- 1Department of Diagnostic Medicine/Pathobiology, Kansas State University, Manhattan, KS, United States
- 2E. coli Reference Center, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, PA, United States
- 3Department of Animal Sciences and Industry/Food Science Institute, Kansas State University, Manhattan, KS, United States
- 4Veterinary Diagnostic Laboratory, Industry/Food Science Institute, Kansas State University, Manhattan, KS, United States
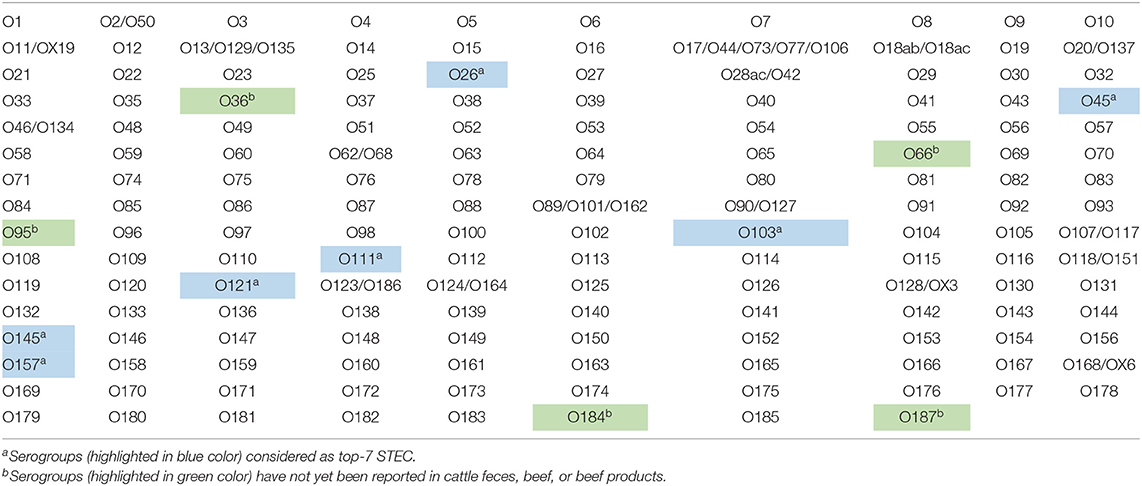
Escherichia coli carrying prophage with genes that encode for Shiga toxins are categorized as Shiga toxin-producing E. coli (STEC) pathotype. Illnesses caused by STEC in humans, which are often foodborne, range from mild to bloody diarrhea with life-threatening complications of renal failure and hemolytic uremic syndrome and even death, particularly in children. As many as 158 of the total 187 serogroups of E. coli are known to carry Shiga toxin genes, which makes STEC a major pathotype of E. coli. Seven STEC serogroups, called top-7, which include O26, O45, O103, O111, O121, O145, and O157, are responsible for the majority of the STEC-associated human illnesses. The STEC serogroups, other than the top-7, called “non-top-7” have also been associated with human illnesses, more often as sporadic infections. Ruminants, particularly cattle, are principal reservoirs of STEC and harbor the organisms in the hindgut and shed in the feces, which serves as a major source of food and water contaminations. A number of studies have reported on the fecal prevalence of top-7 STEC in cattle feces. However, there is paucity of data on the prevalence of non-top-7 STEC serogroups in cattle feces, generally because of lack of validated detection methods. The objective of our study was to develop and validate 14 sets of multiplex PCR (mPCR) assays targeting serogroup-specific genes to detect 137 non-top-7 STEC serogroups previously reported to be present in cattle feces. Each assay included 7–12 serogroups and primers were designed to amplify the target genes with distinct amplicon sizes for each serogroup that can be readily identified within each assay. The assays were validated with 460 strains of known serogroups. The multiplex PCR assays designed in our study can be readily adapted by most laboratories for rapid identification of strains belonging to the non-top-7 STEC serogroups associated with cattle.
Introduction
The polysaccharide portion, called the O-antigen, of the lipopolysaccharide layer of the outer membrane of Escherichia coli provides antigenic specificity and is the basis of serogrouping. As many as 187 E. coli serogroups have been described based on the nucleotide sequences of O-antigen gene clusters (DebRoy et al., 2016). Escherichia coli serogroups that cause disease in humans and animals are categorized into several pathotypes. The serogroups that carry Shiga toxin genes on a prophage are categorized as the Shiga toxin-producing E. coli (STEC) pathotype. As many as 158 serogroups of E. coli are known to carry Shiga toxin gene(s), which make STEC the most predominant E. coli pathotype (Table 1). Illnesses caused by STEC in humans, which are often foodborne, range from mild to bloody diarrhea with life-threatening complications of renal failure and hemolytic uremic syndrome (HUS), and even death, particularly in children (Karmali et al., 2010; Davis et al., 2014). Seven serogroups of STEC, O26, O45, O103, O111, O121, O145, and O157, called “top-7,” are responsible for the majority of human STEC illnesses, including food borne-outbreaks (Brooks et al., 2005; Scallan et al., 2011; Gould et al., 2013; Valilis et al., 2018). However, STEC serogroups other than the top-7, called “non-top-7” have also been reported to cause human illnesses, more often as sporadic infections, although a few are also known to cause severe infections, such as hemorrhagic colitis and HUS (Hussein and Bollinger, 2005; Bettelheim, 2007; Hussein, 2007; Bettelheim and Goldwater, 2014; Valilis et al., 2018). In a recent systematic review done by Valilis et al. (2018), 129 O-serogroups of STEC were identified to be associated with clinical cases of diarrhea in humans.
Ruminants, especially cattle, are a major reservoir of STEC and harbor the organisms in the hindgut and shed them in their feces. A number of studies have reported on the fecal prevalence of the top-7 STEC in cattle because of the availability of detection methods. For these serogroups, culture method involving serogroup-specific immunomagnetic separation and media for selective isolation and PCR assays to identify serogroups of putative isolates have been developed, validated and widely used (Bielaszewska and Karch, 2000; Chapman, 2000; Bettelheim and Beutin, 2003; Noll et al., 2015a). A number of studies have reported shedding of non-top-7 STEC in cattle feces (Table 2). However, not much is known about the prevalence of these STEC serogroups in cattle feces, in terms of their distribution and proportion of animals in a herd positive for various serogroups, largely because of lack of isolation and detection methods. Traditionally, identification of serogroups or serotyping of E. coli, conducted by agglutination reaction using serogroup-specific antisera, is restricted to a few reference laboratories that possess the required antisera. However, the method is time consuming and often exhibits cross-reactions with other serogroups (DebRoy et al., 2011a). A number of PCR-based assays, end point or real time, have been developed and validated for the detection of one or more clinically relevant serogroups of E. coli (Perelle et al., 2004; Monday et al., 2007; Fratamico et al., 2009; Bai et al., 2010, 2012; DebRoy et al., 2011b; Madic et al., 2011; Luedtke et al., 2014; Iguchi et al., 2015b; Noll et al., 2015b; Sanchez et al., 2015; Shridhar et al., 2016a). However, only a few mPCR assays have been described to detect certain STEC serogroups that are non-top-7 (Iguchi et al., 2015b; Sanchez et al., 2015; DebRoy et al., 2018).

Table 2. Serogroups of Shiga toxin-producing Escherichia coli other than the top-7 in gut contents or feces of cattle.
In recent years, DNA microarray and whole genome sequencing have been widely used to identify E. coli serogroups and serotypes (Liu and Fratamico, 2006; Lacher et al., 2014; Joensen et al., 2015; Norman et al., 2015). However, mPCR assays targeting serogroup-specific genes to identify STEC is a simpler, low-cost alternative method, readily adaptable to most laboratories. Iguchi et al. (2015a) and DebRoy et al. (2016) have analyzed the nucleotide sequences of O-antigen gene clusters of 184 serogroups of E. coli and reported remarkable diversity among different serogroups and a high level of conservation of genes within a given serogroup in the O-antigen encoding gene clusters and suggested that these gene sequences can be targeted for serogroup identification. To understand the ecology and prevalence of these STEC serogroups in cattle, it is essential to detect the non-top-7 STEC serogroups shed in cattle feces in order to determine their impact on food safety and human health. Therefore, the objectives of the present study were to develop and validate mPCR assays targeting serogroup-specific genes to detect 137 non-top-7 STEC serogroups known to be associated with cattle.
Materials and Methods
Design of the Assays
A total of 14 mPCR assays, each targeting 7–12 STEC serogroups were designed. The targeted genes to design primers for serogroup detection included: wzx, which encodes for the O-antigen flippase required for O-polysaccharide export (Liu et al., 1996), wzy, which encodes for the O-antigen polymerase required for O antigen biosynthesis (Samuel and Reeves, 2003), gnd, which encodes for 6-phosphogluconate dehydrogenase for O antigen biosynthesis (Nasoff et al., 1984), wzm, which encodes for transport permease for O antigen transport, and orf469 and wbdC, which encode for mannosyltransferase for O antigen biosynthesis (Kido et al., 1995). The primers were designed based on the available nucleotide sequences of the target genes for each of the STEC serogroups from the GenBank database. The sequences for each serogroup were aligned using ClustalX version 2.0. The primers were designed to amplify the target genes with distinct amplicon sizes for each serogroup within an assay for easier visualization. The forward and reverse primer sequences for these serogroups are provided in Supplementary Tables 1A–N.
PCR Assay Conditions
The working concentrations of all primers in a primer mix were 4–7 pM/μl of each primer. The reaction consisted of 1 μL of primer mix, 10 μL of BioRad iQ Multiplex Powermix, 7 μL of sterile PCR grade water, and 2 μL of DNA template. The total reaction volume was 20 μL. The number of PCR cycles and annealing temperatures varied based on optimization for each set (Table 3). The PCR protocol for specific gene target, for sets no. 1–11, included an initial denaturation at 94°C for 5 min, followed by 25 or 30 cycles of denaturation at 94° C for 30 s, annealing for 30 s at 58–68°C, extension for 75 s at 68°C and a final step of extension at 68° C for 7 min. The assay conditions for PCR sets no. 12, 13, and 14 were initial denaturation at 94°C for 1 min, followed by 25 cycles of denaturation at 94°C for 30 s, annealing for 30 s at 58–63°C, extension for 60–80 s at 72°C and final step of extension at 72°C (Table 3). All the other conditions were similar for all 14 sets of assays. Amplicon size of PCR products was determined using a capillary electrophoresis system, QIAxcel Advanced System with QIAxcel DNA Screening Kit (Qiagen, Germantown, MD). DNA extracted from pooled strains of known serogroups for each specific set was used as positive controls and size markers for each set of assay.
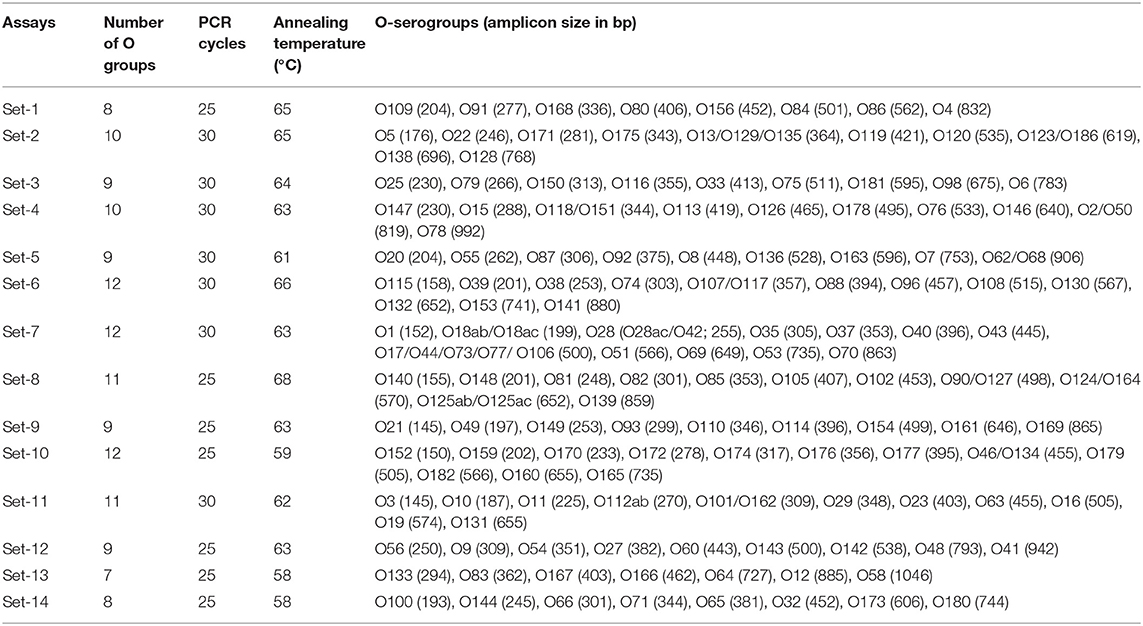
Table 3. Multiplex PCR assays running conditions for the detection of Shiga toxin-producing Escherichia coli (STEC) serogroups, other than top-7 serogroups.
Validation of PCR assays
The specificity of each assay was determined with pooled DNA of the positive controls from the other 13 sets and top-7 STEC plus O104 PCR assays. Additionally, each assay was validated with one or more strains of the targeted serogroups. A total of 460 STEC strains belonging to 137 targeted serogroups were used for the validation of the assays (Table 4; Supplementary Tables 2A–N). The strains were obtained from our culture collection (n = 104), E. coli Reference Center at Pennsylvania State University (n = 223), Michigan State University (n = 42), University of Nebraska (n = 5), and Food and Drug Administration (n = 86). Strains stored in CryoCare beads (CryoCare, Key Scientific Products, Round Rock, TX) at −80°C were streaked onto blood agar plates (Remel, Lenexa, KS) and incubated overnight at 37°C. Following incubation, colonies from the blood agar plates were suspended in 1 ml of distilled water, boiled for 10 min, centrifuged at 9,300 × g for 5 min and the supernatant was used for the PCR assays.
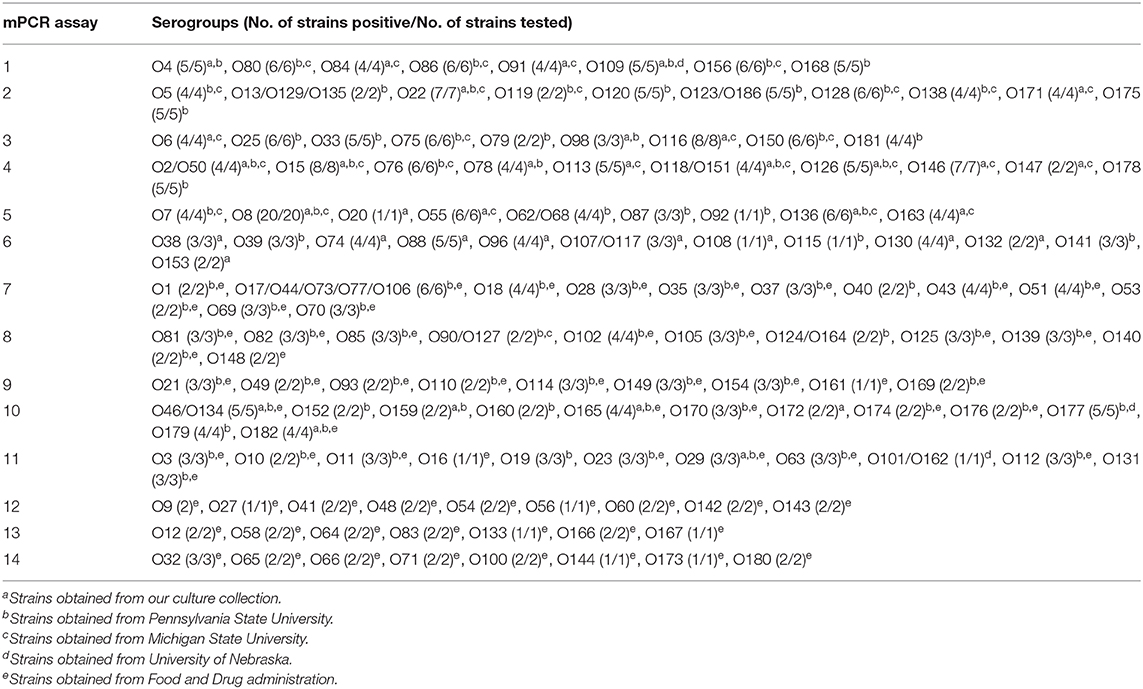
Table 4. Validation of multiplex PCR (mPCR) assays to detect “non-top-7” Shiga toxin-producing Escherichia coli..
Results
Out of the 158 serogroups of STEC, only five, which include O36, O66, O95, O184, and O187, have not been reported to be present in cattle feces, beef or beef products (Table 1). A total of 14 mPCR assays, each targeting 7–12 O-types of 137 non-top-7 serogroups, were designed (Table 3). Each set of mPCR assay contained primer pairs that generated amplicons of different sizes for each target serogroup that were readily differentiated using a capillary electrophoresis system (Table 3; Figures 1A–N). The PCR product size for all the assays ranged from 145 to 1,046 bp (Table 3; Figures 1A–N). The specificity of each assay was confirmed when only the genes of the targeted serogroups were amplified and none of the serogroups targeted by the other 13 sets and top-7 plus O104 PCR assays was amplified (data not shown). The assays were validated with 460 strains of known serogroups, and the results indicated that all the assays correctly identified the target serogroups (Table 4). The 14 sets of mPCR assays did not include the following 14 serogroups: O14, O30, O36, O52, O57, O59, O95, O97, O104, O158, O183, O184, O185, and O187.
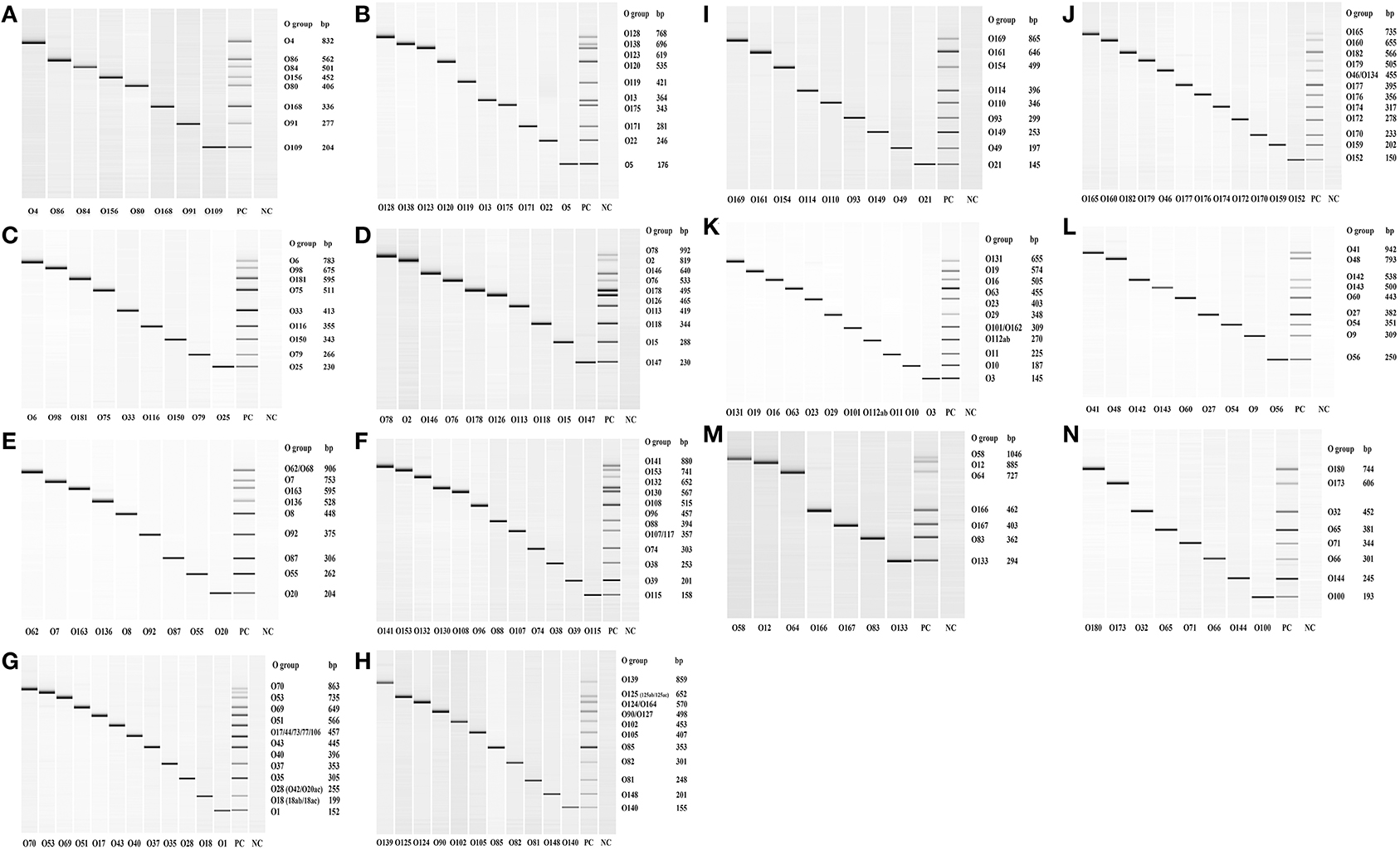
Figure 1. QIAxcel images of the amplicons of serogroup-specific genes of 137 serogroups of Shiga toxin-producing Escherichia coli amplified by 14 sets (A–N) of multiplex PCR assays. Positive control (PC) included pooled cultures of the known serogroups within each assay. Negative control (NC) included all reagents except the DNA template.
Discussion
Of the known 187 serogroups of E. coli, 158 serogroups have been shown to possess genes that encode for Shiga toxin 1, 2 or both. Serogroups, O26, O45, O103, O111, O121, O145, and O157, are top-7 serogroups responsible for a majority of human STEC illness outbreaks (Scallan et al., 2011; Gould et al., 2013). Among the top-7, fecal shedding of the O157 serogroup has been studied extensively, but relatively fewer studies have examined fecal shedding of the other six non-O157 serogroups in cattle, particularly in the United States (Renter et al., 2005; Cernicchiaro et al., 2013; Dargatz et al., 2013; Baltasar et al., 2014; Ekiri et al., 2014; Paddock et al., 2014; Dewsbury et al., 2015; Noll et al., 2015a; Cull et al., 2017). Among the six top-7 non-O157 serogroups, O26, O45, and O103 are the dominant serogroups in cattle feces with prevalence ranging from 40 to 50%. However, only a small proportion of these serogroups (2–6%) carry Shiga toxin genes (Noll et al., 2015a). Because Shiga toxin genes are located on a prophage, it is suggested that the serogroups lacking these genes either have lost the prophage or have the potential to acquire the prophage (Bielaszewska et al., 2007). A majority of the non-O157 top-six STEC have been show to carry Shiga toxin 1 gene (Shridhar et al., 2017). There is evidence that the type of stx gene carried by STEC in cattle is dependent on the age of the animal and season. Shiga toxin gene of STEC strains in adult cattle are predominantly of the stx2 type, whereas the strains from calves primarily possess stx1 type (Cho et al., 2006; Fernández et al., 2012). In a study on E. coli O157 in Argentina, strains of O157 detected in all seasons were predominantly of the stx2 type, the proportion of strains containing stx1 decreased and proportion of strains possessing both types increased in warm seasons (Fernández et al., 2009).
Many PCR assays have been developed and validated, generally targeting top-7 STEC serogroups, and often in combination with major virulence genes (Shiga toxins 1 and 2, intimin, and enterohemolysin: Bai et al., 2010, 2012; DebRoy et al., 2011b; Fratamico et al., 2011; Lin et al., 2011; Anklam et al., 2012; Paddock et al., 2012; Noll et al., 2015b; Shridhar et al., 2016a). There is limited development of PCR assays targeting the non-top-7 STEC in cattle feces. Individual primer pairs have been described and PCR assays have been developed for each of the 187 serogroups of E. coli (DebRoy et al., 2018). However, there are only a few multiplex PCR assays targeting non-top-7 STEC serogroups (Iguchi et al., 2015b; Sanchez et al., 2015). Sanchez et al. (2015) reported the development of three mPCR assays targeting 21 of the most clinically relevant STEC serogroups associated with infections in humans. The assays included, top-7 serogroups and O5, O15, O55, O76, O91, O104, O118, O113, O123, O128, O146, O165, O172, and O177. Iguchi et al. (2015b) designed primer pairs to develop 20 mPCR assays, with each set containing six to nine serogroups, to detect 147 serogroups that included STEC and non-STEC.
Because cattle are a major reservoir of STEC, we designed a series of multiplex PCR assays targeting serogroups, other than the top-7, that have been shown to be associated with feces, beef, or beef products. The nucleotide sequences of some of the targeted serogroups included in our assays have been previously shown to be 98–99.9% identical to other E. coli serogroups (O2/O50, O13/O129/O135, O17/O44/O73/O77/O106, O42/O28ac, O46/O134, O62/O68, O90/O127, O107/O117, O118/O151, O123/O186, O124/O164, O118/O51; DebRoy et al., 2016). Of the 158 known STEC serogroups, only five serogroups, O36, O66, O95, O184, and O187, have not been detected in cattle. The 14 sets of PCR assays did not include O104 because we have published a mPCR assay for the top-7 STEC and O104 (Paddock et al., 2013). The reason for including O104 with the top-7 STEC was because O104:H4, a hybrid pathotype of STEC and enteroaggregative E. coli, was involved in a major foodborne outbreak in Germany in 2011 (Bielaszewska et al., 2011). Cattle have been shown to harbor serogroup O104 in the gut and shed in the feces, however, none of the isolates was the H4 serotype and none possessed traits characteristic of the enteroaggregative E. coli (Paddock et al., 2013; Shridhar et al., 2016b). The 14 sets of mPCR assays did not include the following 13 serogroups: O14, O30, O36, O52, O57, O59, O95, O97, O158, O183, O184, O185, and O187. Of the 13 serogroups, O14 and O57 have been shown to contain no O-antigen biosynthesis gene clusters (Iguchi et al., 2015a; DebRoy et al., 2016). The reason for not including the remaining 11 serogroups (O30, O36, O52, O59, O95, O97, O158, O183, O184, O185, and O187) was because we were unable to procure known strains of the serogroups required for validation.
STEC serogroups other than the top-7 have been reported to be involved in sporadic cases and a few outbreaks of human illness (McLean et al., 2005; Espie et al., 2006; Buchholz et al., 2011; Mingle et al., 2012). Among the non-top-7 STEC, certain serogroups, such as O1, O2, O8, O15, O25, O43, O75, O76, O86, O91, O101, O102, O113, O116, O156, O160, and O165, specifically certain serotypes within these serogroups, have been involved in outbreaks associated with consumption of contaminated beef in the US and European countries (Eklund et al., 2001; Hussein, 2007). Many of the outbreaks included cases of hemorrhagic colitis and HUS. Serogroups O91 (mostly H21 and H14 serotypes) and O113 (mostly H21 serotype) have been associated with severe cases of hemorrhagic colitis and HUS in the US and other countries (Feng et al., 2014, 2017). Obviously, the difference in virulence between serogroups and serotypes is attributable to specific virulence factors encoded by genes in the chromosome, particularly on large horizontally acquired pathogenicity islands, or on plasmids (Levine, 1987; Bolton, 2011).
In contrast to humans, cattle are generally considered to be not susceptible to STEC infections. Only new born calves, particularly those that are immunocompromised because of deprived colostrum, have been shown to exhibit E. coli O157:H7 infections characterized by bloody diarrhea and attaching and effacing lesions (Dean-Nystrom et al., 1998; Moxley and Smith, 2010). Other serogroups that have been associated with diarrheal diseases of calves include O5, O8, O20, O26, O111, and O113 (Mainil and Daube, 2005). The majority of the serotypes causing infections in calves carried only Shiga toxin 1 gene (Mainil and Daube, 2005). Moxley et al. (2015) have reported isolation of STEC O165:H25 from the colonic mucosal tissue of an adult heifer that died of hemorrhagic colitis.
Some of the serogroups detected in cattle feces such as O5, O8, O9, O11, O15, O20, O49, O59, O62, O65, O69, O71, O76, O78, O86, O87, O89, O91, O100, O114, O115, O116, O119, O120, O128, O138, O139, O141, O143, O147, O159, O163, O167, O172, O174, and O180 have also been detected in swine feces (Cha et al., 2018; Peng et al., 2019). A few of the swine STEC serogroups, particularly O8, O138, O139, O141, and O147, are more often implicated in edema disease in weaned piglets and young finishing pigs (Kaper et al., 2004; Melton-Celsa et al., 2012).
Of the 158 STEC serogroups, 130 serogroups have been associated with clinical cases of diarrhea in humans (Mainil and Daube, 2005; Hussein, 2007; Valilis et al., 2018). Therefore, there are 28 STEC serogoups that have not been reported to cause human infections, which is interesting because Shiga toxins are potent virulence factors. Either these STEC have not yet been linked to an illness or they lack other virulence factors, such as those needed for attachment and colonization, necessary to cause infections. A further understanding and assessment of the virulence potential of these serogroups will require sequencing of the whole genome to obtain a comprehensive gene profile.
In conclusion, the multiplex PCR assays designed in our study, which can be readily performed in most microbiology laboratories, will allow for rapid identification of isolates belonging to the non-top-7 E. coli STEC serogroups that are prevalent in cattle feces, beef or beef products.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
JB, TN, and CD conceived and designed the experiments. JL and XS performed the experiments. XS, JB, CD, ER, RP, and TN contributed reagents, materials, and analysis tools. PS, CD, XS, JB, and TN wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This material is based upon work that is supported by the National Institute of Food and Agriculture, U. S. Department of Agriculture, under award number 2012-68003-30155. The funders had no role in the study design, data collection and analyses, preparation of the manuscript or decision to publish.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Shannon Manning (Michigan State University), Dr. Rod Moxley (University of Nebraska) and Ms. Isha Patel (U. S. Food and Drug Administration) for providing us with known serogroups of E. coli and Neil Wallace and Leigh Ann George for assistance in the laboratory. This publication is contribution no. 20-251-J of the Kansas Agricultural Experiment Station.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.00378/full#supplementary-material
References
Anklam, K. S., Kanankege, K. S. T., Gonzales, T. K., Kaspar, C. W., and Döpfer, D. (2012). Rapid and reliable detection of Shiga toxin-producing Escherichia coli by real-time multiplex PCR. J. Food Prot. 75, 643–650. doi: 10.4315/0362-028X.JFP-11-392
Bai, J., Paddock, Z. D., Shi, X., Li, S., An, B., and Nagaraja, T. G. (2012). Applicability of a multiplex PCR to detect the seven major Shiga toxin-producing Escherichia coli based on genes that code for serogroup-specific O-antigens and major virulence factors in cattle feces. Foodborne Pathog. Dis. 9, 541–548. doi: 10.1089/fpd.2011.1082
Bai, J., Shi, X., and Nagaraja, T. G. (2010). A multiplex PCR procedure for the detection of six major virulence genes in Escherichia coli O157:H7. J. Microbiol. Meth. 82, 85–89. doi: 10.1016/j.mimet.2010.05.003
Baltasar, P., Milton, S., Swecker, W., Elvinger, F., and Ponder, M. (2014). Shiga toxin-producing Escherichia coli distribution and characterization in a pasture-based cow-calf production system. J. Food. Prot. 77, 722–731. doi: 10.4315/0362-028X.JFP-13-420
Bettelheim, K. A. (2007). The non-O157 shiga-toxigenic (verocytotoxigenic) Escherichia coli; under-rated pathogens. Crit. Rev. Microbiol. 33, 67–87. doi: 10.1080/10408410601172172
Bettelheim, K. A., and Beutin, L. (2003). Rapid laboratory identification and characterization of verocytotoxigenic (Shiga toxin producing) Escherichia coli (VTEC/STEC). J. Appl. Microbiol. 95, 205–217. doi: 10.1046/j.1365-2672.2003.02031.x
Bettelheim, K. A., and Goldwater, P. N. (2014). Serotypes of non-O157 shigatoxigenic Escherichia coli (STEC). Adv. Microbiol. 04:13. doi: 10.4236/aim.2014.47045
Beutin, L., Geier, D., Steinruck, H., Zimmermann, S., and Scheutz, F. (1993). Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31, 2483–2488. doi: 10.1128/JCM.31.9.2483-2488.1993
Beutin, L., Geier, D., Zimmermann, S., Aleksic, S., Gillespie, H. A., and Whittam, T. S. (1997). Epidemiological relatedness and clonal types of natural populations of Escherichia coli strains producing Shiga toxins in separate populations of cattle and sheep. Appl. Environ. Microbiol. 63, 2175–2180. doi: 10.1128/AEM.63.6.2175-2180.1997
Beutin, L., and Muller, W. (1998). Cattle and verotoxigenic Escherichia coli (VTEC), an old relationship? Vet. Rec. 142, 283–284. doi: 10.1136/vr.142.11.283
Bielaszewska, M., Dobrindt, U., Gärtner, J., Gallitz, I., Hacker, J., Karch, H., et al. (2007). Aspects of genome plasticity in pathogenic Escherichia coli. Int. J. Med. Microbiol. 297, 625–639. doi: 10.1016/j.ijmm.2007.03.001
Bielaszewska, M., and Karch, H. (2000). Non-O157:H7 Shiga toxin (verocytotoxin)-producing Escherichia coli strains: epidemiological significance and microbiological diagnosis. World J. Microbiol. Biotechnol. 16, 711–718. doi: 10.1023/A:1008972605514
Bielaszewska, M., Mellmann, A., Zhang, W., Köck, R., Fruth, A., Bauwens, A., et al. (2011). Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11, 671–676. doi: 10.1016/S1473-3099(11)70165-7
Blanco, M., Blanco, J. E., Blanco, J., Mora, A., Prado, C., Alonso, M. P., et al. (1997). Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet. Microbiol. 54, 309–319. doi: 10.1016/S0378-1135(96)01292-8
Blanco, M., Blanco, J. E., Mora, A., Dahbi, G., Alonso, M. P., Gonzalez, E. A., et al. (2004a). Serotypes, virulence genes, and intimin types of Shiga toxin (Verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-). J. Clin. Microbiol. 42, 645–651. doi: 10.1128/JCM.42.2.645-651.2004
Blanco, M., Padola, N. L., Kruger, A., Sanz, M. E., Blanco, J. E., Gonzalez, E. A., et al. (2004b). Virulence genes and intimin types of Shiga-toxin-producing Escherichia coli isolated from cattle and beef products in Argentina. Int. Microbiol. 7, 269–276.
Blanco, M., Schumacher, S., Tasara, T., Zweifel, C., Blanco, J. E., Dahbi, G., et al. (2005). Serotypes, intimin variants and other virulence factors of eae positive Escherichia coli strains isolated from healthy cattle in Switzerland. Identification of a new intimin variant gene (eae-eta2). BMC Microbiol. 5:23. doi: 10.1186/1471-2180-5-23
Bolton, D. J. (2011). Verocytotoxigenic (Shiga toxin-producing) Escherichia coli: virulence factors and pathogenicity in the farm to fork paradigm. Foodborne Pathog Dis. 8, 357–365. doi: 10.1089/fpd.2010.0699
Bonardi, S., Foni, E., Brindani, F., Bacci, C., Chiapponi, C., and Cavallini, P. (2004). Detection and characterization of verocytotoxin-producing Escherichia coli (VTEC) O157 and non-O157 in cattle at slaughter. New Microbiol. 27, 255–261.
Brooks, J. T., Sowers, E. G., Wells, J. G., Greene, K. D., Griffin, P. M., Hoekstra, R. M., et al. (2005). Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983-2002. J. Infect. Dis. 192, 1422–1429. doi: 10.1086/466536
Buchholz, U., Bernard, H., Werber, D., Böhmer, M. M., Remschmidt, C., Wilking, H., et al. (2011). German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 365, 1763–1770. doi: 10.1056/NEJMoa1106482
Bumunang, E. W., McAllister, T. A., Zaheer, R., Ortega Polo, R., Stanford, K., King, R., et al. (2019). Characterization of non-O157 Escherichia coli from cattle faecal samples in the North-West province of South Africa. Microorganisms 7:8. doi: 10.3390/microorganisms7080272
Cernicchiaro, N., Cull, C. A., Paddock, Z. D., Shi, X., Bai, J., Nagaraja, T. G., et al. (2013). Prevalence of Shiga toxin-producing Escherichia coli and associated virulence genes in feces of commercial feedlot cattle. Foodborne Pathog Dis. 10, 835–841. doi: 10.1089/fpd.2013.1526
Cha, W., Fratamico, P. M., Ruth, L. E., Bowman, A. S., Nolting, J. M., Manning, S. D., et al. (2018). Prevalence and characteristics of Shiga toxin-producing Escherichia coli in finishing pigs: Implications on public health. Int. J. Food Microbiol. 264, 8–15. doi: 10.1016/j.ijfoodmicro.2017.10.017
Chapman, P. A. (2000). Methods available for the detection of Escherichia coli O157 in clinical, food and environmental samples. World J. Microbiol. Biotechnol. 16, 733–740. doi: 10.1023/A:1008985008240
Cho, S., Diez-Gonzales, F., Fossler, C. P., Wells, S. J., Hedberg, C. W., Kaneene, J. B., et al. (2006). Prevalence of Shiga toxin-encoding bacteria and Shiga toxin-producing Escherichia coli isolates from dairy farms and county fairs. Vet. Microbiol. 118, 289–298. doi: 10.1016/j.vetmic.2006.07.021
Cobbold, R., and Desmarchelier, P. (2001). Characterisation and clonal relationships of Shiga-toxigenic Escherichia coli (STEC) isolated from Australian dairy cattle. Vet. Microbiol. 79, 323–335. doi: 10.1016/S0378-1135(00)00366-7
Cull, C. A., Renter, D. G., Dewsbury, D. M., Noll, L. W., Shridhar, P. B., Ives, S. E., et al. (2017). Feedlot- and pen-level prevalence of enterohemorrhagic Escherichia coli in feces of commercial feedlot cattle in two major U.S. cattle feeding areas. Foodborne Pathog. Dis. 14, 309–317. doi: 10.1089/fpd.2016.2227
Dargatz, D. A., Bai, J., Lubbers, B. V., Kopral, C. A., An, B., and Anderson, G. A. (2013). Prevalence of Escherichia coli O-types and Shiga toxin genes in fecal samples from feedlot cattle. Foodborne Pathog. Dis. 10, 392–396. doi: 10.1089/fpd.2012.1289
Davis, T. K., Van De Kar, N. C. A. J., and Tarr, P. I. (2014). Shiga toxin/Verocytotoxin-producing Escherichia coli infections: Practical clinical perspectives. Microbiol. Spect. 2:4. doi: 10.1128/microbiolspec.EHEC-0025-2014
Dean-Nystrom, E. A., Bosworth, B. T., Moon, H. W., and O'Brien, A. D. (1998). “Bovine infection with Shiga toxin-producing Escherichia coli,” in Escherichia coli O157:H7 and Other Shiga Toxin-producing E. coli Strains, eds J. B. Kaper and A. D. O'Brien (Washington, DC: ASM Press), 261–267.
DebRoy, C., Fratamico, P. M., and Roberts, E. (2018). Molecular serogrouping of Escherichia coli. Anim. Hlth. Res. Rev. 19, 1–16. doi: 10.1017/S1466252317000093
DebRoy, C., Fratamico, P. M., Yan, X., Baranzoni, G., Liu, Y., Needleman, D. S., et al. (2016). Comparison of O-antigen gene clusters of all O-serogroups of Escherichia coli and proposal for adopting a new nomenclature for O-typing. PLoS ONE 11:e0147434. doi: 10.1371/journal.pone.0147434
DebRoy, C., Roberts, E., and Fratamico, P. M. (2011a). Detection of O antigens in Escherichia coli. Anim. Health Res. Rev. 12, 169–185. doi: 10.1017/S1466252311000193
DebRoy, C., Roberts, E., Valadez, A. M., Dudley, E. G., and Cutter, C. N. (2011b). Detection of Shiga toxin-producing Escherichia coli O26, O45, O103, O111, O113, O121, O145, and O157 serogroups by multiplex polymerase chain reaction of the wzx gene of the O-antigen gene cluster. Foodborne Pathog. Dis. 8, 651–652. doi: 10.1089/fpd.2010.0769
Dewsbury, D. M. A., Renter, D. G., Shridhar, P. B., Noll, L. W., Shi, X., Nagaraja, T. G., et al. (2015). Summer and winter prevalence of Shiga toxin-producing Escherichia coli (STEC) O26, O45, O103, O111, O121, O145, and O157 in feces of feedlot cattle. Foodborne Pathog. Dis. 12, 726–732. doi: 10.1089/fpd.2015.1987
Diarra, M. S., Giguère, K., Malouin, F., Lefebvre, B., Bach, S., Delaquis, P., et al. (2009). Genotype, serotype, and antibiotic resistance of sorbitol-negative Escherichia coli isolates from feedlot cattle. J. Food Prot. 72, 28–36. doi: 10.4315/0362-028X-72.1.28
Ekiri, A. B., Landblom, D., Doetkott, D., Olet, S., Shelver, W. L., and Khaitsa, M. L. (2014). Isolation and characterization of Shiga toxin-producing Escherichia coli serogroups O26, O45, O103, O111, O113, O121, O145, and O157 shed from range and feedlot cattle from postweaning to slaughter. J. Food Prot. 77, 1052–1061. doi: 10.4315/0362-028X.JFP-13-373
Eklund, M., Scheutz, F., and Siitonen, A. (2001). Clinical isolates of non-O157 Shiga toxin-producing Escherichia coli: serotypes, virulence characteristics, and molecular profiles of strains of the same serotype. J. Clin. Microbiol. 39, 2829–2834. doi: 10.1128/JCM.39.8.2829-2834.2001
Ennis, C., McDowell, D., and Bolton, D. J. (2012). The prevalence, distribution and characterization of Shiga toxin-producing Escherichia coli (STEC) serotypes and virulotypes from a cluster of bovine farms. J. Appl. Microbiol. 113, 1238–1248. doi: 10.1111/j.1365-2672.2012.05421.x
Espie, E., Grimont, F., Vaillant, V., Montet, M. P., Carle, I., Bavai, C., et al. (2006). O148 Shiga toxin-producing Escherichia coli outbreak: microbiological investigation as a useful complement to epidemiological investigation. Clin. Microbiol. Infect. 12, 992–998. doi: 10.1111/j.1469-0691.2006.01468.x
Fan, R., Shao, K., Yang, X., Bai, X., Fu, S., Sun, H., et al. (2019). High prevalence of non-O157 Shiga toxin-producing Escherichia coli in beef cattle detected by combining four selective agars. BMC Microbiology 19:213. doi: 10.1186/s12866-019-1582-8
Feng, P. C. H., Delannoy, S., Lacher, D. W., Bosilavac, J. M., Fach, P., and Beutin, L. (2017). Shiga toxin-producing serogroup O91 Escherichia coli strains isolated from food and environmental samples. Appl. Environ. Microbiol. 83:e01231–e012317. doi: 10.1128/AEM.01231-17
Feng, P. C. H., Delannoy, S., Lacher, D. W., Fernando dos Santos, L., Beutin, L., Fach, P., et al. (2014). Genetic diversity and virulence potential of Shiga toxin-producing Escherichia coli O113:H21 strains isolated from clinical, environmental, and food sources. Appl. Environ. Microbiol. 80, 4757–4763. doi: 10.1128/AEM.01182-14
Fernández, D., Irino, K., Sanz, M. E., Padola, N. L., and Parma, A. E. (2010). Characterization of Shiga toxin-producing Escherichia coli isolated from dairy cows in Argentina. Lett. Appl. Micribiol. 51, 377–382. doi: 10.1111/j.1472-765X.2010.02904.x
Fernández, D., Rodriguez, E. M., Arroyo, G. H., Padola, N. L., and Parma, A. E. (2009). Seasonal variation of Shiga toxin-encoding genes (stx) and detection of E. coli O157 in dairy cattle from Argentina. J. Appl. Micribiol. 106, 1250–1267. doi: 10.1111/j.1365-2672.2008.04088.x
Fernández, D., Sanz, M. E., Parma, A. E., and Padola, N. L. (2012). Short communiaction: Characterization of Shiga toxin-producing Escherichia coli isolated from newborn, milk-fed, and growing calves in Argentina. J. Dairy Sci, 95, 5340–5343. doi: 10.3168/jds.2011-5140
Fratamico, P. M., Bagi, L. K., Cray, W. C., Narang, N., Yan, X., Medina, M., et al. (2011). Detection by multiplex real-time polymerase chain reaction assays and isolation of Shiga toxin-producing Escherichia coli serogroups O26, O45, O103, O111, O121, and O145 in ground beef. Foodborne Pathog. Dis. 8, 601–607. doi: 10.1089/fpd.2010.0773
Fratamico, P. M., DebRoy, C., Miyamoto, T., and Liu, Y. (2009). PCR detection of enterohemorrhagic Escherichia coli O145 in food by targeting genes in the E. coli O145 O-antigen gene cluster and the Shiga toxin 1 and Shiga toxin 2 genes. Foodborne Pathog. Dis. 6, 605–611. doi: 10.1089/fpd.2008.0254
Geue, L., Segura-Alvarez, M., Conraths, F., Kuczius, T., Bockemühl, J., Karch, H., et al. (2002). A long-term study on the prevalence of shiga toxin-producing Escherichia coli (STEC) on four German cattle farms. Epidemiol. Infect. 129, 173–185. doi: 10.1017/S0950268802007288
Gioffré, A., Meichtri, L., Miliwebsky, E., Baschkier, A., Chillemi, G., Romano, M. I., et al. (2002). Detection of Shiga toxin-producing Escherichia coli by PCR in cattle in Argentina: Evaluation of two procedures. Vet. Microbiol. 87, 301–313. doi: 10.1016/S0378-1135(02)00079-2
Gonzalez, A. G., Cerqueira, A. M., Guth, B. E., Coutinho, C. A., Liberal, M. H., Souza, R. M., et al. (2016). Serotypes, virulence markers and cell invasion ability of Shiga toxin-producing Escherichia coli strains isolated from healthy dairy cattle. J. Appl. Microbiol. 121, 1130–1143. doi: 10.1111/jam.13230
Gonzalez, E. A., and Blanco, J. (1989). Serotypes and antibiotic resistance of verotoxigenic (VTEC) and necrotizing (NTEC) Escherichia coli strains isolated from calves with diarrhoea. FEMS Microbiol. Lett. 51, 31–36. doi: 10.1111/j.1574-6968.1989.tb03414.x
Gould, L. H., Mody, R. K., Ong, K. L., Clogher, P., Cronquist, A. B., Garman, K. N., et al. (2013). Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: epidemiologic features and comparison with E. coli O157 Infections. Foodborne Pathog. Dis. 10, 453–460. doi: 10.1089/fpd.2012.1401
Hornitzky, M. A., Bettelheim, K. A., and Djordjevic, S. P. (2000). The isolation of enterohaemorrhagic Escherichia coli O111:H- from Australian cattle. Aust. Vet. J. 78, 636–637. doi: 10.1111/j.1751-0813.2000.tb11941.x
Hornitzky, M. A., Bettelheim, K. A., and Djordjevic, S. P. (2001). The detection of Shiga toxin-producing Escherichia coli in diagnostic bovine faecal samples using vancomycin-cefixime-cefsulodin blood agar and PCR. FEMS Microbiol. Lett. 198, 17–22. doi: 10.1111/j.1574-6968.2001.tb10613.x
Hornitzky, M. A., Mercieca, K., Bettelheim, K. A., and Djordjevic, S. P. (2005). Bovine feces from animals with gastrointestinal infections are a source of serologically diverse atypical enteropathogenic Escherichia coli and Shiga toxin-producing E. coli strains that commonly possess intimin. Appl. Environ. Microbiol. 71, 3405–3412. doi: 10.1128/AEM.71.7.3405-3412.2005
Hornitzky, M. A., Vanselow, B. A., Walker, K., Bettelheim, K. A., Corney, B., Gill, P., et al. (2002). Virulence properties and serotypes of Shiga toxin-producing Escherichia coli from healthy Australian cattle. Appl. Eenviron. Microbiol. 68, 6439–6445. doi: 10.1128/AEM.68.12.6439-6445.2002
Hussein, H. S. (2007). Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 85, E63–E72. doi: 10.2527/jas.2006-421
Hussein, H. S., and Bollinger, L. M. (2005). Prevalence of Shiga toxin–producing Escherichia coli in beef cattle. J. Food Protect. 68, 2224–2241. doi: 10.4315/0362-028X-68.10.2224
Iguchi, A., Iyoda, S., Kikuchi, T., Ogura, Y., Katsura, K., Ohnishi, M., et al. (2015a). A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA Res. 22, 101–107. doi: 10.1093/dnares/dsu043
Iguchi, A., Iyoda, S., Seto, K., Morita-Ishihara, T., Scheutz, F., and Ohnishi, M. (2015b). Escherichia coli O-Genotyping PCR: a comprehensive and practical platform for molecular O serogrouping. J. Clin. Microbiol. 53, 2427–2432. doi: 10.1128/JCM.00321-15
Irino, K., Kato, M. A. M. F., Vaz, T. M. I., Ramos, I. I., Souza, M. A. C., Cruz, A. S., et al. (2005). Serotypes and virulence markers of Shiga toxin-producing Escherichia coli (STEC) isolated from dairy cattle in São Paulo State, Brazil. Vet. Microbiol. 105, 29–36. doi: 10.1016/j.vetmic.2004.08.007
Jajarmi, M., Imani Fooladi, A. A., Badouei, M. A., and Ahmadi, A. (2017). Virulence genes, Shiga toxin subtypes, major O-serogroups, and phylogenetic background of Shiga toxin-producing Escherichia coli strains isolated from cattle in Iran. Microb. Pathog. 109, 274–279. doi: 10.1016/j.micpath.2017.05.041
Joensen, K. G., Tetzschner, A. M., Iguchi, A., Aarestrup, F. M., and Scheutz, F. (2015). Rapid and easy In Silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 53, 2410–2426. doi: 10.1128/JCM.00008-15
Kaper, J. B., Nataro, J. P., and Mobley, H. L. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. doi: 10.1038/nrmicro818
Karmali, M. A., Gannon, V., and Sargeant, J. M. (2010). Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 140, 360–370. doi: 10.1016/j.vetmic.2009.04.011
Kido, N., Torgov, V. I., Sugiyama, T., Uchiya, K., Sugihara, H., Komatsu, T., et al. (1995). Expression of the O9 polysaccharide of Escherichia coli: sequencing of the E. coli O9 rfb gene cluster, characterization of mannosyl transferases, and evidence for an ATP-binding cassette transport system. J. Bacteriol. 177, 2178–2187. doi: 10.1128/JB.177.8.2178-2187.1995
Lacher, D. W., Gangiredla, J., Jackson, S. A., Elkins, C. A., and Feng, P. C. H. (2014). Novel Microarray design for molecular serotyping of shiga toxin-producing Escherichia coli strains isolated from fresh produce. Appl. Environ. Microbiol. 80, 4677–4682. doi: 10.1128/AEM.01049-14
Lee, K., Kusumoto, M., Iwata, T., Iyoda, S., and Akiba, M. (2017). Nationwide investigation of Shiga toxin-producing Escherichia coli among cattle in Japan revealed the risk factors and potentially virulent subgroups. Epidemiol. Infect. 145, 1557–1566. doi: 10.1017/S0950268817000474
Leomil, L., Aidar-Ugrinovich, L., Guth, B. E. C., Irino, K., Vettorato, M. P., Onuma, D. L., et al. (2003). Frequency of Shiga toxin-producing Escherichia coli (STEC) isolates among diarrheic and non-diarrheic calves in Brazil. Vet. Microbiol. 97, 103–109. doi: 10.1016/j.vetmic.2003.08.002
Leung, P. H., Yam, W. C., Ng, W. W., and Peiris, J. S. (2001). The prevalence and characterization of verotoxin-producing Escherichia coli isolated from cattle and pigs in an abattoir in Hong Kong. Epidemiol. Infect. 126, 173–179. doi: 10.1017/S0950268801005210
Levine, M. (1987). Escherichia coli that cause diarrhea - enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. Am. J. Infect. Dis. 155, 377–389. doi: 10.1093/infdis/155.3.377
Lin, A., Sultan, O., Lau, H. K., Wong, E., Hartman, G., and Lauzon, C. R. (2011). O serogroup specific real time PCR assays for the detection and identification of nine clinically relevant non-O157 STECs. Food Microbiol. 28, 478–483. doi: 10.1016/j.fm.2010.10.007
Liu, D., Cole, R. A., and Reeves, P. R. (1996). An O-antigen processing function for Wzx (RfbX): a promising candidate for O-unit flippase. J. Bacteriol. 178, 2102–2107. doi: 10.1128/JB.178.7.2102-2107.1996
Liu, Y., and Fratamico, P. (2006). Escherichia coli O antigen typing using DNA microarrays. Mol. Cell Probes 20, 239–244. doi: 10.1016/j.mcp.2006.01.001
Luedtke, B. E., Bono, J. L., and Bosilevac, J. M. (2014). Evaluation of real time PCR assays for the detection and enumeration of enterohemorrhagic Escherichia coli directly from cattle feces. J. Microbiol. Methods 105, 72–79. doi: 10.1016/j.mimet.2014.07.015
Madic, J., Vingadassalon, N., de Garam, C. P., Marault, M., Scheutz, F., Brugere, H., et al. (2011). Detection of Shiga toxin-producing Escherichia coli serotypes O26:H11, O103:H2, O111:H8, O145:H28, and O157:H7 in raw-milk cheeses by using multiplex real-time PCR. Appl. Environ. Microbiol. 77, 2035–2041. doi: 10.1128/AEM.02089-10
Mainil, J. G., and G., Daube (2005). Verotoxigenic Escherichia coli from animals, humans and foods: who's who? J. Appl. Microbiol. 98, 1332–1344. doi: 10.1111/j.1365-2672.2005.02653.x
Masana, M. O., D'Astek, B. A., Palladino, P. M., Galli, L., and Del Castillo, L. L., and Carbonari, etal. (2011). Genotypic chatcterization of non-O157 Shiga toxin-producing Escherichia coli in beef abattoirs of Argentina. J. Food Prot. 74, 2008–2017. doi: 10.4315/0362-028X.JFP-11-189
McLean, C., Bettelheim, K. A., Kuzevski, A., Falconer, L., and Djordjevic, S. P. (2005). Isolation of Escherichia coli O5:H-, possessing genes for Shiga toxin 1, intimin-beta and enterohaemolysin, from an intestinal biopsy from an adult case of bloody diarrhoea: evidence for two distinct O5:H- pathotypes. J. Med. Microbiol. 54(Pt 6), 605–607. doi: 10.1099/jmm.0.45938-0
Meichtri, L., Miliwebsky, E., Gioffre, A., Chinen, I., Baschkier, A., Chillemi, G., et al. (2004). Shiga toxin-producing Escherichia coli in healthy young beef steers from Argentina: prevalence and virulence properties. Int. J. Food Microbiol. 96, 189–198. doi: 10.1016/j.ijfoodmicro.2004.03.018
Mekata, H., Iguchi, A., Kawano, K., Kirino, Y., Kobayashi, I., and Misawa, N. (2014). Identification of O serotypes, genotypes, and virulotypes of Shiga toxin-producing Escherichia coli isolates, including non-O157 from beef cattle in Japan. J. Food Prot. 77, 1269–1274. doi: 10.4315/0362-028X.JFP-13-506
Melton-Celsa, A., Mohawk, K., Teel, L., and O'Brien, A. (2012). Pathogenesis of Shiga-toxin producing Escherichia coli. Curr Top Microbiol Immunol. 357, 67–103. doi: 10.1007/82_2011_176
Mingle, L. A., Garcia, D. L., Root, T. P., Halse, T. A., Quinlan, T. M., Armstrong, L. R., et al. (2012). Enhanced identification and characterization of non-O157 Shiga toxin-producing Escherichia coli: a six-year study. Foodborne Pathog. Dis. 9, 1028–1036. doi: 10.1089/fpd.2012.1202
Miyao, Y., Kataoka, T., Nomoto, T., Kai, A., Itoh, T., and Itoh, K. (1998). Prevalence of verotoxin-producing Escherichia coli harbored in the intestine of cattle in Japan. Vet. Microbiol. 61, 137–143. doi: 10.1016/S0378-1135(98)00165-5
Monaghan, A., Byrne, B., Fanning, S., Sweeney, T., McDowell, D., and Bolton, D. J. (2011). Serotypes and virulence profiles of non-O157 Shiga toxin-producing Escherichia coli isolates from bovine farms. Appl. Environ. Microbiol. 77, 8662–8668. doi: 10.1128/AEM.06190-11
Monday, S. R., Beisaw, A., and Feng, P. C. H. (2007). Identification of Shiga toxigenic Escherichia coli seropathotypes A and B by multiplex PCR. Mol. Cell Probes. 21, 308–311. doi: 10.1016/j.mcp.2007.02.002
Montenegro, M. A., Bulte, M., Trumpf, T., Aleksic, S., Reuter, G., Bulling, E., et al. (1990). Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J. Clin. Microbiol. 28, 1417–1421. doi: 10.1128/JCM.28.6.1417-1421.1990
Mora, A., Blanco, J., s,.E., Blanco, M., Alonso, M. P., Dhabi, G., et al. (2005). Antimicrobial resistance of Shiga toxin (verotoxin)-producing Escherichia coli O157: H7 and non-O157 strains isolated from humans, cattle, sheep and food in Spain. Res. Microbiol. 156, 793–806. doi: 10.1016/j.resmic.2005.03.006
Moreira, C. N., Pereira, M. A., Brod, C. S., Rodrigues, D. P., Carvalhal, J. B., and Aleixo, J. A. (2003). Shiga toxin-producing Escherichia coli (STEC) isolated from healthy dairy cattle in southern Brazil. Vet. Microbiol. 93, 179–183. doi: 10.1016/S0378-1135(03)00041-5
Moxley, R. A., and Smith, D. R. (2010). Attaching and effacing Escherichia coli infections in cattle. Vet. Clin. North Amer. Food Anim. 26, 29–56. doi: 10.1016/j.cvfa.2009.10.011
Moxley, R. A., Stromberg, Z. R., Lewis, G. L., Loy, J. D., Brodersen, B. W., Patel, I. R., et al. (2015). Haemorrhagic colitis associated with enterohaemorrhagic Escherichia coli O165:H25 infection in a yearling feedlot heifer. J. Microbiol. Mtds Case Rep. 2, 1–6. doi: 10.1099/jmmcr.0.005004
Muniesa, M., Blanco, J. E., de Simon, M., Serra-Moreno, R., Blanch, A. R., and Jofre, J. (2004). Diversity of stx2 converting bacteriophages induced from Shiga-toxin-producing Escherichia coli strains isolated from cattle. Microbiology 150(Pt 9), 2959–2971. doi: 10.1099/mic.0.27188-0
Nasoff, M. S., Baker, H. V. II., and Wolf, R. E. Jr. (1984). DNA sequence of the Escherichia coli gene, gnd, for 6-phosphogluconate dehydrogenase. Gene 27, 253–264. doi: 10.1016/0378-1119(84)90070-2
Navarro, A., Cauich-Sanchez, P. I., Trejo, A., Gutierrez, A., Diaz, S. P., Diaz, C. M., et al. (2018). Characterization of diarrheagenic strains of Escherichia coli isolated from cattle raised in three regions of Mexico. Front. Microbiol. 9:2373. doi: 10.3389/fmicb.2018.02373
Noll, L. W., Shridhar, P. B., Dewsbury, D. M., Shi, X., Cernicchiaro, N., Renter, D. G., et al. (2015a). A comparison of culture- and PCR-based methods to detect six major non-O157 serogroups of Shiga toxin-producing Escherichia coli in cattle feces. PLoS ONE 10:e0135446. doi: 10.1371/journal.pone.0135446
Noll, L. W., Shridhar, P. B., Shi, X., An, B., Cernicchiaro, N., Renter, D. G., et al. (2015b). A four-plex real-time PCR assay, based on rfbE, stx1, stx2, and eae genes, for the detection and quantification of Shiga toxin-producing Escherichia coli O157 in cattle feces. Foodborne Pathog Dis. 12, 787–794. doi: 10.1089/fpd.2015.1951
Norman, K. N., Clawson, M. L., Strockbine, N. A., Mandrell, R. E., Johnson, R., Ziebell, K., et al. (2015). Comparison of whole genome sequences from human and non-human Escherichia coli O26 strains. Front. Cell Infect. Microbiol. 5:21. doi: 10.3389/fcimb.2015.00021
Paddock, Z., Shi, X., Bai, J., and Nagaraja, T. G. (2012). Applicability of a multiplex PCR to detect O26, O45, O103, O111, O121, O145, and O157 serogroups of Escherichia coli in cattle feces. Vet. Microbiol. 156, 381–388. doi: 10.1016/j.vetmic.2011.11.017
Paddock, Z. D., Bai, J., Shi, X., Renter, D. G., and Nagaraja, T. G. (2013). Detection of Escherichia coli O104 in the feces of feedlot cattle by a multiplex PCR assay designed to target major genetic traits of the virulent hybrid strain responsible for the 2011 German outbreak. Appl. Environ. Microbiol. 79, 3522–3525. doi: 10.1128/AEM.00246-13
Paddock, Z. D., Renter, D. G., Cull, C. A., Shi, X., Bai, J., and Nagaraja, T. G. (2014). Escherichia coli O26 in feedlot cattle: fecal prevalence, isolation, characterization, and effects of an E. coli O157 vaccine and a direct-fed microbial. Foodborne Pathog. Dis. 11, 186–193. doi: 10.1089/fpd.2013.1659
Paquette, S.-J., Stanford, K., Thomas, J., and Reuter, T. (2018). Quantitative surveillance of shiga toxins 1 and 2, Escherichia coli O178 and O157 in feces of western-Canadian slaughter cattle enumerated by droplet digital PCR with a focus on seasonality and slaughterhouse location. PLoS ONE 13:e0195880. doi: 10.1371/journal.pone.0195880
Parma, A. E., Sanz, M. E., Blanco, J. E., Blanco, J., Vinas, M. R., Blanco, M., et al. (2000). Virulence genotypes and serotypes of verotoxigenic Escherichia coli isolated from cattle and foods in Argentina. Importance in public health. Eur. J. Epidemiol. 16, 757–762. doi: 10.1023/A:1026746016896
Peng, Z., Liang, W., Hu, Z., Li, X., Guo, R., Hua, L., et al. (2019). O-serogroups, virulence genes, antimicrobial susceptibility, and MLST genotypes of Shiga toxin-producing Escherichia coli from swine and cattle in Central China. BMC Veterinary Res. 15, 427–427. doi: 10.1186/s12917-019-2177-1
Perelle, S., Dilasser, F., Grout, J., and Fach, P. (2004). Detection by 5'-nuclease PCR of Shiga-toxin producing Escherichia coli O26, O55, O91, O103, O111, O113, O145 and O157:H7, associated with the world's most frequent clinical cases. Mol. Cell. Probes 18, 185–192. doi: 10.1016/j.mcp.2003.12.004
Polifroni, R., Etcheverría, A. I., Sanz, M. E., Cepeda, R. E., Krüger, A., Lucchesi, P. M., et al. (2012). Molecular characterization of Shiga toxin-producing Escherichia coli isolated from the environment of a dairy farm. Curr. Microbiol. 3, 337–343. doi: 10.1007/s00284-012-0161-0
Pradel, N., Livrelli, V., De Champs, C., Palcoux, J. B., Reynaud, A., Scheutz, F., et al. (2000). Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J. Clin. Microbiol. 38, 1023–1031. doi: 10.1128/JCM.38.3.1023-1031.2000
Renter, D. G., Morris, J. G., Sargeant, J. M., Hungerford, L. L., Berezowski, J., Ngo, T., et al. (2005). Prevalence, risk factors, O serogroups, and virulence profiles of Shiga toxin-producing bacteria from cattle production environments. J. Food Prot. 68, 1556–1565. doi: 10.4315/0362-028X-68.8.1556
Samuel, G., and Reeves, P. (2003). Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 338, 2503–2519. doi: 10.1016/j.carres.2003.07.009
Sanchez, S., Llorente, M. T., Echeita, M. A., and Herrera-Leon, S. (2015). Development of three multiplex PCR assays targeting the 21 most clinically relevant serogroups associated with Shiga toxin-producing E. coli infection in humans. PLoS ONE 10:e0117660. doi: 10.1371/journal.pone.0117660
Sandhu, K. S., Clarke, R. C., and Gyles, C. L. (1999). Virulence markers in Shiga toxin-producing Escherichia coli isolated from cattle. Can. J. Vet. Res. 63, 177–184.
Sandhu, K. S., Clarke, R. C., McFadden, K., Brouwer, A., Louie, M., Wilson, J., et al. (1996). Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in Southwest Ontario. Epidemiol. Infect. 116, 1–7. doi: 10.1017/S095026880005888X
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M.-A., and Roy, S. L. (2011). Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17, 7–15. doi: 10.3201/eid1701.P11101
Schurman, R. D., Hariharan, H., Heaney, S. B., and Rahn, K. (2000). Prevalence and characteristics of Shiga toxin-producing Escherichia coli in beef cattle slaughtered on Prince Edward Island. J. Food Prot. 63, 1583–1586. doi: 10.4315/0362-028X-63.11.1583
Scott, L., McGee, P., Walsh, C., Fanning, S., Sweeney, T., Blanco, J., et al. (2009). Detection of numerous verotoxigenic E. coli serotypes, with multiple antibiotic resistance from cattle faeces and soil. Vet. Microbiol. 134, 288–293. doi: 10.1016/j.vetmic.2008.08.008
Shridhar, P. B., Noll, L. W., Shi, X., An, B., Cernicchiaro, N., Renter, D. G., et al. (2016a). Multiplex quantitative PCR assays for the detection and quantification of the six major non-O157 Escherichia coli serogroups in cattle feces. J. Food Prot. 79, 66–74. doi: 10.4315/0362-028X.JFP-15-319
Shridhar, P. B., Noll, L. W., Shi, X., Cernicchiaro, N., Renter, D. G., Bai, J., et al. (2016b). Escherichia coli O104 in feedlot cattle feces: prevalence, isolation and characterization. PLoS ONE 11:e0152101. doi: 10.1371/journal.pone.0152101
Shridhar, P. B., Siepker, C., Noll, L. W., Shi, X., Nagaraja, T. G., and Bai, J. (2017). Shiha toxin subtypes of non-O157 Escherichia coli serogroups isolated from cattle feces. Front. Cell. Infect. Microbiol. 7:121. doi: 10.3389/fcimb.2017.00121
Smith, H. R., Scotland, S. M., Willshaw, G. A., Wray, C., McLaren, I. M., Cheasty, T., et al. (1988). Vero cytotoxin production and presence of VT genes in Escherichia coli strains of animal origin. Microbiology 134, 829–834. doi: 10.1099/00221287-134-3-829
Stromberg, Z. R., Lewis, G. L., Schneider, L. G., Erickson, G. E., Patel, I. R., Smith, D. R., et al. (2018). Culture-based quantification with molecular characterization of non-O157 and O157 enterohemorrhagic Escherichia coli isolates from rectoanal mucosal swabs of feedlot cattle. Foodborne Pathog. Dis. 15, 26–32. doi: 10.1089/fpd.2017.2326
Suthienkul, O., Brown, J. E., Seriwatana, J., Tienthongdee, S., Sastravaha, S., and Echeverria, P. (1990). Shiga-like-toxin-producing Escherichia coli in retail meats and cattle in Thailand. Appl. Environ. Microbiol. 56, 1135–1139. doi: 10.1128/AEM.56.4.1135-1139.1990
Tanaro, J. D., Galli, L., Lound, L. H., Leotta, G. A., Piaggio, M. C., Carbonari, C. C., et al. (2012). Non-O157:H7 Shiga toxin-producing Escherichia coli in bovine rectums and surface water streams on a beef cattle farm in Argentina. Foodborne Pathog Dis. 9, 878–884. doi: 10.1089/fpd.2012.1182
Timm, C. D., Irino, K., Gomes, T. A. T., Viera, M. M., Guth, B. E. C., Vaz, T. M. I., et al. (2007). Virulence markers and serotypes of Shiga toxin-producing Escherichia coli isolated from cattle in Rio Grande do Sul, Brazil. Lett. Appl. Microbiol. 44, 419–425. doi: 10.1111/j.1472-765X.2006.02085.x
Urdahl, A. M., Beutin, L., Skjerve, E., Zimmermann, S., and Wasteson, Y. (2003). Animal host associated differences in Shiga toxin-producing Escherichia coli isolated from sheep and cattle on the same farm. J. Appl. Microbiol. 95, 92–101. doi: 10.1046/j.1365-2672.2003.01964.x
Valilis, E., Ramsey, A., Sidiq, S., and DuPont, H. L. (2018). Non-O157 Shiga toxin-producing Escherichia coli-A poorly appreciated enteric pathogen: systematic review. Int. J. Infect. Dis. 76, 82–87. doi: 10.1016/j.ijid.2018.09.002
Wells, J. G., Shipman, L. D., Greene, K. D., Sowers, E. G., Green, J. H., Cameron, D. N., et al. (1991). Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J. Clin. Microbiol. 29, 985–989. doi: 10.1128/JCM.29.5.985-989.1991
Wieler, L., Vieler, E., Erpenstein, C., Schlapp, T., Steinruck, H., Bauerfeind, R., et al. (1996). Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J. Clin. Microbiol. 34, 2980–2984. doi: 10.1128/JCM.34.12.2980-2984.1996
Wieler, L. H., Schwanitz, A., Vieler, E., Busse, B., Steinrück, H., Kaper, J. B., et al. (1998). Virulence properties of Shiga toxin-producing Escherichia coli (STEC) strains of serogroup O118, a major group of STEC pathogens in calves. J. Clin. Microbiol. 36, 1604–1607. doi: 10.1128/JCM.36.6.1604-1607.1998
Wilson, J. B., McEwen, S. A., Clarke, R. C., Leslie, K. E., Wilson, R. A., Waltner-Toews, D., et al. (1992). Distribution and characteristics of verocytotoxigenic Escherichia coli isolated from Ontario dairy cattle. Epidemiol. Infect. 108, 423–439. doi: 10.1017/S0950268800049931
Wray, C., McLaren, I. M., and Carroll, P. J. (1993). Escherichia coli isolated from farm animals in England and Wales between 1986 and 1991. Vet. Rec. 133, 439–442. doi: 10.1136/vr.133.18.439
Keywords: shiga toxin-producing Escherichia coli (STEC), top-7 STEC, non-top-7 STEC, Multiplex PCR assays, cattle, feces
Citation: Ludwig JB, Shi X, Shridhar PB, Roberts EL, DebRoy C, Phebus RK, Bai J and Nagaraja TG (2020) Multiplex PCR Assays for the Detection of One Hundred and Thirty Seven Serogroups of Shiga Toxin-Producing Escherichia coli Associated With Cattle. Front. Cell. Infect. Microbiol. 10:378. doi: 10.3389/fcimb.2020.00378
Received: 06 April 2020; Accepted: 18 June 2020;
Published: 29 July 2020.
Edited by:
Roxane M. Piazza, Butantan Institute, BrazilReviewed by:
Nora Lía Padola, National University of Central Buenos Aires, ArgentinaBeatriz Arellano Reynoso, National Autonomous University of Mexico, Mexico
Copyright © 2020 Ludwig, Shi, Shridhar, Roberts, DebRoy, Phebus, Bai and Nagaraja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfa Bai, amJhaUB2ZXQuay1zdGF0ZS5lZHU=; T. G. Nagaraja, dG5hZ2FyYWpAdmV0Lmstc3RhdGUuZWR1
 Justin B. Ludwig1
Justin B. Ludwig1 Chitrita DebRoy
Chitrita DebRoy T. G. Nagaraja
T. G. Nagaraja