
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell. Infect. Microbiol., 19 March 2021
Sec. Molecular Bacterial Pathogenesis
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.617573
This article is part of the Research TopicHost-pathogen Interaction During Pneumococcal InfectionsView all 14 articles
 Bin Chang1*
Bin Chang1* Yuki Kinjo2,3,4*
Yuki Kinjo2,3,4* Masatomo Morita1
Masatomo Morita1 Kosuke Tamura5
Kosuke Tamura5 Hiroshi Watanabe6
Hiroshi Watanabe6 Yoshinari Tanabe7
Yoshinari Tanabe7 Koji Kuronuma8
Koji Kuronuma8 Jiro Fujita9
Jiro Fujita9 Kengo Oshima10
Kengo Oshima10 Takaya Maruyama11
Takaya Maruyama11 Shuichi Abe12
Shuichi Abe12 Kei Kasahara13
Kei Kasahara13 Junichiro Nishi14
Junichiro Nishi14 Tetsuya Kubota15
Tetsuya Kubota15 Makoto Ohnishi1
Makoto Ohnishi1 Shigeru Suga16
Shigeru Suga16 Kazunori Oishi5 on behalf of the Adult IPD Study Group
Kazunori Oishi5 on behalf of the Adult IPD Study GroupPneumococcal surface protein A (PspA) is a surface protein of Streptococcus pneumoniae that may be a candidate antigen for new pneumococcal vaccines. This study investigates the distribution of PspA clades of the causative strains of adult invasive pneumococcal disease (IPD) in Japan. Of the 1,939 strains isolated from cases of adult IPD during 2014–2019, the PspA clades of 1,932 (99.6%) strains were determined, and no pspA was detected in the remaining 7 strains (0.4%). PspA clades 1–6 were detected in 786 (40.5%), 291 (15.0%), 443 (22.8%), 369 (19.0%), 33 (1.7%), and 6 (0.3%) strains, respectively. New PspA clades (0.2%) were identified in two non-typeable and two serotype 35B pneumococci. The proportions of clade 1 and clade 2 showed significantly decreased and increased trends, respectively. Furthermore, the PspA clade of pneumococcal strains was partially serotype- and sequence type-dependent. The majority of strains belonging to serotypes contained in both the 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23) belonged to PspA clades 1 or 3. In contrast, the distribution of clades in non-vaccine serotypes was wider than that of vaccine serotype pneumococci. Our findings demonstrate that almost all pneumococcal strains from adult IPD express PspA clades 1–4, especially for non-vaccine serotypes. These results may be useful for the development of a new pneumococcal vaccine with PspA.
Streptococcus pneumoniae is the most common cause of pneumonia, bloodstream infections, and meningitis in young children and adults 65 years or older (Centers for Disease Control and Prevention, 2020). To date, 100 pneumococcal capsular serotypes have been identified (Ganaie et al., 2020). Currently, vaccines for the prevention of S. pneumoniae infections include the 23-valent pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13). These vaccines only cover some pneumococcal serotypes and cannot protect against infections due to non-vaccine serotypes and unencapsulated S. pneumoniae (Briles et al., 2019). The 7-valent pneumococcal conjugate vaccine (PCV7) was available for children in Japan in 2010 and was replaced by PCV13 in November 2013. PCVs have been included in the national immunization program (NIP) since April 2013. After the introduction of PCVs, the incidence of IPD in children aged <5 years decreased by >50%; however, this has not decreased further (Suga et al., 2015). PCV13 and PPSV23 have already been licensed for adults, and PPSV23 for those aged ≥65 years has been included in the Japanese NIP from October 2014. We have been studying epidemiology and clinical features of IPD in adults residing in 10 prefectures of Japan (the same area as this study) since 2013 (Fukusumi et al., 2017; Shimbashi et al., 2019; Shimbashi et al., 2020).
The direct and indirect protective effects of PCV have been reported worldwide (Berical et al., 2016). However, an increase in invasive pneumococcal disease (IPD) caused by non-vaccine serotypes (serotype replacement) occurred after the introduction of PCV, especially in children (Hausdorff and Hanage, 2016). The serotype replacement was also observed in adult IPD (Kendall et al., 2016). Because of the limitations of capsular polysaccharide vaccines, there is an urgent need to develop new, effective, and affordable pneumococcal vaccines covering a wide range of serotypes. These candidates include protein-based pneumococcal vaccines using conserved pneumococcal antigens, such as surface-exposed protein, and detoxified pneumolysin.
Pneumococcal surface protein A (PspA) is a choline-binding protein on the cell surface of almost all pneumococcal strains that inhibits the complement-mediated clearance of pneumococci (McDaniel et al., 1984). PspA comprises five domains, namely, a signal peptide, an α-helical highly charged domain, a proline-rich region domain, a choline-binding domain, and a short hydrophobic tail (Hollingshead et al., 2000). The α-helical highly charged domain has an α-helical coiled-coil structure that is further divided into regions A, B, and C (McDaniel et al., 1994). The B region is the clade-defining region, and amino acid residues 192 and 260 of PspA are protection-eliciting epitopes. The sequence variations of this region are used to classify pneumococcal strains into three families and six clades (Pimenta et al., 2006). It was reported that almost 100% of clinical isolates from cases of IPD in adults and non-IPD in children in Japan belonged to either PspA family 1 (clades 1 and 2) or 2 (clades 3–5) (Piao et al., 2014; Kawaguchiya et al., 2018). However, the S. pneumoniae strains analyzed in these studies were from adult cases of IPD before the introduction of PCVs or from noninvasive diseases in children. Therefore, the PspA clade distribution of a relatively large number of S. pneumoniae strains from adult IPD cases after PCV introduction has not yet been reported.
This study determined the PspA clade of 1,932 S. pneumoniae strains isolated from adult IPD cases in Japan between 2014 and 2019 to elucidate whether PCV introduction influenced the PspA clade distribution. We also analyzed the relationships between PspA clade, serotype, and clonal complex (CC).
The adult IPD Study Group implemented population-based surveillance in Japan in 2013 of IPD cases occurring in people over the age of 15 years old who resided in 10 prefectures of Japan (Hokkaido, Miyagi, Yamagata, Niigata, Mie, Nara, Kochi, Fukuoka, Kagoshima, and Okinawa). When IPD occurred, the clinical information and the causative pneumococcal strains were simultaneously collected and sent to the National Institute of Infectious Diseases (NIID). The clinical information included the patient’s sex, age, and history of PCV13 and PPSV23 vaccinations.
A case of IPD was defined as the detection of pneumococci by bacterial culture from normally sterile sites. Our study analyzed S. pneumoniae strains isolated from adult IPD patients from January 2014 to December 2019. One isolate per case was included.
All S. pneumoniae strains were plated on Columbia agar with 5% sheep blood (Nippon Becton Dickinson Co. Ltd., Tokyo, Japan). The quellung reaction was used for serotype determination with pneumococcal antisera (Statens Serum Institut, Copenhagen, Denmark). Serotype 11A/E and non-typeable (NT) were determined as described previously (Shimbashi et al., 2020).
The genomic DNA of the pneumococcal isolates was purified using a High Pure PCR Template Purification Kit (Roche Diagnostics, Tokyo, Japan). MLST was performed as described by Enright and Spratt (1998). Allelic numbers and sequence types (STs) were assigned using the pneumococcal MLST website (https://pubmlst.org/spneumoniae/). Strains where ≥5/7 alleles were identical were classified as a CC (Gertz et al., 2003). PspA clade determination was performed as described by Pimenta et al. (2006). For strains where the PCR fragment was not amplified, an additional PCR reaction was performed using primers upstream (pspA-up: 5′-CACACGAGATTATGCTAGTC-3′) and downstream (pspA-dn: 5′-CTGCTCCTTGAGCAAAAGAG-3′) of pspA, and if a PCR fragment and pspA sequence was then obtained, the PspA clade was determined. For strains having sequences that were <90% identical to the sequences of known clades 1–6, BLAST search in nucleotide database of NCBI was performed.
Genomic DNA libraries of the pneumococcal strains in which pspA could not be detected by PCR were constructed using the Nextera XT DNA sample prep kit (Illumina, San Diego, CA, USA) and then sequenced using an MiSeq (Illumina). After genome assembly was performed using the SPAdes version 3.13.1 with the careful option and a read coverage cutoff value of 10 (Bankevich et al., 2012), homology search of pspA gene on the genomes was performed by GENETYX-MAC (GENETYX, Tokyo, Japan).
The whole genome sequences (accession numbers: DRX251224-DRR251230) as well as new (LC597020-LC597021) and deletion mutant (LC597022-LC597023) pspA sequences of pneumococci have been deposited in the DNA Data Bank of Japan.
The proportions of each clade were compared using χ2-test or Fisher’s exact test. The Mantel–Haenszel test was used to reveal the trend of the proportions for each clade from 2014 to 2019. Multiple comparisons were corrected using Bonferroni’s method. P values <0.05 were considered to be significant. All statistical analyses were performed using IBM SPSS Statistics version 24 (IBM Corp., Armonk, NY, USA).
This study was reviewed and approved by the Ethics Committee of the NIID and was conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was waived because the data did not contain any patient identifiers, and the samples were taken in the course of standard patient care.
A total of 1,963 IPD cases occurred from January 2014 to December 2019 that were reported to the Adult IPD Study Group. Of them, 24 cases were excluded from our study because the S. pneumoniae strains isolated from 20 cases had died, and live bacteria could not be isolated from the remaining 4 cases. The S. pneumoniae strains from the remaining 1,939 IPD cases underwent serotyping, MLST, and PspA clade determination. Another 5 cases were excluded because of incomplete clinical data. The remaining 1,934 patients were 15–103 years old, with a median age of 71. Sixty percent of the patients were men.
The most prevalent serotypes were 3 (230 strains; 11.9%), 12F (195 strains; 10.1%), 19A (166 strains; 8.6%), 10A (148 strains; 7.7%), and 23A (135 strains; 7.0%). In total, 32.5% of them belonged to PCV13 serotypes and 63.0% belonged to PPSV23 serotypes.
The prevalence of each pneumococcal serotype for each year is shown in the Supplementary Figure 1. The number of IPD cases each year was 203, 220, 291, 416, 391, and 418 between 2014 and 2019. The isolation rates of serotypes 3 and 19A peaked in 2015 and decreased from 2016. Conversely, the isolation rate of 12F increased from 2016, peaked in 2017, and decreased in 2018 (Shimbashi et al., 2019) before further decreasing in 2019 (Supplementary Figure 1). During 2014–2019, the isolation rates of pneumococcal serotypes 10A and 23A were consistently high, whereas those of the PCV7 serotypes were low. The coverage rate of the pneumococcal strains by PCV13 decreased from 44.8% to 27.0%. On the other hand, the coverage rate of PPSV23 remained >60% from 2014 to 2018, decreasing by approximately 5% in 2019.
We determined the pspA sequences of 1,932/1,939 (99.6%) S. pneumoniae strains. The sequences of 1,928 strains (99.4%) shared >90% identity with the sequences of clades 1–6. Two serotype 35B CC558 strains, which were isolated in 2018, had deletions between 601 and 948 bp and between 601 and 957 bp of pspA, respectively. Deletion of amino acid residues was noted at positions 201-316 and 201-319, but no stop codons were found. The sequences upstream and downstream of the deleted region of the two strains were identical to each other and also showed high homology with the sequence of clade 4. No similar deletion sequence could be found in the NCBI database. Because PspA between amino acid residues 192 and 260 has been reported to have protection-eliciting epitopes (McDaniel et al., 1994), it is possible that the two strains have different antigenicity with clade 4. Therefore, the pspA genes of the two strains were determined to be a new clade (Table 1).
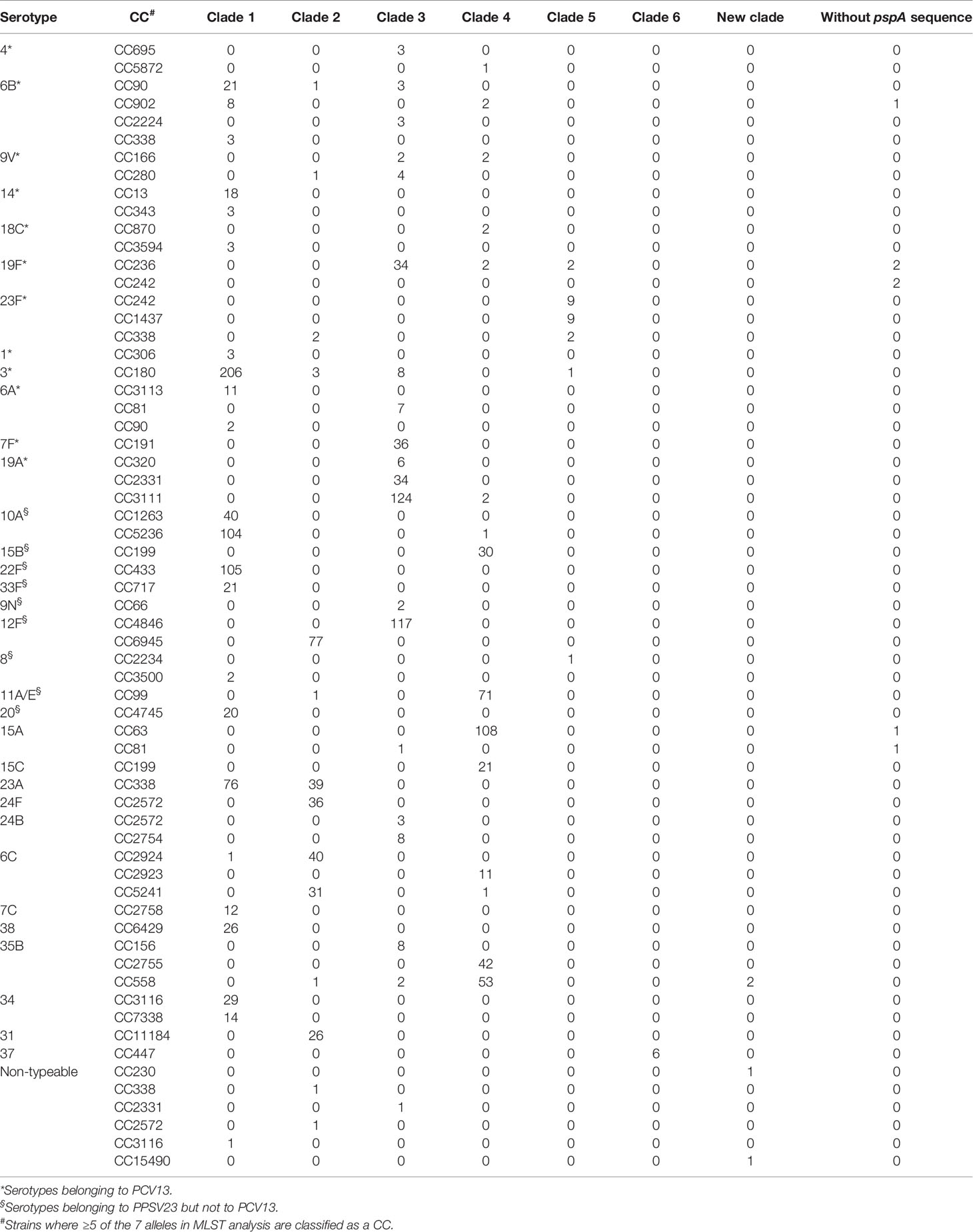
Table 1 Number of the pneumococcal strains with different serotypes and different clonal complexes (CCs).
Additionally, the sequences of two non-typeable strains, which were isolated in 2019, showed less than 90% identity with all sequences of clades 1–6. The sequence of the CC230 strain between primers SKH2 and LSM12 had 76% identity with that of clade 6, and the CC15490 strain was 72% of that of clade 3. The sequences of the two strains also showed low identity (56%) with each other. BLAST in the NCBI database showed that the sequence of the CC230 strain was 99% identical to that of isolate 34YLE (LT669627) and the sequence of CC15490 strain was 99% identical to that of isolate R34-3088 (LT669632) (Croucher et al., 2017). Therefore, the pspA genes of the two strains were determined to be new clades (Table 1).
Besides these strains, the pspA fragment of seven strains (0.4%) was not amplified by PCR using the two primer sets. To identify the presence or absence of pspA, whole genome sequences of these strains were determined and no gene similar to pspA was found by homology search. Therefore, pspA is absent in the seven strains. All seven strains were isolated in 2019, and their serotypes were 19F, 15A, and 6B in four, two, and one strain, respectively (Table 1).
In summary, 786 (40.5%), 291 (15.0%), 443 (22.8%), 369 (19.0%), 33 (1.7%), and 6 (0.3%) strains belonged to clades 1–6, respectively. Four (0.2%) strains possessed new clades. The pspA gene was not detected in seven (0.4%) strains.
The annual distribution of PspA clades of the S. pneumoniae strains is summarized in Figure 1.
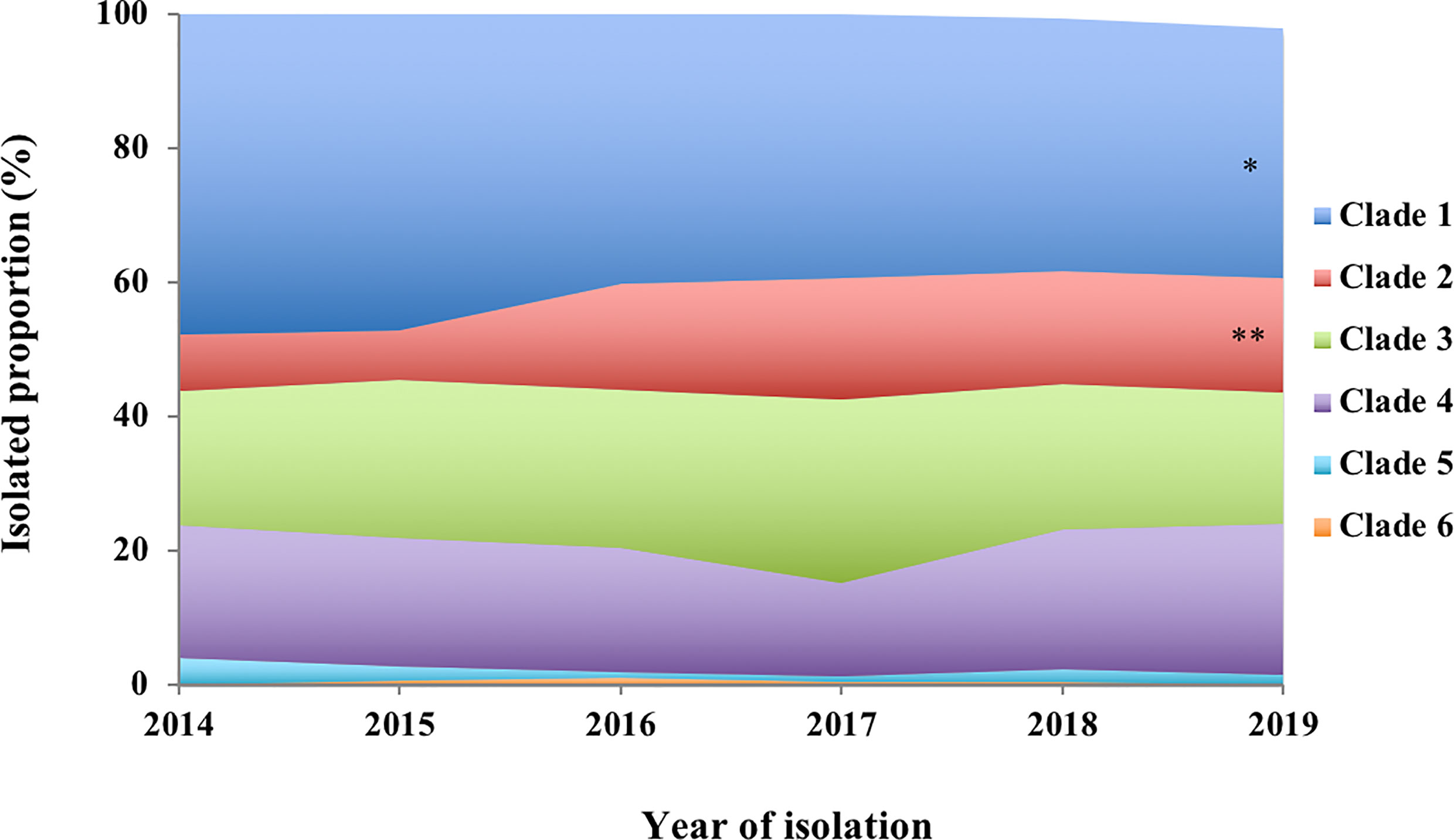
Figure 1 Annual proportion of PspA clades 1–6 among the S. pneumoniae strains isolated from invasive pneumococcal diseases in 2014−2019. Mantel–Haenszel test of trends corrected using Bonferroni’s method to perform an analysis of the trends for the proportions of each clade from 2014 to 2019. *p < 0.05, **p < 0.01.
We compared differences in the proportions of PspA clades of the S. pneumoniae strains belonging to the vaccine and non-vaccine serotypes. The S. pneumoniae strains belonging to PCV13 and PPSV23 had higher rates in PspA clades 1 and 3. On the other hand, the non-PCV13 and non-PPSV23 strains had higher rates in PspA clades 2 and 4 (Figure 2).
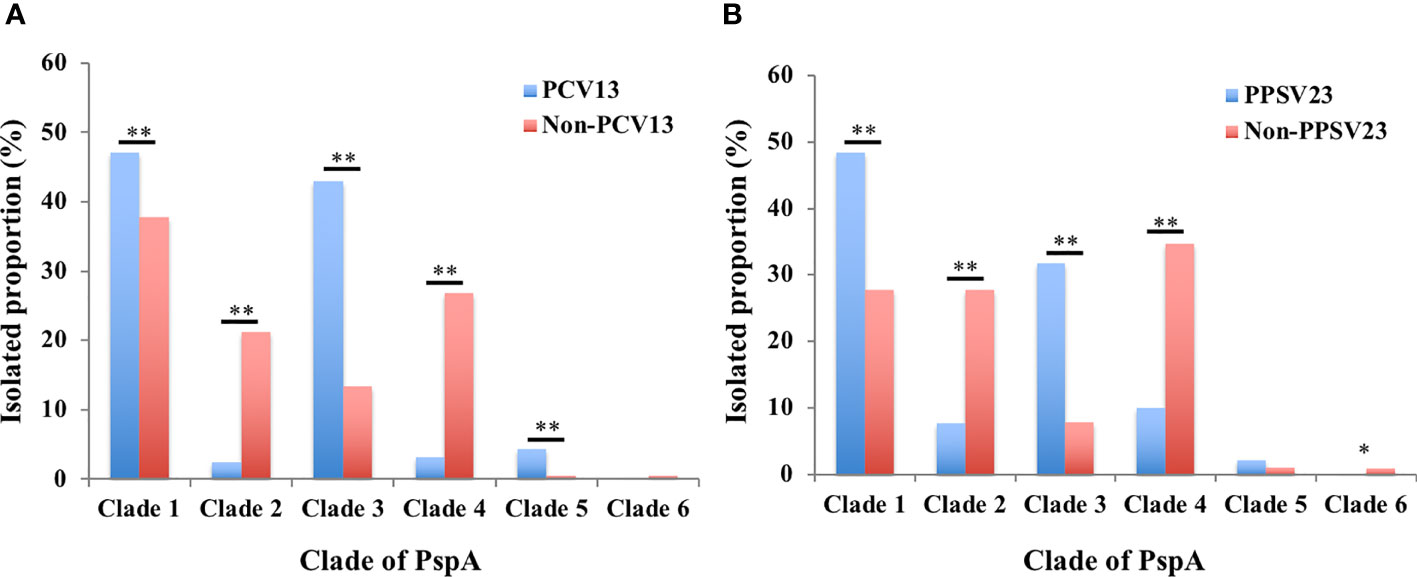
Figure 2 Isolation proportions of PspA clades 1–6 among the S. pneumoniae strains of vaccine and non-vaccine serotypes. (A) PCV13 and non-PCV13 serotypes, (B) PPSV23 and non-PPSV23 serotypes. The proportions of vaccine and non-vaccine serotypes were compared using χ2-test (clades 1–5) or Fisher’s exact test (clade 6) corrected using Bonferroni’s method. * p < 0.05, ** p < 0.01. PCV13: 13-valent pneumococcal conjugate vaccine; PPSV23: 23-valent pneumococcal polysaccharide vaccine.
The PspA clades of the S. pneumoniae strains that belonged to the vaccine serotypes and other major serotypes are summarized in Table 1, and the major CCs of these serotypes are also recorded. All strains of serotype 14, 7F, 15B, 22F, 33F, 9N, 20, 15C, 16F, 21, 24F, 24B, 7C, 38, 34, 29, 31, and 37 comprised a single clade. The PspA clade for serotypes 18C, 6A, and 12F was CC-dependent. Serotypes 6B, 19F, 23F, 3, 8, 11A/E, 15A, 23A, 23B, 6C, 6D, 35B, and non-typeable strains were found in multiple clades.
The CCs of the seven strains that did not possess pspA were CC236 (19F, two strains), CC242 (19F, two strains), CC63 (15A, one strain), CC81 (15A, one strain), and CC902 (6B, one strain). Among the four strains that showed new PspA clades, the two 35B strains were CC558 and the two non-typeable strains were CC230 and CC15490 (Table 1).
Our study summarized the distribution of the PspA clades of 1,928 S. pneumoniae strains isolated from cases of adult IPD in Japan during 2014–2019. This is the first report of PspA clade distribution of S. pneumoniae strains isolated from adult cases of IPD after PCV introduction for children in Japan. The proportions of PspA clades 1–6 were 40.5%, 15.0%, 22.8%, 19.0%, 1.7%, and 0.3%, respectively. A previous study of 68 strains isolated from adult cases of IPD in Japan during 2010–2011 reported that the major PspA clades were 1 (50%) and 3 (28%) (Piao et al., 2014). The other clades 2, 4, and 5 comprised minor proportions of 4%, 9%, and 9% of the cases, respectively. There was no clade 6. The median age of the 68 patients was 68 years, similar to the median age 71 years in this study. Because pneumococcal strains isolated from adult IPD before PCV introduction were limited in Japan, only 68 strains from adult IPD were analyzed in the previous study (Piao et al., 2014). However, we determined PspA clade distribution of 250 pneumococcal strains isolated from pediatric IPD cases that occurred before the introduction of PCVs (Suga et al., 2015), and these data show a trend similar to that obtained from the 68 adult IPD strains. The major PspA clades of the pneumococci from children were 1 (42%) and 3 (32%), whereas the other clades 2, 4, and 5 comprised minor proportions of 4%, 9%, and 13% of the cases, respectively. There was no clade 6 (Supplementary Figure 2). These strains were isolated from pediatric IPD patients who resided in 10 prefectures of Japan (Suga et al., 2015); among the 10 prefectures, seven (Hokkaido, Niigata, Mie, Kochi, Fukuoka, Kagoshima, and Okinawa) were the same as those surveyed in this study. Therefore, the PspA clade distribution of 68 strains from adult patients with IPD and 250 strains from pediatric patients with IPD could be used as controls for this study. Because PCVs have been part of the NIP for children in Japan since 2013, our findings suggested that the PspA clade distribution of strains that caused adult IPD changed considerably after PCV introduction in children. During 2014–2019, our data demonstrated that the annual distributions of PspA clade 1 and clade 2 exhibited significantly decreased and increased trends, respectively (Figure 1). We compared the PspA clade distribution before and after PCV using pediatric IPD and adult IPD data in 2019, respectively. Compared to the data before PCV, clades 2 and 4 were significantly increased and clades 3 and 5 were significantly decreased after PCV. Although there was a tendency for clade 1 to decrease after PCV, the difference was not statistically significant (Supplementary Figure 3). Furthermore, S. pneumoniae strains of PCV13 and PPSV23 serotypes had higher rates in PspA clades 1 and 3 (Figure 2). These results suggest that the serotype replacement might have influenced (Supplementary Figure 1), but only partially, the change in PspA clade distributions.
In this study, 4 strains (0.2%) were classified as new clades because they had the pspA sequences that differed from the well-known clades 1–6 (Table 1). Moreover, there were 7 pspA-negative strains (0.4%). Although the total rate of strains without pspA and having new PspA clade is low (0.6%), continuous surveillance would be important to determine if there will be any changes in the distribution of PspA clades.
The protective effects of PCV have been well-recognized worldwide (Berical et al., 2016; Kim et al., 2016). The introduction of PCV7 and PCV13 in children dramatically decreased the incidence of IPD caused by vaccine serotypes, and annual incidence of IPD in children aged <5 years decreased significantly by 57% in 2013 compared with that in 2008 before PCV introduction in Japan (Suga et al., 2015; Nakano et al., 2020). In addition to direct effect, PCV in national immunization programs for children has had a significant indirect effect on pneumococcal diseases in adults (Berical et al., 2016; Kim et al., 2016). Compared with PCV, the protective effects of PPSV23 are controversial. However, a systematic review and meta-analysis reported the effectiveness of PPSV23 against IPD in adults aged >50 years to be 54% (Kraicer-Melamed et al., 2016). PPSV23 was included for those aged ≥65 years in Japanese national vaccine program in 2014. The adjusted vaccine effectiveness of PPSV23 against adult IPD caused by vaccine serotypes was 42.2% (Shimbashi et al., 2020). Furthermore, it was shown that prior vaccination with either PCV13 or PPSV23 decreased the risk of pneumococcal carriage in adults aged ≥65 years (Branche et al., 2018). These studies indicate that not only PCV13 but also PPSV23 have protective effects against pneumococcal diseases. However, because of the serotype replacement, limitations of the PCV13 and PPSV23 exist. Therefore, there is an urgent need for a new, effective, and affordable pneumococcal vaccine that covers a wide range of serotypes for both children and adults in Japan.
The PspA clades of S. pneumoniae strains from adult IPD cases were partially serotype- and CC-dependent (Table 1). More importantly, our data demonstrated that the isolation rates of the PspA clades 1–4 were maintained at high levels (96.1%–98.8%), and clades 5 and 6 had low rates. The studies of IPD strains from Spain, China, and Korea showed that the rate of PspA clade 5 was 9.1%, 8.2%, and 12.4%, respectively (Rolo et al., 2009; Qian et al., 2012; Yun et al., 2017). The clade 6 was found in only 1 strain from Korea and in no other strains from geographies. However, the number of pneumococci analyzed in these studies was limited as 66, 171, and 190 strains, respectively. Therefore, further studies would be needed to determine the actual rates of clades 5 and 6 among pneumococcal strains from IPD patients in these studies because the rate of clade 5 was 9% in the previous report with 68 adult IPD strains in Japan (Piao et al., 2014). Collectively, our results indicate that new vaccines under development targeting PspA should include at least clades 1–4.
In conclusion, the distribution of the PspA clades of S. pneumoniae strains isolated from adult IPD cases during 2014–2019 in Japan were determined. These data may be useful for designing new PspA-based pneumococcal vaccines. We aim to continue examining the serotypes, STs, and PspA clades for an in-depth understanding of S. pneumoniae strains that cause IPD in adults.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Committee of the National Institute of infectious Diseases. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
BC, YK, and KaO designed the study. HW, YT, KoK, JF, KeO, TM, SA, KeK, JN, and TK collected clinical data and bacterial strains. KT performed statistical analyses. BC carried out the microbiological analysis and analyzed the data. MM and MO carried out the whole genome sequencing. BC and SS provided PspA distribution results of pneumococci from children with IPD. BC and YK drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Ministry of Health and Labour Sciences HA Program Grant Number JPMH20HA1005, the Japan Society for the Promotion of Science KAKENHI Grant number 19H03705 and the Japan Agency for Medical Research and Development (AMED; JP20fk0108139j1901).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We sincerely thank the staff of the local public health centers who collected bacterial isolates and case report forms from the hospitals and the staff of the public health institutes and laboratories who worked for the Adult IPD Study Group. In addition to the authors, the following investigators and the institutions participated in the Adult IPD Study Group: Hokkaido Institute of Public Health; Michiharu Nakano, Sendai City Public Health Center; anonymous, Yamagata Prefectural Bureau of Public Health and Welfare; Tatsuya Ikeda, Yamagata Prefectural Institute of Public Health; Yu Suzuki, Junji Seto, Kazue Yahagi, Welfare and Public Health Bureau of Niigata Prefecture; Kurumi Kazama, Health and Sanitation Department of Niigata City; Satoru Yamazaki and Emiko Kumakura, Department of Health and Welfare, Mie Prefecture; Minoru Hiraoka, Mie Prefecture Health and Environment Research Institute; Yuhki Nagai, Sagako Naraya, Department of Medical Policy, Nara Prefecture; Eri Tsujimoto, The Public Health Institute of Kochi Prefecture; Michiaki Matsumoto, Fukuoka Institute of Health and Environmental Science; Hiroaki Shigemura, Fuyuki Okamoto, Nobuyuki Sera, Center for Environment and Health, Kagoshima Prefecture; Mutsuyo Gokuden, Welfare and Public Health Bureau of Okinawa Prefecture; Toru Itokazu, Okinawa Prefectural Institute of Health and Environment; Jun Kudaka, Kochi Medical School, Kochi University; Akihito Yokoyama, Sapporo Medical University School of Medicine; Hiroki Takahashi, Tohoku University Graduate School of Medicine; Tetsuji Aoyagi, Yamagata Saiseikai Hospital; Hiroaki Takeda, Niigata University Graduate School of Medical and Dental Sciences; Chikako Tsubata, Chikamori Hospital; Masayuki Ishida and University of the Ryukyus; Masashi Nakamatsu.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.617573/full#supplementary-material
Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Berical A. C., Harris D., Dela, Cruz C. S., Possick J. D. (2016). Pneumococcal vaccination strategies. An update and perspective. Ann. Am. Thorac. Soc. 13, 933–944. doi: 10.1513/AnnalsATS.201511-778FR
Branche A. R., Yang H., Java J., Holden-Wiltse J., Topham D. J., Peasley M., et al. (2018). Effect of prior vaccination on carriage rates of Streptococcus pneumoniae in older adults: A longitudinal surveillance study. Vaccine 36, 4304–4310. doi: 10.1016/j.vaccine.2018.05.107
Briles D. E., Paton J. C., Mukerji R., Swiatlo E., Crain M. J. (2019). Pneumococcal Vaccines. Microbiol. Spectr. 7 (6). doi: 10.1128/9781683670131.ch23
Centers for Disease Control and Prevention Pneumococcal disease (2020). Available at: https://www.cdc.gov/pneumococcal/index.html (Accessed 31 December 2020).
Croucher N. J., Campo J. J., Le T. Q., Liang X., Bentley S. D., Hanage W. P., et al. (2017). Diverse evolutionary patterns of pneumococcal antigens identified by pangenome-wide immunological screening. Proc. Natl. Acad. Sci. U. S. A. 114, E357–E366. doi: 10.1073/pnas.1613937114
Enright M. C., Spratt B. G. (1998). A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144, 3049–3060. doi: 10.1099/00221287-144-11-3049
Fukusumi M., Chang B., Tanabe Y., Oshima K., Maruyama T., Watanabe H., et al. (2017). Invasive pneumococcal disease among adults in Japan, April 2013 to March 2015: disease characteristics and serotype distribution. BMC Infect. Dis. 17, 2. doi: 10.1186/s12879-016-2113-y
Ganaie F., Saad J. S., McGee L., van Tonder A. J., Bentley S. D., Lo S. W., et al. (2020). A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. mBio 11, e00937–e00920. doi: 10.1128/mBio.00937-20
Gertz R. E. Jr., McEllistrem M. C., Boxrud D. J., Li Z., Sakota V., Thompson T. A., et al. (2003). Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J. Clin. Microbiol. 41, 4194–4216. doi: 10.1128/JCM.41.9.4194-4216.2003
Hausdorff W. P., Hanage W. P. (2016). Interim results of an ecological experiment – Conjugate vaccination against the pneumococcus and serotype replacement. Hum. Vaccin. Immunother. 12, 358–374. doi: 10.1080/21645515.2015.1118593
Hollingshead S. K., Becker R., Briles D. E. (2000). Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68, 5889–5900. doi: 10.1128/IAI.68.10.5889-5900.2000
Kawaguchiya M., Urushibara N., Aung M. S., Morimoto S., Ito M., Kudo K., et al. (2018). Genetic diversity of pneumococcal surface protein A (PspA) in paediatric isolates of non-conjugate vaccine serotypes in Japan. J. Med. Microbiol. 67, 1130–1138. doi: 10.1099/jmm.0.000775
Kendall B. A., Dascomb K. K., Mehta R. R., Stockmann C., Mason E. O., Ampofo K., et al. (2016). Early Streptococcus pneumoniae serotype changes in Utah adults after the introduction of PCV13 in children. Vaccine 34, 474–478. doi: 10.1016/j.vaccine.2015.12.010
Kim Y. K., LaFon D., Nahm M. H. (2016). Indirect effects of pneumococcal conjugate vaccines in national immunization programs for children on adult pneumococcal disease. Infect. Chemother. 48, 257–266. doi: 10.3947/ic.2016.48.4.257
Kraicer-Melamed H., O’Donnell S., Quach C. (2016). The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: A systematic review and meta-analysis. Vaccine 34, 1540–1550. doi: 10.1016/j.vaccine.2016.02.024
McDaniel L. S., Scott G., Kearney J. F., Briles D. E. (1984). Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J. Exp. Med. 160, 386–397. doi: 10.1084/jem.160.2.386
McDaniel L. S., Ralph B. A., McDaniel D. O., Briles D. E. (1994). Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17, 323–337. doi: 10.1006/mpat.1994.1078
Nakano S., Fujisawa T., Ito Y., Chang B., Matsumura Y., Yamamoto M., et al. (2020). Nationwide surveillance of paediatric invasive and non-invasive pneumococcal disease in Japan after the introduction of the 13-valent conjugated vaccine 2015-2017. Vaccine 38, 1818–1824. doi: 10.1016/j.vaccine.2019.12.022
Piao Z., Akeda Y., Takeuchi D., Ishii K. J., Ubukata K., Briles D. E., et al. (2014). Protective properties of a fusion pneumococcal surface protein A (PspA) vaccine against pneumococcal challenge by five different PspA clades in mice. Vaccine 32, 5607–5613. doi: 10.1016/j.vaccine.2014.07.108
Pimenta F. C., Ribeiro-Dias F., Brandileone M. C., Miyaji E. N., Leite L. C., Sgambatti, et al. (2006). Genetic diversity of PspA types among nasopharyngeal isolates collected during an ongoing surveillance study of children in Brazil. J. Clin. Microbiol. 44, 2838–2843. doi: 10.1128/JCM.00156-06
Qian J., Yao K., Xue L., Xie G., Zheng Y., Wang C., et al. (2012). Diversity of pneumococcal surface protein A (PspA) and relation to sequence typing in Streptococcus pneumoniae causing invasive disease in Chinese children. Eur. J. Clin. Microbiol. Infect. Dis. 31, 217–223. doi: 10.1007/s10096-011-1296-9
Rolo D., Ardanuy C., Fleites A., Martín R., Liñares J. (2009). Diversity of pneumococcal surface protein A (PspA) among prevalent clones in Spain. BMC Microbiol. 9, 80. doi: 10.1186/1471-2180-9-80
Shimbashi R., Chang B., Tanabe Y., Takeda H., Watanabe H., Kubota T., et al. (2019). Epidemiological and clinical features of invasive pneumococcal disease caused by serotype 12F in adults, Japan. PLoS One 14, e0212418. doi: 10.1371/journal.pone.0212418
Shimbashi R., Suzuki M., Chang B., Watanabe H., Tanabe Y., Kuronuma K., et al. (2020). Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in adults, Japan 2013-2017. Emerg. Infect. Dis. 26, 2378–2386. doi: 10.3201/eid2610.191531
Suga S., Chang B., Asada K., Akeda H., Nishi J., Okada K., et al. (2015). Nationwide population-based surveillance of invasive pneumococcal disease in Japanese children: Effects of the seven-valent pneumococcal conjugate vaccine. Vaccine 33, 6054–6060. doi: 10.1016/j.vaccine.2015.07.069
Keywords: Streptococcus pneumoniae, invasive pneumococcal disease, serotype, pneumococcal surface protein A clade, vaccine, Japan, adults
Citation: Chang B, Kinjo Y, Morita M, Tamura K, Watanabe H, Tanabe Y, Kuronuma K, Fujita J, Oshima K, Maruyama T, Abe S, Kasahara K, Nishi J, Kubota T, Ohnishi M, Suga S and Oishi K (2021) Distribution and Variation of Serotypes and Pneumococcal Surface Protein A Clades of Streptococcus pneumoniae Strains Isolated From Adult Patients With Invasive Pneumococcal Disease in Japan. Front. Cell. Infect. Microbiol. 11:617573. doi: 10.3389/fcimb.2021.617573
Received: 21 October 2020; Accepted: 26 February 2021;
Published: 19 March 2021.
Edited by:
Ghassan M. Matar, American University of Beirut, LebanonReviewed by:
Samantha J. King, The Ohio State University, United StatesCopyright © 2021 Chang, Kinjo, Morita, Tamura, Watanabe, Tanabe, Kuronuma, Fujita, Oshima, Maruyama, Abe, Kasahara, Nishi, Kubota, Ohnishi, Suga and Oishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Chang, Yi1jaGFuZ0BuaWguZ28uanA=; Yuki Kinjo, eWtpbmpvQGppa2VpLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.