
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 01 April 2021
Sec. Microbiome in Health and Disease
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.605825
 Shiju Xiao1,2
Shiju Xiao1,2 Guangzhong Zhang1*
Guangzhong Zhang1* Chunyan Jiang1*
Chunyan Jiang1* Xin Liu3
Xin Liu3 Xiaoxu Wang1,2
Xiaoxu Wang1,2 Yafan Li4
Yafan Li4 Meijiao Cheng5
Meijiao Cheng5 Hongpeng Lv4
Hongpeng Lv4 Fuyang Xian4
Fuyang Xian4 Xinwei Guo1,2,6
Xinwei Guo1,2,6 Yong Tan7
Yong Tan7Background: Increasing evidence has shown that alterations in the intestinal microbiota play an important role in the pathogenesis of psoriasis. The existing relevant studies focus on 16S rRNA gene sequencing, but in-depth research on gene functions and comprehensive identification of microbiota is lacking.
Objectives: To comprehensively identify characteristic gut microbial compositions, genetic functions and relative metabolites of patients with psoriasis and to reveal the potential pathogenesis of psoriasis.
Methods: DNA was extracted from the faecal microbiota of 30 psoriatic patients and 15 healthy subjects, and metagenomics sequencing and bioinformatic analyses were performed. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database, cluster of orthologous groups (COG) annotations, and metabolic analyses were used to indicate relative target genes and pathways to reveal the pathogenesis of psoriasis.
Results: Compared with healthy individuals, the gut microbiota of psoriasis patients displayed an alteration in microbial taxa distribution, but no significant difference in microbial diversity. A distinct gut microbial composition in patients with psoriasis was observed, with an increased abundance of the phyla Firmicutes, Actinobacteria and Verrucomicrobia and genera Faecalibacterium, Bacteroides, Bifidobacterium, Megamonas and Roseburia and a decreased abundance of the phyla Bacteroidetes, Euryarchaeota and Proteobacteria and genera Prevotella, Alistipes, and Eubacterium. A total of 134 COGs were predicted with functional analysis, and 15 KEGG pathways, including lipopolysaccharide (LPS) biosynthesis, WNT signaling, apoptosis, bacterial secretion system, and phosphotransferase system, were significantly enriched in psoriasis patients. Five metabolites, hydrogen sulfide (H2S), isovalerate, isobutyrate, hyaluronan and hemicellulose, were significantly dysregulated in the psoriatic cohort. The dysbiosis of gut microbiota, enriched pathways and dysregulated metabolites are relevant to immune and inflammatory response, apoptosis, the vascular endothelial growth factor (VEGF) signaling pathway, gut-brain axis and brain-skin axis that play important roles in the pathogenesis of psoriasis.
Conclusions: A clear dysbiosis was displayed in the gut microbiota profile, genetic functions and relative metabolites of psoriasis patients. This study is beneficial for further understanding the inflammatory pathogenesis of psoriasis and could be used to develop microbiome-based predictions and therapeutic approaches.
Psoriasis is an immune-mediated inflammatory chronic skin disease characterized by chronic inflammation in the dermis, parakeratosis and excessive epidermal growth, and 2-3% of the population are affected by psoriasis (Parisi et al., 2013; Hidalgo-Cantabrana et al., 2019). Less attention has been focused on the relationship between intestinal microbiota dysbiosis and the pathogenesis of psoriasis (Yan et al., 2017). However, recent studies have reported an alteration in the gut microbiota in patients with psoriasis (Scher et al., 2015; Chen et al., 2018; Hidalgo-Cantabrana et al., 2019; Shapiro et al., 2019) that is related to an aberrant inflammatory response (Salem et al., 2018). The intestinal microbiota plays an important role in the maintenance of immune homeostasis, regulating both acquired and innate immune responses (Sommer and Backhed, 2013; Palm et al., 2015; Planer et al., 2016). Gut microbiota alterations may activate T cells, trigger an inflammatory process and induce immune disorders (Littman and Pamer, 2011; Collins et al., 2012; Honda and Littman, 2016). Gut microbiota dysbiosis is related to immune and autoimmune disorders such as rheumatoid arthritis (RA) (Yeoh et al., 2013), allergies (Chen et al., 2016), type 1 diabetes (de Groot et al., 2017), autism (Mangiola et al., 2016; Zhang et al., 2018), inflammatory bowel disease (IBD) (Huttenhower et al., 2014), systemic lupus erythematosus (SLE) (Edwards and Costenbader, 2014), and multiple sclerosis (Rosser and Mauri, 2016). Moreover, intestinal microbiota alteration plays a key role in psoriasis (Shamriz et al., 2016; Luo et al., 2017; Stockinger and Omenetti, 2017), as well as other skin alterations, such as atopic dermatitis and vitiligo (Penders et al., 2013). In addition, the effects of current treatments for psoriasis are limited (Torres and Puig, 2018). Thus, further research on the pathophysiology caused by gut microbiota dysbiosis in psoriasis patients, which may be a breakthrough for pathological mechanism research, is urgently required.
Gut microbiota alterations have been observed in psoriatic patients and psoriatic arthritis patients (Fry and Baker, 2007; Scher et al., 2015; Chen et al., 2018; Hidalgo-Cantabrana et al., 2019; Shapiro et al., 2019). It was also found that gut microbiota dysregulation induced by vancomycin and polymyxin exacerbated psoriasis in an imiquimod (IMQ)-induced mouse model (Zanvit et al., 2015). It has been reported that antibiotic therapy can alleviate the thickness of lesions and reduce the proportion of Th17 cells, γδT cells and IL-17 in an imiquimod-induced psoriasis mouse model (Zákostelská et al., 2016). A predisposition for intestinal bowel diseases among psoriasis patients supports the gut-skin axis theory (Kimball et al., 2014). All of these studies identified the microbiota composition using 16S rRNA sequencing. This method has limitations such as failure to determine most microbes at the strain and species level and the potential for amplification bias (Konstantinidis and Tiedje, 2007; Pinto and Raskin, 2012). In contrast, compared with 16S rRNA sequencing, metagenome sequencing can provide a higher resolution of taxonomic profiles with functional classification of the microbiome (Lee et al., 2018). Nonetheless, few studies on the relationship of the gut microbiome and psoriasis using metagenome sequencing analysis have been conducted.
In this study, to further investigate the intestinal microbiota composition, gene functions and relative metabolites predicted from the microbial composition of psoriasis patients, the profile and composition of the intestinal microbiomes from stool samples of psoriasis patients and healthy controls were compared using metagenomics sequencing analysis. Investigation of the definite role of intestinal microbiome dysbiosis in the immunopathogenesis of inflammatory diseases may boost our realization of psoriasis aetiology and pathological mechanisms and promote targeted and novel predictions and treatments.
Patients with psoriasis (n=30) were identified and collected from the Beijing Hospital of Traditional Chinese Medicine (Table 1). The control group (n=15) comprised age-, sex- and body mass index (BMI)-matched healthy donors. Patients and healthy donors who received antibiotics, probiotics, glucocorticoids, or immunosuppression during the 6 previous months were not included. People with BMI ≥ 35 or ≤ 18, and those who have been suffering from diseases of the digestive system, immune system and other severe illnesses, were excluded. They have no especially dietary habit and belong to the same ethnic’s population (Chinese). According to principle of statistics, the different sample sizes of two groups will not affect the correctness of statistical inference (Pearce, 2016). The study was approved by the Ethical Committee of the Beijing Hospital of Traditional Chinese Medicine and conformed with the Declaration of Helsinki. All study subjects signed informed consent forms. The questionnaires containing personal and clinical information were completed by the patients.
Stool samples were collected in a specimen collection kit and stored at -20°C immediately after defecation and then at -80°C before further manipulation in the laboratory.
DNA from stool samples was extracted using the QIAamp PowerFecal Pro DNA Kit according to the manufacturers’ instructions. DNA was resolved in 10mM Tris-cl buffer and stored at -20°C and at -80°C for short-term and long-term use, respectively. Total DNA quality was measured by using a spectrophotometer with 1% agarose gel electrophoresis. DNA was fragmented to an average size of approximately 350 bp using KAPA HyperPlus PCR-free for library construction. Subsequently, we performed metagenomic sequencing on the Illumina HiSeq platform following the manufacturer’s instructions. The quality control of all raw metagenomic sequencing data was performed using MOCAT2 software. The raw sequence reads were trimmed by SolexaQA package with a length shorter than 30 bp and a quality score lower than 20. The quality control standards are: A260/280 between 1.8-2.0, A260/280>1.5. To remove contaminated reads, the filtered reads were compared to human genome using SOAPaligner, and clean raw reads were obtained. To obtain contigs for the following annotation and prediction, we used SOAPdenovo software to assemble the clean raw reads. Afterwards, the scaftigs from each sample were predicted using MetaGeneMark.
The relative abundances of different level of taxonomic assignment were predicted using MetaPhlAn (metagenomic phylogenetic analysis) 2.0, which includes an expanded set of ~1 million markers from >7,500 species (Truong et al., 2015). We performed all statistical analyses using R software unless otherwise specified. The Shannon indices were used to calculate the α-diversity of the samples. The β-diversity was estimated by taxon-based Bray-Curtis distance via the R package vegan, and compositional differences between psoriasis and non-psoriasis samples (β-diversity) were represented in principal coordinates analysis (PCoA) plots using the R package (Bray and Curtis, 1957; VEGAN, 2003; Dray and Dufour, 2007). The nonparametric Kruskal-Wallis test was used because some of the variables analysed lacked homogeneous variances or were not normally distributed. ANCOM analyses and permutational analysis of variance (PERMANOVA) were also performed (Mandal et al., 2015).
Linear discriminant analysis (LDA) effect size (LEfSe) was applied to identify differences in the relative abundances of genera of gut microbiota between psoriasis and healthy volunteers (Segata et al., 2011). LDA values > 2.0 with a P value < 0.05 were considered significantly enriched.
COG and KEGG pathway annotations were obtained using the DIAMOND database (Version 0.7.9.58) via BLASTP. To determine gene functions that discriminate bacterial compositions between psoriatic patients and healthy donors, the KEGG enrichment pathways were analysed with Generally Applicable Gene-set Enrichment for Pathway Analysis (GAGE) (Luo et al., 2009). The differential expressed bacteria genes were identified by comparing relative abundances between two groups using Wilcoxon test, and P values were adjusted for multiple testing with a Benjamini-Hochberg false discovery rate correction. Adjusted P value < 0.05 were considered significantly differential on gene expression.
The metabolite potential was estimated by the relative abundance of the corresponding species; values were simply added or subtracted if the species produced or consumed, respectively. The production or consumption relationships between metabolites and species were curated from the literature of Sung J et al. and Cecilia Noecker et al. (Noecker et al., 2016; Sung et al., 2017). Heatmaps were generated to represent potential metabolic differences in the gut microbiome between the 2 groups.
The species accumulation curve of each group tended to plateau, indicating that sample biodiversity was adequately covered with the applied sequencing depth (Figure 1). The α-diversity of the gut microbiota (Shannon index) in the healthy group was higher than that in the psoriasis group, but there were no significant differences between the two groups (Figure 2). Moreover, the β-diversity analyses showed through PCoA demonstrated a separate tendency, but no significant clustering of non-psoriasis controls and patients was observed based on Bray-Curtis dissimilarity, indicating that the structural diversity of the intestinal microbiota did not differ between the two groups (Figure 3).
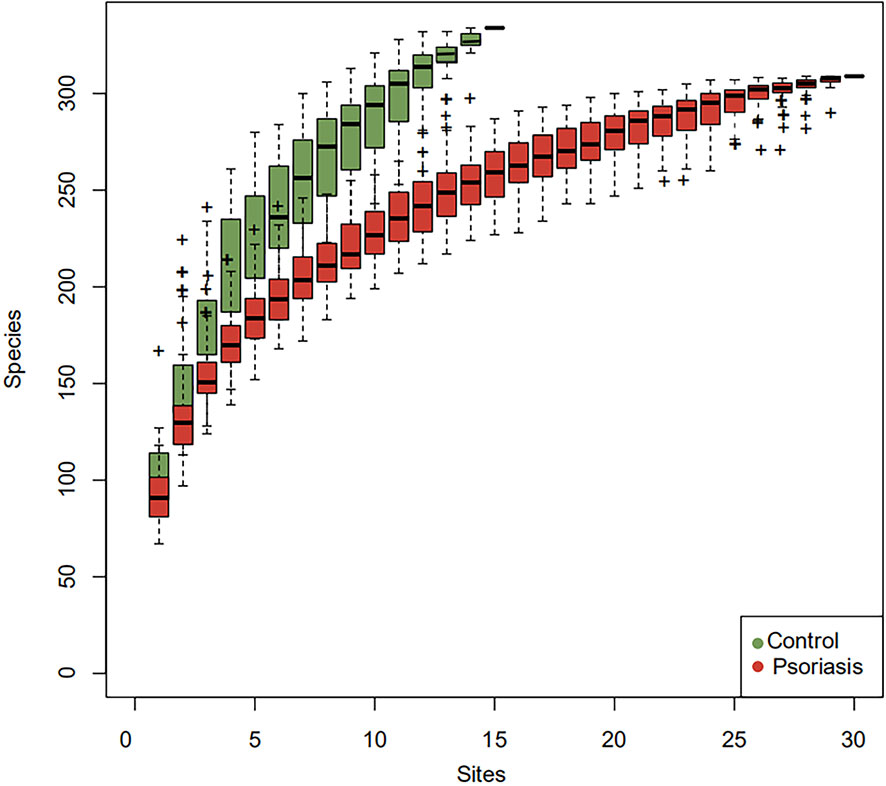
Figure 1 Species accumulation curves. The species accumulation curve of each group tended to plateau, indicating that sample biodiversity was adequately covered with the applied sequencing depth.
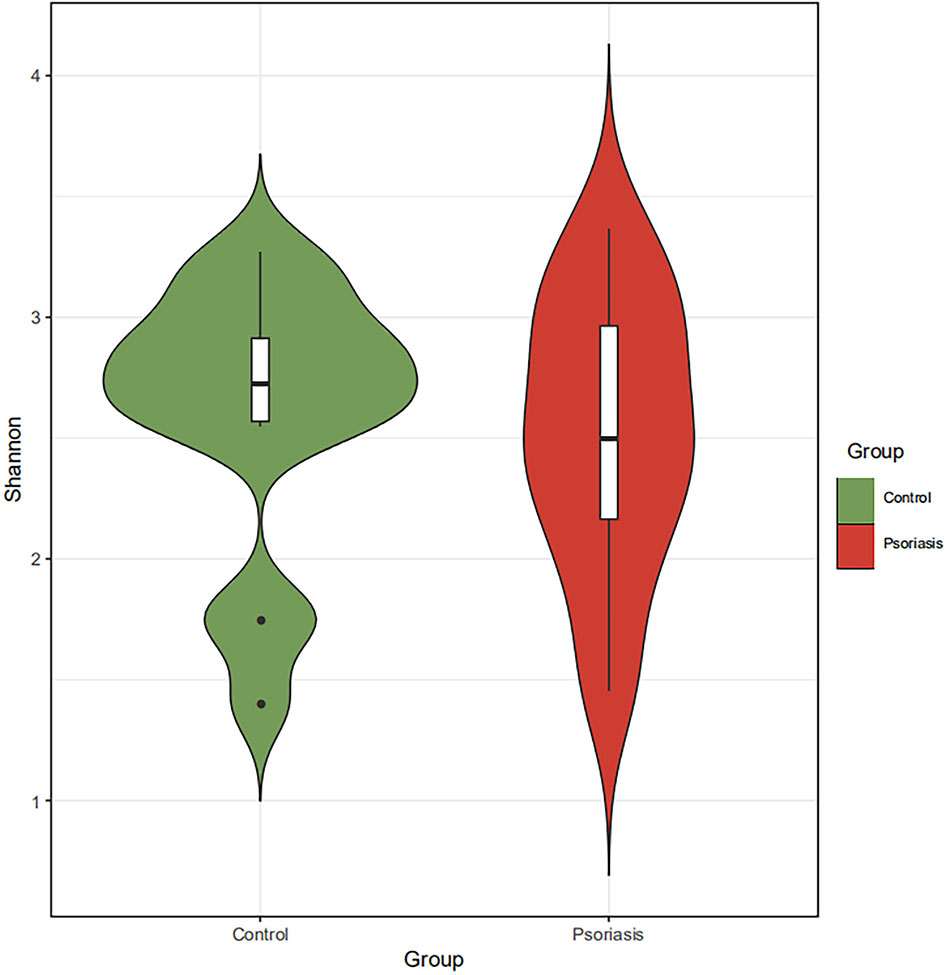
Figure 2 α-Diversity based on Shannon indices displayed no significant differences in the intestinal microbiota between the two groups.
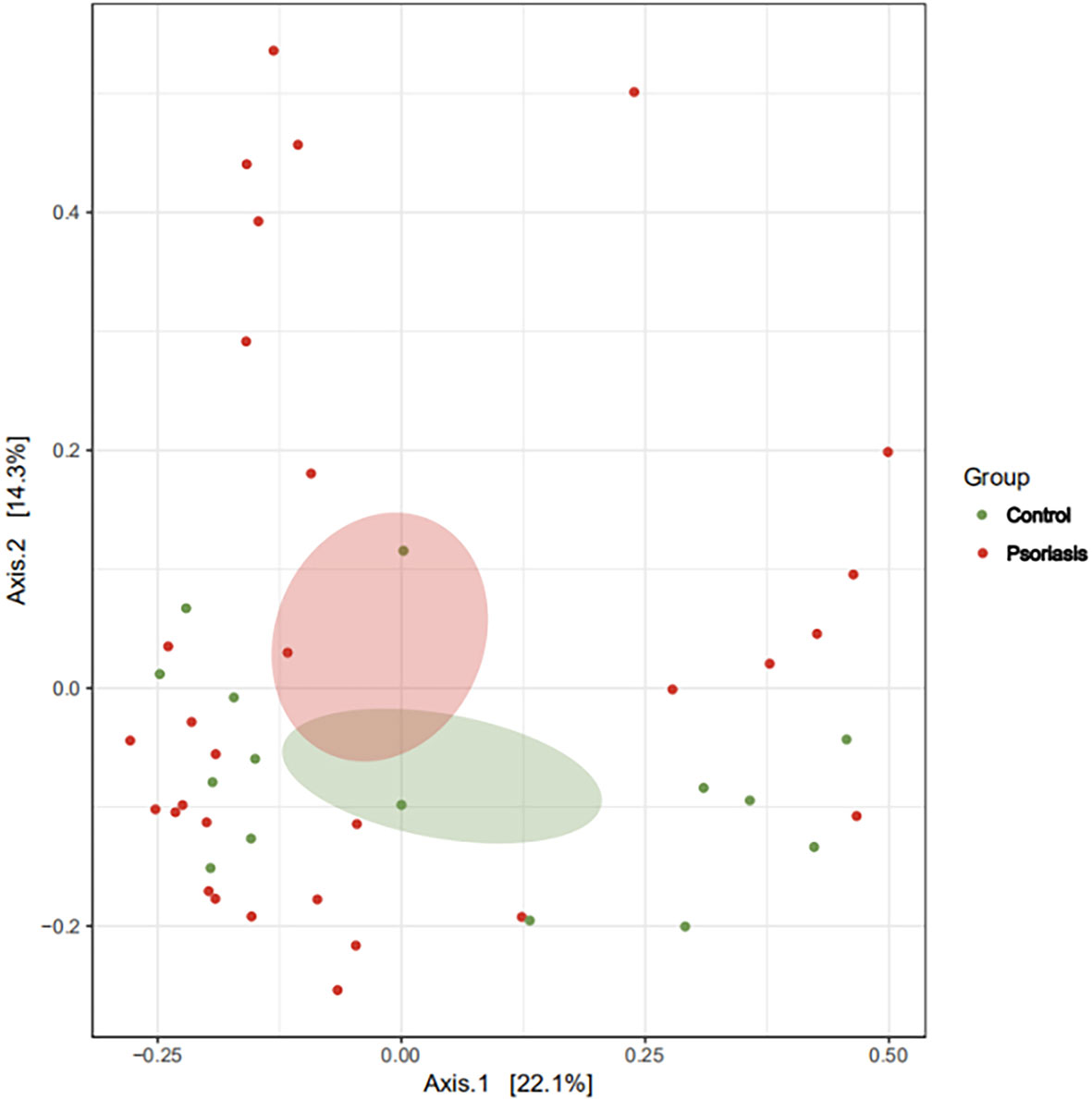
Figure 3 β-Diversity represented as a two-dimensional PCoA showed no differential clustering between the two groups. (The red dots showed the samples from psoriasis patients, whereas the green dots showed the samples from healthy individuals. The closer the dots in one group, the more similar in gut microbiota. The gut microbiota compositions are indicated with red and green circles, respectively. The smaller the overlap of two circles, the more different in the gut microbiota compositions of two groups).
Forty-five faecal samples were collected: 30 (60%) samples were collected from patients suffering from psoriasis, and 15 samples were collected from healthy age-, sex-, BMI-matched individuals. The demographic characteristics details of the two groups are presented in Table 1.
Five dominant phyla based on different relative abundances between psoriasis patients and controls were identified. The phyla Actinobacteria, Firmicutes, and Verrucomicrobia were increased, while Bacteroidetes and Proteobacteria were reduced in the psoriatic patients compared with the controls (Figure 4A, B). Moreover, at the genus level, higher proportions of Faecalibacterium, Bacteroides, Bifidobacterium, Megamonas and Roseburia and lower proportions of Prevotella, Alistipes and Eubacterium were identified in psoriatic patients (Figures 4C, D). The abundance of Faecalibacterium prausnitzii was higher in psoriasis patients than in controls (Figure 4E). It has been reported that Faecalibacterium and Prevotella are associated with anti-inflammatory properties (Shapiro et al., 2019).
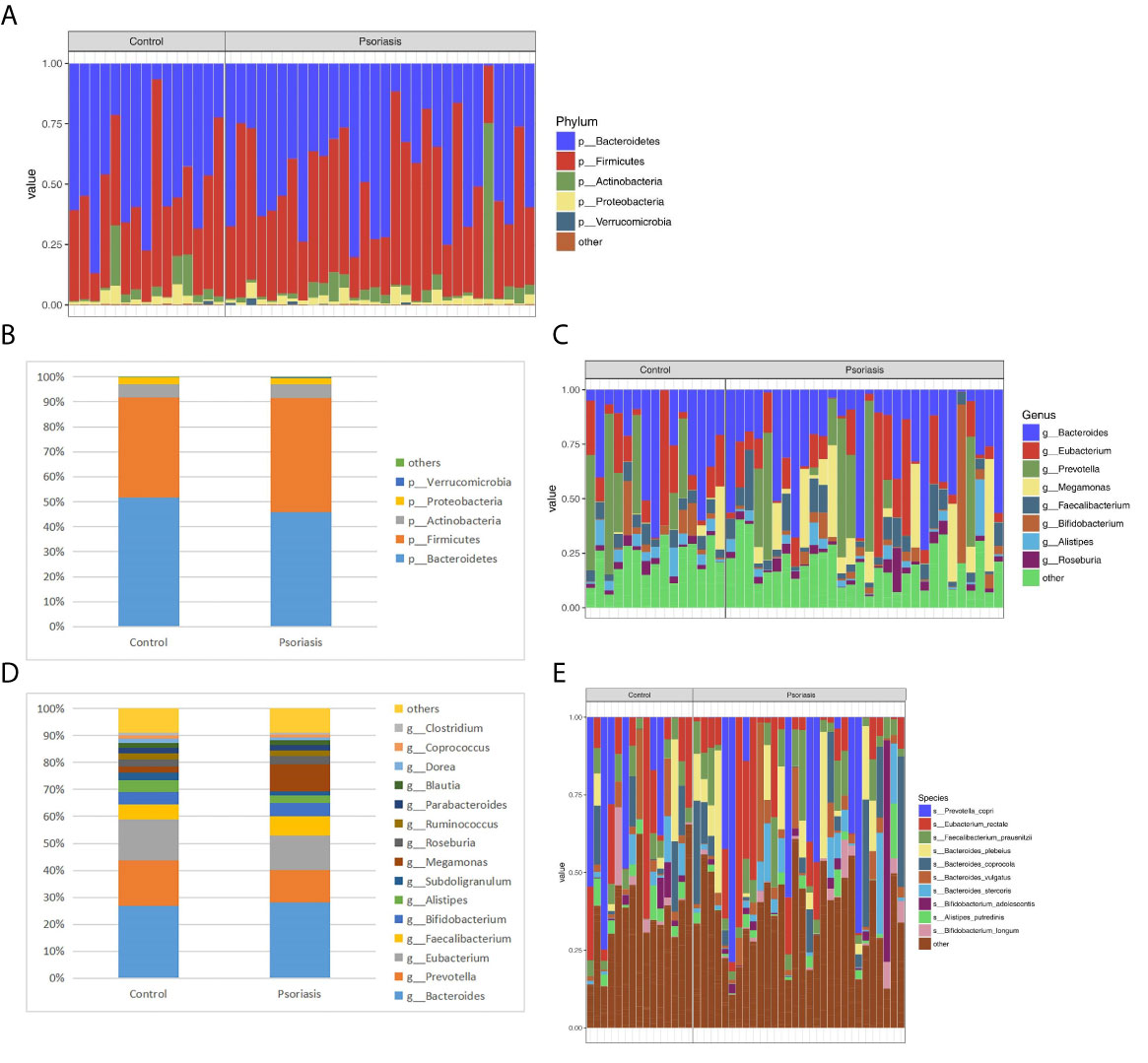
Figure 4 Relative abundance (%) of the intestinal microbiota determined at the phylum (A, B) genus (C, D) and species (E) levels. The columns represent individuals in the psoriasis or control group (A, C, E).
Wilcoxon rank-sum permutation tests were performed to further compare the significant differences in the gut microbiota compositions between the 2 groups. There was a significant decrease in the phylum Euryarchaeota in the psoriasis group (0.031 vs 0.003, P < 0.05). Decreases in the families Oxalobacteraceae, Porphyromonadaceae, Pasteurellaceae, Rikenellaceae, Sphingobacteriaceae and Comamonadaceae were observed in the psoriasis patients (Table 2A). Nine genera, Butyricimonas, Oxalobacter, Actinobacillus, Odoribacter, Anaerotruncus, Alistipes, Pseudoflavonifractor, Pedobacter, and Comamonas, were significantly more abundant in the healthy control individuals than in the psoriasis patients (Table 2B).
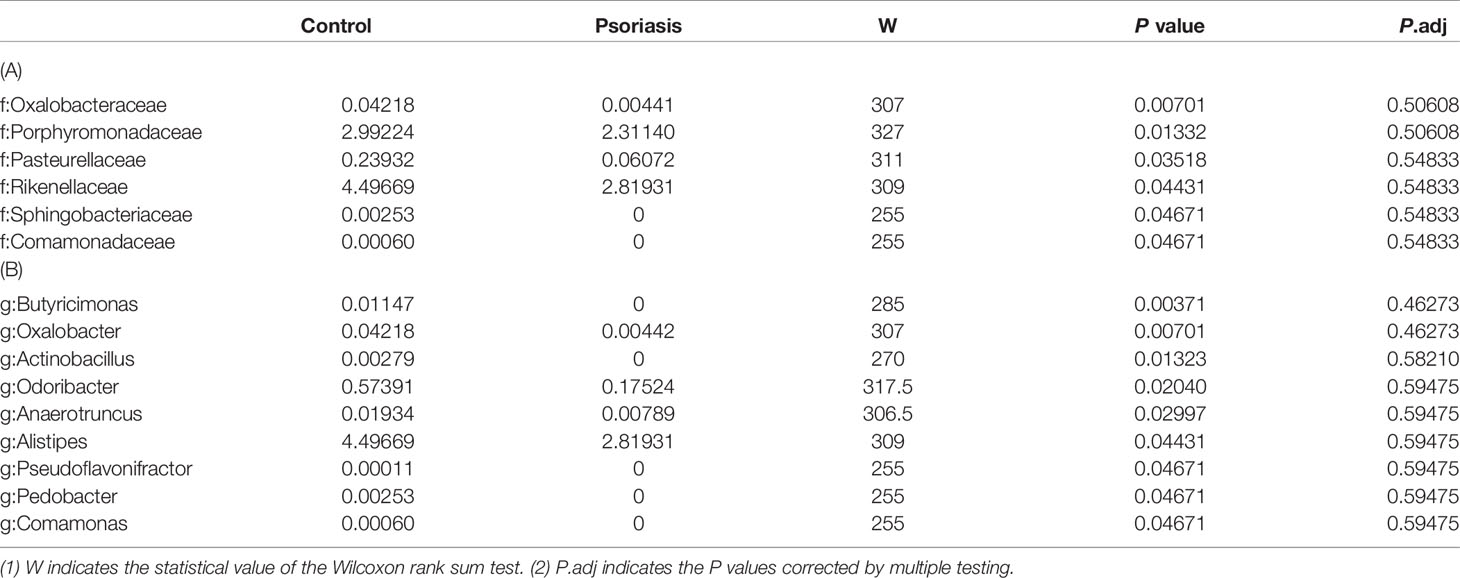
Table 2 Taxonomic differences between the psoriasis patients and controls at the family (A) and genus (B) levels.
LEfSe was used to further determine the specific significantly different bacterial taxa between the two cohorts (Figure 5A). Several genera, including Oscillospiraceae, Oxalobacter, Haemophilus, Odoribacter, Alistipes, and Pedobacter, were significantly enriched in the faeces of controls. The identified taxa were highlighted on the cladogram to indicate significant differences in phylogenetic distributions along with their LDA scores (Figure 5B). These results represented a notable gut microbiota alteration between the 2 groups.
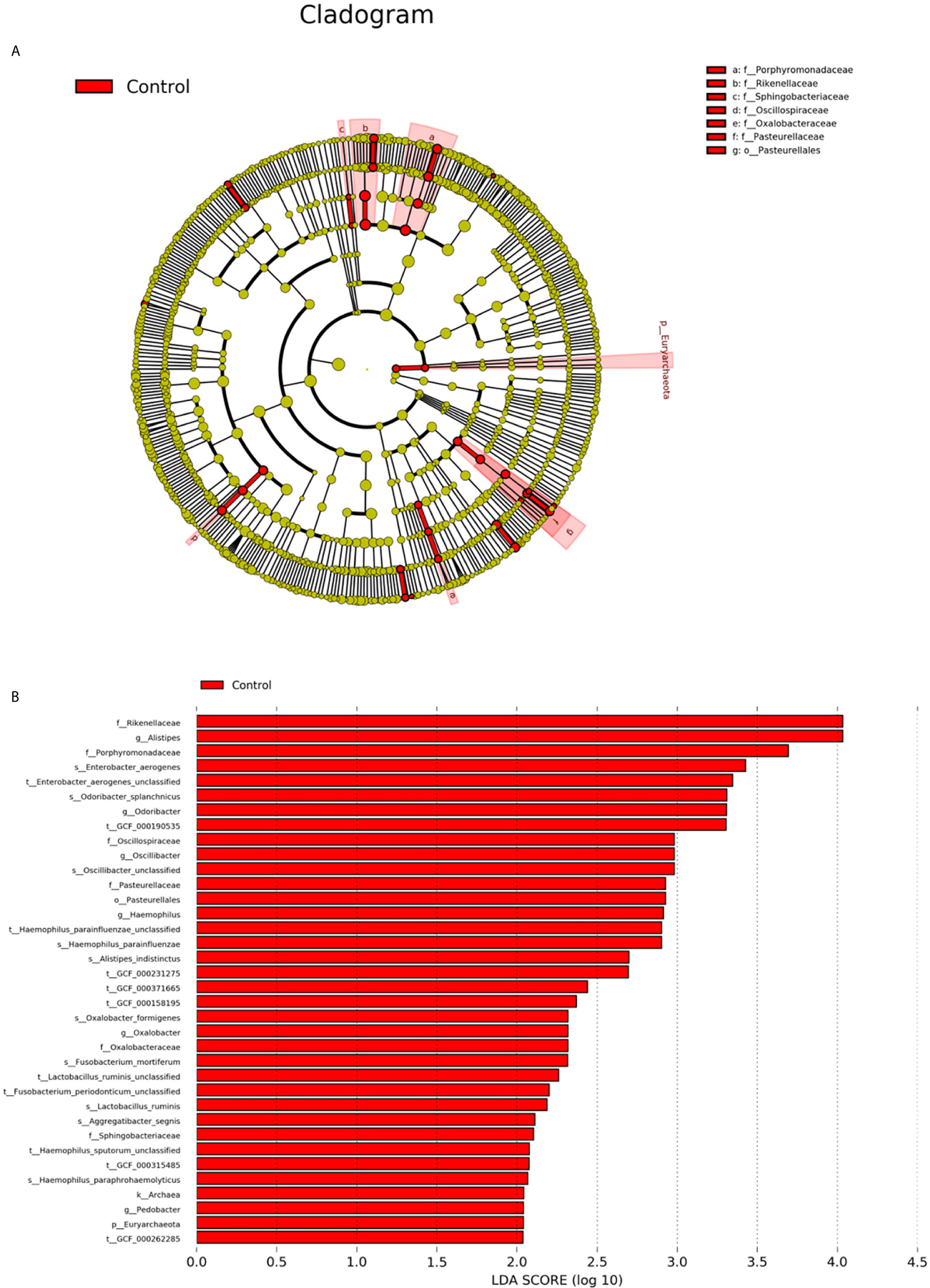
Figure 5 Intestinal microbiota profiles in psoriasis patients. (A) Cladogram generated from the LEfSe analysis indicating the phylogenetic distribution of the microbiota of psoriasis and control groups from phylum to genus. (B) Histogram of LDA scores to identify differentially abundant bacteria between patients and controls (LDA score > 2.0).
A total of 134 significantly different functional COGs between patients and controls were predicted (Table S1). Fifteen KEGG pathways (including LPS biosynthesis, WNT signaling pathway, apoptosis, bacterial secretion system, phosphotransferase system, and others) were significantly enriched in the psoriasis patient group (Table 3). Heatmaps were generated to represent the potential metabolic differences in the gut microbiome between the psoriasis and control groups. We found that 5 metabolites, H2S, isovalerate, isobutyrate, hyaluronan and hemicellulose, were significantly differentially abundant between the two cohorts (Table 4, Figure 6). Figure 6 shows that the colour of the patient individuals was different from that of the control subjects, which indicated the metabolic differences between the two groups.
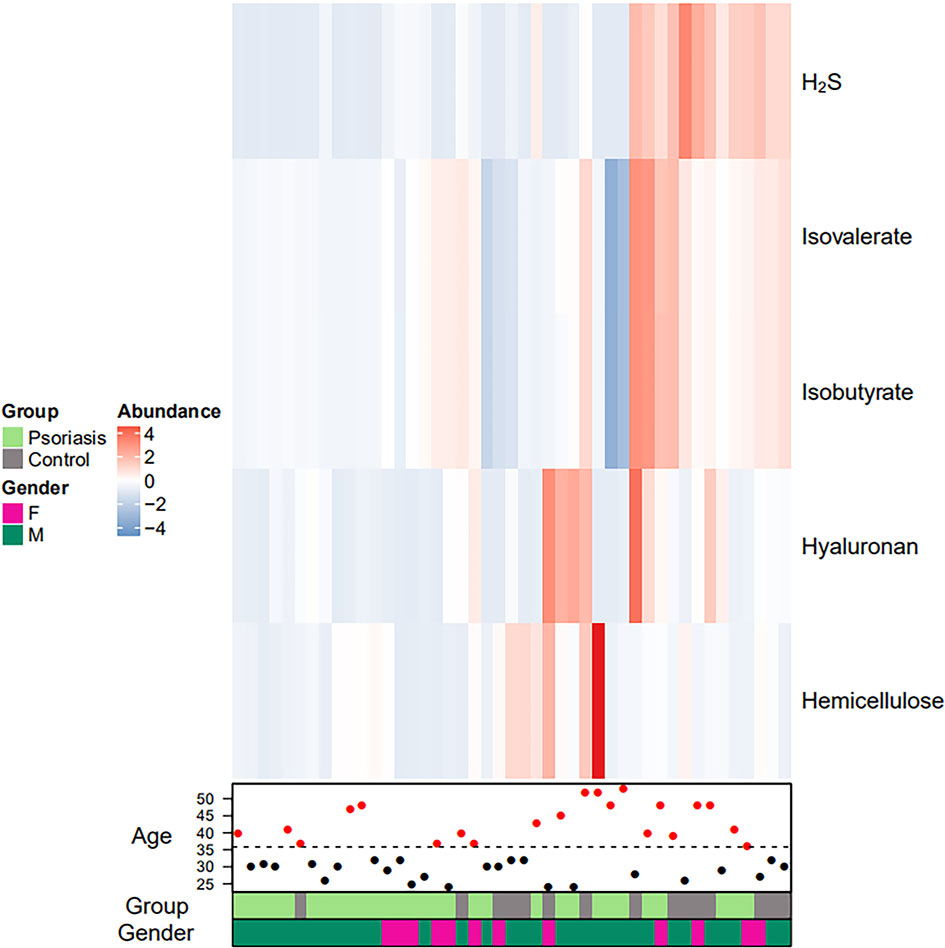
Figure 6 Faecal metabolic heatmap of samples from psoriasis patients. Hierarchical clustering showing the 5 significantly differentially abundant metabolites between the 2 groups (increased and decreased levels of metabolites are indicated by red and blue, respectively). The lines indicate specific metabolites, and the columns represent the individuals.
Evidence linking intestinal microbiota dysbiosis to chronic inflammation and immune system-mediated diseases has been expanding. This study is the first to elucidate gut bacterial changes in patients with psoriasis by metagenomic gene profiling analysis, not 16S rRNA sequencing analysis which has limitations but has been used in most studies. For example, contributions to the metagenome by organisms such as bacteria cannot be predicted by using 16S rRNA primers (Shapiro et al., 2019). Furthermore, in this study, the target gene pathways (KEGG and COG) and metabolic function of the microbiome in patients with psoriasis compared with control subjects were also detected, which are beneficial for in depth explanation of the pathogenesis of psoriasis.
Our results indicated the gut microbiota of psoriasis patients displayed an alteration in microbial taxa distribution, but no significant difference in microbial diversity. The psoriatic patients recruited showed a microbiota profile characterized by increased proportions of the phyla Actinobacteria and Firmicutes and genera Faecalibacterium, Bacteroides, Bifidobacterium and Megamonas and a reduction in the phyla Bacteroidetes, Euryarchaeota and Proteobacteria and genera Prevotella, Alistipes and Eubacterium, which seems to be in accordance with results reported in other studies (Scher et al., 2015; Codoñer et al., 2018; Hidalgo-Cantabrana et al., 2019; Shapiro et al., 2019). Functional analysis suggested that 134 COGs were predicted, and 15 KEGG pathways, including LPS biosynthesis, WNT signaling pathway, apoptosis, bacterial secretion system, and phosphotransferase system, were significantly enriched in psoriasis patients. Five metabolites (H2S, isovalerate, isobutyrate, hyaluronan, and hemicellulose) were significantly downregulated in the psoriatic cohort.
The pathogenesis of psoriasis could be related to immune and inflammatory response due to the dysfunction of medium- and short-chain fatty acids and the compromised colonic mucosal integrity and gut inflammation (Yegorov et al., 2020). Based on these results, the Firmicutes/Bacteroidetes (F/B) ratio increased, and the abundance of Bacteroides and Prevotella was deregulated in the psoriasis group, which seems to be in accordance with results reported in other studies (Visser et al., 2019; Dei-Cas, et al., 2020; Sikora et al., 2020). It has been reported that a high F/B ratio and a deregulated abundance of Bacteroides and Prevotella alter the production of medium- and short-chain fatty acids, such as acetate and butyrate, which are essential in maintaining colonic mucosal integrity (Corrêa-Oliveira et al., 2016; Perry et al., 2016; Komaroff, 2017; Christensen et al., 2018). The compromised integrity of the mucus barrier affects gut immune homeostasis and antigen presentation, resulting in immune responses and chronic inflammation (Bischoff et al., 2014; Christensen et al., 2018). Bacteroides produce polysaccharide A, which activates regulatory T cells and plays an immunomodulatory role in the gut (Mosca et al., 2016). After the addition of arabinoxylans, an elevation in caecal Prevotella was detected; Prevotella can enhance intestinal barrier function and decrease caecal inflammatory markers (Neyrinck et al., 2011; Neyrinck et al., 2012; Hong et al., 2016). Thus, novel therapeutic approaches that lower the F/B ratio and the abundance of Bacteroides, as well higher the abundance of Prevotella may be beneficial for psoriasis.
Except for immune and inflammatory response, psoriasis aetiology and pathological mechanisms may be also related to dysfunction of gut-brain axis and brain-skin axis. The abundance of Faecalibacterium prausnitzii was higher in psoriasis patients than in controls. F. prausnitzii has been observed to be decreased in patients with major depressive diseases, RA, metabolic syndrome, Crohn’s disease and obesity (Sokol et al., 2008; Stoll et al., 2014; Jiang et al., 2015; Remely et al., 2016) and increased in children with atopic dermatitis (Song et al., 2016). It has been reported that psoriasis, associated with both a physical and a psychological burden, is closely related to chronic stress (i.e., depression and anxiety) via the hypothalamic-pituitary-adrenal (HPA) axis which secretes neuroendocrine mediators and triggers skin inflammation in psoriasis (Chen and Lyga, 2014; Alexopoulos and Chrousos, 2016; Mueller et al., 2017; Visser et al., 2019). In addition, gut-brain axis dysfunction may be associated with psoriasis and psoriatic arthritis (Lu and He, 2015; Sikora et al., 2020), and the altered gut microbiota in patients with psoriatic arthritis resembles dysbiosis in inflammatory bowel disease (Scher et al., 2015). Furthermore, F. prausnitzii has effects on cytokine production and can produce butyrate, which inhibits the NF-κB pathway, inhibiting the inflammatory response (Sokol et al., 2008; Jiang et al., 2015; Hiippala et al., 2018). The relative abundances of Alistipes and Parabacteroides were reduced in the psoriasis group. This result is consistent with studies of psoriasis (Scher et al., 2015; Hidalgo-Cantabrana et al., 2019) and Crohn’s disease (Willing et al., 2010). The abundance of members of the genus Sutterella were also found to increase. Sutterella has a potential role in immune regulation and pro-inflammatory properties (Hiippala et al., 2016). Therefore, therapies that can lower the abundance of F. prausnitzii and Sutterella, and higher the abundance of Alistipes and Parabacteroides may be effective for treating psoriasis. Further research of the role of other identified genera in psoriasis is needed in the future.
A total of 15 KEGG pathways were significantly enriched in the patients with psoriasis, including LPS biosynthesis, WNT signaling pathway, bacterial secretion system, phosphotransferase system, and apoptosis, which are relevant to inflammatory response and apoptosis. LPS has been epidemiologically relevant to psoriasis and may maintain chronic inflammation (Shapiro et al., 2007). This inflammagen will activate the production of inflammatory chemokines and cytokines, stimulating the innate and adaptive immune systems (Kell and Pretorius, 2015; Potgieter et al., 2015; Kell and Pretorius, 2018). In addition, the WNT signaling pathway, which is involved in skin inflammation in psoriasis pathogenesis, was enriched in psoriatic patients and psoriatic arthritis patients (Dolcino et al., 2015; Wang et al., 2017). It has been reported that IL-36γ promotes inflammation and suppresses the differentiation of keratinocytes in psoriasis via the WNT signaling pathway (Wang et al., 2017). Moreover, the WNT signaling pathway has been involved in synovial inflammation (Miao et al., 2013). Apoptosis plays a key role in the pathogenesis of psoriasis (Chimenti et al., 2018; Hugh and Weinberg, 2018). T cells cause chronic inflammation and apoptosis via cytokines in psoriasis (Hugh and Weinberg, 2018). It has been reported that topical sunitinib ointment can suppress the proliferation and apoptosis of keratinocytes and reduce psoriasis-like inflammation (Kuang et al., 2018). The associations of other enriched pathways and the pathogenesis of psoriasis may be the direction of further research.
Heat map and hierarchical clustering showed the 5 metabolites (H2S, isovalerate, isobutyrate, hyaluronan, and hemicellulose) that were significantly differentially abundant between psoriasis patients and controls, which are related to inflammation and the VEGF signaling pathway. Studies have shown that H2S is a novel inflammatory mediator (Bhatia, 2015). H2S has been involved in different clinical inflammatory disorders, animal models, and in vitro systems (Bhatia, 2015; Castelblanco et al., 2018). H2S can alleviate LPS-induced lung inflammation in male rats (Zhang et al., 2016; Ali et al., 2018). Remarkable elevation of 3-OH-isovalerate was confirmed in 6 patients with biotinidase deficiency, all of whom presented with some extent of neurological abnormalities and dermatological lesions, and one of them presented with generalized pustular psoriasis (Yang et al., 2003). It has been suggested that butyrate is an intermediate in the anaerobic degradation of isobutyrate (Tholozan et al., 1988). The reduced generation of butyrate in psoriasis patients may compromise the integrity of the intestinal epithelial barrier, resulting in immune responses and chronic inflammation (Bischoff et al., 2014; Christensen et al., 2018; Shapiro et al., 2019). It has been reported that hyaluronan can act as a pro-inflammatory cytokine and induce inflammatory processes in psoriatic arthritis (Hellman et al., 2019). The VEGF signaling pathway plays an important role in psoriasis, and modified hemicellulose suppresses VEGF-induced angiogenesis (Malecic and Young, 2016; Zhu et al., 2017). Chronic inflammation is relevant to alterations of the intestinal microbiota that can be modified with hemicellulose (Konkol et al., 2019).
Lower microbial diversity was detected in some studies (Scher et al., 2015; Hidalgo-Cantabrana et al., 2019), while our results were on their opposite and were consistent with the research of Codoner et al. and Tan et al. (Codoñer et al., 2018; Tan et al., 2018). Difference of those results could be due to the different algorithms used and indices used in the studies, since most studies performed 16S rRNA sequencing and considered the number of OTUs and their relative proportion.
The potential limitations of the study are as follows: (1) The sample size was too small to identify the impact of the length, severity, comorbidities and treatments of psoriasis on the microbiome. (2) The absence of stool supernatant and gut epithelium samples might be beneficial for further research. (3) The microbial metabolite profiling was inferred base on the DNA and the abundance of gut microbiota, not by metabonomics. The actual microbial metabolite profiling may be different from it in the processes of DNA transcription and translation. (4) The gut microbiota profile, genetic functions and relative metabolites of psoriasis patients were performed using metagenomics sequencing. However, the sample marker gene database may include most of the species-and strain-level information, not 100% of all existing species or organisms. (5) The dietary habits have not been screened with specific questionnaire, which may potentially influence the gut microbiota of subjects.
The demonstrated alterations in microbiome composition and corresponding gene function and metabolic dysregulation likely impact the regulation of inflammation and immune responses, the VEGF signaling pathway, apoptosis, gut-brain axis and brain-skin axis that lead to psoriasis. Understanding the association between the intestinal microbiome and psoriasis pathogenesis will be beneficial for the development of novel predictions and therapeutic approaches for psoriasis, including microbial administration via diet, eradication of antibiotics, addition of probiotics or faecal microbial transplantation as a substitute to shift the microbiome towards a healthy status, as has been suggested for IBD (Quraishi et al., 2017).
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra/PRJNA688472.
The studies involving human participants were reviewed and approved by the Ethical Committee of the Beijing Hospital of Traditional Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
GZ and YT designed the study. FX and YL performed the experiments. XL and XW collected and prepared samples for sequencing. MC performed sequencing and sequencing analysis with technical assistance from CJ. HL and XW performed statistical interpretation and analyses with technical assistance from XG, and SX took primary responsibility for writing the manuscript with English improved by YT and GZ. All authors discussed the results and commented on the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (grant number 81673974, 81974572) and the Beijing Municipal Administration of Hospitals Incubating Program (grant number PZ2019024).
MC was employed by the company Beijing QuantiHealth Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.605825/full#supplementary-material
Alexopoulos, A., Chrousos, G. P. (2016). Stress-related skin disorders. Rev. Endocr. Metab. Disord. 17 (3), 295–304. doi: 10.1007/s11154-016-9367-y
Ali, F. F., Abdel-Hamid, H. A., Toni, N. D. (2018). H2S attenuates acute lung inflammation induced by administration of lipopolysaccharide in adult male rats. Gen. Physiol. Biophys. doi: 10.4149/gpb_2018002
Bhatia, M. (2015). H2S and Inflammation: An Overview. Handb. Exp. Pharmacol. 230, 165–180. doi: 10.1007/978-3-319-18144-8_8
Bischoff, S. C., Barbara, G., Buurman, W., Ockhuizen, T., Schulzke, J. D., Serino, M., et al. (2014). Intestinal permeability-a new target for disease prevention and therapy. BMC Gastroenterol. 14:189. doi: 10.1186/s12876-014-0189-7
Bray, J. R., Curtis, J. T. (1957). An ordination of the upland forest communities of Southern Wisconsin. Eco. Monogr. 27, 325–349. doi: 10.2307/1942268
Castelblanco, M., Lugrin, J., Ehirchiou, D., Nasi, S., Ishii, I., So, A., et al. (2018). Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J. Biol. Chem. 293 (7), 2546–2557. doi: 10.1074/jbc.M117.806869
Chen, Y., Lyga, J. (2014). Brain-Skin Connection: Stress, Inflammation and Skin Aging. Inflammation Allergy Drug Targets 13 (3), 177–190. doi: 10.2174/1871528113666140522104422
Chen, C. C., Chen, K. J., Kong, M. S., Chang, H. J., Huang, J. L. (2016). Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr. Allergy Immunol. 27 (3), 254–262. doi: 10.1111/pai.12522
Chen, Y.-J., Ho, H. J., Tseng, C.-H., Lai, Z.-L., Shieh, e.-J., Wu, C.-Y. (2018). Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp. Dermatol. 27 (12), 1336–1343. doi: 10.1111/exd.13786
Chimenti, M. S., Sunzini, F., Fiorucci, L., Botti, E., Fonti, G. L., Conigliaro, P., et al. (2018). Potential role of cytochrome c and tryptase in psoriasis and psoriatic arthritis pathogenesis: focus on resistance to apoptosis and oxidative stress. Front. Immunol. 9, 2363. doi: 10.3389/fimmu.2018.02363
Christensen, L., Roager, H. M., Astrup, A., Hjorth, M. F. (2018). Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 108 (4), 645–651. doi: 10.1093/ajcn/nqy175
Codoñer, F. M., Ramírez-Bosca, A., Climent, E., Carrión-Gutierrez, M., Guerrero, M., Pérez-Orquín, J. M., et al. (2018). Gut microbial composition in patients with psoriasis. Sci. Rep. 8 (1), 3812. doi: 10.1038/s41598-018-22125-y
Collins, S. M., Surette, M., Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 10 (11), 735–742. doi: 10.1038/nrmicro2876
Corrêa-Oliveira, R., Fachi, J. L., Vieira, A., Sato, F. T., Vinolo, M. A. (2016). Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 5 (4), e73. doi: 10.1038/cti.2016.17
de Groot, P. F., Belzer, C., Aydin, Ö., Levin, E., Levels, J. H., Aalvink, S., et al. (2017). Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PloS One 12 (12), e0188475. doi: 10.1371/journal.pone.0188475
Dei-Cas, I., Giliberto, F., Luce, L., Dopazo, H., Penas-Steinhardt, A. (2020). Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new Psoriasis-Microbiome Index. Sci. Rep. 10 (1), 12754. doi: 10.1038/s41598-020-69537-3
Dolcino, M., Ottria, A., Barbieri, A., Patuzzo, G., Tinazzi, E., Argentino, G., et al. (2015). Gene expression profiling in peripheral blood cells and synovial membranes of patients with psoriatic arthritis. PloS One 10 (6), e0128262. doi: 10.1371/journal.pone.0128262
Dray, S., Dufour, A. B. (2007). The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 22 (4), 1–20. doi: 10.18637/jss.v022.i04
Edwards, C. J., Costenbader, K. H. (2014). Epigenetics and the microbiome: developing areas in the understanding of the aetiology of lupus. Lupus 23 (6), 505–506. doi: 10.1177/0961203314531636
Fry, L., Baker, B. S. (2007). Triggering psoriasis: the role of infections and medications. Clin. Dermatol. 25 (6), 606–615. doi: 10.1016/j.clindermatol.2007.08.015
Hellman, U., Engström-Laurent, A., Larsson, A., Lindqvist, U. (2019). Hyaluronan concentration and molecular mass in psoriatic arthritis: biomarkers of disease severity, resistance to treatment, and outcome. Scand. J. Rheumatol. 48 (4), 284–293. doi: 10.1080/03009742.2019.1577490
Hidalgo-Cantabrana, C., Gómez, J., Delgado, S., Requena-López, S., Queiro-Silva, R., Margolles, A., et al. (2019). Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Dermatol. 181 (6), 1287–1295. doi: 10.1111/bjd.17931
Hiippala, K., Kainulainen, V., Kalliomaki, M., Arkkila, P., Satokari, R. (2016) Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front. Microbiol. 7, 1706. doi: 10.3389/fmicb.2016.01706
Hiippala, K., Jouhten, H., Ronkainen, A., Hartikainen, A., Kainulainen, V., Jalanka, J., et al. (2018). The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 10 (8), E988. doi: 10.3390/nu10080988
Honda, K., Littman, D. R. (2016). The microbiota in adaptive immune homeostasis and disease. Nature 535 (7610), 75–84. doi: 10.1038/nature18848
Hong, J., Jia, Y., Pan, S., Jia, L., Li, H., Han, Z., et al. (2016). Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 7 (35), 56071–56082. doi: 10.18632/oncotarget.11267
Hugh, J. M., Weinberg, J. M. (2018). Update on the pathophysiology of psoriasis. Cutis 102 (5S), 6–12.
Huttenhower, C., Kostic, A. D., Xavier, R. J. (2014). Inflammatory bowel disease as a model for translating the microbiome. Immunity 40 (6), 843–854. doi: 10.1016/j.immuni.2014.05.013
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immunol. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Kell, D. B., Pretorius, E. (2015). On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS-induced cell death. Integr. Biol. (Camb.) 7 (11), 1339–1377. doi: 10.1039/c5ib00158g
Kell, D. B., Pretorius, E. (2018). No effects without causes: the iron dysregulation and dormant microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. Camb. Philos. Soc. 93 (3), 1518–1557. doi: 10.1111/brv.12407
Kimball, A. B., Leonardi, C., Stahle, M., Gulliver, W., Chevrier, M., Fakharzadeh, S., et al. (2014). Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR). Br. J. Dermatol. 171 (1), 137–147. doi: 10.1111/bjd.13013
Komaroff, A. L. (2017). The microbiome and risk for obesity and diabetes. JAMA 317 (4), 355–356. doi: 10.1001/jama.2016.20099
Konkol, Y., Keskitalo, A., Vuorikoski, H., Pietilä, S., Elo, L. L., Munukka, E., et al. (2019). Chronic nonbacterial prostate inflammation in a rat model is associated with changes of gut microbiota that can be modified with a galactoglucomannan-rich hemicellulose extract in the diet. BJU Int. 123 (5), 899–908. doi: 10.1111/bju.14553
Konstantinidis, K. T., Tiedje, J. M. (2007). Prokaryotic taxonomy and phylogeny in the genomic era: advancements and challenges ahead. Curr. Opin. Microbiol. 10 (5), 504–509. doi: 10.1016/j.mib.2007.08.006
Kuang, Y.-H., Lu, Y., Liu, Y.-K., Liao, L.-Q., Zhou, X.-C., Qin, Q.-S., et al. (2018). Topical Sunitinib ointment alleviates Psoriasis-like inflammation by inhibiting the proliferation and apoptosis of keratinocytes. Eur. J. Pharmacol. 824, 57–63. doi: 10.1016/j.ejphar.2018.01.048
Lee, M.-J., Kang, M.-J., Lee, S.-Y., Lee, E., Kim, K., Won, S., et al. (2018). Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J. Allergy Clin. Immunol. 141 (4), 1310–1319. doi: 10.1016/j.jaci.2017.11.045
Littman, D. R., Pamer, E. G. (2011). Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10 (4), 311–323. doi: 10.1016/j.chom.2011.10.004
Lu, L., He, L. (2015). Gut-brain axis dysfunction may be associated with psoriatic arthritis: comment on the article by Scher et al. Arthritis Rheumatol. 67 (8), 2280. doi: 10.1002/art.39151
Luo, W., Friedman, M. S., Shedden, K., Hankenson, K. D., Woolf, P. J. (2009). GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinf. 10, 161. doi: 10.1186/1471-2105-10-161
Luo, A., Leach, S. T., Barres, R., Hesson, L. B., Grimm, M. C., Simar, D. (2017). The microbiota and epigenetic regulation of T helper 17/regulatory T cells: in search of a balanced immune system. Front. Immunol. 8, 417. doi: 10.3389/fimmu.2017.00417
Malecic, N., Young, H. S. (2016). Novel investigational vascular endothelial growth factor (VEGF) receptor antagonists for psoriasis. Expert Opin. Investig. Drugs 25 (4), 455–462. doi: 10.1517/13543784.2016.1153064
Mandal, S., Van Treuren, W., White, R. A., Eggesbø, M., Knight, R., Peddada, S. D. (2015). Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. 26, 27663. doi: 10.3402/mehd.v26.27663
Mangiola, F., Ianiro, G., Franceschi, F., Fagiuoli, S., Gasbarrini, G., Gasbarrini, A. (2016). Gut microbiota in autism and mood disorders. World J. Gastroenterol. 22 (1), 361–368. doi: 10.3748/wjg.v22.i1.361
Miao, C.-g., Yang, Y.-y., He, X., Li, X.-f., Huang, C., Huang, Y., et al. (2013). Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal 25 (10), 2069–2078. doi: 10.1016/j.cellsig.2013.04.002
Mosca, A., Leclerc, M., Hugot, J. P. (2016). Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front. Microbiol. 7, 455. doi: 10.3389/fmicb.2016.00455
Mueller, S. M., Hogg, S., Mueller, J., McKie, S., Itin, P., Reinhardt, J., et al. (2017). Functional magnetic resonance imaging in dermatology: The skin, the brain and the invisible. Exp. Dermatol. 26 (10), 845–853. doi: 10.1111/exd.13305
Neyrinck, A. M., Possemiers, S., Druart, C., de Wiele, T. V., De Backer, F., Cani, P. D., et al. (2011). Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PloS One 6, e20944. doi: 10.1371/journal.pone.0020944
Neyrinck, A. M., Van Hée, V. F., Piront, N., De Backer, F., Toussaint, O., Cani, P. D., et al. (2012). Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes 2, e28. doi: 10.1038/nutd.2011.24
Noecker, C., Eng, A., Srinivasan, S., Theriot, C. M., Young, V. B., Jansson, J. K., et al. (2016). Metabolic model-based integration of microbiome taxonomic and metabolomic profiles elucidates mechanistic links between ecological and metabolic variation. mSystems 1 (1), e00013–e00015. doi: 10.1128/mSystems.00013-15
Palm, N. W., de Zoete, M. R., Flavell, R. A. (2015). Immune–microbiota interactions in health and disease. Clin. Immunol. 159 (2), 122–127. doi: 10.1016/j.clim.2015.05.014
Parisi, R., Symmons, D. P., Griffiths, C. E., Ashcroft, D. M., Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team (2013). Global epidemiology of psoriasis: a systemic review of incidence and prevalence. J. Invest. Dermatol. 133 (2), 377–385. doi: 10.1038/jid.2012.339
Penders, J., Gerhold, K., Stobberingh, E. E., Thijs, C., Zimmermann, K., Lau, S., et al. (2013). Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 132 (3), 601–607.e8. doi: 10.1016/j.jaci.2013.05.043
Perry, R. J., Peng, L., Barry, N. A., Cline, G. W., Zhang, D., Cardone, R. L., et al. (2016). Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534 (7606), 213–217. doi: 10.1038/nature18309
Pinto, A. J., Raskin, L. (2012). PCR biases distort bacterial and archaeal community structure in pyrosequencing datasets. PloS One 7, e43093. doi: 10.1371/journal.pone.0043093
Planer, J. D., Peng, Y., Kau, A. L., Blanton, L. V., Ndao, I.M., Tarr, P. I., et al. (2016). Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 534 (7606), 263–266. doi: 10.1038/nature17940
Potgieter, M., Bester, J., Kell, D. B., Pretorius, E. (2015). The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 39 (4), 567–591. doi: 10.1093/femsre/fuv013
Quraishi, M. N., Critchlow, T., Bhala, N., Sharma, N., Iqbal, T. (2017). Faecal transplantation for IBD management-pitfalls and promises. Br. Med. Bull. 124 (1), 181–190. doi: 10.1093/bmb/ldx040
Remely, M., Hippe, B., Zanner, J., Aumueller, E., Brath, H., Haslberger, A. G. (2016). Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausinitzii, Allermansia muciniphila and Peptostreoptococcus anaerobius after weight loss. Endocr. Metab. Immune Disord. Drug Targets 16 (2), 99–106. doi: 10.2174/1871530316666160831093813
Rosser, E. C., Mauri, C. (2016). A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun 74, 85–93. doi: 10.1016/j.jaut.2016.06.009
Salem, I., Ramser, A., Isham, N., Ghannoum, M. A. (2018). The gut microbiome as a major regulator of the gut–skin axis. Front. Microbiol. 9, 1459. doi: 10.3389/fmicb.2018.01459
Scher, J. U., Ubeda, C., Artacho, A., Attur, M., Isaac, S., Reddy, S. M., et al. (2015). Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 67 (1), 128–139. doi: 10.1002/art.38892
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. doi: 10.1186/gb-2011-12-6-r60
Shamriz, O., Mizrahi, H., Werbner, M., Shoenfeld, Y., Avni, O., Koren, O. (2016). Microbiota at the crossroads of autoimmunity. Autoimmun Rev. 15 (9), 859–869. doi: 10.1016/j.autrev.2016.07.012
Shapiro, J., Cohen, A. D., David, M., Hodak, E., Chodik, G., Viner, A., et al. (2007). The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J. Am. Acad. Dermatol. 56 (4), 629–634. doi: 10.1016/j.jaad.2006.09.017
Shapiro, J., Cohen, N. A., Shalev, V., Uzan, A., Koren, O., Maharshak, N. (2019). Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J. Dermatol. 46 (7), 595–603. doi: 10.1111/1346-8138.14933
Sikora, M., Stec, A., Chrabaszcz, M., Knot, A., Waskiel-Burnat, A., Rakowska, A., et al. (2020). Gut microbiome in psoriasis: an updated review. Pathogens 9 (6), 463. doi: 10.3390/pathogens9060463
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U. S. A. 105 (43), 16731–16736. doi: 10.1073/pnas.0804812105
Sommer, F., Backhed, F. (2013). The gut microbiota-masters of host development and physiology. Nat. Rev. Microbiol. 11 (4), 227–238. doi: 10.1038/nrmicro2974
Song, H., Yoo, Y., Hwang, J. H., Na, Y. C., Kim, H. S. (2016). Faecalibacterium prausinitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 137 (3), 852–860. doi: 10.1016/j.jaci.2015.08.021
Stockinger, B., Omenetti, S. (2017). The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 17 (9), 535–544. doi: 10.1038/nri.2017.50
Stoll, M. L., Kumar, R., Morrow, C. D., Lefkowitz, E. J., Cui, X., Genin, A., et al. (2014). Altered microbiota associated with abnormal humoral immune response to commensal organisms in entehsitis-related arthritis. Arthritis Res. Ther. 16 (6), 486. doi: 10.1186/s13075-014-0486-0
Sung, J., Kim, S., Cabatbat, J. J. T., Jang, S., Jin, Y.-S., Jung, G. Y., et al. (2017). Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat. Commun. 8, 15393. doi: 10.1038/ncomms15393
Tan, L., Zhao, S., Zhu, W., Wu, L., Li, J., Shen, M., et al. (2018). The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp. Dermatol. 27 (2), 144–149. doi: 10.1111/exd.13463
Tholozan, J.-L., Samain, E., Grivet, J.-P. (1988). Isomerization between n-butyrate and isobutyrate in enrichment cultures. FEMS Microbiol. Ecol. 4, 187–191. doi: 10.1111/j.1574-6968.1988.tb02663.x
Torres, T., Puig, L. (2018). Apremilast: A Novel Oral Treatment for Psoriasis and Psoriatic Arthritis. Am. J. Clin. Dermatol. 19 (1), 23–32. doi: 10.1007/s40257-017-0302-0
Truong, D. T., Franzosa, E. A., Tickle, T. L., Scholz, M., Weingart, G., Pasolli, E., et al. (2015). MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 12 (10), 902–903. doi: 10.1038/nmeth.3589
Vegan, D. P. (2003). A package of R functions for community ecology. J. Veg. Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Visser, M. J.E., Kell, D. B., Pretorius, E. (2019). Bacterial dysbiosis and translocation in psoriasis vulgaris. Front. Cell Infect. Microbiol. 4, 7. doi: 10.3389/fcimb.2019.00007
Wang, W., Yu, X., Wu, C., Jin, H. (2017). IL-36γ inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int. J. Med. Sci. 14 (10), 1002–1007. doi: 10.7150/ijms.20809
Willing, B. P., Dicksved, J., Halfvarson, J., Andersson, A. F., Lucio, M., Zheng, Z., et al. (2010). A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139 (6), 1844–1854.e1. doi: 10.1053/j.gastro.2010.08.049
Yan, D., Issa, N., Afifi, L., Jeon, C., Chang, H. W., Liao, W. (2017). The role of the skin and gut microbiome in psoriatic disease. Curr. Dermatol. Rep. 6 (2), 94–103. doi: 10.1007/s13671-017-0178-5
Yang, Y. L., Yamaguchi, S., Tagami, Y., Zhang, Y. H., Xiong, H., Hasegawa, Y., et al. (2003). Diagnosis and treatment of biotinidase deficiency-clinical study of six patients. Zhonghua Er Ke Za Zhi 41 (4), 249–251. Chinese.
Yegorov, S., Babenko, D., Kozhakhmetov, S., Akhmaltdinova, L., Kadyrova, I., Nurgozhina, A., et al. (2020). Psoriasis is associated with elevated gut IL-1α and intestinal microbiome alterations. Front. Immunol. 1, 571319. doi: 10.3389/fimmu.2020.571319
Yeoh, N., Burton, J. P., Suppiah, P., Reid, G., Stebbings, S. (2013). The role of the microbiome in rheumatic diseases. Curr. Rheumatol. Rep. 15 (3), 314. doi: 10.1007/s11926-012-0314-y
Zákostelská, Z., Málková, J., Klimešová, K., Rossmann, P., Hornová, M., Novosádová, I., et al. (2016). Intestinal microbiota promotes psoriasis-like skin inflammation by enhancing Th17 response. PloS One 11 (7), e0159539. doi: 10.1371/journal.pone.0159539
Zanvit, P., Konkel, J. E., Jiao, X., Kasagi, S., Zhang, D., Wu, R., et al. (2015). Antibiotics in neonatal life increase murine susceptibility to experimental psoriasis. Nat. Commun. 6, 8424. doi: 10.1038/ncomms9424
Zhang, H.-X., Liu, S.-J., Tang, X.-L., Duan, G.-L., Ni, X., Zhu, X.-Y., et al. (2016). H2S Attenuates LPS-Induced Acute Lung Injury by Reducing Oxidative/Nitrative Stress and Inflammation. Cell Physiol. Biochem. 40 (6), 1603–1612. doi: 10.1159/000453210
Zhang, M., Ma, W., Zhang, J., He, Y., Wang, J. (2018). Analysis of gut microbiota profiles and microbe–disease associations in children with autism spectrum disorders in China. Sci. Rep. 8 (1), 13981. doi: 10.1038/s41598-018-32219-2
Keywords: gut microbiota, genetic functions, metabolites, psoriasis, metagenomics sequencing
Citation: Xiao S, Zhang G, Jiang C, Liu X, Wang X, Li Y, Cheng M, Lv H, Xian F, Guo X and Tan Y (2021) Deciphering Gut Microbiota Dysbiosis and Corresponding Genetic and Metabolic Dysregulation in Psoriasis Patients Using Metagenomics Sequencing. Front. Cell. Infect. Microbiol. 11:605825. doi: 10.3389/fcimb.2021.605825
Received: 15 September 2020; Accepted: 09 March 2021;
Published: 01 April 2021.
Edited by:
Bikash Sahay, University of Florida, United StatesReviewed by:
Simone Garcovich, Catholic University of the Sacred Heart, ItalyCopyright © 2021 Xiao, Zhang, Jiang, Liu, Wang, Li, Cheng, Lv, Xian, Guo and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan Jiang, dGNtamN5QDEyNi5jb20=; Guangzhong Zhang, Z3Vhbmd6aHpoQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.