- 1Department of Infectious Diseases, Jeju National University College of Medicine, Jeju, South Korea
- 2Department of Medical and Biological Sciences, The Catholic University of Korea, Bucheon, South Korea
- 3Department of Medical Nutrition, Graduate School of East-West Medical Science, Kyung Hee University, Yongin, South Korea
- 4Division of Emerging Infectious Diseases and Vector Research, National Institute of Health, Korea Center for Disease Control and Prevention, Osong, South Korea
- 5Department of Microbiology, Seoul National University College of Medicine, Seoul, South Korea
- 6Department of Microbiology, Hanyang University College of Medicine, Seoul, South Korea
Severe fever with thrombocytopenia syndrome (SFTS), a newly emerging tick-borne viral disease, has been detected in Asia since 2009, and person-to-person transmission is possible. SFTS is characterized by atypical signs, including mild to severe febrile illness similar to that associated with hemorrhagic fever, with 16.2 to 30% mortality. We found that the titers of neutralizing antibodies, play an important role in protective immunity, to SFTS virus (SFTSV) in survivors and healthy residents who lived in endemic areas and who were positive for SFTSV IgG, were higher than those in non-survivor patients. Moreover, the titers were maintained in surviving patients and healthy residents but not in non-surviving patients in South Korea.
Introduction
Severe fever with thrombocytopenia syndrome (SFTS), a new tick-borne viral disease with a high mortality rate, was first reported in China in 2009, South Korea in 2010 Japan in 2013, Vietnam in 2017, Myanmar in 2018, and Taiwan in 2019 (Yu et al., 2011; Takahashi et al., 2014; Kim et al., 2018; Tran et al., 2019; Peng et al., 2020; Win et al., 2020). Most SFTS virus (SFTSV) infections occur via bites from the tick Haemaphysalis longicornis; however, SFTSV transmission can also occur through close contact with an infected patient (Yoo et al., 2017). SFTS is characterized by acute high fever, thrombocytopenia, leukopenia, elevated serum hepatic enzyme levels, gastrointestinal symptoms, and multiorgan failure and has a 16.2 to 30% mortality rate (Yu et al., 2011; Takahashi et al., 2014; Peng et al., 2020). Atypical signs and symptoms as well as asymptomatic SFTS infections have also been identified in patients, and SFTS patients in China have been shown to produce and maintain long-lasting neutralizing antibodies to SFTSV (Huang et al., 2016; Yoo et al., 2017; Kim et al., 2018).
In this study, we briefly investigated the levels of neutralizing antibodies to SFTSV in serum among surviving patients, non-surviving patients, and healthy residents living in endemic areas and who were positive for SFTSV IgG antibody in South Korea from 2013 to 2019 (Yoo et al., 2019). We found that the titers of neutralizing antibodies to SFTSV maintained in surviving patients and healthy residents were higher than those detected in non-surviving patients.
Materials and Methods
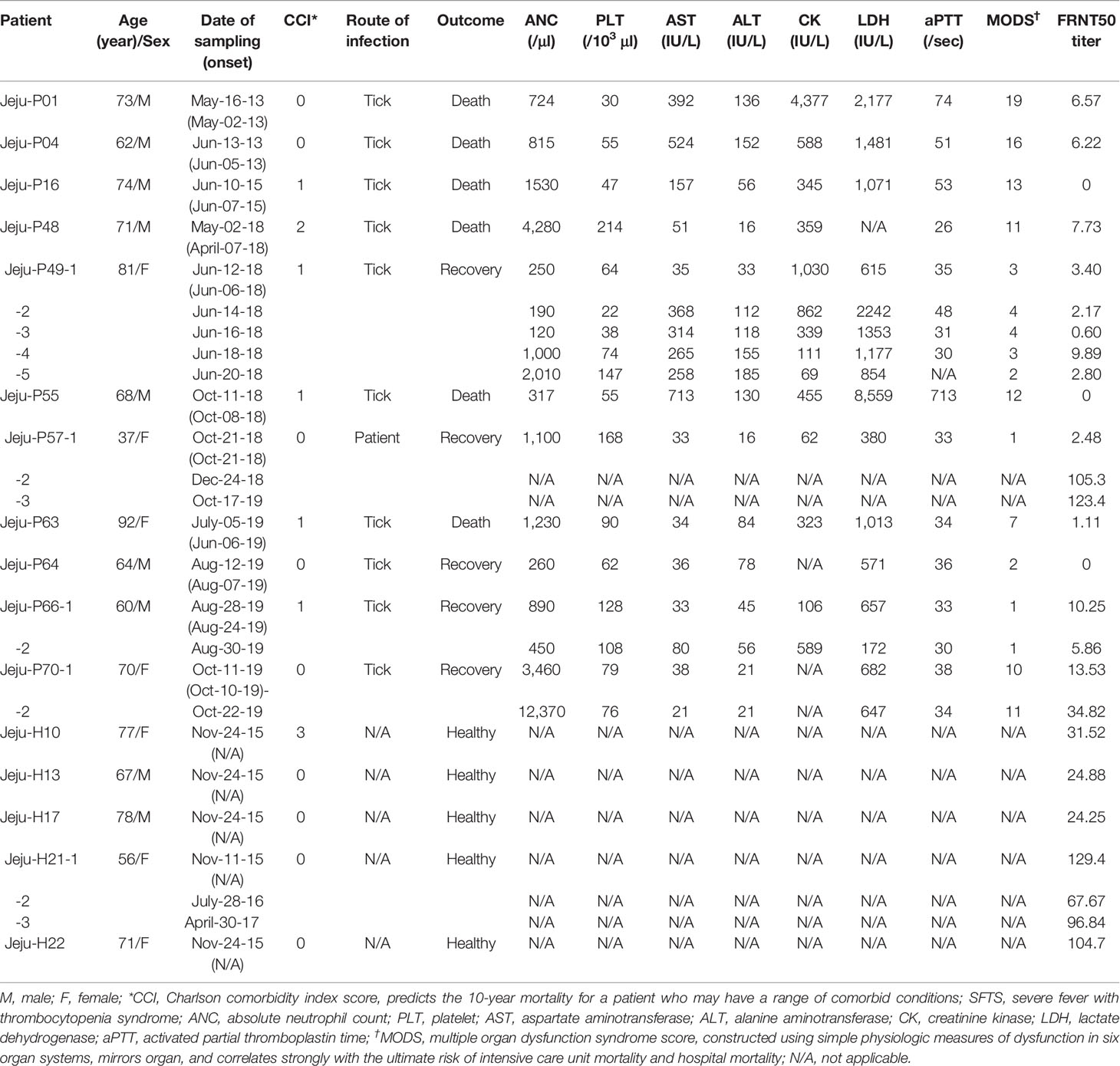
To investigate the levels of neutralizing antibodies to SFTSV, we collected 19 serum samples from 11 patients (non-surviving patients; n=6, mean age: 73.3 and surviving patients; n=5, mean age: 68.8), which were laboratory confirmed at Jeju National University from May 2013 to October 2019, Jeju, South Korea, and 7 serum samples from 5 healthy residents (mean age: 69.8) living in endemic areas who were positive for SFTSV IgG from November 2015 to April 2017 (Yoo et al., 2019) (Table 1). This study was approved by the Institutional Review Board (IRB) of Jeju National University Hospital.
For the molecular diagnosis of SFTSV, RNA was extracted from the stored patient serum using a QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). Real-time RT-PCR was performed to amplify the partial small (S) segment of the viral RNA from the stored serum and confirm SFTSV infection (Zhang et al., 2012; Yun et al., 2015). Real-time RT-PCR showed that all the patients (n=11) were positive for SFTSV, and 6 healthy residents were negative for SFTSV. Antibody detection was performed, and IgG was detected in the serum of all patients and 6 healthy residents (Yoo et al., 2019).
To determine the titers of neutralizing antibody to SFTSV in human sera, the 50% focus reduction neutralization test (FRNT50) assay was performed. Serum samples were heat inactivated at 56°C for 30 min and diluted in 2-fold increments from 1:10 to 1:320. Each dilution was mixed with an equal volume of solution containing SFTSV (1600 ffu/mL). The mixture was inoculated into Vero E6 cells prepared in 24-well plates and then incubated for 1 hour at 37°C. Culture medium was used as a control. After incubation, the cells were overlaid with 1 mL of Dulbecco’s modified Eagle’s medium (DMEM) containing 1.5% carboxymethyl cellulose, and the cells were incubated for an additional 2 days. After 2 days of incubation, cells were fixed with 4% paraformaldehyde and incubated with 500 µl/well of anti-SFTSV NP monoclonal antibodies diluted with 0.5% Triton X-100 in PBS for 90 min at room temperature (RT), followed by incubation with HRP-conjugated secondary antibodies. The visualization of foci of SFTSV-infected cells was performed using a DAB substrate kit. The plaque reduction percentage was calculated by the following formula: [(number of foci of SFTSV diluted without serum)-(number of foci of SFTSV diluted with serum)] x 100/number of foci of SFTSV diluted without serum. From this plaque reduction percentage, the FRTN50 titers were calculated by the [log(inhibitor) vs. normalized response] equation using GraphPad Prism 8.0.
For statistical analysis, data were expressed as medians and 95% confidence intervals. Neutralizing antibodies among three groups (non-surviving patients, surviving patients and healthy residents) according to the dilution factor was examined using Kruskal-Wallis test. All data were analyzed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). A p value < 0.05 was considered statistically significant for two-sided tests.
Results
The FRNT50 results showed that 4 surviving patients developed neutralizing antibodies against SFTSV, with titers ranging from 1:20 to 1:80, and 5 healthy residents were positive for SFTSV IgG. The healthy residents had no typical symptoms or other infectious diseases, had no history of tick bites or contact with SFTS patients during the period of sample collection, and developed neutralizing antibodies against SFTSV at titers ranging from 1:20 to 1:80. However, 6 non-surviving patients did not have well-developed neutralizing antibodies. Neutralizing antibodies to SFTSV differed significantly among surviving patients, non-surviving patients, and healthy residents regardless of dilution factor (p <0.05 for all dilution factor) (Figure 1).
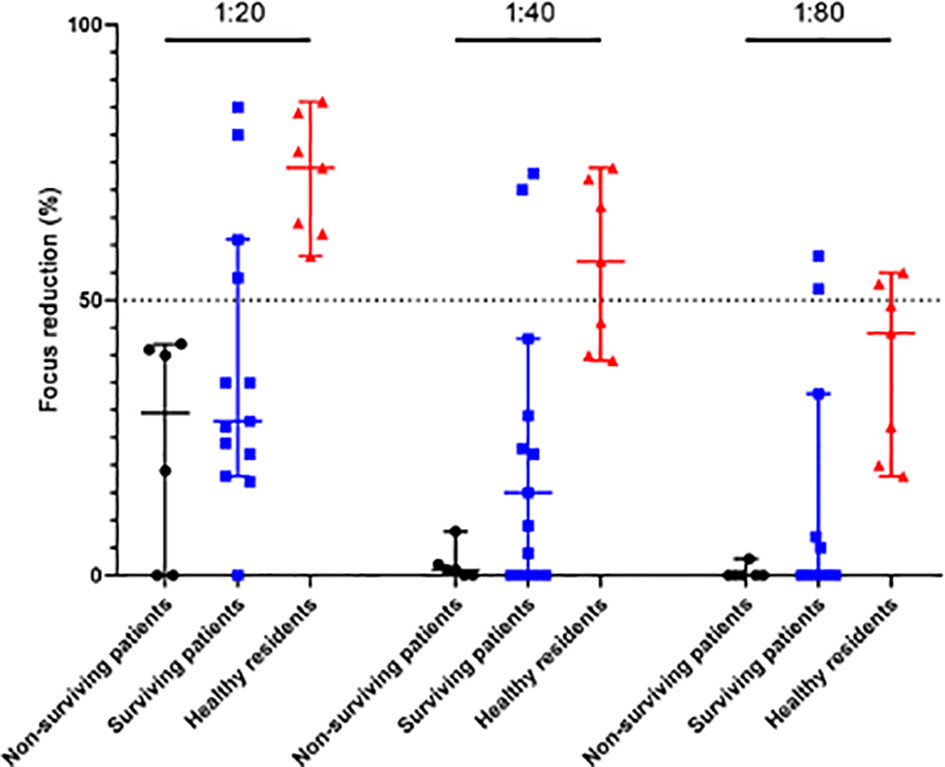
Figure 1 Neutralizing antibody titrations. Neutralizing antibodies to SFTSV were evaluated (FRNT50) in serum samples collected from 6 non-surviving patients, 13 serum samples from 5 surviving patients, and 7 serum samples from 5 healthy residents. Individual samples were serially diluted from 1∶20 to 1∶80. Statistically significant differences were found among surviving patients, non-surviving patients, and healthy residents (p <0.05 for all dilution factor).
Interestingly, neutralizing antibodies one surviving patient (Jeju-P57) appeared at two months after discharge and persisted for one year, and neutralizing antibodies in one healthy resident (Jeju-H21) persisted for the entire study period of 3 years (from 2015 to 2017) (Table 1).
These results show that long-lasting neutralizing antibodies to SFTSV is maintaining in surviving patients (Huang et al., 2016).
Conclusion
Neutralizing antibodies can directly block viral attachment to target cells by interfering with virus-receptor interactions and induce antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity and antibody-dependent cellular phagocytosis (ADCP) (Walker et al., 2018). A previous study showed that SFTS patients produce neutralizing antibodies to SFTSV and can last for four years with a decrease in titers and suggested that neutralizing monoclonal antibody activity blocks the interactions between glycoprotein Gn and cellular receptors (Huang et al., 2016).
In conclusion, we found that surviving patients and healthy residents living in an endemic area had high titers of long-lasting neutralizing antibodies to SFTSV compared with non-surviving patients. A previous study suggested that neutralizing monoclonal antibody activity blocks the interactions between glycoprotein Gn and cellular receptors (Huang et al., 2016). Therefore, we suggest that neutralizing antibodies may play an important role in protective immunity in surviving patients and healthy residents against SFTSV infection.
However, the limitation of this study is that we followed surviving patients for up to one year, whereas we followed healthy residents for up to 3 years and not yet study about the role of long-lasting neutralizing antibodies. Therefore, we do not know how long the neutralizing antibodies in patients last and the characteristics of the neutralizing antibodies to SFTSV.
Therefore, further clinical research on lasting neutralizing antibodies and the characteristics of these antibodies are needed, and this subject deserves further discussion, which will aid in the understanding of the pathogenesis of SFTSV infection.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The Institutional Review Board (IRB) of Jeju National University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: KL, J-HN, SH. Methodology: KL, JHK, J-HN, JY, SH, J-YK, N-HC. Software: J-YK, JHK. Supervision: KL, J-HN, JMK. Validation: JHK. Formal analysis: KL, JHK, J-HN, JY, SH, J-YK, H-JP, J-YL, J-YL, W-JP, N-HC. Funding acquisition: J-HN, KL. Data curation: KL, J-HN, N-HC. Writing-original draft: JY, JHK, J-YK, SH, J-HN, KL. Writing—review and editing: KL, J-HN, JHK, JMK. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by J-HN was supported by SML Biotree Group fund (2019-2021), a fund (HD20A0323) by Research of Korea Center for Disease Control and Prevention, and the research fund of Hanyang University (HY-2020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Huang, Y. T., Zhao, L., Wen, H. L., Hao, Y., Yu, X. J. (2016). Neutralizing Antibodies to Severe Fever with Thrombocytopenia Syndrome Virus 4 Years after Hospitalization, China. Emerg. Infect. Dis. 22 (11), 1985–1987. doi: 10.3201/eid2211.160414
Kim, Y. R., Yun, Y., Bae, S. G., Park, D., Kim, S. H., Lee, J. M., et al. (2018). Severe Fever with Thrombocytopenia Syndrome Virus Infection, South Korea 2010. Emerg. Infect. Dis. 24 (11), 2103–2105. doi: 10.3201/eid2411.170756
Peng, S. H., Yang, S. L., Tang, S. E., Wang, T. C., Hsu, T. C., Su, C. L., et al. (2020). Human Case of Severe Fever with Thrombocytopenia Syndrome Virus Infection, Taiwan 2019. Emerg. Infect. Dis. 26 (7), 1612–1614. doi: 10.3201/eid2607.200104
Takahashi, T., Maeda, K., Suzuki, T., Ishido, A., Shigeoka, T., Tominaga, T., et al. (2014). The First Identification and Retrospective Study of Severe Fever with Thrombocytopenia Syndrome in Japan. J. Infect. Dis. 209, 816–827. doi: 10.1093/infdis/jit603
Tran, X. C., Yun, Y., An, L. V., Kim, S. H., Thao, N. T. P., Man, P. K. C., et al. (2019). Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg. Infect. Dis. 25 (5), 1029–10031. doi: 10.3201/eid2505.181463
Walker, L. M., Burton, D. R. (2018). Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat. Rev. Immunol. 18 (5), 297–308. doi: 10.1038/nri.2017.148
Win, A. M., Nguyen, Y. T. H., Kim, Y., Ha, N. Y., Kang, J. G., Kim, H., et al. (2020). Genotypic Heterogeneity of Orientia tsutsugamushi in Scrub Typhus Patients and Thrombocytopenia Syndrome Co-infection, Myanmar. Emerg. Infect. Dis. 26 (8), 1878–1881. doi: 10.3201/eid2608.200135
Yoo, J. R., Heo, S. T., Park, D., Kim, H., Fukuma, A., Fukushi, S., et al. (2017). Family Cluster Analysis of Severe Fever with Thrombocytopenia Syndrome Virus Infection in Korea. Am. J. Trop. Med. Hyg. 95 (6), 1351–1357. doi: 10.4269/ajtmh.16-0527
Yoo, J. R., Heo, S. T., Kim, M., Song, S. W., Boo, J. W., Lee, K. H. (2019). Seroprevalence of severe fever with thrombocytopenia syndrome in the agricultural population of Jeju Island, Korea 2015-2017. Infect. Chemother. 51 (4), 337–344. doi: 10.3947/ic.2019.51.4.337
Yu, X. J., Liang, M. F., Zhang, S. Y., Liu, Y., Li, J. D., Sun, Y. L., et al. (2011). Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl. J. Med. 364, 1523–1532. doi: 10.1056/NEJMoa1010095
Yun, Y., Heo, S. T., Kim, G., Hewson, R., Kim, H., Park, D., et al. (2015). Phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in South Korea and migratory bird routes between China, South Korea, and Japan. Am. J. Trop. Med. Hyg. 93, 468–474. doi: 10.4269/ajtmh.15-0047
Keywords: SFTSV, neutralizing antibodies, survivors, non-survivors, healthy residents, South Korea
Citation: Yoo JR, Kim J-Y, Heo ST, Kim J, Park H-J, Lee J-Y, Lim H-Y, Park W-J, Cho N-H, Kim JM, Nam J-H and Lee KH (2021) Neutralizing Antibodies to Severe Fever With Thrombocytopenia Syndrome Virus Among Survivors, Non-Survivors and Healthy Residents in South Korea. Front. Cell. Infect. Microbiol. 11:649570. doi: 10.3389/fcimb.2021.649570
Received: 12 January 2021; Accepted: 08 March 2021;
Published: 23 March 2021.
Edited by:
Gaoqian Feng, Burnet Institute, AustraliaReviewed by:
Li Zhao, Shandong University, ChinaChang-jun Bao, Jiangsu Provincial Center for Disease Control And Prevention, China
Copyright © 2021 Yoo, Kim, Heo, Kim, Park, Lee, Lim, Park, Cho, Kim, Nam and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keun Hwa Lee, eW9tdXN0N0BnbWFpbC5jb20=; Jae-Hwan Nam, amhuYW1AY2F0aG9saWMuYWMua3I=; Jung Mogg Kim, anVuZ21vZ2dAaGFueWFuZy5hYy5rcg==
†These authors have contributed equally to this work
 Jeong Rae Yoo
Jeong Rae Yoo Jae-Yong Kim2†
Jae-Yong Kim2† Sang Taek Heo
Sang Taek Heo Jihye Kim
Jihye Kim Woo-Jung Park
Woo-Jung Park Nam-Hyuk Cho
Nam-Hyuk Cho Jung Mogg Kim
Jung Mogg Kim Keun Hwa Lee
Keun Hwa Lee