
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 28 January 2021
Sec. Microbes and Innate Immunity
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.596023
This article is part of the Research Topic Microbial Modulation of Host Programmed Cell Death View all 4 articles
 Jonathan G. Williams1,2
Jonathan G. Williams1,2 Diane Ly1,2
Diane Ly1,2 Nicholas J. Geraghty1,2
Nicholas J. Geraghty1,2 Jason D. McArthur1,2
Jason D. McArthur1,2 Heema K. N. Vyas1,2
Heema K. N. Vyas1,2 Jody Gorman1,2
Jody Gorman1,2 James A. Tsatsaronis3
James A. Tsatsaronis3 Ronald Sluyter1,2
Ronald Sluyter1,2 Martina L. Sanderson-Smith1,2*
Martina L. Sanderson-Smith1,2*Invasive infections due to group A Streptococcus (GAS) advance rapidly causing tissue degradation and unregulated inflammation. Neutrophils are the primary immune cells that respond to GAS. The neutrophil response to GAS was characterised in response to two M1T1 isolates; 5448 and animal passaged variant 5448AP. Co-incubation of neutrophils with 5448AP resulted in proliferation of GAS and lowered the production of reactive oxygen species when compared with 5448. Infection with both strains invoked neutrophil death, however apoptosis was reduced in response to 5448AP. Both strains induced neutrophil caspase-1 and caspase-4 expression in vitro, with inflammatory caspase activation detected in vitro and in vivo. GAS infections involving strains such as 5448AP that promote an inflammatory neutrophil phenotype may contribute to increased inflammation yet ineffective bacterial eradication, contributing to the severity of invasive GAS infections.
Invasive infections due to the obligate human pathogen Streptococcus pyogenes (group A Streptococcus; GAS) are characterised by unregulated inflammation and high mortality (Davies et al., 1996; Svensson et al., 2000; O’Grady et al., 2007; O’Loughlin et al., 2007; Lamagni et al., 2008; Nelson et al., 2016). The highly virulent GAS M1T1 clone 5448 is well studied (Tart et al., 2007; Walker et al., 2007; Aziz and Kotb, 2008). The propensity of M1T1 GAS to cause invasive infections is due, in part, to the increased frequency of spontaneous mutations in the two-component control of virulence regulatory system (covRS) (Walker et al., 2007). CovRS mutations result in differential expression of numerous virulence factors that are used by GAS as a defence against the host immune response (Sumby et al., 2005; Sumby et al., 2006; Walker et al., 2007; Kilsgård et al., 2016). Compelling evidence describes the resistance of covS mutant GAS (mutation to the sensor protein of covRS) to killing by human neutrophils (polymorphonuclear leukocytes, PMNs) (Walker et al., 2007; Ato et al., 2008; Maamary et al., 2010). 5448AP (animal passaged) is one M1T1 covS mutant strain that shows resistance to killing by human neutrophils, has increased bacterial dissemination and results in decreased survival in mouse models of infection (Walker et al., 2007; Maamary et al., 2010; Fiebig et al., 2015). Due to these characteristics, 5448AP can be used as a model strain to explore the host response to invasive GAS infection.
Neutrophils are the primary innate immune cell-type that defend against bacterial pathogens, with their presence contributing to the overarching inflammatory response (Kobayashi et al., 2018). Proteins of neutrophil origin are highly abundant at sites of invasive GAS infection (Edwards et al., 2018). The neutrophil life cycle is tightly regulated to avoid the release of cytotoxic contents that can damage surrounding tissue and contribute further to inflammation (Epstein and Weiss, 1989; Faurschou and Borregaard, 2003). Under normal conditions cytotoxic reactive oxygen species (ROS) produced by neutrophils kill phagocytosed bacteria (Nguyen et al., 2017). An increased production of ROS signals for the induction of the anti-inflammatory cell death pathway apoptosis (Lundqvist-Gustafsson and Bengtsson, 1999). During apoptosis, neutrophils are carefully decommissioned, ensuring no unregulated release of intracellular material, then cleared by a secondary phagocyte, macrophages, through a process known as efferocytosis (Bratton and Henson, 2011). The cleavage of caspase-3 to the p17 form denotes the execution of apoptosis (Nicholson et al., 1995). Increased neutrophil apoptosis is described during GAS infection (Kobayashi et al., 2003). Pro-inflammatory neutrophil death has also been reported during GAS infection (Tsatsaronis et al., 2015). One form of pro-inflammatory death is pyroptosis, involving the formation of an inflammasome and subsequent caspase-1 or caspase-4 activation, culminating in cell-lysis (Sollberger et al., 2012; Man and Kanneganti, 2016). Deregulation of (or excessive) pro-inflammatory neutrophil death may therefore contribute further to inflammatory pathology, as demonstrated during infection with other pathogens, including Pseudomonas aeruginosa (Greenlee-Wacker et al., 2017; Ryu et al., 2017) and Staphylococcus aureus (Ryu et al., 2017; Ghimire et al., 2018). Inflammation is also heavily influenced by cell cytokine production, where increased release can invoke neutrophil dysfunction, affecting neutrophil survival, antibacterial function or even migration (Norrby-Teglund et al., 2000; Brown et al., 2006; Kovach and Standiford, 2012; Kipnis, 2013). Previous work outlines how GAS take advantage of inadequate or exacerbated immune responses and focuses on the bacterial mechanisms involved (Cole et al., 2011). Although models to investigate leukocyte inflammasome activation have been proposed (Tran et al., 2019), a limited number of studies have investigated the neutrophil response to GAS specifically (Kobayashi et al., 2003).
Previous work with emm98 GAS indicates that GAS strains harbouring a covS mutation elicit non-apoptotic neutrophil cell death, and that this is associated with increased inflammatory markers in a mouse model of GAS infection (Tsatsaronis et al., 2015). It is not known if this occurs for other GAS serotypes. Furthermore, the expression of inflammatory caspases by neutrophils, and changes to neutrophil cluster of differentiation (CD) marker expression in response to GAS infection, have not been reported. Here, using an in vitro model, we temporally describe the effect two M1T1 GAS strains have upon human neutrophil death, signalling and inflammatory profile. We further characterise this interaction in murine neutrophils, using an in vivo model of intradermal invasive GAS infection. Additionally, we identify differences in the neutrophil response to GAS due to covS mutation. We hypothesise that changes to neutrophil function by GAS can promote invasive GAS infection by exacerbating the inflammatory response and decreasing antibacterial capacity.
All experiments involving the use of human blood were conducted with informed consent of healthy volunteers, approved and authorised by the University of Wollongong Human Research Ethics Committee (Protocol HE08/250). All experiments involving the use of animals were approved and authorised by the University of Wollongong Animal Ethics Committee (Protocol AE18/10).
Escherichia coli MC1061 were grown in Luria–Bertani Broth (LB) at 37°C with constant shaking. M1T1 S. pyogenes invasive clinical isolate 5448 (emm1) and hypervirulent animal passaged 5448AP (containing a non-functional control of virulence regulator mutation) have been described previously (Chatellier et al., 2000; Aziz et al., 2004). GAS strains were routinely cultured at 37°C on horse-blood agar (Oxoid, Basingstoke, United Kingdom) and enumerated on yeast supplemented (1% w/v) Todd-Hewitt (THY, Bacto Laboratories, Mt Pritchard, Australia) agar. Static overnight cultures were grown at 37°C in THY then sub-inoculated (1:10) into fresh THY and grown to mid-logarithmic phase, antibiotics were added when required (100 μg/ml ampicillin and 200 μg/ml kanamycin). Bacterial pellets were washed twice with phosphate buffered saline (PBS) then resuspended at the specified multiplicity of infection (MOI) and assay specific media conditions.
In brief, stable GFP expression by GAS was created by synthesizing the ribosomal binding site (RBS) and gfp gene from pDCerm-GFP (Ly et al., 2014) into the pUC57 plasmid (GenScript, Piscataway, NJ, USA), resulting in a pUC57-RBSGFP plasmid. RBS and gfp from pUC57-RBSGFP were then sub-cloned using NotI into the toxin–antitoxin (TA) stabilized expression plasmid pLZ12Km2-P23R:TA, kindly provided by Associate Professor Thomas Proft (University of Auckland, New Zealand) (Loh and Proft, 2013), to produce pLZ12Km2-P23R-TA : GFP. The resultant plasmid system utilises the Streptococcal ω–ϵ–ζ TA cassette to achieve segregational plasmid stability under non-selective conditions (Lioy et al., 2010).
Specifically, plasmids pLZ12Km2-P23R:TA and pUC57-RBSGFP (engineered with RBS and gfp from pDCerm-GFP) were first transformed into chemically competent E. coli MC1061 via electroporation. The plasmids were then retrieved and purified from E. coli cultures using a Wizard® Plus SV Minipreps DNA Purification System (Promega, Madison, WI, USA). The pLZ12Km2-P23R:TA plasmid was digested with 20 U NotI enzyme and further treated with 5 U shrimp alkaline phosphatase. The pUC57-RBSGFP plasmid was also incubated with 20 U NotI to excise the RBS and gfp gene from pUC57-RBSGFP, and these were then ligated into digested pLZ12km2-P23R:TA plasmid. The resulting plasmid, pLZ12Km2-P23R-TA : GFP, was then transformed and cloned in MC1061 E. coli, and later extracted and purified. pLZ12Km2-P23R-TA : GFP was transformed into GAS using standard GAS electroporation techniques (McLaughlin and Ferretti, 1995). Transformed GAS was confirmed for GFP expression via flow cytometry.
Venous human blood was drawn into 10 ml lithium heparin-coated Vacutainer (Benton Dickson, Franklin Lakes, NJ, USA) tubes and layered over equal volumes of Polymorphprep (Axis Shield, Oslo, Norway) and centrifuged as per manufacturer’s instructions. The resulting layer of neutrophils was isolated and erythrocytes hypotonically lysed. Isotonic concentration was restored with Hank’s Balanced Salt solution (without Ca2+ or Mg2+, Corning Inc., Corning, NY, USA). Prior to experimentation neutrophils were resuspended at specified concentrations in complete medium, Roswell Park Memorial Institute (RPMI)-1640 medium (Life Technologies, Carlsbad, CA, USA) containing 2% heat-inactivated autologous plasma and 2 mM L-glutamine (Life Technologies), unless otherwise stated. Neutrophil viability was assessed via trypan blue (Sigma-Aldrich, St. Louis, MO, USA) staining and neutrophil purity was assessed using a Benton Dickson LSR Fortessa X-20 flow cytometer via distinct forward and side scatter profiles or CD66b-peridinin chlorophyll protein (PerCP)/Cy5.5 (clone G10F5, BioLegend, San Diego, CA, USA) expression. Neutrophils were maintained at room temperature throughout processing.
Purified human neutrophils were seeded in either 96-well plates at MOI (GAS:neutrophil) 1:10 (survival and proliferation) or 10:1 (ROS production and cytokine release), 24-well plates at 10:1 (phagocytosis, Annexin-V/viability staining, CD expression and FAM-FLICA caspase-1 activation) or six-well plates at 10:1 (immunoblotting) and incubated at 37°C in 5% CO2.
Human neutrophils were co-cultured with GAS or lysed via three freeze-thaw cycles prior to incubation. To block phagocytosis neutrophils were preincubated with 10 μM cytochalasin D (Cayman Chemicals, Ann Arbor, MI, USA) in complete medium at 37°C for 30 min prior to infection. At indicated time points neutrophils were hypotonically lysed and surviving bacteria serially diluted before plating on THY agar.
Human neutrophils were co-cultured with GAS expressing GFP for the times indicated in complete medium. Cells were removed and washed twice with 10% (v/v) heat-inactivated foetal bovine serum (FBS, Bovogen Biologicals, Keilor East, Australia) diluted in PBS, followed by data acquisition via flow cytometry.
ROS production was assessed as previously described (Kobayashi et al., 2003). Briefly, prior to infection, human neutrophils were incubated in complete medium containing 25 μM dichlorofluorescein (DCF, Molecular Probes, Eugene, OR, USA) for 45 min at room temperature (RT). ROS production was measured fluorometrically (ex485 nm em520 nm) using a POLARstar Omega plate reader (BMG Labtech, Ortenberg, Germany).
For human neutrophil in vitro assays, flow cytometry data was acquired using a BD LSR Fortessa X-20 (Benton Dickson) with excitation lasers; violet (405 nm), blue (488 nm), yellow/green (561 nm) and red (640 nm). Bandpass filters for Zombie Aqua Fixable Viability dye (525/50), fluorescein isothiocyanate (FITC)/GFP/FAM FLICA (525/50), PerCP-Cy5.5 (695/40) R-phycoerythrin (PE, 586/15), propidium iodide (PI, 610/20), PE-Cy7 (780/60) and allophycocyanin (APC, 670/30) were used. For murine infection studies, flow cytometry data was acquired using an Invitrogen Attune NxT (Invitrogen, Carlsbad, CA) with excitation lasers; violet (405 nm), blue (488 nm), yellow (561 nm) and red (638 nm). Bandpass filters VL1 (440/50), VL2 (512/25), BL1 (530/30), YL1 (585/16), YL3 (695/40) and RL2 (720/30) were used. Data was analysed using FlowJo software V10.6.1 (Tree Star Inc., Ashland, OR).
To assess cell viability human neutrophils at indicated times were washed with PBS and incubated with Zombie Aqua Fixable Viability dye (BioLegend) for 15 min at RT. Cells were washed again once with PBS, then 10% (v/v) FBS in PBS and stained with Annexin-V-FTIC (BioLegend) for 15 min at RT. Cells were analysed immediately via flow cytometry.
Following co-culture in the presence or absence of GAS, human neutrophil lysates were prepared in RIPA buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris, 1.0% (v/v) Triton X-100, 0.1% (w/v) SDS, 0.5% (w/v) sodium deoxycholate, 2 x complete protease inhibitor cocktail (Roche, Basel, Switzerland), 1 mM phenylmethylsulphonyl fluoride, 5 mM sodium pyrophosphate, 5 mM sodium molybdate and 5 mM β-glycerophosphate) and incubated for 30 min on ice with intermittent vortexing. Soluble fractions were separated via centrifugation at 4°C, snap frozen in liquid N2 and stored at −80°C. Protein concentrations were determined by comparison to bovine serum albumin standards using the DC Protein Assay (Bio-Rad, Hercules, CA, USA) and absorbance (A750nm) measured using a POLARstar Omega plate reader. Neutrophil lysates (20 μg) were separated on 4–20% TGX Stain-Free protein gels (Bio-Rad) as per the manufacturer’s running conditions then activated for 5 min under UV to determine total protein (Bio-Rad ChemiDoc XR, Image Lab Software). Proteins were transferred to PVDF membranes (Bio-Rad), blocked, then incubated overnight at 4°C with caspase-1 polyclonal (1:1000, #2225), caspase-3 polyclonal (1:1,000, #9662), caspase-4 polyclonal (1:1,000, #4450) or caspase-8 monoclonal (1:1000, #4790) antibodies (Cell Signalling Technology, Danvers, MA, USA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal antibody (1:10,000, #ab181602, Abcam, Cambridge, United Kingdom). PVDF membranes were washed thrice between incubations for 5 min with Tris-buffered saline containing 0.1% (v/v) Tween 20. PVDF membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000, Invitrogen) for 1 h at RT and detected using Clarity or Clarity Max Western ECL Blotting Substrate (Bio-Rad) and imaged (Amersham AI600, GE Healthcare, Chicago, IL, USA). Bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA) as area under the peak and normalised over UV determined total protein.
Caspase activation was measured using the FAM-FLICA Caspase-1 Assay Kit (ImmunoChemistry Technologies, Bloomington, MN, USA), which detects active caspase-1, -4 and -5, as per the manufacturer’s instructions. In brief, following co-culture of human neutrophils with GAS, cells were removed and incubated in serum free medium (RPMI-1640) containing 1 x FAM-YVAD-FMK for 60 min at 37°C. Cells were then analysed for caspase-1 activation via flow cytometry.
Human neutrophil supernatants were at indicated times during GAS infection, snap frozen with liquid N2 and stored at −80°C for a period no longer than 5 days. Neutrophils were assessed for the release of cytokines IL-1β, IL-8, IL-18 and TNF-α using the LEGENDplex™ human inflammation panel bead-based immunoassay (BioLegend) and flow cytometry as per manufacturer’s instructions. In brief, samples and standards were incubated with beads and detection antibodies for 120 min at room temperature, with shaking (600 rpm) protected from light. PE-conjugated streptavidin was then added and incubated for a further 30 min at room temperature, with shaking (600 rpm) protected from light. Beads were washed twice with LEGENDplex™ wash buffer and data collected via flow cytometry. Beads were initially gated upon FSC-A and SSC-A (A and B populations). Cytokines have signature APC fluorescence with quantitative expression determined using PE fluorescence in comparison to cytokine standards. Data was analysed using the LEGENDplex™ software V8.0 (VigeneTech Inc., Carlisle, MA, USA).
Human neutrophil cell surface CD expression was assessed during GAS infection. Neutrophils at indicated times were washed with PBS then routinely incubated with Zombie Aqua Fixable Viability dye for 15 min at RT. Cells were washed once again in PBS, followed by 10% (v/v) FBS in PBS. Neutrophils were stained with fluorochrome-conjugated antibodies CD11b-FITC (Clone ICRF44), CD31-PE/Cy7 (Clone WM59), or CD66b-PerCP/Cy5.5 (Clone G10F5) (BioLegend) and CD16-FITC (Clone 3G8, Benton Dickson) for 15 min at RT. Neutrophils were washed with 10% (v/v) FBS in PBS then analysed via flow cytometry.
Intradermal GAS challenge of C57BL/6J mice has been described previously (Maamary et al., 2010; Ly et al., 2014; Tsatsaronis et al., 2014). In brief, equal numbers of 6–8 week old male and female C57BL/6J mice (Australian BioResources, Moss Vale, Australia) were anesthetised via isoflurane inhalation, followed by two intradermal injections into shaved left and right flanks with either 1 x 108 CFU of mid-logarithmic GAS or 100 µl sterile 0.7% (w/v) saline. Mice were euthanised via slow-fill CO2 asphyxiation at 6 or 24 h post-infection. Blood collected via cardiac puncture was separated into two aliquots for serum and flow cytometric analysis. Serum was collected by clotting for 1 h at RT then placed on ice until centrifugation at 1200 x g for 10 min at 4°C, then immediately stored at −80°C until use. Remaining blood samples were mixed with 20 µl 0.5% (w/v) sodium citrate per ml of blood and placed on ice. Equal volume of PBS was added to the blood sample and centrifuged at 350 x g for 5 min. For erythrocyte lysis, samples were incubated with 1 ml of ammonium-chloride-potassium lysing buffer (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2CO3, pH 7.3) for 5 min with gentle agitation and centrifuged at 350 x g for 5 min. This process was repeated, and cells were then washed with PBS and resuspended in 500 µl PBS and placed on ice. Additionally, sites of infection were lavaged with 1 ml of sterile 0.7% (w/v) saline, and fluid collected from both right and left flanks was pooled to increase cell numbers. Pooled samples were kept cold until centrifugation at 350 x g for 5 min, resuspended in 500 µl of PBS and placed on ice. Both blood and lavage fluid samples were analysed via flow cytometry. Alternatively, bacterial load was determined by plating on horse blood agar and CFU determined by colony enumeration.
Cell viability was assessed as described above. To assess caspase-1 activation and CD expression, cells were initially washed with 1 ml of PBS, followed by 1 ml of 10% (v/v) FBS in PBS. Cells were then centrifuged and simultaneously stained with 1 x 660-YVAD-FMK (FLICA 660 Caspase-1 assay kit, ImmunoChemistry Technologies) for inflammatory caspase activation and with fluorochrome-conjugated antibodies CD45-BV421 (clone 30-F11), CD11b-PE/Cy5 (clone M1-70) and Ly-6G-PE (clone 1A8) (BioLegend) and CD16-FITC (clone AT154-2, Bio-Rad) in RPMI 1640 medium for 30 min at RT. Cells were washed with 10% (v/v) FBS in PBS and analysed via flow cytometry.
IL-1β release was measured in murine serum using the LEGENDplex™ mouse inflammation panel bead-based immunoassay (BioLegend) and flow cytometry as described above except shaking was performed at 800 rpm.
Graphs were created using Prism 6 (GraphPad Software Inc., San Diego, CA, USA) and statistical analysis was performed using Prism 6 and IBM SPSS Statistics 25 (IBM® corporation, Armonk, NY, USA). Data was analysed using matched one-way and two-way ANOVA to determine significant differences and adjusted using Tukey HSD corrections or using the Holm-Šídák approach (Figures 1A, C, D, 5F and S1D–E). Two-way ANOVA ‘p’ values represent interaction (treatment*time) or are specified as treatment or time. To account for the nested nature of the human neutrophil Annexin-V/Zombie Aqua and CD data with repeated measures over time in the same donor, a linear mixed model was used to determine significant interaction between treatment and time. Post-hoc tests were performed by multiple one-way ANOVA at each time point where multiple comparisons were adjusted using Tukey HSD corrections. In vivo data was analysed by two-way ANOVA where differences are shown between treatment and time, also using Tukey HSD corrections. Two-way ANOVA ‘p’ values represent interaction (treatment*time) or are specified as treatment or time. Post–hoc tests were only applied to significant factors. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
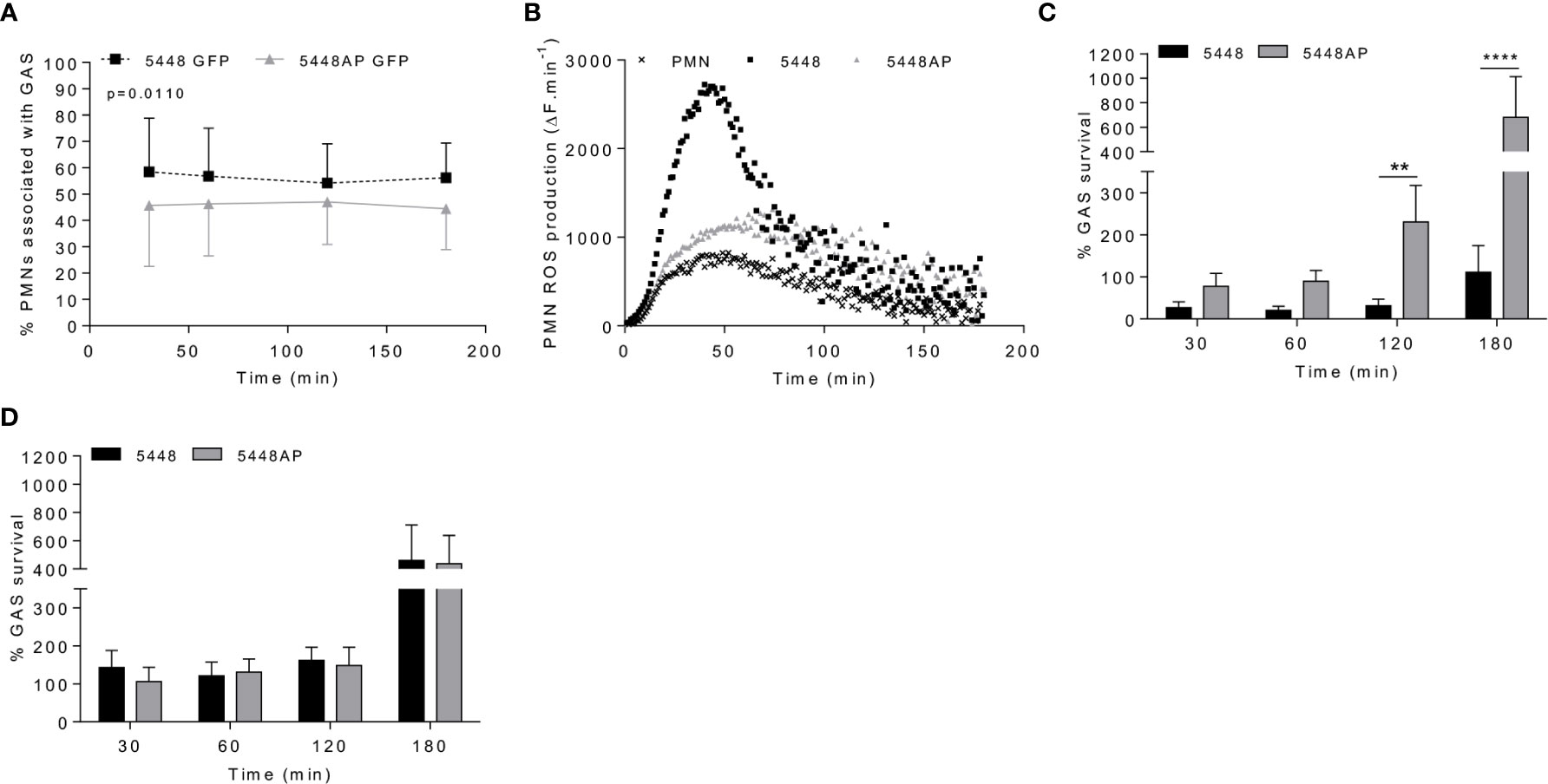
Figure 1 Group A Streptococcus (GAS) persistence and proliferation occurs during a dampened neutrophil reactive oxygen species (ROS) response. (A) Association of fluorescent GAS strains with human neutrophils (PMNs, n=6 donors), analysed via flow cytometry (Figures S1A, B). (B) Infection of human neutrophils with GAS invokes ROS production. Representative of triplicate measurements from three separate experiments (n=3 donors), shown as mean change in fluorescence units over time (ΔF.min-1). GAS strains were incubated in the presence of (C) human neutrophils (n=8 donors) or (D) lysed human neutrophils (n=4 donors) over 180 min with surviving bacterial concentration determined as percentage of inoculum. Results are the pooled means ± SD (of triplicate measurements for panels C, D). Two-way ANOVA ‘p’ values represent interaction (treatment*time) where post-hoc analysis was performed using the Holm-Šídák approach. **p < 0.01 and ****p < 0.0001.
Neutrophil phagocytic ability is a predominant and well-established mechanism of immune defence. Both GAS strains 5448 and 5448AP rapidly associated with human neutrophils, with near maximal association observed within 30 min (Figures 1A and S1A–C). Higher percentages of neutrophils were associated with 5448 compared to 5448AP (Figure 1A). 5448 and 5448AP infection induced ROS production in neutrophils, though increased ROS production was observed during 5448 infection when compared to uninfected and 5448AP infected neutrophils (Figure 1B). As such, the rate of neutrophil ROS production was significantly increased compared to the neutrophil only control during 5448, but not 5448AP, infection (p<0.01, Figure S1D), suggesting dampened antibacterial function in neutrophils in response to 5448AP. This difference in GAS-induced ROS production inversely corresponded to GAS proliferation in the presence of neutrophils, with high numbers of 5448AP observed compared to 5448 over 180 min (Figure 1C). Differential GAS proliferation required viable neutrophils, as 5448 and 5448AP proliferation in the presence of neutrophil lysates was reduced and similar for both strains over 180 min (Figure 1D). Finally, incubation of neutrophils with the phagocytosis inhibitor cytochalasin D significantly increased 5448, but not 5448AP, survival (p<0.001, Figure S1E), indicating that 5448 killing is predominantly mediated via a phagocytic-related pathway. Collectively, these data indicate that compared to 5448, 5448AP associates less readily with human neutrophils and is less readily phagocytosed. In addition, neutrophils show reduced ROS production and bacterial killing in response to 5448AP leading to increased survival of this strain.
GAS can induce death of human neutrophils (Kobayashi et al., 2003; Tsatsaronis et al., 2015), however the mechanism remains poorly described. Phosphatidylserine (PS) exposure is a hallmark of the induction of multiple death pathways including apoptosis and pyroptosis (Wang et al., 2013; Man and Kanneganti, 2016). Human neutrophils were incubated with 5448, 5448AP or in the absence of GAS then the binding of annexin-V (AV), as a measure of PS exposure, and the uptake of Zombie Fixable Viability dye (Z), as a measure of membrane integrity, were analysed by flow cytometry (Figure 2). Cells were defined as viable (AV−Z−), PS exposed (AV+Z−) or dead (AV+Z+) (Figure 2C). Neutrophils incubated without GAS remained viable over 180 min (Figure 2D), with 13% exposing PS (Figure 2E) and 3% dead after this time (Figure 2F). In contrast, incubation of neutrophils with either 5448 or 5448AP resulted in increased PS exposure and neutrophil cell death over 180 min, with significantly more neutrophil cell death during 5448 infection compared to 5448AP infection at 30 and 60 min (p<0.05, Figure 2F). Delay in the initial induction of death in response to 5448AP suggests extended neutrophil viability during the early stages of infection (30–60 min).
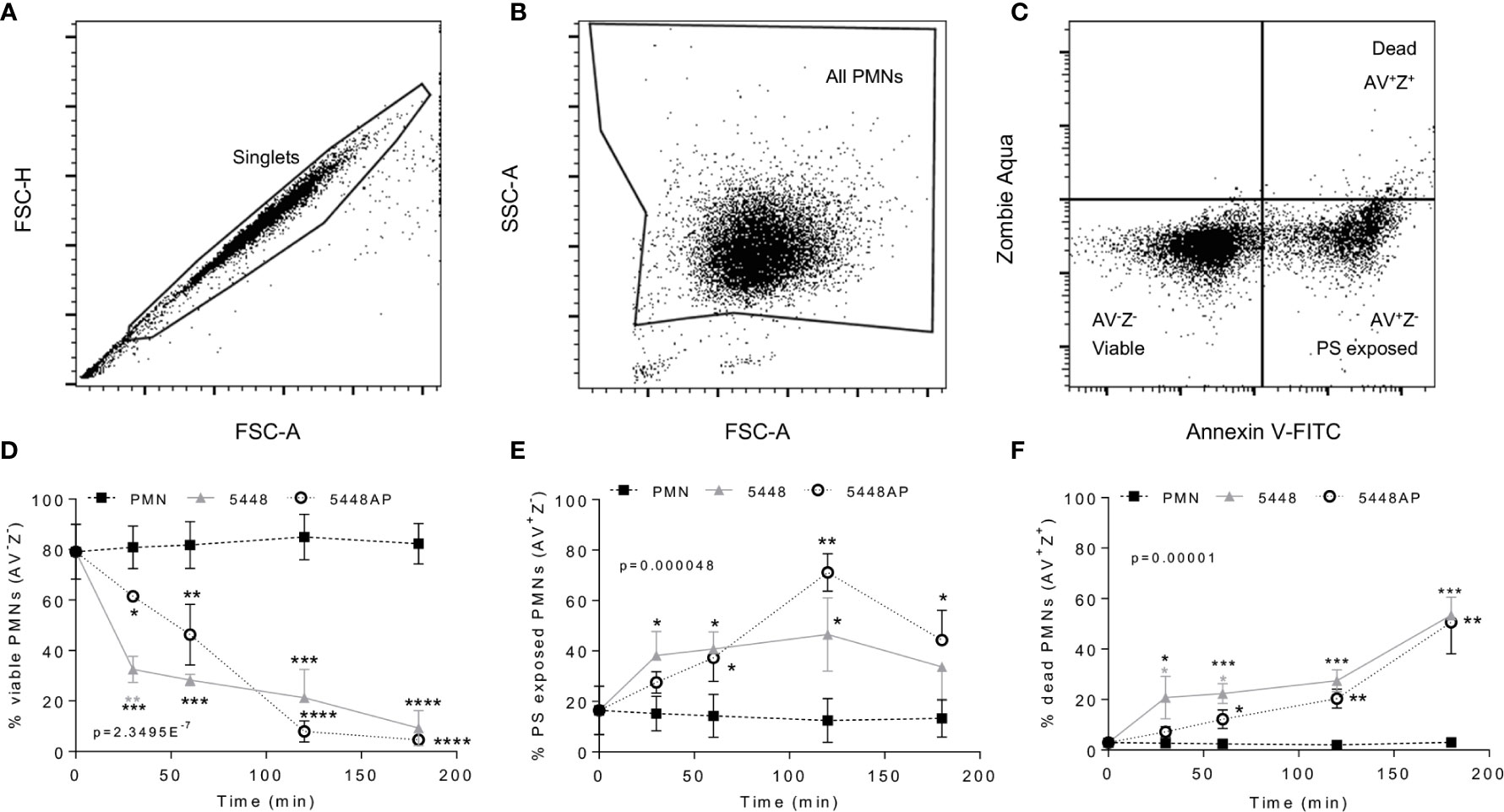
Figure 2 Group A Streptococcus (GAS) infection induced neutrophil death. Human neutrophils (PMNs) were stained with Annexin V-FITC and Zombie Aqua Fixable Viability dye via flow cytometry and initially gated upon (A) ‘singlets’ (FSC-A vs. FSC-H, 10,000 events collected). ‘All PMNs’ (live and dead) were selected using (B) a gate excluding GAS. Cells were then compared for fluorescence of (C) annexin V-FITC/phosphatidylserine (PS) binding against Zombie live/dead viability dye. Purified PMNs were sampled over 180 min to measure (D), ‘viable’ (AV-Z-), (E) ‘PS exposed’ (AV+Z-) and (F) ‘dead’ (AV+Z+), neutrophils during GAS infection (n=3 donors). Results are pooled means ± SD. Linear mixed model ‘p’ values represent interaction (treatment*time) or are stated. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001, with black asterisks denoting significance from control and grey asterisks between 5448 and 5448AP.
The above data (Figure 2) suggests that GAS induce rapid neutrophil death. To explore the mechanism of GAS-induced neutrophil death, human neutrophil lysates were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and screened for the abundance of active caspase-3, caspase-1 and caspase-4 via immunoblotting and quantified using densitometry (Figures 3A and S2A–D). Cleavage of executioner caspase-3 to p17 is a definitive hallmark of apoptosis induction (Nicholson et al., 1995). Conversely, caspase-1 and caspase-4 activity can indicate inflammasome activation, which in turn can cleave downstream effectors of pyroptosis (Sollberger et al., 2012; Man and Kanneganti, 2016). Moreover, a recent report has identified the caspase-1 p46 molecule as the principal active species during inflammasome activation in the cell (Boucher et al., 2018). Neutrophils incubated in the absence of GAS displayed limited evidence of caspase-3 cleavage over 180 min (Figures 3A and S2A). Additionally, in the absence of GAS, neutrophils showed reduced amounts of caspase-1 over 180 min (Figures 3A and S2B, C). Infection of neutrophils with 5448 significantly increased the presence of caspase-3 p17 at 180 min (p>0.0001) compared to both 5448AP-infected and uninfected neutrophils (Figures 3A and S2A). In contrast, incubation of neutrophils with either GAS strain caused an upregulation of caspase-1 (Figures 3A and S2B, C). Compared to uninfected neutrophils, 5448 infection significantly increased pro-caspase-1 (50kDa) at 30 min, 60 min (p<0.05) and 180 min (p<0.01), whilst 5448AP infection only significantly increased pro-caspase-1 at 180 min (p<0.001, Figures 3A and S2B). Further, p46 expression was sustained over 180 min in 5448 and 5448AP infected neutrophils compared with uninfected neutrophils, though this difference was not statistically significant (Figures 3A and S2C). There was no significant difference in expression of caspase-1 when comparing 5448 and 5448AP infected neutrophils. Similar to caspase-1, the abundance of full-length caspase-4 in neutrophils incubated in the absence of GAS declined over time (Figures 3A and S2D). In contrast, 5448 infection increased caspase-4 expression at 30 (p<0.01), 60 (p<0.001) and 180 min compared to uninfected neutrophils (p<0.0001, Figures 2A and S2D). 5448AP infection however, only increased caspase-4 expression at 180 min (p<0.01, Figures 3A and S2D).
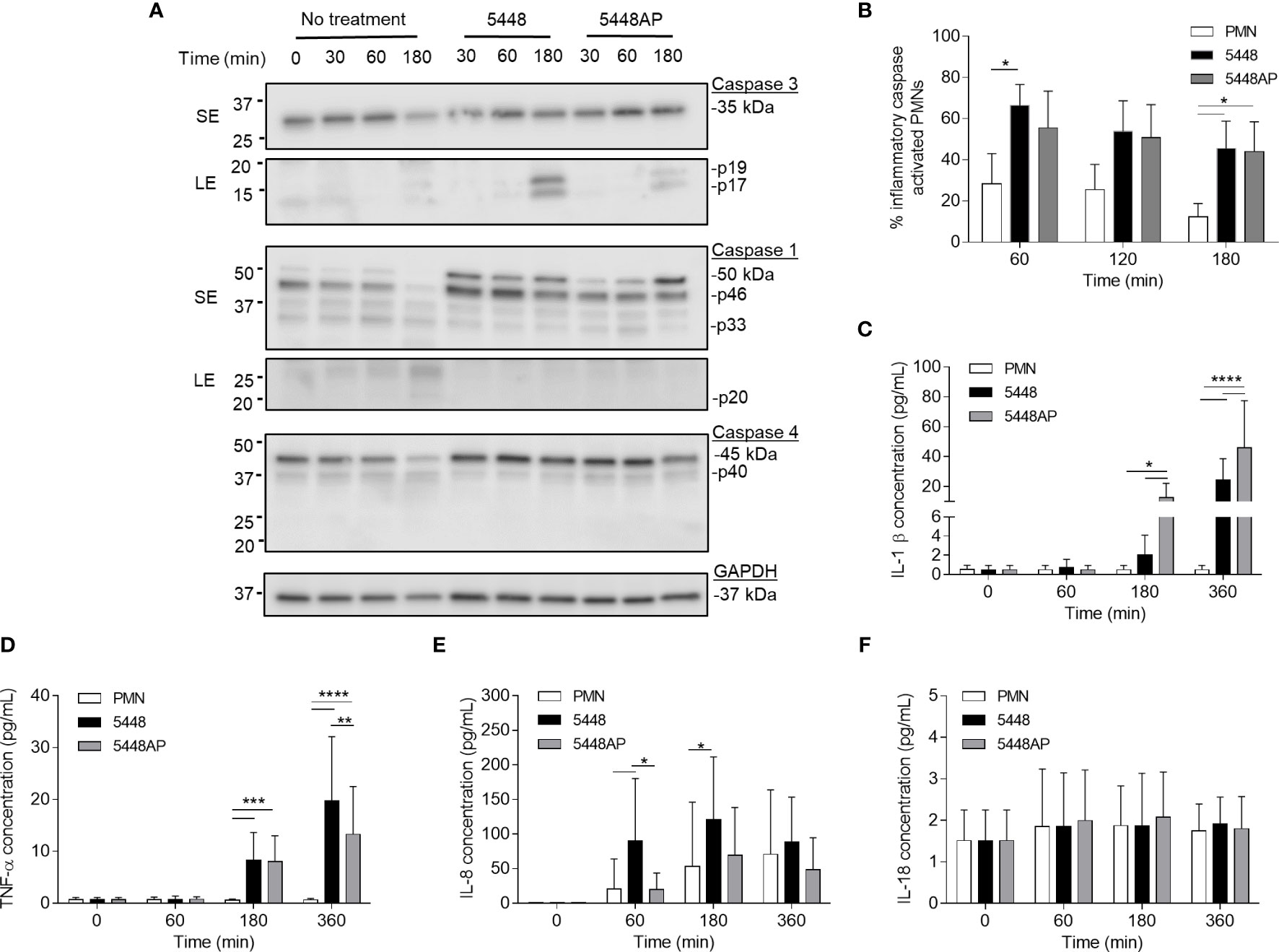
Figure 3 Group A Streptococcus (GAS) infection increases caspase expression in human neutrophils and induces proinflammatory cytokine IL-1β and TNF-α release. (A) Human neutrophil (PMN) lysates were prepared at 30, 60 and 180 min during GAS infection and compared to uninfected neutrophils (0, 30, 60, 180 min) via immunoblotting of caspase-3, caspase-1, caspase-4 and GAPDH. Images shown are from a single donor and are representative of triplicate experiments using different donors. Immunoblot bands were quantified (ImageJ) and normalised over total protein (Figure S2). SE=short exposure, LE=long exposure. (B) Inflammatory caspase activation in human neutrophils due to GAS infection was confirmed via flow cytometry using FLICA (FAM-YVAD-FMK) (n=3 donors). Flow cytometry gating strategy used singlets/PMNs (Figures S3A, B) and FAM-FLICA fluorescence (Figure S3C). The release of cytokines from neutrophils during GAS infection was measured using the LEGENDplex™ human inflammation cytometric bead assay over 360 min. Neutrophils differentially released (C) IL-1β, (D) TNF-α, (E) IL-8 and (F) IL-18 in response to GAS infection (duplicate measurements, n=6 donors). (B–F) Results are the pooled means ± SD. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
To explore the presence of inflammatory caspases further, human neutrophils were incubated with 5448, 5448AP or in the absence of GAS, and inflammatory caspase activity assessed using a fluorogenic flow cytometric assay that detects active caspase-1, -4 and -5 (Figures 3B and S3A–C). Compared to uninfected neutrophils, 5448 infected neutrophils showed increased inflammatory caspase activity at 60 and 180 min (p<0.05), whilst 5448AP infection increased caspase activity at 180 min (p<0.05) (Figure 3B). No significant differences were observed between strains although caspase activity was slightly reduced during 5448AP infection compared to 5448 (Figure 3B), similar to the immunoblot data for caspase-1 p46 (Figures 3A and S2C). Thus, GAS infection of human neutrophils corresponds to increased caspase-1 and caspase-4 expression.
The release of the inflammatory cytokine IL-1β occurs due to caspase-1 activation and is associated with the induction of pyroptosis (Broz et al., 2010). Uncoordinated release of various inflammatory cytokines can exacerbate infection (Tecchio et al., 2014). During GAS infection, disease severity is negatively correlated to cytokine concentration (Norrby-Teglund et al., 2000). Therefore, human neutrophils were incubated with 5448, 5448AP or in the absence of GAS and supernatants assessed for cytokines using a flow cytometric bead assay. Neutrophils incubated in the absence of GAS failed to release IL-1β (Figure 3C) and TNF-α (Figure 3D) despite releasing increasing amounts of IL-8 over 360 min (Figure 3E). Incubation with GAS induced IL-1β release from neutrophils at 180 and 360 min, with significantly greater release during 5448AP infection compared to 5448 at 180 and 360 min (p<0.05, p<0.0001, Figure 3C). Incubation with GAS also induced significant release of TNF-α from neutrophils at 180 min (p<0.001) and 360 min (p<0.0001), with significantly greater release during 5448 infection compared to 5448AP at 360 min (p<0.0001, Figure 3D). Compared to uninfected neutrophils, 5448 induced significant release of IL-8 from neutrophils at 60 and 180 min (p<0.05), with significantly greater release than 5448AP at 60 min (p<0.05, Figure 3E). Neutrophils released minimal amounts of IL-18 over 360 min, and this response did not change following infection with both 5448 or 5448AP (Figure 3F), suggesting that neutrophils have no major role in IL-18 release over 360 min in vitro. These data indicate that both 5448 and 5448AP induce IL-1β release from human neutrophils, consistent with the hypothesis that both GAS strains induce pyroptosis in these cells. Moreover, the results indicate differential cytokine release from human neutrophils infected with GAS, with 5448AP inducing greater IL-1β release and 5448 inducing greater TNF-α and IL-8 release.
Neutrophil adhesion and activation are central to the innate immune response, with CD11b (Mac-1) and CD66b (CEACAM8) playing key roles (Arnaout, 1990; Skubitz et al., 1996; Power et al., 2001). CD16 (FcγRIII) facilitates opsonisation of pathogens and is down-regulated during apoptosis (Dransfield et al., 1994; Fossati et al., 2002), whilst down-regulation of CD31 (PECAM-1) facilitates clearance of apoptotic neutrophils and the resolution of inflammation (Brown et al., 2002; Kurosaka et al., 2003). Therefore, to further explore the impact of GAS on neutrophil function, human neutrophils were incubated with 5448, 5448AP or in the absence of GAS and the expression of cell-surface CD11b, CD66b, CD16 and CD31 was assessed using flow cytometry (Figures 4A–F). In the absence of GAS, neutrophils displayed minor increases in CD11b expression over time. In contrast, incubation with GAS resulted in minor decreases over time, with 5448 inducing a greater loss of CD11b than 5448AP, though these differences were not statistically significant (p=0.289, Figure 4C). Assessing CD66b expression, neutrophils incubated in the absence of GAS revealed minor increases in cell-surface CD66b expression over time (Figure 4D). Incubation with GAS further increased expression of CD66b, with 5448 inducing a slightly greater increase than 5448AP, though these differences were not statistically significant (p=0.162, Figure 4D). Incubation of neutrophils in the absence of GAS resulted in a steady decline in CD16 expression over time, a loss significantly increased by co-incubation with either GAS strain 180 min post-infection (p<0.0001, Figure 4E). Notably, incubation of neutrophils with 5448 induced a significantly greater loss of CD16 expression than 5448AP incubation at 10, 30 and 60 min post-infection (Figure 4E). Similar to CD16, incubation of neutrophils in the absence of GAS resulted in a steady decline in CD31 expression over time, a loss significantly increased by co-incubation with either GAS strain 180 min post-infection (p<0.0001, Figure 4F). Moreover, incubation of neutrophils with 5448 induced a significantly greater loss of CD31 expression than 5448AP incubation at earlier time points. Collectively, these data indicate that GAS has minimal impact upon human neutrophil CD11b and CD66b, but increases the loss of CD16 and CD31 expression, which may impact opsonisation of GAS and subsequent efferocytosis of neutrophils by macrophages, during infection.
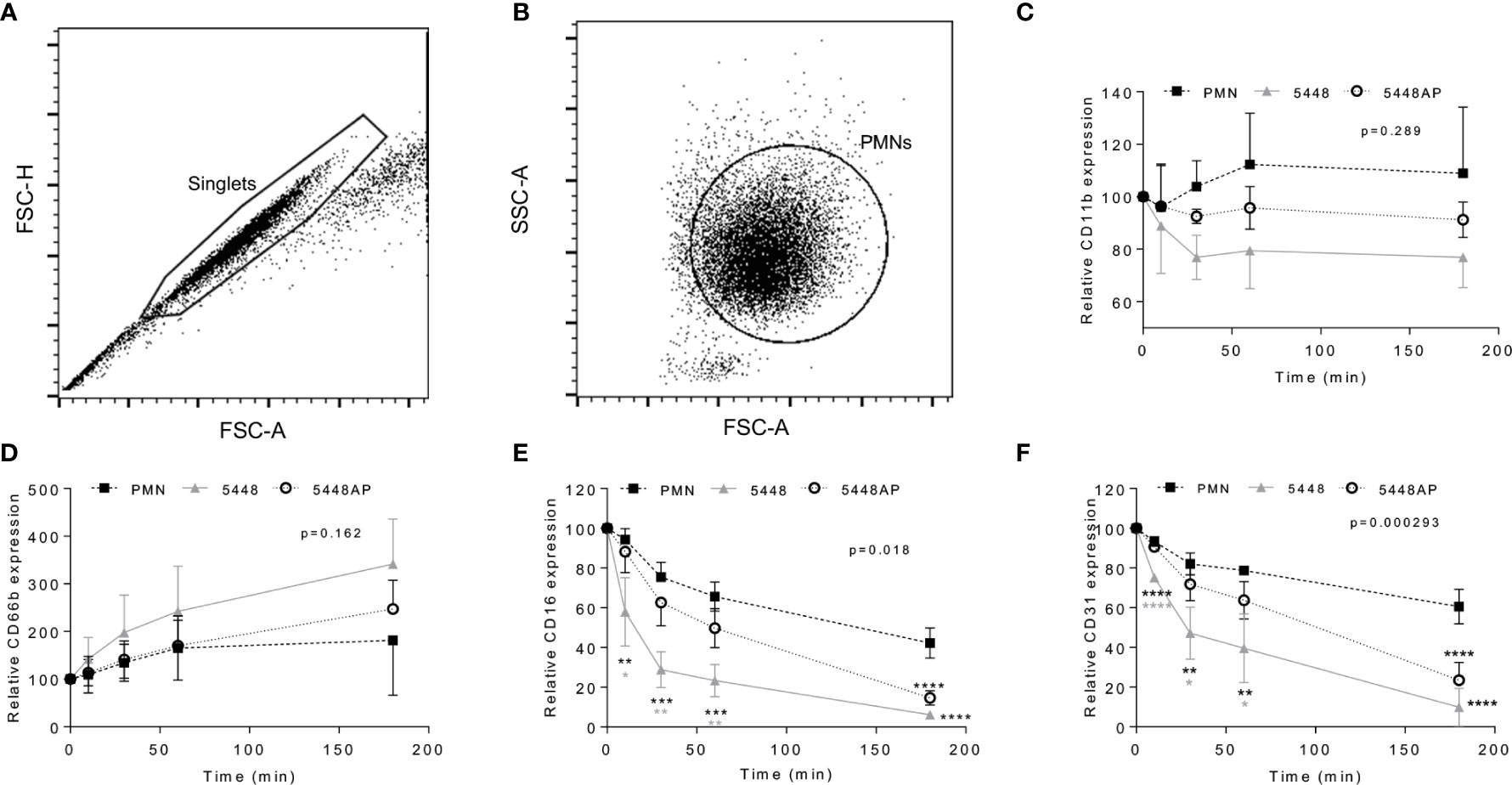
Figure 4 Group A Streptococcus (GAS) infection changes neutrophil functionality. Human neutrophils (PMNs) were analysed using flow cytometry, sequentially gated upon (A) ‘singlets’ (FSC-A vs. FSC-H, 10,000 events collected) then (B) viable ‘PMNs’ (FSC-A vs. FSC-H). Purified neutrophils infected with GAS were sampled over 180 min for the cell-surface expression of (C) CD11b-FITC (n=4 donors), (D) CD66b-PerCP/Cy5.5 (n=5 donors), (E) CD16-FITC (n=4 donors) and (F) CD31-PE/Cy7 (n=4 donors). Results are the pooled means ± SD. Linear mixed model ‘p’ values represent interaction (treatment*time) or are stated. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001, with black asterisks denoting significance from control and grey asterisks between 5448 and 5448AP.
The above data indicate that GAS influences human neutrophils in vitro. To explore if GAS may alter neutrophils in vivo, we assessed the neutrophil response in a mouse model of GAS infection (Maamary et al., 2010; Ly et al., 2014; Tsatsaronis et al., 2014). C57BL/6J mice were injected intradermally with 5448, 5448AP or saline and euthanised at 6 or 24 hours post-infection, with neutrophil populations (CD45+/CD11b+/Ly-6G+), caspase-1 activation and CD16 expression characterised by flow cytometry (Figures 5A–C, E and S4A–F). Infection of mice with either GAS strain significantly increased neutrophil populations at sites of injection at both 6 and 24 h compared to saline control (p<0.001, Figure 5A). The percentage of circulating neutrophils in the blood at 6 h significantly increased during infection with both GAS strains (p<0.01), though the response to 5448AP was significantly greater than 5448 (p<0.05, Figure 5B). Blood neutrophil populations returned to baseline after 24 h, indicating the importance of neutrophils during the early stages of infection (Figure 5B). The percent of murine neutrophils lavaged from sites of saline injection containing active inflammatory caspases was low (Figure 5B). Infection with 5448AP, but not 5448, significantly increased caspase activation at 6 h (p<0.05), whilst both strains increased caspase activation at 24 h (p<0.001, Figure 5C). Cytokines were assessed in murine serum using a flow cytometric bead assay. IL-1β concentrations remained low for saline control and 5448 infected mice but were significantly increased at 24 h during 5448AP infection (p<0.0001, Figure 5D). Neutrophils lavaged from the site of saline and GAS injection displayed CD16 expression, with CD16 expression significantly increased due to 5448 but not 5448AP infection at 6 h when compared to saline control (p<0.05, Figure 5E). The expression of CD16 on neutrophils from mice infected with 5448 significantly decreased between 6 and 24 h (p<0.01), but no significant differences were noted between times for either saline or 5448AP groups (Figure 5E). There was no significant difference in the bacterial load recovered from the site of infection between 5448 and 5448 AP at either time point (Figure 5F).
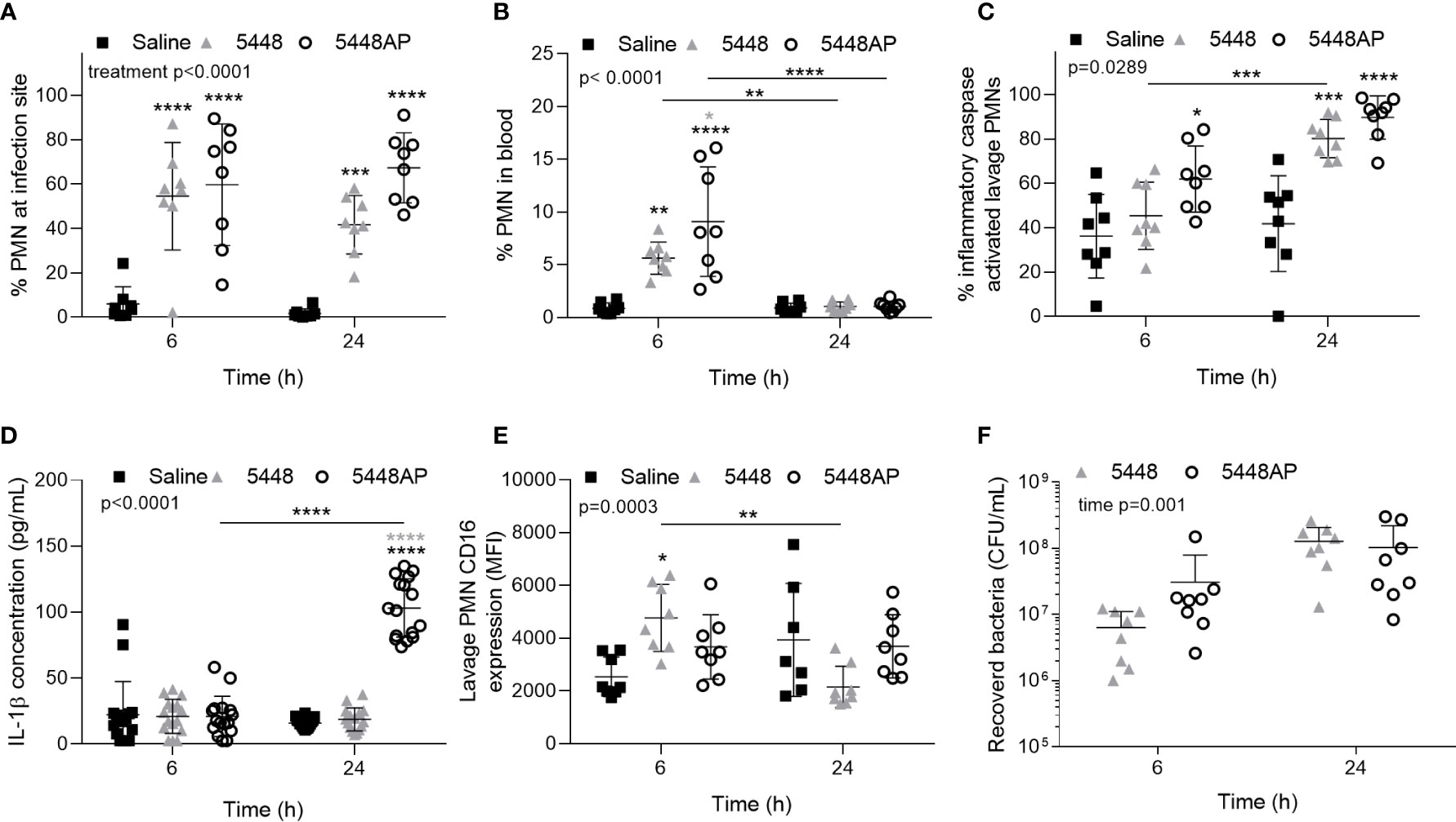
Figure 5 Group A Streptococcus (GAS) infection in vivo activates caspases in murine neutrophils. Percentage of CD45+ cells (A) lavaged from site of injection and in (B) circulating blood characterised as neutrophils (PMNs, CD11b+/Ly-6G+) by flow cytometry (Figures S4A–E). (C) Inflammatory caspase activation in neutrophils at site of GAS infection was confirmed via flow cytometry using FLICA 660 (660-YVAD-FMK, Figure S4F). Each data point represents the results from a single mouse where n=8. (D) The release of IL-1β in mouse serum during GAS infection was measured using the LEGENDplex™ mouse inflammation cytometric bead assay at 6 and 24 h post-infection where mean of duplicate measurements are shown and n=8. (E) CD16 expression of PMNs lavaged from the site of injection and determined using flow cytometry. (F) Bacteria were recovered from the site of infection and concentration determined by plating and colony enumeration where post-hoc analysis was performed using the Holm-Šídák approach. Each data point represents the results from a single mouse where n=8. Results are the means ± SD. Two-way ANOVA ‘p’ values represent interaction (treatment*time) or are stated.*p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001, with black asterisks denoting significance from control and grey asterisks between 5448 and 5448AP, or as indicated by a line.
Here we have mapped the human neutrophil response to GAS, addressing cell death, signalling and the inflammatory profile during the early stages of M1T1 (5448 and 5448AP) infection, whilst also confirming in vitro findings in a murine intradermal GAS infection model. For the first time, we describe an inflammatory neutrophil phenotype, as evidenced by increased caspase-1 and caspase-4 expression and inflammatory caspase activation in vitro and in vivo, that may promote inflammation and exacerbate GAS disease. Most notably, retention of cell-surface proteins CD16 and CD31 during infection with 5548AP may impede the removal of bacteria and clearance of neutrophils at sites of infection, hindering the resolution of inflammation. Further, we elucidate differences between 5448 and 5448AP that aid in understanding the survival and proliferation of GAS harbouring covS mutations in the presence of human neutrophils. We conclude that GAS strains 5448 and 5448AP induce activation of inflammatory caspases 1/4/5 and secretion of IL-1β by neutrophils. For wild type M1 GAS strain 5448, phagocytosis and production of ROS by neutrophils is associated with rapid induction of neutrophil apoptosis as evidenced by cleaved-caspase-3, decreased expression of CD16 and CD31, and loss of viability, which correlates with the lower production and secretion of pro-inflammatory cytokine IL-1β. In contrast, the covS mutant strain 5448AP limits neutrophil activation and production of ROS, which correlates with prolonged neutrophil survival and reduced bacterial killing.
The temporal expression of neutrophil cell surface markers has not previously been investigated in the context of GAS infection. Our data show that significant reductions to both CD16 and CD31 occur in neutrophils due to GAS infection in vitro. Additionally, in vivo data indicates that a reduction in CD16 expression is seen during 5448 infection between 6 and 24 h. CD16 functions as a receptor for opsonised bacteria and immune complexes (Fossati et al., 2002), whilst the loss of CD16 has also been associated with the induction of apoptosis (Dransfield et al., 1994). The induction of apoptosis following bacterial phagocytosis is advantageous to the host as it promotes controlled removal of bacteria and the resolution of inflammation (Kobayashi et al., 2018). We hypothesise that the retention of CD16 during infection promotes a reduction in apoptosis and prolonged degranulation at the site of infection. Further, CD31 expression is reduced during GAS infection, facilitating the eventual removal of neutrophils from sites of infection (Kobayashi et al., 2003). Parallel to CD16, CD31 is retained during 5448AP infection of neutrophils to a greater extent than 5448 infection in vitro. This may result in neutrophils remaining at sites of infection longer, further increasing inflammation. Additionally, we report a trend towards increased cell-surface expression of CD11b and CD66b during GAS infection. Migration of neutrophils to sites of infection, facilitated by CD11b-mediated neutrophil adhesion (Arnaout, 1990), may therefore be affected during GAS infection. Down-regulation of CD11b can prevent accumulation of neutrophils at inflammatory sites (Huston et al., 2009), however retention, such as that seen during 5448AP infection may contribute to prolonged inflammation. Neutrophil activation can be determined through the expression of cell-surface CD66b following stimulation (Skubitz et al., 1996). Reduced cell-surface CD66b expression in response to 5448AP infection may therefore indicate a reduction to neutrophil function and further explain the resistance to neutrophil-mediated killing. Collectively, the neutrophil response is altered and inflammation prolonged at sites of covS mutant GAS infection.
Disruption of the role neutrophils play during the resolution of GAS infection and induction of inflammatory death pathways has been reported previously (Kobayashi et al., 2003; Tsatsaronis et al., 2015). Proinflammatory activation of the NLRP3 inflammasome in macrophages has been demonstrated for M1 GAS (Valderrama et al., 2017), here we provide the first evidence that GAS activates inflammatory caspases in human and murine neutrophils. Western blot analysis showed that caspase-1 expression was consistent during infection with both M1T1 GAS isolates. Flow cytometric analysis using FAM-YVAD-FMK, a probe which preferentially detects active caspase-1, 4 and 5, showed an increase in caspase activation by neutrophils following infection with GAS. However, consistent with previous studies of non-M1 covS mutant GAS (Tsatsaronis et al., 2015), the release of the inflammatory cytokine IL-1β, a hallmark of clinical invasive infection (LaRock and Nizet, 2015), was only significantly increased in response to 5448AP infection in vitro and in vivo. In murine bone marrow-derived dendritic cells caspase-1 activation is essential for IL-1β release (Schneider et al., 2017). The activation of caspase-1 in human and murine neutrophils does not correlate directly to the amount of IL-1β released, and an alternative mechanism may facilitate IL-1β maturation. Type I interferons are known regulators of IL-1β in GAS infected mice (Castiglia et al., 2016), where increased IL-1β release in murine serum seen in this study may also be attributed to disruption of this homeostatic relationship. Alternatively, neutrophils have been shown to express serine proteases that are capable of cleaving IL-1β when infected with S. aureus (Kremserova and Nauseef, 2019). Future work should aim to characterise the mechanism of IL-1β maturation in neutrophils during GAS infection. Regardless of the release mechanism, increased IL-1β, being a neutrophil chemoattractant, may further promote inflammation at sites of infection (Chen et al., 2007), supporting the hypothesis that covS mutant GAS promote greater levels of inflammation during GAS infection.
Previous studies have characterised differences between 5448 and 5448AP, demonstrating resistance to neutrophil-mediated killing in vitro and increased bacterial dissemination and decreased survival in vivo following the acquisition of covS mutations (Walker et al., 2007; Maamary et al., 2010; Fiebig et al., 2015). Here, we show that neutrophil death is delayed in response to 5448AP, suggesting extended neutrophil viability during the early stages of infection. Apoptosis occurs following phagocytic uptake, thus, delayed neutrophil death in response to 5448AP compared to 5448 may be due to reduced neutrophil association and phagocytosis of 5448AP. Alternatively, 5448AP may inhibit cell death pathways via an alternative mechanism. Here, we demonstrate that 5448AP is not only resistant to neutrophil-mediated killing but proliferates in the presence of functional neutrophils. GAS survival has been demonstrated intracellularly in murine neutrophils and human macrophages (Medina et al., 2003a; Medina et al., 2003b; O’Neill et al., 2016) and can even be facilitated by host red blood cells (Wierzbicki et al., 2019). Increased 5448AP proliferation may result from the reduction in neutrophil ROS response during infection. GAS has previously been shown to display resistance to ROS through numerous virulence mechanisms (Henningham et al., 2015). The absence of a hostile environment may further permit bacterial replication. Additionally, the increased production of ROS can induce neutrophil death via apoptosis (Geering and Simon, 2011). Reduced neutrophil ROS production during 5448AP infection may therefore explain the reduction seen in caspase-3 activation (apoptosis). Previously it has been demonstrated that GAS M1T1 strain MGAS5005 modulates neutrophil apoptosis (Kobayashi et al., 2003) and that non-M1T1 GAS harbouring covS mutations elicit non-apoptotic neutrophil cell death (Tsatsaronis et al., 2015), findings that are supported by the current study.
Changes to the function of neutrophils during the initial innate immune response have the potential to hinder mechanisms essential for the resolution of infection. Disruption to these processes during GAS infection may induce this neutrophil phenotype we have described, with increased inflammatory properties and potential to exacerbate infection. Inflammatory caspase expression and activation during GAS infection of neutrophils indicates possible inflammasome activation and death via pyroptosis. However, markers of alternative cell death pathway including necroptosis and NETosis were not explored in the current study, and as such further studies should explore the precise cell death mechanism. A reduction in apoptosis is evident during covS GAS infection, as has been reported for non-M1T1 GAS (Tsatsaronis et al., 2015), whilst modulation of apoptosis has previously been implicated as a factor contributing to GAS survival (Kobayashi et al., 2003). The current study builds on previous findings to show that: M1T1 GAS harbouring covS mutations also elicit reduced apoptosis in neutrophils; M1T1 GAS increase caspase-1 and caspase-4 expression and inflammatory caspase activation in vitro and in vivo; and shows temporal changes in expression of neutrophil cell surface markers during GAS infection. This work aids in understanding the complex host-pathogen interaction identifying host factors that may contribute to severe pathology of invasive GAS infection. Future studies may be able to isolate host-specific targets that could be exploited to control inflammation and tissue damage during invasive GAS infection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Wollongong Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by University of Wollongong Animal Ethics Committee.
MSS, RS, JT, and JW conceptualised the study. MSS, RS, JW, JG, JM, and DL contributed to the methodology. JW, DL, NG, and HV conducted the investigation. JW, DL, RS, and MSS contributed to the data and figure curation. JW wrote the original draft. MSS, RS, JW, DL, NG, JM, HV, JG, and JT wrote, reviewed, and edited the manuscript. MSS, RS and DL acquired the funding. MSS and JM provided the resources. MSS, RS, and JG supervised the study. All authors contributed to the article and approved the submitted version.
Work was funded by the Illawarra Health and Medical Research Institute 2018 NHMRC Near Miss scheme, awarded to MSS and RS. JW and HV are recipients of the Research Training Program Scholarship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Professor Marijka Batterham (University of Wollongong) for her statistical consultation. We thank Associate Professor Thomas Proft (University of Auckland, New Zealand) for pLZ12Km2-P23R:TA. We also thank our generous blood donors for their participation and time. This manuscript has been released as a preprint on bioRxiv (http://www.biorxiv.org) (Williams et al., 2020).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.596023/full#supplementary-material
Arnaout M. A. (1990). Structure and function of the leukocyte adhesion molecule CD11/CD18. Blood 75 (5), 1037–1050. doi: 10.1182/blood.V75.5.1037.bloodjournal7551037
Ato M., Ikebe T., Kawabata H., Takemori T., Watanabe H. (2008). Incompetence of neutrophils to invasive group A streptococcus is attributed to induction of plural virulence factors by dysfunction of a regulator. PLoS One 3 (10), e3455. doi: 10.1371/journal.pone.0003455
Aziz R. K., Kotb M. (2008). Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg. Infect. Dis. 14 (10), 1511–1517. doi: 10.3201/eid1410.071660
Aziz R. K., Pabst M. J., Jeng A., Kansal R., Low D. E., Nizet V., et al. (2004). Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol. Microbiol. 51 (1), 123–134. doi: 10.1046/j.1365-2958.2003.03797.x
Boucher D., Monteleone M., Coll R. C., Chen K. W., Ross C. M., Teo J. L., et al. (2018). Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 215 (3), 827–840. doi: 10.1084/jem.20172222
Bratton D. L., Henson P. M. (2011). Neutrophil clearance: When the party is over, clean-up begins. Trends Immunol. 32 (8), 350–357. doi: 10.1016/j.it.2011.04.009
Brown S., Heinisch I., Ross E., Shaw K., Buckley C. O., Savill J. (2002). Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418 (6894), 200–203. doi: 10.1038/nature00811
Brown K., Brain S., Pearson J., Edgeworth J., Lewis S., Treacher D. (2006). Neutrophils in development of multiple organ failure in sepsis. Lancet 368 (9530), 157–169. doi: 10.1016/S0140-6736(06)69005-3
Broz P., Von Moltke J., Jones J. W., Vance R. E., Monack D. M. (2010). Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8 (6), 471–483. doi: 10.1016/j.chom.2010.11.007
Castiglia V., Piersigilli A., Ebner F., Janos M., Goldmann O., Damböck U., et al. (2016). Type i Interferon Signaling Prevents IL-1β-Driven Lethal Systemic Hyperinflammation during Invasive Bacterial Infection of Soft Tissue. Cell Host Microbe 19 (3), 375–387. doi: 10.1016/j.chom.2016.02.003
Chatellier S., Ihendyane N., Kansal R. G., Khambaty F., Basma H., Norrby-Teglund A., et al. (2000). Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect. Immun. 68 (6), 3523–3534. doi: 10.1128/IAI.68.6.3523-3534.2000
Chen C. J., Kono H., Golenbock D., Reed G., Akira S., Rock K. L. (2007). Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13 (7), 851–856. doi: 10.1038/nm1603
Cole J. N., Barnett T. C., Nizet V., Walker M. J. (2011). Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 9 (10), 724–736. doi: 10.1038/nrmicro2648
Davies H. D., McGeer A., Schwartz B., Green K., Cann D., Simor A. E., et al. (1996). Invasive group A streptococcal infections in Ontario, Canada. New Engl. J. Med. 335 (8), 547–554. doi: 10.1056/NEJM199608223350803
Dransfield I., Buckle A. M., Savill J. S., McDowall A., Haslett C., Hogg N. (1994). Neutrophil apoptosis is associated with a reduction in CD16 (FcγRIII) expression. J. Immunol. 153 (3), 1254–1263.
Edwards R. J., Pyzio M., Gierula M., Turner C. E., Abdul-Salam V. B., Sriskandan S. (2018). Proteomic analysis at the sites of clinical infection with invasive Streptococcus pyogenes. Sci. Rep. 8 (1), 5950. doi: 10.1038/s41598-018-24216-2
Epstein F. H., Weiss S. J. (1989). Tissue Destruction by Neutrophils. N. Engl. J. Med. 320 (6), 365–376. doi: 10.1056/NEJM198902093200606
Faurschou M., Borregaard N. (2003). Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 5 (14), 1317–1327. doi: 10.1016/j.micinf.2003.09.008
Fiebig A., Loof T. G., Babbar A., Itzek A., Koehorst J. J., Schaap P. J., et al. (2015). Comparative Genomics of Streptococcus pyogenes M1 isolates differing in virulence and propensity to cause systemic infection in mice. Int. J. Med. Microbiol 305 (6), 532–543. doi: 10.1016/j.ijmm.2015.06.002
Fossati G., Moots R. J., Bucknall R. C., Edwards S. W. (2002). Differential role of neutrophil Fcγ receptor IIIb (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheumatol. 46 (5), 1351–1361. doi: 10.1002/art.10230
Geering B., Simon H. U. (2011). Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 18 (9), 1457–1469. doi: 10.1038/cdd.2011.75
Ghimire L., Paudel S., Jin L., Baral P., Cai S., Jeyaseelan S. (2018). NLRP6 negatively regulates pulmonary host defense in Gram-positive bacterial infection through modulating neutrophil recruitment and function. PLoS Pathog. 14 (9), e1007308. doi: 10.1371/journal.ppat.1007308
Greenlee-Wacker M. C., Kremserová S., Nauseef W. M. (2017). Lysis of human neutrophils by community-associated methicillin-resistant Staphylococcus aureus. Blood 129 (24), 3237–3244. doi: 10.1182/blood-2017-02-766253
Henningham A., Döhrmann S., Nizet V., Cole J. N. (2015). Mechanisms of group A Streptococcus resistance to reactive oxygen species. FEMS Microbiol Rev. 39 (4), 488–508. doi: 10.1093/femsre/fuu009
Huston J. M., Rosas-Ballina M., Xue X., Dowling O., Ochani K., Ochani M., et al. (2009). Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J. Immunol. 183 (1), 552–559. doi: 10.4049/jimmunol.0802684
Kilsgård O., Karlsson C., Malmström E., Malmström J. (2016). Differential compartmentalization of Streptococcus pyogenes virulence factors and host protein binding properties as a mechanism for host adaptation. Int. J. Med. Microbiol. 306 (7), 504–516. doi: 10.1016/j.ijmm.2016.06.007
Kipnis E. (2013). Neutrophils in sepsis: Battle of the bands. Crit. Care Med. 41 (3), 925–926. doi: 10.1097/CCM.0b013e31828042d8
Kobayashi S. D., Braughton K. R., Whitney A. R., Voyich J. M., Schwan T. G., Musser J. M., et al. (2003). Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. U. S. A. 100 (19), 10948–10953. doi: 10.1073/pnas.1833375100
Kobayashi S. D., Malachowa N., DeLeo F. R. (2018). Neutrophils and Bacterial Immune Evasion. J. Innate Immun. 10 (5–6), 432–441. doi: 10.1159/000487756
Kovach M. A., Standiford T. J. (2012). The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 25 (3), 321–327. doi: 10.1097/QCO.0b013e3283528c9b
Kremserova S., Nauseef W. M. (2019). Frontline Science: Staphylococcus aureus promotes receptor-interacting protein kinase 3- and protease-dependent production of IL-1β in human neutrophils. J. Leukocyte Biol. 105 (3), 437–447. doi: 10.1002/JLB.4HI0918-346R
Kurosaka K., Takahashi M., Watanabe N., Kobayashi Y. (2003). Silent Cleanup of Very Early Apoptotic Cells by Macrophages. J. Immunol. 171 (9), 4672–4679. doi: 10.4049/jimmunol.171.9.4672
Lamagni T. L., Darenberg J., Luca-Harari B., Siljander T., Efstratiou A., Henriques-Normark B., et al. (2008). Epidemiology of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol 46 (7), 2359–2367. doi: 10.1128/JCM.00422-08
LaRock C. N., Nizet V. (2015). Inflammasome/IL-1β responses to streptococcal pathogens. Front. Immunol. 6, 518 (OCT). doi: 10.3389/fimmu.2015.00518
Lioy V. S., Pratto F., de la Hoz A. B., Ayora S., Alonso J. C. (2010). Plasmid pSM19035, a model to study stable maintenance in Firmicutes. Plasmid 64 (1), 1–17. doi: 10.1016/j.plasmid.2010.04.002
Loh J. M. S., Proft T. (2013). Toxin-antitoxin-stabilized reporter plasmids for biophotonic imaging of Group A streptococcus. Appl. Microbiol. Biotechnol. 97 (22), 9737–9745. doi: 10.1007/s00253-013-5200-7
Lundqvist-Gustafsson H., Bengtsson T. (1999). Activation of the granule pool of the NADPH oxidase accelerates apoptosis in human neutrophils. J. Leukocyte Biol. 65 (2), 196–204. doi: 10.1002/jlb.65.2.196
Ly D., Taylor J. M., Tsatsaronis J. A., Monteleone M. M., Skora A. S., Donald C. A., et al. (2014). Plasmin(ogen) Acquisition by Group A Streptococcus Protects against C3b-Mediated Neutrophil Killing. J. Innate Immun. 6 (2), 240–250. doi: 10.1159/000353754
Maamary P. G., Sanderson-Smith M. L., Aziz R. K., Hollands A., Cole J. N., McKay F. C., et al. (2010). Parameters governing invasive disease propensity of non-M1 serotype group A Streptococci. J. Innate Immun. 2 (6), 596–606. doi: 10.1159/000317640
Man S. M., Kanneganti T. D. (2016). Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 16 (1), 7–21. doi: 10.1038/nri.2015.7
McLaughlin R. E., Ferretti J. J. (1995). Electrotransformation of Streptococci. Methods Mol. Biol. 47, 185–193. doi: 10.1385/0-89603-310-4:185
Medina E., Rohde M., Chhatwal G. S. (2003a). Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect. Immun. 71 (9), 5376–5380. doi: 10.1128/IAI.71.9.5376-5380.2003
Medina E., Goldmann O., Toppel A. W., Chhatwal G. S. (2003b). Survival of Streptococcus pyogenes within host phagocytic cells: A pathogenic mechanism for persistence and systemic invasion. J. Infect. Dis. 187 (4), 597–603. doi: 10.1086/373998
Nelson G. E., Pondo T., Toews K. A., Farley M. M., Lindegren M. L., Lynfield R., et al. (2016). Epidemiology of invasive group a streptococcal infections in the United States, 2005-2012. Clin. Infect. Dis. 63 (4), 478–486. doi: 10.1093/cid/ciw248
Nguyen G. T., Green E. R., Mecsas J. (2017). Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell Infect. Microbiol. 7, 373 (AUG). doi: 10.3389/fcimb.2017.00373
Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., et al. (1995). Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376 (6535), 37–43. doi: 10.1038/376037a0
Norrby-Teglund A., Chatellier S., Low D. E., McGeer A., Green K., Kotb M. (2000). Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur. J. Immunol. 30 (11), 3247–3255. doi: 10.1002/1521-4141(200011)30:11<3247::AID-IMMU3247>3.0.CO;2-D
O’Grady K. A. F., Kelpie L., Andrews R. M., Curtis N., Nolan T. M., Selvaraj G., et al. (2007). The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Med. J. Aust. 186 (11), 565–569. doi: 10.5694/j.1326-5377.2007.tb01054.x
O’Loughlin R. E., Roberson A., Cieslak P. R., Lynfield R., Gershman K., Craig A., et al. (2007). The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45 (7), 853–862. doi: 10.1086/521264
O’Neill A. M., Thurston T. L. M., Holden D. W. (2016). Cytosolic replication of group a Streptococcus in human macrophages. mBio 7 (2), e00020–16. doi: 10.1128/mBio.00020-16
Power C., Wang J. H., Sookhai S., Wu Q. D., Redmond H. P. (2001). Proinflammatory effects of bacterial lipoprotein on human neutrophil activation status, function and cytotoxic potential in vitro. Shock 15 (6), 461–466. doi: 10.1097/00024382-200115060-00009
Ryu J. C., Kim M. J., Kwon Y., Oh J. H., Yoon S. S., Shin S. J., et al. (2017). Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol. 10 (3), 757–774. doi: 10.1038/mi.2016.73
Schneider K. S., Groß C. J., Dreier R. F., Saller B. S., Mishra R., Gorka O., et al. (2017). The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep. 21 (13), 3846–3859. doi: 10.1016/j.celrep.2017.12.018
Skubitz K. M., Campbell K. D., Skubitz A. P. N. (1996). CD66a, CD66b, CD66c, and CD66d each independently stimulate neutrophils. J. Leukocyte Biol. 60 (1), 106–117. doi: 10.1002/jlb.60.1.106
Sollberger G., Strittmatter G. E., Kistowska M., French L. E., Beer H. D. (2012). Caspase-4 is required for activation of inflammasomes. J. Immunol. 188 (4), 1992–2000. doi: 10.4049/jimmunol.1101620
Sumby P., Porcella S. F., Madrigal A. G., Barbian K. D., Virtaneva K., Ricklefs S. M., et al. (2005). Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J. Infect. Dis. 192 (5), 771–782. doi: 10.1086/432514
Sumby P., Whitney A. R., Graviss E. A., DeLeo F. R., Musser J. M. (2006). Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PloS Pathog 2 (1), 0041–0049. doi: 10.1371/journal.ppat.0020005
Svensson N., Öberg S., Henriques B., Holm S., Källenius G., Romanus V., et al. (2000). Invasive group A streptococcal infections in Sweden in 1994 and 1995: Epidemiology and clinical spectrum. Scand. J. Infect. Dis. 32 (6), 609–614. doi: 10.1080/003655400459504
Tart A. H., Walker M. J., Musser J. M. (2007). New understanding of the group A Streptococcus pathogenesis cycle. Trends Microbiol. 15 (7), 318–325. doi: 10.1016/j.tim.2007.05.001
Tecchio C., Micheletti A., Cassatella M. A. (2014). Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 5, 508 (OCT). doi: 10.3389/fimmu.2014.00508
Tran T. A. T., Grievink H. W., Lipinska K., Kluft C., Burggraaf J., Moerland M., et al. (2019). Whole blood assay as a model for in vitro evaluation of inflammasome activation and subsequent caspase-mediated interleukin-1 beta release. PLoS One 14 (4), e0214999. doi: 10.1371/journal.pone.0214999
Tsatsaronis J. A., Walker M. J., Sanderson-Smith M. L. (2014). Host Responses to Group A Streptococcus: Cell Death and Inflammation. PLoS Pathog. 10 (8), e1004266. doi: 10.1371/journal.ppat.1004266
Tsatsaronis J. A., Ly D., Pupovac A., Goldmann O., Rohde M., Taylor J. M., et al. (2015). Group A Streptococcus Modulates Host Inflammation by Manipulating Polymorphonuclear Leukocyte Cell Death Responses. J. Innate Immun. 7 (6), 612–622. doi: 10.1159/000430498
Valderrama J. A., Riestra A. M., Gao N. J., Larock C. N., Gupta N., Ali S. R., et al. (2017). Group A streptococcal M protein activates the NLRP3 inflammasome. Nat. Microbiol. 2 (10), 1425–1434. doi: 10.1038/s41564-017-0005-6
Walker M. J., Hollands A., Sanderson-Smith M. L., Cole J. N., Kirk J. K., Henningham A., et al. (2007). DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13 (8), 981–985. doi: 10.1038/nm1612
Wang Q., Imamura R., Motani K., Kushiyama H., Nagata S., Suda T. (2013). Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int. Immunol. 25 (6), 363–372. doi: 10.1093/intimm/dxs161
Wierzbicki I. H., Campeau A., Dehaini D., Holay M., Wei X., Greene T., et al. (2019). Group A Streptococcal S Protein Utilizes Red Blood Cells as Immune Camouflage and Is a Critical Determinant for Immune Evasion. Cell Rep. 29 (10), 2979–2989. doi: 10.1016/j.celrep.2019.11.001
Keywords: inflammation, IL-1β, CD16, CD31, polymorphonuclear leukocyte, covS, Group A streptococcus, neutrophil
Citation: Williams JG, Ly D, Geraghty NJ, McArthur JD, Vyas HKN, Gorman J, Tsatsaronis JA, Sluyter R and Sanderson-Smith ML (2021) Streptococcus pyogenes M1T1 Variants Induce an Inflammatory Neutrophil Phenotype Including Activation of Inflammatory Caspases. Front. Cell. Infect. Microbiol. 10:596023. doi: 10.3389/fcimb.2020.596023
Received: 18 August 2020; Accepted: 07 December 2020;
Published: 28 January 2021.
Edited by:
Sunny Shin, University of Pennsylvania, United StatesReviewed by:
Yuan He, Wayne State University, United StatesCopyright © 2021 Williams, Ly, Geraghty, McArthur, Vyas, Gorman, Tsatsaronis, Sluyter and Sanderson-Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina L. Sanderson-Smith, bWFydGluYUB1b3cuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.