- 1Collaborative to Halt Antibiotic-Resistant Microbes, Department of Pediatrics, University of California, San Diego, La Jolla, CA, United States
- 2Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, La Jolla, CA, United States
Group B Streptococcus (GBS) is a common cause of bacterial urinary tract infections (UTI) in susceptible populations, including pregnant women and the elderly. However, the factors that govern GBS persistence and disease severity in this niche are not fully understood. Here, we report that the presence of the fungus Candida albicans, a common urogenital colonizer, can promote GBS UTI. Co-inoculation of GBS with C. albicans increased bacterial adherence to bladder epithelium and promoted GBS colonization in vivo in a C. albicans adhesin-dependent manner. This study demonstrates that fungal colonization of the urogenital tract may be an important determinant of bacterial pathogenesis during UTI.
Introduction
Streptococcus agalactiae (group B Streptococcus, GBS) is a common inhabitant of the intestinal and vaginal tract in ~18% of the healthy population and can become pathogenic and cause serious disease in neonates and susceptible adults (Meyn et al., 2009). While the implementation of antenatal screening and treatment of maternal GBS colonization prior to birth has significantly reduced the incidence of early-onset neonatal disease, GBS infection in adult populations remains a persistent problem, particularly with respect to urinary tract infection (UTI). Although the presence of GBS in the urine can be asymptomatic, GBS bacteriuria in certain populations can lead to UTI, pyelonephritis, and systemic infection. For example, in the elderly, where the incidence of invasive GBS has increased two- to fourfold in the past 20 years, 39% of GBS bacteremia cases are associated with concurrent GBS bacteriuria (Edwards and Baker, 2005). In pregnancy, GBS bacteriuria increases risk for intrapartum fever, chorioamnionitis, preterm delivery, and vertical transmission of the pathogen to the newborn (Patras and Nizet, 2018). Despite these risks associated with GBS bacteriuria, the mechanisms that drive GBS colonization and pathogenesis in the urinary tract remain poorly understood. The virulence regulator CovR and capsule production are both important during systemic infection and are also crucial for the establishment of bladder colonization (Kline et al., 2011; Kulkarni et al., 2013). However, another well-characterized virulence factor, β-hemolysin/cytolysin, may be dispensable under certain conditions, suggesting that GBS pathogenesis in the urinary tract may be unique compared to other tissues (Kulkarni et al., 2013; Leclercq et al., 2016; Sullivan et al., 2017). Recently, there has been a paradigm shift in the historical understanding that urine is typically sterile. In particular, altered bladder microbiota are observed in lower urinary tract pathologies including symptomatic UTI and incontinence (Nienhouse et al., 2014; Pearce et al., 2014). These data underscore that while a single primary organism, such as GBS, may be the underlying cause of clinical UTI symptoms, infection occurs in a polymicrobial environment that can influence pathogenesis and host response. For example, concurrent exposure of GBS promotes persistent uropathogenic E. coli (UPEC) infection of the urinary tract via modulation of the immune response (Kline et al., 2012). However, significant gaps still exist in our understanding of how the presence or absence of specific microbial organisms during initial inoculation, colonization, and infection may influence the course of disease.
Candida albicans is an opportunistic fungal pathogen estimated to colonize the urogenital tracts of 30% of women (Achkar and Fries, 2010). While certain conditions can predispose a patient toward pathogenic candiduria, such as age, pregnancy, diabetes mellitus, and catheterization, the presence of C. albicans in the urine is usually asymptomatic (Kauffman, 2005). Yet, C. albicans directly interacts with a number of bacterial uropathogens such as E. coli, K. pneumoniae, and GBS (Centeno et al., 1983; Levison and Pitsakis, 1987; Pidwill et al., 2018). In mucosal environments, such as the mouth and vaginal tract, C. albicans-bacterial interaction influences bacterial colonization, susceptibility to antibiotics, biofilm formation, and host responses (Allison et al., 2016), suggesting candiduria may also influence the pathogenesis of bacterial UTI. Moreover, GBS and C. albicans are frequently co-isolated from the vaginal tract, and synergistic interaction between GBS and C. albicans has been described during vaginal epithelial adherence in vitro (Bayó et al., 2002; Altoparlak et al., 2004; Pidwill et al., 2018). In this study, we provide evidence that candiduria can influence GBS colonization of the bladder by enhancing GBS interaction with the bladder epithelium.
Materials and Methods
Fungal Strains
Strains and sources of C. albicans used in this study are as follows: wild-type (WT) SC5314 (American Type Culture Collection, ATCC MYA2876), reference strain DAY185 (Nobile et al., 2008), als3Δ/Δ-mutant CAYF178U (Nobile et al., 2006), and the ALS3-complemented strain CAQTP178U (Nobile et al., 2006). Strains were inoculated into yeast-peptone-extract (YPD) broth (Difco #242720) and passaged twice at 30°C shaking before use in an experiment. On the day of experiment, overnight cultures (14–16 h) were washed twice in sterile phosphate-buffered saline (PBS) before preparing the infectious dose. Cell numbers were determined by counting on a hemocytometer. For all experiments involving C. albicans CFU counts, samples (lysates or mouse organs) were plated on Sabouraud Dextrose Agar with 75 mg/L chloramphenicol (SDA; Difco #210950) and incubated for 24–36 h at 30°C. For C. albicans cells labeled with CellTracker Blue (Thermo Fisher), yeast cells were washed once in PBS, resuspended at a concentration of 2 × 107 cells/mL, and incubated with 30 μM CellTracker Blue for 20 min at 30°C, shaking. After incubation, cells were washed twice in PBS, then resuspended in RPMI prior to infection of HTB-9 cells.
Bacterial Strains
The human serotype III group B Streptococcus strain COH1 (ATCC #BAA-1176) was used for all experiments. The GFP-expressing COH1 strain has been previously described (Cutting et al., 2014). An overnight culture of GBS strain COH1 was diluted 1:10 in 3 mL Todd-Hewitt broth (THB, Hardy Diagnostics #7161D) and incubated at 37°C until mid-log phase (defined as an OD600 of 0.4, ~2 × 108 cells/mL). Erythromycin (5 μg/mL) was added to maintain the plasmid when growing the GFP-expressing GBS. At log-phase, cultures were centrifuged at 3,000×g for 5 min and washed in PBS prior to preparing samples for infection. For all experiments involving GBS CFU counts, samples were plated on CHROMagar StrepB plates (CHROMagar; DRG International, #SB282) and incubated for 24 h at 37°C.
Animal Care
All animal experiments were conducted under veterinary supervision and approved by the University of California, San Diego Institutional Animal Care and Use Committee (IACUC). Eight- to ten-week-old female C57Bl6/J mice were purchased from Jackson Laboratories and allowed to acclimatize for 48 h before experiments. Mice were allowed to eat and drink ad libitum. All efforts were made to minimize suffering of animals employed in this study.
In vivo Urinary Tract Infection
GBS or C. albicans (1 × 107 CFU) in 100 μL PBS was prepared for mono-infection experiments. For co-infection, GBS (1 × 107 CFU) and C. albicans (1 × 107 CFU) were prepared together in a total volume of 100 μl PBS. Mice were infected via transurethral inoculation, as described previously (Coady et al., 2018). Twenty-four hours after infection urine was collected and plated for C. albicans and GBS CFU enumeration. Mice were humanely euthanized via CO2 asphyxiation, and bladders and kidneys were removed and homogenized in PBS using a MagNa Lyser (Roche). Organ lysates were serially diluted and plated for CFU enumeration of C. albicans and GBS.
Adherence Assay
The human bladder epithelial cell line 5637 (ATCC HTB-9) was grown in RPMI-1640 with glutamine and 10% heat-inactivated fetal bovine serum (FBS). Cells were seeded in 24-well plates and grown to confluence overnight (~1 × 105 cells/well). On the day of infection, HTB-9 cells were washed in PBS and media replaced with 400 μL of fresh RPMI-1640 (without FBS). Next, GBS (1 × 106 CFU), C. albicans (1 × 106 CFU), or GBS (1 × 106 CFU) with C. albicans (1 × 106 CFU) were prepared in 100 μL RPMI-1640 and added to cells, resulting in a multiplicity of infection (MOI) of 10 for all organisms. Infected cells were centrifuged at 300 × g for 5 min, then incubated at 37°C with CO2 for 45 min. After incubation, cells were washed 6 × with PBS, incubated in 0.25% trypsin for 10 min, and lysed by vigorous pipetting in 0.025% Triton-X in PBS. Lysate was plated for CFU enumeration of C. albicans and GBS. For experiments involving fixed epithelial cells, HTB-9 cells were fixed for 15 min in 4% paraformaldehyde prior to infection as described above.
Visualization of GBS and C. albicans in Murine Bladders
Mice were infected with 1 × 107 CFU of GFP-expressing COH1 GBS and C. albicans. Mice were euthanized 2 h post infection (as early as logistically feasible) in order to yield the highest possible number of organisms to visualize. Bladders were harvested, stretched, and fixed in 10% neutral-buffered formalin for 24 h and washed in PBS. Bladders were blocked for 15 min in 1% bovine serum albumin (BSA) in PBS prior to staining with 10 μg/mL anti-C. albicans antibody (ThermoFisher, #PA1-27158) for 30 min. Bladders were washed in PBS and stained with 5 μg/mL Alexa Fluor 594 goat anti-rabbit IgG secondary antibody (ThermoFisher, #A-11012) for 30 min, before incubating with 2 μM Hoechst 33342 Solution (ThermoFisher, #62249) for 15 min. Bladders were visualized immediately after staining on a Zeiss AxioObserver D1 microscope.
Visualization of GBS and C. albicans Adherence to Bladder Epithelium in vitro
The human bladder epithelial cell line HTB-9 was grown in RPMI-1640 with glutamine and 10% heat-inactivated FBS. Cells were seeded in 24-well plates that contained sterile 15 mm glass coverslips (Fisherbrand) and grown to confluence overnight (~1 × 105 cells/well). On the day of infection, HTB-9 cells were washed in PBS and media replaced with 400 μL of fresh RPMI-1640 (without FBS). Next, HTB-9 cells were infected with GFP-expressing GBS strain COH1 (GFP-GBS), C. albicans pre-labeled with 2 μM of CellTracker Blue (CTB-Ca), or GFP-GBS with CTB-Ca. For all experiments, the MOI of GFP-GBS was 10 with the CTB-Ca MOI changed as indicated (MOI of 10 or 1). Cells were centrifuged at 300 × g for 5 min, then incubated at 37°C with CO2 for 45 min. After incubation, cells were washed 6×with PBS, fixed for 20 min in 1% paraformaldehyde, and mounted on glass slides with ProLong Diamond AntiFade Mountant (Thermo Fisher). Coverslips were imaged with a Leica SP8 Super Resolution Confocal microscope and attached GFP-GBS and CTB-Ca were quantified using ImageJ.
Statistical Analysis
All in vitro experiments were performed in at least three independent replicates, except for the microscopy experiments, which were repeated twice. All in vivo experiments were performed using at least five mice per group and repeated in at least two independent replicates. Statistical analyses were conducted using GraphPad Prism, version 8.3.0 (GraphPad Software Inc., La Jolla, CA). Mean values from technical replicates were used for statistical analyses, with independent experiment values or biological replicates represented in graphs with medians with or without interquartile ranges as indicated in figure legends. Statistical tests performed include nonparametric Mann-Whitney or Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn's multiple-comparison.
Results
C. albicans Increases GBS Colonization of the Murine Bladder
To determine how C. albicans influences the pathogenesis of GBS UTI, we employed a murine model of polymicrobial infection where GBS and C. albicans are co-inoculated via transurethral injection. Female C57Bl/6J mice were infected with 1 × 107 CFU GBS strain COH1, 1 × 107 CFU WT C. albicans SC5314, or both organisms mixed together. Analysis of bacterial colonization in the bladders of mice at 24 h post-infection revealed that the GBS burden in mice co-infected with GBS and C. albicans was significantly higher (median: 2,400 CFU/bladder) than mice infected with GBS alone (median: 240 CFU/bladder, P = 0.0034) (Figure 1A). Interestingly, bacterial urine CFUs and colonization of the kidney were not enhanced by coinfection with C. albicans (Figures 1B,C). In contrast to bacterial colonization, coinfection had no measurable impact on C. albicans colonization of the bladder, urine, or kidney (Figures 1D–F). Collectively, these data suggest that the presence of C. albicans during GBS infection of the urinary tract promotes bacterial but not fungal colonization of the bladder.
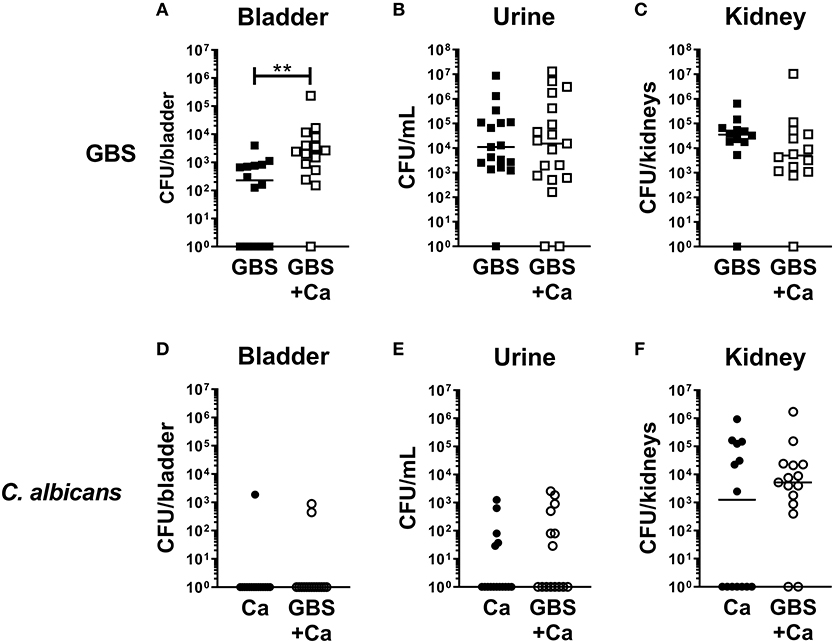
Figure 1. Presence of C. albicans during urinary tract infection increases GBS colonization of the bladder. Female C57Bl/6J mice were infected with 1 × 107 CFU bacteria, fungi or a mixed culture of bacteria and fungi via transurethral inoculation. At 24 h, bladder, urine, and kidneys were harvested and assessed for bacterial (A–C) and fungal (D–F) growth. Squares represent individual mice, and lines represent the median for each group. Experiments were done three times independently and data combined (n = 14–15 mice per group). Statistical analysis between samples was performed using Mann-Whitney test. Significant comparisons are indicated; all others are not significant. **P < 0.01.
C. albicans Promotes GBS Adherence to Bladder Epithelium
To investigate if C. albicans and GBS interact in the bladder in vivo, we visually examined mouse bladders of mice co-infected with WT C. albicans SC5314 and GFP-expressing GBS (Cutting et al., 2014). We found instances where GBS was in close proximity to C. albicans hyphae (Figure 2A). To quantify if the presence of C. albicans can influence GBS interaction with the bladder epithelium, we utilized an in vitro infection model of the human bladder epithelial cell line HTB-9. When co-infected with WT C. albicans SC5314, GBS showed significantly increased adherence to HTB-9 cells (median: 3.6 × 105 CFU) compared to GBS mono-infected cells (median: 1.2 × 105 CFU, P = 0.0286) (Figure 2B). In accordance with our in vivo results, no increase in C. albicans adherence to bladder epithelium was observed with GBS co-infection (Figure 2C). To determine if increased GBS adherence depended upon a bladder epithelial response, adherence to fixed HTB-9 cells during coinfection was measured. Although these data demonstrated a moderate increase in GBS adherence to fixed cells upon coinfection with WT C. albicans SC5314 (Figure 2D, fixed cells, GBS alone median: 1.38 × 105 CFU, GBS+WT C. albicans median: 1.81 × 105 CFU, P = 0.0725), C. albicans promotion of GBS adherence was reduced compared to that seen with live HTB-9 cells (Figure 2D, live cells, GBS alone median: 8 × 104 CFU, GBS + C. albicans median: 2.06 × 105 CFU, P = 0.0007). This reduction in GBS adherence between coinfected live and fixed cells was not significant (Live GBS + C. albicans median: 2.06 × 105 CFU, fixed GBS + C. albicans median: 1.81 × 105, P = 0.1464), nor did C. albicans adherence change under any condition tested (Figure 2E). Collectively, our data suggest that the presence of C. albicans promotes bacterial colonization by increasing GBS adherence to bladder epithelium in a manner that is largely dependent on the host response.
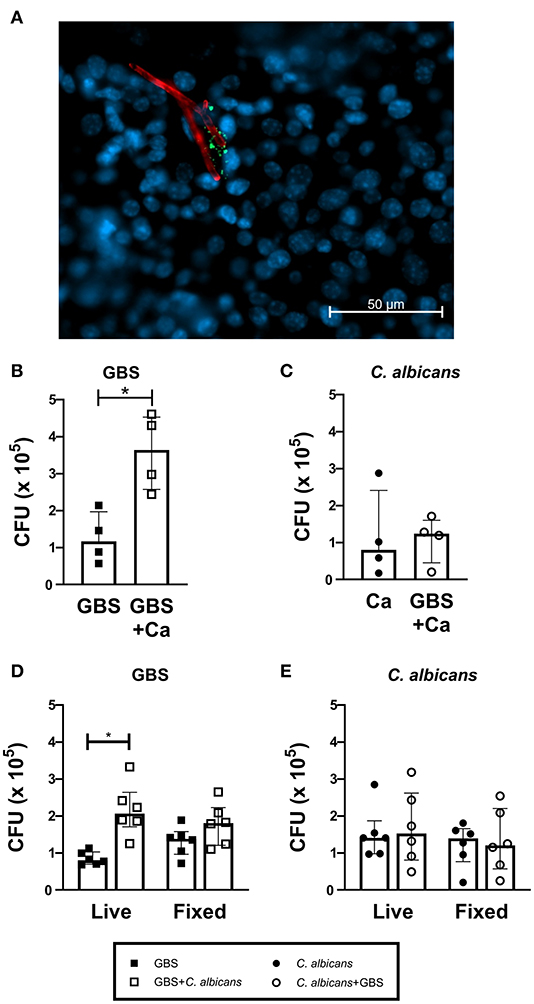
Figure 2. C. albicans and GBS coinfection increases bacterial adherence to bladder cells. (A) WT C. albicans SC5314 and GBS associate in vivo. C57Bl6/J female mice were infected with C. albicans and GFP-expressing GBS. Two hours later, bladders were harvested, fixed and stained for visualization via fluorescent microscopy. GFP-expressing GBS (green), AF594-anti-Ca antibody (red), and Hoechst dye (blue). Adherence of WT C. albicans SC5314 (B) and GBS (C) to human bladder epithelial cell line HTB-9 with single organism or mixed (GBS + C. albicans) inoculum. (D) GBS and (E) WT C. albicans SC5314 adherence of to live or fixed HTB-9 cells with single organism or mixed (GBS + C. albicans) inoculum. Data represent the means from four to five independent experiments performed in technical duplicate and are expressed as medians with interquartile ranges. Statistical analysis between experiments was performed using the Mann-Whitney test *P < 0.05.
The C. albicans Adhesin Protein Als3 Mediates Increased GBS Adherence to Bladder Epithelium
In vaginal colonization, the fungal adhesin Als3 has been shown to mediate C. albicans:GBS interaction and C. albicans adherence to the epithelium (Pidwill et al., 2018). To assess if this adhesin also facilitates C. albicans:GBS interaction during bladder colonization, we compared the ability of an Als3-deficient C. albicans strain (als3Δ/Δ) and a WT C. albicans reference strain DAY185 to promote GBS adherence to bladder epithelium in our HTB-9 in vitro co-infection model. Consistent with our findings using WT C. albicans SC5314, GBS adherence was significantly enhanced in the presence of DAY185 (GBS alone median: 1.4 × 105 CFU, GBS + DAY185 median: 4.2 × 105 CFU, P = 0.0089, Figure 3A), while GBS adherence was not impacted when co-cultured with the als3Δ/Δ mutant (median: 1.7 × 105 CFU, P > 0.9999). Additionally, ALS3-complementation of the als3Δ/Δ mutant restored the WT phenotype (median: 3.5 × 105 CFU, P = 0.0347, Figure 3A). The als3Δ/Δ mutant showed decreased adherence (als3Δ/Δ alone median: 3.75 × 104 CFU) to HTB-9 cells compared to WT C. albicans DAY185 (Figure 3B, DAY185 median: 1.01 × 105 CFU, P = 0.0029). Coinfection with GBS did not alter adherence of either WT C. albicans DAY185 or the als3Δ/Δ mutant to HTB-9 cells (Figure 3B). To examine whether the difference observed in GBS adherence was facilitated by GBS binding to C. albicans or directly to the bladder epithelial cells, we visualized and quantified the amount of GBS adhered to HTB-9 cells directly, as well as the amount of adhered GBS associated with WT C. albicans DAY185 or the als3Δ/Δ mutant. We found a greater number of GBS adhering to bladder cells when coinfected with WT C. albicans DAY185 than with the als3Δ/Δ mutant (Figures 3C–G). In line with a role for Als3 in promoting Candida:GBS interactions, the percent of adherent GBS associated with C. albicans in als3Δ/Δ mutant was also significantly reduced compared to WT DAY185 (Figure 3G). As the als3Δ/Δ mutant is known to display decreased fungal adherence to bladder epithelium, we also repeated these experiments with a lower MOI of WT DAY185 (MOI = 1) to achieve a similar adherence to the als3Δ/Δ mutant (MOI = 10) (Supplemental Figure 1A). In this setting, we found that even at similar levels of C. albicans colonization, WT DAY185 (MOI = 1) had significantly more total GBS and Candida-associated GBS adherence than the als3Δ/Δ mutant (MOI = 10) coinfection (Supplemental Figures 1B,C). In addition, even when accounting for differences in fungal adherence by lowering the MOI, the percent of adherent GBS associated with C. albicans in WT DAY185 (MOI = 1) coinfection was higher than that with als3Δ/Δ mutant (MOI = 10) (Supplemental Figure 1D). Our data suggest that Als3 contributes to GBS:C. albicans association during infection of bladder epithelium and is important in increasing C. albicans-dependent GBS adherence to bladder epithelium.
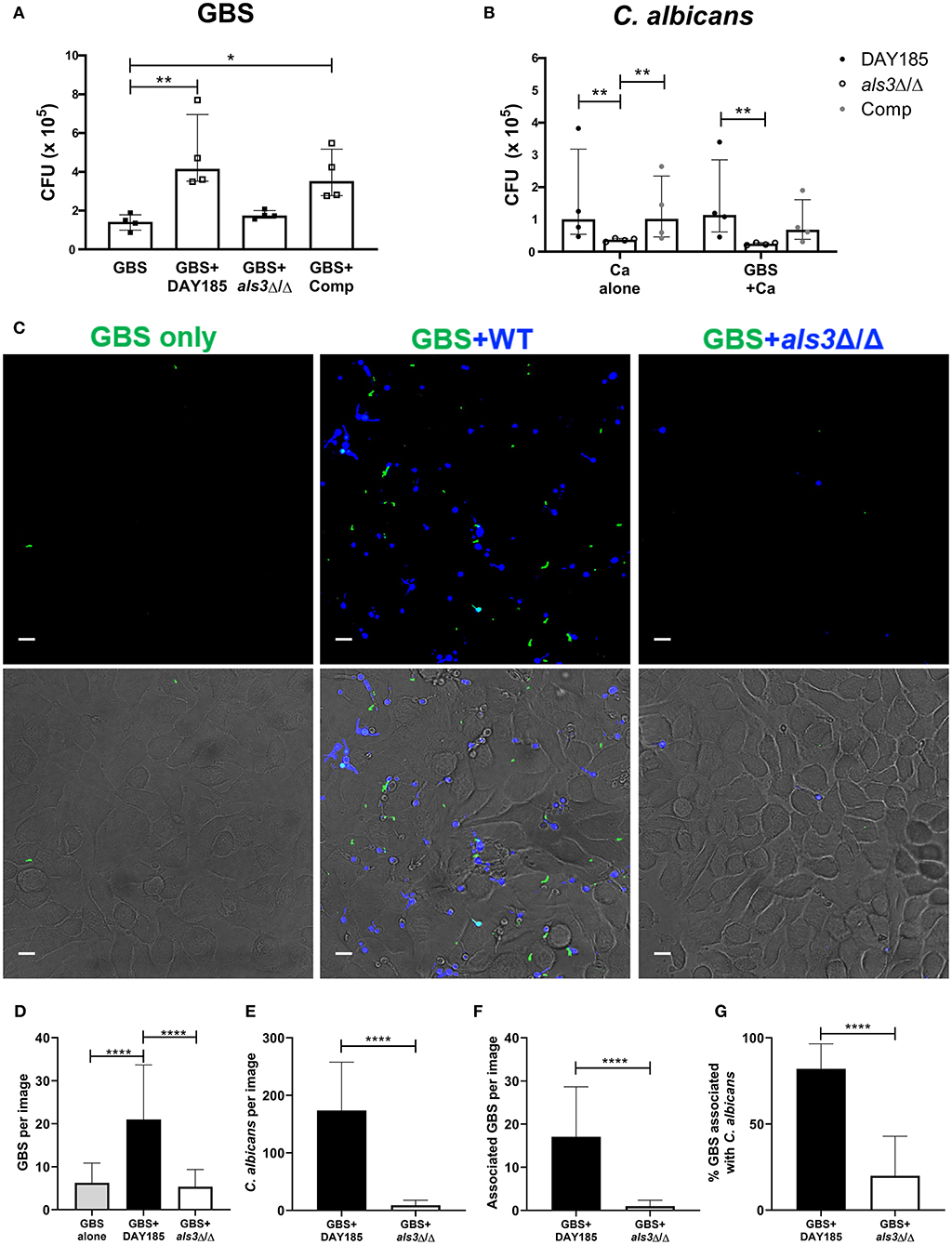
Figure 3. The fungal adhesin Als3 mediates adherence to bladder epithelium. (A) GBS recovery from HTB-9 bladder cells when infected alone or with wild-type (DAY185), als3Δ/Δ mutant, or ALS3-complemented (Comp) C. albicans. (B) Wild-type (DAY185), als3Δ/Δ mutant or ALS3-complemented (comp) C. albicans adherence to HTB-9 cells when infected alone or with GBS. Data represent the means from four independent experiments performed in technical duplicate and are expressed as medians with interquartile ranges. (C–G) HTB-9 cells were infected with GFP-expressing GBS only (green) or with GBS and CellTracker Blue-labeled C. albicans strains (blue). (C) Representative binding of GBS and C. albicans to HTB-9 cells. Merged images of green and blue fluorescence are shown in the top panel and merged fluorescence and phase images are shown in the bottom panel, scale bar = 10 μm. Data represents the quantification of (D) average GBS, (E) C. albicans, and (F) Candida-associated GBS in 15 fields/sample. (G) Percentage of total GBS that associates with indicated C. albicans strain. Data represents the combined image counts of two experiments performed in technical duplicate and are expressed as the mean with standard deviation. Statistical analysis was performed using the Kruskal-Wallis ANOVA with Dunn's multiple comparison or Mann-Whitney test, *P < 0.05, **P < 0.01, ****P < 0.0001.
To validate the importance of Als3 for in vivo colonization, we performed transurethral co-inoculation of GBS with DAY185 or als3Δ/Δ C. albicans. Although not statistically significant, mice inoculated with WT C. albicans DAY185 and GBS display a trend toward increased GBS colonization (Figure 4A, GBS + DAY185 median: 1800 CFU) compared to mice infected with GBS alone (GBS alone median: 1030 CFU, P = 0.0657). als3Δ/Δ C. albicans failed to promote GBS bladder colonization (Figure 4A, GBS + als3Δ/Δ median: 590, P = 0.770). No difference in bacterial colonization was observed in the urine or kidneys of coinfected mice compared to mice infected with GBS alone (Figures 4B,C). Levels of C. albicans colonization of the bladder, kidneys, and urine for both WT DAY185 and the als3Δ/Δ mutant were similar to colonization levels observed for WT C. albicans SC5314 (Figures 4D–F). Collectively, the work outlined in this manuscript demonstrates that the presence of C. albicans during GBS UTI promotes bacterial colonization of the bladder by increasing GBS adherence to the bladder epithelium.
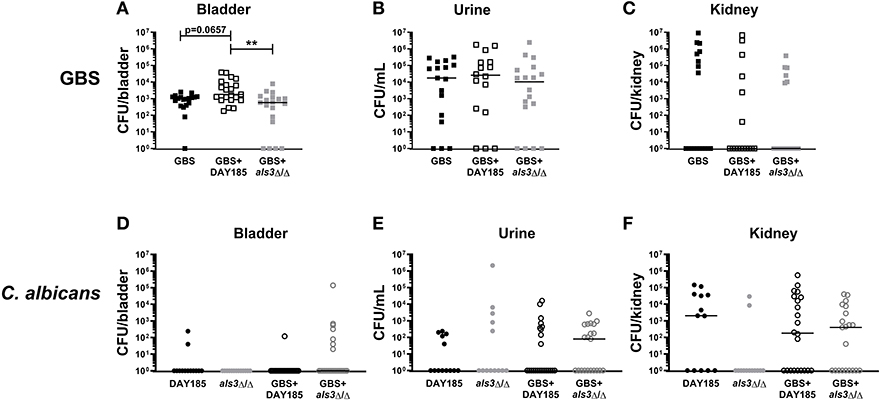
Figure 4. Loss of Als3 decreases bacterial colonization of bladder during in vivo coinfection. Mice were infected with GBS alone, C. albicans DAY185 + GBS, or C. albicans als3Δ/Δ + GBS. At 24 h, bladder, urine and kidneys were harvested and plated for GBS CFUs (A–C) and C. albicans CFU (D–F). Infections were performed three times independently and data combined (n = 16–18 mice per group). Statistical analysis between experiments was performed using the Kruskal-Wallis ANOVA with Dunn's multiple comparison **P < 0.01.
Discussion
Historically, UTI have been studied and treated as monomicrobial; however, this may not accurately reflect the diverse microbial communities present in the urinary tract and in microbial reservoirs, such as the gastrointestinal and vaginal tracts. Here, using both in vitro and in vivo models of coinfection, we show that bacterial co-inoculation with C. albicans promotes GBS bladder colonization through increased adherence to the bladder epithelium. Our work suggests increased adherence involves the C. albicans adhesin Als3, a protein previously described to directly promote GBS:C. albicans interaction (Pidwill et al., 2018). Importantly, this work expands our understanding of how the presence of distinct microbial organisms can influence bacterial colonization and UTI pathogenesis.
Adherence is a critical first step in microbial colonization of the host for both pathogenic and commensal organisms. Interaction between microbial species is one of numerous identified strategies used by both bacteria and fungi to increase adherence to host substrates. For example, C. albicans can associate with multiple Streptococcus and Staphylococcus species, aiding in their adherence and biofilm-formation (Lohse et al., 2017; Koo et al., 2018). For some streptococcal species, like S. gordonii, bacterial surface adhesins bind directly to Ca-Als3 to mediate bacterial-fungal interaction (Silverman et al., 2010). GBS also interacts with C. albicans through recognition of adhesins. In particular, Als3 can bind GBS Bsp proteins, and this interaction is critical for the ability of C. albicans to promote GBS binding to vaginal epithelium (Pidwill et al., 2018). Although multiple Bsp proteins have been described in invasive isolates of GBS (Rego et al., 2016), the presence and diversity of Bsp proteins in urinary isolates of GBS have not been described. Future studies will be necessary to discern if GBS adherence to C. albicans and the epithelium during bladder infection is mediated through specific Bsp proteins.
In this study, we confirmed that Als3 expression is critical for C. albicans binding to bladder epithelium (Coady et al., 2018). Our data also support a role for Ca-Als3 in facilitating GBS adhesion to bladder epithelium. While we observed an increase in GBS associated with WT Candida during infection of bladder epithelial cells (Figure 3), we also found that the C. albicans-induced increase in GBS adhesion to bladder epithelial cells was diminished when the cells were fixed (Figure 2). This result suggests that C. albicans promotes GBS adherence through a manner dependent on both Candida Als3 expression and induction of a host response in the bladder epithelium. However, the extent to which these two phenomena are linked remains uncertain, and the nature of the host response and whether it depends on Als3-driven interactions between GBS and Candida are outstanding questions. Both in vitro and in vivo interaction of C. albicans with epithelial cells induces invasion, cellular damage, and a pro-inflammatory response, largely through the action of the cytolytic peptide toxin candidalysin (Moyes et al., 2016; Naglik et al., 2017). Likewise, GBS deficient in either production or regulation of β-hemolysin/cytolysin are less able to colonize the urogenital tract (Kulkarni et al., 2013; Patras et al., 2013; Leclercq et al., 2016; Sullivan et al., 2017). These data suggest that pathogen control of host cell damage and inflammation are important factors in determining bladder persistence. The mechanisms that influence C. albicans-dependent changes in the host environment to promote GBS colonization remain a research area of interest.
In contrast to adherence to the vaginal epithelium, the GBS:C. albicans interaction did not appear to promote C. albicans adhesion to bladder cells or increase C. albicans colonization of the bladder, indicating that the interaction is not mutually synergistic. This may be an accurate reflection of what occurs in humans, since C. albicans is a common commensal of the gastrointestinal and vaginal tract but rarely observed in the urine of healthy humans. While C. albicans may be introduced into the urinary tract with GBS, it likely results in transient colonization. Populations at risk for infection by C. albicans and GBS include pregnant women, the elderly, diabetes mellitus patients, and catheterized patients (Edwards and Baker, 2005; Achkar and Fries, 2010), but in these susceptible patients, candiduria is often considered benign and non-significant, and thus may be under-reported (Kauffman, 2005). As such, although C. albicans has not been clinically implicated in complications involving GBS, our work suggests that the urogenital carriage of Candida can promote the colonization of GBS, and thus may increase disease incidence and/or severity in these patients. Understanding the associative roles of C. albicans and other colonizing microbes in urine may reveal additional complementary interactions that play a role in establishing UTI.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Author Contributions
SS and AC planned and performed experiments, analyzed data, and wrote the manuscript. ARR, AMR, SM, and KP performed experiments. KP, AMR, and VN provided input into experimental planning and edited the manuscript.
Funding
AC was supported through a postdoctoral fellowship from the A. P. Giannini Foundation, and KP was supported through a postdoctoral fellowship from the Hartwell Foundation. Studies were supported by NIH grants U54-HD090259 and U01-AI124316 to VN.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Scott Filler for providing the C. albicans mutant strains used in this study, and to Dr. Morgan Truitt for helpful discussion and manuscript review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00437/full#supplementary-material
References
Achkar, J. M., and Fries, B. C. (2010). Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 23, 253–273. doi: 10.1128/CMR.00076-09
Allison, D. L., Willems, H. M. E., Jayatilake, J. A. M. S., Bruno, V. M., Peters, B. M., and Shirtliff, M. E. (2016). Candida-bacteria interactions: their impact on human disease. Microbiol. Spectr. 4:3. doi: 10.1128/microbiolspec.VMBF-0030-2016
Altoparlak, U., Kadanali, A., and Kadanali, S. (2004). Genital flora in pregnancy and its association with group B streptococcal colonization. Int. J. Gynaecol. Obstet. 87, 245–246. doi: 10.1016/j.ijgo.2004.08.006
Bayó, M., Berlanga, M., and Agut, M. (2002). Vaginal microbiota in healthy pregnant women and prenatal screening of group B streptococci (GBS). Int. Microbiol. 5, 87–90. doi: 10.1007/s10123-002-0064-1
Centeno, A., Davis, C. P., Cohen, M. S., and Warren, M. M. (1983). Modulation of Candida albicansattachment to human epithelial cells by bacteria and carbohydrates. Infect. Immun. 39, 1354–1360.
Coady, A., Ramos, A. R., Olson, J., Nizet, V., and Patras, K. A. (2018). Tamm-horsfall protein protects the urinary tract against Candida albicans. Infect. Immun. 86, e00451–18. doi: 10.1128/IAI.00451-18
Cutting, A. S., Del Rosario, Y., Mu, R., Rodriguez, A., Till, A., Subramani, S., et al. (2014). The role of autophagy during group B Streptococcus infection of blood-brain barrier endothelium. J. Biol. Chem. 289, 35711–35723. doi: 10.1074/jbc.M114.588657
Edwards, M. S., and Baker, C. J. (2005). Group B streptococcal infections in elderly adults. Clin. Infect. Dis. 41, 839–847. doi: 10.1086/432804
Kline, K. A., Schwartz, D. J., Gilbert, N. M., Hultgren, S. J., and Lewis, A. L. (2012). Immune modulation by group B Streptococcus influences host susceptibility to urinary tract infection by uropathogenic Escherichia coli. Infect. Immun. 80, 4186–4194. doi: 10.1128/IAI.00684-12
Kline, K. A., Schwartz, D. J., Lewis, W. G., Hultgren, S. J., and Lewis, A. L. (2011). Immune activation and suppression by group B Streptococcus in a murine model of urinary tract infection. Infect. Immun. 79, 3588–3595. doi: 10.1128/IAI.00122-11
Koo, H., Andes, D. R., and Krysan, D. J. (2018). Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 14:e1007342. doi: 10.1371/journal.ppat.1007342
Kulkarni, R., Randis, T. M., Antala, S., Wang, A., Amaral, F. E., and Ratner, A. J. (2013). β-Hemolysin/cytolysin of Group B Streptococcus enhances host inflammation but is dispensable for establishment of urinary tract infection. PLoS ONE 8:e59091. doi: 10.1371/journal.pone.0059091
Leclercq, S. Y., Sullivan, M. J., Ipe, D. S., Smith, J. P., Cripps, A. W., and Ulett, G. C. (2016). Pathogenesis of Streptococcus urinary tract infection depends on bacterial strain and β-hemolysin/cytolysin that mediates cytotoxicity, cytokine synthesis, inflammation and virulence. Sci. Rep. 6:29000. doi: 10.1038/srep29000
Levison, M. E., and Pitsakis, P. G. (1987). Susceptibility to experimental Candida albicans urinary tract infection in the rat. J. Infect. Dis. 155, 841–846. doi: 10.1093/infdis/155.5.841
Lohse, M. B., Gulati, M., Johnson, A. D., and Nobile, C. J. (2017). Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 16:19. doi: 10.1038/nrmicro.2017.107
Meyn, L. A., Krohn, M. A., and Hillier, S. L. (2009). Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am. J. Obstet. Gynecol. 201, 76.e1–7. doi: 10.1016/j.ajog.2009.02.011
Moyes, D. L., Wilson, D., Richardson, J. P., Mogavero, S., Tang, S. X., Wernecke, J., et al. (2016). Candida lysin is a fungal peptide toxin critical for mucosal infection. Nature 532, 64–68. doi: 10.1038/nature17625
Naglik, J. R., König, A., Hube, B., and Gaffen, S. L. (2017). Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 40, 104–112. doi: 10.1016/j.mib.2017.10.030
Nienhouse, V., Gao, X., Dong, Q., Nelson, D. E., Toh, E., McKinley, K., et al. (2014). Interplay between bladder microbiota and urinary antimicrobial peptides: mechanisms for human urinary tract infection risk and symptom severity. PLoS ONE 9:e114185. doi: 10.1371/journal.pone.0114185
Nobile, C. J., Andes, D. R., Nett, J. E., Smith, F. J., Yue, F., Phan, Q.-T., et al. (2006). Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. doi: 10.1371/journal.ppat.0020063
Nobile, C. J., Schneider, H. A., Nett, J. E., Sheppard, D. C., Filler, S. G., Andes, D. R., et al. (2008). Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 18, 1017–1024. doi: 10.1016/j.cub.2008.06.034
Patras, K. A., and Nizet, V. (2018). Group B Streptococcal maternal colonization and neonatal disease: molecular mechanisms and preventative approaches. Front. Pediatr. 6:27. doi: 10.3389/fped.2018.00027
Patras, K. A., Wang, N.-Y., Fletcher, E. M., Cavaco, C. K., Jimenez, A., Garg, M., et al. (2013). Group B Streptococcus CovR regulation modulates host immune signalling pathways to promote vaginal colonization. Cell. Microbiol. 15, 1154–1167. doi: 10.1111/cmi.12105
Pearce, M. M., Hilt, E. E., Rosenfeld, A. B., Zilliox, M. J., Thomas-White, K., Fok, C., et al. (2014). The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 5, e01283–e01214. doi: 10.1128/mBio.01283-14
Pidwill, G. R., Rego, S., Jenkinson, H. F., Lamont, R. J., and Nobbs, A. H. (2018). Coassociation between Group B Streptococcus and Candida albicans promotes interactions with vaginal epithelium. Infect. Immun. 86, e00669-17. doi: 10.1128/IAI.00669-17
Rego, S., Heal, T. J., Pidwill, G. R., Till, M., Robson, A., Lamont, R. J., et al. (2016). Structural and functional analysis of cell wall-anchored polypeptide adhesin BspA in Streptococcus agalactiae. J. Biol. Chem. 291, 15985–16000. doi: 10.1074/jbc.M116.726562
Silverman, R. J., Nobbs, A. H., Vickerman, M. M., Barbour, M. E., and Jenkinson, H. F. (2010). Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect. Immun. 78, 4644–4652. doi: 10.1128/IAI.00685-10
Keywords: Streptococcus agalactiae, Candida albicans, urinary tract infection, polymicrobial infection, fungal-bacterial interaction
Citation: Shing SR, Ramos AR, Patras KA, Riestra AM, McCabe S, Nizet V and Coady A (2020) The Fungal Pathogen Candida albicans Promotes Bladder Colonization of Group B Streptococcus. Front. Cell. Infect. Microbiol. 9:437. doi: 10.3389/fcimb.2019.00437
Received: 20 August 2019; Accepted: 05 December 2019;
Published: 10 January 2020.
Edited by:
Ozlem Yilmaz, Medical University of South Carolina, United StatesReviewed by:
Angela Helen Nobbs, University of Bristol, United KingdomSarah Shabayek, Suez Canal University, Egypt
Copyright © 2020 Shing, Ramos, Patras, Riestra, McCabe, Nizet and Coady. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison Coady, YWNvYWR5QHVjc2QuZWR1
 Samuel R. Shing
Samuel R. Shing Anissa R. Ramos
Anissa R. Ramos Kathryn A. Patras
Kathryn A. Patras Angelica M. Riestra
Angelica M. Riestra Sinead McCabe
Sinead McCabe Victor Nizet
Victor Nizet Alison Coady
Alison Coady