
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 21 October 2020
Sec. Clinical Microbiology
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.00479
This article is part of the Research Topic Point-of-Care Testing for Infectious and Foodborne Pathogens View all 16 articles
SARS-Cov-2 was identified in Wuhan, China in December 2019. The World Health Organization (WHO) declared it a pandemic in March of 2020. COVID-19 has now been reported on every continent. In the United States, the total number of confirmed reported cases of COVID-19 has exceeded 1.8 million with the total death exceeding 100,000 people. The most common investigational diagnostics of this disease are RT-PCR and serology testing. The objective of this work was to validate two commercial kits for the detection of IgM and IgG using lateral flow immunoassay tests and to study the effect of the combination of both serology kits for better detection of immunoglobulins. A total of 195 patients presenting with respiratory symptoms suggestive of infection with SARS-Cov-2 were subject to serology and molecular testing. Two lateral flow immunochromatographic assay kits were used: the Healgen Scientific for SARS-CoV-2 IgM/IgG and the Raybiotech for SARS-CoV-2 IgM/IgG. Sensitivity and specificity of each kit alone and in combination were determined and compared. The limit of detection, inter and intra test variations, as well interfering substances and cross reactivity were also studied for both kits. The results show sensitivities for IgM detection varying between 58.9 and 66.2% for the kits alone and 87.7% of the combination of both kits. IgG detection was not significantly affected by this combination. Both kits manifested high specificities (99.2–100%). Both kits showed high clinical performance in terms of cross reactivity and interfering substances. Our results suggest using combinatory testing for the serology of COVID-19 after a full evaluation study, assessing all the parameters affecting their clinical performance before deciding on this combination.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for the coronavirus disease of 2019 (COVID-19). This virus was first identified in Wuhan, China in December 2019, it has since been declared a pandemic by the World Health Organization (WHO) in March of 2020 (Coronavirus disease (COVID-19) pandemic- 2020 https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Coronavirus disease 2019 (COVID-19): situation summary (2020). https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/summary.html). The disease caused by this virus, COVID-19, has now been reported on every continent. In the United States, the total number of confirmed reported cases of Covid-19 has exceeded 1.8 million with total death exceeding 100,000 people. (https://www.worldometers.info/coronavirus/). The World Health Organization (WHO) has criticized countries that have not prioritized testing. The chief executive of the WHO has highlighted the importance of testing on several occasions (WHO, 2020).
The tests most commonly used now for the diagnosis of COVID-19 are RT-PCR and serology testing. Other techniques such as the detection of the viral antigen are also used. The RT-PCR looks for the virus itself (viral RNA) in the nose, throat, or other areas in the respiratory tract to determine if there is an active infection with SARS-CoV-2 (Liua et al., 2020). A positive PCR test suggests that the person being tested has an active COVID-19 infection.
PCR testing only helps determine whether a person has an active infection at the time of testing (Tahamtan and Ardebili, 2020). Unfortunately, it does not help determine who had an infection in the past. It also does not help determine which people who have been exposed to COVID-19 will develop active infection during the two weeks after exposure. In some people, the virus can only be found by PCR for a few days at the beginning of the infection, so the test might not find the virus if the swab is taken more than a few days after the illness starts. Lateral flow immunoassay (LFIA) testing of SARS-Cov-2 (IgM/IgG) is intended for use as a screening test helping in the identification and diagnosis of human subjects who developed antibodies as a result of SARS-CoV-2 infection or exposure. Any reactive specimen with the IgM-SARS-Cov-2 or IgG-SARS-Cov-2 must be confirmed with alternative testing method(s). It is not yet confirmed if the antibodies produced during this infection will be enough to yield immunity and whether this immunity is long or short. The serology testing should not be used alone to diagnose acute SARS-CoV-2 infection. Antibodies against SARS-CoV-2 can be detected in blood a few days after the infection. The production of IgM typically happens 3–5 days post infection, IgG of course are produced at a later stage; this is known as seroconversion. Negative results do not exclude the possibility of a SARS-CoV-2 infection. For this reason, the combination of both serologic and molecular testing to detect the virus is necessary. Cross reactivity of IgM and/or IgG may occur as a result of a previous exposure to other SARS viruses. The serology testing of SARS-CoV-2 (IgM/IgG) is currently permitted only for use under the Food and Drug Administration's emergency use authorization (EUA) (https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html).
As an effective point-of-care tool, paper-based assays offer the advantage of providing supporting results in a timely manner. In addition, these tests are not expensive and allow for faster treatment decisions (Yager et al., 2006). Paper assays have been used in many diagnostic areas. In view of their complexity, many differences can occur between different kits from different manufacturers.
On April 16, 2020, the White House released a document: “Opening Up America Again Guidelines.” This constitutes a road map based on three phases for reopening the society in the States. The Blueprint document released by the White House demonstrates “how the use of two antibody tests rather than one dramatically improves the predictive value of a testing program, particularly in low prevalence environments.” By definition, higher positive predictive values (PPV) are mostly associated with people who contracted the disease and responded with the production of antibodies. Higher negative predictive values (NPV), on the other hand, commonly suggest that one does not have the disease and did not produce the corresponding antibodies (Blueprint for testing plans and rapid response programs- partnering with states to put America back to work https://www.whitehouse.gov/wp-content/uploads/2020/04/Testing-Blueprint.pdf).
In this paper, our intention was to validate two commercial kits for the detection of IgM and IgG using lateral flow immunoassay tests. Based on our results, we propose to combine two serology kits for better detection of immunoglobulins.
Patients and patient sampling: 195 patients presenting with respiratory symptoms suggestive of an infection with SARS-Cov-2 were subject to serology and molecular testing. For serology testing, venous blood was withdrawn by phlebotomy. Blood was collected in EDTA tubes and separated by centrifugation at 2,500 g for 5 min, and plasma was obtained. For molecular testing, a nasopharyngeal swab was obtained from the same patient. RT-PCR experiments were not performed in our lab, they were sent to a reference lab and results were communicated within 24 h.
Lateral flow immunoassay testing: Two different rapid tests were used for the detection IgM and IgG in human plasma. Both test devices utilize lateral flow immunoassay technology that is used for the qualitative, differential detection of both anti-SARS-CoV-2 IgM and IgG antibodies: 1- Healgen Scientific for SARS-CoV-2 IgM/IgG and 2- Raybiotech for SARS-CoV-2 IgM/IgG.
For both kits, the separation of components was performed using capillary force and the specific and rapid binding of an antibody to its antigen. Each cassette consists of a dry medium coated with novel coronavirus N protein and goat anti-chicken IgY antibody (control). Two free colloidal gold-labeled antibodies, mouse anti-human immunoglobulin and chicken IgY, were included in the release pad section. After the addition of plasma, the Ig will bind to coronavirus Ig antibodies if they are present, forming an IgM-IgM complex. The sample and antibodies will then move across the cassette's medium via capillary action. If the coronavirus IgM antibody is present in the sample, the IgM-IgM complex will bind to the test line and develop color.
For the Healgen kit, the test cassette was provided in a sealed foil pouch and laid on a flat surface. Using the plastic dropper provided, 5 μl of plasma specimen was transferred into the sample well (S). Immediately 50 μL of sample buffer was added to the buffer well (B) ensuring that the buffer vial tip did not touch the sample, air bubbles were avoided. After, the control line (C) changed from blue to red in color. One additional drop of sample buffer may be added to the buffer well if migration of the sample has not moved across the test window. The results were read in 10 min, no reading was allowed after 15 min. Both IgM and IgG were reported in the following way: 1- Positive for anti-SARS-CoV-2 immunoglobulins when both the control line (C) and the test line (T) were dark or light pink. 2- Negative for anti-SARS-CoV-2 immunoglobulins when the control line (C) was dark pink and the test line (T) did not develop color or had a faint gray band. 3- Invalid: there was no colored control line (C). Image 1 represents the possible lateral flow device results for the IgM and IgG cassette.
For the Raybiotech kit, one test cassette for each immunoglobulin was provided in a sealed foil pouch (One cassette for IgM and another for IgG). For both immunoglobulins, 25 ul of patient plasma were added to a diluent tube that was mixed by gentle inversion. Using the pipette provided with the cassette, 2–3 drops of the prepared diluent/plasma were added to the release pad of the cassette. If there is no movement of the liquid in the first 30 s, an additional drop may be added to the release pad. Plasma from patients with PCR confirmed positive and negative SARS-CoV-2 infection were used as controls. Positive and negative control plasma were frozen at −20°C for up to 3 months in 35 ul aliquots in labeled plastic bullets. For longer storage periods, controls were frozen at −80°C. These were tested with every new kit lot received, after receipt of a new shipment of the same lot, and on daily basis when running tests from patients. Results were read within 10 min, and no reading was done after 15 min. Both IgM and IgG were reported in the following way: 1- Positive for anti-SARS-CoV-2 immunoglobulins: both the control line (C) and the test line (T) were dark or light pink. 2- Negative for anti-SARS-CoV-2 immunoglobulins: the control line (C) was dark pink and the test line (T) did not develop color or had a faint gray band. 3- Invalid: there was no colored control line (C).
When both tests were combined for interpretation, the following rule was implemented for both IgM and IgG: in the case of agreement between both kits (Raybiotech and Healgen), the agreed upon result was reported. In the case of disagreement between both kits (positive for one kit and negative for the other), the result was reported as positive for the immunoglobulin in question.
Performance parameters
1. Sensitivity or PPA (positive coincidence rate) and specificity or NPA (negative coincidence rate) of the separate and combined kits' results were calculated according to the following formulas:
The total agreement with PCR results was calculated in percent of the total coincidence between serology and molecular tests.
2. Accuracy and limit of detection (LoD) for IgM and IgG: three serial dilutions from three different patients' plasma were prepared separately. The dilution interval from one dilution to another was the double leading to decreasing concentrations by half. Detection of IgM tests were performed on all the dilutions and results were recorded.
3. Intra-assay validation (intra-assay repeatability): intra-assay validation shows the reproducibility between the tubes within one testing time. Data resulting from intra-assay validation helps ensure that samples run in different tubes of the same experiment will give comparable results. Eight plasma samples were run in five repetitions each.
4. Cross-reactivity: we evaluated the cross-reactivity of the SARS-Cov-2 detection rapid test using plasma samples from patients with documented antibodies against the below listed pathogens. Human Coronavirus 229 E, Human Coronavirus NL63 (alpha coronavirus, Human Coronavirus, Respiratory Syncytial Virus, Human Coronavirus NL63+RSV, Human Coronavirus 229+RSV, Human Metapneumovirus (hMPV, Parainfluenza virus (1,3,), Influenza A, Influenza B, Hepatitis C Virus, Hepatitis B Virus, Hemophilus influenzae, Chlamydia pneumoniae, HPV, HIV.
5. Class specificity: we evaluated the potential for: (a) human IgM (0.4 mg/ml of human IgM purified immunoglobulin- Biorad) to cross react and produce false positive results for IgG: using five patients' plasma with IgG negative. Each sample was tested in duplicate, (b) human IgG (8 mg/ml of natural human IgG - Biorad) to cross react and produce false positive results for IgM: using five patients' plasma with IgM negative. Each sample was tested in duplicate, and (c) human IgM 0.4 mg/ml and human IgG 8 mg/ml to compete and produce false negative results for IgM or IgG using five patients' plasma (IgM positive IgG positive). Each sample was tested in duplicate.
6. Potential interfering substances: we prepared low titer SARS-CoV-2 antibody positive serum samples, and SARS-CoV-2 antibody negative serum samples. Then we spiked aliquots of both preparations with one of the below substances to approximate the indicated concentrations and tested triplicates. Hemoglobin (10–20 mg/mL), Bilirubin Conjugated <1 mg/mL, Bilirubin Unconjugated <1 mg/mL, Ciprofloxacin 200 mg/L, Cefotaxime 500 mg/L, Meropenem 200 mg/L, Imipenem 200 mg/L, Amikacin 10 mg/L, and Amphotericin B 200 mg/L.
7. Statistical methods: for the comparison of sensitivity and specificity, the Fisher-Exact test was used for the sample population analysis using a two sided p. Significance was determined according to the value of p.
The results of the detection of IgM and IgG of 195 tests performed by LFIA using the Raybiotech kit and or the Healgen kit are shown in Tables 1, 2. Concerning IgM detection, Tables 3–5 show that the sensitivity of Raybiotech alone was 58.9%, and Healgen alone was 66.2% (p-value for IgM RayBio vs. IgM Healgen = 0.3969). When the rule about disagreement was implemented (refer to Material and Methods), the sensitivity of the combined kits reached 87.7% (p-value for IgM RayBio vs. combination of tests = 0.0001 and p-value for IgM Healgen vs. combination of tests = 0.0003). These results show a clear significance and beneficial added value for the combination of both kits in detecting IgM.
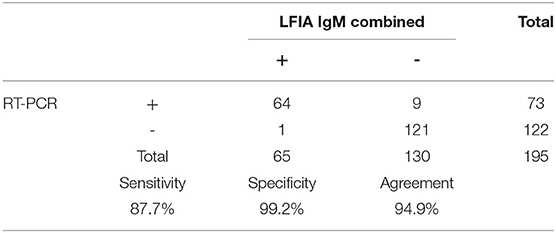
Table 5. Sensitivity, specificity, and agreement of combined Raybiotech+Healgen LFIA IgM with RT-PCR results.
Concerning IgG, as shown in Tables 6–8, it was noted that the sensitivity of Raybiotech alone was 68.9%, and Healgen alone was 74.0% (p-value for IgG RayBio vs. IgG Healgen = 0.5847). When the rule about disagreement was implemented (refer to Material and Methods), the sensitivity of the combined kits was 74.0% (p-value of IgG Raybio vs. combination of both tests = 0.5847). This result does not suggest an added value for the combination of both kits in detecting IgG. The very low number of false positive results (only one patient) resulted in a high specificity varying between 99.2 and 100% for both kits.
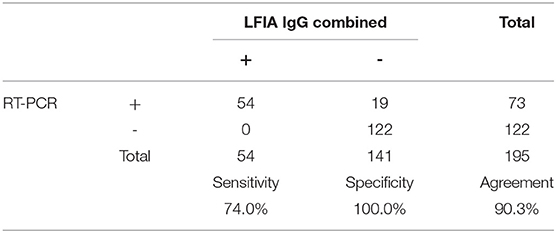
Table 8. Sensitivity, specificity, and agreement of combined Raybiotech+Healgen LFIA IgG with RT-PCR results.
The results of limit of detection show a better detection of IgG at higher dilutions of the sample in both kits than for IgM (Tables 9, 10).
For both tested kits, no cross-reactivity of the SARS-CoV-2 detection rapid tests using plasma samples from patients with documented antibodies against the below listed pathogens was seen: Human Coronavirus 229 E, Human Coronavirus NL63 (alpha coronavirus, Human Coronavirus, Respiratory Syncytial Virus, Human Coronavirus NL63+RSV, Human Coronavirus 229+RSV, Human Metapneumovirus (hMPV, Parainfluenza virus (1,3,), Influenza A, Influenza B, Hepatitis C Virus, Hepatitis B Virus, Hemophilus influenzae, Chlamydia pneumoniae, HPV, HIV.
No class specificity interference with both kits was found using human IgM and human IgG. No false positive or false negative results were recorded.
None of the tested substances (Hemoglobin, Bilirubin Conjugated, Bilirubin Unconjugated, Ciprofloxacin, Cefotaxime, Meropenem, Imipenem, Amikacin, and Amphotericin) were found to interfere with the ability of IgM and IgG detection in both kits. No changes in results were recorded for the inter- and intra-assay tests. All replicates of the same samples in the same experiment as well as the same samples in different experiments yielded the same results.
The pandemic caused by SARS-CoV-2 has been ongoing since the end of 2019. Unfortunately, in the absence of a successful vaccine and a standard efficient treatment, this virus remains invincible in many ways. Lockdown of societies and prevention measures only hope to contain the transmission of the disease until further notice. In this context, massive testing remains a good strategy to identify carriers of the virus. In view of the shortage of material and resources, RT-PCR tests should be part of the algorithm of testing and not the only test. Serology tests investigating the development of IgG and IgM in patients who have been potentially infected is another good addition to the algorithm. Unfortunately, many parameters including the onset of the disease, incubation period, symptomatology, and immunity status of the patient put some limitations on these serological tests regarding their sensitivity and specificity (Haveri et al., 2020). A negative IgM, negative IgG test can be interpreted as no evidence of an increase in human IgM and IgG production against SARS-CoV-2. This negative or non-reactive result might indicate a state of no infection or an incubation period of the virus. In the case of suspicious exposure, the patient should be advised to repeat the test in 7–10 days (Pal et al., 2020). A negative result can also be due to a delayed immune response by the patient. A test showing positive IgM and negative or non-reactive IgG can indicate an early stage of a SARS-CoV-2 infection. The absence of IgG indicates that the patient had not developed acquired immunity yet. A positive IgM and IgG result suggests either an active or an early recovery stage of the infection with SARS-CoV-2. A negative IgM and positive IgG generally indicates a late stage or a past infection with SARS-CoV-2. This suggests that an acquired immunity has developed to the virus.
The detection of immunoglobulins (M and G) is based on immunochromatography where the separation of components in a mixture is accomplished based on the capillary force and the highly specific antigen-antibody binding. IgM antibodies to SARS-CoV-2 generally become positive (detectable in serum) between day 5 and 7 following infection but may occur later. This is the same for IgG antibodies to SARS-CoV-2 that generally become detectable 10–14 days following infection (Huang et al., 2020).
In this work, we have shown that the combination of two lateral flow immunochromatographic tests increase the sensitivity of the assay in detecting IgM, however, this was not the case for IgG. As well as any other testing, the sensitivity and specificity of paper-based assays determines their performance (Tan et al., 1999). Sensitivity assesses and measures the ability of the test to correctly detect the target-substrate. In this context, it is very important to determine the limit of detection (LoD) which can directly affect positively or negatively the sensitivity of the test in question (Wang et al., 2013; Farka et al., 2017). The LoD corresponds to the lowest concentration associated with a positive detectable signal. Several parameters can influence the LoD, such as the affinity of the antigen and antibody, as well as the physical properties of these molecules. In addition, the paper substrate properties, number, printed detector molecules, immunoprobe stability, readout method, and competition with free target molecules are all parameters that can influence the quality of the lateral flow immunochromatography test.
On the other hand, the specificity of a technique relates to the probability of yielding a false positive result. Similar to sensitivity, it is defined by many parameters and factors including the cross-reactivity and nonspecific binding to the immunoprobe. On the other hand, fluidic properties of the paper strip as well as sample preparation are important parameters affecting paper assay performance (Hristov et al., 2019).
It is expected that different manufacturers will produce different products with different performances. When two tests are used for the same sample, there is the possibility of agreement or disagreement on the result. While agreement between tests is considered to “dramatically improve the predictive value of a testing program, particularly in low prevalence environments” (Blueprint for testing plans and rapid response programs- partnering with states to put America back to work https://www.whitehouse.gov/wp-content/uploads/2020/04/Testing-Blueprint.pdf), the disagreement can also be used to dramatically improve the agreement of LFIA with RT-PCT tests, as shown in our paper. This effect has been significantly high with IgM (Tables 3–8) and was insignificant with IgG. Our results suggest that in view of the differences in material and production, and in view of the high specificity of immunoglobulin detection (yielding low levels of false positive results), it is to our advantage to use more than one kit for the detection of immunoglobulins and consider the positive result where the disagreement occurs. The reason why this is true for IgM and not for IgG might be due to the high specificity, smaller size, and higher number of IgGs as compared to IgMs. In any case, the combination of more than one kit should be only advised if this is confirmed by method evaluation and validation.
Regarding the evaluation of LFIA for SARS-CoV-2 detection by Raybiotech and Healgen, our results have shown particularly good performance in terms of the limit of detection, interfering substances, and inter and intra assay variation. These parameters are extremely important in the evaluation process, not only for their direct significance, but also for their influence on the sensitivity and specificity of the tests.
In conclusion, our results are in agreement with others suggesting the use of combinatory testing for the serology of COVID-19 and suggest a full evaluation study including all the parameters affecting their clinical performance before deciding on this combination.
All datasets generated for this study are included in the article/supplementary material.
The studies involving human participants were reviewed and approved by Institution Review Board waived- Michigan Health Clinics IRB approval waived- Only plasma samples previously collected from the patients were used. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
ZD designed the study, participated in the experiments, data analysis, and manuscript writing. JM participated in the experiments and data analysis. DS participated in the study design, data analysis, and manuscript writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Farka, Z. K., Jurík, T. S., Kovár, D., Trnková, L. E., and Skládal, P. (2017). Nanoparticle-based immunochemical biosensors and assays: recent advances and challenges. Chem. Rev. 117, 9973–10042. doi: 10.1021/acs.chemrev.7b00037
Haveri, A., Smura, T., Kuivanen, S., Österlund, P., Hepojoki, J., Ikonen, N., et al. (2020). Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro. Surveill. 25:2000266. doi: 10.2807/1560-7917.ES.2020.25.11.2000266
Hristov, D. R., Rodriguez-Quijada, C., Gomez-Marquez, J., and Hamad-Schifferli, K. (2019). Designing paper-based immunoassays for biomedical applications. Sensors 19:554. doi: 10.3390/s19030554
Huang, C., Wen, T., Shi, F. J., Zeng, X. Y., and Jiao, Y. J. (2020). Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 21, 12550–12556. doi: 10.1021/acsomega.0c01554
Liua, R., Hana, H., Liubc, F., Lva, Z., Wub, K., and Liub, Y. (2020). Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta 505, 172–175. doi: 10.1016/j.cca.2020.03.009
Pal, M., Berhanu, G., Desalegn, C., and Kandi, V. (2020). Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus 12:e7423. doi: 10.7759/cureus.7423
Tahamtan, A., and Ardebili, A. (2020). Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert. Rev. Mol. Diagn. 20, 453–454. doi: 10.1080/14737159.2020.1757437
Tan, E. M., Smolen, J. S., McDougal, J., Butcher, B. T., Conn, D., Dawkins, R., et al. (1999). A critical evaluation of enzyme immunoassays for detection of antinuclear autoantibodies of defined specificities: I. Precision, sensitivity, and specificity. Arthritis Rheum. 42, 455–464.
Wang, Y., Salehi, M., Schütz, M., Rudi, K., and Schlücker, S. (2013). Microspectroscopic SERS detection of interleukin-6 with rationally designed gold/silver nanoshells. Analyst 138, 1764–1771. doi: 10.1039/c3an36610c
WHO (2020). WHODirector-General's opening remarks at the media briefing on COVID-19 - 16March2020. [Online]. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-mediabriefing-on-covid-19 (accessed 16-march, 2020).
Keywords: SARS-CoV-2, IgM, IgG, sensitivity, specificity, serology
Citation: Daoud Z, McLeod J and Stockman DL (2020) Higher Sensitivity Provided by the Combination of Two Lateral Flow Immunoassay Tests for the Detection of COVID-19 Immunoglobulins. Front. Cell. Infect. Microbiol. 10:479. doi: 10.3389/fcimb.2020.00479
Received: 03 June 2020; Accepted: 03 August 2020;
Published: 21 October 2020.
Edited by:
Hongchao Gou, Guangdong Academy of Agricultural Sciences, ChinaReviewed by:
Jing Yuan, Children's Hospital of Capital Institute of Pediatrics, ChinaCopyright © 2020 Daoud, McLeod and Stockman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziad Daoud, emRhb3VkQG1paGVhbHRoY2xpbmljLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.