- 1Department of Infectious Disease, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, China
- 2School of Public Health, Nanjing Medical University, Nanjing, China
- 3Department of Radiology, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, China
- 4Department of Clinical Research Center, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, China
- 5Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
Background: The prevalence of different underlying cryptococcal diseases in human immunodeficiency virus (HIV)-infected patients screened positive for cryptococcal antigenemia and the association between cryptococcal diseases and serum cryptococcal antigen (CrAg) titers were understudied.
Methods: HIV-infected patients with CD4 < 200 cells/ul, admitted to the second hospital of Nanjing, Nanjing, China, from January 2016 to September 2019, were retrospectively analyzed. Integrated into routine HIV care, all these patients were screened for cryptococcal antigenemia with CrAg lateral flow assay. Positive patients received extensive laboratory and radiological studies to evaluate underlying cryptococcal diseases.
Results: A total of 872 HIV inpatients were screened for serum CrAg. The prevalence of cryptococcal antigenemia in the study population was 10.3% (95% CI, 8.3–12.3%), 87.6% of which with cryptococcal antigenemia had clinically cryptococcal diseases. The prevalence of cryptococcal meningitis (CM), cryptococcemia and pulmonary cryptococcosis (PC) in patients with cryptococcal antigenemia were 58.4% (95% CI, 48.0–68.9%), 50.7% (95% CI, 39.1–62.2%), and 68.5% (95% CI, 58.7–78.4%), respectively. The median (range) serum CrAg titers in severe cryptococcal diseases (CM or cryptococcemia), localized PC (without co-existing CM or cryptococcemia) and isolated cryptococcal antigenemia were 1:2560 (1:10–1:2560), 1:20 (1:2–1:320), and 1:5 (1:2–1:320), respectively. Serum CrAg titers ≥1:320 were independently associated with CM (adjusted OR 26.88; 95%CI, 8.36–86.42). Severe cryptococcal diseases were found in all patients with serum CrAg titers ≥1:640. None of the patients with serum CrAg titers ≤ 1:5 had CM.
Conclusion: The prevalence of cryptococcal antigenemia was high in HIV inpatients, supporting routine CrAg screening. Clinical cryptococcal diseases, most commonly the PC, existed in the majority of the patients with cryptococcal antigenemia. Since serum CrAg titer is correlated with cryptococcal disease severity, it may possibly guide anti-fungal treatment.
Background
Cryptococcal diseases are common opportunistic infections in human immunodeficiency virus (HIV)-infected patients. The reported deaths of cryptococcal diseases are generally related to cryptococcal meningitis (CM) which had around 0.22 million incident cases leading to about 0.18 million deaths each year (Rajasingham et al., 2017). Cryptococcal antigenemia precedes symptoms of CM by a median of 22 days (French et al., 2002), suggesting that cryptococcal antigenemia screening may identify early cryptococcal diseases before progressing to CM. This screening strategy has been advocated in routine HIV care and may reduce mortality of HIV-infected patients (Rajasingham et al., 2012; Kaplan et al., 2015; Mfinanga et al., 2015). Recently studies calculated that the prevalence of cryptococcal antigenemia among HIV-infected outpatients with a CD4 cell count <100 was around 6.0% (Rajasingham et al., 2017; Ford et al., 2018; Temfack et al., 2019). Of note, substantial variation of the prevalence existed in different regions, with the highest prevalence reported in Ethiopia (Oromia Region, 15.5%) and lowest in Malawi (1.7%) (Rajasingham et al., 2017). The prevalence of cryptococcal antigenemia was higher among inpatients 9.8%, with also region-related variations (95% CI, 4.0–15.5%) (Ford et al., 2018).
Although the prevalence of cryptococcal antigenemia in HIV-infected out-patients has been widely explored and to a lesser extend in hospitalized HIV patients, the underlying clinical cryptococcal diseases contributing to positive serum cryptococcal antigen (CrAg) and the correlation between different cryptococcal diseases and serum CrAg titers have not been thoroughly studied. The common diagnostic procedure following a positive serum CrAg screening test is lumbar puncture to rule out CM (Ganiem et al., 2014; Mfinanga et al., 2015; Pac et al., 2015; Longley et al., 2016; Mamuye et al., 2016; Vidal et al., 2016; Beyene et al., 2017; Nalintya et al., 2018; Chen et al., 2019). Of note, cryptococcal infection generally begins with inhalation of fungal cells (May et al., 2016). However, it is unclear to what extent pulmonary cryptococcosis (PC) contributes to cryptococcal antigenemia. In the present study, we aimed to determine the prevalence of cryptococcal antigenemia, and to analyze the underlying cryptococcal diseases as well as their correlations with serum CrAg titers in Chinese hospitalized HIV-infected patients.
Methods
Patients
Hospitalized HIV-infected patients with CD4 cell count <200 cells/uL, at the department of infectious diseases, second hospital of Nanjing, Nanjing, China, from January 2016 to September 2019, were retrospectively analyzed. As integrated in routine care, all these patients (HIV infected, CD4 <200 cells/uL) were routinely tested for serum CrAg with lateral flow assay (IMMY Diagnostics, Norman, USA) regardless the symptoms and the severity of the of clinical illness. Patients with positive serum CrAg tests received lumbar punctures, blood cultures and chest CT scans to search for possible clinically relevant cryptococcal diseases. Treatments of the cryptococcal diseases were in accordance with clinical practice guidelines (Perfect et al., 2010). The demographic, clinical and laboratory data of the patients were collected from an electronic health record system. The data were analyzed and presented anonymously. Patients with previous treated cryptococcal diseases were excluded from analysis. This study was approved by the ethics committee of the second hospital of Nanjing (reference number 2019-LS-ky013).
CrAg Lateral Flow Assay
CrAg lateral flow assay was performed per manufacturer's instructions. Patients were firstly screened with qualitative procedure in which undiluted serum samples were mixed with same volume of specimen diluent. When qualitative procedure gave positive result, sample was further measured with semi-quantitative titration procedure that started with an initial dilution of 1:5, followed by 1:2 serial dilutions to 1:2560. In the case of positive qualitative test result but negative semi-quantitative test result, the serum CrAg titer was defined as 1:2. If the specimen is positive at 1:2560, the serum CrAg titer was recorded as ≥1:2560 in clinical lab, however, it was defined as 1:2560 during statistical analysis in this study.
Case Definitions
Cryptococcal diseases were defined as clinical conditions in which Cryptococcus spp. related lesions (such as CM, PC, cryptococcal lymphadenopathy or other cryptococcal lesions) or cryptococcemia were identified. Severe cryptococcal diseases were referred to as CM or cryptococcemia. For simplicity, PC without co-existing CM or cryptococcemia was defined as localized PC. Isolated (or asymptomatic) cryptococcal antigenemia was used to describe the condition that cryptococcal diseases were not found in patients with cryptococcal antigenemia.
A “proven” case of CM should meet one or more of the following criteria: positive cerebrospinal fluid (CSF) India ink staining, positive CSF cryptococcal culture, or positive CSF CrAg test. Cases of PC were classified into three categories, namely “proven,” “probable,” and “possible” PC. “Proven” cases of PC were defined as those in which cryptococcal elements were identified by staining or culture of pleural fluid or lung tissue. “Probable” cases of PC were those with characteristic pulmonary radiological features and cryptococcal elements were identified from sputum or broncholavage fluid. PC was also considered “probable” if patients with cryptococcal antigenemia had characteristic pulmonary radiological features which responded to anti-cryptococcal therapy.
If treatment responses were uncertain in patients with characteristic pulmonary radiographic features and cryptococcal antigenemia, PC was considered “possible.” Possible cases of PC were also discussed by expert panel to evaluate whether those lesions could be explained by infection with other pathogens. Here, the characteristic radiographic features were pulmonary nodules/masses with or without cavitation, as we and others found that those lesions were the most common radiological features of PC in immunocompromised HIV-infected patients (Hu et al., 2013, 2017; Xie et al., 2015). If other forms of PC lesions existed, they were generally accompanied by co-existing pulmonary nodules/masses in our practice (Hu et al., 2017). Therefore, calculating those characteristic lesions may underestimate the prevalence of PC, however, in a mild degree.
Statistical Analysis
Variables were compared across groups using the Mann–Whitney U-test for continuous variables and Chi-Square test for categorical variables. For exploring risk factors associated with positive serum CrAg test, variables of interest included age, sex, antiretroviral therapy (ART) status and CD4 cell count. In addition, serum CrAg titer was also included in analyzing risk factors associated with CM. Adjusted analysis was conducted using binary logistic regression. In multivariable analysis, we included variables that had priori P-value < 0.2 in univariable analysis. Statistical analysis was done by SPSS version 22.0 (IBM). A P-value <0.05 is considered statistically significant.
Results
Prevalence and Risk Factors of Cryptococcal Antigenemia
A total of 872 hospitalized HIV-infected patients were screened for serum CrAg, 21% of whom were on ART with median (interquartile range, IQR) ART duration of 6 (Pac et al., 2015; Rajasingham et al., 2017) months. The prevalence of cryptococcal antigenemia in the study population was 10.3% (90/872; 95% CI, 8.3–12.3%). In unadjusted analysis, there was no difference in risk of cryptococcal antigenemia according to age, sex and ART status (Table 1). Risk of cryptococcal antigenemia was significantly increased for patients with CD4 cell counts < 100 cells/uL (OR 2.98; 95% CI, 1.35, 6.57; P = 0.005). The prevalence of cryptococcal antigenemia in patients with CD4 cell counts < 100 cells/ul and between 100 and 199 cell/ul were 11.7% (95% CI, 9.3–14.1%) and 4.3% (95% CI, 1.1–7.4%), respectively. After adjusted for ART status, the CD4 cell counts < 100 cells/uL had 2.84-fold (95% CI, 1.27–6.30; p = 0.011) risk to have positive serum CrAg tests.
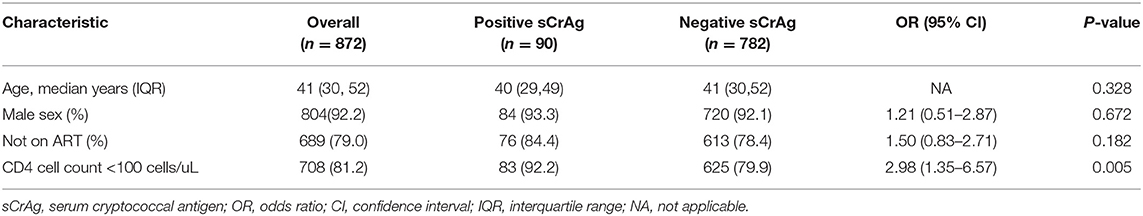
Table 1. Bivariate comparison of risk factors associated with positive serum cryptococcal antigen tests in hospitalized HIV-infected patients.
Cryptococcal Antigenemia Related Clinical Cryptococcal Diseases
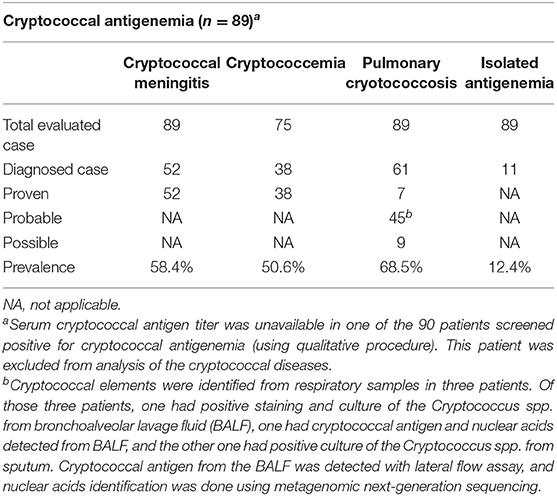
Of the 90 patients with cryptococcal antigenemia (qualitative procedure), 89 patients had serum CrAg titer results and were included for analysis of clinical cryptococcal diseases. Of these 89 patients, 87.6% (95% CI, 80.7–94.6%) had clinical cryptococcal diseases; 12.4% (95% CI, 5.4–19.3%) had isolated cryptococcal antigenemia. The prevalence of CM, cryptococcemia and PC were 58.4% (95% CI, 48.0–68.9%), 50.7% (95% CI, 39.1–62.2%) and 68.5% (95% CI, 58.7–78.4%), respectively (Table 2). One patient (1.1%) had pathologically confirmed cryptococcal lymphadenopathy. Severe cryptococcal diseases (including 3 non-meningeal cryptococcemia with co-existing PC and 52 cases of CM) was identified in 61.8% (95% CI, 51.5–72.1%) of the patients with cryptococcal antigenemia, while localized PC was identified in 25.8% (95% CI, 16.6–35.1%).
The distributions of age, sex, ART status and CD4 cell count were similar between CM and non-meningeal PC (P>0.05), and the proportion of patients with missing data regarding cryptococcemia were comparable between the two groups (5/52 and 5/26, respectively; P = 0.594). When the patients with missing data were excluded from analysis, cryptococcemia occurred in 74.5% (35/47) of the patients with CM and 14.3%(3/21) of the patients with non-meningeal PC, respectively (χ2 = 21.3, p < 0.001).
Distribution of Serum CrAg Titers in Different Cryptococcal Diseases
Of the 89 patients with serum CrAg titer results, the median (range) serum CrAg titer was 1:320 (1:2–1:2560). The median (range) serum CrAg titers in patients with severe cryptococcal diseases (n = 55), localized PC (n = 23) and isolated cryptococcal antigenemia (n = 11) were 1:2560 (1:10–1:2560), 1:20 (1:2–1:320) and 1:5 (1:2–1:320), respectively. Serum CrAg titers in severe cryptococcal diseases were significantly higher than those in localized PC or isolated antigenemia (Mann–Whitney U = 108, n1 =55, n2 = 34, P < 0.001). There were no statistically significant differences in serum CrAg titers between localized PC and isolated antigenemia (Mann–Whitney U = 95, n1 =23, n2 = 11, P =0.240). 75 % (39/52) of the patients with CM and 78.9% (30/38) of the patents with cryptococcemia had serum CrAg titers ≥1:640. 82.6% (19/23) of the patients with localized PC and 90.9% (10/11) of the patients with isolated cryptococcal antigenemia had serum CrAg titers ≤ 1:40.
In unadjusted analysis, there was no difference in risk of CM among patients with cryptococcal antigenemia according to sex and ART status (P = 0.200 and 0.915, respectively, Table 3). The proportions of patients with an age of ≤ 45 years or CD4 cell count of <50 cells/mL were higher in CM patients; however, the differences did not achieve statistical significance (P = 0.087 and P = 0.051, respectively). Serum CrAg titers ≥1:320 were strongly associated with CM (OR 26.88; 95%CI, 8.36–86.42; P < 0.001). Multivariable analysis demonstrated that serum CrAg titer was the only risk factor for CM. After adjusted for age and CD4 cell count, patients with serum CrAg titers ≥1:320 had 25.88-fold (95% CI, 7.65–6.30; p = 0.011) risk to have CM.
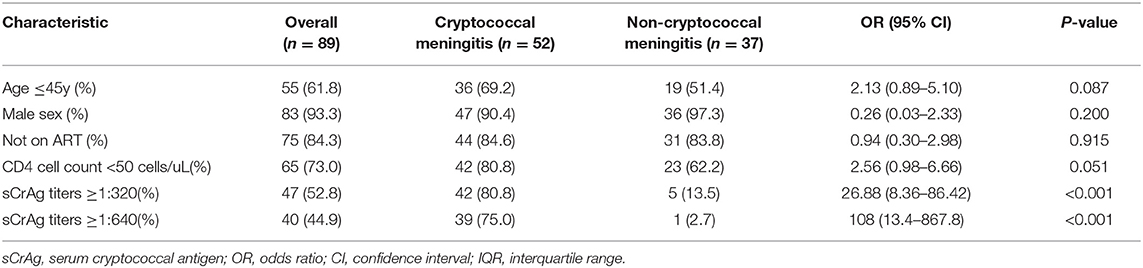
Table 3. Bivariate comparison of risk factors associated with cryptococcal meningitis in hospitalized HIV-infected patients with cryptococcal antigenemia.
When serum CrAg titers were divided into low (≤ 1:20), median (1:40 1:160) and high (≥1:320) titers, the proportion of patients had CM were 12% (3/25), 41.2% (7/17), and 89.4% (42/47), respectively (χ2 =42.784; p < 0.001). All the patients with serum CrAg titers ≥1:640 had severe cryptococcal diseases (39 CM and 1 non-meningeal cryptococcemia). CM occurred in 25.9% (7/27) of the patients with serum CrAg titers between 1:10 and 1:80. None of the patients with serum CrAg titers ≤ 1:5 had CM, although 45.5% (5/11) of whom had PC.
Discussion
A recent meta-analysis showed that the pooled prevalence of cryptococcal antigenemia in hospitalized HIV-infected patients was 9.8% (Ford et al., 2018). Our study in Chinese population had comparable finding that the prevalence was 10.3% (Ford et al., 2018). Of the parameters analyzed, lower CD4 cell count was the only risk factor associated with cryptococcal antigenemia in our study. After adjusted for ART status, CD4 cell counts < 100 cells/uL had 2.84-fold risk to have cryptococcal antigenemia compared with those between 100 and 199 cells/uL. Nevertheless, even in patients with CD4 cell counts between 100 and 199 cells/uL, the prevalence of cryptococcal antigenemia was relatively high (4.3%) exceeding threshold necessitating routine CrAg screening (Rajasingham et al., 2012, 2019). Those results supported routine CrAg screening in hospitalized HIV-infected patients with CD4 cell counts <200 cells/uL.
The vast majority of the patients (87.6%) with cryptococcal antigenemia in our study had clinically relevant cryptococcal diseases. Underlying pulmonary involvement occurred in 68.5% of the patients with cryptococcal antigenemia, which was much higher than previously thought (32% of the inpatients) (Meyohas et al., 1995). An important reason for this discrepancy may be that chest CT scan, which was more sensitive to reveal pulmonary lesions compared with chest radiograph, was routinely performed for patients with cryptococcal antigenemia in our study. As we and others had previously described, the most characteristic radiological features of HIV-associated PC were pulmonary nodules/masses with or without cavitation that may not always be associated with significant respiratory symptoms; clinicians may be unawareness of PC when pulmonary symptoms were not severe (Hu et al., 2013, 2017; Xie et al., 2015). Together, our study suggested that the prevalence of PC in HIV-infected patients may be underestimated.
Previous reported central nervous system (CNS) involvement ranged from 71.3 to 88.9% in hospitalized HIV-infected patients with cryptococcal antigenemia (Wajanga et al., 2011; Mamuye et al., 2016; Chen et al., 2019), and from 19 to 67% in outpatients (Beyene et al., 2017; Wake et al., 2019). In a recent meta-analysis, the pooled prevalence of CM among CrAg-positive outpatients was 33% (95% CI, 21–45%) (Temfack et al., 2019), which was much lower than that in our study. This suggested that inpatients may have more profound cryptococcal diseases compared with outpatients. Nevertheless, in our study, routine cryptococcal antigenemia screening still identified about 40% of the cryptococcal infection before invading the CNS. Of note, PC contributed substantially to non-meningeal cryptococcal antigenemia. For HIV-infected patients with cryptococcal antigenemia, we recommend perform both lumbar puncture and chest CT scan to evaluate possible CNS and respiratory system cryptococcal diseases.
Recent Ethiopian CrAg screening program showed that nearly all HIV-infected patients (97%; 28/29) with plasma CrAg titers ≥1:640 had CM. None of the patients with CrAg titers ≤ 1:80 had CM (Beyene et al., 2017). We found similar result that all the patients with serum CrAg titers ≥1:640 had severe cryptococcal diseases (including 39 CM and 1 non-meningeal cryptococcemia). However, about a quarter of the patients with serum CrAg titers between 1:10 and 1:80 had CM. We recommended that HIV-infected patients with CD4 cell counts <200 cell/ul and serum CrAg titers ≥1:640 should be treated as life-threatening cryptococcal diseases when they are reluctant to lumbar puncture and/or blood culture was unavailable. When the serum CrAg titers ≤ 1:5, lumbar puncture or blood culture may be optional as the risk of CM was very low. However, chest CT scan still needed to be performed as almost half of those patients may have PC.
In our study, 82.6% (19/23) of the patients with localized PC had serum CrAg titers ≤ 1:40. The relatively lower serum CrAg titers in those patients may reflect early pulmonary cryptococcal infection with low fungal burden. Lack of high serum CrAg titers in those immunocompromised patients perhaps implied that cryptococci may early disseminate and invade the CNS system before they substantially multiply and cause extensive damage in the lung. Similar with localized PC, isolated cryptococcal antigenemia was also associated with lower serum CrAg titers. Isolated cryptococcal antigenemia may reflect early or latent cryptococcal infection in which current diagnostic strategies were insensitive to detect any underlying lesions. In this condition, pre-emptive treatment should be administered to prevent life-threatening CM, just as current guidelines recommended (WHO, 2018). However, isolated cryptococcal antigenemia may be also related to a recovery stage of past cryptococcal infection therefore antifungal treatment may not be needed. This hypothesis was supported by the findings in a prospective study on CrAg screening that some of the HIV-infected patients with untreated asymptomatic antigenemia (possibly with low serum CrAg titers) remained alive and none developed CM at 1 year (Longley et al., 2016).
In conclusion, we found a cryptococcal antigenemia prevalence of 10.3% in hospitalized HIV-infected patients. The majority of the hospitalized HIV-infected patients with cryptococcal antigenemia already developed clinical cryptococcal diseases most commonly involving the lung. Routine screening for cryptococcal antigenemia identified about 40% of the cryptococcal infection before progressing to CM. Serum CrAg titers are correlated with the severity of untreated cryptococcal diseases. This may potentially serve as a marker to guide initial anti-fungal treatment.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of the second hospital of Nanjing. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZH designed this study. MX, ZH, and WC collected the data. MX, ZP, CX, ZH, and WC analyzed the data. YChe, YChi, HW, and ZH were involved in the clinical management of the patients and analyzed the clinical data. This manuscript was initially drafted by MX, ZH, and WC and then revised by other authors in this study. All authors approved the final manuscript.
Funding
This study was funded in part by the national natural science foundation of China (NSFC 81701973), Jiangsu natural science foundation (BK20170133), project of Jiangsu province medical youth talent (QNRC2016059), Nanjing medical science and technique development foundation (ZKX17040 and YKK18153).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
HIV, human immunodeficiency virus; CM, cryptococcal meningitis; CrAg, cryptococcal antigen; PC, pulmonary cryptococcosis; CSF, cerebrospinal fluid; ART, antiretroviral therapy; CNS, central nervous system; IQR, interquartile range; CI, confidence interval.
References
Beyene, T., Zewde, A. G., Balcha, A., Hirpo, B., Yitbarik, T., Gebissa, T., et al. (2017). Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)–positive human immunodeficiency virus-infected persons in an ethiopian CrAg screening program. Clin. Infect. Dis. 65, 2126–2129. doi: 10.1093/cid/cix613
Chen, J., Zhang, R., Shen, Y., Liu, L., Qi, T., Wang, Z., et al. (2019). Serum cryptococcal antigen titre as a diagnostic tool and a predictor of mortality in HIV-infected patients with cryptococcal meningitis. HIV Med. 20, 69–73. doi: 10.1111/hiv.12679
Ford, N., Shubber, Z., Jarvis, J. N., Chiller, T., Greene, G., Migone, C., et al. (2018). CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin. Infect. Dis. 66(Suppl_2), S152–S159. doi: 10.1093/cid/cix1143
French, N., Gray, K., Watera, C., Nakiyingi, J., Lugada, E., Moore, M., et al. (2002). Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 16, 1031–1038. doi: 10.1097/00002030–200205030-00009
Ganiem, A. R., Indrati, A. R., Wisaksana, R., Meijerink, H. A, van der Ven Alisjahbana, B., et al. (2014). Asymptomatic cryptococcal antigenemia is associated with mortality among HIV-positive patients in Indonesia. J. Int. AIDS Soc. 17:18821. doi: 10.7448/IAS.17.1.18821
Hu, Z., Chen, J., Wang, J., Xiong, Q., Zhong, Y., Yang, Y., et al. (2017). Radiological characteristics of pulmonary cryptococcosis in HIV-infected patients. PLoS ONE 12:e0173858. doi: 10.1371/journal.pone.0173858
Hu, Z., Xu, C., Wei, H., Zhong, Y., Bo, C., Chi, Y., et al. (2013). Solitary cavitary pulmonary nodule may be a common CT finding in AIDS-associated pulmonary cryptococcosis. Scand. J. Infect. Dis. 45, 378–389. doi: 10.3109/00365548.2012.749422
Kaplan, J. E., Vallabhaneni, S., Smith, R. M., Chideya-Chihota, S., Chehab, J., and Park, B. (2015). Cryptococcal antigen screening and early antifungal treatment to prevent cryptococcal meningitis: a review of the literature. J. Acquir. Immune Defic. Syndr. 68 (Suppl. 3), S331–S339. doi: 10.1097/QAI.0000000000000484
Longley, N., Jarvis, J. N., Meintjes, G., Boulle, A., Cross, A., Kelly, N., et al. (2016). Cryptococcal antigen screening in patients initiating ART in South Africa: A prospective Cohort Study. Clin. Infect. Dis. 62, 581–587. doi: 10.1093/cid/civ936
Mamuye, A. T., Bornstein, E., Temesgen, O., Blumberg, H. M., and Kempker, R. R. (2016). Point-of-care testing for cryptococcal disease among hospitalized human immunodeficiency virus-infected adults in Ethiopia. Am. J. Trop. Med. Hyg. 95, 786–792. doi: 10.4269/ajtmh.15–0857
May, R. C., Stone, N. R., Wiesner, D. L., Bicanic, T., and Nielsen, K. (2016). Cryptococcus: from environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 14, 106–117. doi: 10.1038/nrmicro.2015.6
Meyohas, M. C., Roux, P., Bollens, D., Chouaid, C., Rozenbaum, W., Meynard, J. L., et al. (1995). Pulmonary cryptococcosis: localized and disseminated infections in 27 patients with AIDS. Clin. Infect. Dis. 21, 628–633.
Mfinanga, S., Chanda, D., Kivuyo, S. L., Guinness, L., Bottomley, C., Simms, V., et al. (2015). Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 385, 2173–2182. doi: 10.1016/S0140–6736(15)60164–7
Nalintya, E., Meya, D. B., Lofgren, S., Huppler Hullsiek, K., Boulware, D. R., and Rajasingham, R. (2018). A prospective evaluation of a multisite cryptococcal screening and treatment program in HIV clinics in Uganda. J. Acquir. Immune Defic. Syndr. 78, 231–238. doi: 10.1097/QAI.0000000000001669
Pac, L., Horwitz, M. M., Namutebi, A. M., Auerbach, B. J., Semeere, A., Namulema, T., et al. (2015). Implementation and operational research: integrated pre-antiretroviral therapy screening and treatment for tuberculosis and cryptococcal antigenemia. J. Acquir. Immune Defic. Syndr. 68, e69–e76. doi: 10.1097/qai.0000000000000527
Perfect, J. R., Dismukes, W. E., Dromer, F., Goldman, D. L., Graybill, J. R., Hamill, R. J., et al. (2010). Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 50, 291–322. doi: 10.1086/649858
Rajasingham, R., Meya, D. B., and Boulware, D. R. (2012). Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J. Acquir. Immune Defic. Syndr. 59, e85–e91. doi: 10.1097/QAI.0b013e31824c837e
Rajasingham, R., Meya, D. B., Greene, G. S., Jordan, A., Nakawuka, M., Chiller, T. M., et al. (2019). Evaluation of a national cryptococcal antigen screening program for HIV-infected patients in Uganda: a cost-effectiveness modeling analysis. PLoS ONE 14:e0210105. doi: 10.1371/journal.pone.0210105
Rajasingham, R., Smith, R. M., Park, B. J., Jarvis, J. N., Govender, N. P., Chiller, T. M., et al. (2017). Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17, 873–881. doi: 10.1016/S1473–3099(17)30243–8
Temfack, E., Bigna, J. J., Luma, H. N., Spijker, R., Meintjes, G., Jarvis, J. N., et al. (2019). Impact of routine cryptococcal antigen screening and targeted preemptive fluconazole therapy in antiretroviral-naive human immunodeficiency virus-infected adults with CD4 cell counts <100/muL: a systematic review and meta-analysis. Clin. Infect. Dis 68, 688–698. doi: 10.1093/cid/ciy567
Vidal, J. E., Toniolo, C., Paulino, A., Colombo, A., M. dos Anjos Martins, C., da Silva Meira, V. L., et al. (2016). Asymptomatic cryptococcal antigen prevalence detected by lateral flow assay in hospitalised HIV-infected patients in São Paulo, Brazil. Trop. Med. Int. Health 21, 1539–1544. doi: 10.1111/tmi.12790
Wajanga, B. M., Kalluvya, S., Downs, J. A., Johnson, W. D., Fitzgerald, D. W., and Peck, R. N. (2011). Universal screening of Tanzanian HIV-infected adult inpatients with the serum cryptococcal antigen to improve diagnosis and reduce mortality: an operational study. J. Int. AIDS Soc. 14:48. doi: 10.1186/1758–2652-14–48
Wake, R. M., Govender, N. P., Omar, T., Nel, C., Mazanderani, A. H., Karat, A. S., et al. (2019). Cryptococcal-related mortality despite fluconazole pre-emptive treatment in a cryptococcal antigen (CrAg) screen-and-treat programme. Clin. Infect. Dis. 70, 1683–1690. doi: 10.1093/cid/ciz485
WHO (2018). Guidelines on the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva: World Health Organization (2018).
Keywords: cryptococcal antigenemia, HIV, cryptococcal meningitis, pulmonary cryptococcosis, lateral flow assay
Citation: Xu M, Peng Z, Xu C, Chen Y, Cheng J, Chi Y, Wei H, Chen W and Hu Z (2020) Underlying Cryptococcal Diseases and the Correlation With Serum Cryptococcal Antigen Titers in Hospitalized HIV-Infected Patients Screened Positive for Cryptococcal Antigenemia. Front. Cell. Infect. Microbiol. 10:170. doi: 10.3389/fcimb.2020.00170
Received: 03 December 2019; Accepted: 31 March 2020;
Published: 24 April 2020.
Edited by:
Mari Shinohara, Duke University, United StatesReviewed by:
Andrew Alspaugh, Duke University, United StatesAlexandre Alanio, Université Paris Diderot, France
Copyright © 2020 Xu, Peng, Xu, Chen, Cheng, Chi, Wei, Chen and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiliang Hu, huzhiliangseu@163.com; Wei Chen, weichennannan2017@163.com
 Miaomiao Xu1
Miaomiao Xu1 Wei Chen
Wei Chen Zhiliang Hu
Zhiliang Hu