
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 10 September 2019
Sec. Molecular Bacterial Pathogenesis
Volume 9 - 2019 | https://doi.org/10.3389/fcimb.2019.00324
This article is part of the Research Topic Stress Response Mechanisms of Bacterial Pathogens View all 13 articles
 Uchini S. Kosgodage1
Uchini S. Kosgodage1 Paul Matewele1
Paul Matewele1 Brigitte Awamaria1
Brigitte Awamaria1 Igor Kraev2
Igor Kraev2 Purva Warde3
Purva Warde3 Giulia Mastroianni4
Giulia Mastroianni4 Alistair V. Nunn5
Alistair V. Nunn5 Geoffrey W. Guy6
Geoffrey W. Guy6 Jimmy D. Bell5
Jimmy D. Bell5 Jameel M. Inal3
Jameel M. Inal3 Sigrun Lange7*
Sigrun Lange7*Membrane vesicles (MVs) released from bacteria participate in cell communication and host-pathogen interactions. Roles for MVs in antibiotic resistance are gaining increased attention and in this study we investigated if known anti-bacterial effects of cannabidiol (CBD), a phytocannabinoid from Cannabis sativa, could be in part attributed to effects on bacterial MV profile and MV release. We found that CBD is a strong inhibitor of MV release from Gram-negative bacteria (E. coli VCS257), while inhibitory effect on MV release from Gram-positive bacteria (S. aureus subsp. aureus Rosenbach) was negligible. When used in combination with selected antibiotics, CBD significantly increased the bactericidal action of several antibiotics in the Gram-negative bacteria. In addition, CBD increased antibiotic effects of kanamycin in the Gram-positive bacteria, without affecting MV release. CBD furthermore changed protein profiles of MVs released from E. coli after 1 h CBD treatment. Our findings indicate that CBD may pose as a putative adjuvant agent for tailored co-application with selected antibiotics, depending on bacterial species, to increase antibiotic activity, including via MV inhibition, and help reduce antibiotic resistance.
Outer membrane vesicles (OMVs) and membrane vesicles (MVs) are released from Gram-negative and Gram-positive bacteria and participate in inter-bacterial communication, including via transfer of cargo molecules (Dorward and Garon, 1990; Li et al., 1998; Fulsundar et al., 2014; Jan, 2017; Toyofuku et al., 2019). MVs are released in greater abundance from Gram-negative, than Gram-positive bacteria and their production seems crucial for bacterial survival and forms part of the stress response (McBroom and Kuehn, 2007; Macdonald and Kuehn, 2013; Jan, 2017). Gram-negative bacteria generate, in addition to common one-bilayer vesicles (OMV), also double-bilayer vesicles (O-IMVs), and in some stress conditions other types of MVs (Pérez-Cruz et al., 2016) and therefore we will use the umbrella term “membrane vesicles” (MVs) hereafter. MVs are important in biofilm formation and dissemination of toxins in the host (Wang et al., 2015; Cooke et al., 2019). MVs participate in host-pathogen interactions (Gurung et al., 2011; Koeppen et al., 2016; Bitto et al., 2017, 2018; Codemo et al., 2018; Turner et al., 2018; Cecil et al., 2019) and may also be involved in antibiotic resistance, for instance by protecting biofilms from antibiotics via increased vesiculation (Manning and Kuehn, 2011). Furthermore, MVs from Porphyromonas gingivalis have been linked to metabolic remodeling in the host (Fleetwood et al., 2017), while MVs from Neisseria gonorrhoeae have been shown to target host mitochondria and to induce macrophage death (Deo et al., 2018). Besides roles for cellular and bacterial communication, the use of MVs as nano-carriers for various compounds, including for antibiotic and vaccine delivery, has also raised considerable interest in the research community (Gnopo et al., 2017; Rüter et al., 2018; Tan et al., 2018; Wang et al., 2018).
The regulation of bacterial MV biogenesis and release may therefore be of great importance, both in relation to inter-bacterial communication, including biofilm formation, their host interactions as commensals, as well as in host-pathogen interactions and in antibiotic resistance.
Cannabidiol (CBD) is a phytocannabinoid from Cannabis sativa with anti-inflammatory (Martin-Moreno et al., 2011), anti-cancerous (Pisanti et al., 2017; Kosgodage et al., 2018) and anti-bacterial activity (Hernández-Cervantes et al., 2017). While immunoregulatory roles for cannabinoids have been reported in infectious disease (reviewed in Hernández-Cervantes et al., 2017), and C. sativa has been identified as a natural product with a capability of controlling bacterial infections, including a strong anti-bacterial activity against antibiotic resistant strains (Appendino et al., 2008), a link between CBD and bacterial MV release has hitherto not been investigated.
As our recent work identified CBD as a potent inhibitor of extracellular vesicle (EV) release in eukaryotes (Kosgodage et al., 2018; Gavinho et al., 2019), we sought to investigate whether CBD may work via phylogenetically conserved pathways, involving bacterial MV release from bacteria. As we, and other groups, have previously shown that cancer cells can be sensitized to chemotherapeutic agents via various EV-inhibitors (Jorfi et al., 2015; Koch et al., 2016; Muralidharan-Chari et al., 2016; Kosgodage et al., 2017), including CBD (Kosgodage et al., 2018, 2019), we sought to establish whether in bacteria, similar putative MV modulatory effects could be utilized to sensitize bacteria to antibiotics.
E. coli (VCS257, Agilent, La Jolla, CA) and S. aureus subsp. aureus Rosenbach (ATCC 29247, USA) static cultures were grown in Luria-Bertani (LB) broth for 24 h at 37°C. The growth phase before vesicle isolation was exponential; the volume of the cultures was 20 ml. For MV isolation, ultracentrifugation and nanoparticle tracking analysis (NTA) were used based on previously established methods by other groups (McCaig et al., 2013; Klimentova and Stulik, 2015; Roier et al., 2016).
E. coli and S. aureus cultures were maintained by plating on Mueller-Hinton agar plates and weekly sub-culturing was performed according to previously established methods (Iqbal et al., 2013).
Before MV isolation, all bacterial growth medium (LB broth) was pre-treated before use by ultracentrifugation at 100,000 g for 24 h to ensure minimum contamination with extracellular vesicles (EVs) from the medium (Kosgodage et al., 2017).
For MV isolation, bacteria were grown in EV-free medium (as described above) for 24 h at 37°C, the culture medium was collected and centrifuged once at 400 g for 10 min for removal of cells, followed by centrifugation at 4,000 g for 1 h at 4°C to remove cell debris. The resultant supernatant was then centrifuged for 1 h at 100,000 g at 4°C and the isolated MV pellet was resuspended in Dulbecco's phosphate buffered saline (DPBS; ultracentrifuged and sterile filtered using a 0.22 μm filter) and centrifuged again at 100,000 g for 1 h at 4°C. The resulting MV pellet was sterile filtered (0.45 μm) once and then resuspended in sterile filtered DPBS. The quantitative yield of vesicles was ~6.5 × 109 MVs per liter of culture. The isolated MV pellets were then either used immediately, or stored at −80°C for further experiments.
A suspension of isolated MVs (1.4 × 108 MVs/ml) was used for TEM imaging. MV samples (10 μL) were applied to mesh copper grids, prepared with glow discharged carbon support films, and incubated for 2 min. The grids were then washed five times with 50 μl of 1 % aqueous uranyl acetate. Grids were left to dry for 5 min before being viewed. Micrographs were taken with a JEOL JEM 1230 transmission electron microscope (JEOL, Japan) operated at 80 kV at a magnification of 80,000 to 100,000. Digital images were recorded using a Morada CCD camera (EMSIS, Germany) and processed via iTEM (EMSIS).
Protein was isolated from MV pellets using Bacterial Protein Extraction Reagent (B-PER, ThermoFisher Scientific, U.K.), pipetting gently and shaking the pellets on ice for 2 h, where after samples were centrifuged at 16,000 g at 4°C for 20 min and the resulting supernatant collected for protein analysis. Samples were prepared in 2x Laemmli buffer, boiled at 95°C for 5 min, electrophoresed by SDS-PAGE on 4–20 % TGX gels (BioRad, U.K.), followed by semi-dry Western blotting. Approximately 10 μg of protein was loaded per lane and even protein transfer was assessed by Ponceau S staining (Sigma, U.K.). Blocking of membranes was performed for 1 h at room temperature (RT) in 5 % BSA in TBS-T. The membranes were then incubated with the anti-OmpC (Outer-membrane protein C antibody; orb6940, Biorbyt, U.K.; diluted 1/1000 in TBS-T) overnight at 4°C, followed by washing in TBS-T and incubation for 1 h in anti-rabbit-HRP conjugated secondary antibody at RT. Visualization was performed using ECL (Amersham, U.K.) and the UVP BioDoc-ITTM System (U.K.).
MVs were isolated from control and CBD-treated bacterial cultures as described above. Nanoparticle tracking analysis (NTA) was performed using the Nanosight LM10 (Malvern, U.K.), equipped with a 405 nm diode laser and a sCMOS camera. MV pellets were resuspended in equal volumes (100 μl) of DPBS before NTA analysis to ensure comparable analysis of quantification. Before application, samples were diluted 1:50 in sterile-filtered EV-free DPBS and applied at a constant flow rate, maintaining the number of particles in the field of view in the range of 20–40 with a minimum concentration of samples at 5 × 107 particles/ml. Camera settings were according to the manufacturer's instructions (Malvern), five 60 s videos per sample were recorded and replicate histograms averaged. Each experiment was repeated three times.
E. coli and S. aureus cultures were cultivated using EV-free Müeller-Hinton broth for 24 h. An inoculate of 0.1 ml of bacteria, in a 20 ml culture volume of bacterial growth medium (Luria-Bertani (LB) broth), were grown at exponential phase overnight, as assessed by OD600. The bacterial cells were then washed using DPBS at 4,000 g for 10 min and seeded in 1.5 ml triplicates in micro centrifuge tubes. For treatment with CBD, CBD (GW research Ltd) was applied at concentrations of 1 or 5 μM and incubated with the bacterial cultures for 1 h at 37°C. Treatments were performed in triplicates, including DMSO as a control. MV isolation following CBD and control treatment was carried out using step-wise centrifugation and ultracentrifugation as before. Changes in MV release were assessed by quantifying numbers of MVs by NTA analysis as described above, with each experiment repeated three times. Cell viability was assessed before the start of every experiment and after treatment with CBD compared to controls determined by colony forming unit (CFU) measurement.
Discs were impregnated with the following antibiotics (all from Sigma-Aldrich): colistin (10 μg/ml), rifampicin (15 μg/ml), erythromycin (50 μg/ml), kanamycin (1,000 μg/ml) and vancomycin (5 μg/ml). Concentration of the antibiotics used was based on previously published and established MIC values (Maclayton et al., 2006; Moskowitz et al., 2010; Kshetry et al., 2016; Rojas et al., 2017; Goldstein et al., 2018). E. coli and S. aureus agar plates were prepared for the disc diffusion test (Iqbal et al., 2013) by soaking a sterile paper disc in 5 μM CBD and placing it in the middle of the agar plate, while the impregnated antibiotic discs were placed equidistant to the CBD disc. Zones of inhibition were assessed after 24 h using the Kirby-Bauer test.
To assess differences in E. coli VCS257 MV protein composition in response to CBD treatment, MVs were isolated as before, after 1 h treatment with 1 μM or 5 μM CBD treatment or control untreated, respectively. MVs were assessed by SDS-PAGE (using 4–20 % gradient TGX gels, BioRad, U.K.) and silver staining using the BioRad Silver Stain Plus Kit (1610449, BioRad, U.K.), according to the manufacturer's instructions (BioRad). For assessment of proteomic changes, MVs were subjected to liquid chromatography-mass spectrometry (LC-MS/MS) analysis. MVs from CBD treated, vs. non-treated E. coli were run 1 cm into a SDS-PAGE gel and the whole protein lysate cut out as one band, whereafter it was processed for proteomic analysis (carried out by Cambridge Proteomics, U.K.). Peak list files were submitted to Mascot (in-house, Cambridge Center for Proteomics) using the following database: Uniprot_Escherichia_coli_20180613 (4324 sequences; 1357163 residues).
Histograms and graphs were prepared and statistical analysis was performed using GraphPad Prism version 8 (GraphPad Software, San Diego, U.S.A.). One-way ANOVA and Student's t-test analysis were performed, followed by Tukey's post-hoc analysis. Histograms represent mean of data, with error bars representing standard error of mean (SEM). Significant differences were considered as p ≤ 0.05.
Isolated MVs were assessed by morphology using transmission electron microscopy (TEM), revealing a poly-dispersed population in the size range of mainly 20–230 nm in diameter for E. coli, including MVs showing inner and outer membranes (Figure 1A.1), and characteristic one layer membranes for S. aureus MVs, which were in the 37–300 nm range (Figure 1A.2). Nanoparticle tracking analysis (NTA) verified that the majority of the vesicle population fell in a similar size range under standard culture conditions (mode 143.3 nm; SD ± 72.3 nm for E. coli (Figure 1A.1) and 141.4 nm; SD ± 7.3 nm for S. aureus (Figure 1A.2). Furthermore, Western blotting showed positive for the MV specific marker OmpC (Figure 1A).
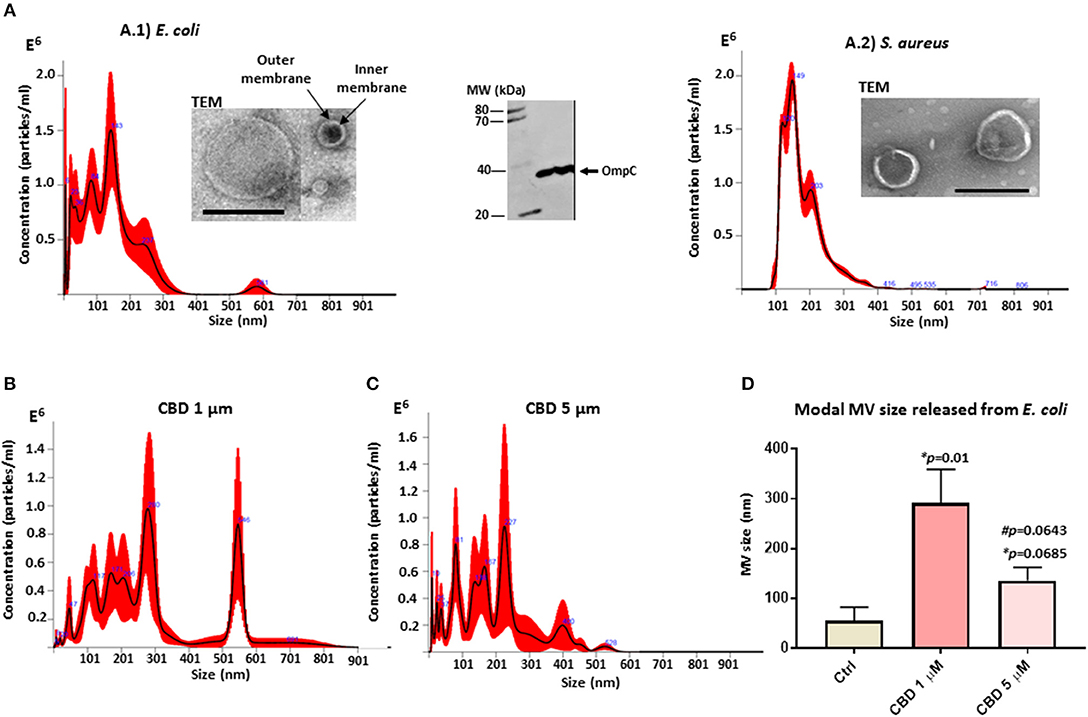
Figure 1. Bacterial MV profile under standard conditions and after CBD treatment. (A) MVs released from untreated E. coli VCS257 (A.1) and S. aureus subsp. aureus Rosenbach (A.2), shown by NTA analysis (Nanosight); Transmission electron microscopy (TEM, scale bar = 200 nm) and Western blotting with the MV-specific marker OmpC. (B) NTA analysis showing MV release from E. coli after 1 h CBD treatment (1 μM). (C) NTA analysis showing MV release from E. coli after 1 h CBD treatment (5 μM). (D) Modal size of MVs released from E. coli under normal culture conditions compared to CBD treatment. Error bars indicate SEM; *p represents p-values compared to control (ctrl) while #p represents p-values compared to 1 μM CBD treatment.
CBD changed the MV release profile from E. coli compared to control treatment (Figures 1B–D). Modal size of MVs released from E. coli was significantly increased (p = 0.01) after 1 μM CBD treatment, compared to control treated cells, while 5 μM CBD treatment did not have statistically significant effects on MV size (p = 0.0685). Effects on modal size of vesicles released from E. coli between the two doses of CBD was also not statistically significant (p = 0.0643; Figure 1D).
CBD had a significant inhibitory effect (p < 0.0001) on total MV release from E. coli VCS257 at both concentrations tested (1 and 5 μM, respectively; Figure 2A). In addition, the lower dose of CBD (1 μM) had stronger MV-inhibitory effects (73 % reduction, p < 0.0001) than 5 μM CBD (54 % reduction, p < 0.0001; Figure 2A) and resulted in a markedly increased peak at 500 nm (Figure 1B), which otherwise was negligible in the control (Figure 1A) and 5 μM CBD (Figure 1C) treated E. coli.
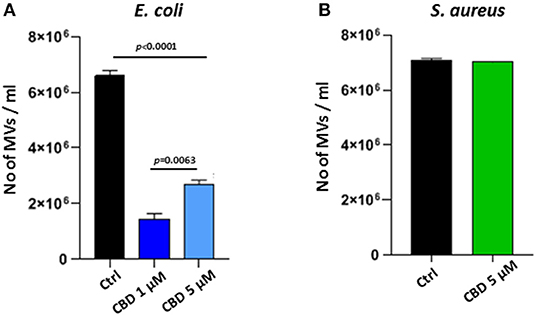
Figure 2. CBD affects MV-release from the Gram-negative bacteria E. coli VCS257 but not Gram-positive S. aureus subsp. aureus Rosenbach. (A) MV release from E. coli was significantly reduced after CBD treatment, with lower dose of CBD being more effective (p = 0.0063). (B) MV release from S. aureus was not significantly affected by CBD treatment. Exact p-values are shown.
Effects of CBD on E. coli VCS257 MVs was furthermore assessed by TEM, verifying the presence of fewer vesicles per field and showing some change in vesicle size and morphology after CBD (Supplementary Figures 2A–C).
Contrary to what was observed for the Gram-negative E. coli, CBD treatment (5 μM) had no significant effect on MV release from the Gram-positive bacterium S. aureus subsp. Aureus Rosenbach (p > 0.1; Figure 2B).
CBD had negligible effect on E. coli cell viability after 24 h incubation with the lower 1 μM dose, while an 11 % (p = 0.0161) reduction in cell viability was observed in response to 5 μM CBD, but no significant effect was observed on S. aureus cell viability, as assessed by disk diffusion test (Supplementary Figure 1).
CBD treatment (5 μM), when applied in combination with a range of antibiotics tested, was found to sensitize E. coli VCS257 to selected antibiotics, as assessed by an increase in the radius of zone of inhibition, using the disk diffusion test (Figure 3). Significantly enhanced antibacterial effects were found for erythromycin (35 % increase; p = 0.006), rifampicin (50 % increase; p = 0.0007) and vancomycin (100 % increase; p < 0.0001), when combined with CBD treatment (5 μM), compared to antibiotic treatment alone. Notably, vancomycin alone did not have bactericidal effects on E. coli, but only in the presence of CBD. Antibacterial effects of kanamycin were increased by 18 % but this was not statistically significant compared to antibiotic alone (p = 0.09). Zone of inhibition with CBD treatment only was also observed in the E. coli plates (Figure 3), but this was significantly lower than when CBD was combined with antibiotics, except for vancomycin. The zone of inhibition for E. coli caused by antibiotic treatment only, vs. CBD alone, differed also significantly for erythromycin (p = 0.0010), vancomycin (p = 0.0158), rifampicin (p = 0.0003) and kanamycin (p = 0.0008), but not for colistin (p = 0.224). Therefore, while CBD showed some anti-bacterial activity against E. coli when applied in isolation, this was significantly lower than observed for the antibiotics alone (except for vancomycin which did not show antibacterial activity while CBD did). However, when applied in combination, CBD increased bactericidal effects of all antibiotics tested, except for colistin.
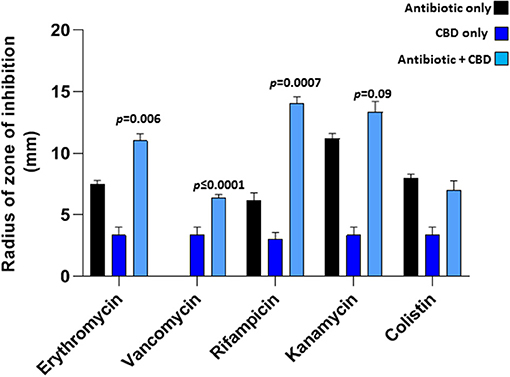
Figure 3. CBD sensitizes Gram-negative bacteria E. coli VCS257 to selected antibiotics. Combinatory treatment of CBD with a range of antibiotics (24 h treatment) showed enhanced CBD-mediated antibacterial effects on E. coli VCS257, as assessed by increased radius of zone around the diffusion disks. CBD was most effective in combination with rifampicin (p = 0.0007), vancomycin (p ≤ 0.0001) and erythromycin (p = 0.006). CBD in isolation also had bactericidal effects on E. coli, while combinatory treatment with the antibiotics was most effective. Exact p-values are shown.
When added to S. aureus subsp. aureus Rosenbach, 5 μM CBD increased the antibiotic activity of kanamycin (30 %; p = 0.0028), as assessed by increased radius of zone around the diffusion disk (Figure 4). CBD did not enhance anti-bacterial activity for the other antibiotics tested and reduced antibacterial effects of both erythromycin and rifampicin (p = 0.0325 and p = 0.0001, respectively). Importantly, there was no halo observed around the diffusion disk containing CBD alone in the S. aureus plates, indicating no bactericidal effects of CBD on this strain of S. aureus.
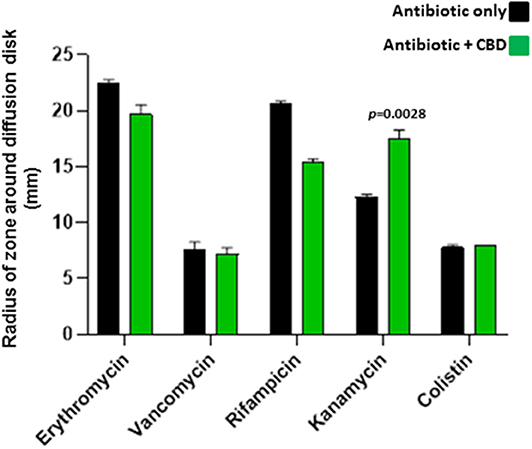
Figure 4. CBD sensitizes Gram-positive bacteria S. aureus subsp. aureus Rosenbach to kanamycin. Combinatory treatment of CBD with a range of antibiotics showed enhanced antibacterial effects of kanamycin only on S. aureus, as assessed by an increased radius of zone around the diffusion disk (p = 0.0028). CBD did not enhance bactericidal activity for the other antibiotics tested and reduced bactericidal effects of both erythromycin (p = 0.0325) and rifampicin (p = 0.0001). CBD application in isolation did not form a halo around the diffusion disk in the S. aureus plates, opposed as to what was observed in E. coli, and CBD treatment in isolation is therefore not included in the histogram. Exact p-values are shown.
Protein composition of MVs was assessed in MVs isolated from E. coli VCS257 after 1 h treatment with 1 μM and 5 μM CBD, respectively, compared to non-treated E. coli MVs, using SDS-PAGE silver stained gels and LC-MS/MS analysis. Silver stained gels revealed some band differences between the three conditions (Figure 5A). Proteins were further analyzed by LC-MS/MS and peak list files submitted to Mascot (in-house, Cambridge Center for Proteomics, Uniprot_Escherichia_coli_20180613). Hits are listed in Tables 1–3. Compared to untreated MVs, five protein hits were absent in MVs released from the 1 μM CBD treated E. coli and four protein hits were absent in MVs released from the 5 μM CBD treated E. coli, respectively (Table 1 and Figure 5B). When comparing the two CBD treatments, 26 protein hits were specific to the E. coli MVs following 1 μM CBD treatment (Table 2 and Figure 5B) while 68 protein hits were unique to the MVs released from E. coli treated with 5 μM CBD (Table 3 and Figure 5B).
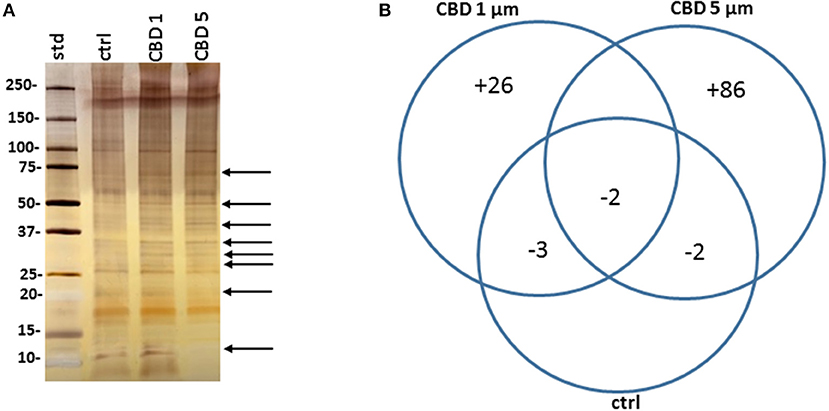
Figure 5. CBD affects protein composition of E. coli VCS257 MVs. (A) A SDS-PAGE silver stained gel reveals banding differences between the CBD treated and non-treated E. coli derived MVs (see arrows highlighting some present and absent bands). (B). Venn diagram showing protein changes in MVs released from CBD treated compared to untreated control E. coli VCS257. Plus (“+”) indicates hits unique to MVs following CBD 1 or 5 μM treatment, respectively; minus (“–“) indicates number of proteins absent in the respective CBD treated MVs, compared to control untreated MVs. For specific protein hits see Tables 1–3.
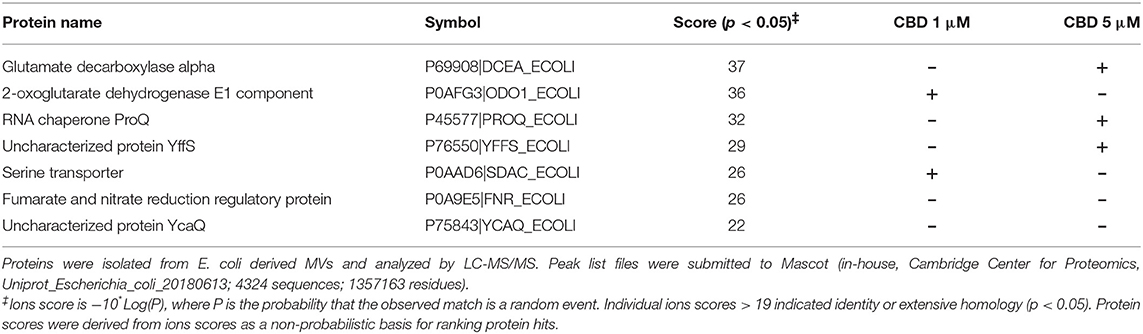
Table 1. Proteins identified as present in E. coli VCS257 control untreated MVs only and absent in MVs from CBD treated E. coli.
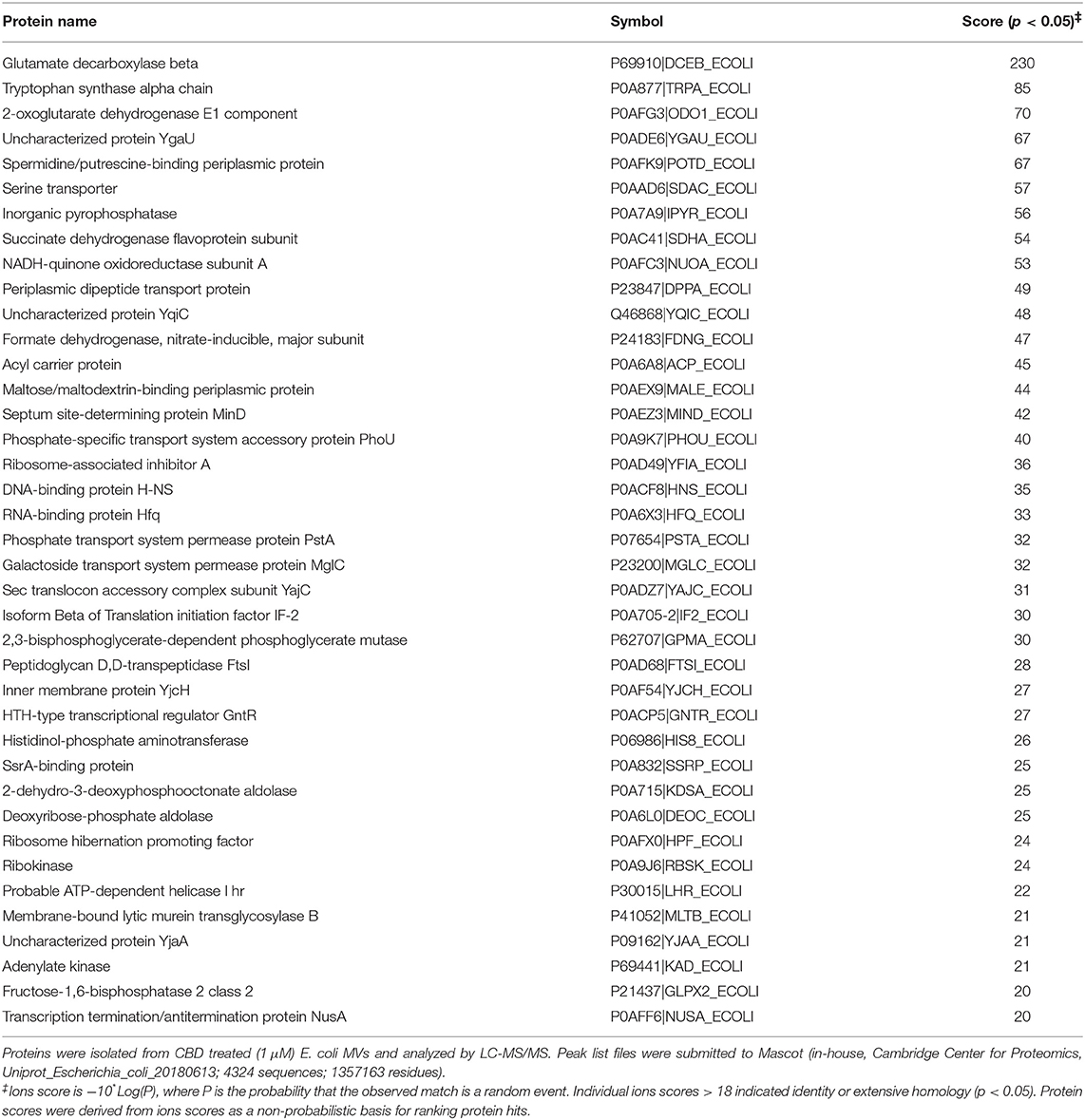
Table 2. Proteins identified as present only in MVs released from E. coli VCS257 following 1 h treatment with 1 μM CBD.
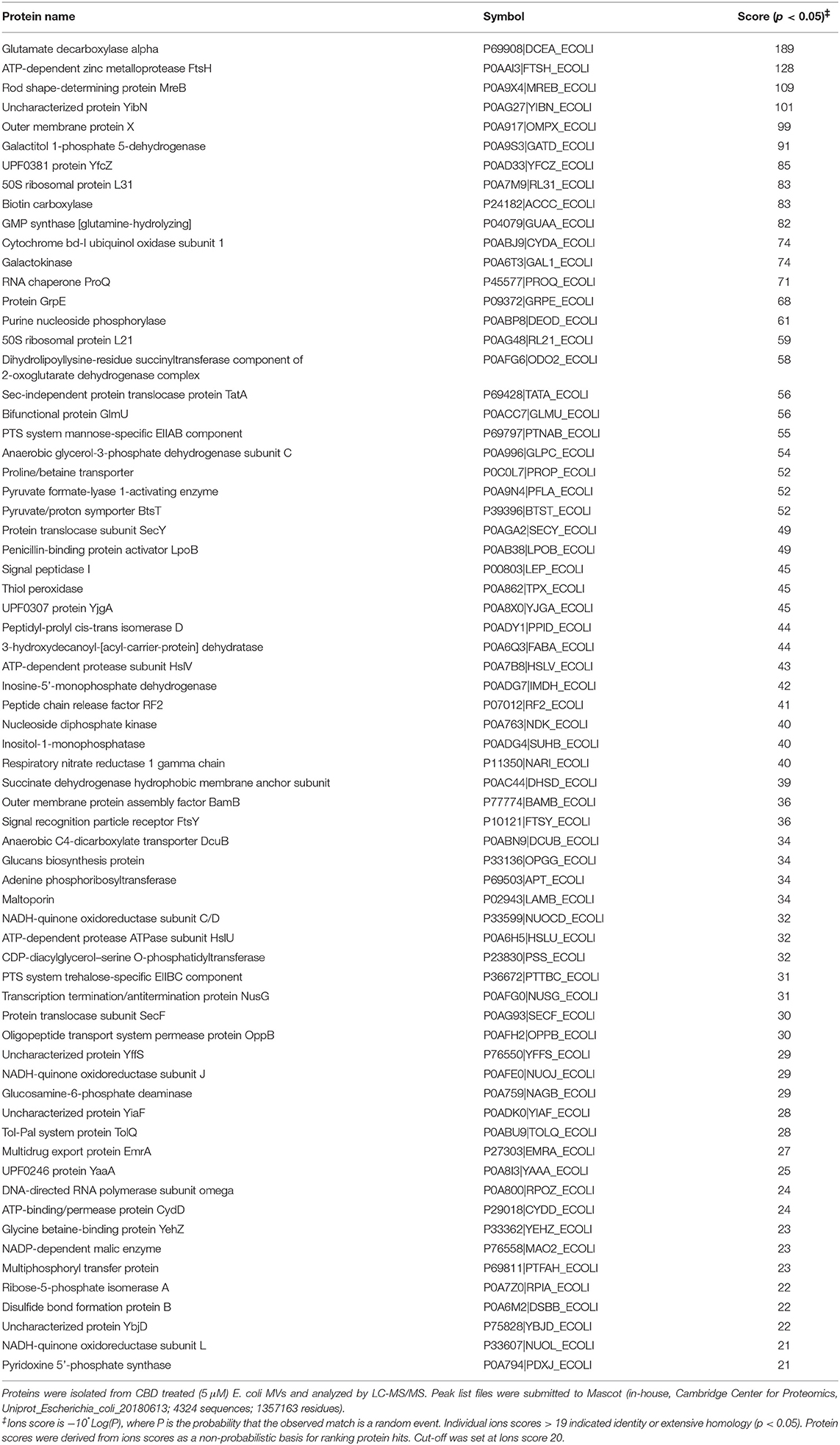
Table 3. Proteins identified as present only in E. coli VCS257 derived MVs following 1 h treatment with 5 μM CBD.
To our knowledge this is the first study to evaluate the putative effects of CBD on the release of membrane vesicles (MVs) from bacteria and effects of CBD on MV profile, including protein composition. In eukaryotic cells, CBD was recently identified as an effective inhibitor of extracellular vesicle (EV) release both in human cancer cells (Kosgodage et al., 2018, 2019) as well as in the intestinal parasite Giardia intestinalis (Gavinho et al., 2019). Therefore, our present findings may indicate phylogenetically conserved pathways of membrane vesicle release from bacteria to mammals that can be modulated via CBD. Moreover, CBD could enhance the anti-bacterial effect of certain antibiotics in some bacterial types, but also inhibit it in others. This indicates that inhibition of MV release and anti-bacterial action are likely linked, as previously suggested (Tashiro et al., 2010). Indeed, a recent study using indole derivatives has revealed a role for MVs in antibiotic resistance/persistence, in particular in Gram-negative bacteria tested (Agarwal et al., 2019).
Here we report that CBD significantly reduced MV release in E. coli VCS257, a Gram-negative bacterium, but had negligible effects on membrane vesicle release in S. aureus subsp. aureus Rosenbach, a Gram-positive bacterium, as assessed here by in vitro analysis. In addition, we also found that lower doses of CBD had a stronger MV inhibitory effect in E. coli VCS257 than a higher 5 μM dose (p = 0.0063), and such an effect has also previously been observed for EVs in certain cancer cell types (Kosgodage et al., 2018). Biphasic effects of CBD are indeed recognized (Bergamaschi et al., 2011) and may be reminiscent of “hormesis,” an effect we have suggested could explain its more general medical benefits as well as effects on mitochondrial dynamics (Nunn et al., 2013). Interestingly, at the lower 1 μM concentration, CBD significantly increased the release of a 500 nm peak of MVs, as observed by NTA analysis, while this peak was negligible both in the control treated bacteria and those treated with 5 μM CBD. Such an effect of CBD on MV profile, and protein MV profile as observed by proteomic analysis here, may be relevant in the light of recent recognition of the importance of MV size for cellular entry and uptake (Turner et al., 2018) and in line with an increased interest in the research community for the identification and characterization of MV sub-populations (Pérez-Cruz et al., 2016; Turner et al., 2018; Cooke et al., 2019; Toyofuku et al., 2019; Zavan et al., 2019). The approximately 6.5-fold and 2.5-fold decreases in MV release observed after CBD (1 and 5 μm, respectively) treatment from E. coli, compared to non-treated controls, also correlated with a trend in shift toward proportionally larger vesicles released according to NTA analysis and change in protein profile. The exact mechanism for packaging proteins and other reagents in MVs is not fully understood and given the plethora of targets for CBD (Ibeas Bih et al., 2015; Hernández-Cervantes et al., 2017; Pisanti et al., 2017), the exact mechanism of this cannabinoid on MV formation remains subject to further extensive studies. In the current study we have indeed identified a range proteins, including proteins involved in metabolism and antibiotic metabolic processing, which differ in MVs released from E. coli VCS257 treated with CBD, compared to MVs released from non-treated E. coli. Previous studies have discussed the use of MVs for example as drug delivery vehicles (Ellis and Kuehn, 2010; Gujrati et al., 2014; Gerritzen et al., 2017; Jain and Pillai, 2017; Jan, 2017; Wang et al., 2018), while MVs have also been tested as delivery vehicles for targeted gene silencing using siRNA-packaged MVs (Alves et al., 2016). Whether CBD may be utilized for combinatory application with such approaches may also be of putative interest, in addition to its observed effects in this study, in effectively reducing MV release.
In relation to antibiotic activity, cannabinoids including CBD, have been widely studied for their anti-bacterial activity (Wasim et al., 1995; Bass et al., 1996; Appendino et al., 2008; Hernández-Cervantes et al., 2017). For example, C. sativa extracts have previously been shown to have microbicidal activity on various Gram-positive bacteria, including several strains of S. aureus, as well as some Gram-negative bacteria (Wasim et al., 1995; Elphick, 2007; Nissen et al., 2010), with the minimum inhibitory concentrations (MIC) for the main phytocannabinoids, such as CBD, being in the 0.5–5 μM range, which is similar to many modern antibiotics (Van Klingeren and Ten Ham, 1976; Appendino et al., 2008). How precisely CBD may be working as an anti-bacterial agent is still not entirely clear (Appendino et al., 2008), particularly in the light of a plethora of targets for CBD (Ibeas Bih et al., 2015; Hernández-Cervantes et al., 2017), while structure-activity studies indicate that the ability of plant-derived phenolic compounds to interact with membranes and the existence of electrophilic functional groups are important (Miklasinska-Majdanik et al., 2018). Hitherto though, no association has been made into a putative regulatory effect of cannabinoids on bacterial membrane vesicle release. Furthermore, as the current study has revealed changes in proteomic profile of MVs released from E. coli VCS257 following CBD treatment, such findings may inform anti-bacterial effects of CBD. Using LC-MS/MS analysis to assess changes in protein profile of MVs from CBD treated and untreated E. coli, respectively, five proteins were found to be absent in the 1 μM CBD treated MVs and 4 proteins were absent in the 5 μM CBD treated MVs, compared to control untreated E. coli MVs. Out of these, 2 proteins overlapped between the two CBD treatments. In addition, comparing 1 and 5 μM CBD treated E. coli MVs, 26 protein hits were unique to MVs released following the 1 μM CBD treatment and 68 protein hits to MVs released following the 5 μM CBD treatment. Using STRING analysis, PPI enrichment p-value was found to be p = 0.0204 for proteins identified as unique to MVs from the 1 μM CBD treatment and p = 1.56 × 10−6 for proteins identified as unique to MVs from the 5 μM CBD. This indicates that for both treatments these proteins have significantly more interactions among themselves, than what would be expected for a random set of proteins of similar size, drawn from the genome. Such enrichment indicates that the proteins are at least partially biologically connected, as a group. Protein networks are represented showing biological GO pathways and KEGG pathways, respectively, in Supplementary Figures 3, 4 for proteins specific to EVs from E. coli after 1 and 5 μM CBD treatment, respectively. Proteins identified are related to metabolic processes, cellular respiration and antibiotic functions (Supplementary Figures 3A,B, 4A,B).
When assessing the effectivity of CBD to enhance susceptibility of Gram-positive and Gram-negative bacterial species to a range of antibiotics, CBD-mediated MV inhibition rendered E. coli VCS257 significantly more sensitive to erythromycin, vancomycin and rifampicin and somewhat to kanamycin, but did not augment the bactericidal effects observed for colistin. This was somewhat unexpected, given a previous study showing that MVs isolated from the E. coli strain MG1655 could protect bacteria against membrane-active antibiotics such as colistin (Kulkarni et al., 2015). Our finding, that CBD did not sensitize E. coli further to colistin, when applied in combination with this antibiotic, may arise from the fact that a different strain of E. coli (VCS257) was used in the current study, compared to in the study by Kulkarni et al. (2015). It has also been previously shown that the presence of calcium decreases the bactericidal effect of colistin on Paenibacillus polymyxa, suggesting a role for Ca2+ in generating a protective barrier against colistin (Yu et al., 2015). As CBD is known to modulate calcium (Rimmerman et al., 2013) it can be postulated that this may interfere with the mode of action of colistin. Our findings also indicate that combinatory application of CBD is not effective for all antibiotics, which may possibly be explained by their different modes of action. Importantly, zones of inhibition were observed in the plates which were only treated with the CBD discs in the presence of E. coli, and this clearly revealed the antibacterial property of CBD.
Interestingly, CBD did increase antibacterial effects of vancomycin on E. coli, in spite of vancomycin's limited effectiveness on Gram-negative species, also seen here by the fact that vancomycin alone did not result in a halo around the diffusion disk for E. coli. Therefore, CBD seems to overcome previously established resistance of E. coli to vancomycin, which has reported to partly be due to its inability to significantly penetrate the outer membrane (Zhou et al., 2015). It may also be important to note that erythromycin, rifampicin and kanamycin inhibit protein synthesis, whereas vancomycin is a glycopeptide that inhibits cell biosynthesis in Gram-positive bacteria, while colistin binds to the outer membrane of Gram-negative bacteria, disrupting it. Thus, these antibiotics display very different modes of action.
In the Gram-positive bacterium S. aureus subsp. aureus Rosenbach, CBD increased bactericidal activity of kanamycin only. The reduced ability of CBD to sensitize this Gram-positive bacterium to antibiotics, compared to the significantly higher effects in the Gram-negative bacterium, tallied in with CBD's ability to regulate MV-release, indicating a relevant contribution of MVs to antibiotic resistance. Roles for MVs in protecting biofilms via adsorption of antimicrobial agents have indeed been previously recognized (Schooling and Beveridge, 2006; Manning and Kuehn, 2011; Toyofuku et al., 2019). This also indicates that MV-inhibitors that target membrane vesicles from specific bacteria species, such as CBD here, could be applied in combination with selected antibiotics for tailored antibiotic treatment to tackle antibiotic resistance.
CBD effectively inhibited MV release from the Gram-negative bacterium E. coli VCS257, exhibiting a stronger MV-inhibiting effect at lower dose. In addition, CBD modulated MV protein profiles of E. coli following 1 h treatment. CBD did not have significant effects on MV release in the Gram-positive bacterium S. aureus subsp. aureus Rosenbach. When applied in combination with a range of antibiotics, CBD increased anti-bacterial effects of selected antibiotics, depending on bacteria type. CBD, in combination with specific antibiotics, may thus possibly be used as an adjuvant to selectively target bacteria to sensitize them to antibiotic treatment and reduce antibiotic resistance.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
UK, PM, BA, IK, PW, and SL performed the experiments. UK, JB, AN, JI, and SL analyzed the data. PM, GM, GG, IK, SL, and JI provided resources. UK, SL, and JI designed the study. SL, UK, and AN wrote the manuscript. All authors critically reviewed the manuscript.
GG is founder and chairman of GW Pharmaceuticals. AN is a scientific advisor to GW Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported in parts by a University of Westminster Start-up Grant to SL and an unrestricted grant from GW Pharmaceuticals. Thanks are due to the Guy Foundation for funding the purchase of equipment utilized in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00324/full#supplementary-material
Supplementary Figure 1. Effects of CBD on bacterial growth in (A) E. coli VCS257 and (B) S. aureus subsp. aureus Rosenbach, after 24 h incubation, as assessed by disk diffusion test. Exact p-values are shown.
Supplementary Figure 2. TEM of MV released from E. coli VCS257 following 1 or 5 μM CBD treated for 1 h, compared to MVs isolated from control, untreated E. coli. (A) A composite image showing MVs released from control, untreated E. coli. (B) A composite image showing MVs released from E. coli treated with 1 μM CBD for 1 h. (C) A composite image showing MVs released from E. coli treated with 5 μM CBD for 1 h. Scale bars indicate 100 nm, respectively, and are included in the individual figures.
Supplementary Figure 3. Protein-protein interaction networks of protein hits identified in MVs from 1 μM CBD treated E. coli VCS257. Reconstruction of protein-protein interactions based on known and predicted interactions using STRING analysis. Colored nodes represent query proteins and first shell of interactors; white nodes are second shell of interactors. (A) Biological GO processes are highlighted as follows: red, citrate metabolic process; green, antibiotic metabolic process; yellow, regulation of cellular amide metabolic process; purple, carboxylic acid metabolic process; dark green, regulation of phosphate metabolic process; light blue, cellular respiration; orange, small molecule metabolic process; dark red, negative regulation of translational elongation; dark blue, generation of precursor metabolites and energy. (B) KEGG pathways are highlighted as follows: dark green, oxidative phosphorylation; dark red, citrate cycle (TCA cycle); red, biosynthesis of antibiotics; purple, butanoate metabolism; dark blue, biosynthesis of secondary metabolites; light blue, carbon metabolism; orange, phenylalanine, tyrosine and tryptophan biosynthesis; light green, Microbial metabolism in diverse environments; yellow, Metabolic pathways; violet, glycine, serine, and threonine metabolism. Colored lines indicate whether protein interactions are identified via known interactions (curated databases, experimentally determined), predicted interactions (gene neighborhood, gene fusion, gene co-occurrence) or via text mining, co-expression or protein homology (see color key for connective lines).
Supplementary Figure 4. Protein-protein interaction networks of protein hits identified in MVs from 5 μM CBD treated E. coli VCS257. Reconstruction of protein-protein interactions based on known and predicted interactions using STRING analysis. Colored nodes represent query proteins and first shell of interactors; white nodes are second shell of interactors. (A) Biological GO processes are highlighted as follows: red, cellular respiration; green, purine-containing compound metabolic process; yellow, electron transport chain; purple, ribose phosphate metabolic process; dark green, purine ribonucleotide metabolic process; light blue, generation of precursor metabolites and energy; orange, nucleobase-containing small molecule metabolic process; dark red, purine ribonucleoside metabolic process; dark blue, organophosphate metabolic process. (B) KEGG pathways are highlighted as follows: red, bacterial secretion system; light green, metabolic pathways; yellow, oxidative phosphorylation; purple, butanoate metabolism; dark green, quorum sensing; light blue, amino sugar and nucelotide sugar metabolism; dark blue, protein export; violet, purine metabolism. Colored lines indicate whether protein interactions are identified via known interactions (curated databases, experimentally determined), predicted interactions (gene neighborhood, gene fusion, gene co-occurrence) or via text mining, co-expression or protein homology (see color key for connective lines).
Agarwal, B., Karthikeyan, R., Gayathri, P., RameshBabu, B., Ahmed, G., and Jagannadham, M. V. (2019). Studies on the mechanism of multidrug resistance of Acinetobacter baumannii by proteomic analysis of the outer membrane vesicles of the bacterium. Proteins Proteom. 10, 1–15. doi: 10.1007/s42485-018-0001-4
Alves, N. J., Turner, K. B., Mendints, I. L., and Walper, S. A. (2016). Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci. Rep. 6:24866. doi: 10.1038/srep24866
Appendino, G., Gibbons, S., Giana, A., Pagani, A., Grassi, G., Stavri, M., Smith, et al. (2008). Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J. Nat. Prod. 71, 1427–1430. doi: 10.1021/np8002673
Bass, R., Engelhard, D., Trembovler, V., and Shohami, E. (1996). A novel nonpsychotropic cannabinoid, HU-211, in the treatment of experimental pneumococcal meningitis. J. Infect Dis. 173, 735–738. doi: 10.1093/infdis/173.3.735
Bergamaschi, M. M., Queiroz, R. H., Zuardi, A. W., and Crippa, J. A. (2011). Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 6, 237–249. doi: 10.2174/157488611798280924
Bitto, N. J., Baker, P. J., Dowling, J. K., Wray-McCann, G., De Paoli, A., Tran, L. S., et al. (2018). Membrane vesicles from Pseudomonas aeruginosa activate the noncanonical inflammasome through caspase-5 in human monocytes. Immunol. Cell Biol. 96, 1120–1130. doi: 10.1111/imcb.12190
Bitto, N. J., Chapman, R., Pidot, S., Costin, A., Lo, C., Choi, J., et al. (2017). Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep. 7:7072. doi: 10.1038/s41598-017-07288-4
Cecil, J. D., Sirisaengtaksin, N., O'Brien-Simpson, N. M., and Krachler, A. M. (2019). Outer membrane vesicle-host cell Interactions. Microbiol. Spectr. 7:PSIB–0001–2018. doi: 10.1128/microbiolspec.PSIB-0001-2018
Codemo, M., Muschiol, S., Iovino, F., Nannapaneni, P., Plant, L., Wai, S. N., et al. (2018). Immunomodulatory effects of pneumococcal extracellular vesicles on cellular and humoral host defenses. MBio. 9:e00559–18. doi: 10.1128/mBio.00559-18
Cooke, A. C., Nello, A. V., Ernst, R. K., and Schertzer, J. W. (2019). Analysis of Pseudomonas aeruginosa biofilm membrane vesicles supports multiple mechanisms of biogenesis. PLoS ONE. 14:e0212275. doi: 10.1371/journal.pone.0212275
Deo, P., Chow, S. H., Hay, I. D., Kleifeld, O., Costin, A., Elgass, K. D., et al. (2018). Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog. 14:e1006945. doi: 10.1371/journal.ppat.1006945
Dorward, D. W., and Garon, C. F. (1990). DNA is packaged within membrane- derived vesicles of gram- negative but not gram- positive bacteria. Appl. Environ. Microbiol. 56, 1960–1962.
Ellis, T. N., and Kuehn, M. J. (2010). Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94. doi: 10.1128/MMBR.00031-09
Elphick, M. R. (2007). BfCBR: a cannabinoid receptor ortholog in the cephalochordate Branchiostoma floridae (amphioxus). Gene 399, 65–71. doi: 10.1016/j.gene.2007.04.025
Fleetwood, A. J., Lee, M. K. S., Singleton, W., Achuthan, A., Lee, M. C., O'Brien-Simpson, N. M., et al. (2017). Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front. Cell Infect Microbiol. 7:351. doi: 10.3389/fcimb.2017.00351
Fulsundar, S., Harms, K., Flaten, G. E., Johnsen, P. J., Chopade, B. A., and Nielsen, K. M. (2014). Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl. Environ. Microbiol. 80, 3469–3483. doi: 10.1128/AEM.04248-13
Gavinho, B., Rossi, I. V., Evans-Osses, I., Lange, S., and Ramirez, M. I. (2019). Peptidylarginine deiminase inhibition abolishes the production of large extracellular vesicles from Giardia intestinalis, affecting host-pathogen interactions by hindering adhesion to host cells. Biorxiv 586438. doi: 10.1101/586438
Gerritzen, M. J. H., Martens, D. E., Wijffels, R. H., van der Pol, L., and Stork, M. (2017). Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 5, 565–574. doi: 10.1016/j.biotechadv.2017.05.003
Gnopo, Y. M. D., Watkins, H. C., Stevenson, T. C., DeLisa, M. P., and Putnam, D. (2017). Designer outer membrane vesicles as immunomodulatory systems - Reprogramming bacteria for vaccine delivery. Adv. Drug Deliv. Rev. 114, 132–142. doi: 10.1016/j.addr.2017.05.003
Goldstein, E. J. C., Citron, D. M., Tyrrell, K. L., and Leoncio, E. (2018). In vitro stability of three oral vancomycin preparations stored at 2- 5 2- 5 °C and ambient room temperature for up to 60 days against 100 Clostridioides (Clostridium) difficile and 51Staphylococcus aureus strains. Anaerobe. 52, 83–85. doi: 10.1016/j.anaerobe.2018.06.003
Gujrati, V., Kim, S., Kim, S. H., Min, J. J., Choy, H. E., Kim, S. C., et al. (2014). Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 8, 1525–37. doi: 10.1021/nn405724x
Gurung, M., Moon, D. C., Choi, C. W., Lee, J. H., Bae, Y. C., Kim, J., et al. (2011). Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE. 6:e27958. doi: 10.1371/journal.pone.0027958
Hernández-Cervantes, R., Méndez-Díaz, M., Prospéro-García, Ó., and Morales-Montor, J. (2017). Immunoregulatory role of cannabinoids during infectious disease. Neuroimmunomodulation. 24, 183–199. doi: 10.1159/000481824
Ibeas Bih, C., Chen, T., Nunn, A. V., Bazelot, M., Dallas, M., and Whalley, B. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12, 699–730. doi: 10.1007/s13311-015-0377-3
Iqbal, J., Siddiqui, R., Kazmi, S. U., and Khan, N. A. (2013). A simple assay to screen antimicrobial compounds potentiating the activity of current antibiotics. Bio. Med. Res. Int. 2013:927323. doi: 10.1155/2013/927323
Jain, S., and Pillai, J. (2017). Bacterial membrane vesicles as novel nanosystems for drug delivery. Int. J. Nanomed. 12, 6329–6341. doi: 10.2147/IJN.S137368
Jan, A. T. (2017). Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front. Microbiol. 8:1053. doi: 10.3389/fmicb.2017.01053
Jorfi, S., Ansa-Addo, E. A., Kholia, S., Stratton, D., Valley, S., Lange, S., et al. (2015). Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci. Rep. 5:13006. doi: 10.1038/srep13006
Klimentova, J., and Stulik, J. (2015). Methods of isolation and purification of outer membrane vesicles from gram negative bacteria. Microbiol. Res. 170, 1–9. doi: 10.1016/j.micres.2014.09.006
Koch, R., Aung, T., Vogel, D., Chapuy, B., Wenzel, D., Becker, S., et al. (2016). Nuclear trapping through inhibition of exosomal export by indomethacin increases cytostatic efficacy of doxorubicin and pixantrone. Clin. Cancer Res. 22, 395–404. doi: 10.1158/1078-0432.CCR-15-0577
Koeppen, K., Hampton, T. H., Jarek, M., Scharfe, M., Gerber, S. A., Mielcarz, D. W., et al. (2016). A novel mechanism of host-pathogen interaction through srna in bacterial outer membrane vesicles. PLoS Pathog. 12:e1005672. doi: 10.1371/journal.ppat.1005672
Kosgodage, U. S., Mould, R., Henley, A. B., Nunn, A. V., Guy, G. W., Thomas, E. L., et al. (2018). Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 9:889. doi: 10.3389/fphar.2018.00889
Kosgodage, U. S., Trindade, R. P., Thompson, P. R., Inal, J. M., and Lange, S. (2017). Chloramidine/bisindolylmaleimide-i-mediated inhibition of exosome and microvesicle release and enhanced efficacy of cancer chemotherapy. Int. J. Mol. Sci. 18:E1007. doi: 10.3390/ijms18051007
Kosgodage, U. S., Uysal-Onganer, P., MacLatchy, A., Mould, R., Nunn, A. V., Guy, G. W., et al. (2019). Cannabidiol affects extracellular vesicle release, miR21 and miR126, and reduces prohibitin protein in glioblastoma multiforme cells. Transl. Oncol. 12, 513–522. doi: 10.1016/j.tranon.2018.12.004
Kshetry, A. O., Pant, N. D., Bhandari, R., Khatri, S., Shrestha, K. L., Upadhaya, S. K., et al. (2016). Minimum inhibitory concentration of vancomycin to methicillin resistant Staphylococcus aureus isolated from different clinical samples at a tertiary care hospital in Nepal. Antimicrob. Resist. Infect. Control. 5:27. doi: 10.1186/s13756-016-0126-3
Kulkarni, H. M., Nagaraj, R., and Jagannadham, M. V. (2015). Protective role of E. coli outer membrane vesicles against antibiotics. Microbiol. Res. 181, 1–7. doi: 10.1016/j.micres.2015.07.008
Li, Z., Clarke, A. J., and Beveridge, T. J. (1998). Gram- negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180, 5478–5483.
Macdonald, I. A., and Kuehn, M. J. (2013). Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bacteriol. 195, 2971–2981. doi: 10.1128/JB.02267-12
Maclayton, D. O., Suda, K. J., Coval, K. A., York, C. B., and Garey, K. W. (2006). Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 μg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin. Therapeut. 28, 1208–1216. doi: 10.1016/j.clinthera.2006.08.003
Manning, A. J., and Kuehn, M. J. (2011). Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 11:258. doi: 10.1186/1471-2180-11-258
Martin-Moreno, A. M., Reigada, D., Ramírez, B. G., Mechoulam, R., Innamorato, N., Cuadrado, A., et al. (2011). Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer's disease. Mol. Pharmacol. 791, 964–973. doi: 10.1124/mol.111.071290
McBroom, A. J., and Kuehn, M. J. (2007). Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63, 545–558. doi: 10.1111/j.1365-2958.2006.05522.x
McCaig, W. D., Koller, A., and Thanassi, D. G (2013). Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol. 195, 1120–1132. doi: 10.1128/JB.02007-12
Miklasinska-Majdanik, M., Kepa, M., Wojtyczka, R. D., Idzik, D., and Wasik, T. J. (2018). Phenolic compounds diminish antibiotic resistance of Staphylococcus Aureus clinical strains. Int. J. Environ. Res. Publ. Health. 15:E2321. doi: 10.3390/ijerph15102321
Moskowitz, S. M., Garber, E., Chen, Y., Clock, S. A., Tabibi, S., Miller, A. K., et al. (2010). Colistin susceptibility testing: evaluation of reliability for cystic fibrosis isolates of Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 65, 1416–1423. doi: 10.1093/jac/dkq131
Muralidharan-Chari, V., Kohan, H. G., Asimakopoulos, A. G., Sudha, T., Sell, S., Kannan, K., et al. (2016). Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget 7, 50365–50379. doi: 10.18632/oncotarget.10395
Nissen, L., Zatta, A., Stefanini, I., Grandi, S., Sgorbati, B., Biavati, B., et al. (2010). Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 81, 413–419. doi: 10.1016/j.fitote.2009.11.010
Nunn, A. V. W., Henley, A., Brody, L., and Bell, J. D. (2013). “Phytocannabinoids modulate mitochondrial dynamics in cell lines; stress adaptation,”in Abstract, 6th European Workshop on Cannabinoid Research. Available online at: www.pA2online.org 11.
Pérez-Cruz, C., Cañas, M. A., Giménez, R., Badia, J., Mercade, E., Baldom,à, L., et al. (2016). Membrane vesicles released by a hypervesiculating escherichia coli nissle 1917 tolr mutant are highly heterogeneous and show reduced capacity for epithelial cell interaction and entry. PLoS ONE. 11:e0169186. doi: 10.1371/journal.pone.0169186
Pisanti, S., Malfitano, A. M., Ciaglia, E., Lamberti, A., Ranieri, R., Cuomo, G., et al. (2017). Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol. Ther. 175, 133–150. doi: 10.1016/j.pharmthera.2017.02.041
Rimmerman, N., Ben-Hail, D., Porat, Z., Juknat, A., Kozela, E., Daniels, M. P., et al. (2013). Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 4:e949. doi: 10.1038/cddis.2013.471
Roier, S., Zingl, F. G., Cakar, F., Durakovic, S., Kohl, P., Eichmann, T. O., et al. (2016). A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 7:10515. doi: 10.1038/ncomms10515
Rojas, L. J., Salim, M., Cober, E., Richter, S. S., Perez, F., Salata, R. A., et al. (2017). Colistin resistance in carbapenem-resistant klebsiella pneumoniae: laboratory detection and impact on mortality. Clin. Infect. Dis.. 64, 711–718. doi: 10.1093/cid/ciw805
Rüter, C., Lubos, M. L., Norkowski, S., and Schmidt, M. A. (2018). All in-Multiple parallel strategies for intracellular delivery by bacterial pathogens. Int. J. Med. Microbiol. 308, 872–881. doi: 10.1016/j.ijmm.2018.06.007
Schooling, S. R., and Beveridge, T. J. (2006). Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957. doi: 10.1128/JB.00257-06
Tan, K., Li, R., Huang, X., and Liu, Q. (2018). Outer membrane vesicles: current status and future direction of these novel vaccine adjuvants. Front. Microbiol. 9:783. doi: 10.3389/fmicb.2018.00783
Tashiro, Y., Ichikawa, S., Shimizu, M., Toyofuku, M., Takaya, N., Nakajima-Kambe, T., et al. (2010). Variation of physiochemical properties and cell association activity of membrane vesicles with growth phase in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 76, 3732–3739. doi: 10.1128/AEM.02794-09
Toyofuku, M., Nomura, N., and Eberl, L. (2019). Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24. doi: 10.1038/s41579-018-0112-2
Turner, L., Bitto, N. J., Steer, D. L., Lo, C., D'Costa, K., Ramm, G., et al. (2018). Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front. Immunol. 9:1466. doi: 10.3389/fimmu.2018.01466
Van Klingeren, B., and Ten Ham, M. (1976). Antibacterial activity of delta9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwen. 42, 9–12. doi: 10.1007/BF00399444
Wang, W., Chanda, W., and Zhong, M. (2015). The relationship between biofilm and outer membrane vesicles: a novel therapy overview. FEMS Microbiol. Lett. 362:fnv117. doi: 10.1093/femsle/fnv117
Wang, X., Thompson, C. D., Weidenmaier, C., and Lee, J. C. (2018). Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun. 9:1379. doi: 10.1038/s41467-018-03847-z
Wasim, K., Haq, I., and Ashraf, M. (1995). Antimicrobial studies of the leaf of Cannabis sativa L. Pak. J. Pharm. Sci. 8, 29–38.
Yu, Z., Cai, Y., Qin, W., Lin, J., and Qiu, J. (2015). Polymyxin E induces rapid Paenibacillus polymyxa death by damaging cell membrane while Ca2+ can protect cells from damage. PLoS ONE 10:e0135198. doi: 10.1371/journal.pone.0135198
Zavan, L., Bitto, N. J., Johnston, E. L., Greening, D. W., and Kaparakis-Liaskos, M. (2019). Helicobacter pylori growth stage determines the size, protein composition, and preferential cargo packaging of outer membrane vesicles. Proteomics 19:e1800209. doi: 10.1002/pmic.201970004
Zhou, A., Kang, T. M., Yuan, J., Beppler, C., Nguyen, C., Mao, Z., et al. (2015). Synergistic interactions of vancomycin with different antibiotics against Escherichia coli: trimethoprim and nitrofurantoin display strong synergies with vancomycin against wild-type E. coli. Antimicrob. Agents Chemother. 59, 276–281. doi: 10.1128/AAC.03502-14
Keywords: bacterial membrane vesicles (MVs), cannabidiol (CBD), antibiotic resistance, gram-negative, gram-positive, E. coli VCS257, S. aureus subsp. aureus Rosenbach
Citation: Kosgodage US, Matewele P, Awamaria B, Kraev I, Warde P, Mastroianni G, Nunn AV, Guy GW, Bell JD, Inal JM and Lange S (2019) Cannabidiol Is a Novel Modulator of Bacterial Membrane Vesicles. Front. Cell. Infect. Microbiol. 9:324. doi: 10.3389/fcimb.2019.00324
Received: 04 April 2019; Accepted: 28 August 2019;
Published: 10 September 2019.
Edited by:
Jyl S. Matson, University of Toledo, United StatesReviewed by:
Medicharla Venkata Jagannadham, Centre for Cellular Molecular Biology (CCMB), IndiaCopyright © 2019 Kosgodage, Matewele, Awamaria, Kraev, Warde, Mastroianni, Nunn, Guy, Bell, Inal and Lange. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sigrun Lange, cy5sYW5nZUB3ZXN0bWluc3Rlci5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.