
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 20 July 2021
Sec. Clinical Microbiology
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.658070
The emergence and prevalence of carbapenem-resistant Enterobacteriaceae (CRE) have drawn worldwide attention. Ceftazidime/avibactam (CAZ/AVI) gives us a valuable alternative strategy to treat CRE infections. Unfortunately, CAZ/AVI resistance could occur during CAZ/AVI treatment. The CAZ/AVI-resistant Carbapenem-resistant Klebsiella pneumoniae (CR-KP) (KP137060) and earlier CAZ/AVI-susceptible isolate (KP135194) from the same hospitalized patient were collected at Fujian Medical University Union Hospital between October and November 2019. In this study, CAZ/AVI MICs of CAZ/AVI-susceptible and -resistant isolates (KP135194 and KP137060) were 4 mg/L and 128 mg/L, respectively; and the two isolates had the same antibiotic resistance pattern to other carbapenems. Two strains were then submitted for whole-genome sequencing and bioinformatic analysis. ompK36 was not detected in two isolates. No mutation was observed in blaKPC-2, ompK35 and ompK37 in this study and there was no significant difference of the expression in blaKPC-2, ompK35 and ompK37 between the two isolates (p>0.05). Two isolates were sequence type 11 and harbored blaKPC-2, blaSHV-182 and blaTEM-1B. Compared with KP135194, KP137060 harbored an additional blaNDM-5 positive plasmid. blaNDM-5 gene could be successfully transferred into E. coli J53 at a conjugation frequency of 1.14×10-4. Plasmid stability testing showed that blaKPC-2- and blaNDM-5-harboring plasmids were still stably maintained in the hosts. Growth assay and growth competition experiments showed there was no significant difference in fitness cost between two CR-KP isolates. Our study described the acquisition of a blaNDM-5-harboring plasmid leading to resistance to ceftazidime/avibactam in KPC-2-producing Klebsiella pneumoniae during treatment. This phenomenon deserves further exploration.
The emergence and prevalence of carbapenem-resistant Enterobacteriaceae (CRE) have attracted extensive attention (Potter et al., 2016). Carbapenem-resistant Klebsiella pneumoniae (CR-KP) is the most common CRE worldwide (Grundmann et al., 2017; Han et al., 2020). CR-KP can lead to many kinds of infections, such as pneumonia, bloodstream infections and urinary tract infections, resulting in high morbidity and mortality (Huang et al., 2018). CR-KP is an extremely severe public health challenge nowadays (Zheng et al., 2017; Jung et al., 2019). Patients with CR-KP infections had few effective treatment options, including polymyxins, tigecycline, aminoglycosides and fosfomycin (Zhang et al., 2019). However, these antibiotics are limited by efficacy and safety (Satlin et al., 2011).
Ceftazidime/avibactam (CAZ/AVI) is a valuable alternative strategy to treat KPC-producing K. pneumoniae infections (Tumbarello et al., 2019). CAZ/AVI is a novel combination of ceftazidime and the β-lactamase inhibitor avibactam with activity against class A, class C and some class D carbapenemases, including the Klebsiella pneumoniae carbapenemase (KPC) (Zasowski et al., 2015; Shirley, 2018). Furthermore, CAZ/AVI has no activity against class B carbapenemases, such as New Delhi metallo-β-lactamase (NDM) (Thaden et al., 2017; Zhang et al., 2020). NDM from Klebsiella pneumoniae was first found in 2009 (Kumarasamy et al., 2010). Up to now, a total of 31 variants (NDM-1 to NDM-31) have been detected globally and the sequences were deposited in NCBI [https://www.ncbi.nlm.nih.gov/pathogens/refgene/#gene_family: (blaNDM)]. Among these variants, NDM-5 was first identified in an Escherichia coli strain in the UK in 2011 and has drawn worldwide attention due to its rapid dissemination (Hornsey et al., 2011). Furthermore, NDM-5-producing strains have been usually identified from humans, animals and hospital environments’ sewage water (Hornsey et al., 2011; Yousfi et al., 2015; Parvez and Khan, 2018). Worryingly, NDM-5 has been identified in various species of Enterobacterales across many cities in China (Mao et al., 2018; Sun et al., 2018; Guo et al., 2019). Like other variants of NDM, NDM-5 can lead to CAZ/AVI resistance (Wei et al., 2020).
Many studies found that CAZ/AVI resistance could occur during CAZ/AVI treatment, such as mutations in blaKPC, the increased copy number of the variation of blaKPC (blaKPC-3) and overexpression of blaKPC (Shields et al., 2017; Hemarajata and Humphries, 2019; Räisänen et al., 2019; Coppi et al., 2020; Zhang et al., 2020). However, there has been no report of a case of acquiring metallo-β-Lactamase genes during CAZ/AVI treatment. In this study, we reported the emergence of CAZ/AVI-resistance due to acquisition of a metallo-β-lactamase gene during treatment of KPC-2-producing Klebsiella pneumoniae infections and identify and validate the reason for CAZ/AVI-resistance emergence using genomic and molecular genetic approaches.
The CAZ/AVI-resistant CR-KP (KP137060) and earlier CAZ/AVI-susceptible isolate (KP135194) from the same hospitalized patient were collected at Fujian Medical University Union Hospital between October and November 2019. The bacterial species were identified using Vitek-2 (GN cards).
Following a traffic accident, a 67-year-old man was admitted to our ICU with pulmonary infections. Treatment with tigecycline (50 mg every 12 h) and polymyxin B (0.5 miu every 12 h) was initiated after obtaining postbronchoscopic sputum cultures, which yielded CR-KP. However, postbronchoscopic sputum cultures were still positive for CR-KP (KP135194) after 16 days of treatment, susceptible to CAZ/AVI and resistant to carbapenems. Eight CR-KPs have been isolated before KP135194 and they had the same MICs in CAZ/AVI, MEM, ETP and IMP (4µg/ml, ≥16µg/ml, ≥16µg/ml and ≥8µg/ml, respectively). Then the patient was switched to CAZ/AVI treatment. The treatment with CAZ/AVI in monotherapy (2.5 g every 8 h) was administered for 11 days. However, postbronchoscopic sputum cultures were positive for CR-KP (KP137060) again after seven days end of the CAZ/AVI treatment, resistant to both CAZ/AVI and carbapenems. No other CR-KP was isolated between KP135194 and KP137060. Stenotrophomonas maltophilia had been recovered in postbronchoscopic sputum cultures on hospital day 19, day 21, day 37 and day 42. The patient was subsequently discharged and turned to another hospital. Microbiologic details, timelines and antibiotic therapies used were summarized in Figure 1.
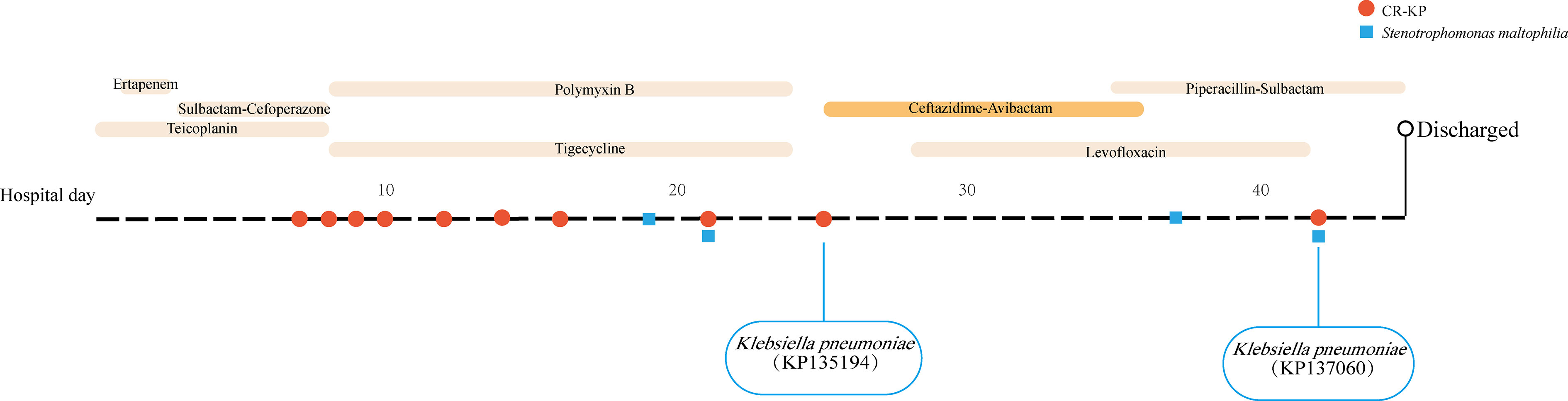
Figure 1 Time courses of infection and treatment of the patient with carbapenem-resistant K. pneumoniae infection.
The antimicrobial susceptibility testing was performed by Vitek-2 (Vitek-AST-GN16 and Vitek-AST-GN09) according to the Clinical and Laboratory Standards Institute guidelines (CLSI, 2020). CAZ powder was kindly provided by Pfizer (China) and AVI powder was purchased from GlpBio (Montclair, CA, USA). CAZ/AVI susceptibility testing was performed by agar dilution method. Avibactam was tested at a fixed concentration of 4µg/ml, in combination with 2-fold dilutions of ceftazidime. MICs were interpreted according to CLSI susceptible breakpoint of ≤ 8/4 μg/ml (CLSI, 2020). E. coli ATCC 25922 was used as quality control.
The presence of carbapenemase genes (blaKPC, blaNDM, blaVIM and blaIMP) was identified by PCR as described previously (Li et al., 2014). The complete sequence of blaKPC was determined by Sanger sequencing and subsequently submitted to BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi) against the blaKPC reference sequence (GenBank accession number: NG_049253.1). The primers of carbapenemase genes are listed in Table S1.
Clonal relatedness of two CR-KPs was analyzed by ERIC-PCR as described previously (Smith et al., 2007).
Overnight cultures of two CR-KP strains were grown till the logarithmic growth phase in LB broth medium and cells were harvested at an optical density of 1 at 600 nm (OD600). Total RNA was extracted from the two isolates using Trizol reagent (Sigma-Aldrich, USA) according to the manufacturer’s instructions. The expression levels of RNA coding for blaKPC-2, ompK35 and ompK37 were estimated by qRT-PCR using SYBR Green detection reagents (Tiangen, Beijing) on ABI 7500 Real-Time PCR system. The Relative gene expression levels were calculated using the 2-ΔCT formula with the rpsL gene as the internal control (Haeili et al., 2017). All samples were performed in triplicate. rpsL, blaKPC-2, ompK35 and ompK37 were amplified using primers listed in Table S1.
Conjugation experiments were performed to test the transferability of blaKPC-2 and blaNDM-5-harboring plasmids using filter mating (Shintani et al., 2019). E. coli J53 AzR was used as the recipient. The mating mixture was washed from the filter and spread onto MH agar containing sodium azide at 100 mg/liter and imipenem at 1 mg/liter. The grown isolates were selected and identified by PCR (Li et al., 2014). The antimicrobial susceptibility testing of the recipient and transconjugant was performed as described above. The conjugation frequency (CF) was calculated as follow (Shintani et al., 2019):
Plasmid stability testing was conducted to evaluate the stability of blaKPC-2 and blaNDM-5-harboring plasmids as described previously (Johnson et al., 2016).
Growth assay and in vitro growth competition experiments were tested to assess the fitness cost of KP135194 and KP137060. Growth assay was performed as described previously (Fernández et al., 2012). In vitro competition experiments were performed as described previously (Li et al., 2017).
The genomic DNA samples were extracted from the CAZ/AVI-susceptible and –resistant isolates by TIANamp Bacteria DNA Kit (Tiangen, Beijing), sequenced using the Illumina NovaSeq platform at Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). The raw data were assembled using SPAdesv3.12.0. The genetic relationship of the two strains was determined by average nucleotide identity (ANI) and core genome SNPs analysis (Richter et al., 2016). The Center for Genomic Epidemiology (https://cge.cbs.dtu.dk/services/) was used to identify resistance genes, major outer membrane porin genes (ompK35, ompK36 and ompK37) and plasmid types. The multilocus-sequence typing was identified on MLST. VFDB (http://www.mgc.ac.cn/VFs/main.htm) was used to predict the virulence genes. The K-type prediction was carried out by Kaptive (http://kaptive.holtlab.net).
For plasmids, the putative extrachromosomal contigs combining the identification with the genes encoding plasmid replication, partition, conjugative transfer or resistance, were recognized as potential plasmid contigs (Botelho et al., 2017; Xu et al., 2018). The blaKPC-2 and blaNDM-5 were identified on those plasmid-associated contigs in assembled genomes, determining in silico the blaKPC-2 (or blaNDM-5) location on plasmids. The putative plasmid contigs were analyzed and re-assembled to build single circular plasmids by mapping reference complete plasmid genome. Additional sets of PCRs were performed to confirm the circular status of predicted plasmids as reported previously (Carattoli et al., 2018; Xu et al., 2018). Genes were predicted with GeneMarkS™ and further annotated by BLASTP and BLASTN against NR databases and SwissProt. The BLASTN program was used to compare similar plasmids in the international databases.
Statistical analysis was performed using SAS 9.4. Chi-square test or Fisher’s exact test (two-tailed) or Mann-Whitney test was performed for data comparison. Only p < 0.05 was set as the significance level.
ERIC-PCR analysis demonstrated that two CR-KP isolates were clonally related (Figure S1). KP137060 had higher MICs of CAZ/AVI (5-doubling dilution difference) and TGC (1-doubling dilution difference) than KP135194. They had the same MICs in MEM, ETP and IMP. Antimicrobial susceptibility profiles of the two CR-KPs were shown in Table 1. blaKPC-2 was found in both isolates, and blaNDM-5 was also detected in KP137060.
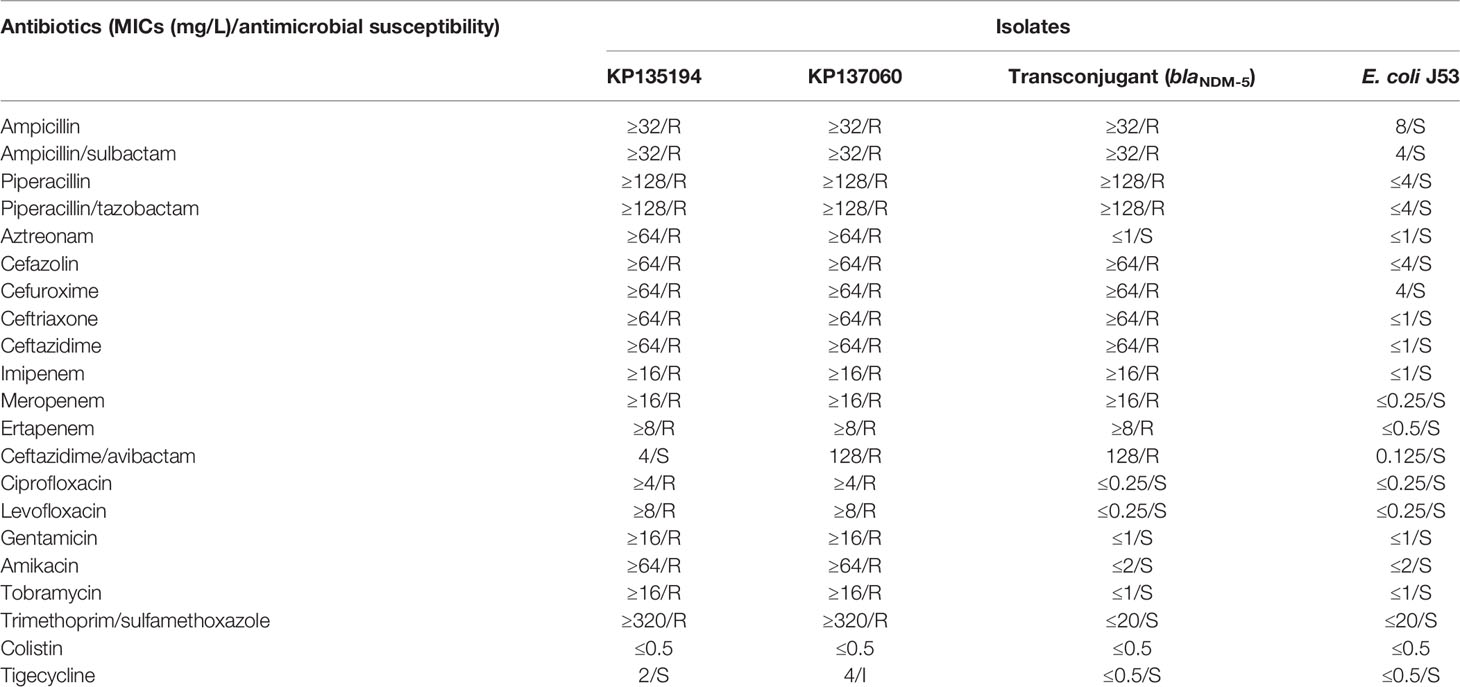
Table 1 Minimal inhibitory concentrations (MICs) of two CR-KP isolates, the blaNDM-5-positive E. coli transconjugant and E. coli J53 recipient strains.
The results of PCR amplification and sequencing of blaKPC-2 suggested that no mutation was found in blaKPC-2 in two CR-KP isolates (Table 2). We did not detect mutations in ompK35 and ompK37 using ResFinder, respectively. ompK36 was not present in two isolates in this study (Table 2). Meanwhile, there were no significant differences in the expression of blaKPC-2, ompK35 and ompK37 between the two isolates (p=0.2209, p=0.4217 and p=0.8626, respectively) (Figure S2).
In this study, the blaNDM-5 gene was transferred successfully into E. coli J53 at a conjugation frequency of 1.14×10-4, but blaKPC-2 failed. The presence of blaKPC-2 and blaNDM-5 was confirmed by PCR. The results of antimicrobial susceptibility testing among recipient cell and transconjugant were shown in Table 1. Plasmid stability testing showed that blaKPC-2- and blaNDM-5-harboring plasmids were still stably maintained in the CR-KP during 150 generations (Figure S3).
As shown in Figure S4, no significant difference (p>0.05) in growth was observed in two CR-KP isolates. The result of in vitro growth competition experiments showed that the relative fitness cost was 1.01.
General genome features of the sequenced genomes of two strains were shown in Table S2. Genetic analysis demonstrated that the two CR-KP isolates were sequence type (ST) 11 and harbored beta-lactamase genes. The whole-genome analysis revealed that KP135194 and KP137060 were highly homologous, showing 99.99% ANI values between them. The result of core genome SNPs analysis showed that the number of nonsynonymous SNPs in the core genome was 23 between the two strains. In this study, KP137060 harbored an additional blaNDM-5. Other resistance genes were detected in both isolates, which were shown in Table 2, including rmtB, fosA, catA2, qnrS1, sul2, tet (A) and dfrA14. Virulence genes (rmpA and rmpA2) and capsule types (K1 and K2) genes associated with hypervirulent K. pneumoniae (hvKp) were not found (Table 2).
Compared with the KP135194, KP137060 had an additional plasmid (IncX3) (Table 2). Additionally, the results of whole-genome sequencing suggested that blaKPC-2 (IncFII/IncR) and blaNDM-5 (IncX3) located in two different plasmids, respectively. The complete nucleotide sequences of blaKPC-2- and blaNDM-5-harboring plasmids and sizes of plasmids were shown in Figure 2. Table S3 showed the results of blaNDM-5-harboring plasmid and its sequences producing significant alignments using the BLAST tool.
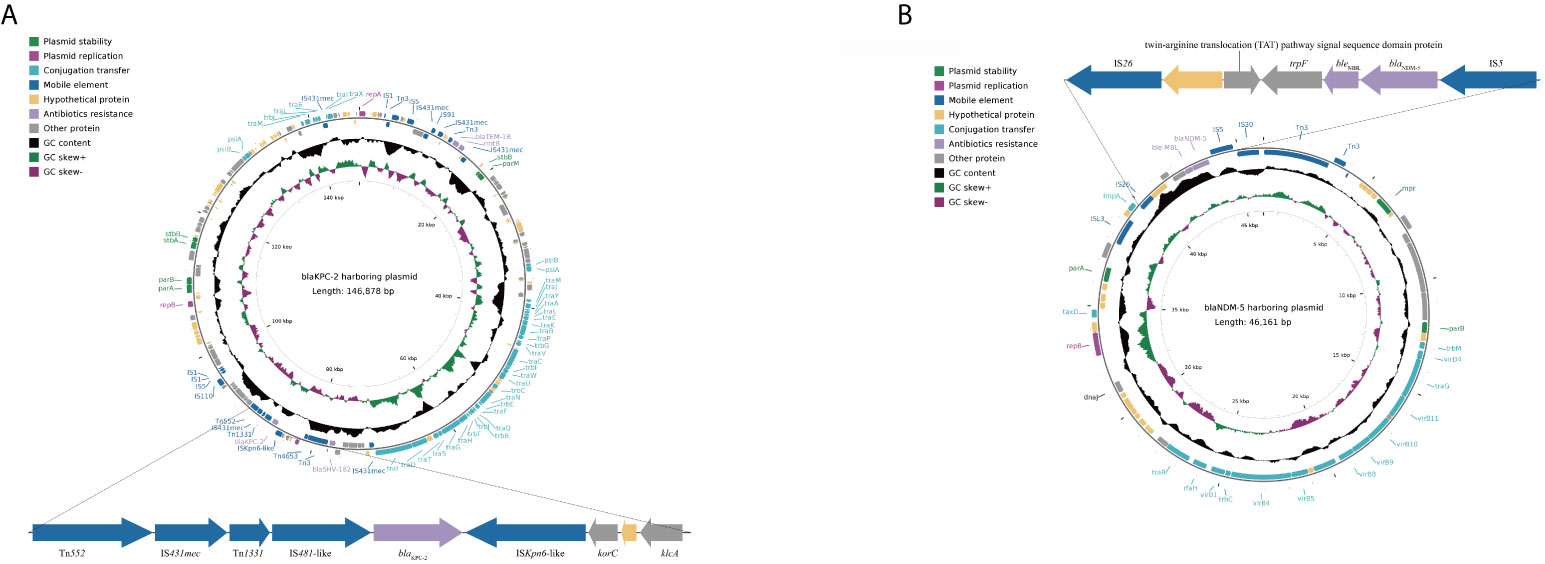
Figure 2 Schematic maps of the blaKPC-2-harboring plasmid (A) and the blaNDM-5-harboring plasmid (B). Genes are denoted by arrows and colored based on gene function classification. The innermost circle represents GC-Skew [G-C)/G+C]. The black circle represents GC content. The pKPC-CR-hvKP-C789 plasmid was used to reconstruct blaKPC-2-harboring plasmid as reference plasmid (accession number: CP034417.1). The pCREC-591_4 plasmid was used to reconstruct blaNDM-5-harboring plasmid as reference plasmid (accession number: CP024825.1).
For the genetic environment of the blaKPC-2gene, upstream of the blaKPC-2 gene was located in a Tn1331 element. Furthermore, a putative ISKpn6-like element was located downstream of the blaKPC-2 gene, followed by the korC gene (a gene encoding transcriptional repressor protein), a gene encoding hypothetical proteins and the klcA gene. For blaNDM-5, upstream of the blaNDM-5 was located a putative IS26 element, a gene encoding twin-arginine translocation (TAT) pathway signal sequence domain protein, a gene encoding hypothetical proteins and a trpF gene (a gene encoding phosphorybosilanthranilate isomerase gene), were inserted between them. In addition, an IS5 element was located downstream of blaNDM-5.
CAZ/AVI has been considered a promising treatment strategy for CRKP infections after approval by the U.S. Food & Drug Administration (FDA) in 2015 (van Duin and Bonomo, 2016). Unfortunately, many reports found CAZ/AVI resistance could occur during CAZ/AVI treatment (Shields et al., 2017; Räisänen et al., 2019; Zhang et al., 2020). According to the rapid risk assessment published by the European Centre for Disease Prevention and Control (ECDC) in 2018, the emergence of CAZ-AVI resistance is an important cross-border threat that should be monitored carefully (Räisänen et al., 2019). Here, in this study, we reported the acquisition of a metallo-β-lactamase gene leading to resistance to ceftazidime/avibactam in KPC-2-producing K. pneumoniae during treatment.
CAZ/AVI resistance has been linked to the specific mutations in blaKPC, blaCTX-M and outer membrane protein genes, the increased copy number of the variation of blaKPC (blaKPC-3), overexpression of blaKPC and outer membrane protein genes and acquisition of MBL genes (Galani et al., 2019; Hemarajata and Humphries, 2019; Coppi et al., 2020; Zhang et al., 2020). In this study, a MBL gene, blaNDM-5, was detected in KP137060. Our results indicated that the acquisition of blaNDM-5 during CAZ/AVI treatment was the possible reason of its resistance to CAZ/AVI. Meanwhile, KP137060 had higher MICs of CAZ/AVI (5-doubling dilution difference) than KP135194 (Table 1), which was supported by the fact that CAZ/AVI has no activity against class B carbapenemases (Mueller et al., 2019). In addition, recent study found that the MBL-producing CR-KP isolates, including VIM-producing and VIM/KPC-co-producing isolates, increased after the introduction of CAZ/AVI treatment in an ICU ward (Papadimitriou-Olivgeris et al., 2019). Therefore, our study provided evidence to the fact that widespread CAZ/AVI use might lead to a change in carbapenemases palettes with the substitution of MBL-producing CR-KP isolates for KPC (Papadimitriou-Olivgeris et al., 2019).
In this study, blaKPC-2 and blaNDM-5 located in two different plasmids, respectively (Figure 2). Our results indicated that blaNDM-5 was contained on IncX3-type plasmid in KP137060. IncX3 plasmids were usually detected in clinical blaNDM-5-positive bacteria in China (Cao et al., 2020; Gao et al., 2020). The results of BLAST demonstrated that the blaNDM-5-harboring plasmid showed >99% sequence identity to the corresponding regions of other plasmids identified in other countries and isolated from different hosts (Table S3), suggesting that this type of plasmid was widespread dissemination worldwide. Meanwhile, many studies have characterized the genetic environments of the blaNDM-5 and various mobile genetic elements played a critical role in the rapid spread of blaNDM-5 (Pitart et al., 2015; Reynolds et al., 2019). In this study, we described the genetic environment of blaNDM-5 (Figure 2). It is noteworth that such a genetic environment was found in different plasmids in China, probably indicating that they have a common origin (Liu et al., 2019; Cao et al., 2020). Additionally, the genetic environment of blaNDM-5 was similar to those in previously reported clinical K. pneumoniae isolates in India and Spain (Krishnaraju et al., 2015; Pitart et al., 2015). The results of this study suggested that the genetic environment of blaNDM-5could contribute to the widespread of CAZ/AVI-resistant CR-KPs.
In addition, our study demonstrated that the blaNDM-5-harboring plasmid was transferrable and stable. Given the high transferability and stability of blaNDM-5-harboring plasmid in clinical CR-KP, the spread of blaNDM-5-harboring plasmids into other K. pneumoniae had the potential to cause refractory infections due to limited therapeutic options. Futhermore, there were no significant fitness cost compared with KP135194 in this study (Figure S4), indicating that no considerable fitness costs were identified in carriage of the plasmid containing blaNDM-5 could be accepted as an important advantage of CAZ/AVI-resistant CR-KP clonal propagation, increasing the risk of dissemination.
In the present case, the patient was treated with 11 days of CAZ/AVI (2.5 g; administered intravenously every 8 h) treatment. After that, CAZ/AVI-resistant CR-KP was identified and clonally related to earlier CAZ/AVI-susceptible CR-KP. Risk factors associated with acquired CR-KP colonization included previous exposure to tigecycline or β-lactam/β-lactamases inhibitor, ICU stays and invasive processes or surgical operations (Qin et al., 2020). In current study, the patient stayed in ICU for up to 30 days and received tigecycline treatment before CAZ/AVI treatment. CR-KP was not isolated when the patient was admitted to the hospital. Additionally, CR-KP was most common among CRE in our hospital (He et al., 2016; Li et al., 2019). Based on the above-mention facts, we speculated the patient might get CR-KP colonization during his stay at ICU and acquisition of a blaNDM-5-harboring plasmid in KP137096 was caused mainly by the selective pressure of CAZ/AVI usage.
There were some limitations to our study. First, the CRE-screening tests were not carried out to exclude the presence of the NDM-5 producer as colonizer using specimens from sputum or/and rectal swabs. Meanwhile, because the determination was not performed to detect the carbapenem genes in clinical CRE strains in our hospital, we could not know the origin of blaNDM-5. Second, the CAZ/AVI-resistant CR-KP strain was isolated after CAZ/AVI treatment. Therefore, we could not exclude the probability that KPC-2-producing K.pneumonia has harbored the blaNDM-5 from different bacteria that carried it through HGT during the patient’s hospitalization, rather than the CAZ-AVI treatment. Third, we only used additional PCR sets to confirm the circular status of predicted plasmids in this study. The results of plasmid contents should be more reliable if the S1-PFGE coupled with hybridization could be used in this study.
Our study demonstrated the acquisition of a blaNDM-5-harboring plasmid leading to resistance to ceftazidime/avibactam in KPC-2-producing Klebsiella pneumoniae during treatment. Meanwhile, the blaNDM-5-harboring plasmid was transferrable and the conjugation frequency was 1.14×10-4. Furthermore, no considerable fitness costs were identified in the carriage of the plasmid containing blaNDM-5. Therefore, careful monitoring of resistance development by bacterial cultures and subsequent susceptibility testing is significant. Continued surveillance is required to avoid CAZ/AVI-resistance CR-KP dissemination.
The datasets presented in this study can be found in online repositories. The complete genome sequence of CR-KP strains KP135194 and KP137060 were deposited in GenBank with accession numbers JABJTP000000000 and JABJWR000000000 under BioProject PRJNA633438. The complete sequences of blaNDM-5-harboring plasmid and blaKPC-2-harboring plasmid were submitted to GenBank under accession numbers MW218142 and MW218143, respectively.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Fujian Medical University Union Hospital (No.2020KY088). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JH and SZ performed experiments. JH and ZZ designed the study and drafted the manuscript. SZ analyzed the data. YC and MC supervised the study. BL revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
This work was supported by the Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2017Y9049, 2017Y9051) and the Educational and Scientific Research Project for Young and Middle-Aged Teachers of Fujian Province (Grant number: JAT190191).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.658070/full#supplementary-material
Botelho, J., Grosso, F., Quinteira, S., Mabrouk, A., Peixe, L. (2017). The Complete Nucleotide Sequence of an IncP-2 Megaplasmid Unveils a Mosaic Architecture Comprising a Putative Novel blaVIM-2-Harbouring Transposon in Pseudomonas Aeruginosa. J. Antimicrob. Chemother. 72 (8), 2225–2229. doi: 10.1093/jac/dkx143
Cao, T., Liu, Y., Li, Y., Wang, Y., Shen, Z., Shao, B., et al. (2020). A Public Health Concern: Emergence of Carbapenem-Resistant Klebsiella Pneumoniae in a Public Transportation Environment. J. Antimicrob. Chemother. 75 (10), 2769–2772. doi: 10.1093/jac/dkaa260
Carattoli, A., Carretto, E., Brovarone, F., Sarti, M., Villa, L. (2018). Comparative Analysis of an Mcr-4 Salmonella Enterica Subsp. Enterica Monophasic Variant of Human and Animal Origin. J. Antimicrob. Chemother. 73 (12), 3332–3335. doi: 10.1093/jac/dky340
CLSI (2020). M100-S28. Performance Standards for Antimicrobial Susceptibility Testing: 30th Informational Supplement (Wayne, PA: Clinical and Laboratory Standards Institute).
Coppi, M., Di Pilato, V., Monaco, F., Giani, T., Conaldi, P. G., Rossolini, G. M. (2020). Ceftazidime-Avibactam Resistance Associated With Increased blaKPC-3 Gene Copy Number Mediated by Pkpqil Plasmid Derivatives in Sequence Type 258 Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 64 (4), e01816–e01819. doi: 10.1128/aac.01816-19
Fernández, A., Pérez, A., Ayala, J. A., Mallo, S., Rumbo-Feal, S., Tomás, M., et al. (2012). Expression of OXA-Type and SFO-1 β-Lactamases Induces Changes in Peptidoglycan Composition and Affects Bacterial Fitness. Antimicrob. Agents Chemother. 56 (4), 1877–1884. doi: 10.1128/aac.05402-11
Galani, I., Antoniadou, A., Karaiskos, I., Kontopoulou, K., Giamarellou, H., Souli, M. (2019). Genomic Characterization of a KPC-23-Producing Klebsiella Pneumoniae ST258 Clinical Isolate Resistant to Ceftazidime-Avibactam. Clin. Microbiol. Infect. 25 (6), 763.e765–763.e768. doi: 10.1016/j.cmi.2019.03.011
Gao, Y., Wen, J., Wang, S., Xu, X., Zhan, Z., Chen, Z., et al. (2020). Plasmid-Encoded blaNDM-5 Gene That Confers High-Level Carbapenem Resistance in Salmonella Typhimurium of Pork Origin. Infect. Drug Resist. 13, 1485–1490. doi: 10.2147/idr.S249357
Grundmann, H., Glasner, C., Albiger, B., Aanensen, D. M., Tomlinson, C. T., Andrasević, A. T., et al. (2017). Occurrence of Carbapenemase-Producing Klebsiella Pneumoniae and Escherichia Coli in the European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE): A Prospective, Multinational Study. Lancet Infect. Dis. 17 (2), 153–163. doi: 10.1016/s1473-3099(16)30257-2
Guo, X., Rao, Y., Guo, L., Xu, H., Lv, T., Yu, X., et al. (2019). Detection and Genomic Characterization of a Morganella Morganii Isolate From China That Produces NDM-5. Front. Microbiol. 10, 1156. doi: 10.3389/fmicb.2019.01156
Haeili, M., Javani, A., Moradi, J., Jafari, Z., Feizabadi, M. M., Babaei, E. (2017). MgrB Alterations Mediate Colistin Resistance in Klebsiella Pneumoniae Isolates From Iran. Front. Microbiol. 8, 2470. doi: 10.3389/fmicb.2017.02470
Han, R., Shi, Q., Wu, S., Yin, D., Peng, M., Dong, D., et al. (2020). Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) Among Carbapenem-Resistant Enterobacteriaceae Isolated From Adult and Children Patients in China. Front. Cell Infect. Microbiol. 10, 314. doi: 10.3389/fcimb.2020.00314
He, Q., Chen, W., Huang, L., Lin, Q., Zhang, J., Liu, R., et al. (2016). Performance Evaluation of Three Automated Identification Systems in Detecting Carbapenem-Resistant Enterobacteriaceae. Ann. Clin. Microbiol. Antimicrob. 15 (1), 40. doi: 10.1186/s12941-016-0154-0
Hemarajata, P., Humphries, R. M. (2019). Ceftazidime/avibactam Resistance Associated With L169P Mutation in the Omega Loop of KPC-2. J. Antimicrob. Chemother. 74 (5), 1241–1243. doi: 10.1093/jac/dkz026
Hornsey, M., Phee, L., Wareham, D. W. (2011). A Novel Variant, NDM-5, of the New Delhi Metallo-β-Lactamase in a Multidrug-Resistant Escherichia Coli ST648 Isolate Recovered From a Patient in the United Kingdom. Antimicrob. Agents Chemother. 55 (12), 5952–5954. doi: 10.1128/aac.05108-11
Huang, W., Qiao, F., Zhang, Y., Huang, J., Deng, Y., Li, J., et al. (2018). In-Hospital Medical Costs of Infections Caused by Carbapenem-Resistant Klebsiella Pneumoniae. Clin. Infect. Dis. 67 (suppl_2), S225–s230. doi: 10.1093/cid/ciy642
Johnson, T. J., Danzeisen, J. L., Youmans, B., Case, K., Llop, K., Munoz-Aguayo, J., et al. (2016). Separate F-Type Plasmids Have Shaped the Evolution of the H30 Subclone of Escherichia Coli Sequence Type 131. mSphere 1 (4), e00121–e00116. doi: 10.1128/mSphere.00121-16
Jung, H. J., Littmann, E. R., Seok, R., Leiner, I. M., Taur, Y., Peled, J., et al. (2019). Genome-Wide Screening for Enteric Colonization Factors in Carbapenem-Resistant ST258 Klebsiella Pneumoniae. mBio 10 (2), e02663–e02618. doi: 10.1128/mBio.02663-18
Krishnaraju, M., Kamatchi, C., Jha, A. K., Devasena, N., Vennila, R., Sumathi, G., et al. (2015). Complete Sequencing of an IncX3 Plasmid Carrying blaNDM-5 Allele Reveals an Early Stage in the Dissemination of the blaNDM Gene. Indian J. Med. Microbiol. 33 (1), 30–38. doi: 10.4103/0255-0857.148373
Kumarasamy, K. K., Toleman, M. A., Walsh, T. R., Bagaria, J., Butt, F., Balakrishnan, R., et al. (2010). Emergence of a New Antibiotic Resistance Mechanism in India, Pakistan, and the UK: A Molecular, Biological, and Epidemiological Study. Lancet Infect. Dis. 10 (9), 597–602. doi: 10.1016/s1473-3099(10)70143-2
Li, C., Li, Y., Zhao, Z., Liu, Q., Li, B. (2019). Treatment Options and Clinical Outcomes for Carbapenem-Resistant Enterobacteriaceae Bloodstream Infection in a Chinese University Hospital. J. Infect. Public Health 12 (1), 26–31. doi: 10.1016/j.jiph.2018.08.002
Liu, Z., Xiao, X., Li, Y., Liu, Y., Li, R., Wang, Z. (2019). Emergence of IncX3 Plasmid-Harboring blaNDM-5 Dominated by Escherichia Coli ST48 in a Goose Farm in Jiangsu, China. Front. Microbiol. 10, 2002. doi: 10.3389/fmicb.2019.02002
Li, B., Xu, X. H., Zhao, Z. C., Wang, M. H., Cao, Y. P. (2014). High Prevalence of Metallo-β-Lactamase Among Carbapenem-Resistant Klebsiella Pneumoniae in a Teaching Hospital in China. Can. J. Microbiol. 60 (10), 691–695. doi: 10.1139/cjm-2014-0291
Li, S., Yin, Y., Chen, H., Wang, Q., Wang, X., Wang, H. (2017). Fitness Cost of Daptomycin-Resistant Staphylococcus Aureus Obtained From In Vitro Daptomycin Selection Pressure. Front. Microbiol. 8, 2199. doi: 10.3389/fmicb.2017.02199
Mao, J., Liu, W., Wang, W., Sun, J., Lei, S., Feng, Y. (2018). Antibiotic Exposure Elicits the Emergence of Colistin- and Carbapenem-Resistant Escherichia Coli Coharboring MCR-1 and NDM-5 in a Patient. Virulence 9 (1), 1001–1007. doi: 10.1080/21505594.2018.1486140
Mueller, L., Masseron, A., Prod’Hom, G., Galperine, T., Greub, G., Poirel, L., et al. (2019). Phenotypic, Biochemical and Genetic Analysis of KPC-41, a KPC-3 Variant Conferring Resistance to Ceftazidime-Avibactam and Exhibiting Reduced Carbapenemase Activity. Antimicrob. Agents Chemother. 63 (12), e01111–e01119. doi: 10.1128/aac.01111-19
Papadimitriou-Olivgeris, M., Bartzavali, C., Lambropoulou, A., Solomou, A., Tsiata, E., Anastassiou, E. D., et al. (2019). Reversal of Carbapenemase-Producing Klebsiella Pneumoniae Epidemiology From blaKPC- to blaVIM-Harbouring Isolates in a Greek ICU After Introduction of Ceftazidime/Avibactam. J. Antimicrob. Chemother. 74 (7), 2051–2054. doi: 10.1093/jac/dkz125
Parvez, S., Khan, A. U. (2018). Hospital Sewage Water: A Reservoir for Variants of New Delhi Metallo-β-Lactamase (NDM)- and Extended-Spectrum β-Lactamase (ESBL)-Producing Enterobacteriaceae. Int. J. Antimicrob. Agents 51 (1), 82–88. doi: 10.1016/j.ijantimicag.2017.08.032
Pitart, C., Solé, M., Roca, I., Román, A., Moreno, A., Vila, J., et al. (2015). Molecular Characterization of blaNDM-5 Carried on an IncFII Plasmid in an Escherichia Coli Isolate From a Nontraveler Patient in Spain. Antimicrob. Agents Chemother. 59 (1), 659–662. doi: 10.1128/aac.04040-14
Potter, R. F., D’Souza, A. W., Dantas, G. (2016). The Rapid Spread of Carbapenem-Resistant Enterobacteriaceae. Drug Resist. Update 29, 30–46. doi: 10.1016/j.drup.2016.09.002
Qin, X., Wu, S., Hao, M., Zhu, J., Ding, B., Yang, Y., et al. (2020). The Colonization of Carbapenem-Resistant Klebsiella Pneumoniae: Epidemiology, Resistance Mechanisms, and Risk Factors in Patients Admitted to Intensive Care Units in China. J. Infect. Dis. 221 (Suppl 2), S206–s214. doi: 10.1093/infdis/jiz622
Räisänen, K., Koivula, I., Ilmavirta, H., Puranen, S., Kallonen, T., Lyytikäinen, O., et al. (2019). Emergence of Ceftazidime-Avibactam-Resistant Klebsiella Pneumoniae During Treatment, Finland, December 2018. Euro Surveill 24 (19), 1900256. doi: 10.2807/1560-7917.Es.2019.24.19.1900256
Reynolds, M. E., Phan, H. T. T., George, S., Hubbard, A. T. M., Stoesser, N., Maciuca, I. E., et al. (2019). Occurrence and Characterization of Escherichia Coli ST410 Co-Harbouring blaNDM-5, blaCMY-42 and blaTEM-190 in a Dog From the UK. J. Antimicrob. Chemother. 74 (5), 1207–1211. doi: 10.1093/jac/dkz017
Richter, M., Rosselló-Móra, R., Oliver Glöckner, F., Peplies, J. (2016). JSpeciesWS: A Web Server for Prokaryotic Species Circumscription Based on Pairwise Genome Comparison. Bioinformatics 32 (6), 929–931. doi: 10.1093/bioinformatics/btv681
Satlin, M. J., Kubin, C. J., Blumenthal, J. S., Cohen, A. B., Furuya, E. Y., Wilson, S. J., et al. (2011). Comparative Effectiveness of Aminoglycosides, Polymyxin B, and Tigecycline for Clearance of Carbapenem-Resistant Klebsiella Pneumoniae From Urine. Antimicrob. Agents Chemother. 55 (12), 5893–5899. doi: 10.1128/aac.00387-11
Shields, R. K., Chen, L., Cheng, S., Chavda, K. D., Press, E. G., Snyder, A., et al. (2017). Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations During Treatment of Carbapenem-Resistant Klebsiella Pneumoniae Infections. Antimicrob. Agents Chemother. 61 (3), e02097–e02016. doi: 10.1128/aac.02097-16
Shintani, M., Ohkuma, M., Kimbara, K. (2019). High-Resolution Comparison of Bacterial Conjugation Frequencies. J. Vis. Exp. 143, e57812. doi: 10.3791/57812
Shirley, M. (2018). Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 78 (6), 675–692. doi: 10.1007/s40265-018-0902-x
Smith, J. L., Drum, D. J., Dai, Y., Kim, J. M., Sanchez, S., Maurer, J. J., et al. (2007). Impact of Antimicrobial Usage on Antimicrobial Resistance in Commensal Escherichia Coli Strains Colonizing Broiler Chickens. Appl. Environ. Microbiol. 73 (5), 1404–1414. doi: 10.1128/aem.01193-06
Sun, L., Xu, J., He, F. (2018). Draft Genome Sequence of an NDM-5, CTX-M-15 and OXA-1 Co-Producing Escherichia Coli ST167 Clinical Strain Isolated From a Urine Sample. J. Glob Antimicrob. Resist. 14, 284–286. doi: 10.1016/j.jgar.2018.08.005
Thaden, J. T., Pogue, J. M., Kaye, K. S. (2017). Role of Newer and Re-Emerging Older Agents in the Treatment of Infections Caused by Carbapenem-Resistant Enterobacteriaceae. Virulence 8 (4), 403–416. doi: 10.1080/21505594.2016.1207834
Tumbarello, M., Trecarichi, E. M., Corona, A., De Rosa, F. G., Bassetti, M., Mussini, C., et al. (2019). Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella Pneumoniae Carbapenemase-Producing K. Pneumoniae. Clin. Infect. Dis. 68 (3), 355–364. doi: 10.1093/cid/ciy492
van Duin, D., Bonomo, R. A. (2016). Ceftazidime/avibactam and Ceftolozane/Tazobactam: Second-Generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 63 (2), 234–241. doi: 10.1093/cid/ciw243
Wei, J., Zou, C., Wang, D., Huang, A., Niu, S. (2020). Genetic Diversity and In Vitro Activity of Ceftazidime/Avibactam and Aztreonam/Avibactam Against Imipenem-Resistant Enterobacteriaceae Isolates in Southwest China: A Single-Centre Study. J. Glob Antimicrob. Resist. 22, 448–451. doi: 10.1016/j.jgar.2020.04.023
Xu, Z., Xie, J., Yang, L., Chen, D., Peters, B. M., Shirtliff, M. E. (2018). Complete Sequence of pCY-CTX, a Plasmid Carrying a Phage-Like Region and an ISEcp1-Mediated Tn2 Element From Enterobacter Cloacae. Microb. Drug Resist. 24 (3), 307–313. doi: 10.1089/mdr.2017.0146
Yousfi, M., Mairi, A., Bakour, S., Touati, A., Hassissen, L., Hadjadj, L., et al. (2015). First Report of NDM-5-Producing Escherichia Coli ST1284 Isolated From Dog in Bejaia, Algeria. New Microbes New Infect. 8, 17–18. doi: 10.1016/j.nmni.2015.09.002
Zasowski, E. J., Rybak, J. M., Rybak, M. J. (2015). The β-Lactams Strike Back: Ceftazidime-Avibactam. Pharmacotherapy 35 (8), 755–770. doi: 10.1002/phar.1622
Zhang, P., Shi, Q., Hu, H., Hong, B., Wu, X., Du, X., et al. (2020). Emergence of Ceftazidime/Avibactam Resistance in Carbapenem-Resistant Klebsiella Pneumoniae in China. Clin. Microbiol. Infect. 26 (1), 124.e121–124.e124. doi: 10.1016/j.cmi.2019.08.020
Zhang, J., Yu, L., Fu, Y., Zhao, Y., Wang, Y., Zhao, J., et al. (2019). Tigecycline in Combination With Other Antibiotics Against Clinical Isolates of Carbapenem-Resistant Klebsiella Pneumoniae In Vitro. Ann. Palliat Med. 8 (5), 622–631. doi: 10.21037/apm.2019.09.11
Keywords: ceftazidime/avibactam, carbapenem-resistant Klebsiella pneumoniae, whole-genome sequencing, fitness cost, NDM
Citation: Huang J, Zhang S, Zhao Z, Chen M, Cao Y and Li B (2021) Acquisition of a Stable and Transferable blaNDM-5-Positive Plasmid With Low Fitness Cost Leading to Ceftazidime/Avibactam Resistance in KPC-2-Producing Klebsiella pneumoniae During Treatment. Front. Cell. Infect. Microbiol. 11:658070. doi: 10.3389/fcimb.2021.658070
Received: 25 January 2021; Accepted: 29 June 2021;
Published: 20 July 2021.
Edited by:
Krisztina M. Papp-Wallace, Louis Stokes Cleveland VA Medical Center, United StatesReviewed by:
Maria Fernanda Mojica, Case Western Reserve University, United StatesCopyright © 2021 Huang, Zhang, Zhao, Chen, Cao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingping Cao, Y2FveWluZ3BpbmdAYWxpeXVuLmNvbQ==; Bin Li, bGVvbmxlZTMwN0Bob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.