking, Time to Detection and W-Beijing Genotype. PloS One 7 (1), e29588. doi: 10.1371/journal.pone.0029588
Vongthilath-Moeung, R., Plojoux, J., Poncet, A., Renzi, G., Veziris, N., Schrenzel, J., et al. (2021). Nontuberculous Mycobacteria Under Scrutiny in the Geneva Area (2015-2020) Respiration, accepted for publication.
Keywords: mycobacteria, Mycobacterium tuberculosis, time to detection of growth, mycobacterium growth indicator tube, nuclear acid amplification techniques
Citation: Vongthilath-Moeung R, Poncet A, Renzi G, Schrenzel J and Janssens J-P (2021) Time to Detection of Growth for Mycobacterium tuberculosis in a Low Incidence Area. Front. Cell. Infect. Microbiol. 11:704169. doi: 10.3389/fcimb.2021.704169
Received: 30 May 2021; Accepted: 04 August 2021;
Published: 19 August 2021.
Edited by:
Alexandra Aubry, Sorbonne Universités, FranceReviewed by:
Tuhina Banerjee, Banaras Hindu University, IndiaCopyright © 2021 Vongthilath-Moeung, Poncet, Renzi, Schrenzel and Janssens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Paul Janssens, amVhbi1wYXVsLmphbnNzZW5zQGhjdWdlLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.