- 1Research Department of Infection, Division of Infection and Immunity, University College London, London, United Kingdom
- 2Department of Parasitology, Leiden University Medical Center, Leiden, Netherlands
- 3Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, United Kingdom
- 4Tropical and Infectious Diseases Unit, Liverpool University Hospitals National Health Service (NHS) Foundation Trust, Liverpool, United Kingdom
- 5Respiratory Medicine, University College London, London, United Kingdom
In humans, nasopharyngeal carriage of Streptococcus pneumoniae is common and although primarily asymptomatic, is a pre-requisite for pneumonia and invasive pneumococcal disease (IPD). Together, these kill over 500,000 people over the age of 70 years worldwide every year. Pneumococcal conjugate vaccines have been largely successful in reducing IPD in young children and have had considerable indirect impact in protection of older people in industrialized country settings (herd immunity). However, serotype replacement continues to threaten vulnerable populations, particularly older people in whom direct vaccine efficacy is reduced. The early control of pneumococcal colonization at the mucosal surface is mediated through a complex array of epithelial and innate immune cell interactions. Older people often display a state of chronic inflammation, which is associated with an increased mortality risk and has been termed ‘Inflammageing’. In this review, we discuss the contribution of an altered microbiome, the impact of inflammageing on human epithelial and innate immunity to S. pneumoniae, and how the resulting dysregulation may affect the outcome of pneumococcal infection in older individuals. We describe the impact of the pneumococcal vaccine and highlight potential research approaches which may improve our understanding of respiratory mucosal immunity during pneumococcal colonization in older individuals.
Introduction
William Osler, a Canadian physician, who himself died of pneumonia, wrote in his book The Principles and Practice of Medicine: “In the aged, the chances are against recovery. So fatal that it has been termed the natural end of the old man” (Osler, 1892).
Much has changed since, with a huge global public health effort to reduce the burden of pneumonia and invasive pneumococcal disease (IPD), particularly in young children. However, the 1.5 billion people worldwide who are >65yrs (older individuals) now outnumber those <5yrs and, by 2050, will outnumber those aged 15–24yrs when there is predicted to be 426 million people >80yrs (U.N, 2019). Community Acquired Pneumonia (CAP) is common in older individuals, particularly men, with infection by Streptococcus pneumoniae as the leading cause (Kaplan et al., 2002; Janssens and Krause, 2004; Stupka et al., 2009). In 2016, pneumococcal pneumonia was responsible for ~494,340 deaths globally in individuals >70yrs (Collaborators, 2018).
Why older people are so vulnerable to disease caused by S. pneumoniae is likely to be multifactorial including co-morbidities, relative immunodeficiency, malnutrition and defective swallowing (Janssens and Krause, 2004; Zalacain, 2004; Arndt, 2015). Disease follows pneumococcal carriage and reported nasopharyngeal and oropharyngeal carriage rates in older people vary between 0–39% (Krone et al., 2015; Adler et al., 2020; Almeida et al., 2020; Smith et al., 2020; Yasuda et al., 2020). Unlike adults aged 18–64yrs, older adults do not appear to benefit from the natural immune effects of pneumococcal colonization events that are thought to protect against re-colonization and disease (Ferreira et al., 2013; Adler et al., 2020). Older people often display a disorganised inflammatory state, which is associated with an increased mortality risk and has been termed ‘Inflammageing’ (Franceschi and Campisi, 2014; Ferrucci and Fabbri, 2018). This may compromise upper respiratory mucosal immunity, mediated by the nasopharyngeal epithelium and other cellular and soluble innate immune components (Simell et al., 2012; Wilson et al., 2015; Jochems et al., 2018; Jochems et al., 2019a; Weight et al., 2019).
In this review we discuss the impact of inflammageing on innate immunity to S. pneumoniae in older people, summarised in Figure 1. We outline the impacts of the pneumococcal vaccine in older individuals and experimental approaches which may lead to deeper understanding of how the pneumococcus affects this vulnerable population.
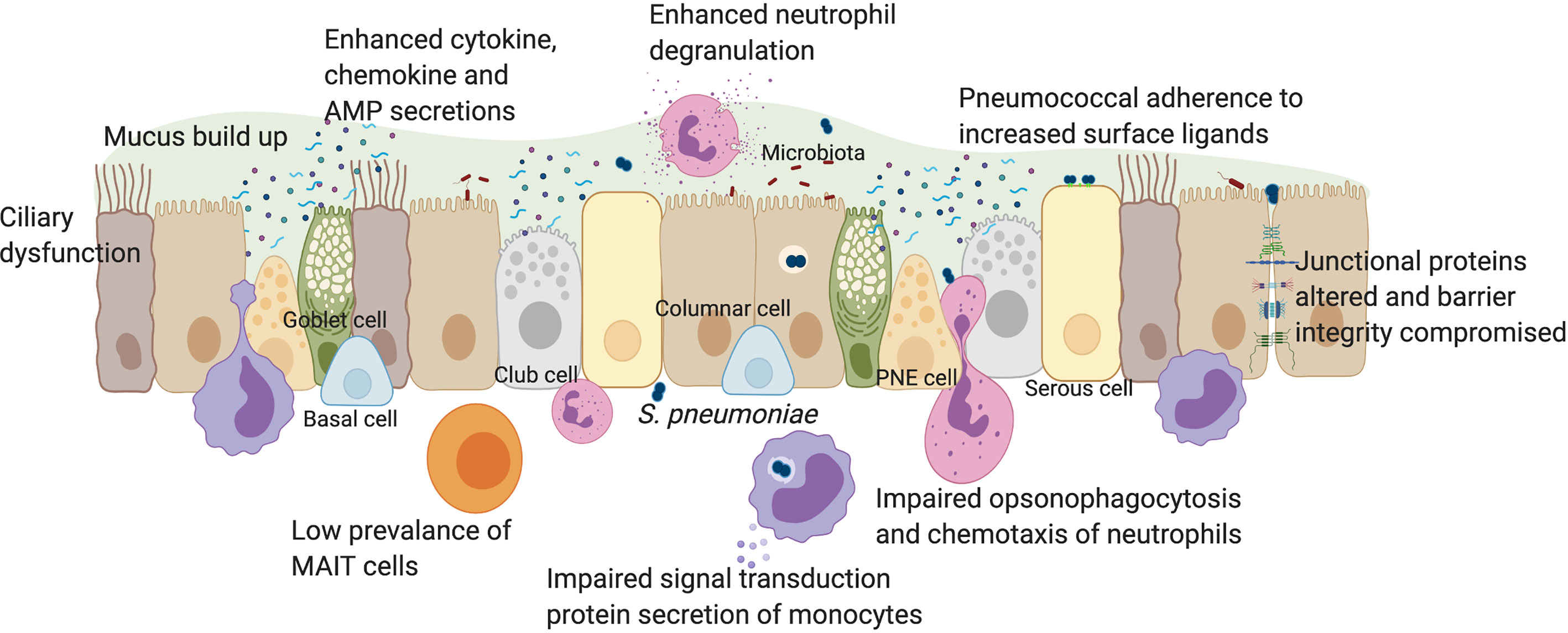
Figure 1 The impact of pneumococcal infection on mucosal immunity in older individuals. The human respiratory epithelium includes many cell types all of which contribute to the development of innate immunity through a physical barrier held together by junctional proteins, and a chemical barrier via secretions of mucus, cytokines, chemokines, antimicrobial peptides, vitamin D and retinoic acid. Innate immune cells such as monocytes, neutrophils and MAIT cells are also present in the mucosa. In older individuals, there is a loss of physical movement both at a mechanical elasticity level and a lack of cilia beating which impacts on mucus clearance. This contributes towards increased prevalence of luminal factors such as cell debris, secreted factors, microbiota and pathogens such as S. pneumoniae which trigger already elevated baseline levels of cytokines such as IL-6, IL-8 and TNFα. In younger adults, epithelial-derived secretion of anti-microbial peptides such as cathelicidin and NFκB activation, leads to autophagy. Impaired autophagy and type 1 interferon responses in older individuals may lead to suppressed IFNβ levels, increasing pneumococcal load. Increased expression of epithelial senescence markers and pneumococcal ligands such as PAFr in older people enhances pneumococcal colonization, influencing adhesion, micro-invasion and transmigration potential. Vitamin D deficiency in older people may affect epithelial barrier integrity. In younger adults, disruption to barrier function after pneumococcal infection affected the expression of junctional proteins such as Claudins. In older adults, dysregulation of barrier may enhance rates of pneumococcal transmigration, infiltration of innate immune cells and inflammation. Although MAIT cells are rare in the airway of older individuals, neutrophil prevalence is enhanced, which elevates degranulation and reactive oxygen species levels following pneumococcal infection. However, neutrophil ability for opsonophagocytosis and chemotaxis is impaired in older individuals. Monocyte function may also be impaired in signal transduction and secrete less IL-6, IL-8 and TNFα during infection, in comparison to younger adults. PNE cell, pulmonary neuroendocrine cell; AMP, anti-microbial peptides. Created with Biorender.com.
Physiology and Ageing
Pulmonary Physiology
In humans, there is a decrease in pulmonary elasticity, loss of respiratory muscular strength, decreased ciliary beating and mucus velocity with age (Ho et al., 2001; Janssens and Krause, 2004). These changes occur from a combination of genetic predisposition, inflammageing and environmental exposure, which involve a wide range of molecular and cellular changes and impairment of cell-cell communications (Brandenberger and Muhlfeld, 2017).
Microbiome
It is becoming increasingly apparent that the microbiome is an important determinant of lung and gut homeostasis and the development of disease, particularly in older individuals (de Steenhuijsen Piters et al., 2016; Schenck et al., 2016; Man et al., 2017; Ragonnaud and Biragyn, 2021). It remains uncertain whether changes in the composition and diversity of microbiota represent a cause or consequence of pneumonia (de Steenhuijsen Piters et al., 2016). Older individuals with pneumonia exhibit increased abundance of species such as S. pneumoniae, Rothia and Lactobacilli, but decreased overall anaerobic bacterial diversity in the upper respiratory tract (URT) (de Steenhuijsen Piters et al., 2016). In a human experimental pneumococcal challenge model (EHPC), low density pneumococcal carriage was associated with a stable mucosal microbiome (baseline presence of Corynebacterium/Dolosigranulum species) and a less pro-inflammatory phenotype (de Steenhuijsen Piters et al., 2019). Whether different pneumococcal strains interact differently with the respiratory microbiome during colonization (Cremers et al., 2014) and how this affects older individuals, remains to be determined. The role of intestinal microbiota on lung susceptibility to pneumococcal infection also warrants further investigation in humans as murine studies suggest that Nod-stimulating microbiota in the gut induce GMCSF-dependent immunity, which influences alveolar macrophage function during pneumococcal infection (Schuijt et al., 2016; Brown et al., 2017).
Inflammageing
An imbalance of cytokine expression in older individuals is referred to as “inflammageing”, where damage to the tissue, changes in composition of the microbiome and cellular and immune senescence, all contribute to this state of chronic inflammation (Franceschi and Campisi, 2014). The contributors to inflammageing may include microbial translocation, chronic infections, mitochondrial dysfunction and accumulation of DNA damage (Fulop et al., 2017; Ferrucci and Fabbri, 2018). This increased inflammation extends to the lungs, as healthy individuals >65yrs have elevated levels of IL-6, IL-8 and higher numbers of neutrophils in bronchoalveolar lavage (BAL) samples (Thompson et al., 1992; Meyer et al., 1996). The increased susceptibility to respiratory tract infections has also been linked to the heightened inflammatory status in older people. In a large prospective study of people aged 70-79yrs, being in the highest tertile for systemic IL-6 and TNF levels was associated with 1.6-1.7-fold increased risk for developing CAP (Yende et al., 2005). Inflammation, in particular IL-6 levels, at time of admission to hospital is also associated with CAP disease severity (Glynn et al., 1999; Antunes et al., 2002). Whether this represents more severe disease, or a pre-existing heightened inflammatory state is uncertain. For example, at admission, in a cohort of 22 patients with pneumonia, of which 19 had confirmed S. pneumoniae, patients <55yrs had increased levels of IL-6 compared to patients >68yrs (Bruunsgaard et al., 1999). However, at 7 days post admission, pro-inflammatory cytokines TNF and soluble TNF receptor I remained elevated in older adults, while they had returned to baseline in young adults. This increased inflammatory state with age may therefore contribute to dysregulation of immunomodulation of innate immune cells such as neutrophils, cytokines and chemokines (Williams et al., 2015) and ultimately, pneumococcal colonization, increasing the chance of IPD in older individuals.
Epithelial Cell Function and Ageing
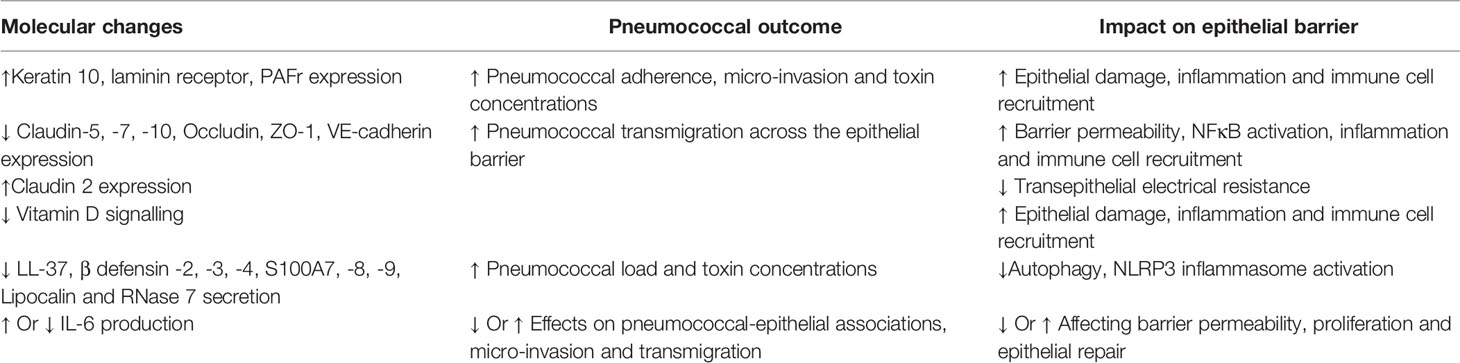
The nasopharyngeal epithelium provides the first line of defense against respiratory pathogens. An intact physical barrier, together with epithelial secretions of mucus, anti-microbial peptides and proteins, chemokines and cytokines, forms the basis of epithelial-derived innate immunity (Figure 1). Hence, age related alterations in epithelial responses would have profound effects on pneumococcal colonization, as summarized in Table 1.
Epithelial Cell Activation
S. pneumoniae binds to a variety of epithelial receptors including Keratin 10, laminin receptor and platelet-activating factor receptor (PAFr), the expression of which is altered in older individuals, thus potentially influencing pneumococcal adherence and susceptibility towards disease. For example, levels of Keratin 10, laminin receptor and PAFr are elevated in aged mice, human lung tissue and senescent A549 cells (Hinojosa et al., 2009; Shivshankar et al., 2011) which could contribute to altered outcomes after pneumococcal colonization. Pneumococcal micro-invasion of the epithelium in vitro is also associated with epithelial secretion of cytokines and chemokines and a transcriptomic enrichment of innate signaling pathways including Toll receptor cascades, NFκB and MAPK activation (Weight et al., 2019). In the EHPC model, an epithelial transcriptomic signature that was associated with bacterial clearance has been identified, indicating the involvement of epithelial activation in the control of pneumococcal colonization (Weight et al., 2019). Furthermore, in vitro epithelial cell models reveal that activation of p65, upregulation of the histone demethylase KDM6B and IL-11 secretion are associated with protection from epithelial damage following pneumococcal infection (Connor et al., 2020). We therefore speculate that in older adults, where pneumococcal micro-invasion and cellular senescence may be enhanced, dysregulation of these pathways and regulatory mechanisms could exacerbate invasive disease.
Mucosal Barrier Function: The expression profiles of intercellular junctional proteins such as Claudins, ZO-1 and E-Cadherin, determine the permissiveness of the respiratory epithelium to the passage of microbes across the barrier. Changes in epithelial junctional expression during pneumococcal infection leads to structural reorganization of the barrier. For example, in vitro infection of human nasopharyngeal cells with S. pneumoniae is initially associated with decreased permeability to 4kDa FITC-dextran, indicating a strengthened barrier (Weight et al., 2019). However, over time (>8 hours post-infection, when bacterial replication and autolysis have also occurred) barrier integrity is altered, demonstrated through decreased expression of Occludin, ZO-1, Claudin-5 and VE-cadherin in the alveoli epithelium and endothelium in younger adults lung explants (Peter et al., 2017). Furthermore, downregulation of Claudin-7 and Claudin-10 in human and murine epithelial cells, led to increased pneumococcal transmigration across the epithelial barrier (Clarke et al., 2011).
The vitamin D receptor (VDR) regulates epithelial barrier function (Chen et al., 2018). In Salmonella infected VDR-/- mice, Claudin 2 upregulation was associated with leaky intestinal barrier, increased pathology and upregulation of NFκB (Zhang et al., 2019), a critical regulator of inflammageing (Salminen et al., 2008) and tight junction protein expression (Ward et al., 2015). In the older human intestine, Claudin-2 upregulation has been detected, which was accompanied by decreased transepithelial electrical resistance and increased permeability (Man et al., 2015). Activation of NFκB following pneumococcal infection is widely reported (Malley et al., 2003; Weight et al., 2019), and Vitamin D deficiency is more severe in older generations (Hirani and Primatesta, 2005; Jolliffe et al., 2013), and there is evidence to suggest that supplementation of Vitamin D could be beneficial in boosting immunity and reducing acute respiratory infections (Martineau et al., 2017; Chambers et al., 2021). Whether regulation and junctional protein responses in the URT are altered with age in humans, and how this contributes to control of pneumococcal colonization, remains to be determined.
Antimicrobial Peptides and Proteins (AMPs)
An important factor of epithelial innate immunity includes AMPs that neutralize toxins and eliminate pathogens (Hiemstra, 2001). Infection of human corneal epithelial cells with pneumococcus induced NFκB activation leading to the secretion of LL-37, β defensin -2, -3, -4, S100A7, S100A8, S100A9, Lipocalin and RNase 7 (Sharma et al., 2019). LL-37 plays a role in wound healing, can induce autophagy in a 1,25-dihydroxyvitamin D3 dependent manner, can activate the NLRP3 inflammasome in a model of P. aeruginosa and, is bactericidal against S. pneumoniae and Mycobacterium tuberculosis (Nijnik and Hancock, 2009; Yuk et al., 2009; McHugh et al., 2019; Sharma et al., 2019). Older individuals maintain similar levels of baseline production of cathelicidins and β defensin 2 in serum compared to younger adults (Castaneda-Delgado et al., 2013). However, in aged mice, CRAMP expression, the murine homolog of LL-37, was not upregulated following pneumococcal infection, compared to younger adults (Krone et al., 2013). This suggests a potential dysregulation of AMPs in older individuals and the implications for the control of S. pneumoniae at the mucosal surface warrants further investigation.
Cytokines and Chemokines
AMPs also induce the secretion of cytokines and chemokines like IL-6 and IL-8 from nasal epithelial cells, in an NFκB dependent manner (Pistolic et al., 2009). One might predict that given elevated levels of cytokines such as IL-6 and TNFα in older individuals (Yende et al., 2005; Man et al., 2015), epithelial cell responses may also differ in the response to pneumococcal carriage. IL-6 is a pleiotropic cytokine and so elevated baseline secretion in older individuals may either enhance or weaken barrier integrity upon pathogenic challenge. For example, IL-6 regulates the expression of tight junction proteins such as Claudin 2 and increases intestinal barrier permeability (Suzuki et al., 2011; Man et al., 2015). This could also occur in the respiratory setting, which may increase pneumococcal transmigration across the epithelial barrier. Alternatively, IL-6 is also known to confer epithelial repair and promote proliferation (Kuhn et al., 2014), which may inhibit pneumococcal adherence to the epithelium. For example, co-infection with Influenza A increases susceptibility to S. pneumoniae in both adult and older mice and in younger adults, characterized by increased bacterial burden in the URT (Mina et al., 2014; Jochems et al., 2018; Gou et al., 2019). In the murine study, IL-6 production was required to maintain barrier function and macrophage phagocytic function, which played a role in pneumococcal control and clearance (Gou et al., 2019). Although secreted by infected human nasopharyngeal cells in vitro (Weight et al., 2019), levels of epithelial IL-6 secretion in vivo have not been directly investigated in adults or older individuals.
Innate Immunity and Ageing
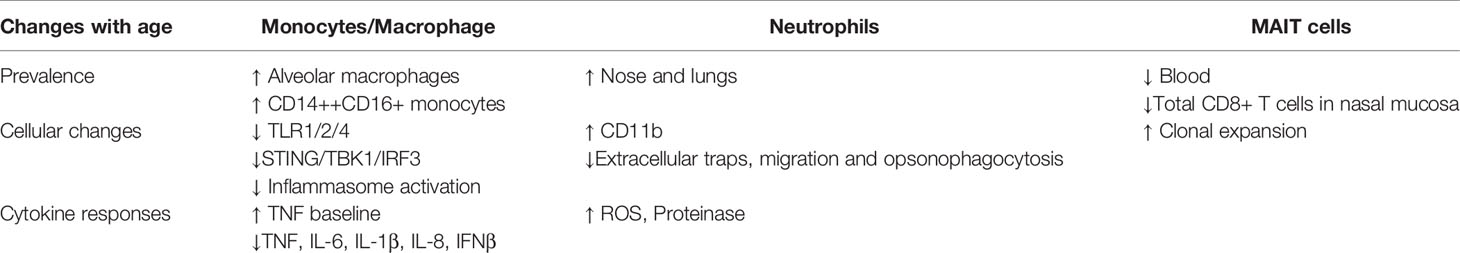
The degree of inflammageing likely influences the functional responses of monocytes/macrophages, neutrophils and Mucosal-associated invariant T (MAIT) cells to S. pneumoniae, which in turn, may be detrimental in controlling the outcome of nasopharyngeal pneumococcal colonization in older people, as summarized in Table 2.
Monocytes and Macrophages
TLR1 levels are reduced on monocytes from older adults and TLR1/2 specific stimulation using Pam2SCK4 is associated with reduced responses in monocytes from older people (van Duin et al., 2007). Indeed, cytokine responses to pneumococcal or relevant ligands also appear decreased with age. Frail older individuals have increased baseline production of TNF by intermediate monocytes in particular, but show an impaired induction upon TLR1/2 or TLR4 stimulation (Hearps et al., 2012; Verschoor et al., 2014b). Upon heat-killed pneumococcal stimulation however, monocyte-derived macrophages in frail older people produce less TNF, IL-6, IL-1β and IL-8 and have a reduced capacity to kill S. pneumoniae. This is possibly related to defective PI3K-AKT signaling (Verschoor et al., 2014a), and/or insufficient activation of the NLRP3 inflammasome, as demonstrated in bone marrow derived macrophages from aged mice (Cho et al., 2018).
In younger adults, infiltration of classical monocytes into the nasal mucosa in the EHPC model coincides with initiation of pneumococcal clearance, while nasal myeloid cytokines correlate with clearance of colonization (Jochems et al., 2018). The impact of the respiratory monocyte/macrophage dysfunction described above on pneumococcal control in older people is not fully understood. However, altered monocyte subsets are an important contributor to a reduced ability to prevent pneumococcal infection in older people (Puchta et al., 2016). For example, reduced cytokine production to TLR1/2 agonists seem to be mediated by changes in CD14++ CD16+ intermediate and CD14+ CD16+ non-classical monocytes (Nyugen et al., 2010). In addition, alveolar macrophage numbers are higher in BAL samples from healthy older adults compared to younger adults (Thompson et al., 1992; Meyer et al., 1996), although how this affects innate immune responses to S. pneumoniae is unknown.
Murine studies have also identified age-related functional differences in monocyte/macrophage interactions with S. pneumoniae that may be relevant for disease pathogenesis. Puchta et al. demonstrated that TNF is a crucial mediator of the susceptibility to pneumococcal infections in inflammageing, as well as the alterations in monocyte subsets (Puchta et al., 2016). Increasing TNF with age led to premature egress of pro-inflammatory monocytes from bone marrow and increased levels of intermediate CD14++ CD16+ monocytes. Specific depletion of these monocytes or reduction in TNF levels enhanced immunity to pneumococcal infection and increased clearance in old mice (Puchta et al., 2016). Koppe found that following sensing of pneumococcal dsDNA by murine macrophages, STING (“stimulator of IFN genes”) binds to TBK1 (TANK-binding Kinase 1), leading to IRF3 (Interferon Regulatory Transcription Factor 3) activation and production of IFNβ, which assists pneumococcal clearance (Koppe et al., 2012). In aged mice, there is less STING/TBK1/IRF3 mRNA and protein expression compared to young mice infected with S. pneumoniae (Mitzel et al., 2014). This was associated with lower levels of IFNβ and higher bacterial burden in the lung, thought to be due to age-associated stress of the endoplasmic reticulum, resulting in increased autophagy-related protein 9a-STING complex formation (Mitzel et al., 2014), preventing STING complex formation with TBK1 (Saitoh et al., 2009).
Neutrophils
There is a large body of evidence from both murine and human studies showing the importance of neutrophils in protecting against pneumococcal colonization of the nasopharynx (Lu et al., 2008; Weinberger et al., 2009; Jochems et al., 2018; Nikolaou et al., 2018). Impaired functional responses of neutrophils in older individuals may therefore also be detrimental during pneumococcal infection.
In the nose and lungs of healthy older people, neutrophils are highly abundant (Thompson et al., 1992; Meyer et al., 1996; Reiné et al., 2019). Excessive neutrophil recruitment to the lung following infection can mediate tissue damage and may exacerbate inflammation in older people (Menter et al., 2014). In frail older individuals, neutrophils have an immature profile with increased levels of intracellular reactive oxygen species (ROS) and cell surface expression of proinflammatory markers like CD11b (Verschoor et al., 2015). They have an impaired capacity to produce neutrophil extracellular traps (Hazeldine et al., 2014) and altered chemotaxis responses to respiratory infection, which leads to prolonged production of proteinase and a pro-inflammatory milieu (Sapey et al., 2014; Sapey et al., 2017). Neutrophils in older individuals may also be impaired in their opsonophagocytotic ability to several bacterial pathogens including S. pneumoniae and S. aureus (Wenisch et al., 2000; Butcher et al., 2001; Simell et al., 2011). Interestingly, vitamin E supplementation in aged mice prevented neutrophil migration and mortality during pneumococcal pneumonia (Ghanem et al., 2015), suggesting that regulating neutrophil activity in older individuals could be beneficial to the host.
MAIT cells
Unconventional, innate-like T cells called MAIT cells, play important roles in the defense against bacterial and viral infections. Recently, studies with the EHPC model demonstrated that blood and nasal MAIT cells were associated with protection from pneumococcal colonization (Jochems et al., 2019b). They can be activated by conserved bacterial ligands derived from vitamin B (riboflavin) biosynthesis or indirectly via cytokines (Godfrey et al., 2019; Toubal et al., 2019). MAIT cells recognize precursors of the riboflavin synthesis pathway after presentation via MHC class I–related protein 1 (MR-1). This pathway is highly conserved in the pneumococcal genome, and MAIT cells can respond to pneumococcal isolates in both MR-1 dependent and independent manners (Kurioka et al., 2018). MAIT cells are depleted from blood in old age, with levels progressively dropping from 3-5% of T cells in young adulthood to below 1% in older adults (Novak et al., 2014; Walker et al., 2014; Loh et al., 2020). Remaining MAIT cells in this population show clonal expansion, similar to conventional CD8 T cells, and increased basal inflammation, although they retain potent anti-microbial function (Loh et al., 2020). There appears to be a substantial depletion of total CD8+ T cells from the nasal mucosa in older adults (Reiné et al., 2019) and the ratio of CD4/CD8 T cells increases in the lung in middle age (Meyer et al., 1996). Therefore, as MAIT cells are predominantly found within the CD8+ T cell compartment, we postulate that there is loss of MAIT cells at the mucosal surface in older individuals which negatively impacts on the control of pneumococcal colonization. This warrants further investigation.
Th17 and T Regulatory Cells (Tregs)
In murine models, a Th17-mediated recruitment of monocytes and neutrophils leads to clearance of S. pneumoniae colonization (Lu et al., 2008; Zhang et al., 2009). Some human studies also suggest a role for Th17 cells in protection against colonization showing increased ratios of pneumococcal-specific Th17/Tregs with increasing age as carriage rates decrease (Mubarak et al., 2016) and a SNP in the IL17A gene associated with ~2-fold increased risk of pneumococcal colonization in children in the first year of life (Vuononvirta et al., 2015). However, a protective role for Th17 cells in humans has not been fully substantiated. We have shown acquisition of pneumococcal antigen-specific tonsillar Th1 T cells but not Th17 cells with age (Pido-Lopez et al., 2011). In the EHPC model, Th17 cells were found in the lung after colonization, which associated with increased bacterial killing in macrophages (Wright et al., 2013), but were not identified in the nasopharynx. Higher Th17 responses were found in children from Bangladesh with high carriage rates, compared to children from Sweden with lower carriage rates (Lundgren et al., 2012). In HIV+ individuals in Malawi where carriage rates are high, there was no evidence of a Th17 protective phenotype (Glennie et al., 2013). Furthermore, colonization by S. pneumoniae has been associated with decreased Th17/Treg ratios in children, possibly mediated by TGF-β induction leading to regulatory responses (Zhang et al., 2011; Neill et al., 2014; Jiang et al., 2015). Stimulation of PBMCs from individuals of different ages with three different pneumococcal proteins revealed a non-significant decrease in responses of older adults, although polyfunctionality (co-production of IFNγ by same donor) was decreased (Schmid et al., 2011). In older adults, total Th17 numbers in blood are decreased and total Treg numbers increased (van der Geest et al., 2014). Together, these observations highlight that a role of Th17 cells in conferring protection against colonization in humans remains unclear. Further investigations of mucosal Th17 and Tregs responses in older people using longitudinal studies and the EHPC model are required.
Pneumococcal Vaccines in Older People
With the high risk of S. pneumoniae infection in the older population and an ageing population, there is an urgent need for effective vaccine approaches to protect this vulnerable group. The 23-valent pneumococcal polysaccharide vaccine (PPV23) is widely used in richer countries to prevent pneumococcal disease in older people and often administered alongside the influenza vaccine. Observational studies have suggested that PPV23 reduces the incidence of pneumococcal pneumonia and vaccine-serotype IPD and mortality in older individuals by 29% - 57% (Christenson et al., 2001; Andrews et al., 2004; Spindler et al., 2008; Suzuki et al., 2017). However, meta-analyses have suggested that PPV23 may only be beneficial against IPD, with no effect against the far more common non-bacteremic pneumonia (Moberley et al., 2013; Latifi-Navid et al., 2018). Along with its disputed efficacy against pneumonia, PPV23 has no protective effect against pneumococcal colonization (Adler et al., 2020).
PPV23 induces the production of anti-capsular antibodies via a T-cell-independent mechanism. Pneumococcal conjugate vaccines (PCV) induce higher antibody levels and longer-term immune memory via carrier protein mediated T-cell-dependent mechanisms. In controlled infection studies of young adults, PCV13 reduced pneumococcal colonization (Collins et al., 2015; German et al., 2019). Together with routine vaccination of children with PCV, which reduces carriage and transmission, they protect older people from S. pneumoniae infections through herd immunity. However, as serotype replacement threatens the efficacy of the vaccine (Lewnard and Hanage, 2019), new strategies to protect older individuals are required.
Clinical trials have confirmed that PCV13 can reduce the incidence of vaccine-serotype colonization in older adults, though the effect did not persist beyond six months (van Deursen et al., 2018). This impact of PCV13 on pneumococcal colonization may therefore be expected to overcome some of the deficiencies of PPV23 in its activity against pneumonia and, in adults >65yrs, PCV13 indeed demonstrated a 45% efficacy against non-bacteremic CAP caused by the vaccine serotypes, along with a 75% efficacy against IPD (Bonten et al., 2015). PCV20 represents a potential further advance due to the increase in serotype coverage, and results of phase 3 trials in older individuals are currently awaited (clinical trial NCT03835975) (Pfizer, 2019).
The process of inflammageing in older individuals which includes physical airway alterations, shifts in the microbiome coupled with effects on the epithelium and innate immunity, will likely contribute to a decreased efficacy of the pneumococcal vaccines. Therefore, understanding in more detail the underlying molecular and cellular mechanisms could help identify interventions to enhance immune responses in this population group. Novel vaccine approaches such as targeting pneumococcal proteins, using whole cell inactivated or attenuated strains, and with new adjuvants or immunomodulating agents to overcome the effects of inflammageing, may enhance protection against IPD in older individuals (Feldman and Anderson, 2014; Ramos-Sevillano et al., 2020; Wagner and Weinberger, 2020). Potential novel mucosal immunomodulating interventions include statins, vitamin supplementation and changes to the nasal microbiome to boost mucosal immunity, but these need to be first supported by high quality clinical trial data. In the meantime, high levels of pneumococcal vaccine uptake by adults alongside PCV vaccination of younger individuals to generate herd immunity will have the greatest effect on the morbidity and mortality associated with S. pneumoniae in older individuals. Additional important preventative strategies include annual health checks and smoking cessation as heart disease, diabetes mellitus and smoking increase the incidence of CAP (Torres et al., 2015).
Discussion
The immune dysregulation associated with inflammageing has wide ranging effects on the control of pneumococcal colonization and the transition to invasive disease. To better define these processes, in parallel with murine models and in vitro culture systems, the EHPC model provides a unique and safe opportunity to investigate in detail the cellular and molecular changes involved. The model also enables investigation of pneumococcal-epithelial-innate immune cell interactions and activation. For example, the model can be used to assess changes in pneumococcal micro-invasion of the epithelium and characterize subsequent alterations in epithelial-derived innate immune responses following pneumococcal infection in older individuals. Transcriptomic and metabolomic approaches applied to these systems will lead to further understanding of molecular changes that occur during inflammageing and how they influence pneumococcal infection (Valdes et al., 2013; Giamarellos-Bourboulis et al., 2020).
In the last 128 years since William Osler’s observations on pneumonia, advances in the understanding of mucosal immune protection against pneumococcal disease in older individuals has progressed considerably. However, it is evident that there is still much more that needs to be discovered if we are to reduce the burden of pneumococcal disease in this vulnerable population.
Author Contributions
CW and RH conceptualized the review. CW planned, wrote and revised the manuscript. SJ and HA wrote and revised the manuscript. JB, DF, and RH critically read and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
CW and RH are supported by the Medical Research Council, Grant Ref: MR/T016329/1. RH is a National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article are those of the authors and not necessarily those of the NIHR, or the Department of Health and Social Care.
Disclaimer
The views expressed in this article are those of the authors and not necessarily those of the MRC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adler, H., German, E. L., Mitsi, E., Nikolaou, E., Pojar, S., Hales, C., et al. (2020). Experimental Human Pneumococcal Colonisation in Older Adults is Feasible and Safe, Not Immunogenic. Am. J. Respir. Crit. Care Med. 203 (5), 604–613. doi: 10.1164/rccm.202004-1483OC
Almeida, S. T., Pedro, T., Paulo, A. C., de Lencastre, H., Sa-Leao, R. (2020). Re-Evaluation of Streptococcus Pneumoniae Carriage in Portuguese Elderly by qPCR Increases Carriage Estimates and Unveils an Expanded Pool of Serotypes. Sci. Rep. 10 (1), 8373. doi: 10.1038/s41598-020-65399-x
Andrews, R. M., Counahan, M. L., Hogg, G. G., McIntyre, P. B. (2004). Effectiveness of a Publicly Funded Pneumococcal Vaccination Program Against Invasive Pneumococcal Disease Among the Elderly in Victoria, Australia. Vaccine 23 (2), 132–138. doi: 10.1016/j.vaccine.2004.06.016
Antunes, G., Evans, S. A., Lordan, J. L., Frew, A. J. (2002). Systemic Cytokine Levels in Community-Acquired Pneumonia and Their Association With Disease Severity. Eur. Respir. J. 20 (4), 990–995. doi: 10.1183/09031936.02.00295102
Arndt, P. (2015). Pneumonia and Host Defense in the Elderly. Clin. Pulm. Med. 22 (6), 271–278. doi: 10.1097/CPM.0000000000000100
Bonten, M. J., Huijts, S. M., Bolkenbaas, M., Webber, C., Patterson, S., Gault, S., et al. (2015). Polysaccharide Conjugate Vaccine Against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 372 (12), 1114–1125. doi: 10.1056/NEJMoa1408544
Brandenberger, C., Muhlfeld, C. (2017). Mechanisms of Lung Aging. Cell Tissue Res. 367 (3), 469–480. doi: 10.1007/s00441-016-2511-x
Brown, R. L., Sequeira, R. P., Clarke, T. B. (2017). The Microbiota Protects Against Respiratory Infection Via GM-CSF Signaling. Nat. Commun. 8 (1), 1512. doi: 10.1038/s41467-017-01803-x
Bruunsgaard, H., Skinhoj, P., Qvist, J., Pedersen, B. K. (1999). Elderly Humans Show Prolonged In Vivo Inflammatory Activity During Pneumococcal Infections. J. Infect. Dis. 180 (2), 551–554. doi: 10.1086/314873
Butcher, S. K., Chahal, H., Nayak, L., Sinclair, A., Henriquez, N. V., Sapey, E., et al. (2001). Senescence in Innate Immune Responses: Reduced Neutrophil Phagocytic Capacity and CD16 Expression in Elderly Humans. J. Leukoc. Biol. 70 (6), 881–886. doi: 10.1189/jlb.70.6.881
Castaneda-Delgado, J. E., Miranda-Castro, N. Y., Gonzalez-Amaro, R., Gonzalez-Curiel, I., Montoya-Rosales, A., Rivas-Calderon, B., et al. (2013). Production of Antimicrobial Peptides is Preserved in Aging. Clin. Immunol. 148 (2), 198–205. doi: 10.1016/j.clim.2013.05.015
Chambers, E. S., Vukmanovic-Stejic, M., Turner, C. T., Shih, B. B., Trahair, H., Pollara, G., et al. (2021). Vitamin D3 Replacement Enhances Antigen-Specific Immunity in Older Adults. Immunother. Adv. 1 (1), 1–13. doi: 10.1093/immadv/ltaa008
Chen, H., Lu, R., Zhang, Y. G., Sun, J. (2018). Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs. Tissue Barriers 6 (4), 1–13. doi: 10.1080/21688370.2018.1540904
Cho, S. J., Rooney, K., Choi, A. M. K., Stout-Delgado, H. W. (2018). NLRP3 Inflammasome Activation in Aged Macrophages is Diminished During Streptococcus Pneumoniae Infection. Am. J. Physiol. Lung Cell Mol. Physiol. 314 (3), L372–L387. doi: 10.1152/ajplung.00393.2017
Christenson, B., Lundbergh, P., Hedlund, J., Ortqvist, A. (2001). Effects of a Large-Scale Intervention With Influenza and 23-Valent Pneumococcal Vaccines in Adults Aged 65 Years or Older: A Prospective Study. Lancet 357 (9261), 1008–1011. doi: 10.1016/S0140-6736(00)04237-9
Clarke, T. B., Francella, N., Huegel, A., Weiser, J. N. (2011). Invasive Bacterial Pathogens Exploit TLR-mediated Downregulation of Tight Junction Components to Facilitate Translocation Across the Epithelium. Cell Host Microbe 9 (5), 404–414. doi: 10.1016/j.chom.2011.04.012
Collaborators, G.B.D.L.R.I. (2018). Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Lower Respiratory Infections in 195 Countrie-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18 (11), 1191–1210. doi: 10.1016/S1473-3099(18)30310-4
Collins, A. M., Wright, A. D., Mitsi, E., Gritzfeld, J. F., Hancock, C. A., Pennington, S. H., et al. (2015). First Human Challenge Testing of a Pneumococcal Vaccine. Double-blind Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 192 (7), 853–858. doi: 10.1164/rccm.201503-0542OC
Connor, M. G., Camarasa, T. M. N., Patey, E., Rasid, O., Barrio, L., Weight, C. M., et al. (2020). The Histone Demethylase KDM6B Fine-Tunes the Host Response to Streptococcus Pneumoniae. Nat. Microbiol. 6 (2), 257–269. doi: 10.1038/s41564-020-00805-8
Cremers, A. J., Zomer, A. L., Gritzfeld, J. F., Ferwerda, G., van Hijum, S. A., Ferreira, D. M., et al. (2014). The Adult Nasopharyngeal Microbiome as a Determinant of Pneumococcal Acquisition. Microbiome 2:44. doi: 10.1186/2049-2618-2-44
de Steenhuijsen Piters, W. A., Huijskens, E. G., Wyllie, A. L., Biesbroek, G., van den Bergh, M. R., Veenhoven, R. H., et al. (2016). Dysbiosis of Upper Respiratory Tract Microbiota in Elderly Pneumonia Patients. ISME J. 10 (1), 97–108. doi: 10.1038/ismej.2015.99
de Steenhuijsen Piters, W. A. A., Jochems, S. P., Mitsi, E., Rylance, J., Pojar, S., Nikolaou, E., et al. (2019). Interaction Between the Nasal Microbiota and S. Pneumoniae in the Context of Live-Attenuated Influenza Vaccine. Nat. Commun. 10 (1), 2981. doi: 10.1038/s41467-019-10814-9
Feldman, C., Anderson, R. (2014). Review: Current and New Generation Pneumococcal Vaccines. J. Infect. 69 (4), 309–325. doi: 10.1016/j.jinf.2014.06.006
Ferreira, D. M., Neill, D. R., Bangert, M., Gritzfeld, J. F., Green, N., Wright, A. K., et al. (2013). Controlled Human Infection and Rechallenge With Streptococcus Pneumoniae Reveals the Protective Efficacy of Carriage in Healthy Adults. Am. J. Respir. Crit. Care Med. 187 (8), 855–864. doi: 10.1164/rccm.201212-2277OC
Ferrucci, L., Fabbri, E. (2018). Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 15 (9), 505–522. doi: 10.1038/s41569-018-0064-2
Franceschi, C., Campisi, J. (2014). Chronic Inflammation (Inflammaging) and its Potential Contribution to Age-Associated Diseases. J. Gerontol. A. Biol. Sci. Med. Sci. 69 Suppl 1, S4–S9. doi: 10.1093/gerona/glu057
Fulop, T., Larbi, A., Dupuis, G., Le Page, A., Frost, E. H., Cohen, A. A., et al. (2017). Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 8, 1960. doi: 10.3389/fimmu.2017.01960
German, E. L., Solorzano, C., Sunny, S., Dunne, F., Gritzfeld, J. F., Mitsi, E., et al. (2019). Protective Effect of PCV Vaccine Against Experimental Pneumococcal Challenge in Adults is Primarily Mediated by Controlling Colonisation Density. Vaccine 37 (30), 3953–3956. doi: 10.1016/j.vaccine.2019.05.080
Ghanem, E. N. B., Clark, S., Du, X. G., Wu, D. Y., Camilli, A., Leong, J. M., et al. (2015). The Alpha-Tocopherol Form of Vitamin E Reverses Age-Associated Susceptibility to Streptococcus Pneumoniae Lung Infection by Modulating Pulmonary Neutrophil Recruitment. J. Immunol. 194 (3), 1090–1099. doi: 10.4049/jimmunol.1402401
Giamarellos-Bourboulis, E. J., Tsilika, M., Moorlag, S., Antonakos, N., Kotsaki, A., Dominguez-Andres, J., et al. (2020). Activate: Randomized Clinical Trial of BCG Vaccination Against Infection in the Elderly. Cell 183 (2), 315–323.e319. doi: 10.1016/j.cell.2020.08.051
Glennie, S. J., Banda, D., Gould, K., Hinds, J., Kamngona, A., Everett, D. D., et al. (2013). Defective Pneumococcal-Specific Th1 Responses in HIV-Infected Adults Precedes a Loss of Control of Pneumococcal Colonization. Clin. Infect. Dis. 56 (2), 291–299. doi: 10.1093/cid/cis842
Glynn, P., Coakley, R., Kilgallen, I., Murphy, N., O’Neill, S. (1999). Circulating Interleukin 6 and Interleukin 10 in Community Acquired Pneumonia. Thorax 54 (1), 51–55. doi: 10.1136/thx.54.1.51
Godfrey, D. I., Koay, H. F., McCluskey, J., Gherardin, N. A. (2019). The Biology and Functional Importance of MAIT Cells. Nat. Immunol. 20 (9), 1110–1128. doi: 10.1038/s41590-019-0444-8
Gou, X., Yuan, J., Wang, H., Wang, X., Xiao, J., Chen, J., et al. (2019). Il-6 During Influenza-Streptococcus Pneumoniae Co-Infected Pneumonia-a Protector. Front. Immunol. 10, 3102. doi: 10.3389/fimmu.2019.03102
Hazeldine, J., Harris, P., Chapple, I. L., Grant, M., Greenwood, H., Livesey, A., et al. (2014). Impaired Neutrophil Extracellular Trap Formation: A Novel Defect in the Innate Immune System of Aged Individuals. Aging Cell 13 (4), 690–698. doi: 10.1111/acel.12222
Hearps, A. C., Martin, G. E., Angelovich, T. A., Cheng, W. J., Maisa, A., Landay, A. L., et al. (2012). Aging is Associated With Chronic Innate Immune Activation and Dysregulation of Monocyte Phenotype and Function. Aging Cell 11 (5), 867–875. doi: 10.1111/j.1474-9726.2012.00851.x
Hiemstra, P. S. (2001). Epithelial Antimicrobial Peptides and Proteins: Their Role in Host Defence and Inflammation. Paediatr. Respir. Rev. 2 (4), 306–310. doi: 10.1053/prrv.2001.0165
Hinojosa, E., Boyd, A. R., Orihuela, C. J. (2009). Age-Associated Inflammation and Toll-Like Receptor Dysfunction Prime the Lungs for Pneumococcal Pneumonia. J. Infect. Dis. 200 (4), 546–554. doi: 10.1086/600870
Hirani, V., Primatesta, P. (2005). Vitamin D Concentrations Among People Aged 65 Years and Over Living in Private Households and Institutions in England: Population Survey. Age Ageing 34 (5), 485–491. doi: 10.1093/ageing/afi153
Ho, J. C., Chan, K. N., Hu, W. H., Lam, W. K., Zheng, L., Tipoe, G. L., et al. (2001). The Effect of Aging on Nasal Mucociliary Clearance, Beat Frequency, and Ultrastructure of Respiratory Cilia. Am. J. Respir. Crit. Care Med. 163 (4), 983–988. doi: 10.1164/ajrccm.163.4.9909121
Janssens, J. P., Krause, K. H. (2004). Pneumonia in the Very Old. Lancet Infect. Dis. 4 (2), 112–124. doi: 10.1016/S1473-3099(04)00931-4
Jiang, X. L., Zhang, G. L., Yang, T., Yang, B. H., Wang, L. J., Wang, Q. H., et al. (2015). Association of Pneumococcal Carriage and Expression of Foxp3+ Regulatory T Cells and Th17 Cells in the Adenoids of Children. Respiration 90 (1), 25–32. doi: 10.1159/000381724
Jochems, S. P., de Ruiter, K., Solorzano, C., Voskamp, A., Mitsi, E., Nikolaou, E., et al. (2019a). Innate and Adaptive Nasal Mucosal Immune Responses Following Experimental Human Pneumococcal Colonization. J. Clin. Invest. 129 (10), 4523–4538. doi: 10.1172/JCI128865
Jochems, S. P., de Ruiter, K., Solorzano, C., Voskamp, A., Mitsi, E., Nikolaou, E., et al. (2019b). Innate and Adaptive Nasal Mucosal Immune Responses Following Experimental Human Pneumococcal Colonization. J. Clin. Invest. 130, 4523–4538. doi: 10.1172/JCI128865
Jochems, S. P., Marcon, F., Carniel, B. F., Holloway, M., Mitsi, E., Smith, E., et al. (2018). Inflammation Induced by Influenza Virus Impairs Human Innate Immune Control of Pneumococcus. Nat. Immunol. 19 (12), 1299–1308. doi: 10.1038/s41590-018-0231-y
Jolliffe, D. A., Griffiths, C. J., Martineau, A. R. (2013). Vitamin D in the Prevention of Acute Respiratory Infection: Systematic Review of Clinical Studies. J. Steroid Biochem. Mol. Biol. 136, 321–329. doi: 10.1016/j.jsbmb.2012.11.017
Kaplan, V., Angus, D. C., Griffin, M. F., Clermont, G., Scott Watson, R., Linde-Zwirble, W. T. (2002). Hospitalized Community-Acquired Pneumonia in the Elderly: Age- and Sex-Related Patterns of Care and Outcome in the United States. Am. J. Respir. Crit. Care Med. 165 (6), 766–772. doi: 10.1164/ajrccm.165.6.2103038
Koppe, U., Hogner, K., Doehn, J. M., Muller, H. C., Witzenrath, M., Gutbier, B., et al. (2012). Streptococcus Pneumoniae Stimulates a STING- and IFN Regulatory Factor 3-Dependent Type I IFN Production in Macrophages, Which Regulates RANTES Production in Macrophages, Cocultured Alveolar Epithelial Cells, and Mouse Lungs. J. Immunol. 188 (2), 811–817. doi: 10.4049/jimmunol.1004143
Krone, C. L., Trzcinski, K., Zborowski, T., Sanders, E. A., Bogaert, D. (2013). Impaired Innate Mucosal Immunity in Aged Mice Permits Prolonged Streptococcus Pneumoniae Colonization. Infect. Immun. 81 (12), 4615–4625. doi: 10.1128/IAI.00618-13
Krone, C. L., Wyllie, A. L., van Beek, J., Rots, N. Y., Oja, A. E., Chu, M. L., et al. (2015). Carriage of Streptococcus Pneumoniae in Aged Adults With Influenza-Like-Illness. PLoS One 10 (3), e0119875. doi: 10.1371/journal.pone.0119875
Kuhn, K. A., Manieri, N. A., Liu, T. C., Stappenbeck, T. S. (2014). IL-6 Stimulates Intestinal Epithelial Proliferation and Repair After Injury. PLoS One 9 (12), e114195. doi: 10.1371/journal.pone.0114195
Kurioka, A., van Wilgenburg, B., Javan, R. R., Hoyle, R., van Tonder, A. J., Harrold, C. L., et al. (2018). Diverse Streptococcus Pneumoniae Strains Drive a Mucosal-Associated Invariant T-Cell Response Through Major Histocompatibility Complex Class I-Related Molecule-Dependent and Cytokine-Driven Pathways. J. Infect. Dis. 217 (6), 988–999. doi: 10.1093/infdis/jix647
Latifi-Navid, H., Latifi-Navid, S., Mostafaiy, B., Jamalkandi, S. A., Ahmadi, A. (2018). Pneumococcal Disease and the Effectiveness of the PPV23 Vaccine in Adults: A Two-Stage Bayesian Meta-Analysis of Observational and RCT Reports. Sci. Rep. 8 (1), 11051. doi: 10.1038/s41598-018-29280-2
Lewnard, J. A., Hanage, W. P. (2019). Making Sense of Differences in Pneumococcal Serotype Replacement. Lancet Infect. Dis. 19 (6), e213–e220. doi: 10.1016/S1473-3099(18)30660-1
Loh, L., Gherardin, N. A., Sant, S., Grzelak, L., Crawford, J. C., Bird, N. L., et al. (2020). Human Mucosal-Associated Invariant T Cells in Older Individuals Display Expanded TCRalphabeta Clonotypes With Potent Antimicrobial Responses. J. Immunol. 204 (5), 1119–1133. doi: 10.4049/jimmunol.1900774
Lu, Y. J., Gross, J., Bogaert, D., Finn, A., Bagrade, L., Zhang, Q., et al. (2008). Interleukin-17A Mediates Acquired Immunity to Pneumococcal Colonization. PLoS Pathog. 4 (9), e1000159. doi: 10.1371/journal.ppat.1000159
Lundgren, A., Bhuiyan, T. R., Novak, D., Kaim, J., Reske, A., Lu, Y. J., et al. (2012). Characterization of Th17 Responses to Streptococcus Pneumoniae in Humans: Comparisons Between Adults and Children in a Developed and a Developing Country. Vaccine 30 (26), 3897–3907. doi: 10.1016/j.vaccine.2012.03.082
Malley, R., Henneke, P., Morse, S. C., Cieslewicz, M. J., Lipsitch, M., Thompson, C. M., et al. (2003). Recognition of Pneumolysin by Toll-like Receptor 4 Confers Resistance to Pneumococcal Infection. Proc. Natl. Acad. Sci. U. S. A. 100 (4), 1966–1971. doi: 10.1073/pnas.0435928100
Man, A. L., Bertelli, E., Rentini, S., Regoli, M., Briars, G., Marini, M., et al. (2015). Age-Associated Modifications of Intestinal Permeability and Innate Immunity in Human Small Intestine. Clin. Sci. (Lond) 129 (7), 515–527. doi: 10.1042/CS20150046
Man, W. H., de Steenhuijsen Piters, W. A., Bogaert, D. (2017). The Microbiota of the Respiratory Tract: Gatekeeper to Respiratory Health. Nat. Rev. Microbiol. 15 (5), 259–270. doi: 10.1038/nrmicro.2017.14
Martineau, A. R., Jolliffe, D. A., Hooper, R. L., Greenberg, L., Aloia, J. F., Bergman, P., et al. (2017). Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 356, i6583. doi: 10.1136/bmj.i6583
McHugh, B. J., Wang, R., Li, H. N., Beaumont, P. E., Kells, R., Stevens, H., et al. (2019). Cathelicidin is a “Fire Alarm”, Generating Protective NLRP3-dependent Airway Epithelial Cell Inflammatory Responses During Infection With Pseudomonas Aeruginosa. PLoS Pathog. 15 (4), e1007694. doi: 10.1371/journal.ppat.1007694
Menter, T., Giefing-Kroell, C., Grubeck-Loebenstein, B., Tzankov, A. (2014). Characterization of the Inflammatory Infiltrate in Streptococcus Pneumoniae Pneumonia in Young and Elderly Patients. Pathobiology 81 (3), 160–167. doi: 10.1159/000360165
Meyer, K. C., Ershler, W., Rosenthal, N. S., Lu, X. G., Peterson, K. (1996). Immune Dysregulation in the Aging Human Lung. Am. J. Respir. Crit. Care Med. 153 (3), 1072–1079. doi: 10.1164/ajrccm.153.3.8630547
Mina, M. J., McCullers, J. A., Klugman, K. P. (2014). Live Attenuated Influenza Vaccine Enhances Colonization of Streptococcus Pneumoniae and Staphylococcus Aureus in Mice. mBio 5 (1), e01040-13. doi: 10.1128/mBio.01040-13
Mitzel, D. N., Lowry, V., Shirali, A. C., Liu, Y., Stout-Delgado, H. W. (2014). Age-Enhanced Endoplasmic Reticulum Stress Contributes to Increased Atg9A Inhibition of STING-mediated IFN-Beta Production During Streptococcus Pneumoniae Infection. J. Immunol. 192 (9), 4273–4283. doi: 10.4049/jimmunol.1303090
Moberley, S., Holden, J., Tatham, D. P., Andrews, R. M. (2013). Vaccines for Preventing Pneumococcal Infection in Adults. Cochrane Database Syst. Rev. 23 (1), CD000422. doi: 10.1002/14651858.CD000422.pub3
Mubarak, A., Ahmed, M. S., Upile, N., Vaughan, C., Xie, C., Sharma, R., et al. (2016). A Dynamic Relationship Between Mucosal T Helper Type 17 and Regulatory T-cell Populations in Nasopharynx Evolves With Age and Associates With the Clearance of Pneumococcal Carriage in Humans. Clin. Microbiol. Infect. 22736 (8), e731–e737. doi: 10.1016/j.cmi.2016.05.017
Neill, D. R., Coward, W. R., Gritzfeld, J. F., Richards, L., Garcia-Garcia, F. J., Dotor, J., et al. (2014). Density and Duration of Pneumococcal Carriage is Maintained by Transforming Growth Factor Beta1 and T Regulatory Cells. Am. J. Respir. Crit. Care Med. 189 (10), 1250–1259. doi: 10.1164/rccm.201401-0128OC
Nijnik, A., Hancock, R. E. (2009). The Roles of Cathelicidin LL-37 in Immune Defences and Novel Clinical Applications. Curr. Opin. Hematol. 16 (1), 41–47. doi: 10.1097/moh.0b013e32831ac517
Nikolaou, E., Jochems, S. P., Mitsi, E., Pojar, S., Negera, E., Reine, J., et al. (2021). Experimental Human Challenge Defines Distinct Pneumococcal Kinetic Profiles and Mucosal Responses between Colonized and Non-Colonized Adults. mBio 12 (1), e02020–20. doi: 10.1101/459495
Novak, J., Dobrovolny, J., Novakova, L., Kozak, T. (2014). The Decrease in Number and Change in Phenotype of Mucosal-Associated Invariant T Cells in the Elderly and Differences in Men and Women of Reproductive Age. Scand. J. Immunol. 80 (4), 271–275. doi: 10.1111/sji.12193
Nyugen, J., Agrawal, S., Gollapudi, S., Gupta, S. (2010). Impaired Functions of Peripheral Blood Monocyte Subpopulations in Aged Humans. J. Clin. Immunol. 30 (6), 806–813. doi: 10.1007/s10875-010-9448-8
Osler, W. (1892). The Principles and Practice of Medicine : Designed for the Use of Practitioners and Students of Medicine (New York: D. Appleton and Company).
Peter, A., Fatykhova, D., Kershaw, O., Gruber, A. D., Rueckert, J., Neudecker, J., et al. (2017). Localization and Pneumococcal Alteration of Junction Proteins in the Human Alveolar-Capillary Compartment. Histochem. Cell Biol. 147 (6), 707–719. doi: 10.1007/s00418-017-1551-y
Pfizer, I. (2019). Pfizer Announces Serotypes Included in 20-Valent Pneumococcal Conjugate Vaccine Candidate Being Investigated for the Prevention of Invasive Pneumococcal Disease in Adults Aged 18 and Older.
Pido-Lopez, J., Kwok, W. W., Mitchell, T. J., Heyderman, R. S., Williams, N. A. (2011). Acquisition of Pneumococci Specific Effector and Regulatory Cd4+ T Cells Localising Within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue. PLoS Pathog. 7 (12), e1002396. doi: 10.1371/journal.ppat.1002396
Pistolic, J., Cosseau, C., Li, Y., Yu, J. J., Filewod, N. C., Gellatly, S., et al. (2009). Host Defence Peptide LL-37 Induces IL-6 Expression in Human Bronchial Epithelial Cells by Activation of the NF-kappaB Signaling Pathway. J. Innate Immun. 1 (3), 254–267. doi: 10.1159/000171533
Puchta, A., Naidoo, A., Verschoor, C. P., Loukov, D., Thevaranjan, N., Mandur, T. S., et al. (2016). Tnf Drives Monocyte Dysfunction With Age and Results in Impaired Anti-Pneumococcal Immunity. PLoS Pathog. 12 (1), e1005368. doi: 10.1371/journal.ppat.1005368
Ragonnaud, E., Biragyn, A. (2021). Gut Microbiota as the Key Controllers of “Healthy” Aging of Elderly People. Immun. Ageing 18 (1), 2. doi: 10.1186/s12979-020-00213-w
Ramos-Sevillano, E., Ercoli, G., Felgner, P., Ramiro de Assis, R., Nakajima, R., Goldblatt, D., et al. (2020). Preclinical Development of Virulence Attenuated Streptococcus Pneumoniae Strains Able to Enhance Protective Immunity Against Pneumococcal Infection. Am. J. Respir. Crit. Care Med. 203 (8), 1037–1041. doi: 10.1164/rccm.202011-4161LE
Reiné, J., Carniel, B. F., Solórzano, C., Mitsi, E., Pojar, S., Nikolaou, E., et al. (2019). Dynamic Changes in Innate Immune and T Cell Function and Composition At the Nasal Mucosa Across the Human Lifespan. bioRxiv. doi: 10.1101/576744
Saitoh, T., Fujita, N., Hayashi, T., Takahara, K., Satoh, T., Lee, H., et al. (2009). Atg9a Controls dsDNA-driven Dynamic Translocation of STING and the Innate Immune Response. Proc. Natl. Acad. Sci. U. S. A. 106 (49), 20842–20846. doi: 10.1073/pnas.0911267106
Salminen, A., Huuskonen, J., Ojala, J., Kauppinen, A., Kaarniranta, K., Suuronen, T. (2008). Activation of Innate Immunity System During Aging: NF-kB Signaling is the Molecular Culprit of Inflamm-Aging. Ageing Res. Rev. 7 (2), 83–105. doi: 10.1016/j.arr.2007.09.002
Sapey, E., Greenwood, H., Walton, G., Mann, E., Love, A., Aaronson, N., et al. (2014). Phosphoinositide 3-Kinase Inhibition Restores Neutrophil Accuracy in the Elderly: Toward Targeted Treatments for Immunosenescence. Blood 123 (2), 239–248. doi: 10.1182/blood-2013-08-519520
Sapey, E., Patel, J. M., Greenwood, H. L., Walton, G. M., Hazeldine, J., Sadhra, C., et al. (2017). Pulmonary Infections in the Elderly Lead to Impaired Neutrophil Targeting, Which Is Improved by Simvastatin. Am. J. Respir. Crit. Care Med. 196 (10), 1325–1336. doi: 10.1164/rccm.201704-0814OC
Schenck, L. P., Surette, M. G., Bowdish, D. M. (2016). Composition and Immunological Significance of the Upper Respiratory Tract Microbiota. FEBS Lett. 590 (21), 3705–3720. doi: 10.1002/1873-3468.12455
Schmid, P., Selak, S., Keller, M., Luhan, B., Magyarics, Z., Seidel, S., et al. (2011). Th17/Th1 Biased Immunity to the Pneumococcal Proteins PcsB, Stkp and PsaA in Adults of Different Age. Vaccine 29 (23), 3982–3989. doi: 10.1016/j.vaccine.2011.03.081
Schuijt, T. J., Lankelma, J. M., Scicluna, B. P., de Sousa e Melo, F., Roelofs, J. J., de Boer, J. D., et al. (2016). The Gut Microbiota Plays a Protective Role in the Host Defence Against Pneumococcal Pneumonia. Gut 65 (4), 575–583. doi: 10.1136/gutjnl-2015-309728
Sharma, P., Sharma, N., Mishra, P., Joseph, J., Mishra, D. K., Garg, P., et al. (2019). Differential Expression of Antimicrobial Peptides in Streptococcus Pneumoniae Keratitis and STAT3-Dependent Expression of LL-37 by Streptococcus Pneumoniae in Human Corneal Epithelial Cells. Pathogens 8 (1), 31. doi: 10.3390/pathogens8010031
Shivshankar, P., Boyd, A. R., Le Saux, C. J., Yeh, I. T., Orihuela, C. J. (2011). Cellular Senescence Increases Expression of Bacterial Ligands in the Lungs and is Positively Correlated With Increased Susceptibility to Pneumococcal Pneumonia. Aging Cell 10 (5), 798–806. doi: 10.1111/j.1474-9726.2011.00720.x
Simell, B., Auranen, K., Kayhty, H., Goldblatt, D., Dagan, R., O’Brien, K. L., et al. (2012). The Fundamental Link Between Pneumococcal Carriage and Disease. Expert Rev. Vaccines 11 (7), 841–855. doi: 10.1586/erv.12.53
Simell, B., Vuorela, A., Ekstrom, N., Palmu, A., Reunanen, A., Meri, S., et al. (2011). Aging Reduces the Functionality of Anti-Pneumococcal Antibodies and the Killing of Streptococcus Pneumoniae by Neutrophil Phagocytosis. Vaccine 29 (10), 1929–1934. doi: 10.1016/j.vaccine.2010.12.121
Smith, E. L., Wheeler, I., Adler, H., Ferreira, D. M., Sa-Leao, R., Abdullahi, O., et al. (2020). Upper Airways Colonisation of Streptococcus Pneumoniae in Adults Aged 60 Years and Older: A Systematic Review of Prevalence and Individual Participant Data Meta-Analysis of Risk Factors. J. Infect. 81 (4), 540–548. doi: 10.1016/j.jinf.2020.06.028
Spindler, C., Hedlund, J., Jasir, A., Normark, B. H., Ortqvist, A. (2008). Effects of a Large-Scale Introduction of the Pneumococcal Polysaccharide Vaccine Among Elderly Persons in Stockholm, Sweden. Vaccine 26 (43), 5541–5546. doi: 10.1016/j.vaccine.2008.06.073
Stupka, J. E., Mortensen, E. M., Anzueto, A., Restrepo, M. I. (2009). Community-Acquired Pneumonia in Elderly Patients. Aging Health 5 (6), 763–774. doi: 10.2217/ahe.09.74
Suzuki, M., Dhoubhadel, B. G., Ishifuji, T., Yasunami, M., Yaegashi, M., Asoh, N., et al. (2017). Serotype-Specific Effectiveness of 23-Valent Pneumococcal Polysaccharide Vaccine Against Pneumococcal Pneumonia in Adults Aged 65 Years or Older: A Multicentre, Prospective, Test-Negative Design Study. Lancet Infect. Dis. 17 (3), 313–321. doi: 10.1016/S1473-3099(17)30049-X
Suzuki, T., Yoshinaga, N., Tanabe, S. (2011). Interleukin-6 (IL-6) Regulates Claudin-2 Expression and Tight Junction Permeability in Intestinal Epithelium. J. Biol. Chem. 286 (36), 31263–31271. doi: 10.1074/jbc.M111.238147
Thompson, A. B., Scholer, S. G., Daughton, D. M., Potter, J. F., Rennard, S. I. (1992). Altered Epithelial Lining Fluid Parameters in Old Normal Individuals. J. Gerontol. 47 (5), M171–M176. doi: 10.1093/geronj/47.5.m171
Torres, A., Blasi, F., Dartois, N., Akova, M. (2015). Which Individuals are At Increased Risk of Pneumococcal Disease and Why? Impact of COPD, Asthma, Smoking, Diabetes, and/or Chronic Heart Disease on Community-Acquired Pneumonia and Invasive Pneumococcal Disease. Thorax 70 (10), 984–989. doi: 10.1136/thoraxjnl-2015-206780
Toubal, A., Nel, I., Lotersztajn, S., Lehuen, A. (2019). Mucosal-Associated Invariant T Cells and Disease. Nat. Rev. Immunol. 19 (10), 643–657. doi: 10.1038/s41577-019-0191-y
Valdes, A. M., Glass, D., Spector, T. D. (2013). Omics Technologies and the Study of Human Ageing. Nat. Rev. Genet. 14 (9), 601–607. doi: 10.1038/nrg3553
van der Geest, K. S., Abdulahad, W. H., Tete, S. M., Lorencetti, P. G., Horst, G., Bos, N. A., et al. (2014). Aging Disturbs the Balance Between Effector and Regulatory CD4+ T Cells. Exp. Gerontol. 60, 190–196. doi: 10.1016/j.exger.2014.11.005
van Deursen, A. M. M., van Houten, M. A., Webber, C., Patton, M., Scott, D., Patterson, S., et al. (2018). The Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Carriage in the Community Acquired Pneumonia Immunization Trial in Adults (CapiTA) Study. Clin. Infect. Dis. 67 (1), 42–49. doi: 10.1093/cid/ciy009
van Duin, D., Mohanty, S., Thomas, V., Ginter, S., Montgomery, R. R., Fikrig, E., et al. (2007). Age-Associated Defect in Human TLR-1/2 Function. J. Immunol. 178 (2), 970–975. doi: 10.4049/jimmunol.178.2.970
Verschoor, C. P., Johnstone, J., Loeb, M., Bramson, J. L., Bowdish, D. M. E. (2014a). Anti-Pneumococcal Deficits of Monocyte-Derived Macrophages From the Advanced-Age, Frail Elderly and Related Impairments in PI3K-AKT Signaling. Hum. Immunol. 75 (12), 1192–1196. doi: 10.1016/j.humimm.2014.10.004
Verschoor, C. P., Johnstone, J., Millar, J., Parsons, R., Lelic, A., Loeb, M., et al. (2014b). Alterations to the Frequency and Function of Peripheral Blood Monocytes and Associations With Chronic Disease in the Advanced-Age, Frail Elderly. PLoS One 9 (8), ARTN e104522. doi: 10.1371/journal.pone.0104522
Verschoor, C. P., Loukov, D., Naidoo, A., Puchta, A., Johnstone, J., Millar, J., et al. (2015). Circulating TNF and Mitochondrial DNA are Major Determinants of Neutrophil Phenotype in the Advanced-Age, Frail Elderly. Mol. Immunol. 65 (1), 148–156. doi: 10.1016/j.molimm.2015.01.015
Vuononvirta, J., Peltola, V., Ilonen, J., Mertsola, J., He, Q. (2015). The Gene Polymorphism of IL-17 G-152A is Associated With Increased Colonization of Streptococcus Pneumoniae in Young Finnish Children. Pediatr. Infect. Dis. J. 34 (9), 928–932. doi: 10.1097/INF.0000000000000691
Wagner, A., Weinberger, B. (2020). Vaccines to Prevent Infectious Diseases in the Older Population: Immunological Challenges and Future Perspectives. Front. Immunol. 11, 717. doi: 10.3389/fimmu.2020.00717
Walker, L. J., Tharmalingam, H., Klenerman, P. (2014). The Rise and Fall of MAIT Cells With Age. Scand. J. Immunol. 80 (6), 462–463. doi: 10.1111/sji.12237
Ward, C., Schlingmann, B., Stecenko, A. A., Guidot, D. M., Koval, M. (2015). NF-Kappab Inhibitors Impair Lung Epithelial Tight Junctions in the Absence of Inflammation. Tissue Barriers 3 (1-2), e982424. doi: 10.4161/21688370.2014.982424
Weight, C. M., Venturini, C., Pojar, S., Jochems, S. P., Reine, J., Nikolaou, E., et al. (2019). Microinvasion by Streptococcus Pneumoniae Induces Epithelial Innate Immunity During Colonisation At the Human Mucosal Surface. Nat. Commun. 10 (1), 3060. doi: 10.1038/s41467-019-11005-2
Weinberger, D. M., Trzcinski, K., Lu, Y. J., Bogaert, D., Brandes, A., Galagan, J., et al. (2009). Pneumococcal Capsular Polysaccharide Structure Predicts Serotype Prevalence. PLoS Pathog. 5 (6), ARTN e1000476. doi: 10.1371/journal.ppat.1000476
Wenisch, C., Patruta, S., Daxbock, F., Krause, R., Horl, W. (2000). Effect of Age on Human Neutrophil Function. J. Leukoc. Biol. 67 (1), 40–45. doi: 10.1002/jlb.67.1.40
Williams, A. E., Jose, R. J., Brown, J. S., Chambers, R. C. (2015). Enhanced Inflammation in Aged Mice Following Infection With Streptococcus Pneumoniae Is Associated With Decreased IL-10 and Augmented Chemokine Production. Am. J. Physiol. Lung Cell Mol. Physiol. 308 (6), L539–L549. doi: 10.1152/ajplung.00141.2014
Wilson, R., Cohen, J. M., Jose, R. J., de Vogel, C., Baxendale, H., Brown, J. S. (2015). Protection Against Streptococcus Pneumoniae Lung Infection After Nasopharyngeal Colonization Requires Both Humoral and Cellular Immune Responses. Mucosal Immunol. 8 (3), 627–639. doi: 10.1038/mi.2014.95
Wright, A. K., Bangert, M., Gritzfeld, J. F., Ferreira, D. M., Jambo, K. C., Wright, A. D., et al. (2013). Experimental Human Pneumococcal Carriage Augments IL-17A-dependent T-Cell Defence of the Lung. PLoS Pathog. 9 (3), e1003274. doi: 10.1371/journal.ppat.1003274
Yasuda, I., Suzuki, M., Dhoubhadel, B. G., Terada, M., Satoh, A., Sando, E., et al. (2020). The Low Carriage Prevalence of Pneumococcus Among Community-Dwelling Older People: A Cross-Sectional Study in Japan. Vaccine 38 (21), 3752–3758. doi: 10.1016/j.vaccine.2020.03.033
Yende, S., Tuomanen, E. I., Wunderink, R., Kanaya, A., Newman, A. B., Harris, T., et al. (2005). Preinfection Systemic Inflammatory Markers and Risk of Hospitalization Due to Pneumonia. Am. J. Respir. Crit. Care Med. 172 (11), 1440–1446. doi: 10.1164/rccm.200506-888OC
Yuk, J. M., Shin, D. M., Lee, H. M., Yang, C. S., Jin, H. S., Kim, K. K., et al. (2009). Vitamin D3 Induces Autophagy in Human Monocytes/Macrophages Via Cathelicidin. Cell Host Microbe 6 (3), 231–243. doi: 10.1016/j.chom.2009.08.004
Zalacain, R. T. A. (2004). Pneumonia in the Elderly. Clin. Pulm. Med. 11, 210–218. doi: 10.1097/01.cpm.0000132888.49928.86
Zhang, Q., Leong, S. C., McNamara, P. S., Mubarak, A., Malley, R., Finn, A. (2011). Characterisation of Regulatory T Cells in Nasal Associated Lymphoid Tissue in Children: Relationships With Pneumococcal Colonization. PLoS Pathog. 7 (8), e1002175. doi: 10.1371/journal.ppat.1002175
Zhang, Y. G., Lu, R., Xia, Y., Zhou, D., Petrof, E., Claud, E. C., et al. (2019). Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflammation Bowel Dis. 25 (1), 97–110. doi: 10.1093/ibd/izy292
Keywords: epithelium, pneumococcus (Streptococcus pneumoniae), innate immunity, inflammageing, older individuals
Citation: Weight CM, Jochems SP, Adler H, Ferreira DM, Brown JS and Heyderman RS (2021) Insights Into the Effects of Mucosal Epithelial and Innate Immune Dysfunction in Older People on Host Interactions With Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 11:651474. doi: 10.3389/fcimb.2021.651474
Received: 09 January 2021; Accepted: 10 May 2021;
Published: 25 May 2021.
Edited by:
Masaya Yamaguchi, Osaka University, JapanReviewed by:
Daniel Neill, University of Liverpool, United KingdomStephen I. Pelton, Boston University, United States
Soichiro Kimura, Toho University, Japan
Copyright © 2021 Weight, Jochems, Adler, Ferreira, Brown and Heyderman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline M. Weight, Yy53ZWlnaHRAdWNsLmFjLnVr
 Caroline M. Weight
Caroline M. Weight Simon P. Jochems
Simon P. Jochems Hugh Adler3,4
Hugh Adler3,4 Jeremy S. Brown
Jeremy S. Brown Robert S. Heyderman
Robert S. Heyderman