- 1Infection Control Center, Xiangya Hospital, Central South University, Changsha, China
- 2Changsha Hospital of Traditional Chinese Medicine, Changsha, China
The gut microbiota composition of intensive care unit (ICU) patients suffering from Clostridium difficile-positive diarrhea (CDpD) is poorly understood. This prospective study aims to use 16S rDNA (and metagenome) sequencing to compare the microbiota composition of 58 (and 5) ICU patients with CDpD (CDpD group), 33 (and 4) ICU patients with C. difficile-negative diarrhea (CDnD group), and 21 (and 5) healthy control subjects (control group), as well as CDpD patients in the A+B+ (N = 34; A/B: C. difficile TcdA/B), A−B+ (N = 7), and A−B− (N = 17) subgroups. For 16S rDNA data, OTU clustering (tool: UPARSE), taxonomic assignment (tool: RDP classifier), α-diversity, and β-diversity analyses (tool: QIIME) were conducted. For metagenome data, metagenome assembly (tool: SOAPdenovo), gene calling (tools: MetaGeneMark, CD-HIT, and SoapAligner), unigene alignment (tool: DIAMOND), taxon difference analysis (tool: Metastats), and gene annotation (tool: DIAMOND) were performed. The microbial diversity of the CDpD group was lower than that of the CDnD and control groups. The abundances of 10 taxa (e.g., Deferribacteres, Cryptomycota, Acetothermia) were significantly higher in the CDpD group than in the CDnD group. The abundances of Saccharomycetes and Clostridia were significantly lower in CDpD in comparison with control. Some taxa were significantly different between the A+B+ and A−B− subgroups. CDpD might relate to a decrease in beneficial taxa (i.e., Saccharomycetes and Clostridia) and an increase in harmful taxa (e.g., Deferribacteres, Cryptomycota, Acetothermia) in gut microbiota of ICU patients. C. difficile toxin type might be slightly associated with gut microbiota composition.
Introduction
Antibiotics are often used in treating intensive care unit (ICU) patients (Vincent et al., 2016). However, antibiotic treatment is the most crucial risk factor associated Clostridium difficile (also known as Peptoclostridium difficile) infection (CDI) (Stevens et al., 2011), as antibiotics adversely affect the indigenous gut microbiota composition and decrease colonization resistance to C. difficile (Britton and Young, 2012). The clinical symptoms of CDI range from mild diarrhea to severe complications such as pseudomembranous colitis, toxic megacolon, bowel perforation, and death (Yassin et al., 2001; Rupnik et al., 2009; Shivashankar et al., 2013; Leffler and Lamont, 2015). The occurrence of CDI is 7.4~14.1 cases per 10,000 patient-days in ICU (Centers for Disease Control Prevention (CDC), 2012; Lee et al., 2015; Li et al., 2018).
Nevertheless, current antibiotic therapies for CDI such as vancomycin have limited efficacy (Bagdasarian et al., 2015), and fecal microbiota transplantation (FMT) is recommended as an alternative therapy in treating highly recurrent CDI that has failed to respond to vancomycin treatment (Kelly et al., 2015). The success of FMT emphasizes the importance of restoring the gut microbiome in CDI patients.
In recent years, high-throughput deep-sequencing of 16S rDNA and the metagenome has been applied to investigating microbiota composition. Milani et al. used 16S rDNA and metagenome sequencing to study the gut microbiota compositions of three groups of elderly (age ≥ 65) hospitalized patients, involving 30 CDI-negative patients not exposed to antibiotics, 29 CDI-negative patients exposed to antibiotics, and 25 CDI-positive patients (Milani et al., 2016). The microbial diversity of the CDI-positive group was significantly lower than that of the CDI-negative group. CDI was associated with the decrease in gut commensal bacteria such as Bacteroides, Alistipes, Lachnospira, and Barnesiella, while antibiotic treatment in CDI-negative patients might lead to the depletion of commensal bacteria such as Alistipes (Milani et al., 2016). Schubert et al. utilized 16S rDNA sequencing to study the gut microbiota compositions of CDI cases, diarrheal controls, and non-diarrheal controls (Schubert et al., 2014). Statistical models were developed for CDI and diarrhea by incorporating clinical and demographic data with microbiome data, and loss of several species in Ruminococcaceae, Lachnospiraceae, Bacteroides and Porphyromonadaceae might be associate with CDI (Schubert et al., 2014). However, the microbiome alterations related with diarrhea and C. difficile-positive diarrhea (CDpD) have not been completely elucidated in ICU patients. Generally, only C. difficile that produces toxin A (an intestinotoxin, TcdA) and/or toxin B (a cytotoxin, TcdB) can cause gastrointestinal diseases in humans. However, relationships between C. difficile type (i.e., A+B+, A−B+, and A−B−) and the gut microbiota composition have not been investigated.
In this prospective study, we utilized 16S rDNA and metagenome deep-sequencing to characterize the gut microbiota composition in healthy subjects, CDpD ICU patients, and C. difficile negative diarrhea (CDnD) ICU patients, as well as the gut microbiota composition in ICU patients with A+B+ CDpD, A−B+ CDpD, and A−B− CDpD. Our results may help to elucidate the mechanisms underlying diarrhea and CDpD development in ICU patients and identify potential candidates for curative or preventive microbe therapy.
Materials and Methods
Study Population and Sample Collection
Xiangya Hospital is a 3,500-bed urban tertiary teaching hospital in Changsha, Hunan Province, China, and admits ~90,000 patients per annum. The hospital has a General ICU (35 beds). In this study, a total of 112 subjects were enrolled between March 2014 and December 2014. Subjects included 21 non-pregnant healthy individuals (to whom antibiotics had not been administered within 1 month and without any signs of diarrhea within 7 days before sample collection) and 91 non-pregnant ICU patients with signs of hospital-onset diarrhea (where diarrhea occurred 48 h after hospital admission with stool three or more times within 24 h). Subjects with inflammatory bowel disease and diarrhea that occurred less than 48 h after hospital admission were excluded from the study. For the ICU patients with hospital-onset diarrhea, C. difficile was screened within 24 h after sampling (at least 300-μl diarrheal stool samples frozen at−20°C for PCR) using 16S rDNA-PCR (PS13: 5′-GGAGGCAGCAGTGGGGAATA-3′, PS14: 5′-TGACGGGCGGTGTGTACAAG-3′) (Persson et al., 2011), sequencing, and BLAST alignment. Patients with positive results were classified into the CDpD group (N = 58), and patients with negative results were classified into the CDnD group (N = 33).
Diarrheal stool samples (at least 300 μl) obtained from patients and non-diarrheal stool samples (at least 300 μl) obtained from healthy individuals were frozen at −80°C before sequencing in 2015. Then, 16S rDNA V4 sequencing-based microbiota analysis was performed for stool samples in the CDpD, CDnD, and healthy control groups (CDpD-CDnD-control 16S rDNA V4 sequencing analysis). Moreover, 14 samples were selected from these groups (five samples in the CDpD group, four samples in the CDnD group, and five samples in the control group) to conduct genome sequencing-based metagenomics analysis (CDpD-CDnD-control metagenome sequencing analysis).
According to the PCR results of C. difficile TcdA (gene for toxin A; Forward primer: 5′-AGATTCCTATATTTACATGACAATAT-3′, Reverse primer: 5′-GTATCAGGCATAAAGTAATATACTTT-3′) (Lemee et al., 2004) and TcdB (gene for toxin B; NK104: 5′-GTGTAGCAATGAAAGTCCAAGTTTACGC-3′, NK105: 5′-CACTTAGCTCTTTGATTGCTGCACCT-3′) (Kato et al., 1998), patients in the CDpD group were classified into the A+B+ subgroup (N = 34), A−B+ subgroup (N = 7), and A−B− subgroup (N = 17), and 16S rDNA V4 sequencing-based microbiota analysis was then performed for stool specimens in the A+B+, A−B+, and A−B− subgroups (CDpD subgroups 16S rDNA V4 sequencing analysis).
Clinical Data Collection
For each subject, clinical data were collected, including age, gender, duration of hospital stay, C. difficile detection, other diseases (i.e., diabetes, cancer, hematopathy, respiratory failure, renal insufficiency, and tuberculosis), surgery, and exposure to immunosuppressants, glucocorticoids, and antibiotics.
Amplification and Sequencing of 16S rDNA
As previously described (Schubert et al., 2014; Milani et al., 2016), DNA was extracted from stool samples, and the V4-region (primers: 515F and 806R) of 16S rDNA were amplified and sequenced using an Illumina HiSeq2500 PE250 Paired-end sequencer. As detailed in the Supplemental Materials, 16S rDNA-based microbiota analysis was performed, including chimera sequence removal, operational taxonomic unit (OTU) clustering, taxonomic assignment, phylogenetic relationship, α-diversity analysis, and β-diversity analysis [unweighted unifrac distance, weighted unifrac distance, sample clustering tree, and principal coordinate analysis (PCoA)].
Genome Sequencing
Total metagenomic DNA was isolated from each stool sample (Milani et al., 2016). After obtaining fragments of 300 bp (ultrasonic method), end repairing, adding poly-A, adding sequencing adapters, purification, and PCR, DNA libraries were obtained and then sequenced using an Illumina HiSeq2500 Paired-end sequencer with a 2 × 150 bp read length. Metagenomic data were analyzed (Supplemental Materials), including metagenome assembly de novo, gene calling, alignment of unigenes to reference genomes (microbial reference genomes of bacteria, fungi, archaea, and viruses in the NR database of the National Center for Biological Information), taxonomical analysis and pathway annotation.
Statistical Analyses
Statistical analyses were performed using SPSS 19.0 software to determine the differences in clinical characteristics among groups (i.e., CDpD, CDnD, and control groups), as well as subgroups (i.e., A+B+, A−B+, and A−B− subgroups). For continuous variables, comparison among three groups was performed using one-way ANOVA, whereas comparison between two groups was conducted using independent-samples t-test. In one-way ANOVA, least-significant-difference test and Dunnett's T3 method were used under the conditions of equal variance and unequal variance, respectively. P < 0.05 was set as the criterion for statistical difference. For categorical variables, Fisher's exact test and Pearson's chi-square test were performed.
Results
Clinical Features of Subjects
For CDpD-CDnD-control 16S rDNA V4 sequencing analysis, subjects in the CDpD group (N = 58), CDnD group (N = 33), and control group (N = 21) were significantly different for age, but similar for gender. Subjects in the CDpD and CDnD groups were significantly different for antibiotics usage (i.e., exposure to antibiotics within 1 month before diarrhea) but similar for the other clinical features (Table 1).
For the CDpD subgroups 16S rDNA V4 sequencing analysis, subjects in the A+B+ subgroup (N = 34), A−B+ subgroup (N = 7), and A−B− subgroup (N = 17) of the CDpD group were significantly different for antibiotics usage but similar for the other clinical features (Table 1).
For CDpD-CDnD-control metagenome sequencing analysis, subjects in the CDpD group (N = 5), CDnD group (N = 4), and control group (N = 5) were significantly different for age but similar for gender. Subjects in the CDpD and CDnD groups were significantly different for antibiotics usage but similar for the other clinical features (Table 1).
CDpD-CDnD-Control 16S rDNA V4 Sequencing Analysis
In α-diversity analysis, the Chao1 index, Shannon index, and number of observed species were assessed. The Chao1 index (i.e., community richness) in the CDpD group was lower than that in the CDnD and control groups. The Shannon index (i.e., community diversity) in the CDpD group was similar to that in the CDnD group but much lower than that in the control group. The number of observed species in the CDpD group was much lower than in the CDnD and control groups. A total of 2,125 16S rDNA-based OTUs were clustered, among which 513 OTUs were shared by the three groups.
In β-diversity analysis, the un-weighted unifrac distances were 0.282, 0.617, and 0.61 between CDnD and control, between CDpD and control, and between CDpD and CDnD, respectively (Figure 1A). PCoA was conducted based on un-weighted unifrac distance, and CDpD samples could be clearly separated from CDnD samples and controls (Figure 1B). Besides, the CDpD, CDnD, and control groups could be distinguished from each other based on unweighted/weighted unifrac distance and relative abundance at the Phylum level (Figures 1C,D).
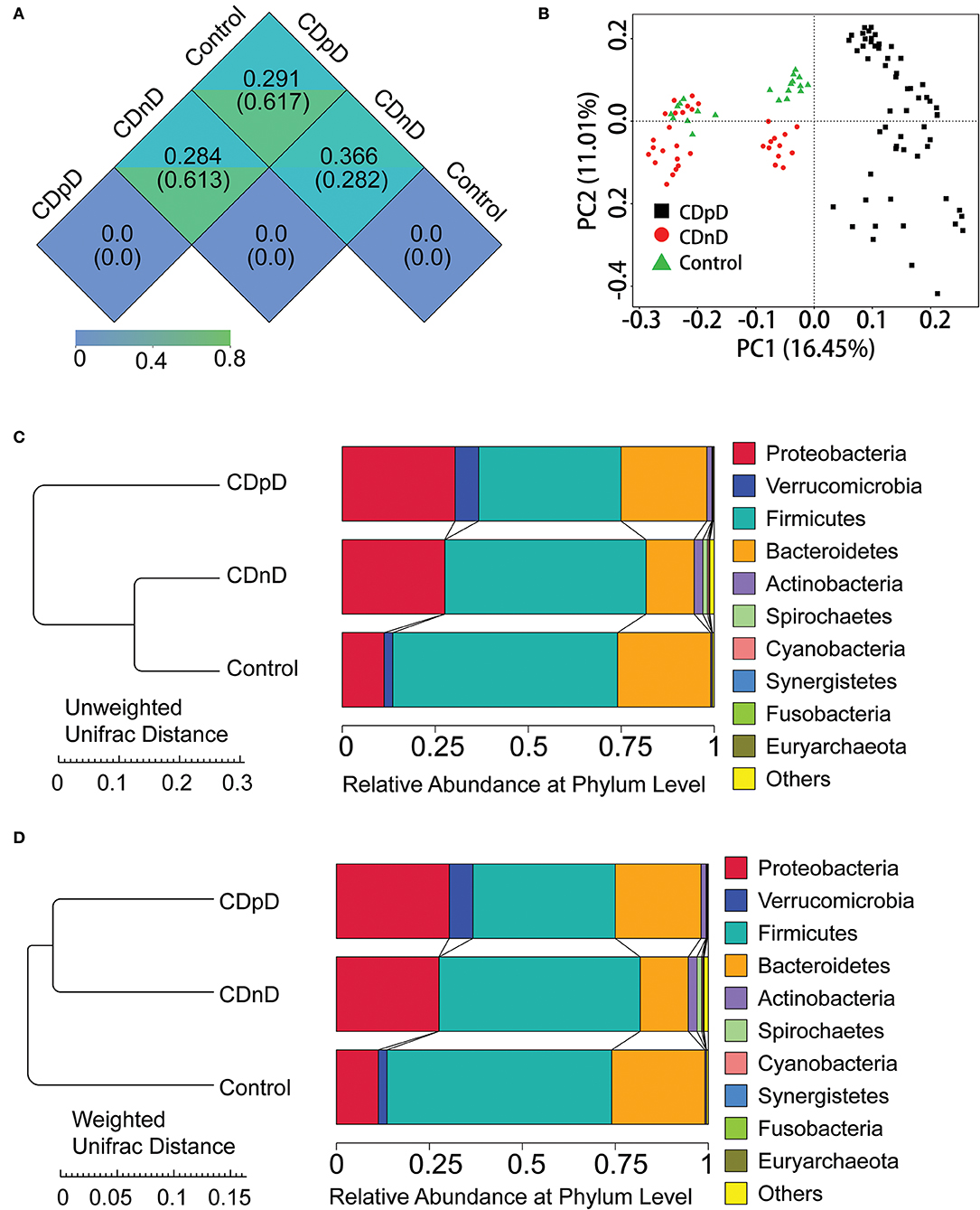
Figure 1. α-diversity and β-diversity analysis in CDpD-CDnD-control 16S rDNA V4 sequencing analysis. (A) Unifrac distance between groups. Upper number: weighted unifrac distance; lower number: un-weighted unifrac distance. Both numbers represent the index for differences in taxon-diversity between groups. (B) PCoA based on unweighted unifrac distance. (C) Sample clustering based on unweighted unifrac distance and relative abundance at Phylum level. (D) Sample clustering based on weighted unifrac distance and relative abundance at Phylum level.
Relative abundances of the top 10 taxa in terms of Phylum, Class, Order, Family, and Genus were studied. At the Phylum level, the relative abundance of Firmicutes was lower whereas those of Proteobacteria and Verrucomicrobia were higher in the CDpD group when compared with the CDnD and control groups (Figures 1C,D). At the Class level, the relative abundances of Verrucomicrobiae and Gammaproteobacteria were higher in the CDpD group (Figure 2A). At the Order level, the relative abundances of Verrucomicrobiales and Enterobacteriales were higher in the CDpD group (Figure 2B). At the Family level, the relative abundances of Porphyromonadaceae, Verrucomicrobiaceae, and Enterobacteriaceae were higher in the CDpD group (Figure 2C). At the Genus level, the relative abundances of Parabacteroides and Akkermansia were higher in the CDpD group (Figure 2D).
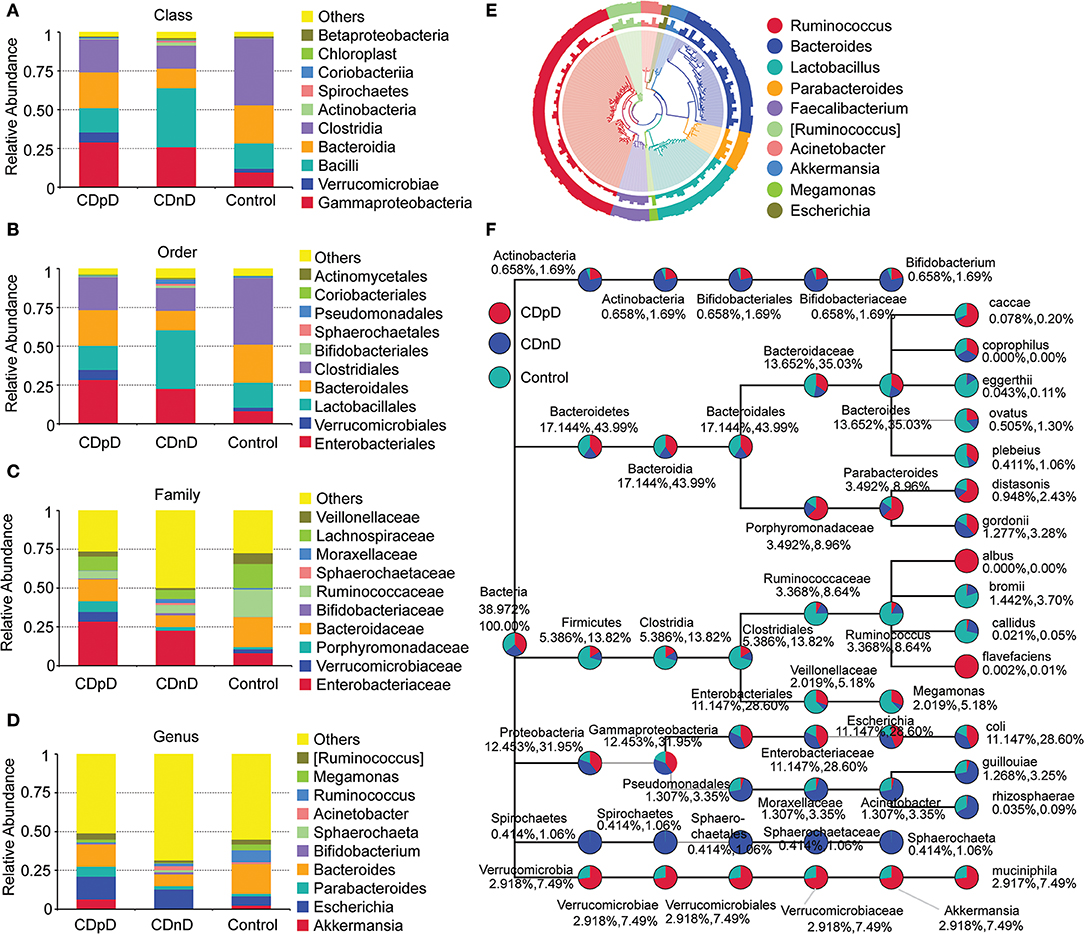
Figure 2. Taxonomic differences and phylogenetic relationships in CDpD-CDnD-control 16S rDNA V4 sequencing analysis. (A) Taxonomic differences at Class level. (B) Taxonomic differences at Order level. (C) Taxonomic differences at Family level. (D) Taxonomic differences at Genus level. (E) Phylogenetic relationships between OTUs annotated into top 10 Genera. Inner cycle, phylogenetic tree based on representative sequences of OTUs; middle cycle, relative abundances of OTUs; outer cycle, reliability of OUT annotation. (F) Taxonomic differences in selected taxa at the levels of Phylum, Class, Order, Family, and Genus. The former (later) number = [mean relative abundance of this taxon in all samples]/[mean relative abundance of all (selected) taxa in all samples] × 100%.
The phylogenetic relationships between the representative sequences of all OTUs corresponding to the top 10 Genera are shown in Figure 2E. Additionally, a taxonomic tree was constructed (Figure 2F). The abundances of Porphyromonadaceae, Parabacteroides, Parabacteroides distasonis, and Bacteroides caccae in the CDpD group were greater than those in the CDnD and control groups. These taxa might be associated with CDpD. Firmicutes, Clostridia, Clostridiales, Ruminococcaceae, Ruminococcus, Ruminococcus Bromii, and Ruminococcus callidus as well as Veillonellaceae and Megamonas were more abundant in the control group than in the CDnD and CDpD groups. These taxa might be associated with diarrhea.
CDpD-CDnD-Control Metagenome Sequencing Analysis
In core-pan gene analysis, the number of non-redundant genes declined along with the increase in sample number in the core-gene curve, whereas it increased along with the increase in sample number in the pan-gene curve. These results supported a further detailed analysis of metagenome sequencing data. A total of 591,552 genes were predicted based on metagenome sequences.
In CDpD-CDnD-control metagenome sequencing analysis, significant differences were identified between the CDpD and CDnD groups in the abundances of 10 taxa (Dictyoglomi, Acetothermia, Cryptomycota, Chytridiomycota, Glomeromycota, Planctomycetes, Aquificae, Deferribacteres, Poribacteria, and Armatimonadetes; Figures 3A,B). Also, significant differences were found between the CDpD and control groups in the abundances of Holophagae, Bacteroidia, Saccharomycetes, and Clostridia (Figures 3C,D). In both CDpD-CDnD-control 16S rDNA V4 sequencing analysis and CDpD-CDnD-control metagenome sequencing analysis, the relative abundance of Clostridia in the CDpD group was lower than in the control group.
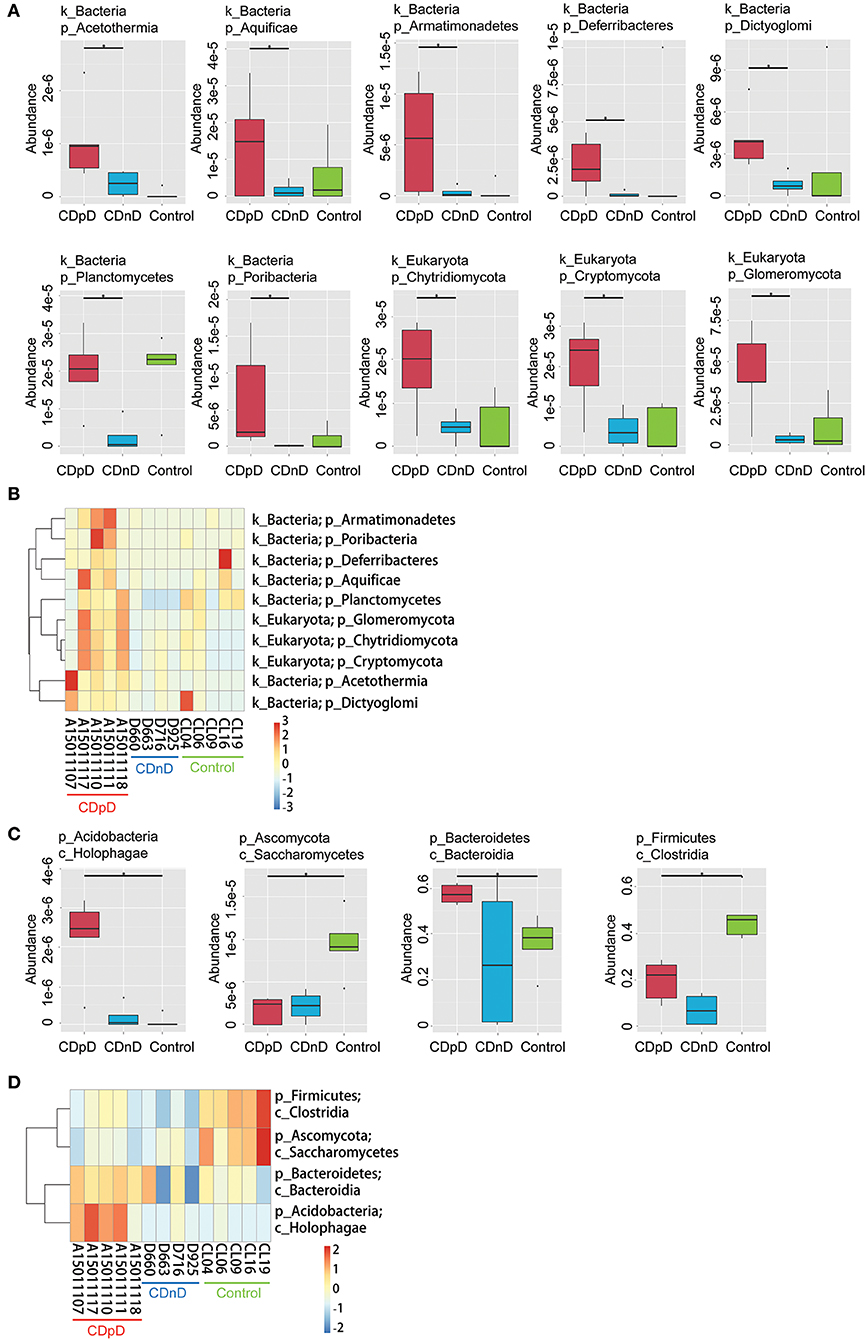
Figure 3. Significant taxonomic differences in CDpD-CDnD-control metagenome sequencing analysis. (A) *Significant taxonomic differences between groups (Phylum level). (B) Clustering based on the significant taxonomic differences at Phylum level. (C) *Significant taxonomic differences between groups (Class level). (D) Clustering based on the significant taxonomic differences at Class level.
Based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, most of the predicted genes were related with Metabolism (e.g., Carbohydrate metabolism, Amino acid metabolism), Environmental Information Processing (e.g., Membrane transport), and Genetic Information Processing (e.g., Translation) (Figure 4A). Samples were clustered based on Bray-Curtis distances and gene abundances (KEGG level 1), and control samples were closely clustered (Figure 4B). The top-35 pathways with the highest gene abundance were identified (KEGG level 2, Figure 4C). Compared with CDnD and control samples, CDpD samples showed significantly higher abundances in pathways such as “Cellular community,” “Excretory system,” and “Circulatory system.” Besides, “Lysine biosynthesis” and “Coenzyme B biosynthesis” were only annotated in CDpD samples when compared with controls.
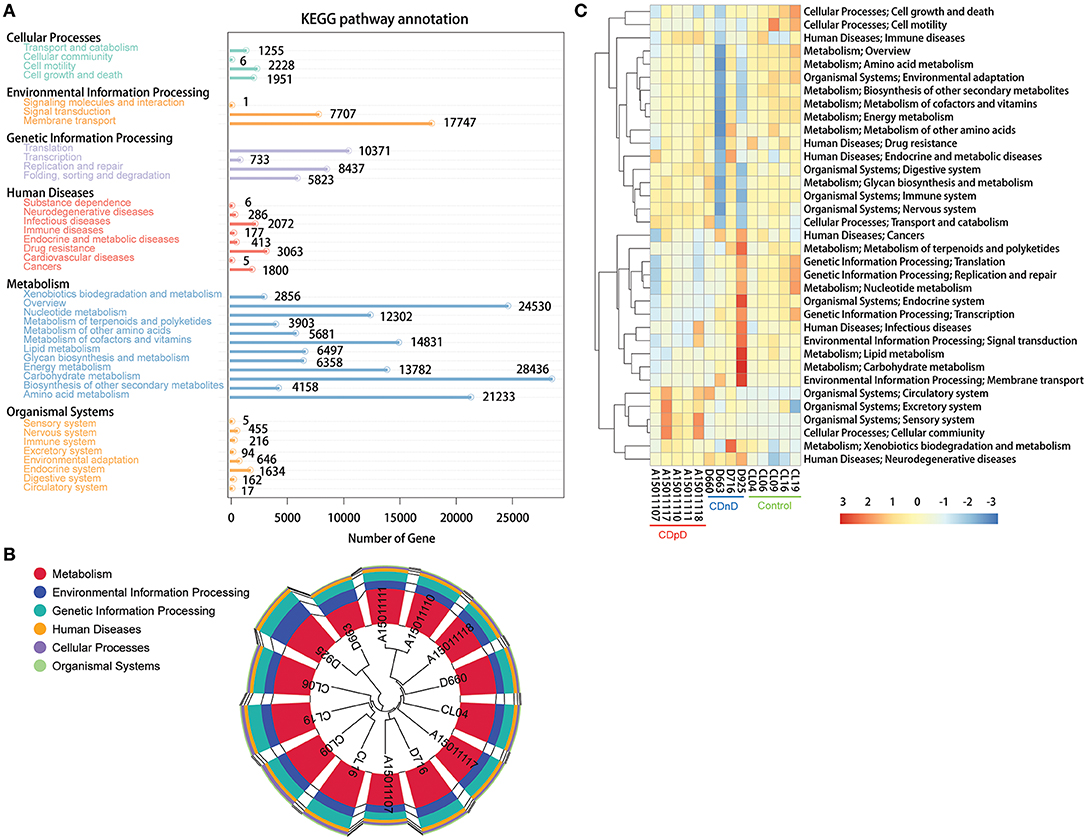
Figure 4. Functional annotation based on the KEGG database in CDpD-CDnD-control metagenome sequencing analysis. (A) Distribution of genes in KEGG pathways. (B) Sample clustering based on Bray-Curtis distances and gene abundances (Level 1). (C) Top-35 pathways (Level 2) with highest gene abundance.
CDpD Subgroups 16S rDNA V4 Sequencing Analysis
In α-diversity analysis, the Chao1 index, Shannon index, and number of observed species in the A+B+ subgroup was much higher than in the A−B+ and A−B− subgroups.
In β-diversity analysis, the unweighted unifrac distances were 0.471, 0.429, and 0.466 between A−B+ and A−B−, between A+B+ and A−B−, and between A+B+ and A−B+ (Figure 5A). In β-diversity analysis, the weighted unifrac distances were 0.257, 0.254, and 0.252 between A−B+ and A−B−, between A+B+ and A−B−, and between A+B+ and A−B+ (Figure 5A). These results indicated that the differences in taxon diversity between these subgroups were similar.
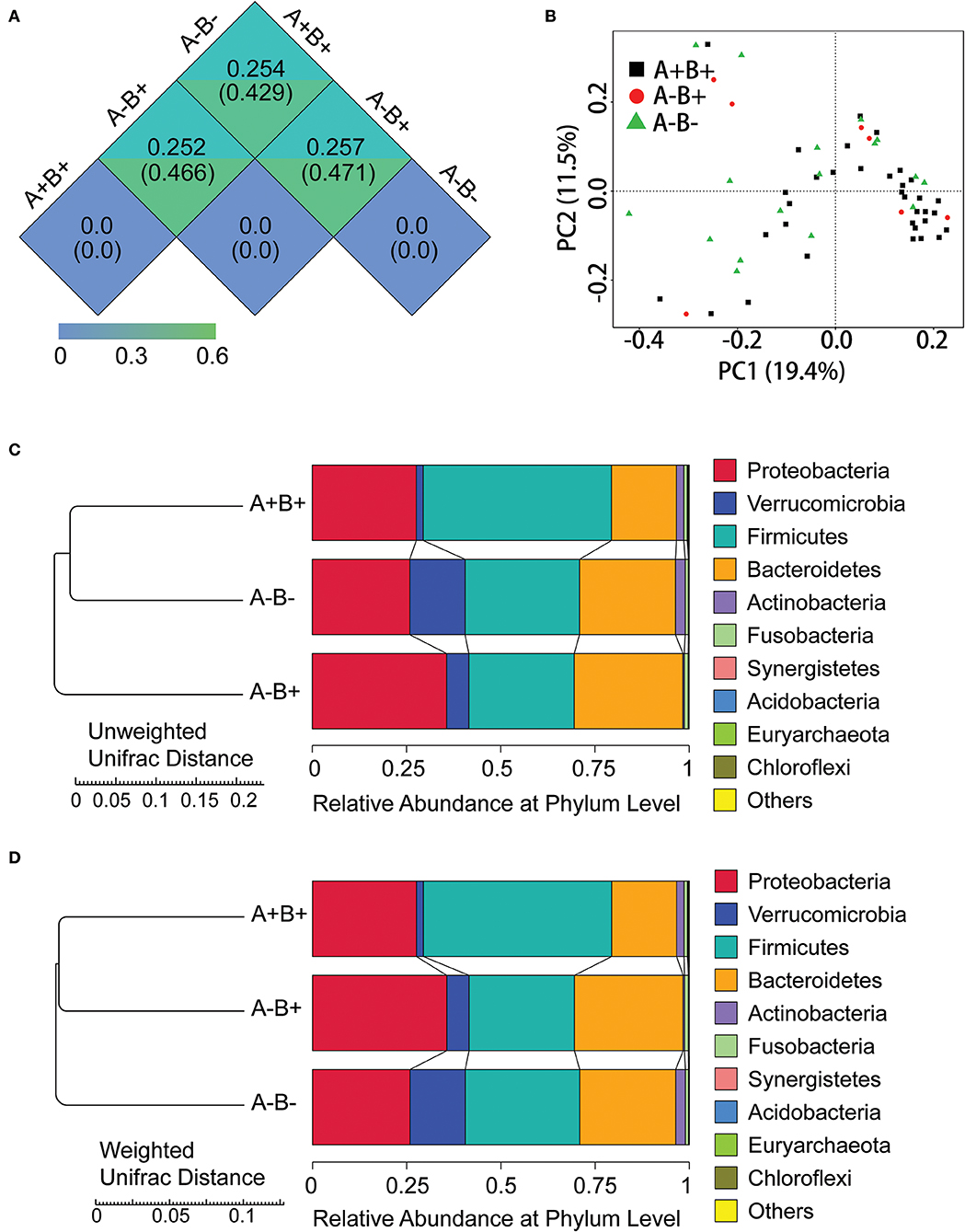
Figure 5. α-diversity and β-diversity analysis in subgroups 16S rDNA V4 sequencing analysis. (A) Unifrac distance between groups. Upper number: weighted unifrac distance; lower number: un-weighted unifrac distance. Both numbers represent the index for differences in taxon-diversity between groups. (B) PCoA based on unweighted unifrac distance. (C) Sample clustering based on unweighted unifrac distance and relative abundance at Phylum level. (D) Sample clustering based on weighted unifrac distance and relative abundance at Phylum level.
In PCoA based on unweighted unifrac distance, high inter-sample variability was found across groups, and A+B+ samples could not be distinguished from A−B+ or A−B− samples (Figure 5B). However, the A+B+, A−B+, and A−B− subgroups could be distinguished from each other based on the unweighted/weighted unifrac distance and relative abundance at the Phylum level (Figures 5C,D).
At the Phylum level, the relative abundances of Bacteroidetes and Verrucomicrobia were lower whereas the relative abundance of Firmicutes was higher in the A+B+ subgroup when compared with the A−B+ and A−B− subgroups (Figures 5C,D). At the Class level, the relative abundances of Bacteroidia and Verrucomicrobiae were lower and the relative abundances of Clostridia and Bacilli were higher in the A+B+ subgroup (Figure 6A). At the Order level, the relative abundances of Bacteroidales and Verrucomicrobiales were lower and the relative abundances of Clostridiales and Lactobacillales were higher in the A+B+ subgroup (Figure 6B). At the Family level, the relative abundance of Verrucomicrobiaceae was lower and the relative abundances of Enterococcaceae and Ruminococcaceae were higher in the A+B+ subgroup (Figure 6C). At the Genus level, the relative abundance of Akkermansia was lower in the A+B+ subgroup (Figure 6D). Moreover, Methylococcaceae, Geobacteraceae, Peptococcaceae, Crenotrichaceae, Mogibacteriaceae, Acetobacter, Dialister, and Crenothrix significantly increased in the A+B+ subgroup in comparison with the A−B− subgroup. Exiguobacteraceae, Rickettsiaceae, Promicromonosporaceae, Procabacteriaceae, Providencia, Cellulosimicrobium, Wolbachia, and Saccharopolyspora significantly decreased in the A+B+ subgroup in comparison with the A−B− subgroup.
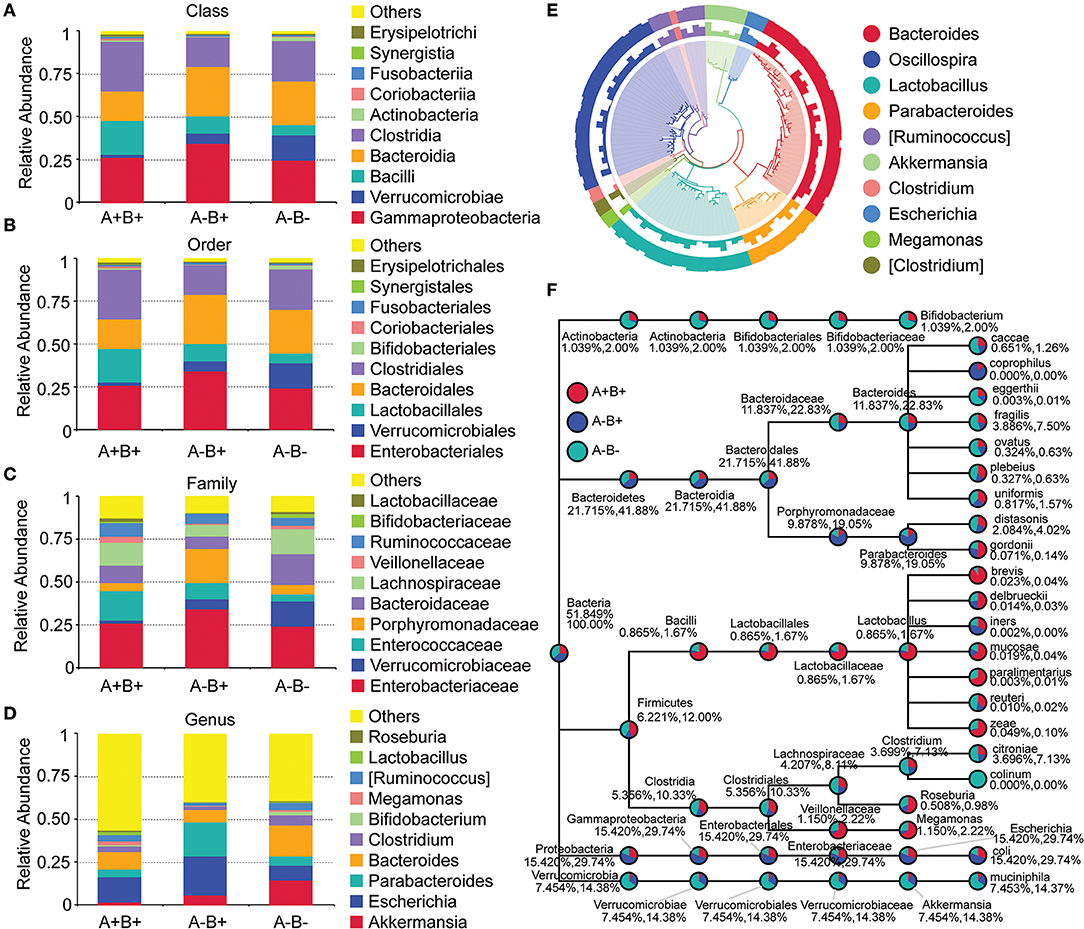
Figure 6. Taxonomic differences and phylogenetic relationships in subgroups 16S rDNA V4 sequencing analysis. (A) Taxonomic differences between A+B+, A−B+, and A−B− subgroups (Class level). (B) Taxonomic differences between A+B+, A−B+, and A−B− subgroups (Order level). (C) Taxonomic differences between A+B+, A−B+, and A−B− subgroups (Family level). (D) Taxonomic differences between A+B+, A−B+, and A−B− subgroups (Genus level). (E) Phylogenetic relationships between OTUs annotated into top 10 Genera. Inner cycle, phylogenetic tree based on representative sequences of OTUs; middle cycle, relative abundances of OTUs; outer cycle, reliability of OUT annotation. (F) Taxonomic differences in selected taxa at the levels of Phylum, Class, Order, Family, and Genus. The former number = (mean relative abundance of this taxon in all samples)/(mean relative abundance of all taxa in all samples) × 100%; the later number = (mean relative abundance of this taxon in all samples)/(mean relative abundance of selected taxa in all samples) × 100%.
Phylogenetic relationships between the representative sequences of all OTUs corresponding to the top 10 taxa in Genus were studied (Figure 6E), and a taxonomic tree was constructed (Figure 6F). Bacteroides eggerthii, Bacteroides fragilis, Bacteroides ovatus, and Clostridium citroniae were more abundant in the A−B− subgroup than in the A−B+ and A+B+ subgroups. These taxa might be suppressed by the presence of toxins. The abundances of Proteobacteria, Gammaproteobacteria, Enterobacteriales, Enterobacteriaceae, Escherichia, and Escherichia coli in the A−B− subgroup A−B+ subgroup were much lower than in the A+B+ and A−B− subgroups. These taxa might be associated with A−B+.
Discussion
To better understand how the microbiome is associated with diarrhea, CDpD, and C. difficile type, we characterized the gut microbiota of healthy individuals and ICU individuals with CDpD or CDnD. Consequently, we found gut microbiota compositions with potential associations with CDpD and C. difficile type in ICU patients.
Analyses of the Shannon index and number of observed species indicated that the microbial diversity of the CDpD group was lower than that of the CDnD and control groups, and this agreed well with a previous study in elderly (age ≥ 65) hospitalized patients (Milani et al., 2016). The unweighted unifrac distance between CDpD and control/CDnD was higher than that between CDnD and control. In PCoA, CDpD samples could be distinguished from CDnD samples and controls, whereas CDnD samples could not be separated from controls. These results indicated that the differences in taxon diversity between CDpD and control/CDnD were much higher than those between CDnD and control and that the appearance of C. difficile was strongly associated with the decrease in diversity of the gut microbiota.
Differences in community composition between groups were investigated. Metagenome analysis revealed that the abundances of 10 taxa (e.g., Deferribacteres, Cryptomycota, Acetothermia) in the CDpD group were significantly higher than in the CDnD group. Additionally, the abundances of two taxa (Saccharomycetes and Clostridia) were significantly lower in CDpD in comparison with control. Saccharomyces boulardii is a nonpathogenic yeast that promotes intestinal immunoglobulin-A immune response to C. difficile toxin A and thus protects against recurrent C. difficile colitis and diarrhea (Qamar et al., 2001). A balanced gut microbiota is characterized by conserved features like the predominance of Bacteroidia and Clostridia (Winter and Baumler, 2014). Therefore, CDpD might be associated with a decrease in beneficial taxa (i.e., Saccharomycetes and Clostridia) and an increase in harmful taxa (e.g., Deferribacteres, Cryptomycota, Acetothermia) in gut microbiota. Also, Saccharomycetes and Clostridia might serve as future candidate targets in ICU CDpD patients to help them re-establish healthy gut microbiota. Saccharomyces boulardii has been utilized to prevent antibiotic-associated diarrhea in adult hospitalized patients in clinical trials (Ehrhardt et al., 2016).
Moreover, based on 16S rDNA sequencing data, relative abundances of some taxa (e.g., Gammaproteobacteria, Enterobacteriaceae, Porphyromonadaceae, and Akkermansia) were higher in the CDpD group in comparison with the CDnD and control groups. Our results were partially consistent with previous study, in which Gammaproteobacteria and Enterobacteriaceae were significantly over-represented in CDI samples, whereas Porphyromonadaceae and Akkermansia were significantly under-represented in CDI samples, in comparison with non-CDI samples. Reportedly, A. muciniphila is an intestinal representative of the Verrucomicrobia, and it associates with health in humans. Reduction of A. muciniphila was observed in patients with inflammatory bowel diseases, which led to the hypothesis that A. muciniphila possesses health-promoting activities and anti-inflammatory properties (Derrien et al., 2016). Although Akkermansia may improve barrier function, its over-representation in CDpD may be caused by modifications in the gut micro-environment and reflects enteric mucosa inflammation (Zwielehner et al., 2011). Thus, CDpD might also correlate with increase in Akkermansia.
Furthermore, differences in microbiota pathways between the three groups were studied using metagenome sequencing data. “Lysine biosynthesis” and “Coenzyme B biosynthesis” were only annotated in CDpD samples. Therefore, CDpD might be related with these pathways in gut microbiota, and drugs inhibiting these pathways or the colonization of Deferribacteres, Cryptomycota, Acetothermia, Gammaproteobacteria, and Enterobacteriaceae might be effective in limiting C. difficile blooming.
In the CDpD group, the community richness and microbial diversity of the A+B+ subgroup were higher than those of the A−B+ and A−B− subgroups. The similar unweighted unifrac distances (or weighted unifrac distances) between A−B+ and A−B−, between A+B+ and A−B−, and between A+B+ and A−B+ indicated similar differences in taxon diversity between these subgroups. High inter-sample variability was found in PCoA, and A+B+ samples could not be distinguished from A−B+ or A−B− samples. Besides, some taxa were significantly different between the A+B+ and A−B− subgroups (e.g., Acetobacter, Saccharopolyspora). These results suggested that higher sample numbers in the A+B+, A−B+, and A−B− subgroups are required for conclusive results.
Unfortunately, some limitations were found in this study. Firstly, we did not collect the reasons for diarrhea in the CDnD group. Secondly, age and antibiotic usage were not matched among the three groups, as the clinical complexity of ICU patients and the application of polypharmacy did not allow us to identify a sufficient number of subjects for each group or to standardize antibiotic treatment. In our future studies, more subjects will be enrolled to obtain a better stratification of patient features at baseline, and more clinical data (e.g., the reasons for diarrhea in the CDnD group) will be collected.
Conclusions
In ICU patients, the development of CDpD might be associated with alterations in gut microbiota, including decreases in beneficial taxa (i.e., Saccharomycetes and Clostridia) and increases in Deferribacteres, Cryptomycota, Acetothermia, Gammaproteobacteria, and Enterobacteriaceae. Besides, C. difficile toxin types might slightly influence gut microbiota composition in ICU patients with CDpD. These results provided a further understanding of the mechanism of CDpD in ICU patients and shed light on future directions for developing novel therapies to re-construct healthy gut microbiota.
Data Availability Statement
The data supporting the conclusion of this article were submitted to SRA NCBI; the SRA accession information is PRJNA597086 (16s rDNA) and PRJNA591064 (metagenome).
Ethics Statement
This study was approved by the Ethics Committee of Xiangya Hospital Central South University, China (ID 201212027). All participants granted written informed consent before enrollment, and all investigations followed the principles of the Declaration of Helsinki.
Author Contributions
AW, JD, and CL conceived and designed the experiments. JD, SL, CZ, and XM performed the experiments. CF, PZ, QD, and CL analyzed the data. CL and AW wrote the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81601803), the Research Fund of Hunan Provincial Health and Family Planning Commission (No. B2016107), Young Scientists Fund of Xiangya Hospital (2014Q05), and Xiangya Sinobio Way Health Research Fund (No. xywm2015I11). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a Pre-Print at bioRxiv.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.00190/full#supplementary-material
Abbreviations
CDI, C. difficile infection; CDpD, C. difficile-positive diarrhea; FMT, Fecal microbiota transplantation; ICU, Intensive care unit; OTU, Operational taxonomic unit.
References
Bagdasarian, N., Rao, K., and Malani, P. N. (2015). Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 313, 398–408. doi: 10.1001/jama.2014.17103
Britton, R. A., and Young, V. B. (2012). Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 20, 313–319. doi: 10.1016/j.tim.2012.04.001
Centers for Disease Control and Prevention (CDC) (2012). Vital signs: preventing Clostridium difficile infections. MMWR Morb. Mortal Wkly. Rep. 61, 157–162.
Derrien, M., Belzer, C., and De Vos, W. M. (2016). Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 106, 171–181. doi: 10.1016/j.micpath.2016.02.005
Ehrhardt, S., Guo, N., Hinz, R., Schoppen, S., May, J., Reiser, M., et al. (2016). Saccharomyces boulardii to prevent antibiotic-associated diarrhea: a randomized, double-masked, placebo-controlled trial. Open Forum. Infect. Dis. 3:ofw011. doi: 10.1093/ofid/ofw011
Kato, H., Kato, N., Watanabe, K., Iwai, N., Nakamura, H., Yamamoto, T., et al. (1998). Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 36, 2178–2182.
Kelly, C. R., Kahn, S., Kashyap, P., Laine, L., Rubin, D., Atreja, A., et al. (2015). Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237. doi: 10.1053/j.gastro.2015.05.008
Lee, J.-C., Hung, Y.-P., Tsai, P.-J., and Ko, W.-C. (2015). Clostridium difficile-associated diarrhea in medical intensive care units of a medical center in southern Taiwan: a prospective surveillance study. J. Microbiol. Immunol. Infect. 48:S182. doi: 10.1016/j.jmii.2015.02.637
Leffler, D. A., and Lamont, J. T. (2015). Clostridium difficile infection. N. Engl. J. Med. 373, 287–288. doi: 10.1056/NEJMc1506004
Lemee, L., Dhalluin, A., Testelin, S., Mattrat, M. A., Maillard, K., Lemeland, J. F., et al. (2004). Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (Toxin A), and tcdB (Toxin B) genes for toxigenic culture of Clostridium difficile. J. Clin. Microbiol. 42, 5710–5714. doi: 10.1128/JCM.42.12.5710-5714.2004
Li, C., Li, Y., Huai, Y., Liu, S., Meng, X., Duan, J., et al. (2018). Incidence and outbreak of healthcare-onset healthcare-associated clostridioides difficile infections among intensive care patients in a large teaching hospital in China. Front. Microbiol. 9:566. doi: 10.3389/fmicb.2018.00566
Milani, C., Ticinesi, A., Gerritsen, J., Nouvenne, A., Lugli, G. A., Mancabelli, L., et al. (2016). Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci. Rep. 6:25945. doi: 10.1038/srep25945
Persson, S., Jensen, J. N., and Olsen, K. E. (2011). Multiplex PCR method for detection of Clostridium difficile tcdA, tcdB, cdtA, and cdtB and internal in-frame deletion of tcdC. J. Clin. Microbiol. 49, 4299–4300. doi: 10.1128/JCM.05161-11
Qamar, A., Aboudola, S., Warny, M., Michetti, P., Pothoulakis, C., Lamont, J. T., et al. (2001). Saccharomyces boulardii stimulates intestinal immunoglobulin a immune response to Clostridium difficile toxin a in mice. Infect. Immun. 69, 2762–2765. doi: 10.1128/iai.69.4.2762-2765.2001
Rupnik, M., Wilcox, M. H., and Gerding, D. N. (2009). Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7, 526–536. doi: 10.1038/nrmicro2164
Schubert, A., Rogers, M., Ring, C., Mogle, J., Petrosino, J., Young, V., et al. (2014). Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. mBio 5, e01021–e01014. doi: 10.1128/mBio.01021-14
Shivashankar, R., Khanna, S., Kammer, P. P., Harmsen, W. S., Zinsmeister, A. R., Baddour, L. M., et al. (2013). Clinical factors associated with development of severe-complicated Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 11, 1466–1471. doi: 10.1016/j.cgh.2013.04.050
Stevens, V., Dumyati, G., Fine, L. S., Fisher, S. G., and Van Wijngaarden, E. (2011). Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin. Infect. Dis. 53, 42–48. doi: 10.1093/cid/cir301
Vincent, J. L., Bassetti, M., Francois, B., Karam, G., Chastre, J., Torres, A., et al. (2016). Advances in antibiotic therapy in the critically ill. Crit. Care 20:133. doi: 10.1186/s13054-016-1285-6
Winter, S. E., and Baumler, A. J. (2014). Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut. Microbes 5, 71–73. doi: 10.4161/gmic.27129
Yassin, S. F., Young-Fadok, T. M., Zein, N. N., and Pardi, D. S. (2001). Clostridium difficile-associated diarrhea and colitis. Mayo Clin. Proc. 76, 725–730. doi: 10.4065/76.7.725
Keywords: 16S rDNA sequencing, Clostridium difficile-associated diarrhea, gut microbiota, metagenome sequencing, TcdA/TcdB, Clostridium difficile infection, CDI, Clostridium difficile-positive diarrhea
Citation: Duan J, Meng X, Liu S, Zhou P, Zeng C, Fu C, Dou Q, Wu A and Li C (2020) Gut Microbiota Composition Associated With Clostridium difficile-Positive Diarrhea and C. difficile Type in ICU Patients. Front. Cell. Infect. Microbiol. 10:190. doi: 10.3389/fcimb.2020.00190
Received: 13 January 2020; Accepted: 09 April 2020;
Published: 11 May 2020.
Edited by:
Gianluca Ianiro, Agostino Gemelli University Polyclinic, ItalyReviewed by:
Christopher Staley, University of Minnesota Twin Cities, United StatesGiusi Desirè Sciumè, University of Pisa, Italy
Copyright © 2020 Duan, Meng, Liu, Zhou, Zeng, Fu, Dou, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunhui Li, bGljaHVuaHVpJiN4MDAwNDA7Y3N1LmVkdS5jbg==; Anhua Wu, eHl3dWFuaHVhJiN4MDAwNDA7Y3N1LmVkdS5jbg==
 Juping Duan1,2
Juping Duan1,2 Anhua Wu
Anhua Wu Chunhui Li
Chunhui Li