
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 24 March 2020
Sec. Microbiome in Health and Disease
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.00098
This article is part of the Research Topic The Microbiota and Interventions View all 6 articles
 Karuna E. W. Vendrik1,2,3
Karuna E. W. Vendrik1,2,3 Rogier E. Ooijevaar2,4
Rogier E. Ooijevaar2,4 Pieter R. C. de Jong5
Pieter R. C. de Jong5 Jon D. Laman6
Jon D. Laman6 Bob W. van Oosten7
Bob W. van Oosten7 Jacobus J. van Hilten5
Jacobus J. van Hilten5 Quinten R. Ducarmon1,8
Quinten R. Ducarmon1,8 Josbert J. Keller2,9,10
Josbert J. Keller2,9,10 Eduard J. Kuijper1,2,3,8*
Eduard J. Kuijper1,2,3,8* Maria Fiorella Contarino5,11
Maria Fiorella Contarino5,11Background: Several studies suggested an important role of the gut microbiota in the pathophysiology of neurological disorders, implying that alteration of the gut microbiota might serve as a treatment strategy. Fecal microbiota transplantation (FMT) is currently the most effective gut microbiota intervention and an accepted treatment for recurrent Clostridioides difficile infections. To evaluate indications of FMT for patients with neurological disorders, we summarized the available literature on FMT. In addition, we provide suggestions for future directions.
Methods: In July 2019, five main databases were searched for studies and case descriptions on FMT in neurological disorders in humans or animal models. In addition, the ClinicalTrials.gov website was consulted for registered planned and ongoing trials.
Results: Of 541 identified studies, 34 were included in the analysis. Clinical trials with FMT have been performed in patients with autism spectrum disorder and showed beneficial effects on neurological symptoms. For multiple sclerosis and Parkinson's disease, several animal studies suggested a positive effect of FMT, supported by some human case reports. For epilepsy, Tourette syndrome, and diabetic neuropathy some studies suggested a beneficial effect of FMT, but evidence was restricted to case reports and limited numbers of animal studies. For stroke, Alzheimer's disease and Guillain-Barré syndrome only studies with animal models were identified. These studies suggested a potential beneficial effect of healthy donor FMT. In contrast, one study with an animal model for stroke showed increased mortality after FMT. For Guillain-Barré only one study was identified. Whether positive findings from animal studies can be confirmed in the treatment of human diseases awaits to be seen. Several trials with FMT as treatment for the above mentioned neurological disorders are planned or ongoing, as well as for amyotrophic lateral sclerosis.
Conclusions: Preliminary literature suggests that FMT may be a promising treatment option for several neurological disorders. However, available evidence is still scanty and some contrasting results were observed. A limited number of studies in humans have been performed or are ongoing, while for some disorders only animal experiments have been conducted. Large double-blinded randomized controlled trials are needed to further elucidate the effect of FMT in neurological disorders.
The bidirectional communication between the gut and the central nervous system, often referred to as the gut-brain axis, has been a topic of great interest in the past decade. Several studies suggest an important role of the gut microbiota in the pathophysiology of neurological disorders. A different human gut microbiota composition compared to healthy controls has been reported for several neurological disorders, such as Parkinson's disease (Hasegawa et al., 2015; Keshavarzian et al., 2015; Scheperjans et al., 2015; Unger et al., 2016), multiple sclerosis (Miyake et al., 2015; Chen et al., 2016; Jangi et al., 2016; Cosorich et al., 2017), autism spectrum disorder (Finegold et al., 2002, 2010; De Angelis et al., 2013, 2015; Kang et al., 2013; Ma et al., 2019), Alzheimer's disease (Vogt et al., 2017; Zhuang et al., 2018; Haran et al., 2019; Li B. et al., 2019; Liu et al., 2019), neuromyelitis optica (Cree et al., 2016), Rett syndrome (Strati et al., 2016), epilepsy (Xie et al., 2017; Peng et al., 2018; Lindefeldt et al., 2019), amyotrophic lateral sclerosis (Fang et al., 2016; Rowin et al., 2017; Mazzini et al., 2018), cerebral infarction (Karlsson et al., 2012; Yin et al., 2015), spinal cord injury (Gungor et al., 2016), and multiple system atrophy (Tan et al., 2018). However, data on microbiota composition are frequently inconsistent and numerous potential confounders are involved. Interestingly, patients with these neurological disorders often experience gastrointestinal symptoms (Poewe, 2008; Adams et al., 2011; Postuma et al., 2012; McElhanon et al., 2014; Willison et al., 2016), which could imply that the intestinal tract is involved in disease pathophysiology. Onset, clinical characteristics and progression of these neurological disorders may potentially be modulated by gut microbiota interventions. Gut microbiota interventions could also affect availability and pharmacokinetics of medication for neurological disorders, which may lead to an increased efficacy and a different side effect profile. There are multiple gut microbiota interventions, e.g., the administration of antibiotics, probiotics, prebiotics, synbiotics, or fecal microbiota transplantation (FMT). Antibiotic treatment has been reported to change disease course in a few neurological disorders (Sandler et al., 2000; Laake and Oeksengaard, 2002; Fasano et al., 2013; Ghanizadeh and Berk, 2015; Angelucci et al., 2019; Lum et al., 2019). Probiotics may improve disease symptoms, but results are inconsistent (Parracho et al., 2010; Kaluzna-Czaplinska and Blaszczyk, 2012; West et al., 2013; Partty et al., 2015; Jiang et al., 2017; Shaaban et al., 2018; Gazerani, 2019; Kobayashi et al., 2019a,b; Tamtaji et al., 2019). The most effective option in modulation of the gut microbiota is FMT, which includes administration of a solution of fecal matter from a donor into the intestinal tract of a recipient. FMT is an efficacious treatment for recurrent Clostridioides difficile infections (van Nood et al., 2013; Kelly et al., 2016). It is currently under investigation for several neurological disorders. Publications on FMT in humans with and animal models of neurological disorders are discussed in this narrative review.
In July 2019, a literature search on FMT in neurological disorders was performed in five main databases, including Pubmed, Embase, Web of Science, COCHRANE library and Academic Search Premier database, using appropriate keywords (Appendix 1). Meeting and congress abstracts were also included. Furthermore, the reference lists of some recent reviews were consulted to detect relevant additional publications. In addition, the website ClinicalTrials.gov was searched (June 27th 2019) for ongoing or planned clinical trials with FMT in neurological disorders. Further details of the search strategy are provided in Appendix 1.
Eligibility was assessed by screening titles and abstracts. The following inclusion criteria were applied: (1) in vivo studies or case descriptions with FMT in humans or animal models; (2) FMT with feces from healthy humans or animals transferred to humans with, or animal models of, individual neurological disorders; or FMT with feces from humans with, or animal models of, individual neurological disorders transferred to healthy humans or animals; (3) original research; (4) articles in English.
Two exclusion criteria were applied: (1) Use of individual bacteria, bacterial groups or bacterial metabolites instead of feces; (2) the effect of FMT on neurological symptoms/features was not described.
The initial search yielded 541 articles and abstracts. After exclusion of articles or abstracts not meeting the abovementioned criteria, 34 articles and abstracts remained. All included FMT studies are reported in Tables 1–9. Figure 1 shows the most important effects of FMT in humans and animals for neurological disorders. An overview of planned and ongoing studies, found on the website ClinicalTrials.gov, is provided in Appendix 2. Abbreviations and terms are explained in Appendix 3.
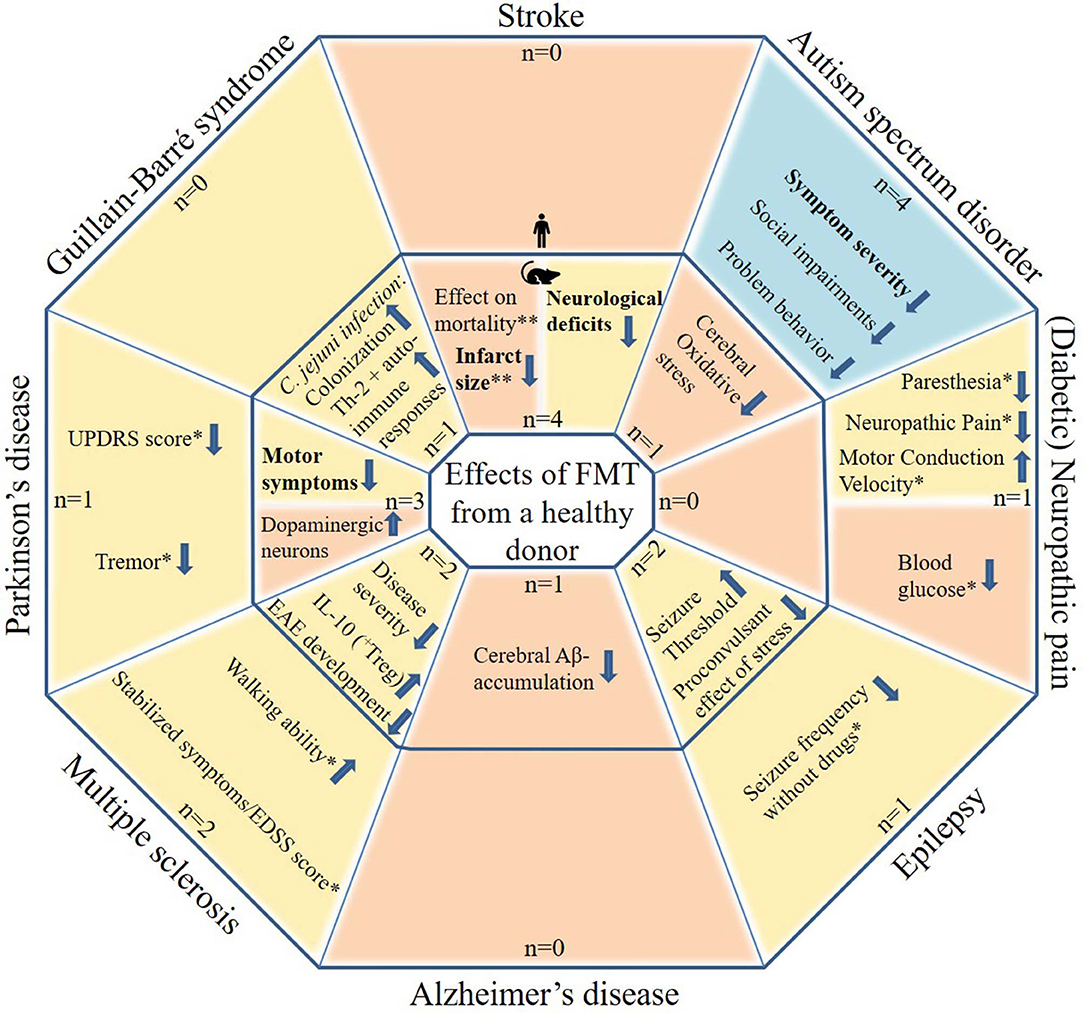
Figure 1. Potential effects of FMT in patients with neurological disorders and in animal models for neurological disorders. The figure includes studies in which patients with a neurological disorder or animal models for a neurological disorder received FMT with feces from a healthy donor. Tourette syndrome was not included as this contains only one case report. Blue areas include cognitive symptoms, yellow areas include motor and sensory symptoms or effects and orange areas include other effects. The outer parts contain results from human studies and the inner parts from animal studies. Statements in bold are found by more than one study, excluding case descriptions. N: the number of studies identified per neurological disorder, subdivided in human and animal studies. *based on case reports/series only (very limited evidence). **inconsistent results.
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders, characterized by altered social communication and interaction as well as repetitive, stereotyped behavior.
The exact etiology is unknown: a combination of genetic and environmental risk factors, dysregulation of the immune system, inflammation and also maternal factors is proposed (Fattorusso et al., 2019). Increased systemic (Ashwood et al., 2011) and neuroinflammation (Vargas et al., 2005; Li et al., 2009) and even brain-specific autoantibodies (Vojdani et al., 2002; Silva et al., 2004; Connolly et al., 2006; Cabanlit et al., 2007; Wills et al., 2009), though not confirmed in another study (Todd et al., 1988), have been observed in ASD patients. Hyperserotoninemia in ASD patients may also contribute to the etiology (Fattorusso et al., 2019).
ASD patients have a different gut microbiota composition and different gut metabolomes (including neurotransmitters) compared to healthy controls (Finegold et al., 2002, 2010; De Angelis et al., 2013, 2015; Kang et al., 2013; Ma et al., 2019). Relatively higher levels of the phylum Bacteroidetes, which produces short-chain fatty acids (SCFA), are observed in ASD subjects. Furthermore, decreased levels of the anti-inflammatory genus Bifidobacterium or increased levels of the genus Clostridium, which is known to produce potentially toxic metabolites such as phenols and p-cresols, may play a role in pathogenesis (Fattorusso et al., 2019). Altered production of gut-microbial metabolites, such as p-cresol and SCFA, are associated with ASD symptoms (MacFabe et al., 2007; Fattorusso et al., 2019). An increased intestinal production of serotonin and decreased cerebral serotonin synthesis in ASD may also be caused by alterations in the gut microbiota, but evidence is inconsistent (Fattorusso et al., 2019). Data on α-diversity in gut microbiota of ASD patients are also contrasting (Finegold et al., 2010; Williams et al., 2011; De Angelis et al., 2013; Kang et al., 2013; Ma et al., 2019). An altered gut microbiota composition may influence the immune system, inflammation and metabolism and thereby increase the risk for ASD (Park, 2003; Fattorusso et al., 2019). Diet, which is known to shape the gut microbiota (David et al., 2014), is also thought to modulate ASD behavior (Knivsberg et al., 2002; Cermak et al., 2010; Whiteley et al., 2010; de Theije et al., 2014; Fattorusso et al., 2019).
Gastrointestinal symptoms, such as abdominal pain, constipation, diarrhea, and bloating, are more frequently described in ASD patients than controls, with a corresponding odds ratio of 4.42 (McElhanon et al., 2014). Some studies found an association between gastrointestinal symptoms and severity of ASD symptoms (Adams et al., 2011; Wang L. W. et al., 2011; Mazurek et al., 2013), but others could not reproduce these findings (Kang et al., 2013; Son et al., 2015). ASD patients appear to have a higher prevalence of IBD (Doshi-Velez et al., 2015) and a higher number of pro-inflammatory immune cells in the intestinal wall (Navarro et al., 2016), although intestinal inflammatory markers, such as fecal calprotectin, appear normal (Navarro et al., 2016). The gastrointestinal symptoms may be caused by the presence of more pro-inflammatory gut bacteria (Fattorusso et al., 2019), but other factors may also be involved (Mayer et al., 2014). Studies have also reported altered gastrointestinal motility and increased intestinal permeability in ASD (D'Eufemia et al., 1996; Boukthir et al., 2010; de Magistris et al., 2010), which may increase the risk of translocation of bacteria or neurotoxic peptides, such as lipopolysaccharide (LPS). However, inconsistency is again observed (Navarro et al., 2016).
Another important finding that supports a role for the gut microbiota is a temporary improvement of ASD and gastrointestinal symptoms after 8 weeks of oral vancomycin treatment (Sandler et al., 2000). Furthermore, several studies on the effect of probiotics in ASD patients and ASD animal models showed a positive effect on ASD symptoms (Parracho et al., 2010; Kaluzna-Czaplinska and Blaszczyk, 2012; Hsiao et al., 2013; West et al., 2013; Partty et al., 2015; Shaaban et al., 2018). This included improvement of neurobehavioral symptoms, such as anxiety or problems with concentration, and/or gastrointestinal symptoms (Parracho et al., 2010; Kaluzna-Czaplinska and Blaszczyk, 2012; Hsiao et al., 2013; West et al., 2013; Partty et al., 2015; Shaaban et al., 2018). One human study reported the absence of onset of Asperger syndrome in a group of 40 children of which the mothers during pregnancy or post-partum and, in case there was no breastfeeding, the children themselves had received probiotics (Lactobacillus rhamnosus GG) for 6 months as opposed to 3 out of 35 children who developed Asperger syndrome in the placebo group (Partty et al., 2015).
In animal studies, germ-free male mice showed increased social impairments compared to conventionally colonized mice, which suggests an important role for gut microbiota in this behavior (Desbonnet et al., 2014).
Sharon et al. (2019) performed FMT in germ-free wild-type mice with feces from children with ASD or normally developing children. The ASD group and their offspring had ASD-like symptoms. Furthermore, brains of offspring displayed alternative splicing of ASD-relevant genes. When gamma-aminobutyric acid (GABA)A receptor agonists, reduced in the ASD-group colon, were administered to an ASD mouse model, ASD symptoms decreased. Another study (Aabed et al., 2019) observed decreased cerebral oxidative stress after FMT with feces from a normal hamster in an ASD hamster model. This effect was stronger after administration of Lactobacillus paracaseii.
In an open-label clinical trial (Kang et al., 2017, 2019), 18 children with ASD and gastrointestinal symptoms received daily FMT for 7–8 weeks by mixing standardized human gut microbiota with a drink or via enema. Gastrointestinal and behavioral ASD symptoms improved, which persisted until 2 years after treatment. FMT appeared safe, since most adverse events were temporary and observed at start of vancomycin pre-treatment (e.g., mild to moderate tantrums/aggression and hyperactivity) and 5% suffered from nausea/vomiting. Furthermore, there was a correlation between ASD symptoms and gastrointestinal symptoms. However, this was an open-label study without a placebo group in a heterogenous group of 18 participants, in which 12 changed their medication, diet, or nutritional supplements during the study. Furthermore, there was no vancomycin-only group and no information on adverse events in the long-term follow-up was provided (Kang et al., 2017, 2019).
An abstract (Zhao et al., 2019) reporting an open-label, randomized waitlist-controlled trial showed improvements of ASD symptoms and changes in gastrointestinal symptoms 2 months after two FMTs in 24 ASD-children compared to 24 control ASD-children. However, improvement of ASD symptoms was temporary. Seven FMT-patients reported adverse events, such as nausea, fever and allergy, but these were all mild and transient. There was no placebo-group and there was lack of information on α- and β-diversity of the gut microbiota, pre-treatment and amount of donor feces (Zhao et al., 2019).
In a case series described in an abstract (Ward et al., 2016), ASD symptoms did not change in a 21-year-old man, but were improved in eight younger subjects. Regression of symptoms often occurred, mostly after antibiotics post-FMT, but often improved again after re-FMT. In another case series (Urbonas and Cervinskiene, 2018) (abstract only), the authors described that the parent global impression score (PGI-R) and gastrointestinal symptoms improved after three FMTs in five boys with ASD and mild gastrointestinal symptoms. However, PGI-R pre-FMT scores were not shown and scores did not appear to improve over time.
One placebo-controlled randomized clinical trial (RCT) with CP101, a drug that contains a full community of gut bacteria, is planned and two RCT, of which one is placebo-controlled, with FMT in human ASD subjects are ongoing (Appendix 2).
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system (CNS). The pathophysiology of MS is complex and has not been fully elucidated. Genetic, infectious and environmental factors (e.g., Epstein-Barr virus infection, smoking, and sunlight/vitamin D) play pivotal roles (Olsson et al., 2017). The interplay between these factors appears to lead to immune dysregulation, but a true autoimmune origin of the disease remains elusive. Nevertheless, the relative efficacy of therapies targeting inflammation supports a critical role of the immune system, in particular T-cell mediated mechanisms. This includes limiting leukocyte egress from secondary lymphoid organs and their entry into the CNS (Dendrou et al., 2015; Thompson et al., 2018). Peripheral activation of CD8+ T-cells and CD4+ T-helper cells (Th) type 1 and 17 allows for these cells to infiltrate the CNS and cause inflammation. This peripheral activation is thought to be caused by a reduction of regulatory T cells (Treg) and antigen presentation of brain antigens in secondary lymphoid organs (Dendrou et al., 2015). Infiltrating macrophages are also critical to CNS inflammation.
The gut microbiota influences and modulates the equilibrium between pro- and anti-inflammatory T-cells in the gut-associated lymphoid tissue (Rooks and Garrett, 2016; Yissachar et al., 2017). Several studies have been performed which characterized the gut microbiota profile of patients suffering from different forms of MS. Similar subtle alterations in gut microbiota composition were found throughout these studies via analyses of fecal samples (Miyake et al., 2015; Chen et al., 2016; Jangi et al., 2016). One study found a similar perturbed microbiota composition within duodenal mucosal biopsies (Cosorich et al., 2017). Based on these studies, it could be hypothesized that MS patients' gut microbiota harbors less bacterial species that can induce Treg cells, which may contribute to elevated peripheral levels of Th1 and 17 (Jangi et al., 2016; Cekanaviciute et al., 2017). It is hypothesized that subsequently elevated Th1 and 17 cause inflammation in the CNS and increased blood-brain barrier permeability, leading in turn to exacerbation of the inflammation of the CNS (Dendrou et al., 2015). Modulation of the gut microbiota to induce more Treg cells could lead to less activation of pathogenic T-cells (Berer et al., 2014). Interestingly, gavage with a human gut-derived commensal strain Prevotella histicola resulted in a decreased incidence of disease in a mouse model of MS (Mangalam et al., 2017). Moreover, a decrease in Th1 and 17 cell numbers and an increase in Treg cells were found.
Experimental autoimmune encephalomyelitis (EAE) is an animal model that mimics aspects of pathophysiology and symptoms of MS (Goverman et al., 1993). The gut microbiota is required to induce EAE, as germ-free mice did not develop spontaneous EAE in a transgenic model (Berer et al., 2011). Two mouse EAE studies used gavage to transplant MS patients' or healthy human controls' microbiota. The transplanted MS microbiota resulted in an increased EAE incidence, a more severe disease course and a decrease in expression of anti-inflammatory cytokine IL-10 (Berer et al., 2017; Cekanaviciute et al., 2017). In general, these findings seem to correlate with current interpretations of the distinct immunological findings in MS-patients (Dendrou et al., 2015).
Currently, there are only two case reports/series on effects of FMT on MS symptoms and disease progression (Borody et al., 2011; Makkawi et al., 2018). Both claim sustained beneficial effects following FMT. One secondary progressive MS patient was treated with a single FMT for concomitant recurrent Clostridioides difficile infections. The FMT resolved the recurrent C. difficile infections and was suggested to prevent MS disease progression for over 10 years (Makkawi et al., 2018). In the case-series amelioration of MS symptoms following repeated FMTs was observed in three patients (Borody et al., 2011).
ClinicalTrials.gov lists one ongoing RCT, one ongoing non-randomized trial, and one planned prospective case-only observational study in which MS patients receive FMT as an experimental treatment (Appendix 2).
Parkinson's disease (PD) is a progressive neurodegenerative disorder, characterized by neuron degeneration in the CNS, enteric nervous system and peripheral autonomic nervous system, and by the presence of Lewy bodies and Lewy neurites in affected neurons (Pakkenberg et al., 1991). The etiology and pathogenesis of PD is still largely unknown and possibly heterogeneous. Both genetic and environmental factors might play a role, at least in some forms of the disease. The gut-brain axis in PD has been intensively studied. Gastrointestinal symptoms (including obstipation and delayed transit) are frequently observed in patients with PD. In some cases they precede the onset of motor symptoms and represent the first clinical manifestation of PD (Poewe, 2008; Postuma et al., 2012).
An important factor in the etiology of PD is the aggregation of the protein alpha-synuclein (αSyn), a major component of Lewy bodies (Spillantini et al., 1997). Several studies demonstrated that the enteric nervous system and vagus nerve are affected in an early, and even in the prodromal, phase of disease (Braak et al., 2003; Kuo et al., 2010; Hallett et al., 2012; Shannon et al., 2012; Stokholm et al., 2016). It has been suggested that the disease may start in the gut, with a neurotropic substance with prion-like properties, possibly misfolded αSyn, that is transported from the gastrointestinal tract to the CNS (Liautard, 1991; Braak et al., 2003). Mice studies indeed confirmed that αSyn forms could be transported to the brain and pass the blood-brain barrier (Pan-Montojo et al., 2010; Ulusoy et al., 2013; Holmqvist et al., 2014; Kim et al., 2019). Moreover, aggregation of αSyn in the brain, and possibly the gut, of PD patients could be a consequence of inflammation-induced oxidative stress (Shults, 2006; Keshavarzian et al., 2015). It is indeed observed that PD patients have increased expression of pro-inflammatory cytokines and glial markers in the colonic biopsies compared to healthy controls (Devos et al., 2013). PD patients also appear to have increased intestinal permeability (Forsyth et al., 2011) and small intestine bacterial overgrowth (Gabrielli et al., 2011; Fasano et al., 2013; Tan et al., 2014). The latter is associated with more motor fluctuations when using levodopa, which improves after antibiotics (Fasano et al., 2013). Furthermore, several studies indicate that the gut microbiota composition and the metabolome in PD patients are different from healthy individuals (Hasegawa et al., 2015; Keshavarzian et al., 2015; Scheperjans et al., 2015; Unger et al., 2016). Overall, more pro-inflammatory gut bacteria, such as LPS-producing Proteobacteria, and less anti-inflammatory butyrate-producing gut bacteria are found in PD patients (Keshavarzian et al., 2015). Scheperjans et al. (2015) found that the relative abundance of the family Enterobacteriaceae in PD patients is positively associated with postural instability and gait difficulty. Two other important studies suggested that gut bacterial tyrosine decarboxylases can metabolize levodopa to dopamine without being susceptible for aromatic amino acid decarboxylase inhibitors, such as carbidopa. Increased presence of gut bacterial tyrosine decarboxylases may thereby cause response fluctuations in levodopa/carbidopa-treated PD patients, as dopamine cannot cross the blood-brain barrier (Maini Rekdal et al., 2019; van Kessel et al., 2019). Furthermore, probiotics may improve PD symptoms, but this includes mainly improvement of constipation (Gazerani, 2019).
A recent study (Sampson et al., 2016) demonstrated that the presence of gut microbiota is necessary for the development of PD characteristics in alpha-synuclein-overexpressing (ASO) mice. Germ-free ASO mice showed less motor symptoms, constipation, alpha-synucleinopathy, and microglia activation compared to specific-pathogen-free ASO mice, while colonization with specific-pathogen-free microbiota led to an increase of symptoms. When ASO mice received feces from PD patients, motor symptoms increased compared to mice that received healthy human feces (Sampson et al., 2016). Another study (Sun et al., 2018) showed that a PD mouse model had improved motor function, increased striatal neurotransmitters, and decreased neuroinflammation after receiving feces from healthy mice. Healthy mice that received feces from PD mice had deteriorated motor function and decreased striatal neurotransmitters compared to controls. Zhou et al. (2019) observed less motor function decline and loss of dopaminergic neurons in the substantia nigra in PD mice that received a fasting mimicking diet (FMD) compared to ad-libitum-fed PD mice. Furthermore, they observed a higher striatal dopamine and serotonin concentration in PD mice that had received feces from FMD-fed control mice compared to phosphate-buffered solution (PBS)-gavaged or ad-libitum microbiota-gavaged PD mice.
There is only one case report (Huang H. et al., 2019) describing a PD patient that received FMT in whom temporary improvement of leg tremors and other PD symptoms was observed 1 week after three FMTs. Unfortunately, leg tremors recurred 2 months post-FMT and other PD symptoms had also returned to baseline levels 1 month later. On the other hand, constipation had also improved and this improvement lasted until the end of follow-up 3 months post-FMT. No further studies on FMT in PD were identified, except for one communication in a divulgative magazine in which improvement of PD symptoms after FMT was mentioned without further details (Ananthaswamy, 2011).
On ClinicalTrials.gov, one RCT and one non-randomized trial with FMT in PD patients are registered as ongoing trials, and one placebo-controlled RCT with PRIM-DJ2727, an orally administered lyophilized fecal microbiota product, is planned (Appendix 2).
In epilepsy, both genetic and environmental factors are thought to be involved in individual predisposition, but exact etiology of most cases remains unknown. A link between the gut microbiota and the pathophysiology of epilepsy has been proposed by some studies.
A difference in gut microbiota profiles between patients with different types of therapy refractory epilepsy and healthy controls was found in several studies. All these studies reported increased abundance of the phyla Firmicutes relative to Bacteroidetes in subjects with refractory epilepsy (Xie et al., 2017; Peng et al., 2018; Lindefeldt et al., 2019). Some bacteria of the phylum Firmicutes may alter neurotransmitter levels (Peng et al., 2018). Further microbiota analysis outcomes differed considerably between these studies, including α-diversity measures (Xie et al., 2017; Peng et al., 2018; Lindefeldt et al., 2019). One study (Peng et al., 2018) found an increased Firmicutes/Bacteroidetes ratio and α-diversity in drug-resistant patients compared to drug-sensitive patients, with the latter being similar to healthy controls. Importantly, α-diversity was probably increased due to the abnormal increased abundance of rare bacteria. On genus level, several differences were also found. Based on these results, one could hypothesize a role for bacteria in the effectivity of medication for epilepsy, but no causal statements can be made (Peng et al., 2018). Interestingly, zonisamide, an anticonvulsant drug, is metabolized by gut bacteria (Kitamura et al., 1997). Furthermore, an increase in Bifidobacteria and Lactobacillus was correlated with four or less seizures per year (Peng et al., 2018). Another important finding in patients with epilepsy is that a ketogenic diet reduces the number of seizures and that a ketogenic diet is associated with an altered gut microbiota composition and function (Dahlin and Prast-Nielsen, 2019).
Sewal et al. (2017) found increased seizure susceptibility after intraperitoneal administration of LPS in rats, which was accompanied by increased blood-brain barrier permeability and increased levels of pro-inflammatory cytokines in the brain. Furthermore, contrasting results on whether antibiotic treatment provides protective or inducing effects on seizures are observed in animal and human studies (Lum et al., 2019). Importantly, potential direct neurotoxic effects of the antibiotics themselves or pro-epileptogenic effects of the underlying disease (e.g., infection) that is treated might rather be involved. Furthermore, some studies found a positive effect of probiotics in epilepsy (Gomez-Eguilaz et al., 2018; Yeom et al., 2019).
Medel-Matus et al. (2018) found that transfer of feces from a stressed rat donor increased progression and duration of kindled seizures (i.e., rearing, with or without falling) in sham-stressed rats. The pro-epileptic effects were counteracted in stressed recipients of donor feces from sham-stressed rats. Another study (Olson et al., 2018) observed that a germ-free temporal lobe epilepsy mouse model did not show ketogenic diet-mediated seizure protection. They also observed that the seizure threshold in specific-pathogen-free mice increased after transplantation with ketogenic diet microbiota or long-term administration of species Akkermansia muciniphila, Parabacteroides merdae, and P. distasonis (associated with a ketogenic diet).
There is one case report (He et al., 2017) of a patient with generalized epilepsy and Crohn's disease that received three FMTs. Before FMTs, she experienced frequent seizures when not using sodium valproate treatment, and after FMTs, the patient was seizure-free without antiepileptic drugs for 20 months. Furthermore, the Crohn's disease activity index improved (He et al., 2017).
One registered interventional study with a single group assignment is ongoing with FMT in patients with epilepsy (Appendix 2).
Neuropathic pain is pain that is caused by damage (e.g., nerve trauma or chemotherapeutic damage) or diseases (e.g., diabetes mellitus) of the peripheral or central somatosensory nervous system. It is characterized by abnormal sensations or pain following normally non-painful stimulation (Guo et al., 2019). A potential complication of diabetes mellitus is peripheral neuropathy with accompanying neuropathic pain and peripheral neuropathy is positively associated with insulin resistance (Han et al., 2015). Interestingly, patients with diabetes mellitus have a different gut microbiota composition and function compared to controls (Qin et al., 2012; Karlsson et al., 2013; Jamshidi et al., 2019). FMT may alter insulin resistance and thereby neuropathic pain. An increase in insulin resistance was observed in germ-free wild-type mice after FMT with feces from conventionally raised mice (Backhed et al., 2004). In humans, FMT with feces from lean donors in subjects with metabolic syndrome led to increased insulin sensitivity (Vrieze et al., 2012).
The gut microbiota may also regulate pain by directly modulating neuronal excitability of dorsal root ganglia or indirectly by regulating neuroinflammation in the peripheral and central nervous system (Guo et al., 2019). Microbiota depletion by antibiotics or the complete absence of gut microbiota in mice had a protective effect on pain in oxaliplatin-induced peripheral neuropathy, accompanied by decreased infiltration of macrophages and cytokines in the dorsal root ganglia. The effect could be reversed by gut microbiota restoration and this suggests an influence of the gut microbiota on neuropathic pain (Shen et al., 2017). Another study demonstrated a positive effect of probiotics in vitro on paclitaxel-induced neuropathic pain features (Castelli et al., 2018). However, when probiotics L. reuteri LR06 or Bifidobacterium BL5b were administered to rats with chronic constriction injury-induced neuropathic pain, no effect on pain sensation was observed (Huang J. et al., 2019).
One study (Yang C. et al., 2019) observed an increased pain-like phenotype in antibiotic-treated mice. Furthermore, mice that received antibiotics and FMT with feces from a neuropathic pain rat model with anhedonia-like phenotype developed more pain-symptoms compared to antibiotic- and PBS-treated mice. Mice that received antibiotics and non-anhedonia microbiota showed less pain-symptoms compared to PBS-treated mice, comparable to mice receiving feces from sham-operated rats.
In a case report, a woman with poorly regulated type 2 diabetes mellitus and diabetic neuropathy experienced improvement of limb pain and paresthesia after two FMTs. Visual analog scale (VAS) pain score decreased. There was improvement of motor conduction velocity in the tibial nerve without improvement of sensory dysfunction on electromyogram. Furthermore, fasting blood glucose levels decreased and stabilized, and HbA1c decreased from 7.5 to 6.3. However, length of follow-up was unclear and therefore it is unknown how long the improvements lasted (Cai et al., 2018).
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by the presence of motor and phonic tics with onset during childhood. It is considered to be caused by an interplay between genetic, environmental and social factors. Reports on an association of the gut microbiota with TS or tic disorders in general are scarce. Liao et al. (2019) observed a decrease of tic-like behaviors in a rat model after administration of Lactobacillus plantarum PS128 which coincided with improved dopamine metabolism and norepinephrine levels in the striatum and prefrontal cortex. A study of 30 patients with pediatric acute-onset neuropsychiatric syndrome (PANS) and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections syndrome (PANDAS), in which tic disorders may appear following a streptococcal infection, revealed a different gut microbiota composition compared to healthy controls. In addition, a decrease in pathways involved in brain function and an increase of some pathways involved in modulation of the antibody response to intestinal inflammation were observed in younger PANS/PANDAS patients, although the sample size was small (Quagliariello et al., 2018). Another study (Snider et al., 2005) found that penicillin plus azithromycin prophylaxis was effective in decreasing streptococcal infections and the associated neuropsychiatric disorders, including tic disorders, in PANDAS patients. However, the role of the gut microbiota is unclear in this case, as the improvement of the neuropsychiatric symptoms could also be related simply to the concomitant suppression of streptococcal infections. Moreover, the sample size was small and the applied methodology was questionable (Budman et al., 2005).
In an open-label clinical trial, described in an abstract (Ding et al., 2019), a transient decrease of tic severity was observed in 11 males with TS after treatment with three administrations of a mixed bacterial community.
No animal studies on FMT and TS were identified.
Zhao H. et al. (2017) described a case report of a child with TS that had decreased tic severity at the follow-up moment 8 weeks after FMT. The parents reported that they had observed disappearance of involuntary phonation, a decrease in involuntary shrugging and improved attention in the 8 weeks after FMT. Further follow-up was not described.
In some studies, patients with stroke appeared to have an altered gut microbiota composition compared to healthy controls (Karlsson et al., 2012; Yin et al., 2015), although some other studies reported that no change occurred or that change was only temporary (Koren et al., 2011; Swidsinski et al., 2012). However, these results may be confounded by factors, such as age, type 2 diabetes mellitus or obesity, which are risk factors for stroke that are associated with a different gut microbiota composition (Qin et al., 2012; Karlsson et al., 2013; Torres-Fuentes et al., 2017; An et al., 2018). For α-diversity, studies are also contradictory (Yin et al., 2015; Li N. et al., 2019). Furthermore, decreased neuronal injury and improved cognitive performance was observed in diabetic mice with bilateral common carotid arteries occlusion after receiving Clostridium butyricum suspension intragastrically (Sun et al., 2016).
Studies on the role of the gut microbiota in stroke are frequently contradictory. It has been hypothesized that the gut microbiota influences the severity of ischemic brain injury following stroke. After stroke, reduced intestinal motility combined with reduced α-diversity of bacterial species, bacterial overgrowth and impaired intestinal barrier may lead to formation of proinflammatory immune cells in the gut-associated lymphoid tissue and subsequent infiltration of the brain with increased infarct volume (Singh et al., 2016). Possibly, the translocation of gut bacteria and their metabolites is also involved (Caso et al., 2009; Chen et al., 2019). However, gut microbiota depletion with bacterial outgrowth of certain taxa and reduced α-diversity may also have a protective effect on ischemic brain injury by suppression of trafficking of effector T cells from gut to brain (Benakis et al., 2016). The immune function appears to be reduced after stroke, with impairment of the gut-associated lymphoid tissue (Meisel et al., 2005; Schulte-Herbruggen et al., 2009). Furthermore, the reduced intestinal motility in stroke patients is reflected by the increased frequency of constipation (Camara-Lemarroy et al., 2014).
Another role of the gut microbiota may be hypothesized in the development of atherosclerosis and subsequent stroke. Several studies in animal models and humans suggest that the gut microbiota influences the formation of atherosclerotic plaques (Stepankova et al., 2010; Koren et al., 2011; Wang Z. et al., 2011; Gregory et al., 2015; Zhu et al., 2016). Symptomatic atherosclerosis in humans is indeed associated with a different gut microbiota composition and functional capacity (Karlsson et al., 2012). Production of trimethylamine-N-oxide by the gut microbiota may be associated with cardiovascular events, including stroke (Nam et al., 2019; Yang S. et al., 2019), whereas others studies suggest a protective effect (Yin et al., 2015; Collins et al., 2016; Arduini et al., 2019). Nevertheless, atherosclerosis is not the only determinant of stroke.
Winek et al. (2016) found similar infarct volumes but increased mortality in temporarily antibiotic-treated mice after stroke induction compared to stroke mice without antibiotics or continuous antibiotics and sham-operated antibiotic-treated mice. Temporarily antibiotic-treated mice also developed severe colitis. When these mice received gavage with specific-pathogen-free microbiota before stroke-induction, a similar mortality and infarct volume was observed, but sample size was low. However, colitis was prevented. This means colitis could not have caused the increased mortality in the FMT-group. Antibiotics may have played a role as mortality in the FMT-group was similar to the antibiotic treated-stroke group (Winek et al., 2016). Two other studies found more functional impairment, larger cerebral infarct volume, and increased intestinal, systemic and cerebral inflammation in (pseudo-)GF stroke mice that had received dysbiotic post-stroke mouse or human microbiota compared to mice receiving normal microbiota (Singh et al., 2016; Xia et al., 2019). In addition, gavage with normal microbiota led to reduced infarct volumes (Singh et al., 2016). In contrast, Benakis et al. (2016) found a protective effect of microbiota depletion by antibiotics on infarct volume. Reduced ischemic brain injury and sensorimotor deficits were observed in amoxicillin/clavulanic acid (AC)-sensitive mice treated with AC compared to AC-resistant mice. AC-treated mice that had received AC-sensitive microbiota revealed a decreased infarct volume compared to mice receiving AC-resistant microbiota.
Another study found that gut microbiota in young mice is altered after experimental stroke and resembles that of uninjured aged mice. They found improved performance in several behavioral tests, decreased mortality and infarct size and decreased pro-inflammatory cytokines after FMT with young microbiota compared to those receiving aged microbiota (Spychala et al., 2018).
Alzheimer's disease (AD) is a neurodegenerative disease with a progressive decline in cognitive function and loss of neurons and synapses. A combination of genetic and environmental factors is thought to be involved in the etiology (Angelucci et al., 2019). Pathology is characterized by intraneuronal deposits of neurofibrillary tangles and extracellular accumulations of abnormally folded amyloid beta (Aβ) proteins. Aβ proteins are thought to be pro-inflammatory neurotoxic proteins (Calsolaro and Edison, 2016). However, the hypothesis that Aβ proteins depositions lead to synaptic dysfunction and subsequently symptoms has been questioned (Selkoe and Hardy, 2016). It is unclear whether Aβ protein deposition may be the cause or result of AD. There could be a vicious cycle between Aβ accumulation leading to microglia activation and microglia dysfunction leading to Aβ accumulation (Cai et al., 2014). Neuroinflammation is thought to play a key role in AD (Calsolaro and Edison, 2016).
In recent years, numerous publications on the relation between AD and the gut microbiota have become available. AD patients have a different gut microbiota composition compared to healthy controls or elderly without dementia (Vogt et al., 2017; Zhuang et al., 2018; Haran et al., 2019; Li B. et al., 2019; Liu et al., 2019), but α-diversity measures show contrasting results (Vogt et al., 2017; Li B. et al., 2019; Liu et al., 2019; Saji et al., 2019). Hypotheses on the role of the gut microbiota include direct actions of bacteria, indirect actions or aging-related processes (Angelucci et al., 2019).
Studies have demonstrated the presence of microorganisms in brains of both AD patients and healthy controls. However, increased LPS levels (Zhan et al., 2016; Zhao Y. et al., 2017) and an increased presence of several bacteria and fungi, including some gut commensals, were observed in brains of AD patients compared to controls (Mawanda and Wallace, 2013; Pisa et al., 2017; Alonso et al., 2018; Dominy et al., 2019). The presence of bacterial LPS or endotoxin-mediated inflammation contributes to amyloid neurotoxicity (Zhao et al., 2015). A study found that LPS in the brain colocalizes with AD-related Aβ proteins in amyloid plaques (Zhan et al., 2016). Interestingly, the LPS- and microorganism-detecting receptor CD14, important in the neutralization of invading microorganisms, is also stimulated by Aβ fibrils (Zhao et al., 2015). Furthermore, feces of patients with brain amyloidosis and cognitive impairment contain more pro-inflammatory gut bacteria and blood more pro-inflammatory cytokines compared to patients with cognitive impairment without amyloidosis or controls. In addition, less anti-inflammatory bacteria and cytokines are observed (Cattaneo et al., 2017). Interestingly, certain bacteria can produce extracellular bacterial amyloids known as “curli fibers,” a main component of biofilms for these bacteria. Some bacteria that produce these curli fibers are recognized by the same Toll-like receptor as the Aβ42 peptide that accumulates in AD (Zhao et al., 2015; Tursi and Tükel, 2018). Increased levels of E. coli, a curli fiber producer, were indeed found in AD brains compared to controls (Zhan et al., 2016). However, it is unknown whether bacterial amyloids co-localize with the amyloid deposits or other insoluble lesions that are observed in AD (Zhao et al., 2015; Tursi and Tükel, 2018). Bacteria may also contribute to AD by production of neurotransmitters and altering proteins and receptors involved in synaptic plasticity (Barrett et al., 2012; Maqsood and Stone, 2016).
Apart from the direct action of bacteria, it is suggested that certain gut microbiota alterations may stimulate inflammatory pathways and thereby neuroinflammation (Marques et al., 2016). Some researchers hypothesized that Aβ depositions are part of an innate immune response that normally protects against microbial infections in the brain (Moir et al., 2018). A decrease of Aβ accumulation and microglia activation after antibiotic treatment has indeed been observed. Conversely, other studies suggested that microbiota depletion may negatively affect cognitive function and microglia and synaptic function in mice and contrasting results were also observed in humans (Laake and Oeksengaard, 2002; Angelucci et al., 2019). Furthermore, a positive effect of probiotics on cognitive function was observed in animal models and AD patients or adults with mild cognitive impairment (Davari et al., 2013; Jiang et al., 2017; Kobayashi et al., 2017, 2019a; Rezaei Asl et al., 2019; Tamtaji et al., 2019), whereas one study reported no to little effect (Kobayashi et al., 2019b).
Erny et al. (2015) observed defects of microglia in germ-free mice leading to impaired innate immune responses. This is in line with the hygiene hypothesis, that proposes that AD patients have reduced microbial diversity due to environmental sanitation and therefore an impaired response to pathogens, with an important role for T cells, in particular Treg cells (Larbi et al., 2009; Browne et al., 2013; Dansokho et al., 2016; Hu et al., 2016). Furthermore, with increasing age, an increase in proinflammatory cytokines and decrease in anti-inflammatory gut bacteria is observed (Biagi et al., 2010). This inflamm-aging may also be associated with cognitive decline (Franceschi et al., 2000).
Zhan et al. (2018) observed that wild-type mice showed decreased cognitive function after broad-spectrum antibiotic treatment. Subsequently, spatial learning and memory improved after receiving feces from senescence-resistant mice (similar to control) compared to mice receiving feces from senescence-prone mice (similar to antibiotics plus vehicle). Using diabetic mice with and without cognitive deterioration gave a similar result (Yu et al., 2019), although this is not a mouse model of AD but cognitive deterioration secondary to diabetes. These two studies suggest that antibiotics decrease cognitive function in mice, which may be reversed by FMT. In contrast, two studies (Harach et al., 2017; Dodiya et al., 2019) observed reduced Aβ-pathology and neuroinflammation in male mice (not in female mice) when the gut microbiota was depleted. When an AB-treated AD mouse model received FMT with feces from age- and sex-matched AD mice without antibiotics, the positive effect of antibiotics was partially reversed (Dodiya et al., 2019). In a germ-free AD mouse model, FMT with conventional microbiota or conventional AD microbiota caused an increase of pathology, with the latter showing a stronger effect (Harach et al., 2017). Another study with germ-free wild-type mice found a deterioration of cognitive function with lower fecal metabolites related to the nervous system, such as GABA, in aged, and not young, mice that received AD feces compared to healthy control feces (Fujii et al., 2019).
Cui et al. (2018) found that the chronic noise exposure-associated increased risk for AD in mice may be mediated by the gut microbiota, and that chronic noise causes age-related neurochemical and inflammatory dysregulation. Gavage with feces from high-intensity noise AD mice to age-matched recipient AD mice led to increased cerebral Aβ and affected intestinal and blood-brain barrier compared to control-microbiota recipients.
No published FMT-studies in humans with AD were found, but ClinicalTrials.gov showed an ongoing RCT with FMT in patients with AD.
Guillain-Barré syndrome (GBS) is a paralytic neuropathy, assumed to be caused by an auto-immune response after infection or other immune stimulation, predominantly preceded by gastrointestinal infection with Campylobacter jejuni. The disease is frequently characterized by rapidly progressing bilateral weakness. This may be accompanied by additional neurologic or autonomic symptoms. Symptom severity varies among different patients. Eventually, most patients improve, but permanent disability can occur (Willison et al., 2016).
Several studies reported an association between C. jejuni colonization/infection and the gut microbiota. C. jejuni colonization may be inhibited by gut microbiota-mediated colonization resistance (Brooks and Mansfield, 2017). However, several studies suggest that C. jejuni may overcome this colonization resistance in several ways, such as increasing acetinogenesis gene expression, which leads to the conversion of pyruvate to acetate (Ducarmon et al., 2019). Inoculation of mice with C. jejuni strains from GBS patients causes less colitis but more autoantibodies with increased peripheral nerve lesions compared to inoculation with C. jejuni strains from colitis patients (Malik et al., 2014; St Charles et al., 2017). This is exacerbated after antibiotic treatment (St Charles et al., 2017; Brooks et al., 2019). The production of cross-reactive anti-ganglioside antibodies during C. jejuni infection due to molecular mimicry between bacterial LPS of GBS-associated C. jejuni strains and peripheral nerve gangliosides have been considered as one of the factors associated with the development of GBS (Jacobs et al., 1997; Yuki, 1997; Ang et al., 2002). This molecular mimicry is more frequently observed in GBS-associated C. jejuni strains compared to colitis-associated C. jejuni strains.
Antibiotic-treated and gnotobiotic mice display increased colonization and gastrointestinal inflammation after C. jejuni inoculation (Chang and Miller, 2006; Stahl et al., 2014; O'Loughlin et al., 2015), with a potential protective role for Enterococcus faecalis (O'Loughlin et al., 2015). Furthermore, a considerably decreased C. jejuni clearance time is observed in mice that receive antibiotics and gavage with murine microbiota compared to human microbiota-receiving mice and gnotobiotic mice, that showed a more pro-inflammatory immune response (Bereswill et al., 2011).
Moreover, probiotics were successful at reducing C. jejuni load in poultry (Morishita et al., 1997; Willis and Reid, 2008). This was also confirmed by one mouse study (Wagner et al., 2009) and one study showed that a probiotic inhibited C. jejuni invasion of human intestinal epithelial cells (Wine et al., 2009).
Brooks et al. (2017) observed increased colonization, Th-2 responses (independent of inoculation status) and autoimmune responses after C. jejuni injection in a human microbiota-treated mouse group compared to a conventional mouse microbiota group. However, this should be interpreted with caution as C. jejuni strains may have been adapted to the human microbiota, while human and animal gut microbiota differ. This study suggests that a particular gut microbiota composition may enhance susceptibility to GBS after C. jejuni infection.
Although no FMT-studies are available yet for amyotrophic lateral sclerosis, one placebo-controlled RCT with FMT in human amyotrophic lateral sclerosis patients is registered on ClinicalTrials.gov.
Neurological disorders are complex, often involving cognitive, motor and systemic aspects. A combination of genetic and environmental factors is mostly thought to be involved in the pathogenesis, with the gut microbiota being one potential factor. Gut microbiota manipulation by FMT may influence symptoms or progression of neurological disorders by gut microbiota-mediated immunological, endocrine, metabolic and/or neural pathways. Gut microbiota-produced metabolites and cytokines may influence the level of intestinal and systemic inflammation and may alter intestinal barrier function. The vagus nerve provides a direct neural connection between the gut and the brain and may play an important role. Furthermore, gut microbiota interventions may influence the availability and efficacy of medication by directly or indirectly interacting with the processing of drugs or by modulation of the immune response.
Gut microbiota manipulation by FMT could be a promising treatment approach for several neurological disorders, but the evidence is limited. The neurological disorder with the most evidence on efficacy of a healthy donor FMT is ASD. Various articles, including clinical trials in humans, suggest that FMT leads to decreased symptom severity. FMT may alter production of gut microbial metabolites, such as serotonin, SCFA or GABA receptor agonists, whereas a reduction of pro-inflammatory gut bacteria may decrease neuro- and systemic inflammation and cerebral oxidative stress. However, the reliability of conclusions in humans might be undermined by the lack of a blind design, as a placebo-effect may play an important role. More and larger double-blind controlled studies are needed. For PD, MS, AD and stroke, several animal studies suggest a positive effect of FMT, supported by some case reports in humans. The potential beneficial effects of FMT for patients with PD could be mediated through a decreased α-syn accumulation in the intestinal wall and subsequently in the brain, by reduced inflammation-induced oxidative stress. Beneficial effects on Parkinson symptoms could be further strengthened by an increased availability and efficacy of levodopa following FMT with feces from donors with less bacterial tyrosine decarboxylases in their feces (Maini Rekdal et al., 2019; van Kessel et al., 2019). Remarkably, one mouse study showed that administration of PD feces is enough to induce PD symptoms (Sun et al., 2018), whereas another study showed that genetic abnormalities in addition to a certain microbiota composition is necessary to induce PD symptoms (Sampson et al., 2016). In MS patients, an increase in Treg cells after FMT may attenuate auto-immunity with demyelination and possibly disease progression. Furthermore, FMT with feces from young healthy donors may decrease AD progression by reducing translocation of pro-inflammatory gut bacteria from gut to brain and consequently neuroinflammation processes mediated by them. However, most animal studies only examined the effects of FMT with feces from AD patients or feces from animal models of AD in healthy mice and not the opposite. The effects of FMT in animal models of stroke are less clear. Performing FMT in patients with high-risk for stroke may potentially decrease infarct size when they develop stroke by a decrease of pro-inflammatory immune cells trafficking to the infarct area. When this mechanism is confirmed by future studies, this suggests that FMT performed immediately after a stroke may also potentially improve infarct size, but no studies have assessed this yet. Interestingly, one mouse study (Winek et al., 2016) reported an increased mortality rate, but similar infarct volume, after FMT. The increased mortality may have been linked to increased infarct size due to FMT, since infarct size was only measured on day 1 after stroke-induction, but it could also be linked to the antibiotic treatment. Remarkably, contrasting effects of microbiota depletion by antibiotics were observed for stroke and AD. The experimental results on FMT for epilepsy are even more difficult to interpret, considering that there are several different subtypes of epilepsy with different pathophysiology. Some, but very limited evidence is available that suggests a potential role of the gut microbiota in seizure susceptibility. Animal studies may imply that the gut microbiota mediates a stress-induced increase and ketogenic diet-induced decrease in seizure susceptibility. For other neurological disorders, such as TS, diabetic neuropathy and Guillain-Barré, the available evidence is very limited. For these disorders and for epilepsy, animal studies need to be replicated and expanded in future studies before an attempt in humans.
Numerous trials on FMT in neurological disorders are currently planned or ongoing and it is expected that the bulk of evidence on the efficacy of FMT in neurological disorders will grow.
For many disorders, research to date has been limited to animal models. It should be noticed that in this field, as in other fields, it is often not known whether results obtained in animal studies are directly applicable to human patients. Robustness of quality and mechanistic depth of animal models differ widely for neurological disorders. Furthermore, behavior and cognition tests in animal models may be subjective or difficult to interpret and not reproducible. Another important consideration is that the immunoreactivity of animal models could be very different from that observed in patients. In some animal studies, feces from humans were transferred to animal models. This may not provide a good model for diseased or healthy humans, considering the differences in gut microbiota and subsequent host-microbiota interactions between humans and animals (Hugenholtz and de Vos, 2018). For this reason, assessment of gut microbiota composition in animal models of disease may also not be reliable. Coprophagy, behavior that is frequently observed in mice (Ebino et al., 1987), may also affect the results of gut microbiota analyses and efficacy of FMT. Several studies attempted to avoid this by caging mice separately, but autologous FMT by coprophagy may still occur. This must all be appraised, when translating findings in animal models into human neurological disorders.
There are also other important limitations on both the human and animal studies with FMT. These limitations are related to the, often complex, study design, including for example the use of different antibiotics, different FMT procedures, the choice of different donors, and the lack of long term follow-up or appropriate control groups.
For human studies, neuropsychiatric diseases analyzed with subjective outcome measures are prone to strong placebo effects after such an invasive treatment (Belcher et al., 2018). A double-blinded design including a control group (for example with autologous FMT) is desirable. Moreover, it is important to acknowledge the possibility of publication bias, especially regarding case reports. Case descriptions and studies with little or no effect of FMT on disease symptoms or characteristics were rare. In addition, several neurological disorders tend to fluctuate in disease severity, which implicates that assessment of FMT efficacy might be difficult. The mechanism of action of FMT might be different in different disorders: while in its consolidated use for recurrent C. difficile infections usually a single FMT is sufficient to produce sustained benefit, for neurological disorders, which are progressive in nature, multiple FMT may be necessary to achieve a sustained response; the required number of FMTs and effective interval between multiple FMTs would then need to be evaluated. For a few case descriptions and studies on ASD and PD, indeed only transient positive effects were observed, but this may also be explained by a placebo effect or fluctuations in disease severity.
For some neurological disorders examined in this review, contrasting evidence was observed in gut microbiota analysis results. Various non-standardized methods could be used for the assessment of the gut microbiota composition. The results of microbiota analyses can be affected by a wide variety of factors throughout the entire workflow of a microbiota study, starting with sample collection and DNA extraction and ending with choice of statistical tests. In addition, due to the influence of many other patient- or subject related factors, including medication use, diet, or age, inconsistency in gut microbiota composition and α- and β-diversity analyses is often observed. Furthermore, functional analyses of the gut microbiome, using metagenomics and metabolomics, are emerging and may be more important than the detection of taxa, exclusively reported by many studies. What the gut bacteria produce and the concomitant effects on processes in the human body is more important information than which bacteria are present.
The safety of this experimental treatment should also be better elucidated. Potential benefits of FMT should be carefully weighed against the potential risks, and future studies should focus primarily on safety, with effectivity of FMT as a secondary endpoint. Indeed, not all human and animal studies mentioned assessment of adverse events in subjects undergoing FMT and potential long-term negative effects were rarely examined. Side effects could be related to the administration route (e.g., gastroscopy or colonoscopy) (Wang et al., 2016), to the required pretreatment (antibiotics and bowel lavage), or even to the administration of feces itself, which could theoretically influence the pathogenesis of the disorder in a negative way. Transferring certain taxa or inducing an increase in α-diversity may turn out to be beneficial or detrimental, and this may differ between individual disorders. Whether all healthy donors or only a few donors with certain gut microbiome characteristics are suitable, is unknown. Rational feces donor selection, based on available literature, may be crucial, but more knowledge on the pathophysiology of several neurological disorders and the relevant characteristics of the microbiome is required. Also, future studies should answer the question whether gut microbiota baseline profiles can predict who will benefit from FMT. Herein, the use of appropriate methods for the (preparation of) microbiota analysis and assessment of possible confounding factors are crucial. Confounding factors should be corrected for or prevented. Concomitantly, functional analyses of the gut microbiome will have a crucial role in determining which taxa are important in each neurological disorder. Also important is that more evidence has to become available on the number of FMTs required for each neurological disorder. Furthermore, it is unclear whether pre-treatment with antibiotics and bowel lavage is necessary in neurological disorders. The current view in C. difficile infections is that pre-treatment improves donor feces engraftment. In our opinion, a similar pre-treatment as in C. difficile infections may also be required to foster the effects of FMT in neurological disorders. In the future, capsules that contain beneficial bacterial consortia may replace FMT, thus increasing the comfort for patients and reducing the potential side effects associated with the administration route.
In conclusion, although some evidence is available, well-designed large double-blinded randomized controlled trials in human patients are needed to further elucidate the effect of FMT in neurological disorders.
KV reviewed all literature and wrote the manuscript. PJ assembled the figure, performed the initial search, and participated in writing the manuscript. RO, JL, and BO reviewed the literature on MS and wrote the section of the manuscript on MS. EK and MC supported and supervised the writing of the manuscript. QD aided in the interpretation of microbiota data. All authors critically reviewed the manuscript.
KV, RO, JK, and EK are members of the Netherlands Donor Feces Bank (https://www.ndfb.nl/), which received an unrestricted grant from Vedanta Biosciences in Boston (https://www.vedantabio.com/).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.00098/full#supplementary-material
3AIBA, 3-aminoisobutyric acid; 3-CST, three-chamber sociability test; 5-HT, serotonin or 5-hydroxytryptamine; A, aged 18–20 months old mice; AB, antibiotics; ABC, aberrant behavior checklist; AC, amoxicillin/clavulanate; AC Res, amoxicilline/clavulanate-resistant; AC Sens, amoxicilline/clavulanate-sensitive; AD, Alzheimer's disease; AE, adverse event; Ampho-B, amphotericin B; APP, Aβ precursor protein; APPPS1, co-expression of KM670/671NL Swedish mutation of human amyloid precursor protein (APP) and L166P mutation of human presenilin 1 (PS1) under control of the Thy-1 promoter with age-dependent Aβ parenchymal accumulation and minimal vascular Aβ amyloid, restricted to the pial vessels; APPPS1-21, amyloid precursor proteinSWE/ presenilin 1L166P mouse model of amyloid-β amyloidosis/Alzheimer's disease that express familial AD–linked APPSWE and PS1L166P transgenes driven by the neuron-specific Thy1 promoter; ASD, autism spectrum disorder; ASO, alpha-synuclein overexpression; Aβ, amyloid beta; cAPPPS1, APPPS1 mice with conventional microbiota; CARS, childhood autism rating scale; CD, cognitive dysfunction; CDi, control diet; CD11b, a type of mouse antigen-specific antibodies; CDAI, Crohn's disease activity index; CFA, complete Freud's adjuvant; CLDN1, claudin 1; cMCAO, permanent occlusion of MCA distal of lenticulostriate arteries (cause small cortical lesions); CNS, central nervous system; Conv-11168, mice are infected with C. jejuni from enteric disease patient; Conv-260.94, mice are infected with C. jejuni from GBS patient; Conv-TSB, mice are inoculated with tryptic soy broth; d, day(s); DA, striatal dopamine; DSI, direct social interaction test; DSR, daily stool records; EAE, experimental autoimmune encephalomyelitis; EDSS, expanded disability status scale; fMCAO, transient occlusion of MCA by temporarily placing a filament in the internal carotid artery; FMD, fasting mimicking diet; FMT, fecal microbiota transplantation; FPD, Faith's phylogenetic diversity; FST, forced swimming test; GABA, gamma-aminobutyric acid; GBS, Guillain-Barré syndrome; GF, germ-free; GI, gastrointestinal; GSI, gastrointestinal severity index; GSRS, gastrointestinal symptom rating scale; HC, healthy control; HHC, human healthy household control; HK, heat-killed; HN, high intensity noise; Hu, humanized; HWT, hang wire test; i.c., ileocecocolic; i.p., intraperitoneal; IL, interleukin; KCNA1, Potassium Voltage-Gated Channel Subfamily A Member 1; KDi, ketogenic diet; LN, low-intensity noise; LPS, lipopolysaccharide; m, month(s); MB, marble burying test; MCAO, middle cerebral artery occlusion; mNSS, modified neurological severity score; MOG, myelin oligodendrocyte glycoprotein; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; (SP)MS, (secondary progressive) multiple sclerosis; MSFC, Modified Multiple Sclerosis Functional Composite; MWMT, morris water maze test; MWT, mechanical withdrawal test; N, normal; NA, data not available; ND, normally developing; NDS, neurological deficit score; Non-anh, spared nerve injury without developing a anhedonia-like phenotype; NF, mice that were treated with normal saline by intraperitoneal injection and fasting mimicking diet; non-sign., non-significant; NS, normal saline; OFT, open-field testing; OLT, object location test; ORT, novel object recognition test; OTU, operational taxonomic unit; PAC-QOL, patient assessment of constipation–quality of life; PANDAS, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections syndrome; PANS, pediatric acute-onset neuropsychiatric syndrome; PBS, phosphate-buffered solution; PBS/G, mice that were treated with 20% glycerol in sterile phosphate-buffered solution; PCoA, principal coordinates analysis; PD, Parkinson's disease; PGI-III, parent global impressions-III; PGI-R, parent global impressions-revised; phylog. div, phylogenetic diversity; PLS-DA, partial least squares discrimination analysis; PPA, propionic acid; Qol, quality of life; RR, relapsing-remitting; SAE, serious adverse event(s); SAMP8, senescence-accelerated mouse prone 8; SAMR-1, senescence-accelerated mouse resistant 1; SC, sodium citrate buffer (control for FMT); SCFA, short chain fatty acids; SD, Sprague Dawley; SDI, stroke dysbiosis index; SDI-H, stroke patients with a high stroke dysbiosis index; SDI-L, stroke patients with a low stroke dysbiosis index; SGHM, Standardized Human Gut Microbiota; SNI, spared nerve injury; SPF, specific-pathogen-free; SPT, Sucrose preference test; SRS, Social Responsiveness Scale; STER, mice are handled sterile; SW, Swiss-Webster; T1D, type 1 diabetes mellitus; TET, Transendoscopic enteral tubing; TFT, Tail-flick test; Th, T-helper cells, Thy1-αSyn alpha-synuclein-overexpression mouse model; TLR, toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cells; TS, Tourette syndrome; TSB, tryptic soy broth; TST, tail suspension test; UPDRS, unified Parkinson's disease rating scale; USV, ultrasonic vocalizations test; VABS-II, Vineland Adaptive Behavior Scale II; VAS, Visual analog scale; w, week(s); w., weighted; WT, wild-type; y, year(s); y.o., year old; Y, young 8-12 weeks old mice; YGTSS, Yale Global Tic Severity Scale; ZO-1, tight junction protein 1.
Aabed, K., Shafi Bhat, R., Moubayed, N., Al-Mutiri, M., Al-Marshoud, M., Al-Qahtani, A., et al. (2019). Ameliorative effect of probiotics [Lactobacillus paracaseii and Protexin(R)] and prebiotics (propolis and bee pollen) on clindamycin and propionic acid-induced oxidative stress and altered gut microbiota in a rodent model of autism. Cell. Mol. Biol. 65, 1–7. doi: 10.14715/cmb/2019.65.1.1
Adams, J. B., Johansen, L. J., Powell, L. D., Quig, D., and Rubin, R. A. (2011). Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11:22. doi: 10.1186/1471-230X-11-22
Alonso, R., Pisa, D., Fernandez-Fernandez, A. M., and Carrasco, L. (2018). Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer's disease. Front. Aging Neurosci. 10:159. doi: 10.3389/fnagi.2018.00159
An, R., Wilms, E., Masclee, A. A. M., Smidt, H., Zoetendal, E. G., and Jonkers, D. (2018). Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut 67, 2213–2222. doi: 10.1136/gutjnl-2017-315542
Ananthaswamy, A. (2011). Faecal transplant eases symptoms of Parkinson's. NewScientist 209, 8–9. doi: 10.1016/S0262-4079(11)60124-3
Ang, C. W., Laman, J. D., Willison, H. J., Wagner, E. R., Endtz, H. P., De Klerk, M. A., et al. (2002). Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barre and Miller Fisher patients. Infect. Immun. 70, 1202–1208. doi: 10.1128/IAI.70.3.1202-1208.2002
Angelucci, F., Cechova, K., Amlerova, J., and Hort, J. (2019). Antibiotics, gut microbiota, and Alzheimer's disease. J. Neuroinflamm. 16:108. doi: 10.1186/s12974-019-1494-4
Arduini, A., Zammit, V. A., and Bonomini, M. (2019). Identification of trimethylamine N-oxide (TMAO)-producer phenotype is interesting, but is it helpful? Gut 69, 400–401. doi: 10.1136/gutjnl-2018-318000
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I., and Van de Water, J. (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immunity 25, 40–45. doi: 10.1016/j.bbi.2010.08.003
Backhed, F., Ding, H., Wang, T., Hooper, L. V., Koh, G. Y., Nagy, A., et al. (2004). The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A. 101, 15718–15723. doi: 10.1073/pnas.0407076101
Barrett, E., Ross, R. P., O'Toole, P. W., Fitzgerald, G. F., and Stanton, C. (2012). gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417. doi: 10.1111/j.1365-2672.2012.05344.x
Belcher, A. M., Ferre, S., Martinez, P. E., and Colloca, L. (2018). Role of placebo effects in pain and neuropsychiatric disorders. Progress Neuro Psychopharmacol. Biol. Psychiatry 87(Pt B), 298–306. doi: 10.1016/j.pnpbp.2017.06.003
Benakis, C., Brea, D., Caballero, S., Faraco, G., Moore, J., Murphy, M., et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 22, 516–523. doi: 10.1038/nm.4068
Berer, K., Boziki, M., and Krishnamoorthy, G. (2014). Selective accumulation of pro-inflammatory T cells in the intestine contributes to the resistance to autoimmune demyelinating disease. PLoS ONE. 9:e87876. doi: 10.1371/journal.pone.0087876
Berer, K., Gerdes, L. A., Cekanaviciute, E., Jia, X., Xiao, L., Xia, Z., et al. (2017). Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. U.S.A. 114, 10719–10724. doi: 10.1073/pnas.1711233114
Berer, K., Mues, M., Koutrolos, M., Rasbi, Z. A., Boziki, M., Johner, C., et al. (2011). Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. doi: 10.1038/nature10554
Bereswill, S., Fischer, A., Plickert, R., Haag, L. M., Otto, B., Kuhl, A. A., et al. (2011). Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 6:e20953. doi: 10.1371/annotation/5247af81-4595-44b7-9c3f-2e45ad85abfa
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., et al. (2010). Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 5:e10667. doi: 10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d
Borody, T. L. S., Campbell, J., Torres, M., and Nowak, A. (2011). Fecal Microbiota Transplantation (FMT) in Multiple Sclerosis (MS). Am. J. Gastroenterol. 106:S352. doi: 10.14309/00000434-201110002-00942
Boukthir, S., Matoussi, N., Belhadj, A., Mammou, S., Dlala, S. B., Helayem, M., et al. (2010). Abnormal intestinal permeability in children with autism. La Tunisie Med. 88, 685–686.
Braak, H., Rub, U., Gai, W. P., and Del Tredici, K. (2003). Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Trans. 110, 517–536. doi: 10.1007/s00702-002-0808-2
Brooks, P. T., Bell, J. A., Bejcek, C. E., Malik, A., and Mansfield, L. S. (2019). An antibiotic depleted microbiome drives severe Campylobacter jejuni-mediated Type 1/17 colitis, Type 2 autoimmunity and neurologic sequelae in a mouse model. J. Neuroimmunol. 337:577048. doi: 10.1016/j.jneuroim.2019.577048
Brooks, P. T., Brakel, K. A., Bell, J. A., Bejcek, C. E., Gilpin, T., Brudvig, J. M., et al. (2017). Transplanted human fecal microbiota enhanced Guillain Barre syndrome autoantibody responses after Campylobacter jejuni infection in C57BL/6 mice. Microbiome 5:92. doi: 10.1186/s40168-017-0284-4
Brooks, P. T., and Mansfield, L. S. (2017). Effects of antibiotic resistance (AR) and microbiota shifts on Campylobacter jejuni-mediated diseases. Anim. Health. Res. Rev. 18, 99–111. doi: 10.1017/S1466252318000014
Browne, T. C., McQuillan, K., McManus, R. M., O'Reilly, J. A., Mills, K. H., and Lynch, M. A. (2013). IFN-gamma Production by amyloid beta-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. J. Immunol. 190, 2241–2251. doi: 10.4049/jimmunol.1200947
Budman, C., Coffey, B., Dure, L., Gilbert, D., Juncos, J., Kaplan, E., et al. (2005). Regarding “antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders”. Biol. Psychiatry 58:917; author reply 8–9. doi: 10.1016/j.biopsych.2005.08.005
Cabanlit, M., Wills, S., Goines, P., Ashwood, P., and Van de Water, J. (2007). Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann. N. Y. Acad. Sci. 1107, 92–103. doi: 10.1196/annals.1381.010
Cai, T. T., Ye, X. L., Yong, H. J., Song, B., Zheng, X. L., Cui, B. T., et al. (2018). Fecal microbiota transplantation relieve painful diabetic neuropathy: a case report. Medicine 97:e13543. doi: 10.1097/MD.0000000000013543
Cai, Z., Hussain, M. D., and Yan, L. J. (2014). Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer's disease. Int. J. Neurosci. 124, 307–321. doi: 10.3109/00207454.2013.833510
Calsolaro, V., and Edison, P. (2016). Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimer's Dement. 12, 719–732. doi: 10.1016/j.jalz.2016.02.010
Camara-Lemarroy, C. R., Ibarra-Yruegas, B. E., and Gongora-Rivera, F. (2014). Gastrointestinal complications after ischemic stroke. J. Neurol. Sci. 346, 20–25. doi: 10.1016/j.jns.2014.08.027
Caso, J. R., Hurtado, O., Pereira, M. P., Garcia-Bueno, B., Menchen, L., Alou, L., et al. (2009). Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am. J. Physiol. Regul. Integr. Compar. Physiol. 296, R979–R985. doi: 10.1152/ajpregu.90825.2008
Castelli, V., Palumbo, P., d'Angelo, M., Moorthy, N. K., Antonosante, A., Catanesi, M., et al. (2018). Probiotic DSF counteracts chemotherapy induced neuropathic pain. Oncotarget 9, 27998–28008. doi: 10.18632/oncotarget.25524
Cattaneo, A., Cattane, N., Galluzzi, S., Provasi, S., Lopizzo, N., Festari, C., et al. (2017). Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68. doi: 10.1016/j.neurobiolaging.2016.08.019
Cekanaviciute, E., Yoo, B. B., Runia, T. F., Debelius, J. W., Singh, S., Nelson, C. A., et al. (2017). Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. U.S.A. 114, 10713–10718. doi: 10.1073/pnas.1711235114
Cermak, S. A., Curtin, C., and Bandini, L. G. (2010). Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Dietetic Assoc. 110, 238–246. doi: 10.1016/j.jada.2009.10.032
Chang, C., and Miller, J. F. (2006). Campylobacter jejuni colonization of mice with limited enteric flora. Infect. Immun. 74, 5261–5271. doi: 10.1128/IAI.01094-05
Chen, J., Chia, N., Kalari, K. R., Yao, J. Z., Novotna, M., Paz Soldan, M. M., et al. (2016). Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6:28484. doi: 10.1038/srep28484
Chen, R., Wu, P., Cai, Z., Fang, Y., Zhou, H., Lasanajak, Y., et al. (2019). Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J. Nutr. Biochem. 65, 101–114. doi: 10.1016/j.jnutbio.2018.12.004
Collins, H. L., Drazul-Schrader, D., Sulpizio, A. C., Koster, P. D., Williamson, Y., Adelman, S. J., et al. (2016). L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis 244, 29–37. doi: 10.1016/j.atherosclerosis.2015.10.108
Connolly, A. M., Chez, M., Streif, E. M., Keeling, R. M., Golumbek, P. T., Kwon, J. M., et al. (2006). Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol. Psychiatry 59, 354–363. doi: 10.1016/j.biopsych.2005.07.004
Cosorich, I., Dalla-Costa, G., Sorini, C., Ferrarese, R., Messina, M. J., Dolpady, J., et al. (2017). High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 3:e1700492. doi: 10.1126/sciadv.1700492
Cree, B. A., Spencer, C. M., Varrin-Doyer, M., Baranzini, S. E., and Zamvil, S. S. (2016). Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann. Neurol. 80, 443–447. doi: 10.1002/ana.24718
Cui, B., Su, D., Li, W., She, X., Zhang, M., Wang, R., et al. (2018). Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer's disease. J. Neuroinflamm. 15:190. doi: 10.1186/s12974-018-1223-4
Dahlin, M., and Prast-Nielsen, S. (2019). The gut microbiome and epilepsy. EBioMedicine 44, 741–746. doi: 10.1016/j.ebiom.2019.05.024
Dansokho, C., Ait Ahmed, D., Aid, S., Toly-Ndour, C., Chaigneau, T., Calle, V., et al. (2016). Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain J. Neurol. 139(Pt 4), 1237–1251. doi: 10.1093/brain/awv408
Davari, S., Talaei, S. A., Alaei, H., and Salami, M. (2013). Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 240, 287–296. doi: 10.1016/j.neuroscience.2013.02.055
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
De Angelis, M., Francavilla, R., Piccolo, M., De Giacomo, A., and Gobbetti, M. (2015). Autism spectrum disorders and intestinal microbiota. Gut Microbes 6, 207–213. doi: 10.1080/19490976.2015.1035855
De Angelis, M., Piccolo, M., Vannini, L., Siragusa, S., De Giacomo, A., Serrazzanetti, D. I., et al. (2013). Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS. ONE 8:e76993. doi: 10.1371/journal.pone.0076993
de Magistris, L., Familiari, V., Pascotto, A., Sapone, A., Frolli, A., Iardino, P., et al. (2010). Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatric Gastroenterol. Nutr. 51, 418–424. doi: 10.1097/MPG.0b013e3181dcc4a5
de Theije, C. G., Wu, J., Koelink, P. J., Korte-Bouws, G. A., Borre, Y., Kas, M. J., et al. (2014). Autistic-like behavioural and neurochemical changes in a mouse model of food allergy. Behavio. Brain. Res. 261, 265–274. doi: 10.1016/j.bbr.2013.12.008
Dendrou, C. A., Fugger, L., and Friese, M. A. (2015). Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558. doi: 10.1038/nri3871
Desbonnet, L., Clarke, G., Shanahan, F., Dinan, T. G., and Cryan, J. F. (2014). Microbiota is essential for social development in the mouse. Mol. Psychiatry 19, 146–148. doi: 10.1038/mp.2013.65
D'Eufemia, P., Celli, M., Finocchiaro, R., Pacifico, L., Viozzi, L., Zaccagnini, M., et al. (1996). Abnormal intestinal permeability in children with autism. Acta Paediatrica 85, 1076–1079. doi: 10.1111/j.1651-2227.1996.tb14220.x
Devos, D., Lebouvier, T., Lardeux, B., Biraud, M., Rouaud, T., Pouclet, H., et al. (2013). Colonic inflammation in Parkinson's disease. Neurobiol. Dis. 50, 42–48. doi: 10.1016/j.nbd.2012.09.007
Ding, X., Zhang, F., Li, Q., Ting, Z., Cui, B., and Li, P. (2019). Selective microbiota transplantation is effective for controlling tourette's syndrome. Gastroenterology 156, S-456–S-457. doi: 10.1016/S0016-5085(19)37992-2
Dodiya, H. B., Kuntz, T., Shaik, S. M., Baufeld, C., Leibowitz, J., Zhang, X., et al. (2019). Sex-specific effects of microbiome perturbations on cerebral Abeta amyloidosis and microglia phenotypes. J. Exp. Med. 216, 1542–1560. doi: 10.1084/jem.20182386
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5:eaau3333. doi: 10.1126/sciadv.aau3333
Doshi-Velez, F., Avillach, P., Palmer, N., Bousvaros, A., Ge, Y., Fox, K., et al. (2015). Prevalence of inflammatory bowel disease among patients with autism spectrum disorders. Inflamm. Bowel Dis. 21, 2281–2288. doi: 10.1097/MIB.0000000000000502
Ducarmon, Q. R., Zwittink, R. D., Hornung, B. V. H., van Schaik, W., Young, V. B., and Kuijper, E. J. (2019). Gut microbiota and colonization resistance against bacterial enteric infection. Microb. Mol. Biol. Rev. 83:e00007-19. doi: 10.1128/MMBR.00007-19
Ebino, K. Y., Amao, H., Suwa, T., Kuwabara, Y., Saito, T. R., and Takahashi, K. W. (1987). Coprophagy in the germfree mouse. Jikken Dobutsu 36, 33–37. doi: 10.1538/expanim1978.36.1_33
Erny, D., Hrabe de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Fang, X., Wang, X., Yang, S., Meng, F., Wang, X., Wei, H., et al. (2016). Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front. Microbiol. 7:1479. doi: 10.3389/fmicb.2016.01479
Fasano, A., Bove, F., Gabrielli, M., Petracca, M., Zocco, M. A., Ragazzoni, E., et al. (2013). The role of small intestinal bacterial overgrowth in Parkinson's disease. Mov. Disord. 28, 1241–1249. doi: 10.1002/mds.25522
Fattorusso, A., Di Genova, L., Dell'Isola, G. B., Mencaroni, E., and Esposito, S. (2019). Autism spectrum disorders and the gut microbiota. Nutrients 11:521. doi: 10.3390/nu11030521
Finegold, S. M., Dowd, S. E., Gontcharova, V., Liu, C., Henley, K. E., Wolcott, R. D., et al. (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453. doi: 10.1016/j.anaerobe.2010.06.008
Finegold, S. M., Molitoris, D., Song, Y., Liu, C., Vaisanen, M. L., Bolte, E., et al. (2002). Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 35(Suppl. 1), S6–s16. doi: 10.1086/341914
Forsyth, C. B., Shannon, K. M., Kordower, J. H., Voigt, R. M., Shaikh, M., Jaglin, J. A., et al. (2011). Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS ONE 6:e28032. doi: 10.1371/journal.pone.0028032
Franceschi, C., Bonafe, M., Valensin, S., Olivieri, F., De Luca, M., Ottaviani, E., et al. (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x
Fujii, Y., Nguyen, T. T. T., Fujimura, Y., Kameya, N., Nakamura, S., Arakawa, K., et al. (2019). Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer's disease. Biosci. Biotechnol. Biochem. 83, 1–9. doi: 10.1080/09168451.2019.1644149
Gabrielli, M., Bonazzi, P., Scarpellini, E., Bendia, E., Lauritano, E. C., Fasano, A., et al. (2011). Prevalence of small intestinal bacterial overgrowth in Parkinson's disease. Mov. Dis. 26, 889–892. doi: 10.1002/mds.23566
Gazerani, P. (2019). Probiotics for Parkinson's disease. Int. J. Mol. Sci. 20:E4121. doi: 10.3390/ijms20174121
Ghanizadeh, A., and Berk, M. (2015). Beta-lactam antibiotics as a possible novel therapy for managing epilepsy and autism, a case report and review of literature. Iran. J. Child Neurol. 9, 99–102. doi: 10.22037/ijcn.v9i1.5493
Gomez-Eguilaz, M., Ramon-Trapero, J. L., Perez-Martinez, L., and Blanco, J. R. (2018). The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Beneficial Microbes 9, 875–881. doi: 10.3920/BM2018.0018
Goverman, J., Woods, A., Larson, L., Weiner, L. P., Hood, L., and Zaller, D. M. (1993). Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 72, 551–560. doi: 10.1016/0092-8674(93)90074-Z
Gregory, J. C., Buffa, J. A., Org, E., Wang, Z., Levison, B. S., Zhu, W., et al. (2015). Transmission of atherosclerosis susceptibility with gut microbial transplantation. J. Biol. Chem. 290, 5647–5660. doi: 10.1074/jbc.M114.618249
Gungor, B., Adiguzel, E., Gursel, I., Yilmaz, B., and Gursel, M. (2016). intestinal microbiota in patients with spinal cord injury. PLoS ONE 11:e0145878. doi: 10.1371/journal.pone.0145878
Guo, R., Chen, L. H., Xing, C., and Liu, T. (2019). Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br. J. Anaesth. 123, 637–654. doi: 10.1016/j.bja.2019.07.026
Hallett, P. J., McLean, J. R., Kartunen, A., Langston, J. W., and Isacson, O. (2012). alpha-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol. Dis. 47, 258–267. doi: 10.1016/j.nbd.2012.04.009
Han, L., Ji, L., Chang, J., Wen, J., Zhao, W., Shi, H., et al. (2015). Peripheral neuropathy is associated with insulin resistance independent of metabolic syndrome. Diabetol. Metab. Syndrome 7:14. doi: 10.1186/s13098-015-0010-y
Harach, T., Marungruang, N., Duthilleul, N., Cheatham, V., Mc Coy, K. D., Frisoni, G., et al. (2017). Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7:41802. doi: 10.1038/srep46856
Haran, J. P., Bhattarai, S. K., Foley, S. E., Dutta, P., Ward, D. V., Bucci, V., et al. (2019). Alzheimer's disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio 10:e00632-19. doi: 10.1128/mBio.00632-19
Hasegawa, S., Goto, S., Tsuji, H., Okuno, T., Asahara, T., Nomoto, K., et al. (2015). Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson's disease. PLoS ONE 10:e0142164. doi: 10.1371/journal.pone.0142164
He, Z., Cui, B. T., Zhang, T., Li, P., Long, C. Y., Ji, G. Z., et al. (2017). Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World. J. Gastroenterol. 23, 3565–3568. doi: 10.3748/wjg.v23.i19.3565
Holmqvist, S., Chutna, O., Bousset, L., Aldrin-Kirk, P., Li, W., Bjorklund, T., et al. (2014). Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820. doi: 10.1007/s00401-014-1343-6
Hsiao, E. Y., McBride, S. W., Hsien, S., Sharon, G., Hyde, E. R., McCue, T., et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. doi: 10.1016/j.cell.2013.11.024
Hu, X., Wang, T., and Jin, F. (2016). Alzheimer's disease and gut microbiota. Sci. China Life Sci. 59, 1006–1023. doi: 10.1007/s11427-016-5083-9
Huang, H., Xu, H., Luo, Q., He, J., Li, M., Chen, H., et al. (2019). Fecal microbiota transplantation to treat Parkinson's disease with constipation: a case report. Medicine 98:e16163. doi: 10.1097/MD.0000000000016163
Huang, J., Zhang, C., Wang, J., Guo, Q., and Zou, W. (2019). Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain. Behav. 9:e01260. doi: 10.1002/brb3.1260
Hugenholtz, F., and de Vos, W. M. (2018). Mouse models for human intestinal microbiota research: a critical evaluation. Cell. Mol. Life. Sci. 75, 149–160. doi: 10.1007/s00018-017-2693-8
Jacobs, B. C., Hazenberg, M. P., van Doorn, P. A., Endtz, H. P., and van der Meche, F. G. (1997). Cross-reactive antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in patients with Guillain-Barre or Miller Fisher syndrome. J. Infect. Dis. 175, 729–733. doi: 10.1093/infdis/175.3.729
Jamshidi, P., Hasanzadeh, S., Tahvildari, A., Farsi, Y., Arbabi, M., Mota, J. F., et al. (2019). Is there any association between gut microbiota and type 1 diabetes? A systematic review. Gut. Pathog. 11:49. doi: 10.1186/s13099-019-0332-7
Jangi, S., Gandhi, R., Cox, L. M., Li, N., von Glehn, F., Yan, R., et al. (2016). Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7:12015. doi: 10.1038/ncomms12015
Jiang, C., Li, G., Huang, P., Liu, Z., and Zhao, B. (2017). The gut microbiota and Alzheimer's disease. J. Alzheimer's. Dis. 58, 1–15. doi: 10.3233/JAD-161141
Kaluzna-Czaplinska, J., and Blaszczyk, S. (2012). The level of arabinitol in autistic children after probiotic therapy. Nutrition 28, 124–126. doi: 10.1016/j.nut.2011.08.002
Kang, D. W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., McDonough-Means, S., et al. (2019). Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 9:5821. doi: 10.1038/s41598-019-42183-0
Kang, D. W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., et al. (2017). Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. doi: 10.1186/s40168-016-0225-7
Kang, D. W., Park, J. G., Ilhan, Z. E., Wallstrom, G., Labaer, J., Adams, J. B., et al. (2013). Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 8:e68322. doi: 10.1371/journal.pone.0068322
Karlsson, F. H., Fak, F., Nookaew, I., Tremaroli, V., Fagerberg, B., Petranovic, D., et al. (2012). Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 3:1245. doi: 10.1038/ncomms2266
Karlsson, F. H., Tremaroli, V., Nookaew, I., Bergstrom, G., Behre, C. J., Fagerberg, B., et al. (2013). Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103. doi: 10.1038/nature12198
Kelly, C. R., Khoruts, A., Staley, C., Sadowsky, M. J., Abd, M., Alani, M., et al. (2016). Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann. Int. Med. 165, 609–616. doi: 10.7326/M16-0271
Keshavarzian, A., Green, S. J., Engen, P. A., Voigt, R. M., Naqib, A., Forsyth, C. B., et al. (2015). Colonic bacterial composition in Parkinson's disease. Mov. Disord. 30, 1351–1360. doi: 10.1002/mds.26307
Kim, S., Kwon, S. H., Kam, T. I., Panicker, N., Karuppagounder, S. S., Lee, S., et al. (2019). Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson's disease. Neuron 103, 627–641.e7. doi: 10.1016/j.neuron.2019.05.035
Kitamura, S., Sugihara, K., Kuwasako, M., and Tatsumi, K. (1997). The role of mammalian intestinal bacteria in the reductive metabolism of zonisamide. J. Pharm. Pharmacol. 49, 253–256. doi: 10.1111/j.2042-7158.1997.tb06790.x
Knivsberg, A. M., Reichelt, K. L., Hoien, T., and Nodland, M. (2002). A randomised, controlled study of dietary intervention in autistic syndromes. Nutr. Neurosci. 5, 251–261. doi: 10.1080/10284150290028945
Kobayashi, Y., Kinoshita, T., Matsumoto, A., Yoshino, K., Saito, I., and Xiao, J. Z. (2019a). Bifidobacterium Breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: an open-label, single-arm study. J. Prev. Alzheimer's. Dis. 6, 70–75. doi: 10.14283/jpad.2018.32
Kobayashi, Y., Kuhara, T., Oki, M., and Xiao, J. Z. (2019b). Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef. Microbes10, 511–520. doi: 10.3920/BM2018.0170
Kobayashi, Y., Sugahara, H., Shimada, K., Mitsuyama, E., Kuhara, T., Yasuoka, A., et al. (2017). Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease. Sci. Rep. 7:13510. doi: 10.1038/s41598-017-13368-2
Koren, O., Spor, A., Felin, J., Fak, F., Stombaugh, J., Tremaroli, V., et al. (2011). Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl 1), 4592–4598. doi: 10.1073/pnas.1011383107
Kuo, Y. M., Li, Z., Jiao, Y., Gaborit, N., Pani, A. K., Orrison, B. M., et al. (2010). Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 19, 1633–1650. doi: 10.1093/hmg/ddq038
Laake, K., and Oeksengaard, A. R. (2002). D-cycloserine for Alzheimer's disease. Cochrane Database Syst. Rev. Cd003153. doi: 10.1002/14651858.CD003153
Larbi, A., Pawelec, G., Witkowski, J. M., Schipper, H. M., Derhovanessian, E., Goldeck, D., et al. (2009). Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer's disease. J. Alzheimer's Di. 17, 91–103. doi: 10.3233/JAD-2009-1015
Li, B., He, Y., Ma, J., Huang, P., Du, J., Cao, L., et al. (2019). Mild cognitive impairment has similar alterations as Alzheimer's disease in gut microbiota. Alzheimer's Dement. 15, 1357–1366. doi: 10.1016/j.jalz.2019.07.002
Li, N., Wang, X., Sun, C., Wu, X., Lu, M., Si, Y., et al. (2019). Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 19:191. doi: 10.1186/s12866-019-1552-1
Li, X., Chauhan, A., Sheikh, A. M., Patil, S., Chauhan, V., Li, X. M., et al. (2009). Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 207, 111–116. doi: 10.1016/j.jneuroim.2008.12.002
Liao, J. F., Cheng, Y. F., Li, S. W., Lee, W. T., Hsu, C. C., Wu, C. C., et al. (2019). Lactobacillus plantarum PS128 ameliorates 2,5-Dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Res. Bull. 153, 59–73. doi: 10.1016/j.brainresbull.2019.07.027
Liautard, J. P. (1991). Are prions misfolded molecular chaperones? FEBS Lett. 294, 155–157. doi: 10.1016/0014-5793(91)80657-O
Lindefeldt, M., Eng, A., Darban, H., Bjerkner, A., Zetterstrom, C. K., Allander, T., et al. (2019). The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 5:5. doi: 10.1038/s41522-018-0073-2
Liu, P., Wu, L., Peng, G., Han, Y., Tang, R., Ge, J., et al. (2019). Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 80, 633–643. doi: 10.1016/j.bbi.2019.05.008
Lum, G. R., Olson, C. A., and Hsiao, E. Y. (2019). Emerging roles for the intestinal microbiome in epilepsy. Neurobiol. Dis. 135:104576. doi: 10.1016/j.nbd.2019.104576
Ma, B., Liang, J., Dai, M., Wang, J., Luo, J., Zhang, Z., et al. (2019). Altered gut microbiota in chinese children with autism spectrum disorders. Front. Cell. Infect. Microbiol. 9:40. doi: 10.3389/fcimb.2019.00040
MacFabe, D. F., Cain, D. P., Rodriguez-Capote, K., Franklin, A. E., Hoffman, J. E., Boon, F., et al. (2007). Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 176, 149–169. doi: 10.1016/j.bbr.2006.07.025
Maini Rekdal, V., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J., and Balskus, E. P. (2019). Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364:eaau6323. doi: 10.1126/science.aau6323
Makkawi, S., Camara-Lemarroy, C., and Metz, L. (2018). Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflamm. 5:e459. doi: 10.1212/NXI.0000000000000459
Malik, A., Sharma, D., St Charles, J., Dybas, L. A., and Mansfield, L. S. (2014). Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol. 7, 802–817. doi: 10.1038/mi.2013.97
Mangalam, A., Shahi, S. K., Luckey, D., Karau, M., Marietta, E., Luo, N., et al. (2017). Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 20, 1269–1277. doi: 10.1016/j.celrep.2017.07.031
Maqsood, R., and Stone, T. W. (2016). The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem. Res. 41, 2819–2835. doi: 10.1007/s11064-016-2039-1
Marques, C., Meireles, M., Faria, A., and Calhau, C. (2016). High-fat diet-induced dysbiosis as a cause of neuroinflammation. Biol. Psychiatry 80, e3–4. doi: 10.1016/j.biopsych.2015.10.027
Mawanda, F., and Wallace, R. (2013). Can infections cause Alzheimer's disease? Epidemiol. Rev. 35, 161–180. doi: 10.1093/epirev/mxs007
Mayer, E. A., Padua, D., and Tillisch, K. (2014). Altered brain-gut axis in autism: comorbidity or causative mechanisms? BioEssays. 36, 933–939. doi: 10.1002/bies.201400075
Mazurek, M. O., Vasa, R. A., Kalb, L. G., Kanne, S. M., Rosenberg, D., Keefer, A., et al. (2013). Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J. Abnormal Child Psychol. 41, 165–176. doi: 10.1007/s10802-012-9668-x
Mazzini, L., Mogna, L., De Marchi, F., Amoruso, A., Pane, M., Aloisio, I., et al. (2018). Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J. Clin. Gastroenterol. 52(Suppl. 1), S68–S70. doi: 10.1097/MCG.0000000000001042
McElhanon, B. O., McCracken, C., Karpen, S., and Sharp, W. G. (2014). Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 133, 872–883. doi: 10.1542/peds.2013-3995
Medel-Matus, J. S., Shin, D., Dorfman, E., Sankar, R., and Mazarati, A. (2018). Facilitation of kindling epileptogenesis by chronic stress may be mediated by intestinal microbiome. Epilepsia Open 3, 290–294. doi: 10.1002/epi4.12114
Meisel, C., Schwab, J. M., Prass, K., Meisel, A., and Dirnagl, U. (2005). Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 6, 775–786. doi: 10.1038/nrn1765
Miyake, S., Kim, S., Suda, W., Oshima, K., Nakamura, M., Matsuoka, T., et al. (2015). Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS ONE 10:e0137429. doi: 10.1371/journal.pone.0137429
Moir, R. D., Lathe, R., and Tanzi, R. E. (2018). The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimer's Dement. 14, 1602–1614. doi: 10.1016/j.jalz.2018.06.3040
Morishita, T. Y., Aye, P. P., Harr, B. S., Cobb, C. W., and Clifford, J. R. (1997). Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian. Dis. 41, 850–855. doi: 10.2307/1592338
Nam, H. S., Ha, J., Ji, D., Kwon, I., Lee, H. S., Han, M., et al. (2019). Elevation of the gut microbiota metabolite trimethylamine N-oxide predicts stroke outcome. J. Stroke 21, 350–352. doi: 10.5853/jos.2019.00850
Navarro, F., Liu, Y., and Rhoads, J. M. (2016). Can probiotics benefit children with autism spectrum disorders? World J. Gastroenterol. 22, 10093–10102. doi: 10.3748/wjg.v22.i46.10093
O'Loughlin, J. L., Samuelson, D. R., Braundmeier-Fleming, A. G., White, B. A., Haldorson, G. J., Stone, J. B., et al. (2015). The intestinal microbiota influences Campylobacter jejuni colonization and extraintestinal dissemination in mice. Appl. Environ. Microbiol. 81, 4642–4650. doi: 10.1128/AEM.00281-15
Olson, C. A., Vuong, H. E., Yano, J. M., Liang, Q. Y., Nusbaum, D. J., and Hsiao, E. Y. (2018). The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–1741. doi: 10.1016/j.cell.2018.04.027
Olsson, T., Barcellos, L. F., and Alfredsson, L. (2017). Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36. doi: 10.1038/nrneurol.2016.187
Pakkenberg, B., Moller, A., Gundersen, H. J., Mouritzen Dam, A., and Pakkenberg, H. (1991). The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J. Neurol. Neurosurg. Psychiatry 54, 30–33. doi: 10.1136/jnnp.54.1.30
Pan-Montojo, F., Anichtchik, O., Dening, Y., Knels, L., Pursche, S., Jung, R., et al. (2010). Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 5:e8762. doi: 10.1371/journal.pone.0008762
Park, Y. D. (2003). The effects of vagus nerve stimulation therapy on patients with intractable seizures and either Landau-Kleffner syndrome or autism. Epilepsy Behav. 4, 286–290. doi: 10.1016/S1525-5050(03)00080-5
Parracho, H. M. R. T., Gibson, G. R., Knott, F., Bosscher, D., Kleerebezem, M., and McCartney, A. (2010). A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int. J. Probiotics Prebiotics 5, 69–74.
Partty, A., Kalliomaki, M., Wacklin, P., Salminen, S., and Isolauri, E. (2015). A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr. Res. 77, 823–828. doi: 10.1038/pr.2015.51
Peng, A., Qiu, X., Lai, W., Li, W., Zhang, L., Zhu, X., et al. (2018). Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 147, 102–107. doi: 10.1016/j.eplepsyres.2018.09.013
Pisa, D., Alonso, R., Fernandez-Fernandez, A. M., Rabano, A., and Carrasco, L. (2017). Polymicrobial infections in brain tissue from Alzheimer's disease patients. Sci. Rep. 7:5559. doi: 10.1038/s41598-017-05903-y
Poewe, W. (2008). Non-motor symptoms in Parkinson's disease. Eur. J. Neurol. 15(Suppl. 1), 14–20. doi: 10.1111/j.1468-1331.2008.02056.x
Postuma, R. B., Aarsland, D., Barone, P., Burn, D. J., Hawkes, C. H., Oertel, W., et al. (2012). Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov. Disord. 27, 617–626. doi: 10.1002/mds.24996
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Quagliariello, A., Del Chierico, F., Russo, A., Reddel, S., Conte, G., Lopetuso, L. R., et al. (2018). Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front. Microb. 9:675. doi: 10.3389/fmicb.2018.00675
Rezaei Asl, Z., Sepehri, G., and Salami, M. (2019). Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer's disease. Behav. Brain Res. 376:112183. doi: 10.1016/j.bbr.2019.112183
Rooks, M. G., and Garrett, W. S. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352. doi: 10.1038/nri.2016.42
Rowin, J., Xia, Y., Jung, B., and Sun, J. (2017). Gut inflammation and dysbiosis in human motor neuron disease. Physiol. Rep. 5:e13443. doi: 10.14814/phy2.13443
Saji, N., Niida, S., Murotani, K., Hisada, T., Tsuduki, T., Sugimoto, T., et al. (2019). Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci. Rep. 9:1008. doi: 10.1038/s41598-018-38218-7
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell 167, 1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
Sandler, R. H., Finegold, S. M., Bolte, E. R., Buchanan, C. P., Maxwell, A. P., Vaisanen, M. L., et al. (2000). Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 15, 429–435. doi: 10.1177/088307380001500701
Scheperjans, F., Aho, V., Pereira, P. A., Koskinen, K., Paulin, L., Pekkonen, E., et al. (2015). Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov. Disord. 30, 350–358. doi: 10.1002/mds.26069
Schulte-Herbruggen, O., Quarcoo, D., Meisel, A., and Meisel, C. (2009). Differential affection of intestinal immune cell populations after cerebral ischemia in mice. Neuroimmunomodulation 16, 213–218. doi: 10.1159/000205514
Selkoe, D. J., and Hardy, J. (2016). The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 8, 595–608. doi: 10.15252/emmm.201606210
Sewal, R. K., Modi, M., Saikia, U. N., Chakrabarti, A., and Medhi, B. (2017). Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res. 135, 176–186. doi: 10.1016/j.eplepsyres.2017.05.012
Shaaban, S. Y., El Gendy, Y. G., Mehanna, N. S., El-Senousy, W. M., El-Feki, H. S. A., Saad, K., et al. (2018). The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr. Neurosci. 21, 676–681. doi: 10.1080/1028415X.2017.1347746
Shannon, K. M., Keshavarzian, A., Dodiya, H. B., Jakate, S., and Kordower, J. H. (2012). Is alpha-synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov. Disord. 27, 716–719. doi: 10.1002/mds.25020
Sharon, G., Cruz, N. J., Kang, D. W., Gandal, M. J., Wang, B., Kim, Y. M., et al. (2019). Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618.e17. doi: 10.1016/j.cell.2019.05.004
Shen, S., Lim, G., You, Z., Ding, W., Huang, P., Ran, C., et al. (2017). Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 20, 1213–1216. doi: 10.1038/nn.4606
Shults, C. W. (2006). Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 103, 1661–1668. doi: 10.1073/pnas.0509567103
Silva, S. C., Correia, C., Fesel, C., Barreto, M., Coutinho, A. M., Marques, C., et al. (2004). Autoantibody repertoires to brain tissue in autism nuclear families. J. Neuroimmunol. 152, 176–182. doi: 10.1016/j.jneuroim.2004.03.015
Singh, V., Roth, S., Llovera, G., Sadler, R., Garzetti, D., Stecher, B., et al. (2016). Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016
Snider, L. A., Lougee, L., Slattery, M., Grant, P., and Swedo, S. E. (2005). Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol. Psychiatry 57, 788–792. doi: 10.1016/j.biopsych.2004.12.035
Son, J. S., Zheng, L. J., Rowehl, L. M., Tian, X., Zhang, Y., Zhu, W., et al. (2015). Comparison of fecal microbiota in children with autism spectrum disorders and neurotypical siblings in the simons simplex collection. PLoS ONE 10:e0137725. doi: 10.1371/journal.pone.0137725
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997). Alpha-synuclein in Lewy bodies. Nature 388, 839–840. doi: 10.1038/42166
Spychala, M. S., Venna, V. R., Jandzinski, M., Doran, S. J., Durgan, D. J., Ganesh, B. P., et al. (2018). Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 84, 23–36. doi: 10.1002/ana.25250
St Charles, J. L., Bell, J. A., Gadsden, B. J., Malik, A., Cooke, H., Van de Grift, L. K., et al. (2017). Guillain barre syndrome is induced in non-obese diabetic (NOD) mice following Campylobacter jejuni infection and is exacerbated by antibiotics. J. Autoimmunity 77, 11–38. doi: 10.1016/j.jaut.2016.09.003
Stahl, M., Ries, J., Vermeulen, J., Yang, H., Sham, H. P., Crowley, S. M., et al. (2014). A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS. Pathog. 10:e1004264. doi: 10.1371/journal.ppat.1004264
Stepankova, R., Tonar, Z., Bartova, J., Nedorost, L., Rossman, P., Poledne, R., et al. (2010). Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J. Atheroscl. Thrombosis 17, 796–804. doi: 10.5551/jat.3285
Stokholm, M. G., Danielsen, E. H., Hamilton-Dutoit, S. J., and Borghammer, P. (2016). Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 79, 940–949. doi: 10.1002/ana.24648
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2016). Altered gut microbiota in Rett syndrome. Microbiome 4:41. doi: 10.1186/s40168-016-0185-y
Sun, J., Wang, F., Ling, Z., Yu, X., Chen, W., Li, H., et al. (2016). Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 1642, 180–188. doi: 10.1016/j.brainres.2016.03.042
Sun, M. F., Zhu, Y. L., Zhou, Z. L., Jia, X. B., Xu, Y. D., Yang, Q., et al. (2018). Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 70, 48–60. doi: 10.1016/j.bbi.2018.02.005
Swidsinski, A., Loening-Baucke, V., Krüger, M., and Kirsch, S. (2012). Central nervous system and the colonic bioreactor: analysis of colonic microbiota in patients with stroke unravels unknown mechanisms of the host defense after brain injury. Intestinal Res. 10, 332–342. doi: 10.5217/ir.2012.10.4.332
Tamtaji, O. R., Heidari-Soureshjani, R., Mirhosseini, N., Kouchaki, E., Bahmani, F., Aghadavod, E., et al. (2019). Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin. Nutr. 38, 2569–2575. doi: 10.1016/j.clnu.2018.11.034
Tan, A. H., Chong, C. W., Song, S. L., Teh, C. S. J., Yap, I. K. S., Loke, M. F., et al. (2018). Altered gut microbiome and metabolome in patients with multiple system atrophy. Mov. Disord. 33, 174–176. doi: 10.1002/mds.27203
Tan, A. H., Mahadeva, S., Thalha, A. M., Gibson, P. R., Kiew, C. K., Yeat, C. M., et al. (2014). Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism Relat. Disord. 20, 535–540. doi: 10.1016/j.parkreldis.2014.02.019
Thompson, A. J., Baranzini, S. E., Geurts, J., Hemmer, B., and Ciccarelli, O. (2018). Multiple sclerosis. Lancet 391, 1622–1636. doi: 10.1016/S0140-6736(18)30481-1
Todd, R. D., Hickok, J. M., Anderson, G. M., and Cohen, D. J. (1988). Antibrain antibodies in infantile autism. Biol. Psychiatry 23, 644–647. doi: 10.1016/0006-3223(88)90012-1
Torres-Fuentes, C., Schellekens, H., Dinan, T. G., and Cryan, J. F. (2017). The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2, 747–756. doi: 10.1016/S2468-1253(17)30147-4
Tursi, S. A., and Tükel, C. (2018). Curli-containing enteric biofilms inside and out: matrix composition, immune recognition, and disease implications. Microbiol. Mol. Biol. Rev. 82:e00028-18. doi: 10.1128/MMBR.00028-18
Ulusoy, A., Rusconi, R., Perez-Revuelta, B. I., Musgrove, R. E., Helwig, M., Winzen-Reichert, B., et al. (2013). Caudo-rostral brain spreading of alpha-synuclein through vagal connections. EMBO Mol. Med. 5, 1119–1127. doi: 10.1002/emmm.201302475
Unger, M. M., Spiegel, J., Dillmann, K. U., Grundmann, D., Philippeit, H., Burmann, J., et al. (2016). Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72. doi: 10.1016/j.parkreldis.2016.08.019
Urbonas, V., and Cervinskiene, J. (2018). “Fecal transplantation and its role in autism spectrum disorders,” in EHMSG – XXXIst International Workshop on Helicobacter and Microbiota in Inflammation and Cancer (Kaunas: Helicobacter), 17.
van Kessel, S. P., Frye, A. K., El-Gendy, A. O., Castejon, M., Keshavarzian, A., van Dijk, G., et al. (2019). Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson's disease. Nat. Commun. 10:310. doi: 10.1038/s41467-019-08294-y
van Nood, E., Vrieze, A., Nieuwdorp, M., Fuentes, S., Zoetendal, E. G., de Vos, W. M., et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415. doi: 10.1056/NEJMoa1205037
Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W., and Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57, 67–81. doi: 10.1002/ana.20315
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer's disease. Sci. Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
Vojdani, A., Campbell, A. W., Anyanwu, E., Kashanian, A., Bock, K., and Vojdani, E. (2002). Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J. Neuroimmunol. 129, 168–177. doi: 10.1016/S0165-5728(02)00180-7
Vrieze, A., Van Nood, E., Holleman, F., Salojarvi, J., Kootte, R. S., Bartelsman, J. F., et al. (2012). Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7. doi: 10.1053/j.gastro.2012.06.031
Wagner, R. D., Johnson, S. J., and Kurniasih Rubin, D. (2009). Probiotic bacteria are antagonistic to Salmonella enterica and Campylobacter jejuni and influence host lymphocyte responses in human microbiota-associated immunodeficient and immunocompetent mice. Mol. Nutr. Food Res. 53, 377–388. doi: 10.1002/mnfr.200800101
Wang, L. W., Tancredi, D. J., and Thomas, D. W. (2011). The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J. Dev. Behav. Pediatrics 32, 351–360. doi: 10.1097/DBP.0b013e31821bd06a
Wang, S., Xu, M., Wang, W., Cao, X., Piao, M., Khan, S., et al. (2016). Systematic review: adverse events of fecal microbiota transplantation. PLoS ONE 11:e0161174. doi: 10.1371/journal.pone.0161174
Wang, Z., Klipfell, E., Bennett, B. J., Koeth, R., Levison, B. S., Dugar, B., et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63. doi: 10.1038/nature09922
Ward, L., O'Grady, H., Wu, K., Cannon, K., Workentine, M., and Louie, T. (2016). “Combined oral fecal capsules plus fecal enema as treatment of late onset autism spectrum disorder in children: report of a small case series,” in IDweek (New Orleans, LA). Available online at: https://idsa.confex.com/idsa/2016/webprogram/Paper60261.html
West, R., Roberts, E., Sichel, L. S., and Sichel, J. (2013). Improvements in gastrointestinal symptoms among children with autism spectrum disorder receiving the delpro® probiotic and immunomodulator formulation. J. Prob. Health 1:102. doi: 10.4172/2329-8901.1000102
Whiteley, P., Haracopos, D., Knivsberg, A. M., Reichelt, K. L., Parlar, S., Jacobsen, J., et al. (2010). The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr. Neurosci. 13, 87–100. doi: 10.1179/147683010X12611460763922
Williams, B. L., Hornig, M., Buie, T., Bauman, M. L., Cho Paik, M., Wick, I., et al. (2011). Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 6:e24585. doi: 10.1371/journal.pone.0024585
Willis, W. L., and Reid, L. (2008). Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poultry Sci. 87, 606–611. doi: 10.3382/ps.2006-00458
Willison, H. J., Jacobs, B. C., and van Doorn, P. A. (2016). Guillain-Barre syndrome. Lancet 388, 717–727. doi: 10.1016/S0140-6736(16)00339-1
Wills, S., Cabanlit, M., Bennett, J., Ashwood, P., Amaral, D. G., and Van de Water, J. (2009). Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav. Immun. 23, 64–74. doi: 10.1016/j.bbi.2008.07.007
Wine, E., Gareau, M. G., Johnson-Henry, K., and Sherman, P. M. (2009). Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 300, 146–152. doi: 10.1111/j.1574-6968.2009.01781.x
Winek, K., Engel, O., Koduah, P., Heimesaat, M. M., Fischer, A., Bereswill, S., et al. (2016). Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 47, 1354–1363. doi: 10.1161/STROKEAHA.115.011800
Xia, G. H., You, C., Gao, X. X., Zeng, X. L., Zhu, J. J., Xu, K. Y., et al. (2019). Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front. Neurol. 10:397. doi: 10.3389/fneur.2019.00397
Xie, G., Zhou, Q., Qiu, C. Z., Dai, W. K., Wang, H. P., Li, Y. H., et al. (2017). Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 23, 6164–6171. doi: 10.3748/wjg.v23.i33.6164
Yang, C., Fang, X., Zhan, G., Huang, N., Li, S., Bi, J., et al. (2019). Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 9:57. doi: 10.1038/s41398-019-0379-8
Yang, S., Li, X., Yang, F., Zhao, R., Pan, X., Liang, J., et al. (2019). gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front. Pharmacol. 10:1360. doi: 10.3389/fphar.2019.01360
Yeom, J. S., Park, J. S., Kim, Y. S., Kim, R. B., Choi, D. S., Chung, J. Y., et al. (2019). Neonatal seizures and white matter injury: Role of rotavirus infection and probiotics. Brain Dev. 41, 19–28. doi: 10.1016/j.braindev.2018.07.001
Yin, J., Liao, S. X., He, Y., Wang, S., Xia, G. H., Liu, F. T., et al. (2015). Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 4:e002699. doi: 10.1161/JAHA.115.002699
Yissachar, N., Zhou, Y., Ung, L., Lai, N. Y., Mohan, J. F., Ehrlicher, A., et al. (2017). An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 168, 1135–48.e12. doi: 10.1016/j.cell.2017.02.009.
Yu, F., Han, W., Zhan, G., Li, S., Xiang, S., Zhu, B., et al. (2019). Abnormal gut microbiota composition contributes to cognitive dysfunction in streptozotocin-induced diabetic mice. Aging 11, 3262–3279. doi: 10.18632/aging.101978
Yuki, N. (1997). Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barre syndrome and Miller Fisher syndrome. J. Infect. Dis. 176(Suppl. 2), S150–S153. doi: 10.1086/513800
Zhan, G., Yang, N., Li, S., Huang, N., Fang, X., Zhang, J., et al. (2018). Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 10, 1257–1267. doi: 10.18632/aging.101464
Zhan, X., Stamova, B., Jin, L. W., DeCarli, C., Phinney, B., and Sharp, F. R. (2016). Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87, 2324–2332. doi: 10.1212/WNL.0000000000003391
Zhao, H., Gao, X., Xi, L., Shi, Y., Peng, L., Wang, C., et al. (2019). “Fecal microbiota transplantation for children with autism spectrum disorder,” in DDW 2019 ASGE Program and Abstracts, Gastrointestinal Endoscopy, ed M. B. Wallace (San Diego, CA), AB512–AB513.
Zhao, H., Shi, Y., Luo, X., Peng, L., Yang, Y., and Zou, L. (2017). The effect of fecal microbiota transplantation on a child with tourette syndrome. Case. reports. in. medicine. 2017:6165239. doi: 10.1155/2017/6165239
Zhao, Y., Dua, P., and Lukiw, W. J. (2015). Microbial Sources of amyloid and relevance to amyloidogenesis and Alzheimer's disease (AD). J. Alzheimer's. Dis. Parkinsonism 5:177. doi: 10.4172/2161-0460.1000177
Zhao, Y., Jaber, V., and Lukiw, W. J. (2017). Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front. Cell. Infect. Microbiol. 7:318. doi: 10.3389/fcimb.2017.00318
Zhou, Z. L., Jia, X. B., Sun, M. F., Zhu, Y. L., Qiao, C. M., Zhang, B. P., et al. (2019). Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson's disease mice via gut microbiota and metabolites. Neurotherapeutics 16, 741–760. doi: 10.1007/s13311-019-00719-2
Zhu, W., Gregory, J. C., Org, E., Buffa, J. A., Gupta, N., Wang, Z., et al. (2016). Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124. doi: 10.1016/j.cell.2016.02.011
Keywords: fecal microbiota transplantation, nervous system diseases, gastrointestinal microbiome, neurodegenerative, autoimmunity, gut-brain axis, Parkinson's disease, autism spectrum disorder
Citation: Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, Keller JJ, Kuijper EJ and Contarino MF (2020) Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 10:98. doi: 10.3389/fcimb.2020.00098
Received: 23 December 2019; Accepted: 26 February 2020;
Published: 24 March 2020.
Edited by:
Andrew T. Gewirtz, Georgia State University, United StatesReviewed by:
Gianluca Ianiro, Agostino Gemelli University Polyclinic, ItalyCopyright © 2020 Vendrik, Ooijevaar, de Jong, Laman, van Oosten, van Hilten, Ducarmon, Keller, Kuijper and Contarino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduard J. Kuijper, ZWprdWlqcGVyQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.