- Department of Pediatric Pneumology, Immunology and Intensive Care Medicine, CF Center, Charité—Universitätsmedizin Berlin, Berlin, Germany
Background: In Cystic Fibrosis (CF), the airways are often colonized by opportunistic fungi. The most frequently detected mold is Aspergillus fumigatus (Af). Af diseases are associated with significant morbidity and mortality. The most common clinical picture caused by Af is allergic bronchopulmonary aspergillosis (ABPA), triggered by an immunological reaction against Af. Af bronchitis and invasive aspergillosis rarely occur in CF as a result of spore colonization and germination. Since pulmonary mycoses and exacerbations by other pathogens overlap in clinical, radiological, and immunological characteristics, diagnosis still remains a challenge. The search for reliable, widely available biomarkers for Af diseases is therefore still an important task today.
Objectives: Af-specific IgG m3 is broadly available. Sensitivity and specificity data are contradictory and differ depending on the study population. In our prospective study on pulmonary Af diseases in CF, we determined specific IgG m3 in order to test its suitability as a biomarker for acute Af diseases and as a follow-up parameter.
Methods: In this prospective single center study, 109 patients with CF were screened from 2016 to 2019 for Af-associated diseases. According to diagnostic criteria, they were divided into four groups (control, bronchitis, ABPA, pneumonia). The groups were compared with respect to the level of Af-specific IgG (ImmunoCAP Gm3). We performed a receiver operating characteristic (ROC) curve analysis to determine cut-off, sensitivity and specificity. Twenty-one patients could be enrolled for a follow-up examination.
Results: Of the 109 patients, 36 were classified as acute Af-disease (Af bronchitis, ABPA, Af pneumonia). Of these, 21 patients completed follow up-screening. The median Af-specific Gm3 was higher in the acute Af-disease groups. There was a significant difference in Af-specific IgG m3 compared to the control group without acute Af-disease. Overall, there was a large interindividual distribution of Gm3. A cut-off value of 78.05 mg/L for Gm3 was calculated to discriminate controls and patients with ABPA/pneumonia with a specificity of 75% and a sensitivity of 74.6%. The follow up examination of 21 patients showed a decrease of Gm3 in most patients without statistical significance due to the small number of follow up patients.
Conclusion: Af specific IgG may be a useful biomarker for acute ABPA and Af pneumonia, but not for Af bronchitis in CF. However, due to the large interindividual variability of Gm3, it should only be interpreted alongside other biomarkers. Therefore, due to its broad availability, it could be suitable as a biomarker for ABPA and Af pneumonia in CF, if the results can be supported by a larger multicenter cohort.
Introduction
Cystic fibrosis (CF) is the most common lethal genetic disease in Caucasians and affects approximately between 70,000 and 100,000 people worldwide. The leading cause of death is the progressive lung disease, driven by a complex and diverse inflammatory immune syndrome induced by recurrent and chronic bacterial and fungal infections of the lung (Stephenson et al., 2015; Bell et al., 2020).
A. fumigatus (Af), the most frequently detected mold fungus in CF airways, is the fungal pathogen with the most impact on morbidity and mortality in CF (Pihet et al., 2009; Amin et al., 2010; Middleton et al., 2013; Schwarz et al., 2018a). The defective chloride channel in CF together with impaired mucociliary clearance and structural changes resulting from infectious complications (e.g. cavitary lung disease, bronchiectasis) result in a local mucosal immunodeficiency (Cohen and Prince, 2012), which allows airway pathogens like Af conidia to colonize and germinate in the airways. Allergic bronchopulmonary aspergillosis (ABPA), bronchitis, aspergilloma and invasive infection (pneumonia) are the known disease entities caused by Af in CF (Moss, 2010; Baxter et al., 2013a; Middleton et al., 2013; Hong et al., 2018).
ABPA is an immune mediated lung disease with a prevalence of up to 15% in CF. It manifests with a poorly controlled obstructive disease and recurrent pulmonary infiltrates with or without bronchiectasis (Stevens, 2003). According to the 2003 CFF (Cystic Fibrosis Foundation) consensus criteria, classic diagnosis of ABPA comprises clinical deterioration not attributed to other causes, serum IgE >1000 IU/ml, a positive skin prick test or positive specific IgE, antibodies against Af, precipitating or IgG antibodies against Af, and radiological changes that are not treatable by antibiotics. For the minimal ABPA criteria, serum IgE >500 IU/ml and fewer of the criteria mentioned above are sufficient to suspect ABPA (Stevens, 2003). Invasive pulmonary Af infections primarily occur in severely immunocompromised patients, but they also can occur in CF if additional risk factors are present (e.g. severe lung disease, diabetes, steroid therapy) (Kousha et al., 2011; Hong et al., 2018). The prevalence is extremely low, thus precise data are not available. In CF, invasive pulmonary Af infections often show a subacute, slowly progressing clinical course. and is induced by local invasion of Af (Schwarz et al., 2018b). Radiographic imaging and clinical courses are often non-specific (Felton and Simmonds, 2014; Kosmidis and Denning, 2015). This bears the risk of delayed diagnosis in CF. Af bronchitis is another Af entity with an estimated prevalence of 9% in CF. Increasing evidence exists that Af in CF can cause airway symptoms without hypersensitivity and without tissue invasion of fungal hyphae (Felton and Simmonds, 2014; Brandt et al., 2018). Shoseyov et al. were the first to report on Af bronchitis in six patients (Shoseyov et al., 2006). The group suggested that bronchitis should be considered, and antifungal therapy initiated when deteriorating respiratory function is not responding to antibacterial therapy, Af is growing in sputum cultures and ABPA was excluded. Chrdle et al. proposed a definition using clinical criteria: symptomatic chronic lower airway disease, detection of Af in sputum or bronchial alveolar lavage (BAL) by culture or PCR, and detection of Aspergillus-specific IgG antibodies (Chrdle et al., 2012). In summary, repeatedly detected Af in sputum samples and persistent respiratory symptoms without signs of Af hypersensitivity and without radiographic evidence of infection are characteristics of Af bronchitis in patients with CF (Felton and Simmonds, 2014; Kosmidis and Denning, 2015). Due to the polymicrobial colonization, overlapping clinical, radiographic, and laboratory characteristics, it is still difficult to clearly distinguish Af conditions from other causes of bronchopulmonary exacerbation in CF (Stevens, 2003). This bears the risk of Af diseases being missed and can lead to situations in which a potentially harmful differential therapeutic strategy must be attempted in order to achieve successful treatment. Therefore, the search for reliable, widely available biomarkers for Af diseases is still an important task today (el-Dahr et al., 1994; Kurup, 2005; Latzin et al., 2008; Hafen GM et al., 2009; Delhaes et al., 2010; Scheffold et al., 2018; Keown et al., 2019). In this light, it seems useful to evaluate single biomarkers in terms of their significance for Af diseases. Af IgG antibodies are used for diagnosis of chronic pulmonary aspergillosis (CPA) and aspergillus diseases in patients with chronic lung disorders and are referred to as diagnostic criteria in many guidelines (Stevens, 2003; Delhaes et al., 2010; Agarwal et al., 2017). They are found in many patients with ABPA, bronchitis and invasive aspergillosis, and several assays are available. In the clinical routine, in recent years, technologies like ImmunoCAP have continuously replaced older methods like immunoprecipitation (Van Hoeyveld et al., 2006; Baxter et al., 2013b). The ImmunoCAP technique provides quantitative measures and reproducible results and therefore a higher sensitivity and specificity in detecting aspergillus conditions (Baxter et al., 2013b). However, there is still a lack of standardization in terms of cut-off values, as several working groups examining Af IgG in Af related conditions obtained discrepant results, which is in part due to different study designs and different laboratory methods used, but this may also reflect the different host-pathogen-interaction of heterogeneous study populations with different underlying diseases (Agarwal et al., 2017; Sehgal et al., 2018; Alghamdi et al., 2019; Sehgal et al., 2019). We therefore evaluated specific Af IgG in ABPA, Af bronchitis and invasive aspergillosis in CF.
Materials and Methods
This prospective study was conducted between January 2016 and December 2019 at Christiane Herzog Cystic Fibrosis Center, Charité—Universitätsmedizin Berlin, Germany. Ethical aspects were considered and approval for the study was gained by the Ethics Committee of Charité—Universitätsmedizin Berlin, Germany (Number EA2/121/16).
We collected sera from 109 patients with CF, ages 6–69 years, independent of their history of Af related conditions. To obtain both a control group and a disease group, Af-specific IgG was determined either as part of a diagnostic workup when Af conditions were suspected or without such suspicion in pulmonary exacerbated and in clinically stable patients during routine checkup. Af-specific IgG (ImmunoCAP Gm3) was determined in our routine laboratory. Depending on the diagnosis criteria for Af related conditions in CF, the patients were categorized into four groups: control, Af bronchitis, ABPA, and Af pneumonia. As a few patients were assessed several times during the course of the study, some follow-up measurements for Gm3 are available and listed below.
Diagnosis Criteria for Af Related Conditions
Diagnosis of ABPA was based on the minimal 2003 Cystic Fibrosis Foundation (CFF) consensus criteria: i) serum IgE >500 IU/ml; ii) Af-specific IgE >0.35 kU/L; iii) clinical deterioration not attributed to other causes; and one of the following criteria: i) presence of Af IgG (Gm3 ImmunoCAP) antibodies in serum; ii) new or recent abnormalities on chest radiography or chest CT that have not cleared with antibiotics and standard physiotherapy. In one patient, diagnosis of ABPA was assumed despite a total IgE <500 kU/L due to a threefold increase in total IgE and positive diagnosis criteria.
Af bronchitis was defined as: i) clinical deterioration with repeatedly positive sputum and Af PCR or repeatedly positive sputum cultures (≥2 during the past 6 months) for Af, ii) no antibiotic treatment response, iii) total serum IgE level <200 kU/L, iv) no observation of new pulmonary infiltrates, and v) appropriate antifungal treatment response.
Criteria for diagnosis of Af pneumonia were i) positive sputum Af PCR or repeatedly positive sputum cultures for Af, ii) new or recent abnormalities on chest radiography or chest CT that have not cleared with antibiotics and standard physiotherapy, iii) no antibiotic treatment response, and iv) total serum IgE level <200 kU/L. In two patients, diagnosis of pneumonia was postulated due to repeatedly positive sputum cultures for Af combined with typical radiographic features.
Subjects were excluded if they met any of the following criteria: i) intake of systemic glucocorticoids >5 days for ABPA diagnosis and/or antifungal therapy >5 days within the last 4 weeks; ii) failure to provide informed consent. Patients on standard therapy for Af diseases in CF (corticosteroid therapy and/or antifungal treatment) were excluded to enable a clear diagnosis and follow up examination after therapy.
For the available follow up measurements of Gm3, data >30 and <180 days after diagnosis and initiation of therapy were included if the patients were stable at that time and did not meet the diagnostic criteria for Af disease.
Af-Specific IgG (Gm3)
Af-specific IgG (Gm3) concentration was measured in our routine laboratory by the commercial ImmunoCAP system (Phadia, Thermo Fisher Scientific, USA), which uses automated fluorescent enzyme immunoassay (FEIA) technology. The m3 antigen is a crude extract from whole Af conidia and mycelia. According to manufacturer data, the reference value is <39 mg/L.
Statistical Analysis
Median and ranges were calculated for metrical variables. Distribution of data was assessed with Shapiro–Wilk test for normal distribution. For comparison of two groups t test or Mann–Whitney test was applied as appropriate. Comparison of more than two groups with normally distributed data sets was performed with one-way ANOVA including Tukey´s multiple comparison test. Not normally distributed data were analyzed by Kruskal Wallis test. Frequency and percentage were used for categorical variables. Associations of categorical variables were analyzed using chi-square test. Cut-off value determination for IgGm3 was based on receiver operating characteristic (ROC) analysis. A P value < 0.05 was accepted to indicate statistical significance. Data analysis were performed with GraphPad Prism version 8 (GraphPad Software).
Results
Baseline Characteristics
One hundred nine sera of patients with CF were analyzed for Af IgG (Gm3 ImmunoCAP), with 63 patients assigned to the control group, 22 patients to the bronchitis group, 18 patients to the ABPA group and six patients to the pneumonia group. The median age of all participants was 26 years (range: 6–69 years). The median BMI was 19.8 kg/m2, with the lowest BMI in the pneumonia group (17.5 kg/m2). The median percent predicted FEV1 was 54 (range: 16.0–133.1), with the highest median percent predicted FEV1 in the ABPA group (60) and the lowest median percent predicted FEV1 in the pneumonia group (22, p < 0,01). 24.8% of the patients had diabetes, with the highest proportion in the pneumonia group (66.7%, no statistical significance). The mean total IgG was 13.2 kU/L and was markedly higher in the pneumonia group (16.3 kU/L, no statistical significance). Co-colonization with Candida spp. was documented in 85.3% of all patients (control group 84.1%, bronchitis 90.9%, ABPA and pneumonia each 83.3%, no statistical significance). Detection of other fungal pathogens was documented in 11% of all patients (22.7% bronchitis, 5.6% ABPA, 0% pneumonia, no statistical significance) (see Table 1).
Af-Specific IgG (Gm3) Results
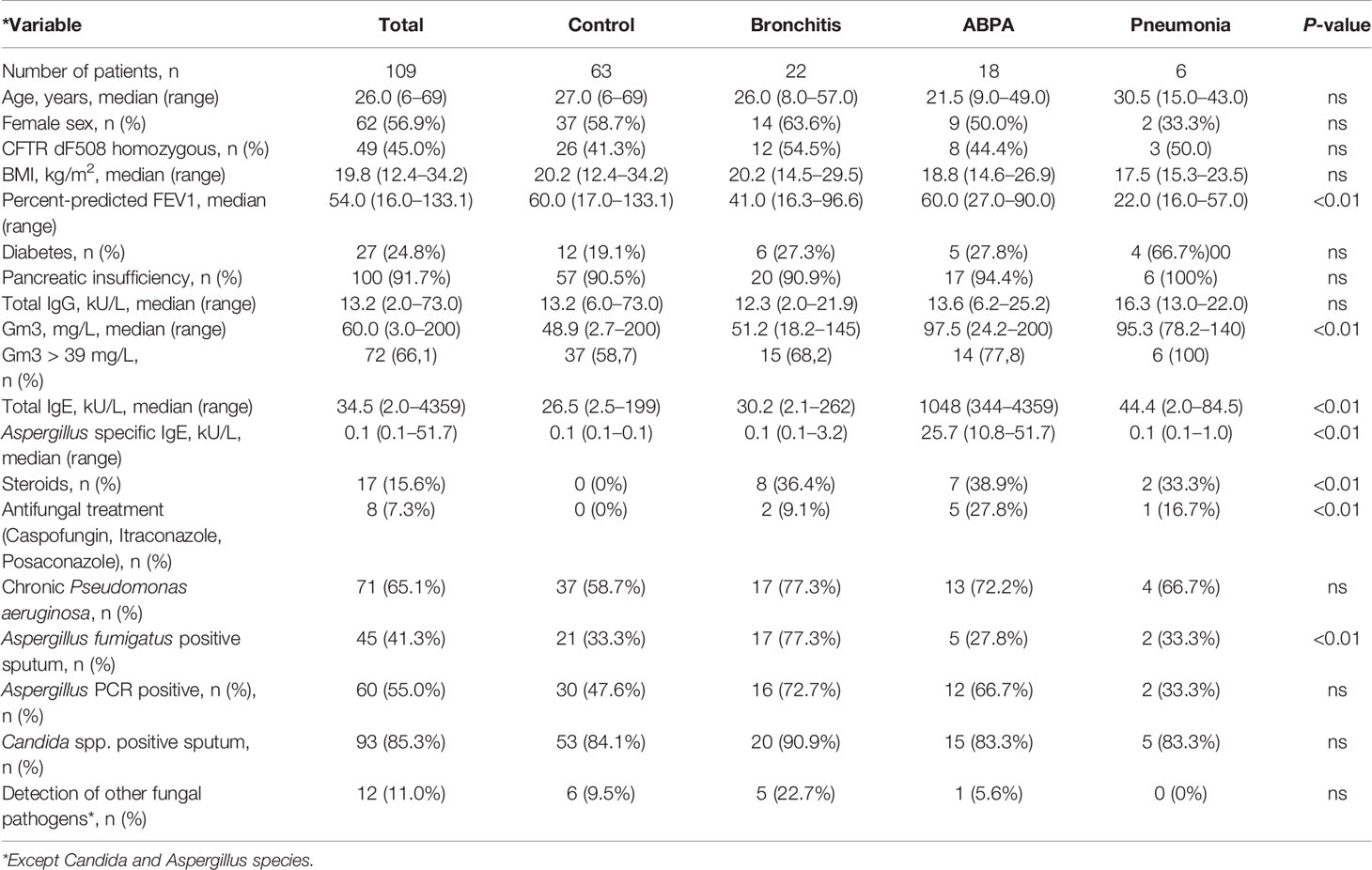
Median Gm3 for the control group was 48.9 mg/L ± 40.15 (2.7 - >200 mg/L), for the bronchitis group 51.2 mg/L ± 38.6 (18.2 - 145 mg/L), for the ABPA group 97.5 mg/L ± 56,34 (24.2 - >200 mg/L), and for the pneumonia group 95.3 mg/L ± 22.5 (78.2 - 140 mg/L, p < 0.01, (see Figure 1).
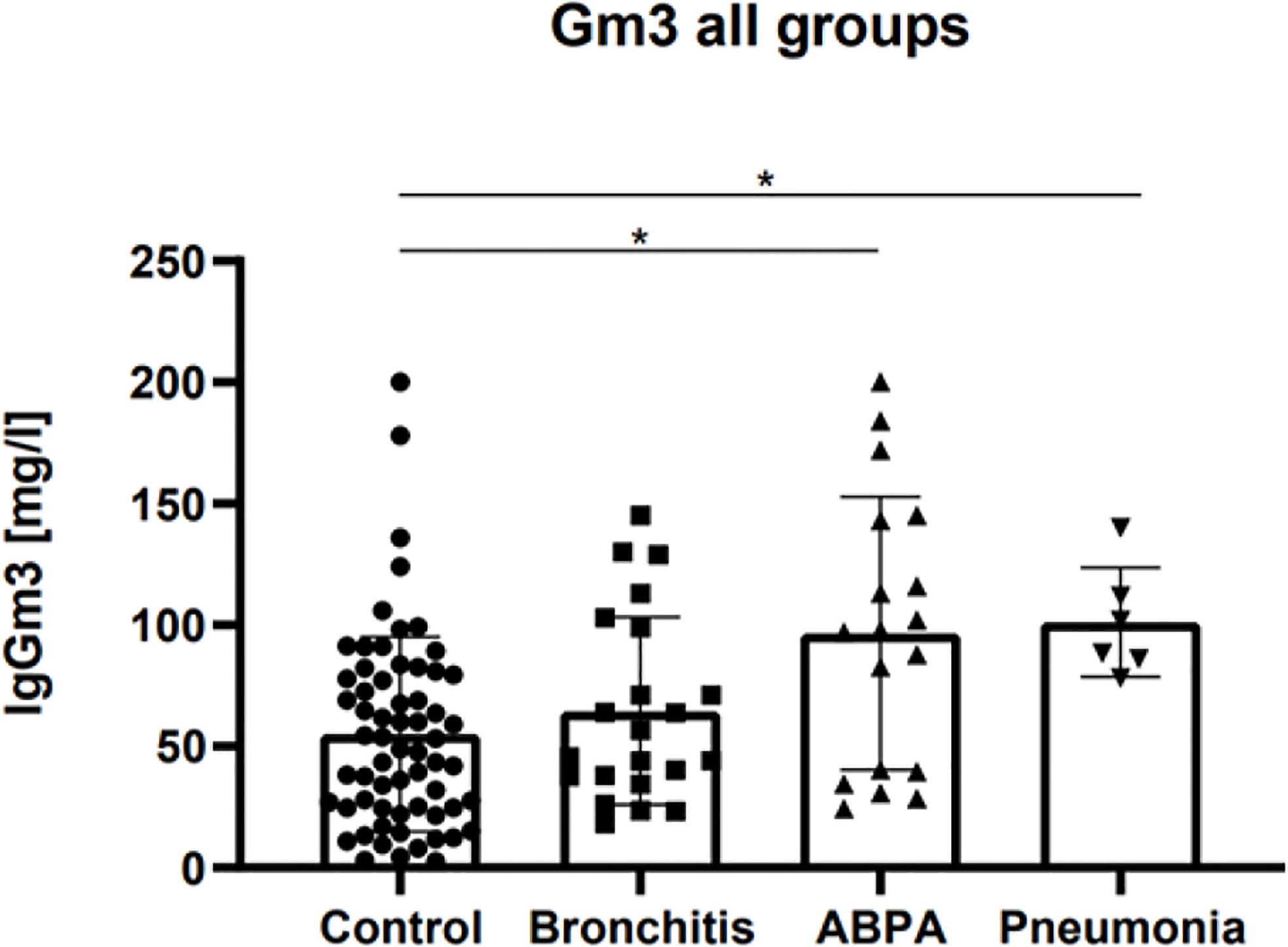
Figure 1 Gm3 (mg/L) for control group, Af bronchitis group, ABPA group and Af pneumonia group before treatment. Adjusted p-values: control vs. bronchitis: ns, control vs. ABPA: p <0.05, control vs. pneumonia: p <0.05. * represents the level of significance (p ≤ 0.05).
A statistically significant difference was found between median Gm3 of control vs. ABPA group (p < 0.05), and between median Gm3 of control vs Af pneumonia group (p < 0.05). No significant difference was found between median Gm3 of control vs. bronchitis group (see Figure 1).
In the Af bronchitis and ABPA group, several patients did not meet the manufacturer’s cut-off value of >39 mg/L: in the bronchitis group, 31.8% (n=7) patients had Gm3 levels below the cutoff, and in the ABPA group, 22.2% (n=4) patients had Gm3 levels below the cutoff. In contrast, in the control group, a majority of 58.7% (n=37) patients had Gm3 levels above the cutoff. In the Af pneumonia group, all patients had Gm3 levels above the manufacturer`s cut-off of >39 mg/L. (see Figure 1).
Follow-Up Gm3
Twenty-one patients could be enrolled for a follow-up examination. In the Af disease groups, it was assessed between 30 and 180 days after diagnosis. For the control group, mean Gm3 was assessed at two arbitrary points in time. The mean difference of the control group was 1.6 mg/L. For the bronchitis group, the mean Gm3 difference after initiation of therapy was 13.4 mg/L, and for the ABPA group, it was 12.6 mg/L (no statistical significance). In both the bronchitis and ABPA group, single patients showed a rise in Gm3 in the follow up (see Figure 2).
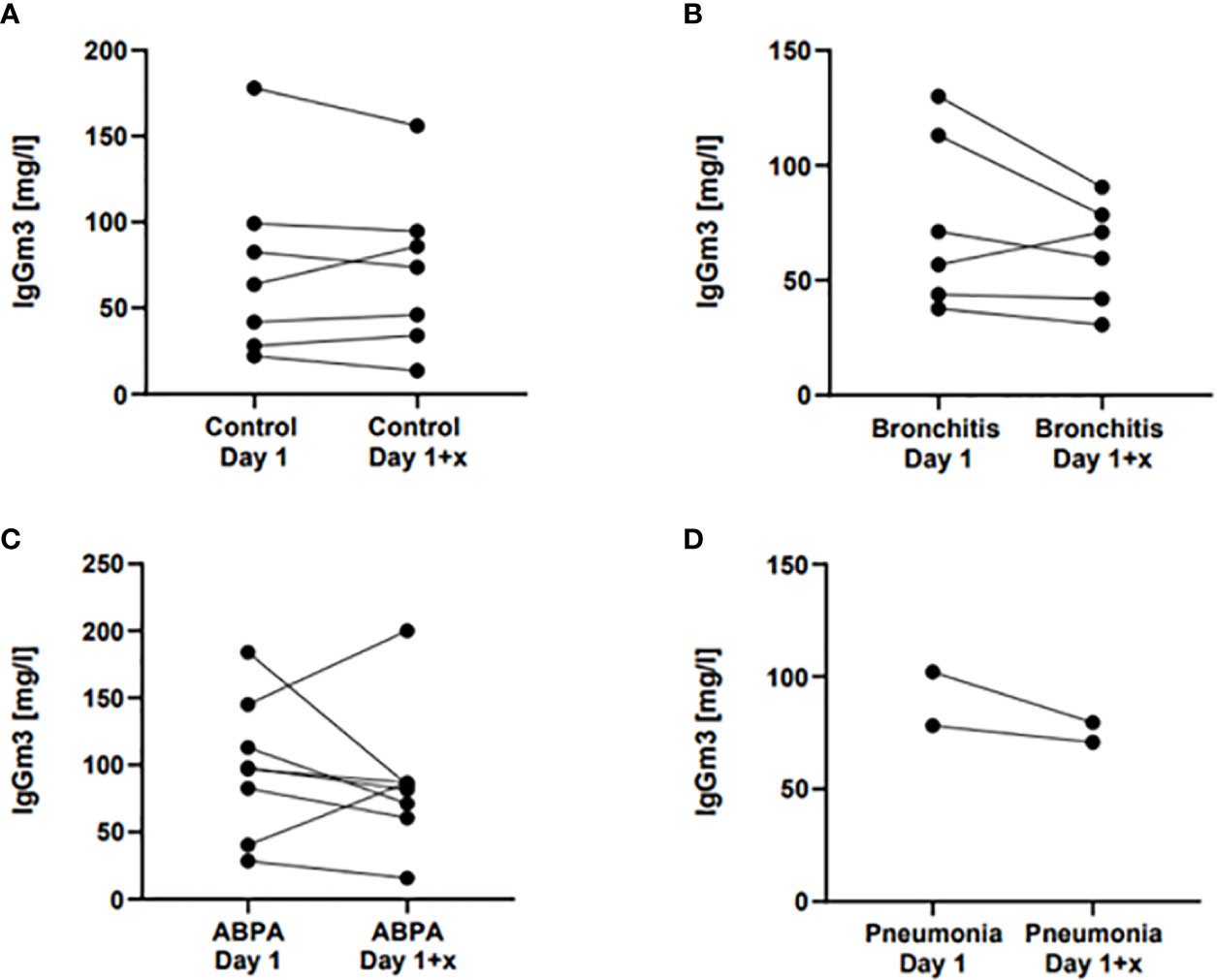
Figure 2 Gm3 follow-up measurements for (A) control group, (B) Af bronchitis group, (C) ABPA group and (D) Af pneumonia group, for each group at time of diagnosis (Day 1) and after treatment (Day 1 +x), for control group at any two consecutive times. Adjusted p-values: no statistical significance.
Cut-Off Determination
To determinate a cut-off value, only CF control subjects (n = 63) and CF patients with ABPA or pneumonia (n = 24) were included. ROC curve analysis showed an area under the curve of 0.7675 (95% Confidence Interval = 0.6524–0.88826, see Figure 3) A cut-off value of 78.05 mg/L for Gm3 was calculated to discriminate controls and patients with ABPA/pneumonia with a specificity (white square line) of 75% and a sensitivity (black square line) of 74.6% (see Figure 4).
Figure 3 ROC curve analysis showing the performance of Gm3 for distinguishing control group from ABPA/Af pneumonia group.
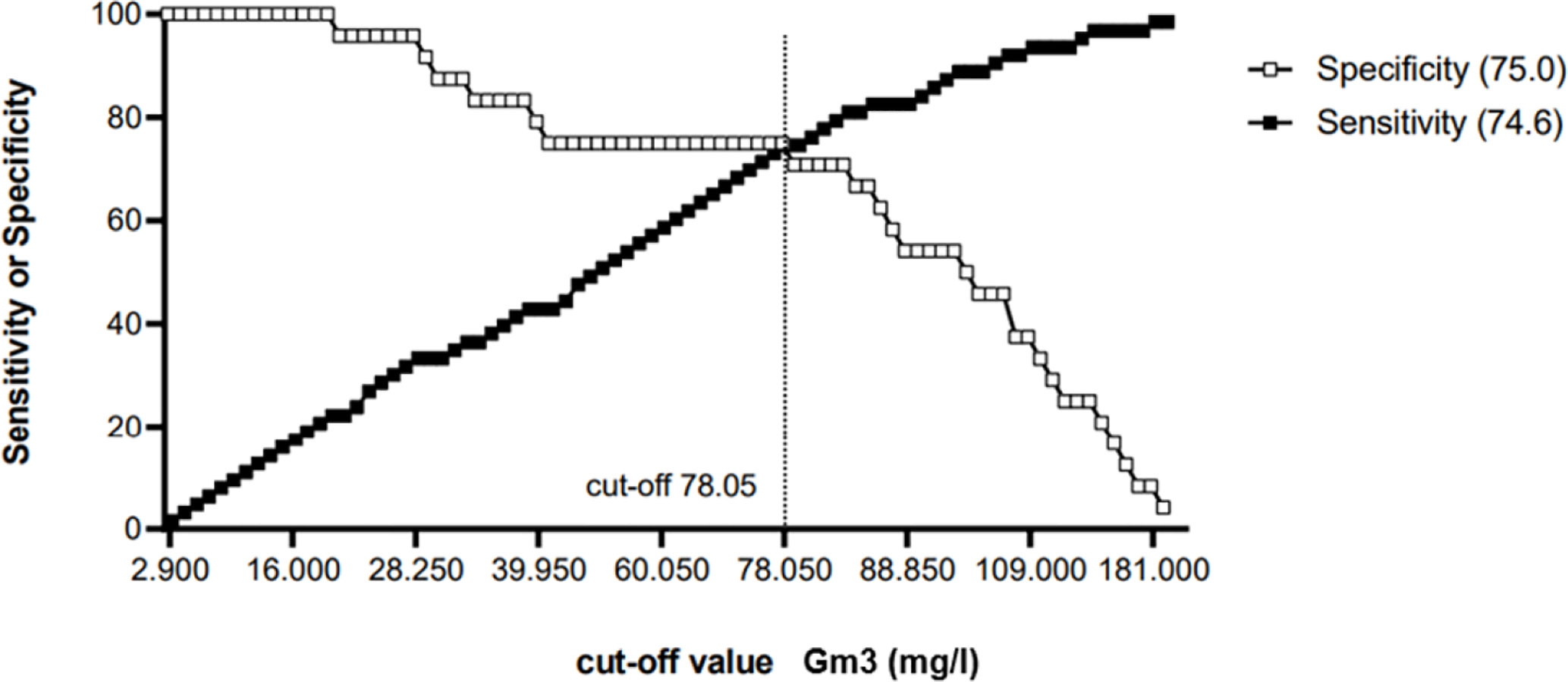
Figure 4 Cut-off-determination: Gm3 = 78.05 mg/L with a specificity (white square line) of 75% and a sensitivity (black square line) of 74.6%.
Discussion
In this study, we evaluated the utility of Af specific Gm3 for the diagnosis of Af associated diseases in CF. Mean Gm3 levels revealed a significant difference between control group vs. ABPA and vs. pneumonia group, but not vs. bronchitis group. Af specific IgG may therefore be a useful biomarker for acute ABPA and Af pneumonia in cystic fibrosis, but not for Af bronchitis in cystic fibrosis.
However, we also found a wide distribution of Gm3 in the control, bronchitis and ABPA group. Thus, for the diagnosis of ABPA and Af pneumonia in CF, Gm3 should only be interpreted alongside other biomarkers.
Interestingly, a significant proportion of the Gm3 of our control group exceeded the manufacturer`s cut-off-level of 39 mg/L. Due to the high exposure of the CF lung to respiratory pathogens, the immune response in CF—here in the form of Gm3—may fundamentally differ from that of healthy individuals. Specific cut-offs may therefore be required in CF to describe pathogen-mediated conditions. Positive and even high titers of Gm3 do not necessarily represent Af-associated disease. On the other hand, the pathogenic impact and the need for treatment of chronic colonization with Af in CF is still under discussion. In our cohort, 33% of the control group patients had Af positive sputum (see Table 1). There could be cases of bronchitis in the control group that did not meet the definition of bronchitis. Another reason for high Gm3 levels in the control group may be cross reactivity to other fungi colonizing the respiratory tract in CF, as m3 is the crude Af antigen and shows cross reactivities to other opportunistic fungi (Fukutomi et al., 2016). In our cohort 85.3% were co-colonized with Candida spp. and 11% with other fungi (see Table 1). If this problem could be overcome by using recombinant antigens, is unclear, because so far, no investigation has been performed under this aspect. The studies conducted with recombinant antigens to date show vastly different results (Nikolaizik et al., 1995; Kurup, 2005; Fricker-Hidalgo et al., 2010; Alghamdi et al., 2019). Finally, in some patients in the cohort of acute Af diseases, Gm3 exceeds the measurement range of 200 mg/L. Quantification above 200 mg/L could therefore improve sensitivity and specificity.
The non-significant difference in median Gm3 levels between control and bronchitis group could be explained by a predominantly endobronchial host-pathogen interaction in Af bronchitis, which may less likely lead to IgG production than in the more invasive forms of aspergillosis. This may also explain why a significant proportion of the bronchitis group had Gm3 levels below the manufacturer`s cut-off. On the other hand, also a small but still significant proportion of the ABPA group had Gm3 levels below the manufacturer`s cut-off, which may be due to a predominant Type 1 hypersensitivity reaction in these cases.
Our results of a Gm3 cut-off-level of 78.05 mg/L with a sensitivity of 75% and a specificity of 74.6% in Af pneumonia and ABPA in CF differ substantially from those found in other populations. Agarwal et al. examined Af specific Gm3 in asthmatic patients with ABPA and Af sensitization without ABPA and found a sensitivity of 88% and specificity of 100% when using a cut-off level of 26.9 mg/L ImmunoCAP Gm3 (Agarwal et al., 2017). In chronic pulmonary aspergillosis in non-CF-patients, Sehgal et al. found a sensitivity and specificity of 95.6% and 100% using a Gm3 cut-off of 27.3 mg/L (Sehgal et al., 2018). For simple aspergilloma in pulmonary tuberculosis patients, the same working group found a sensitivity of 63.5% and a specificity of 98.3% using a cut-off of 27.3 mg/L. However, the Gm3 cut-off-level is still under discussion, as a recent multicentric study found a median Gm3 of 21.3 mg/L and a 90% quantile for Gm3 of 78 mg/L in a population of 121 healthy donors (Raulf et al., 2019). The working group suggested that IgG values higher than at least 90% of the healthy donor group indicate elevated levels, which is more consistent with our findings of a cut-off-level of 78.05 mg/L.
Our follow-up data, where some patients showed a rise in Gm3 at a second point of time after diagnosis are consistent with the data of Agarwal et al. in asthmatic patients with ABPA, who observed an increase of Gm3 in 23.1% of the patients and a decrease in 23.1% (Agarwal et al., 2017). However, immunoglobulins are often maintained long-time following antigen contact, and, so far, no longitudinal data exist for titer progression of Gm3. Considering the small number of cases in our cohort, no statement can be made if and after what time Gm3 is suitable as a follow-up marker. Due to the low prevalence of Af diseases in CF, the suitability of Gm3 as a screening and outcome parameter should be investigated in a multicenter study.
Conclusion
Af-specific IgG may be a useful biomarker for acute ABPA and Af pneumonia, but not for Af bronchitis in CF. However, due to the large interindividual variability of Gm3, it should only be interpreted alongside other biomarkers. Therefore, due to its broad availability, it could be suitable as a biomarker for ABPA and Af pneumonia in CF, if the results can be supported by a larger multicenter cohort. Standardized, longitudinal studies and a better knowledge of underlying immunological mechanisms may improve its usability.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethikkommission Charité Universitätsmedizin Berlin. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
PE, CG, and CS contributed to the conception and design of the study. PE and CG organized the database. CG performed the statistical analysis. PE, CG, and CS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the German Federal Ministry of Education and Science (BMBF)—Project InfectControl 2020 (“ART4Fun”; 03ZZ0813E).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all participating patients and members of the CF centers involved in this study and the ARTForFun working group.
References
Agarwal R., Dua D., Choudhary H., Aggarwal A. N., Sehgal I. S., Dhooria S., et al. (2017). Role of Aspergillus fumigatus-specific IgG in diagnosis and monitoring treatment response in allergic bronchopulmonary aspergillosis. Mycoses 60, 33–39. doi: 10.1111/myc.12541
Alghamdi N. S., Barton R., Wilcox M., Peckham D. (2019). Serum IgE and IgG reactivity to Aspergillus recombinant antigens in patients with cystic fibrosis. J. Med. Microbiol 68, 924–929. doi: 10.1099/jmm.0.000991
Amin R., Dupuis A., Aaron S. D., Ratjen F. (2010). The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 137, 171–176. doi: 10.1378/chest.09-1103
Baxter C. G., Dunn G., Jones A. M., Webb K., Gore R., Richardson M. D., et al. (2013a). Novel immunologic classification of aspergillosis in adult cystic fibrosis. J. Allergy Clin. Immunol. 132, 560–566. doi: 10.1016/j.jaci.2013.04.007
Baxter C. G., Denning D. W., Jones A. M., Todd A., Moore C. B., Richardson M. D. (2013b). Performance of two Aspergillus IgG EIA assays compared with the precipitin test in chronic and allergic aspergillosis. Clin. Microbiol Infect. 19, E197–E204. doi: 10.1111/1469-0691.12133
Bell S. C., Mall M. A., Gutierrez H., Macek M., Madge S., Davies J. C., et al. (2020). The future of cystic fibrosis care: a global perspective. Lancet Respiratory Med. 8, 65–124. doi: 10.1016/S2213-2600(19)30337-6
Brandt C., Roehmel J., Rickerts V., Melichar V., Niemann N., Schwarz C. (2018). Aspergillus Bronchitis in Patients with Cystic Fibrosis. Mycopathologia 183, 61–69. doi: 10.1007/s11046-017-0190-0
Chrdle A., Mustakim S., Bright-Thomas R. J., Baxter C. G., Felton T., Denning D. W. (2012). Aspergillus bronchitis without significant immunocompromise. Ann. N Y Acad. Sci. 1272, 73–85. doi: 10.1111/j.1749-6632.2012.06816.x
Cohen T. S., Prince A. (2012). Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat. Med. 18, 509–519. doi: 10.1038/nm.2715
Delhaes L., Frealle E., Pinel C. (2010). Serum markers for allergic bronchopulmonary aspergillosis in cystic fibrosis: State of the art and further challenges. Med. Mycol 48 Suppl 1, S77–S87. doi: 10.3109/13693786.2010.514301
el-Dahr J. M., et al. (1994). Development of Immune Responses to Aspergillus at an Early Age in Children with Cystic Fibrosis. Am. J. Respir. Crlt Care Med. 150 (6 Pt 1), 1513–8. doi: 10.1164/ajrccm.150.6.7952609
Felton I. C., Simmonds N. J. (2014). Aspergillus and cystic fibrosis: old disease - new classifications. Curr. Opin. Pulm Med. 20, 632–638. doi: 10.1097/MCP.0000000000000106
Fricker-Hidalgo H., Coltey B., Llerena C., Renversez J. C., Grillot R., Pin I., et al. (2010). Recombinant allergens combined with biological markers in the diagnosis of allergic bronchopulmonary aspergillosis in cystic fibrosis patients. Clin. Vaccine Immunol. 17, 1330–1336. doi: 10.1128/CVI.00200-10
Fukutomi Y., Tanimoto H., Yasueda H., Taniguchi M. (2016). Serological diagnosis of allergic bronchopulmonary mycosis: Progress and challenges. Allergol Int. 65, 30–36. doi: 10.1016/j.alit.2015.08.004
Hafen GM H. D., Regamey N., Casaulta C., Latzin P. (2009). Allergic bronchopulmonary aspergillosis: the hunt for a diagnostic serological marker in cystic fibrosis patients. Expert Rev. Mol. Diagn 9, 157–164. doi: 10.1586/14737159.9.2.157
Hong G., Psoter K. J., Jennings M. T., Merlo C. A., Boyle M. P., Hadjiliadis D., et al. (2018). Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J. Cystic Fibrosis Off. J. Eur. Cystic Fibrosis Soc. 17, 624–630. doi: 10.1016/j.jcf.2018.01.008
Keown K., Abbott S., Kuzeljevic B., Rayment J. H., Chilvers M. A., Yang C. L. (2019). An investigation into biomarkers for the diagnosis of ABPA and aspergillus disease in cystic fibrosis. Pediatr. Pulmonol 54, 1787–1793. doi: 10.1002/ppul.24465
Kosmidis C., Denning D. W. (2015). The clinical spectrum of pulmonary aspergillosis. Thorax 70, 270–277. doi: 10.1136/thoraxjnl-2014-206291
Kousha M., Tadi R., Soubani A. O. (2011). Pulmonary aspergillosis: a clinical review. Eur. Respir. Rev. 20, 156–174. doi: 10.1183/09059180.00001011
Kurup V. P. (2005). Aspergillus antigens: which are important? Med. Mycol 43 Suppl 1, S189–S196. doi: 10.1080/13693780500064763
Latzin P., Hartl D., Regamey N., Frey U., Schoeni M. H., Casaulta C. (2008). Comparison of serum markers for allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur. Respiratory J. 31, 36–42. doi: 10.1183/09031936.00078107
Middleton P. G., Chen S. C., Meyer W. (2013). Fungal infections and treatment in cystic fibrosis. Curr. Opin. Pulm Med. 19, 670–675. doi: 10.1097/MCP.0b013e328365ab74
Moss R. B. (2010). Allergic bronchopulmonary aspergillosis and Aspergillus infection in cystic fibrosis. Curr. Opin. Pulmonary Med. 16, 598–603. doi: 10.1097/MCP.0b013e32833e24a6
Nikolaizik W. H., et al. (1995). Identification of allergic bronchopulmonary aspergillosis in cystic fibrosis patients by recombinant Aspergillus fumigatus I_a-specific serology. Am. J. Respir. Crlt Care Med. 152 (2), 634–9. doi: 10.1164/ajrccm.152.2.7633719
Pihet M., Carrere J., Cimon B., Chabasse D., Delhaes L., Symoens F., et al. (2009). Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis–a review. Med. Mycol 47, 387–397. doi: 10.1080/13693780802609604
Raulf M., Joest M., Sander I., Hoffmeyer F., Nowak D., Ochmann U., et al. (2019). Update of reference values for IgG antibodies against typical antigens of hypersensitivity pneumonitis. Allergo J. Int. 28, 192–203. doi: 10.1007/s40629-019-0099-x
Scheffold A., Schwarz C., Bacher P. (2018). Fungus-Specific CD4 T Cells as Specific Sensors for Identification of Pulmonary Fungal Infections. Mycopathologia 183, 213–226. doi: 10.1007/s11046-017-0229-2
Schwarz C., Bouchara J. P., Buzina W., Chrenkova V., Dmenska H., de la Pedrosa E. G. G., et al. (2018a). Organization of Patient Management and Fungal Epidemiology in Cystic Fibrosis. Mycopathologia 183, 7–19. doi: 10.1007/s11046-017-0205-x
Schwarz C., Brandt C., Whitaker P., Sutharsan S., Skopnik H., Gartner S., et al. (2018b). Invasive Pulmonary Fungal Infections in Cystic Fibrosis. Mycopathologia 183, 33–43. doi: 10.1007/s11046-017-0199-4
Sehgal I. S., Choudhary H., Dhooria S., Aggarwal A. N., Garg M., Chakrabarti A., et al. (2018). Diagnostic cut-off of Aspergillus fumigatus-specific IgG in the diagnosis of chronic pulmonary aspergillosis. Mycoses 61, 770–776. doi: 10.1111/myc.12815
Sehgal I. S., Dhooria S., Choudhary H., Aggarwal A. N., Garg M., Chakrabarti A., et al. (2019). Efficiency of A fumigatus-specific IgG and galactomannan testing in the diagnosis of simple aspergilloma. Mycoses 62, 1108–1115. doi: 10.1111/myc.12987
Shoseyov D. B. K., Conway S. P., Kerem E. (2006). Aspergillus Bronchitis in Cystic Fibrosis. Chest 130, 222–226. doi: 10.1378/chest.130.1.222
Stephenson A. L., Tom M., Berthiaume Y., Singer L. G., Aaron S. D., Whitmore G. A., et al. (2015). A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur. Respiratory J. 45, 670–679. doi: 10.1183/09031936.00119714
Stevens A. (2003). Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis—State of the Art: Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 37:Suppl 3:S225–64. doi: 10.1086/376525
Keywords: cystic fibrosis, aspergillosis, Aspergillus fumigatus, allergic bronchopulmonary aspergillosis, ABPA, Gm3, Aspergillus fumigatus-specific IgG
Citation: Eschenhagen P, Grehn C and Schwarz C (2021) Prospective Evaluation of Aspergillus fumigatus-Specific IgG in Patients With Cystic Fibrosis. Front. Cell. Infect. Microbiol. 10:602836. doi: 10.3389/fcimb.2020.602836
Received: 04 September 2020; Accepted: 03 December 2020;
Published: 22 January 2021.
Edited by:
Andy Mark Borman, Public Health England, United KingdomReviewed by:
Vishukumar Aimanianda, Institut Pasteur, FranceAdilia Warris, University of Exeter, United Kingdom
Copyright © 2021 Eschenhagen, Grehn and Schwarz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patience Eschenhagen, cGF0aWVuY2UuZXNjaGVuaGFnZW5AY2hhcml0ZS5kZQ==
 Patience Eschenhagen
Patience Eschenhagen Claudia Grehn
Claudia Grehn Carsten Schwarz
Carsten Schwarz