- 1Infectious Disease Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, South Korea
- 2Department of Biomolecular Science, KRIBB School of Bioscience, Korea University of Science and Technology (UST), Daejeon, South Korea
- 3Environmental Diseases Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, South Korea
Following infection with certain strains of Shiga toxin-producing Escherichia coli (STEC), particularly enterohemorrhagic ones, patients are at elevated risk for developing life-threatening extraintestinal complications, such as acute renal failure. Hence, these bacteria represent a public health concern in both developed and developing countries. Shiga toxins (Stxs) expressed by STEC are highly cytotoxic class II ribosome-inactivating proteins and primary virulence factors responsible for major clinical signs of Stx-mediated pathogenesis, including bloody diarrhea, hemolytic uremic syndrome (HUS), and neurological complications. Ruminant animals are thought to serve as critical environmental reservoirs of Stx-producing Escherichia coli (STEC), but other emerging or arising reservoirs of the toxin-producing bacteria have been overlooked. In particular, a number of new animal species from wildlife and aquaculture industries have recently been identified as unexpected reservoir or spillover hosts of STEC. Here, we summarize recent findings about reservoirs of STEC and review outbreaks of these bacteria both within and outside the United States. A better understanding of environmental transmission to humans will facilitate the development of novel strategies for preventing zoonotic STEC infection.
Introduction
Escherichia coli is a component of the normal flora in the human gut, but some strains are pathogenic. Based on its pathotypes, intestinal pathogenic E. coli can be classified into six groups: Shiga toxin (Stx)-producing [STEC, also referred to as verocytotoxin-producing (VTEC) or enterohemorrhagic (EHEC)], enterotoxigenic (ETEC), enteropathogenic (EPEC), enteroaggregative (EAEC), enteroinvasive (EIEC), and diffusely adherent (DAEC) (Kaper et al., 2004). Among those, STEC tends to be a clonal group characterized by somatic (O) antigen, and more than 200 serotypes of E. coli have been known to produce Stxs based on their molecular and genetic features. In addition, a new classification scheme of five seropathotypes (A–E) based on virulence, serological and genetic features has been suggested due to the various symptoms and severity of clinical STEC infections (Frankel et al., 1998; Nataro and Kaper, 1998; Boerlin et al., 1999; Karmali et al., 2003). However, a recent massive outbreak in Germany raised questions about the efficacy of this categorization because the strain involved was not classified as type A or B based on its genetics (specifically, it was negative for the LEE Island). Hence, in this review, we summarize outbreaks and STEC isolates by serotype, not seropathotype, based on surveillance reports.
Stxs are a family of bacterial exotoxins expressed by Shigella dysenteriae serotype 1 and STEC (Fraser et al., 1994; Sandvig, 2001). These toxins are primary virulence factors responsible for bloody diarrheal disease that can progress to life-threatening systemic sequelae, such as an acute renal failure syndrome (also known as hemolytic uremic syndrome, HUS), as well as central nervous system (CNS) abnormalities (Tarr et al., 2005; Lee et al., 2016; Lee and Tesh, 2019). The toxins produced by STEC are classified as type 1 (Stx1) and type 2 (Stx2), and several Stx1/Stx2 subtypes and variants have been reported based on the receptor preference and toxin potency (Scheutz et al., 2012; Melton-Celsa, 2014). And among those, Stx2, which is more potent than Stx1, causes clinically severe weight loss and renal injury (Lentz et al., 2011; Pradhan et al., 2016).
Multiple studies have focused on revealing the source and transmission route of STEC infections in humans and the food chain (Erickson and Doyle, 2007; Kintz et al., 2017). Animals are undoubtedly the most important carriers of STEC, as these strains have been isolated from a wide variety of domestic and human-associated animal species (Persad and LeJeune, 2014; Espinosa et al., 2018). Several lines of evidence have confirmed zoonotic human infections caused by contact with companion and domestic animals (Chalmers et al., 1997; Luna et al., 2018). In addition, work in recent decades has emphasized the importance of wildlife surveillance, as a large proportion of emerging zoonotic pathogens are of wildlife origin (Jones et al., 2013), and increasing numbers of wild animals have been shown to be potential STEC reservoirs (Espinosa et al., 2018). Although the need for the One Health approach has been continuously emphasized in STEC research, surveillance studies have generally been limited to domestic animals (Garcia et al., 2010). However, a recent STEC surveillance study revealed that more distantly related fields, such as aquaculture, should be included as important areas of interest and monitored accordingly. In this review, we update the list of animal species recently reported as STEC reservoirs. In so doing, our goal is to emphasize the importance of applying the interdisciplinary One Health approach in surveillance systems by strengthening multi-sectorial collaboration between agriculture, aquaculture, and wildlife science, as well as to provide a broad perspective on industrial fields relevant to food production.
STEC Global Outbreaks and Clinical Isolates
Historically, Stxs and verotoxin were studied separately. Stxs was discovered by Kiyoshi Shiga in 1898 as a factor involved in bacterial dysentery caused by S. dysenteriae serotype I (Kaper and O'Brien, 2014). Independently, in 1977, verotoxin was discovered by Konowalchuck in diarrheagenic E. coli strains (Konowalchuk et al., 1977). In 1983, Johnson et al. confirmed that two toxins belonged to the same family (Johnson et al., 1983), and they began to be considered together in studies of the first STEC outbreak strains from 1982. Shiga toxin-producing bacteria, including STEC and S. dysenteriae serotype 1, are agents of hemorrhagic colitis, which can progress to potentially lethal complications, such as bloody diarrhea-associated HUS (D + HUS) with acute renal dysfunction (Figure 1) and CNS disorders, such as seizure or paralysis. Investigations of major outbreaks have focused on STEC, rather than on S. dysenteriae serotype 1 because STEC infections are more common in the broader community than Shigella infections.
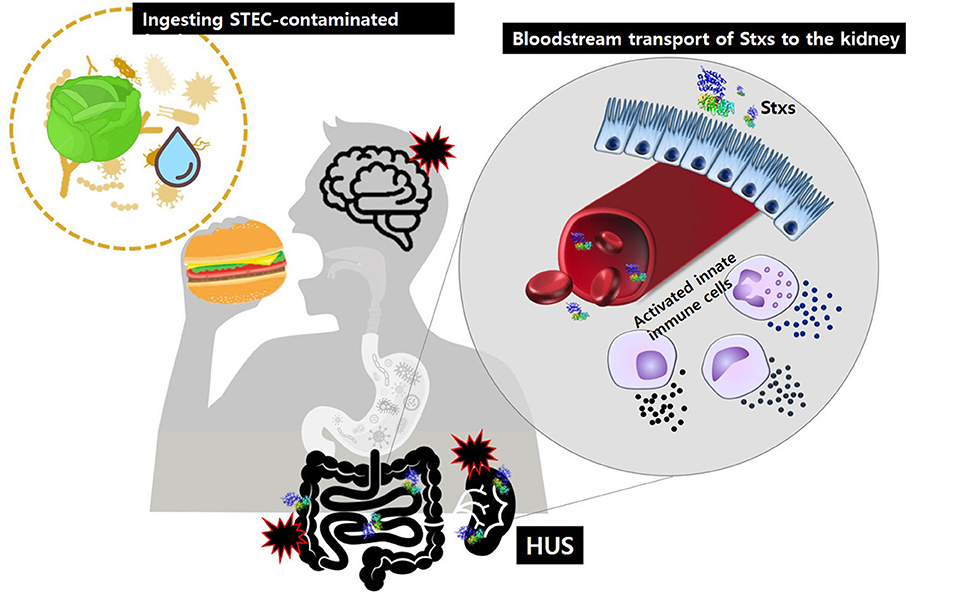
Figure 1. After ingestion of food or water contaminated with pathogenic STEC, Stxs may cross the intestinal epithelial barrier via M-cell uptake and transcytosis or paracellular transport. Once in the submucosa, the toxins activate innate immune cells, such as neutrophils or monocytes that act as “carrier” cells to deliver Stxs in the bloodstream and may also further exacerbate tissue injury via localized production of proinflammatory cytokines. Ultimately, the toxins are transferred to glomerular endothelial cells and tubular epithelial cells, which are rich in the toxin receptor Gb3. Damage to the kidney, the primary target organ, leads to D + HUS (diarrhea-associated hemolytic uremic syndrome).
In the United States
In 1982, two severe outbreaks that caused HUS occurred in Oregon and Michigan. E. coli O157:H7 was isolated from the stool specimens of patients and determined to be the cause of disease (Centers for Disease, 1982). After a year, production of Stxs was confirmed by comparing toxins purified from S. dysenteriae and three E. coli isolates from the outbreaks (O'Brien et al., 1983). Since then, STEC O157 has rapidly emerged as a major problem in the food industry and clinics. In the 30 years since the first report, a total of 740 outbreaks caused by STEC O157:H7 and O157:NM were reported in the United States. A total of 13,526 cases resulted in 2,765 hospitalizations (20%), 653 HUS (4.8%), and 73 deaths (0.5%) (Rangel et al., 2005; Heiman et al., 2015). In all years since 1994 except for 1997, the annual outbreak size rose above 30 cases a year.
Food is the best-known transmission route of STEC O157. The frequency of foodborne outbreaks has increased dramatically over the past three decades: 183 out of a total of 350 outbreaks (52%) in the first 20 years (1982–2002) vs. 255 out of a total of 390 outbreaks (65%) in the last 10 years (2003–2012). Over the same period, the incidence of outbreaks via other routes has decreased: person-to-person (14–10%), water (9–4%), and other or unknown reasons (21–11%). Interestingly, STEC outbreaks due to animal contact have also become more common, from 11 (3%) in the first 20 years to 39 (10%) in the last 10 years, indicating that animal resources represent important STEC reservoirs (Rangel et al., 2005; Heiman et al., 2015) (see Environmental Transmission section).
Although STEC O157 was the first E. coli strain involved in Stx–related disease and remains the most important strain in this regard, non-O157 STEC strains also represent a major public health concern. The Centers for Disease Control and Prevention estimates that 265,000 STEC infections occur each year in the United States, of which STEC O157 causes 36%; thus 64% of STEC infections are non-O157 (Scallan et al., 2011). More than 50 non-O157 STEC serogroups are involved in human illness. The first US outbreak of non-O157 STEC, caused by STEC O111, was reported in 1990; over the next 20 years (1990–2010), 46 outbreaks caused 1,727 illnesses, 144 hospitalizations, and one death. As with O157, food (n = 20, 43%) is a major transmission route in non-O157 outbreaks (Luna-Gierke et al., 2014).
Since the first outbreak in 1990, 11 serotypes and one undetermined type have been observed in non-O157 outbreaks. The most commonly isolated serotype is O111, followed by O26; together, O111 and O26 account for more than 60% of outbreaks (Brooks et al., 2005; Luna-Gierke et al., 2014). O103, O121, O45, O145, O104, O165, O69, O84, and O141 are also frequently isolated from outbreak patients. Interestingly, although non-O157 infection is almost twice as common as O157 infection, non-O157 strains cause fewer outbreaks than O157 (Scallan et al., 2011). This might be due to the greater severity of O157 (more hospitalization) or issues with subtyping techniques (e.g., it is difficult to subtype non-O157 strains) (Gould et al., 2013).
Outside the United States
The World Health Organization (WHO) estimated that STEC infection caused more than 1 million illnesses and 100 deaths in 2010 (Havelaar et al., 2015). Between 1998 and 2016, the European region (EUR) and Western Pacific region (WPR) reported 211 STEC outbreaks (EUR: 176, WPR: 35), far fewer than the number of outbreaks in the Americas (708) (FAO/WHO, 2018).
The largest O157 STEC outbreak ever recorded occurred in the WPR (Japan, 1996) (Fukushima et al., 1999). Of 12,680 symptomatic patients, 121 (0.95%) developed HUS, and three died. After that massive outbreak, the frequency of STEC cases increased dramatically: from 1999 to 2012, more than 3,000 cases were reported in Japan, whereas during the previous 5 years (1991–1995) the annual average was only 105 cases. Following O157, the most frequent serotype, other common serogroups of STEC are O26, O111, O103, O121, and O145 (Terajima et al., 2014).
The most severe outbreak of non-O157 STEC (O104) occurred in EUR (Germany, 2011): over a 3 months period, 3,816 cases were reported. Despite the smaller number of cases relative to the Sakai outbreak, the rates of HUS (n = 845, 22.4%) and death (n = 54) made the German outbreak historic (Frank et al., 2011). According to surveillance reports from Food- and Waterborne Diseases and Zoonoses and the European Centre for Disease Prevention and Control, the total number of confirmed STEC infections was 3,573 (doi: 10.2903/j.efsa.2011.2090) in 2009, increasing dramatically to 6,073 cases in 2017 (https://doi.org/10.2903/j.efsa.2018.5500). As in other regions, the most commonly reported serogroup from 2009 to 2017 was O157, followed by O26, O103, O91, O145, and O146. However, the proportion of O157 dropped from 51.7 to 31.9%, whereas the proportion of non-O157 infections increased accordingly. Among the 31 countries in Europe, Germany and the United Kingdom had the highest human STEC infection rates.
Environmental Transmission of STEC
Over the past decade, interest in zoonotic pathogens of wildlife origin has increased because those pathogens were shown to constitute the primary source (>60%) of emerging infectious diseases (Jones et al., 2008). Moreover, adaptation of certain urban exploiter animal species has increased contact between wild animals and humans, potentiating the transmission of zoonotic pathogens by fecal contamination of agri-food, the environment, or the water chain (Rothenburger et al., 2017). Although most E. coli are commensal organisms of both humans and animals, the emergence of STEC has been reported in almost all parts of the world and from a wide variety of animal species, including mammals, birds, amphibians, fish, and invertebrates (Persad and LeJeune, 2014; Espinosa et al., 2018). We have updated the list of animal species reported to be reservoir or spillover hosts for, or to be contaminated by, STEC strains (Table 1).
Domestic Animals Are Indisputable Reservoirs of STEC
Ruminants are recognized as principal reservoirs of STEC, especially O157 (Gyles, 2007; La Ragione et al., 2009). As with humans, ruminants are exposed to STEC through contaminated feed and drinking water, or by exposure to the feces of other animals that are shedding the bacteria (LeJeune et al., 2001; Persad and LeJeune, 2014). Among ruminants, cattle (especially ruminating post-weaning calves and heifers) are considered to be the most important STEC reservoirs without symptomatic colonization (Caprioli et al., 2005; Gyles, 2007; Ferens and Hovde, 2011). The natural absence of vascular receptors (globotriaosylceramide) in the intestinal vasculature of the cattle inhibits endocytosis and transportation of Stxs to other organs that might be sensitive to the toxins, resulting in asymptomatic colonization in the large intestine (Pruimboom-Brees et al., 2000; Naylor et al., 2003; Nguyen and Sperandio, 2012). Like cattle, smaller ruminants, such as sheep and goats are also recognized as significant carriers due to their ability to harbor STEC O157 and other serotypes; these animals are important asymptomatic shedders in the epidemiology of bacterial infections in the United States, Australia, and Europe (Beutin et al., 1993; Cortes et al., 2005; Gyles, 2007; La Ragione et al., 2009; Brandal et al., 2012). Also as in cattle, the asymptomatic nature of STEC colonization in smaller ruminants might be due to their lack of vascular receptors for Stx (Persad and LeJeune, 2014). In addition, STEC O157 and non-O157 strains have been reported in other domestic or captive ruminant species, such as alpacas, antelopes, American bison, various deer species, elk, llamas, moose, water buffalo, and yaks (Galiero et al., 2005; French et al., 2010; Chandran and Mazumder, 2013; Mohammed Hamzah et al., 2013; Nyholm et al., 2015).
Several recent surveillance studies have provided strong evidence that monogastric farm animals should now be considered as important reservoir or spillover hosts of STEC. Although the prevalence of STEC O157 and other serotypes varies in swine (Fairbrother and Nadeau, 2006; Ferens and Hovde, 2011), pigs have been shown to harbor and shed STEC for up to 2 months post-infection (Booher et al., 2002). Moreover, because pigs possess Stx-sensitive vascular receptors (globotetraosylceramide) in their intestines, they are susceptible to STEC strains possessing Stx2e, which cause edema with apparent clinical signs and mortality (Waddell et al., 1998; Pruimboom-Brees et al., 2000; Fratamico et al., 2004; Steil et al., 2016). Moreover, although horses are not considered reservoirs for STEC due to its low prevalence in that species (Hancock et al., 1998; Pritchard et al., 2009; Lengacher et al., 2010), some cases of clinical infection from equine contact have been reported (Chalmers et al., 1997; Luna et al., 2018); therefore, horses should be considered as a potential source of infection. Domestic poultry, such as chicken, duck, and turkeys have also been reported to carry STEC (Doane et al., 2007; Ferens and Hovde, 2011; Koochakzadeh et al., 2015). In particular, chickens which were experimentally inoculated with STEC O157 can harbor and shed the bacteria in their feces for almost a year (Schoeni and Doyle, 1994).
The importance of companion animals (pets) in the epidemiology of STEC infection should not be underestimated. Via their feces, pets, such as dogs and cats can serve as asymptomatic shedders in the epidemiology of a wide range of STEC serotypes (Beutin, 1999; Roopnarine et al., 2007; Hogg et al., 2009; Rumi et al., 2012). Accordingly, several clinical infections due to canine and feline exposure have been reported (Busch et al., 2007; Persad and LeJeune, 2014; McFarland et al., 2017). STEC has also been found from the feces of wild canids but not felids (Mora et al., 2012; Persad and LeJeune, 2014).
Wild Animals Are Important Reservoir or Spillover Hosts of STEC
The number of STEC outbreaks associated with the consumption of fruits and vegetables contaminated with wild animal feces is increasing (World Health Organization, 2016). Hence, from a global public health standpoint, it is important to investigate the prevalence of STEC in urban exploiter and wild animals that can transmit the bacteria to human by direct and/or indirect contact. Therefore, several studies have investigated the prevalence of STEC among urban exploiter species, such as rats (Himsworth et al., 2015), pigeons (Gargiulo et al., 2014; Murakami et al., 2014), and flies (Kobayashi et al., 1999; Alam and Zurek, 2004; Keen et al., 2006). In fact, rodents are capable of harboring and shedding STEC, and various serogroups have been recovered from animals living in urban areas and farms (Blanco Crivelli et al., 2012; Kilonzo et al., 2013). Moreover, many wild bird species found in close proximity to livestock operations, waste disposal landfill sites, and human habitation areas have also been identified as potential sources of STEC infection (Cizek et al., 2000; Pedersen and Clark, 2007). In addition, houseflies can harbor and transmit STEC O157 to other animals, demonstrating that insects can be important vectors in the dissemination of STEC within the environment (Kobayashi et al., 1999; Alam and Zurek, 2004; Keen et al., 2006). Because domestic animal feed represents an easy food source for rodents, birds, and insects, these animals are attracted to farms and may transmit STEC between livestock and humans or vice versa.
Likewise, wild animals residing in close proximity to livestock facilities can be contaminated (or harbored) with STEC (Espinosa et al., 2018). Several recent studies emphasized the urgent need to investigate the prevalence of STEC in wild animals, as some large STEC outbreaks were closely related to or originated from contamination from wild animal feces (Laidler et al., 2013; Crook and Senior, 2017; Soderqvist et al., 2019). Although wild animals were identified as a source of STEC in the 1990s, more than 70% of relevant studies were published since the turn of the century, and an increasing number of wild animal species have been identified as reservoir or spillover hosts for STEC (Espinosa et al., 2018). Nevertheless, very little published research has addressed the role of wild animals in the transmission of STEC to humans, domestic animals, and within the food chain. Animals, such as wild boars, deer, birds, and rodents might be involved in direct interspecies contact between humans, domestic, and wild animals, thereby creating a circle of transmission that increases the prevalence of STEC. These species should be thoroughly monitored, as they could potentially cause a spillover or spillback to humans and other animals (Daszak et al., 2000).
Emerging Reservoirs of STEC and Needs for the One Health Approach
Numerous studies have reported both O157 and non-O157 STEC in fresh fish and shellfish, and their ready-to-eat products in retail markets (Thampuran et al., 2005; Surendraraj et al., 2010; Prakasan et al., 2018), suggesting that human activities, such as handling, processing, and ingestion of the products might be a major source of STEC contamination. Interestingly, fish and shellfish residing in coastal areas, some cultured fish, and those in close proximity to or downstream of animal livestock facilities have been found to be contaminated with STEC (Gourmelon et al., 2006; Sekhar et al., 2017; Cardozo et al., 2018; Siddhnath et al., 2018; Hussein et al., 2019). These results strongly indicate that fish and shellfish are a potential reservoir or spillover hosts of STEC, and that effluent water from STEC-contaminated culture ponds might also be an additional potential source of transmission, emphasizing the need for further investigations of the aquaculture industry.
Based on the findings of recent surveillance approaches, a wide range of domestic, captive, and wild animals, including aquatic animals, can transmit STEC to humans directly by ingestion or contact at farms and petting zoos, or indirectly through fecal contaminations in water sources, vegetable fields, or meats and milks. Moreover, STEC is closely associated with human activities; therefore, the broad expansion of human activities due to technological advances will expand contaminations to an increasingly wider variety of wild organisms and foodstuffs in the future. Therefore, a detailed identification of the prevalence of STEC in various animal species will be essential for epidemiological investigations and the development of proper risk mitigation strategies (Persad and LeJeune, 2014). The integration of human and animal health was appreciated in ancient times, but this idea was comprehensively revisited through the One Health perspective, which proposes a unification of human and veterinary medicine to protect against zoonotic pathogens (King et al., 2008; Zinsstag et al., 2011). Investigations of STEC outbreaks in humans also clearly demonstrate the relevance of the One Health concept (Jay et al., 2007; Laidler et al., 2013; McFarland et al., 2017). Moreover, the importance of a One Health approach for control or prevention of STEC infection has already been emphasized in practical cases (Garcia et al., 2010). A number of new animal species, including those of aquatic origin, have been identified as unexpected reservoir or spillover hosts of STEC. Therefore, we propose an alternative One Health approach in which coordinated multidisciplinary efforts integrate terrestrial and aquatic animal medicine within future STEC surveillance. These efforts should facilitate the development of novel strategies to prevent, control, and treat zoonotic STEC infections.
Conclusion
Since the advent of systematic and efficient diagnostic techniques, reports of national STEC outbreaks have increased dramatically. The current world-wide surveillance system reveals the impact of STEC infection, the diversity of STEC, and sources of contamination. Although contaminated food is the most prominent source of STEC outbreaks, infections caused by contact with animals has increased over the past 10 years. Hence, understanding of animals as potential STEC reservoir and their transmission is essential for preventing the occurrence of STEC infections and outbreaks. Multiple complex studies aimed at discovering numerous STEC in the various animals have revealed a wide range of strains capable of producing Stxs, however, it remains to be determined to what extent these newly identified reservoirs are involved in the pathogenesis and transmission of the bacteria. In particular, several animals in more distantly related fields, such as fish produced by the aquaculture industry and a wide range of underestimated wild animal species have been reported as potential STEC reservoirs. Therefore, we propose an alternative One Health approach in which coordinated multidisciplinary efforts integrate terrestrial and aquatic animal medicine in the context of future STEC surveillance efforts.
Author Contributions
Written and revised by J-SK, M-SL, and JK.
Funding
This work was supported by the KRIBB Research Initiative Programs, the Collaborative Genome Program of the Korea Institute of Marine Science and Technology Promotion (20180430) funded by the Ministry of Oceans and Fisheries, and Basic Science Research Program (2019R1I1A2A01041221) through the National Research Foundation of Korea (NRF), funded by the Ministry of Education.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alam, M. J., and Zurek, L. (2004). Association of Escherichia coli O157:H7 with houseflies on a cattle farm. Appl. Environ. Microbiol. 70, 7578–7580. doi: 10.1128/AEM.70.12.7578-7580.2004
Bardiau, M., Gregoire, F., Muylaert, A., Nahayo, A., Duprez, J. N., Mainil, J., et al. (2010). Enteropathogenic (EPEC), enterohaemorragic (EHEC) and verotoxigenic (VTEC) Escherichia coli in wild cervids. J. Appl. Microbiol. 109, 2214–2222. doi: 10.1111/j.1365-2672.2010.04855.x
Beutin, L., Geier, D., Steinruck, H., Zimmermann, S., and Scheutz, F. (1993). Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31, 2483–2488. doi: 10.1128/JCM.31.9.2483-2488.1993
Blanco Crivelli, X., Rumi, M. V., Carfagnini, J. C., Degregorio, O., and Bentancor, A. B. (2012). Synanthropic rodents as possible reservoirs of shigatoxigenic Escherichia coli strains. Front. Cell. Infect Microbiol. 2:134. doi: 10.3389/fcimb.2012.00134
Boerlin, P., McEwen, S. A., Boerlin-Petzold, F., Wilson, J. B., Johnson, R. P., and Gyles, C. L. (1999). Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37, 497–503. doi: 10.1128/JCM.37.3.497-503.1999
Booher, S. L., Cornick, N. A., and Moon, H. W. (2002). Persistence of Escherichia coli O157:H7 in experimentally infected swine. Vet. Microbiol. 89, 69–81. doi: 10.1016/S0378-1135(02)00176-1
Borges, C. A., Cardozo, M. V., Beraldo, L. G., Oliveira, E. S., Maluta, R. P., Barboza, K. B., et al. (2017). Wild birds and urban pigeons as reservoirs for diarrheagenic Escherichia coli with zoonotic potential. J. Microbiol. 55, 344–348. doi: 10.1007/s12275-017-6523-3
Brandal, L. T., Sekse, C., Lindstedt, B. A., Sunde, M., Lobersli, I., Urdahl, A. M., et al. (2012). Norwegian sheep are an important reservoir for human-pathogenic Escherichia coli O26:H11. Appl. Environ. Microbiol. 78, 4083–4091. doi: 10.1128/AEM.00186-12
Brooks, J. T., Sowers, E. G., Wells, J. G., Greene, K. D., Griffin, P. M., Hoekstra, R. M., et al. (2005). Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 192, 1422–1429. doi: 10.1086/466536
Busch, U., Hormansdorfer, S., Schranner, S., Huber, I., Bogner, K. H., and Sing, A. (2007). Enterohemorrhagic Escherichia coli excretion by child and her cat. Emerg. Infect. Dis. 13, 348–349. doi: 10.3201/eid1302.061106
Caprioli, A., Morabito, S., Brugere, H., and Oswald, E. (2005). Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36, 289–311. doi: 10.1051/vetres:2005002
Cardozo, M. V., Borges, C. A., Beraldo, L. G., Maluta, R. P., Pollo, A. S., Borzi, M. M., et al. (2018). Shigatoxigenic and atypical enteropathogenic Escherichia coli in fish for human consumption. Braz. J. Microbiol. 49, 936–941. doi: 10.1016/j.bjm.2018.02.013
Centers for Disease, C. (1982). Isolation of E. coli O157:H7 from sporadic cases of hemorrhagic colitis–United States. MMWR Morb. Mortal. Wkly Rep. 31, 580–585.
Chalmers, R. M., Salmon, R. L., Willshaw, G. A., Cheasty, T., Looker, N., Davies, I., et al. (1997). Vero-cytotoxin-producing Escherichia coli O157 in a farmer handling horses. Lancet 349:1816. doi: 10.1016/S0140-6736(05)61697-2
Chandran, A., and Mazumder, A. (2013). Prevalence of diarrhea-associated virulence genes and genetic diversity in Escherichia coli isolates from fecal material of various animal hosts. Appl. Environ. Microbiol. 79, 7371–7380. doi: 10.1128/AEM.02653-13
Cizek, A., Literak, I., and Scheer, P. (2000). Survival of Escherichia coli O157 in faeces of experimentally infected rats and domestic pigeons. Lett. Appl. Microbiol. 31, 349–352. doi: 10.1046/j.1472-765x.2000.00820.x
Cortes, C., De La Fuente, R., Blanco, J., Blanco, M., Blanco, J. E., Dhabi, G., et al. (2005). Serotypes, virulence genes and intimin types of verotoxin-producing Escherichia coli and enteropathogenic E. coli isolated from healthy dairy goats in Spain. Vet. Microbiol. 110, 67–76. doi: 10.1016/j.vetmic.2005.06.009
Crook, B., and Senior, H. (2017). Wildlife as source of human Escherichia coli O157 infection. Emerg. Infect. Dis. 23:2122. doi: 10.3201/eid2312.171210
Daszak, P., Cunningham, A. A., and Hyatt, A. D. (2000). Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science 287, 443–449. doi: 10.1126/science.287.5452.443
De Oliveira, M. C. V., Camargo, B. Q., Cunha, M. P. V., Saidenberg, A. B., Teixeira, R. H. F., Matajira, C. E. C., et al. (2018). Free-ranging synanthropic birds (Ardea alba and Columba livia domestica) as carriers of Salmonella spp. and diarrheagenic Escherichia coli in the vicinity of an Urban Zoo. Vector Borne Zoonotic. Dis. 18, 65–69. doi: 10.1089/vbz.2017.2174
Dipineto, L., Gargiulo, A., Russo, T. P., De Luca Bossa, L. M., Borrelli, L., D'ovidio, D., et al. (2010). Survey of Escherichia coli O157 in captive frogs. J. Wildl. Dis. 46, 944–946. doi: 10.7589/0090-3558-46.3.944
Doane, C. A., Pangloli, P., Richards, H. A., Mount, J. R., Golden, D. A., and Draughon, F. A. (2007). Occurrence of Escherichia coli O157:H7 in diverse farm environments. J. Food Prot. 70, 6–10. doi: 10.4315/0362-028X-70.1.6
Erickson, M. C., and Doyle, M. P. (2007). Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J. Food Prot. 70, 2426–2449. doi: 10.4315/0362-028X-70.10.2426
Espinosa, L., Gray, A., Duffy, G., Fanning, S., and Mcmahon, B. J. (2018). A scoping review on the prevalence of shiga-toxigenic Escherichia coli in wild animal species. Zoonoses Public Health 65, 911–920. doi: 10.1111/zph.12508
Fadel, H. M., Afifi, R., and Al-Qabili, D. M. (2017). Characterization and zoonotic impact of Shiga toxin producing Escherichia coli in some wild bird species. Vet. World 10, 1118–1128. doi: 10.14202/vetworld.2017.1118-1128
Fairbrother, J. M., and Nadeau, E. (2006). Escherichia coli: on-farm contamination of animals. Rev. Sci. Tech. 25, 555–569. doi: 10.20506/rst.25.2.1682
FAO/WHO (2018). “Shiga toxin-producing Escherichia coli (STEC) and food: attribution, characterization, and monitoring,” in Microbiological Risk Assessment Series (FAO/WHO) (Rome: FAO).
Ferens, W. A., and Hovde, C. J. (2011). Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog. Dis. 8, 465–487. doi: 10.1089/fpd.2010.0673
Foster, G., Evans, J., Knight, H. I., Smith, A. W., Gunn, G. J., Allison, L. J., et al. (2006). Analysis of feces samples collected from a wild-bird garden feeding station in Scotland for the presence of verocytotoxin-producing Escherichia coli O157. Appl. Environ. Microbiol. 72, 2265–2267. doi: 10.1128/AEM.72.3.2265-2267.2006
Frank, C., Werber, D., Cramer, J. P., Askar, M., Faber, M., An Der Heiden, M., et al. (2011). Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365, 1771–1780. doi: 10.1056/NEJMoa1106483
Frankel, G., Phillips, A. D., Rosenshine, I., Dougan, G., Kaper, J. B., and Knutton, S. (1998). Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30, 911–921. doi: 10.1046/j.1365-2958.1998.01144.x
Franklin, A. B., Vercauteren, K. C., Maguire, H., Cichon, M. K., Fischer, J. W., Lavelle, M. J., et al. (2013). Wild ungulates as disseminators of Shiga toxin-producing Escherichia coli in urban areas. PLoS ONE 8:e81512. doi: 10.1371/journal.pone.0081512
Fraser, M. E., Chernaia, M. M., Kozlov, Y. V., and James, M. N. (1994). Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 a resolution. Nat. Struct. Biol. 1, 59–64. doi: 10.1038/nsb0194-59
Fratamico, P. M., Bagi, L. K., Bush, E. J., and Solow, B. T. (2004). Prevalence and characterization of Shiga toxin-producing Escherichia coli in swine feces recovered in the national animal health monitoring system's Swine 2000 study. Appl. Environ. Microbiol. 70, 7173–7178. doi: 10.1128/AEM.70.12.7173-7178.2004
French, E., Rodriguez-Palacios, A., and LeJeune, J. T. (2010). Enteric bacterial pathogens with zoonotic potential isolated from farm-raised deer. Foodborne Pathog. Dis. 7, 1031–1037. doi: 10.1089/fpd.2009.0486
Fukushima, H., Hashizume, T., Morita, Y., Tanaka, J., Azuma, K., Mizumoto, Y., et al. (1999). Clinical experiences in sakai city hospital during the massive outbreak of enterohemorrhagic Escherichia coli O157 infections in Sakai City, 1996. Pediatr. Int. 41, 213–217. doi: 10.1046/j.1442-200X.1999.4121041.x
Galiero, G., Conedera, G., Alfano, D., and Caprioli, A. (2005). Isolation of verocytotoxin-producing Escherichia coli O157 from water buffaloes (Bubalus bubalis) in southern Italy. Vet. Rec. 156, 382–383. doi: 10.1136/vr.156.12.382
Garcia, A., Fox, J. G., and Besser, T. E. (2010). Zoonotic enterohemorrhagic Escherichia coli: a one health perspective. ILAR J. 51, 221–232. doi: 10.1093/ilar.51.3.221
Gargiulo, A., Russo, T. P., Schettini, R., Mallardo, K., Calabria, M., Menna, L. F., et al. (2014). Occurrence of enteropathogenic bacteria in urban pigeons (columba livia) in Italy. Vector Borne Zoonotic Dis. 14, 251–255. doi: 10.1089/vbz.2011.0943
Gioia-Di Chiacchio, R. M., Cunha, M. P. V., De Sa, L. R. M., Davies, Y. M., Pereira, C. B. P., Martins, F. H., et al. (2018). Novel hybrid of typical enteropathogenic Escherichia coli and shiga-toxin-producing E. coli (tEPEC/STEC) emerging from pet birds. Front. Microbiol. 9:2975. doi: 10.3389/fmicb.2018.02975
Gould, L. H., Mody, R. K., Ong, K. L., Clogher, P., Cronquist, A. B., Garman, K. N., et al. (2013). Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog. Dis. 10, 453–460. doi: 10.1089/fpd.2012.1401
Gourmelon, M., Montet, M. P., Lozach, S., Le Mennec, C., Pommepuy, M., Beutin, L., et al. (2006). First isolation of Shiga toxin 1d producing Escherichia coli variant strains in shellfish from coastal areas in France. J. Appl. Microbiol. 100, 85–97. doi: 10.1111/j.1365-2672.2005.02753.x
Gyles, C. L. (2007). Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 85, E45–62. doi: 10.2527/jas.2006-508
Hancock, D. D., Besser, T. E., Rice, D. H., Ebel, E. D., Herriott, D. E., and Carpenter, L. V. (1998). Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev. Vet. Med. 35, 11–19. doi: 10.1016/S0167-5877(98)00050-6
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12:e1001923. doi: 10.1371/journal.pmed.1001923
Heiman, K. E., Mody, R. K., Johnson, S. D., Griffin, P. M., and Gould, L. H. (2015). Escherichia coli O157 outbreaks in the United States, 2003–2012. Emerg. Infect. Dis. 21, 1293–1301. doi: 10.3201/eid2108.141364
Himsworth, C. G., Zabek, E., Desruisseau, A., Parmley, E. J., Reid-Smith, R., Jardine, C. M., et al. (2015). Prevalence and characteristics of Escherichia coli and Salmonella spp. In the feces of wild urban Norway and Black Rats (Rattus norvegicus and Rattus rattus) from an inner-city neighborhood of Vancouver. Can. J. Wildl. Dis. 51, 589–600. doi: 10.7589/2014-09-242
Hofer, E., Cernela, N., and Stephan, R. (2012). Shiga toxin subtypes associated with Shiga toxin-producing Escherichia coli strains isolated from red deer, roe deer, chamois, and ibex. Foodborne Pathog. Dis. 9, 792–795. doi: 10.1089/fpd.2012.1156
Hogg, R. A., Holmes, J. P., Ghebrehewet, S., Elders, K., Hart, J., Whiteside, C., et al. (2009). Probable zoonotic transmission of verocytotoxigenic Escherichia coli O 157 by dogs. Vet. Rec. 164, 304–305. doi: 10.1136/vr.164.10.304
Hussein, M. A., Merwad, A. M. A., Elabbasy, M. T., Suelam, I. I. A., Abdelwahab, A. M., and Taha, M. A. (2019). Prevalence of enterotoxigenic Staphylococcus aureus and Shiga toxin producing Escherichia coli in fish in Egypt: quality parameters and public health hazard. Vector Borne. Zoonotic. Dis. 19, 255–264. doi: 10.1089/vbz.2018.2346
Jay, M. T., Cooley, M., Carychao, D., Wiscomb, G. W., Sweitzer, R. A., Crawford-Miksza, L., et al. (2007). Escherichia coli O157:H7 in feral swine near spinach fields and cattle, central California coast. Emerg. Infect. Dis. 13, 1908–1911. doi: 10.3201/eid1312.070763
Johnson, W. M., Lior, H., and Bezanson, G. S. (1983). Cytotoxic Escherichia coli O157:H7 associated with haemorrhagic colitis in Canada. Lancet 1:76. doi: 10.1016/S0140-6736(83)91616-1
Jones, B. A., Grace, D., Kock, R., Alonso, S., Rushton, J., Said, M. Y., et al. (2013). Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. U.S.A. 110, 8399–8404. doi: 10.1073/pnas.1208059110
Jones, K. E., Patel, N. G., Levy, M. A., Storeygard, A., Balk, D., Gittleman, J. L., et al. (2008). Global trends in emerging infectious diseases. Nature 451, 990–993. doi: 10.1038/nature06536
Kaper, J. B., Nataro, J. P., and Mobley, H. L. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. doi: 10.1038/nrmicro818
Kaper, J. B., and O'Brien, A. D. (2014). Overview and historical perspectives. Microbiol. Spectr. 2, 1–2. doi: 10.1128/microbiolspec.EHEC-0028-2014
Karmali, M. A., Mascarenhas, M., Shen, S., Ziebell, K., Johnson, S., Reid-Smith, R., et al. (2003). Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41, 4930–4940. doi: 10.1128/JCM.41.11.4930-4940.2003
Keen, J. E., Wittum, T. E., Dunn, J. R., Bono, J. L., and Durso, L. M. (2006). Shiga-toxigenic Escherichia coli O157 in agricultural fair livestock, United States. Emerg. Infect. Dis. 12, 780–786. doi: 10.3201/eid1205.050984
Kilonzo, C., Li, X., Vivas, E. J., Jay-Russell, M. T., Fernandez, K. L., and Atwill, E. R. (2013). Fecal shedding of zoonotic food-borne pathogens by wild rodents in a major agricultural region of the central California coast. Appl. Environ. Microbiol. 79, 6337–6344. doi: 10.1128/AEM.01503-13
King, L. J., Anderson, L. R., Blackmore, C. G., Blackwell, M. J., Lautner, E. A., Marcus, L. C., et al. (2008). Executive summary of the AVMA one health initiative task force report. J. Am. Vet. Med. Assoc. 233, 259–261. doi: 10.2460/javma.233.2.259
Kintz, E., Brainard, J., Hooper, L., and Hunter, P. (2017). Transmission pathways for sporadic Shiga-toxin producing E. coli infections: a systematic review and meta-analysis. Int. J. Hyg. Environ. Health 220, 57–67. doi: 10.1016/j.ijheh.2016.10.011
Kobayashi, H., Kanazaki, M., Hata, E., and Kubo, M. (2009). Prevalence and characteristics of eae- and stx-positive strains of Escherichia coli from wild birds in the immediate environment of Tokyo Bay. Appl. Environ. Microbiol. 75, 292–295. doi: 10.1128/AEM.01534-08
Kobayashi, M., Sasaki, T., Saito, N., Tamura, K., Suzuki, K., Watanabe, H., et al. (1999). Houseflies: not simple mechanical vectors of enterohemorrhagic Escherichia coli O157:H7. Am. J. Trop. Med. Hyg. 61, 625–629. doi: 10.4269/ajtmh.1999.61.625
Konowalchuk, J., Speirs, J. I., and Stavric, S. (1977). Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18, 775–779. doi: 10.1128/IAI.18.3.775-779.1977
Koochakzadeh, A., Askari Badouei, M., Zahraei Salehi, T., Aghasharif, S., Soltani, M., and Ehsan, M. R. (2015). Prevalence of Shiga toxin-producing and enteropathogenic Escherichia coli in wild and pet birds in Iran. Braz. J. Poult. Sci. 17, 445–450. doi: 10.1590/1516-635X1704445-450
Kullas, H., Coles, M., Rhyan, J., and Clark, L. (2002). Prevalence of Escherichia coli serogroups and human virulence factors in faeces of urban Canada geese (branta canadensis). Int. J. Environ. Health Res. 12, 153–162. doi: 10.1080/09603120220129319
La Ragione, R. M., Best, A., Woodward, M. J., and Wales, A. D. (2009). Escherichia coli O157:H7 colonization in small domestic ruminants. FEMS Microbiol. Rev. 33, 394–410. doi: 10.1111/j.1574-6976.2008.00138.x
Laidler, M. R., Tourdjman, M., Buser, G. L., Hostetler, T., Repp, K. K., Leman, R., et al. (2013). Escherichia coli O157:H7 infections associated with consumption of locally grown strawberries contaminated by deer. Clin. Infect. Dis. 57, 1129–1134. doi: 10.1093/cid/cit468
Lee, M. S., Koo, S., Jeong, D. G., and Tesh, V. L. (2016). Shiga toxins as multi-functional proteins: induction of host cellular stress responses, role in pathogenesis and therapeutic applications. Toxins (Basel). 8:77. doi: 10.3390/toxins8030077
Lee, M. S., and Tesh, V. L. (2019). Roles of Shiga toxins in immunopathology. Toxins (Basel). 11:212. doi: 10.3390/toxins11040212
LeJeune, J. T., Besser, T. E., Merrill, N. L., Rice, D. H., and Hancock, D. D. (2001). Livestock drinking water microbiology and the factors influencing the quality of drinking water offered to cattle. J. Dairy Sci. 84, 1856–1862. doi: 10.3168/jds.S0022-0302(01)74626-7
Lengacher, B., Kline, T. R., Harpster, L., Williams, M. L., and LeJeune, J. T. (2010). Low prevalence of Escherichia coli O157:H7 in horses in Ohio, USA. J. Food Prot. 73, 2089–2092. doi: 10.4315/0362-028X-73.11.2089
Lentz, E. K., Leyva-Illades, D., Lee, M. S., Cherla, R. P., and Tesh, V. L. (2011). Differential response of the human renal proximal tubular epithelial cell line HK-2 to Shiga toxin types 1 and 2. Infect. Immun. 79, 3527–3540. doi: 10.1128/IAI.05139-11
Leotta, G. A., Deza, N., Origlia, J., Toma, C., Chinen, I., Miliwebsky, E., et al. (2006). Detection and characterization of Shiga toxin-producing Escherichia coli in captive non-domestic mammals. Vet. Microbiol. 118, 151–157. doi: 10.1016/j.vetmic.2006.07.006
Luna, S., Krishnasamy, V., Saw, L., Smith, L., Wagner, J., Weigand, J., et al. (2018). Outbreak of E. coli O157:H7 infections associated with exposure to animal manure in a rural community–Arizona and Utah, June–July 2017. MMWR Morb. Mortal. Wkly. Rep. 67, 659–662. doi: 10.15585/mmwr.mm6723a2
Luna-Gierke, R. E., Griffin, P. M., Gould, L. H., Herman, K., Bopp, C. A., Strockbine, N., et al. (2014). Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol. Infect. 142, 2270–2280. doi: 10.1017/S0950268813003233
Makino, S., Kobori, H., Asakura, H., Watarai, M., Shirahata, T., Ikeda, T., et al. (2000). Detection and characterization of Shiga toxin-producing Escherichia coli from seagulls. Epidemiol. Infect. 125, 55–61. doi: 10.1017/S0950268899004100
Martin, C. C., Svanevik, C. S., Lunestad, B. T., Sekse, C., and Johannessen, G. S. (2019). Isolation and characterisation of Shiga toxin-producing Escherichia coli from Norwegian bivalves. Food Microbiol. 84:103268. doi: 10.1016/j.fm.2019.103268
McFarland, N., Bundle, N., Jenkins, C., Godbole, G., Mikhail, A., Dallman, T., et al. (2017). Recurrent seasonal outbreak of an emerging serotype of Shiga toxin-producing Escherichia coli (STEC O55:H7 Stx2a) in the south west of England, July 2014 to September 2015. Euro. Surveill. 22, 1–10. doi: 10.2807/1560-7917.ES.2017.22.36.30610
Melton-Celsa, A. R. (2014). Shiga toxin (Stx) classification, structure and function. Microbiol. Spectr. 2:EHEC-0024-2013. doi: 10.1128/microbiolspec.EHEC-0024-2013
Mercado, E. C., Rodriguez, S. M., Elizondo, A. M., Marcoppido, G., and Parreno, V. (2004). Isolation of Shiga toxin-producing Escherichia coli from a South American camelid (lama guanicoe) with diarrhea. J. Clin. Microbiol. 42, 4809–4811. doi: 10.1128/JCM.42.10.4809-4811.2004
Milton, A. P., Agarwal, R. K., Priya, G. B., Aravind, M., Athira, C. K., et al. (2019). Captive wildlife from India as carriers of Shiga toxin-producing, enteropathogenic and enterotoxigenic Escherichia coli. J. Vet. Med. Sci. 81, 321–327. doi: 10.1292/jvms.18-0488
Mohammed Hamzah, A., Mohammed Hussein, A., and Mahmoud Khalef, J. (2013). Isolation of Escherichia coli 0157:H7 strain from fecal samples of zoo animal. Sci. World J. 2013:843968. doi: 10.1155/2013/843968
Mora, A., Lopez, C., Dhabi, G., Lopez-Beceiro, A. M., Fidalgo, L. E., Diaz, E. A., et al. (2012). Seropathotypes, phylogroups, stx subtypes, and intimin types of wildlife-carried, Shiga toxin-producing Escherichia coli strains with the same characteristics as human-pathogenic isolates. Appl. Environ. Microbiol. 78, 2578–2585. doi: 10.1128/AEM.07520-11
Murakami, K., Etoh, Y., Ichihara, S., Maeda, E., Takenaka, S., Horikawa, K., et al. (2014). Isolation and characteristics of Shiga toxin 2f-producing Escherichia coli among pigeons in Kyushu, Japan. PLoS ONE 9:e86076. doi: 10.1371/journal.pone.0086076
Nataro, J. P., and Kaper, J. B. (1998). Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201. doi: 10.1128/CMR.11.1.142
Naylor, S. W., Low, J. C., Besser, T. E., Mahajan, A., Gunn, G. J., Pearce, M. C., et al. (2003). Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71, 1505–1512. doi: 10.1128/IAI.71.3.1505-1512.2003
Nguyen, Y., and Sperandio, V. (2012). Enterohemorrhagic E. coli (EHEC) pathogenesis. Front. Cell. Infect. Microbiol. 2:90. doi: 10.3389/fcimb.2012.00090
Nielsen, E. M., Skov, M. N., Madsen, J. J., Lodal, J., Jespersen, J. B., and Baggesen, D. L. (2004). Verocytotoxin-producing Escherichia coli in wild birds and rodents in close proximity to farms. Appl. Environ. Microbiol. 70, 6944–6947. doi: 10.1128/AEM.70.11.6944-6947.2004
Nyholm, O., Heinikainen, S., Pelkonen, S., Hallanvuo, S., Haukka, K., and Siitonen, A. (2015). Hybrids of shigatoxigenic and enterotoxigenic Escherichia coli (STEC/ETEC) among human and animal isolates in Finland. Zoonoses Public Health 62, 518–524. doi: 10.1111/zph.12177
O'Brien, A. O., Lively, T. A., Chen, M. E., Rothman, S. W., and Formal, S. B. (1983). Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet 1:702. doi: 10.1016/S0140-6736(83)91987-6
Pedersen, K., and Clark, L. (2007). A review of Shiga toxin Escherichia coli and salmonella enterica in cattle and free-ranging birds: potential association and epidemiological links. Hum. Wildl. Confl. 1, 68–77.
Persad, A. K., and LeJeune, J. T. (2014). Animal reservoirs of Shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2:EHEC-0027-2014. doi: 10.1128/microbiolspec.EHEC-0027-2014
Pradhan, S., Pellino, C., Macmaster, K., Coyle, D., and Weiss, A. A. (2016). Shiga toxin mediated neurologic changes in murine model of disease. Front. Cell. Infect. Microbiol. 6:114. doi: 10.3389/fcimb.2016.00114
Prakasan, S., Prabhakar, P., Lekshmi, M., Kumar, S., and Nayak, B. B. (2018). Isolation of Shiga toxin-producing Escherichia coli harboring variant Shiga toxin genes from seafood. Vet. World 11, 379–385. doi: 10.14202/vetworld.2018.379-385
Pritchard, G. C., Smith, R., Ellis-Iversen, J., Cheasty, T., and Willshaw, G. A. (2009). Verocytotoxigenic Escherichia coli O157 in animals on public amenity premises in England and Wales, 1997 to 2007. Vet. Rec. 164, 545–549. doi: 10.1136/vr.164.18.545
Pritchard, G. C., Williamson, S., Carson, T., Bailey, J. R., Warner, L., Willshaw, G., et al. (2001). Wild rabbits-a novel vector for verocytotoxigenic Escherichia coli O157. Vet. Rec. 149:567.
Pruimboom-Brees, I. M., Morgan, T. W., Ackermann, M. R., Nystrom, E. D., Samuel, J. E., Cornick, N. A., et al. (2000). Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. U.S.A. 97, 10325–10329. doi: 10.1073/pnas.190329997
Rangel, J. M., Sparling, P. H., Crowe, C., Griffin, P. M., and Swerdlow, D. L. (2005). Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 11, 603–609. doi: 10.3201/eid1104.040739
Reinstein, S., Fox, J. T., Shi, X., Alam, M. J., and Nagaraja, T. G. (2007). Prevalence of Escherichia coli O157:H7 in the American bison (Bison bison). J. Food Prot. 70, 2555–2560. doi: 10.4315/0362-028X-70.11.2555
Roopnarine, R. R., Ammons, D., Rampersad, J., and Adesiyun, A. A. (2007). Occurrence and characterization of verocytotoxigenic Escherichia coli (VTEC) strains from dairy farms in Trinidad. Zoonoses Public Health 54, 78–85. doi: 10.1111/j.1863-2378.2007.01024.x
Rothenburger, J. L., Himsworth, C. H., Nemeth, N. M., Pearl, D. L., and Jardine, C. M. (2017). Environmental factors and zoonotic pathogen ecology in urban exploiter species. Ecohealth 14, 630–641. doi: 10.1007/s10393-017-1258-5
Rumi, M. V., Irino, K., Deza, N., Huguet, M. J., and Bentancor, A. B. (2012). First isolation in argentina of a highly virulent Shiga toxin-producing Escherichia coli O145:NM from a domestic cat. J. Infect. Dev. Ctries. 6, 358–363. doi: 10.3855/jidc.2225
Sargeant, J. M., Hafer, D. J., Gillespie, J. R., Oberst, R. D., and Flood, S. J. (1999). Prevalence of Escherichia coli O157:H7 in white-tailed deer sharing rangeland with cattle. J. Am. Vet. Med. Assoc. 215, 792–794.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 17, 7–15. doi: 10.3201/eid1701.P11101
Scheutz, F., Teel, L. D., Beutin, L., Piérard, D., Buvens, G., Karch, H., et al. (2012). Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 50, 2951–2963. doi: 10.1128/JCM.00860-12
Schoeni, J. L., and Doyle, M. P. (1994). Variable colonization of chickens perorally inoculated with Escherichia coli O157:H7 and subsequent contamination of eggs. Appl. Environ. Microbiol. 60, 2958–2962. doi: 10.1128/AEM.60.8.2958-2962.1994
Sekhar, M. S., Sharif, N. M., and Rao, T. S. (2017). Serotypes of sorbitol-positive shiga toxigenic Escherichia coli (SP-STEC) isolated from freshwater fish. Int. J. Fish. Aquatic Sci. 5, 503–505.
Shere, J. A., Bartlett, K. J., and Kaspar, C. W. (1998). Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in wisconsin. Appl. Environ. Microbiol. 64, 1390–1399. doi: 10.1128/AEM.64.4.1390-1399.1998
Siddhnath, K., Majumdar, R. K., Parhi, J., Sharma, S., Mehta, N. K., and Laishram, M. (2018). Detection and characterization of Shiga toxin-producing Escherichia coli from carps from integrated aquaculture system. Aquaculture 487, 97–101. doi: 10.1016/j.aquaculture.2018.01.008
Soderqvist, K., Rosberg, A. K., Boqvist, S., Alsanius, B., Mogren, L., and Vagsholm, I. (2019). Season and species: two possible hurdles for reducing the food safety risk of Escherichia coli O157 contamination of leafy vegetables. J. Food Prot. 82, 247–255. doi: 10.4315/0362-028X.JFP-18-292
Steil, D., Bonse, R., Meisen, I., Pohlentz, G., Vallejo, G., Karch, H., et al. (2016). A Topographical atlas of Shiga toxin 2e receptor distribution in the tissues of weaned piglets. Toxins (Basel). 8:357. doi: 10.3390/toxins8120357
Surendraraj, A., Thampuran, N., and Joseph, T. C. (2010). Molecular screening, isolation, and characterization of enterohemorrhagic Escherichia coli O157:H7 from retail shrimp. J. Food Prot. 73, 97–103. doi: 10.4315/0362-028X-73.1.97
Szalanski, A. L., Owens, C. B., Mckay, T., and Steelman, C. D. (2004). Detection of campylobacter and Escherichia coli O157:H7 from filth flies by polymerase chain reaction. Med. Vet. Entomol. 18, 241–246. doi: 10.1111/j.0269-283X.2004.00502.x
Tarr, P. I., Gordon, C. A., and Chandler, W. L. (2005). Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365, 1073–1086. doi: 10.1016/S0140-6736(05)71144-2
Terajima, J., Iyoda, S., Ohnishi, M., and Watanabe, H. (2014). Shiga toxin (verotoxin)-producing Escherichia coli in Japan. Microbiol. Spectr. 2, 1–9. doi: 10.1128/microbiolspec.EHEC-0011-2013
Thampuran, N., Surendraraj, A., and Surendran, P. K. (2005). Prevalence and characterization of typical and atypical Escherichia coli from fish sold at retail in Cochin, India. J. Food Prot. 68, 2208–2211. doi: 10.4315/0362-028X-68.10.2208
Vasan, A., Leong, W. M., Ingham, S. C., and Ingham, B. H. (2013). Thermal tolerance characteristics of non-O157 shiga toxigenic strains of Escherichia coli (STEC) in a beef broth model system are similar to those of O157:H7 STEC. J. Food Prot. 76, 1120–1128. doi: 10.4315/0362-028X.JFP-12-500
Wacheck, S., Fredriksson-Ahomaa, M., Konig, M., Stolle, A., and Stephan, R. (2010). Wild boars as an important reservoir for foodborne pathogens. Foodborne. Pathog. Dis. 7, 307–312. doi: 10.1089/fpd.2009.0367
Waddell, T. E., Coomber, B. L., and Gyles, C. L. (1998). Localization of potential binding sites for the edema disease verotoxin (VT2e) in pigs. Can. J. Vet. Res. 62, 81–86.
Wadolkowski, E. A., Burris, J. A., and O'Brien, A. D. (1990). Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58, 2438–2445. doi: 10.1128/IAI.58.8.2438-2445.1990
Woods, J. B., Schmitt, C. K., Darnell, S. C., Meysick, K. C., and O'Brien, A. D. (2002). Ferrets as a model system for renal disease secondary to intestinal infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. J. Infect. Dis. 185, 550–554. doi: 10.1086/338633
World Health Organization (2016). E. coli Fact Sheet. Retrieved from: https://www.who.int/mediacentre/factsheets/fs125/en/
Xu, J., Liu, Q., Jing, H., Pang, B., Yang, J., Zhao, G., et al. (2003). Isolation of Escherichia coli O157:H7 from dung beetles catharsius molossus. Microbiol. Immunol. 47, 45–49. doi: 10.1111/j.1348-0421.2003.tb02784.x
Keywords: Shiga toxin-producing Escherichia coli, Shiga toxin, STEC reservoir, HUS, environmental transmission
Citation: Kim J-S, Lee M-S and Kim JH (2020) Recent Updates on Outbreaks of Shiga Toxin-Producing Escherichia coli and Its Potential Reservoirs. Front. Cell. Infect. Microbiol. 10:273. doi: 10.3389/fcimb.2020.00273
Received: 29 January 2020; Accepted: 07 May 2020;
Published: 04 June 2020.
Edited by:
Nora Lía Padola, National University of Central Buenos Aires, ArgentinaReviewed by:
Emily Mallick, Fluid-Screen, United StatesLucia Galli, National University of La Plata, Argentina
Copyright © 2020 Kim, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moo-Seung Lee, bXNsMDMxMDAwQGtyaWJiLnJlLmty; Ji Hyung Kim, a3poODFAa3JpYmIucmUua3I=
 Jun-Seob Kim
Jun-Seob Kim Moo-Seung Lee
Moo-Seung Lee Ji Hyung Kim
Ji Hyung Kim