
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 15 July 2021
Sec. Fungal Pathogenesis
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.693522
This article is part of the Research Topic Fungal diagnostics: culture, molecular, mass spectrometry View all 6 articles
 Samaneh Halvaee1
Samaneh Halvaee1 Roshanak Daie-Ghazvini1*
Roshanak Daie-Ghazvini1* Seyed Jamal Hashemi1,2
Seyed Jamal Hashemi1,2 Sadegh Khodavaisy1
Sadegh Khodavaisy1 Abbas Rahimi-Foroushani3
Abbas Rahimi-Foroushani3 Heidar Bakhshi1
Heidar Bakhshi1 Zahra Rafat4
Zahra Rafat4 Pegah Ardi1
Pegah Ardi1 Mahdi Abastabar5
Mahdi Abastabar5 Mahdi Zareei1
Mahdi Zareei1 Zeinab Borjian-Boroujeni1
Zeinab Borjian-Boroujeni1 Hasti Kamali Sarvestani1
Hasti Kamali Sarvestani1Background: Onychomycosis is one of the most common and recurrent dermatological diseases worldwide. The antimycotic activity of prescribed medications varies according to the causative agents, and treatment failure rates exceeding 30%. This study aimed to assess the epidemiological profile of onychomycosis in Iran. Also, the susceptibilities to conventional and new antifungals were investigated.
Methods: In this descriptive cross-sectional study, during the period of 18 months starting from September 2019 until March 2020, 594 nail specimens were obtained from patients who presented nail changes compatible with a clinical diagnosis of onychomycosis. The patients were referred from different cities, including Tehran, Kermanshah, Arak, Kashan, Rasht, Qom, Urmia, Zahedan, Hamadan, Zanjan, Borujerd, Bushehr, and Yazd. All the samples were subjected to microscopic examination and fungal culture. Fungi identified were confirmed through the PCR-sequencing method. The susceptibility to itraconazole, fluconazole, terbinafine, griseofulvin, posaconazole, ravuconazole, efinaconazole, luliconazole, and tavaborole was evaluated according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, document M38-A2 for filamentous fungi, and document M27-A3 for yeasts.
Results: 594 patients were included. Of these, in 179 cases (30.1%) (95% CI:0.3 ± 0.037) onychomycosis was confirmed. The majority of patients were ≥ 60 years of age (n=58, 32.6%) and female (n=113, 63.1%). Saprophytic fungi accounted for the vast majority of the nail isolates (n=92, 51.4%) (95% CI:0.051 ± 0.0.073), followed by dermatophytes (n=45, 25.1%) (95% CI:0.25 ± 0.063), and yeasts (n=42, 23.5%) (95% CI:0.23 ± 0.061). Diabetes mellitus (77.3%), hypothyroidism (18.2%), and solid tumors (4.5%) were documented as the most prevalent underlying conditions. Antifungal susceptibility testing was performed against 60 fungal isolates (20 each of Candida species, saprophytic fungi, and dermatophytes). Efinaconazole, ravuconazole, and luliconazole were the most active agents against Candida species. Also, luliconazole, posaconazole, and efinaconazole were most potent against dermatophytes. Luliconazole had the greatest antifungal activity against saprophytic fungi.
Conclusions: The prevalence of onychomycosis in Iranian patients was relatively high. LUL exhibited potent antifungal activity against the three groups of fungi tested, determining its broad-spectrum antimycotic activity and its probable use as the first-line therapy for onychomycosis.
Finger and toenails serve as visual advertisements of an individual’s overall health and have unquestionable effects on patients’ psychological, physical, social, and business activities. Onychomycosis defined as a fungal infection of the fingernails or toenails caused by yeasts, dermatophytes, and non-dermatophytic molds (Kaur et al., 2008; Rafat et al., 2019). Usually, it manifests as nail plate thickening (onychogryphosis), white or yellow nail discoloration, and separation of the nail from the nail bed (onycholysis). It is the most prevalent of all nail diseases and is considered an important public health problem affecting an increasing number of countries worldwide because of its prevalence and healthcare costs (Faergemann and Baran, 2003; Kaur et al., 2008; Westerberg and Voyack, 2013; Rafat et al., 2019). Its prevalence is estimated at more than 10% among healthy persons, and 40% in individuals older than 60 years, and accounts for 50% of all nail disorders seen in clinical practice (Baran et al., 1999). Several factors, including old age, underlying conditions (peripheral vascular disease, diabetes, compromised immune system, psoriasis, obesity, smoking), and walking shoeless in moist environments like public swimming pools and bathing places, are associated with an increased risk of this infection (Jacobsen and Tosti, 2017). There is also evidence suggesting that some people have a genetic predisposition to onychomycosis (Faergeman et al., 2005). Furthermore, different occupational and exercise activities (i.e., ballet dance, gymnastics, water sports, tennis, soccer, and cricket), the incidence of nail injuries, the frequent use of synthetic clothing that retains moisture (sweat or precipitation), and communal bath/shower rooms are associated with a high risk for developing onychomycosis (Blake Steele et al., 2020). The accurate treatment of onychomycosis is essential as this infection has an important impact on the quality of life and could lead to a more serious infection and complication if left untreated (Scher and Baran, 2003). Due to the composition of the human nail plates, it acts as a formidable barrier against permeation and diffusion of all drugs. In addition, the nail has a slow growth rate, requiring a long duration of therapy, usually 8-12 months or longer, until the nail has grown back (Thomas et al., 2010). Conventional therapeutics (e.g., terbinafine, voriconazole, itraconazole, fluconazole, griseofulvin) have been the preferred treatment because of their accessibility and efficacy. Therapy failure is a substantial clinical problem occurring in 25–40% of patients with onychomycosis. This failure has been attributed to emerging resistant strains of dermatophytes, low bioavailability, the increasing prevalence of onychomycosis due to non-dermatophytes, the inability of topical antifungals to pass through the nail plate, and drug interactions (Buen et al., 2010). The new antifungals (posaconazole, ravuconazole, efinaconazole, luliconazole, tavaborole) serve as a further group of curative agents that might play important roles in the treatment of onychomycosis (Gupta et al., 2005). Lack of knowledge about antifungal susceptibility profiles of fungal elements causing onychomycosis against new antifungals among Iranian patients prompted us to conduct a comprehensive study to fill this gap. Therefore, the present study aimed to assess the epidemiological profile of onychomycosis in Iran and determine the susceptibilities to conventional and new antifungals. Clarifying this factor will aid in better clinical management and can help to select the best treatment protocols.
The study was approved by the Research Ethics Committee of Tehran University of Medical Sciences (the number of ethics committee protocol: IR.TUMS.SPH.REC.1399.114). All patients who agreed to participate in the investigation signed a written consent form.
This descriptive cross-sectional study was carried out over a period of 18 months from September 2019 to March 2020, in the Referral Medical Mycology Laboratory of Tehran University of Medical Sciences, Tehran, Iran. It is the main referral center of medical mycology in Iran, where especially patients with fungal infections who do not respond to routine treatments are referred for antifungal susceptibility testing to select the best treatment protocol. The patients were referred from different cities, including Tehran (n=61), Kermanshah (n=32), Arak (n=23), Kashan (n=18), Rasht (n=15), Qom (n=8), Urmia (n=5), Zahedan (n=4), Hamadan (n=3), Zanjan (n=3), Borujerd (n=3), Bushehr (n=2), and Yazd (n=2). Sampling was performed on patients who presented nail changes compatible with a clinical diagnosis of onychomycosis, including yellow or white discoloration of the nail, thickening or thinning of the nail, nail plate brittleness, and/or detached nail. The exclusion criteria were as follow: patients using topical and/or systemic antifungals at the time of sampling or up to 15 days before collecting the specimen, and patients whose clinical specimens were insufficient for complete analysis. Demographic features including age, gender, and underlying conditions were recorded. Scrapings were collected from the nails. The outermost debris collected was discarded, and fragments were taken from the site closest to the cuticle, where contains a greater concentration of fungal elements.
The specimens were subjected to direct microscopy using 15% potassium hydroxide (KOH microscopy) and cultured on Sabouraud’s dextrose agar (SDA) containing 0.05 g/L chloramphenicol (Merck, Germany). The culture tubes were incubated at 30°C for up to 30 days. Fungal growth was assessed daily. Any growth obtained was identified by its identification characteristics include colony morphology, growth rate, and colony pigmentation.
Yeast isolates were identified based on formation and structural arrangement of chlamydospores on corn meal agar (Becton, France) and color of the colonies on CHROMagar™ Candida medium (CHROMagar, HiMedia, India). Potato-dextrose agar (PDA; Merck, Darmstadt, Germany) and Czapek agar (CZ, Micro media, Hungary) were used as differential media for the initial identification of isolated dermatophytes and non-dermatophytic filamentous fungi through colonial morphology and microscopic characteristics using lactophenol cotton blue and slide culture (Abe et al., 2009; Procop et al., 2017). For confirmation of identified species all isolates were subjected to PCR-sequencing as below.
DNA was extracted using the High Pure PCR Template Preparation kit (Roche, Germany) according to the recommended instructions of the manufacturer.
To discriminate Aspergillus isolates at the species level the Beta tubulin gene of Aspergillus species was amplified using the forward (Bt2a: 5’-GGTAACCAAATCGGTGCTGCTTTC-3’) and reverse (Bt2b: 5-ACCCTCAGTGTAGTGACCCTTGGC-3) primers. Also, other fungal species were identified to the species level using the universal primers: ITS1 (5′TCC GTA GGT GAA CCT GCG G 3′), which hybridizes at the end of 18S rDNA, and ITS4 (5′TCC TCC GCT TAT TGA TAT GC 3), which hybridizes at the beginning of 28S rDNA (Life Technologies, Barcelona, Spain). The following thermal conditions were used: 95°C for 5 min, followed by 35 cycles of 30 s at 94°C, 60°C for 45 s, and 72°C for 1 min, followed by one final extension at 72°C for 5 min.
Positive PCR products were subjected to single-direction sequencing using a forward primer (Bioneer, South Korea). The amplicons were sequenced and results were analyzed using NCBI BLAST (https://www.blast.ncbi.nlm.nih.gov/Blast.cgi)n (http://its.mycologylab.org) database.
In vitro antifungal susceptibility testing was performed against isolated strains according to the protocols described by the Clinical and Laboratory Standards Institute (CLSI) guidelines, document M38-A2 for filamentous fungi (Wayne and Clinical and Laboratory Standards Institute, 2008), and document M27-A3 for yeasts (Wayne, 2008). AFST included the following antifungal drugs: terbinafine (TER), voriconazole (VCZ), itraconazole (ITR), fluconazole (FLZ), griseofulvin (GSF), posaconazole (PSZ), ravuconazole (RAV), efinaconazole (EFIN), luliconazole (LUL), and tavaborole (TAVA) (all from Sigma-Aldrich, St. Louis, MO, U.S.A). The drug dilution ranges tested were 0.125–64 µg/mL for fluconazole and griseofulvin, 0.0625–32 µg/mL for tavaborole, 0.03125–16 µg/mL for voriconazole and itraconazole, 0.01562–8 µg/mL for terbinafine, and 0.003906-2 µg/mL for posaconazole, ravuconazole, efinaconazole, and luliconazole. Reference strains of C. parapsilosis (ATCC 22019) and C. krusei (ATCC 6258) were used for quality control purposes. Briefly, homogeneous conidial suspensions were spectrophotometrically measured at the 530 nm wavelength and a percent transmission within the range of 75-77%. The final inoculum suspension adjusted to 0.5-2.5 × 103 conidia/mL in RPMI 1640 medium (GIBCO, UK) buffered at pH 7.0 with 0.165 M morpholino propanesulfonic acid (MOPS, Sigma-Aldrich, St. Louis, MO, USA). After adding 100 µl of the inoculum suspension the microdilution plates were incubated at 35°C for 48 h; the plates were read visually according to the recommendations proposed by the CLSI M27-A3 and M38-A2 documents.
We used SPSS software version 11 (SPSS Inc., Chicago, IL, USA) to analyze the data obtained in this study. The Relative percentages, 95% confidence interval, and Chi-square test were used to describe the antifungal susceptibility profile. We considered P values ≤ 0.05 to be statistically significant.
Between September 2019 and March 2020, 594 patients who passed the eligibility criteria were included in this study. Of this population, 179 (30.1%) (95% CI:0.3 ± 0.037) cases were diagnosed to suffer from onychomycosis. The 179 patients comprised 66 men (36.9%) and 113 women (63.1%) with a median age of 50 years (range 3 - 85 years).
Saprophytic fungi accounted for the vast majority of the nail isolates (n=92, 51.4%) (95% CI:0.051 ± 0.0.073), followed by dermatophytes (n=45, 25.1%) (95% CI:0.25 ± 0.063), and yeasts (n=42, 23.5%) (95% CI:0.23 ± 0.061). The highest prevalence of onychomycosis was found in the age group of ≥60 years (n=58, 32.6%) (Table 1). In the age group under 15 years only yeasts were the causative fungal agents of onychomycosis and in the age group above 60 years, dermatophytes were the predominant causative agent of onychomycosis (Table 1). The results showed the age of subjects was significantly effective on the prevalence of onychomycosis (P = 0.001).
Results of the present study showed that there was no difference in the incidence of onychomycosis due to yeasts between females and males (P = 0.850958). On the other hand, significant gender differences in dermatophytic onychomycosis (P = 0. 000046) and onychomycosis due to saprophytic agents (P = 0.00022) were found between the male and female patients. Statistical analysis found a significant association between the causative agent of onychomycosis and the patient’s gender (P< 0.00001) (Table 2 and Figure 1).
Trichphyton mentagrophytes was the most frequently recovered dermatophyte (n=22, 48.9%), followed by Trichphyton rubrum (n=19, 42.2%), and Trichphyton verrucosum (n=4, 8.9%). Also, among yeast isolates responsible for onychomycosis, Candida albicans (n=22, 53.6%) was the most frequent detected species, followed by Candida parapsilosis (n=10, 24.4%), Candidia tropicalis (n=6, 13.9%), Candida krusei (n=2, 4.6%), and Trichosporon asahii (n=2, 4.6%). Furthermore, among saprophytic agents Aspergillus flavus (n=56, 60.9%) was the predominant etiologic agent of onychomycosis, followed by Aspergillus niger (n=13, 14.2%), Aspergillus terreus (n=8, 8.7%), Fusarium oxysporum (n=11, 11.9%), Aspergillus candidus (n=2, 2.2%), and Nattrassia mangiferae (n= 2, 2.2%). Phenotypic identification and sequencing results for the 179 fungal isolates included in the present study are shown in Table 3.
Diabetes mellitus, hypothyroidism, and solid tumors were found in 77.3%, 18.2%, and 4.5% of patients, respectively. These predisposing factors were not significantly associated with the prevalence of onychomycosis (P = 0.087).
In vitro activity based on MIC ranges, geometric mean (GM), 50% MIC (MIC50), and 90% MIC (MIC90) of all tested antifungals against the isolated yeast species responsible for onychomycosis is presented in Table 4. When all Candida strains were considered together, it was found that they were highly susceptible to EFIN (MIC range: 0. 0078-0.5 µg/mL), LUL (MIC range: 0.0125-2µg/mL), and RAV (MIC range: 0.0039-0.25/25 µg/mL) whereas TER (MIC range: 4->8 µg/mL), TAVA (MIC range: 2-8 µg/mL), and FLZ (MIC range: 0.25-16 µg/mL) had the lowest antifungal activity against Candida species (Figure 2).
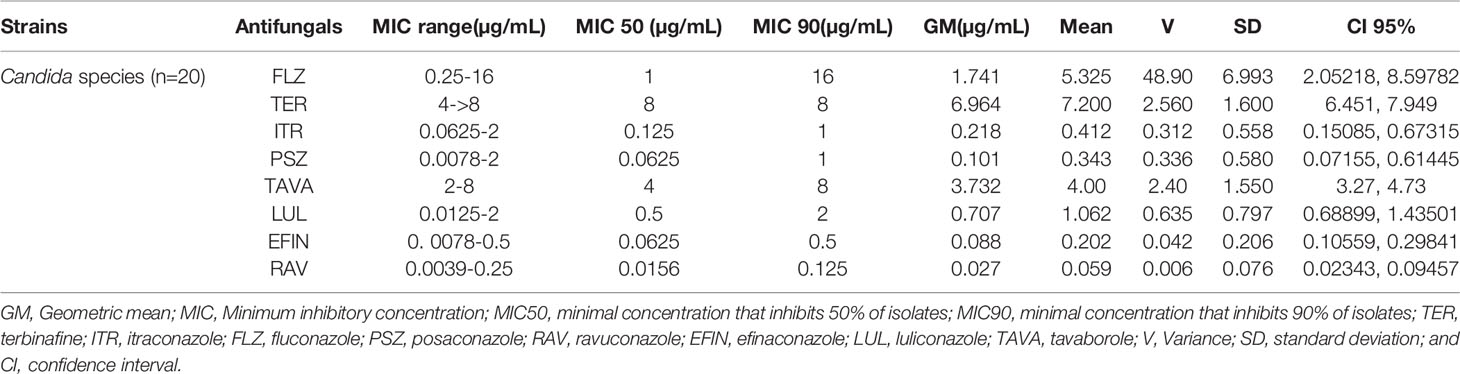
Table 4 The geometric mean, MIC ranges, MIC50 and MIC90 values obtained by testing the susceptibility of yeast isolates to each antifungal agent.
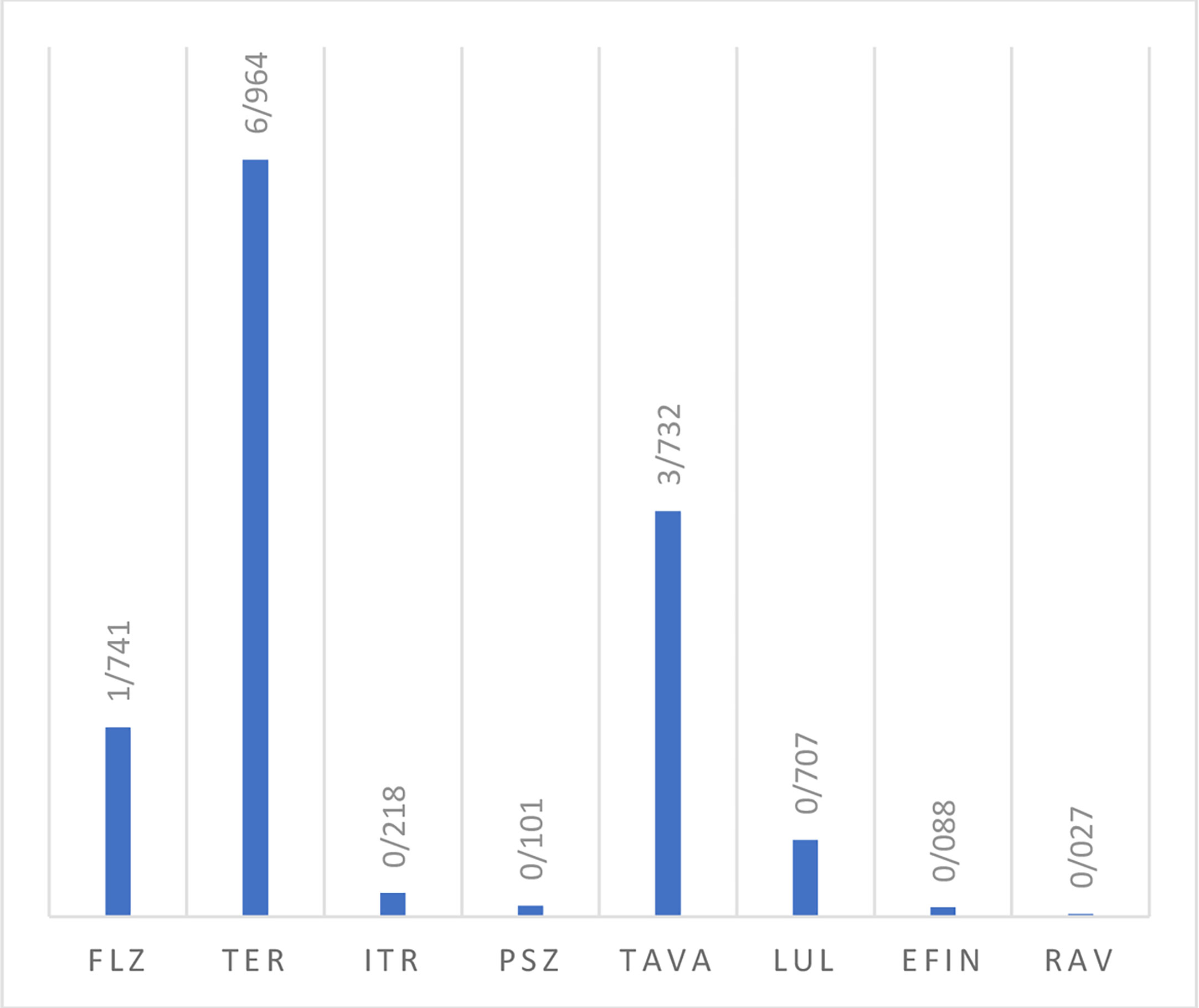
Figure 2 The geometric mean obtained by testing the susceptibility of yeast isolates to each antifungal agent.
Table 5 lists the GM, MIC50, and MIC90 of each antifungal agent against the isolated dermatophytes responsible for onychomycosis in the present study. Totally, dermatophyte species were highly susceptible to LUL (MIC range: <0.0039 µg/mL), PSZ (MIC range: 0. 0078-0.0625 µg/mL), EFIN (MIC range: 0. 0625-0.0156 µg/mL), ITR (MIC range: 0. 125-0.5 µg/mL) and GSF (MIC range: 0.25-1µg/mL) whereas TER (MIC range: 1-4 µg/mL), TAVA (MIC range: 2-8 µg/mL), and FLZ (MIC range: 1-32 µg/mL) had the lowest antifungal activity against these fungal species (Figure 3).
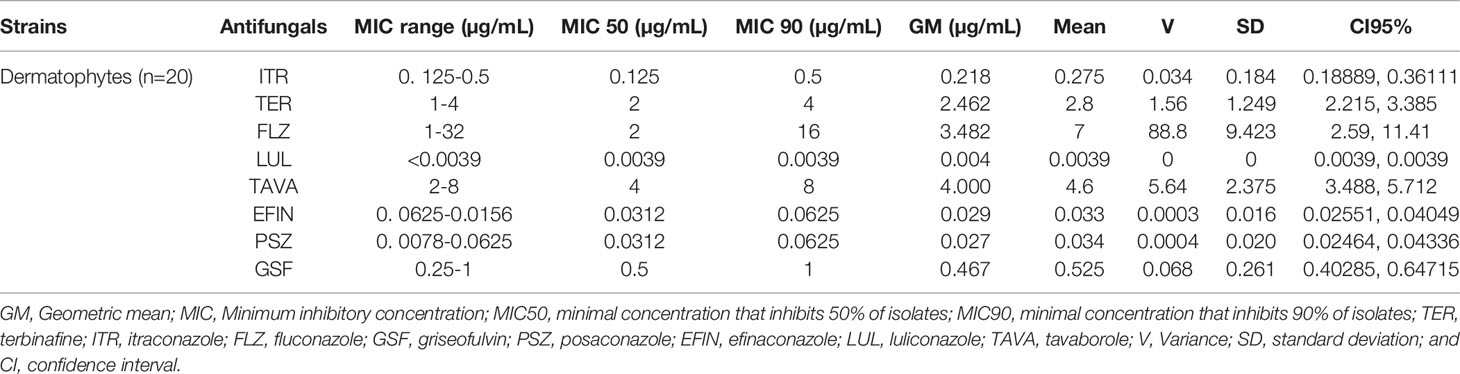
Table 5 The geometric mean, MIC ranges, MIC50 and MIC90 values obtained by testing the susceptibility of dermatophytes to each antifungal agent.
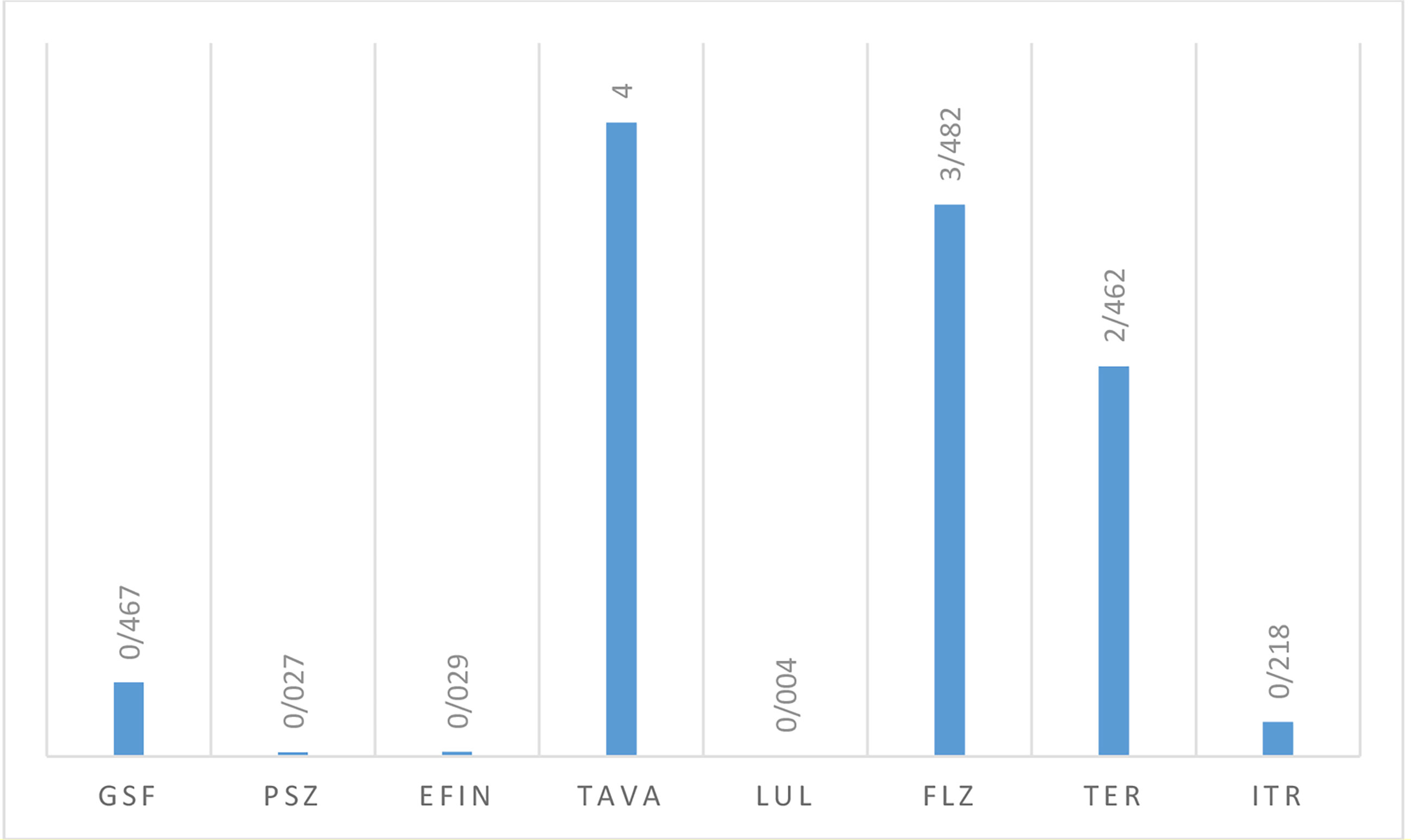
Figure 3 The geometric mean obtained by testing the susceptibility of dermatophytic isolates to each antifungal agent.
In vitro activity based on MIC ranges, geometric mean (GM), 50% MIC (MIC50), and 90% MIC (MIC90) of all antifungals against saprophytic fungi responsible for onychomycosis is shown in Table 6. The results showed that LUL (MIC range: <0.0039 µg/mL) had the greatest and TER (MIC range: 1->8 µg/mL), and TAVA (MIC range: 2-8 µg/mL) had the lowest antifungal activity against saprophytic fungi (Figure 4).
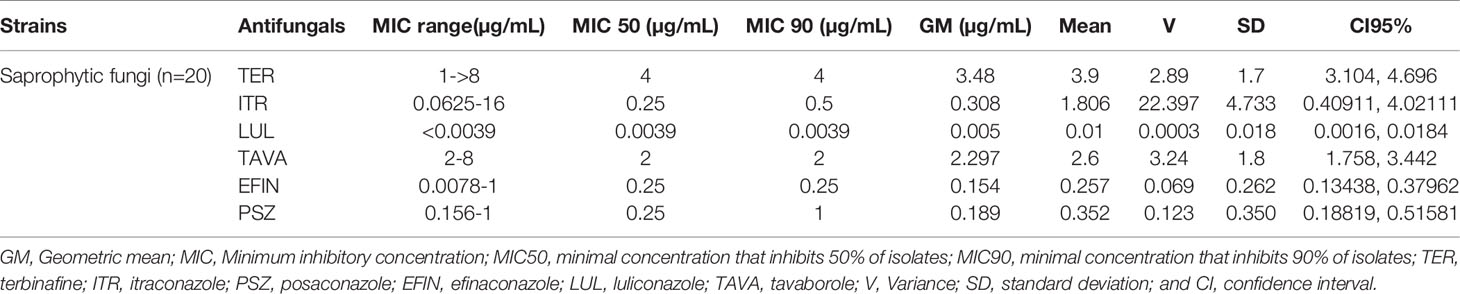
Table 6 The geometric mean, MIC ranges, MIC50 and MIC90 values obtained by testing the susceptibility of saprophytic fungi to each antifungal agent.
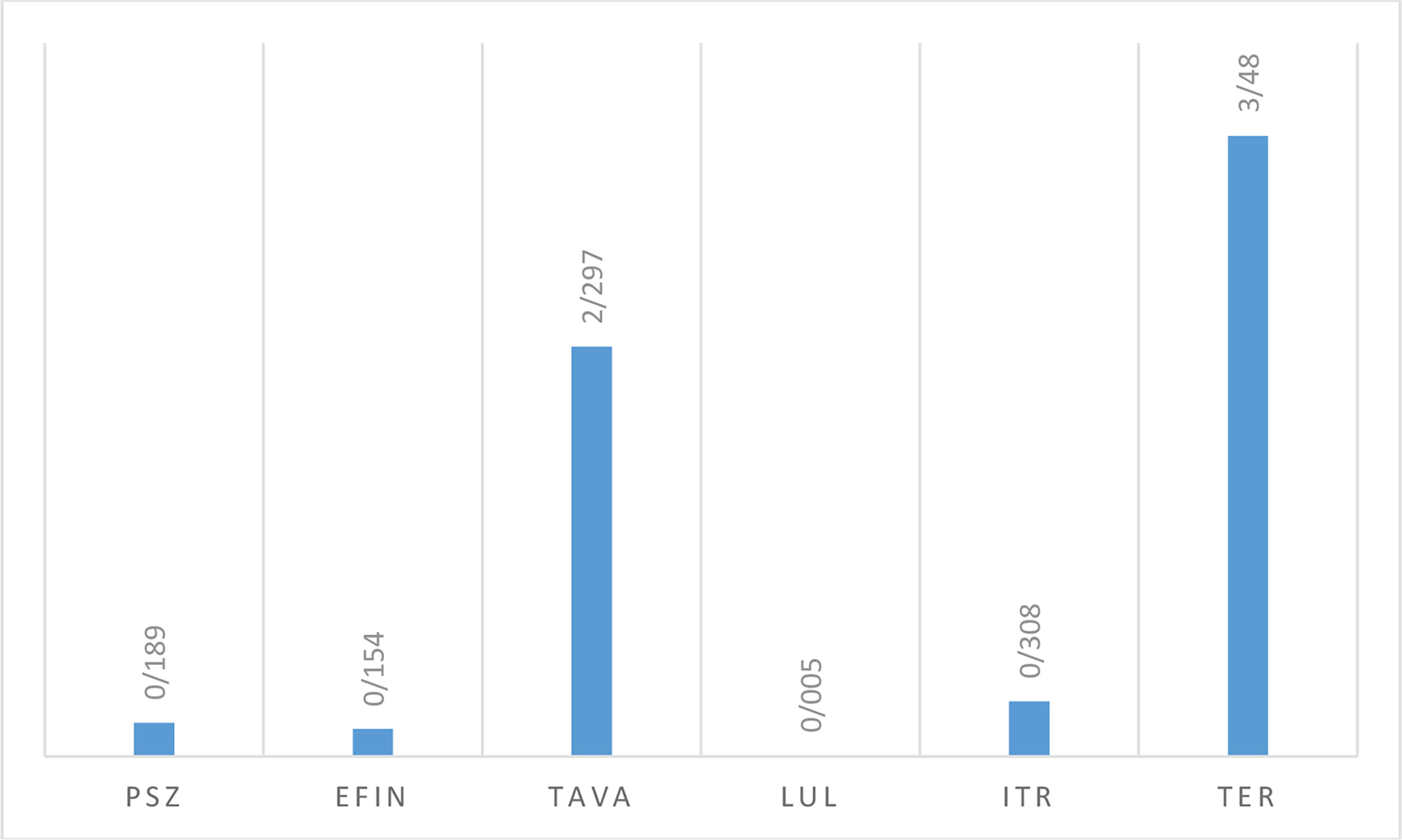
Figure 4 The geometric mean obtained by testing the susceptibility of saprophytic isolates to each antifungal agent.
Onychomycosis is an important global disease and represents 20% to 40% of all onychopathies. Although it has worldwide occurrence, its frequency is variable which depends on different climatic, ethics, professional, and socio-economic conditions. In the present study, from 594 patients suspected to onychomycosis, 179 cases (30.1%) were positive based on laboratory finding, while a comprehensive survey conducted by Pönnighaus in Africa did not find any cases of onychomycosis (Pönnighaus et al., 1996), also in a study conducted by Roberts et al., in the United Kingdom the estimated prevalence of onychomycosis was 1.3 to 4.7% (Roberts, 1992). The present study assessed that onychomycosis is extremely rare in children, common in adults, and very frequent in the elderly. The finding is according to other studies done in the world (Smith et al., 2001; Htwe et al., 2007; Abdullah and Abbas, 2011). This increased prevalence of onychomycosis in the elderly (≥ 60 years of age) may be associated with the presence of arthritis and other related conditions that weaken physical activity and flexibility. It should be noted that this age group is more susceptible to colonization by infectious organisms resulting from inadequate foot and nail hygiene.
The results of the present study showed that females were affected more than males by onychomycosis. One reason is that for any nail disorder, females go to the doctor more often than males. Also, prolonged contact with water and soap or other detergents in females is another risk factor that decreases the local immunity at the nail complex (Jayatilake et al., 2009).
Our results on the distribution of onychomycosis etiological agents differ from other published reports. In the present study, saprophytic fungi accounted for the vast majority of the nail isolates, while an Iranian meta-analysis study speculated that yeasts are the predominant leading agent of onychomycosis (Rafat et al., 2019). Also, a multicenter study performed in the USA reported that the commonest causative agents of onychomycosis are dermatophytes (Ghannoum et al., 2000). As our results show, among yeasts, Candida albicans was the most prevalent species isolated from patients with onychomycosis, and among dermatophytes, Trichphyton mentagrophytes was the main species isolated from the patients. Furthermore, the predominant isolated saprophytic fungi in the present study was Aspergillus flavus. These findings are in accordance with the results of a systematic review and meta-analysis conducted by Rafat et al., in Iran (Rafat et al., 2019). Furthermore, diabetes mellitus and hypothyroidism were observed as the risk factors for developing onychomycosis in the present study. Previous studies indicated that endocrine disorders (hypothyroidism and diabetes mellitus) are some of the common systemic diseases that facilitate fungal nail infections via suppression of host immunity (Chi et al., 2005; Jayatilake et al., 2009).
Itraconazole, terbinafine, and fluconazole are the most widely available antifungal agents used for the systemic treatment of onychomycosis. Historically, the treatment of onychomycosis has been challenging (Gupta and Versteeg, 2016). In the current study, we used a panel of different species of dermatophytes, yeasts, and saprophytic fungi isolated from patients with onychomycosis to evaluate the in vitro activity of new antifungals (posaconazole, ravuconazole, efinaconazole, luliconazole, tavaborole) in comparison with conventional therapeutics (terbinafine, itraconazole, fluconazole, and griseofulvin). The high MICs obtained for Candida species with terbinafine, tavaborole, and fluconazole are consistent with those previously reported (Darkes et al., 2003; Sojakova et al., 2004; Buen et al., 2010; Abastabar et al., 2018) therefore, we would recommend cautious use of these antifungals against different Candida strains causing onychomycosis. Also, in the present study, 10% of Candida isolates were resistant to posaconazole and itraconazole. In contrast, the results of a study conducted by Sabatelli et al., showed that posaconazole was frequently more active than itraconazole, fluconazole, voriconazole, and amphotericin B against approximately 7,000 isolates of Candida and Cryptococcus spp (Sabatelli et al., 2006). Furthermore, in the present study, LUL, EFIN, and RAV had low MICs against Candida species and it was found that they were highly susceptible to these antifungals. This finding is consistent with the results from previous reports (Pfaller et al., 2002; Uchida et al., 2004; Siu et al., 2013).
Relative to the other agents tested, LUL possessed the highest antifungal activity against all dermatophytes. This finding is in agreement with previous studies (Uchida et al., 2004; Baghi et al., 2016). Also, the results of the present study showed that dermatophytes were highly susceptible to PSZ, EFIN, ITR, and GSF, whereas TER, TAVA, and FLZ had the lowest antifungal activity against these fungal species. In accordance, in previous studies, against dermatophytes, low MICs were obtained with EFIN, and PSZ (Gupta et al., 2005; Siu et al., 2013). Results of a study conducted by Baghi et al., demonstrated TER, and ITR displayed excellent activity against all dermatophyte isolates, although they reported that the majority of dermatophyte isolates showed low susceptibility to GSF and very low to FLZ (Baghi et al., 2016).
Our results showed that LUL had the greatest and, TER and TAVA had the lowest antifungal activity against saprophytic fungal strains isolated from onychomycosis. In accordance, another study has been reported that LUL had strong in vitro antifungal activity against Aspergillus fumigatus (Ghannoum et al., 2000). In the present study, MICs of TAVA against saprophytic fungal strains were similar to those reported by Abastabar et al. (2018), and MICs of TER against these fungal isolates were similar to those reported by Mao et al. (2006). Also, the resistance of saprophytic fungi to ITR and PSZ was accounted for 10% and 20%, respectively. In a study conducted by Gupta et al., the lowest MICs for saprophytic fungal strains was obtained with posaconazole, followed by, ravuconazole, terbinafine, and itraconazole (Jayatilake et al., 2009).
In conclusion, our results provide further evidence for the spectrum and potency of luliconazole, a novel topical imidazole, in the treatment of onychomycosis. In the present study, luliconazole had low MICs against the three groups of fungi tested (yeasts, dermatophytes, and non-dermatophytic molds) determining its broad-spectrum antimycotic activity and its probable use as the first-line therapy for onychomycosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Tehran University of Medical Sciences. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
SH, PA, ZB, MZ, MA, and HKS performed experiments. RD, SJH, and SK supervised the study. HB and AR performed the statistical analysis. ZR wrote the manuscript. SH, PA, ZB, MZ, MA, and HKS performed experiments. RD obtained study funding. All authors contributed to the article and approved the submitted version.
This work was supported by the funding from Tehran University of Medical Sciences, Tehran, Iran.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abastabar, M., Haghani, I., Shokohi, T., Hedayati, M. T., Aghili, S. R., Jedi, A., et al. (2018). Low In Vitro Antifungal Activity of Tavaborole Against Yeasts and Molds From Onychomycosis. Antimicrob. Agents Chemother. 62, e01632–e01618. doi: 10.1128/AAC.01632-18
Abdullah, L., Abbas, O. (2011). Common Nail Changes and Disorders in Older People: Diagnosis and Management. Can. Fam. Physician 57, 173–181.
Abe, M., Ogawa, Z., Tanuma, H., Kume, H. (2009). Study of Mycological Examination Methods in Clinical Laboratories-Specimen Pretreatment and Isolation. Jpn. J. Med. Mycol. 50, 235–242. doi: 10.3314/jjmm.50.235
Baghi, N., Shokohi, T., Badali, H., Makimura, K., Rezaei-Matehkolaei, A., Abdollahi, M., et al. (2016). In Vitro Activity of New Azoles Luliconazole and Lanoconazole Compared With Ten Other Antifungal Drugs Against Clinical Dermatophyte Isolates. J. Med. Mycol. 7, 757–763. doi: 10.1093/mmy/myw016
Baran, R., Haneke, E., Hay, R. J., Piraccini, B. M., Tosti, A. (1999). Onychomycosis: The Current Approach to Diagnosis and Therapy. CRC Press. 25 (2), 170. doi: 10.1201/b14710
Blake Steele, R., Taylor, J. S., Aneja, S. (2020). Skin Disorders in Athletes: Professional and Recreational Sports. In: John, S., Johansen, J., Rustemeyer, T., Elsner, P., Maibach, H. (eds) Kanerva’s Occupational Dermatology. Springer, Cham. doi: 10.1007/978-3-319-68617-2_186
Buen, J. G., Martinez, C., Zapata, B., Sanclemente, G., Gallego, M., Mesa, A. C. (2010). In Vitro Activity of Fluconazole, Itraconazole, Voriconazole and Terbinafine Against Fungi Causing Onychomycosis, Clin. Exp. Dermatol. 35, 658–663. doi: 10.1111/j.1365-2230.2009.03698.x
Chi, C. C., Wang, S. H., Chou, M. C. (2005). The Causative Pathogens of Onychomycosis in Southern Taiwan. Mycoses 48, 413–420. doi: 10.1111/j.1439-0507.2005.01152.x
Darkes, M. J., Scott, L. J., Goa, K. L. (2003). Terbinafine: A Review of its Use in Onychomycosis in Adults, am. J. Clin. Dermatol. 4, 39–65. doi: 10.2165/00128071-200304010-00005
Faergeman, J., Correia, O., Nowicki, R., Ro, B. I. (2005). Genetic Predisposition–Understanding Underlying Mechanisms of Onychomycosis. J. Eur. Acad. Dermatol. Venereol. 19, 17–19. doi: 10.1111/j.1468-3083.2005.01283.x
Faergemann, J., Baran, R. (2003). Epidemiology, Clinical Presentation and Diagnosis of Onychomycosis. Br. J. Dermatol. 149, 1–4. doi: 10.1046/j.1365-2133.149.s65.4.x
Ghannoum, M. A., Hajjeh, R. A., Scher R, R., Konnikov, N., Gupta, A. K., Summerbell, R., et al. (2000). A Large-Scale North American Study of Fungal Isolates From Nails: The Frequency of Onychomycosis, Fungal Distribution, and Antifungal Susceptibility Patterns. J. Am. Acad. Dermatol. 43, 641–648. doi: 10.1067/mjd.2000.107754
Gupta, A. K., Kohli, Y., Batra, R. (2005). In Vitro Activities of Posaconazole, Ravuconazole, Terbinafine, Itraconazole and Fluconazole Against Dermatophyte, Yeast and non-Dermatophyte Species. Med. Mycol. J. 2, 179–185. doi: 10.1080/13693780410001731583
Gupta, A. K., Versteeg, S. G. (2016). Tavaborole – a Treatment for Onychomycosis of the Toenails, Expert. Rev. Clin. Pharmacol. 9, 1145–1152. doi: 10.1080/17512433.2016.1206467
Htwe, T. H., Mushtaq, A., Robinson, S. B., Rosher, R. B., Khardori, N. (2007). Infection in the Elderly. Infect. Dis. Clin. North. Am. 21, 711–743. doi: 10.1016/j.idc.2007.07.006
Jacobsen, A. A., Tosti, A. (2017). Predisposing Factors for Onychomycosis, Onychomycosis (Miami, USA: Springer), 11–19.
Jayatilake, J. A., Tilakaratne, W. M., Panagoda, G. J. (2009). Candidal Onychomycosis: A Mini-Review. Mycopathologia 168, 165–173. doi: 10.1007/s11046-009-9212-x
Kaur, R., Kashyap, B., Bhalla, P. (2008). Onychomycosis-Epidemiology, Diagnosis and Management, Indian J. Med. Microbiol. 26, 108–116. doi: 10.4103/0255-0857.40522
Mao, W., Rock, F. L., Alley, M. R. (2006). Initial Characterization of Resistant Mutants to AN2690, a New Antifungal Agent in Development for the Topical Treatment of Onychomycosis, Predicts a Novel Mechanism of Action: P1607. J. Am. Acad. Dermatol. 3, 6185–6190. doi: 10.2147/DDDT.S81944
Pfaller, M. A., Messer, S. A., Hollis, R. J., Jones, R. N., Diekema, D. J. (2002). In Vitro Activities of Ravuconazole and Voriconazole Compared With Those of Four Approved Systemic Antifungal Agents Against 6,970 Clinical Isolates of Candida Spp. Antimicrob. Agents Chemother. 6, 1723–1727. doi: 10.1128/aac.46.6.1723-1727.2002
Pönnighaus, J. M., Clayton, Y., Warndorff, D. (1996). The Spectrum of Dermatophytes in Northern Malawi (Africa) Das Dermatophytenspektrum in Nord-Malawi (Afrika). Mycoses 39, 293–297. doi: 10.1111/j.1439-0507.1996.tb00141.x
Procop, G. W., Church, D., Hall, G., William, G. M. J., Elmer, W. K., Paul, C. S., et al. (2017). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 7th ed (Philadelphia: Wolters Kluwer).
Rafat, Z., Hashemi S, S. J., Saboor-Yaraghi, A. A., Pouragha, B., Taheriniya, A., Moosavi, A., et al. (2019). A Systematic Review and Meta-Analysis on the Epidemiology, Casual Agents and Demographic Characteristics of Onychomycosis in Iran. J. Mycol. Med. 29, 265–272. doi: 10.1016/j.mycmed.2019.05.004
Roberts, D. T. (1992). Prevalence of Dermatophyte Onychomycosis in the United Kingdom: Results of an Omnibus Survey. Br. J. Dermatol. 126, 23–27. doi: 10.1111/j.1365-2133.1992.tb00005.x
Sabatelli, F., Patel, R., Mann, P. A., Mendrick, C. A., Norris, C. C., Hare, R., et al. (2006). In Vitro Activities of Posaconazole, Fluconazole, Itraconazole, Voriconazole, and Amphotericin B Against a Large Collection of Clinically Important Molds and Yeasts. Antimicrob. Agents Chemother. 6, 2009–2015. doi: 10.1128/AAC.00163-06
Scher, R., Baran, R. (2003). Onychomycosis in Clinical Practice: Factors Contributing to Recurrence. Br. J. Dermatol. 149, 5–9. doi: 10.1046/j.1365-2133.149.s65.5.x
Siu, W. J., Tatsumi, Y., Senda, H., Pillai, R., Nakamura, T., Sone, D., et al. (2013). Comparison of In Vitro Antifungal Activities of Efinaconazole and Currently Available Antifungal Agents Against a Variety of Pathogenic Fungi Associated With Onych1omycosis. Antimicrob. Agents Chemother. 4, 1610–1616. doi: 10.1128/AAC.02056-12
Smith, E. S., Fleischer, A. B., Feldman, S. R. (2001). Demographics of Aging and Skin Disease. Clin. Geriatr. Med. 17, 631–641. doi: 10.1016/s0749-0690(05)70090-2
Sojakova, M., Liptajova, D., Borovsky, M., Subik, J. (2004). Fluconazole and Itraconazole Susceptibility of Vaginal Yeast Isolates From Slovakia. Mycopathologia 2, 163–169. doi: 10.1023/B:MYCO.0000020594.35357.b0
Thomas, J., Jacobson, G., Narkowicz, C., Peterson, G., Burnet, H., Sharpe, C. (2010). Toenail Onychomycosis: An Important Global Disease Burden. J. Clin. Pharm. Ther. 35, 497–519. doi: 10.1111/j.1365-2710.2009.01107.x
Uchida, K., Nishiyama, Y., Yamaguchi, H. (2004). In Vitro Antifungal Activity of Luliconazole (NND-502), a Novel Imidazole Antifungal Agent. J. Infect. Chemother. 4, 216–219. doi: 10.1007/s10156-004-0327-1
Wayne, P. A. (2008). Clinical and Laboratory Standards Institute: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. CLSI document M27-A3 Supplement CLSI 3, 6–12.
Wayne, P. A., Clinical and Laboratory Standards Institute (2008). “Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard,” in Document M38-A2, 2nd ed (Pennsylvania, USA: CLSI).
Keywords: Onychomycosis, yeasts, dermatophytes, saprophytic agents, antifungal susceptibility testing, new antifungals, conventional antifungals
Citation: Halvaee S, Daie-Ghazvini R, Hashemi SJ, Khodavaisy S, Rahimi-Foroushani A, Bakhshi H, Rafat Z, Ardi P, Abastabar M, Zareei M, Borjian-Boroujeni Z and Kamali Sarvestani H (2021) A Mycological and Molecular Epidemiologic Study on Onychomycosis and Determination In Vitro Susceptibilities of Isolated Fungal Strains to Conventional and New Antifungals. Front. Cell. Infect. Microbiol. 11:693522. doi: 10.3389/fcimb.2021.693522
Received: 01 May 2021; Accepted: 29 June 2021;
Published: 15 July 2021.
Edited by:
Priya Uppuluri, University of California, Los Angeles, United StatesReviewed by:
Hadis Jafarian, Shiraz University of Medical Sciences, IranCopyright © 2021 Halvaee, Daie-Ghazvini, Hashemi, Khodavaisy, Rahimi-Foroushani, Bakhshi, Rafat, Ardi, Abastabar, Zareei, Borjian-Boroujeni and Kamali Sarvestani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roshanak Daie-Ghazvini, cmRhaWVAdHVtcy5hYy5pcg==; cm9zaGFuYWsxMDQzQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.