
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 08 May 2020
Sec. Clinical Microbiology
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.00204
This article is part of the Research Topic Advances in Diagnosis and Therapeutic Intervention for Foodborne Parasitic Diseases View all 10 articles
Toxoplasmosis is a widely distributed zoonotic infection caused by the obligate intracellular apicomplexan parasite Toxoplasma gondii. It is mainly transmitted through the ingestion of oocysts shed by an infected cat acting as its definitive host. The key to effective control and treatment of toxoplasmosis is prompt and accurate detection of T. gondii infection. Several laboratory diagnostic methods have been established, including the most commonly used serological assays such as the dye test (DT), direct or modified agglutination test (DAT/MAT), indirect hemagglutination test (IHA), latex agglutination test (LAT), indirect immunofluorescent test (IFAT), enzyme-linked immunosorbent assays (ELISA), immunochromatographic tests (ICT), and the western blot. Nonetheless, creating specific and reliable approaches for serodiagnosis of T. gondii infection, and differentiating between acute and chronic phases of infection remains a challenge. This review provides information on the current trends in the serodiagnosis of human toxoplasmosis. It highlights the advantages of the use of recombinant proteins for serological testing and provides insight into the possible future direction of these methods.
Toxoplasmosis is a widely distributed zoonotic infection caused by the obligate intracellular apicomplexan parasite Toxoplasma gondii. T. gondii infects humans and almost all warm-blooded animals, making it one of the important parasites affecting public health and animal production. It is mainly transmitted through the ingestion of oocysts shed by an infected cat as its definitive host (Dubey and Beattie, 1988; Dubey and Jones, 2008; Torrey and Yolken, 2013). Toxoplasmosis affects approximately one-third of the world's human population. However, it is generally asymptomatic in immunocompetent individuals, or it may manifest flu-like symptoms and other non-specific clinical signs (Dubey, 1991). The disease may even be severe or fatal in immunocompromized patients (Montoya and Liesenfeld, 2004). Vertical transmission of the parasite through the placenta from the infected mother may compromise the life of the fetus and the infected mothers (Gatkowska et al., 2006; Elmore and Jones, 2010; Sun et al., 2013). The key to effective control and treatment of toxoplasmosis depends on accurate detection of T. gondii infection. The utilization of highly sensitive and specific diagnostic methods is a vital step in the prevention and treatment of the disease (Terkawi et al., 2013).
Due to its non-specificity of clinic signs, the diagnosis of T. gondii infection cannot be made through the assessment of clinical manifestations (Tenter et al., 2000). T. gondii diagnosis for immunocompromized patients is usually done using polymerase chain reaction (PCR), hybridization assays, isolation, and histological analysis. For congenital cases, diagnosis is through direct detection of the organism through mouse inoculation, cell culture or PCR from samples collected from amniotic fluid (Cazenave et al., 1991), cerebrospinal fluid, blood and urine (Fuentes et al., 1996), and through ophthalmologic and radiological examinations (Montoya, 2002; Pomares and Montoya, 2016). However, the most common form of T. gondii infection is latent, wherein the parasites are usually not found in the circulation, and isolating the parasites are particularly challenging (Robert-Gangneux and Dardé, 2012). However, as T. gondii induces an intense and often persistent humoral immune response with detectable antibody titers, regardless of the clinical manifestations in the infected individuals (Parmley et al., 1992; Dubey, 2008), serological tests that detect specific antibody responses are deemed useful.
Over the years, there have been several serological methods established for the diagnosis of toxoplasmosis, and many have produced satisfactory results. However, the development of specific and reliable approaches for T. gondii infection serodiagnosis, which could ideally differentiate between acute and chronic phases of infection, remains very complicated. This review offers updated knowledge on the current trends in human toxoplasmosis serodiagnosis. It emphasizes the advantages of the use of recombinant proteins for serological testing. Moreover, insight into the possible future direction of these methods is also provided.
As a direct demonstration of the T. gondii parasite is often difficult, several serodiagnostic methods have been developed. These methods, which detect different antibodies (Montoya, 2002; Sudan et al., 2013) or antigens (Desmonts et al., 1981) have been used to achieve reliable diagnosis. In most epidemiological studies of toxoplasmosis, serological tests have been mainly preferred (Montoya, 2002; Robert-Gangneux and Dardé, 2012) and appear to be the primary approach in satisfactorily evaluating disease investigations (Rorman et al., 2006). The generation of each isotype antibodies is directly related to the humoral immune response after the infection. Hence, determining whether or not the host has Toxoplasma infection can be achieved by monitoring these responses. Due to the non-specificity of clinical signs of toxoplasmosis, serological test results have been paired with clinical signs evaluation in diagnosing toxoplasmosis (Montoya, 2002; Lopes et al., 2007).
The levels of different types of antibodies, including IgM, IgG, IgA, and IgE, are measured by the tests, which increases and decreases during or after infection (Rorman et al., 2006; Dubey, 2008). IgM is serologically detected 1 week after infection, and hence, is considered as an early and sensitive diagnostic marker for acute toxoplasmosis. However, it may also be serologically present for several months or years (Liu et al., 2015). In an infected pregnancy, IgM antibodies in the maternal circulation can be detected even 18 months after infection and may confuse interpretation whether the detected antibody is from active or previous infection (Bortoletti Filho et al., 2013). If an antibody is from an earlier infection, usually, no consequences for the fetus occur. However, if the infection occurs during pregnancy, the clinician should decide on administering anti-parasitic treatment to avoid disease complications in the unborn child (Montoya, 2002; Lopes et al., 2007). Results interpretation based on IgM levels can, therefore, be sometimes tricky and insufficient (Liu et al., 2015).
IgG antibodies against T. gondii can be detected 1–2 weeks following infection. It peaks typically within 1–2 months and declines at various rates. As it can persist lifelong at residual titers, this antibody is an indicator of a previous infection. It has since been used as a standard diagnostic marker for chronic infection. However, this antibody still has difficulty in differentiating previous and recent infections. An auxiliary IgG based-test has been established to differentiate acute from chronic infection in an asymptomatic patient (Montoya, 2002; Lopes et al., 2007). Other tests based on IgE and IgA have been developed. These antibodies are produced during the first weeks of infection and disappear early (Robert-Gangneux and Dardé, 2012). Various serological procedures have already been established to determine recent and previous exposures: Sabin-Feldman dye test (SFDT) (Sabin and Feldman, 1948), agglutination tests (Dubey, 1997, 2008; Robert-Gangneux and Dardé, 2012; Liu et al., 2015), indirect fluorescent assay (IFA) (Rorman et al., 2006; Saraei et al., 2010), and enzyme-linked immunosorbent assays (ELISAs) (Voller et al., 1976; Döskaya et al., 2014; Liu et al., 2015), or a combination of these methods (Rorman et al., 2006; Dubey, 2008; Robert-Gangneux and Dardé, 2012).
The Sabin-Feldman dye test (SFDT) was developed more than seven decades ago (Sabin and Feldman, 1948) for the investigation of T. gondii infection in the laboratory (Rorman et al., 2006). SFDT has high sensitivity and specificity and is still considered as the “gold standard” (Reiter-Owona et al., 1999). It utilizes complementation of live tachyzoite incubation with patient serum. If the serum has specific antibodies against T. gondii, the parasites will be subsequently coated and lyzed by the complement system, and staining with dye methylene blue will not happen. The number of stained (live) and unstained (dead) tachyzoites are counted to determine the end-point titer (Reiter-Owona et al., 1999; Rorman et al., 2006; Udonsom et al., 2010). While SFDT can detect both IgM and IgG, the antibody titers cannot accurately differentiate between acute or chronic infection. Moreover, SFDT entails using live parasites, which is a biohazard, thereby limiting its application to only a few laboratories (Reiter-Owona et al., 1999; Udonsom et al., 2010).
Agglutination tests require particulate antigens that can bind with antibodies. Multivalent antibodies (called agglutinins) form large clumps or aggregates with suspended particulate antigens when present, which can be visually seen without magnification. These tests are used to determine concentrations of specific antibodies. In toxoplasmosis diagnosis in humans and animals, different agglutination tests, including direct agglutination test (DAT), modified agglutination test (MAT), indirect hemagglutination test (IHAT), and latex agglutination test (LAT), have been used (Dubey, 1997, 2008; Robert-Gangneux and Dardé, 2012; Liu et al., 2015). DAT was developed in 1965 and has since been very useful in detecting anti-T. gondii antibodies in humans and animals (Dubey, 2008). It is only used for the detection of IgG antibodies. It is very simple as it does not require a secondary antibody and specialized equipment. In DAT, diluted patient sera are added to microtiter plates that are coated with formalinized Toxoplasma tachyzoites. Subsequent agglutination happens if anti-Toxoplasma antibodies are present in the sera. If the sample is negative, precipitated tachyzoites will be found at the bottom of the wells (Desmonts and Remington, 1980). While DAT is considered very sensitive and economical, it requires a large antigen amount. Moreover, the presence of IgM antibodies in the sera causes non-specific agglutination (Dubey, 2008). The MAT is an adaptation of the DAT with some adjustments involving the preparation of the antigen and incubation period of the test plates (Dubey, 1997; Al-Adhami et al., 2016).
IHAT utilizes red blood cells (RBCs) that are sensitized with T. gondii soluble antigen. The sensitized cells will subsequently agglutinate if the sera contain anti-T. gondii antibodies. IHAT is also considered very simple and inexpensive (Liu et al., 2015). On the other hand, LAT utilizes covalently bonded tachyzoite particles that are coated to latex beads. A visible agglutination reaction is observed when the sera contain specific IgG antibody (Mazumder et al., 1988). The sensitivity and specificity of LAT in humans and animals range from low to high. Same with other agglutination tests, non-specific IgM agglutinations can also happen in LAT which can generate false-positive results (Ohshima et al., 1981; Mazumder et al., 1988; Oncel et al., 2005; Robert-Gangneux and Dardé, 2012). LAT has been used often as a screening test for epidemiological studies before other serological tests are utilized for further examination (Holliman et al., 1990).
IFA is an alternative simple and safe diagnostic method that does not use live tachyzoites (Rorman et al., 2006; Saraei et al., 2010). This assay is based on the specific antigen-antibody interaction from diluted serum specimens with killed Toxoplasma tachyzoites. The interaction will then be detected by the addition of fluorescent-labeled anti-human IgG or IgM antibodies under a fluorescence microscope (Pappas et al., 1986). Among the limitations of IFA include the individual differences in result reading and the chances of false-positive results in case the sera contain rheumatoid factors or antinuclear antibodies (Rorman et al., 2006). Nonetheless, high levels of T. gondii-specific IgG in some recently acquired toxoplasmosis patients may interfere with the IgM antibodies and cause false-negative results (Remington et al., 1985).
Even after four decades since it was established in toxoplasmosis diagnosis (Voller et al., 1976), ELISA is still considered one of the most common techniques with high sensitivity and specificity in the quantitative detection of antibodies and all antigenically active molecules (Döskaya et al., 2014; Liu et al., 2015). The ELISA system typically consists of a solid phase antigen or antibody, enzyme-labeled antigen or antibody, and a substrate for the enzyme reaction, which can be modified to test both antibodies and antigens (Liu et al., 2015). There are different kinds of ELISA developed to detect T. gondii antibodies or antigens, namely indirect ELISA, sandwich ELISA, and dot-ELISA. The indirect ELISA involves coating a microtiter plate with antigens and the application of sera, which contains antibodies. The presence of anti-Toxoplasma antibodies leads to consequent binding with the coated antigen and is detected by using an anti-human enzyme-conjugate (secondary antibody). The subsequent washing steps will remove any unbound reagents, and when the substrate is finally added, color reaction develops. This type of ELISA is mostly used to detect anti-T. gondii IgG, IgM, and IgA antibodies rather than antigens (Tomasi et al., 1986). The conventional indirect ELISAs using T. gondii tachyzoite lysate antigen (TLA) as coating antigen revealed a high degree of agreement with SFDT, MAT or IFAT detecting IgG or IgM antibodies in humans and animals (Filice et al., 1983; Tomasi et al., 1986; Obwaller et al., 1995). In the sandwich ELISA, capture antibodies are coated onto a microtiter plate, and a serum sample containing T. gondii antigens is added. After incubation and washing, the capture antibody-antigen reaction is also detected by the addition of enzyme-conjugated secondary antibody. Following subsequent washings, the substrate is added for a color reaction to develop. The sandwich ELISA with TLA is more sensitive and more specific to detect human IgM antibodies than IFAT (Tomasi et al., 1986). The dot-ELISA is a modified ELISA where the antigen-antibody reaction is done on nitrocellulose instead of the microtiter plate. This test is sensitive to detect T. gondii antigens and antibodies (Pappas et al., 1986; Jafar Pour Azami et al., 2011) and does not require any special equipment, thus easier to perform than standard ELISAs (Pappas et al., 1986; Youssef et al., 1992). The quantity of antibodies detected in the sera using ELISA is shown to be positively correlated with the intensity of the color reaction. Results interpretation usually is dependent on the qualitative assessment of color change spectrophotometrically. Deciding samples to be positive or negative is achieved by correlating the optical density of the serum with the control after establishing a threshold value (Seefeldt et al., 1989).
The ELISA is primarily utilized for routine screening of T. gondii infections because it is highly sensitive (allowing quantitative and semi-quantitative antibody measurements), easily adopted, and inexpensive (Shaapan et al., 2008). It can be simply used to test large populations in a short period of time (Sudan et al., 2013), with the capability to detect both IgG and IgM (Seefeldt et al., 1989). This method is also primarily used to evaluate the efficacy of different recombinant proteins as antigens for serodiagnosis. However, standardization of used antigens in ELISA has been challenging (Shaapan et al., 2008). In cases of a weak positive reaction, a photometer is required to differentiate it from a negative reaction, thereby increasing the cost (Seefeldt et al., 1989). False-positive results can also happen in IgM-based ELISA (Fuccillo et al., 1986; Liesenfeld et al., 1997), possibly due to rheumatoid factors in the serum, while IgG-based ELISA can result in false-negative results possibly due to specific IgG competitive inhibition (Fuccillo et al., 1986). The low-level IgG detection is a problem, i.e. IgG results from the “gray zone” in ELISA. According to Robert-Gangneux and Dardé (2012), these low titers must be confirmed by using a dye test or a sensitive Western blot (WB) assay.
ICT is a rapid lateral flow test intended to detect the presence or absence of the target analyte. The principle of ICT is based on a dye-labeled antibody or colloidal gold-labeled antigen that is specific for the target analyte in the liquid sample, which is present on the lower end of the nitrocellulose strip or in the plastic well along with the strip. The sample is placed at the designated pad on the nitrocellulose membrane, which will slowly infiltrate the conjugated pad through capillary action, and subsequent antibody-antigen complexes will demonstrate color reaction (Wang et al., 2011). It is simple because specialized and costly equipment may not be needed, although several laboratory-based applications and reading equipment may exist. It is believed to be a low-cost test which facilitates the rapid identification of analytes at the point of care (Weiss, 1999; Zhang et al., 2009; Thobhani et al., 2010; Goni et al., 2012; Yetisen et al., 2013). Its ease of application and rapidity of test results with no special equipment required makes the ICT suitable for field application. In toxoplasmosis, this technology has been used to diagnose human (Lévêque et al., 2019; Taha et al., 2019; Wassef and Abdel-Malek, 2019) and animal cases (Khan and Noordin, 2019). ICT has been shown to have a high agreement with ELISA in terms of sensitivity and specificity (Terkawi et al., 2013; Ybañez and Nishikawa, 2020; Ybañez, Kyan and Nishikawa, 2020). ICT based on GRA7 (Terkawi et al., 2013; Ybañez and Nishikawa, 2020; Ybañez, Kyan and Nishikawa, 2020) and SAG2 (Huang et al., 2004) show high consistency with results obtained from LAT and ELISA.
The western blot (sometimes referred to as immunoblot) aids conventional serological tests and shows the reaction of sera with T. gondii antigen on a membrane transferred from a polyacrylamide gel, and the resulting banding patterns that are matched with known molecular weight. An immunoblot test can have varying reliability of its specificity and sensitivity depending on the type of sample used (Villard et al., 2003; Stroehle et al., 2005). Western blot is also complementary for the early postnatal diagnosis of congenital toxoplasmosis (Robert-Gangneux et al., 1999), diagnosis of human patients (Gay et al., 2019) and characterization and evaluation of T. gondii proteins (Appiah-Kwarteng et al., 2019; Liu et al., 2019).
While T. gondii diagnosis based on serology are generally satisfactory, it has been challenged with producing specific and standard antigens that are usually crudely prepared through mouse passages or cell culture systems in commercial tests (Titilincu et al., 2009; Cóceres et al., 2010; Dai et al., 2012; Sudan et al., 2013; Sun et al., 2013). It has been shown that the different processes of producing and purifying native antigens may lead to contamination with non-parasitic materials (Holec-Gąsior, 2013). These processes also utilize live pathogens that require extra care because of biological hazards (Sonaimuthu et al., 2014) and are, therefore, difficult to standardize (Holec-Gąsior, 2013). With the limitations posed by the native antigens and the need to improve serodiagnostic tests, recombinant antigens have been considered as an alternative diagnostic marker to replace the native antigens (Cai et al., 2015).
There are already several studies that have documented that recombinant antigens improve the serological diagnosis of toxoplasmosis (Harning et al., 1996; Martin et al., 1998; Jacobs et al., 1999; Aubert et al., 2000; Lecordier et al., 2000; Beghetto et al., 2003, 2006; Pietkiewicz et al., 2004; Buffolano et al., 2005; Hiszczyńska-Sawicka et al., 2005; Pfrepper et al., 2005; Holec et al., 2007; Lau and Fong, 2008; Holec-Gąsior et al., 2009; Wu et al., 2009; Holec-Gąsior and Kur, 2010; Sonaimuthu et al., 2014). However, the critical goal is not only to enhance T. gondii diagnosis with the use of recombinant antigens but also to improve ways to discriminate different stages of toxoplasmosis. Primary infection during pregnancy predisposes the child to serious medical problems (Petersen, 2007). The accurate diagnosis of the acute stage of the disease in pregnant women ensures early care for the mother and the fetus to prevent serious medical problems; thus, the differentiation between acute and chronic phases of T. gondii infection is vital (Ciardelli et al., 2008; Márquez-Contreras, 2018).
Through the years, several genes encoding T. gondii proteins have been cloned and expressed using various expression systems to produce recombinant antigens. The antigens that have been widely utilized for the improvement of T. gondii serodiagnosis include the surface antigens SAG1 (P30) (Burg et al., 1988; Chen et al., 2001; Hiszczyńska-Sawicka et al., 2003; Kotresha et al., 2012), SAG2 (previously P22) (Prince et al., 1990; Parmley et al., 1992; Hiszczyńska-Sawicka et al., 2005), SAG3 (previously P43) (Khanaliha et al., 2012); the rhoptries ROP1 (previously P66) (Holec-Gąsior et al., 2009) and ROP2 (previously P54) (Saavedra et al., 1991; van Gelder et al., 1993; Martin et al., 1998); the dense granule antigens GRA1 (previously P24) (Cesbron-Delauw et al., 1989; Hiszczyńska-Sawicka et al., 2003), GRA2 (previously P28) (Prince et al., 1989; Murray et al., 1993; Holec-Gąsior et al., 2009; Lau et al., 2012; Ching et al., 2013), GRA4 (Mévélec et al., 1992; Lau et al., 2010), GRA5 (Holec-Gąsior and Kur, 2010), GRA6 (previously P32) (Lecordier et al., 1995; Redlich and Müller, 1998; Hiszczyńska-Sawicka et al., 2005), and GRA7 (previously P29) (Bonhomme et al., 1998; Fischer et al., 1998; Jacobs et al., 1998; Hiszczyńska-Sawicka et al., 2003; Selseleh et al., 2012); GRA8 (previously P35) (Hiszczyńska-Sawicka et al., 2005), and GRA9 (previously B10/P41) (Nockemann et al., 1998), the matrix antigen MAG1 (Holec et al., 2007), and the micronene protein MIC1 (Holec et al., 2008).
The use of specific molecular markers is another option adapted in T. gondii serodiagnosis. These proteins are distinctive of the parasite's tachyzoite or bradyzoite stage that could recognize specific antibodies from acute or chronic human infections. Recently, many papers have reported positive results on the utility of specific recombinant proteins that identify the phase of infection during the testing of human sera. Table 1 details the application of T. gondii recombinant antigens in diagnostic studies, and their potential to recognize the clinical phases of the disease. Collectively, these results suggest that adequately selected recombinant antigens can be employed to investigate acute or chronic toxoplasmosis. In T. gondii, dense granule (GRA) proteins are vastly secreted into the parasitophorous vacuole (PV) shortly following host invasion. These proteins are the major components of T. gondii excretory-secretory antigens (ESA) expressed by both the tachyzoites and encysted bradyzoites and circulate in the bloodstream during the first few hours after infection (Hughes and van Knapen, 1982; Cesbron-Delauw, 1994) It has been shown that ESAs are highly immunogenic (Darcy et al., 1988; Prigione et al., 2000), and can stimulate an antibody-dependent or cell-mediated immunity (Zenner et al., 1999). GRA antigens such as the GRA1, 2, 3, 4, 5, 6, 7, and 8 have been evaluated for its potential as molecular markers for the detection of antibodies against T. gondii, and all showed high sensitivities for the detection of anti-Toxoplasma antibodies (Table 1). However, GRA2 (Holec-Gąsior et al., 2009), GRA6 (Redlich and Müller, 1998; Hiszczyńska-Sawicka et al., 2005; Golkar et al., 2008), GRA7 (Pietkiewicz et al., 2004; Kotresha et al., 2012), and GRA8 (Li et al., 2000b; Suzuki et al., 2000; Lu et al., 2006) proved to be valuable markers for the diagnosis of acute toxoplasmosis, while GRA5 showed high sensitivity to detect IgG antibodies from individuals with chronic toxoplasmosis (Holec-Gąsior and Kur, 2010). The surface antigens (SAG) of T. gondii are abundant on the surface of both extracellular and intracellular tachyzoites (Burg et al., 1988). The SAG1 is one of the most immunogenic T. gondii antigens widely used for its diagnostic ability (Pietkiewicz et al., 2004; Jalallou et al., 2010; Bel-Ochi et al., 2013). It is reported to be stage-specific, being detected only in the tachyzoite stage, and not in the sporozoite and bradyzoite stages (Burg et al., 1988; Windeck and Gross, 1996). However, previous serodiagnosis studies revealed that SAG1 is a useful antigen for the diagnosis of chronic toxoplasmosis (Pietkiewicz et al., 2004; Selseleh et al., 2012). Contrastingly, a study using another surface antigen, SAG2, revealed that IgG from patients with acute phase of toxoplasmosis reacted much more with SAG2A antigens than sera from patients with a chronic phase, confirming its potential as a marker for diagnosis of human acute toxoplasmosis (Béla et al., 2008). The rhoptry proteins (ROP) are another secretory antigens of T. gondii that are reported to be also involved in the formation of the PV and the clustering with host cell organelles (Sam-Yellowe, 1996). Among the ROP antigens evaluated for its serodiagnostic utility, the ROP1 (Holec-Gąsior et al., 2009, 2010), ROP8 (Sonaimuthu et al., 2014), and ROP18 (Grzybowski et al., 2015) showed higher antibody reactions, with ROP1 and ROP18 being able to detect antibodies from individuals with acute toxoplasmosis.
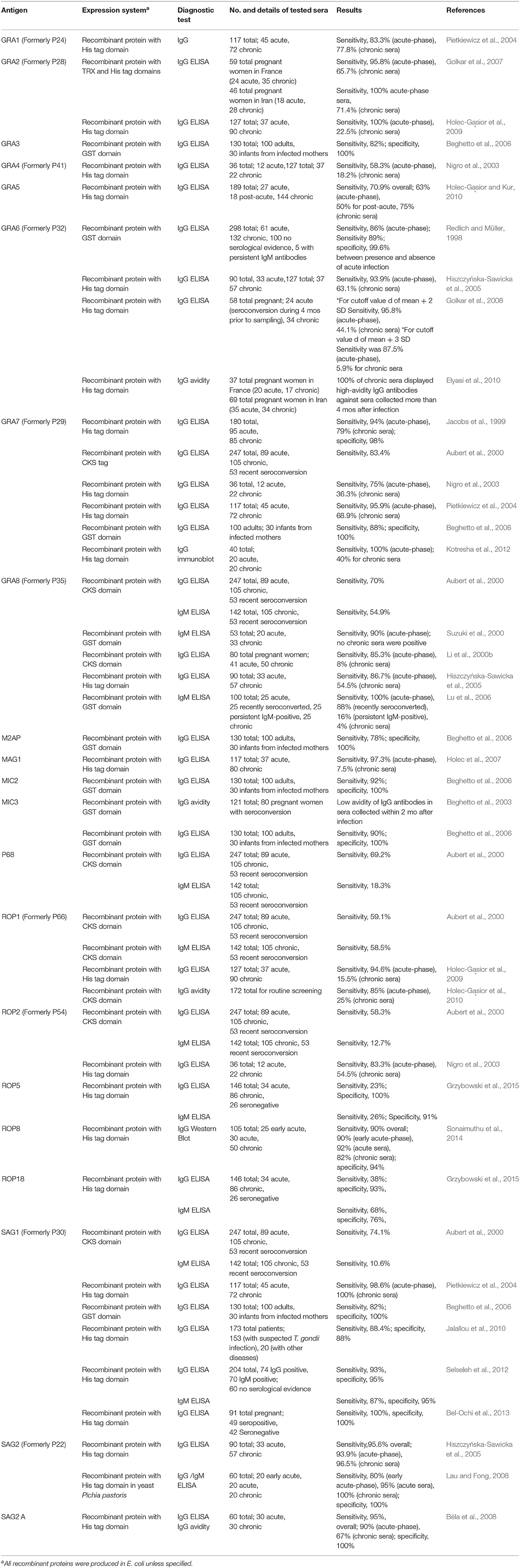
Table 1. Updated list of T. gondii recombinant antigens used as molecular markers for the serodiagnosis of human toxoplasmosis.
The use of specific recombinant antigens appears to be promising and sensitive enough in differentiating acute vs. chronic infection (Ferrandiz et al., 2004; Ching et al., 2014). Despite no clear definition of either chronic or acute infection, detection of IgM antibodies is usually employed for the diagnosis of acute infection in immunocompetent patients (Remington et al., 2011). IgM antibodies appear earlier in infection but decline more rapidly than IgG antibodies, enabling its detection to determine recent or previously acquired infection. Nevertheless, IgM might remain for a long time post-infection, even detectable 2 years after infection (Bobić et al., 1991). Therefore, the results may not be correct to prove recent infection, unless the serum is tested further using another method such as the IgG avidity. The IgG avidity assay, based on functional affinity of IgG antibodies (Hedman et al., 1993), may be used to detect recently acquired (acute) or latent (chronic) infection that has been in the body for a longer period (Hedman et al., 1993; Rahbari et al., 2012) The avidity of IgG is low in acute phase and high in chronic phase of toxoplasmosis (Iqbal and Khalid, 2007); high IgG avidity excludes infection three to five months prior, while a low avidity indicates recent infection (Flori et al., 2008).
In 2013, the diagnostic performances of four commercially available Toxoplasma IgG avidity tests, ARCHITECT Toxo IgG Avidity (Abbott, Wiesbaden, Germany), Vidas Toxo IgG Avidity (bioMérieux, Marcy l'Étoile, France), Platelia Toxo IgG Avidity (Bio-Rad, Marnes la Coquette, France), and Liaison Toxo IgG Avidity II (DiaSorin, Saluggia, Italy), were assessed in immunocompetent and immunocompromised patients with acute and latent toxoplasmosis in France (Villard et al., 2013). These fully automated assays are the most commonly used in French biology laboratories and reference laboratories abroad. They were developed based on the exclusion of acute infection. Among the four tests, the Vidas Toxo IgG Avidity showed the best performance for the diagnosis of latent toxoplasmosis. The ARCHITECT assay, which utilizes tachyzoite-specific surface antigen P30 (SAG1) and P35 (GRA8) recombinant antigens, performed best in the detection of latent infection in the presence of persistent IgM. The test detects low-avidity IgG by blocking high-avidity IgG in the sample with a soluble recombinant antigen (Curdt et al., 2009). Sickinger et al. (2008) also confirmed that the ARCHITECT Toxo IgG and IgG avidity panel can be used to rule out acute T. gondii infection in pregnant women as it was able to detect 100% (124/124) of acute-phase sera (four months after infection) as low avidity, compared to the 98.9% detected by the Vidas Toxo IgG avidity assay. Based on these findings, new prospectives for T. gondii serodiagnosis can be offered by the application of recombinant antigens in an IgG avidity assay. Previously, proteins P16, P32, P38, P40, P43, P54, P60, P66, and P97 were selected as valuable antigens in an avidity assay to potentially discriminate between phases of toxoplasmosis (Marcolino et al., 2000). Beghetto et al. (2003) showed that MIC3 is an excellent molecular marker that distinguishes infection based on avidity results between sera from patients infected with T. gondii within or more than 2 months after infection (Table 1). An avidity test was constructed by applying ROP1, MAG1, SAG1, GRA7, and GRA8 antigens onto the recom-Line Toxoplasma IgG strip test (Mikrogen GmbH, Nueried, Germany). Results revealed that IgG antibodies against antigens recognized early (i.e., GRA7, GRA8, and ROP1) matured significantly earlier than those IgGs directed against antigens that were recognized later (i.e., SAG1 and MAG1) (Pfrepper et al., 2005). Another IgG avidity test that applied recombinant ROP1 antigen detected specific low-avidity antibodies in most of the sera from individuals with acute toxoplasmosis, high-avidity antibodies were detected in sera from patients with chronic infection (Holec-Gąsior et al., 2010). Meanwhile, a GRA6 avidity testing (Table 1) among pregnant women may be useful to rule out recent infections occurring 4 months prior (Elyasi et al., 2010). Moreover, a study utilizing a mixture of recombinant proteins, including GRA7, SAG1, and GRA1, for IgG avidity testing reported that IgG avidity maturation against this mixture is different from that received against TLAs. This finding corroborates with previous reports stating that the development of IgG avidity maturation varies depending on the stimulating antigen and antibodies mature at different rates than the Toxoplasma native antigens (Marcolino et al., 2000; Pfrepper et al., 2005; Pietkiewicz et al., 2007). All these findings highlight the enormous potential of recombinant antigens to replace TLAs in IgG avidity assays and enhance the current methods for serodiagnosis, especially for acute T. gondii infections.
Aside from ease of standardization, another advantage of using recombinant proteins over whole parasite lysates is that more than one antigen can be applied at the same time, like in ELISA. In 1992, the diagnostic value of combining two recombinant T. gondii proteins for the detection of T. gondii specific IgM was evaluated for the first time (Johnson et al., 1992). An ELISA based on the combination of H4/GST and H11/GST revealed higher sensitivity (81.3%) for the detection of IgM ELISA as compared to when H4/GST and H11/GST were tested separately (54% and 61%, respectively) (Tenter and Johnson, 1991). Jacobs et al. (1999) evaluated the performance of recombinant GRA7 and Tg34AR (ROP2 C-terminal fragment) for the detection of IgG-specific antibodies. When used separately, the sensitivity of the ELISA was 81 and 88%, respectively, but the mixture of the two proteins improved the sensitivity to 96%. Similarly, Lecordier et al. (2000) discovered that GRA1 may complement GRA6-Nt to attain an overall IgG ELISA sensitivity of 98%. Low sensitivities were obtained when GRA1 and GRA6-Nt were applied individually (68 and 96%, respectively).
Table 2 shows different combinations of recombinant proteins that were recommended for the detection of IgM and IgG antibodies against T. gondii. A cocktail of GRA7, GRA8, and ROP1 recombinant proteins was reported beneficial to detect IgM antibodies in human sera (Aubert et al., 2000). Furthermore, other antigen mixtures such as MAG1 or GRA2 or ROP1 supplemented with SAG1 and GRA5 (Holec-Gąsior and Kur, 2010); MIC1ex2, MAG1, and MIC3, or P35 (GRA8), SAG2, and GRA6, (Holec et al., 2008); SAG1, GRA1, and GRA7 (Pietkiewicz et al., 2004); P22 (SAG2), P25 (H4), P29 (GRA7), and P35 (GRA8) (Li et al., 2000a); and GRA7, SAG1, and GRA8 (Aubert et al., 2000) were documented to be sufficient for the detection of IgG antibodies against T. gondii. All these data presented in the previous studies mentioned above have validated the potential of using two or more complementary recombinant antigens to improve the sensitivity of immunoassays comparable to that obtained with using crude antigens. It is noteworthy that the combinations of recombinant antigens mentioned above include any of the GRA5, GRA7, GRA8, SAG2, and H4 proteins that have been reported to be valuable in differentiating a recently acquired infection from one acquired in the past (Li et al., 2000a; Holec-Gąsior and Kur, 2010). The antigen concoctions presented above were established to be antigenic, with sensitivity for specific IgG or IgM detection comparable to native antigens of T. gondii. However, to optimize the detection of antibodies from different stages of the toxoplasmosis, the assay necessitates proportion of highly reactive antigens, such as SAG1 (Jalallou et al., 2010; Bel-Ochi et al., 2013), and specific molecular markers for acute-stage toxoplasmosis, like the GRA2 (Holec-Gąsior et al., 2009), GRA6 (Hiszczyńska-Sawicka et al., 2005), GRA7 (Pietkiewicz et al., 2004), GRA8 (Lu et al., 2006), MAG1 (Holec et al., 2007), and ROP1 (Holec-Gąsior et al., 2009, 2010); and GRA5 (Holec-Gąsior and Kur, 2010) and SAG1 (Pietkiewicz et al., 2004) for chronic stage serodetection. Therefore, a well-defined component of antigen mixtures is vital in obtaining a preparation that is essential for any serodiagnostic application.
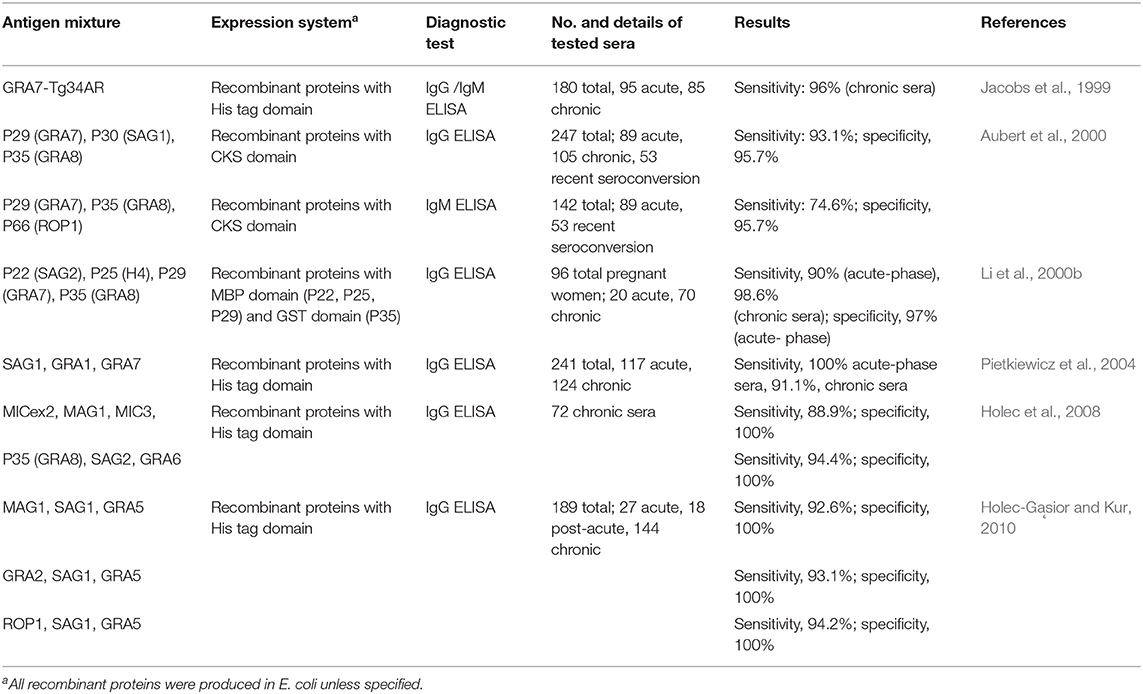
Table 2. List of combinations of T. gondii recombinant antigens used for the serodiagnosis of human toxoplasmosis.
In recent years, the use of multi-epitope or chimeric antigens has been recommended as an alternative approach to address the need for standardizing and increasing the sensitivity and specificity of serodiagnostic tests of toxoplasmosis. Moreover, the capability to discriminate previous from recently acquired infections can also be accomplished (Wang et al., 2014). A recombinant chimera contains different immunoreactive epitopes from several properly selected T. gondii antigens that are generally well-exposed on the protein surface. The epitope or antigenic determinant is a part of a protein with an ability to be recognized by T cell and B cell receptors or the antibody binding sites (Saha and Raghava, 2006). The use of diagnostic markers with a high density of antibody binding sites increases the chances of antibody detection in serum samples and thereby enhances the degree of specificity and sensitivity of the assay (Camussone et al., 2009; De Souza et al., 2013). Numerous bioinformatic tools have been handy in the prediction and identification of immunodominant epitopes. The epitopes of several T. gondii antigens can be predicted, and their antigenicity can be evaluated by employing software-based prediction techniques (Dai et al., 2012; Wang et al., 2014). Phage display of cDNA libraries (Beghetto et al., 2001), epitope mapping (Cardona et al., 2009; Reineke, 2009), and reactivity with monoclonal antibodies (Mévélec et al., 1998) are among the experimental approaches applied to identify epitopes.
Presently, only minimal studies have described the diagnostic usefulness of different chimeric proteins for the serodiagnosis of toxoplasmosis in human sera (Table 3). Beghetto et al. (2006) first explored the application of two chimeric antigens namely, GST-EC2 and GST-EC3, which contains antigenic regions of MIC2, MIC3, SAG1, GRA3, GRA7, and M2AP proteins. The study revealed that both chimeric antigens obtained an improved serodiagnosis of toxoplasmosis in adults with acquired infection and infants born to mothers with a primary T. gondii infection. Additionally, the performance of the IgG and IgM Rec-ELISAs based on the two chimeric antigens were comparable to those of the commercial assays. Moreover, the IgM Rec-ELISAs using the GST-EC2 and GST-EC3 chimeric antigens improved the capacity to diagnose congenital toxoplasmosis postnatally compared to standard assays. In 2011, the specificity of the recombinant chimeric SAG1/2 antigen to detect IgG and IgM in T. gondii infection was confirmed using Western blot. Results revealed that it was immunogenic enough to stimulate a humoral response and protection in a mouse model and might be considered a good vaccine candidate (Lau et al., 2011). Meanwhile, Holec-Gąsior et al. (2012a) designed a chimera containing antigenic regions of MIC1 and MAG1 proteins. A high sensitivity using MIC1-MAG1 chimeric protein (90.9%) was attained, which was almost as high as that for the TLA (91.8%), and higher than the sensitivities of the assays using the recombinant proteins individually or a mixture of both which ranged 60–75.5% only. Furthermore, another chimeric protein containing immunodominant regions from MIC1, MAG1, and SAG1 (Holec-Gąsior et al., 2012b) was developed by the same research group and generated better results than the chimeric antigen containing fragments only from MIC1 and MAG1 proteins (Table 3). The addition of a fragment of SAG1, one of the most immunogenic proteins of T. gondii, to the chimeric antigen increased the reactivity with specific IgG antibodies from patients with chronic toxoplasmosis. These suggest that a properly designed chimeric antigen containing numerous different immunogenic regions is better than a mixture of recombinant proteins and may be used instead of TLAs for optimal serodiagnosis human T. gondii infections. Using the software, SAG1, SAG2, SAG3, GRA5, GRA6, and P35 were analyzed to identify immunodominant epitopes for the serodiagnosis of T. gondii infection. Two potential epitopes with high predicted antigenicity and reactivity were selected for each antigen. T. gondii-positive human sera strongly recognized three recombinant epitopes (rEPs), cloned from SAG1 (rSAG1_EP2), SAG2 (rSAG2_EP1), and SAG3 (rSAG3_EP2) antigens. A recombinant multi-epitope fusion peptide (rMEP) consisting of these three epitopes was then developed and assessed using IgG and IgM ELISAs (Dai et al., 2012, 2013). The results revealed that the rMEP successfully discriminated sera of pregnant women with recent and past infections, and obtained similar serodiagnostic efficiency as the two commercially available ELISA kits. In 2015, a chimeric antigen made up of antigenic fragments of SAG2, GRA1, and ROP1 (large fragment, ROP1L) achieved 100% sensitivity and specificity when utilized in an IgG ELISA assay (Ferra et al., 2015). In the same year, a synthetic gene called USM.TOXO1 that encodes multi-immunodominant epitopes of SAG1, GRA2, and GRA7 was constructed by assembly PCR (Hajissa et al., 2015). Initial ELISA and Western blot analyses using 80 human serum samples showed 100% sensitivity and specificity. The efficacy of USM.TOXO1 was further validated by testing 157 human sera and obtained sensitivity and specificity of 85.43 and 81.25%, respectively (Hajissa et al., 2017). The latest addition to the pool of suitable chimeric proteins for the detection of specific anti-T. gondii antibodies are the recombinant tetravalent chimeric proteins containing fragments of SAG2, GRA1, ROP1, and AMA1 antigens (Ferra et al., 2019). In this study, four tetravalent recombinant chimeric proteins (SAG2-GRA1-ROP1-AMA1N, AMA1N-SAG2-GRA1-ROP1, AMA1C-SAG2-GRA1-ROP1, and AMA1-SAG2-GRA1-ROP1) acquired through genetic engineering were evaluated for their efficacy in detecting specific IgM and IgG antibodies from T. gondii-infected human sera. All chimeric proteins showed 100% sensitivity and specificity in the IgG ELISAs. Avidity assay results suggested the usefulness of the chimeric antigens for avidity assessment, with results comparable to commercial assays. Furthermore, the AMA1-SAG2-GRA1-ROP1 chimeric protein displayed great potential in distinguishing specific antibodies from the sera of individuals with acute and chronic T. gondii infections. These findings exhibit the potential application of these recombinant chimeric antigens as replacements for TLA in standardized commercial tests for toxoplasmosis serodiagnosis.
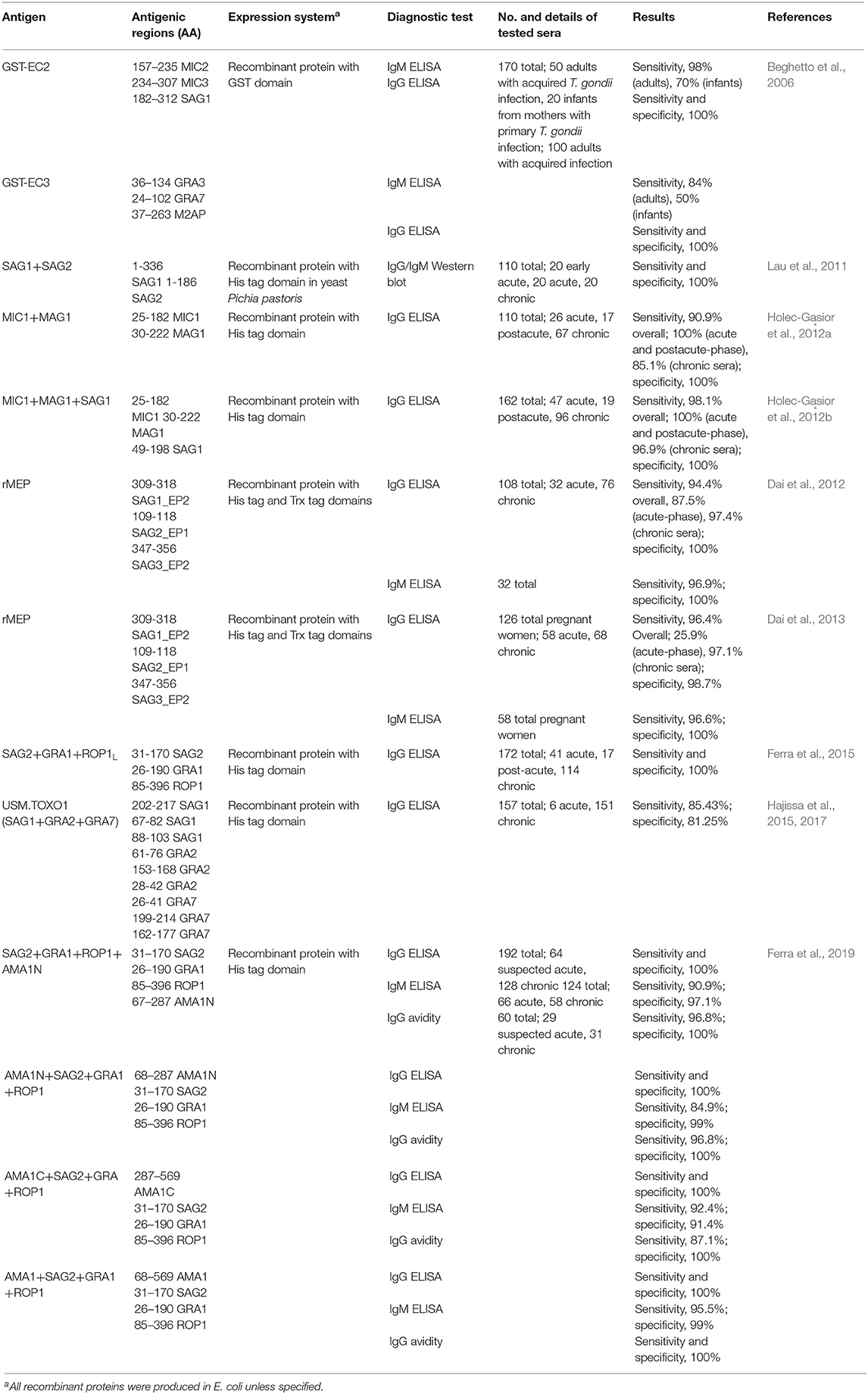
Table 3. Updated list of multi-epitope and chimeric T. gondii recombinant antigens used for the serodiagnosis of human toxoplasmosis.
All the studies mentioned above proposed that multi-epitopes and peptide proteins augment the test sensitivity, thus opening new possibilities in the serodiagnosis of T. gondii infection. The utilization of recombinant chimeras will not only enable the development of more precise and reliable diagnostic systems but will pave the way for the discovery of more promising tests that are capable of distinguishing early or recently acquired infections from the chronic ones.
The diagnosis of T. gondii infection remains a big challenge. Currently, serological diagnosis plays a crucial role in the identification of T. gondii infections in both humans and other animals. But due to the inadequacy of accuracy and reliability of the current diagnostic tests brought about by the lack of standardization in the production of the T. gondii whole-cell lysates, the consideration of other diagnostic options is compelling. An increasing number of studies have presented the growing advantage of using recombinant proteins, used singly or in combination, or chimeric antigens for the serological detection of T. gondii infections, such as improving the standardization of detection kits (Kotresha and Noordin, 2010), reducing the costs of production (Holec-Gąsior, 2013) and increasing the probability of differentiating different phases of toxoplasmic infection (Sickinger et al., 2008). Nevertheless, there are still many issues to be resolved when using recombinant antigens as diagnostic antigens. The sensitivity of assays using recombinant antigens has been reported to be lower than that of assays using native antigens. Differences in cloning strategies, methods of recombinant protein purification, and criteria used in the data analyses could also lead to variance in sensitivities and specificities of diagnostic tests. These important points should be considered when interpreting the results of various reports (Holec-Gąsior, 2013; Zhang et al., 2016). Another concern is in the use of E. coli expression system where the recombinant antigens produced often lose their antigenic value due to incorrect folding, and some contamination with E. coli antigens in partially purified recombinant proteins are reported. One resolution to these problems is the production of recombinant proteins in eukaryotic expression systems. Studies have revealed that they have post-translational modification mechanisms that enable the production of recombinant proteins that have conformations almost identical to that of the native proteins. Moreover, they do not contain bacteria-derived contaminants, thus, avoiding cross-reactions with human sera (Biemans et al., 1998; Zhou et al., 2007; Lau et al., 2010, 2011, 2012; Chang et al., 2011; Thiruvengadam et al., 2011).
Meanwhile, bioinformatics has been useful in the analysis of biological data by employing various methods and technologies ranging from mathematics, statistics, and computer sciences to biology and medicine. It presents valuable results from the analysis of large amounts of raw data (Romano et al., 2011). One goal of bioinformatics is to effectively and promptly organize, analyze, and translate information from the genome, transcriptome, and/or proteome (Brusic and Flower, 2004). It has been extensively used to predict protein structures, functions, and other biological characteristics (Romano et al., 2011). Bioinformatics tools and online software have been extensively used to analyze gene and protein expression and predict the structure, immunogenicity, and general features of T. gondii proteins (Bai et al., 2012; Shaddel et al., 2018). Prediction of epitopes can show the pathogenesis and immune mechanisms of pathogens, thus vital data can be obtained to identify immunogenic peptides and useful for the development of diagnostic reagents and new vaccines (Bai et al., 2012). Likewise, approach to integrating multiple omics technologies—such as genomics, transcriptomics, proteomics, and metabolomics has been adapted to obtain a more comprehensive insight of the biology and disease for a better and holistic understanding of diagnosis and treatment protocols (Karczewski et al., 2018). In T. gondii, proteomic and genomic analyses and molecular modeling were utilized to characterize new rhoptry proteins of the ROP2 family to elucidate the specific roles of the proteins, especially in the early interaction with the host cell upon invasion (El Hajj et al., 2006). Molecular technologies including microsatellite analyses (Ajzenberg et al., 2010), multilocus sequence typing to recognize single nucleotide polymorphisms (Ajzenberg et al., 2002; Khan et al., 2005, 2007; Lehmann et al., 2006; Su et al., 2006) and polymorphic polypeptides from T. gondii antigens (Kong et al., 2003; Xiao et al., 2009) have been developed and applied for genotyping and serotyping T. gondii infections.
After consolidating all the findings presented by the different studies we reviewed in this paper, we conclude that the utility of recombinant proteins in the serodiagnosis of T. gondii infections is highly advantageous in improving the standardization of the tests and lessen their production costs. Combining several recombinant antigens with multiple immunodominant epitopes, as either a mixture or a chimeric product, significantly increases the probability of detecting T. gondii antibodies at different stages of the infection. The diagnosis of T. gondii infection continues to be challenging until relatively rapid and highly sensitive, and specific methods are developed. The direction now is to integrate genomic, transcriptomic, and proteomic technologies and multi-locus genotyping methods with molecular and bioinformatics tools for the advancement of detection methods utilizing these recombinant antigens. These new techniques help demonstrate the genetic diversity of Toxoplasma strains as well as the stage of infection, which would aid better in the diagnosis of toxoplasmic infection.
RY, AY, and YN have contributed equally during the conceptualization, writing, editing, and finalization of this review paper.
This study was supported by the Research Program on Emerging and Re-emerging Infectious Diseases (20fk0108137h0001 [YN]) from the Japan Agency for Medical Research and Development (AMED).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ajzenberg, D., Cogne, N., Paris, L., Bessieres, M. H., Thulliez, P., Filisetti, D., et al. (2002). Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J. Infect. Dis. 186, 684–689. doi: 10.1086/342663
Ajzenberg, D., Collinet, F., Mercier, A., Vignoles, P., and Dardé, M. L. (2010). Genotyping of Toxoplasma gondii isolates with 15 microsatellite markers in a single multiplex PCR assay. J. Clin. Microbiol. 48, 4641–4645. doi: 10.1128/JCM.01152-10
Al-Adhami, B. H., Simard, M., Hernández-Ortiz, A., Boireau, C., and Gajadhar, A. A. (2016). Development and evaluation of a modified agglutination test for diagnosis of Toxoplasma infection using tachyzoites cultivated in cell culture. Food Waterborne Parasitol. 2, 15–21. doi: 10.1016/j.fawpar.2015.12.001
Appiah-Kwarteng, C., Saito, T., Toda, N., Kitoh, K., Nishikawa, Y., Adenyo, C., et al. (2019). Native SAG1 in Toxoplasma gondii lysates is superior to recombinant SAG1 for serodiagnosis of T. gondii infections in chickens. Parasitol. Int. 69, 114–120. doi: 10.1016/j.parint.2019.01.001
Aubert, D., Maine, G. T., Villena, I., Hunt, J. C., Howard, L., Sheu, M., et al. (2000). Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin in human sera by enzyme immunoassay. J. Clin. Microbiol. 38, 1144–1150. doi: 10.1128/JCM.38.3.1144-1150.2000
Bai, Y., He, S., Zhao, G., Chen, L., Shi, N., Zhou, H., et al. (2012). Toxoplasma gondii: bioinformatics analysis, cloning and expression of a novel protein TgIMP1. Exp. Parasitol. 132, 458–464. doi: 10.1016/j.exppara.2012.09.015
Beghetto, E., Buffolano, W., Spadoni, A., Del Pezzo, M., Di Cristina, M., Minenkova, O., et al. (2003). Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 41, 5414–5418. doi: 10.1128/JCM.41.12.5414-5418.2003
Beghetto, E., Pucci, A., Minenkova, O., Spadoni, A., Bruno, L., Buffolano, W., et al. (2001). Identification of a human immunodominant B-cell epitope within the GRA1 antigen of Toxoplasma gondii by phage display of cDNA libraries. Int. J. Parasitol. 31, 1659–1668. doi: 10.1016/S0020-7519(01)00288-0
Beghetto, E., Spadoni, A., Bruno, L., Buffolano, W., and Gargano, N. (2006). Chimeric antigens of Toxoplasma gondii: toward standardization of toxoplasmosis serodiagnosis using recombinant products. J. Clin. Microbiol. 44, 2133–2140. doi: 10.1128/JCM.00237-06
Béla, S. R., Oliveira Silva, D. A., Cunha-Júnior Pirovani, J. P., Pirovani, C. P., Chaves-Borges, F. A., de Carvalho, F. R., et al. (2008). Use of SAG2A recombinant Toxoplasma gondii surface antigen as a diagnostic marker for human acute toxoplasmosis: analysis of titers and avidity of IgG and IgG1 antibodies. Diagn. Microbiol. Infect. Dis. 62, 245–254. doi: 10.1016/j.diagmicrobio.2008.05.017
Bel-Ochi, N. C., Bouratbine, A., and Mousli, M. (2013). Enzyme-linked immunosorbent assay using recombinant SAG1 antigen to detect Toxoplasma gondii-specific immunoglobulin G antibodies in human sera and saliva. Clin. Vaccine Immunol. 20, 468–473. doi: 10.1128/CVI.00512-12
Biemans, R., Grégoire, D., Haumont, M., Bosseloir, A., Garcia, L., Jacquet, A., et al. (1998). The conformation of purified Toxoplasma gondii SAG1 antigen, secreted from engineered Pichia pastoris, is adequate for serorecognition and cell proliferation. J. Biotechnol. 66, 137–146. doi: 10.1016/S0168-1656(98)00143-6
Bobić, B., Sibalić, D., and Djurković-Djaković, O. (1991). High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary Toxoplasma infection. Case report. Gynecol. Obstet. Invest. 31, 182–184. doi: 10.1159/000293151
Bonhomme, A., Maine, G. T., Beorchia, A., Burlet, H., Aubert, D., Villena, I., et al. (1998). Quantitative immunolocalization of a P29 protein (GRA7), a new antigen of Toxoplasma gondii. J. Histochem. Cytochem. 46, 1411–1422. doi: 10.1177/002215549804601210
Bortoletti Filho, J., Araujo Júnior, E., da Silva Carvalho, N., Helfer, T. M., de Oliveira Nogueira Serni, P., Nardozza, L. M. M., et al. (2013). The importance of IgG avidity and the polymerase chain reaction in treating toxoplasmosis during pregnancy: current knowledge. Interdiscip. Perspect. Infect. Dis. 2013:370769. doi: 10.1155/2013/370769
Brusic, V., and Flower, D. R. (2004). Bioinformatics tools for identifying T-cell epitopes. Drug Disc. Today Biosilico. 2, 8–23. doi: 10.1016/S1741-8364(04)02374-1
Buffolano, W., Beghetto, E., Del Pezzo, M., Spadoni, A., Di Cristina, M., Petersen, E., et al. (2005). Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 43, 5916–5924. doi: 10.1128/JCM.43.12.5916-5924.2005
Burg, J. L., Perelman, D., Kasper, L. H., Ware, P. L., and Boothroyd, J. C. (1988). Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141, 3584–3591.
Cai, Y., Wang, Z., Li, J., Li, N., Wei, F., and Liu, Q. (2015). Evaluation of an indirect ELISA using recombinant granule antigen GRA7 for serodiagnosis of Toxoplasma gondii infection in cats. J. Parasitol. 101, 37–40. doi: 10.1645/14-575.1
Camussone, C., Gonzalez, V., Belluzo, M. S., Pujato, N., Ribone, M. E., Lagier, C. M., et al. (2009). Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clin. Vaccine Immunol. 16, 899–905. doi: 10.1128/CVI.00005-09
Cardona, N., de la Torre, A., Siachoque, H., Patarroyo, M. A., and Gomez-Marin, J. E. (2009). Toxoplasma gondii: P30 peptides recognition pattern in human toxoplasmosis. Exp. Parasitol. 123, 199–202. doi: 10.1016/j.exppara.2009.06.017
Cazenave, J., Cheyrou, A., and Blouin, P. (1991). Use of polymerase chain reaction to detect Toxoplasma. J. Clin. Pathol. 44:1037. doi: 10.1136/jcp.44.12.1037-a
Cesbron-Delauw, M. F. (1994). Dense granule organelles of Toxoplasma gondii: their role in the host parasite relationship. Parasitol. Today 10, 293–296. doi: 10.1016/0169-4758(94)90078-7
Cesbron-Delauw, M. F., Guy, B., Torpier, G., Pierce, R. J., Lenzen, G., Cesbron, J. Y., et al. (1989). Molecular characterization of a 23-kilodalton major antigen secreted by Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 86, 7537–7541. doi: 10.1073/pnas.86.19.7537
Chang, P. Y., Fong, M. Y., Nissapatorn, V., and Lau, Y. L. (2011). Evaluation of pichia pastoris-expressed recombinant rhoptry protein 2 of Toxoplasma gondii for its application in diagnosis of toxoplasmosis. Am. J. Trop. Med. Hyg. 85, 485–489. doi: 10.4269/ajtmh.2011.11-0351
Chen, X. G., Gong, Y., Hua-Li Lun, Z. R., and Fung, M. C. (2001). High-level expression and purification of immunogenic recombinant SAG1 (P30) of Toxoplasma gondii in Escherichia coli. Protein Expr. Purif. 23, 33–37. doi: 10.1006/prep.2001.1483
Ching, X. T., Lau, Y. L., Fong, M. Y., and Nissapatorn, V. (2013). Evaluation of Toxoplasma gondii-recombinant dense granular protein (GRA2) for serodiagnosis by western blot. Parasitol. Res. 112, 1229–1236. doi: 10.1007/s00436-012-3255-5
Ching, X. T., Lau, Y. L., Fong, M. Y., Nissapatorn, V., and Andiappan, H. (2014). Recombinant dense granular protein (GRA5) for detection of human toxoplasmosis by western blot. Biomed. Res. Int. 2014:690529. doi: 10.1155/2014/690529
Ciardelli, L., Meroni, V., Avanzini, M., Bollani, L., Tinelli, C., Garofoli, F., et al. (2008). Early and accurate diagnosis of congenital toxoplasmosis. Pediatr. Infect. Dis. J. 27, 125–129. doi: 10.1097/INF.0b013e3181586052
Cóceres, V. M., Becher, M. L., De Napoli, M. G., Corvi, M. M., Clemente, M., and Angel, S. O. (2010). Evaluation of the antigenic value of recombinant Toxoplasma gondii HSP20 to detect specific immunoglobulin G antibodies in Toxoplasma infected humans. Exp. Parasitol. 126, 263–266. doi: 10.1016/j.exppara.2010.04.013
Curdt, I., Praast, G., Sickinger, E., Schultess, J., Herold, I., Braun, H. B., et al. (2009). Development of fully automated determination of marker-specific immunoglobulin G (IgG) avidity based on the avidity competition assay format: application for abbott architect cytomegalovirus and Toxo IgG avidity assays. J. Clin. Microbiol. 47, 603–613. doi: 10.1128/JCM.01076-08
Dai, J., Jiang, M., Wang, Y., Qu, L., Gong, R., and Si, J. (2012). Evaluation of a recombinant multiepitope peptide for serodiagnosis of Toxoplasma gondii infection. Clin. Vaccine Immunol. 19, 338–342. doi: 10.1128/CVI.05553-11
Dai, J. F., Jiang, M., Qu, L. L., Sun, L., Wang, Y. Y., Gong, L. L., et al. (2013). Toxoplasma gondii: enzyme-linked immunosorbent assay based on a recombinant multi-epitope peptide for distinguishing recent from past infection in human sera. Exp. Parasitol. 133, 95–100. doi: 10.1016/j.exppara.2012.10.016
Darcy, F., Deslee, D., Santoro, F., Charif, H., Auriault, C., Decoster, A., et al. (1988). Induction of a protective antibody-dependent response against toxoplasmosis by in vitro excreted/secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol. 10, 553–567. doi: 10.1111/j.1365-3024.1988.tb00242.x
De Souza, M. Q., Galdino, A. S., dos Santos, J. C., Soares, M. V., de Nóbrega, Y. C., da Cunha Morales Alvares, A., et al. (2013). A recombinant multiepitope protein for hepatitis B diagnosis. Biomed. Res. Int. 2013:148317. doi: 10.1155/2013/148317
Desmonts, G., Naot, Y., and Remington, J. S. (1981). Immunoglobulin M-immunosorbent agglutination assay for diagnosis of infectious diseases: diagnosis of acute congenital and acquired Toxoplasma infections. J. Clin. Microbiol. 14, 486–491. doi: 10.1128/JCM.14.5.486-491.1981
Desmonts, G., and Remington, J. S. (1980). Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J. Clin. Microbiol. 11, 562–568. doi: 10.1128/JCM.11.6.562-568.1980
Döskaya, M., Caner, A., Can, H., Iz, S. G., Gedik, Y., Döskaya, A. D., et al. (2014). Diagnostic value of a Rec-ELISA using Toxoplasma gondii recombinant SporoSAG, BAG1, and GRA1 proteins in murine models infected orally with tissue cysts and oocysts. PLoS ONE 9:e108329. doi: 10.1371/journal.pone.0108329
Dubey, J. P. (1991). Toxoplasmosis - an overview. Southeast Asian J. Trop. Med. Public Health 22, 88–92.
Dubey, J. P. (1997). Validation of the specificity of the modified agglutination test for toxoplasmosis in pigs. Vet. Parasitol. 71, 307–310. doi: 10.1016/S0304-4017(97)00016-2
Dubey, J. P. (2008). The history of Toxoplasma gondii- the first 100 years. J. Eukaryot. Microbiol. 55, 467–475. doi: 10.1111/j.1550-7408.2008.00345.x
Dubey, J. P., and Beattie, C. P. (1988). Toxoplasmosis of Animals and Man. Boca Raton: CRC Press, 320.
Dubey, J. P., and Jones, J. L. (2008). Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 38, 1257–1278. doi: 10.1016/j.ijpara.2008.03.007
El Hajj, H., Demey, E., Poncet, J., Lebrun, M., Wu, B., Galéotti, N., et al. (2006). The ROP2 family of Toxoplasma gondii rhoptry proteins: proteomic and genomic characterization and molecular modeling. Proteomics 6, 5773–5784. doi: 10.1002/pmic.200600187
Elmore, S. A., and Jones, J. L. (2010). Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 26, 190–196. doi: 10.1016/j.pt.2010.01.009
Elyasi, H., Babaie, J., Fricker-Hidalgo, H., Brenier-Pinchart, M. P., Zare, M., Sadeghiani, G., et al. (2010). Use of dense granule antigen GRA6 in an immunoglobulin G avidity test to exclude acute Toxoplasma gondii infection during pregnancy. Clin. Vaccine Immunol. 17, 1349–1355. doi: 10.1128/CVI.00199-10
Ferra, B., Holec-Gąsior, L., and Kur, J. (2015). A new Toxoplasma gondii chimeric antigen containing fragments of SAG2, GRA1, and ROP1 proteins - impact of immunodominant sequences size on its diagnostic usefulness. Parasitol. Res. 114, 3291–3299. doi: 10.1007/s00436-015-4552-6
Ferra, B. T., Holec-Gąsior, L., Gatkowska, J., Dziadek, B., Dzitko, K., Grazlewska, W., et al. (2019). The first study on the usefulness of recombinant tetravalent chimeric proteins containing fragments of SAG2, GRA1, ROP1 and AMA1 antigens in the detection of specific anti-Toxoplasma gondii antibodies in mouse and human sera. PLoS ONE 14:e0217866. doi: 10.1371/journal.pone.0217866
Ferrandiz, J., Mercier, C., Wallon, M., Picot, S., Cesbron-Delauw, M. F., and Peyron, F. (2004). Limited value of assays using detection of immunoglobulin G antibodies to the two recombinant dense granule antigens, GRA1 and GRA6 Nt of Toxoplasma gondii, for distinguishing between acute and chronic infections in pregnant women. Clin. Diagn. Lab. Immunol. 11, 1016–1021. doi: 10.1128/CDLI.11.6.1016-1021.2004
Filice, G., Meroni, V., Carnevale, G., Olliaro, P., and Carosi, G. (1983). Comparison of ELISA and indirect immunofluorescence in the detection of IgG and IgM antiToxoplasma antibodies. Boll. Ist. Sieroter Milan. 62, 445–450.
Fischer, H. G., Stachelhaus, S., Sahm, M., Meyer, H. E., and Reichmann, G. (1998). GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol. Biochem. Parasitol. 91, 251–262. doi: 10.1016/S0166-6851(97)00227-2
Flori, P., Bellete, B., Crampe, C., Maudry, A., Patural, H., Chauleur, C., et al. (2008). A technique for dating toxoplasmosis in pregnancy and comparison with the Vidas anti-Toxoplasma IgG avidity test. Clin. Microbiol. Infect. 14, 242–249. doi: 10.1111/j.1469-0691.2007.01905.x
Fuccillo, D., Madden, D., Tzan, N., and Sever, J. (1986). Difficulties associated with serological diagnosis of Toxoplasma gondii infections. Diagn. Clin. Immunol. 5, 8–13.
Fuentes, I., Rodriguez, M., Domingo, C., Del Castillo, F., Juncosa, T., and Alvar, J. (1996). Urine sample used for congenital toxoplasmosis diagnosis by PCR. J. Clin. Microbiol. 34, 2368–2371. doi: 10.1128/JCM.34.10.2368-2371.1996
Gatkowska, J., Hiszczynska-Sawicka, E., Kur, J., Holec, L., and Dlugonska, H. (2006). Toxoplasma gondii: an evaluation of diagnostic value of recombinant antigens in a murine model. Exp. Parasitol. 114, 220–227. doi: 10.1016/j.exppara.2006.03.011
Gay, J., Gendron, N., Verney, C., Joste, V., Dardé, M. L., Loheac, C., et al. (2019). Disseminated toxoplasmosis associated with hemophagocytic syndrome after kidney transplantation: a case report and review. Transpl. Infect. Dis. 21:e13154. doi: 10.1111/tid.13154
Golkar, M., Azadmanesh, K., Khalili, G., Khoshkholgh-Sima, B., Babaie, J., Mercier, C., et al. (2008). Serodiagnosis of recently acquired Toxoplasma gondii infection in pregnant women using enzyme-linked immunosorbent assays with a recombinant dense granule GRA6 protein. Diagn. Microbiol. Infect. Dis. 61, 31–39. doi: 10.1016/j.diagmicrobio.2007.09.003
Golkar, M., Rafati, S., Abdel-Latif, M. S., Brenier-Pinchart, M. P., Fricker-Hidalgo, H., Sima, B. K., et al. (2007). The dense granule protein GRA2, a new marker for the serodiagnosis of acute Toxoplasma infection: comparison of sera collected in both France and Iran from pregnant women. Diagn. Microbiol. Infect. Dis. 58, 419–426. doi: 10.1016/j.diagmicrobio.2007.03.003
Goni, P., Martin, B., Villacampa, M., Garcia, A., Seral, C., Castillo, F. J., et al. (2012). Evaluation of an immunochromatographic dip strip test for simultaneous detection of Cryptosporidium spp, Giardia duodenalis, and Entamoeba histolytica antigens in human faecal samples. Eur. J. Clin. Microbiol. 31, 2077–2082. doi: 10.1007/s10096-012-1544-7
Grzybowski, M. M., Gatkowska, J. M., Dziadek, B., Dzitko, K., and Długonska, H. (2015). Human toxoplasmosis: a comparative evaluation of the diagnostic potential of recombinant Toxoplasma gondii ROP5 and ROP18 antigens. Exp. Parasitol. 66, 1201–1207. doi: 10.1099/jmm.0.000148
Hajissa, H., Zakaria, R., Suppian, R., and Mohamed, Z. (2015). Design and evaluation of a recombinant multi-epitope antigen for serodiagnosis of Toxoplasma gondii infection in humans. Parasit. Vector 8:315. doi: 10.1186/s13071-015-0932-0
Hajissa, H., Zakaria, R., Suppian, R., and Mohamed, Z. (2017). An evaluation of a recombinant multiepitope based antigen for detection of Toxoplasma gondii specific antibodies. BMC Infect. Dis. 17:807. doi: 10.1186/s12879-017-2920-9
Harning, D., Spenter, J., Metsis, A., Vuust, J., and Petersen, E. (1996). Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin. Diagn. Lab. Immunol. 3, 355–357. doi: 10.1128/CDLI.3.3.355-357.1996
Hedman, K., Lappalainen, M., Söderlund, M., and Hedman, L. A. (1993). Avidity of IgG in the serodiagnosis of infectious diseases. Rev. Med. Microbiol. 4, 123–129. doi: 10.1097/00013542-199307000-00001
Hiszczyńska-Sawicka, E., Brillowska-Dabrowska, A., Dabrowski, S., Pietkiewicz, H., Myjak, P., and Kur, J. (2003). High yield expression and single-step purification of Toxoplasma gondii SAG1, GRA1, and GRA7 antigens in Escherichia coli. Protein Exp. Purif. 27, 150–157. doi: 10.1016/S1046-5928(02)00593-4
Hiszczyńska-Sawicka, E., Kur, J., Pietkiewicz, H., Holec, L., Gąsior, A., and Myjak, P. (2005). Efficient production of the Toxoplasma gondii GRA6, P35 and SAG2 recombinant antigens and their applications in the serodiagnosis of toxoplasmosis. Acta Parasitol. 50, 249–254.
Holec, L., Gąsior, A., Brillowska-Dabrowska, A., and Kur, J. (2008). Toxoplasma gondii: enzyme-linked immunosorbent assay using different fragments of recombinant microneme protein (MIC1) for detection of immunoglobulin G antibodies. Exp. Parasitol. 119, 1–6. doi: 10.1016/j.exppara.2007.12.002
Holec, L., Hiszczyńska-Sawicka, E., Gąsior, A., Brillowska-Dabrowska, A., and Kur, J. (2007). Use of MAG1 recombinant antigen for detection of Toxoplasma gondii infection in humans. Clin. Vaccine Immunol. 14, 220–225. doi: 10.1128/CVI.00419-06
Holec-Gąsior, L. (2013). Toxoplasma gondii recombinant antigens as tools for serodiagnosis of human toxoplasmosis-the current status of studies. Clin. Vaccine Immunol. 20, 1343–1351. doi: 10.1128/CVI.00117-13
Holec-Gąsior, L., Drapała, D., Lautenbach, D., and Kur, J. (2010). Toxoplasma gondii: usefulness of ROP1 recombinant antigen in an immunoglobulin G avidity assay for diagnosis of acute toxoplasmosis in humans. Pol. J. Microbiol. 59, 307–310. doi: 10.33073/pjm-2010-046
Holec-Gąsior, L., Ferra, B., and Drapała, D. (2012b). MIC1-MAG1-SAG1 chimeric protein, a most effective antigen for detection of human toxoplasmosis. Clin. Vaccine Immunol. 19, 1977–1979. doi: 10.1128/CVI.00452-12
Holec-Gąsior, L., Ferra, B., Drapała, D., Lautenbach, D., and Kur, J. (2012a). A new MIC1-MAG1 recombinant chimeric antigen can be used instead of the Toxoplasma gondii lysate antigen in serodiagnosis of human toxoplasmosis. Clin. Vaccine Immunol. 19, 57–63. doi: 10.1128/CVI.05433-11
Holec-Gąsior, L., and Kur, J. (2010). Toxoplasma gondii: recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Exp. Parasitol. 124, 272–279. doi: 10.1016/j.exppara.2009.10.010
Holec-Gąsior, L., Kur, J., and Hiszczynska-Sawicka, E. (2009). GRA2 and ROP1 recombinant antigens as potential markers for detection of Toxoplasma gondii-specific immunoglobulin G in human with acute toxoplasmosis. Clin. Vaccine Immunol. 16, 510–514. doi: 10.1128/CVI.00341-08
Holliman, R. E., Barker, K. F., and Johnson, J. D. (1990). Selective antenatal screening for toxoplasmosis and the latex agglutination test. Epidemiol. Infect. 105, 409–414. doi: 10.1017/S0950268800047981
Huang, X., Xuan, X., Hirata, H., Yokoyama, N., Xu, L., Suzuki, N., et al. (2004). Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J. Clin. Microbiol. 42, 351–353. doi: 10.1128/JCM.42.1.351-353.2004
Hughes, H. P., and van Knapen, F. (1982). Characterisation of a secretory antigen from Toxoplasma gondii and its role in circulating antigen production. Int. J. Parasitol. 125, 433–437. doi: 10.1016/0020-7519(82)90073-X
Iqbal, J., and Khalid, N. (2007). Detection of acute Toxoplasma gondii infection in early pregnancy by IgG avidity and PCR analysis. J. Med. Microbiol. 56, 1459–1499. doi: 10.1099/jmm.0.47260-0
Jacobs, D., Dubremetz, J. F., Loyens, A., Bosman, F., and Saman, E. (1998). Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol. Biochem. Parasitol. 91, 237–249. doi: 10.1016/S0166-6851(97)00204-1
Jacobs, D., Vercammen, M., and Saman, E. (1999). Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin. Diagn. Lab. Immunol. 6, 24–29. doi: 10.1128/CDLI.6.1.24-29.1999
Jafar Pour Azami, S., Keshavarz, H., Rezaian, M., Mohebali, M., and Shojaee, S. (2011). Rapid detection of Toxoplasma gondii antigen in experimentally infected mice by Dot- ELISA. Iran. J. Parasitol. 6, 28–33.
Jalallou, N., Bandepour, M., Khazan, H., Haghighi, A., Abdollahi, S. H., and Kazemi, B. (2010). Recombinant SAG1 antigen to detect Toxoplasma gondii specific immunoglobulin G in human sera by ELISA test. Iran. J. Parasitol. 5, 1–9.
Johnson, A. M., Roberts, H., and Tenter, A. M. (1992). Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J. Med. Microbiol. 37, 404–409. doi: 10.1099/00222615-37-6-404
Karczewski, K. J., Michael, P., and Snyder, M. P. (2018). Integrative omics for health and disease. Nat. Rev. Genet. 19, 299–310. doi: 10.1038/nrg.2018.4
Khan, A., Fux, B., Su, C., Dubey, J. P., Dardé, M. L., Ajioka, J. W., et al. (2007). Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc. Natl. Acad. Sci. U.S.A, 104, 14872–14877. doi: 10.1073/pnas.0702356104
Khan, A., Su, C., German, M., Storch, G. A., Clifford, D. B., and Sibley, L. D. (2005). Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J. Clin. Microbiol. 43, 5881–5887. doi: 10.1128/JCM.43.12.5881-5887.2005
Khan, A. H., and Noordin, R. (2019). Serological and molecular rapid diagnostic tests for Toxoplasma infection in humans and animals. Eur. J. Clin. Microbiol. Infect. Dis. 39, 19–30. doi: 10.1007/s10096-019-03680-2
Khanaliha, K., Motazedian, M., Sarkari, B., Bandehpour, M., Sharifnia, Z., and Kazemi, B. (2012). Expression and purification of P43 Toxoplasma gondii surface antigen. Iran. J. Parasitol. 7, 48–53.
Kong, J. T., Grigg, M. E., Uyetake, L., Parmley, S., and Boothroyd, J. C. (2003). Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J. Infect. Dis. 187, 1484–1495. doi: 10.1086/374647
Kotresha, D., and Noordin, R. (2010). Recombinant proteins in the diagnosis of toxoplasmosis. APMIS. 118, 529–542. doi: 10.1111/j.1600-0463.2010.02629.x
Kotresha, D., Poonam, D., Muhammad Hafiznur, Y., Saadatnia, G., Nurulhasanah, O., Sabariah, O., et al. (2012). Recombinant proteins from new constructs of SAG1 and GRA7 sequences and their usefulness to detect acute toxoplasmosis. Trop. Biomed. 29, 129–137.
Lau, Y. L., and Fong, M. Y. (2008). Toxoplasma gondii: serological characterization and immunogenicity of recombinant surface antigen 2 (SAG2) expressed in the yeast Pichia pastoris. Exp. Parasitol. 119, 373–378. doi: 10.1016/j.exppara.2008.03.016
Lau, Y. L., Fong, M. Y., Idris, M. M., and Ching, X. T. (2012). Cloning and expression of Toxoplasma gondii dense granule antigen 2 (GRA2) gene by Pichia pastoris. Southeast Asian J. Trop. Med. Public Health 43, 10–16.
Lau, Y. L., Hasan, M. T., Thiruvengadam, G., Idris, M. M., and Init, I. (2010). Cloning and expression of Toxoplasma gondii dense granular protein 4 (GRA4) in Pichia pastoris. Trop. Biomed. 27, 525–533.
Lau, Y. L., Thiruvengadam, G., Lee, W. W., and Fong, M. F. (2011). Immunogenic characterization of the chimeric surface antigen 1 and 2 (SAG1/2) of Toxoplasma gondii expressed in the yeast Pichia pastoris. Parasitol. Res. 109, 871–878. doi: 10.1007/s00436-011-2315-6
Lecordier, L., Fourmaux, M. P., Mercier, C., Dehecq, E., Masy, E., and Cesbron-Delauw, M. F. (2000). Enzyme-linked immunosorbent assay using recombinant dense granule antigens GRA6 and GRA1 of Toxoplasma gondii for detection of immunoglobulin G antibodies. Clin. Diagn. Lab. Immunol. 7, 607–611. doi: 10.1128/CDLI.7.4.607-611.2000
Lecordier, L., Moleon-Borodowsky, I., Dubremetz, J. F., Tourvieille, B., Mercier, C., Deslee, D., et al. (1995). Characterization of a dense granule antigen of Toxoplasma gondii (GRA6) associated to the network of the parasitophorous vacuole. Mol. Biochem. Parasitol. 70, 85–94. doi: 10.1016/0166-6851(95)00010-X
Lehmann, T., Marcet, P. L., Graham, D. H., Dahl, E. R., and Dubey, J. P. (2006). Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 103, 11423–11428. doi: 10.1073/pnas.0601438103
Lévêque, M. F., Chiffré, D., Galtier, C., Albaba, S., Ravel, C., Lachaud, L., et al. (2019). Molecular diagnosis of toxoplasmosis at the onset of symptomatic primary infection: a straightforward alternative to serological examinations. Int. J. Infect. Dis. 79, 131–133. doi: 10.1016/j.ijid.2018.11.368
Li, S., Galvan, G., Araujo, F. G., Suzuki, Y., Remington, J. S., and Parmley, S. (2000a). Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 7, 781–787. doi: 10.1128/CDLI.7.5.781-787.2000
Li, S., Maine, G., Suzuki, Y., Araujo, F. G., Galvan, G., Remington, J. S., et al. (2000b). Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J. Clin. Microbiol. 38, 179–184.
Liesenfeld, O., Press, C., Montoya, J. G., Gill, R., Isaac-Renton, J. L., Hedman, K., et al. (1997). False-positive results in immunoglobulin M (IgM) Toxoplasma antibody tests and importance of confirmatory testing: the platelia toxo IgM test. J. Clin. Microbiol. 35, 174–178. doi: 10.1128/JCM.35.1.174-178.1997
Liu, M., Li, F. X., Li, C. Y., Li, X. C., Chen, L. F., Wu, K., et al. (2019). Characterization of protein arginine methyltransferase of TgPRMT5 in Toxoplasma gondii. Parasit. Vectors. 12:221. doi: 10.1186/s13071-019-3464-1
Liu, Q., Wang, Z. D., Huang, S. Y., and Zhu, X. Q. (2015). Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit. Vectors 8:292. doi: 10.1186/s13071-015-0902-6
Lopes, F. M. R., Gonçalves, D. D., Mitsuka-Breganó, R., Freire, R. L., and Navarro, I. T. (2007). Toxoplasma gondii infection in pregnancy. Braz. J. Infect. Dis. 11, 496–506. doi: 10.1590/S1413-86702007000500011
Lu, B., Wu, S., Shi, Y., Zhang, R., Zou, L., Gao, S., et al. (2006). Toxoplasma gondii: expression pattern and detection of infection using full-length recombinant P35 antigen. Exp. Parasitol. 113, 83–90. doi: 10.1016/j.exppara.2005.12.014
Marcolino, P. T., Silva, D. A., Leser, P. G., Camargo, M. E., and Mineo, J. R. (2000). Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by western blotting. Clin. Diagn. Lab. Immunol. 7, 384–389. doi: 10.1128/CDLI.7.3.384-389.2000
Márquez-Contreras, M. E. (2018). Diagnosis serological of toxoplasmosis using recombinants antigens. Arch. Parasitol. 2:116.
Martin, V., Arcavi, M., Santillan, G., Amendoeira, M. R. R., De Souza Neves, E., Griemberg, G., et al. (1998). Detection of human Toxoplasma-specific immunoglobulins A, M, and G with a recombinant Toxoplasma gondii ROP2 protein. Clin. Diagn. Lab. Immunol. 5, 627–631. doi: 10.1128/CDLI.5.5.627-631.1998
Mazumder, P., Chuang, H., Wentz, M. W., and Wiedbrauk, D. L. (1988). Latex agglutination test for detection of antibodies to Toxoplasma gondii. J. Clin. Microbiol. 26, 2444–2446. doi: 10.1128/JCM.26.11.2444-2446.1988
Mévélec, M. N., Chardès, T., Mercereau-Puijalon, O., Bourguin, I., Achbarou, A., Dubremetz, J. F., et al. (1992). Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein, recognized by mucosal IgA antibodies. Mol. Biochem. Parasitol. 56, 227–238. doi: 10.1016/0166-6851(92)90172-G
Mévélec, M. N., Mercereau-Puijalon, O., Buzoni-Gatel, D., Bourguin, I., Chardès, T., Dubremetz, J. F., et al. (1998). Mapping of B epitopes in GRA4, a dense granule antigen of Toxoplasma gondii and protection studies using recombinant proteins administered by the oral route. Parasit. Immunol. 20, 183–195.
Montoya, J. G. (2002). Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 185(Suppl. 1), S73–S82. doi: 10.1086/338827
Montoya, J. G., and Liesenfeld, O. (2004). Toxoplasmosis. Lancet. 363, 1965–1976. doi: 10.1016/S0140-6736(04)16412-X
Murray, A., Mercier, C., Decoster, A., Lecordier, L., Capron, A., and Cesbron- Delauw, M. F. (1993). Multiple B-cell epitopes in a recombinant GRA2 secreted antigen of Toxoplasma gondii. Appl. Parasitol. 34, 235–244.
Nigro, M., Gutierrez, A., Hoffer, A. M., Clemente, M., Kaufer, F., Carral, L., et al. (2003). Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn. Microbiol. Infect. Dis. 47, 609–613. doi: 10.1016/S0732-8893(03)00156-1
Nockemann, S. H., Dlugonska, H., Henrich, B., Kitzerow, A., and Daubener, W. (1998). Expression, characterization and serological reactivity of a 41 kDa excreted-secreted antigen (ESA) from Toxoplasma gondii. Mol. Biochem. Parasitol. 97, 109–121. doi: 10.1016/S0166-6851(98)00138-8
Obwaller, A., Hassl, A., Picher, O., and Aspock, H. (1995). An enzyme-linked immunosorbent assay with whole trophozoites of Toxoplasma gondii from serum-free tissue culture for detection of specific antibodies. Parasitol. Res. 81, 361–364. doi: 10.1007/BF00931494
Ohshima, S., Tsubota, N., and Hiraoka, K. (1981). Latex agglutination microtiter test for diagnosis of Toxoplasma infection in animals. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 250, 376–382. doi: 10.1016/S0174-3031(81)80130-8
Oncel, T., Vural, G., Babür, C., and Kiliç, S. (2005). Detection of Toxoplasmosis gondii seropositivity in sheep in Yalova by Sabin Feldman dye test and latex agglutination test. Turkiye Parazitol. Derg. 29, 10–12.
Pappas, M. G., Lunde, M. N., Hajkowski, R., and McMahon, J. (1986). Determination of IgM and IgG antibodies to Toxoplasma using the IFA test, ELISA, and Dot-ELISA procedures. Vet. Parasitol. 20, 31–42. doi: 10.1016/0304-4017(86)90090-7
Parmley, S. F., Sgarlato, G. D., Mark, J., Prince, J. B., and Remington, J. S. (1992). Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J. Clin. Microbiol. 30, 1127–1133. doi: 10.1128/JCM.30.5.1127-1133.1992
Petersen, E. (2007). Toxoplasmosis. Semin. Fetal Neonat. M. 12, 214–223. doi: 10.1016/j.siny.2007.01.011
Pfrepper, K. I., Enders, G., Gohl, M., Krczal, D., Hlobil, H., Wassenberg, D., et al. (2005). Seroreactivity to and avidity for recombinant antigens in toxoplasmosis. Clin. Diagn. Lab. Immunol. 12, 977–982. doi: 10.1128/CDLI.12.8.977-982.2005
Pietkiewicz, H., Hiszczyńska-Sawicka, E., Kur, J., Petersen, E., Nielsen, H. V., Paul, M., et al. (2007). Usefulness of Toxoplasma gondii recombinant antigens (GRA1, GRA7, SAG1) in an immunoglobulin G avidity test for serodiagnosis of toxoplasmosis. Parasitol. Res. 100, 333–337. doi: 10.1007/s00436-006-0265-1
Pietkiewicz, H., Hiszczyńska-Sawicka, E., Kur, J., Petersen, E., Nielsen, H. V., Stankiewicz, M., et al. (2004). Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J. Clin. Microbiol. 42, 1779–1781. doi: 10.1128/JCM.42.4.1779-1781.2004
Pomares, C., and Montoya, J. G. (2016). Laboratory diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 54, 2448–2454. doi: 10.1128/JCM.00487-16
Prigione, I., Facchetti, P., Lecordier, L., Deslée, D., Chiesa, S., Cesbron-Delauw, M. F., et al. (2000). T cell clones raised from chronically infected healthy humans by stimulation with Toxoplasma gondii excretory-secretory antigens cross-react with live tachyzoites: characterization of the fine antigenic specificity of the clones and implications for vaccine development. J. Immunol. 164, 3741–3748. doi: 10.4049/jimmunol.164.7.3741
Prince, J. B., Araujo, F. G., Remington, J. S., Burg, J. L., Boothroyd, J. C., and Sharma, S. D. (1989). Cloning of cDNAs encoding a 28 kDa antigen of Toxoplasma gondii. Mol. Biochem. Parasitol. 34, 3–13. doi: 10.1016/0166-6851(89)90014-5
Prince, J. B., Auer, K. L., Huskinson, J., Parmley, S. F., Araujo, F. G., and Remington, J. S. (1990). Cloning, expression, and cDNA sequence of surface antigen P22 from Toxoplasma gondii. Mol. Biochem. Parasitol. 43, 97–106. doi: 10.1016/0166-6851(90)90134-8
Rahbari, A., Keshavarz, H., Shoejaee, S., Mohebali, M., and Rezaeian, M. (2012). IgG avidity ELISA test for diagnosis of acute toxoplasmosis in humans. Korean J. Parasitol. 50, 99–102. doi: 10.3347/kjp.2012.50.2.99
Redlich, A., and Müller, W. A. (1998). Serodiagnosis of acute toxoplasmosis using a recombinant form of the dense granule antigen GRA6 in an enzyme-linked immunosorbent assay. Parasitol. Res. 84, 700–706. doi: 10.1007/s004360050473
Reineke, U. (2009). Antibody epitope mapping using De Novo generated synthetic peptide libraries. Methods Mol. Biol. 524, 203–211. doi: 10.1007/978-1-59745-450-6_14
Reiter-Owona, I., Petersen, E., Joynson, D., Aspöck, H., Dardé, M. L., Disko, R., et al. (1999). The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull. World Health Organ. 77, 929–935.
Remington, J., McLeod, R., Wilson, C. B., and Desmonts, G. (2011). “Toxoplasmosis” in Infectious Diseases of the Fetus and the Newborn Infant, 7th ed. (Philadelphia, PA: Saunders-Elsevier), 918–1041. doi: 10.1016/B978-1-4160-6400-8.00031-6
Remington, J. S., Araujo, F. G., and Desmonts, G. (1985). Recognition of different Toxoplasma antigens by IgM and IgG antibodies in mothers and their congenitally infected newborns. J. Infect. Dis. 152,1020–1024. doi: 10.1093/infdis/152.5.1020
Robert-Gangneux, F., Commerce, V., Tourte-Schaefer, C., and Dupouy-Camet, J. (1999). Performance of a western blot assay to compare mother and newborn anti-Toxoplasma antibodies for the early neonatal diagnosis of congenital toxoplasmosis. Eur. J. Clin. Microbiol. 18, 648–654. doi: 10.1007/s100960050366
Robert-Gangneux, F., and Dardé, M. L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296. doi: 10.1128/CMR.05013-11
Romano, P., Giugno, R., and Pulvirenti, A. (2011). Tools and collaborative environments for bioinformatics research. Brief. Bioinform. 12, 549–561. doi: 10.1093/bib/bbr055
Rorman, E., Zamir, C. S., Rilkis, I., and Ben-David, H. (2006). Congenital toxoplasmosis-prenatal aspects of Toxoplasma gondii infection. Reprod. Toxicol. 21, 458–472. doi: 10.1016/j.reprotox.2005.10.006
Saavedra, R., De Meuter, F., Decourt, J. L., and Hérion, P. (1991). Human T cell clone identifies a potentially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J. Immunol. 147, 1975–1982.
Sabin, A. B., and Feldman, H. A. (1948). Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoon parasite (Toxoplasma). Science 108, 660–663. doi: 10.1126/science.108.2815.660
Saha, S., and Raghava, G. (2006). Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 65, 40–48. doi: 10.1002/prot.21078
Sam-Yellowe, T. Y. (1996). Rhoptry organelles of the apicomplexa: their role in host cell invasion and intracellular survival. Parasitol. Today 12, 308–316. doi: 10.1016/0169-4758(96)10030-2
Saraei, M., Shojaee, S., Esmaeli, A., Jahani-Hashemi, H., and Keshavarz, H. (2010). Evaluation of confounders in toxoplasmosis indirect fluorescent antibody assay. Iran J. Parasitol. 5, 55–62.
Seefeldt, S. L., Kirkbride, C. A., and Dubey, J. P. (1989). Comparison of enzyme-linked immunosorbent assay, indirect fluorescent antibody test, and direct agglutination test for detecting Toxoplasma gondii antibodies in naturally aborted ovine fetuses. J. Vet. Diagn. Invest. 1, 124–127. doi: 10.1177/104063878900100206
Selseleh, M., Keshavarz, H., Mohebali, M., Shojaee, S., Selseleh, M., Eshragian, M. R., et al. (2012). Production and evaluation of Toxoplasma gondii recombinant GRA7 for serodiagnosis of human infections. Korean J. Parasitol. 50, 233–238. doi: 10.3347/kjp.2012.50.3.233
Shaapan, R., El-Nawawi, F., and Tawfik, M. (2008). Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Vet. Parasitol. 153, 359–362. doi: 10.1016/j.vetpar.2008.02.016
Shaddel, M., Ebrahimi, M., and Tabandeh, M. R. (2018). Bioinformatics analysis of single and multi-hybrid epitopes of GRA-1, GRA-4, GRA-6 and GRA-7 proteins to improve DNA vaccine design against Toxoplasma gondii. J. Parasit. Dis. 42, 269–276. doi: 10.1007/s12639-018-0996-9
Sickinger, E., Gay-Andrieu, F., Jonas, G., Schultess, J., Stieler, M., Smith, D., et al. (2008). Performance characteristics of the new ARCHITECT Toxo IgG and Toxo IgG avidity assays. Diagn. Microbiol. Infect. Dis. 62, 235–244. doi: 10.1016/j.diagmicrobio.2008.07.005
Sonaimuthu, P., Fong, M. Y., Kalyanasundaram, R., Mahmud, R., and Lau, Y. L. (2014). Serodiagnostic evaluation of Toxoplasma gondii recombinant rhoptry antigen 8 expressed in E. coli. Parasit. Vectors 7:297. doi: 10.1186/1756-3305-7-297
Stroehle, A., Schmid, K., Heinzer, I., Naguleswaran, A., and Hemphill, A. (2005). Performance of a western immunoblot assay to detect specific anti-Toxoplasma gondii IgG antibodies in human saliva. J. Parasitol. 91, 561–563. doi: 10.1645/GE-423R
Su, C., Zhang, X., and Dubey, J. P. (2006). Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int. J. Parasitol. 36, 841–848. doi: 10.1016/j.ijpara.2006.03.003
Sudan, V., Jaiswal, A. K., and Shanker, D. (2013). Recent trends in the diagnosis of toxoplasmosis. Clin. Rev. Opin. 5, 11–17. doi: 10.5897/CRO11.022
Sun, X., Lu, H., Jia, B., Chang, Z., Peng, S., Yin, J., et al. (2013). A comparative study of Toxoplasma gondii seroprevalence in three healthy Chinese populations detected using native and recombinant antigens. Parasit. Vectors 6:241. doi: 10.1186/1756-3305-6-241
Suzuki, Y., Ramirez, R., Press, C., Li, S., Parmley, S., Thulliez, P., et al. (2000). Detection of immunoglobulin M antibodies to P35 antigen of Toxoplasma gondii for serodiagnosis of recently acquired infection in pregnant women. J. Clin. Microbiol. 38, 3967–3970. doi: 10.1128/JCM.38.11.3967-3970.2000
Taha, R. K. M., Hamad, M. N. M., and Taha, K. M. (2019). Seroprevalence of toxoplasmosis between aborted ladies in Atbara district, Sudan. MOJ Womens Health 8, 86–87. doi: 10.15406/mojwh.2019.08.00217
Tenter, A., Heckeroth, A., and Weiss, L. (2000). Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–58. doi: 10.1016/S0020-7519(00)00124-7
Tenter, A. M., and Johnson, A. M. (1991). Recognition of recombinant Toxoplasma gondii antigens by human sera in an ELISA. Parasitol. Res. 77, 197–203. doi: 10.1007/BF00930858
Terkawi, M. A., Kameyama, K., Rasul, N. H., Xuan, X., and Nishikawa, Y. (2013). Development of an immunochromatographic assay based on dense granule protein 7 for serological detection of Toxoplasma gondii infection. Clin. Vaccine Immunol. 20, 596–601. doi: 10.1128/CVI.00747-12
Thiruvengadam, G., Init, I., Fong, M. Y., and Lau, Y. L. (2011). Optimization of the expression of surface antigen SAG1/2 of Toxoplasma gondii in the yeast Pichia pastoris. Trop. Biomed. 28, 506–513.
Thobhani, S., Attree, S., Boyd, R., Kumarswami, N., Noble, J., Szymanski, M., et al. (2010). Bioconjugation and characterisation of gold colloid-labelled proteins. J. Immunol. Methods. 356, 60–69. doi: 10.1016/j.jim.2010.02.007
Titilincu, A., Mircean, V., Anamaria, I., and Cozma, V. (2009). Development of an indirect ELISA test using tachyzoite crude antigen for sero-diagnosis of sheep Toxoplasma gondii infection. Vet. Parasitol. 66, 137–141. doi: 10.1016/j.vetpar.2009.05.029
Tomasi, J. P., Schlit, A. F., and Stadtsbaeder, S. (1986). Rapid double-sandwich enzyme-linked immunosorbent assay for detection of human immunoglobulin M anti-Toxoplasma gondii antibodies. J. Clin. Microbiol. 24, 849–850. doi: 10.1128/JCM.24.5.849-850.1986
Torrey, E. F., and Yolken, R. (2013). Toxoplasma oocysts as a public health problem, Trends Parasitol. 29, 380–384. doi: 10.1016/j.pt.2013.06.001
Udonsom, R., Buddhirongawatr, R., and Sukthana, Y. (2010). Is Sabin-Feldman dye test using T. gondii tachyzoites from animal inoculation still the best method for detecting Toxoplasma gondii antibodies? Southeast Asian J. Trop. Med. Public Health. 41, 1059–1064.
van Gelder, V., Bosman, F., De Meuter, F., Van Heuverswyn, H., and Hérion, P. (1993). Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli. J. Clin. Microbiol. 31, 9–15. doi: 10.1128/JCM.31.1.9-15.1993
Villard, O., Breit, L., Cimon, B., Franck, J., Fricker-Hidalgo, H., Godineau, N., et al. (2013). Comparison of four commercially available avidity tests for Toxoplasma gondii-specific IgG antibodies. Clin. Vaccine Immunol. 20, 197–204. doi: 10.1128/CVI.00356-12
Villard, O., Filisetti, D., Roch-Deries, F., Garweg, J., Flament, J., and Candolfi, E. (2003). Comparison of enzyme-linked immunosorbent assay, immunoblotting, and PCR for diagnosis of toxoplasmic chorioretinitis. J. Clin. Microbiol. 41, 3537–3541. doi: 10.1128/JCM.41.8.3537-3541.2003
Voller, A., Bidwell, D., Bartlett, A., Fleck, D., Perkins, M., and Oladehin, B. (1976). A microplate enzyme immunoassay for Toxoplasma antibody. J. Clin. Pathol. 29, 150–153. doi: 10.1136/jcp.29.2.150
Wang, Y., Wang, G., Ou, J., Yin, H., and Zhang, D. (2014). Analyzing and identifying novel B cell epitopes within Toxoplasma gondii GRA4. Parasit. Vectors 7:474. doi: 10.1186/s13071-014-0474-x
Wang, Y. H., Li, X. R., Wang, G. X., Yin, H., Cai, X. P., Fu, B. Q., et al. (2011). Development of an immunochromatographic strip for the rapid detection of Toxoplasma gondii circulating antigens. Parasitol. Int. 60, 105–107. doi: 10.1016/j.parint.2010.11.002
Wassef, R., and Abdel-Malek, R. (2019). Validity of a new immunochromatographic test in detection of Toxoplasma gondii in cancer patients. J. Parasit. Dis. 43, 83–86. doi: 10.1007/s12639-018-1063-2
Windeck, T., and Gross, U. (1996). Toxoplasma gondii strain-specific transcript levels of SAG1 and their association with virulence. Parasitol. Res. 82, 715–719. doi: 10.1007/s004360050190
Wu, K., Chen, X. G., Li, H., Yan, H., Yang, P. L., Lun, Z. R., et al. (2009). Diagnosis of human toxoplasmosis by using the recombinant truncated surface antigen 1 of Toxoplasma gondii. Diagn. Microbiol. Infect. Dis. 64, 261–266. doi: 10.1016/j.diagmicrobio.2009.02.009
Xiao, J., Buka, S. L., Cannon, T. D., Suzuki, Y., Viscidi, R. P., Torrey, E. F., et al. (2009). Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 11, 1011–1018. doi: 10.1016/j.micinf.2009.07.007
Ybañez, R. H. D., Kyan, H., and Nishikawa, Y. (2020). Detection of antibodies against Toxoplasma gondii in cats using an immunochromatographic test based on GRA7 antigen. J. Vet. Med. Sci. 82, 441–445. doi: 10.1292/jvms.19-0654
Ybañez, R. H. D., and Nishikawa, Y. (2020). Serological detection of T. gondii infection in humans using an immunochromatographic assay based on dense granule protein 7. Parasitol. Int. 76:102089. doi: 10.1016/j.parint.2020.102089
Yetisen, A. K., Akram, M. S., and Lowe, C. R. (2013). Paper-based microfluidic point-of-care diagnostic devices. Lab. Chip 13, 2210–2251. doi: 10.1039/c3lc50169h
Youssef, M. M., El-Ganayni, G. A., and El-Shazly, A. M. (1992). The efficacy of IHAT, IFAT and Dot-ELISA in serodiagnosis of toxoplasmosis in complicated pregnancies. J. Egypt Soc. Parasitol. 22, 343–347.
Zenner, L., Estaquier, J., Darcy, F., Maes, P., Capron, A., and Cesbron-Delauw, M. F. (1999). Protective immunity in the rat model of congenital toxoplasmosis and the potential of excreted-secreted antigens as vaccine components. Parasit. Immunol. 21, 261–272. doi: 10.1046/j.1365-3024.1999.00229.x
Zhang, G., Guo, J., and Wang, X. (2009). Immunochromatographic lateral flow strip tests. Methods Mol. Biol. 504, 169–183. doi: 10.1007/978-1-60327-569-9_12
Zhang, K., Lin, G., Han, Y., and Li, J. (2016). Serological diagnosis of toxoplasmosis and standardization. Clin. Chim. Acta. 461, 83–89. doi: 10.1016/j.cca.2016.07.018
Keywords: Toxoplasma gondii, toxoplasmosis, serodiagnosis, recombinant antigens, human
Citation: Ybañez RHD, Ybañez AP and Nishikawa Y (2020) Review on the Current Trends of Toxoplasmosis Serodiagnosis in Humans. Front. Cell. Infect. Microbiol. 10:204. doi: 10.3389/fcimb.2020.00204
Received: 27 January 2020; Accepted: 16 April 2020;
Published: 08 May 2020.
Edited by:
Guo-Hua Liu, Hunan Agricultural University, ChinaReviewed by:
Veeranoot Nissapatorn, Walailak University, ThailandCopyright © 2020 Ybañez, Ybañez and Nishikawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshifumi Nishikawa, bmlzaWthd2FAb2JpaGlyby5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.