
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol., 17 June 2021
Sec. Microbes and Innate Immunity
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.678961
This article is part of the Research TopicVulvodynia and Beyond: Innate Immune Sensing, Microbes, Inflammation, and Chronic PainView all 5 articles
 Jorma Paavonen1*
Jorma Paavonen1* David A. Eschenbach2
David A. Eschenbach2Localized provoked vulvodynia (LPV) causes dyspareunia among reproductive aged women. We review the pathogenesis of LPV and suggest that LPV is an inflammatory pain syndrome of the vestibular mucosa triggered by microbial antigens in a susceptible host. Tissue inflammation and hyperinnervation are characteristic findings which explain symptoms and clinical signs. Education of health care providers of LPV is important since this condition is common, often unrecognized, and patients often become frustrated users of health care. Research is needed on the antigen triggers of the syndrome. Randomized clinical trials are needed to evaluate treatment modalities.
Localized provoked vulvodynia is a major cause of dyspareunia and is triggered by inflammation and hyperinnervation of the vestibular mucosa. Education of health care providers is important since LPV is common, but often remains unrecognized.
Localized provoked vulvodynia (LPV) is defined as vulvar pain induced by touching the vulvar vestibular epithelium that has lasted a minimum of 3 months, in the absence of another recognizable vulvar disease (American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and American Society for Colposcopy and Cervical Pathology (ASCCP), 2016; Bornstein et al., 2016). The onset can be primary (present at the first attempted sexual intercourse) or secondary appearing later in life after a period of painless sexual intercourse. The dyspareunia associated with LPV causes sexual dysfunction, and severely impacts sexual, mental and social health. The prevalence of LPV varies between 8 and 18% in population based studies (Harlow et al., 2014). LPV is a poorly recognized condition. For instance, in one study only 50% of symptomatic women sought care, 30% had three or more physician visits, and 40% remained undiagnosed (Harlow et al., 2001). The term localized provoked vulvodynia (LPV) has been universally accepted and holds priority over the former term vulvar vestibulitis syndrome (Bornstein et al., 2016).
The clinical diagnostic test is known as the Q-tip test and is performed using a simple cotton swab gently touching the vestibular epithelium (Figure 1) (American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and American Society for Colposcopy and Cervical Pathology (ASCCP), 2016; Bornstein et al., 2016). The most characteristic painful areas are around the ductal openings of the vestibular glands, most commonly around the posteriorly located Bartholin’s glands and less commonly around the anteriorly located Skene’s glands (Goldstein et al., 2016). Patients with LPV in the anterior vestibule often have external dysuria that should be differentiated from cystitis or urethritis. Cervicovaginal infections and other gynecological or dermatological conditions causing vulvar pain should be excluded. Dermatoses such as lichen sclerosus, lichen planus or lichen simplex chronicus can usually be diagnosed by their characteristic appearance or by biopsy.
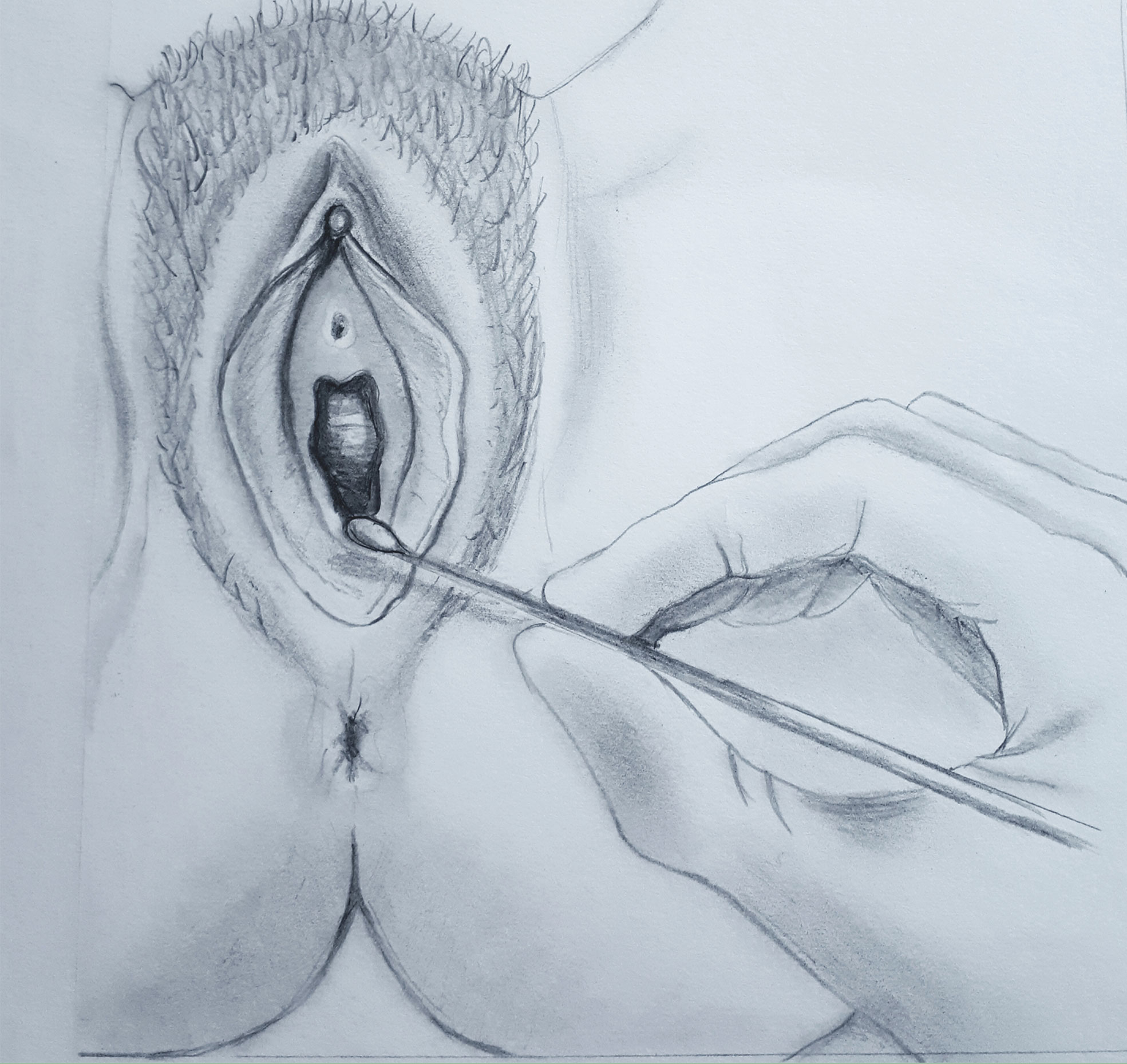
Figure 1 Q-tip test. Vestibular point tenderness is tested at 2-, 5-, 6-, 7-, and 10-o’clock positions, and quantified as mild, moderate, or severe.
The pathogenesis of LPV involves microbial, immunological and genetic components. Inflammation and hyperesthesia within well-defined areas of the vestibule well explain the symptoms and signs of LPV (Tommola et al., 2015; Tommola et al., 2016). Many lines of evidence suggest that Candida is one trigger antigen causing LPV. Women with LPV have a history of recurrent vulvovaginal candidiasis (RVVC) more often than control women. This finding has been consistently replicated in several case-control studies of LPV (Leusink et al., 2018). Further, women with LPV often have cutaneous hypersensitivity to Candida albicans as detected by patch skin testing. Ramirez De Knott et al. reported that 10 of 27 LPV patients tested positive for C. albicans cutaneous hypersensitivity compared to 0 of 13 control women (p=0.01) (Ramirez De Knott et al., 2005). A familiarity analysis of LPV patients treated by vestibulectomy suggests a genetic predisposition (Morgan et al., 2016). A mouse model of repeated vulvovaginal C. albicans infections replicates the clinical findings of vulvodynia and its characteristic histopathological changes (Farmer et al., 2011). In vitro studies also demonstrate that vestibular fibroblasts from LPV patients produce more proinflammatory cytokines such as interleukin (IL)-1 beta, IL-6 and IL-8, as well as prostaglandin E2 when stimulated with Candida antigens than fibroblasts from control women (Foster et al., 2007; Foster et al., 2015). Fibroblasts from LPV patients expressed higher levels of dectin-1 fungal antigen receptor and recognized lower concentrations of Candida antigen than fibroblasts from control subjects (Falsetta et al., 2015).
Other less extensively studied trigger antigens may also play a role in the pathogenesis of LPV. The histopathology of the vulvar vestibule in LPV demonstrates two distinctive pathologic features, infiltration with lymphocytic inflammatory cells and proliferation of sensory nerve fibers. Several studies report chronic inflammation in LPV (Halperin et al., 2005; Goetsch et al., 2010). Increased CD4-positive T helper cell densities (Leclair et al., 2014) and elevated tissue levels of IL-1 beta and tumor necrosis factor-alpha (TNF-α) were found (Foster and Hasday, 1997). In areas with the highest densities of inflammation, T and B cells form lymphoid aggregates that represent secondary lymphoid tissue (Tommola et al., 2015; Figures 2A, B). The presence of lymphoid aggregates and increased density of B cells, including activated plasma cells are the major differences between the vestibular tissue from women with LPV and samples from control women (Leusink et al., 2018). Overall, the total number of inflammatory cells is markedly increased in the tender areas of the vestibule (Tommola et al., 2015). The first reports on neural tissue changes in LPV originated from immunohistochemistry studies that reported more intraepithelial nerve fibers in LPV than in controls (Bohm-Starke et al., 1998; Bohm-Starke et al., 1999). Nerve fiber proliferation and hyperinnervation is consistently observed in virtually all histopathological studies of the vestibular areas of LPV patients. The density of intraepithelial nerve fibers was higher in vestibular samples with lymphoid aggregates than in vestibular samples without lymphoid aggregates (Tommola et al., 2016). Glandular epithelium also showed proliferation of epithelial nerve fibers with higher densities in glands surrounded by B cell infiltrates than in glands without such infiltrates. This hyperinnervation is derived by sprouting sensory axons (Liao et al., 2017). Thus, tender zone hyperinnervation is due at least in part to proliferation of nerve fibers known to detect mechanical pain contributing to the hypersensitivity to touch in LPV. (Liao et al. (2017) demonstrated that inflammatory hypersensitivity and hyperinnervation also occur in concert with a local renin-angiotensin system (RAS), and that tender tissue shows a markedly robust RAS.
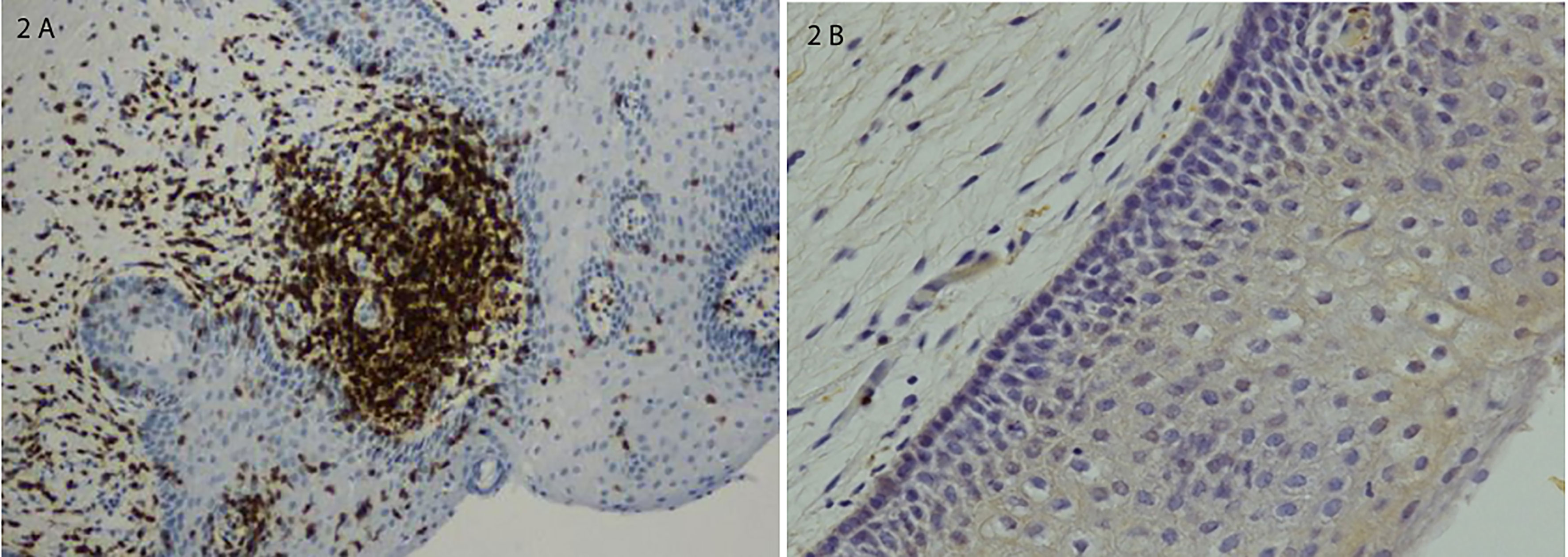
Figure 2 (A) Lymphoid aggregate in a vestibular biopsy from a patient with severe LPV; immunostaining for T cells and B cells. (B) Normal vestibular epithelium without inflammatory changes. Histological sections were counterstained with hematoxylin and photomicrographed using x10 objective. Adapted from Tommola P., et al., Am J Obstet Gynecol7; 2015;212(4):476.e1-8 (by permission).
In aggregate, the pathophysiological evidence suggests that LPV has a biological basis and that microbial antigens such as Candida can induce a prolonged cutaneous hypersensitivity response in the vulvar vestibular epithelial cells in a subset of susceptible women. However, the overall evidence is still relatively limited, and some studies question the role of inflammation in driving LPV (Chalmers et al., 2016). Stress-induced life factors (Harlow and Gunther Stewart, 2005; Khandker et al., 2011; Khandker et al., 2014; Khandker et al., 2019) can also trigger an immunological tissue response. The pathogenesis of primary LPV and secondary LPV may also differ so that the microbial cause is more likely related to secondary LPV. Clearly, LPV is a clinical diagnosis and may have multiple etiologies.
Recent reviews of the management of LPV (Spoelstra et al., 2013; Goldstein et al., 2016; Miranda Varella Pereira et al., 2018) have concluded that most studies are underpowered and fail to demonstrate beneficial effects of an intervention. An expert committee reviewed the evidence on the treatment of women’s vulvar pain syndrome and recommended the following treatments for the management of LPV: psychological interventions, pelvic floor physical therapy, and vestibulectomy (Goldstein et al., 2016). Psychological interventions, such as cognitive behavioral therapy or psychosexual counseling, may help patients to cope with the symptoms and reduce central pain hypersensitivity. In a randomized study, cognitive behavioral therapy proved more effective in LPV than traditional support or topical corticosteroids (Bergeron et al., 2016). Biofeedback therapy and myofascial trigger point manipulation reduces pelvic floor muscle spasm and dysfunction and normalizes muscle tone by pain desensitization. Physical therapy and psychosexual counseling are often combined. However, most studies evaluating these therapeutic approaches lack comparison groups. Botulinum toxin has been used to decrease peripheral and central sensitization and pelvic floor dysfunction associated with LPV. Although case-series and case reports have demonstrated efficacy, botulinum toxin is not recommended as first-line treatment for LPV, pending more robust scientific support (Goldstein et al., 2016).
Surgical treatment of LPV consists of removal of the posterior vestibular epithelium by a vestibulectomy. In clinical practice, surgery has been only recommended for patients with severe LPV refractory to conservative treatment (Tommola et al., 2010; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and American Society for Colposcopy and Cervical Pathology (ASCCP), 2016; Goldstein et al., 2016). Long-term patient satisfaction after surgery can be explained by the mucosal pathophysiology since surgery removes the localized tender mucosa superficially and replaces it with advanced vaginal mucosa. Overall, 80-90% of the patients reported a partial or complete response (Tommola et al., 2010; Tommola and Unkila-Kallio Paavonen, 2011). However, randomized trials of the effectiveness of surgery are needed. Table 1 shows a summary of selected LPV treatment modalities by strength of evidence.
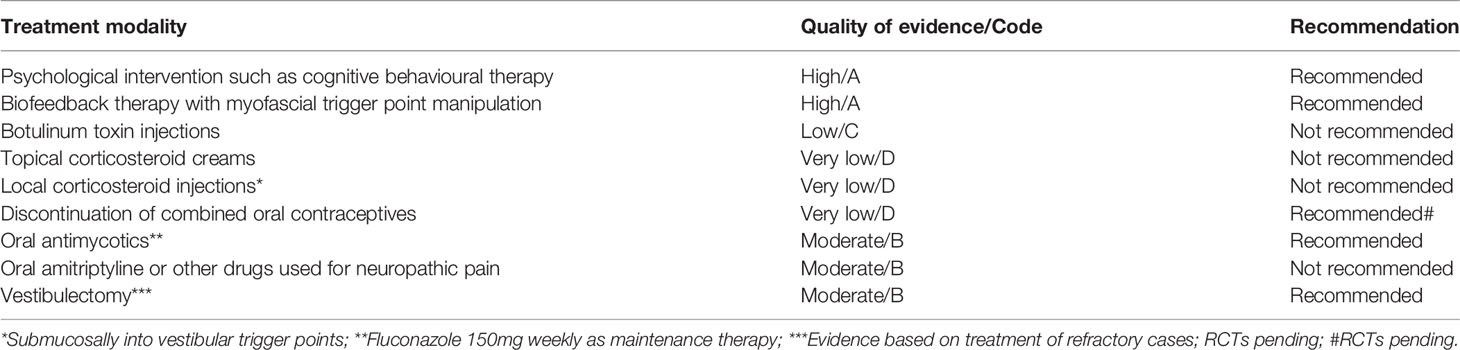
Table 1 Selected treatment modalities for localised provoked vulvodynia (LPV) by quality of evidence.
A useful clinical management algorithm has been developed by the American College of Obstetricians and Gynecologists and the American Society for Colposcopy and Cervical Pathology (Figure 3) (American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and American Society for Colposcopy and Cervical Pathology (ASCCP), 2016). One additional step in the management is to encourage women to discontinue the use of combined oral contraceptives. This is based on expert opinion and clinical experience of alleviation of allodynia with return of natural menstrual cycle. Women using oral contraceptives may have decreased mechanical pain threshold in the vestibular mucosa (Bohm-Starke et al., 2004). However, without better evidence this recommandation is not universally accepted.
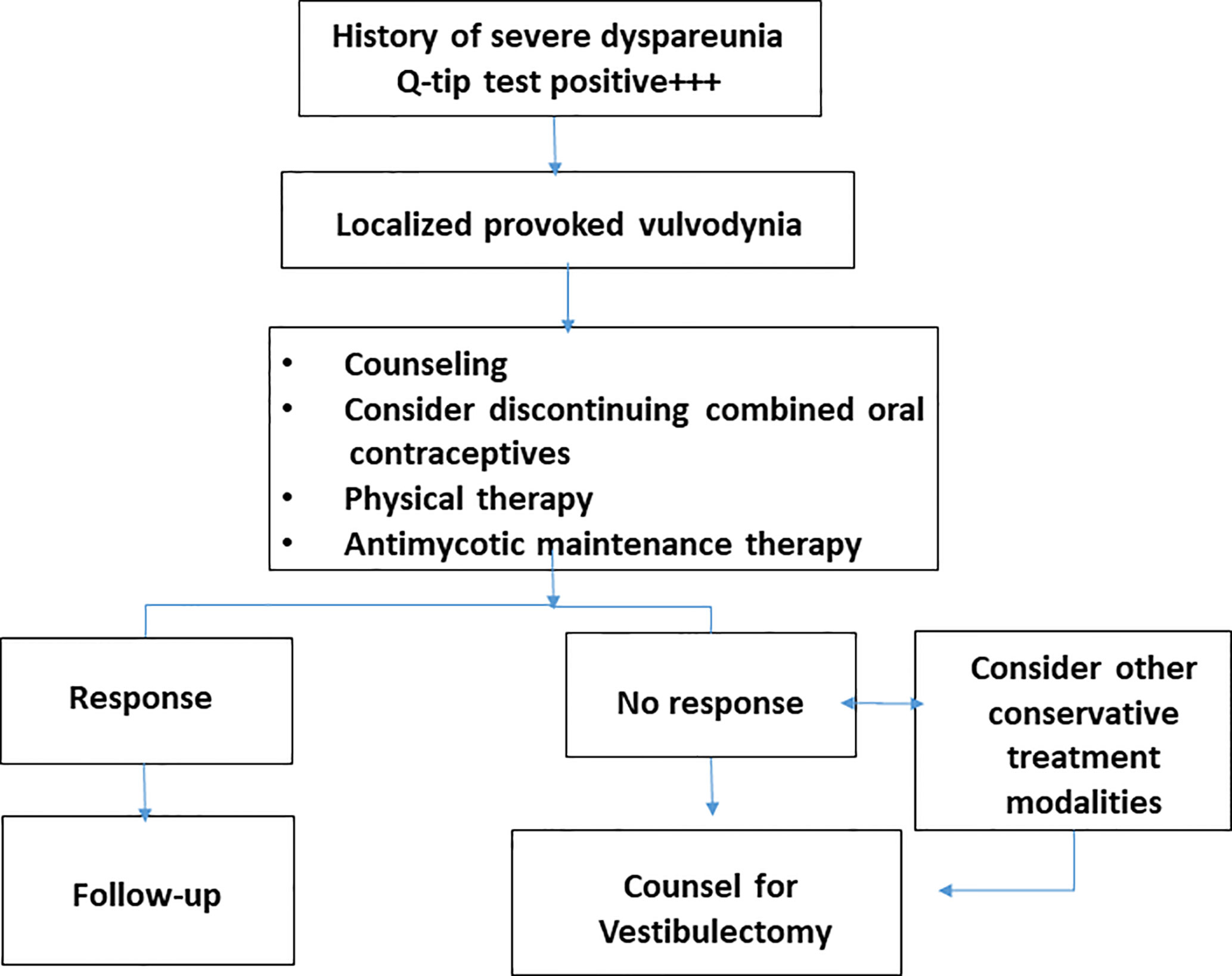
Figure 3 Clinical algorithm developed by the authors and recommended for the management of patients with severe vulvar vestibulitis syndrome.
In conclusion, the landmark histopathologic findings of chronic inflammation and hyperinnervation in LPV well explain the clinical symptoms and signs. Inflammation in the vestibular tissue and its association with hyperinnervation derived by mechanical nociceptor axons results in the altered pain sensation and hypersensitivity. The vestibular mucosa is a key mucocutaneous surface with an important role in immune responses in general (Robert and Kupper, 1999). Education of health care providers on the diagnosis and management of LPV is important, since such patients often are frustrated users of the health care system.
The authors report equal roles in the conception, planning, carrying out, and writing up the work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors want to thank Dr. Robert C. Brunham, MD, for reviewing the manuscript. The authors also want to thank Dr. Päivi Tommola, MD, PhD, Dr. Leila Unkila-Kallio, MD, PhD, Prof. Seppo Meri, MD, PhD, and Dr. Ralf Bützow, MD, PhD for research collaboration. The authors also want to thank Mrs. Pia Nevalainen for technical assistance.
American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, American Society for Colposcopy and Cervical Pathology (ASCCP) (2016). Committee Opinion No 673: Persistent Vulvar Pain. Obstet. Gynecol. 128 (3), e78–e84.
Bergeron, S., Khalife, S., Dupuis, M. J., McDuff, P. (2016). A Randomized Clinical Trial Comparing Group Cognitive-Behavioral Therapy and a Topical Steroid for Women With Dyspareunia. J. Consult. Clin. Psychol. 3, 259–268. doi: 10.1037/ccp0000072
Bohm-Starke, N., Hilliges, M., Falconer, C., Rylander, E. (1998). Increased Intraepithelial Innervation in Women With Vulvar Vestibulitis Syndrome. Gynecol. Obstet. Invest. 4, 256–260. doi: 10.1159/000010045
Bohm-Starke, N., Hilliges, M., Falconer, C., Rylander, E. (1999). Neurochemical Characterization of the Vestibular Nerves in Women With Vulvar Vestibulitis Syndrome. Gynecol. Obstet. Invest. 48 (4), 270–275. doi: 10.1159/000010198
Bohm-Starke, N., Johannesson, U., Hilliges, M., Rylander, E., Torebjörk, E. (2004). Decreased Mechanical Pain Threshold in the Vestibular Mucosa of Women Using Oral Contraceptives: A Contributing Factor in Vulvar Vestibulitis? J. Reprod. Med. 49, 888–892.
Bornstein, J., Goldstein, A. T., Stockdale, C. K., Bergeron, S., Pukall, C., Zolnoun, D., et al. (2016). Consensus Vulvar Pain Terminology Committee of the International Society for the Study of Vulvovaginal Disease (ISSVD), the International Society for the Study of Women's Sexual Health (ISSWSH), and the International Pelvic Pain Society CIPPS). Obstet. Gynecol. 127 (4), 745–751. doi: 10.1097/AOG.0000000000001359
Chalmers, K. J., Madden, V. J., Hutchinson, M. R., Moseley, G. L. (2016). Local and Systemic Inflammation in Localized, Provoked Vestibulodynia: A Systematic Review. Obstet. Gynecol. 128 (2), 337–347. doi: 10.1097/AOG.0000000000001510
Falsetta, M. L., Foster, D. C., Woeller, C. F., Pollock, S. J., Song, K., Bonham, A., et al. (2015). Identification of Novel Mechanisms Involved in Generating Localized Vulvodynia Pain. Am. J. Obstet. Gynecol. 213 (1), 38.e1–38.12. doi: 10.1016/j.ajog.2015.02.002
Farmer, M. A., Taylor, A. M., Bailey, A. L., Tuttle, A. H., McIntyre, L. C., Milagrosa, Z. E., et al. (2011). Repeated Vulvovaginal Fungal Infections Cause Persistent Pain in a Mouse Model of Vulvodynia. Sci. Transl. Med. 21;3 (101), 101ra91. doi: 10.1126/scitranslmed.3002613
Foster, D. C., Falsetta, M. L., Woeller, C. F., Pollock, S. J., Bonham, A. D., Haidaris, C. G., et al. (2015). Site-Specific Mesenchymal Control of Inflammatory Pain to Yeast Challenge in Vulvodynia-Afflicted and Pain-Free Women. Pain 3, 386–396. doi: 10.1097/01.j.pain.0000460320.95267.5d
Foster, D. C., Hasday, J. D. (1997). Elevated Tissue Levels of Interleukin-L Beta and Tumor Necrosis Factor-Alpha in Vulvar Vestibulitis. Obstet. Gynecol. 89 (2), 291– 296. doi: 10.1016/S0029-7844(96)00447-4
Foster, D. C., Piekarz, K. H., Murant, TI, LaPoint, R., Haidaris, C. G., Phipps, R. P. (2007). Enhanced Synthesis of Proinflammatory Cytokines by Vulvar Vestibular Fibroblasts: Implications for Vulvar Vestibulitis. Am. J. Obstet. Gynecol. 196;4, 346.e1–3346e8. doi: 10.1016/j.ajog.2006.12.038
Goetsch, M. F., Morgan, T. K., Korcheva, V. B., Li, H., Peters, D., Leclair, C. M. (2010). Histologic and Receptor Analysis of Primary and Secondary Vestibulodynia and Controls: A Prospective Study. Am. J. Obstet. Gynecol. 202 (6), 614.e1–614.e8. doi: 10.1016/j.ajog.2010.01.028
Goldstein, A. T., Pukall, C. F., Browm, C., Bergeron, S., Stein, A., Kellogg-Spadt, S. (2016). Vulvodynia: Assessment and Treatment. J. Sex Med. 4, 572–590. doi: 10.1016/j.jsxm.2016.01.020
Halperin, R., Zehavi, S., Vaknin, Z., Ben-Ami, I., Pansky, M., Schneider, D. (2005). The Major Histopathologic Characteristics in the Vulvar Vestibulitis Syndrome. Gynecol. Obstet. Invest. 2, 75–79. doi: 10.1159/000082112
Harlow, B. L., Gunther Stewart, E. (2005). Adult-Onset Vulvodynia in Relation to Childhood Violence Victimization. Am. J. Epidemiol. 161 (9), 871–880. doi: 10.1093/aje/kwi108
Harlow, B. L., Kunitz, C. G., Nguyen, R. H., Rydell, S. A., Turner, R. M., MacLehose, R. F. (2014). Prevalence of Symptoms Consistent With a Diagnosis of Vulvodynia: Population- Based Estimates From 2 Geographic Regions. Am. J. Obstet. Gynecol. 210 (1), 40.e1–40.e8. doi: 10.1016/j.ajog.2013.09.033
Harlow, B. L., Wise, L. A., Stewart, E. G. (2001). Prevalence and Predictors of Chronic Lower Genital Tract Discomfort. Am. J. Obstet. Gynecol. 185 (3), 545–550. doi: 10.1067/mob.2001.116748
Khandker, M., Brady, S. S., Rydell, S. A., Turner, R. M., Schreiner, P. J., Harlow, B. L. (2019). Early-Life Chronic Stressors, Rumination, and the Onset of Vulvodynia. J. Sex Med. 16 (6), 880–890. doi: 10.1016/j.jsxm.2019.03.010
Khandker, M., Brady, S. S., Stewart, E. G., Harlow, B. L. (2014). Is Chronic Stress During Childhood Associated With Adult Onset Vulvodynia? J. Womens Health 23, 649–656. doi: 10.1089/jwh.2013.4484
Khandker, M., Brady, S. S., Vitonis, A. F., MacLehose, R. F., Stewart, E. G., Harlow, B. L. (2011). The Influence of Depression and Anxiety on Risk of Adult Onset Vulvodynia. J. Womens Health 20 (10), 1445–1451. doi: 10.1089/jwh.2010.2661
Leclair, C. M., Leeborg, N. J., Jacobson-Dunlop, E., Goetsch, M. F., Morgan, T. K. (2014). CD4- Positive T-Cell Recruitment in Primary-Provoked Localized Vulvodynia: Potential Insights Into Disease Triggers. J. Low. Genit. Tract Dis. 18 (2), 195–201. doi: 10.1097/LGT.0b013e3182a55591
Leusink, P., van de Pasch, S., Teunissen, D., Laan, E. T., Lagro-Janssen, A. L. (2018). The Relationship Between Vulvovaginal Candidiasis and Provoked Vulvodynia: A Systematic Review. J. Sex Med. 15 (9), 1310– 1321. doi: 10.1016/j.jsxm.2018.07.011
Liao, Z., Chakrabarty, A., Mu, Y., Bhattacherjee, A., Goestch, M., Leclair, C. M., et al. (2017). A Local Inflammatory Renin-Angiotensin System Drives Sensory Axon Sprouting in Provoked Vestibulodynia. J. Pain 18 (5), 511–525. doi: 10.1016/j.jpain.2016.12.008
Miranda Varella Pereira, G., Soriano Marcolino, M., Silveira Nogueira Reis, Z., Vale de Castro Monteiro, M. (2018). A Systematic Review of Drug Treatment of Vulvodynia: Evidence of a Strong Placebo Effect. BJOG 125 (10), 1216–1224. doi: 10.1111/1471-0528.15223
Morgan, T. K., Allen-Brady, K. L., Monson, M. A., Leclair, C. M., Sharp, H. T., Cannon-Albright, L. A. (2016). Familiality Analysis of Provoked Vestibulodynia Treated by Vestibulectomy Supports Genetic Predisposition. Am. J. Obstet. Gynecol. 214, 609.e1–609.e7. doi: 10.1016/j.ajog.2015.11.019
Ramirez De Knott, H. M., McCormick, T. S., Do, S. O., Goodman, W., Ghannoum, M. A., Cooper, K. D., et al. (2005). Cutaneous Hypersensitivity to Candida Albicans in Idiopathic Vulvodynia. Contact Dermatitis 53 (4), 214–218. doi: 10.1111/j.0105-1873.2005.00685.x
Robert, C., Kupper, T. S. (1999). Inflammatory Skin Diseases, T Cells, and Immune Surveillance. N. Engl. J. Med. 341 (24), 1817–1828. doi: 10.1056/NEJM199912093412407
Spoelstra, S. K., Borg, C., Weijmar Schultz, W. C. M. (2013). Anticonvulsant Pharmacotherapy for Generalized and Localized Vulvodynia: A Critical Review of the Literature. J. Psychosom. Obstet. Gynaecol. 3, 133–138. doi: 10.3109/0167482X.2013.823942
Tommola, P., Bützow, R., Unkila-Kallio, L., Paavonen, J., Meri, S. (2015). Activation of Vestibule-Associated Lymphoid Tissue in Localized Provoked Vulvodynia. Am. J. Obstet. Gynecol. 212 (4), 476.e1–476.e8. doi: 10.1016/j.ajog.2014.10.1098
Tommola, P., Unkila-Kallio, L., Paavonen, J. (2010). Surgical Treatment of Vulvar Vestibulitis: A Review. Acta Obstet. Gynecol. Scand. 89 (11), 1385–1395. doi: 10.3109/00016349.2010.512071
Tommola, P., Unkila-Kallio Paavonen, J. (2011). Long-Term Follow Up of Posterior Vestibulectomy for Treating Vulvar Vestibulitis. Acta Obstet. Gynecol. Scand. 90 (11), 1225–1231. doi: 10.1111/j.1600-0412.2011.01248.x
Keywords: localized provoked vulvodynia, vulvar pain syndrome, vulvar vestibulitis syndrome, vulvodynia, vulvar pain
Citation: Paavonen J and Eschenbach DA (2021) Localized Provoked Vulvodynia-An Ignored Vulvar Pain Syndrome. Front. Cell. Infect. Microbiol. 11:678961. doi: 10.3389/fcimb.2021.678961
Received: 10 March 2021; Accepted: 03 June 2021;
Published: 17 June 2021.
Edited by:
Megan L. Falsetta, University of Rochester, United StatesReviewed by:
Surya Prakash Pandey, University of Pittsburgh, United StatesCopyright © 2021 Paavonen and Eschenbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jorma Paavonen, am9ybWEucGFhdm9uZW5AaGVsc2lua2kuZmk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.